Distribution Characteristics of High-Background Elements and Assessment of Ecological Element Activity in Typical Profiles of Ultramafic Rock Area
Abstract
1. Introduction
2. Study Area Setting
3. Materials and Methods
3.1. Distribution of Sampling Sites and Sample Collection
3.2. Evaluation Methods
3.2.1. Migration Coefficient Method
3.2.2. Bioavailable Fraction Ratio (F1 + F2)
3.2.3. Secondary Phase to Primary Phase Ratio Method (RSP)
3.2.4. Correlation Analysis
4. Results and Discussion
4.1. Heavy Metal Distribution and Enrichment Patterns in Weathering Crust Profile Horizons
4.2. Heavy Metal Migration Characteristics in Weathering Crust Profile Horizons
4.3. Morphological Distribution of High-Background Elements Across Horizons in Weathering Crust Profiles
4.4. Ecological Element Activity Assessment of High-Background Elements in Weathering Crust Profiles
4.5. Influencing Factors of Heavy Metal Migration and Transformation in Weathering Crust Profiles
4.6. Regional Comparison and Countermeasure Proposals
5. Conclusions
- (1)
- In the weathering crust profile, the concentrations of eight heavy metals exhibit substantial variability. Cr and Ni show significantly elevated concentrations compared to background values, with their distributions strongly influenced by pedogenic parent materials and mining activities. Cr and Ni are preferentially enriched in the saprolite layers of serpentine and pyroxenite, demonstrating a decreasing trend from bedrock to topsoil; conversely, the concentrations of the remaining six elements decline with increasing depth. Mining activities have promoted a growth trend in the occurrence amounts of most heavy metal elements, with the notable exceptions of Zn and Hg.
- (2)
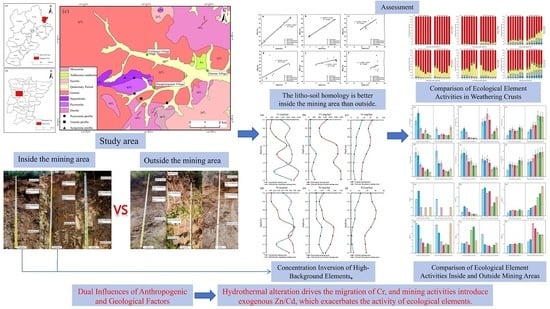
- Lithogenic homology between rocks and soils is more pronounced within mining areas than outside, suggesting that direct mining activities have not introduced exogenous materials, while indirect mining has caused a substantial material influx. Cr exhibits depletion in serpentine and pyroxenite but enrichment in granite, whereas Ni demonstrates mobility patterns similar to yet distinct from Cr. Particular attention should be paid to the excessive activity of ecological elements of Cr around ore-forming plutons and Ni in saprolite layers of ore-hosting bedrock.
- (3)
- In serpentine, Cr is predominantly present in residual fractions, whereas in the extra-mining pyroxenite zone, Cr shows preferential adsorption by metal (hydr)oxides. In granite, the proportion of carbonate-bound Cr increases significantly. Ni exhibits distinct speciation patterns compared to Cr and presents the phenomenon of excessive activity of ecological elements. The degree of ecological element activity is granite > pyroxenite > serpentine, with external areas demonstrating higher element activity than internal mining zones. The excessive activity of ecological elements associated with bioavailable fractions decreases systematically with increasing profile depth.
- (4)
- Within mining areas, soil heavy metals are predominantly derived from the parent material layer, where Cr and Ni exhibit mutual promotion, while other elements exert inhibitory effects on their accumulation. In contrast, indirect mining activities outside these areas disrupt elemental evolution pathways, introducing exogenous heavy metals and enhancing Cr/Ni enrichment. Specifically, indirect mining weakens the regulatory influence of major elements on Cr and diminishes their control over Ni.
- (5)
- Optimized Translation Marked disparities exist in the impacts of anthropogenic and geological factors on metal behavior: Geologically, hydrothermal alteration drives Cr migration, climatic conditions govern Cr speciation, and weathering intensity dictates Ni release. Anthropogenically, mining introduces exogenous Zn/Cd, elevating active Ni fractions in the outer mining area. Additionally, mining-induced degradation of parent rock structures increases the migration coefficients of Cr in serpentinite and Ni in granite, thereby exacerbating ecological element activity.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tepanosyan, G.; Sahakyan, L.; Belyaeva, O.; Asmaryan, S.; Saghatelyan, A. Continuous impact of mining activities on soil heavy metals levels and human health. Sci. Total Environ. 2018, 639, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Concas, A.; Ardau, C.; Cristini, A.; Zuddas, P.; Cao, G. Mobility of heavy metals from tailings to stream waters in a mining activity contaminated site. Chemosphere 2006, 63, 244–253. [Google Scholar] [CrossRef]
- Liao, G.L.; Liao, D.X.; Li, Q.M. Heavy metals contamination characteristics in soil of different mining activity zones. Trans. Nonferrous Met. Soc. China 2008, 18, 207–211. [Google Scholar] [CrossRef]
- Abdul Rashid, S.R.; Wan Yaacob, W.Z.; Umor, M.R. Assessments of heavy metals accumulation, bioavailability, mobility, and toxicity in serpentine soils. Sustainability 2023, 15, 1218. [Google Scholar] [CrossRef]
- Dilshara, P.; Abeysinghe, B.; Premasiri, R.; Dushyantha, N.; Ratnayake, N.; Senarath, S.; Ratnayake, A.S.; Batapola, N. The role of nickel (Ni) as a critical metal in clean energy transition: Applications, global distribution and occurrences, production-demand and phytomining. J. Asian Earth Sci. 2024, 259, 105912. [Google Scholar] [CrossRef]
- Rajakaruna, N.; Harris, T.B.; Alexander, E.B. Serpentine geoecology of eastern North America: A review. Rhodora 2009, 111, 21–108. [Google Scholar] [CrossRef]
- Meindl, G.A.; Bain, D.J.; Ashman, T.L. Nickel accumulation in leaves, floral organs and rewards varies by serpentine soil affinity. AoB Plants 2014, 6, plu036. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Sodhi, K.K.; Kumar, M.; Singh, D.K. Heavy metal pollution: Insights into chromium eco-toxicity and recent advancement in its remediation. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100388. [Google Scholar] [CrossRef]
- Yang, C.Y.; Nguyen, D.Q.; Ngo, H.T.T.; Navarrete, I.A.; Nakao, A.; Huang, S.T.; Hseu, Z.Y. Increases in Ca/Mg ratios caused the increases in the mobile fractions of Cr and Ni in serpentinite-derived soils in humid Asia. Catena 2022, 216, 106418. [Google Scholar] [CrossRef]
- Oze, C.; Fendorf, S.; Bird, D.K.; Coleman, R.G. Chromium geochemistry of serpentine soils. Int. Geol. Rev. 2004, 46, 97–126. [Google Scholar] [CrossRef]
- Cheng, C.H.; Jien, S.H.; Iizuka, Y.; Tsai, H.; Chang, Y.H.; Hseu, Z.Y. Pedogenic chromium and nickel partitioning in serpentine soils along a toposequence. Soil Sci. Soc. Am. J. 2011, 75, 659–668. [Google Scholar] [CrossRef]
- Proctor, J.; Woodell, S.R. The ecology of serpentine soils. Adv. Ecol. Res. 1975, 9, 255–366. [Google Scholar] [CrossRef]
- Kierczak, J.; Pietranik, A.; Pędziwiatr, A. Ultramafic geoecosystems as a natural source of Ni, Cr, and Co to the environment: A review. Sci. Total Environ. 2021, 755, 142620. [Google Scholar] [CrossRef] [PubMed]
- Pędziwiatr, A.; Kierczak, J.; Waroszewski, J.; Ratié, G.; Quantin, G.; Ponzevera, M. Rock-type control of Ni, Cr, and Co phytoavailability in ultramafic soils. Plant Soil 2018, 423, 339–362. [Google Scholar] [CrossRef]
- Kierczak, J.; Pędziwiatr, A.; Waroszewski, J.; Modelska, M. Mobility of Ni, Cr and Co in serpentine soils derived on various ultrabasic bedrocks under temperate climate. Geoderma 2016, 268, 78–91. [Google Scholar] [CrossRef]
- Mistikawy, J.A.; Mackowiak, T.J.; Butler, M.J.; Mischenko, I.C.; Cernak, R.S.; Richardson, J.B. Chromium, manganese, nickel, and cobalt mobility and bioavailability from mafic-to-ultramafic mine spoil weathering in western Massachusetts, USA. Environ. Geochem. Health 2020, 42, 3263–3279. [Google Scholar] [CrossRef]
- Zhong, X.; Chen, Z.; Li, Y.; Ding, K.; Liu, W.; Liu, Y.; Yuan, Y.; Zhang, M.; Baker, A.J.M.; Yang, W.; et al. Factors influencing heavy metal availability and risk assessment of soils at typical metal mines in Eastern China. J. Hazard. Mater. 2020, 400, 123289. [Google Scholar] [CrossRef]
- Kierczak, J.; Neel, C.; Bril, H.; Puziewicz, J. Effect of mineralogy and pedoclimatic variations on Ni and Cr distribution in serpentine soils under temperate climate. Geoderma 2007, 142, 165–177. [Google Scholar] [CrossRef]
- Li, F.; Li, X.; Hou, L.; Shao, A. Impact of the coal mining on the spatial distribution of potentially toxic metals in farmland tillage soil. Sci. Rep. 2018, 8, 14925. [Google Scholar] [CrossRef]
- Liu, H.; Qu, M.; Chen, J.; Guang, X.; Zhang, J.; Liu, M.; Kang, J.; Zhao, Y.; Huang, B. Heavy metal accumulation in the surrounding areas affected by mining in China: Spatial distribution patterns, risk assessment, and influencing factors. Sci. Total Environ. 2022, 825, 154004. [Google Scholar] [CrossRef]
- Baleeiro, A.; Fiol, S.; Otero-Fariña, A.; Antelo, J. Surface chemistry of iron oxides formed by neutralization of acidic mine waters: Removal of trace metals. Appl. Geochem. 2018, 89, 129–137. [Google Scholar] [CrossRef]
- Ettler, V.; Pipková, Z.; Kvapil, J.; Mihaljevič, M.; Drahota, P.; Vaněk, V.; Penížek, V. Nickel, chromium, and cobalt in soils developed on nickel laterites near an abandoned mining area in southern Czech Republic. J. Geochem. Explor. 2024, 264, 107529. [Google Scholar] [CrossRef]
- Wang, C.; Xu, D.; Li, Y.; Zhou, W.; Bian, P.; Zhang, S. Source and Migration Pathways of Heavy Metals in Soils from an Iron Mine in Baotou City, China. Minerals 2024, 14, 506. [Google Scholar] [CrossRef]
- Shaheen, S.M.; Chen, H.Y.; Song, H.; Rinklebe, J.; Hseu, Z.Y. Release and mobilization of Ni, Co, and Cr under dynamic redox changes in a geogenic contaminated soil: Assessing the potential risk in serpentine paddy environments. Sci. Total Environ. 2022, 850, 158087. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.T.; Liu, J.J.; Zhang, J.C.; Wang, J.Y.L.; Jiang, Y.G.; Wang, M.; Li, H.F.; Yang, W.H.; Yan, X.J. Analysis of soil heavy metal influencing factors and sources in typical small watersheds in shallow mountainous area. Geophys. Geochem. Explor. 2024, 48, 834–846. (In Chinese) [Google Scholar] [CrossRef]
- Wu, Y.F.; Li, X.; Yu, L.; Wang, T.Q.; Wang, J.N.; Liu, T.T. Review of soil heavy metal pollution in China: Spatial distribution, primary sources, and remediation alternatives. Resour. Conserv. Recycl. 2022, 181, 106261. [Google Scholar] [CrossRef]
- Haghighizadeh, A.; Rajabi, O.; Nezarat, A.; Hajyani, Z.; Haghmohammadi, M.; Hedayatikhah, S.; Asl, S.D.; Beni, A.A. Comprehensive analysis of heavy metal soil contamination in mining Environments: Impacts, monitoring Techniques, and remediation strategies. Arab. J. Chem. 2024, 17, 105777. [Google Scholar] [CrossRef]
- Rouhani, A.; Skousen, J.; Tack, F.M. An overview of soil pollution and remediation strategies in coal mining regions. Minerals 2023, 13, 1064. [Google Scholar] [CrossRef]
- DZ/T 0279-2016; Analysis Methods for Regional Geochemical Samples. Ministry of Land and Resources of the People’s Republic of China: Beijing, China, 2016.
- GB/T 14506-2010; Rock and Mineral Analysis. National Research Center for Geoanalysis, China Geological Survey: Beijing, China, 2010.
- Gao, J.; Gong, J.; Yang, J.; Wang, Z.; Fu, Y.; Tang, S.; Ma, S. Spatial distribution and ecological risk assessment of soil heavy metals in a typical volcanic area: Influence of parent materials. Heliyon 2023, 9, e12993. [Google Scholar] [CrossRef]
- Anju, M.; Banerjee, D.K. Comparison of two sequential extraction procedures for heavy metal partitioning in mine tailings. Chemosphere 2010, 78, 1393–1402. [Google Scholar] [CrossRef]
- Arenas-Lago, D.; Andrade, M.L.; Vega, F.A.; Singh, B.R. TOF-SIMS and FE-SEM/EDS to verify the heavy metal fractionation in serpentinite quarry soils. Catena 2016, 136, 30–43. [Google Scholar] [CrossRef]
- Engvik, A.K.; Austrheim, H.; Erambert, M. Interaction between fluid flow, fracturing and mineral growth during eclogitization, an example from the Sunnfjord area, Western Gneiss Region, Norway. Lithos 2001, 57, 111–141. [Google Scholar] [CrossRef]
- Wang, Z.; Luo, Y.; Zheng, C.; An, C.; Mi, Z. Spatial distribution, source identification, and risk assessment of heavy metals in the soils from a mining region: A case study of Bayan Obo in northwestern China. Hum. Ecol. Risk Assess Int. J. 2020, 27, 1276–1295. [Google Scholar] [CrossRef]
- Wang, F.; Wang, W.; Wu, B.; Bai, Q.; Perera, M.S.A. Mechanism, Cause, and Control of Water, Solutes, and Gas Migration Triggered by Mining Activities. Geofluids 2019. [Google Scholar] [CrossRef]
- Liao, J.; Wen, Z.; Ru, X.; Chen, J.; Wu, H.; Wei, C. Distribution and migration of heavy metals in soil and crops affected by acid mine drainage: Public health implications in Guangdong Province, China. Ecotoxicol. Environ. Saf. 2016, 124, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, C. Natural and human factors affect the distribution of soil heavy metal pollution: A review. Water Air Soil Pollut. 2020, 231, 350. [Google Scholar] [CrossRef]
- Bini, C.; Maleci, L.; Wahsha, M. Potentially toxic elements in serpentine soils and plants from Tuscany (Central Italy). A proxy for soil remediation. Catena 2017, 148, 60–66. [Google Scholar] [CrossRef]
- Martin, J.M.; Nirel, P.; Thomas, A.J. Sequential extraction techniques: Promises and problems. Mar. Chem. 1987, 22, 313–341. [Google Scholar] [CrossRef]
- Botsou, F.; Koutsopoulou, E.; Andrioti, A.; Dassenakis, M.; Scoullos, M.; Karageorgis, A.P. Chromium speciation, mobility, and Cr (VI) retention–release processes in ultramafic rocks and Fe–Ni lateritic deposits of Greece. Environ. Geochem. Health 2022, 44, 2815–2834. [Google Scholar] [CrossRef]
- Wang, Y.L.; Tsou, M.C.; Liao, H.T.; Hseu, Z.Y.; Dang, W.; Hsi, H.C.; Chien, L.C. Influence of soil properties on the bioaccessibility of Cr and Ni in geologic serpentine and anthropogenically contaminated non-serpentine soils in Taiwan. Sci. Total Environ. 2020, 714, 136761. [Google Scholar] [CrossRef]
- Bonifacio, E.; Falsone, G.; Piazza, S. Linking Ni and Cr concentrations to soil mineralogy: Does it help to assess metal contamination when the natural background is high? J. Soils Sediments 2010, 10, 1475–1486. [Google Scholar] [CrossRef]
- Sun, Z.; Xie, X.; Wang, P.; Hu, Y.; Cheng, H. Heavy metal pollution caused by small-scale metal ore mining activities: A case study from a polymetallic mine in South China. Sci. Total Environ. 2018, 639, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Fazekašová, D.; Fazekaš, J. Soil quality and heavy metal pollution assessment of iron ore mines in Nizna Slana (Slovakia). Sustainability 2020, 12, 2549. [Google Scholar] [CrossRef]
- Kashtabeh, R.; Akbari, M.; Heidari, A.; Najafpour, A. Impact of Iron Ore Mining on the Concentration of some Heavy Metals and Soil Pollution Zoning (Case Study: Sangan Iron Ore Mine, Khaf-Iran). Water Soil 2023, 37, 77–94. [Google Scholar] [CrossRef]
- Kanianska, R.; Varga, J.; Benková, N.; Kizeková, M.; Jančová, L. Floodplain soils contamination assessment using the sequential extraction method of heavy metals from past mining activities. Sci. Rep. 2022, 12, 2927. [Google Scholar] [CrossRef]
- Saedpanah, S.; Amanollahi, J. Environmental pollution and geo-ecological risk assessment of the Qhorveh mining area in western Iran. Environ. Pollut. 2019, 253, 811–820. [Google Scholar] [CrossRef]
- Orosun, M.M.; Nwabachili, S.; Alshehri, R.F.; Omeje, M.; Alshdoukhi, I.F.; Okoro, H.K.; Ogunkunle, C.O.; Louis, H.; Abdulhamid, F.A.; Osahon, S.E.; et al. Potentially toxic metals in irrigation water, soil, and vegetables and their health risks using Monte Carlo models. Sci. Rep. 2023, 13, 21220. [Google Scholar] [CrossRef] [PubMed]
- Niero, M.; Pizzol, M.; Bruun, H.G.; Thomsen, M. Comparative life cycle assessment of wastewater treatment in Denmark including sensitivity and uncertainty analysis. J. Clean. Prod. 2014, 68, 25–35. [Google Scholar] [CrossRef]
- Wu, J.; Zhu, Q.; Qiao, N.; Wang, Z.; Sha, W.; Luo, K.; Wang, H.; Feng, Z. Ecological risk assessment of coal mine area based on “source-sink” landscape theory–A case study of Pingshuo mining area. J. Clean. Prod. 2021, 295, 126371. [Google Scholar] [CrossRef]
- Gupta, S.S.; Bhattacharyya, K.G. Adsorption of Ni (II) on clays. J. Colloid Interface Sci. 2006, 295, 21–32. [Google Scholar] [CrossRef]
- Ugwu, I.M.; Igbokwe, O.A. Sorption of heavy metals on clay minerals and oxides: A review. Adv. Sorpt. Process Appl. 2019, 2019, 1–23. [Google Scholar] [CrossRef]
- Hao, W.; Chen, N.; Sun, W.; Mänd, K.; Kirsimäe, K.; Teitler, Y.; Somelar, P.; Robbins, L.J.; Babechuk, M.J.; Planavsky, N.J.; et al. Binding and transport of Cr (III) by clay minerals during the Great Oxidation Event. Earth Planet. Sci. Lett. 2022, 584, 117503. [Google Scholar] [CrossRef]
- Gupta, S.S.; Bhattacharyya, K.G. Adsorption of metal ions by clays and inorganic solids. RSC Adv. 2014, 4, 28537–28586. [Google Scholar] [CrossRef]
- Dube, A.; Zbytniewski, R.; Kowalkowski, T.; Cukrowska, E.; Buszewski, B. Adsorption and migration of heavy metals in soil. Pol. J. Environ. Stud. 2001, 10, 1–10. [Google Scholar]








| Lithostratigraphic Unit | Geological Features | ||||
|---|---|---|---|---|---|
| Geological time | Code | Lithology | Macrogeological characteristics | Contact relationship with the surrounding rocks | Isotopic age (Ma) |
| Late Triassic | ξγT3 | Granite | The weathering is intense, with some mineral grains protruding on the weathered surface, presenting a sugary texture. | Hypabyssal intrusion νδT3, ψωPt1, ψιPt1 | 175 ± 10 |
| ξοT3 | Syenite | Flesh-red in color, with pits on the weathered surface and scarce quartz. | 235 ± 10 | ||
| ηοT3 | Monzonite | Dark gray in color, rich in dark minerals, with scarce and unevenly distributed quartz. | |||
| Late Triassic–Middle Triassic | νδT3 | Diorite | Gray in color, with well-developed vein rocks. | It is hypabysally intruded by νδT3, ψωPt1, and ψιPt1. | 200.2 ± 2.4 |
| Paleoproterozoic Era | ψωPt1 | Serpentinite | Purplish-black in color, with iron ores embedded in it. | It is hypabysally intruded by νδT3. | 1796–1837 |
| ψιPt1 | Pyroxenite | Dark green | |||
| Indicator | Analytical Methods | Detection Limits | Indicator | Analytical Methods | Quantitation Ranges |
|---|---|---|---|---|---|
| As | Atomic Fluorescence Spectrometry | 0.2 mg/kg | CaO | Total Chemical Analysis Method | 0.1% to 15% |
| Cd | Inductively Coupled Plasma Optical Emission Spectrometry | 0.021 mg/kg | MgO | Total Chemical Analysis Method | 0.01% to 10% |
| Cr | Inductively Coupled Plasma Optical Emission Spectrometry | 0.61 mg/kg | K2O | X-ray Fluorescence Spectrometry | 0.05% to 8% |
| Ni | Inductively Coupled Plasma Optical Emission Spectrometry | 0.6 mg/kg | Na2O | X-ray Fluorescence Spectrometry | 0.05% to 8% |
| Cu | Inductively Coupled Plasma Optical Emission Spectrometry | 0.6 mg/kg | Fe2O3 | Total Chemical Analysis Method | ≥0.05% |
| Pb | Inductively Coupled Plasma Optical Emission Spectrometry | 0.5 mg/kg | Al2O3 | X-ray Fluorescence Spectrometry | ≥0.05% |
| Hg | Atomic Fluorescence Spectrometry | 0.005 mg/kg | SiO2 | Total Chemical Analysis Method | ≥5% |
| Zn | Inductively Coupled Plasma Optical Emission Spectrometry | 1.1 mg/kg | TiO2 | Total Chemical Analysis Method | 0.2% to 10% |
| Analysis Steps | Speciation Analysis | Extractant | Operation Steps |
|---|---|---|---|
| F1 | exchangeable | 16 mL of 1 mol/L MgCl2 solution | Adjust the pH to 7.0, then shake continuously at 25 °C for 1 h. Centrifuge for 20 min, aspirate the supernatant, and dilute the solution to 25 mL in a volumetric flask. Wash the residue with deionized water. Following centrifugation, filter the entire supernatant and then measure the heavy metal concentration. |
| F2 | carbonate-bound | 16 mL of 1 mol/L NaAc solution | Adjust the pH to 5.0, then shake continuously at 25 °C for 8 h. Centrifuge for 20 min. Aspirate the upper supernatant and dilute to a 25 mL volumetric flask. Wash the residue with deionized water. Following centrifugation, filter the entire supernatant and then measure the heavy metal concentration. |
| F3 | Fe/Mn oxide-bound | 16 mL of 0.04 mol/L NH2OH·HCl and 25% (v/v) HAc mixed solution | Incubate at (96 ± 3) °C with intermittent shaking for 4 h, then centrifuge for 20 min. Aspirate the upper supernatant and dilute to a 25 mL volumetric flask. Wash the residue with deionized water. Following centrifugation, filter the entire supernatant and then measure the heavy metal concentration. |
| F4 | organic-bound | 3 mL of 0.01 mol/L HNO3, 5 mL of 30% (v/v) H2O2, and 5 mL of 3.2 mol/L CH3COONH4 | Adjust the pH to 2 with HNO3, heat in a water bath to (85 ± 2) °C, and shake intermittently for 2 h. Then add 5 mL of H2O2, adjust the pH to 2 again, heat at (85 ± 2) °C for another 2 h with intermittent shaking. Cool to (25 ± 1) °C, add 5 mL of 3.2 mol/L NH4Ac in 20% HNO3 solution, dilute to 20 mL, shake continuously for 30 min, and centrifuge for 20 min. Aspirate the upper supernatant and dilute to a 25 mL volumetric flask. Wash the residue with deionized water. Following centrifugation, filter the entire supernatant and then measure the heavy metal concentration. |
| F5 | residual | HCl + HNO3 + HF + HClO4 | After digestion, transfer the solution to a 50 mL volumetric flask and dilute to the mark. Use this solution as a sample for heavy metal concentration determination. |
| Weathered Crust Type | Sampling Horizon | Data Evaluation Metrics | Cr | Ni | Cu | Zn | Cd | Pb | Hg | As |
|---|---|---|---|---|---|---|---|---|---|---|
| Serpentine profile | topsoil layer | Measured value (mg/kg) | 417 (1072) | 129 (419) | 22.1 (36.8) | 75.6 (129) | 0.13 (0.25) | 19.9 (35.9) | 0.020 (0.026) | 10.1 (13.5) |
| fully weathered layer | Mean value (mg/kg) | 842 (1615) | 240 (605) | 23.6 (19.2) | 80.0 (122) | 0.12 (0.16) | 16.6 (16.0) | 0.019 (0.013) | 10.2 (6.7) | |
| Mean Relative Error (%) | 53.5 (8.43) | 32.5 (5.74) | 2.31 (10.6) | 6.66 (6.04) | 2.70 (17.1) | 12.4 (13.2) | 4.91 (36.8) | 0.98 (24.8) | ||
| semi-weathered layer | Mean value (mg/kg) | 1553 (1412) | 629 (507) | 23.6 (10.8) | 87.1 (116) | 0.07 (0.14) | 5.9 (15.5) | 0.009 (0.005) | 2.3 (3.6) | |
| Mean Relative Error (%) | 36.1 (39.8) | 9.30 (34.3) | 47.2 (5.03) | 13.2 (4.25) | 12.4 (70.5) | 12.4 (75.3) | 15.9 (13.6) | 31.9 (41.9) | ||
| bedrock layer | Measured value (mg/kg) | 2047 (1869) | 681 (560) | 9.6 (5.9) | 86.1 (99.5) | 0.04 (0.06) | 3.16 (6.16) | 0.005 (0.005) | 0.6 (2.7) | |
| Pyroxeniteprofile | topsoil layer | Measured value (mg/kg) | 1357 (135) | 114 (55) | 23.5 (75.2) | 68.5 (119) | 0.09 (0.24) | 21.5 (15.6) | 0.019 (0.017) | 5.6 (7.4) |
| fully weathered layer | Mean value (mg/kg) | 1272 (126) | 97 (58) | 19.8 (88.6) | 59.1 (139) | 0.08 (0.28) | 17.1 (18.7) | 0.010 (0.017) | 5.7 (6.9) | |
| Mean Relative Error (%) | 42.1 (2.14) | 12.4 (1.47) | 33.8 (6.72) | 26.4 (5.12) | 15.6 (8.08) | 38.0 (3.19) | 33.2 (13.1) | 49.8 (4.20) | ||
| semi-weathered layer | Mean value (mg/kg) | 2117 (377) | 180 (88) | 17.3 (75.2) | 63.0 (110) | 0.12 (0.21) | 13.0 (14.8) | 0.006 (0.013) | 1.9 (5.4) | |
| Mean Relative Error (%) | 8.70 (30.3) | 5.38 (8.11) | 8.70 (16.1) | 13.7 (2.07) | 8.96 (6.26) | 17.7 (4.14) | 18.8 (11.5) | 13.7 (8.89) | ||
| bedrock layer | Measured value (mg/kg) | 2388 (443) | 127 (110) | 9.3 (25.0) | 70.4 (62.7) | 0.09 (0.11) | 14.1 (6.92) | 0.005 (0.009) | 0.8 (2.9) | |
| Granite profile | topsoil layer | Measured value (mg/kg) | 66 (35) | 24.1 (11.9) | 21.0 (13.0) | 69.0 (81.1) | 0.16 (0.12) | 29.8 (18.4) | 0.016 (0.019) | 7.7 (3.5) |
| fully weathered layer | Mean value (mg/kg) | 57 (19) | 28.6 (6.3) | 22.5 (8.9) | 79.7 (90.0) | 0.15 (0.10) | 28.0 (17.8) | 0.019 (0.013) | 9.3 (1.8) | |
| Mean Relative Error (%) | 21.4 (4.12) | 27.4 (6.04) | 17.8 (5.96) | 1.74 (2.72) | 6.00 (18.0) | 2.06 (3.77) | 10.3 (8.25) | 33.5 (5.70) | ||
| semi-weathered layer | Mean value (mg/kg) | 39 (9) | 23.7 (1.6) | 18.7 (4.1) | 92.3 (83.0) | 0.14 (0.07) | 24.8 (18.1) | 0.014 (0.009) | 3.8 (0.3) | |
| Mean Relative Error (%) | 8.22 (13.4) | 26.0 (18.4) | 8.21 (14.9) | 4.42 (9.26) | 5.24 (17.6) | 8.29 (5.46) | 3.70 (22.6) | 21.1 (33.2) | ||
| bedrock layer | Measured value (mg/kg) | 31 (12) | 18.8 (3.24) | 7.8 (5.1) | 150 (75.7) | 0.69 (0.14) | 25.4 (15.2) | 0.004 (0.010) | 1.8 (0.4) |
| Layer | Cr | Ni | Cu | Zn | Cd | Pb | Hg | As | |
|---|---|---|---|---|---|---|---|---|---|
| soil layer | Cr | 1 | 0.939 ** | 0.448 | 0.501 | 0.404 | −0.269 | 0.136 | 0.739 ** |
| Ni | 0.622 * | 1 | 0.49 | 0.538 | 0.435 | −0.189 | 0.05 | 0.622 * | |
| Cu | 0.105 | 0.35 | 1 | 0.825 ** | 0.961 ** | 0.196 | 0.457 | 0.713 ** | |
| Zn | −0.322 | 0.168 | 0.406 | 1 | 0.835 ** | 0.263 | 0.243 | 0.720 ** | |
| Cd | −0.719 ** | −0.26 | −0.133 | 0.607 * | 1 | 0.414 | 0.489 | 0.751 ** | |
| Pb | −0.734 ** | −0.853 ** | −0.049 | 0.119 | 0.526 | 1 | 0.19 | 0.193 | |
| Hg | −0.571 | −0.305 | 0.014 | 0.406 | 0.587 * | 0.504 | 1 | 0.529 | |
| As | −0.3 | 0.462 | 0.24 | 0.656 * | 0.471 | −0.212 | 0.018 | 1 | |
| parent material layer | Cr | 1 | 0.979 ** | 0.462 | 0.552 | −0.245 | −0.671 * | −0.278 | 0.531 |
| Ni | 0.538 | 1 | 0.455 | 0.531 | −0.259 | −0.689 * | −0.216 | 0.538 | |
| Cu | −0.147 | −0.147 | 1 | 0.462 | 0.517 | −0.334 | 0.49 | 0.902 ** | |
| Zn | −0.51 | −0.252 | −0.364 | 1 | 0.196 | 0.021 | 0.015 | 0.629 * | |
| Cd | −0.552 | −0.811 ** | 0.196 | 0.231 | 1 | 0.545 | 0.45 | 0.259 | |
| Pb | −0.559 | −0.881 ** | 0.308 | 0.217 | 0.902 ** | 1 | −0.026 | −0.432 | |
| Hg | −0.385 | −0.084 | 0.783 ** | 0.049 | −0.035 | 0.14 | 1 | 0.516 | |
| As | −0.476 | −0.287 | 0.769 ** | 0.21 | 0.42 | 0.517 | 0.762 ** | 1 |
| Placement | Migration Factor | Layer | SiO2 | CaO | MgO | Al2O3 | Fe2O3 | K2O | Na2O | TiO2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Inside the mining area | Cr′ | soil layer | 0.494 | −0.304 | −0.508 | 0.425 | −0.683 * | 0.449 | −0.06 | 0.435 |
| Cr′ | pedogenic soil matrix | 0.967 ** | 0.05 | −0.912 ** | 0.883 ** | −0.633 | 0.619 | 0.883 ** | 0.883 ** | |
| Ni′ | soil layer | 0.463 | −0.34 | −0.474 | 0.459 | −0.642 * | 0.418 | −0.102 | 0.467 | |
| Ni′ | pedogenic soil matrix | 0.483 | 0.517 | −0.477 | 0.5 | −0.633 | −0.008 | 0.517 | 0.4 | |
| Outside the mining area | Cr′ | soil layer | 0.593 * | −0.24 | −0.582 * | 0.589 * | −0.594 * | 0.583 * | 0.600 * | 0.085 |
| Cr′ | pedogenic soil matrix | 0.433 | 0 | −0.433 | 0.45 | −0.65 | 0.567 | 0.467 | −0.333 | |
| Ni′ | soil layer | 0.781 ** | −0.088 | −0.746 ** | 0.743 ** | −0.777 ** | 0.742 ** | 0.708 * | 0.217 | |
| Ni′ | pedogenic soil matrix | −0.05 | 0.033 | 0.05 | 0.05 | −0.233 | 0.2 | 0.05 | −0.283 |
| Indicator | Inside the Mining Area | Outside the Mining Area | ||
|---|---|---|---|---|
| PC1 | PC2 | PC1 | PC2 | |
| SiO2 | 0.084 | 0.077 | 0.127 | 0.020 |
| CaO | 0.097 | −0.138 | −0.057 | −0.168 |
| MgO | −0.163 | 0.028 | −0.124 | −0.041 |
| Al2O3 | 0.124 | −0.022 | 0.134 | 0.101 |
| Fe2O3 | −0.109 | −0.022 | −0.118 | −0.049 |
| K2O | 0.145 | −0.071 | 0.124 | −0.049 |
| Na2O | 0.257 | −0.154 | 0.116 | −0.041 |
| TiO2 | 0.226 | −0.166 | 0.053 | 0.041 |
| Cr | −0.038 | −0.049 | −0.101 | 0.039 |
| Ni | −0.208 | 0.096 | −0.092 | 0.032 |
| Cu | −0.093 | 0.345 | 0.001 | 0.261 |
| Zn | 0.044 | −0.127 | −0.034 | 0.208 |
| Cd | 0.052 | −0.062 | 0.008 | 0.103 |
| Pb | 0.046 | 0.051 | 0.084 | −0.150 |
| Hg | −0.153 | 0.352 | 0.089 | 0.324 |
| As | −0.177 | 0.423 | 0.003 | 0.313 |
| Eigenvalue | 10.808 | 2.953 | 8.827 | 4.727 |
| Variance/% | 60.045 | 15.404 | 49.038 | 26.263 |
| Region | Parent Material Type | Cr Concentration (mg/kg) | Ni Concentration (mg/kg) | Key Influencing Factors | Ref. |
|---|---|---|---|---|---|
| Polymetallic Mining Areas in Southern China | Serpentinite, Peridotite | 42.3–72.4 | 25.9–42.6 | Mining activities combined with weathering of ultramafic rocks have led to significant Ni and Cr pollution in downstream farmland. | [20,44] |
| Hualien, Taiwan, China | Serpentinite | 540 | 2440 | Serpentinite directly weathers into high-Ni soil, and the paddy soil environment enhances Ni activity. | [44] |
| Eastern Mining Areas of China | Ultramafic rock contact zone | 61.0–72.4 | 26.9–42.6 | Mining disturbances accelerate the alteration of ultramafic rocks, leading to the diffusion of Ni and Cr to the surrounding areas. | [20] |
| Humid Areas in Asia (Japan, Taiwan of China, the Philippines, Vietnam) | Serpentinite | 1000–3000 | 1000–2000 | The higher the degree of serpentinization, the more Mg is lost, the Ca/Mg ratio increases, and the concentrations of mobile forms (PMFs) of Cr and Ni increase. | [9,34] |
| Poland | Serpentinized peridotite | 2196–2915 | 1577–3944 | The degree of serpentinization determines the amount of Ni released, and the surface soil is enriched in Ni due to weathering. | [18] |
| Red Clay Mining Area in Southern Czechia | Weathered laterite of ultramafic rocks | 4500–5700 | 3600–7700 | Tropical weathering forms laterite, and Ni and Cr are fixed by oxides. | [18] |
| Tuscany, Italy | Serpentinite | 3502 | 2342 | Weathering is weak in temperate climates, and Cr and Ni exist in residual forms. | [39] |
| The Italian Alps region | Serpentinite | 1649–3428 | 750–2370 | The concentrations of Cr and Ni are influenced by parent rock types, soil pH, organic carbon, and mineral composition. | [15] |
| New Caledonia | Serpentinite | >1000 | 10,000–25,500 | In tropical climates, ultramafic rocks undergo intense weathering, and Ni and Cr are enriched through the pedogenesis process. | [13] |
| The Klamath Mountains, California, USA | Serpentinite | 1000–4000 | 100–1000 | In soils formed from ultramafic rocks (including peridotite and serpentinite), the distribution of Cr and Ni is controlled by topography and the degree of weathering, with mobility increasing under acidic conditions. | [13] |
| Galicia, Spain | Serpentinite | 1499–4309 | 76–373 | In soils formed by weathering of ultramafic rocks (serpentinite), Cr and Ni are mainly hosted in magnesium silicates and iron oxides. | [33] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, J.; Liu, J.; Hu, S.; Wang, J. Distribution Characteristics of High-Background Elements and Assessment of Ecological Element Activity in Typical Profiles of Ultramafic Rock Area. Toxics 2025, 13, 558. https://doi.org/10.3390/toxics13070558
Shi J, Liu J, Hu S, Wang J. Distribution Characteristics of High-Background Elements and Assessment of Ecological Element Activity in Typical Profiles of Ultramafic Rock Area. Toxics. 2025; 13(7):558. https://doi.org/10.3390/toxics13070558
Chicago/Turabian StyleShi, Jingtao, Junjian Liu, Suduan Hu, and Jiangyulong Wang. 2025. "Distribution Characteristics of High-Background Elements and Assessment of Ecological Element Activity in Typical Profiles of Ultramafic Rock Area" Toxics 13, no. 7: 558. https://doi.org/10.3390/toxics13070558
APA StyleShi, J., Liu, J., Hu, S., & Wang, J. (2025). Distribution Characteristics of High-Background Elements and Assessment of Ecological Element Activity in Typical Profiles of Ultramafic Rock Area. Toxics, 13(7), 558. https://doi.org/10.3390/toxics13070558