Chemical Risks, Genotoxicity, and Oxidative Stress in Healthcare Workers
Abstract
1. Introduction
2. Materials and Methods
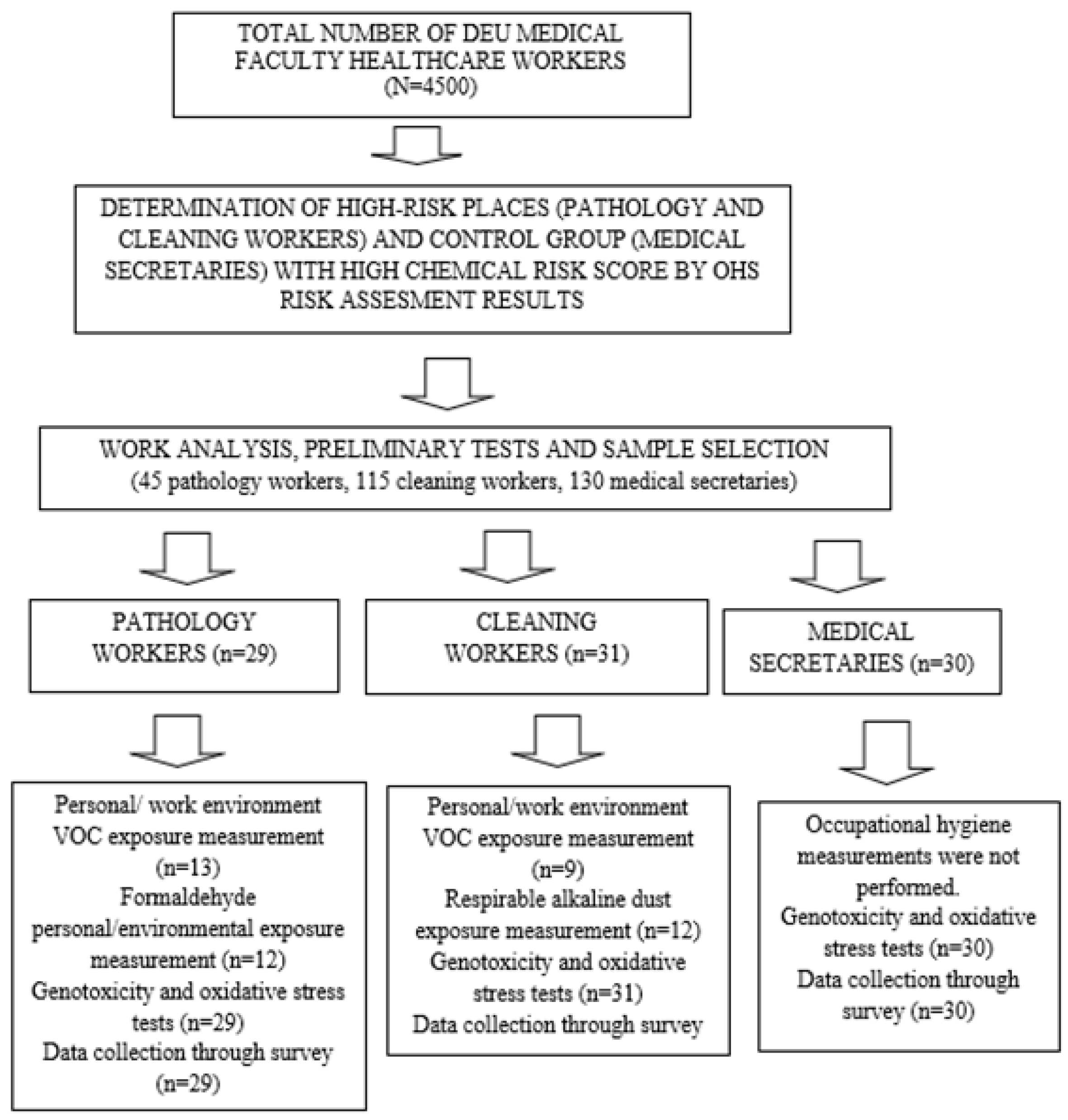
2.1. Population and Sample Selection
2.2. Sample Selection, Inclusion, and Exclusion Criteria
2.3. Occupational Hygiene Measurements [VOCs and Alkaline Dust Measurement]
2.4. Genotoxicity Tests
2.4.1. Buccal MN Method
Collection and Analysis of Exfoliated Buccal Cells
Alkaline Comet Assay
Oxidative Stress Tests
Collection and Analysis of Oxidative Stress Indicators
2.5. Statistics
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| VOC | Volatile Organic Compounds |
| ROS | Reactive oxygen species |
| MN | Micronucleus |
| NIOSH | National Institute for Occupational Safety and Health |
| PAH | Polycyclic aromatic hydrocarbons |
| IARC | International Agency for Research on Cancer |
References
- NIOSH. Chemical Hazards for Healthcare Workers. Available online: https://www.cdc.gov/niosh/healthcare/index.html (accessed on 22 November 2024).
- Beyan, A.; Demiral, Y. Mesleksel Uçucu Organik Bileşik Maruz Kalımı ve Genototoksisite. Çalışma Ve Toplum. 2021, 3, 1901–1916. [Google Scholar]
- Ahmad, I.M.; Abdalla, M.Y.; Moore, T.A.; Bartenhagen, L.; Case, A.J.; Zimmerman, M.C. Healthcare Workers Occupationally Exposed to Ionizing Radiation Exhibit Altered Levels of Inflammatory Cytokines and Redox Parameters. Antioxidants 2019, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, D.; Tranfo, G.; Ursini, C.L.; Fresegna, A.M.; Ciervo, A.; Maiello, R.; Paci, E.; Pigini, D.; Gherardi, M.; Gatto, M.P.; et al. Biomarkers of early genotoxicity and oxidative stress for occupational risk assessment of exposure to styrene in the fibreglass reinforced plastic industry. Toxicol. Lett. 2018, 298, 53–59. [Google Scholar] [CrossRef]
- Al Zabadi, H.; Ferrari, L.; Laurent, A.M.; Tiberguent, A.; Paris, C.; Zmirou-Navier, D. Biomonitoring of complex occupational exposures to carcinogens: The case of sewage workers in Paris. BMC Cancer 2008, 8, 67. [Google Scholar] [CrossRef]
- Chang, F.-K.; Mao, I.-F.; Chen, M.-L.; Cheng, S.-F. Urinary 8-Hydroxydeoxyguanosine as a Biomarker of Oxidative DNA Damage in Workers Exposed to Ethylbenzene. Ann. Occup. Hyg. 2011, 55, 519–525. [Google Scholar] [PubMed]
- Lamplugh, A.; Harries, M.; Xiang, F.; Trinh, J.; Hecobian, A.; Montoya, L.D. Occupational exposure to volatile organic compounds and health risks in Colorado nail salons. Environ. Pollut. 2019, 249, 518–526. [Google Scholar] [CrossRef]
- Souza, K.M.; Braz, L.G.; Nogueira, F.R.; Souza, M.B.; Bincoleto, L.F.; Aun, A.G.; Corrente, J.E.; Carvalho, L.R.; Braz, J.R.C.; Braz, M.G. Occupational exposure to anesthetics leads to genomic instability, cytotoxicity and proliferative changes. Mutat. Res. Mol. Mech. Mutagen. 2016, 791-792, 42–48. [Google Scholar] [CrossRef]
- Londoño-Velasco, E.; Martínez-Perafán, F.; Carvajal-Varona, S.; García-Vallejo, F.; Hoyos-Giraldo, L.S. Assessment of DNA damage in car spray painters exposed to organic solvents by the high-throughput comet assay. Toxicol. Mech. Methods 2016, 26, 238–242. [Google Scholar] [CrossRef]
- Aktaş-Şüküroglu, S.B. Occupational exposure to the chemicals in hairdressing salons and health risk. Türk Hij. Deney. Biyol. Derg. 2018, 75, 195–212. [Google Scholar] [CrossRef]
- Sisto, R.; Cavallo, D.; Ursini, C.L.; Fresegna, A.M.; Ciervo, A.; Maiello, R.; Paci, E.; Pigini, D.; Gherardi, M.; Gordiani, A.; et al. Direct and Oxidative DNA Damage in a Group of Painters Exposed to VOCs: Dose—Response Relationship. Front. Public Health 2020, 1, 445. [Google Scholar] [CrossRef]
- Wrońska-Nofer, T.; Palus, J.; Krajewski, W.; Jajte, J.; Kucharska, M.; Stetkiewicz, J.; Wąsowicz, W.; Rydzyński, K. DNA damage induced by nitrous oxide: Study in medical personnel of operating rooms. Mutat. Res. Mol. Mech. Mutagen. 2009, 666, 39–43. [Google Scholar]
- Ursini, C.L.; Cavallo, D.; Colombi, A.; Giglio, M.; Marinaccio, A.; Iavicoli, S. Evaluation of early DNA damage in healthcare workers handling antineoplastic drugs. Int. Arch. Occup. Environ. Health 2006, 80, 134–140. [Google Scholar] [PubMed]
- Dokuz Eylül Üniversitesi. Dokuz Eylül Üniversitesi Risk Yönetimi Yönergesi. 2014. Available online: https://strateji.deu.edu.tr/wp-content/uploads/2014/12/Dokuz-Eyl%C3%BCl-%C3%9Cniversitesi-Risk-Y%C3%B6netimi-Y%C3%B6nergesi.pdf (accessed on 22 November 2024).
- Republic of Turkey Ministry of Family, Labor and Social Services. Regulation on Health and Safety Measures in Working with Chemical Substances. Ankara. Available online: https://www.mevzuat.gov.tr/mevzuat?MevzuatNo=18709&MevzuatTur=7&MevzuatTertip=5 (accessed on 22 November 2024).
- NIOSH Pocket Guide to Chemical Hazards; US Department of Health and Human Services, Centers for Disease Control and Prevention: Cincinnati, OH, USA, 2007.
- ISO 16200-2:2000; Workplace Air Quality-Sampling and Analysis of Volatile Organic Compounds by Solvent Desorption/Gas Chromatography. ISO: Geneva, Switzerland, 2000.
- NIOSH Manual of Analytical Methods; US Department of Health and Human Services, Centers for Disease Control and Prevention: Cincinnati, OH, USA, 2020.
- Bolognesi, C.; Knasmueller, S.; Nersesyan, A.; Thomas, P.; Fenech, M. The HUMNxl scoring criteria for different cell types and nuclear anomalies in the buccal micronucleus cytome assay—An update and expanded photogallery. Mutat. Res. 2013, 753, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Dizdaroglu, M.; Jaruga, P.; Birincioglu, M.; Rodriguez, H. Free radical-induced damage to DNA: Mechanisms and measurement. Free Radic. Biol. Med. 2002, 32, 1102–1115. [Google Scholar]
- Reddy, P.T.; Jaruga, P.; Kirkali, G.; Tuna, G.; Nelson, B.C.; Dizdaroglu, M. Identification and quantification of human DNA repair protein NEIL1 by liquid chromatography/isotope-dilution tandem mass spectrometry. J. Proteome Res. 2013, 12, 1049–1061. [Google Scholar]
- Peeters, W.; Peng, Z. An approach towards global standardization of the risk matrix. J. Space Saf. Eng. 2015, 2, 31–38. [Google Scholar]
- IARC. IARC Monographs on the Identification of Carcinogenic Hazards to Humans. 2024. Available online: https://publications.iarc.fr/Book-And-Report-Series/Iarc-Monographs-On-The-Identification-Of-Carcinogenic-Hazards-To-Humans (accessed on 24 February 2024).
- Kramer, A.; Kramer, K.Z. The potential impact of the Covid-19 pandemic on occupational status, work from home, and occupational mobility. J. Vocat. Behav. 2020, 119, 103442. [Google Scholar] [CrossRef]
- Kasi, V.; Elango, N.; Ananth, S.; Vembhu, B.; Poornima, J.G. Occupational exposure to photocopiers and their toners cause genotoxicity. Hum. Exp. Toxicol. 2018, 37, 205–217. [Google Scholar] [CrossRef]
- Peteffi, G.P.; da Silva, L.B.; Antunes, M.V.; Wilhelm, C.; Valandro, E.T.; Glaeser, J.; Kaefer, D.; Linden, R. Evaluation of genotoxicity in workers exposed to low levels of formaldehyde in a furniture manufacturing facility. Toxicol. Ind. Health 2016, 32, 1763–1773. [Google Scholar]
- Everatt, R.; Slapšyte, G.; Mierauskiene, J.; Dedonyte, V.; Bakiene, L. Biomonitoring study of dry cleaning workers using cytogenetic tests and the comet assay. J. Occup. Environ. Hyg. 2013, 10, 609–621. [Google Scholar]
- Akhlaghi, G.; Shahsavari, F.; Ghorbanpour, M. Formalin Induced Micronucleus Formation in the Buccal Mucosa of Pathology Laboratory Workers. Iran. J. Pathol. 2023, 18, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Jannuzzi, A.T.; Alpertunga, B. Evaluation of DNA damage and DNA repair capacity in occupationally lead-exposed workers. Toxicol. Ind. Health 2016, 32, 1859–1865. [Google Scholar] [CrossRef] [PubMed]
- Feinendegen, L.E. Evidence for beneficial low level radiation effects and radiation hormesis. Br. J. Radiol. 2005, 78, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Oltulu, C.; Devecïoglu, T.Y.; Akïncï, M.; Olmez, S.G.A.; Obeïdïn, S.V.; Beceren, A. Evaluation of Genotoxicity Risk in Health Care Workers Exposed to Antineoplastic Drugs. Clin. Exp. Health Sci. 2019, 9, 166–170. [Google Scholar] [CrossRef]
- de Aquino, T.; Zenkner, F.F.; Ellwanger, J.H.; Prá, D.; Rieger, A. DNA damage and cytotoxicity in pathology laboratory technicians exposed to organic solvents. An. Acad. Bras. Cienc. 2016, 88, 227–236. [Google Scholar] [CrossRef]
| All Workers (n:90) (%) | Pathology Worker (n = 29) (%) | Cleaning Worker (n = 31) (%) | Medical Secretary (n = 30) (%) | p | |
|---|---|---|---|---|---|
| Age (years) Mean ± SD (min–max) Median | 40.9 ± 8.7 (22.0–59.0) 40.0 | 40.1 ± 8.3 (27.0–59.0) 39.0 | 41.2 ± 9.6 (22.0–55.0) 43.0 | 41.3 ± 8.5 (25.0–55.0) 43.0 | 0.590 * |
| Gender Female Male | 75 (83.3) 15 (16.7) | 21 (72.4) 8 (27.6) | 27 (87.1) 4 (12.9) | 27 (90.0) 3 (10.0) | 0.100 ** |
| Smoking Yes No | 47 (52.8) 11 (12.4) | 9 (31.0) 20 (69.0) | 10 (32.3) 21 (67.7) | 12 (41.4) 17 (58.6) | 0.700 ** |
| Alcohol use Yes No | 42 (47.8) 47 (52.2) | 18 (62.1) 11 (37.9) | 8 (25.8) *** 23 (74.2) | 16 (53.3) 14 (46.7) | 0.013 ** |
| Chronic disease Yes No | 45 (50.0) 44 (48.9) | 16 (55.2) 13 (44.8) | 17 (54.8) 14 (45.2) | 12 (40.0) 18 (60.0) | 0.400 ** |
| Doing sports Yes No | 17 (18.9) 73 (81.1) | 11 (37.9) *** 18 (62.1) | 2 (6.5) 29 (93.5) | 4 (13.3) 26 (86.7) | 0.005 ** |
| Cigarettes (pack*year) Mean ± SD (min–max) Median | 8.1 ± 7.4 (0.6–31.0) 5.1 | 8.6 ± 7.4 (1.0–22.5) 6.5 | 8.9 ± 9.6 (0.5–30.0) 4.3 | 6.4 ± 5.3 (0.1–15.0) 7.5 | 0.727 * |
| Year of work (year) Mean ± SD (min–max) Median | 10.4 ± 8.3 (2.0–38.0) 10.4 | 11.3 ± 10.6 (2.0–19.5) 10.0 | 6.1 ± 5.9 (1.0–10.0) 5.0 | 7.7 ± 7.1 (1.0–25.0) 5.5 | 0.200 * |
| Weekly working time (hours) Mean ± SD (min–max) Median | 41.6 ± 7.8 (8.0–60.0) 45.0 | 42.3 ± 8.1 (090–60.0) 45.0 | 41.7 ± 8.8 (10.0–48.0) 45.0 | 40.7 ± 6.7 (8.0–45.0) 40.0 | 0.100 * |
| Use of latex gloves Yes No | 53 (58.9) 36 (40.0) | 25 (86.2) 4 (13.8) | 27 (87.1) 4 (12.9) | 1 (3.3) 29 (96.7) ** | <0.001 * |
| Use of non-latex gloves Yes No | 13 (14.4) 77 (86.5) | 5 (17.2) 24 (82.8) | 8 (25.8) 23 (74.2) | 0 (0.0) ** 30 (100.0) | 0.014 * |
| Mask use Yes No | 82 (91.1) 8 (8.9) | 25 (86.2) 4 (13.8) | 28 (90.3) 3 (9.7) | 29 (96.7) 1 (3.3) | 0.400 * |
| Apron use Yes No | 30 (33.3) 60 (66.7) | 19 (65.5) ** 10 (34.5) | 10 (32.3) ** 21 (67.7) | 1 (3.3) ** 29 (96.7) | <0.001 * |
| n * | Mean ± SD | (Min–Max) | Median (1st–3rd Quart) | IARC Class of the Chemical |
|---|---|---|---|---|
| Isopropylbenzene (n = 13) | 1.0 ± 1.3 | (0.4–5.1) | 0.5 (0.5–0.9) | 2B |
| Toluene (n = 13) | 2.7 ± 4.2 | (0.4–16.4) | 1.9 (0.9–2.4) | 3 |
| 2chlorotoluene (n = 13) | 0.9 ± 0.2 | (0.6–1.2) | 0.8 (0.7–1.0) | 3 |
| Trichlorobenzene (n = 13) | 0.9 ± 0.1 | (0.7–1.3) | 0.7 (0.8–1.0) | not listed by IARC |
| Oxylene (n = 13) | 693.4 ± 2417.1 | (3.7–8737.2) | 16.3 (5.3–24.0) | 3 |
| Pmxylene (n = 13) | 1858.0 ± 6354.7 | (20.7–230.1) | 103.4 (47.7–154.1) | 3 |
| Ethanol (n = 11) | 29.7 ± 3.5 | (23.3–34.4) | 30.6 (27.6–33.0) | 1 |
| Dichlorobenzene (n = 11) | 0.9 ± 0.1 | (0.7–1.2) | 0.9 (0.7–0.9) | 2B |
| Tertbutylbenzene (n = 9) | 2.9 ± 0.3 | (2.4–3.4) | 2.8 (2.6–3.2) | not listed by IARC |
| Methanol (n = 9) | 7.5 ± 4.5 | (2.2–15.1) | 5.7 (4.8–11.2) | not listed by IARC |
| Formaldehyde-environmental (n = 8) | 0.02 ± 0.01 | (0.01–0.05) | 0.01 (0.01–0.04) | 1 |
| Formaldehyde-personal (n = 4) | 0.03 ± 0.01 | (0.02–0.05) | 0.03 (0.01–0.05) | 1 |
| Ethylbenzene (n = 1) | 1.6 | 1.6 | 1.6 | 2B |
| Occupational Hygiene Measurements Personal measurement Work environment measurement | 6 7 | - | ||
| Number of Measurements on PEL ** | 3 |
| n | Mean ± SD | (Min–Max) | Median (1st–3rd Quart) | IARC Class of the Chemical |
|---|---|---|---|---|
| Alkali Dust (n = 12) | 1.4 ± 0.3 | (0.8–1.8) | 1.3 (1.1–1.6) | not listed by IARC |
| Isopropylbenzene (n = 9) | 0.6 ± 0.2 | (0.4–0.8) | 0.6 (0.4–0.8) | 2B |
| Toluene (n = 9) | 1.2 ± 0.8 | (0.2–2.4) | 1.2 (0.3–2) | 3 |
| Dichlorobenzene (n = 9) | 1.1 ± 1.1 | (0.4–3.8) | 0.7 (0.6–1.3) | 2B |
| Pmxylene (n = 8) | 72.9 ± 65.3 | (6.6–174.2) | 66.5 (7.8–127.1) | 3 |
| 2chlorotoluene (n = 7) | 0.8 ± 0.1 | (0.6–0.9) | 0.8 (0.7–0.9) | 3 |
| Trichlorobenzene (n = 7) | 0.8 ± 0.05 | (0.7–0.9) | 0.8 (0.8–0.8) | not listed by IARC |
| Oxylene (n = 6) | 5.9 ± 3.5 | (3.4–12.2) | 4.1 (3.6–8.8) | 3 |
| Tertbutylbenzene (n = 4) | 2.5 ± 0.1 | (2.3–2.7) | 2.5 (2.4–2.7) | not listed by IARC |
| Ethanol (n = 4) | 27.1 ± 5.4 | (24.1–35.1) | 24.5 (24.2–32.5) | 1 |
| Methanol (n = 3) | 2.2 ± 0.4 | (1.8–2.6) | 2.2 (1.8–) | not listed by IARC |
| Ethylbenzene (n = 1) | 1.1 | 1.1 | 1.1 | 2B |
| Occupational Hygiene Measurements VOC measurement Alkali dust measurement | 9 12 | |||
| Number of Measurements on PEL * | - | - |
| Whole Group (N:90) | 8-OH-dG in Urine (nmol/mmol Creatinine) Mean ± SD (min–max) Median | p | S-cdA in Urine (nmol/mmol Creatinine) Mean ± SD (min–max) Median | p | R-cdA in Urine (nmol/mmol Creatinine) Mean ± SD (min–max) Median | p | Tail Intensity% Mean ± SD (min–max) Median | p | MN Frequency (MN/1000 Cells) Mean ± SD (min–max) Median | p | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Exposure status | Exposure group (n = 60) | 2.33 ± 2.20 (0.31–17.61) 1.91 | 0.306 * | 0.04 ± 0.03 (0.00–0.21) 0.04 | 0.849 * | 0.06 ± 0.05 (0.00–0.33) 0.05 | 0.659 * | 17.67 ± 9.15 (3.43–44.23) 16.92 | 0.053 * | 2.00 ± 1.82 2.00 (0.00–8.00) | 0.279 * |
| Control group (n = 30) | 2.31 ± 0.93 (0.97–5.03) 2.21 | 0.04 ± 0.03 (0.00–0.11) 0.04 | 0.05 ± 0.02 (0.02–0.10) 0.05 | 14.14 ± 6.52 (5.65–31.62) 11.91 | 1.46 ± 1.25 (0.00–8.00) 1.00 | ||||||
| Gender | Female (n = 75) | 2.38 ± 2.01 (0.31–17.61) 2.19 | 0.555 * | 0.04 ± 0.03 (0.00–0.21) 0.04 | 0.001 * | 0.06 ± 0.04 (0.00–0.33) 0.05 | 0.772 * | 16.30 ± 7.99 (3.43–44.23) 15.38 | 0.987 * | 1.70 ± 1.46 (0.00–7.00) 2.00 | 0.494 * |
| Male (n = 25) | 2.05 ± 0.87 (1.17–4.18) 1.91 | 0.02 ± 0.01 (0.00–0.06) 0.02 | 0.06 ± 0.04 (0.02–0.16) 0.05 | 17.45 ± 10.95 (4.21–36.63) 14.83 | 2.40 ± 2.41 (0.00–8.00) 1.00 | ||||||
| Age group | under 40 (n = 35) | 2.65 ± 2.75 (0.88–17.61) 2.16 | 0.484 * | 0.04 ± 0.03 (0.01–0.21) 0.04 | 0.890 * | 0.07 ± 0.06 (0.02–0.33) 0.05 | 0.564 * | 14.55 ± 7.74 (3.57–35.12) 12.97 | 0.095 * | 1.68 ± 1.65 (0.00–8.00) 1.00 | 0.500 * |
| 40 and above (n = 55) | 2.12 ± 0.93 (0.31–5.32) 1.93 | 0.04 ± 0.03 (0.00–0.16) 0.04 | 0.05 ± 0.03 (0.00–0.17) 0.05 | 17.37 ± 8.78 (3.43–44.23) 16.32 | 1.90 ± 1.68 (0.00–7.00) 2.00 | ||||||
| Glove use | Yes (n = 53) | 2.34 ± 2.32 (0.31–17.61) 1.91 | 0.233 * | 0.04 ± 0.03 (0.00–0.21) 0.04 | 0.385 * | 0.06 ± 0.05 (0.00–0.33) 0.05 | 0.256 * | 17.37 ± 9.38 (3.43–44.23) 16.46 | 0.281 * | 2.01 ± 1.87 (0.00–8.00) 2.00 | 0.371 * |
| No (n = 37) | 2.31 ± 0.93 (0.97–5.03) 2.19 | 0.04 ± 0.03 (0.00–0.11) 0.04 | 0.06 ± 0.03 (0.02–0.17) 0.05 | 15.24 ± 6.97 (5.65–31.62) 12.19 | 1.54 ± 1.30 (0.00–5.00) 1.00 | ||||||
| Mask use | Yes (n = 82) | 2.38 ± 1.94 (0.31–17.61) 2.17 | 0.275 * | 0.04 ± 0.03 (0.00–0.21) 0.04 | 0.726 * | 0.06 ± 0.04 (0.00–0.33) 0.05 | 0.251 * | 16.28 ± 8.36 (3.43–44.23) 14.98 | 0.514 * | 1.74 ± 1.61 (0.00–8.00) 1.00 | 0.186 * |
| No (n = 8) | 1.80 ± 0.50 (1.19–2.79) 1.73 | 0.04 ± 0.03 (0.02–0.11) 0.04 | 0.05 ± 0.04 (0.02–0.16) 0.03 | 18.70 ± 10.10 (7.13–36.63) 18.24 | 2.62 ± 2.06 (0.00–6.00) 2.50 | ||||||
| Apron use | Yes (n = 30) | 2.03 ± 0.97 (0.31–5.32) 1.84 | 0.290 * | 0.04 ± 0.02 (0.01–0.12) 0.04 | 0.546 * | 0.05 ± 0.04 (0.00–0.27) 0.05 | 0.043 * | 14.87 ± 9.72 (3.43–35.12) 12.78 | 0.108 * | 1.75 ± 1.80 (0.00–8.00) 1.00 | 0.616 * |
| No (n = 60) | 2.47 ± 2.17 (0.94–17.61) 2.20 | 0.04 ± 0.03 (0.00–0.21) 0.04 | 0.06 ± 0.04 (0.02–0.33) 0.05 | 17.31 ± 7.77 (5.62–44.23) 15.85 | 1.85 ± 1.61 (0.00–7.00) 2.00 | ||||||
| Presence of chronic disease | Yes (n = 32) | 2.16 ± 0.98 (0.31–5.32) 2.02 | 0.326 * | 0.04 ± 0.03 (0.00–0.16) 0.04 | 0.288 * | 0.06 ± 0.05 (0.00–0.27) 0.05 | 0.621 * | 17.19 ± 9.27 (4.21–44.23) 16.46 | 0.617 * | 1.86 ± 1.67 (0.00–8.00) 2.00 | 0.669 * |
| No (n = 58) | 2.49 ± 2.45 (0.94–17.61) 1.92 | 0.04 ± 0.03 (0.00–0.21) 0.04 | 0.06 ± 0.04 (0.02–0.33) 0.05 | 15.86 ± 7.75 (3.43–36.63) 14.98 | 1.77 ± 1.69 (0.00–7.00) 1.00 | ||||||
| Continuous drug use | Yes (n = 42) | 2.05 ± 0.95 (0.31–5.32) 1.86 | 0.872 * | 0.04 ± 0.03 (0.00–0.16) 0.03 | 0.386 * | 0.06 ± 0.05 (0.00–0.27) 0.05 | 0.958 * | 17.18 ± 9.13 (4.53–44.23) 15.88 | 0.673 * | 1.71 ± 1.41 (0.00–4.00) 2.00 | 0.993 * |
| No (n = 48) | 2.48 ± 2.21 (0.88–17.61) 2.24 | 0.04 ± 0.03 (0.00–0.21) 0.04 | 0.06 ± 0.04 (0.02–0.33) 0.05 | 16.12 ± 8.17 (3.43–36.63) 14.98 | 1.87 ± 1.80 (0.00–8.00) 1.00 | ||||||
| Doing sports | Yes (n = 17) | 2.04 ± 0.75 (1.17–3.86) 1.86 | 0.610 * | 0.03 ± 0.02 (0.02–0.11) 0.03 | 0.321 * | 0.07 ± 0.06 (0.02–0.27) 0.05 | 0.241 * | 14.69 ± 7.71 (5.65–35.12) 12.60 | 0.330 * | 2.29 ± 2.49 (0.00–8.00) 1.00 | 0.785 * |
| No (n = 73) | 2.39 ± 2.04 (0.31–17.61) 2.02 | 0.04 ± 0.03 (0.00–0.21) 0.04 | 0.06 ± 0.04 (0.00–0.33) 0.05 | 16.92 ± 8.66 (3.43–44.23) 15.44 | 1.70 ± 1.40 (0.00–6.00) 2.00 | ||||||
| Alcohol use | Yes | 2.61 ± 2.54 (1.01–17.61) 2.24 | 0.302 * | 0.04 ± 0.03 (0.00–0.21) 0.04 | 0.568 * | 0.06 ± 0.06 (0.02–0.33) 0.05 | 0.589 * | 17.26 ± 9.02 (3.43–44.23) 15.29 | 0.518 * | 2.02 ± 2.05 (0.00–8.00) 1.00 | 0.833 * |
| No | 2.07 ± 0.91 (0.31–4.18) 1.89 | 0.04 ± 0.02 (0.00–0.16) 0.04 | 0.06 ± 0.03 (0.00–0.17) 0.05 | 15.83 ± 8.03 (3.57–34.60) 15.04 | 1.63 ± 1.22 (0.00–5.00) 2.00 | ||||||
| Smoking | Yes (n = 31) | 2.68 ± 2.88 (1.19–17.61) 2.24 | 0.627 ** | 0.06 ± 0.04 (0.01–0.21) 0.05 | 0.006 ** | 0.06 ± 0.05 (0.02–0.33) 0.05 | 0.996 ** | 16.45 ± 7.51 (3.43–36.63) 15.38 | 0.481 ** | 2.30 ± 2.05 (0.00–8.00) 2.00 | 0.155 ** |
| No (n = 48) | 2.10 ± 0.91 (0.88–5.03) 1.91 | 0.03 ± 0.02 (0.00–0.16) 0.04 | 0.06 ± 0.02 (0.00–0.16) 0.04 | 15.68 ± 7.95 (3.57–31.62) 14.83 | 1.46 ± 1.34 (0.00–5.00) 1.00 | ||||||
| Quit (n = 11) | 2.36 ± 1.26 (0.31–5.32) 2.37 | 0.03 ± 0.03 (0.00–0.11) 0.02 | 0.06 ± 0.04 (0.00–0.16) 0.05 | 20.80 ± 12.35 (4.53–44.23) 20.21 | 2.09 ± 1.57 (0.00–4.00) 2.00 | ||||||
| Working Years | <5 years (n =34) | 2.62 ± 2.78 (0.88–17.61) 2.19 | 0.647 * | 0.05 ± 0.04 (0.00–0.21) 0.04 | 0.074 * | 0.06 ± 0.05 (0.02–0.33) 0.05 | 0.431 * | 17.17 ± 7.49 (3.57–31.62) 16.36 | 0.314 * | 1.94 ± 1.63 (0.00–7.00) 1.50 | 0.509 * |
| >5 years (n = 56) | 2.15 ± 0.96 (0.31–5.32) 1.92 | 0.04 ± 0.02 (0.00–0.16) 0.04 | 0.06 ± 0.04 (0.00–0.27) 0.05 | 16.08 ± 9.09 (3.43–44.23) 12.83 | 1.74 ± 1.70 (0.00–8.00) 2.00 | ||||||
| Pathology Worker (n = 29) Mean ± SD (Min–Max) Median | Cleaning Worker (n = 31) Mean ± SD (Min–Max) Median | Medical Secretary (n = 30) Mean ± SD (Min–Max) Median | |
|---|---|---|---|
| 8-OH-dG in urine (nmol/mmol creatinine) | 2.4 ± 3.1 (0.3–17.6) 1.7 | 2.2 ± 0.8 (0.9–4.0) 2.0 | 2.3 ± 0.9 (0.9–5.0) 2.2 |
| S-cdA in urine (nmol/mmol creatinine) | 0.04 ± 0.03 (0–0.2) 0.03 | 0.04 ± 0.03 (0.01–0.2) 0.04 | 0.04 ± 0.03 (0.0–0.1) 0.04 |
| R-cdA in urine (nmol/mmol creatinine) | 0.06 ± 0.07 (0–0.3) ** 0.05 | 0.07 ± 0.03 (0.02–0.17) 0.06 | 0.05 ± 0.02 (0.02–0.1) 0.05 |
| Tail intensity % | 16.3 ± 10.7 (3.4–44.2) 14.8 | 18.9 ± 7.4 (3.6–0.1) ** 20.2 | 14.1 ± 6.5 (5.6–31.6) 11.9 |
| MN Frequency (MN/1000 cells) | 2.3 ± 2.2 (0.0–8.0) 2.0 | 1.7 ± 1.3 (0.0–5.0) 2.0 | 1.5 ± 1.2 (0.0–5.0) 1.0 |
| Exposure Group (n = 60) | Control Group (n = 30) | p * | |
|---|---|---|---|
| 8-OH-dG in urine (nmol/mmol) Mean ± SD (min–max) Median | 2.33 ± 2.20 (0.31–17.61) 1.91 | 2.31 ± 0.93 (0.97–5.03) 2.21 | 0.306 |
| S-cdA in urine (nmol/mmol) Mean ± SD (min–max) Median | 0.04 ± 0.03 (0.00–0.21) 0.04 | 0.04 ± 0.03 (0.00–0.11) 0.04 | 0.849 |
| R-cdA in urine (nmol/mmol) Mean ± SD (min–max) Median | 0.06 ± 0.05 (0.00–0.33) 0.05 | 0.05 ± 0.02 (0.02–0.10) 0.05 | 0.659 |
| Tail intensity % | 17.67 ± 9.15 (3.43–44.23) 16.92 | 14.14 ± 6.52 (5.65–31.62) 11.91 | 0.053 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beyan, A.C.; Emerce, E.; Tuna, G.; İşlekel, G.H. Chemical Risks, Genotoxicity, and Oxidative Stress in Healthcare Workers. Toxics 2025, 13, 189. https://doi.org/10.3390/toxics13030189
Beyan AC, Emerce E, Tuna G, İşlekel GH. Chemical Risks, Genotoxicity, and Oxidative Stress in Healthcare Workers. Toxics. 2025; 13(3):189. https://doi.org/10.3390/toxics13030189
Chicago/Turabian StyleBeyan, Ayşe Coşkun, Esra Emerce, Gamze Tuna, and Gül Hüray İşlekel. 2025. "Chemical Risks, Genotoxicity, and Oxidative Stress in Healthcare Workers" Toxics 13, no. 3: 189. https://doi.org/10.3390/toxics13030189
APA StyleBeyan, A. C., Emerce, E., Tuna, G., & İşlekel, G. H. (2025). Chemical Risks, Genotoxicity, and Oxidative Stress in Healthcare Workers. Toxics, 13(3), 189. https://doi.org/10.3390/toxics13030189






