Abstract
Hydrogen is seen as the new energy carrier for sustainable energy systems of the future. Meanwhile, proton exchange membrane fuel cell (PEMFC) stacks are considered the most promising alternative to the internal combustion engines for a number of transportation applications. Nevertheless, PEMFCs need high-grade hydrogen, which is difficultly stored and transported. To solve these issues, generating hydrogen using membrane reactor (MR) systems has gained great attention. In recent years, the role of silica membranes and MRs for hydrogen production and separation attracted particular interest, and a consistent literature is addressed in this field. Although most of the scientific publications focus on silica MRs from an experimental point of view, this review describes the progress done in the last two decades in terms of the theoretical approach to simulate silica MR performances in the field of hydrogen generation. Furthermore, future trends and current challenges about silica membrane and MR applications are also discussed.
1. Introduction
One of the biggest challenges for humanity is to find practical solutions to the effect of greenhouse gas emissions on climate change. CO2 represents one of the main causes of global warming, and its concentration increase in the atmosphere mainly depends on human activities, as a consequence of the large use of fossil fuels. In this regard, CO2 capture and sequestration attracted strong interest [1,2]. It is widely accepted that carbon capture and storage (CCS), large exploitation of renewable sources, and alternative processes may represent the most viable solutions to global warming [3]. Among a number of possible strategies, PEMFCs represent one of the most promising technologies to effectively reduce CO2/greenhouse emissions. These devices are electrochemical MRs combining hydrogen (as a fuel) and oxygen (from air) to produce electrical power in an efficient way, exhausting water vapour [4,5,6]. However, hydrogen from fossil fuels may be also useful to enhance the system efficiency. In automotive applications, electromotors combined to hydrogen-powered fuel cells may show an overall efficiency ranging from 40 to 55%, significantly higher than internal combustion engines (13–30%).
Consequently, many countries are seriously considering the implications of a shift towards a hydrogen-oriented economy [7]. Furthermore, the growing interest in hydrogen is due to its potentiality in solving two major challenges: achieving the energy independence while minimizing the environmental impact. Unfortunately, there are four critical aspects needing development before hydrogen economy could be realistically pursued [8]:
- Cost-effective hydrogen generation in a carbon constrained global energy system: the challenges in this area involve hydrogen production from fossil fuels combined to carbon sequestration, in parallel with an increase in renewable sources exploitation.
- Hydrogen purification and storage technologies able to separate and purify hydrogen streams to the requirements of the end-users: efficient hydrogen separation and storage devices will have to match the United States—Department of Energy (US DOE) targets, such as hydrogen permeability and permselectivity (2015, last up-date), recognized worldwide as reference values for any hydrogen permselective membrane worthy of interest for its potential utilization in industrial applications.
- An efficient, widely available, and well-managed hydrogen delivery and distribution infrastructure.
- Efficient fuel cells and other energy conversion technologies fueled by hydrogen.
Nowadays, referring to the production and separation of hydrogen, membrane technology is considered a promising alternative with respect to the traditional reactors [9,10,11,12].
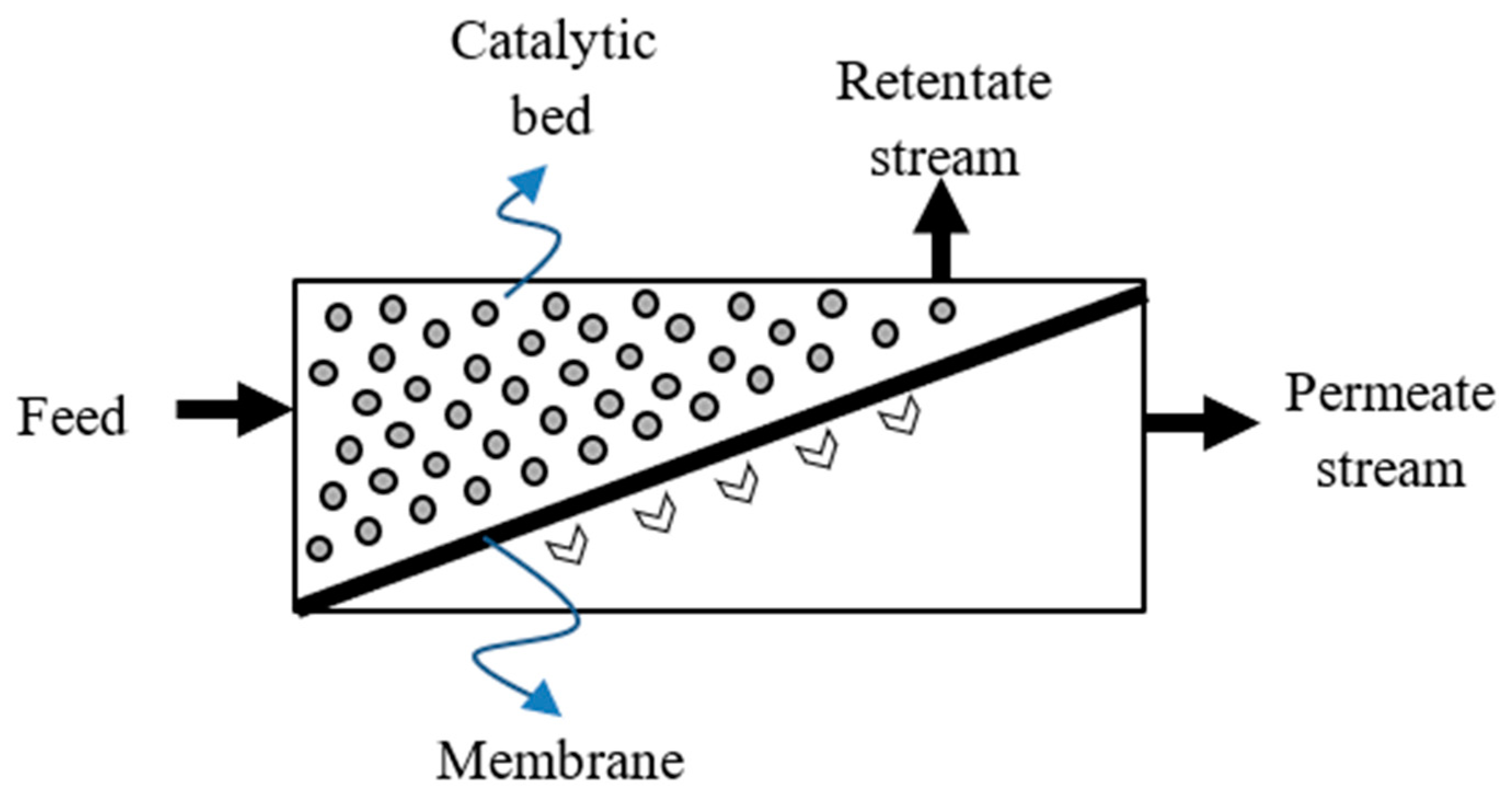
On the other hand, the specific thermodynamic constrains limiting the performance of the TRs can be circumvented by using innovative integrated systems such as MRs, which represent an integrated system in which a catalytic reaction and the hydrogen separation take place simultaneously (Figure 1) [13].
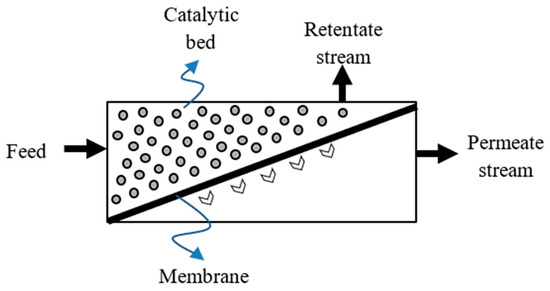
Figure 1.
Conceptual scheme of a membrane reactor (MR) system.
Palladium membrane technology played the largest role in the field of hydrogen separation and purification [10,11,12,13], even though in recent years, silica membranes attracted a relevant interest due to their low cost, high permeability, and easy manufacturing. To our best knowledge, no comprehensive reviews on modeling of silica MRs performance for hydrogen production/separation have been noticed. Therefore, the aim of this review is to provide the recent advances on silica MRs applied in the field of hydrogen production and purification, analyzing the state-of-the-art from a modeling point of view. Therefore, in this work the most up-dated modeling studies are reported to favor a better understanding of the prevailing phenomena behind the MR behavior, with the advantage of lowering and the optimizing the experimental tests for such a reaction process.
2. Silica Membranes
The singular characteristics of silica (SiO2) are related to the ability of its elemental bricks (SiO4 tetrahedral) to be strictly connected, in order to increase the amount of various amorphous or crystallized solids, commonly macroporous, mesoporous, or microporous (Table 1). Compared to other common single oxides such as Al2O3, TiO2, or ZrO2, silica may be more easily synthesized as an ultra-microporous or super-microporous amorphous thin layer, and then used for gas separation processes [14,15,16].

Table 1.
Standard classification of porous materials as a function of their pore size.
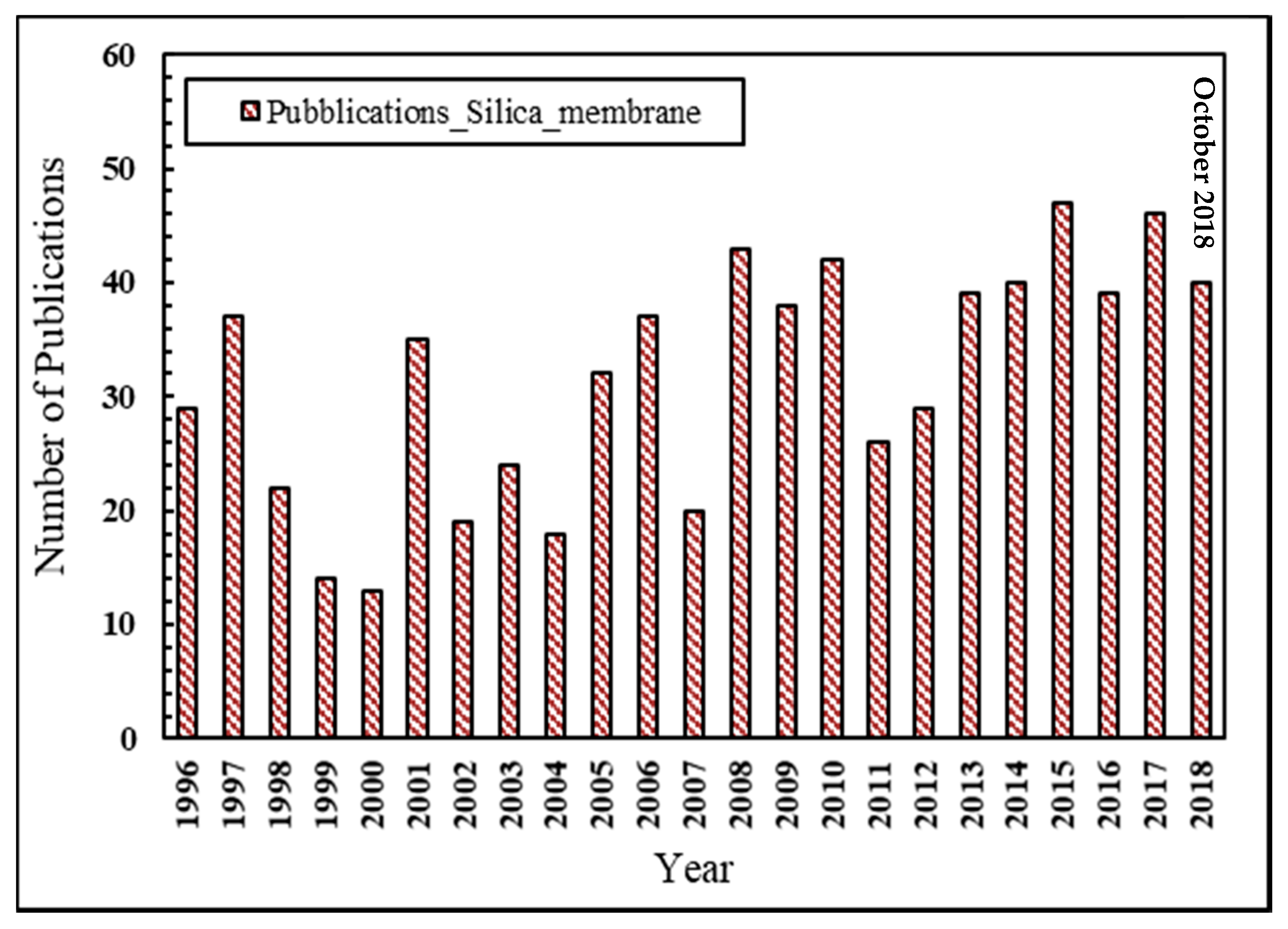
In the last two decades, the scientific interest towards silica membranes applied to hydrogen separation/purification may be testified by the growing number of scientific publications in this field, as represented in Figure 2.
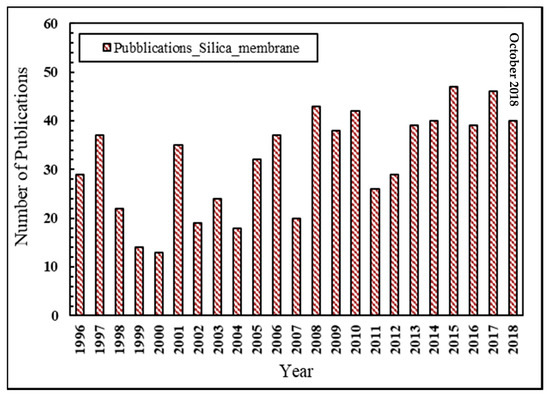
Figure 2.
Number of scientific publications about silica membranes for hydrogen separation vs. year, Scopus database: www.scopus.com.
As a general consideration, the transport mechanisms of various species may be different, depending on the pore size. In membrane gas separation via porous membranes, the highest permselectivities are reached by using microporous ones [17,18]. Oyama et al. [15] described in depth how the transport mechanisms regulate the gas permeation through inorganic porous membranes, while Table 2 shows basically the different transport mechanism adopted for silica membranes. Microporous amorphous silica exhibits a weak stability in humid environment, which limits its application as separative membrane. Indeed, Fotou et al. [18] stated that microporous silica materials are not hydrothermally stable, since their exposure to humid environment for prolonged periods at T > 400 °C (which represents the calcination temperature of silica) induces rapid densification. Consequently, due to the pore structure changes, both hydrogen permeability and permselectivity are reduced. The same authors reported that steam may catalyze the surface diffusion of silica, moving along the surface of larger pores to fill in pores of smaller dimensions.

Table 2.
Gas transport mechanism inside porous materials and their perm-selectivity.
2.1. Silica Structure
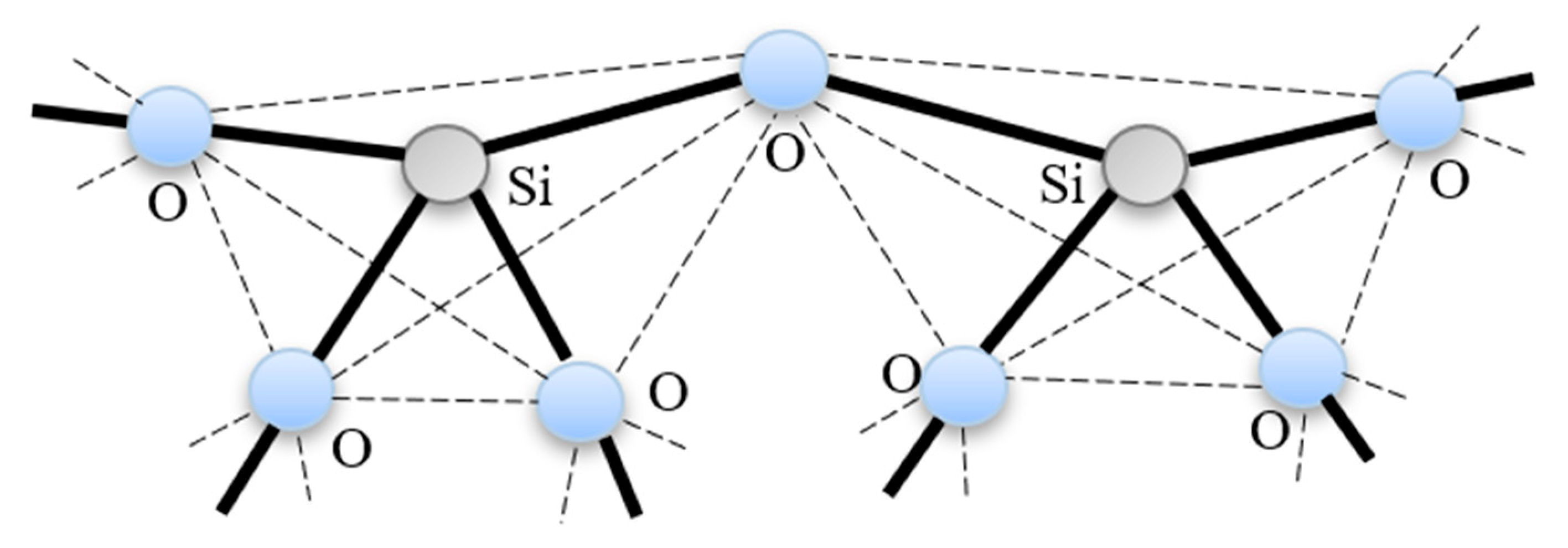
As shown in Figure 3, the configuration of silica material consists of a corner sharing SiO4 covalently connected in a continuous 3-D network having no long-range order.
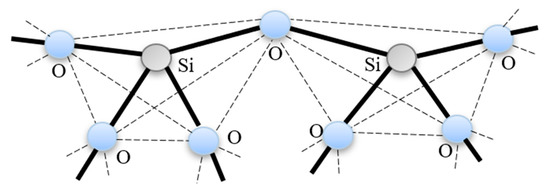
Figure 3.
Silica structure; corner-sharing SiO4 tetrahedral.
Conventional preparation of silica glasses is implemented by cooling melted solids, but amorphous phases can be achieved at lower temperatures from liquid solutions or gaseous mixtures. Furthermore, amorphous phases are intrinsically metastable, whereas crystallization is systematically associated with a microstructural change. Nevertheless, in the case of microporous solids such as silica, it commonly results in a significant enhancement of pore sizes [19].
2.2. Transport Mechanism in Microporous Silica Membrane
One of the most relevant behaviors of microporous membranes is represented by the activated gas transport, which may be also referred to as a molecular sieving mechanism. It was phenomenologically demonstrated that the permeating flux of such a species through microporous materials increases as a function of temperature, according to the following Arrhenious like Equation [19] (1):
De Lange et al. [20] described the gas transport and separation in microporous membrane materials. Hence, the derived activated transport may be expressed as follows (2):
where D0 (m2·s−1) is the mean intrinsic diffusion coefficient for micropore diffusion, K0 the intrinsic Henry constant, ε the membrane porosity, l the membrane thickness, the bulk density, qst the isosteric heat adsorption, Ei the activation energy for gas species, R the universal gas constant, and T the temperature.
3. Membrane Reactor Technology
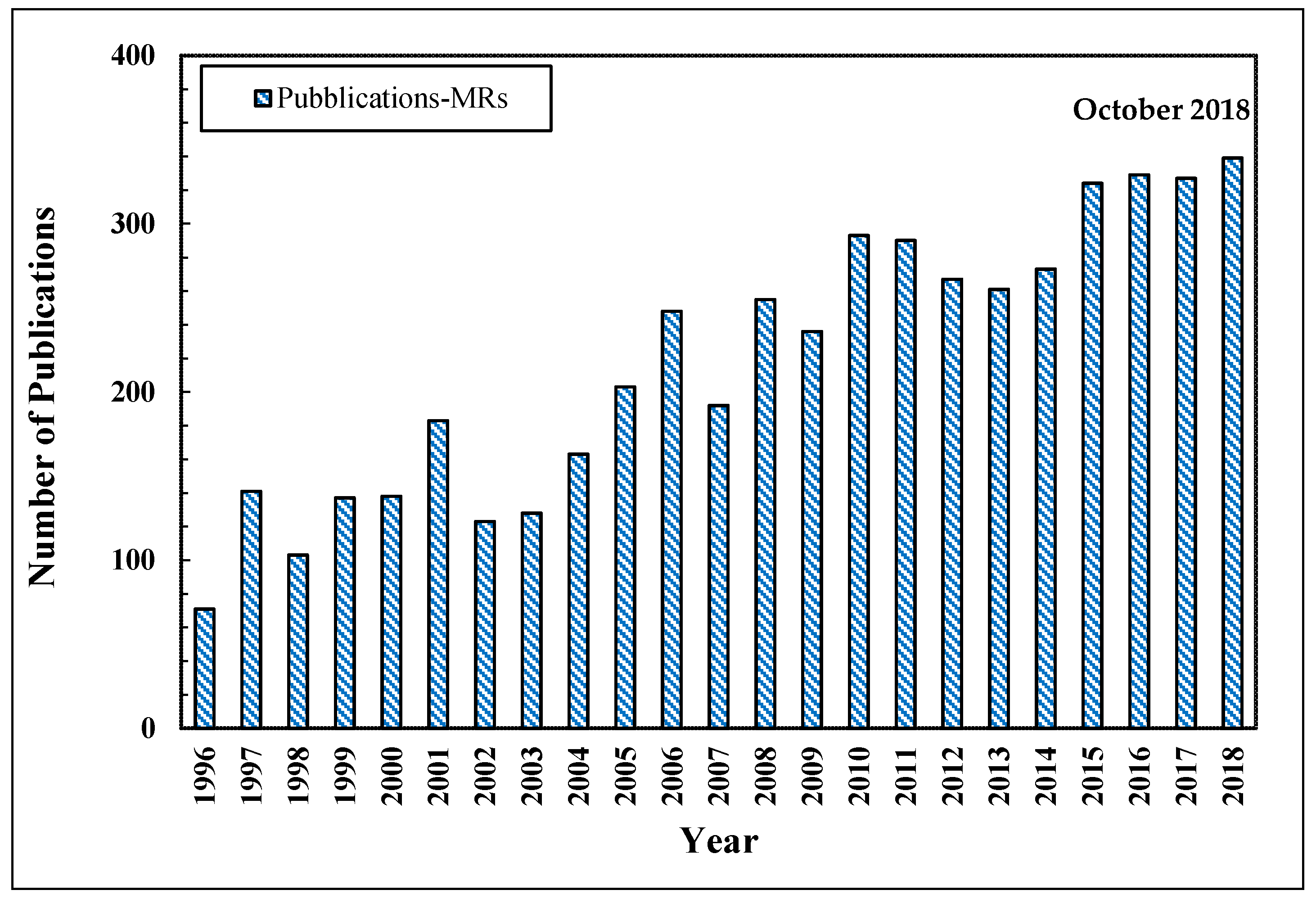
As stated above, MRs simultaneously combine a chemical reaction with the selective separation of such a product. The significant progress about MRs technology is testified by the growing number of publications as reported in Figure 4.
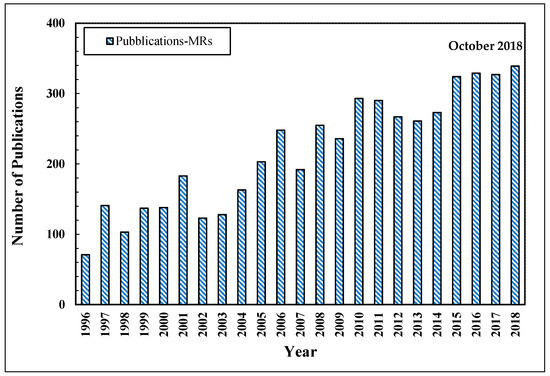
Figure 4.
Number of scientific publications about the MRs vs. year. Scopus database: www.scopus.com.
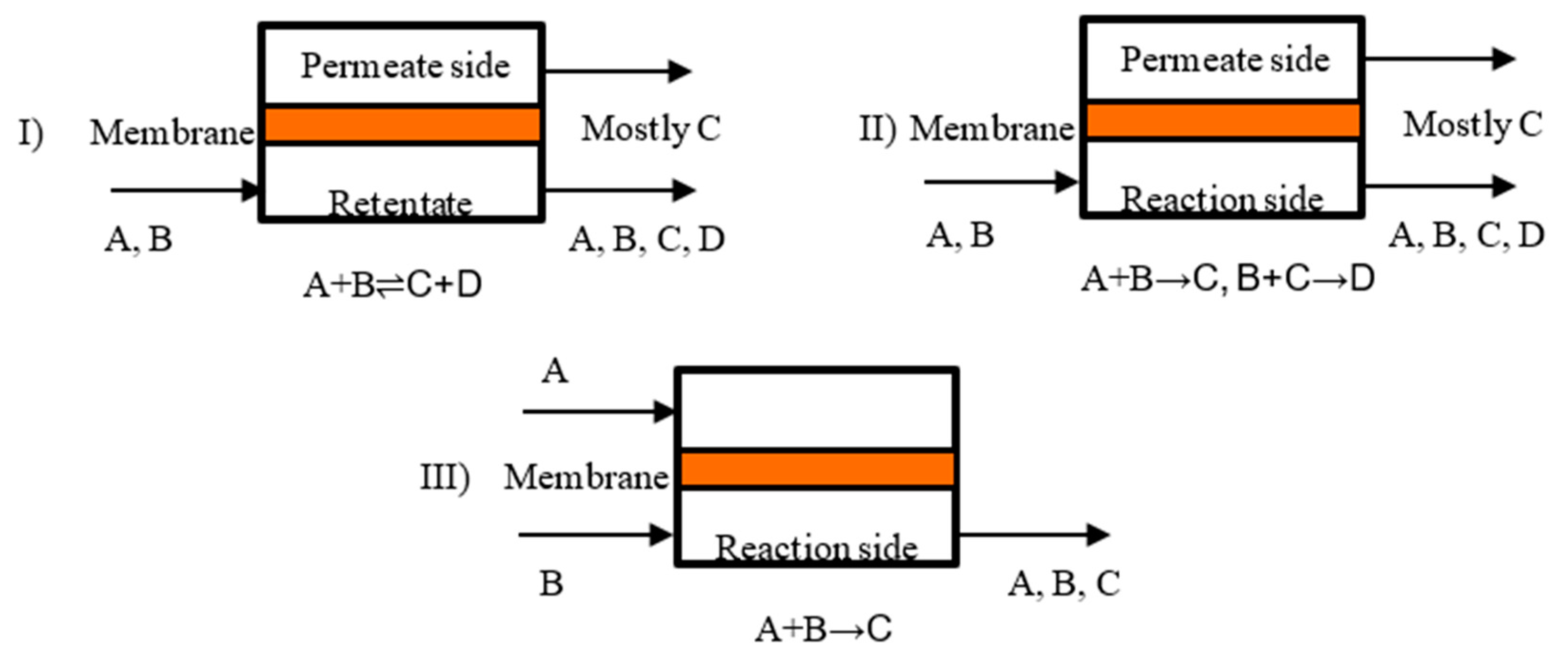
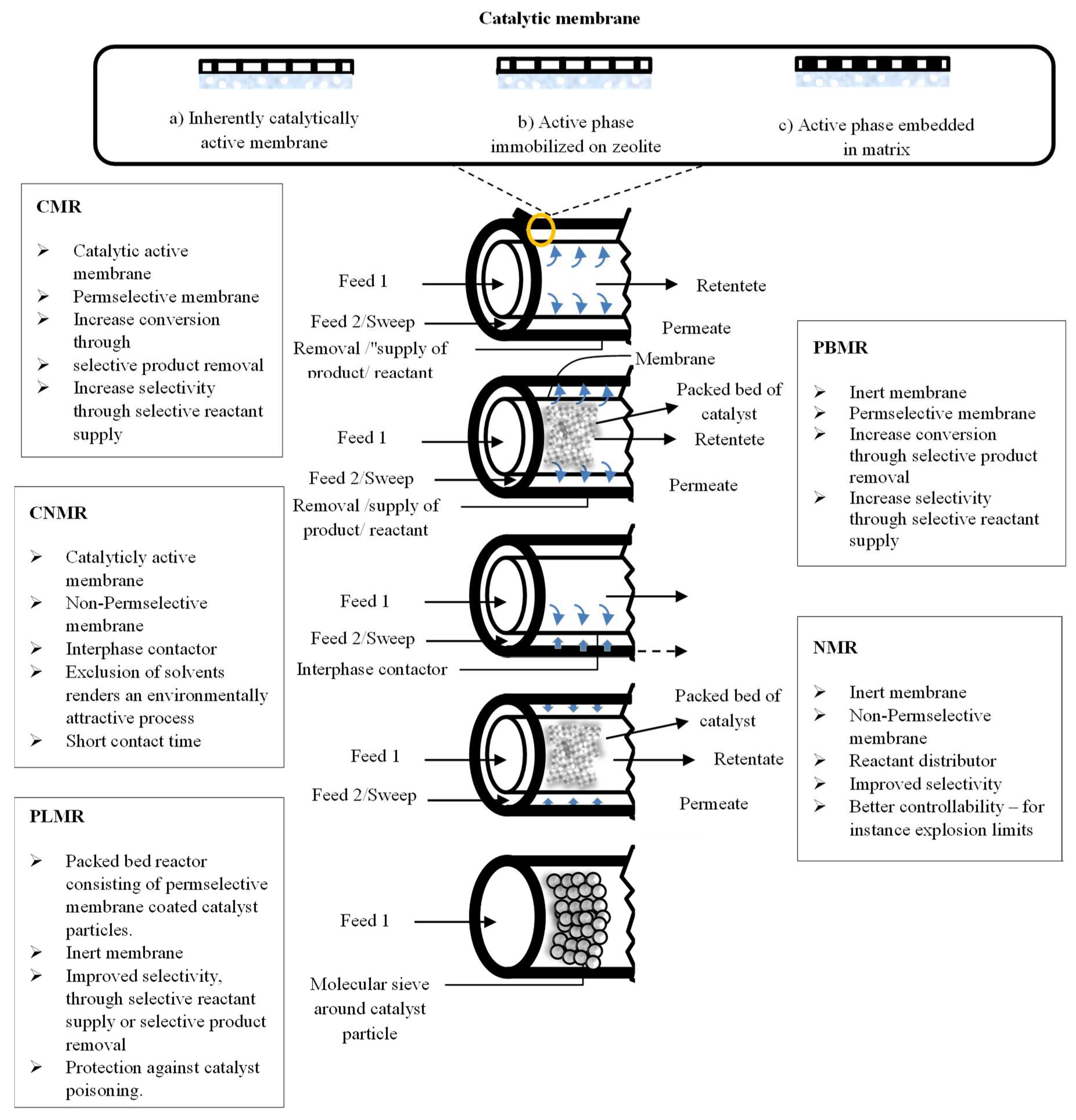
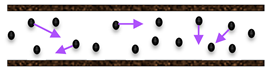
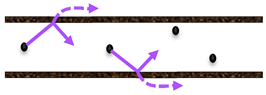
Various heterogeneous gas–solid catalytic processes of industrial application include the combination of operations at high temperatures and in chemically harsh conditions. Consequently, by considering these two factors, inorganic membranes are more favorable than the polymeric ones. Generally, an MR may be operated in flat (Figure 5) or tubular geometry (Figure 6).
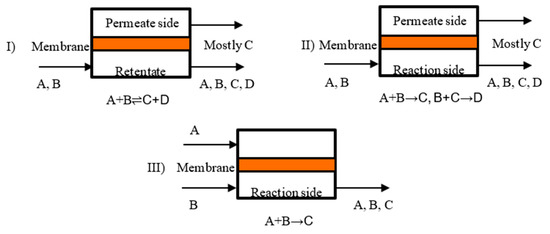
Figure 5.
Scheme of a MR operated in flat configuration: (I) Product removal to overcome thermodynamic limitation; (II) selective removal of a desired product; (III) controlled addition of a reactant for selective permeation.
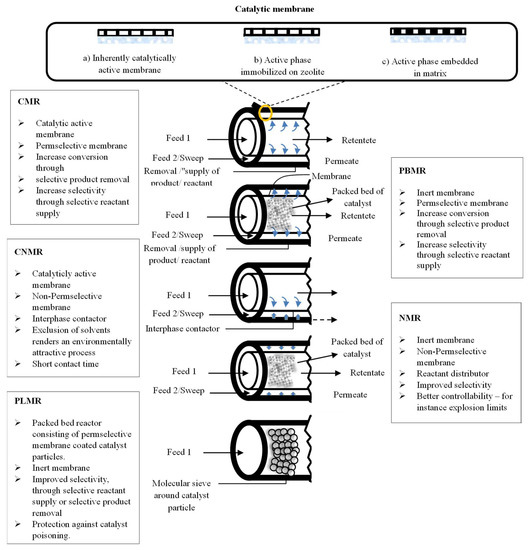
Figure 6.
Tubular MRs classification based on the membrane function and its position.
In tubular MRs, the density of the packed bed could be enhanced using multichannel tubular monoliths and depositing the catalyst inside the pores [21].
A further MRs classification can be summarized as reported below, and also schematically represented in Figure 6:
- catalytic membrane reactors (CMR);
- packed bed membrane reactors (PBMR);
- catalytic non-permselective membrane reactors (CNMR),
- non-permselective membrane reactors (NMR);
- reactant-selective packed bed reactors (RSPBR).
4. Application of Silica Membranes in MR Systems
Among different technologies involved in hydrogen generation and purification, MRs seem to be the most promising [10,11,12]. As stated above, the specific thermodynamic constrains which limit the TR’s performance can be circumvented by using the MRs. Being an open system, an MR allows the selective removal of a desired product from the reaction side for permeation through the membrane while shifting the reaction system towards the products. Consequently, the reaction conversion is enhanced, with the possibility of overcoming the thermodynamic conversion of the correspondent TR [13].
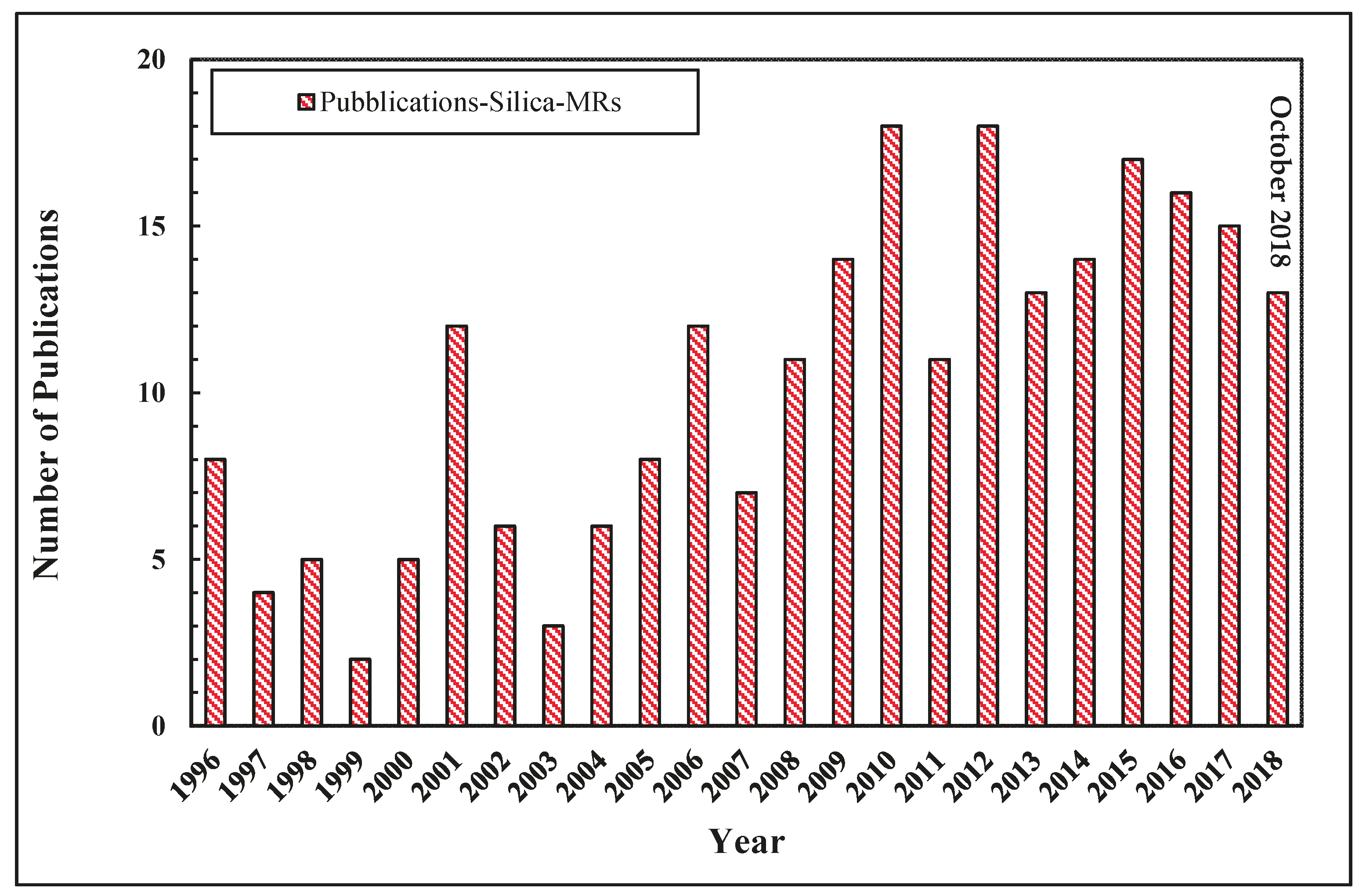
Palladium membranes were mostly applied in MRs for high-grade hydrogen generation [22,23,24,25,26,27,28,29], but in recent years several scientists investigated other membrane materials to evaluate the effectiveness of non Pd-based membranes applied to MRs, with the main purpose of achieving more economical solutions than Pd-based membranes utilization [30,31]. Compared to other membranes, silica membranes and their applications in MRs for hydrogen generation received particular attention since they are cheaper, show higher permeability and result in more robust than Pd-based membranes [30,32,33,34,35]. As illustrated in Figure 7, the interest towards silica MRs from both an experimental and modeling point of view is testified by an increasing number of scientific publications in this field.
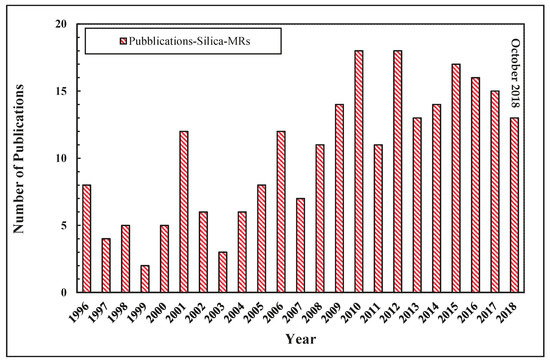
Figure 7.
Trend of scientific publications for the silica MRs versus year, Scopus database: www.scopus.com.
As summarized in Table 3, silica MRs utilization makes possible several benefits over the TRs, although they also present some disadvantages. The most significative advantage is represented by the cost, which is substantially reduced with respect to the TRs, whereas the most critical issue is represented by the not full H2 perm-selectivity of silica membranes, making them useless for high-grade hydrogen separation/purification instead of Pd-based membranes.

Table 3.
Advantages and disadvantages of silica MRs utilization over TRs.
In recent years, one of the most promising fields of silica MRs application was that of greenhouse gases reduction combined to hydrogen production. Hence, in the following paragraph, a literature overview about the theoretical approach on silica MRs to evaluate their performance in the field of hydrogen generation from reforming reactions is detailed, reported, and discussed.
Modeling of Silica MRs
It is generally accepted that the main benefit deriving from the modeling of such a process is due to cost saving, originated by the reduction of the experimental tests number. Concerning silica membranes and their applications in MRs, computational fluid dynamics (CFD), black box and molecular dynamic (MD) models were applied in various studies as addressed in literature [32,33,34], but most of them are based on 1-D model with a mass balance equation; in other cases, 2-D models were based on the utilization of CFD method. Table 4 reports some of the most representative modeling studies on silica MRs, highlighting the main details about them.

Table 4.
A summary of evaluation for silica MR performance from modeling viewpoints.
Koukou et al. [36] developed two mathematical models with different levels of complexity as tools for designing and optimizing silica MRs, adopted for operations at industrial scale. In particular, they theoretically evaluated the design of a silica MR with high hydrogen perm-selectivity, integrated in a gasification combined cycle unit for better controlling CO2 emissions and the whole process energy efficiency, adopting both a simple and a CFD-based model. These authors reached a good understanding of the factors determining the silica MR performance during a water gas shift (WGS) process, observing that:
- the simple model represents a useful tool for a preliminary evaluation of the WGS-MR performance.
- The CFD-based model can be adopted for designing the WGS-MR and for optimising the silica MR performance.
- The desired MR performance can be provided with a high perm-selective silica membrane.
Prabhu et al. [37] proposed a 1-D mass balance-based model to evaluate the performance of three reactor configurations: a fixed-bed reactor, a partially selective MR, and a totally selective MR. Methane dry reforming process was carried out at ambient pressure over a Rh/Al2O3 catalyst. The experimental data were obtained by using a perm-selective silica membrane (Nanosil) and a semipermeable Vycor glass membrane, observing that methane conversion increased in the following order: plug-flow < Vycor glass < Nanosil. Furthermore, simulation results highlighted that pressure drop and axial temperature gradients across the catalytic bed were not noticeable.
In particular, Table 5 reports the good agreement between theoretical and experimental values for the reverse WGS reaction.

Table 5.
Reverse WGS reaction: comparison between theoretical and experimental results for fixed bed reactor, Vycor, and Nanosil MRs. With permission of reprint from Elsevier by Prabhu et al. [37].
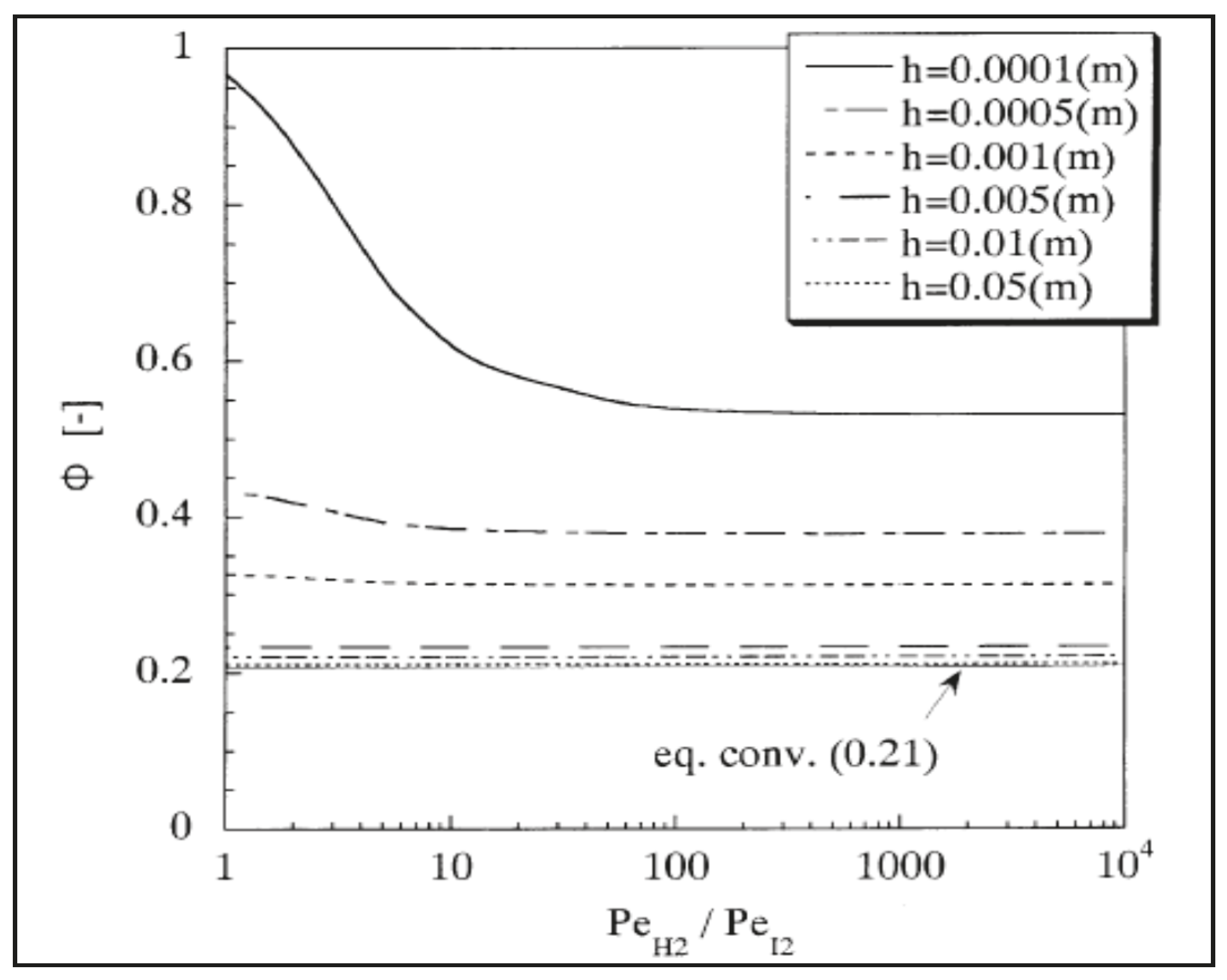
Hwang and Onuki [38] theoretically investigated hydrogen iodide decomposition in a silica MR to produce hydrogen for further utilization in a thermochemical iodine-sulfur process. The simulations evidenced a conversion higher than 90%. In particular, Figure 8 shows HI conversion versus H2/I2 perm-selectivity at different h values (h value represents the ratio between reaction zone volume and silica membrane surface area).
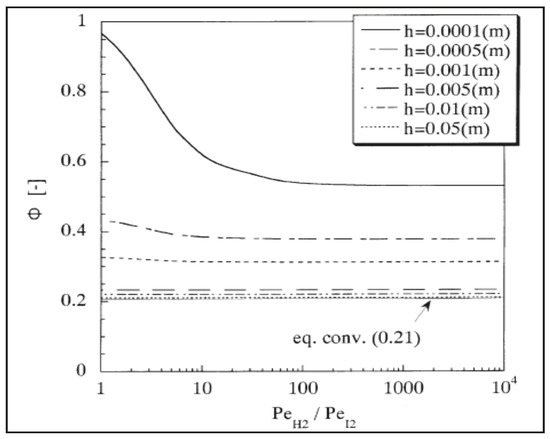
Figure 8.
HI conversion versus H2/I2 perm-selectivity for silica membrane at various h values. With permission of reprint from Elsevier by Hwang and Onuki [38].
At h = 0.05, 0.01, 0.005, 0.001, and 0.0005, HI conversions were simulated to be comparable by increasing H2/I2 permselectivity. At h = 0.0001, HI conversion would be high at very low selectivity (H2/I2 perm-selectivity ~1). At higher h values (>0.0005), the permeation rate of the products becomes lower than the reaction rate due to DH2 being lower than 1. In summary, these authors theoretically demonstrated that H2/I2 perm-selectivities above 100 do not substantially induce further shift effect on the MR conversion, confirming that silica membranes could represent a better solution than more hydrogen perm-selective membranes.
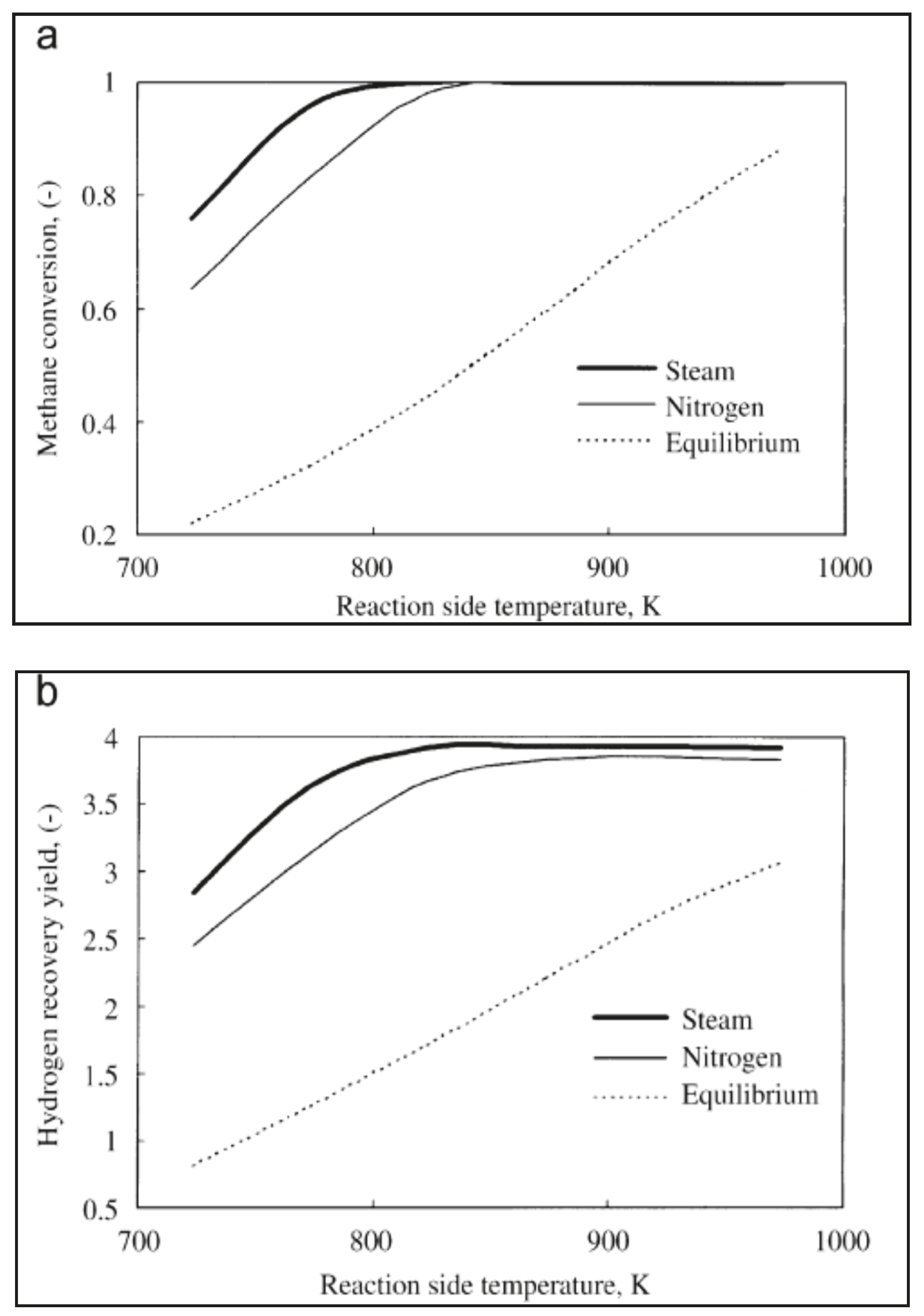
Yu et al. [39] used a 1-D mathematical model to study methane steam reforming reaction performed in a silica MR, analyzing the effect of various parameters, such as reaction temperature, feed flow rate, typology of sweep gas, and its flow rate as well as its flow pattern configuration.
A counter-current flow pattern was indicated as better modality to carry out this process, while Figure 9a,b points out how steam, when used as sweep gas, allowed better methane conversion and hydrogen recovery over nitrogen. This was described with the different partial pressure profiles of components on the reaction and permeation sides.
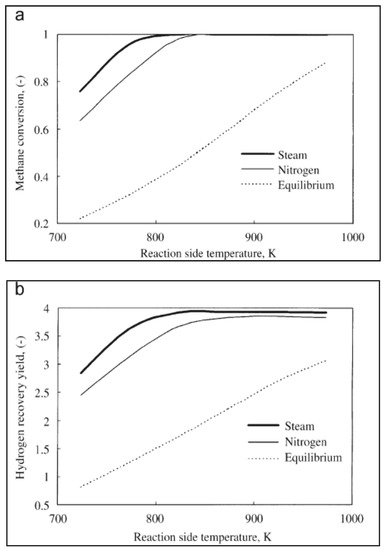
Figure 9.
Effects of different sweep gas utilization on silica MR performance: (a) methane conversion vs. reaction temperature; (b) hydrogen recovery vs. reaction temperature. With permission of reprint from Elsevier by Yu et al. [39].
For the same reaction, Oyama and Hacarlioglu [40] developed both 1-D and 2-D models without adjustable parameters, useful for describing the performance of another silica MR. These authors evaluated when a 2-D model should be applied instead of a 1-D model for MR performance evaluation, stating that a 2-D model is essential when both deviations from plug-flow MR behavior and permeation rate are higher than reaction rate take place. Table 6 shows the predicted production yields of H2, CO and CO2 using a 2-D model versus experimental data, simulating both a conventional packed-bed reactor (PBR) and a silica MR, operated at 600 and 650 °C. At higher pressures, the theoretical results were slightly higher than the experimental values. The 2-D model effectively predicted an increase of production yields as a consequence of the reaction temperature increase from 600 to 650 °C.

Table 6.
Estimation of product Yields by 2-D model versus experimental data. With permission to reprint from Elsevier by Oyama and Hacarlioglu [40].
Tsuru et al. [41] studied the effects of various parameters such as silica membrane perm-selectivity, permeation, and reaction rates on WGS reaction carried out in a silica MR. According to their theoretical results, when the Damkhöler number was approximatively equal to the permeation number, maximum CO conversion improvement was achieved. However, enhancements in CO conversion may be attained even when silica membranes show low perm-selectivity values (~10).
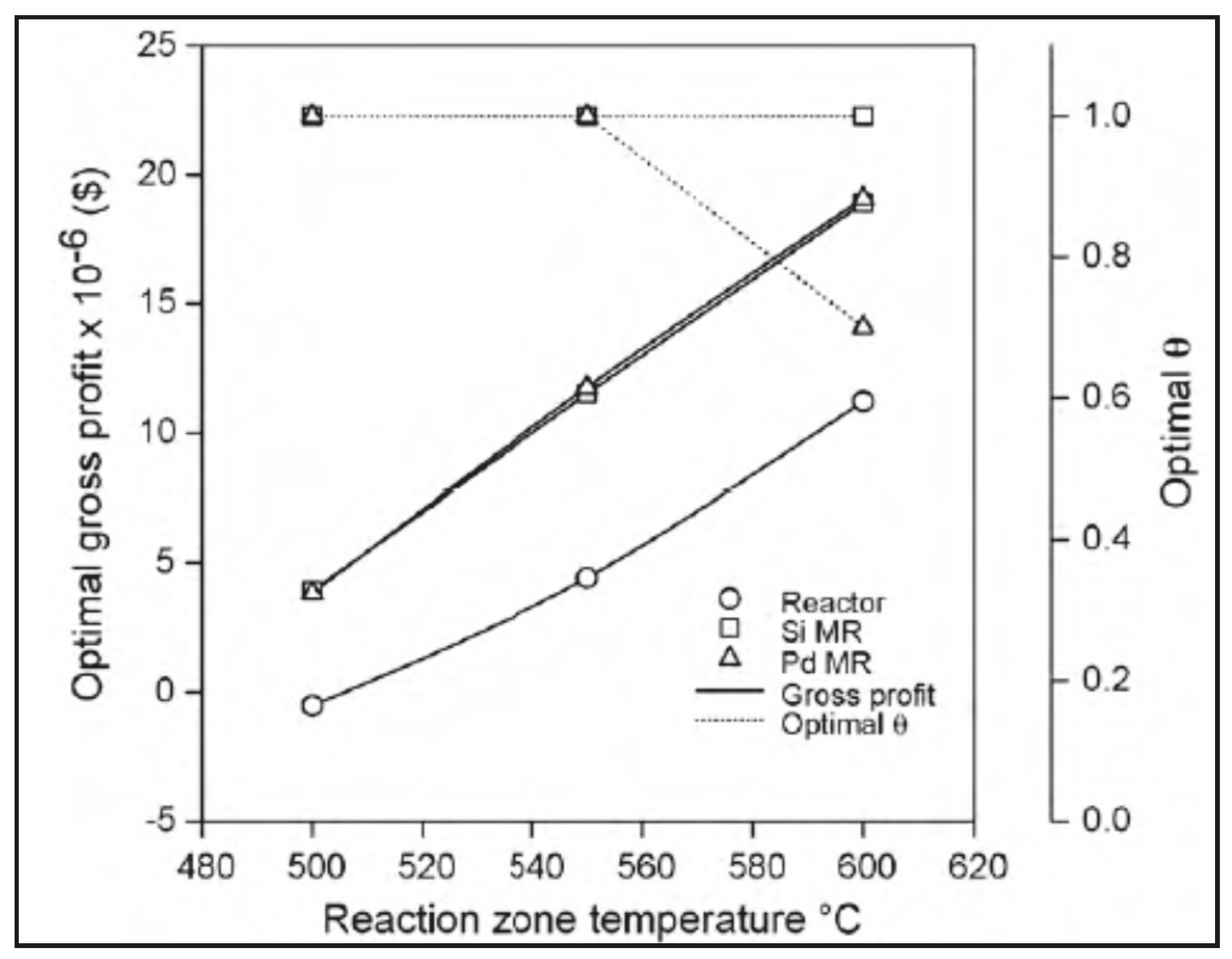
Moparthi et al. [42] theoretically analysed the economic feasibility of silica and palladium MRs used for performing dehydrogenation reactions. Hence, they used a theoretical design-based simulation strategy for the comparative economic assessment of MRs and TRs. In details, the propylene production process was studied to provide 60–70% extra profits using MRs in comparison to TRs. The gross profit profiles for both MR and TR schemes were found to be similar to the case of styrene production. In both cases, it was estimated that the cost contribution of membranes and other auxiliary equipments did not exceed 20% of the total costs. It was concluded that the industrial applicability of silica/Pd-based MRs was economically feasible for those dehydrogenation reactions that enable higher conversions than those of the equivalent TRs. Figure 10 illustrates an example of a comparative economic performance of TR, silica, and Pd-based MRs at different temperatures. As indicated, the TR configuration provided an optimal gross profit of −0.52 M$ at 500 °C, which increased to 4.4 M$ at 550 °C, and 11.2 M$ at 600 °C, with the recommendation that industrial operations should not consider temperatures higher than 550 °C due to possible coking process effects. Both silica and palladium MRs provided higher optimal gross profit values, varying from 4.5 (at 500 °C) to 18 M$ (at 600 °C). In other words, it can be detected from this figure that both silica and Pd MRs perform better than the TR.
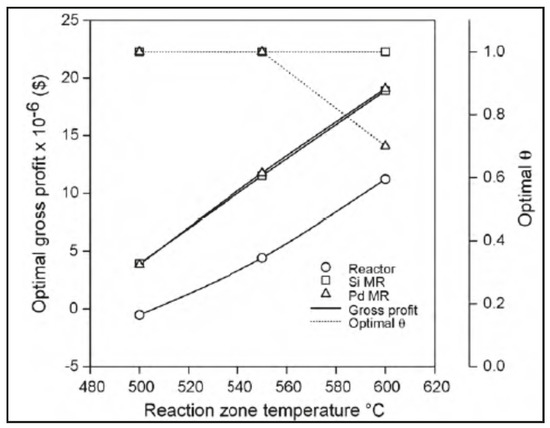
Figure 10.
Economic evaluation silica and Pd-based MRs with TR vs. reaction temperature. With permission to reprint from Elsevier by Moparthi et al. [42].
Furthermore, the economic evaluation of both silica and palladium membranes performance indicated that, while palladium membrane provided higher values of hydrogen flux and selectivity at higher cost, silica membrane provided moderate combinations of hydrogen flux and selectivity at lower costs.
Ghasemzadeh et al. [43,44] developed a 1-D isothermal model to compare, from a modeling point of view, a silica MR with a Pd-Ag MR, both used in a methanol steam reforming process for producing hydrogen. The simulations indicated that silica MR performance was comparable to that of the Pd-Ag MR in terms of methanol conversion operating at low temperature (200 °C) and high space velocity (>2000 h−1). As reported in Table 7, at a higher silica membrane permselectivity, both higher methanol conversion and hydrogen recovery were reached. For example, adopting a silica membrane showing H2/N2 ideal selectivity = 600, ~75% methanol conversion and ~67% hydrogen recovery could be obtained, whereas ~81% methanol conversion and ~52% hydrogen recovery could be reached using a dense hydrogen fully perm-selective Pd-Ag membrane.

Table 7.
Evaluation of the perm-selectivity effects on silica MR performance over to the dense Pd-Ag MR (at 523 K, 2 bar, sweep factor = 2.5, H2O/CH3OH = 1 and gas hourly space velocity = 1700 h−1). With permission to reprint from Elsevier from Ghasemzadeh et al. [43].
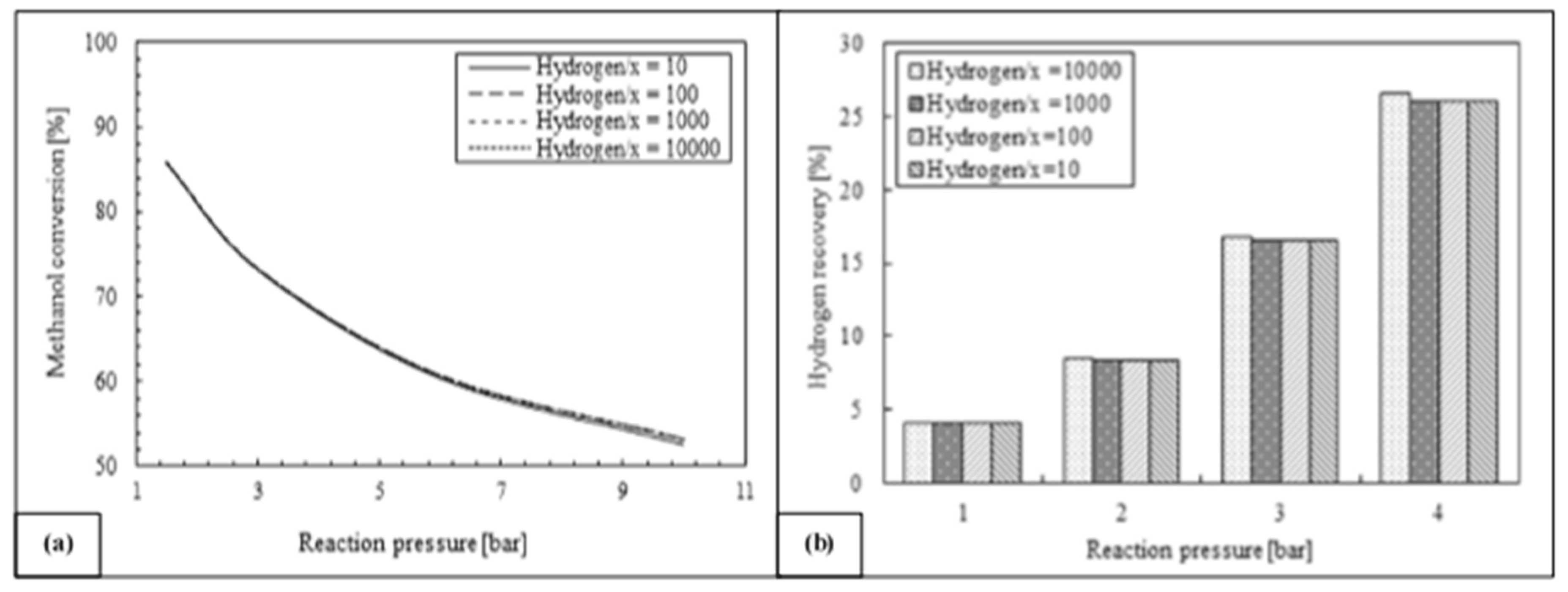
Furthermore, Ghasemzadeh et al. [45] presented a further detailed quantitative analysis of a silica MR performance during methanol steam reforming reaction for hydrogen production. Figure 11 shows how higher hydrogen perm-selectivities of silica membrane can improve the hydrogen recovery, leaving a substantial constant trend of methanol conversion. Nevertheless, higher hydrogen selectivity in lower ranges of hydrogen permeance could not be more effective on silica MR performance during the reaction process.
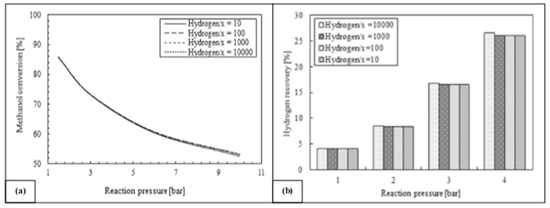
Figure 11.
Methanol conversion (a) and hydrogen recovery (b) vs. reaction pressure at various membrane hydrogen perm-selectivity, 523 K, H2O/CH3OH = 3, 0.03 ml/min and 10−8 order of hydrogen permeance. With permission to reprint from Elsevier by Ghasemzadeh et al. [45].
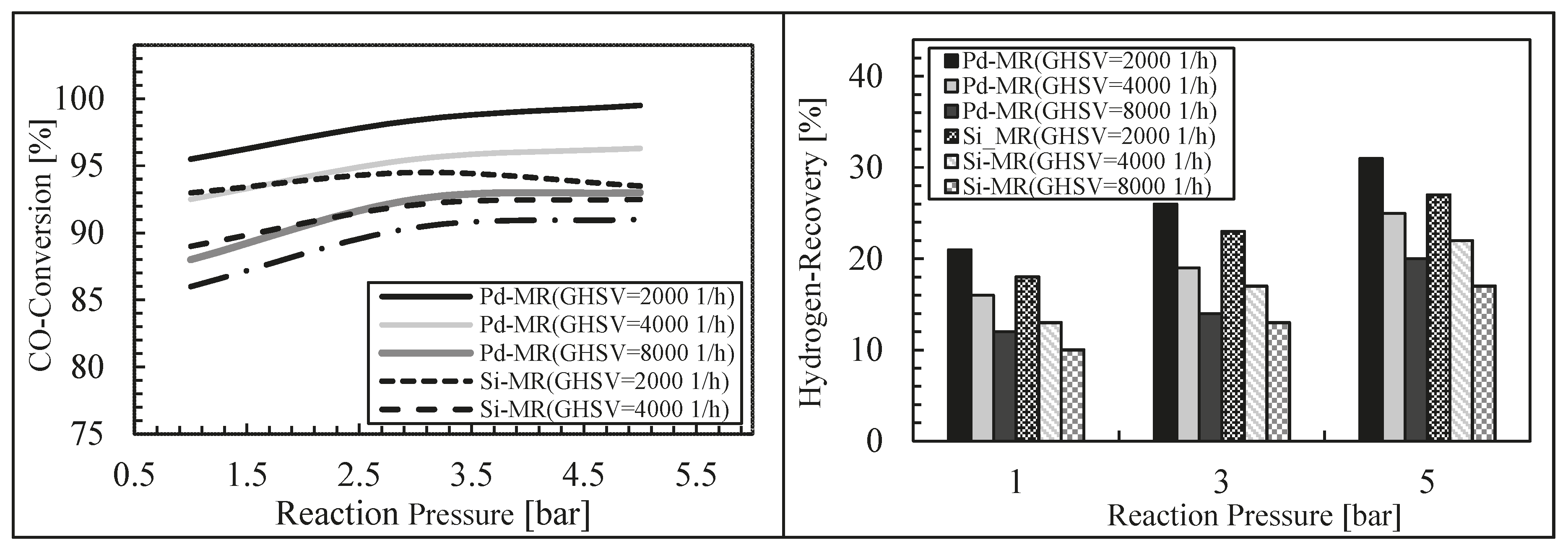
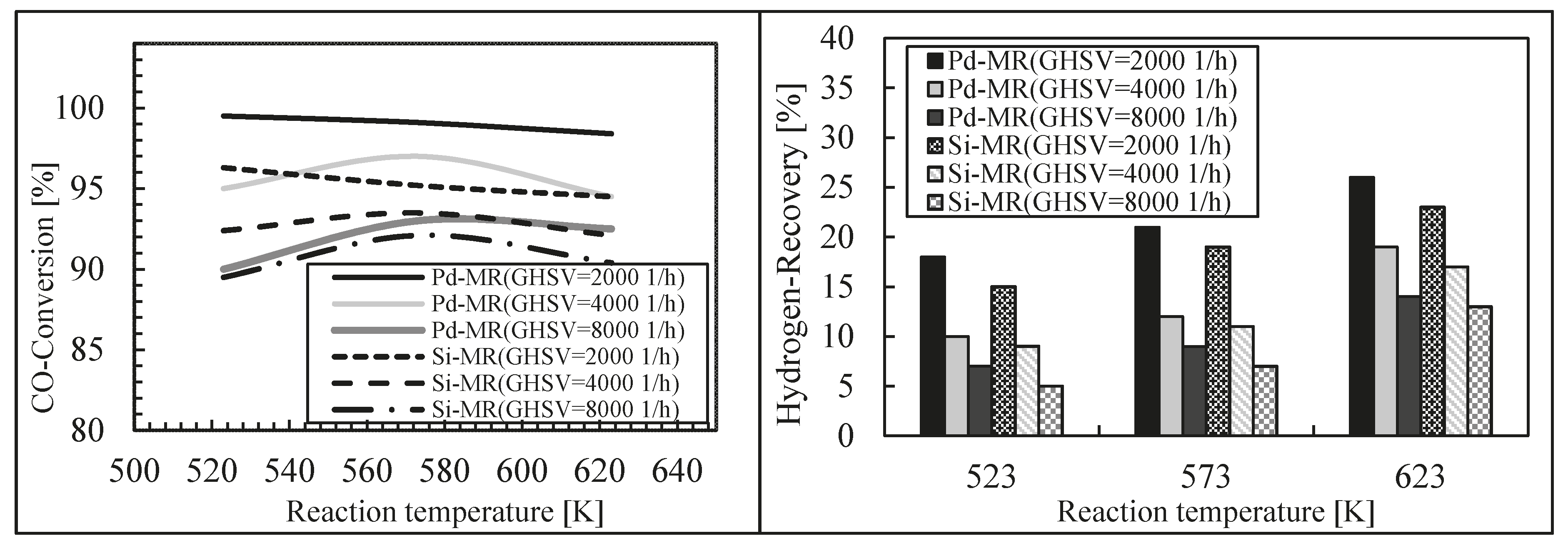
Finally, these authors modeled a silica MR during an WGS reaction, theoretically comparing its performance with a Pd-Ag MR [46]. For this purpose, a 1-D isothermal mathematical model was developed and its validation was carried out by using experimental data coming from literature, achieving a good matching between simulation and experimental results. After model validation, the effects of some significant operating parameters on the performance of both MRs were studied in terms of hydrogen recovery and CO conversion. The simulations showed lower performance for the silica MR in terms of CO conversion and hydrogen recovery with respect to those of the Pd-Ag MR, while the reaction temperature evidenced dual effects at various space velocities for both MRs (Figure 12 and Figure 13).
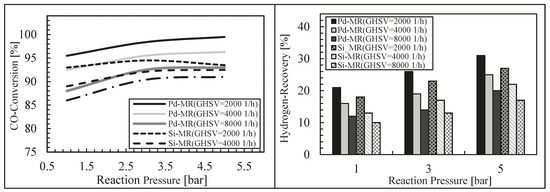
Figure 12.
CO conversion and hydrogen recovery vs. reaction pressure for the dense Pd-Ag and silica MRs and TR at 623 K, H2O/CO = 1 and three different space velocity values. With permission to reprint from Elsevier by Ghasemzadeh et al. [46].
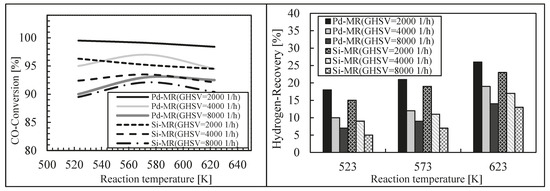
Figure 13.
CO conversion and hydrogen recovery vs. reaction temperature for the dense Pd-Ag and silica MRs and TR at 3 atm, H2O/CO = 1 and three different space velocity values. With permission to reprint from Elsevier by Ghasemzadeh et al. [46].
Nevertheless, this theoretical study evidenced how the silica MR during WGS reaction presented an acceptable performance in comparison with a Pd-Ag MR in the case where its hydrogen permselectivity is higher than 400 and possessing a hydrogen permeance higher than 5 × 10−7 mol/m2·Pa·s.
5. Conclusions and Future Trends
This review highlighted the recent advances in silica MRs for hydrogen generation from a modeling point of view. Taking into account that both the scientific community and industrial companies are constantly interested and devoted to investigate hydrogen production in more technically, environmentally, and economically attractive routes, silica MRs can be suggested as a viable alternative for hydrogen generation over the Pd-based MRs. Indeed, the development of low-cost, defect free, and effective silica membranes could be a chance for realistic applications of MRs at industrial scale, although it is still a challenge regarding their scale up due to the disadvantages illustrated in the previous paragraphs and for the limited operation times due to the aforementioned stability problems. In this review, several modeling works of silica MRs were analysed, pointing out the growing interest in the optimization of the processes themselves, with the purpose of proposing them as a viable alternative to Pd-based MRs and conventional reactors application. However, great attention should be paid in evaluating the effective balance between advantages and disadvantages when applying silica MR technology to produce hydrogen. The scale up of silica MRs will represent one of the most important challenges for the future. Specially, more research should be devoted to study the effects of operating and/or capital costs related to high reaction pressure and temperature in order to increase the silica MR’s performance.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
List of Acronyms
| ANN | Artificial Neural Network |
| CCS | Carbon capture and storage |
| CFD | Computational fluid dynamics |
| CMR | Catalytic membrane reactor |
| CNMR | Catalytic non perm-selective membrane reactors |
| FBR | Fixed bed reactor |
| MD | Molecular dynamics |
| MR | Membrane reactor |
| NMR | Non perm-selective membrane reactors |
| PBMR | Packed bed membrane reactors |
| PEMFC | Proton exchange membrane fuel cell |
| RSPBR | Reactant-selective packed bed reactors |
| TR | Traditional reactor |
| WGS | Water gas shift |
List of Symbols
| D0 | mean intrinsic diffusion coefficient for micropore diffusion (m2·s−1) |
| K0 | intrinsic Henry constant (-) |
| ε | membrane porosity (-) |
| L | membrane thickness (m) |
| bulk density (Kg/m3) | |
| qst | isosteric heat adsorption (J/mol) |
| Ei | activation energy for gas species (KJ/mol) |
| R | universal gas constant (J/mol·K) |
| T | temperature (K) |
References
- Bujnicki, J.; Dykstra, P.; Fortunato, E.; Heuer, R.-D.; Keskitalo, C.; Nurse, P. Novel carbon capture and utilisation technologies, Scientific Opinion 4/2018 (Supported by SAPEA Evidence Review Report No 2), European Commission—Directorate-General for Research and Innovation Unit RTD.DDG1.02—Scientific Advice Mechanism, Brussel. 23 May 2018. Available online: https://ec.europa.eu/research/sam/pdf/sam_ccu_report.pdf (accessed on 18 October 2018).
- Ji, G.; Zhao, M. Membrane separation technology in carbon capture. In Recent Advances in Carbon Capture and Storage; Yun, Y., Ed.; Intech Open Science: Rijeka, Croatia, 2017; pp. 59–90. [Google Scholar]
- Qiu, H.H.; Liu, L.G. A Study on the evolution of carbon capture and storage technology based on knowledge mapping. Energy 2018, 11, 1103. [Google Scholar] [CrossRef]
- AIulianelli, A.; Basile, A. Sulfonated PEEK-based polymers in PEMFC and DMFC applications: A review. Int. J. Hydrogen Eng. 2012, 37, 15241–15255. [Google Scholar] [CrossRef]
- Vivek, R.; Muthukumar, M.D. Performance improvement of Proton Exchange Membrane Fuel Cell. Innov. Energy Res. 2018, 7, 1–5. [Google Scholar]
- Basu, S. Proton Exchange Membrane Fuel Cell Technology: India’s Perspective. Proc. Indian Natl. Sci. Acad. 2015, 81, 865–890. [Google Scholar] [CrossRef]
- Basile, A.; Iulianelli, A. Advances in Hydrogen Production, Storage and Distribution, 1st ed.; Woodhead Publishing: Sawston, UK, 7 July 2014; pp. 1–546. ISBN 9780857097682. [Google Scholar]
- Voitic, G.; Pichler, B.; Basile, A.; Iulianelli, A.; Malli, K.; Bock, S.; Hacker, V. Chapter 10—Hydrogen Production. In Fuel Cells and Hydrogen; Hacker, V., Mitsushima, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 215–241. ISBN 9780128114599. [Google Scholar]
- Lu, N.; Xie, D. Novel membrane reactor concepts for hydrogen production from hydrocarbons: A review. Int. J. Chem. React. Eng. 2015, 14, 1–31. [Google Scholar] [CrossRef]
- Gallucci, F.; Fernandez, E.; Corengia, P.; van Sint, M. Annaland, Recent advances on membranes and membrane reactors for hydrogen production. Chem. Eng. Sci. 2013, 92, 40–66. [Google Scholar] [CrossRef]
- Lu, G.Q.; da Costa, J.C.D.; Duke, M.; Giessler, S.; Socolow, R.; Williams, R.H.; Kreutz, T. Inorganic membranes for hydrogen production and purification: A critical review and perspective. J. Coll. Interface Sci. 2007, 314, 589–603. [Google Scholar] [CrossRef]
- Pacheco, D.T.; van Sint Annaland, M.; Gallucci, F. Recent advances in Pd-based membranes for membrane reactors. Molecules 2017, 22, 51. [Google Scholar]
- Abdallah, H. A review on catalytic membranes production and applications. Bull. Chem. React. Eng. Catal. 2017, 12, 136–156. [Google Scholar] [CrossRef]
- Ayral, A.; Julbe, A.; Roualdes, S.; Rouessac, V.; Durand, J.; Sala, B. Silica membranes-basic principles. Chem. Eng. 2006, 50, 67–79. [Google Scholar]
- Sugawara, T.; Takagaki, A.; Kikuchi, R. Review on mechanisms of gas permeation through inorganic membranes. J. Jpn. Petrol. Inst. 2011, 54, 298–309. [Google Scholar]
- Lee, P.S.; Lee, K.H. Inorganic membranes for gas separation. In Membrane Engineering for the Treatment of Gases: Volume 2: Gas-Separation Issues Combined with Membrane Reactors, 2nd ed.; Drioli, E., Barbieri, G., Brunetti, A., Eds.; Royal Society of Chemistry: London, UK, 2017; Chapter 10; pp. 303–357. ISBN 978-1-78262-875-0. [Google Scholar]
- Li, C.; Meckler, S.M.; Smith, Z.P.; Bachman, J.E.; Maserati, L.; Long, J.R.; Helms, B.A. Engineered transport in microporous materials and membranes for clean energy technologies. Adv. Mater. 2018, 30, 1704953–1704986. [Google Scholar] [CrossRef] [PubMed]
- Fotou, G.P.; Lin, Y.S.; Pratsinis, S.E. Hydrothermal stability of pure and modified microporous silica membranes. J. Mater. Sci. 1995, 30, 2803–2808. [Google Scholar] [CrossRef]
- Rowe, B.W.; Robeson, L.M.; Freeman, B.D.; Paul, D.R. Influence of temperature on the upper bound: Theoretical considerations and comparison with experimental results. J. Membr. Sci. 2010, 360, 58–69. [Google Scholar] [CrossRef]
- De Lange, R.; Keizer, K.; Burggraf, A.J. Analysis and theory of gas transport in microporous sol–gel derived ceramic membranes. J. Membr. Sci. 1995, 104, 81–100. [Google Scholar] [CrossRef]
- Dixon, A.G. Recent research in catalytic inorganic membrane reactors. Int. J. Chem. React. Eng. 2003, 1, 1–35. [Google Scholar] [CrossRef]
- Alique, D.; Martinez-Diaz, D.; Sanz, R.; Calles, J. Review of supported Pd-based membranes preparation by electroless plating for ultra-pure hydrogen production. Membranes 2018, 8, 5–44. [Google Scholar] [CrossRef]
- Cardoso, S.P.; Azenha, I.S.; Lin, Z.; Portugal, I.; Rodrigues, A.E.; Silva, C.M. Inorganic membranes for hydrogen separation. Sep. Purif. Rev. 2018, 47, 229–266. [Google Scholar] [CrossRef]
- Rahimpour, M.R.; Samimi, F.; Babapoor, A.; Tohidian, T.; Mohebi, S. Palladium membranes applications in reaction systems for hydrogen separation and purification: A review. Chem. Eng. Proc.: Process Intens. 2017, 121, 24–49. [Google Scholar] [CrossRef]
- Dolan, M.D. Non-Pd BCC alloy membranes for industrial hydrogen separation. J. Membr. Sci. 2010, 362, 12–28. [Google Scholar] [CrossRef]
- Murmura, M.A.; Cerbelli, S.; Annesini, M.C. Modeling fixed bed membrane reactors for hydrogen production through steam reforming reactions: A critical analysis. Membranes 2018, 19, 8. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh, M.; Aghili, F. Recent advances in membrane reactors for hydrogen production by steam reforming of ethanol as a renewable resource. Rev. Chem. Eng. 2018, in press. [Google Scholar] [CrossRef]
- Iulianelli, A.; Longo, T.; Liguori, S.; Basile, A. Production of hydrogen via glycerol steam reforming in a Pd-Ag membrane reactor over Co-Al2O3 catalyst. Asia-Pacific J. Chem. Eng. 2010, 5, 138–145. [Google Scholar] [CrossRef]
- Gallucci, F.; Medrano, J.; Fernandez, E.; Melendez, J.; Annaland, M.V.; Pacheco, A. Advances on high temperature Pd-based membranes and membrane reactors for hydrogen purification and production. J. Membr. Sci. Res. 2017, 3, 142–156. [Google Scholar]
- Ghasemzadeh, K.; Aghaeinejad-Meybodi, A.; Iulianelli, A.; Basile, A. Theoretical performance evaluation of not Pd-based membranes for hydrogen separation. J. Membr. Sci. Res. 2018, 4, 198–203. [Google Scholar]
- Yin, H.; Yip, A.C.K. A review on the production and purification of biomass-derived hydrogen using emerging membrane technologies. Catalysts 2018, 7, 297–327. [Google Scholar] [CrossRef]
- Ghasemzadeh, K.; Tilebon, S.M.S.; Basile, A. Modeling of silica membranes. In Current Trends and Future Developments on (Bio-) Membranes; Basile, A., Ghasemzadeh, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Chapter 6; pp. 135–153. ISBN 9780444638663. [Google Scholar]
- Ghasemzadeh, K.; Aghaeinejad-Meybodi, A.; Basile, A. Hydrogen production as a green fuel in silica membrane reactor: Experimental analysis and artificial neural network modeling. Fuel 2018, 222, 114–124. [Google Scholar] [CrossRef]
- Ghasemzadeh, K.; Zeynali, R.; Basile, A.; Iulianelli, A. CFD analysis of a hybrid sorption-enhanced membrane reactor for hydrogen production during WGS reaction. Int. J. Hydrog. Eng. 2017, 42, 26914–26923. [Google Scholar] [CrossRef]
- Meng, L.; Tsuru, T. Hydrogen production from energy carriers by silica-based catalytic membrane reactors, Catal. Today 2016, 268, 3–11. [Google Scholar] [CrossRef]
- Koukou, M.K.; Papayannakos, N.; Markatos, N.C.; Bracht, M.; Alderliesten, P.T. Simulation tools for the design of industrial-scale membrane reactors. Chem. Eng. Res. Des. 1998, 76, 911–920. [Google Scholar] [CrossRef]
- Prabhu, A.K.; Liu, A.; Lovell, L.G.; Ted Oyama, S. Modeling of the methane reforming reaction in hydrogen selective membrane reactors. J. Membr. Sci. 2000, 177, 83–95. [Google Scholar] [CrossRef]
- Hwang, G.J.; Onuki, K. Simulation study on the catalytic decomposition of hydrogen iodide in a membrane reactor with a silica membrane for the thermochemical water-splitting IS process. J. Membr. Sci. 2001, 194, 207–215. [Google Scholar] [CrossRef]
- Yu, W.; Ohmori, T.; Kataoka, S.; Yamamoto, T. A comparative simulation study of methane steam reforming in a porous ceramic membrane reactor using nitrogen and steam as sweep gases. Int. J. Hydrog. Eng. 2008, 33, 685–692. [Google Scholar] [CrossRef]
- Oyama, S.T.; Hacarlioglu, P. The boundary between simple and complex descriptions of membrane reactors: The transition between 1-D and 2-D analysis. J. Membr. Sci. 2009, 337, 188–199. [Google Scholar] [CrossRef]
- Tsuru, T.; Morita, T.; Shintani, H.; Yoshioka, T. Membrane reactor performance of steam reforming of methane using hydrogen-permselective catalytic SiO2 membranes. J. Membr. Sci. 2008, 316, 53–62. [Google Scholar] [CrossRef]
- Moparthi, A.; Uppaluri, R.; Gill, B.S. Economic feasibility of silica and palladium composite membranes for industrial dehydrogenation reactions. Chem. Eng. Res. Des. 2010, 88, 1088–1101. [Google Scholar] [CrossRef]
- Ghasemzadeh, K.; Morrone, P.; Liguori, S.; Babaluo, A.A.; Basile, A. Evaluation of silica membrane reactor performance for hydrogen production via methanol steam reforming: Modeling study. Int. J. Hydrog. Eng. 2013, 38, 16698–16709. [Google Scholar] [CrossRef]
- Ghasemzadeh, K.; Morrone, P.; Iulianelli, A.; Liguori, S.; Babaluo, A.A.; Basile, A. H2 production in silica membrane reactor via methanol steam reforming: Modeling and HAZOP analysis. Int. J. Hydrog. Eng. 2013, 38, 10315–10326. [Google Scholar] [CrossRef]
- Ghasemzadeh, K.; Morrone, P.; Babaluo, A.A.; Basile, A. A simulation study on methanol steam reforming in the silica membrane reactor for hydrogen production. Int. J. Hydrog. Eng. 2015, 39, 2–11. [Google Scholar] [CrossRef]
- Ghasemzadeh, K.; Zeynali, R.; Basile, A. Theoretical study of hydrogen production using inorganic membrane reactors during WGS reaction. Int. J. Hydrog. Eng. 2016, 41, 8696–8705. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).