Comprehensive Risk Evaluation of Perfluoroalkyl Substance Pollution in Urban Riverine Systems: Ecotoxicological and Human Health Perspectives
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Standards
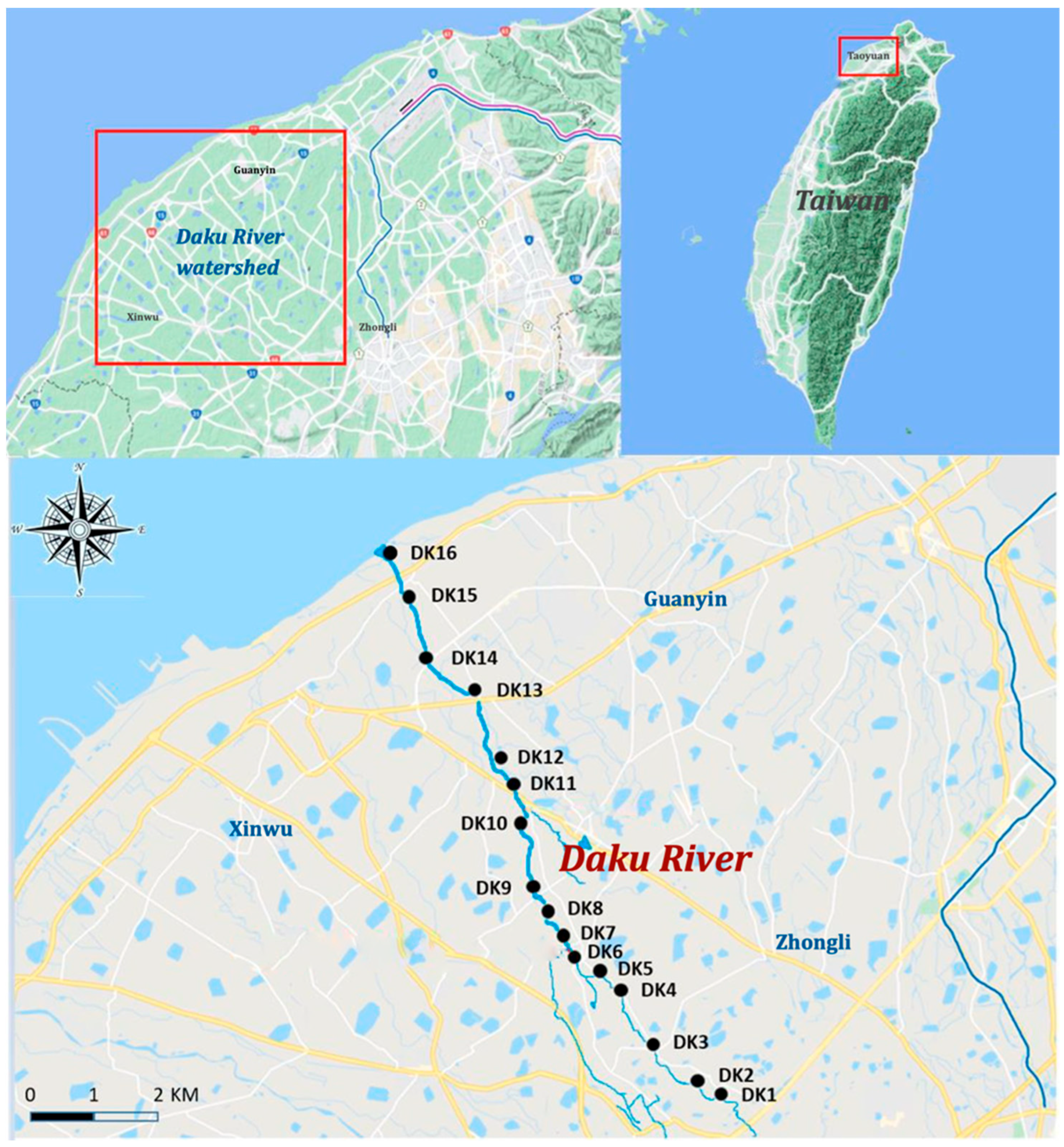
2.2. Study Area and Sample Collection
2.3. Sample Pretreatment
2.4. Instrumental Analysis
2.5. Quality Assurance and Quality Control (QA/QC)
2.6. Statistical Analysis
2.7. Risk Characterization
3. Results and Discussion
3.1. Occurrence of PFASs in Daku River
3.2. Source Identifications
3.3. Mass Loadings of PFASs
3.4. Health Risk Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barzen-Hanson, K.A.; Roberts, S.C.; Choyke, S.; Oetjen, K.; McAlees, A.; Riddell, N.; McCrindle, R.; Ferguson, P.L.; Higgins, C.P.; Field, J.A. Discovery of 40 Classes of Per- and Polyfluoroalkyl Substances in Historical Aqueous Film-Forming Foams (AFFFs) and AFFF-Impacted Groundwater. Environ. Sci. Technol. 2017, 51, 2047–2057. [Google Scholar] [CrossRef] [PubMed]
- Lindstrom, A.B.; Strynar, M.J.; Libelo, E.L. Polyfluorinated Compounds: Past, Present, and Future. Environ. Sci. Technol. 2011, 45, 7954–7961. [Google Scholar] [CrossRef] [PubMed]
- Prevedouros, K.; Cousins, I.T.; Buck, R.C.; Korzeniowski, S.H. Sources, Fate and Transport of Perfluorocarboxylates. Environ. Sci. Technol. 2006, 40, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Kedikoglou, K.; Costopoulou, D.; Vassiliadou, I.; Leondiadis, L. Preliminary Assessment of General Population Exposure to Perfluoroalkyl Substances through Diet in Greece. Environ. Res. 2019, 177, 108617. [Google Scholar] [CrossRef]
- Schiavone, C.; Portesi, C. PFAS: A Review of the State of the Art, from Legislation to Analytical Approaches and Toxicological Aspects for Assessing Contamination in Food and Environment and Related Risks. Appl. Sci. 2023, 13, 6696. [Google Scholar] [CrossRef]
- Zafeiraki, E.; Gebbink, W.A.; Hoogenboom, R.L.A.P.; Kotterman, M.; Kwadijk, C.; Dassenakis, E.; van Leeuwen, S.P.J. Occurrence of Perfluoroalkyl Substances (PFASs) in a Large Number of Wild and Farmed Aquatic Animals Collected in the Netherlands. Chemosphere 2019, 232, 415–423. [Google Scholar] [CrossRef]
- Barry, V.; Winquist, A.; Steenland, K. Perfluorooctanoic Acid (PFOA) Exposures and Incident Cancers among Adults Living near a Chemical Plant. Environ. Health Perspect. 2013, 121, 1313–1318. [Google Scholar] [CrossRef] [PubMed]
- Sagiv, S.K.; Rifas-Shiman, S.L.; Webster, T.F.; Mora, A.M.; Harris, M.H.; Calafat, A.M.; Ye, X.; Gillman, M.W.; Oken, E. Sociodemographic and Perinatal Predictors of Early Pregnancy Per- and Polyfluoroalkyl Substance (PFAS) Concentrations. Environ. Sci. Technol. 2015, 49, 11849–11858. [Google Scholar] [CrossRef]
- Fiedler, H.; Kallenborn, R.; de Boer, J.; Sydnes, L.K. The Stockholm Convention: A Tool for the Global Regulation of Persistent Organic Pollutants. Chem. Int. 2019, 41, 4–11. [Google Scholar] [CrossRef]
- Kunacheva, C.; Boontanon, S.K.; Fujii, S.; Tanaka, S.; Musirat, C.; Artsalee, C.; Wongwattana, T. Contamination of Perfluorinated Compounds (PFCs) in Chao Phraya River and Bangpakong River, Thailand. Water Sci. Technol. 2009, 60, 975–982. [Google Scholar] [CrossRef]
- Pan, C.-G.; Ying, G.-G.; Zhao, J.-L.; Liu, Y.-S.; Jiang, Y.-X.; Zhang, Q.-Q. Spatiotemporal Distribution and Mass Loadings of Perfluoroalkyl Substances in the Yangtze River of China. Sci. Total Environ. 2014, 493, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Zushi, Y.; Ye, F.; Motegi, M.; Nojiri, K.; Hosono, S.; Suzuki, T.; Kosugi, Y.; Yaguchi, K.; Masunaga, S. Spatially Detailed Survey on Pollution by Multiple Perfluorinated Compounds in the Tokyo Bay Basin of Japan. Environ. Sci. Technol. 2011, 45, 2887–2893. [Google Scholar] [CrossRef]
- Zainuddin, K.; Zakaria, M.P.; Al-Odaini, N.A.; Bakhtiari, A.R.; Latif, P.A. Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonate (PFOS) in Surface Water from the Langat River, Peninsular Malaysia. Environ. Forensics 2012, 13, 82–92. [Google Scholar] [CrossRef]
- Nakayama, S.F.; Strynar, M.J.; Reiner, J.L.; Delinsky, A.D.; Lindstrom, A.B. Determination of Perfluorinated Compounds in the Upper Mississippi River Basin. Environ. Sci. Technol. 2010, 44, 4103–4109. [Google Scholar] [CrossRef]
- Lin, Y.; Jiang, J.-J.; Rodenburg, L.A.; Cai, M.; Wu, Z.; Ke, H.; Chitsaz, M. Perfluoroalkyl Substances in Sediments from the Bering Sea to the Western Arctic: Source and Pathway Analysis. Environ. Int. 2020, 139, 105699. [Google Scholar] [CrossRef]
- Jiang, J.J.; Okvitasari, A.R.; Huang, F.Y.; Tsai, C.S. Characteristics, Pollution Patterns and Risks of Perfluoroalkyl Substances in Drinking Water Sources of Taiwan. Chemosphere 2021, 264, 128579. [Google Scholar] [CrossRef] [PubMed]
- U.S. Environmental Protection Agency Drinking Water Health Advisory for Perfluorooctanoic Acid (PFOA). Available online: https://www.epa.gov/sites/default/files/2016-05/documents/pfoa_health_advisory_final_508.pdf (accessed on 3 March 2017).
- U.S. Environmental Protection Agency Drinking Water Health Advisory for Perfluorooctane Sulfonate (PFOS). Available online: https://www.epa.gov/sites/default/files/2016-05/documents/pfos_health_advisory_final_508.pdf (accessed on 3 March 2017).
- Brown, J.B.; Conder, J.M.; Arblaster, J.A.; Higgins, C.P. Assessing Human Health Risks from Per- And Polyfluoroalkyl Substance (PFAS)-Impacted Vegetable Consumption: A Tiered Modeling Approach. Environ. Sci. Technol. 2020, 54, 15202–15214. [Google Scholar] [CrossRef]
- Xu, J.; Shi, G.-L.; Guo, C.-S.; Wang, H.-T.; Tian, Y.-Z.; Huangfu, Y.-Q.; Zhang, Y.; Feng, Y.-C.; Xu, J. A New Method to Quantify the Health Risks from Sources of Perfluoroalkyl Substances, Combined with Positive Matrix Factorization and Risk Assessment Models. Environ. Toxicol. Chem. 2018, 37, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Pang, Y.; Luo, M.; Jiang, X.; Huang, J.; Li, Z. Multi-Media Distribution and Risk Assessment of per- and Polyfluoroalkyl Substances in the Huai River Basin, China. Sci. Total Environ. 2024, 914. [Google Scholar] [CrossRef]
- Li, B.B.; Hu, L.X.; Yang, Y.Y.; Wang, T.T.; Liu, C.; Ying, G.G. Contamination Profiles and Health Risks of PFASs in Groundwater of the Maozhou River Basin. Environ. Pollut. 2020, 260, 113996. [Google Scholar] [CrossRef]
- Viticoski, R.L.; Wang, D.; Feltman, M.A.; Mulabagal, V.; Rogers, S.R.; Blersch, D.M.; Hayworth, J.S. Spatial Distribution and Mass Transport of Perfluoroalkyl Substances (PFAS) in Surface Water: A Statewide Evaluation of PFAS Occurrence and Fate in Alabama. Sci. Total Environ. 2022, 836, 155524. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhao, Z.; Li, J.; Hua, X.; Zhang, B.; Tang, C.; An, X.; Lin, T. Emerging and Legacy Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS) in Surface Water around Three International Airports in China. Chemosphere 2023, 344, 140360. [Google Scholar] [CrossRef]
- Tang, W.Q.; Wang, T.T.; Miao, J.W.; Tan, H.D.; Zhang, H.J.; Guo, T.Q.; Chen, Z.B.; Wu, C.Y.; Mo, L.; Mai, B.X.; et al. Presence and Sources of Per- and Polyfluoroalkyl Substances (PFASs) in the Three Major Rivers on Hainan Island. Environ. Res. 2025, 266, 120590. [Google Scholar] [CrossRef]
- Duong, H.T.; Kadokami, K.; Shirasaka, H.; Hidaka, R.; Chau, H.T.C.; Kong, L.; Nguyen, T.Q.; Nguyen, T.T. Occurrence of Perfluoroalkyl Acids in Environmental Waters in Vietnam. Chemosphere 2015, 122, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.M.; Bharat, G.K.; Tayal, S.; Larssen, T.; Bečanová, J.; Karásková, P.; Whitehead, P.G.; Futter, M.N.; Butterfield, D.; Nizzetto, L. Perfluoroalkyl Substances (PFAS) in River and Ground/Drinking Water of the Ganges River Basin: Emissions and Implications for Human Exposure. Environ. Pollut. 2016, 208, 704–713. [Google Scholar] [CrossRef]
- Ramírez-Canon, A.; Paola Becerra-Quiroz, A.; Herrera-Jacquelin, F. Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS): First Survey in Water Samples from the Bogotá River, Colombia. Environ. Adv. 2022, 8. [Google Scholar] [CrossRef]
- Teymoorian, T.; Delon, L.; Munoz, G.; Sauvé, S. Target and Suspect Screening Reveal PFAS Exceeding European Union Guideline in Various Water Sources South of Lyon, France. Environ. Sci. Technol. Lett. 2025, 12, 327–333. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Li, J.; Wu, N.; Li, W.; Niu, Z. Distribution, Partitioning Behavior and Positive Matrix Factorization-Based Source Analysis of Legacy and Emerging Polyfluorinated Alkyl Substances in the Dissolved Phase, Surface Sediment and Suspended Particulate Matter around Coastal Areas of Bohai Bay. Environ. Pollut. 2019, 246, 34–44. [Google Scholar] [CrossRef]
- Lin, A.Y.-C.; Panchangam, S.C.; Lo, C.-C. The Impact of Semiconductor, Electronics and Optoelectronic Industries on Downstream Perfluorinated Chemical Contamination in Taiwanese Rivers. Environ. Pollut. 2009, 157, 1365–1372. [Google Scholar] [CrossRef]
- Sun, R.; Wu, M.; Tang, L.; Li, J.; Qian, Z.; Han, T.; Xu, G. Perfluorinated Compounds in Surface Waters of Shanghai, China: Source Analysis and Risk Assessment. Ecotoxicol. Environ. Saf. 2018, 149, 88–95. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Taniyasu, S.; Yeung, L.W.Y.; Lam, P.K.S.; Wang, J.; Li, X.; Yamashita, N.; Dai, J. Distribution and Fate of Perfluoroalkyl Substances in Municipal Wastewater Treatment Plants in Economically Developed Areas of China. Environ. Pollut. 2013, 176, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.H.; Wu, W.; Huang, T.Y.; Cheng, S.Q.; Xiang, S.; Pang, Y. Characteristics, Sources, and Risk Assessment of Perlyfluoroalkyl Substances in Surface Water and Sediment of Luoma Lake. Huanjing Kexue/Environ. Sci. 2022, 43, 3562–3574. [Google Scholar] [CrossRef]
- Zhang, Y.; Lai, S.; Zhao, Z.; Liu, F.; Chen, H.; Zou, S.; Xie, Z.; Ebinghaus, R. Spatial Distribution of Perfluoroalkyl Acids in the Pearl River of Southern China. Chemosphere 2013, 93, 1519–1525. [Google Scholar] [CrossRef]
- Liu, L.; Qu, Y.; Huang, J.; Weber, R. Per- and Polyfluoroalkyl Substances (PFASs) in Chinese Drinking Water: Risk Assessment and Geographical Distribution. Environ. Sci. Eur. 2021, 33, 1–12. [Google Scholar] [CrossRef]
- Sun, H.; Li, F.; Zhang, T.; Zhang, X.; He, N.; Song, Q.; Zhao, L.; Sun, L.; Sun, T. Perfluorinated Compounds in Surface Waters and WWTPs in Shenyang, China: Mass Flows and Source Analysis. Water Res. 2011, 45, 4483–4490. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.S.; Li, M.H.; Chen, S.W. Estimation and Modeling the Surface Flow System for Irrigation Regions. J. Taiwan Agric. Eng. 2011, 57, 76–91. [Google Scholar]
- Olsen, G.W.; Burris, J.M.; Ehresman, D.J.; Froehlich, J.W.; Seacat, A.M.; Butenhoff, J.L.; Zobel, L.R. Half-Life of Serum Elimination of Perfluorooctanesulfonate, Perfluorohexanesulfonate, and Perfluorooctanoate in Retired Fluorochemical Production Workers. Environ. Health Perspect. 2007, 115, 1298–1305. [Google Scholar] [CrossRef]
- Wang, Z.; Cousins, I.T.; Scheringer, M.; Buck, R.C.; Hungerbühler, K. Global Emission Inventories for C4-C14 Perfluoroalkyl Carboxylic Acid (PFCA) Homologues from 1951 to 2030, Part I: Production and Emissions from Quantifiable Sources. Environ. Int. 2014, 70, 62–75. [Google Scholar] [CrossRef]
- Park, M.; Daniels, K.D.; Wu, S.; Ziska, A.D.; Snyder, S.A. Magnetic Ion-Exchange (MIEX) Resin for Perfluorinated Alkylsubstance (PFAS) Removal in Groundwater: Roles of Atomic Charges for Adsorption. Water Res. 2020, 181, 115897. [Google Scholar] [CrossRef]
- National Library of Medicine PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 3 March 2017).
- Surveillance and Epidemiology Branch Center for Health Protection Departement of Health Report of the 2014–2015 Demographic Health Survey; 2017. Available online: https://www.chp.gov.hk/files/pdf/dh_phs_2014_15_full_report_eng.pdf (accessed on 3 March 2017).
- Food and Drug Administration; National Health Research Institute National Food Consumption Database. Available online: https://tnfcds.nhri.edu.tw/ (accessed on 3 March 2017).
- Arcadis U.S. Inc. Review of Models for Evaluating Per- and Polyfluoroalkyl Substances in Land Applied Residuals and Biosolids; v. 1.1.; National Council for Air and Stream Improvement, Inc.: Cary, NC, USA, 2020. [Google Scholar]
- Costello, M.C.S.; Lee, L.S. Sources, Fate, and Plant Uptake in Agricultural Systems of Per- and Polyfluoroalkyl Substances. Curr. Pollut. Rep. 2024, 10, 799–819. [Google Scholar] [CrossRef]




| Sampling Location | Location Type | Concentration (ng/L) | References | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PFBS | PFHxS | PFOS | PFHpA | PFOA | PFNA | PFDA | |||
| Daku River, Taiwan | River | n.d.–111.58 | n.d.–56.5 | n.d.–20.7 | n.d.–47.1 | 0.08–185 | n.d.–67.76 | 0.16–46.39 | This study |
| Yangtze River, China | River | n.d.–41.9 | n.d.–18.0 | n.d.–3.93 | n.d.–2.59 | 0.52–18.0 | n.d.–0.86 | n.d.–0.33 | [11] |
| Red River, Vietnam | River | n.d. | n.d.–0,22 | n.d.–0.94 | n.d.–1.51 | n.d.–0.51 | n.d.–0.61 | [26] | |
| Ganges River, India | River | n.d.–10.2 | n.d.–0.304 | n.d.–1.73 | 0.340–3.27 | 0.079–1.18 | n.d.–0.19 | n.d.–0.19 | [27] |
| Maozhou River, China | River | 16.4–42.5 | 0.13–11.9 | 1.7–195.8 | 1.5–17.0 | 3.2–27.0 | 0.4–1.6 | 0.18–0.83 | [22] |
| Baoshan reservoir, Taiwan | Reservoir | 0.02–7.94 | n.d.–30.5 | 0.02–61.2 | n.d. | 0.02–68.9 | 0.02–33.4 | n.d. | [16] |
| Alabama River, US (Mean ± SD) | River | 28.24 ± 2.31–45.06 ± 3.00 | 10.65 ± 1.01–19.55 ± 2.14 | 7.55 ± 0.38–9.43 ± 0.78 | 14.12 ± 0.39–19.20 ± 1.52 | [23] | |||
| Tunjuelo River, Colombia | River | 0.08 | 0.52 | 0.24 | 0.03 | 0.06 | <0.02 | <0.02 | [28] |
| River around Shanghai Pudong Intl’ Airport, China | River | n.d.–37.08 | 2.31–12.91 | 0.83–6.72 | 1.32–14.68 | 8.71–222.17 | 0.59–3.86 | 0.62–2.33 | [24] |
| Huai River, China (Mean ± SD) | River | n.d.–9.83 (1.80 ± 1.57) | n.d.–4.76 (1.08 ± 0.89) | 0.14–3.42 (1.21 ± 0.66) | 0.06–5.94 (3.21 ± 1.21) | 4.62–81.31 (16.85 ± 12.90) | 0.46–1.93 (1.10 ± 0.30) | n.d.–0.61 (0.22 ± 0.12) | [21] |
| Southern Lyon, France | River | 0.064–9.9 | 0.053–19 | 0.052–76 | 0.07–62 | 0.071–61 | 0.05–14 | 0.034–1.8 | [29] |
| Nandu River, China | River | 0.01–0.89 | 0.01–0.89 | 0.00–0.39 | 0.00–1.61 | 0.35–0.72 | 0.11–0.41 | 0.02–0.10 | [25] |
| Changhua River, China | River | 0.01–0.41 | 0.00–0.34 | 0.02–0.57 | 0.02–3.10 | 0.30–3.36 | 0.11–0.33 | 0.00–0.08 | [25] |
| Wanquan River, China | River | 0.04–1.17 | 0.00–0.85 | 0.04–1.48 | 0.12–1.21 | 0.35–8.55 | 0.14–0.35 | 0.02–0.13 | [25] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satriaji, F.V.; Le Tong, C.T.; Marlina, N.; Lin, Y.; Dat, N.D.; Bui, H.M.; Horie, Y.; Jiang, J.-J. Comprehensive Risk Evaluation of Perfluoroalkyl Substance Pollution in Urban Riverine Systems: Ecotoxicological and Human Health Perspectives. Toxics 2025, 13, 435. https://doi.org/10.3390/toxics13060435
Satriaji FV, Le Tong CT, Marlina N, Lin Y, Dat ND, Bui HM, Horie Y, Jiang J-J. Comprehensive Risk Evaluation of Perfluoroalkyl Substance Pollution in Urban Riverine Systems: Ecotoxicological and Human Health Perspectives. Toxics. 2025; 13(6):435. https://doi.org/10.3390/toxics13060435
Chicago/Turabian StyleSatriaji, Ferlian Vida, Cat Tuong Le Tong, Nelly Marlina, Yan Lin, Nguyen Duy Dat, Ha Manh Bui, Yoshifumi Horie, and Jheng-Jie Jiang. 2025. "Comprehensive Risk Evaluation of Perfluoroalkyl Substance Pollution in Urban Riverine Systems: Ecotoxicological and Human Health Perspectives" Toxics 13, no. 6: 435. https://doi.org/10.3390/toxics13060435
APA StyleSatriaji, F. V., Le Tong, C. T., Marlina, N., Lin, Y., Dat, N. D., Bui, H. M., Horie, Y., & Jiang, J.-J. (2025). Comprehensive Risk Evaluation of Perfluoroalkyl Substance Pollution in Urban Riverine Systems: Ecotoxicological and Human Health Perspectives. Toxics, 13(6), 435. https://doi.org/10.3390/toxics13060435








