Photocatalytic and Antimicrobial Properties of Ag2O/TiO2 Heterojunction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Ag2O/TiO2 Photocatalysts
2.2. Characterization
2.3. Photocatalytic Activity Tests
2.4. Bactericidal Activity Test
2.5. Fungicidal Activity Test
2.5.1. Suspension Method
2.5.2. Spore Counting Method
3. Results and Discussion
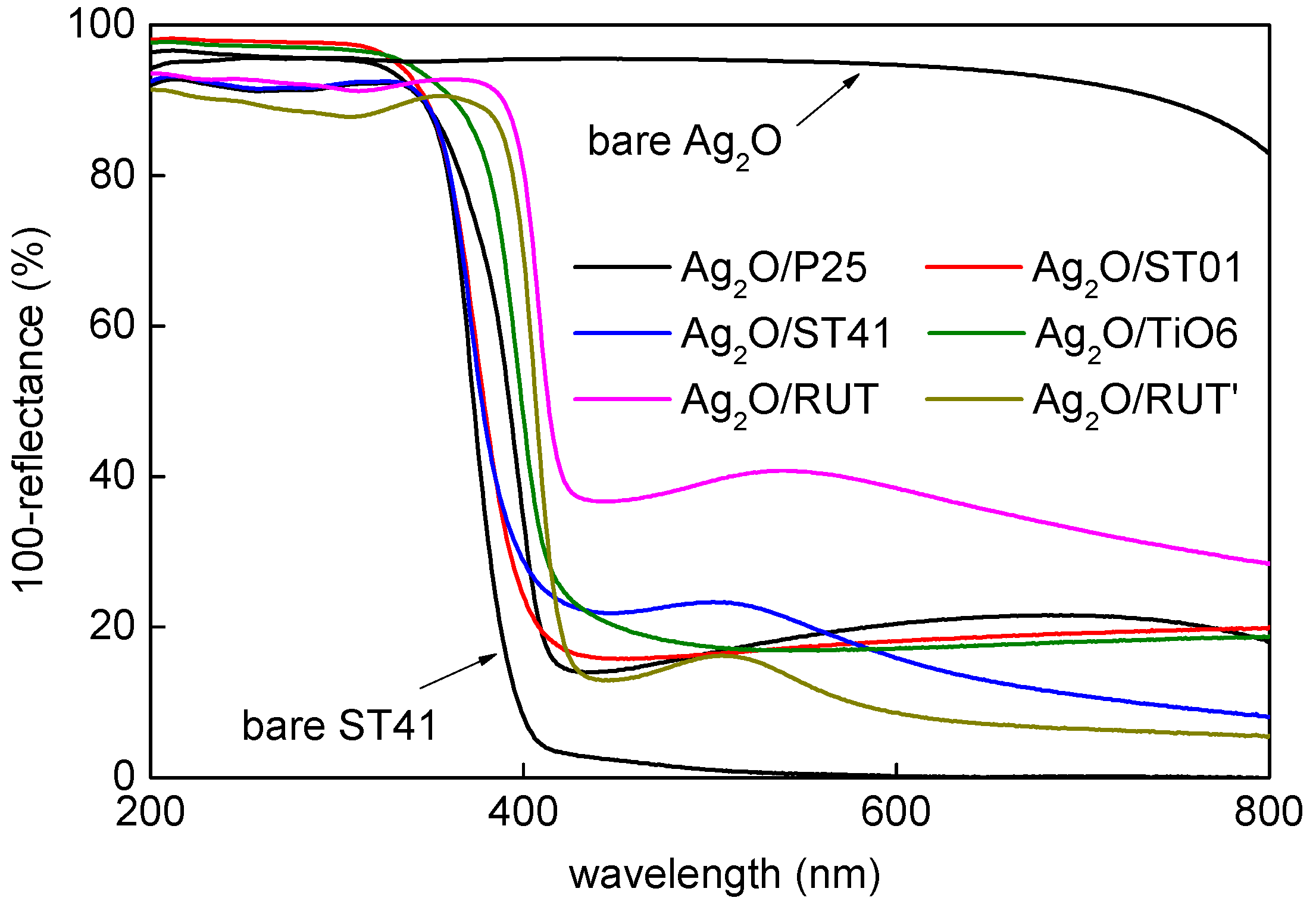
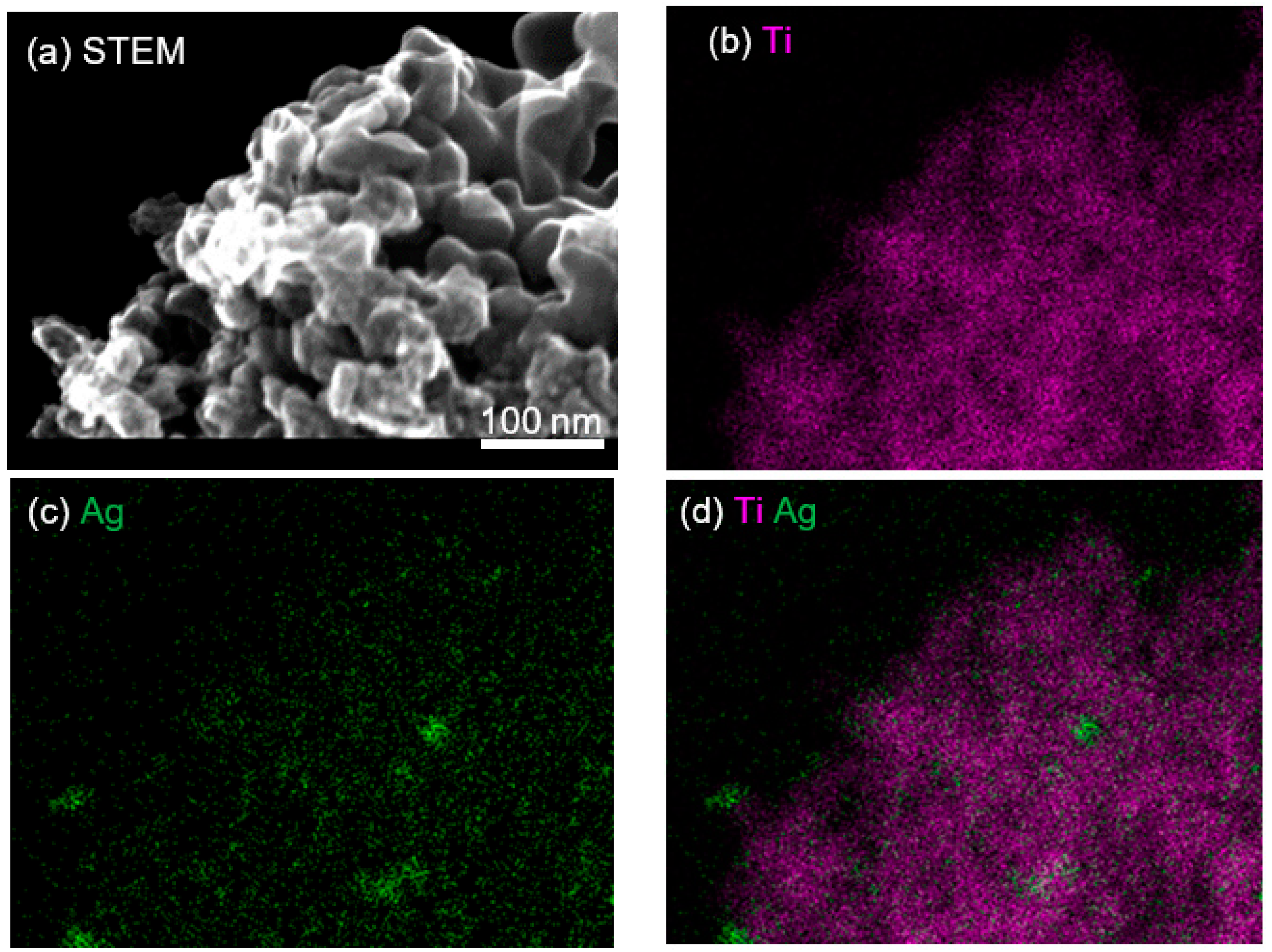
3.1. Characterization of Ag2O/TiO2 Samples
3.2. Photocatalytic Activity
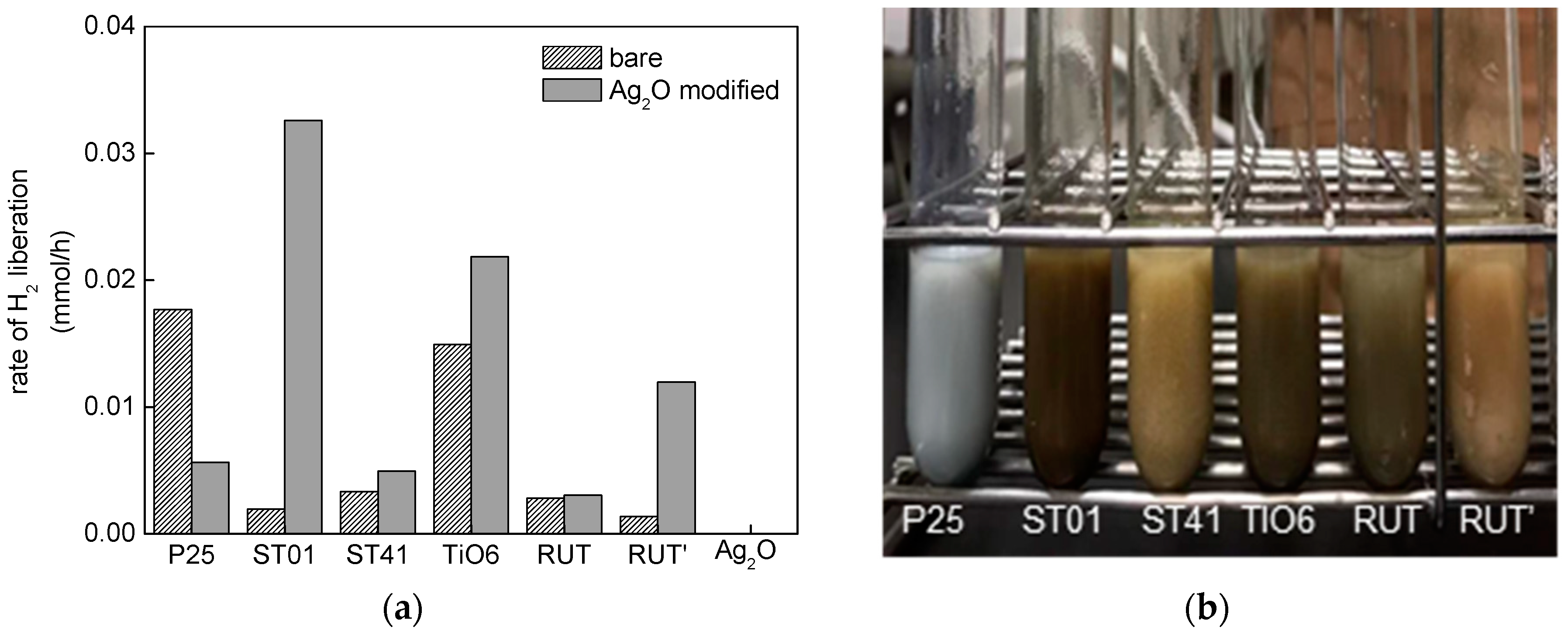
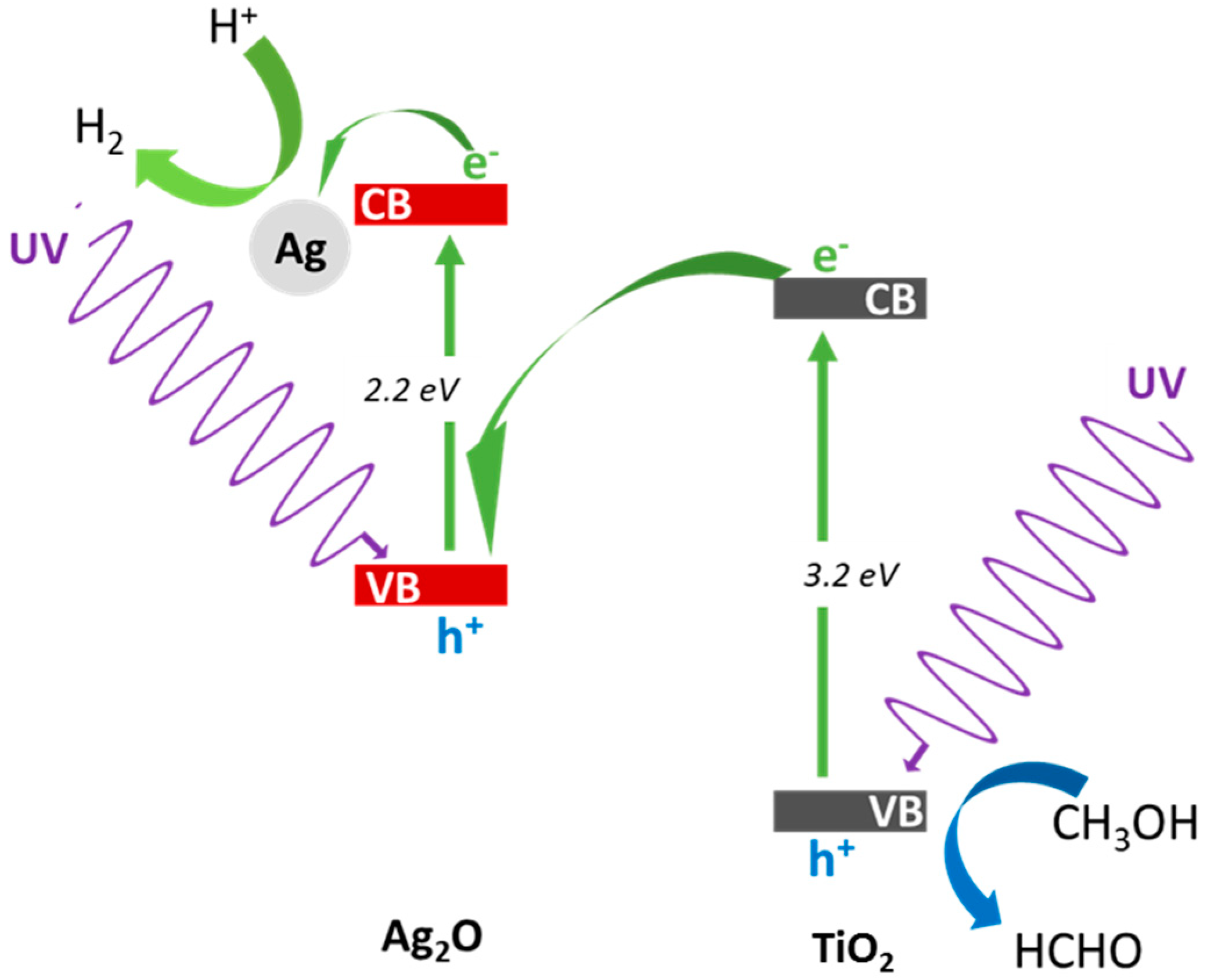
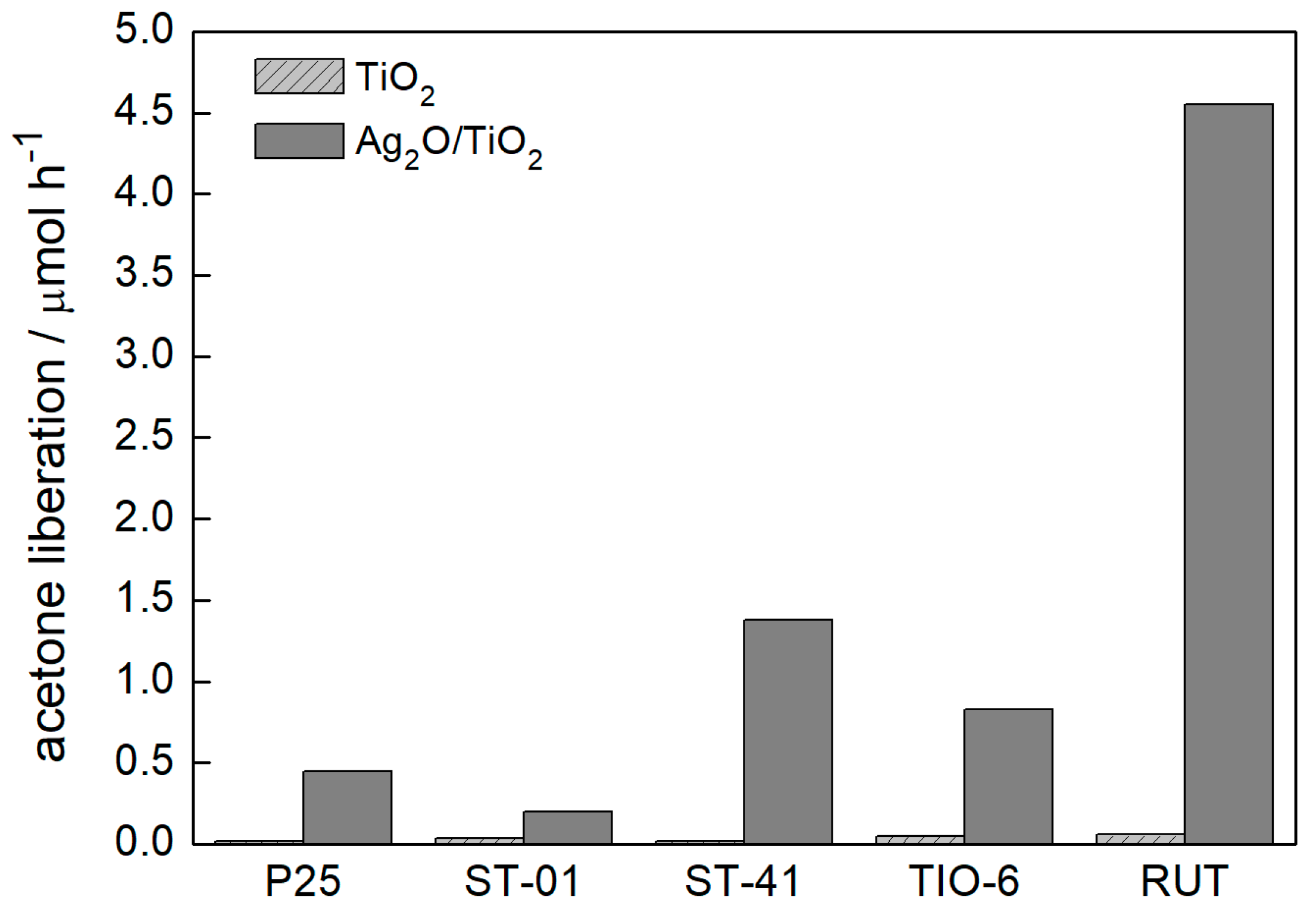
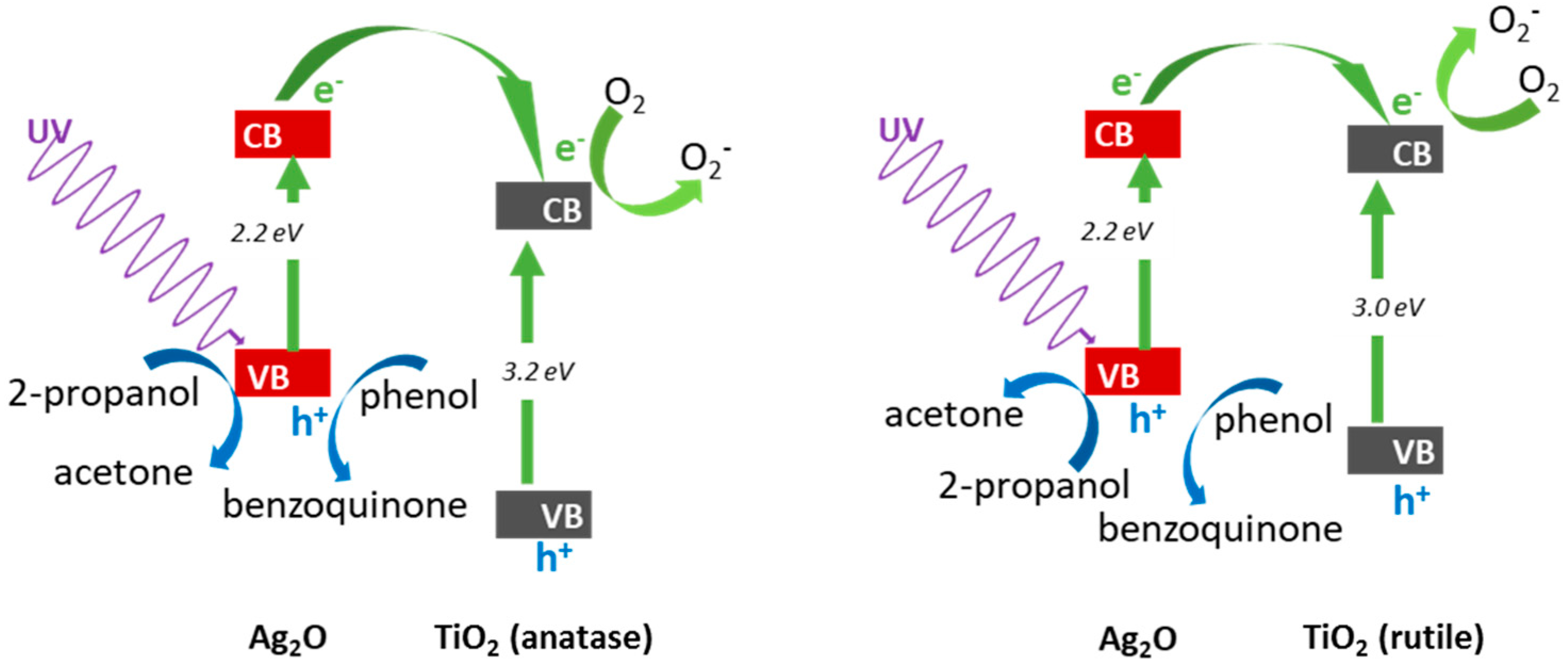
3.2.1. UV/Vis-Induced Methanol Dehydrogenation
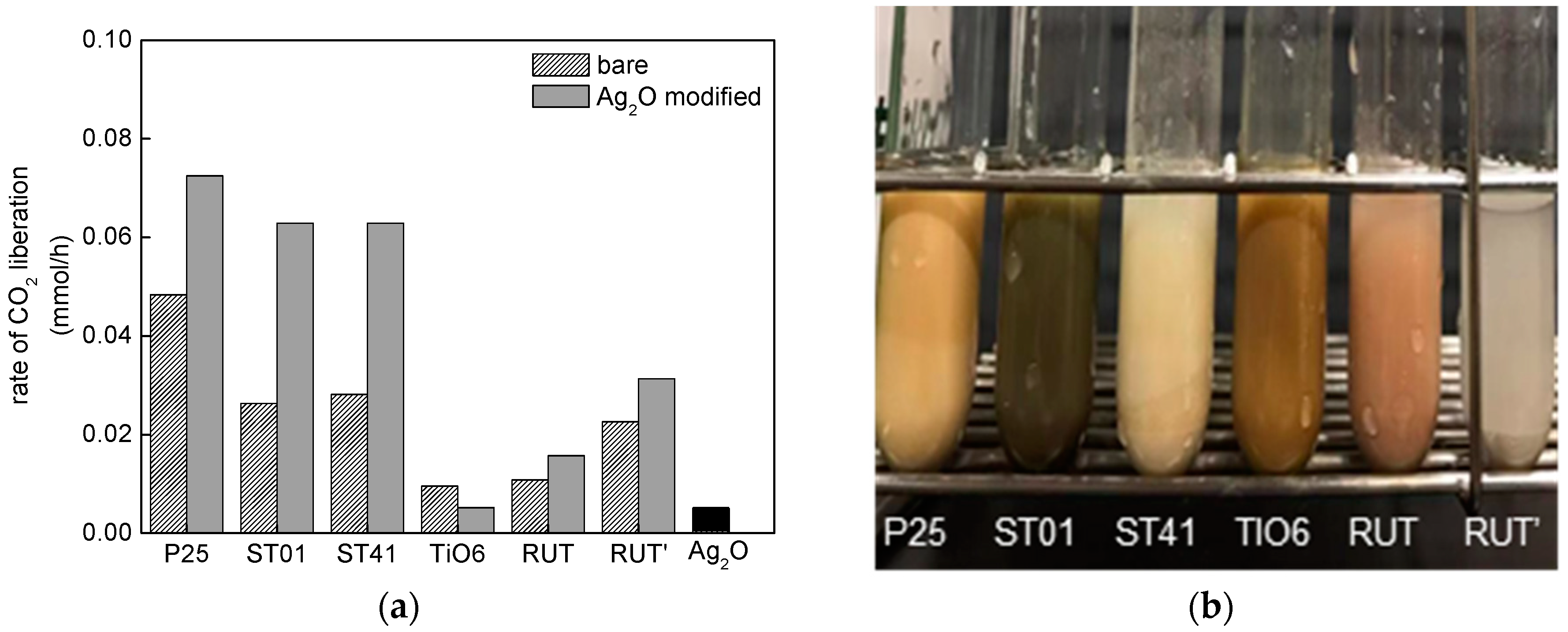
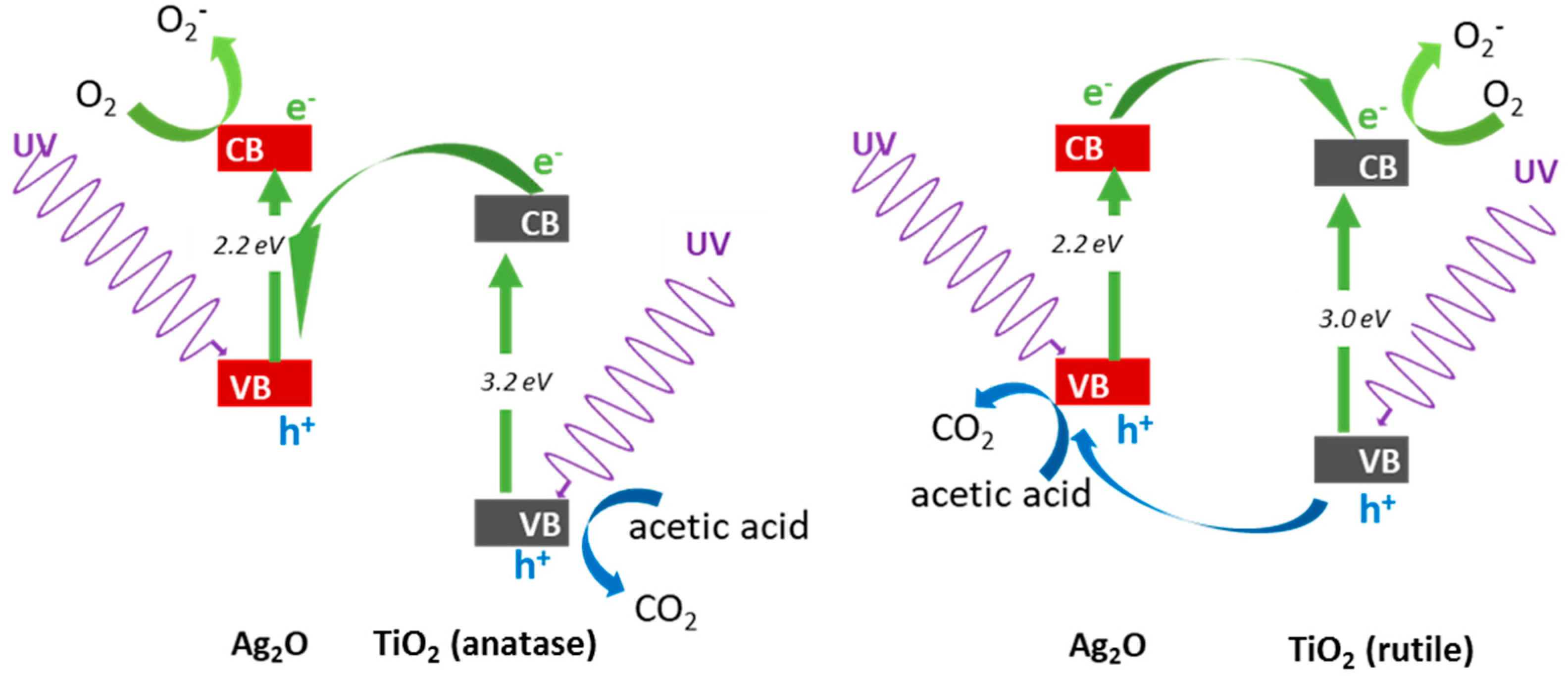
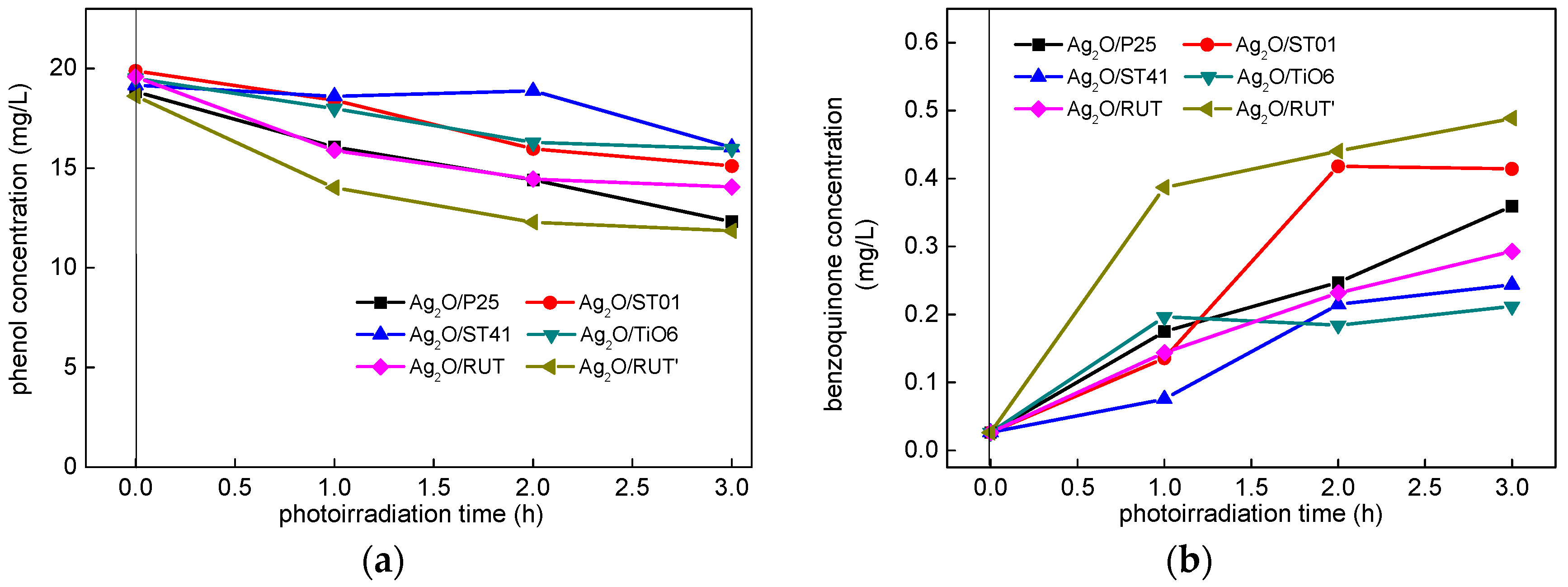
3.2.2. UV/Vis-Induced Acetic Acid Oxidation
3.2.3. Visible Light Photocatalytic Activity
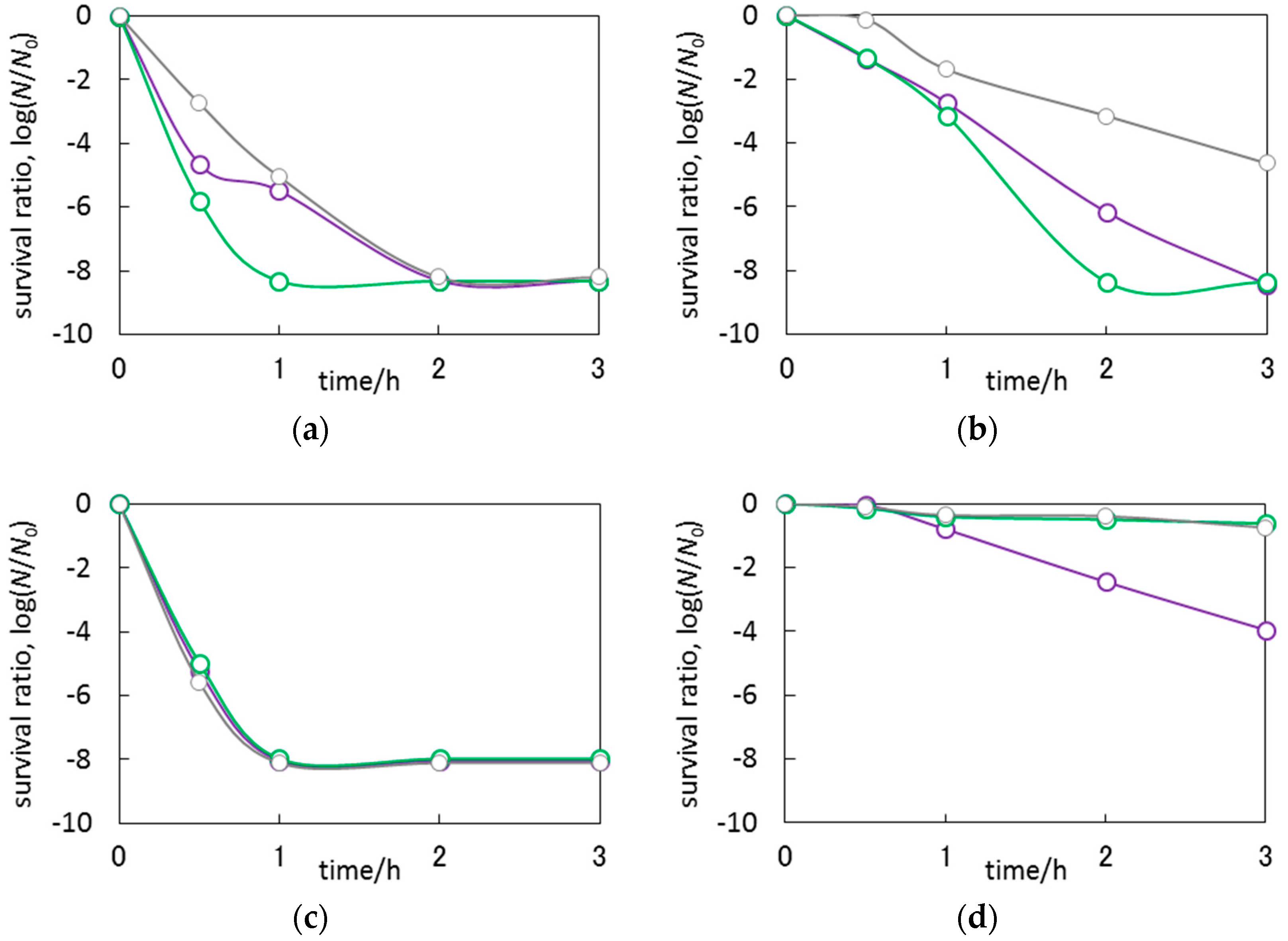
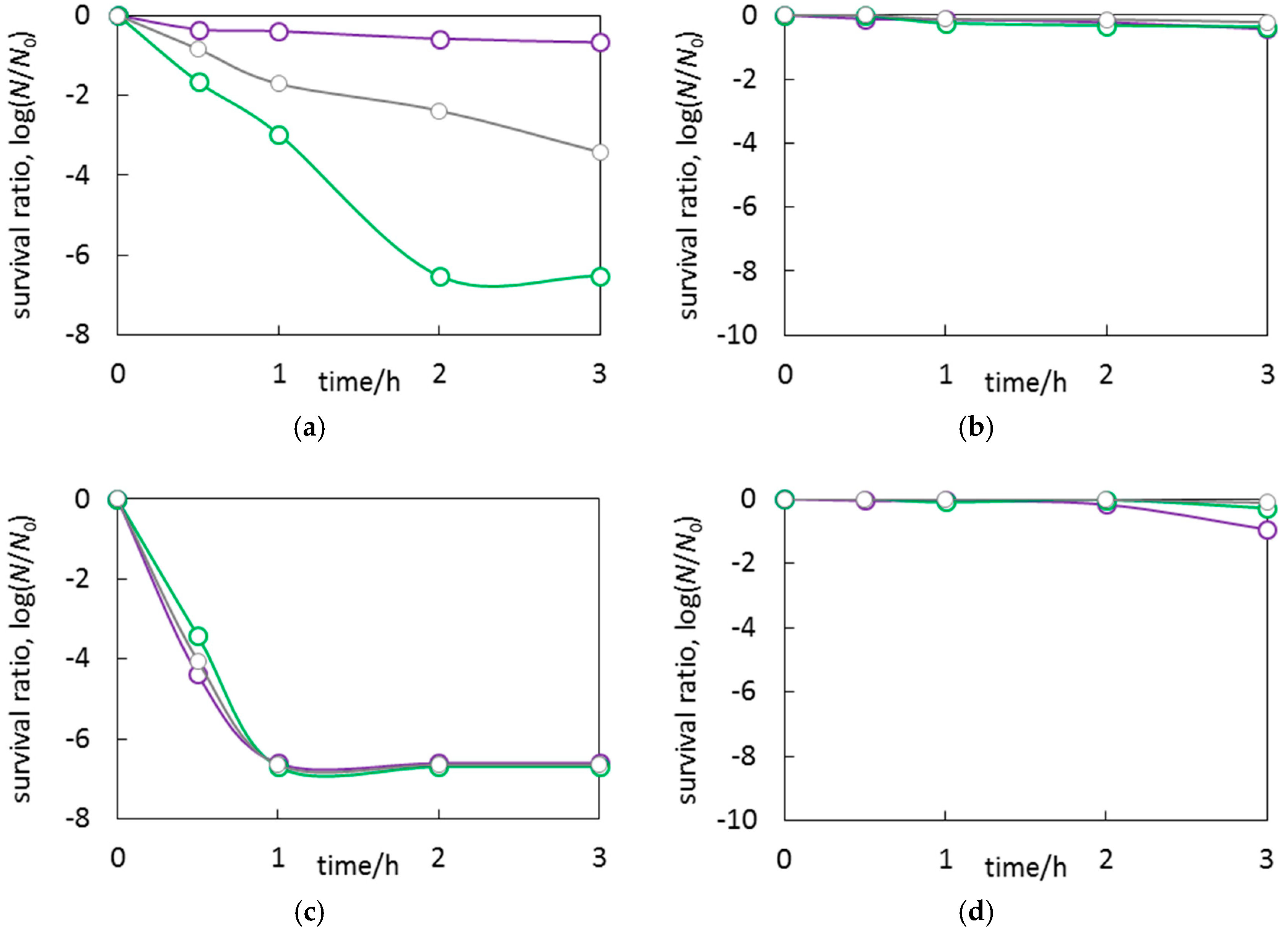
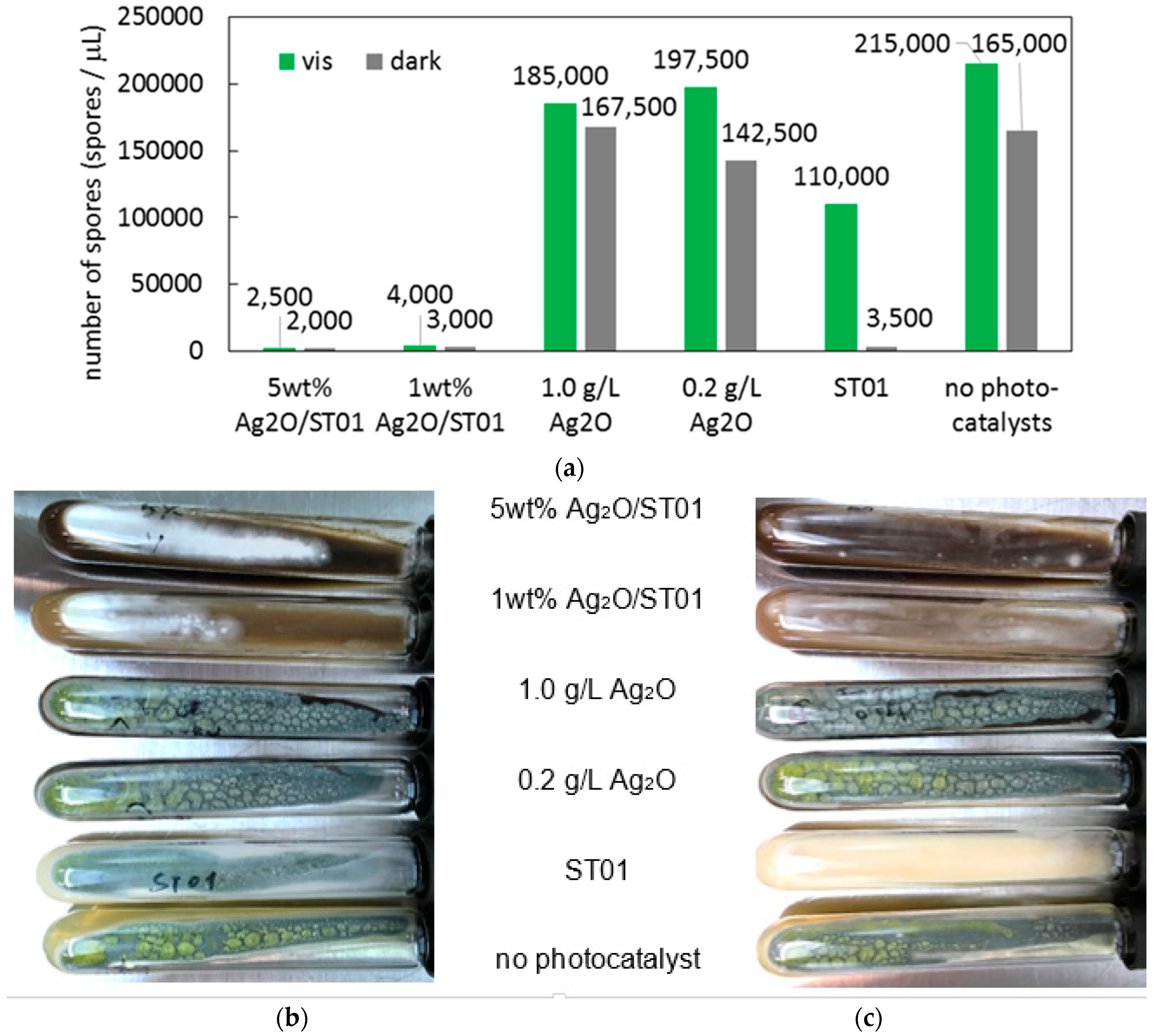
3.3. Antimicrobial Properties of Ag2O/TiO2
4. Summary and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hoffmann, M.R.; Martin, S.T.; Choi, W.Y.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Abe, R.; Higashi, M.; Domen, K. Overall water splitting under visible light through a two-step photoexcitation between TaON and WO3 in the presence of an iodate-iodide shuttle redox mediator. ChemSusChem 2011, 4, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Fattakhova-Rohlfing, D.; Zaleska, A.; Bein, T. Three-dimensional titanium dioxide nanomaterials. Chem. Rev. 2014, 114, 9487–9558. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, B.; Mahaney, O.O.P.; Amano, F.; Murakami, N.; Abe, R. What are titania photocatalysts? An exploratory correlation of photocatalytic activity with structural and physical properties. J. Adv. Oxid. Technol. 2010, 13, 247–261. [Google Scholar] [CrossRef]
- D’Oliveira, J.-C.; Al-Sayyed, G.; Pichat, P. Photodegradation of 2- and 3-chlorophenol in TiO2 aqueous suspensions. Environ. Sci. Technol. 1990, 24, 990–996. [Google Scholar] [CrossRef]
- Kisch, H. Semiconductor photocatalysis—Mechanistic and synthetic aspects. Angew. Chem. Int. Ed. 2013, 52, 812–847. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef] [Green Version]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Byrne, J.A.; Dunlop, P.S.M.; Hamilton, J.W.J.; Fernandez-Ibanez, P.A.; Polo-Lopez, I.; Sharma, P.K.; Vennard, A.S.M. A review of heterogeneous photocatalysis for water and surface disinfection. Molecules 2015, 20, 5574–5615. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, E.; Wei, Z.; Janczarek, M. Band-gap engineering of photocatalysts: Surface modification versus doping. In Visible-Light-Active Photocatalysis: Nanostructured Catalyst Design, Mechanisms and Applications; Ghosh, S., Ed.; Wiley: Weinheim, Germany, 2018; pp. 449–484. [Google Scholar]
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor heterojunction photocatalysts: Design, construction, and photocatalytic performances. Chem. Soc. Rev. 2014, 43, 5234–5244. [Google Scholar] [CrossRef] [PubMed]
- Tjeng, L.H.; Meinders, M.B.J.; Elp, J.; Ghijsen, J.; Sawatzky, G.A.; Johnson, R.L. Electronic structure of Ag2O. Phys. Rev. B 1990, 41, 3190–3199. [Google Scholar] [CrossRef]
- Wang, X.; Li, S.; Yu, H.; Yu, J.; Liu, S. Ag2O as a new visible-light photocatalyst: Self-stability and high photocatalytic activity. Chem. Eur. J. 2011, 17, 7777–7780. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Ma, X.; Huang, B.; Cheng, H.; Wang, Z.; Zhan, J.; Qin, X.; Zhang, X.; Dai, Y. Controlled synthesis of Ag2O microcrystals with facet-dependent photocatalytic activities. J. Mater. Chem. 2012, 22, 21189–21194. [Google Scholar] [CrossRef]
- Chen, Y.J.; Chiang, Y.W.; Huang, M.H. Synthesis of diverse Ag2O crystals and their facet-dependent photocatalytic activity examination. ACS Appl. Mater. Interfaces 2016, 8, 19672–19679. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Wang, X.; Wu, Z.; Yue, X.; Yuan, S.; Lu, H.; Liang, B. Silver oxide as superb and stable photocatalyst under visible and near-infrared light irradiation and its photocatalytic mechanism. Ind. Eng. Chem. Res. 2014, 54, 832–841. [Google Scholar] [CrossRef]
- Wang, X.; Wu, H.F.; Huang, R.B.; Xie, Z.X.; Zheng, L.S. Shape-dependent antibacterial activities of Ag2O polyhedral particles. Langmuir 2010, 26, 2774–2778. [Google Scholar] [CrossRef] [PubMed]
- Lyu, L.M.; Huang, M.H. Investigation of relative stability of different facets of Ag2O nanocrystals through face-selective etching. J. Phys. Chem. C 2011, 115, 17768–17773. [Google Scholar] [CrossRef]
- Wodka, D.; Bielanska, E.; Socha, R.P.; Elzbieciak-Wodka, M.; Gurgul, J.; Nowak, P.; Warszynski, P.; Kumakiri, I. Photocatalytic activity of titanium dioxide modified by silver nanoparticles. ACS Appl. Mater. Interfaces 2010, 2, 1945–1953. [Google Scholar] [CrossRef]
- Kowalska, E.; Wei, Z.; Karabiyik, B.; Herissan, A.; Janczarek, M.; Endo, M.; Markowska-Szczupak, A.; Remita, H.; Ohtani, B. Silver-modified titania with enhanced photocatalytic and antimicrobial properties under UV and visible light irradiation. Catal. Today 2015, 252, 136–142. [Google Scholar] [CrossRef] [Green Version]
- Janczarek, M.; Wei, Z.; Endo, M.; Ohtani, B.; Kowalska, E. Silver- and copper-modified decahedral anatase titania particles as visible light-responsive plasmonic photocatalyst. J. Photonics Energy 2017, 7, 012008. [Google Scholar] [CrossRef]
- Wei, Z.; Janczarek, M.; Endo, M.; Colbeau-Justin, C.; Ohtani, B.; Kowalska, E. Silver-modified octahedral anatase particles as plasmonic photocatalyst. Catal. Today 2018, 310, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, G.; Chen, D.; Lv, X.; Li, J. Tuning photoelectrochemical performances of Ag-TiO2 nanocomposites via reduction/oxidation of Ag. Chem. Mater. 2008, 20, 6543–6549. [Google Scholar] [CrossRef]
- Priya, R.; Baiju, K.V.; Shukla, S.; Biju, S.; Reddy, M.L.P.; Patil, K.; Warrier, K.G.K. Comparing ultraviolet and chemical reduction techniques for enhancing photocatalytic activity of silver oxide/silver deposited nanocrystalline anatase titania. J. Phys. Chem. C 2009, 113, 6243–6255. [Google Scholar] [CrossRef]
- Kang, J.G.; Sohn, Y. Interfacial nature of Ag nanoparticles supported on TiO2 photocatalysts. J. Mater. Sci. 2012, 47, 824–832. [Google Scholar] [CrossRef]
- Liu, C.; Cao, C.; Luo, X.; Luo, S. Ag-bridged Ag2O nanowire network/TiO2 nanotube array p–n heterojunction as a highly efficient and stable visible light photocatalyst. J. Hazard. Mater. 2015, 285, 319–324. [Google Scholar] [CrossRef]
- Cui, Y.; Ma, Q.; Deng, X.; Meng, Q.; Cheng, X.; Xie, M.; Li, X.; Cheng, Q.; Liu, H. Fabrication of Ag-Ag2O/reduced TiO2 nanophotocatalyst and its enhanced visible light driven photocatalytic performance for degradation of diclofenac solution. Appl. Catal. B Environ. 2017, 206, 136–145. [Google Scholar] [CrossRef]
- Grabowska, E.; Zaleska, A.; Sorgues, S.; Kunst, M.; Etcheberry, A.; Colbeau-Justin, C.; Remita, H. Modification of titanium(IV) dioxide with small silver nanoparticles: Application in photocatalysis. J. Phys. Chem. C 2013, 117, 1955–1962. [Google Scholar] [CrossRef]
- Mendez-Medrano, M.G.; Kowalska, E.; Lehoux, A.; Herissan, A.; Ohtani, B.; Bahena, D.; Briois, V.; Colbeau-Justin, C.; Rodriguez-Lopez, J.; Remita, H. Surface modification of TiO2 with Ag nanoparticles and CuO nanoclusters for applications in photocatalysis. J. Phys. Chem. C 2016, 120, 5143–5154. [Google Scholar] [CrossRef]
- Zielinska, A.; Kowalska, E.; Sobczak, J.W.; Lacka, I.; Gazda, M.; Ohtani, B.; Hupka, J.; Zaleska, A. Silver-doped TiO2 prepared by microemulsion method: Surface properties, bio- and photoactivity. Sep. Purif. Technol. 2010, 72, 309–318. [Google Scholar] [CrossRef]
- Sclafani, A.; Herrmann, J.-M. Influence of metallic silver and of platinum-silver bimetallic deposits on the photocatalytic activity of titania (anatase and rutile) in organic and aqueous media. J. Photochem. Photobiol. A 1998, 113, 181–188. [Google Scholar] [CrossRef]
- Herrmann, J.M.; Tahiri, H.; AitIchou, Y.; Lassaletta, G.; GonzalezElipe, A.R.; Fernandez, A. Characterization and photocatalytic activity in aqueous medium of TiO2 and Ag-TiO2 coatings on quartz. Appl. Catal. B Environ. 1997, 13, 219–228. [Google Scholar] [CrossRef]
- Sclafani, A.; Mozzanega, M.N.; Pichat, P. Effect of silver deposits on the photocatalytic activity of titanium dioxide samples for the dehydrogenation or oxidation of 2-propanol. J. Photochem. Photobiol. A 1991, 59, 181–189. [Google Scholar] [CrossRef]
- Ohtani, B.; Kakimoto, M.; Miyadzu, H.; Nishimoto, S.; Kagiya, T. Effect of surface-adsorbed 2-propanol on the photocatalytic reduction of silver and/or nitrate ions in acidic TiO2 suspensions. J. Phys. Chem. 1988, 92, 5773–5777. [Google Scholar] [CrossRef]
- Nishimoto, S.; Ohtani, B.; Kajiwara, H.; Kagiya, T. Photoinduced oxygen formation and silver metal deposition in aqueous solutions of various silver salts by suspended titanium dioxide powder. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1983, 79, 2685–2694. [Google Scholar] [CrossRef]
- Lalitha, K.; Reddy, J.K.; Sharma, M.V.P.; Kumari, V.D.; Subrahmanyam, M. Continuous hydrogen production activity over finely dispersed Ag2O/TiO2 catalysts from methanol:water mixtures under solar irradiation: A structure–activity correlation. Int. J. Hydrogen Energy 2010, 35, 3991–4001. [Google Scholar] [CrossRef]
- You, Y.; Wan, L.; Zhang, S.; Xu, D. Effect of different doping methods on microstructure and photo-catalytic activity of Ag2O–TiO2 nanofibers. Mater. Res. Bull. 2010, 45, 1850–1854. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, H.; Liu, D.; Du, G.; Cui, J. Ag2O/TiO2 nanobelts heterostructure with enhanced ultraviolet and visible photocatalytic activity. ACS Appl. Mater. Interfaces 2010, 2, 2385–2392. [Google Scholar] [CrossRef]
- Chen, F.; Liu, Z.; Liu, Y.; Fang, P.; Dai, Y. Enhanced adsorption and photocatalytic degradation of high-concentration methylene blue on Ag2O-modified TiO2-based nanosheet. Chem. Eng. J. 2013, 221, 283–291. [Google Scholar] [CrossRef]
- Sarkar, D.; Ghosh, C.K.; Mukherjee, S.; Chattopadhyay, K.K. Three dimensional Ag2O/TiO2 Type-II (p−n) nanoheterojunctions for superior photocatalytic ctivity. ACS Appl. Mater. Interfaces 2013, 5, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Jiang, L.; Shi, X.; Wang, W.; Li, G.; Zhu, F.; Zhang, D. Ag2O/TiO2 nanorods heterojunctions as a strong visible-light photocatalyst for phenol treatment. J. Sol-Gel Sci. Technol. 2015, 73, 314–321. [Google Scholar] [CrossRef]
- Ren, H.T.; Jia, S.Y.; Zou, J.J.; Wu, S.H.; Han, X. A facile preparation of Ag2O/P25 photocatalyst for selective reductionof nitrate. Appl. Catal. B Environ. 2015, 176, 53–61. [Google Scholar] [CrossRef]
- Sadanandam, G.; Kumari, V.D.; Scurrell, M.S. Highly stabilized Ag2O-loaded nano TiO2 for hydrogen production from glycerol: Water mixtures under solar light irradiation. Int. J. Hydrogen Energy 2016, 42, 807–820. [Google Scholar] [CrossRef]
- Wei, N.; Cui, H.; Song, Q.; Zhang, L.; Song, X.; Wang, K.; Zhang, Y.; Li, J.; Wen, J.; Tian, J. Ag2O nanoparticle/TiO2 nanobelt heterostructures with remarkable photo-response and photocatalytic properties under UV, visible and near-infrared irradiation. Appl. Catal. B Environ. 2016, 198, 83–90. [Google Scholar] [CrossRef]
- Liu, B.; Mu, L.; Han, B.; Zhang, J.; Shi, H. Fabrication of TiO2/Ag2O heterostructure with enhanced photocatalytic and antibacterial activities under visible light irradiation. Appl. Surf. Sci. 2017, 396, 1596–1603. [Google Scholar] [CrossRef]
- Zelekew, O.A.; Kuo, D.H.; Yassin, J.M.; Ahmed, K.E.; Abdullah, H. Synthesis of efficient silica supported TiO2/Ag2O heterostructured catalyst with enhanced photocatalytic performance. Appl. Surf. Sci. 2017, 410, 454–463. [Google Scholar] [CrossRef]
- Liu, G.; Wang, G.; Hu, Z.; Su, Y.; Zhao, L. Ag2O nanoparticles decorated TiO2 nanofibers as a p-n heterojunction for enhanced photocatalytic decomposition of RhB under visible light irradiation. Appl. Surf. Sci. 2019, 465, 902–910. [Google Scholar] [CrossRef]
- Jiang, B.; Hou, Z.; Tian, C.; Zhou, W.; Zhang, X.; Wu, A.; Tian, G.; Pan, K.; Ren, Z.; Fu, H. A facile and green synthesis route towards two-dimensional TiO2@Ag heterojunction structure with enhanced visible light photocatalytic activity. Cryst. Eng. Commun. 2013, 15, 5821–5827. [Google Scholar] [CrossRef]
- Wang, K.L.; Wei, Z.S.; Ohtani, B.; Kowalska, E. Interparticle electron transfer in methanol dehydrogenation on platinum-loaded titania particles prepared from P25. Catal. Today 2018, 303, 327–333. [Google Scholar] [CrossRef]
- Yan, X.; Ohno, T.; Nishijima, K.; Abe, R.; Ohtani, B. Is methylene blue an appropriate substrate for a photocatalytic activity test? A study with visible-light responsive titania. Chem. Phys. Lett. 2006, 429, 606–610. [Google Scholar] [CrossRef] [Green Version]
- Kowalska, E.; Mahaney, O.O.P.; Abe, R.; Ohtani, B. Visible-light-induced photocatalysis through surface plasmon excitation of gold on titania surfaces. Phys. Chem. Chem. Phys. 2010, 12, 2344–2355. [Google Scholar] [CrossRef] [PubMed]
- Souri, D.; Honarvar, F.; Tahan, Z.E. Characterization of semiconducting mixed electronic-ionic TeO2-V2O5-Ag2O glasses by employing ultrasonic measurements and Vicker’s microhardness. J. Alloys Compd. 2017, 699, 601–610. [Google Scholar] [CrossRef]
- Peyser, L.A.; Vinson, A.E.; Bartko, A.P.; Dickson, R.M. Photoactivated fluorescence from individual silver nanoclusters. Science 2001, 291, 103–106. [Google Scholar] [CrossRef]
- Ren, H.T.; Yang, Q. Fabrication of Ag2O/TiO2 with enhanced photocatalytic performances for dye pollutants degradation by a pH-induced method. Appl. Surf. Sci. 2017, 396, 530–538. [Google Scholar] [CrossRef]
- Wei, Z.; Endo, M.; Wang, K.; Charbit, E.; Markowska-Szczupak, A.; Ohtani, B.; Kowalska, E. Noble metal-modified octahedral anatase titania particles with enhanced activity for decomposition of chemical and microbiological pollutants. Chem. Eng. J. 2017, 318, 121–134. [Google Scholar] [CrossRef] [Green Version]
- Janczarek, M.; Endo, M.; Zhang, D.; Wang, K.; Kowalska, E. Enhanced photocatalytic and antimicrobial prformance of cuprous oxide/titania: The effect of titania matrix. Materials. 2018, 11, 2069. [Google Scholar] [CrossRef]
- Buchalska, M.; Kobielusz, M.; Matuszek, A.; Pacia, A.; Wojtyla, S.; Macyk, W. On oxygen activation at rutile- and anatase-TiO2. ACS Catal. 2015, 5, 7424–7431. [Google Scholar] [CrossRef]
- Scanlon, D.O.; Dunnill, C.W.; Buckeridge, J.; Shevlin, S.A.; Logsdail, A.J.; Woodley, S.M.; Catlow, C.R.A.; Powell, M.J.; Palgrave, R.G.; Parkin, I.P.; et al. Band alignment of rutile and anatase TiO2. Nat. Mater. 2013, 12, 798–801. [Google Scholar] [CrossRef]
- Allen, J.P.; Scanlon, D.O.; Watson, G.W. Electronic structures of silver oxides. Phys. Rev. B 2011, 84, 115141. [Google Scholar] [CrossRef]
- Lok, C.N.; Ho, C.M.; Chen, R.; He, Q.Y.; Yu, W.Y.; Sun, H.Z.; Tam, P.K.H.; Chiu, J.F.; Che, C.M. Proteomic analysis of the mode of antibacterial action of silver nanoparticles. J. Proteome Res. 2006, 5, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Egger, S.; Lehmann, R.P.; Height, M.J.; Loessner, M.J.; Schuppler, M. Antimicrobial properties of a novel silver-silica nanocomposite material. Appl. Environ. Microbiol. 2009, 75, 2973–2976. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C Mater. 2014, 44, 278–284. [Google Scholar] [CrossRef]
- Allahverdiyev, A.M.; Abamor, E.S.; Bagirova, M.; Rafailovich, M. Antimicrobial effects of TiO2 and Ag2O nanoparticles against drug-resistant bacteria and leishmania parasites. Future Microbiol. 2011, 6, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Archana, D.; Singh, B.K.; Dutta, J.; Dutta, P.K. Chitosan-PVP-nano silver oxide wound dressing: In vitro and in vivo evaluation. Int. J. Biol. Macromol. 2015, 73, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-Y.; Kim, H.-J.; Chung, C.-H. Photocatalytic antifungla activity against Candida albicans by TiO2 coated acrylic resign denture base. J. Korean Acad. Prosthodont. 2006, 44, 284–294. [Google Scholar]
- Jones, L.; Oshea, P. The electrostatic nature of the cell-surface of Candida albicans—A role in adhesion. Exp. Mycol. 1994, 18, 111–120. [Google Scholar] [CrossRef]
- Carlile, M.J. The Photobiology of Fungi. Annu. Rev. Plant Physiol. 1965, 16, 175–202. [Google Scholar] [CrossRef]
- Hill, E.P. Effect of light on growth and sporulation of Aspergillus ornatus. J. Gen. Microbiol. 1976, 95, 39–44. [Google Scholar] [CrossRef]
- Kopke, K.; Hoff, B.; Bloemendal, S.; Katschorowski, A.; Kamerewerd, J.; Kuck, U. Members of the Penicillium chrysogenum velvet complex play functionally opposing roles in the regulation of penicillin biosynthesis and conidiation. Eukaryot. Cell 2013, 12, 299–310. [Google Scholar] [CrossRef]
- Markowska-Szczupak, A.; Ulfig, K.; Morawski, A.W. Antifungal effect of titanium dioxide, indoor light and the photocatalytic process in vitro test on different media. J. Adv. Oxid. Technol. 2012, 15, 30–33. [Google Scholar] [CrossRef]
- Markowska-Szczupak, A.; Wang, K.L.; Rokicka, P.; Endo, M.; Wei, Z.S.; Ohtani, B.; Morawski, A.W.; Kowalska, E. The effect of anatase and rutile crystallites isolated from titania P25 photocatalyst on growth of selected mould fungi. J. Photochem. Photobiol. B 2015, 151, 54–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raliya, R.; Biswas, P.; Tarafdar, J.C. TiO2 nanoparticle biosynthesis and its physiological effect on mung bean (Vigna radiata L.). Biotechnol. Rep. 2015, 5, 22–26. [Google Scholar] [CrossRef] [PubMed]

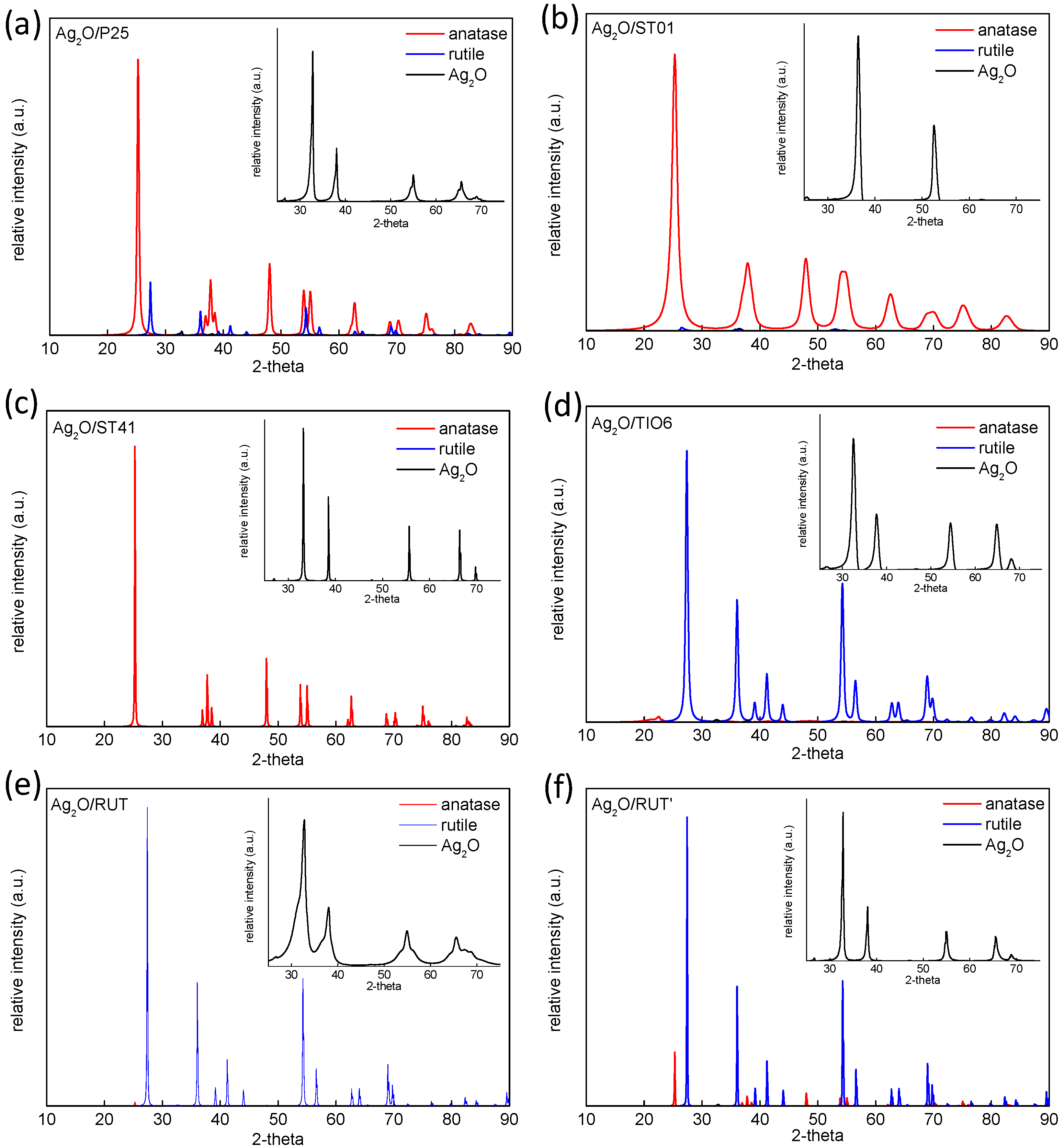
| Sample Name | Crystalline Content * (%) | Crystallite Size (nm) | ||||
|---|---|---|---|---|---|---|
| Anatase | Rutile | Ag2O | Anatase | Rutile | Ag2O | |
| Ag2O/P25 | 82.5 | 16.9 | 0.6 | 21.8 | 38.5 | 18.3 |
| Ag2O/ST01 | 99.9 | - | 0.1 | 7.8 | - | 3.2 |
| Ag2O/ST41 | 99.6 | - | 0.4 | 75.7 | - | 7.8 |
| Ag2O/TiO6 | 7.3 | 92.2 | 0.5 | 6.3 | 15.9 | 13.0 |
| Ag2O/RUT | 2.0 | 97.4 | 0.6 | 38.9 | 86.1 | 29.8 |
| Ag2O/RUT’ | 11.5 | 87.4 | 0.7 | 109.2 | 170.1 | 41.3 |
| Ag2O | - | - | 100 | - | - | 58.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Endo-Kimura, M.; Janczarek, M.; Bielan, Z.; Zhang, D.; Wang, K.; Markowska-Szczupak, A.; Kowalska, E. Photocatalytic and Antimicrobial Properties of Ag2O/TiO2 Heterojunction. ChemEngineering 2019, 3, 3. https://doi.org/10.3390/chemengineering3010003
Endo-Kimura M, Janczarek M, Bielan Z, Zhang D, Wang K, Markowska-Szczupak A, Kowalska E. Photocatalytic and Antimicrobial Properties of Ag2O/TiO2 Heterojunction. ChemEngineering. 2019; 3(1):3. https://doi.org/10.3390/chemengineering3010003
Chicago/Turabian StyleEndo-Kimura, Maya, Marcin Janczarek, Zuzanna Bielan, Dong Zhang, Kunlei Wang, Agata Markowska-Szczupak, and Ewa Kowalska. 2019. "Photocatalytic and Antimicrobial Properties of Ag2O/TiO2 Heterojunction" ChemEngineering 3, no. 1: 3. https://doi.org/10.3390/chemengineering3010003