Empirical Kinetic Modelling of the Effect of l-Ascorbic Acid on the Cu(II)-Induced Oxidation of Quercetin
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Treatment, Sampling and UV-Vis Measurements
2.3. Large-Scale Treatment and Sample Preparation
2.4. Experimental Design
2.5. Liquid Chromatography-Diode Array-Mass Spectrometry (LC-DAD-MS)
2.6. Statistics
3. Results and Discussion
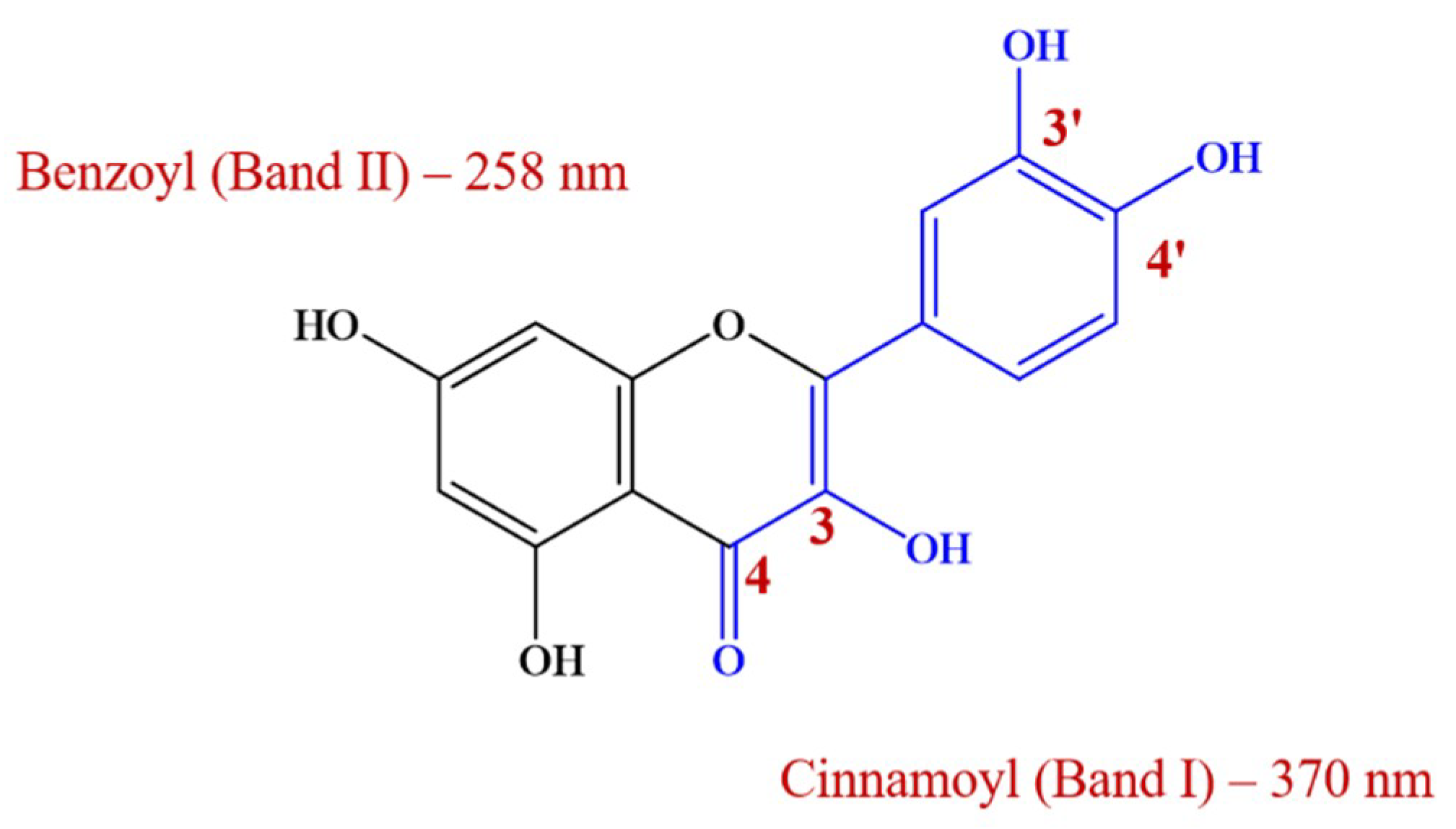
3.1. Initial Spectrophotometric Examination
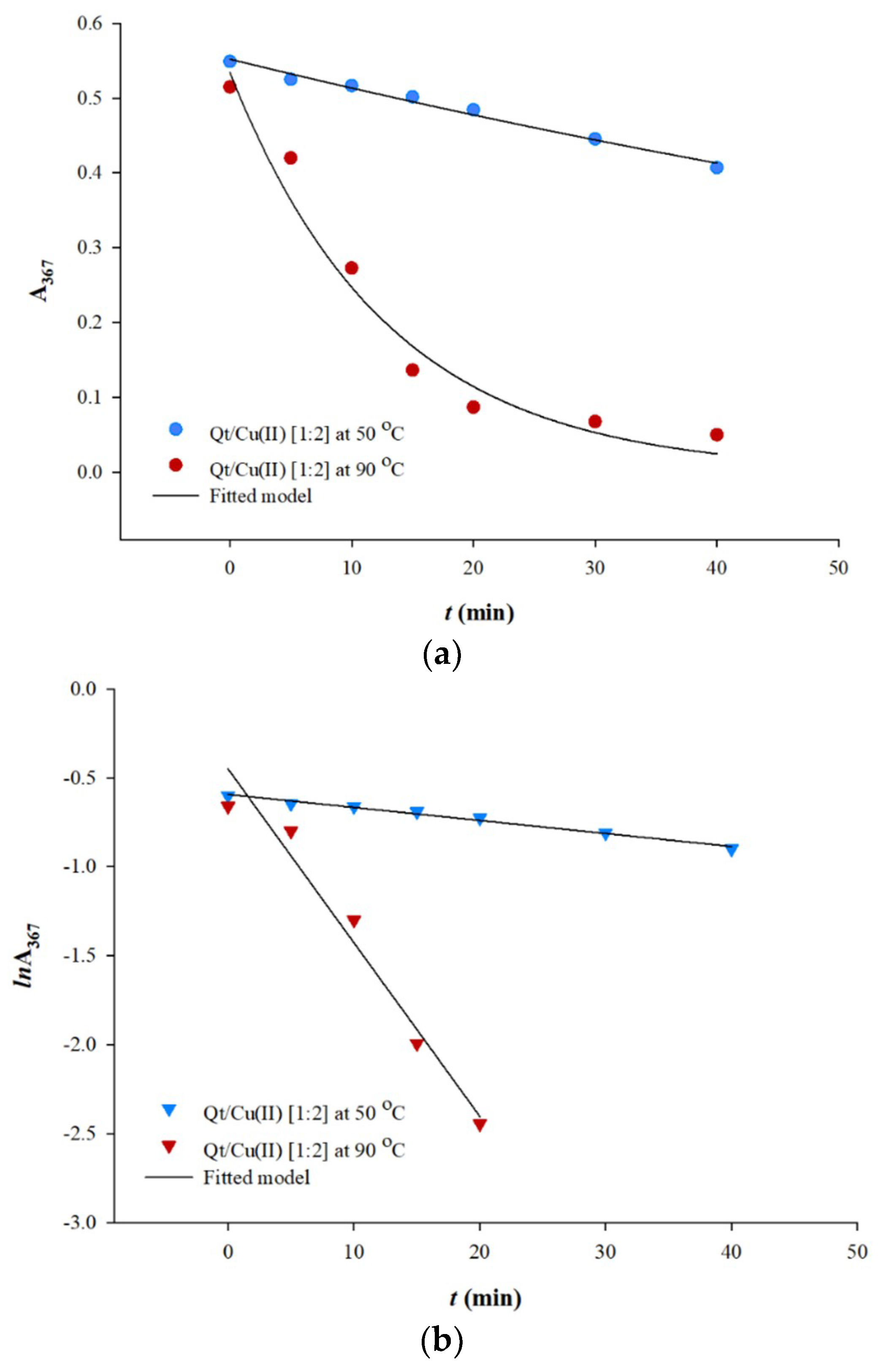
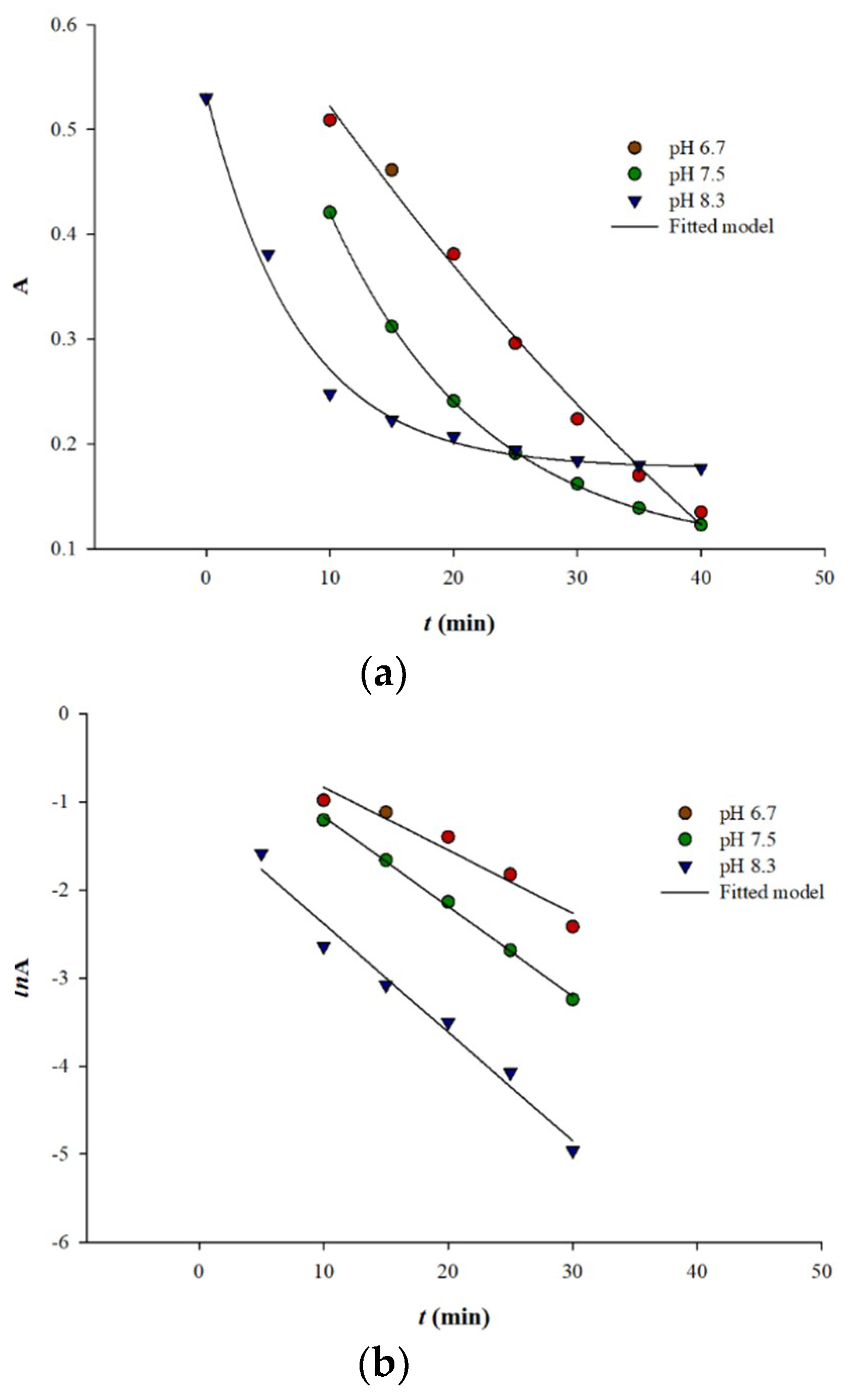
3.2. Preliminary Oxidation Kinetics and Temperature Effects
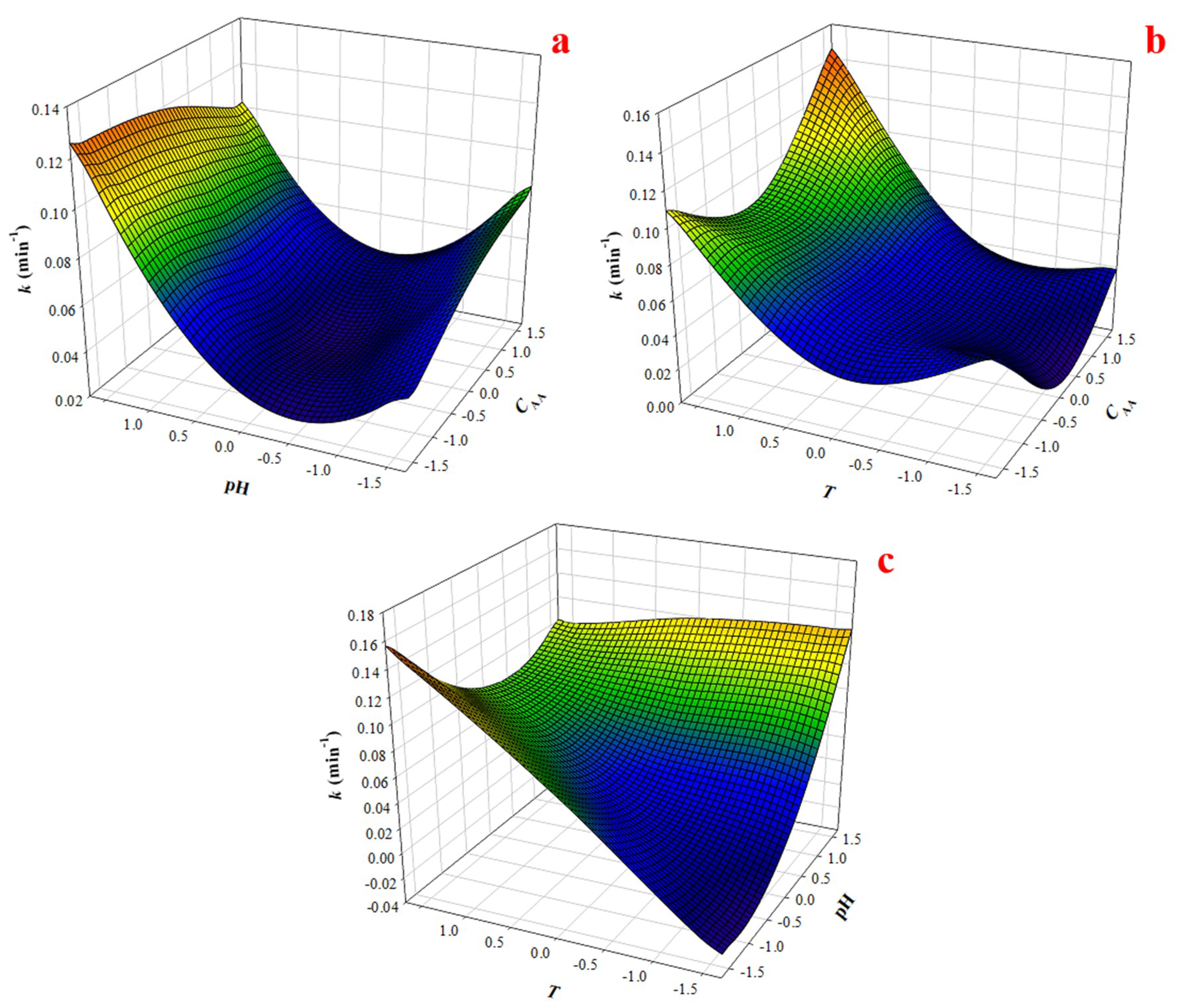
3.3. Response Surface Modelling
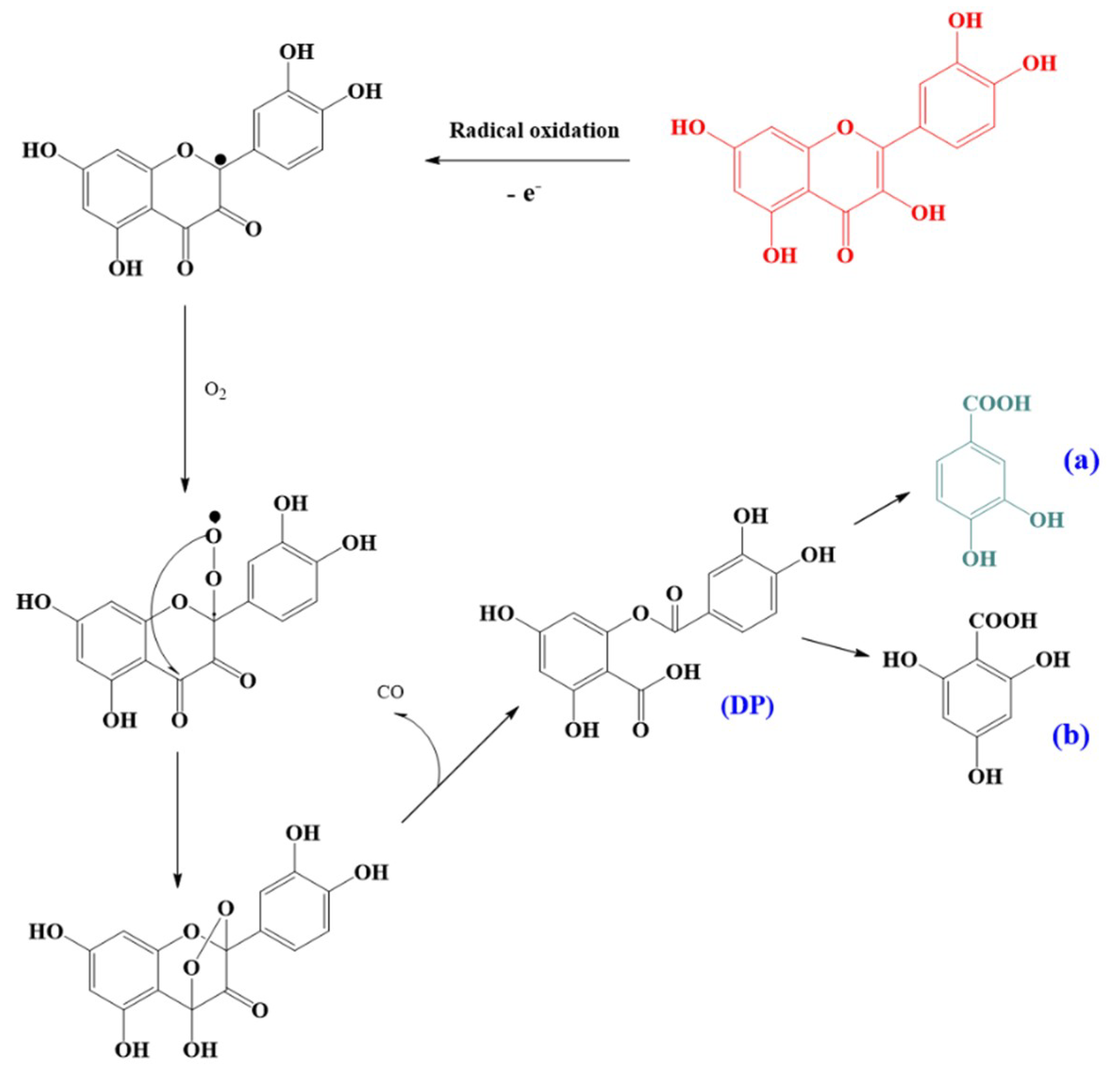
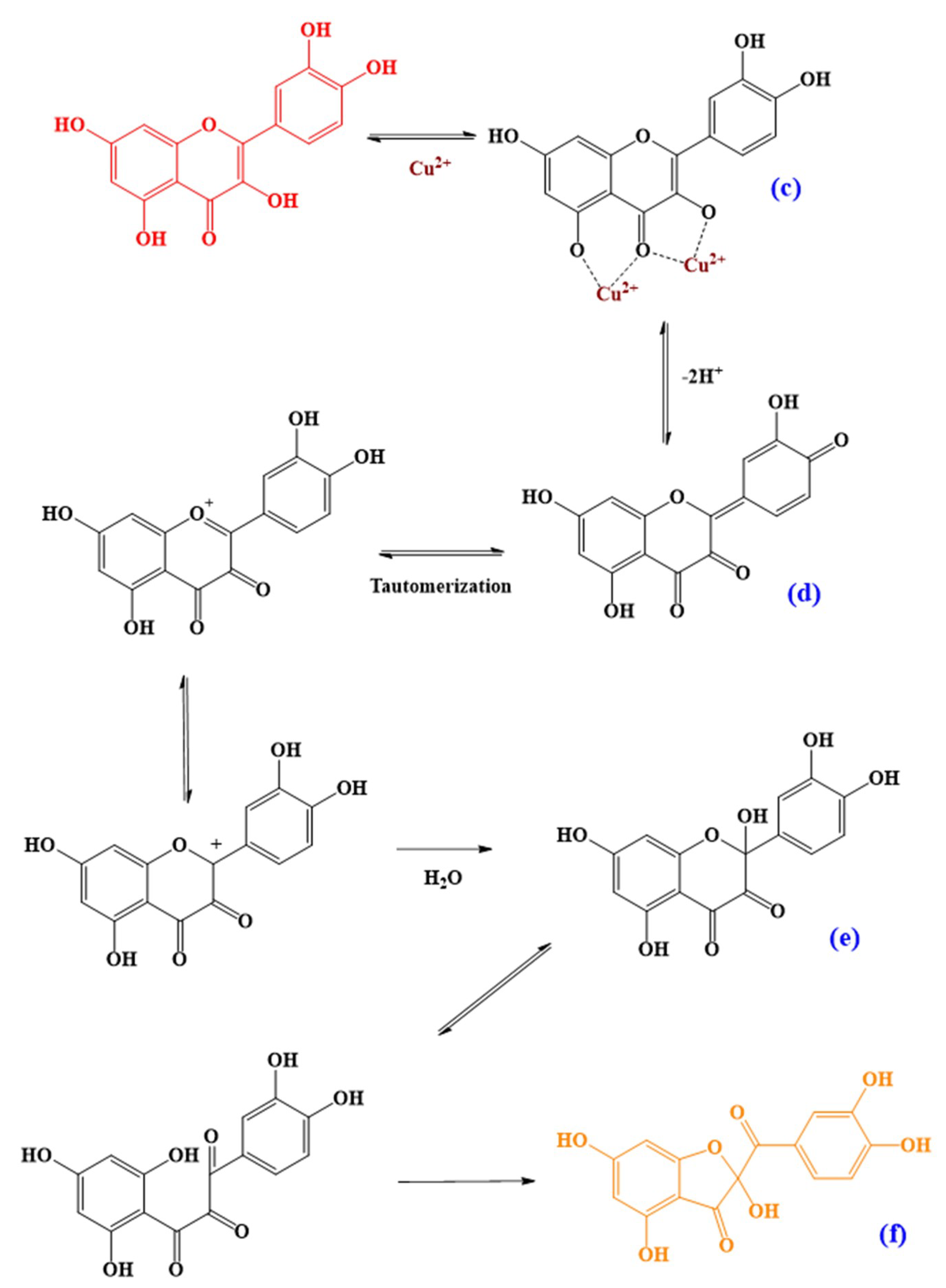
3.4. Oxidation Products and Putative Pathways
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| k | first-order quercetin decay constant (min−1) |
| CAA | l-ascorbic acid concentration (μM) |
| Ea | activation energy (kJ·mol−1) |
| R | universal gas constant (J·K−1·mol−1) |
| T | temperature (°C or K) |
| t | time (min) |
| t½ | half-life of quercetin (min) |
References
- Wang, W.; Sun, C.; Mao, L.; Ma, P.; Liu, F.; Yang, J.; Gao, Y. The biological activities, chemical stability, metabolism and delivery systems of quercetin: A review. Trends Food Sci. Technol. 2016, 56, 21–38. [Google Scholar] [CrossRef]
- Calani, L.; Dall’Asta, M.; Bruni, R.; Rio, D.D. Flavonoid occurrence, bioavailability, metabolism, and protective effects in humans: Focus on Flavan-3-ols and Flavonols. In Recent Advances in Polyphenol Research; Romani, A., Lattanzio, V., Quideau, S., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2014; Volume 4, pp. 239–279. [Google Scholar]
- Jan, A.T.; Kamli, M.R.; Murtaza, I.; Singh, J.B.; Ali, A.; Haq, Q. Dietary flavonoid quercetin and associated health benefits—An overview. Food Rev. Int. 2010, 26, 302–317. [Google Scholar] [CrossRef]
- Zhou, A.; Sadik, O.A. Comparative analysis of quercetin oxidation by electrochemical, enzymatic, autoxidation, and free radical generation techniques: a mechanistic study. J. Agric. Food Chem. 2008, 56, 12081–12091. [Google Scholar] [CrossRef] [PubMed]
- Makris, D.P.; Rossiter, J.T. Effect of natural antioxidants on heat-induced, copper (II)-catalysed, oxidative degradation of quercetin and rutin (quercetin 3-O-rutinoside) in aqueous model systems. J. Sci. Food Agric. 2002, 82, 1147–1153. [Google Scholar] [CrossRef]
- Gülşen, A.; Turan, B.; Makris, D.P.; Kefalas, P. Copper (II)-mediated biomimetic oxidation of quercetin: generation of a naturally occurring oxidation product and evaluation of its in vitro antioxidant properties. Eur. Food Res. Technol. 2007, 225, 435. [Google Scholar] [CrossRef]
- Gülşen, A.; Makris, D.P.; Kefalas, P. Biomimetic oxidation of quercetin: isolation of a naturally occurring quercetin heterodimer and evaluation of its in vitro antioxidant properties. Food Res. Int. 2007, 40, 7–14. [Google Scholar] [CrossRef]
- Fuentes, J.; Atala, E.; Pastene, E.; Carrasco-Pozo, C.; Speisky, H. Quercetin oxidation paradoxically enhances its antioxidant and cytoprotective properties. J. Agric. Food Chem. 2017, 65, 11002–11010. [Google Scholar] [CrossRef] [PubMed]
- Cort, W.M. Antioxidant properties of ascorbic acid in foods. In Ascorbic Acid: Chemistry, Metabolism, and Uses; Seib, P.A., Tolbert, B.M., Eds.; American Chemical Society: Washington, DC, USA, 2007; Volume 200, pp. 533–550. ISBN 9780841206328. [Google Scholar]
- Liao, M.-L.; Seib, P.A. Chemistry of l-ascorbic acid related to foods. Food Chem. 1988, 30, 289–312. [Google Scholar] [CrossRef]
- Takamura, K.; Ito, M. Effects of metal ions and flavonoids on the oxidation of ascorbic acid. Chem. Pharmaceut. Bul. 1977, 25, 3218–3225. [Google Scholar] [CrossRef]
- Beker, B.Y.; Sönmezoğlu, İ.; İmer, F.; Apak, R. Protection of ascorbic acid from copper (II)-catalyzed oxidative degradation in the presence of flavonoids: Quercetin, catechin and morin. Inter. J. Food Sci. Nutr. 2011, 62, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Mabry, T.J.; Markham, K.R.; Thomas, M.B. The ultraviolet spectra of flavones and flavonols. In The Systematic Identification of Flavonoids; Springer: Heidelberg, Germany, 1970; pp. 41–164. [Google Scholar]
- Makris, D.P.; Rossiter, J.T. Heat-induced, metal-catalyzed oxidative degradation of quercetin and rutin (quercetin 3-O-rhamnosylglucoside) in aqueous model systems. J. Agric. Food Chem. 2000, 48, 3830–3838. [Google Scholar] [CrossRef] [PubMed]
- Pękal, A.; Biesaga, M.; Pyrzynska, K. Interaction of quercetin with copper ions: complexation, oxidation and reactivity towards radicals. Biometals 2011, 24, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Hajji, H.E.; Nkhili, E.; Tomao, V.; Dangles, O. Interactions of quercetin with iron and copper ions: Complexation and autoxidation. Free Radic. Res. 2006, 40, 303–320. [Google Scholar] [CrossRef] [PubMed]
- Jungbluth, G.; Rühling, I.; Ternes, W. Oxidation of flavonols with Cu(II), Fe(II) and Fe(III) in aqueous media. J. Chem. Soc. Perkin Trans. 2000, 2, 1946–1952. [Google Scholar] [CrossRef]
- Jurasekova, Z.; Domingo, C.; Garcia-Ramos, J.; Sanchez-Cortes, S. Effect of pH on the chemical modification of quercetin and structurally related flavonoids characterized by optical (UV-visible and Raman) spectroscopy. Phys. Chem. Chem. Phys. 2014, 16, 12802–12811. [Google Scholar] [CrossRef] [PubMed]
- Mocek, M.; Richardson, P.J. Kinetics and mechanism of quercetin oxidation. J. Inst. Brew. 1972, 78, 459–465. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, X.-H. Degradation kinetics of fisetin and quercetin in solutions as effected by pH, temperature and coexisted proteins. J. Serb. Chem. Soc. 2016, 81, 243. [Google Scholar] [CrossRef]
- House, J.E. Principles of Chemical Kinetics, 2nd ed.; Academic Press: Cambridge, MA, USA, 2007. [Google Scholar]
- Samra, M.A.; Chedea, V.S.; Economou, A.; Calokerinos, A.; Kefalas, P. Antioxidant/prooxidant properties of model phenolic compounds: Part I. Studies on equimolar mixtures by chemiluminescence and cyclic voltammetry. Food Chem. 2011, 125, 622–629. [Google Scholar] [CrossRef]
- Letan, A. The relation of structure to antioxidant activity of quercetin and some of its derivatives I. Primary activity. J. Food Sci. 1966, 31, 518–523. [Google Scholar] [CrossRef]
- Hayakawa, K.; Minami, S.; Nakamura, S. Kinetics of the oxidation of ascorbic acid by the copper (II) ion in an acetate buffer solution. Bull. Chem. Soc. Jpn. 1973, 46, 2788–2791. [Google Scholar] [CrossRef]
- Kimura, M.; Kobayashi, A.; Boku, K. Kinetic studies of the oxidation of l-ascorbic acid by the peroxodisulfate ion, and of copper (II)-catalysis. Bull. Chem. Soc. Jpn. 1982, 55, 2068–2073. [Google Scholar] [CrossRef]
- Gutteridge, J.; Wilkins, S. Copper-dependent hydroxyl radical damage to ascorbic acid. FEBS Let. 1982, 137, 327–330. [Google Scholar] [CrossRef]
- Kaizer, J.; Speier, G. Radical-initiated oxygenation of flavonols by dioxygen. J. Mol. Catal. A Chem. 2001, 171, 33–36. [Google Scholar] [CrossRef]
- Kano, K.; Mabuchi, T.; Uno, B.; Esaka, Y.; Tanaka, T.; Linuma, M. Superoxide anion radical-induced dioxygenolysis of quercetin as a mimic of quercetinase. J. Chem. Soc. Chem. Commun. 1994, 0, 593–594. [Google Scholar] [CrossRef]
- Osman, A.; Makris, D.P.; Kefalas, P. Investigation on biocatalytic properties of a peroxidase-active homogenate from onion solid wastes: An insight into quercetin oxidation mechanism. Process Biochem. 2008, 43, 861–867. [Google Scholar] [CrossRef]
- Sokolová, R.; Degano, I.; Ramešová, Š.; Bulíčková, J.; Hromadová, M.; Gál, M.; Fiedler, J.; Valášek, M. The oxidation mechanism of the antioxidant quercetin in nonaqueous media. Electrochim. Acta 2011, 56, 7421–7427. [Google Scholar] [CrossRef]
- Zhou, A.; Kikandi, S.; Sadik, O.A. Electrochemical degradation of quercetin: Isolation and structural elucidation of the degradation products. Electrochem. Commun. 2007, 9, 2246–2255. [Google Scholar] [CrossRef]
- Sokolová, R.; Ramešová, Š.; Kocábová, J.; Kolivoška, V.; Degano, I.; Pitzalis, E. On the difference in decomposition of taxifolin and luteolin vs. fisetin and quercetin in aqueous media. Monats. Chem. Chem. Mon. 2016, 147, 1375–1383. [Google Scholar] [CrossRef]
- Zenkevich, I.G.; Eshchenko, A.Y.; Makarova, S.V.; Vitenberg, A.G.; Dobryakov, Y.G.; Utsal, V.A. Identification of the products of oxidation of quercetin by air oxygenat ambient temperature. Molecules 2007, 12, 654–672. [Google Scholar] [CrossRef] [PubMed]
- Pham, A.N.; Xing, G.; Miller, C.J.; Waite, T.D. Fenton-like copper redox chemistry revisited: Hydrogen peroxide and superoxide mediation of copper-catalyzed oxidant production. J. Catal. 2013, 301, 54–64. [Google Scholar] [CrossRef]
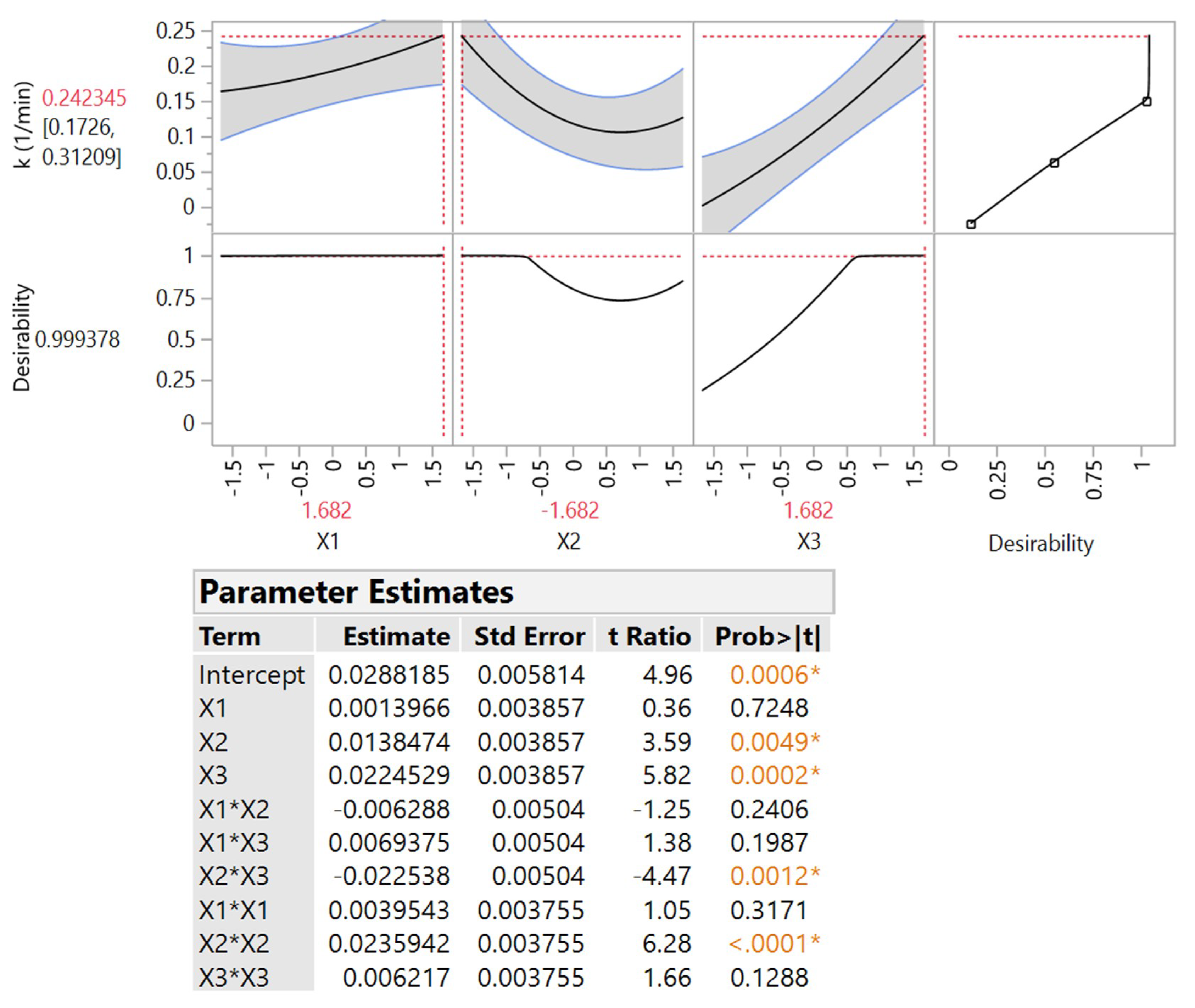

| Independent Variable | Code | Coded Variable Level | ||||
|---|---|---|---|---|---|---|
| −1.682 | −1 | 0 | 1 | 1.682 | ||
| CAA (μM) | X1 | 6.4 | 20 | 40 | 60 | 73.6 |
| pH | X2 | 6.7 | 7 | 7.5 | 8 | 8.3 |
| T (°C) | X3 | 53 | 60 | 70 | 80 | 87 |
| Design Point | Independent Variable Level | Response (k, min−1) | |||
|---|---|---|---|---|---|
| X1 | X2 | X3 | Measured | Predicted | |
| 1 | −1 | −1 | −1 | 0.0019 | 0.0030 |
| 2 | −1 | −1 | 1 | 0.0871 | 0.0791 |
| 3 | −1 | 1 | −1 | 0.0700 | 0.0883 |
| 4 | −1 | 1 | 1 | 0.0769 | 0.0743 |
| 5 | 1 | −1 | −1 | 0.0002 | 0.0045 |
| 6 | 1 | −1 | 1 | 0.1250 | 0.1084 |
| 7 | 1 | 1 | −1 | 0.0550 | 0.0647 |
| 8 | 1 | 1 | 1 | 0.0778 | 0.0784 |
| 9 | −1.682 | 0 | 0 | 0.0421 | 0.0377 |
| 10 | 1.682 | 0 | 0 | 0.0403 | 0.0424 |
| 11 | 0 | −1.682 | 0 | 0.0600 | 0.0723 |
| 12 | 0 | 1.682 | 0 | 0.1335 | 0.1188 |
| 13 | 0 | 0 | −1.682 | 0.0277 | 0.0086 |
| 14 | 0 | 0 | 1.682 | 0.0675 | 0.0842 |
| 15 | 0 | 0 | 0 | 0.0256 | 0.0288 |
| 16 | 0 | 0 | 0 | 0.0215 | 0.0288 |
| 17 | 0 | 0 | 0 | 0.0254 | 0.0288 |
| 18 | 0 | 0 | 0 | 0.0401 | 0.0288 |
| 19 | 0 | 0 | 0 | 0.0300 | 0.0288 |
| 20 | 0 | 0 | 0 | 0.0321 | 0.0288 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bobolaki, N.; Photiades, A.; Grigorakis, S.; Makris, D.P. Empirical Kinetic Modelling of the Effect of l-Ascorbic Acid on the Cu(II)-Induced Oxidation of Quercetin. ChemEngineering 2018, 2, 46. https://doi.org/10.3390/chemengineering2040046
Bobolaki N, Photiades A, Grigorakis S, Makris DP. Empirical Kinetic Modelling of the Effect of l-Ascorbic Acid on the Cu(II)-Induced Oxidation of Quercetin. ChemEngineering. 2018; 2(4):46. https://doi.org/10.3390/chemengineering2040046
Chicago/Turabian StyleBobolaki, Nikoletta, Angelos Photiades, Spyros Grigorakis, and Dimitris P. Makris. 2018. "Empirical Kinetic Modelling of the Effect of l-Ascorbic Acid on the Cu(II)-Induced Oxidation of Quercetin" ChemEngineering 2, no. 4: 46. https://doi.org/10.3390/chemengineering2040046
APA StyleBobolaki, N., Photiades, A., Grigorakis, S., & Makris, D. P. (2018). Empirical Kinetic Modelling of the Effect of l-Ascorbic Acid on the Cu(II)-Induced Oxidation of Quercetin. ChemEngineering, 2(4), 46. https://doi.org/10.3390/chemengineering2040046







