The Role of Oxidative Stress in the Pathogenesis and Treatment of Leishmaniasis: Impact on Drug Toxicity and Therapeutic Potential of Natural Products
Abstract
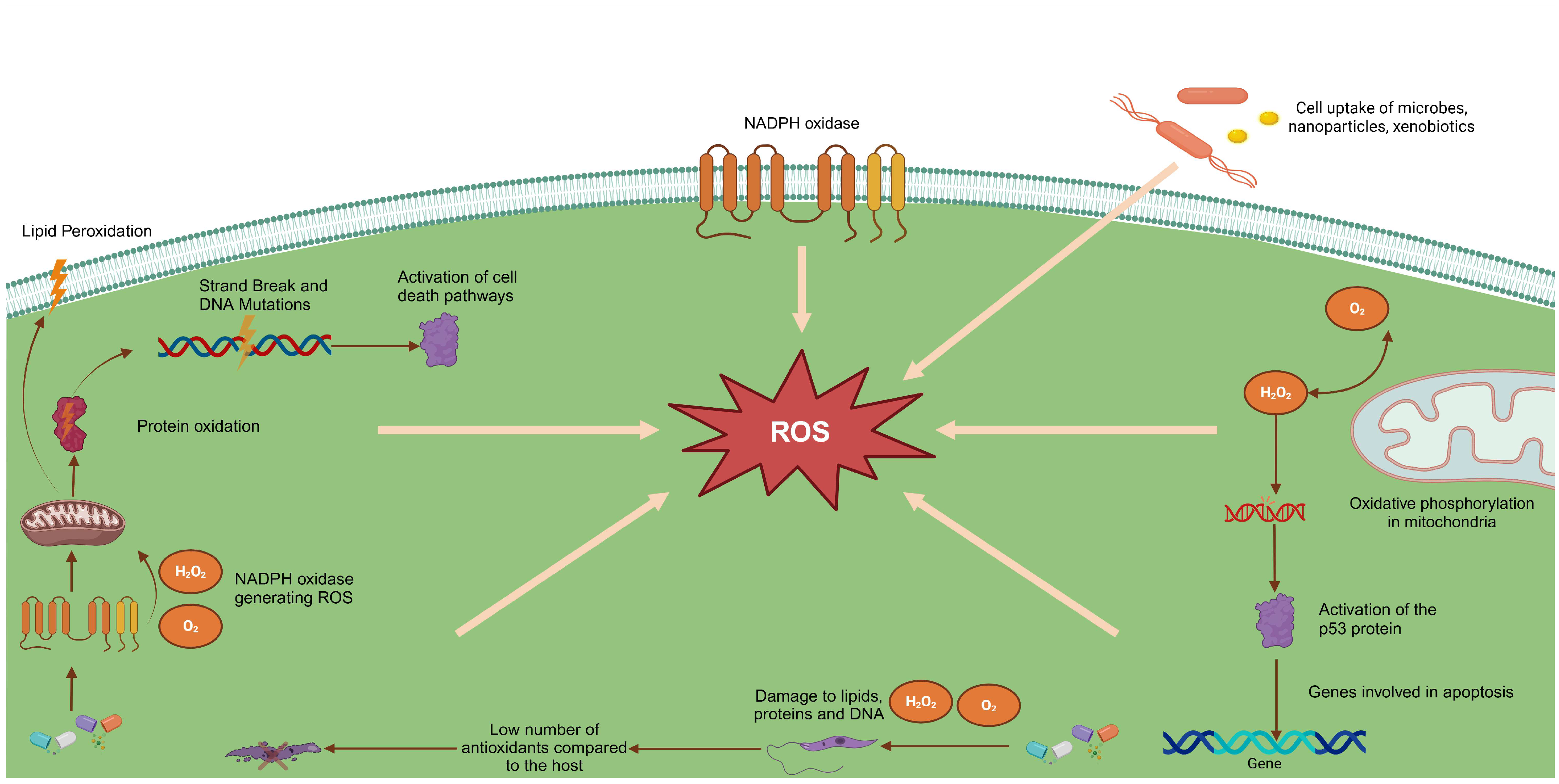
1. Introduction
2. Human Infection by the Leishmania Parasite, Immune Response, and Oxidative Stress
3. Treatment of Leishmaniasis and Possible Involvement of Oxidative Stress
4. New Therapeutic Alternatives for the Treatment of Leishmaniasis and Their Toxic Potential
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LC | Cutaneous leishmaniasis |
| LV | Visceral leishmaniasis |
| GLP | Glycosylphosphatidylinositol |
| CRP | C-reactive protein |
| CR4 | Complement receptor 4 |
| PKC | Protein kinase C |
| CL | Langerhans cells |
| LTA | American tegumentary leishmaniasis |
| NO | Nitric oxide |
| NOS2 | Nitric oxide synthase type 2 |
| ROS | Reactive oxygen species |
| O2− | Superoxide anions |
| H2O2 | Hydrogen peroxide |
| NADPH oxidase | Nicotinamide Adenine Dinucleotide Phosphate Oxidase |
| O2 | Molecular oxygen |
| RNS | Reactive nitrogen species |
| TXNPx | Thioredoxin peroxidase |
| FeSOD | Iron superoxide dismutase |
| MA | Meglumine antimoniate |
| ZnNPs | Zinc nanoparticles synthesized by green synthesis |
| CC50 | 50% cytotoxic concentration |
| HEVS | Hexane extract |
| SI | Selectivity index |
| IC50 | 50% inhibitory concentration |
References
- WHO World Health Organization. Leishmaniasis. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (accessed on 2 February 2025).
- Gontijo, C.M.F.; Melo, M.N. Leishmaniose Visceral No Brasil: Quadro Atual, Desafios e Perspectivas. Rev. Bras. Epidemiol. 2004, 7, 338–349. [Google Scholar] [CrossRef]
- Ornellas-Garcia, U.; Cuervo, P.; Ribeiro-Gomes, F.L. Malaria and Leishmaniasis: Updates on Co-Infection. Front. Immunol. 2023, 14, 1122411. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, A.C. Parasitic Adaptive Mechanisms in Infection by Leishmania. Exp. Mol. Pathol. 2002, 72, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Dos-Santos, A.L.A.; Carvalho-Kelly, L.F.; Dick, C.F.; Meyer-Fernandes, J.R. Innate Immunomodulation to Trypanosomatid Parasite Infections. Exp. Parasitol. 2016, 167, 67–75. [Google Scholar] [CrossRef]
- Culley, F.J.; Harris, R.A.; Kaye, P.M.; McAdam, K.P.; Raynes, J.G. C-Reactive Protein Binds to a Novel Ligand on Leishmania donovani and Increases Uptake into Human Macrophages. J. Immunol. 1996, 156, 4691–4696. [Google Scholar] [CrossRef]
- Brittingham, A.; Chen, G.; Mcgwire, B.S.; Chang, K.-P.; Mosser, D.M. Interaction of Leishmania Gp63 with Cellular Receptors for Fibronectin. Infect. Immun. 1999, 67, 4477–4484. [Google Scholar] [CrossRef]
- Tejle, K.; Magnusson, K.-E.; Rasmusson, B. Phagocytosis and Phagosome Maturation Are Regulated by Calcium in J774 Macrophages Interacting with Unopsonized Prey. Biosci. Rep. 2002, 22, 529–540. [Google Scholar] [CrossRef]
- Holm, Å.; Tejle, K.; Gunnarsson, T.; Magnusson, K.-E.; Descoteaux, A.; Rasmusson, B. Role of Protein Kinase C α for Uptake of Unopsonized Prey and Phagosomal Maturation in Macrophages. Biochem. Biophys. Res. Commun. 2003, 302, 653–658. [Google Scholar] [CrossRef]
- Blank, C.; Fuchs, H.; Rappersberger, K.; Rollinghoff, M.; Moll, H. Parasitism of Epidermal Langerhans Cells in Experimental Cutaneous Leishmaniasis with Leishmania Major. J. Infect. Dis. 1993, 167, 418–425. [Google Scholar] [CrossRef]
- Moll, H. Epidermal Langerhans Cells Are Critical for Immunoregulation of Cutaneous Leishmaniasis. Immunol. Today 1993, 14, 383–387. [Google Scholar] [CrossRef]
- Axelrod, O.; Klaus, S.; Frankenburg, S. Antigen Presentation by Epidermal Langerhans Cells in Experimental Cutaneous Leishmaniasis. Parasite Immunol. 1994, 16, 593–598. [Google Scholar] [CrossRef]
- Moll, H.; Flohé, S.; Röllinghoff, M. Dendritic Cells in Leishmania Major-Immune Mice Harbor Persistent Parasites and Mediate an Antigen-Specific T Cell Immune Response. Eur. J. Immunol. 1995, 25, 693–699. [Google Scholar] [CrossRef]
- Silveira, F.T.; Müller, S.R.; de Souza, A.A.A.; Lainson, R.; Gomes, C.; Laurenti, M.D.; Corbett, C.E.P. Revisão Sobre a Patogenia Da Leishmaniose Tegumentar Americana Na Amazônia, Com Ênfase à Doença Causada Por Leishmania (V.) Braziliensis e Leishmania (L.) amazonensis. Rev. Para. Med. 2008, 22, 9–20. [Google Scholar]
- Machado, P.R.L.; Araújo, M.I.A.S.; Carvalho, L.; Carvalho, E.M. Mecanismos de Resposta Imune Às Infecções. An. Bras. Dermatol. 2004, 79, 647–662. [Google Scholar] [CrossRef]
- Silveira, F.T. What Makes Mucosal and Anergic Diffuse Cutaneous Leishmaniases So Clinically and Immunopathogically Different? A Review in Brazil. Trans. R. Soc. Trop. Med. Hyg. 2019, 113, 505–516. [Google Scholar] [CrossRef]
- Bogdan, C.; Röllinghoff, M.; Diefenbach, A. The Role of Nitric Oxide in Innate Immunity. Immunol. Rev. 2000, 173, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Vouldoukis, I.; Drapier, J.C.; Nüssler, A.K.; Tselentis, Y.; Da Silva, O.A.; Gentilini, M.; Mossalayi, D.M.; Monjour, L.; Dugas, B. Canine Visceral Leishmaniasis: Successful Chemotherapy Induces Macrophage Antileishmanial Activity via the L-Arginine Nitric Oxide Pathway. Antimicrob. Agents Chemother. 1996, 40, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Panaro, M.A.; Lisi, S.; Mitolo, V.; Acquafredda, A.; Fasanella, A.; Carelli, M.G.; Brandonisio, O. Evaluation of Killing, Superoxide Anion and Nitric Oxide Production by Leishmania Infantum-Infected Dog Monocytes. Cytobios 1998, 95, 151–160. [Google Scholar]
- Olivier, M.; Gregory, D.J.; Forget, G. Subversion Mechanisms by Which Leishmania Parasites Can Escape the Host Immune Response: A Signaling Point of View. Clin. Microbiol. Rev. 2005, 18, 293–305. [Google Scholar] [CrossRef]
- Olivier, M.; Brownsey, R.W.; Reiner, N.E. Defective Stimulus-Response Coupling in Human Monocytes Infected with Leishmania donovani Is Associated with Altered Activation and Translocation of Protein Kinase C. Proc. Natl. Acad. Sci. USA 1992, 89, 7481–7485. [Google Scholar] [CrossRef]
- Fairlamb, A.H.; Blackburn, P.; Ulrich, P.; Chait, B.T.; Cerami, A. Trypanothione: A Novel Bis (Glutathionyl) Spermidine Cofactor for Glutathione Reductase in Trypanosomatids. Science (1979) 1985, 227, 1485–1487. [Google Scholar] [CrossRef] [PubMed]
- Spies, H.S.C.; Steenkamp, D.J. Thiols of Intracellular Pathogens: Identification of Ovothiol A in Leishmania donovani and Structural Analysis of a Novel Thiol from Mycobacterium Bovis. Eur. J. Biochem. 1994, 224, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Ariyanayagam, M.R.; Fairlamb, A.H. Ovothiol and Trypanothione as Antioxidants in Trypanosomatids. Mol. Biochem. Parasitol. 2001, 115, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Krauth-Siegel, L.R.; Comini, M.A.; Schlecker, T. The Trypanothione System. In Peroxiredoxin Systems; Structures and Functions; Springer: Dordrecht, The Netherlands, 2007; pp. 231–251. [Google Scholar]
- Irigoín, F.; Cibils, L.; Comini, M.A.; Wilkinson, S.R.; Flohé, L.; Radi, R. Insights into the Redox Biology of Trypanosoma Cruzi: Trypanothione Metabolism and Oxidant Detoxification. Free Radic. Biol. Med. 2008, 45, 733–742. [Google Scholar] [CrossRef]
- Plewes, K.A.; Barr, S.D.; Gedamu, L. Iron Superoxide Dismutases Targeted to the Glycosomes of Leishmania Chagasi Are Important for Survival. Infect. Immun. 2003, 71, 5910–5920. [Google Scholar] [CrossRef]
- Wilkinson, S.R.; Prathalingam, S.R.; Taylor, M.C.; Ahmed, A.; Horn, D.; Kelly, J.M. Functional Characterisation of the Iron Superoxide Dismutase Gene Repertoire in Trypanosoma Brucei. Free Radic. Biol. Med. 2006, 40, 198–209. [Google Scholar] [CrossRef]
- Chan, J.; Fujiwara, T.; Brennan, P.; McNeil, M.; Turco, S.J.; Sibille, J.-C.; Snapper, M.; Aisen, P.; Bloom, B.R. Microbial Glycolipids: Possible Virulence Factors That Scavenge Oxygen Radicals. Proc. Natl. Acad. Sci. USA 1989, 86, 2453–2457. [Google Scholar] [CrossRef]
- Lodge, R.; Diallo, T.O.; Descoteaux, A. Leishmania donovani Lipophosphoglycan Blocks NADPH Oxidase Assembly at the Phagosome Membrane. Cell. Microbiol. 2006, 8, 1922–1931. [Google Scholar] [CrossRef]
- Miller, M.A.; McGowan, S.E.; Gantt, K.R.; Champion, M.; Novick, S.L.; Andersen, K.A.; Bacchi, C.J.; Yarlett, N.; Britigan, B.E.; Wilson, M.E. Inducible Resistance to Oxidant Stress in the Protozoan Leishmania Chagasi. J. Biol. Chem. 2000, 275, 33883–33889. [Google Scholar] [CrossRef]
- Madusanka, R.K.; Silva, H.; Karunaweera, N.D. Treatment of Cutaneous Leishmaniasis and Insights into Species-Specific Responses: A Narrative Review. Infect. Dis. Ther. 2022, 11, 695–711. [Google Scholar] [CrossRef]
- Solomon, M.; Ollech, A.; Pavlotsky, F.; Barzilai, A.; Schwartz, E.; Sharon, B.; Astman, N. Comparison of Intralesional Sodium Stibogluconate versus Intralesional Meglumine Antimoniate for the Treatment of Leishmania Major Cutaneous Leishmaniasis. Acta Derm. Venereol. 2024, 104, adv35089. [Google Scholar] [CrossRef] [PubMed]
- Bento, D.B.; De Souza, B.; Steckert, A.V.; Dias, R.O.; Leffa, D.D.; Moreno, S.E.; Petronilho, F.; de Andrade, V.M.; Dal-Pizzol, F.; Romão, P.R. Oxidative Stress in Mice Treated with Antileishmanial Meglumine Antimoniate. Res. Vet. Sci. 2013, 95, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Yadegari, J.G.; Khalaf, A.K.; Ezzatkhah, F.; Shakibaie, M.; Mohammadi, H.R.; Mahmoudvand, H. Antileishmanial, Cellular Mechanisms, and Cytotoxic Effects of Green Synthesized Zinc Nanoparticles Alone and in Combined with Glucantime against Leishmania Major Infection. Biomed. Pharmacother. 2023, 164, 114984. [Google Scholar]
- Laniado-Laborín, R.; Cabrales-Vargas, M.N. Amphotericin B: Side Effects and Toxicity. Rev. Iberoam. Micol. 2009, 26, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Baginski, M.; Czub, J.; Sternal, K. Interaction of Amphotericin B and Its Selected Derivatives with Membranes: Molecular Modeling Studies. Chem. Rec. 2006, 6, 320–332. [Google Scholar] [CrossRef]
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative Stress, Prooxidants, and Antioxidants: The Interplay. Biomed. Res. Int. 2014, 2014, 761264. [Google Scholar] [CrossRef]
- Pokharel, P.; Ghimire, R.; Lamichhane, P. Efficacy and Safety of Paromomycin for Visceral Leishmaniasis: A Systematic Review. J. Trop. Med. 2021, 2021, 8629039. [Google Scholar] [CrossRef]
- Henderson, D.; Salvi, R.J.; Quaranta, A.; McFadden, S.L.; Burkard, R.F. Ototoxicity: Basic Science and Clinical Applications; New York Academy of Sciences: New York, NY, USA, 1999. [Google Scholar]
- Armstrong, D.; Stratton, R.D. (Eds.) Oxidative Stress and Antioxidant Protection: The Science of Free Radical Biology and Disease; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Berra, C.M. Estudos de Reparo de DNA Por Excisão de Nucleotídeos Em Lesões Oxidativas Em Células de Mamíferos. Ph.D. Thesis, Universidade de São Paulo, São Paulo, Brazil, 2008. [Google Scholar]
- Grivicich, I.; Regner, A.; Brondani Da Rocha, A. Morte Celular Por Apoptose. Rev. Bras. Cancerol. 2007, 53, 335–343. [Google Scholar] [CrossRef]
- Kajimoto, Y.; Kaneto, H. Role of Oxidative Stress in Pancreatic β-Cell Dysfunction. Mitochondrial Pathog. Genes. Apoptosis Aging Dis. 2004, 1011, 168–176. [Google Scholar]
- Kalil Filho, R.; Hajjar, L.A.; Bacal, F.; Hoff, P.M.; Diz, M.d.P.; Galas, F. I Diretriz Brasileira de Cardio-Oncologia Da Sociedade Brasileira de Cardiologia. Arq. Bras. Cardiol. 2011, 96, 1–52. [Google Scholar] [CrossRef]
- Teixeira, V.C.; Amorim, A.C.O.; Rodrigues, C.R.; Sampaio Filho, H.C.; Cambuy, M.R.T.; Machado, R.D.; De Cayres, T.B.; Dos Santos, Y.R.A. Miltefosina No Tratamento Da Leishmaniose Tegumentar: Eficácia e Limitações Da Primeira Terapia Oral Autorizada No Brasil. Braz. J. Health Rev. 2023, 6, 17261–17272. [Google Scholar] [CrossRef]
- Pinheiro, B.M.K.; Granzoto, A.C.G. Uma Visão Biomédica Sobre a Leishmaniose Tegumentar Americana:: Revisão de Literatura. Rev. Mato-Grossense Saúde 2023, 1, 143–157. [Google Scholar]
- Silva, L.R.S.d.; Silva, L.R.S.d.; Silva, F.P.B.d.; Rocha, F.H.B.P.d.; Costa, Y.K.d.S.; Bezerra, D.C.B.; Lima, L.d.L.S.; Chiacchio, A.D. Análise Dos Benefícios Do Desenvolvimento de Vacinas Contra Leishmaniose Em Relação Ao Tratamento Clássico. Contrib. A Las Cienc. Soc. 2024, 17, e7407. [Google Scholar] [CrossRef]
- Pinto-Martinez, A.K.; Rodriguez-Durán, J.; Serrano-Martin, X.; Hernandez-Rodriguez, V.; Benaim, G. Mechanism of Action of Miltefosine on Leishmania donovani Involves the Impairment of Acidocalcisome Function and the Activation of the Sphingosine-Dependent Plasma Membrane Ca2+ Channel. Antimicrob. Agents Chemother. 2018, 62, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Sundar, S.; Chakravarty, J. An Update on Pharmacotherapy for Leishmaniasis. Expert. Opin. Pharmacother. 2015, 16, 237–252. [Google Scholar] [CrossRef]
- Veiga, A.; Albuquerque, K.; Corrêa, M.E.; Brigido, H.; Silva e Silva, J.; Campos, M.; Silveira, F.; Santos, L.; Dolabela, M. Leishmania amazonensis and Leishmania chagasi: In Vitro Leishmanicide Activity of Virola surinamensis (Rol.) Warb. Exp. Parasitol. 2017, 175, 68–73. [Google Scholar] [CrossRef]
- Paes, S.S.; Silva-Silva, J.V.; Portal Gomes, P.W.; Silva, L.O.d.; Costa, A.P.L.d.; Lopes Júnior, M.L.; Hardoim, D.d.J.; Moragas-Tellis, C.J.; Taniwaki, N.N.; Bertho, A.L. (-)-5-Demethoxygrandisin B a New Lignan from Virola surinamensis (Rol.) Warb. Leaves: Evaluation of the Leishmanicidal Activity by In Vitro and In Silico Approaches. Pharmaceutics 2023, 15, 2292. [Google Scholar] [CrossRef]
- Pramanik, P.K.; Chakraborti, S.; Bagchi, A.; Chakraborti, T. Bioassay-Based Corchorus Capsularis L. Leaf-Derived β-Sitosterol Exerts Antileishmanial Effects against Leishmania donovani by Targeting Trypanothione Reductase. Sci. Rep. 2020, 10, 20440. [Google Scholar] [CrossRef]
- Das, A.; Das, M.C.; Das, N.; Bhattacharjee, S. Evaluation of the Antileishmanial Potency, Toxicity and Phytochemical Constituents of Methanol Bark Extract of Sterculia Villosa. Pharm. Biol. 2017, 55, 998–1009. [Google Scholar] [CrossRef]
- da Silva e Silva, J.V.; Cordovil Brigido, H.P.; Oliveira de Albuquerque, K.C.; Miranda Carvalho, J.; Ferreira Reis, J.; Vinhal Faria, L.; Coelho-Ferreira, M.R.; Silveira, F.T.; da Silva Carneiro, A.; Percário, S. Flavopereirine—An Alkaloid Derived from Geissospermum vellosii—Presents Leishmanicidal Activity In Vitro. Molecules 2019, 24, 785. [Google Scholar] [CrossRef]
- Brígido, H.P.C.; Varela, E.L.P.; Gomes, A.R.Q.; Bastos, M.L.C.; de Oliveira Feitosa, A.; do Rosário Marinho, A.M.; Carneiro, L.A.; Coelho-Ferreira, M.R.; Dolabela, M.F.; Percário, S. Evaluation of Acute and Subacute Toxicity of Ethanolic Extract and Fraction of Alkaloids from Bark of Aspidosperma nitidum in Mice. Sci. Rep. 2021, 11, 18283. [Google Scholar] [CrossRef] [PubMed]
- da Veiga, A.d.S.S.; Silveira, F.T.; do Rosario Marinho, A.M.; da Trindade, R.C.d.S.; Campos, M.B.; Dolabela, M.F. Atividade Leishmanicida de Aspidosperma nitidum Benth. Ex Müll. Arg. Res. Soc. Dev. 2021, 10, e50210212646. [Google Scholar] [CrossRef]
- do Socorro Silva da Veiga, A.; Silveira, F.T.; da Silva, E.O.; Júnior, J.A.P.D.; Araújo, S.C.; Campos, M.B.; do Rosário Marinho, A.M.; Brandão, G.C.; Vale, V.V.; Percário, S. Activity of Alkaloids from Aspidosperma nitidum against Leishmania (Leishmania) amazonensis. Sci. Rep. 2022, 12, 8662. [Google Scholar] [CrossRef] [PubMed]
- Brígido, H.P.C.; Varela, E.L.P.; Quadros Gomes, A.R.; Neves Cruz, J.; Correa-Barbosa, J.; Siqueira, J.E.d.S.; Chagas, C.K.S.; Marinho, A.M.d.R.; Almeida Carneiro, L.; Coelho-Ferreira, M.R. Aspidosperma nitidum Reduces Parasite Load and Modulates Cytokines in BALB/c Mice Infected with Leishmania (Leishmania) amazonensis. Front. Chem. 2024, 12, 1492770. [Google Scholar] [CrossRef]
- Geroldinger, G.; Tonner, M.; Fudickar, W.; De Sarkar, S.; Dighal, A.; Monzote, L.; Staniek, K.; Linker, T.; Chatterjee, M.; Gille, L. Activation of Anthracene Endoperoxides in Leishmania and Impairment of Mitochondrial Functions. Molecules 2018, 23, 1680. [Google Scholar] [CrossRef]
- Khazaei, M.; Rahnama, V.; Motazedian, M.H.; Samani, S.M.; Hatam, G. In Vitro Effect of Artemether-Loaded Nanostructured Lipid Carrier (NLC) on Leishmania Infantum. J. Parasit. Dis. 2021, 45, 964–971. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Feng, X.-C.; Lian, L.-H.; Jiang, Y.-Z.; Nan, J.-X. The Protective Effects of Total Saponins from Ornithogalum Saundersiae (Liliaceae) on Acute Hepatic Failure Induced by Lipopolysaccharide and D-Galactosamine in Mice. J. Ethnopharmacol. 2010, 132, 450–455. [Google Scholar]
- Wang, Y.; Xu, P.; Wang, Y.; Liu, H.; Zhou, Y.; Cao, X. The Protection of Salidroside of the Heart against Acute Exhaustive Injury and Molecular Mechanism in Rat. Oxid. Med. Cell Longev. 2013, 2013, 507832. [Google Scholar] [CrossRef]
- Xie, H.; Zhu, D.H. Advance in Studies on Pharmacological Effect of Salidroside on Nervous System Diseases. Zhongguo Zhong Yao Za Zhi 2012, 37, 2505–2509. [Google Scholar]
- Wu, D.; Yuan, P.; Ke, C.; Xiong, H.; Chen, J.; Guo, J.; Lu, M.; Ding, Y.; Fan, X.; Duan, Q. Salidroside Suppresses Solar Ultraviolet-Induced Skin Inflammation by Targeting Cyclooxygenase-2. Oncotarget 2016, 7, 25971. [Google Scholar] [CrossRef]
- Chauhan, K.; Kaur, G.; Kaur, S. Evaluation of Antileishmanial Efficacy of Salidroside against the SSG-Sensitive and Resistant Strain of Leishmania donovani. Parasitol. Int. 2019, 72, 101928. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.K.; Patra, S.; Dubey, V.K. Iridoid Glucosides from Nyctanthes Arbortristis Result in Increased Reactive Oxygen Species and Cellular Redox Homeostasis Imbalance in Leishmania Parasite. Eur. J. Med. Chem. 2012, 54, 49–58. [Google Scholar] [CrossRef]
- Selvendiran, K.; Tong, L.; Bratasz, A.; Kuppusamy, M.L.; Ahmed, S.; Ravi, Y.; Trigg, N.J.; Rivera, B.K.; Kálai, T.; Hideg, K. Anticancer Efficacy of a Difluorodiarylidenyl Piperidone (HO-3867) in Human Ovarian Cancer Cells and Tumor Xenografts. Mol. Cancer Ther. 2010, 9, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Tierney, B.J.; McCann, G.A.; Cohn, D.E.; Eisenhauer, E.; Sudhakar, M.; Kuppusamy, P.; Hideg, K.; Selvendiran, K. HO-3867, a STAT3 Inhibitor Induces Apoptosis by Inactivation of STAT3 Activity in BRCA1-Mutated Ovarian Cancer Cells. Cancer Biol. Ther. 2012, 13, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Rath, K.S.; Naidu, S.K.; Lata, P.; Bid, H.K.; Rivera, B.K.; McCann, G.A.; Tierney, B.J.; ElNaggar, A.C.; Bravo, V.; Leone, G. HO-3867, a Safe STAT3 Inhibitor, Is Selectively Cytotoxic to Ovarian Cancer. Cancer Res. 2014, 74, 2316–2327. [Google Scholar] [CrossRef]
- Madan, E.; Parker, T.M.; Bauer, M.R.; Dhiman, A.; Pelham, C.J.; Nagane, M.; Kuppusamy, M.L.; Holmes, M.; Holmes, T.R.; Shaik, K. The Curcumin Analog HO-3867 Selectively Kills Cancer Cells by Converting Mutant P53 Protein to Transcriptionally Active Wildtype P53. J. Biol. Chem. 2018, 293, 4262–4276. [Google Scholar] [CrossRef]
- Mast, J.M.; Tse, D.; Shee, K.; Lakshmi Kuppusamy, M.; Kmiec, M.M.; Kálai, T.; Kuppusamy, P. Diarylidenylpiperidones, H-4073 and HO-3867, Induce G2/M Cell-Cycle Arrest, Apoptosis and Inhibit STAT3 Phosphorylation in Human Pancreatic Cancer Cells. Cell Biochem. Biophys. 2019, 77, 109–119. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of Curcumin: Problems and Promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Sinjari, B.; Pizzicannella, J.; D’Aurora, M.; Zappacosta, R.; Gatta, V.; Fontana, A.; Trubiani, O.; Diomede, F. Curcumin/Liposome Nanotechnology as Delivery Platform for Anti-Inflammatory Activities via NFkB/ERK/PERK Pathway in Human Dental Pulp Treated with 2-Hydroxyethyl Methacrylate (HEMA). Front. Physiol. 2019, 10, 633. [Google Scholar] [CrossRef]
- Das, A.; Kamran, M.; Ali, N. HO-3867 Induces ROS-Dependent Stress Response and Apoptotic Cell Death in Leishmania donovani. Front. Cell. Infect. Microbiol. 2021, 11, 774899. [Google Scholar] [CrossRef]
| Samples | Antileishmania Activity | Toxicity Profile | SI | Mechanism of Action | |
|---|---|---|---|---|---|
| Promastigote | Amastigote | ||||
| Virola surinamensis (hexanic extract) | IC50 = 86.40 µg/mL (L. chagasi), 79.7 ± 1.3 µg/mL (L. amazonensis) | Inactive | (CC50 > 500 µg/mL) Low toxicity | >5.78 >6.27 | Modulation of oxidative stress and apoptosis in the parasite |
| (-)-5-Demethoxygrandisin B | IC50 = 7.0 µM | IC50 = 26.04 µM | (CC50 = 26.04 µM) High selectivity, low toxicity | 3.7 | Mitochondrial damage, interaction with TryR |
| β-Sitosterol | IC50 = 17.7 ± 0.43 µg/mL | Induction of apoptosis | (>500 µg/mL) Low toxicity | >28.2 | Increased ROS and mitochondrial depolarization |
| Sterculia villosa (methanolic extract) | IC50 = 17.5 µg/mL | DNA fragmentation | Low toxicity | ND | ROS overproduction and oxidative stress |
| Flavopereirine (Geissospermum vellosii) | IC50 = 0.23 µg/mL (24 h) | IC50 = 0.15 µg/mL (72 h) | (CC50 = 499.3 µg/mL) High selectivity | 3328.7 | Inhibition of oligopeptidase B |
| Aspidosperma nitidum (ethanol extract) | IC50 = 23.87 µg/mL | Reduction of parasite load in vivo | (CC50 = 500 µg/mL) in vitro No toxicity in vivo | 21 | Inhibition of trypanothione reductase, apoptosis |
| Aspidosperma nitidum (alkaloidal fraction) | IC50 = 18.5 µg/mL | Reduction of parasite load in vivo | (CC50 = 200 µg/mL) in vitro No toxicity in vivo | 11 | Inhibition of trypanothione reductase, apoptosis |
| Artemether (ART) | IC50 = 16.43 µg/mL | IC50 = 37.12 µg/mL | Reduced toxicity | ND | Interference in mitochondrial phosphorylation |
| Artemether (NLC-ART) | IC50 = 15.42 µg/mL | IC50 = 32.1 µg/mL | Reduced toxicity | ND | Interference in mitochondrial phosphorylation |
| Salidroside (Rhodiola spp.) | Reduction of promastigote growth | Reduction of parasite load | Low liver and kidney toxicity | ND | Modulation of the immune response, increased NO and ROS |
| Iridoid glycosides (Nyctanthes arbortristis) | Induction of apoptosis via oxidative stress | ROS-induced cell death | Low cytotoxicity in normal cells | ND | Mitochondrial oxidative damage, apoptosis |
| HO-3867 (Curcumin analogue) | Cell cycle arrest | Interruption of intracellular charge | Low cytotoxicity to macrophages | ND | Disruption of the STAT3 pathway and activation of apoptotic pathways |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brígido, H.P.C.; dos Santos, L.G.A.; de Barros, R.C.; Correa-Barbosa, J.; Santos, P.V.B.d.; Paz, R.F.L.; Pereira, A.R.; Albuquerque, K.C.O.d.; Campos, M.B.; Silveira, F.T.; et al. The Role of Oxidative Stress in the Pathogenesis and Treatment of Leishmaniasis: Impact on Drug Toxicity and Therapeutic Potential of Natural Products. Toxics 2025, 13, 190. https://doi.org/10.3390/toxics13030190
Brígido HPC, dos Santos LGA, de Barros RC, Correa-Barbosa J, Santos PVBd, Paz RFL, Pereira AR, Albuquerque KCOd, Campos MB, Silveira FT, et al. The Role of Oxidative Stress in the Pathogenesis and Treatment of Leishmaniasis: Impact on Drug Toxicity and Therapeutic Potential of Natural Products. Toxics. 2025; 13(3):190. https://doi.org/10.3390/toxics13030190
Chicago/Turabian StyleBrígido, Heliton Patrick Cordovil, Laís Gabrielly Abreu dos Santos, Renilson Castro de Barros, Juliana Correa-Barbosa, Paulo Victor Barbosa dos Santos, Rayana Franciele Lopes Paz, Amanda Ramos Pereira, Kelly Cristina Oliveira de Albuquerque, Marliane Batista Campos, Fernando Tobias Silveira, and et al. 2025. "The Role of Oxidative Stress in the Pathogenesis and Treatment of Leishmaniasis: Impact on Drug Toxicity and Therapeutic Potential of Natural Products" Toxics 13, no. 3: 190. https://doi.org/10.3390/toxics13030190
APA StyleBrígido, H. P. C., dos Santos, L. G. A., de Barros, R. C., Correa-Barbosa, J., Santos, P. V. B. d., Paz, R. F. L., Pereira, A. R., Albuquerque, K. C. O. d., Campos, M. B., Silveira, F. T., Percário, S., & Dolabela, M. F. (2025). The Role of Oxidative Stress in the Pathogenesis and Treatment of Leishmaniasis: Impact on Drug Toxicity and Therapeutic Potential of Natural Products. Toxics, 13(3), 190. https://doi.org/10.3390/toxics13030190








