Clinical Application of Cell-Based Approaches in Maxillary Sinus Floor Augmentation: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Eligibility Criteria
- Studies involving sinus augmentation and stem cells.
- Cases with dental implantation.
- Studies focused on maxillary posterior area.
- Studies on sinus augmentation that do not involve the use of stem cells.
- Patients who have not undergone dental implant procedures.
- Patients with maxillary dentures.
- Studies that did not provide the necessary data as mean and standard deviation for conducting a meta-analysis.
2.2. Information Sources and Search Strategy
2.3. Study Selection and Data Extraction
2.4. Risk of Bias Assessment
2.5. Data Synthesis and Analysis
2.6. Assessment of Certainty of Evidence
3. Results
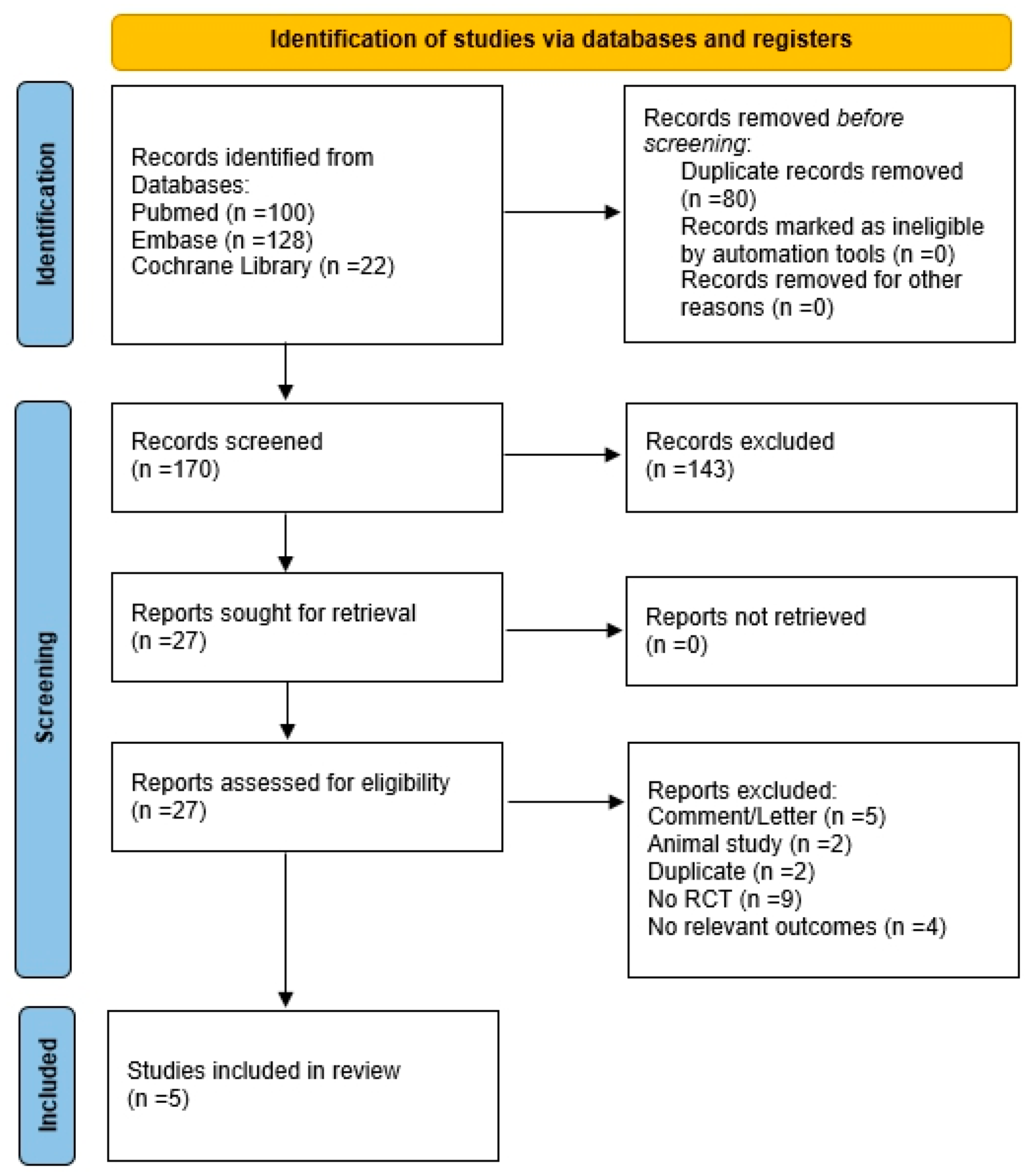
3.1. Study Selection and Data Extraction
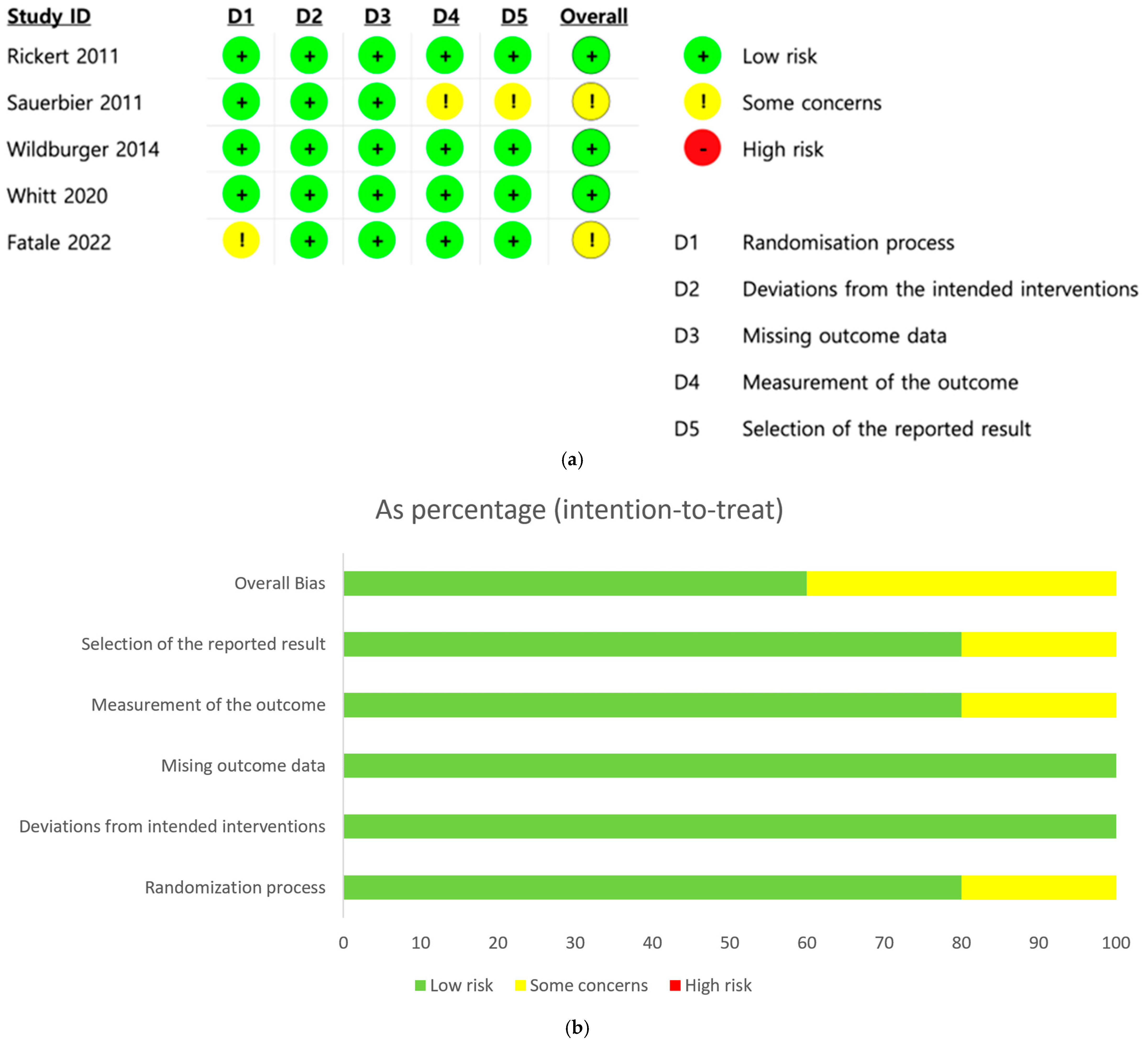
3.2. Risk of Bias Assessment
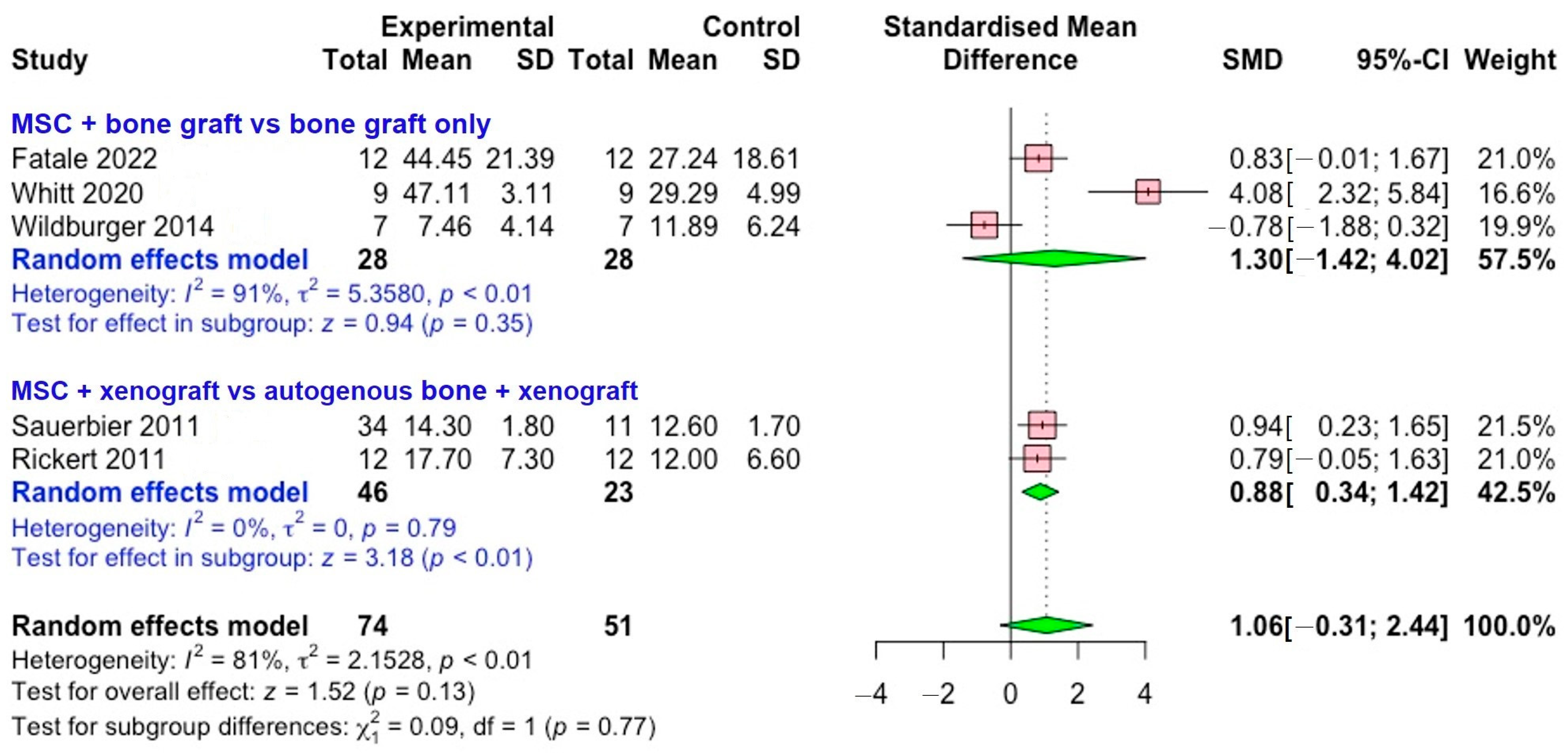
3.3. Meta-Analysis
3.3.1. New Bone Formation
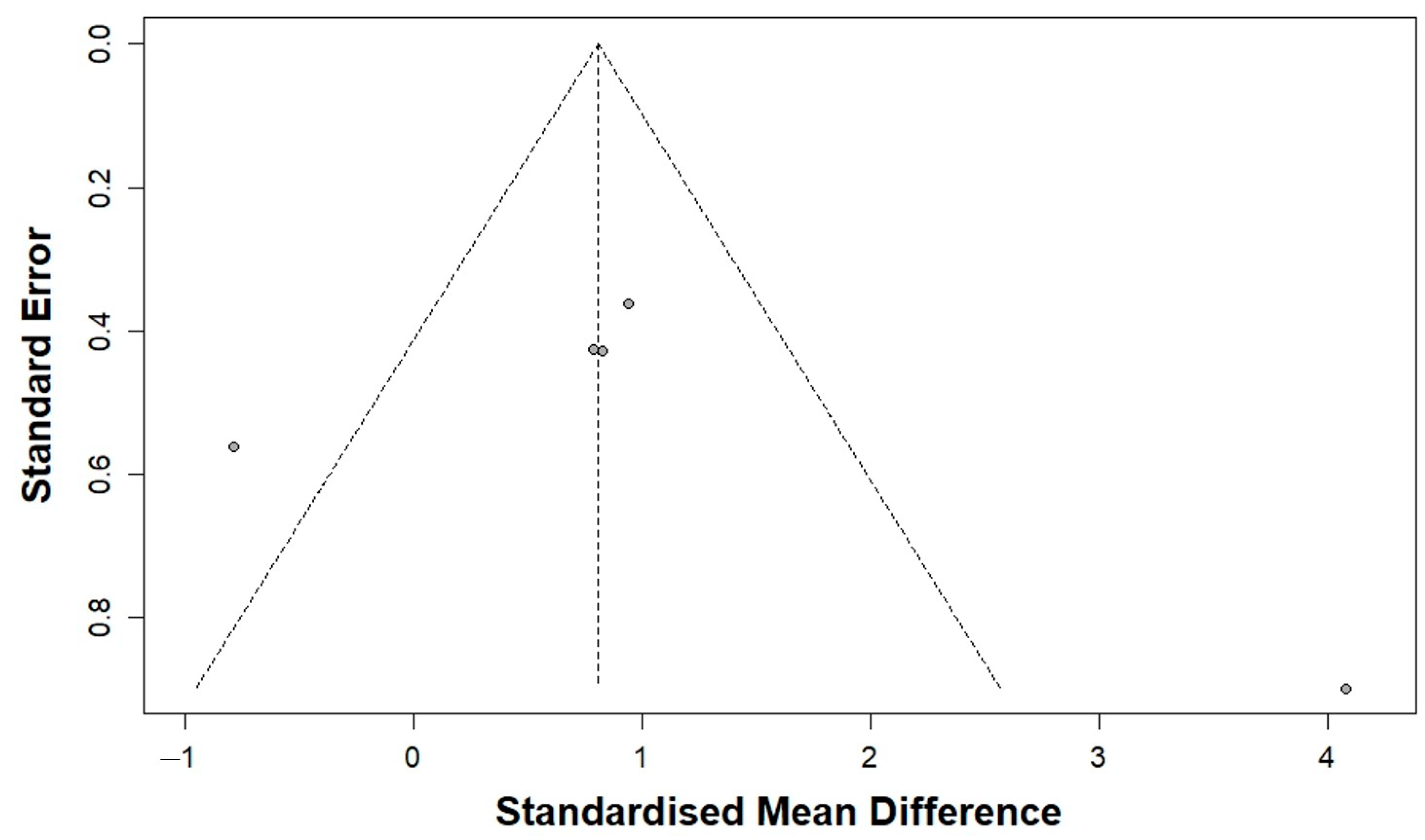
3.3.2. Publication Bias Analysis
3.4. Certainty of Evidence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SMD | standardized mean difference |
| CI | confidence interval |
References
- Yang, G.; Liu, X.; Huang, T.; Ding, R.; Wang, Y. Combined Application of Dentin Noncollagenous Proteins and Odontogenic Biphasic Calcium Phosphate in Rabbit Maxillary Sinus Lifting. Tissue Eng. Regen. Med. 2023, 20, 93–109. [Google Scholar] [CrossRef]
- Alshamrani, A.M.; Mubarki, M.; Alsager, A.S.; Alsharif, H.K.; AlHumaidan, S.A.; Al-Omar, A. Maxillary Sinus Lift Procedures: An Overview of Current Techniques, Presurgical Evaluation, and Complications. Cureus 2023, 15, e49553. [Google Scholar] [CrossRef]
- Fatale, V.; Pagnoni, S.; Pagnoni, A.E.; Passarelli, P.C.; Netti, A.; Lajolo, C.; Santacroce, L.; D’Addona, A. Histomorphometric Comparison of New Bone Formed After Maxillary Sinus Lift with Lateral and Crestal Approaches Using Periosteal Mesenchymal Stem Cells and Beta-Tricalcium Phosphate: A Controlled Clinical Trial. J. Craniofac. Surg. 2022, 33, 1607–1613. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.H.; Almansoori, A.A.; Kwon, O.J.; Seo, Y.K.; Kim, B.; Kim, Y.K.; Lee, J.H.; Pang, K. Sinus augmentation with poly(ε)caprolactone-β tricalcium phosphate scaffolds, mesenchymal stem cells and platelet rich plasma for one-stage dental implantation in minipigs. J. Periodontal Implant Sci. 2023, 53, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Starch-Jensen, T.; Aludden, H.; Dahlin, C.; Bruun, N.H.; Fink, T. Histomorphometric outcome following sinus floor augmentation with allogeneic adipose tissue-derived stem cells. A randomized controlled experimental study. J. Craniomaxillofac. Surg. 2025, 53, 104–113. [Google Scholar] [CrossRef]
- Niño-Sandoval, T.C.; Vasconcelos, B.C.; SL, D.M.; CA, A.L.; Pellizzer, E.P. Efficacy of stem cells in maxillary sinus floor augmentation: Systematic review and meta-analysis. Int. J. Oral Maxillofac. Surg. 2019, 48, 1355–1366. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef]
- Whitt, J.; Al-Sabbagh, M.; Dawson, D.; Shehata, E.; Housley-Smith, M.; Tezanos, A.; Kutkut, A. Efficacy of stem cell allograft in maxillary sinus bone regeneration: A randomized controlled clinical and blinded histomorphometric study. Int. J. Implant Dent. 2020, 6, 25. [Google Scholar] [CrossRef]
- Wildburger, A.; Payer, M.; Jakse, N.; Strunk, D.; Etchard-Liechtenstein, N.; Sauerbier, S. Impact of autogenous concentrated bone marrow aspirate on bone regeneration after sinus floor augmentation with a bovine bone substitute—A split-mouth pilot study. Clin. Oral Implant. Res. 2014, 25, 1175–1181. [Google Scholar] [CrossRef]
- Sauerbier, S.; Rickert, D.; Gutwald, R.; Nagursky, H.; Oshima, T.; Xavier, S.P.; Christmann, J.; Kurz, P.; Menne, D.; Vissink, A.; et al. Bone marrow concentrate and bovine bone mineral for sinus floor augmentation: A controlled, randomized, single-blinded clinical and histological trial—Per-protocol analysis. Tissue Eng. Part A 2011, 17, 2187–2197. [Google Scholar] [CrossRef]
- Rickert, D.; Sauerbier, S.; Nagursky, H.; Menne, D.; Vissink, A.; Raghoebar, G.M. Maxillary sinus floor elevation with bovine bone mineral combined with either autogenous bone or autogenous stem cells: A prospective randomized clinical trial. Clin. Oral Implant. Res. 2011, 22, 251–258. [Google Scholar] [CrossRef]
- Jeong, T.M.; Lee, J.K. The Efficacy of the Graft Materials after Sinus Elevation: Retrospective Comparative Study Using Panoramic Radiography. Maxillofac. Plast. Reconstr. Surg. 2014, 36, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.H. Autologous bone graft: Is it still the gold standard? Injury 2021, 52 (Suppl. 2), S18–S22. [Google Scholar] [CrossRef] [PubMed]
- Betz, R.R. Limitations of autograft and allograft: New synthetic solutions. Orthopedics 2002, 25, s561–s570. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; An, H.W.; Im, J.S.; Kim, W.J.; Lee, D.W.; Yun, J.H. Evaluation of the clinical and radiographic effectiveness of treating peri-implant bone defects with a new biphasic calcium phosphate bone graft: A prospective, multicenter randomized controlled trial. J. Periodontal Implant Sci. 2023, 53, 306–317. [Google Scholar] [CrossRef]
- Kudsi, R.; Dawson, D.R.; Gonzalez, O.A.; Kutkut, A.; Tucci, M.A.; Porras-Aguilar, R.; Momox, A.E.; Al-Sabbagh, M. Relationship between bone quality and shrinkage in maxillary sinus augmentation using synthetic alloplast versus xenograft. J. Periodontal Implant Sci. 2025, 55, 306–320. [Google Scholar] [CrossRef]
- Cheah, C.W.; Al-Namnam, N.M.; Lau, M.N.; Lim, G.S.; Raman, R.; Fairbairn, P.; Ngeow, W.C. Synthetic Material for Bone, Periodontal, and Dental Tissue Regeneration: Where Are We Now, and Where Are We Heading Next? Materials 2021, 14, 6123. [Google Scholar] [CrossRef]
- Rickert, D.; Vissink, A.; Slot, W.J.; Sauerbier, S.; Meijer, H.J.; Raghoebar, G.M. Maxillary sinus floor elevation surgery with BioOss® mixed with a bone marrow concentrate or autogenous bone: Test of principle on implant survival and clinical performance. Int. J. Oral Maxillofac. Surg. 2014, 43, 243–247. [Google Scholar] [CrossRef]
- Parnia, F.; Yazdani, J.; Maleki Dizaj, S. Applications of Mesenchymal Stem Cells in Sinus Lift Augmentation as a Dental Implant Technology. Stem Cells Int. 2018, 2018, 3080139. [Google Scholar] [CrossRef]
- Kim, Y.K.; Nakata, H.; Yamamoto, M.; Miyasaka, M.; Kasugai, S.; Kuroda, S. Osteogenic Potential of Mouse Periosteum-Derived Cells Sorted for CD90 In Vitro and In Vivo. Stem Cells Transl. Med. 2016, 5, 227–234. [Google Scholar] [CrossRef]
- Liao, H.T.; Chen, C.T. Osteogenic potential: Comparison between bone marrow and adipose-derived mesenchymal stem cells. World J. Stem Cells 2014, 6, 288–295. [Google Scholar] [CrossRef]
- Boopathy, K.; Palaniyandi, T.; Ravi, M.; Wahab, M.R.A.; Baskar, G.; Rab, S.O.; Saeed, M.; Balaramnavar, V.M. Exploring the potential of stem cell therapy: Applications, types, and future directions. Acta Histochem. 2025, 127, 152237. [Google Scholar] [CrossRef]
- Oliva, S.; Capogreco, M.; Murmura, G.; Lupi, E.; Mariachiara, D.C.; D’Amario, M. The socket shield technique and its complications, implant survival rate, and clinical outcomes: A systematic review. J. Periodontal Implant Sci. 2023, 53, 99–109. [Google Scholar] [CrossRef]
- Cruz, I.B.; Severo, A.L.; Azzolin, V.F.; Garcia, L.F.; Kuhn, A.; Lech, O. Regenerative potential of the cartilaginous tissue in mesenchymal stem cells: Update, limitations, and challenges. Rev. Bras. Ortop. 2017, 52, 2–10. [Google Scholar] [CrossRef][Green Version]
- Schroll, J.B.; Moustgaard, R.; Gøtzsche, P.C. Dealing with substantial heterogeneity in Cochrane reviews. Cross-sectional study. BMC Med. Res. Methodol. 2011, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Han, S.-H.; Song, H.-J.; Kim, N.J.; Park, W.-J.; Park, J.-B. Long-Term Outcomes of Collagen Matrix versus Subepithelial Connective Tissue in Root Coverage for Multiple Teeth: A Systematic Review and Meta-Analysis. Appl. Sci. 2024, 14, 8049. [Google Scholar] [CrossRef]
- Tzur, E.; Rozen, N.; David, D.B.; Barzilai, M.G.; Novak, A.; Kivity, V.; Bronshtein, T.; Meretzki, S. ADVANCING MESENCHYMAL CELL-BASED BONE TISSUE ENGINEERING: COMPREHENSIVE PHASE II CLINICAL TRIAL OUTCOMES OF BONOFILL™ IN MAXILLOFACIAL RECONSTRUCTION. Cytotherapy 2024, 26, S20–S21. [Google Scholar] [CrossRef]
- Tzur, E.; Bronshtein, T. COMPLICATED LARGE MAXILLOFACIAL BONE DEFICIENCIES SUCCESSFULLY RECONSTRUCTED USING BONOFILL: A NOVEL TISSUE-ENGINEERED BONE GRAFT BASED ON OSTEOPROGENITOR CELLS FROM THE PATIENT’S ADIPOSE TISSUE. Int. J. Oral Maxillofac. Surg. 2024, 52, 12–13. [Google Scholar] [CrossRef]
- Nulend, J.K.; Guasch, E.F.; Bravenboer, N.; Helder, M.N.; Bruggenkate, C.M.T.; Schulten, E.A.J.M. Blood vessel formation and bone regeneration potential of human adipose stem cells for jaw bone augmentation. BioImpacts 2018, 8, 25. [Google Scholar]
- Forouzanfar, T.; Nulend, J.K.; Prins, H.J.; Schulten, E.A.J.M.; Bruggenkate, C.M.T.; Helder, M.N. Clinical implementation of the one-step surgical procedure for craniofacial dental implantation in the maxillary sinus floor elevation (MSFE) model. BioImpacts 2018, 8, 6. [Google Scholar]
- Schulten, E.A.J.M.; Prins, H.J.; Ten Bruggenkate, C.M.; Klein Nulend, J.; Helder, M.N. Bone regeneration with adipose stem cells and calcium phosphate ceramics in the human maxillary sinus floor elevation model using a one-step surgical procedure. Int. J. Oral Maxillofac. Surg. 2017, 46, 214. [Google Scholar] [CrossRef]
- Jensen, T. RADIOGRAPHIC OUTCOME AFTER MAXILLARY SINUS FLOOR AUGMENTATION WITH ALLOGENEIC ADIPOSE TISSUE-DERIVED STEM CELLS SEEDED ON DEPROTEINIZED BOVINE BONE MINERAL. A RANDOMIZED CONTROLLED TRIAL IN MINIPIGS. Int. J. Oral Maxillofac. Surg. 2024, 52, 15. [Google Scholar] [CrossRef]
- Prins, H.J.; Schulten, E.A.J.M.; Ten Bruggenkate, C.M.; Klein-Nulend, J.; Helder, M.N. Bone regeneration using the freshly isolated autologous stromal vascular fraction of adipose tissue in combination with calcium phosphate ceramics. Stem Cells Transl. Med. 2016, 5, 1362–1374. [Google Scholar] [CrossRef] [PubMed]
- Shayesteh, Y.S.; Khojasteh, A.; Soleimani, M.; Alikhasi, M.; Khoshzaban, A.; Ahmadbeigi, N. Sinus augmentation using human mesenchymal stem cells loaded into a β-tricalcium phosphate/hydroxyapatite scaffold. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2008, 106, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Tzur, E.; Ben-David, D.; Gur Barzilai, M.; Rozen, N.; Meretzki, S. Safety and Efficacy Results of BonoFill First-in-Human, Phase I/IIa Clinical Trial for the Maxillofacial Indication of Sinus Augmentation and Mandibular Bone Void Filling. J. Oral Maxillofac. Surg. 2021, 79, 787–798.e2. [Google Scholar] [CrossRef] [PubMed]
- Farré-Guasch, E.; Bravenboer, N.; Helder, M.N.; Schulten, E.; Ten Bruggenkate, C.M.; Klein-Nulend, J. Blood Vessel Formation and Bone Regeneration Potential of the Stromal Vascular Fraction Seeded on a Calcium Phosphate Scaffold in the Human Maxillary Sinus Floor Elevation Model. Materials 2018, 11, 161. [Google Scholar] [CrossRef]
- Katagiri, W.; Watanabe, J.; Toyama, N.; Osugi, M.; Sakaguchi, K.; Hibi, H. Clinical Study of Bone Regeneration by Conditioned Medium from Mesenchymal Stem Cells After Maxillary Sinus Floor Elevation. Implant Dent. 2017, 26, 607–612. [Google Scholar] [CrossRef]
- Duttenhoefer, F.; Hieber, S.F.; Stricker, A.; Schmelzeisen, R.; Gutwald, R.; Sauerbier, S. Follow-up of implant survival comparing ficoll and bone marrow aspirate concentrate methods for hard tissue regeneration with mesenchymal stem cells in humans. BioResearch Open Access 2014, 3, 75–76. [Google Scholar] [CrossRef]
- Yamada, Y.; Nakamura, S.; Ueda, M.; Ito, K. Osteotome technique with injectable tissue-engineered bone and simultaneous implant placement by cell therapy. Clin. Oral Implant. Res. 2013, 24, 468–474. [Google Scholar] [CrossRef]
- Gonshor, A.; McAllister, B.S.; Wallace, S.S.; Prasad, H. Histologic and histomorphometric evaluation of an allograft stem cell-based matrix sinus augmentation procedure. Int. J. Oral Maxillofac. Implant. 2011, 26, 123–131. [Google Scholar]
- Sauerbier, S.; Stricker, A.; Kuschnierz, J.; Bühler, F.; Oshima, T.; Xavier, S.P.; Schmelzeisen, R.; Gutwald, R. In vivo comparison of hard tissue regeneration with human mesenchymal stem cells processed with either the FICOLL method or the BMAC method. Tissue Eng. Part C Methods 2010, 16, 215–223. [Google Scholar] [CrossRef]
- Wu, V.; Klein-Nulend, J.; Bravenboer, N.; Ten Bruggenkate, C.M.; Helder, M.N.; Schulten, E. Long-Term Safety of Bone Regeneration Using Autologous Stromal Vascular Fraction and Calcium Phosphate Ceramics: A 10-Year Prospective Cohort Study. Stem Cells Transl. Med. 2023, 12, 617–630. [Google Scholar] [CrossRef]
- Gupta, A.S.; Aurora, J.K.; Dubey, K.N.; Chauhan, H.; Saxena, M.; Ganvir, S.R. A comparative evaluation of bone regeneration using mesenchymal stem cells versus blood coagulum in sinus augmentation procedures. Natl. J. Maxillofac. Surg. 2021, 12, 349–356. [Google Scholar] [CrossRef]
- Bertolai, R.; Catelani, C.; Aversa, A.; Rossi, A.; Giannini, D.; Bani, D. Bone graft and mesenchimal stem cells: Clinical observations and histological analysis. Clin. Cases Miner. Bone Metab. 2015, 12, 183–187. [Google Scholar] [CrossRef] [PubMed]
| Study Author (Year) | Study Design | Sample Size (Test:Control) (Sites) | Test Procedure | Control Procedure | Results | Follow-Up |
|---|---|---|---|---|---|---|
| Fatale (2022) [3] | Controlled clinical trial | 12:12 | Mesenchymal stem cells (periosteal progenitor cells) + synthetic resorbable biphasic calcium phosphate | Synthetic resorbable biphasic calcium phosphate | Patients who received grafts with MSCs experienced a 63.18% increase in bone formation compared to those who received grafts without MSCs. | 3 months |
| Whitt (2020) [8] | Single-center randomized controlled trial | 9:9 | Stem cell-based allograft (Osteocel Plus; NuVasive Therapeutics, San Diego, CA, USA), | Cortico-cancellous allograft | The findings indicated a statistically significant variation in the percentage of vital bone between the test and control groups at the posterior grafted locations. | 3 months |
| Wildburger (2014) [9] | Split-mouth study | 7:7 | Mesenchymal stem cells (autogenous concentrated bone marrow aspirate) + pure bovine bone material | Pure bovine bone material | In the control group, new bone formation was recorded at 11.8% (SD 6.2%) after three months, while the test group exhibited 7.4% (SD 4.1%). At the six-month mark, the control group had 13.9% (SD 8.5%) new bone growth, compared to 13.5% (SD 5.4%) in the test group. | 3 months, 6 months |
| Sauerbier (2011) [10] | Multicentric, randomized, controlled, clinical trial | 11:34 | Mesenchymal stem cells (bone marrow aspirate concentrate) + bovine bone mineral | 30% autogenous bone + 70% bovine bone mineral | After a period of 3–4 months, the formation of new bone in the sinus is comparable when enhanced with either bone marrow aspirate concentrate and bovine bone mineral or a combination of autogenous bone and bovine bone mineral. | 3–4 months |
| Rickert (2011) [11] | Randomized, controlled, split-mouth design study | 12:12 | Mesenchymal stem cells (iliac crest bone marrow concentrate) + bovine bone material | 30% autogenous bone + 70% bovine bone material | Radiographic examination analysis revealed a marginal bone loss of 0.47 ± 0.31 mm on the test side and 0.41 ± 0.25 mm on the control side. | 12 months |
| Original Analysis | Trim-and-Fill Analysis | Egger’s Regression Test p-Value | |||
|---|---|---|---|---|---|
| SMD (95% CI) | p-Value | SMD (95% CI) | Trimmed Studies/Total Studies | ||
| New bone formation | 0.94 (0.38 to 1.51) | p < 0.01 | 0.94 (0.38 to 1.51) | 0/6 | 0.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, S.-H.; Han, S.-B.; Park, G.S.; Kim, N.J.; Park, W.-J.; Park, J.-B. Clinical Application of Cell-Based Approaches in Maxillary Sinus Floor Augmentation: A Systematic Review and Meta-Analysis. Bioengineering 2025, 12, 1209. https://doi.org/10.3390/bioengineering12111209
Han S-H, Han S-B, Park GS, Kim NJ, Park W-J, Park J-B. Clinical Application of Cell-Based Approaches in Maxillary Sinus Floor Augmentation: A Systematic Review and Meta-Analysis. Bioengineering. 2025; 12(11):1209. https://doi.org/10.3390/bioengineering12111209
Chicago/Turabian StyleHan, Sung-Hoon, Saet-Byeol Han, Greg Shinho Park, Na Jin Kim, Won-Jong Park, and Jun-Beom Park. 2025. "Clinical Application of Cell-Based Approaches in Maxillary Sinus Floor Augmentation: A Systematic Review and Meta-Analysis" Bioengineering 12, no. 11: 1209. https://doi.org/10.3390/bioengineering12111209
APA StyleHan, S.-H., Han, S.-B., Park, G. S., Kim, N. J., Park, W.-J., & Park, J.-B. (2025). Clinical Application of Cell-Based Approaches in Maxillary Sinus Floor Augmentation: A Systematic Review and Meta-Analysis. Bioengineering, 12(11), 1209. https://doi.org/10.3390/bioengineering12111209







