Research Progress on Chemical Compositions, Pharmacological Activities, and Toxicities of Quinone Compounds in Traditional Chinese Medicines
Abstract
1. Introduction
2. Progress in Chemical Composition Research
2.1. Structure Type and Distribution
2.1.1. Benzoquinones

| No. | Name | Resource | Molecular | Classification | Ref. |
|---|---|---|---|---|---|
| 1 | 2-methyl-p-quinone | Blaps rynchopetera Fairmaire | C7H6O2 | small molecule benzoquinone | [13] |
| 2 | 2,5-dimethyl-3-methoxy-p-benzoquinone | Fluridobulus penneri | C9H10O3 | small molecule benzoquinone | [14] |
| 3 | 2, 6-dimethoxy-1, 4-benzoquinone | Atractylodes macrocephala Koidz | C8H8O4 | small molecule benzoquinone | [15] |
| 4 | aurantiogliocladin | Arnebia euchroma (Royle) I.M. Johnst. | C10H12O4 | small molecule benzoquinone | [16] |
| 5 | 2-hydroxy-3-methoxy-5-methyl-p-benzoquinone | Antrodia cinnamomea T. T. Chang & W. N. Chou | C8H8O4 | small molecule benzoquinone | [17] |
| 6 | 2-methoxy-6-methyl-p-benzoquinone | Antrodia cinnamomea T. T. Chang & W. N. Chou | C8H8O3 | small molecule benzoquinone | [17] |
| 7 | 2,3-dimethoxy-5-methyl-p-benzoquinone | Antrodia cinnamomea T. T. Chang & W. N. Chou | C9H10O4 | small molecule benzoquinone | [17] |
| 8 | 2-hydroxy-5-methoxy-3-methyl-p-benzoquinone | Antrodia cinnamomea T. T. Chang & W. N. Chou | C8H8O4 | small molecule benzoquinone | [17] |
| 9 | anserinone A | Podospora anserina (Rabenh.) Niessl | C11H12O4 | small molecule benzoquinone | [18] |
| 10 | anserinone B | Podospora anserina (Rabenh.) Niessl | C11H14O4 | small molecule benzoquinone | [18] |
| 11 | 2-hydroxy-3-methyl-5-methoxy-p-benzoquinone | Pterospermum heterophyllum Hance | C8H8O4 | small molecule benzoquinone | [14] |
| 12 | 2.3-dimethyl-5, 6-dimethoxy-p-benzoquinone | Gliocladium penicilloides Corda | C10H12O4 | small molecule benzoquinone | [14] |
| 13 | 2, 5-dimethoxy-3, 6-dimethyl-p-benzoquinone | Neonectria fuckeliana (C. Booth) Castl. & Rossman | C10H12O4 | small molecule benzoquinone | [14] |
| 14 | thymoquinone | Nigella sativa L. | C10H12O2 | small molecule benzoquinone | [19] |
| 15 | primin | Miconia lepidota DC. | C12H16O3 | advanced straight-chain hydrocarbon benzoquinone | [20] |
| 16 | embelin | Embelia ribes Burm. f | C17H26O4 | advanced straight-chain hydrocarbon benzoquinone | [21] |
| 17 | 2,5-dihydroxy-3-tridecyl-1, 4-benzoquinone | Embelia ribes Burm. f. | C19H30O4 | advanced straight-chain hydrocarbon benzoquinone | [21] |
| 18 | myrsinone | Myrsine africana L. var. acuminata C. Y. Wu et C. Chen (synonym) | C17H26O4 | advanced straight-chain hydrocarbon benzoquinone | [14] |
| 19 | idebenone | - | C19H30O5 | advanced straight-chain hydrocarbon benzoquinone | [22] |
| 20 | 2-methoxy-6-nonadecyl-1,4-benzoquinone | Miconia lepidota DC. | C26H44O3 | advanced straight-chain hydrocarbon benzoquinone | [23] |
| 21 | (-)-a-tocospirone | Gynura japonica (Thunb.) Juel | C29H50O4 | advanced straight-chain hydrocarbon benzoquinone | [24] |
| 22 | maesaquinone | Maesa japonica (Thunb.) Moritzi | C26H42O4 | advanced straight-chain hydrocarbon benzoquinone | [25] |
| 23 | paphionone | Paphiopedilum exul (Ridl.) Rolfe | C20H30O5 | advanced straight-chain hydrocarbon benzoquinone | [26] |
| 24 | isopentenyl p-benzoquinone | Phagnalon purpurescens Sch. Bip. | C11H12O2 | isopentenyl benzoquinone | [14] |
| 25 | 3,5,6-trimethoxy-2-isopentene-p-benzoquinone | Dendrobium nobile Lindl. | C14H18O5 | isopentenyl benzoquinone | [14] |
| 26 | omphalone | Lentinellus micheneri (Berk. & M. A. Curtis) Pegler | C11H8O3 | isopentenyl benzoquinone | [27] |
| 27 | 2(E) -2-geranyl-6-methyl p-benzoquinone | Atractylodes koreana (Nakai) Kita. | C17H22O2 | isopentenyl benzoquinone | [14] |
| 28 | 2-(Z) -2-geranyl-6-methyl p-benzoquinone | Atractylodes koreana (Nakai) Kita. | C17H22O2 | isopentenyl benzoquinone | [14] |
| 29 | amebifuranone | Arnebia euchroma (Royle) I.M. Johnst | C18H20O5 | isopentenyl benzoquinone | [14] |
| 30 | arnebinone | Arnebia euchroma (Royle) I.M. Johnst | C18H22O4 | isopentenyl benzoquinone | [14] |
| 31 | chabrolobenzoquinone E | Nephthea chabrolii Audouin | C27H38O3 | isopentenyl benzoquinone | [28] |
| 32 | chabrolobenzoquinone F | Nephthea chabrolii Audouin | C29H40O4 | isopentenyl benzoquinone | [28] |
| 33 | chabrolobenzoquinone G | Nephthea chabrolii Audouin | C27H38O3 | isopentenyl benzoquinone | [28] |
| 34 | chabrolobenzoquinone H | Nephthea chabrolii Audouin | C29H42O5 | isopentenyl benzoquinone | [28] |
| 35 | atrovirinone | Garcinia atroviridis Griffith ex T. Anderson | C25H28O8 | isopentenyl benzoquinone | [29] |
| 36 | cyperaquinone | Cyperus nipponicus Franch. & Sav. | C14H10O4 | furanobenzoquinone | [30] |
| 37 | albidin | Penicillium albidum Sopp | C10H8O4 | furanobenzoquinone | [14] |
| 38 | graphisquinone | Graphis scripta (L.) Ach. | C11H10O5 | furanobenzoquinone | [14] |
| 39 | chrysoquinane | Euphorbia esula L. | C19H16O9 | flavonoid benzoquinone | [14] |
| 40 | claussequinone | Dalbergia odorifera T.Chen | C16H16O5 | flavonoid benzoquinone | [14] |
| 41 | bowdichione | Dalbergia odorifera T.Chen | C16H10O6 | flavonoid benzoquinone | [14] |
| 42 | donoherbivol-cyclocledoquinone | Dalbergia odorifera T.Chen | C32H28O9 | flavonoid benzoquinone | [14] |
| 43 | 3-Acetoxymo-quinone | Cordia oncocalyx (Allemão) Baill. | C12H14O4 | terpenebenzoquinone | [31] |
| 44 | glanduline A | Helianthus annuus L. | C15H20O2 | terpenebenzoquinone | [14] |
| 45 | glanduline B | Helianthus annuus L. | C15H18O2 | terpenebenzoquinone | [14] |
| 46 | methylvilangin | Myrsine africana L. var. acuminata C. Y. Wu et C. Chen (synonym) | C36H54O8 | biphenylquinone | [25] |
| 47 | methylanhydrovilangin | Myrsine africana L. var. acuminata C. Y. Wu et C. Chen (synonym) | C16H52O7 | biphenylquinone | [25] |
| 48 | lanciaquinone | Ardisia japonica (Thunb.) Bl. | C27H36O7 | biphenylquinone | [32] |
| 49 | neonambiquinone A | Neonothopanus nambi (Speg.) R. H. Petersen & Krisai | C19H14O6 | biphenylquinone | [33] |
| 50 | volucrisporin | Volucrispora aurantiaca Haskins | C18H12O4 | biphenylquinone | [34] |
| 51 | oosporein | Beauveria bassiana (Bals.-Criv.) Vuill. | C14H18O8 | biphenylquinone | [35] |
| 52 | biembelin | Rapanea melanophloeos (L.) Meisn. | C34H50O8 | biphenylquinone | [14] |
| 53 | embenones A | Knema globularia (Lam.) Warb. | C15H18O4 | other | [35] |
| 54 | embenones B | Knema globularia (Lam.) Warb. | C15H20O4 | other | [35] |
| 55 | triaziquone | Artemisia sieberi. J | C12H13N3O2 | other | [36] |
| 56 | aziridyl benzoquinone | - | C16H22N2O6 | other | [37] |
| 57 | erectquione B | Hypericum erectum Sol. ex R.Br. | C29H40O6 | other | [38] |
| 58 | erectquione C | Hypericum erectum Sol. ex R.Br. | C25H34O6 | other | [38] |
| 59 | Atromentin | Ascocoryne sarcoides | C18H12O6 | other | [39] |
| 60 | Erectquione A | Hypericum erectum Sol. ex R.Br. | C21H28O4 | ortho-benzoquinone | [38] |


2.1.2. Naphthoquinones


| No. | Name | Resource | Formula | Classification | Ref. |
|---|---|---|---|---|---|
| 61 | 3-bromoplumbagin | Diospyros maritima Blume | C11H7BrO3 | small molecule naphthoquinones | [42] |
| 62 | 3-(2-hydroxyethyl)plumbagin | Diospyros maritima Blume | C13H12O4 | small molecule naphthoquinones | [42] |
| 63 | 6-(1-ethoxyethyl)plumbagin | Diospyros maritima Blume | C15H16O4 | small molecule naphthoquinones | [43] |
| 64 | juglone | Juglans regia L. | C10H6O3 | small molecule naphthoquinones | [14] |
| 65 | 2-methyl-1, 4-naphthoquinone | Juglans regia L. | C11H8O2 | small molecule naphthoquinones | [14] |
| 66 | lawsone | Lythrum salicaria L. | C10H6O4 | small molecule naphthoquinones | [14] |
| 67 | 2-amino-1.4-naphthoquinone | Laurus nobilis L. | C10H7NO3 | small molecule naphthoquinones | [14] |
| 68 | plumbagin | Plumbago zeylanica L. | C11H8O3 | small molecule naphthoquinones | [14] |
| 69 | isoplumbagin | Impatiens balsamina L. | C11H8O3 | small molecule naphthoquinones | [14] |
| 70 | chimaphilin | Pyrola soldanellifolia Andres | C12H10O3 | small molecule naphthoquinones | [14] |
| 71 | 7-methyl juglone | Diospyros usambarensis Engl. | C11H8O3 | small molecule naphthoquinones | [14] |
| 72 | 2-methoxy-6-acetyl-7-methyljuglone | Pleuropterus multiflorus (Thunb.) Nakai | C13H12O5 | small molecule naphthoquinones | [44] |
| 73 | 2-methoxystypandrone | Rumex japonicus Houtt | C14H12O5 | small molecule naphthoquinones | [45] |
| 74 | 2-butanoyl-3,6,8-trihydroxy-1,4-naphthoquinone 6-O-sulfate | Oxycomanthus japonicus J. F. W. Mller | C14H11NaO9S | small molecule naphthoquinones | [46] |
| 75 | 2-butanoyl-3,6,8-trihydroxy-1,4-naphthoquinone | Oxycomanthus japonicus J. F. W. Mller | C14H12O6 | small molecule naphthoquinones | [46] |
| 76 | cribrarione B | Cribraria cancellata (Batsch) Nann.-Bremek. | C12H10O6 | small molecule naphthoquinones | [47] |
| 77 | fusarnaphthoquinoe A | Fusarium spp. | C15H18O7 | small molecule naphthoquinones | [48] |
| 78 | 7-carbomethoxy-2,8-dimethoxy-5-hydroxy-l,4-naphthoquinone | Penicillium raistrickii Stolk & Scott | C14H13O7 | small molecule naphthoquinones | [49] |
| 79 | 2,7-dimethoxy-5-hydroxy-1,4-naphthoquinone | Penicillium raistrickii Stolk & Scott | C12H10O5 | small molecule naphthoquinones | [49] |
| 80 | 8-formyl-7-hydroxy-5-isopropyl-2-methoxy-3-methyl-1,4-naphthoquinone | Ceiba pentandra (L.) Gaertn. | C16H16O5 | small molecule naphthoquinones | [50] |
| 81 | 2,7-dihydroxy-8-formyl-5-isopropyl-3-methyl-1.4-naphthoquinone | Ceiba pentandra (L.) Gaertn. | C15H14O5 | small molecule naphthoquinones | [50] |
| 82 | 7-hydroxy-5-isopropyl-2-methoxy-3-methylnaphthoquinone | Bombax malabaricum DC. | C15H16O4 | small molecule naphthoquinones | [51] |
| 83 | lanigerone | Salvia lanigera Poir. (Lamiaceae) | C14H14O3 | small molecule naphthoquinones | [52] |
| 84 | salvigerone | Salvia lanigera Poir. (Lamiaceae) | C21H26O4 | small molecule naphthoquinones | [52] |
| 85 | droserone | Plumbago capensis Thunb | C11H8O4 | small molecule naphthoquinones | [53] |
| 86 | davidianone A | Ulmus davidiana Planch. | C15H12O4 | benzoisochromanquinone | [54] |
| 87 | davidianone B | Ulmus davidiana Planch. | C16H12O5 | benzoisochromanquinone | [54] |
| 88 | davidianone C | Ulmus davidiana Planch. | C17H16O5 | benzoisochromanquinone | [54] |
| 89 | mansonone E | Ulmus pumila L. | C15H14O3 | benzoisochromanquinone | [55] |
| 90 | mansonone F | Ulmus pumila L. | C15H12O3 | benzoisochromanquinone | [55] |
| 91 | mansonone H | Ulmus pumila L. | C15H14O4 | benzoisochromanquinone | [56] |
| 92 | mansonone I | Ulmus pumila L. | C15H14O4 | benzoisochromanquinone | [57] |
| 93 | rhinacanthone | Rhinacanthus nasutus (L.) Kurz | C15H14O3 | benzoisochromanquinone | [58] |
| 94 | rhinacanthin A | Rhinacanthus nasutus (L.) Kurz | C15H14O4 | benzoisochromanquinone | [59] |
| 95 | rhinacanthin O | Rhinacanthus nasutus (L.) Kurz | C24H26O5 | benzoisochromanquinone | [58] |
| 96 | rhinacanthin P | Rhinacanthus nasutus (L.) Kurz | C24H26O5 | benzoisochromanquinone | [58] |
| 97 | rhinacanthin S | Rhinacanthus nasutus (L.) Kurz | C24H24O5 | benzoisochromanquinone | [58] |
| 98 | rhinacanthin T | Rhinacanthus nasutus (L.) Kurz | C24H26O5 | benzoisochromanquinone | [60] |
| 99 | mansonin A | Mansonia altissima A. Chev. | C17H18O5 | benzoisochromanquinone | [60] |
| 100 | mansonin B | Mansonia altissima A. Chev. | C17H18O6 | benzoisochromanquinone | [60] |
| 101 | 5-methoxy-3,4-dehydroxanthomegnin | Paepalanthus latipes Silveira | C16H12O7 | benzoisochromanquinone | [61] |
| 102 | pyranokunthone A | Stereospermum kunthianum Cham. | C20H20O4 | benzoisochromanquinone | [62] |
| 103 | 4-O-methyl erythrostominone | Cordyceps unilateralis (Tul.) Sacc. var. clavata (Y. Kobayasi) | C18H18O8 | benzoisochromanquinone | [63] |
| 104 | halawanone A | Streptomyces Schröter | C23H22O9 | benzoisochromanquinone | [64] |
| 105 | pyranokunthone B | Stereospermum kunthianum Cham. | C20H20O4 | benzoisochromanquinone | [62] |
| 106 | (3a,3′a,4β,β)-3,3′-dimethoxy-cis-[4,4′-bis(3,4,5,10-tetra-hydro-1H-naphtho(2,3-clpyran)]-5.5.10,10-tetraone | Pentas longiflora Oliv. | C28H22O8 | benzoisochromanquinone | [65] |
| 107 | arthoniafurone B | Arthonia cinnabarina Ach. | C14H10O5 | furanonaphthoquinone | [66] |
| 108 | fusarnaphthoquinone B | Fusarium Link | C15H16O5 | furanonaphthoquinone | [48] |
| 109 | arthoniafurone A | Arthonia cinnabarina (DC.) Wallr. | C14H8O5 | furanonaphthoquinone | [66] |
| 110 | cribrarione A | Cribraria purpurea Schwein. | C13H10O7 | furanonaphthoquinone | [67] |
| 111 | 8-hydroxy-1-methylnaphtho[2,3-c]furan-4,9-dione | Bulbine capitata Poelln. | C13H8O4 | furanonaphthoquinone | [68] |
| 112 | 5,8-dihydroxy-1-methylnaphtho[2,3-c]furan-4,9-dione | Aloe ferox Mill. | C13H8O5 | furanonaphthoquinone | [69] |
| 113 | 5,8-dihydroxy-1-hydroxymethylnaphtho[2,3-c]furan-4,9-dione | Aloe ferox Mill. | C13H8O6 | furanonaphthoquinone | [69] |
| 114 | avicequinone A | Avicennia alba Blume | C15H14O5 | furanonaphthoquinone | [70] |
| 115 | avicequinone B | Avicennia alba Blume | C12H6O3 | furanonaphthoquinone | [70] |
| 116 | avicequinone C | Avicennia alba Blume | C15H12O4 | furanonaphthoquinone | [70] |
| 117 | avicequinone D | Avicennia alba Blume | C15H12O5 | furanonaphthoquinone | [70] |
| 118 | avicequinone E | Mendoncia cowanii (S. Moore) Benoist | C15H14O5 | furanonaphthoquinone | [71] |
| 119 | 2-(1′-methylethenyl)naphtho[2,3-b]furan-4,9-dione | Newbouldia laevis (P. Beauv.) Seem. ex Bureau | C15H10O3 | furanonaphthoquinone | [72] |
| 120 | 2-isopropenyl-9-methaxy-1,8-dioxa-dicyclopenta[b,g]naphthal-ene-4,10-dione | Plumbago zeylanica L. | C18H12O5 | furanonaphthoquinone | [73] |
| 121 | 9-hydroxy-2-isopropenyl-1,8-dioxa-dicyclopenta[b,g]naphthal-ene-4,10-dione | Plumbago zeylanica L. | C17H10O5 | furanonaphthoquinone | [74] |
| 122 | 2-(1-hydroxy-l-methyl-ethyl)-9-methoxy-1,8-dioxa-dicyclo-penta[b,g]naphthalene-4,10-dione | Plumbago zeylanica L. | C18H14O6 | furanonaphthoquinone | [73] |
| 123 | (R)-7-hydroxy-a-dunnione | Chirita eburnea Hance | C15H14O4 | furanonaphthoquinone | [74] |
| 124 | (R)-8-hydroxy-a-dunnione | Chirita eburnea Hance | C15H14O4 | furanonaphthoquinone | [74] |
| 125 | (R)-a-7,8-dihydroxy-a-dunnione | Chirita eburnea Hance | C15H14O5 | furanonaphthoquinone | [74] |
| 126 | (R)-7-methoxy-6,8-dihydroxy-a-dunnione | Chirita eburnea Hance | C16H16O6 | furanonaphthoquinone | [74] |
| 127 | 7,8-dimethoxydunnione | Sinningia leucotricha (Hoehne) H. E. Moore | C17H18O5 | furanonaphthoquinone | [75] |
| 128 | dehydro-a-isodunnione | Tectona grandis L. f. | C15H12O3 | furanonaphthoquinone | [76] |
| 129 | 5-hydroxy-7-methoxydehydroiso-a-lapachone | Newbouldia laevis (P. Beauv.) Seemann ex Bureau | C16H14O5 | furanonaphthoquinone | [77] |
| 130 | glycoquinone | Glycosmis pentaphylla (Retz.) Corrêa | C20H24O4 | furanonaphthoquinone | [78] |
| 131 | (2R)-6,8-dihydroxy-a-dunnione | Lysionotus pauciflorus Maxim. | C15H14O5 | furanonaphthoquinone | [79] |
| 132 | balsaminone D | Impatiens balsamina L. | C20H14O7 | furanonaphthoquinone | [80] |
| 133 | (2R)-6-hydroxy-7-methoxy-dehydroiso-α-lapachone | Spermacoce latifolia Aubl. | C15H14O5 | furanonaphthoquinone | [81] |
| 134 | crassiflorone | Diospyros crassiflora Hiern | C21H12O6 | furanonaphthoquinone | [82] |
| 135 | lapachol | Tabebuia avellanedae Lorentz ex Griseb. | C15H14O3 | isopentenyl naphthoquinone | [83] |
| 136 | hydroxysesamone | Sesamum indicum L. | C15H14O5 | isopentenyl naphthoquinone | [84] |
| 137 | 2,3-epoxysesamone | Sesamum indicum L. | C15H14O5 | isopentenyl naphthoquinone | [84] |
| 138 | lantalucratin D | Lantana involucrata L. | C17H18O5 | isopentenyl naphthoquinone | [85] |
| 139 | lantalucratin E | Lantana involucrata L. | C17H18O6 | isopentenyl naphthoquinone | [85] |
| 140 | lantalucratin F | Lantana involucrata L. | C17H18O7 | isopentenyl naphthoquinone | [85] |
| 141 | butylalkannin | Arnebia hispidissima (Sieber ex Lehm.) A.DC. | C20H22O6 | isopentenyl naphthoquinone | [86] |
| 142 | alkannin | Arnebia hispidissima (Sieber ex Lehm.) A.DC. | C6H16O5 | isopentenyl naphthoquinone | [86] |
| 143 | rhinacanthin B | Rhinacanthus nasutus (L.) Kurz | C25H28O5 | isopentenyl naphthoquinone | [59] |
| 144 | rhinacanthin C | Rhinacanthus nasutus (L.) Kurz | C25H30O5 | isopentenyl naphthoquinone | [58] |
| 145 | rhinacanthin G | Rhinacanthus nasutus (L.) Kurz | C25H30O6 | isopentenyl naphthoquinone | [58] |
| 146 | rhinacanthin H | Rhinacanthus nasutus (L.) Kurz | C25H30O6 | isopentenyl naphthoquinone | [58] |
| 147 | rhinacanthin I | Rhinacanthus nasutus (L.) Kurz | C25H30O6 | isopentenyl naphthoquinone | [58] |
| 148 | rhinacanthin J | Rhinacanthus nasutus (L.) Kurz | C25H28O6 | isopentenyl naphthoquinone | [58] |
| 149 | rhinacanthin K | Rhinacanthus nasutus (L.) Kurz | C25H32O7 | isopentenyl naphthoquinone | [58] |
| 150 | rhinacanthin L | Rhinacanthus nasutus (L.) Kurz | C25H32O8 | isopentenyl naphthoquinone | [58] |
| 151 | cordiaquinone A | Cordia curassavica (Jacq.) Roem. & Schult | C21H26O3 | isopentenyl naphthoquinone | [87] |
| 152 | chabrolonaphthoquinone A | Nephthea chabrolii Milne Edwards & Haime | C27H32O4 | isopentenyl naphthoquinone | [88] |
| 153 | chabrolonaphthoquinone B | Nephthea chabrolii Milne Edwards & Haime | C29H38O5 | isopentenyl naphthoquinone | [28] |
| 154 | 6,8-dihydroxy-2,7-dimethoxy-3-(1,1-dimethylprop-2-enyl)-1,4-naphthoquinones | Lysionotus pauciflorus Maxim. | C17H18O6 | isopentenyl naphthoquinone | [79] |
| 155 | 7-hydroxy-2-O-methyldunniol | Sinningia conspicua (Seem.) Focke | C16H15O4 | isopentenyl naphthoquinone | [89] |
| 156 | 7-methoxy-2-O-methyldunniol | Sinningia conspicua (Seem.) Focke | C17H17O4 | isopentenyl naphthoquinone | [89] |
| 157 | 3,5,8-tribydroxy-6-methoxy-2-(5-oxohexa- 1,3-dienyl-1.4-naphthoquinone | Cordyceps unilateralis (Tul.) Petch | C17H14O7 | isopentenyl naphthoquinone | [63] |
| 158 | rhinacanthin D | Rhinacanthus nasutus (L.) Kurz | C23H20O7 | other | [58] |
| 159 | rhinacanthin M | Rhinacanthus nasutus (L.) Kurz | C22H20O5 | other | [90] |
| 160 | rhinacanthin N | Rhinacanthus nasutus (L.) Kurz | C27H24O7 | other | [58] |
| 161 | rhinacanthin Q | Rhinacanthus nasutus (L.) Kurz | C28H26O7 | other | [58] |
| 162 | rhinacanthin U | Rhinacanthus nasutus (L.) Kurz | C17H18O5 | other | [58] |
| 163 | rhinacanthin V | Rhinacanthus nasutus (L.) Kurz | C25H22O6 | other | [58] |
| 164 | cordiaquinone E | Cordia curassavica (Jacq.) Roemer&Schultes | C21H24O3 | other | [87] |
| 165 | cordiaquinone B | Cordia curassavica (Jacq.) Roemer&Schultes | C21H24O3 | other | [87] |
| 166 | cordiaquinone K | Cordia curassavica (Jacq.) Roemer&Schultes | C21H22O3 | other | [87] |
| 167 | cordiaquinone F | Cordia curassavica (Jacq.) Roemer&Schultes | C26H30O5 | other | [87] |
| 168 | cordiaquinone G | Cordia curassavica (Jacq.) Roemer&Schultes | C21H26O4 | other | [87] |
| 169 | cordiaquinone H | Cordia curassavica (Jacq.) Roemer&Schultes | C21H26O4 | other | [87] |
| 170 | cordiaquinone J | Cordia curassavica (Jacq.) Roemer&Schultes | C21H24O3 | other | [87] |
| 171 | isagarin | Pentas longiflora | C15H12O4 | other | [91] |
| 172 | 3-hydroxy-2-metoxy-8,8,10-trimethyl-8H-antracen-1,4,5-trione | Byrsonima microphylla A.Juss. | C18H16O5 | other | [92] |
| 173 | 3,7-dihydroxy-2-methoxy-8,8,10-trimethyl- 7,8-dihydro-6H-antracen-1,4,5-trione | Byrsonima microphylla A.Juss. | C18H18O6 | other | [92] |
| 174 | sterekunthal A | Stereospermum kunthianum Cham. | C20H18O5 | other | [62] |
| 175 | stereiqunone C | Stereospermum kunthianum Cham. | C19H16O3 | other | [93] |
| 176 | sterequinone E | Stereospermum personatum (Hassk.) Chatterjee | C19H16O4 | other | [93] |
| 177 | sterekunthal B | Stereospermum personatum (Hassk.) Chatterjee | C20H18O4 | other | [62] |
| 178 | sterequinone B | Stereospermum personatum (Hassk.) Chatterjee | C21H20O5 | other | [93] |
| 179 | 3,8′-biplumbagin | Diospyros maritima Blume | C22H14O6 | other | [43] |
| 180 | isozeylanone | Plumbago zeylanica L. | C22H14O6 | other | [94] |
| 181 | ethylidene-3,3′-biplumbagin | Diospyros maritima Blume | C24H18O6 | other | [43] |
| 182 | ethylidene-3,6′-biplumbagin | Diospyros maritima Blume | C24H18O6 | other | [43] |
| 183 | ethylidene-6,6′-biplumbagin | Diospyros maritima Blume | C24H18O6 | other | [95] |
| 184 | balsaminone E | Impatiens balsamina L. | C22H16O5 | other | [80] |
| 185 | adenophyllone | Heterophragma adenophyllum Seem | C30H22O5 | other | [96] |
| 186 | dilapachone | Heterophragma adenophyllum Seem | C30H26O6 | other | [96] |
| 187 | fusarnaphthoquinone C | Fusarium spp. | C29H26O11 | other | [48] |
| 188 | hygrocin A | Streptomyces hygroscopicus Jensen | C28H31NO8 | other | [97] |
| 189 | hygrocin B | Streptomyces hygroscopicus Jensen | C28H29NO8 | other | [97] |
| 190 | lippisidoquinone | Lippia sidoides Cham. | C30H26O5 | other | [98] |
| 191 | phytonadione | Anethum graveolens L. | C31H46O2 | other | [99] |
| 192 | maritinone | Diospyros anisandra S.F.Blake | C22H14O6 | other | [100] |






2.1.3. Phenanthrenequinones


| No. | Name | Resource | Formula | Classification | Ref. |
|---|---|---|---|---|---|
| 193 | trijuganone A | Salvia trijuga Diels. | C18H14O4 | para-phenanthrenequinone | [103] |
| 194 | bauhinione | Bauhinia variegata L. | C17H16O4 | para-phenanthrenequinone | [104] |
| 195 | ochrone A | Coelogyne ochracea Lindl. | C13H12O4 | para-phenanthrenequinone | [105] |
| 196 | stemanthraquinone | Stemona tuberosa Lour. | C16H14O4 | para-phenanthrenequinone | [106] |
| 197 | dioscoreanone | Dioscorea membranacea Pierre | C16H12O5 | para-phenanthrenequinone | [107] |
| 198 | denbinobin | Dendrobium nobile Lindl. | C16H12O5 | para-phenanthrenequinone | [108] |
| 199 | 7-hydroxy-5,6-dimethoxy-1,4-phenanthrenequinone | Dendrobium moniliforme (L.) Sw. | C16H12O5 | para-phenanthrenequinone | [109] |
| 200 | moniliformin | Fusarium verticillioides (Sacc.) Nirenberg | C16H10O6 | para-phenanthrenequinone | [110] |
| 201 | phenanobiles A | Dendrobium nobile Lindl. | C14H8O5 | para-phenanthrenequinone | [101] |
| 202 | phenanobiles B | Dendrobium nobile Lindl. | C16H13O5 | para-phenanthrenequinone | [101] |
| 203 | phenanobiles C | Dendrobium nobile Lindl. | C14H10O4 | para-phenanthrenequinone | [101] |
| 204 | 6,7-dihydroxy-2-methoxy-1,4-phenanthrenedione | Dioscorea opposita Thunb. | C15H10O5 | para-phenanthrenequinone | [101] |
| 205 | pyranospiranthoquinone | Spiranthes sinensis (Pers.) Ames | C20H18O5 | para-phenanthrenequinone | [14] |
| 206 | ephemeranthoquinone | Flickingeria comata (Bl.) Hawkes. | C15H12O4 | para-phenanthrenequinone | [111] |
| 207 | annoquinone A | Annona montana Macfad. | C15H10O3 | para-phenanthrenequinone | [112] |
| 208 | danshenxinkun C | Salvia miltiorrhiza Bunge | C21H20O4 | para-phenanthrenequinone | [110] |
| 209 | cypripediquinone A | Cypripedium macranthum Sw. | C17H14O5 | o-phenanthrenequinone I | [111] |
| 210 | bulbophyllanthrone | Bulbophyllum odoratissimum (J. E. Sm.) Lindl. | C17H14O6 | o-phenanthrenequinone I | [112] |
| 211 | Sch6 86 31 | Spiromyces sp. | C19H16O4 | o-phenanthrenequinone I | [14] |
| 212 | biruloquinone | Mycosphaerella rubella (Westend.) | C17H10O7 | o-phenanthrenequinone I | [14] |
| 213 | danshenxinkun A | Salvia miltiorrhiza Bunge | C18H16O4 | o-phenanthrenequinone II | [113] |
| 214 | danshenxinkun B | Salvia miltiorrhiza Bunge | C16H12O3 | o-phenanthrenequinone II | [113] |
| 215 | danshenxinkun D | Salvia miltiorrhiza Bunge | C18H16O3 | o-phenanthrenequinone II | [113] |
| 216 | cryptotanshinone | Salvia miltiorrhiza Bunge | C19H20O3 | o-phenanthrenequinone II | [113] |
| 217 | tanshinone I | Salvia miltiorrhiza Bunge | C18H12O3 | o-phenanthrenequinone II | [113] |
| 218 | dihydrotanshinone I | Salvia miltiorrhiza Bunge | C18H14O3 | o-phenanthrenequinone II | [113] |
| 219 | tanshinone IIA | Salvia miltiorrhiza Bunge | C19H18O3 | o-phenanthrenequinone II | [113] |
| 220 | hydroxytanshinone IIA | Salvia miltiorrhiza Bunge | C19H18O4 | o-phenanthrenequinone II | [113] |
| 221 | tanshinone IIB | Salvia miltiorrhiza Bunge | C19H18O4 | o-phenanthrenequinone II | [113] |
| 222 | miltirone | Salvia miltiorrhiza Bunge | C18H17O2 | o-phenanthrenequinone II | [113] |
| 223 | trijuganone B | Salvia trijuga Diels. | C18H16O3 | o-phenanthrenequinone II | [103] |
| 224 | trijuganone C | Salvia trijuga Diels. | C20H20O5 | o-phenanthrenequinone II | [103] |


2.1.4. Anthraquinones

Monoanthraquinones

| No. | Name | Resource | Formula | Classification | Ref. |
|---|---|---|---|---|---|
| 225 | chrysazin | Rheum palmatum L. | C14H8O4 | rhodopsin-type anthraquinone | [14] |
| 226 | chrysophanol | Rheum palmatum L. | C15H10O4 | rhodopsin-type anthraquinone | [14] |
| 227 | emodin | Rheum palmatum L. | C15H10O5 | rhodopsin-type anthraquinone | [120] |
| 228 | isochrysophanol | Rheum palmatum L. | C15H12O4 | rhodopsin-type anthraquinone | [14] |
| 229 | Rhein | Rheum palmatum L. | C15H8O6 | rhodopsin-type anthraquinone | [14] |
| 230 | 4-hydroxymethyl chrysazin | Tripterygium wilfordii Hook. f | C15H12O5 | rhodopsin-type anthraquinone | [14] |
| 231 | 1,8-dihydroxy-4-methylanthraquinone | cyanobacterium | C15H10O4 | rhodopsin-type anthraquinone | [121] |
| 232 | monodictyquinone A | Monodictys cerebriformis G. Z. Zhao & T. Y. Zhang | C16H12O5 | rhodopsin-type anthraquinone | [122] |
| 233 | carviolin | Penicillium Link ex Fr. | C16H12O6 | rhodopsin-type anthraquinone | [123] |
| 234 | 1-O-methylemodin | Senna obtusifolia (L.) H. S. Irwin & Barneby. | C16H12O5 | rhodopsin-type anthraquinone | [124] |
| 235 | ω-acetylcarviolin | Zopfiella longicaudata (Ces.) Sacc. | C18H14O7 | rhodopsin-type anthraquinone | [125] |
| 236 | ω-hydroxyemodin | Zopfiella longicaudata (Ces.) Sacc. | C15H10O6 | rhodopsin-type anthraquinone | [46] |
| 237 | lunatin | Curvularia lunata (Wakker) Boedijn | C15H10O6 | rhodopsin-type anthraquinone | [125] |
| 238 | ptilometric acid 6-O-sulfate | Tropiometra afra macrodiscus (Hartlaub) | C18H13NaO10S | rhodopsin-type anthraquinone | [46] |
| 239 | ptilometric acid | Tropiometra afra macrodiscus (Hartlaub) | C18H14O7 | rhodopsin-type anthraquinone | [46] |
| 240 | cassanthraquinone A | Cassia siamea Lam. | C20H14O6 | rhodopsin-type anthraquinone | [126] |
| 241 | ventilanone L | Ventilago denticulata Willd. | C18H14O7 | rhodopsin-type anthraquinone | [127] |
| 242 | ventilanone M | Ventilago denticulata Willd. | C18H16O6 | rhodopsin-type anthraquinone | [127] |
| 243 | 1,8-dihydroxy-3-succinic acid monoethyl ester-6-methylanthraquinone | - | C19H13O8 | rhodopsin-type anthraquinone | [128] |
| 244 | Aloe emodin | Pleuropterus multiflorus (Thunb.) Nakai | C15H10O5 | rhodopsin-type anthraquinone | [44] |
| 245 | emodin methyl ether | Pleuropterus multiflorus (Thunb.) Nakai | C16H12O5 | rhodopsin-type anthraquinone | [44] |
| 246 | ω-hydroxyemodin 8-methyl ether | Pleuropterus multiflorus (Thunb.) Nakai | C16H12O6 | rhodopsin-type anthraquinone | [44] |
| 247 | emodin 8-methyl ether | Pleuropterus multiflorus (Thunb.) Nakai | C16H12O5 | rhodopsin-type anthraquinone | [44] |
| 248 | vismiaquinone C | Vismia martiana Rchb.f. | C21H20O5 | rhodopsin-type anthraquinone | [129] |
| 249 | asparasone A | Aspergillus parasiticus Speare | C18H14O8 | rhodopsin-type anthraquinone | [130] |
| 250 | laurentiquinone A | Vismia laurentii De Wild. | C22H20O7 | rhodopsin-type anthraquinone | [131] |
| 251 | laurenquinone A | Vismia laurentii De Wild. | C22H20O7 | rhodopsin-type anthraquinone | [132] |
| 252 | 3-O-(2-hydroxy-3-methylbut-3-enyl)-emodin | Vismia guineensis (L.) Choisy | C20H18O6 | rhodopsin-type anthraquinone | [133] |
| 253 | 3-O-(2-methoxy-3-methylbut-3-enyl)-emodin | Vismia guineensis (L.) Choisy | C21H20O6 | rhodopsin-type anthraquinone | [133] |
| 254 | 3-O-(E-3-hydroxymethylbut-2-enyl)-emodin | Vismia guineensis (L.) Choisy | C20H18O6 | rhodopsin-type anthraquinone | [133] |
| 255 | 3-O-(3-hydroxymethyl-4-hydroxybut-2-enyl)-emodin | Vismia guineensis (L.) Choisy | C20H18O7 | rhodopsin-type anthraquinone | [133] |
| 256 | pruniflorone J | Cratoxylum formosum (Jack) Dyer | C25H26O6 | rhodopsin-type anthraquinone | [134] |
| 257 | araliorhamnone A | Araliorhamnus vaginata H.Perrier | C18H12O8 | rhodopsin-type anthraquinone | [135] |
| 258 | laurenquinone B | Vismia laurentii De Wild. | C22H18O7 | rhodopsin-type anthraquinone | [132] |
| 259 | laurentiquinone C | Vismia laurentii De Wild. | C24H20O9 | rhodopsin-type anthraquinone | [136] |
| 260 | ploiariquinone A | Ploiarium alternifolium (Szyszył.) Melch. | C25H24O5 | rhodopsin-type anthraquinone | [137] |
| 261 | 4′-demethylknipholone | Bulbine capitata Poelln. | C23H16O8 | rhodopsin-type anthraquinone | [138] |
| 262 | knipholone | Kniphofia foliosa Hochst. | C24H18O8 | rhodopsin-type anthraquinone | [139] |
| 263 | isoknipholone | Kniphofia foliosa Hochst. | C24H18O8 | rhodopsin-type anthraquinone | [140] |
| 264 | knipholone-6-methyl ether | Bulbine capitata Poelln. | C25H20O8 | rhodopsin-type anthraquinone | [68] |
| 265 | gaboroquinone A | Bulbine frutescens (L.) Willd. | C24H18O9 | rhodopsin-type anthraquinone | [141] |
| 266 | gaboroquinone B | Bulbine frutescens (L.) Willd. | C24H18O9 | rhodopsin-type anthraquinone | [141] |
| 267 | sodium ent-knipholone 6′-O-sulfate | Bulbine frutescens (L.) Willd. | C24H17NaO11S | rhodopsin-type anthraquinone | [142] |
| 268 | sodium 4′-O-demethylknipholone 6′-O-sulfate | Bulbine frutescens (L.) Willd. | C23H15NaO11S | rhodopsin-type anthraquinone | [142] |
| 269 | sodium isoknipholone 6-O-sulfate | Bulbine frutescens (L.) Willd. | C24H17NaO11S | rhodopsin-type anthraquinone | [142] |
| 270 | 11-hydroxysulfurmycinone | Streptomyces sp. | C23H20O10 | rhodopsin-type anthraquinone | [143] |
| 271 | blanchaquinone | Streptomyces sp. | C22H20O7 | rhodopsin-type anthraquinone | [143] |
| 272 | brasiliquinone D | Nocardia brasiliensis Lindenberg & Cohn | C28H29NO8 | rhodopsin-type anthraquinone | [144] |
| 273 | cratoxyarborequinone A | Cratoxylum sumatranum (Jack) Blume | C44H46O9 | rhodopsin-type anthraquinone | [144] |
| 274 | cratoxyarborequinone B | Cratoxylum sumatranum(Jack) Blume | C49H54O9 | rhodopsin-type anthraquinone | [145] |
| 275 | floribundone | Senna septemtrionalis (Viv.) H. S. Irwin & Barneby. | C32H22O10 | rhodopsin-type anthraquinone | [146] |
| 276 | phaeosphenone | Phaeosphaeria sp. | C30H26O10 | rhodopsin-type anthraquinone | [147] |
| 277 | R-(-)-skyrin-6-O-β-xylopyranoside | Hypericum perforatum L. | C35H26O14 | rhodopsin-type anthraquinone | [148] |
| 278 | 8-O-β-D-glucopyranosyl-1,1′,8′-trihydroxy- 3,3′-dimethyl-2,7′-bianthraquinone | Eremurus chinensis O.Fedtsch. | C36H28O13 | rhodopsin-type anthraquinone | [149] |
| 279 | floribundiquinone A | Berchemia polyphylla var. leioclada (Hand.-Mazz.) Hand.-Mazz. | C32H26O10 | rhodopsin-type anthraquinone | [150] |
| 280 | floribundiquinone B | Berchemia polyphylla var. leioclada (Hand.-Mazz.) Hand.-Mazz. | C32H26O10 | rhodopsin-type anthraquinone | [150] |
| 281 | floribundiquinone C | Berchemia polyphylla var. leioclada (Hand.-Mazz.) Hand.-Mazz. | C31H24O9 | rhodopsin-type anthraquinone | [150] |
| 282 | floribundiquinone D | Berchemia polyphylla var. leioclada (Hand.-Mazz.) Hand.-Mazz. | C32H26O10 | rhodopsin-type anthraquinone | [150] |
| 283 | anhydrophlegmacin-9′,10′-quinone | Cassia torosa Cav. | C32H26O10 | rhodopsin-type anthraquinone | [151] |
| 284 | isosengulone | Senna multiglandulosa (Jacq.) H.S.Irwin & Barneby. | C32H22O10 | rhodopsin-type anthraquinone | [152] |
| 285 | icterinoidin A | Dermocybe icterinoides (Peck) Hesler & A.H. Sm. | C30H22O10 | rhodopsin-type anthraquinone | [153] |
| 286 | icterinoidin B | Dermocybe icterinoides (Peck) Hesler & A.H. Sm. | C30H22O10 | rhodopsin-type anthraquinone | [153] |
| 287 | febrifuquinoe | Psorospermum febrifugum Spach. | C40H38O10 | rhodopsin-type anthraquinone | [154] |
| 288 | chaetomanone | Chaetomium globosum Kunze | C31H24O12 | rhodopsin-type anthraquinone | [155] |
| 289 | bulbineloneside A | Bulbinella floribunda (Aiton) T.Durand & Schinz. | C30H28O13 | rhodopsin-type anthraquinone | [156] |
| 290 | bulbineloneside B | Bulbinella floribunda (Aiton) T.Durand & Schinz. | C28H24O12 | rhodopsin-type anthraquinone | [156] |
| 291 | bulbineloneside C | Bulbinella floribunda (Aiton) T.Durand & Schinz. | C28H24O12 | rhodopsin-type anthraquinone | [156] |
| 292 | bulbineloneside D | Bulbinella floribunda (Aiton) T.Durand & Schinz. | C29H26O13 | rhodopsin-type anthraquinone | [156] |
| 293 | alizarin | Rubia cordifolial L. | C14H8O4 | alizarin-type anthraquinone | [14] |
| 294 | alizarin 2-methyl ether | Rubia cordifolia L. | C15H10O4 | alizarin-type anthraquinone | [14] |
| 295 | digitolutein | Ventilago goughii Gamble | C16H14O4 | alizarin-type anthraquinone | [14] |
| 296 | 6-ethylalizarin | Galium spurium L. | C15H12O4 | Alizarin-type anthraquinone | [14] |
| 297 | altersolanol A | Stemphylium botryosum var. lactucum | C16H13O7 | alizarin-type anthraquinone | [14] |
| 298 | rubiawallin A | Rubia wallichiana Decne | C16H12O5 | alizarin-type anthraquinone | [157] |
| 299 | 1,4-dihydroxy-2,3-dimethoxyanthraquinone | Hedyotis herbacea L. | C16H12O6 | alizarin-type anthraquinone | [158] |
| 300 | 2-methoxy-1,3,6-trihydroxyanthraquinone | Morinda citrifolia L. | C15H10O6 | alizarin-type anthraquinone | [159] |
| 301 | 6-methylanthragallol 3-methyl ether | Galium sinaicum (Delile ex Decne.) Boiss. | C16H12O5 | alizarin-type anthraquinone | [160] |
| 302 | 7-methylanthragallol 1,3-dimethyl ether | Galium sinaicum (Delile ex Decne.) Boiss. | C17H14O5 | alizarin-type anthraquinone | [160] |
| 303 | 7-methylanthragallol 2-methyl ether | Galium sinaicum (Delile ex Decne.) Boiss. | C16H12O5 | alizarin-type anthraquinone | [160] |
| 304 | 7-formylanthragallol 1,3-dimethyl ether | Galium sinaicum (Delile ex Decne.) Boiss. | C17H12O6 | alizarin-type anthraquinone | [160] |
| 305 | 8-hydroxy-6,7-dimethoxy-2-methyl-9,10-anthraquinone | Prismatomeris tetrandra (Roxb.) K. Schum. | C17H14O5 | alizarin-type anthraquinone | [161] |
| 306 | 1,3-dihydroxy-5,6-dimethoxy-2-methyl-9,10-anthraquinone | Prismatomeris tetrandra (Roxb.) K. Schum. | C17H14O6 | alizarin-type anthraquinone | [162] |
| 307 | 3-dihydroxy-1,5,6-trimethoxy-2-methyl-9,10-anthraquinone | Prismatomeris tetrandra (Roxb.) K. Schum. | C18H16O6 | alizarin-type anthraquinone | [162] |
| 308 | 6-hydroxy-1, 2, 3-trimethoxy-7-methylanthracene-9, 10-dione | Prismatomeris tetrandra (Roxb.) K. Schum. | C18H16O6 | alizarin-type anthraquinone | [162] |
| 309 | 6-(hydroxymethyl)-1, 2,3-trimethoxyanthracene-9, 10-dione | Prismatomeris tetrandra (Roxb.) K. Schum. | C18H16O6 | alizarin-type anthraquinone | [163] |
| 310 | 7-hydroxy-6-(hydroxymethyl)-1, 2-dimethoxyanthracene-9,10-dione | Prismatomeris tetrandra (Roxb.) K. Schum. | C17H14O6 | alizarin-type anthraquinone | [163] |
| 311 | 8-hydroxyanthragallol 2,3-dimethyl ether | Galium sinaicum (Delile ex Decne.) Boiss. | C16H12O6 | alizarin-type anthraquinone | [160] |
| 312 | copareolatin 5,7-dimethyl ether | Galium sinaicum (Delile ex Decne.) Boiss. | C17H14O6 | alizarin-type anthraquinone | [160] |
| 313 | copareolatin 6,7-dimethyl ether | Galium sinaicum (Delile ex Decne.) Boiss. | C17H14O6 | alizarin-type anthraquinone | [160] |
| 314 | 5,15-dimethylmorindol | Morinda citrifolia L. | C17H14O6 | alizarin-type anthraquinone | [164] |
| 315 | 1,5,15-tri-O-methylmorindol | Morinda citrifolia L. | C18H16O6 | alizarin-type anthraquinone | [165] |
| 316 | (2R)-6-hydroxy-7-methoxy-dehydroiso-α-lapachone | Spermacoce alata Aubl. | C15H10O6 | alizarin-type anthraquinone | [81] |
| 317 | ventilanone N | Ventilago denticulata Willd. | C16H12O6 | alizarin-type anthraquinone | [127] |
| 318 | 3,4,8-trihydroxy-1-methylanthra-9,10-quinone-2-carboxylic acid methyl ester | Eleutherine plicata Herb. | C17H12O7 | alizarin-type anthraquinone | [166] |
| 319 | 4,8-dihydroxy-3-methoxy-1-methylanthra-9,10-quinone-2-carboxylic acid methyl ester | Eleutherine plicata Herb. | C18H14O7 | alizarin-type anthraquinone | [167] |
| 320 | 2-hydroxyemodin 1-methyl ether | Senna tora (L.) Roxb. | C16H12O6 | alizarin-type anthraquinone | [168] |
| 321 | araliorhamnone B | Araliorhamnus vaginata H.Perrier | C19H14O8 | alizarin-type anthraquinone | [135] |
| 322 | bostrycoidin | Fusarium solani (Mart.) Sacc. | C15H11NO5 | alizarin-type anthraquinone | [169] |
| 323 | 6-methoxylucidinω-ethyl ether | Prismatomeris tetrandra (Roxb.) K. Schum. | C18H16O6 | other | [161] |
| 324 | guinizarin | Galium sinaicum (Delile ex Decne.) Boiss. | C14H8O4 | other | [14] |
| 325 | pachybasin | Rheum moorcroftianum Royle | C15H10O3 | other | [14] |
| 326 | 2-hydroxy-3-methyl-anthraquinone | Hedyotis diffusa Willd. | C15H10O3 | other | [14] |
| 327 | tectoquinone | Acatypha india L. | C15H10O2 | other | [14] |
| 328 | 1-hydroxyanthraquinone | Morinda officinalis How | C15H10O2 | other | [14] |
| 329 | 2-methylol anthraquinone | Morinda parvifolia Bartl. ex DC. | C15H10O3 | other | [14] |
| 330 | 5-hydroxy-2-methyl-anthraquinone | Rubia tinctorum Linn. | C15H10O3 | other | [14] |
| 331 | barleriaquinone I | Barleria buxifolia L. | C15H10O3 | other | [14] |
| 332 | barleriaquinone II | Barleria buxifolia L. | C16H10O5 | other | [14] |
| 333 | 2-methylquinizarin | Galium sinaicum (Delile ex Decne.) Boiss. | C15H12O4 | other | [14] |
| 334 | damnacanthol | Damnacanthus major Siebold & Zucc. | C16H14O5 | other | [14] |
| 335 | ziganein | Salvia przewalskii Maxim. | C15H10O4 | other | [14] |
| 336 | 1-amino-2,4-dibromoanthraquinone | - | C14H7Br2NO2 | other | [14] |
| 337 | munjistin methyl ester | Salvia miltiorrhiza Bunge | C16H10O6 | other | [116] |
| 338 | fridamycin E | Spiroplectammina parvula Schwager | C20H20O7 | other | [14] |
| 339 | soranjidiol | Morinda elliptica (Hook.f.) Ridl. | C15H10O4 | other | [14] |
| 340 | ω-hydroxy-phomarin | Digitalis cariensis Boiss. ex Jaub. & Spach | C15H10O5 | other | [14] |
| 341 | rubiawallin C | Rubia wallichiana Decne | C16H10O5 | other | [157] |
| 342 | 2-formyl-1-hydroxyanthraquinone | Morinda elliptica (Hook.f.) Ridl. | C15H8O4 | other | [170] |
| 343 | sterequinone F | Stereospermum colais (Buch.-Ham. ex Dillwyn) Mabb. | C19H16O3 | other | [170] |
| 344 | sterequinone H | Stereospermum colais (Buch.-Ham. ex Dillwyn) Mabb. | C19H18O3 | other | [171] |
| 345 | 1-acetoxy-3-methoxy-9,10-anthraquinone | Rubia cordifolia L. | C17H12O5 | other | [172] |
| 346 | ophiohayatone C | Ophiorrhiza hayatana Ohwi | C15H8O5 | other | [173] |
| 347 | munjistin-1-O-methyl ether | Rhynchotechum vestitum Wall. ex Clatke | C16H10O6 | other | [174] |
| 348 | 1,3-dimethoxy-2-methoxymethylanthraquinone | Coussarea macrophylla (Mart.) Müll.Arg. | C18H16O5 | other | [175] |
| 349 | 1-hydroxy-2-hydroxymethyl-3-methoxyanthraquinone | Rubia wallichiana Decne | C16H12O5 | other | [157] |
| 350 | 2-n-butoxymethyl-1,3-dihydroxyanthraquinone | Morinda angustifolia Roxb. | C19H18O5 | other | [176] |
| 351 | 1-methoxy-3-hydroxy-2-carbomethoxy-9,10-anthraquinone | Saprosma scortechinii King & Gamble | C17H12O6 | other | [177] |
| 352 | rubiawallin B | Rubia wallichiana Decne | C16H12O4 | other | [157] |
| 353 | 1,7-dihydroxy-2-hydroxymethyl-9,10-anthraquinone | Hemiboea subcapitata Clarke | C15H10O5 | other | [178] |
| 354 | sterequinone G | Stereospermum colais (Buch.-Ham. ex Dillwyn) Mabb. | C20H18O4 | other | [171] |
| 355 | anthrakunthone | Stereospermum kunthianum Cham. | C19H16O4 | other | [62] |
| 356 | 3,6-dihydroxy-2-hydroxymethyl-9,10-anthraquinone | Knoxia valerianoides Thorel ex Pitard | C15H10O5 | other | [179] |
| 357 | ophiohayatone A | Ophiorrhiza hayatana Ohwi | C16H12O5 | other | [173] |
| 358 | pustuline | Heterophyllaea pustulata Hook.f. | C16H12O4 | other | [180] |
| 359 | 6-hydroxyxanthopurpurin | Galium sinaicum (Delile ex Decne.) Boiss. | C14H8O5 | other | [160] |
| 360 | 3-methoxycarbonyl-1,5-dihydroxyanthraquinone | Engelhardia roxburghiana Wall. | C16H10O6 | other | [181] |
| 361 | 1,3,6-trihydroxy-2-methoxymethyl-9,10-anthraquinone | Saprosma scortechinii King & Gamble | C16H12O6 | other | [177] |
| 362 | 1-methoxy-3,6-dihydroxy-2-hydroxymethyl-9,10-anthra-quinone | Saprosma scortechinii King & Gamble | C16H12O6 | other | [177] |
| 363 | aloesaponarin I | Aloe camperi Schweinf. | C17H12O6 | other | [182] |
| 364 | aloesaponarin I 3-methyl ether | Aloe camperi Schweinf. | C18H14O6 | other | [183] |
| 365 | alatinone | Cassia alata L. | C15H10O5 | other | [184] |
| 366 | przewalskinone B | Cassia italica Mill. | C16H12O5 | other | [185] |
| 367 | 2-Methyl-1-nitroanthraquinone | - | C15H9NO4 | other | [186] |
| 368 | 3,8-dihydroxy-6-methoxy-1-methylanthra-9,10-quinone-2-carboxylic acid methyl ester | Gladiolus gandavensis Van Houtte | C18H14O7 | other | [187] |
| 369 | ventilanone O | Ventilago denticulata Willd. | C16H12O6 | other | [127] |
| 370 | scorpinone | Amorosia littoralis Mantle & D.Hawksw. B.R. | C16H13NO4 | other | [188] |
| 371 | 1-amino-2-methylanthraquinone | - | C15H11NO2 | other | [189] |
| 372 | dielsiquinone | Guatteria dielsiana R.E.Fr. | C15H11NO4 | other | [190] |
| 373 | marcanine B | Goniothalamus marcanii Craib | C16H13NO4 | other | [129] |
| 374 | marcanine C | Goniothalamus marcanii Craib | C16H13NO5 | other | [123] |
| 375 | marcanine D | Goniothalamus marcanii Craib | C15H11NO5 | other | [129] |
| 376 | marcanine E | Goniothalamus marcanii Craib | C16H13NO5 | other | [129] |
| 377 | araliorhamnone C | Araliorhamnus vaginata H.Perrier | C17H10O7 | other | [135] |
| 378 | laurentiquinone B | Vismia laurentii De Wild. | C22H18O7 | other | [136] |
| 379 | sterequinone I | Stereospermum personatum (Hassk.) Chatterjee | C20H18O4 | other | [171] |
| 380 | sterequinone A | Stereospermum colais (Buch.-Ham. ex Dillwyn) Mabb. | C19H14O2 | other | [93] |
| 381 | sterequinone D | Stereospermum colais (Buch.-Ham. ex Dillwyn) Mabb. | C20H16O3 | other | [93] |
| 382 | 2-hydroxymethyl-10-hydroxy-1,4-anthraquinone | Hedyotis herbacea Lour. | C15H10O4 | other | [190] |
| 383 | 2,3-dimethoxy-9-hydroxy-1,4-anthraquinone | Hedyotis herbacea Lour. | C16H12O5 | other | [163] |
| 384 | 9,10-dimethoxy-2-methylanthra-1,4-quinone | - | C17H14O4 | other | [191] |
| 385 | physcion | Rheum palmatum L. | C16H12O5 | other | [192] |
| 386 | 2-aminoanthraquinone | - | C14H9NO2 | other | [193] |
| 387 | kengaquinone | Harungana madagascariensis Lam. ex Poir. | C25H26O5 | other | [194] |
| 388 | newbouldiaquinone | Newbouldia laevis (P.Beauv.) Seem. ex Bureau | C25H14O5 | other | [195] |
| 389 | newbouldiaquinone A | Newbouldia laevis (P.Beauv.) Seem. ex Bureau | C25H14O6 | other | [196] |
| 390 | tectograndone | Tectona grandis L. f. | C30H20O10 | other | [197] |
| 391 | (S)-5,5′-bisoranjidiol | Heterophyllaea pustulata Hook.f. | C30H18O8 | other | [180] |
| 392 | presengulone | Senna sophera (L.) Roxb. | C32H26O10 | other | [198] |
| 393 | scutianthraquinone A | Scutia myrtina (L.) Roxb. | C39H32O13 | other | [199] |
| 394 | scutianthraquinone B | Scutia myrtina (L.) Roxb. | C38H30O13 | other | [199] |
| 395 | scutianthraquinone C | Scutia myrtina (L.) Roxb. | C34H24O12 | other | [199] |
| 396 | scutianthraquinone D | Scutia myrtina (L.) Roxb. | C61H53O20 | other | [199] |
| 397 | mitoxantrone | - | C22H28N4O6 | Other | [200] |
| 398 | sulfemodin 8-O-β-D-glucoside | Rheum palmatum L. | C21H20O13S | anthraquinone glycosides of rhodopsin type | [201] |
| 399 | 1-methyl-8-hydroxyl-9,10-anthraquinone-3-O-β-D-glucopyranoside | Rheum palmatum L. | C22H19O11 | anthraquinone glycosides of rhodopsin type | [202] |
| 400 | 4′-O-demethylknipholone-4′-O-β-D-glucoside | Bulbine frutescens (L.) Willd. | C29H26O13 | anthraquinone glycosides of rhodopsin type | [142] |
| 401 | sodium-4′-O-demethylknipholone-4′-β-D-gluc-opyranoside 6′-O-sulfate | Bulbine frutescens (L.) Willd. | C29H25NaO16S | anthraquinone glycosides of rhodopsin type | [142] |
| 402 | aloin | Aloe vera (L.) Burm.f. | C21H22O9 | anthraquinone glycosides of rhodopsin type | [203] |
| 403 | emodin-1-O-β-gentiobioside | Cassia obtusifolia | C27H30O15 | anthraquinone glycosides of rhodopsin type | [204] |
| 404 | knipholone-8-β-D-gentiobioside | Bulbine narcissifolia | C36H38O18 | anthraquinone glycosides of rhodopsin type | [205] |
| 405 | bulbineloneside E | Bulbinella floribunda | C34H34O17 | anthraquinone glycosides of rhodopsin type | [156] |
| 406 | emodin-8-O-β-D-glucopyranoside | Pleuropterus multiflorus (Thunb.) Nakai | C21H20O10 | anthraquinone glucoside | [44] |
| 407 | emodin methyl ether-8-O-β-D-glucopyranoside | Pleuropterus multiflorus (Thunb.) Nakai | C22H22O10 | anthraquinone glucoside | [44] |
| 408 | polygonum multiflorum ethyl | Pleuropterus multiflorus (Thunb.) Nakai | C21H22O9 | anthraquinone glucoside | [44] |
| 409 | halawanone C | Streptomycete | C21H20O7 | anthraquinone glucoside | [64] |
| 410 | nepalenside A | Rumex nepalensis Spreng. | C21H22O11 | anthraquinone glucoside | [206] |
| 411 | nepalenside B | Rumex nepalensis Spreng. | C21H22O11 | anthraquinone glucoside | [206] |
| 412 | rubiadin-3-O-β-glucoside | Rhynchotechum vestitum Wall. ex C. B. Clarke | C21H20O9 | anthraquinone glucoside | [174] |
| 413 | lucidin-3-O-β-glucoside | Rhynchotechum vestitum Wall. ex C. B. Clarke | C21H20O10 | anthraquinone glucoside | [174] |
| 414 | lasianthuoside A | Lasianthus acuminatissimus Miq. | C22H22O10 | anthraquinone glucoside | [207] |
| 415 | lasianthuoside B | Lasianthus acuminatissimus Miq. | C23H24O10 | anthraquinone glucoside | [207] |
| 416 | lasianthuoside C | Lasianthus acuminatissimus Miq. | C28H32O14 | anthraquinone glucoside | [208] |
| 417 | putorinoside A | Putoria calabrica Pers. | C22H22O12 | anthraquinone glucoside | [209] |
| 418 | putorinoside B | Putoria calabrica Pers. | C22H22O11 | anthraquinone glucoside | [209] |
| 419 | 1,3-dihydroxy-2-carbomethoxy-9,10-anthraquinone3-O-β-primeveroside | Saprosma scortechinii King & Gamble | C27H28O15 | anthraquinone glucoside | [177] |
| 420 | 1.3,6-trihydroxy-2-hydroxymethyl-9,10-anthraquinone 3-O-β-primeveroside | Saprosma scortechinii King & Gamble | C26H28O15 | anthraquinone glucoside | [177] |
| 421 | emodin-6-O-β-D-glucopyranoside | Reynoutria japonica Houtt. | C21H20O10 | anthraquinone glucoside | [210] |

| No. | Name | Resource | Formula | Classification | Ref. |
|---|---|---|---|---|---|
| 422 | rubiasin A | Rubia cordifolia L. | C15H16O2 | oxyanthrone | [211] |
| 423 | rubiasin B | Rubia cordifolia L. | C15H16O2 | oxyanthrone | [211] |
| 424 | rubiasin C | Rubia cordifolia L. | C15H16O2 | oxyanthrone | [211] |
| 425 | 1-oxo-4(S),9-dihydroxy-8-methoxy-6-hydroxymethyl-1,2,3,4-tetrahydroanthracene | Eremurus chinensis O.Fedtsch. | C16H16O5 | oxyanthrone | [149] |
| 426 | aloesaponol III-8-methyl ether | Eremurus persicus (Jaub. & Spach) Boiss. | C16H16O4 | oxyanthrone | [212] |
| 427 | kenganthranol A | Harungana madagascariensis Lam. ex Poir. | C30H36O5 | oxyanthrone | [194] |
| 428 | kenganthranol B | Harungana madagascariensis Lam. ex Poir. | C25H28O5 | oxyanthrone | [194] |
| 429 | kenganthranol C | Harungana madagascariensis Lam. ex Poir. | C26H30O6 | oxyanthrone | [194] |
| 430 | 10-hydroxycascaroside C | Rheum australe D. Don | C27H32O14 | oxyanthrone glycoside | [213] |
| 431 | 10-hydroxycascaroside D | Rheum australe D. Don | C27H32O14 | oxyanthrone glycoside | [213] |
| 432 | mayoside | Mycobacterium microti | C26H24O11 | oxyanthrone glycoside | [214] |
| 433 | mayoside B | Mycobacterium microti | C26H24O11 | oxyanthrone glycoside | [214] |
| 434 | mayoside C | Picramnia teapensis Tul. | C33H34O16 | oxyanthrone glycoside | [215] |
| 435 | mayoside E | Picramnia latifolia Tul. | C27H24O9 | oxyanthrone glycoside | [216] |
| 436 | rubanthrone A | Rubus ulmifolius Schott | C17H14O10 | anthrone | [217] |
| 437 | rubanthrone B | Rubus ulmifolius Schott | C17H16O9 | anthrone | [217] |
| 438 | rubanthrone C | Rubus ulmifolius Schott | C16H12O10 | anthrone | [217] |
| 439 | knipholone anthrone | Kniphofia foliosa Hochst. | C24H20O7 | anthrone | [218] |
| 440 | isoknipholone anthrone | Kniphofia foliosa Hochst. | C24H20O7 | anthrone | [218] |
| 441 | harunganol A | Harungana madagascariensis Lam. ex Poir. | C25H28O4 | anthrone | [219] |
| 442 | harunganol B | Harungana madagascariensis Lam. ex Poir. | C30H36O4 | anthrone | [219] |
| 443 | harungin anthrone | Harungana madagascariensis Lam. ex Poir. | C30H36O4 | anthrone | [194] |
| 444 | bazouanthrone | Harungana madagascariensis Lam. ex Poir. | C30H36O5 | anthrone | [194] |
| 445 | harunmadagascarin A | Harungana madagascariensis Lam. ex Poir. | C30H34O4 | anthrone | [194] |
| 446 | harunmadagascarin B | Harungana madagascariensis Lam. ex Poir. | C35H42O4 | anthrone | [194] |
| 447 | harunmadagascarin C | Harungana madagascariensis Lam. ex Poir. | C30H36O4 | anthrone | [220] |
| 448 | harunmadagascarin D | Harungana madagascariensis Lam. ex Poir. | C30H36O5 | anthrone | [220] |
| 449 | kenganthranol D | Harungana madagascariensis Lam. ex Poir. | C30H32O6 | anthrone | [220] |
| 450 | abyquinone C | Bulbine abyssinica A.Rich. | C30H24O8 | anthrone | [221] |
| 451 | (R)-prechrysophanol | Streptomyces Waksman & Henrici | C15H14O4 | anthrone | [222] |
| 452 | torosachrysone | Dermocybe splendida E. Horak | C16H16O5 | anthrone | [223] |
| 453 | atrochrysone | Aspergillus oryzae (Ahlburg) Cohn | C15H14O5 | anthrone | [224] |
| 454 | aloe barbendol | Aloe vera (L.) Burm. f. | C15H14O4 | anthrone | [225] |
| 455 | acetyltorosachrysone | Psorospermum glaberrimum Hochr. | C18H18O6 | anthrone | [226] |
| 456 | vismione H | Psorospermum glaberrimum Hochr. | C22H24O6 | anthrone | [227] |
| 457 | vismione D | Vismia orientalis (Engl.) Byng & Christenh. | C25H30O5 | anthrone | [228] |
| 458 | vismione L | Psorospermum aurantiacum Engl. | C25H30O5 | anthrone | [229] |
| 459 | vismione M | Psorospermum aurantiacum Engl | C26H32O5 | anthrone | [229] |
| 460 | asperflavin | Microsporum sp. | C21H24O9 | anthrone | [230] |
| 461 | 5-hydroxyaloin A | Aloe nobilis A.Berger | C21H22O10 | anthrone glycoside | [231] |
| 462 | 5-hydroxyaloin A 6′-O-acetate | Aloe nobilis A.Berger | C23H24O11 | anthrone glycoside | [231] |
| 463 | picramnioside A | Picramnia antidesma Sieber ex Steud. | C27H24O10 | anthrone glycoside | [232] |
| 464 | picramnioside B | Picramnia antidesma Sieber ex Steud. | C22H22O10 | anthrone glycoside | [232] |
| 465 | picramnioside C | Picramnia antidesma Sieber ex Steud. | C22H22O10 | anthrone glycoside | [232] |
| 466 | 10-epi-uveoside | Picramnia antidesma Sieber ex Steud. | C27H24O9 | anthrone glycoside | [233] |
| 467 | uveoside | Picramnia antidesma Sieber ex Steud. | C27H24O9 | anthrone glycoside | [233] |
| 468 | microstigmin A | Aloe microstigma Salm-Dyck | C30H28O13 | anthrone glycoside | [234] |
| 469 | microdontin A | Aloe microdonta Salm-Dyck | C30H28O11 | anthrone glycoside | [234] |
| 470 | microdontin B | Aloe microdonta Salm-Dyck | C30H28O13 | anthrone glycoside | [235] |
| 471 | cascaroside E | Rhamnus purshiana DC. | C27H32O14 | anthrone glycoside | [236] |
| 472 | cascaroside F | Rhamnus purshiana DC. | C27H32O14 | anthrone glycoside | [236] |
| 473 | 10R-chrysaloin 1-O-β-D-glucopyranoside | Rheum emodi D. Don | C27H32O13 | anthrone glycoside | [213] |
| 474 | isofoliosone | Bulbine capitata Poelln. | C24H20O8 | anthrone glycoside | [138] |
| 475 | picramnioside D | Picramnia teapensis Tul. | C26H24O10 | anthrone glycoside | [237] |
| 476 | picramnioside E | Picramnia teapensis Tul. | C26H24O10 | anthrone glycoside | [237] |
| 477 | picramnioside F | Picramnia teapensis Tul. | C33H34O15 | anthrone glycoside | [215] |
| 478 | picramniosdie G | Picramnia latifolia Tul. | C27H24O8 | anthrone glycoside | [216] |
| 479 | picramnioside H | Picramnia latifolia Tul. | C27H24O8 | anthrone glycoside | [216] |
| 480 | mayoside D | Picramnia latifolia Tul. | C27H24O9 | anthrone glycoside | [216] |















Dithranones





2.2. Extraction and Separation Methods
2.2.1. Extraction
Alkali Extraction and Acid Precipitation Method
Organic Solvent Extraction Methods
Physical Field Enhanced Extraction
Steam Distillation Method
Lead Salt Method
Supercritical Fluid Extraction Methods
Solid-Phase Extraction Method
Pressurized Liquid Extraction Method
2.2.2. Separation
pH Gradient Extraction Method
Chromatographic Methods
Macroporous Adsorption Resin Method
2.3. Structural Identification Methods
2.3.1. Benzoquinones
2.3.2. Naphthoquinones
2.3.3. Phenanthrenequinones
2.3.4. Anthraquinones
3. Progress in Pharmacological Activity Research
3.1. Immunomodulatory Effects
3.2. Anti-Tumor Activity
3.3. Antioxidant Activity
3.4. Anti-Inflammatory Activity
3.5. Antimicrobial Activity
3.6. Anti-Fibrotic Effect
3.7. Laxative Effect
3.8. Antidepressant Effects
4. Progress in Toxicity Studies
4.1. Digestive System Toxicity
4.1.1. Hepatotoxicity
4.1.2. Enterotoxicity
4.2. Urinary Toxicity
4.3. Reproductive Toxicity
4.4. Carcinogenicity
5. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peng, Y.H.; Liu, X.Q.; Lv, H.Y. Natural Medicinal Chemistry, 2nd ed.; Chemical Industry Press: Beijing, China, 2020. [Google Scholar]
- Yang, Y.T.; Zhang, G.Y.; Yang, D.F.; Zhang, Y.Q.; Zhang, L.; Wu, S.; Li, X.; Zhou, Y.Z. Research progress on the regulation of lipid metabolism in the body by active components of traditional Chinese medicine. Chin. Herb. Med. 2024, 55, 3127–3136. [Google Scholar]
- Wu, R.; Li, K.F.; Wang, W.F. Efficacy of sodium tanshinone IIA sulfonate combined with metoprolol in the treatment of senile coronary heart disease and its effect on ventricular remodeling. Clin. Med. Res. Pract. 2025, 10, 53–56. [Google Scholar] [CrossRef]
- Yuan, H.L.; Zhao, Y.L.; Fan, D.S.; He, W.L.; Zhao, P.P.; Cai, Y. Improved synthesis method of buparvaquone. Chem. Bull. 2021, 84, 952–957. [Google Scholar] [CrossRef]
- Tao, M.B.; Zhang, L.; Liu, F.; Chen, L.; Liu, Y.P.; Chen, H.P. Research progress on the safety of traditional Chinese medicines containing anthraquinone components. Pharmacol. Clin. Chin. Mater. Med. 2016, 32, 238–243. [Google Scholar] [CrossRef]
- Zhou, X.J. Analysis of clinical characteristics of 130 cases of melanosis coli. Jilin Med. J. 2014, 35, 3487–3488. [Google Scholar]
- Wang, X.; Sun, K.W.; Tian, T.; Zeng, W.T.; Yuan, W. Clinical analysis of 12 cases of drug-induced liver injury caused by Polygonum multiflorum and its related preparations. Liver 2024, 29, 1538–1540. [Google Scholar] [CrossRef]
- Duan, Y.H. Literature analysis of traditional Chinese medicine varieties causing kidney damage. Strait. Pharm. J. 2014, 26, 138–139. [Google Scholar]
- Zhao, F.B.; Li, T.J.; Zhao, H.; Guo, J.W.; Zhang, J.G. Clinical analysis of 356 cases of acute kidney injury in inpatients of a nephrology-specialized hospital. J. Intern. Med. Theor. Pract. 2015, 10, 177–180. [Google Scholar] [CrossRef]
- Holliday, A.E.; Walker, F.M.; Brodie, E.D., III; Formica, V.A. Differences in defensive volatiles of the forked fungus beetle, Bolitotherus cornutus, living on two species of fungus. J. Chem. Ecol. 2009, 35, 1302–1308. [Google Scholar] [CrossRef]
- Dong, M.; Ming, X.; Xiang, T.; Feng, N.; Zhang, M.; Ye, X.; He, Y.; Zhou, M.; Wu, Q. Recent research on the physicochemical properties and biological activities of quinones and their practical applications: A comprehensive review. Food Funct. 2024, 15, 8973–8997. [Google Scholar] [CrossRef]
- Qiu, Y.F.; Lu, B.; Yan, Y.Y.; Luo, W.Y.; Gao, Z.Q.; Wang, J. A convenient synthesis of 1,4-benzoquinones. J. Chem. Res. 2019, 43, 124–126. [Google Scholar] [CrossRef]
- Shi, Z.M.; Wang, Q.; Lu, X.X.; Zeng, H.P.; Xiao, H.; Zhang, C.G.; Liu, H. Study on the physicochemical properties and druglikeness prediction of methyl-p-benzoquinone. J. Dali. Univ. 2024, 9, 26–32. [Google Scholar]
- Lu, Y. Chemistry of Quinones (Series of Natural Product Chemistry), 2nd ed.; Chemical Industry Press: Beijing, China, 2009; ISBN 978-7-122-04505-8. [Google Scholar]
- Yang, D.Y.; Yu, H.; Wu, X.Y.; Zhu, Y.H.; Xiao, X.L.; Xu, W.A.; Chen, Y.X.; Gong, Q.F. Research progress on chemical components and biological activities of Atractylodes macrocephala Koidz. Chin. Arch. Tradit. Chin. Med. 2023, 41, 171–182. [Google Scholar] [CrossRef]
- Zhang, W.Q. Study on the Chemical Components and Anti-Inflammatory Activities of Arnebia euchroma (Royle) Johnst. Master’s Thesis, Southern Medical University, Guangzhou, China, 2022. [Google Scholar]
- Chen, C.Y.; Wang, J.J.; Kao, C.L.; Li, H.T.; Wu, M.D.; Cheng, M.J. A New Benzoquinone from Antrodia camphorata. Chem. Nat. Compd. 2022, 58, 614–616. [Google Scholar] [CrossRef]
- Wang, H.J.; Gloer, K.B.; Gloer, J.B.; Scott, J.A.; Malloch, D. Anserinones A and B: New antifungal and antibacterial benzoquinones from the coprophilous fungus Podospora anserina. J. Nat. Prod. 1997, 60, 629–631. [Google Scholar] [CrossRef]
- Gupta, I.; Peddha, M.S. Anti-adipogenic activity of oleoresin from Nigella sativa L. seeds via modulation of PPAR-γ and C/EBP-α expression in 3T3-L1 adipocytes. Adv. Tradit. Med. 2024. [Google Scholar] [CrossRef]
- Ko, J.-H.; Lee, S.-G.; Yang, W.M.; Um, J.-Y.; Sethi, G.; Mishra, S.; Shanmugam, M.K.; Ahn, K.S. The Application of Embelin for Cancer Prevention and Therapy. Molecules 2018, 23, 621. [Google Scholar] [CrossRef]
- Liu, J. Study on the Chemical Components and Biological Activities of Embelia ribes Burm.f. and Hypericum spathulatum Hook.f. & Thomson ex Dyer. Master’s Thesis, Dali Univercity, China, 2018. [Google Scholar]
- De Gaetano, F.; Mannino, D.; Celesti, C.; Bulzomí, M.; Iraci, N.; Giofrè, S.V.; Esposito, E.; Paterniti, I.; Ventura, C.A. Randomly methylated β-cyclodextrin improves water-solubility, cellular protection and mucosa permeability of idebenone. Int. J. Pharm. 2024, 665, 124718. [Google Scholar] [CrossRef]
- Gunatilaka, A.A.; Berger, J.M.; Evans, R.; Miller, J.S.; Wisse, J.H.; Neddermann, K.M.; Bursuker, I.; Kingston, D.G. Isolation, synthesis, and structure-activity relationships of bioactive benzoquinones from Miconia lepidota from the Suriname rainforest. J. Nat. Prod. 2001, 64, 2–5. [Google Scholar] [CrossRef]
- Lin, W.Y.; Kuo, Y.H.; Chang, Y.L.; Teng, C.M.; Wang, E.C.; Ishikawa, T.; Chen, I.S. Anti-platelet aggregation and chemical constituents from the rhizome of Gynura japonica. Planta Med. 2003, 69, 757–764. [Google Scholar] [CrossRef]
- Arot Manguro, L.O.; Midiwo, J.O.; Kraus, W.; Kraus, W.; Ugi, I. Benzoquinone derivatives of Myrsine africana and Maesa lanceolata. Phytochemistry 2003, 64, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Lertnitikul, N.; Teerasukpimol, L.; Aekanantakul, P.; Pooreecharurot, N.; Sukrong, S.; Boonyong, C.; Poldorn, P.; Rungrotmongkol, T.; Sukandar, E.R.; Aonbangkhen, C.; et al. A new benzoquinone and a new stilbenoid from Paphiopedilum exul (Ridl.) Rolfe. Nat. Prod. Res. 2024, 22, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.Y.; Li, N.; Luo, W.Y.; Wang, L.L.; Zhang, Y.Y.; Wang, J. A Simple and Convenient Two-step Synthesis of Idebenone. Org. Prep. Proced. Int. 2021, 53, 397–401. [Google Scholar] [CrossRef]
- Su, J.H.; Ahmed, A.F.; Sung, P.J.; Wu, Y.C.; Sheu, J.H. Meroditerpenoids from a Formosan soft coral Nephthea chabrolii. J. Nat. Prod. 2005, 68, 1651–1655. [Google Scholar] [CrossRef] [PubMed]
- Permana, D.; Lajis, N.H.; Mackeen, M.M.; Ali, A.M.; Aimi, N.; Kitajima, M.; Takayama, H. Isolation and bioactivities of constitutents of the roots of Garcinia atroviridis. J. Nat. Prod. 2001, 64, 976–979. [Google Scholar] [CrossRef]
- Pirouz, M.; Abaee, M.S.; Harris, P.; Mojtahedi, M.M. One-pot synthesis of benzofurans via heteroannulation of benzoquinones. Heterocycl. Commun. 2021, 27, 24–31. [Google Scholar] [CrossRef]
- Ferreira, P.M.P.; De Almeida, A.A.C.; Conceição, M.L.P.; Pessoa, O.D.L.; Marques, L.G.A.; Capasso, R.; Pessoa, C. Cordia oncocalyx and oncocalyxones: From the phytochemistry to the anticancer action and therapeutic benefits against chronic diseases. Fitoterapia 2023, 169, 105624. [Google Scholar] [CrossRef]
- Guillonneau, L.; Taddei, D.; Moody, C.J. Synthesis of the reported structure of the bisbenzoquinone lanciaquinone, isolated from Maesa lanceolata. Org. Lett. 2008, 10, 4505–4508. [Google Scholar] [CrossRef]
- Sangsopha, W.; Lekphrom, R.; Schevenels, F.T.; Saksirirat, W.; Bua-Art, S.; Kanokmedhakul, K.; Kanokmedhakul, S. New p-terphenyl and benzoquinone metabolites from the bioluminescent mushroom Neonothopanus nambi. Nat. Prod. Res. 2020, 34, 2186–2193. [Google Scholar] [CrossRef]
- Chandra, P.; Read, G.; Vining, L.C. Studies on the biosynthesis of volucrisporin. II Metabolism of some phenylpropanoid compounds by Volucrispora aurantiaca Haskins. Can. J. Biochem. 1966, 44, 403–413. [Google Scholar] [CrossRef]
- Truong Nguyen, H.; Duong, T.H.; Dang, M.K.; Pham, M.D.; Pham, N.K.; Tri Mai, D.; Son Dang, V.; Nguyen, N.H.; Sichaem, J. Two New Benzoquinone Derivatives from Vietnamese Knema globularia Stems. Chem. Biodivers. 2024, 21, 4. [Google Scholar] [CrossRef]
- Lin, Y.L.; Su, Y.T.; Chen, B.H. A study on inhibition mechanism of breast cancer cells by bis-type triaziquone. Eur. J. Pharmacol. 2010, 637, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Prins, B.; Koster, A.S.; Verboom, W.; Reinhoudt, D.N. Microsomal superoxide anion production and NADPH-oxidation in a series of 22 aziridinylbenzoquinones. Biochem. Pharmacol. 1989, 38, 3753–3757. [Google Scholar] [CrossRef] [PubMed]
- An, T.-Y.; Shan, M.-D.; Hu, L.-H.; Liu, S.-J.; Chen, Z.-L. Polyprenylated phloroglucinol derivatives from Hypericum erectum. Phytochemistry 2002, 59, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Wieder, C.; Peres da Silva, R.; Witts, J.; Jäger, C.M.; Geib, E.; Brock, M. Characterisation of ascocorynin biosynthesis in the purple jellydisc fungus Ascocoryne sarcoides. Fungal. Biol. Biotechnol. 2022, 9, 8. [Google Scholar] [CrossRef]
- Pei, J.; Hsu, C.-C.; Wang, Y.; Yu, K.F. Corona discharge-induced reduction of quinones in negative electrospray ionization mass spectrometry. RSC Adv. 2017, 7, 43540–43545. [Google Scholar] [CrossRef]
- Ding, H.; Sun, B.; Jin, C. Review on the synthesis methods of 2-methyl-1,4-naphthoquinone. Zhejiang Chem. Ind. 2023, 54, 23–26. [Google Scholar]
- Higa, M.; Ogihara, K.; Yogi, S. Bioactive naphthoquinone derivatives from Diospyros maritima BLUME. Chem. Pharm. Bull. 1998, 46, 1189–1193. [Google Scholar] [CrossRef]
- Higa, M.; Noha, N.; Yokaryo, H.; Ogihara, K.; Yogi, S. Three new naphthoquinone derivatives from Diospyros maritima Blume. Chem. Pharm. Bull. 2002, 50, 590–593. [Google Scholar] [CrossRef]
- Yao, R.; Guo, H.; Zhang, X.S.; Wang, Y.; Guo, X.H.; Chen, J.; Li, J.H.; Xu, L.; Yang, J.B.; Jing, W.G.; et al. Research progress on the processing technology, chemical components and pharmacological activities of processed Polygonum multiflorum. Front. Pharm. 2024, 28, 523–535. [Google Scholar]
- Nishina, A.; Kubota, K.; Osawa, T. Antimicrobial components, trachrysone and 2-methoxystypandrone, in Rumex japonicus Houtt. J. Agric. Food Chem. 1993, 41, 1772–1775. [Google Scholar] [CrossRef]
- Takahashi, D.; Maoka, T.; Tsushima, M.; Fujitani, K.; Kozuka, M.; Matsuno, T.; Shingu, T. New Quinone Sulfates from the Crinoids Tropiometra afra macrodiscus and Oxycomanthus japonicus. Chem. Pharm. Bull. 2002, 50, 1609–1612. [Google Scholar] [CrossRef] [PubMed]
- Iwata, D.; Ishibashi, M.; Yamamoto, Y.; Cribrarione, B. A new naphthoquinone pigment from the myxomycete Cribraria cancellata. J. Nat. Prod. 2003, 66, 1611–1612. [Google Scholar] [CrossRef] [PubMed]
- Trisuwan, K.; Khamthong, N.; Rukachaisirikul, V.; Phongpaichit, S.; Preedanon, S.; Sakayaroj, J. Anthraquinone, Cyclopentanone, and Naphthoquinone Derivatives from the Sea Fan-Derived Fungi Fusarium spp. PSU-F14 and PSU-F135. J. Nat. Prod. 2010, 73, 1507–1511. [Google Scholar] [CrossRef]
- Rong, X.G.; Ma, L.Y.; Liu, W.Z.; Liu, D.S. A new naphthoquinone compound from Penicillium raistrickii. Chin. J. Mar. Drugs 2024, 43, 10–16. [Google Scholar] [CrossRef]
- Kishore, P.H.; Reddy, M.V.; Gunasekar, D.; Caux, C.; Bodo, B. A new naphthoquinone from Ceiba pentandra. J. Asian Nat. Prod. Res. 2003, 5, 227–230. [Google Scholar] [CrossRef]
- Sreeramulu, K.; Rao, K.V.; Rao, C.V.; Gunasekar, D. A new naphthoquinone from Bombax malabaricum. J. Asian Nat. Prod. Res. 2001, 3, 261–265. [Google Scholar] [CrossRef]
- Lee, I.S.; Kaneda, N.; Suttisri, R.; El-Lakany, A.M.; Sabri, N.N.; Kinghorn, A.D. New orthoquinones from the roots of Salvia lanigera. Planta Med. 1998, 64, 632–634. [Google Scholar] [CrossRef]
- Sreelatha, T.; Hymavathi, A.; Murthy, J.M.; Rani, P.U.; Rao, J.M.; Babu, K.S. Bioactivity-guided isolation of mosquitocidal constituents from the rhizomes of Plumbago capensis Thunb. Bioorg. Med. Chem. Lett. 2010, 20, 2974–2977. [Google Scholar] [CrossRef]
- Kim, J.P.; Kim, W.G.; Koshino, H.; Jung, J.; Yoo, I.D. Sesquiterpene O-naphthoquinones from the root bark of Ulmus davidiana. Phytochemistry 1996, 43, 425–430. [Google Scholar] [CrossRef]
- Wang, D.; Xia, M.Y.; Cui, Z.; Tashiro, S.; Onodera, S.; Ikejima, T. Cytotoxic effects of mansonone E and F isolated from Ulmus pumila. Biol. Pharm. Bull. 2004, 27, 1025–1030. [Google Scholar] [CrossRef]
- Changwong, N.; Sabphon, C.; Ingkaninan, K.; Sawasdee, P. Acetyl- and Butyryl-cholinesterase Inhibitory Activities of Mansorins and Mansonones. Phytother. Res. 2012, 26, 392–396. [Google Scholar] [CrossRef] [PubMed]
- El-Halawany, A.M.; Chung, M.H.; Ma, C.-M.; Komatsu, K.; Nishihara, T.; Hattori, M. Anti-estrogenic activity of mansorins and mansonones from the heartwood of Mansonia gagei DRUMM. Chem. Pharm. Bull. 2007, 55, 1332–1337. [Google Scholar] [CrossRef] [PubMed]
- Panichayupakaranant, P.; Charoonratana, T.; Sirikatitham, A. RP-HPLC Analysis of Rhinacanthins in Rhinacanthus nasutus: Validation and Application for the Preparation of Rhinacanthin High-Yielding Extract. J. Chromatogr. Sci. 2009, 47, 705–708. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.-S.; Tien, H.-J.; Yeh, M.-Y.; Lee, K.-H. Isolation and cytotoxicity of rhinacanthin-A and -B, two naphthoquinones, from Rhinacanthus nasutus. Phytochemistry 1988, 27, 3787–3788. [Google Scholar] [CrossRef]
- Luo, J.; Wu, Z.L.; Huang, X.J.; Zhang, X.Q.; Fan, C.L.; Ye, W.C.; Wang, Y. Two new pyranonaphthoquinone compounds from Mansoa alliacea (Lam.) A.H.Gentry. China J. Chin. Mat. Med. 2021, 46, 3364–3367. [Google Scholar] [CrossRef]
- Kitagawa, R.R.; Villegas, W.; Carlos, I.Z.; Raddi, M.S.G. Antitumor and immunomodulatory effects of the naphthoquinone 5-methoxy-3,4-dehydroxanthomegnin. Rev. Bras. Farmacogn. 2011, 21, 1084–1088. [Google Scholar] [CrossRef]
- Onegi, B.; Kraft, C.; Köhler, I.; Freund, M.; Jenett-Siems, K.; Siems, K.; Beyer, G.; Melzig, M.F.; Bienzle, U.; Eich, E. Herbal remedies traditionally used against malaria part 6 -: Antiplasmodial activity of napthoquinones and one anthraquinone from Stereospermum kunthianum. Phytochemistry 2002, 60, 39–44. [Google Scholar] [CrossRef]
- Kittakoop, P.; Punya, J.; Kongsaeree, P.; Lertwerawat, Y.; Jintasirikul, A.; Tanticharoen, M.; Thebtaranonth, Y. Bioactive naphthoquinones from Cordyceps unilateralis. Phytochemistry 1999, 52, 453–457. [Google Scholar] [CrossRef]
- Ford, P.W.; Gadepelli, M.; Davidson, B.S. Halawanones A-D, New polycyclic quinones from a marine-derived streptomycete. J. Nat. Prod. 1998, 61, 1232–1236. [Google Scholar] [CrossRef]
- El-Hady, S.; Bukuru, J.; Kesteleyn, B.; Van Puyvelde, L.; Nguyen, T.V.; De Kimpe, N. New pyranonaphthoquinone and pyranonaphthohydroquinone from the roots of Pentas longiflora. J. Nat. Prod. 2002, 65, 1377–1379. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Kinoshita, Y.; Thor, G.R.; Hasumi, M.; Kinoshita, K.; Koyama, K.; Takahashi, K.; Yoshimura, I. Isofuranonaphthoquinone derivatives from cultures of the lichen Arthonia cinnabarina (DC.) Wallr. Phytochemistry 2002, 60, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Naoe, A.; Ishibashi, M.; Yamamoto, Y. Cribrarione A, a new antimicrobial naphthoquinone pigment from a myxomycete Cribraria purpurea. Tetrahedron 2003, 59, 3433–3435. [Google Scholar] [CrossRef]
- Bezabih, M.; Motlhagodi, S.; Abegaz, B.M. Isofuranonaphthoquinones and phenolic and knipholone derivatives from the roots of Bulbine capitata. Phytochemistry 1997, 46, 1063–1067. [Google Scholar] [CrossRef]
- Piggott, M.J.; Wege, D. The synthesis of 5-hydroxy-3-methylnaphtho[2,3-c]furan-4,9-dione and 5,8-dihydroxy-1-methylnaphtho[2,3-c]furan-4,9-dione. Aust. J. Chem. 2003, 56, 691–702. [Google Scholar] [CrossRef]
- Ito, C.; Katsuno, S.; Kondo, Y.; Tan, H.T.; Furukawa, H. Chemical constituents of Avicennia alba. Isolation and structural elucidation of new naphthoquinones and their analogues. Chem. Pharm. Bull. 2000, 48, 339–343. [Google Scholar] [CrossRef]
- Williams, R.B.; Norris, A.; Miller, J.S.; Razafitsalama, L.J.; Andriantsiferana, R.; Rasamison, V.E.; Kingston, D.G.I. Two new cytotoxic naphthoquinones from Mendoncia cowanii from the rainforest of Madagascar. Planta Med. 2006, 72, 564–566. [Google Scholar] [CrossRef]
- Gormann, R.; Kaloga, M.; Li, X.C.; Ferreira, D.; Bergenthal, D.; Kolodziej, H. Furanonaphthoquinones, atraric acid and a benzofuran from the stem barks of Newbouldia laevis. Phytochemistry 2003, 64, 583–587. [Google Scholar] [CrossRef]
- Kishore, N.; Mishra, B.B.; Tiwari, V.K.; Tripathi, V. Difuranonaphthoquinones from Plumbago zeylanica roots. Phytochem. Lett. 2010, 3, 62–65. [Google Scholar] [CrossRef]
- Cai, X.H.; Luo, X.D.; Zhou, J.; Hao, X.J. Quinones from Chirita eburnea. J. Nat. Prod. 2005, 68, 797–799. [Google Scholar] [CrossRef]
- Verdán, M.H.; Koolen, H.H.F.; Salvador, M.J.; Barison, A.; Stefanello, M.E.A. A New Naphthoquinone from Sinningia leucotricha (Gesneriaceae). Nat. Prod. Commun. 2015, 10, 625–626. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.K.; Singh, P. A naphthoquinone derivative from Tectona grandis (Linn.). J. Asian Nat. Prod. Res. 2004, 6, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Gafner, S.; Wolfender, J.-L.; Nianga, M.; Hostettmann, K. A naphthoquinone from Newbouldia laevis roots. Phytochemistry 1998, 48, 215–216. [Google Scholar] [CrossRef]
- Ito, C.; Kondo, Y.; Rao, K.S.; Tokuda, H.; Nishino, H.; Furukawa, H. Chemical constituents of Glycosmis pentaphylla: Isolation of a novel naphthoquinone and a new acridone alkaloid. Chem. Pharm. Bull. 1999, 47, 1579–1581. [Google Scholar] [CrossRef]
- Zhong, Y.J.; Wen, Q.F.; Li, C.Y.; Su, X.H.; Yuan, Z.P.; Li, Y.F. Two New Naphthoquinone Derivatives from Lysionotus pauciflorus. Helv. Chim. Acta 2013, 96, 1750–1756. [Google Scholar] [CrossRef]
- Li, Q.; Guo, Z.H.; Wang, K.B.; Zhang, X.S.; Lou, Y.T.; Zhao, Y.Q. Two new 1,4-naphthoquinone derivatives from Impatiens balsamina L. flowers. Phytochem. Lett. 2015, 14, 8–11. [Google Scholar] [CrossRef]
- Luo, Y.; Shen, H.Y.; Shen, Q.X.; Cao, Z.H.; Zhang, M.; Long, S.Y.; Wang, Z.B.; Tan, J.W. A new anthraquinone and a new naphthoquinone from the whole plant of Spermacoce latifolia. J. Asian Nat. Prod. Res. 2017, 19, 869–876. [Google Scholar] [CrossRef]
- Tangmouo, J.G.; Meli, A.L.; Komguem, J.; Kuete, V.; Ngounou, F.N.; Lontsi, D.; Beng, V.P.; Choudhary, M.I.; Sondengam, B.L. Crassiflorone, a new naphthoquinone from Diospyros crassiflora (Hien). Tetrahedron Lett. 2006, 47, 3067–3070. [Google Scholar] [CrossRef]
- Hussain, H.; Krohn, K.; Ahmad, V.U.; Miana, G.A.; Green, I.R. Lapachol: An overview. Arkivoc 2007, 2007, 145–171. [Google Scholar] [CrossRef]
- Hasan, A.; Furumoto, T.; Begum, S.; Fukui, H. Hydroxysesamone and 2,3-epoxysesamone from roots of Sesamum indicum. Phytochemistry 2001, 58, 1225–1228. [Google Scholar] [CrossRef]
- Hayashi, K.; Chang, F.R.; Nakanishi, Y.; Bastow, K.F.; Cragg, G.; McPhail, A.T.; Nozaki, H.; Lee, K.H. Antitumor agents. 233. Lantalueratins A–F, new cytotoxic naphthoquinones from Lantana involucrata. J. Nat. Prod. 2004, 67, 990–993. [Google Scholar] [CrossRef] [PubMed]
- Shukla, Y.N.; Srivastava, A.; Singh, S.C.; Kumar, S. New naphthoquinones from Arnebia hispidissima roots. Planta Med. 2001, 67, 575–577. [Google Scholar] [CrossRef] [PubMed]
- Ioset, J.R.; Marston, A.; Gupta, M.P.; Hostettmann, K. Antifungal and larvicidal cordiaquinones from the roots of Cordia curassavica. Phytochemistry 2000, 53, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Sheu, J.H.; Su, J.H.; Sung, P.J.; Wang, G.H.; Dai, C.F. Novel meroditerpenoid—related metabolites from the formosan soft coral Nephthea chabrolii. J. Nat. Prod. 2004, 67, 2048–2052. [Google Scholar] [CrossRef]
- Winiewski, V.; Verdán, M.H.; Oliveira, C.S.; Su, X.H.; Yuan, Z.P.; Li, Y.F. Two new naphthoquinone derivatives from Sinningia conspicua (Gesneriaceae). Nat. Prod. Res. 2023, 39, 88–93. [Google Scholar] [CrossRef]
- Kongkathip, N.; Luangkamin, S.; Kongkathip, B.; Sangma, C.; Grigg, R.; Kongsaeree, P.; Prabpai, S.; Pradidphol, N.; Pitaviriyagul, S.; Siripong, P. Synthesis of novel rhinacanthins and related anticancer naphthoquinone esters. J. Med. Chem. 2004, 47, 4427–4438. [Google Scholar] [CrossRef]
- Van Puyvelde, L.; El Hady, S.; De Kimpe, N.; Feneau-Dupont, J.; Declercq, J.P. Isagarin, a new type of tetracyclic naphthoquinone from the roots of Pentas longiflora. J. Nat. Prod. 1998, 61, 1020–1021. [Google Scholar] [CrossRef]
- Aguiar, R.M.; David, J.P.; David, J.M. Unusual naphthoquinones, catechin and triterpene from Byrsonima microphylla. Phytochemistry 2005, 66, 2388–2392. [Google Scholar] [CrossRef]
- Kumar, U.S.; Aparna, P.; Rao, R.J.; Rao, T.P.; Rao, J.M. 1-methyl anthraquinones and their biogenetic precursors from Stereospermum personatum. Phytochemistry 2003, 63, 925–929. [Google Scholar] [CrossRef]
- Sankaram, A.V.B.; Rao, A.S.; Shoolery, J.N. Zeylanone and Isozeylanone, two novel quinones from Plumbago zeylanica. Tetrahedron 1979, 35, 1777–1782. [Google Scholar] [CrossRef]
- Higa, M. A new binaphthoquinone from Diospyros maritima Blume. Chem. Pharm. Bull. 1988, 36, 3234. [Google Scholar] [CrossRef]
- Jassbi, A.R.; Singh, P.; Jain, S.; Tahara, S. Novel naphthoquinones from Heterophragma adenophyllum. Helv. Chim. Acta 2004, 87, 820–824. [Google Scholar] [CrossRef]
- Cai, P.; Kong, F.M.; Ruppen, M.E.; Glasier, G.; Carter, G.T. Hygrocins A and B, naphthoquinone macrolides from Streptomyces hygroscopicus. J. Nat. Prod. 2005, 68, 1736–1742. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.M.O.; Lemos, T.L.G.; Pessoa, O.D.L.; Assunção, J.C.C.; Braz-Filho, R. Constituintes químicos de Lippia es (Cham.) Verbenaceae. Rev. Bras. Farmacogn. 2002, 12 (Suppl. S1), 7. [Google Scholar] [CrossRef]
- Bryshten, I.; Paprotny, Ł.; Olszowy-Tomczyk, M.; Wianowska, D. Quantitative Study of Vitamin K in Plants by Pressurized Liquid Extraction and LC-MS/MS. Molecules 2024, 29, 4420. [Google Scholar] [CrossRef]
- Uc-Cachón, A.H.; Borges-Argáez, R.; Said-Fernández, S.; Vargas-Villarreal, J.; González-Salazar, F.; Méndez-González, M.; Cáceres-Farfán, M.; Molina-Salinas, G.M. Naphthoquinones isolated from Diospyros anisandra exhibit potent activity against pan-resistant first-line drugs Mycobacterium tuberculosis strains. Pulm. Pharmacol. Ther. 2014, 27, 114–120. [Google Scholar] [CrossRef]
- Liu, Y.H.; Lin, F.X.; Tan, Y.F.; Yang, J.Y.; Zhang, B.; Zhou, X.M.; Song, X.M. Three new phenanthrenequinone compounds from the roots of Dendrobium. Chin. J. Org. Chem. 2021, 41, 2112–2115. [Google Scholar] [CrossRef]
- Sarkar, P.; Ahmed, A.; Ray, J.K. Suzuki cross coupling followed by cross dehydrogenative coupling: An efficient one pot synthesis of Phenanthrenequinones and analogues. Tetrahedron Lett. 2020, 61, 151701. [Google Scholar] [CrossRef]
- Xuezhao, L.; Houwei, L.; Masatake, N. Trijuganone A and B: Two New Phenanthrenequinones from Roots of Salvia trijuga. Planta Med. 1990, 56, 87–88. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Cui, C.B.; Cai, B.; Han, B.; Sun, Q.S. A new phenanthraquinone from the stems of Bauhinia variegata L. J. Asian Nat. Prod. Res. 2005, 7, 835–838. [Google Scholar] [CrossRef]
- Bhaskar, M.U.; Rao, L.J.M.; Rao, N.S.P.; Rao, P.R.M. Ochrone A, a novel 9,10-dihydro-1,4-phenanthraquinone from Coelogyne ochracea. J. Nat. Prod. 1991, 54, 386. [Google Scholar] [CrossRef]
- Lin, L.-G.; Yang, X.-Z.; Tang, C.-P.; Ke, C.Q.; Zhang, J.B.; Ye, Y. Antibacterial stilbenoids from the roots of Stemona tuberosa. Phytochemistry 2007, 69, 457–463. [Google Scholar] [CrossRef]
- Itharat, A.; Plubrukarn, A.; Kongsaeree, P.; Bui, T.; Keawpradub, N.; Houghton, P.J. Dioscorealides and dioscoreanone, novel cytotoxic naphthofuranoxepins, and 1,4-phenanthraquinone from Dioscorea membranacea Pierre. Org. Lett. 2003, 5, 2879–2882. [Google Scholar] [CrossRef]
- Talapatra, B.; Mukhopadhyay, P.; Chaudhury, P.; Talapatra, S.K. Denbinobin, a new phenanthraquinone from Dendrobium nobile Lindl (Orchidaceae). Indian J. Chem. Sect. B 1982, 21, 386. [Google Scholar]
- Bae, E.Y.; Oh, H.; Oh, W.K.; Kim, M.S.; Kim, B.S.; Kim, B.Y.; Sohn, C.B.; Osada, H.; Ahn, J.S. A new VHR dual-specificity protein tyrosine phosphatase inhibitor from Dendrobium moniliforme. Planta Med. 2004, 70, 869–870. [Google Scholar] [CrossRef]
- Harvey, R.B.; Edrington, T.S.; Kubena, L.F.; Rottinghaus, G.E.; Turk, J.R.; Genovese, K.J.; Ziprin, R.L.; Nisbet, D.J. Toxicity of fumonisin from Fusarium verticillioides culture material and moniliformin from Fusarium fujikuroi culture material when fed singly and in combination to growing barrows. J. Food Prot. 2002, 65, 373–377. [Google Scholar] [CrossRef]
- Chen, D.N.; Wu, Y.P.; Chen, Y.J.; Liu, W.J.; Wang, J.X.; He, F.; Jiang, L. Two new stilbenoids from aerial parts of Flickingeria fimbriata. J. Asian Nat. Prod. Res. 2019, 21, 117–122. [Google Scholar] [CrossRef]
- Wu, T.S.; Jong, T.T.; Tien, H.J.; Kuoh, C.S.; Furukawa, H.; Lee, K.H. Annoquinone-A, an antimicrobial and cytotoxic principle from Annona montana. Phytochemistry 1987, 26, 1623–1625. [Google Scholar] [CrossRef]
- Tezuka, Y.; Kasimu, R.; Basnet, P.; Namba, T.; Kadota, S. Aldose reductase inhibitory constituents of the root of Salvia miltiorhiza Bunge. Chem. Pharm. Bull. 1997, 45, 1306–1311. [Google Scholar] [CrossRef]
- Ju, J.H.; Yang, J.S.; Li, J.; Xiao, P.G. Cypripediquinone A, a new phenanthraquinone from Cypripedium macranthum (Orchidaceae). Chin. Chem. Lett. 2000, 11, 37–38. [Google Scholar]
- Majumder, P.L.; Sen, R.C. Bulbophyllanthrone, a phenanthraquinone from Bulbophyllum odoratissimum. Phytochemistry 1991, 30, 2092. [Google Scholar] [CrossRef]
- Zhao, L.Y. Study on the Chemical Constituents of Salvia bowleyana Dunn. Master’s Thesis, Hubei University of Science and Technology, China, 2023. [Google Scholar]
- Liang, W.; Sun, J.C.; Guo, F.X.; Zhang, X.M.; Xu, B.; Chen, Y.; Li, X. Research progress on the synthesis of anthraquinones based on the shikimic acid/o-succinylbenzoic acid pathway and polyketide pathway. Chin. Herb. Med. 2020, 51, 1939–1950. [Google Scholar]
- Lu, J.; Guo, Y.S.; Shi, C. Research progress on the sources and pharmacological activities of anthraquinones in medicinal plants. Guiding J. Tradit. Chin. Med. Pharm. 2024, 30, 111–116. [Google Scholar] [CrossRef]
- He, X.Q. Re-Evaluation of the Quality of Rhubarb Chinese Medicine Formula Granules and Comparison of Laxative Effects with Rhubarb Chinese Medicine Decoction Pieces. Master’s Thesis, Zhejiang Chinese Medical University, Hangzhou, China, 2024. [Google Scholar] [CrossRef]
- Dong, X.; Fu, J.; Yin, X.; Cao, S.L.; Li, X.C.; Lin, L.F.; Huyiligeqi, N.J. Emodin: A Review of its Pharmacology, Toxicity and Pharmacokinetics. Phytother. Res. 2016, 30, 1207–1218. [Google Scholar] [CrossRef]
- Wang, A.; Wu, C.-H.; Biehl, E. Unambiguous synthesis and spectral characterization of 1,8-dihydroxy-4-methylanthraquinone. Arkivoc 2002, 2002, 80–84. [Google Scholar] [CrossRef]
- El-Beih, A.A.; Kawabata, T.; Koimaru, K.; Ohta, T.; Tsukamoto, S. Monodictyquinone A: A new antimicrobial anthraquinone from a sea urchin-derived fungus Monodictys sp. Chem. Pharm. Bull. 2007, 55, 1097–1098. [Google Scholar] [CrossRef]
- Hind, H.G. A coloring matter of Penicillium carmino-violaceum Biourge—The constitution of carviolin. Biochem. J. 1940, 34, 577. [Google Scholar] [CrossRef]
- Fujimoto, H.; Nakamura, E.; Okuyama, E.; Ishibashi, M. Six immunosuppressive features from an ascomycete, Zopfiella longicaudata, found in a screening study monitored by immunomodulatory activity. Chem. Pharm. Bull. 2004, 52, 1005–1008. [Google Scholar] [CrossRef]
- Jadulco, R.; Brauers, G.; Edrada, R.A.; Ebel, R.; Wray, V.; Sudarsono; Proksch, P. New metabolites from sponge-derived fungi Curvularia lunata and Cladosporium herbarum. J. Nat. Prod. 2002, 65, 730–733. [Google Scholar] [CrossRef]
- Wang, Y.D.; Dong, W.; Zhou, K.; Liu, G.Y.; Li, L.M.; Lou, J.; Hu, Q.F.; Yang, H.Y. A new anthraquinone compound from the branches of Cassia siamea Lam., a Dai ethnic medicine. Chin. Herb. Med. 2015, 46, 1727–1729. [Google Scholar]
- Hangsamai, N.; Photai, K.; Mahaamnart, T.; Kanokmedhakul, S.; Kanokmedhakul, K.; Senawong, T.; Pitchuanchom, S.; Nontakitticharoen, M. Four New Anthraquinones with Histone Deacetylase Inhibitory Activity from Ventilago denticulata Roots. Molecules 2022, 27, 1088. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hu, X.L.; Pan, T.F.; Dong, L.; Ding, L.L.; Wang, Z.Z.; Song, R.; Wang, X.Z.; Wang, N.; Zhang, Y.; et al. Kanglexin, a new anthraquinone compound, attenuates lipid accumulation by activating the AMPK/SREBP-2/PCSK9/LDLR signalling pathway. Biomed. Pharmacother. 2021, 133, 110802. [Google Scholar] [CrossRef] [PubMed]
- Soonthornchareonnon, N.; Suwanborirux, K.; Bavovada, R.; Patarapanich, C.; Cassady, J.M. New cytotoxic 1-azaanthraquinones and 3-aminonaphthoquinone from the stem bark of Goniothalamus marcanii. J. Nat. Prod. 1999, 62, 1390–1394. [Google Scholar] [CrossRef] [PubMed]
- Malysheva, S.V.; Arroyo-Manzanares, N.; Cary, J.W.; Ehrlich, K.C.; Vanden Bussche, J.; Vanhaecke, L.; Bhatnagar, D.; Di Mavungu, J.D.; De Saeger, S. Identification of novel metabolites from Aspergillus flavus by high resolution and multiple stage mass spectrometry. Food Addit. Contam. Part A 2014, 31, 111–120. [Google Scholar] [CrossRef]
- Kamnaing, P.; Free, S.N.Y.F.; Nkengfack, A.E.; Folefoc, G.; Fomum, Z.T. An isoflavan—quinone and a flavonol from Millettia laurentii. Phytochemistry 1999, 51, 829–832. [Google Scholar] [CrossRef]
- Wabo, H.K.; Kouam, S.F.; Krohn, K.; Hussain, H.; Tala, M.F.; Tane, P.; van Ree, T.; Hu, Q.X.; Schulz, B. Prenylated anthraquinones and other constituents from the seeds of Vismia laurentii. Chem. Pharm. Bull. 2007, 55, 1640–1642. [Google Scholar] [CrossRef]
- Bilia, A.R.; Yusuf, A.W.; Bracà, A.; Keita, A.; Morelli, I. New prenylated anthraquinones and xanthones from Vismia guineensis. J. Nat. Prod. 2000, 63, 16–21. [Google Scholar] [CrossRef]
- Boonnak, N.; Karalai, C.; Chantrapromma, S.; Ponglimanont, C.; Fun, H.K.; Kanjana-Opas, A. Bioactive prenylated xanthones and anthraquinones from Cratoxylum formosum ssp. pruniflorum. Tetrahedron 2006, 62, 8850–8859. [Google Scholar] [CrossRef]
- Mammo, W.; Dagne, E.; Casser, I.; Steglich, W. Unusual anthraquinone lactones from Araliorhamnus vaginata. Phytochemistry 1990, 29, 2637. [Google Scholar] [CrossRef]
- Noungoue, D.T.; Antheaume, C.; Chaabi, M.; Lenta Ndjakou, B.; Ngouela, S.; Lobstein, A.; Tsamo, E. Anthraquinones from the fruits of Vismia laurentii. Phytochemistry 2008, 69, 1024–1028. [Google Scholar] [CrossRef]
- Tchizhova, A.Y.; Anufriev, V.P.; Denisenko, V.A.; Novikov, V.L. Synthesis of (±)-ploiariquinones A and B. J. Nat. Prod. 1995, 58, 1772. [Google Scholar] [CrossRef]
- Bezabih, M.; Abegaz, B.M. 4′-Demethylknipholone from aerial parts of Bulbine capitata. Phytochemistry 1998, 48, 1071–1073. [Google Scholar] [CrossRef]
- Dagne, E.; Steglich, W. Knipholone: A unique anthraquinone derivative from Kniphofia foliosa. Phytochemistry 1984, 23, 1729. [Google Scholar] [CrossRef]
- Yenew, A.; Dagne, E.; Müller, M.; Steglich, W. An anthrone, an anthraquinone and two oxanthrones from Kniphofia foliosa. Phytochemistry 1994, 37, 525. [Google Scholar] [CrossRef]
- Abegaz, B.M.; Bezabih, M.; Msuta, T.; Brun, R.; Menche, D.; Muehlbacher, J.; Bringmann, G. Gaboroquinones A and B and 4′-O-Demethylknipholone-4′-O-β-D-glucopyranoside, phenylanthraquinones from the roots of Bulbine frutescens. J. Nat. Prod. 2002, 65, 1117–1121. [Google Scholar] [CrossRef]
- Mutanyatta, J.; Bezabih, M.; Abegaz, B.M.; Dreyer, M.; Brun, R.; Kocher, N.; Bringmann, G. The first 6′-O-sulfated phenylanthraquinones: Isolation from Bulbine frutescens, structural elucidation, enantiomeric purity, and partial synthesis. Tetrahedron 2005, 61, 8475–8484. [Google Scholar] [CrossRef]
- Clark, B.; Capon, R.J.; Stewart, M.; Lacey, E.; Tennant, S.; Gill, J.H. Blanchaquinone: A new anthraquinone from an Australian Streptomyces sp. J. Nat. Prod. 2004, 67, 1729–1731. [Google Scholar] [CrossRef]
- Tsuda, M.; Nemoto, A.; Komaki, H.; Tanaka, Y.; Yazawa, K.; Mikami, Y.; Kobayashi, J. Nocarasins A-C and Brasiliquinone D, new metabolites from the actinomycete Nocardia brasiliensis. J. Nat. Prod. 1999, 62, 1640–1642. [Google Scholar] [CrossRef]
- Seo, E.-K.; Kim, N.-C.; Wani, M.C.; Wall, M.E.; Navarro, H.A.; Burgess, J.P.; Kawanishi, K.; Kardono, L.B.S.; Riswan, S.; Rose, W.C.; et al. Cytotoxic prenylated xanthones and the unusual compounds anthraquinobenzophenones from Cratoxylum sumatranum. J. Nat. Prod. 2002, 65, 299–305. [Google Scholar] [CrossRef]
- Alemayehu, G.; Woldeyesus, B.; Abegaz, B.M. (+)-Floribundone 3 from the pods of Senna septentrionalis. Bull. Chem. Soc. Ethiop. 1997, 11, 25–29. [Google Scholar] [CrossRef]
- Zhang, C.; Ondeyka, J.G.; Zink, D.L.; Basilio, A.; Vicente, F.; Collado, J.; Platas, G.; Bills, G.; Huber, J.; Dorso, K.; et al. Isolation, structure, and antibacterial activity of phaeosphenone from a Phaeosphaeria sp. discovered by antisense strategy. J. Nat. Prod. 2008, 71, 1304–1307. [Google Scholar] [CrossRef] [PubMed]
- Wirz, A.; Simmen, U.; Heilmann, J.; Calis, I.; Meier, B.; Sticher, O. Bisanthraquinone glycosides of Hypericum perforatum with binding inhibition to CRH-1 receptors. Phytochemistry 2000, 55, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Shi, J.-G.; Zhang, Y.-P.; Zhang, C.-Z. Constituents of Eremurus chinensis. J. Nat. Prod. 2000, 63, 653–656. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Yang, J.; Wang, Y.; Yang, X.S.; Mu, S.Z. Anthraquinone-benzisochromanquinone dimers from Berchemia polyphylla var. leioclada. Chin. Pharm. J. 2011, 46, 661–664. [Google Scholar]
- Takahashi, S.; Kitanaka, S.; Takido, M.; Sankawa, U.; Shibata, S. Phlegmacins and anhydrophlegmacinquinones: Dimeric hydroanthracenes from seedlings of Cassia torosa. Phytochemistry 1977, 16, 999–1002. [Google Scholar] [CrossRef]
- Alemayehu, G.; Abegaz, B.M. Bianthraquinones from the seeds of Senna multiglandulosa. Phytochemistry 1996, 41, 919. [Google Scholar] [CrossRef]
- Gill, M.; Morgan, P.M. Pigments of fungal. Part 73. Absolute stereochemistry of fungal metabolites: Icterinoidins A1 and B1, and atrovirins B1 and B2. Arkivoc 2004, 2004, 152–165. [Google Scholar] [CrossRef]
- Tsaffack, M.; Nguemeving, J.R.; Kuete, V.; Ndejouong Tchize Ble, S.; Mkounga, P.; Penlap Beng, V.; Hultin, P.G.; Tsamo, E.; Nkengfack, A.E. Two new antimicrobial dimeric compounds: Febrifuquinone, a vismione-anthraquinone coupled pigment and adamabianthrone, from two Psorospermum species. Chem. Pharm. Bull. 2009, 57, 1113–1118. [Google Scholar] [CrossRef]
- Kanokmedhakul, S.; Kanokmedhakul, K.; Phonkerd, N.; Soytong, K.; Kongsaeree, P.; Suksamrarn, A. Antimycobacterial anthraquinone-chromanone compound and diketopiperazine alkaloid from the fungus Chaetomium globosum KMITL-N0802. Planta Med. 2002, 68, 834–836. [Google Scholar] [CrossRef]
- Kuroda, M.; Mimaki, Y.; Sakagami, H.; Sashida, Y. Bulbinelonesides A-E, phenylanthraquinone glycosides from the roots of Bulbinella floribunda. J. Nat. Prod. 2003, 66, 894–897. [Google Scholar] [CrossRef]
- Wu, T.-S.; Lin, D.-M.; Shi, L.-S.; Damu, A.G.; Kuo, P.-C.; Kuo, Y.-H. Cytotoxic anthraquinones from the stems of Rubia wallichiana Decne. Chem. Pharm. Bull. 2003, 51, 948–950. [Google Scholar] [CrossRef] [PubMed]
- Perman, D.; Lajis, N.H.; Othman, A.G.; Ali, A.M.; Aimi, N.; Kitajima, M.; Takayama, H. Anthraquinones from Hedyotis herbacea. J. Nat. Prod. 1999, 62, 1430–1431. [Google Scholar] [CrossRef] [PubMed]
- Pawlus, A.D.; Su, B.-N.; Keller, W.J.; Kinghorn, A.D. An anthraquinone with potent quinone reductase-inducing activity and other constituents of the fruits of Morinda citrifolia (Noni). J. Nat. Prod. 2005, 68, 1720–1722. [Google Scholar] [CrossRef]
- El-Gamal, A.A.; Takeya, K.; Itokawa, H.; Falim, A.F.; Amer, M.M.; Saad, H.-E.A.; Awad, S.A. Anthraquinones from Galium sinaicum. Phytochemistry 1995, 40, 245. [Google Scholar] [CrossRef]
- Zhang, C.L.; Guan, H.; Xi, P.Z.; Deng, T.; Gao, J.M. Anthraquinones from the roots of Prismatomeris tetrandra. Nat. Prod. Commun. 2010, 5, 1251–1252. [Google Scholar] [CrossRef]
- Feng, Z.M.; Jiang, J.S.; Wang, Y.H.; Zhang, P.C. Anthraquinones from the roots of Prismatomeris tetrandra. Chem. Pharm. Bull. 2005, 53, 1330–1332. [Google Scholar] [CrossRef]
- Chen, X.Y.; Zhang, C.; Dong, Y.R.; Zang, Y.D.; Chen, J.Q.; Jin, H.T.; Zhang, D.M. Three new anthraquinoids from Radix Dalbergiae (Huanggen) and their neuroprotective effects on nerve cells. Acta Pharm. Sin. B 2023, 58, 3710–3714. [Google Scholar] [CrossRef]
- Deng, S.; West, B.J.; Jensen, C.J.; Basar, S.; Westendorf, J. Development and validation of an RP-HPLC method for the analysis of anthraquinones in noni fruits and leaves. Food Chem. 2009, 116, 505–508. [Google Scholar] [CrossRef]
- Akihisa, T.; Matsumoto, K.; Tokuda, H.; Yasukawa, K.; Seino, K.-i.; Nakamoto, K.; Kuninaga, H.; Suzuki, T.; Kimura, Y. Anti-inflammatory and potential cancer chemopreventive constituents of the fruits of Morinda citrifolia (Noni). J. Nat. Prod. 2007, 70, 754–757. [Google Scholar] [CrossRef]
- Komura, K.; Mizukawa, Y.; Minami, H.; Qin, G.W.; Xu, R.S. New anthraquinones from Eleutherine americana. Chem. Pharm. Bull. 1983, 31, 4206–4208. [Google Scholar] [CrossRef]
- Mahabusarakam, W.; Hemtasin, C.; Chakthong, S.; Voravuthikunchai, S.P.; Olawumi, I.B. Naphthoquinones, anthraquinones and naphthalene derivatives from the bulbs of Eleutherine americana. Planta Med. 2010, 76, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Paudel, P.; Kim, D.H.; Jeon, J.; Park, S.E.; Seong, S.H.; Jung, H.A.; Choi, J.S. Neuroprotective Effect of Aurantio-Obtusin, a Putative Vasopressin V(1A) Receptor Antagonist, on Transient Forebrain Ischemia Mice Model. Int. J. Mol. Sci. 2021, 22, 3335. [Google Scholar] [CrossRef] [PubMed]
- Arsenault, G.P. Fungal metabolites II. Structure of bostrycoidin, a β-azaanthraquinone from Fusarium solani D2 purple. Tetrahedron Lett. 1965, 6, 4033. [Google Scholar] [CrossRef]
- Ismail, N.H.; Ali, A.M.; Aimi, N.; Kitajima, M.; Takayama, H.; Lajis, N.H. Anthraquinones from Morinda elliptica. Phytochemistry 1997, 45, 1723–1725. [Google Scholar] [CrossRef]
- Kumar, U.S.; Tiwari, A.K.; Reddy, S.V.; Aparna, P.; Rao, R.J.; Ali, A.Z.; Rao, J.M. Free-radical-scavenging and xanthine oxidase inhibitory constituents from Stereospermum personatum. J. Nat. Prod. 2005, 68, 1615–1621. [Google Scholar] [CrossRef]
- Son, J.K.; Jung, S.J.; Jung, J.H.; Fang, Z.; Lee, C.S.; Seo, C.S.; Moon, D.C.; Min, B.S.; Kim, M.R.; Woo, M.H. Anticancer constituents from the roots of Rubia cordifolia L. Chem. Pharm. Bull. 2008, 56, 213–216. [Google Scholar] [CrossRef]
- Chan, H.-H.; Li, C.-Y.; Damu, A.G.; Wu, T.S. Anthraquinones from Ophiorrhiza hayatana Ohwi. Chem Pharm. Bull. 2005, 53, 1232–1235. [Google Scholar] [CrossRef]
- Yang, L.; Pei-Juan, X.; Ze-Nai, C.; Liu, G.-M. The anthraquinones of Rhynchotechum vestitum. Phytochemistry 1998, 47, 315–317. [Google Scholar] [CrossRef]
- Chiriboga, X.; Gilardoni, G.; Magnaghi, I.; Finzi, P.V.; Zanoni, G.; Vidari, G. New anthracene derivatives from Coussarea macrophylla. J. Nat. Prod. 2003, 66, 905–909. [Google Scholar] [CrossRef]
- Xiang, W.; Song, Q.S.; Zhang, H.J.; Guo, S.P. Antimicrobial anthraquinones from Morinda angustifolia. Fitoterapia 2008, 79, 501–504. [Google Scholar] [CrossRef]
- Ling, S.-K.; Komorita, A.; Tanaka, T.; Fujioka, T.; Mihashi, K.; Kouno, I. Iridoids and anthraquinones from the Malaysian medicinal plant, Saprosma scortechinii (Rubiaceae). Chem. Pharm. Bull. 2002, 50, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.-L.; Xu, L.-X.; Wei, X.-Y.; Kang, M.; Lin, L.-d. Anthraquinones from Hemiboea subcapitata. Redai Yaredai Zhiwu Xuebao (J. Trop. Subtrop. Bot.) 2014, 22, 507–510. [Google Scholar]
- Yoo, N.H.; Jang, D.S.; Lee, Y.M.; Jeong, I.H.; Cho, J.-H.; Kim, J.-H.; Kim, J.S. Anthraquinones from the roots of Knoxia valerianoides inhibit the formation of advanced glycation end products and rat lens aldose reductase in vitro. Arch. Pharmacal. Res. 2010, 33, 209–214. [Google Scholar] [CrossRef]
- Núñez Montoya, S.C.; Agnese, A.M.; Cabrera, J.L. Anthraquinone derivatives from Heterophyllaea pustulata. J. Nat. Prod. 2006, 69, 801–803. [Google Scholar] [CrossRef]
- Lin, W.Y.; Peng, C.F.; Tsai, I.L.; Chen, J.J.; Cheng, M.J.; Chen, I.S. Antitubercular constituents from the roots of Engelhardia roxburghiana. Planta Med. 2005, 71, 171–175. [Google Scholar] [CrossRef]
- Nagaoka, S.-I.; Uno, H.; Huppert, D. Ultrafast excited-state intramolecular proton transfer of aloesaponarin I. J. Phys. Chem. B 2013, 117, 4347–4353. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Yang, D.; Li, H.; Jiang, K.; Sun, J.F. Photoinduced excited state intramolecular proton transfer and spectral behaviors of aloesaponarin 1. Spectrochim. Acta Part A 2015, 151, 814–820. [Google Scholar] [CrossRef]
- Hemlata, K.; Kalidhar, S.B. Alatinone, an anthraquinone from Cassia alata. Phytochemistry 1993, 32, 1616. [Google Scholar] [CrossRef]
- Kelly, T.R.; Ma, Z.; Xu, W. Revision of the structure of przewalskinone B. Tetrahedron Lett. 1992, 33, 7713. [Google Scholar] [CrossRef]
- Gruen, H.; Görner, H. Photoreduction of 2-methyl-1-nitro-9,10-anthraquinone in the presence of 1-phenylethanol. Photochem. Photobiol. Sci. 2008, 7, 1344–1352. [Google Scholar] [CrossRef]
- Chen, B.; Wang, D.Y.; Ye, Q.; Li, B.G.; Zhang, G.L. Anthraquinones from Gladiolus gandavensis. J. Asian Nat. Prod. Res. 2005, 7, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Van Wagoner, R.M.; Mantle, P.G.; Wright, J.L.C. Biosynthesis of scorpinone, a 2-azaanthraquinone from Amorosia littoralis, a fungus from marine sediment. J. Nat. Prod. 2008, 71, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Scoles, D.R.; Gandelman, M.; Paul, S.; Dexheimer, T.; Dansithong, W.; Figueroa, K.P.; Pflieger, L.T.; Redlin, S.; Kales, S.C.; Sun, H. A quantitative high-throughput screen identifies compounds that lower expression of the SCA2- and ALS-associated gene ATXN2. J. Biol. Chem. 2022, 298, 102228. [Google Scholar] [CrossRef]
- Goulart, M.O.F.; Sant’Anna, A.E.G.; de Oliveira, A.B.; De Oliveira, G.G.; Maia, J.G.S. Azafluorenones and azaanthraquinone from Guatteria dielsiana. Phytochemistry 1986, 25, 1691. [Google Scholar] [CrossRef]
- Mal, D.; Ray, S. First synthesis of 9,10-dimethoxy-2-methyl-1,4-anthraquinone: A naturally occurring unusual anthraquinone. Eur. J. Org. Chem. 2008, 2008, 3014–3020. [Google Scholar] [CrossRef]
- Qi, F.; Zhang, W.; Xue, Y.; Geng, C.; Jin, Z.G.; Li, J.B.; Guo, Q.; Huang, X.N.; Lu, X.F. Microbial production of the plant-derived fungicide physcion. Metab. Eng. 2022, 74, 130–138. [Google Scholar] [CrossRef]
- Sardashti-Birjandi, A.; Mollashahi, E.; Maghsoodlou, M.T.; Yazdani-Elah-Abadi, A. Green and catalyst-free synthesis of aminoanthraquinone derivatives in solvent-free conditions. Res. Chem. Intermed. 2021, 47, 3597–3608. [Google Scholar] [CrossRef]
- Kouam, S.F.; Khan, S.N.; Krohn, K.; Ngadjui, B.T.; Kapche, D.G.; Yapna, D.B.; Zareem, S.; Moustafa, A.M.; Choudhary, M.I. Alpha-glucosidase inhibitory anthranols, kenganthranols A-C, from the stem bark of Harungana madagascariensis. J. Nat. Prod. 2006, 69, 229–233. [Google Scholar] [CrossRef]
- Eyong, K.O.; Krohn, K.; Hussain, H.; Folefoc, G.N.; Nkengfack, A.E.; Schulz, B.; Hu, Q.X. Newbouldiaquinone and newbouldiamide: A new naphthoquinone-anthraquinone coupled pigment and a new ceramide from Newbouldia laevis. Chem. Pharm. Bull. 2005, 53, 616–619. [Google Scholar] [CrossRef]
- Eyong, K.O.; Folefoc, G.N.; Kuete, V.; Beng, V.P.; Krohn, K.; Hussain, H.; Nkengfack, A.E.; Saeftel, M.; Sarite, S.R.; Hoerauf, A. Newbouldiaquinone A: A naphthoquinone-anthraquinone ether coupled pigment, as a potential antimicrobial and antimalarial agent from Newbouldia laevis. Phytochemistry 2006, 67, 605–609. [Google Scholar] [CrossRef]
- Aguinaldo, A.M.; Ocampo, O.P.M.; Bowden, B.F.; Gray, A.I.; Waterman, P.G. Tectograndone, an anthraquinone-naphthoquinone pigment from the leaves of Tectona grandis. Phytochemistry 1993, 33, 933. [Google Scholar] [CrossRef]
- Alemayehu, G.; Abegaz, B.; Kraus, W. A 1,4-anthraquinone-dihydroanthracenone dimer from Senna sophera. Phytochemistry 1998, 48, 699–702. [Google Scholar] [CrossRef]
- Hou, Y.P.; Cao, S.G.; Brodie, P.J.; Callmander, M.W.; Ratovoson, F.; Rakotobe, E.A.; Rasamison, V.E.; Ratsimbason, M.; Alumasa, J.N.; Roepe, P.D.; et al. Antiproliferative and antimalarial anthraquinones of Scutia myrtina from the Madagascar forest. Bioorg. Med. Chem. 2009, 17, 2871–2876. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, H.; Cai, W.; Chen, S.; Sheng, P.; Fu, X. CaCO3-complexed pH-responsive nanoparticles encapsulating mitoxantrone and celastrol enhance tumor chemoimmunotherapy. Int. J. Pharm. 2024, 667 Pt A, 124860. [Google Scholar] [CrossRef]
- Krenn, L.; Presser, A.; Pradhan, R.; Bahr, B.; Paper, D.H.; Mayer, K.K.; Kopp, B. Sulfemodin 8-O-β-D-glucoside, a new sulfated anthraquinone glycoside, and antioxidant phenolic compounds from Rheum emodi. J. Nat. Prod. 2003, 66, 1107–1109. [Google Scholar] [CrossRef]
- Xia, Z.X.; Tang, Z.Y.; An, R.; Chen, Y.; Zhang, Y.Z.; Wang, X.H. A new anthraquinone glycoside from Rheum officinale. Acta Pharm. Sin. B 2012, 47, 1183–1186. [Google Scholar] [CrossRef]
- Tomasin, R.; Ferreira, I.C.; Sawaya, A.C.H.F.; Mazzafera, P.; Pascoal, A.C.R.F.; Salvador, M.J.; Gomes-Marcondes, M.C.C. Honey and Aloe vera Solution Increases Survival and Modulates the Tumor Size In Vivo. Mol. Nutr. Food Res. 2024, 68, e2400378. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, R.; Liu, B.; Tu, G. Structure elucidation of a sodium salified anthraquinone from the seeds of Cassia obtusifolia by NMR technique assisted with acid-alkali titration. Magn. Reson. Chem. 2011, 49, 529–532. [Google Scholar] [CrossRef]
- Qhotsokoane-Lusunzi, M.E.A.; Karuso, P. Secondary metabolites from Basotho medicinal plants. I Bulbine narcissifolia. J. Nat. Prod. 2001, 64, 1368–1372. [Google Scholar] [CrossRef]
- Mei, R.; Liang, H.; Wang, J.; Zeng, L.H.; Lu, Q.; Cheng, Y.X. New seco-anthraquinone glucosides from Rumex nepalensis. Planta Med. 2009, 75, 1162–1164. [Google Scholar] [CrossRef]
- Li, B.; Zhang, D.-M.; Luo, Y.-M.; Chen, X.-G. Three new and antitumor anthraquinone glycosides from Lasianthus acuminatissimus Merr. Chem. Pharm. Bull. 2006, 54, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Ming, J.; Zhong, J.; Zhong, Y.; Wu, H.; Liu, H.; Li, B. Three new anthraquinones, one new benzochromene and one new furfural glycoside from Lasianthus acuminatissimus. Nat. Prod. Res. 2019, 33, 1916–1923. [Google Scholar] [CrossRef] [PubMed]
- Calis, I.; Tasdemir, D.; Ireland, C.M.; Sticher, O. Lucidin type anthraquinone glycosides from Putoria calabrica. Chem. Pharm. Bull. 2002, 50, 701–702. [Google Scholar] [CrossRef]
- Lee, W.; Ku, S.-K.; Kim, T.H.; Bae, J.S. Emodin-6-O-β-D-glucoside inhibits HMGB1-induced inflammatory responses in vitro and in vivo. Food Chem. Toxicol. 2013, 52, 97–104. [Google Scholar] [CrossRef]
- Chang, L.C.; Chávez, D.; Gills, J.J.; Fong, H.H.S.; Pezzuto, J.M.; Kinghorn, A.D. Rubiasins A-C, new anthracene derivatives from the roots and stems of Rubia cordifolia. Tetrahedron Lett. 2000, 41, 7157–7162. [Google Scholar] [CrossRef]
- Rossi, D.; Ahmed, K.M.; Gaggeri, R.; Della Volpe, S.; Maggi, L.; Mazzeo, G.; Longhi, G.; Abbate, S.; Corana, F.; Martino, E.; et al. (R)-(−)-Aloesaponol III 8-Methyl Ether from Eremurus persicus: A Novel Compound against Leishmaniosis. Molecules 2017, 22, 519. [Google Scholar] [CrossRef]
- Krenn, L.; Pradhan, R.; Presser, A.; Reznicek, G.; Kopp, B. Anthrone C-glucosides from Rheum emodi. Chem. Pharm. Bull. 2004, 52, 391–393. [Google Scholar] [CrossRef]
- Thurman, P.F.; Chai, W.; Rosankiewicz, J.R.; Rogers, H.J.; Lawson, A.M.; Draper, P. Possible intermediates in the biosynthesis of mycoside B by Mycobacterium microti. Eur. J. Biochem. 1993, 212, 705–711. [Google Scholar] [CrossRef]
- Rodríguez-Gamboa, T.; Victor, S.R.; Fernandes, J.B.; Fo, E.R.; Silva, M.d.G.d.; Vieira, P.C.; Pagnocca, F.C.; Bueno, O.C.; Hebling, M.J.A.; Castro, O.C. Anthrone and oxanthrone C,O-diglycosides from Picramnia teapensis. Phytochemistry 2000, 55, 837–841. [Google Scholar] [CrossRef]
- Diaz, F.; Chai, H.B.; Mi, Q.; Su, B.-N.; Vigo, J.S.; Graham, J.G.; Cabieses, F.; Farnsworth, N.R.; Cordell, G.A.; Pezzuto, J.M.; et al. Anthrone and oxanthrone C-glycosides from Picramnia latifolia collected in Peru. J. Nat. Prod. 2004, 67, 352–356. [Google Scholar] [CrossRef]
- Flamini, G.; Catalano, S.; Caponi, C.; Panizzi, L.; Morelli, I. Three anthrones from Rubus ulmifolius. Phytochemistry 2002, 59, 873–876. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Knipholone anthrone from Kniphofia foliosa induces a rapid onset of necrotic cell death in cancer cells. Fitoterapia 2010, 81, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Ndjakou Lenta, B.; Ngouela, S.; Fekam Boyom, F.; Tantangmo, F.; Tchouya, G.R.F.; Tsamo, E.; Gut, J.; Rosenthal, P.J.; Connolly, J.D. Anti-plasmodial activity of some constituents of the root bark of Harungana madagascariensis Lam. (Hypericaceae). Chem. Pharm. Bull. 2007, 55, 464–467. [Google Scholar] [CrossRef]
- Kouam, S.F.; Yapna, D.B.; Krohn, K.; Ngadjui, B.T.; Ngoupayo, J.; Choudhary, M.I.; Schulz, B. Antimicrobial prenylated anthracene derivatives from the leaves of Harungana madagascariensis. J. Nat. Prod. 2007, 70, 600–603. [Google Scholar] [CrossRef]
- Wanjohi, J.M.; Yenew, A.; MIDIWO, J.O.; Heydenreich, M.; Peter, M.G.; Dreyer, M.; Reichert, M.; Bringmann, G. Three dimeric anthracene derivatives from the fruits of Bulbine abyssinica. Tetrahedron 2005, 61, 2667–2674. [Google Scholar] [CrossRef]
- Fiedler, H.P.; Dieter, A.; Gulder, T.A.; Kajahn, I.; Hamm, A.; Brown, R.; Jones, A.L.; Goodfellow, M.; Müller, W.E.; Bringmann, G. Genoketides A1 and A2, new octaketides and biosynthetic intermediates of chrysophanol produced by Streptomyces sp. AK 671. J. Antibiot. 2008, 61, 464–473. [Google Scholar] [CrossRef]
- Elsworth, C.; Gill, M.; Saubern, S. Biosynthesis of tetrahydroanthraquinones in fungi. Phytochemistry 2000, 55, 23–27. [Google Scholar] [CrossRef]
- Kan, E.; Katsuyama, Y.; Maruyama, J.I.; Tamano, K.; Koyama, Y.; Ohnishi, Y. Efficient heterologous production of atrochrysone carboxylic acid-related polyketides in an Aspergillus oryzae host with enhanced malonyl-coenzyme A supply. J. Gen. Appl. Microbiol. 2020, 66, 195–199. [Google Scholar] [CrossRef]
- Sánchez, M.; González-Burgos, E.; Iglesias, I.; Gómez-Serranillos, M.P. Pharmacological Update Properties of Aloe Vera and its Major Active Constituents. Molecules 2020, 25, 1324. [Google Scholar] [CrossRef]
- Botta, B.; Delle Monache, F.; Delle Monache, G.; Menichini, F. Psorolactones and other metabolites from Psorospermum glaberrimum. Tetrahedron 1988, 44, 7193–7198. [Google Scholar] [CrossRef]
- Chevalier, Q.; Gallé, J.B.; Wasser, N.; Mazan, V.; Villette, C.; Mutterer, J.; Elustondo, M.M.; Girard, N.; Elhabiri, M.; Schaller, H.; et al. Unravelling the puzzle of anthranoid metabolism in living plant cells using spectral imaging coupled to mass spectrometry. Metabolites 2021, 11, 571. [Google Scholar] [CrossRef] [PubMed]
- Mbwambo, Z.H.; Apers, S.; Moshi, M.J.; Kapingu, M.C.; Van Miert, S.; Claeys, M.; Brun, R.; Cos, P.; Pieters, L.; Vlietinck, A. Anthranoid compounds with antiprotozoal activity from Vismia orientalis. Planta Med. 2004, 70, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Tiani, G.M.; Ahmed, I.; Krohn, K.; Green, I.R.; Nkengfack, A.E. Kenganthranol F, a new anthranol from Psorospermum aurantiacum. Nat. Prod. Commun. 2013, 8, 103–104. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, X.; Lee, U.; Kang, J.S.; Choi, H.D.; Sona, B.W. A new radical scavenging anthracene glycoside, asperflavin ribofuranoside, and polyketides from a marine isolate of the fungus Microsporum. Chem. Pharm. Bull. 2006, 54, 882–883. [Google Scholar] [CrossRef]
- Lv, L.; Tian, X.Y.; Fang, W.S. Three new antioxidant C-glucosylanthrones from Aloe nobilis. J. Asian Nat. Prod. Res. 2010, 12, 443–447. [Google Scholar] [CrossRef]
- Solis, P.N.; Ravelo, A.G.; Gonzalez, A.G.; Gupta, M.P.; Phillipson, J.D. Bioactive anthraquinone glycosides from Picramnia antidesma spp. fessonia. Phytochemistry 1995, 38, 477–480. [Google Scholar] [CrossRef]
- Hernández-Medel, R.; Pereda-Miranda, R. Cytotoxic anthraquinone derivatives from Picramnia antidesma. Planta Med. 2002, 68, 556–558. [Google Scholar] [CrossRef]
- Dagne, E.; Bisrat, D.; Van Wyk, B.E.; Viljoen, A.; Hellwig, V.; Steglich, W. Anthrones from Aloe microstigma. Phytochemistry 1997, 44, 1271–1274. [Google Scholar] [CrossRef]
- Farah, M.H.; Andersson, R.; Samuelsson, G. Microdontin A and B: Two new aloin derivatives from Aloe microdonta. Planta Med. 1992, 58, 88–93. [Google Scholar] [CrossRef]
- Rho, T.; Kil, H.W.; Seo, Y.J.; Shin, K.J.; Wang, D.; Yoon, K.D. Isolation of six anthraquinone diglucosides from cascara sagrada bark by high-performance countercurrent chromatography. J. Sep. Sci. 2020, 43, 4036–4046. [Google Scholar] [CrossRef]
- Rodríguez-Gamboa, T.; Fernandes, J.B.; Fo, E.R.; da Silva, M.F.D.G.F.; Vieira, P.C.; Castro, O.C. Two anthrones and one oxanthrone from Picramnia teapensis. Phytochemistry 1999, 51, 583–586. [Google Scholar] [CrossRef]
- Yang, J.B.; Li, L.; Dai, Z.; Wu, Y.; Geng, X.C.; Li, B.; Ma, S.C.; Wang, A.G.; Su, Y.L. Polygonumnolides C1-C4; minor dianthrone glycosides from the roots of Polygonum multiflorum Thunb. J. Asian Nat. Prod. Res. 2016, 18, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Rideout, J.; Sutherland, I. Pigments of marine animals. XV Bianthrones and related polyketides from Lamprometra palmata gyges and other species of crinoids. Aust. J. Chem. 1985, 38, 793–808. [Google Scholar] [CrossRef]
- Overy, D.P.; Berrue, F.; Correa, H.; Hanif, N.; Hay, K.; Lanteigne, M.; Mquilian, K.; Duffy, S.; Boland, P.; Jagannathan, R.; et al. Sea foam as a source of fungal inoculum for the isolation of biologically active natural products. Mycology 2014, 5, 130–144. [Google Scholar] [CrossRef]
- Cohen, P.A.; Towers, G.H.N. The anthraquinones of Heterodermia obscurata. Phytochemistry 1995, 40, 911–915. [Google Scholar] [CrossRef]
- Politi, M.; Sanogo, R.; Ndjoko, K.; Guilet, D.; Wolfender, J.L.; Hostettmann, K.; Morelli, I. HPLC-UV/PAD and HPLC-MS(n) analyses of leaf and root extracts of Vismia guineensis and ion and identification of two new bianthrones. Phytochem. Anal. 2010, 15, 364. [Google Scholar] [CrossRef]
- Form, I.C.; Bonus, M.; Gohlke, H.; Lin, W.; Daletos, G.; Proksch, P. Xanthone, benzophenone and bianthrone derivatives from the hypersaline lake-derived fungus Aspergillus wentii. Bioorg. Med. Chem. 2019, 27, 115005. [Google Scholar] [CrossRef]
- Meirelles, G.D.C.; Bridi, H.; Rates, S.M.K.; Poser, G.L.V. Southern Brazilian Hypericum species, promising sources of bioactive metabolites. Stud. Nat. Prod. Chem. 2018, 59, 491–507. [Google Scholar]
- Aly, A.H.; Debbab, A.; Clements, C.; Edrada-Ebel, R.A.; Orlikova, B.; Diederich, M.; Wray, V.; Lin, W.H.; Proksch, P. NF-κB inhibitors and antitrypanosomal metabolites from endophytic fungus Penicillium sp. isolated from Limonium tubiflorum. Bioorg. Med. Chem. 2011, 19, 414–421. [Google Scholar] [CrossRef]
- Yang, J.; Yan, Z.; Ren, J.; Su, Y. Polygonumnolides A1-B3, minor dianthrone derivatives from the roots of Polygonum multiflorum Thunb. Arch. Pharm. Res. 2018, 41, 617–624. [Google Scholar] [CrossRef]
- Yang, J.B.; Tian, J.Y.; Dai, Z.; Ye, F.; Ma, S.C.; Wang, A.G. α-Glucosidase inhibitors extracted from the roots of Polygonum multiflorum Thunb. Fitoterapia 2017, 117, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Lenta, B.N.; Devkota, K.P.; Ngouela, S.; Boyom, F.F.; Naz, Q.; Choudhary, M.I.; Tsamo, E.; Rosenthal, P.J.; Sewald, N. Anti-plasmodial and cholinesterase inhibiting activities of some constituents of Psorospermum glaberrimum. Chem. Pharm. Bull. 2010, 56, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Le, P.; Mai, F.; Guéritte, V.; Dumontet, M.; Van, T. Cytotoxicity of rhamnosylanthraquinones and rhamnosylanthrones from Rhamnus nepalensis. J. Nat. Prod. 2001, 64, 1162–1168. [Google Scholar] [CrossRef]
- Botta, B.; Dall’Olio, G.; Ferrari, F.; Vinciguerra, V.; Scurria, R.; Iacomacci, P.; Ferrari, F.; Delle Monache, G.; Misiti, D. Cell suspension cultures of Cassia didymobotrya: Mization of growth and secondary metabolite production by application of the orthogonal n method. J. Plant Physiol. 1989, 135, 290–294. [Google Scholar] [CrossRef]
- Nawong, B.; Karalai, C.; Chantrapromma, S.; Ponglimanont, C.; Kanjana-Opas, A.; Chantrapromma, K.; Fun, H.-K. Quinonoids from the barks of Cratoxylum formosum subsp. pruniflorum. Can. J. Chem. 2007, 85, 341–345. [Google Scholar] [CrossRef]
- Sibanda, S.; Nyanyira, C.; Nicoletti, M.; Galefi, C. Ochnabianthrone: A trans-9,9′-bianthrone from Ochna pulchra. Phytochemistry 1990, 29, 3974–3976. [Google Scholar] [CrossRef]
- Sasakl, K.N.N.; Yamauchi, K.; Kuwano, S. Isolation of a new aloe-emodin dianthrone diglucoside from senna and its potentiating effect on the purgative activity of sennoside A in mice. J. Pharm. Pharmacol. 1985, 37, 703–706. [Google Scholar] [CrossRef]
- Oshio, H.; Imai, S.; Fujioka, S.; Sugawara, T.; Miyamoto, M.; Tsukui, M. Investigation of Rhubarbs. III New purgative constituents, sennosides E and F. Chem. Pharm. Bull. 1974, 22, 823–831. [Google Scholar] [CrossRef]
- Lemli, J.; Bequeker, R.; Cuveele, J. Sennidin C, reidin B and reidin C, heterodianthrones of the fresh roots of rhubarb. Pharm. Weekbl. 1964, 99, 613–616. [Google Scholar]
- Gao, W.; Jin, L.; Liu, C.; Zhang, N.; Zhang, R.; Bednarikova, Z.; Gazova, Z.; Bhunia, A.; Siebert, H.C.; Dong, H. Inhibition behavior of sennoside A and sennoside C on amyloid fibrillation of human lysozyme and its possible mechanism. Int. J. Biol. Macromol. 2021, 178, 424–433. [Google Scholar] [CrossRef]
- Alemayehu, G.; Abegaz, B.; Snatzke, G.; Duddeck, H. Bianthrones from Senna longiracemosa. Phytochemistry 1993, 32, 1273–1277. [Google Scholar] [CrossRef]
- Macedo, E.M.S.d.; Wiggers, H.J.; Silva, M.G.V.; Braz-Filho, R.; Andricopulo, A.D.; Montanari, C.A. A new bianthron glycoside as itor of Trypanosoma cruzi glyceraldehyde 3-phosphate dehydrogenase activity. J. Braz. Chem. 2009, 20, 947–953. [Google Scholar] [CrossRef]
- Xiang, W.; Long, Z.; Zeng, J.; Zhu, X.; Yuan, M.; Wu, J.; Wu, Y.; Liu, L. Mechanism of Radix Rhei et rhizome intervention in cerebral infarction: A research based on chemoinformatics and systematic pharmacology. Evid.-Based Complement. Alternat. Med. 2021, 2021, 6789835. [Google Scholar] [CrossRef]
- Sehgal, V.N.; Verma, P.; Khurana, A. Anthralin/dithranol in dermatology. Int. J. Dermatol. 2014, 53, e449-60. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Wang, Y.R.; Wang, Z.Y.; Ma, C.K.; Shi, Y.J. Optimization of the extraction process for rutin from corn silk by alkali extraction and acid precipitation. Chem. Biol. Eng. 2021, 38, 27–31. [Google Scholar]
- Zhang, L.M.; Tian, B.; Mao, H.; Su, Y.Q.; Lv, D. Optimization of the extraction process for anthraquinones from Cassia obtusifolia. Biochem. Eng. J. 2024, 10, 93–96+108. [Google Scholar]
- Cao, L.L.; Ruan, C.Q.; Gao, J.L.; Li, X.; Zhao, M.; Li, Q. Study on the extraction process of anthraquinones from Rubia cordifolia rhizomes. Liaoning Chem. Ind. 2024, 53, 501–505+529. [Google Scholar] [CrossRef]
- Du, Z.X. Determination of plumbagin content in fresh and dry stems of Plumbago zeylanica. Anhui Agric. Sci. 2009, 37, 16363–16364. [Google Scholar] [CrossRef]
- Li, M.J.; Deng, Q.G.; Andong, Z.Y. Overview of separation and purification processes for active ingredients in Chinese medicine. J. Qiqihar Univ. 2006, 2, 7–10. [Google Scholar]
- Dong, J.X.; Jia, A.L.; Dong, X.L.; Qiu, Z.D. Study on the extraction process of anthraquinones from Polygonum multiflorum. J. Changchun Univ. Chin. Med. 2012, 28, 150–151. [Google Scholar] [CrossRef]
- Zhu, K.; Yu, Y.; Dong, X.L.; Qiu, Y.; Zhang, X.Y.; Qiu, Z.D. Study on the extraction process of anthraquinones from Rheum officinale by CO2 supercritical fluid extraction. J. Jilin Chin. Med. 2013, 33, 1261–1263. [Google Scholar] [CrossRef]
- Zhao, J.L.; Kang, Y.; Sun, H.; Li, X.Q. Determination of hydroquinone and phenol in cosmetics by solid-phase extraction-high performance liquid chromatography. Chin. J. Health Lab. Sci. 2019, 29, 16–18. [Google Scholar]
- Chen, D.M. Key Technologies for Quantitative and Confirmatory Analysis of Veterinary Drug Residues in Animal-Derived Foods. Ph.D. Thesis, Huazhong Agricultural University, Wuhan, China, 2010. [Google Scholar]
- Ong, E.S.; Len, S.M. Evaluation of pressurized liquid extraction and pressurized hot water extraction for tanshinone I and IIA in Salvia miltiorrhiza using LC and LC-ESI-MS. J. Chromatogr. Sci. 2004, 42, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Wang, P. Isolation and Identification of Polyphenols and Anthraquinones from Smilax scobinicaulis. Master’s Thesis, Northwest Agriculture and Forestry University, Xianyang, China, 2013. [Google Scholar] [CrossRef]
- He, Y.; Zhang, Y.L.; Zhang, G.R. Extraction, separation and structure identification of browning products from pomegranate peel. Sci. Technol. Food Ind. 2008, 4, 133–136. [Google Scholar] [CrossRef]
- Huang, J.; Yu, L.; Peng, X.S.; Zheng, R.; Xu, Y.; Ni, L.J. Determination and mass spectrometric confirmation of lawsone in cosmetics by high performance liquid chromatography. Chin. J. Health Lab. Sci. 2022, 32, 1182–1186. [Google Scholar]
- Tian, G.; Zhang, T.Y.; Zhang, Y.B.; Ito, Y. Separation of tanshinones from Salvia miltiorrhiza Bunge by multidimensional counter-current chromatography. J. Chromatogr. A 2002, 945, 281–285. [Google Scholar] [CrossRef]
- Lu, C.G. Study on the Extraction Process of Effective Components from Aloe by Supercritical CO2. Master’s Thesis, Zhengzhou University, Zhengzhou, China, 2007. [Google Scholar]
- Yang, N.; Zhou, C.J.; Wen, R. Research progress in extraction and separation technologies of Chinese herbal medicines. J. Baotou Med. Coll. 2015, 31, 143–145. [Google Scholar] [CrossRef]
- Lu, Z.K. Component Analysis and Biological Activity Study of Green Walnut Peel Pigment. Master’s Thesis, Shihezi University, Shihezi, China, 2022. [Google Scholar] [CrossRef]
- Zhang, Z.J. Study on the Synthesis Methods of Resveratrol Compounds and Their Derivatives. Master’s Thesis, Hunan University, Shihezi, China, 2008. [Google Scholar]
- Qiu, B.L. Synthesis and Property Study of Emodin Derivatives. Master’s Thesis, Fuzhou University, Shihezi, China, 2010. [Google Scholar]
- Wang, Y.; Chen, J.W.; Li, F.; Bian, H.T. Acute phototoxicity mechanism and QSAR study of anthraquinones to Daphnia magna. In Proceedings of the 5th National Conference on Environmental Chemistry, Dalian, China, 10 May 2009. [Google Scholar]
- Shen, J.; Zhang, M.Y.; Guo, Z.T.; Han, S.H.; Li, H.T.; Zhou, Z.Y.; Peng, M.Y. Immunomodulatory and therapeutic effects of embelin on systemic lupus erythematosus mice. J. Army Med. Univ. 2022, 44, 363–370. [Google Scholar] [CrossRef]
- Siegelin, M.D.; Gaiser, T.; Siegelin, Y. The XIAP inhibitor Embelin enhances TRAIL-mediated apoptosis in malignant glioma cells by down-regulation of the short isoform of FLIP. Neurochem. Int. 2009, 55, 423–430. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Li, J.; Hu, J.D.; Zheng, J.; Zheng, Z.H.; Zhu, L.F.; Chen, X.J.; Lin, Z.X. Study on the reversal effect of emodin on multidrug resistance in HL-60/ADR resistant cells. J. Exp. Hematol. 2013, 21, 1413–1422. [Google Scholar]
- Chen, S.-C.; Chen, Q.-W.; Ko, C.-Y. Chrysophanol induces cell death and inhibits invasiveness through alteration of calcium levels in HepG2 human liver cancer cells. Chin. J. Integr. Med. 2024, 31, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, S.; Crauwels, P.; Bohn, R.; Radzimski, C.; Szaszak, M.; Klinger, M.; Rupp, J.; van Zandbergen, G. AP-1 transcription factor serves as a molecular switch between Chlamydia pneumoniae replication and persistence. Infect. Immun. 2015, 83, 2651–2660. [Google Scholar] [CrossRef] [PubMed]
- Avci, E.; Arikoglu, H.; Kaya, D.E. Investigation of juglone effects on metastasis and angiogenesis in pancreatic cancer cells. Gene 2016, 588, 74–78. [Google Scholar] [CrossRef]
- Zhu, F.; Wu, G.; He, Y.Q.; Li, Z.Y.; Peng, G.; Ren, J.H. Effects of plumbagin on proliferation of hepatoma cell 2 and expression of vascular endothelial growth factor. Chin. Herbal Med. 2010, 41, 775–778. [Google Scholar]
- Yang, J.G.; Wang, H.Y.; Gao, X.S.; Li, X.H. Effects of aloe-emodin on the biological behaviors of cervical cancer HeLa cells. Hebei Med. J. 2018, 40, 814–818. [Google Scholar]
- Itharat, A.; Plubrukan, A.; Kaewpradub, N.; Chuchom, T.; Ratanasuwan, P.; Houghton, P.J. Selective cytotoxicity and antioxidant effects of compounds from Dioscorea membranacea rhizomes. Nat. Prod. Commun. 2007, 2, 643–648. [Google Scholar] [CrossRef]
- Thangaraj, S.; Tsao, W.-S.; Luo, Y.-W.; Lee, Y.-J.; Chang, C.-F.; Lin, C.-C.; Uang, B.-J.; Yu, C.-C.; Guh, J.-H.; Teng, C.-M. Total synthesis of moniliformediquinone and calanquinone A as potent inhibitors for breast cancer. Tetrahedron 2011, 67, 6166–6172. [Google Scholar] [CrossRef]
- Zhang, X.W.; Cheng, M.; Wang, X.J.; Ji, Y.L.; Zhou, C.S. Inhibitory effects of phenanthrenequinone from Dendrobium nobile on the proliferation and metastasis of human ovarian cancer cells. Pharmacol. Clin. Chin. Mat. Med. 2016, 32, 72–75+19. [Google Scholar] [CrossRef]
- Yang, N.; Li, C.; Li, H.L.; Liu, M.; Cai, X.J.; Cao, F.J.; Feng, Y.B.; Li, M.L.; Wang, X.B. Emodin induced SREBP1-dependent and SREBP1-independent apoptosis in hepatocellular carcinoma cells. Front. Pharmacol. 2019, 10, 709. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Liu, Y.; Pan, D.F.; Wang, Y.; Yang, N.; Xiang, L.C.; Cai, X.J.; Feng, Y.B. Hepatoprotection and hepatotoxicity of Polygonum multiflorum, a Chinese medicinal herb: Context of the paradoxical effect. Food Chem. Toxicol. 2017, 108, 407–418. [Google Scholar] [CrossRef]
- Xu, H. Idebenone Protects Against Oxidative Stress-Induced Neuronal Cell Apoptosis by Regulating the CD38-SIRT3-P53 Pathway. Master’s Thesis, Jilin University, Ürümqi, China, 2023. [Google Scholar]
- Kumar, S.; Gautam, S.; Sharma, A. Antimutagenic and antioxidant properties of plumbagin and other naphthoquinones. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2013, 755, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Liu, T. Study on the Antioxidant and Antibacterial Activities and Antibacterial Mechanism of Juglone. Master’s Thesis, Shanxi Normal University, Ürümqi, China, 2018. [Google Scholar]
- Hou, Y.S. Analysis of Naphthoquinones in Arnebia euchroma and Their Antioxidant Ntitumor Activities In Vitro. Master’s Thesis, Xinjiang Medical University, Ürümqi, China, 2020. [Google Scholar] [CrossRef]
- Chen, J.; Yin, Z.; Yu, N.; Ou, S.S.; Wang, X.; Li, H.P.; Zhu, H.L. Tanshinone alleviates UVA-induced melanogenesis in melanocytes via the Nrf2-regulated antioxidant defense signaling pathway. Curr. Mol. Med. 2024, 24, 1529–1539. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Huang, D.R.; Zhang, J.W.; Liao, W.J.; Wu, F.; Liu, Y.W. Tanshinone IIA inhibits oxidative stress and exerts anti-hepatocellular carcinoma effects by regulating the PI3K/Akt and Nrf2/HO-1 signaling pathways. China J. Chin. Mater. Med. 2024, 49, 6724–6734. [Google Scholar] [CrossRef]
- Mellado, M.; Madrid, A.; Peña-Cortés, H.; López, R.; Jara, C.; Espinoza, L. Antioxidant activity of anthraquinones isolated from leaves of Muehlenbeckia hastulata (J.E.Sm.) Johnst. (Polygonaceae). J. Chil. Chem. Soc. 2013, 58, 1767–1770. [Google Scholar] [CrossRef]
- Liu, M.Y.; Pan, X.L.; Li, X.B.; Wu, B. Study on the antioxidant activity of metal complexes of aloe-emodin. J. Sichuan Univ. Med. Sci. Ed. 2021, 52, 241–247. [Google Scholar] [CrossRef]
- Hu, T.; Feng, D.; Teng, L.; Zhou, G.F.; Liu, S. Extraction of naphthoquinones from the leaves of Juglans mandshurica and their antioxidant activities in vitro. Food Ind. 2022, 43, 29–32. [Google Scholar]
- Pan, J. Extraction of Total Anthraquinones from Rubia cordifolia and Preliminary Study on Their Antioxidant and Anti-Inflammatory Activities. Master’s Thesis, Guangzhou University of Chinese Medicine, Guangzhou, China, 2017. [Google Scholar] [CrossRef]
- Liu, M.X.; Yang, S.Q.; Xia, X.K.; Tan, W.J.; Xiang, M.X. Optimization of extraction process and anti-inflammatory study of naphthoquinones from Arnebia euchroma. J. Wuhan Inst. Technol. 2023, 45, 401–406. [Google Scholar] [CrossRef]
- Wang, Q. Study on the Necrosis-Targeting Property of Mono-Anthraquinones and Their Application in the Evaluation of Myocardial Activity. Master’s Thesis, Nanjing University of Chinese Medicine, Nanjing, China, 2016. [Google Scholar]
- Hu, X.H.; Meng, K.; Wang, Z.; Di, M.J. Research progress on the antitumor activity of anthraquinones. Chin. J. Med. Guid. 2023, 25, 1223–1229. [Google Scholar]
- Lu, Z.K.; Wu, Q.Z.; Zhang, J.; Mao, X.Y. Antibacterial effect and mechanism of juglone green walnut peel against Escherichia coli. Food Sci. 2023, 44, 65–73. [Google Scholar]
- Yang, H.; Lee, P.J.; Jeong, E.J.; Kim, H.P.; Kim, Y.C. Selective apoptosis in hepatic stellate cells ates the antifibrotic effect of phenanthrenes from Dendrobium nobile. Phytother. Res. 2012, 26, 980. [Google Scholar] [CrossRef]
- Tang, D.X.; Tan, Z.H.; Liang, Y.Y.; Cheng, L.; Huang, L. Preliminary study on the cathartic effect and anism of anthraquinones from Rheum officinale. Lishizhen Med. Mater. Med. Res. 2007, 6, 1314. [Google Scholar]
- Chen, Y.Y.; Cao, Y.J.; Tang, Y.P.; Yue, S.J.; Duan, J.A. Comparative pharmacodynamic, pharmacokinetic and tissue distribution of Dahuang-Gancao decoction in normal and experimental constipation mice. Chin. J. Nat. Med. 2019, 17, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Yang, L.; Huang, X.Y.; Wang, M.X.; Ma, Z.C.; Tang, X.L.; Wang, Y.G.; Gao, Y. Regulatory effects of THSG and anthraquinones from Polygonum multiflorum on CYP3A4 mediated by human pregnane X receptor. China J. Chin. Mater. Med. 2017, 42, 4827–4833. [Google Scholar] [CrossRef]
- Hu, X.Q.; Geng, Z.Y.; Li, Q.L.; Fang, H.L.; Zhang, X.Q. Experimental study on different doses of processed Polygonum multiflorum and the degree of liver injury in rats. Shaanxi J. Tradit. Chin. Med. 2007, 10, 1420–1421. [Google Scholar]
- Mao, Z.H.; Liu, Q.; Wang, X.; Wen, H.R. Study on the hepatotoxicity of anthraquinones from Rheum officinale. In Proceedings of the 6th National Annual Conference on Pharmacotoxicology, Chongqing, China; 2016. [Google Scholar]
- Wen, H.R.; Wang, Y.N.; Yang, Y.; Zhao, T.T.; Ma, S.C.; Wang, Q. Risk assessment of gene mutations induced by emodin-type monoanthrones. Chin. J. Pharmacovigil. 2020, 17, 455–460. [Google Scholar] [CrossRef]
- Li, C.L.; Ma, J.; Li, H.J. Research progress on the absorption and metabolism of anthraquinones. Pharm. Biotechnol. 2012, 19, 557–560. [Google Scholar] [CrossRef]
- Huang, H.; Li, X.Y. Research progress of melanosis coli. Yunnan Med. J. 2008, 29, 595–597. [Google Scholar]
- Steer, H.W.; Colin-Jones, D.G. Melanosis coli: Studies of the toxic effects of irritant purgatives. J. Pathol. 1975, 115, 199–205. [Google Scholar] [CrossRef]
- Cheng, Y. Study on the Potential Toxic Effects of Metabolic Products and Monomer Components of Anthraquinones from Rheum officinale on Human Colon Cells. Master’s Thesis, Northwestern University, Xi’an, China, 2021. [Google Scholar] [CrossRef]
- Lan, J.; Wen, H.R.; Huang, Z.Y.; Wang, Q.; Ma, S.C. Study on the cytotoxicity of anthraquinone monomer components from Polygonum multiflorum to HK-2 cells. Chin. J. Pharmacovigil. 2023, 20, 616–622+628. [Google Scholar] [CrossRef]
- Chang, M.H.; Huang, F.J.; Chan, W.H. Emodin induces embryonic toxicity in mouse blastocysts through apoptosis. Toxicology 2012, 299, 25–32. [Google Scholar] [CrossRef]
- Yao, K.Z.; Kang, Q.M.; Liu, W.B.; Chen, D.N.; Wang, L.F.; Li, S. Chronic exposure to tire rubber-derived contaminant 6PPD-quinone impairs sperm quality and induces the damage of reproductive capacity in male mice. J. Hazard. Mater. 2024, 470, 134165. [Google Scholar] [CrossRef] [PubMed]
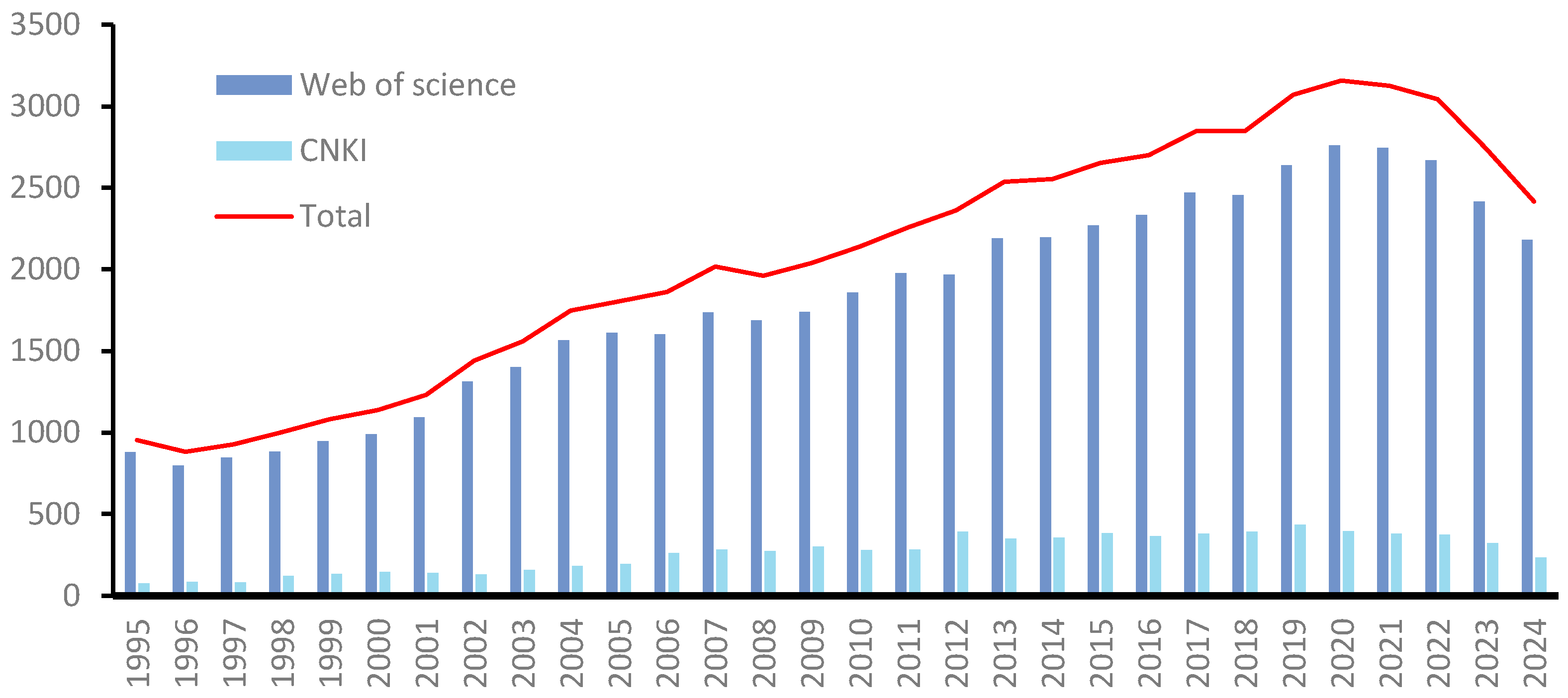




| No. | Name | Resource | Formula | Type | Ref. |
|---|---|---|---|---|---|
| 481 | polygonumnolide C1 | Pleuropterus multiflorus (Thunb.) Nakai | C36H32O13 | type I | [238] |
| 482 | polygonumnolide C2 | Pleuropterus multiflorus (Thunb.) Nakai | C36H32O13 | type I | [238] |
| 483 | polygonumnolide C3 | Pleuropterus multiflorus (Thunb.) Nakai | C36H32O13 | type I | [238] |
| 484 | polygonumnolide C4 | Pleuropterus multiflorus (Thunb.) Nakai | C36H32O13 | type I | [238] |
| 485 | trans-emodin dianthrones | Pleuropterus multiflorus (Thunb.) Nakai | C30H22O8 | type I | [238] |
| 486 | cis-emodin dianthrones | Pleuropterus multiflorus (Thunb.) Nakai | C30H22O8 | type I | [238] |
| 487 | (+)-crinemodin-rhodoptilometrin dianthrone | Himerometra magnipinna AH Clark | C35H32O8 | type I | [239] |
| 488 | 7,7′-dichlorohypericin | Heterodermia obscurata (Nyl.) Trevis. | C30H14Cl2O8 | type I | [240] |
| 489 | nephrolaevigatin A | Nephroma laevigatum Ach. | C30H20Cl2O8 | type I | [241] |
| 490 | nephrolaevigatin B | Nephroma laevigatum Ach. | C30H20ClO8 | type I | [241] |
| 491 | bioanthrone 1 | Vismia guineensis (L.) Choisy | C50H54O8 | type I | [242] |
| 492 | flavoobscurin B | Heterodermia obscurata (Nyl.) Trevis. | C30H19Cl4O8 | type I | [241] |
| 493 | 8,8′-dihydroxy-1,1′,3,3′-tetramethoxy-6,6′-dimethyl-10,10′-dianthrone | Aspergillus wentii Wehmer | C34H30O8 | type I | [243] |
| 494 | hypericin | Hypericum monogynum L. | C30H16O8 | type I | [244] |
| 495 | pseudohypericin | Hypericum monogynum L. | C30H16O9 | type I | [244] |
| 496 | neobulgarone E | Limonium tubiflorum (Delile) Kuntze | C32H24Cl2O8 | type I | [245] |
| 497 | polygonumnolide A1 | Pleuropterus multiflorus (Thunb.) Nakai | C37H34O13 | type II | [246] |
| 498 | polygonumnolide A2 | Pleuropterus multiflorus (Thunb.) Nakai | C37H34O13 | type II | [246] |
| 499 | polygonumnolide A3 | Pleuropterus multiflorus (Thunb.) Nakai | C37H34O13 | type II | [246] |
| 500 | polygonumnolide A4 | Pleuropterus multiflorus (Thunb.) Nakai | C37H34O13 | type II | [246] |
| 501 | polygonumnolide B1 | Pleuropterus multiflorus (Thunb.) Nakai | C43H44O18 | type II | [246] |
| 502 | polygonumnolide B2 | Pleuropterus multiflorus (Thunb.) Nakai | C43H44O18 | type II | [246] |
| 503 | polygonumnolide B3 | Pleuropterus multiflorus (Thunb.) Nakai | C43H44O18 | type II | [246] |
| 504 | polygonumnolide E | Pleuropterus multiflorus (Thunb.) Nakai | C37H34O13 | type II | [247] |
| 505 | adamadianthrone | Psorospermum febrifugum Spach | C45H46O8 | type II | [154] |
| 506 | bioanthrone 2 | Vismia guineensis (L.) Choisy | C30H20O11 | type II | [242] |
| 507 | glaberianthrone | Psorospermum glaberrimum Hochr. | C45H46O8 | type II | [248] |
| 508 | prinoidin-emodin dianthrones | Rhamnus napalensis (Wall.) Lawson | C40H37O14 | type II | [249] |
| 509 | (S)-2-hydroxybutyl-4,4′,5,5′,7-pentahydroxy-2′-methoxy-2,7′-dimethyl-10,10′-dioxo-9,9′,10,10′-tetrahydro-[9,9′-bianthracene]-3-carboxylate | Aspergillus wentii Wehmer | C36H32O11 | type II | [249] |
| 510 | (S)-2-hydroxybutyl 4,4′,5,7-tetrahydroxy-5′,7′-dimethoxy-2,2′-dimethyl-10,10′-dioxo-9,9′,10,10′-tetrahydro-[9,9′-bianthracene]-3-carboxylate | Aspergillus wentii Wehmer | C37H34O11 | type II | [249] |
| 511 | 2,4′,5-trihydroxy-4,5′,7′-trimethoxy-2′,7-dimethyl-[9,9′-bianthracene]-10,10′(9H,9′H)-dione | Aspergillus wentii Wehmer | C33H28O8 | type II | [249] |
| 512 | dianthrone A1 | Psorospermum febrifugum Spach | C50H54O8 | type III | [154] |
| 513 | bioanthrone 3 | Vismia guineensis | C30H20O12 | type III | [242] |
| 514 | dianthrone A2a | Psorospermum glaberrimum Hochr. | C45H46O8 | type III | [242] |
| 515 | dianthrone A2b | Psorospermum glaberrimum Hochr. | C40H38O8 | type III | [248] |
| 516 | prinoidin dianthrones rhamnepalins | Rhamnus napalensis (Wall.) M.A.Lawson | C50H51O20 | type III | [249] |
| 517 | 8,8′-dihydroxy-1,1′,3,3′-tetramethoxy-6,6′-dimethyl-10,10′-dianthrone | Aspergillus wentii Wehmer | C34H30O8 | type III | [243] |
| 518 | physcion-10,10′-bianthrone | Cassia didymobotrya Fresen. | C32H28O8 | type III | [250] |
| 519 | dianthrone J | Cratoxylum formosum subsp. pruniflorum (Kurz) Gogelein | C42H42O8 | type III | [251] |
| 520 | (−)-trans-2,2′-Digeranyloxy-7,7′-dimethyl-4,4′,5,5′-tetrahydroxy-9,9′-dianthrone | Ochna pulchra Hook. | C50H54O8 | type III | [252] |
| 521 | trans aloe-emodin dianthrone diglucoside | Cassia angustifolia Vahl | C42H42O18 | type IV | [253] |
| 522 | sennoside B | Senna alexandrina Milll. | C42H38O20 | type V | [254] |
| 523 | (−)-ochnadianthrone | Ochna pulchra Hook. | C50H54O8 | type V | [255] |
| 524 | sennidin C | Rheum palmatum L. | C30H20O9 | type VI | [255] |
| 525 | sennoside A | Senna alexandrina Milll. | C42H40O19 | type VI | [254] |
| 526 | sennoside D | Senna alexandrina Milll. | C48H44O25 | type VI | [256] |
| 527 | sennoside E | Senna alexandrina Milll. | C48H44O25 | type VI | [254] |
| 528 | sennoside F | Senna alexandrina Milll. | C48H44O25 | type VI | [254] |
| 529 | chrysophanol dianthrone | Heterodermia obscurata (Nyl.) Trevis. | C30H21O6 | type VII | [240] |
| 530 | chrysophanol-l0,l0′-dianthrone | Cassia didymobotrya Fresen. | C30H22O6 | type VII | [250] |
| 531 | chrysophanol-isophyscion dianthrone | Senna longiracemosa (Vatke) Lock | C31H25O7 | type VII | [257] |
| 532 | isophyscion dianthrone | Senna longiracemosa (Vatke) Lock | C32H28O8 | type VII | [257] |
| 533 | martianine 1 | Senna martiana (Benth.) H. S. Irwin & Barneby | C43H44O16 | type VII | [258] |
| 534 | palmidin B | Rheum palmatum L. | C30H22O7 | type VII | [258] |
| 535 | palmidin C | Rheum palmatum L. | C30H22O7 | type VIII | [259] |
| 536 | neobulgarone G | Limonium tubiflorum (Delile) Kuntze | C32H24Cl2O9 | other | [245] |
| 537 | chrysophanol-physcion-l0,l0′-dianthrone | Cassia didymobotrya Fresen. | C31H25O7 | other | [250] |
| 538 | 1,8,1′,8′-tetrahydroxy-10,10′-dianthrone | Hypericum Tourn. ex L. | C28H18O6 | other | [260] |
| 539 | palmidin A | Rheum palmatum L. | C30H22O8 | other | [259] |
| 540 | rendin A | Rheum palmatum L. | C30H20O9 | other | [255] |
| 541 | rendin B | Rheum palmatum L. | C30H20O8 | other | [255] |
| 542 | rendin C | Rheum palmatum L. | C31H22O9 | other | [255] |
| No. | Name | Cell Line | IC50 |
|---|---|---|---|
| 15 | embelin | PC-3 | 3.7 μmol/L |
| LNCaP | 5.7 μmol/L | ||
| HeLa | 5–7 μmol/L | ||
| 64 | juglone | BxPC-3 | 21.05 μmol/L |
| 68 | plumbagin | HepG2 | (27.08 ± 0.40) μmol/L |
| HL-60 | 0.8 μmol/L | ||
| 197 | dioscoreanone | MCF-7 | 20 μmol/L |
| 198 | denbinobin | K562 | 1.84 μmol/L |
| GSK5182 | 1.6 μmol/L | ||
| 219 | tanshinone IIA | A549 | 42.45 μmol |
| BGC-823 | 61.46 μmol/L | ||
| Hep-2 | 9.6 μmol/L | ||
| 226 | chrysophanol | HepG2 | 30 μmol/L |
| MCF-7 | 25 μmol/L | ||
| A549 | 18 μmol/L | ||
| 227 | emodin | HL-60/ADR | 5.79 μmol/L |
| SMMC-7721 | 21.6 μmol/L | ||
| HL-60 | 20 μmol/L | ||
| L02 | 135 μmol/L | ||
| 521 | aloe-emodin | HeLa | 58.3 μmol/L |
| HepG2 | 10 μmol/L | ||
| HCT116 | 8.7 μmol/L |
| No. | Name | DPPH | ABTS |
|---|---|---|---|
| 68 | plumbagin | IC50 = 50 μmol/L | |
| 64 | juglone | IC50 = 0.498 mg/mL | IC50 = 0.189 mg/mL |
| 142 | alkannin | IC50 = 40 μg/mL | |
| 217 | tanshinone I | IC50 = 0.07 μmol/L | |
| 227 | emodin | EC50 = 147.87 mg/L IC50 = 112.32 mg/mL | |
| 385 | physcion | IC50 = 56.05 mg/mL | |
| 521 | aloe-emodin | EC50 = 6.03 mg/L |
| No. | Name | Classification |
|---|---|---|
| 225 | dantron(chrysazin;1,8-dihydroxyanthraquinone) | 2B |
| 328 | 1-hydroxyanthraquinone | 2B |
| 336 | 1-amino-2,4-dibromoanthraquinone | 2B |
| 337 | 2-methyl-1-nitroanthraquinone | 2B |
| 397 | mitoxantrone | 2B |
| 59 | tris(aziridinyl)-para-benzoquinone (triaziquone) | 3 |
| 60 | aziridyl benzoquinone | 3 |
| 371 | 1-amino-2-methylanthraquinone | 3 |
| 386 | 2-aminoanthraquinone | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Yao, R.; Guo, H.; Jing, W.; Guo, X.; Liu, X.; Pan, Y.; Cao, P.; Zhang, L.; Yang, J.; et al. Research Progress on Chemical Compositions, Pharmacological Activities, and Toxicities of Quinone Compounds in Traditional Chinese Medicines. Toxics 2025, 13, 559. https://doi.org/10.3390/toxics13070559
Li Z, Yao R, Guo H, Jing W, Guo X, Liu X, Pan Y, Cao P, Zhang L, Yang J, et al. Research Progress on Chemical Compositions, Pharmacological Activities, and Toxicities of Quinone Compounds in Traditional Chinese Medicines. Toxics. 2025; 13(7):559. https://doi.org/10.3390/toxics13070559
Chicago/Turabian StyleLi, Zhe, Rui Yao, Hong Guo, Wenguang Jing, Xiaohan Guo, Xiaoqiu Liu, Yingni Pan, Pei Cao, Lei Zhang, Jianbo Yang, and et al. 2025. "Research Progress on Chemical Compositions, Pharmacological Activities, and Toxicities of Quinone Compounds in Traditional Chinese Medicines" Toxics 13, no. 7: 559. https://doi.org/10.3390/toxics13070559
APA StyleLi, Z., Yao, R., Guo, H., Jing, W., Guo, X., Liu, X., Pan, Y., Cao, P., Zhang, L., Yang, J., Cheng, X., & Wei, F. (2025). Research Progress on Chemical Compositions, Pharmacological Activities, and Toxicities of Quinone Compounds in Traditional Chinese Medicines. Toxics, 13(7), 559. https://doi.org/10.3390/toxics13070559







