Abstract
Background: Acorus calamus (sweet flag) is widely used in traditional medicine, yet its dermal safety profile remains insufficiently defined under modern regulatory standards. Objective: To comprehensively evaluate the skin irritation, corrosion, and sensitisation potential of A. calamus rhizome oil using new approach methodologies’ (NAMs) test batteries under GLP conditions. Results: The A. calamus rhizome oil was predicted as a Category 2 skin irritant, non-corrosive and GHS Category 1B skin sensitiser. Chemical analysis revealed β-asarone as the major constituent (~40.75%). The reconstructed human epidermis models established reversible irritation without corrosion. Mechanistic concordance across the Direct Peptide Reactivity Assay, KeratinoSens™, and Human Cell Line Activation Test showed activation of the three key events of the skin sensitisation adverse outcome pathway. Using the “2-out-of-3” Defined Approach with the KE 3/1 sequential strategy allowed for hazard classification into GHS Category 1B. Quantitative risk modelling using SARA-ICE models and SCCS parameters yielded conservative safe-use concentrations ranging from 0.13 to 0.78% (w/w) for leave-on products and up to 7.46% (w/w) for rinse-off formulations. Conclusions: The combined evidence from the NAM-based assays showed that A. calamus rhizome oil is a moderate sensitiser and irritant but not corrosive, providing critical data for risk assessment and regulatory decision-making, which was previously unknown. The SARA-ICE PoD-derived safe-use concentrations provide guidance for cosmetic formulators to ensure consumer safety, particularly in leave-on applications such as face and hand creams, where sensitisation risk is highest. This study demonstrates the utility of NAMs for botanical safety assessment and regulatory decision-making.
1. Introduction
Acorus calamus, commonly known as calamus, sweet flag, or Pillai Marunthu, is widely recognised for its diverse medicinal properties, attributed primarily to the oil extracted from its rhizome []. This plant has a vast record of herbal uses []. The oil extracted from the rhizome has been traditionally used to treat diseases such as mental disorders, epilepsy, dysentery, chronic diarrhoea, abdominal tumours, fever, kidney and liver issues, and rheumatism [,]. Additionally, the mature leaves of A. calamus possess anthelmintic, antifungal, insecticidal, and antibacterial properties, further emphasising its therapeutic significance [,,]. Beyond its medicinal uses, rhizome oil finds applications in the pharmaceutical industry []. Moreover, lectins in the rhizomes exhibit mitogenic activity []. A. calamus is also taken for a range of health conditions such as oral and throat diseases, epilepsy, bronchitis, fevers, delirium, tumours, hysteria, excessive thirst, memory impairment, rat bites, earworms, general fatigue, tooth pain, chest and kidney discomfort, asthma, diarrhoea, dysentery, flatulence, dyspepsia, chronic ulcers, and rheumatism []. It is also used as a remedy for snakebites and remittent fever []. Inhalation of the oil may alleviate low-grade fever and dyspepsia and enhance vocal clarity. Traditional use of A. calamus for colic and diarrhoea is supported by its calcium channel blocking activity demonstrated in rabbit jejunum [,].
Plant-based materials, despite their therapeutic benefits, may lead to adverse dermal reactions such as skin irritation, sensitisation, phototoxicity, and allergic responses []. Herbal ingredients in cosmetics, pharmaceutical formulations, and over-the-counter products are often associated with allergic contact dermatitis, a significant dermatological condition caused by chemicals interacting with living cells [,]. Results of published studies show that indeed, some herbs have toxic effects, with major hepatotoxic herbs being Cimicifuga racemosa, Larrea tridentata, Teucrium chamaedrys, Scutellaria lateriflora, and Scutellaria baicalensis, etc. [,]. Different herbal medicines also affect the heart, which comprises drugs obtained from plants such as Digitalis purpurea []. Several common plants used in herbal medicines have probable neurotoxic effects, e.g., Papaver somniferum (opium) []. This raises concerns about the potential toxic effects from both short-term and long-term use of these plants. Therefore, the evaluation of the toxicological outcomes of any herbal extract proposed to be used in humans is of paramount significance. Phytochemical characterisation, comprising the plant source, data on contamination, adulteration, and hazardous residues, is the critical issue in the safety assessment of plant materials in personal care products. The Defined Approach (DA), combining the Direct Peptide Reactivity Assay (DPRA), KeratinoSens™, and the human Cell Line Activation Test (h-CLAT), has been applied to a broad set of 181 regulatory-interest substances, including complex or otherwise challenging chemistries, to produce hazard and potency predictions suitable for downstream risk assessment and regulatory consideration, and highlights practical limits for mixtures and poorly soluble materials []. These authors recommended that DA-derived outcomes for multi-constituent botanical extracts should be interpreted alongside robust compositional characterisation and exposure information.
Skin irritation and skin sensitisation are critical aspects for the evaluation of the materials exposed to the skin. Allergic contact dermatitis, affecting an estimated 15–20.1% of the general population, is a prevalent condition, with cosmetics being the second leading cause of contact allergies due to the fragrances and preservatives they contain []. Given the widespread use of these products, avoiding exposure is challenging. Furthermore, many topically applied botanical products contain compounds capable of inducing contact dermatitis [,], necessitating systematic toxicological assessments. The skin toxicity is simplest to identify as the reaction is instantaneously noticed.
Plant materials or extracts comprise several adverse dermal effects, including skin irritation, skin sensitisation, phototoxicity, and immediate-type allergies. The increasing reliance on herbal products underscores the need for comprehensive safety and efficacy data []. Photosensitisation dermatitis is a toxic skin reaction triggered by exposure to sunlight when a photosensitiser is present in the body, leading to sunburn-like reactions in non-pigmented skin areas. Plant materials from Ficus carica, Heracleum mantegazzianum, Tetradymia species, Hypericum species, and Lantana camara, have been proven to trigger photosensitisation reactions []. Food materials from some plants also show skin irritation, e.g., Pastinaca sativa (parsnip) and Agaricus bisporus (mushrooms) [].
Nonetheless, despite the number of ethnobotanical uses and reported bioactivities, the toxicological profile of A. calamus is heterogeneous and chemotype-dependent [], and its toxicological profile remains insufficiently studied []. The pharmacological and toxicological impacts of these plant materials are reviewed in a publication that suggests the overall toxicity profile of the extract of A. calamus has demonstrated the cardiotoxic, hepatotoxic, reproductive toxic, mutagenic, and carcinogenic potential for propenyl asarone isomers [].
Regulatory bodies and expert committees have therefore restricted or advised limits on the use of calamus/asarone-containing preparations: the United States Food and Drug Administration lists A. calamus among substances disallowed for direct addition or use as food []. European scientific reviews have concluded that β-asarone is carcinogenic in rodents and should be minimised in flavouring/food uses and fragrance-industry guidance (IFRA) sets conservative maximum levels for cis/trans-asarone in finished consumer products, with reported guidance for finished product concentrations being ≈100 ppm/0.01% []. These toxicological and regulatory data make compositional analysis (β-asarone content and chemotype identification) a critical component of any safety assessment of calamus rhizome oils []. The ban on A. calamus was centred on the carcinogenic effects observed in laboratory animals exposed to sweet flag extracts of the diploid variety, containing higher quantities of β-asarone []. Based on these developments, limits for β-asarone content in non-alcoholic and alcoholic drinks were set by the European Council to 0.1 mg/kg and 1 mg/kg, respectively [,]. Still, very limited or insufficient information is available to ascertain the toxic dose of A. calamus oil.
Although several studies conducted using the essential oils obtained from rhizomes of A. calamus have demonstrated numerous pharmacologic actions, very few or rare scientific studies have been published with recently innovated advanced technologies and models to prove these oils’ safety for different endpoints, such as their genotoxicity, skin sensitisation, or skin irritation effects. A. calamus was chosen for reasons directly relevant to risk management for skin sensitisation including the following: (1) its rhizome oil features in traditional remedies and contemporary leave-on and rinse-off personal-care formats, creating broad potential for consumer exposure [,]; (2) β-asarone is a prominent constituent with historical toxicological flags [], making a transparent, point-of-departure (PoD)-based evaluation especially important; (3) the asarone isomer content varies by plant chemotype, geography [], increasing uncertainty in extrapolating safety across supply chains; and (4) despite its traditional ethnomedicinal use, no single study that integrates a fully OECD-compliant battery with quantitative risk translation to a product-type specific maximum in use concentrations was found. Addressing these gaps for A. calamus, therefore, offers both ingredient-specific clarity and a general template for botanical oils with similar data deficiencies.
This prompted the current investigation to evaluate the rhizome extract for skin safety evaluations using new approach methodologies (NAMs), involving skin irritation, skin corrosion, and skin sensitisation assays. Modern regulatory practice and the adverse outcome pathway (AOP) for skin sensitisation emphasise mechanism-based, animal-free approaches that map to the key events of the sensitisation cascade with three key events (KEs); KE1, covalent protein binding (haptenation), assessed by the Direct Peptide Reactivity Assay (DPRA; OECD Test Guideline No. 442D); KE2, keratinocyte activation via Nrf2-ARE signalling, measured by the KeratinoSens™ assay (OECD Test Guideline No. 442D); and KE3, dendritic-cell activation, characterised by the human Cell Line Activation Test (h-CLAT; OECD Test Guideline No. 442E). Together, these assays form the foundation of DAs and Integrated Approaches to Testing and Assessment (IATA) that enable hazard identification and potency categorisation without reliance on animal models such as the Local Lymph Node Assay (LLNA) or Guinea Pig Maximisation Test (GPMT). Similarly, the use of reconstructed human epidermis models for assessing skin irritation and corrosion provides validated, regulatory-accepted tools for evaluating dermal toxicity. Collectively, these OECD-validated NAMs now provide a clear, mechanistically proven pathway not only for the classification of skin sensitisers, irritants, and corrosive agents, supporting both hazard assessment but also the quantitative next-generation risk management (NGRA) of cosmetic and botanical ingredients. Accordingly, the present study provides the first integrated, NAM-based toxicological profile for A. calamus rhizome oil, addressing a critical data gap and establishing its dermal hazard potential within a modern regulatory context.
Test Guidelines and GLP Compliance
All experiments were conducted following the relevant OECD Test Guidelines (TGs). Skin sensitisation was determined using the skin sensitisation DA as highlighted in OECD TG No. 497 [] following performance of OECD TG No. 442C for peptide reactivity using the DPRA [], OECD TG No. 442D for keratinocyte activation using the KeratinoSens™ assay [] and OECD TG No. 442E for dendritic cell activation using the hCLAT assay []. Skin irritation was assessed using the SkinEthic reconstructed human epithelium (RhE) model following OECD TG No. 439 []. Skin corrosion was assessed using the RhE model following OECD TG No. 431 []. All studies made a formal claim of GLP compliance.
2. Materials and Methods
2.1. Plant Material and Extraction of Rhizome Oil
The A. calamus rhizomes were collected from the Pampore, Pulwama District, Jammu and Kashmir, India (Geographical coordinates, 33.92882 74.90524 34.11682 75.09093). Post-collection, the rhizomes were air-dried and finely ground using an auto mixer (Figure S1). Dried rhizome powder was mixed with distilled water and subjected to hydro-distillation using a Clevenger-type apparatus, Borosil, Mumbai, India [] (Figure S2). The mixture was heated to 100–120 °C for 4 h, facilitating efficient extraction of the oil.
The oil was separated from the aqueous solution after no further condensing oil was visible, and after extraction, the resultant essential oil was stored at 4 °C for subsequent analysis and experimental use.
2.2. Chemicals and Reagents
Unless otherwise specified, all chemicals and materials adhered to the requirements described in the OECD test guidelines (TG Nos. 442C, 442D, 442E, 439, and 431). The following chemicals and reagents were used: Dulbecco’s Phosphate-Buffered Saline (DPBS), Foetal Bovine Serum (FBS), Trypsin-EDTA (0.05%), Dulbecco’s Modified Eagle Medium with Glutamax, and Geneticin; all were sourced from Gibco, Grand Island, NY, USA. Maintenance Medium and Growth Medium were obtained from SkinEthic Laboratories, Lyon Cedex 7, France. Dimethyl sulfoxide (DMSO), sodium hydroxide, isopropanol, and nickel sulphate were purchased from Qualigens, Mumbai, India. Trans-cinnamaldehyde, 2,4-dinitrochlorobenzene (DNCB), and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were sourced from Sigma-Aldrich, St. Louise, MO, USA. IgG1 and anti-CD54 was obtained from BD Pharmingen, Franklin Lakes, NJ, USA, while anti-CD86 was obtained from Dako, Carpinteria, CA, USA. Propidium iodide was purchased from Sigma-Aldrich, St. Louise, MO, USA. Penicillin-streptomycin was obtained from Thermo Fisher Scientific, Darmstadt, Germany. Passive lysis buffer was obtained from Promega, Madison, WI, USA.
2.3. Determination and Quantification of α-, β-, and γ-Asarone
2.3.1. Chemicals and Reference Standards
Certified analytical reference standards of α-asarone (TCI, Haryana, India; purity = 99.7%), β-asarone (Phytolab, Vestenbergsgreuth, Germany; purity = 96.41%), and γ-asarone (Biorbyt, Cambridge, UK; purity = 99.35%) were used. Individual stock solutions (1000 mg/L) were prepared in HPLC-grade methanol by accurately weighing α-asarone (1.004 mg), β-asarone (1.038 mg), and γ-asarone (1.007 mg) into separate 1 mL volumetric flasks. Ten-fold dilutions in methanol yielded 100 mg/L working standards for calibration, retention time confirmation, and mass-spectral identification.
2.3.2. Sample Preparation for HPLC Analysis
A weighed quantity of 20 mg of hydro-distilled A. calamus oil was made up to 1 mL, to prepare the stock solution (20,000 mg/L) for HPLC analysis. This stock was diluted 10 times in methanol to obtain 2000 mg/L. Aliquots were further diluted to prepare analyte-specific test concentrations: α-asarone (100 mg/L); β-asarone (1250 mg/L); and γ-asarone (1000 mg/L). The final test solutions were injected directly into HPLC and LC-MS/MS systems without additional treatment.
2.3.3. HPLC Quantification
Quantitative analysis was performed on a i-series LC-2050C system equipped with a UV detector, sourced from Shimadzu, Kyoto, Japan. Separation was achieved on an Agilent Eclipse C18 column (150 mm × 4.6 mm, 3.5 µm), sourced from Bengaluru, Karnataka, India, maintained at 40 °C. The mobile phase comprised formic acid in methanol (0.1%)–water (60:40, v/v) delivered isocratically at 0.7 mL/min. The autosampler was maintained at 15 °C, the injection volume was 5 µL, and UV detection was set at 254 nm.
2.3.4. LC-MS/MS Confirmation
Analyte identity was confirmed on a 8050 LC-MS/MS coupled to a Nexera-X2 UHPLC sourced from Shimadzu, Kyoto, Japan. Chromatographic separation employed a Union column with methanol ammonium formate (5 mM) in water (85:15, v/v) at 0.6 mL/min. Injections (2 µL) were analysed with the autosampler at 6 °C and the column at 40 °C. Mass spectrometry was performed in positive electrospray ionisation mode using a Q1 scan range of 270–300 m/z. Interface, desolvation line, and heat-block temperatures were 300 °C, 250 °C, and 400 °C, respectively.
2.3.5. Quantification and Data Analysis
Analyte concentrations in the test samples were calculated based on the area responses of standards (AS) and samples (AT), reference standard concentration (RC), volume (mL) of reference standard taken from stock solution (RT), final volume (mL) of working solution of reference standard (RV), concentration of sample stock solution (SC), final volume (mL) of working solution of sample solution (ST), and volume (mL) of stock solution of sample taken, according to the following equation:
2.3.6. Solubility Test
The solubility of A. calamus rhizome oil in DMSO was checked and was found to be appropriate for use. Dose formulations for the hCLAT and KeratinoSensTM assays were prepared in DMSO and then diluted in relevant media. For the DPRA test, the rhizome oil was dissolved in acetonitrile. The highest concentrations of the extracted oil used for testing were 500 mg/mL (h-CLAT), 40 mg/mL (KeratinoSens™), and 20 mg/mL (DPRA), with the DMSO concentration limited to no greater than 0.2–1% (v/v). For the skin Irritation and skin corrosion test, hydro-distilled A. calamus rhizome extract was used without any further dilution.
2.3.7. In Vitro Skin Irritation and Corrosion Tests
To evaluate the skin irritation and corrosion potential of A. calamus rhizome oil, in vitro tests were conducted using the SkinEthic™ RhE model. This model replicates the multi-layered, highly differentiated, and stratified epidermis structure of human skin. The skin irritation test identifies irritants (UN GHS Category 2) and non-irritants (UN GHS No Category), while the skin corrosion test distinguishes corrosive substances (UN GHS Category 1) from non-corrosive substances. Both tests rely on measuring cell viability through enzymatic conversion of the vital dye, MTT, to formazan, which is quantitatively measured to assess tissue damage.
2.3.8. Skin Irritation Test
Skin irritation was assessed using the SkinEthic™ reconstructed human epidermis (RhE) model in accordance with OECD TG No. 439. Tissues were pre-incubated for at least 2 h at 37 ± 1 °C, 5% CO2. A. calamus rhizome oil was applied directly onto tissues (16 µL/0.5 cm2) with concurrent negative (DPBS) and positive (5% SDS) controls; freeze-killed tissues were used to correct for non-specific MTT reduction. After the prescribed exposure and post-incubation intervals, the tissues were processed for an MTT viability assessment, and absorbance was read at 570 nm. Mean tissue viability (%) relative to the negative control was used to determine irritancy according to the OECD TG No. 439 acceptance criteria. Detailed experimental conditions are provided in Supplementary Materials (Tests S1–S3).
2.3.9. Skin Corrosion Test
Skin corrosion was evaluated using the SkinEthic™ RhE model in accordance with OECD Test Guideline No. 431. Tissues were pre-incubated for at least 2 h at 37 ± 1 °C, 5% CO2. A. calamus rhizome oil was applied directly onto tissues (40 µL/0.5 cm2) for 3 min and 60 min alongside negative (sterile water) and positive (8 N KOH) controls. Freeze-killed tissues were included to correct for non-specific MTT reductions. After exposure, tissues were rinsed, incubated with MTT, and formazan extraction and absorbance measurements at 570 nm were performed as described for the irritation assay. Tissue viability relative to negative controls determined corrosion potential according to the OECD TG No. 431 criteria. The detailed experimental conditions are provided in Supplementary Materials (Texts S1–S3).
2.3.10. The Direct Peptide Reactivity Assay (DPRA)
The Direct Peptide Reactivity Assay (DPRA) addresses the first key event (KE1) of the skin sensitisation AOP, assessing the covalent binding of electrophilic test items to nucleophilic residues in model peptides containing cysteine or lysine. The method was conducted in accordance with OECD TG No. 442C and DB-ALM Protocol 154. Synthetic cysteine and lysine peptides (0.667 mM) were incubated individually with A. calamus rhizome oil (20 mg/mL in acetonitrile) at defined 1:10 and 1:50 molar ratios, respectively, for 24 ± 2 h at room temperature in dark. Cinnamaldehyde (100 mM) served as the positive control, and acetonitrile as the vehicle control. Following incubation, peptide depletion was quantified by HPLC-UV at 220 nm using a reverse-phase C-18 column and expressed as percentage loss relative to control samples. The assay’s acceptance criteria, including reference control stability and linearity, were met for all runs. Classification of sensitising potential was based on the mean percent depletion of cysteine and lysine peptides, using the prediction model threshold of 6.38% to discriminate sensitisers from non-sensitisers. Reactivity classes (low, moderate, and high) were assigned according to OECD-defined depletion ranges for integration into the DA (OECD TG No. 497). The detailed procedure for the assay is described in Supplementary Materials (Texts S1–S3).
2.3.11. KeratinoSens™ Assay
To further assess the skin sensitisation potential of A. calamus rhizome oil, the KeratinoSens™ assay was conducted. This assay evaluates keratinocyte activation, the second key event in the AOP for skin sensitisation, by measuring the Keap1-Nrf2-ARE-mediated activation of antioxidant response element (ARE)-dependent genes. The assay utilises the stably transfected HaCaT cell line, KeratinoSensTM (acCELLerate GmbH, Osterfeldstraße 12–14, 22529 Hamburg, Germany), and quantifies luciferase activity as an indicator of Nrf2 pathway activation. A significant increase in luciferase activity, exceeding predefined thresholds and occurring at non-cytotoxic concentrations, was considered indicative of a positive sensitisation response.
The cells were seeded in 96-well plates at a density of 10,000 cells/well, one day prior to exposure. Plates were incubated at 37 ± 1 °C in a 5 ± 1% CO2 atmosphere for 24 h. A. calamus rhizome oil was tested at concentrations ranging from 0.20 to 400 µg/mL in DMSO. Trans-cinnamaldehyde was used as the positive control, and cells with DMSO were used as the negative control. One well with untreated cells was kept as a blank. Four plates were seeded for testing; three 96-well white assay plates for the luciferase assay, and one 96-well flat-bottom transparent plate for the cytotoxicity (MTT) assay was prepared. Following treatment, plates were incubated for 48 ± 2 h at 37 ± 1 °C, in a 5 ± 1% CO2 atmosphere. After incubation, cytotoxicity was assessed using the MTT assay. The MTT solution (5 mg/mL in DPBS) was mixed in DMEM containing FBS (1%, v/v). After the addition of MTT, plates were incubated for 4 h before adding isopropanol (50 µL) to dissolve the formazan crystals. Plates were shaken for 30 min and absorbance was measured at 570 nm using a Synergy HT microplate reader (BioTek Instruments, Winooski, VT, USA).
The cytotoxicity test was performed to make sure the observed luciferase induction was not due to adverse effects resulting from cell death.
For the luminescence measurement, the medium was removed, and the cells were washed once with DPBS. Passive lysis buffer was added to each well and plates were incubated for 20 min at room temperature. The luciferase substrate was added and luminescence was measured.
The assay evaluates the luciferase induction at various concentrations of the A. calamus rhizome oil. Results exceeding the predefined thresholds of EC1.5 (i.e., Imax ≥ 1.5-fold compared to solvent control) were considered positive for skin sensitisation response.
2.3.12. Human Cell Line Activation Test (h-CLAT)
The skin sensitisation potential of A. calamus rhizome oil was evaluated using the h-CLAT test method. This assay, based on the THP-1 cell line (ATCC: TIB-202), is a surrogate model for dendritic cells (DCs) and is described in OECD TG No. 442E for assessing skin sensitisers. The method quantifies the upregulation of CD86/CD54 on the THP-1 cell line (mimicking the activation of DCs), which correlates with the substance being a sensitiser. The h-CLAT method is widely adopted by regulatory authorities for differentiating between skin sensitisers and non-sensitisers.
THP-1 cells were cultured in RPMI-1640 medium supplemented with (FBS, 10% v/v), 2-mercaptoethanol (0.05 mM), and penicillin (100 U/mL)–streptomycin (100 µg/mL). Before the experiments, cells were tested for their ability to express CD86 and CD54 surface markers in response to the positive controls (reactivity check).
In the DRF experiments, the cells were exposed to ten (1.75–1000 µg/mL), 2-fold serial dilutions of A. calamus rhizome oil in DMSO across two independent experiments. The CV75 value (the concentration resulting in 75% cell viability) was determined using propidium iodide staining and flow cytometry. Working solutions of the test items and controls were prepared in a 1:1 (v/v) ratio with cell suspensions in 96-well flat-bottom plates.
Based on the results of the dose range-finding assay, eight concentrations were selected at 1.2-fold dilutions for assessing CD86 and CD54 expression. The highest dose corresponded to CV75 × 1.2-fold. Cells were exposed to the final concentration of 17–61 µg/mL of A. calamus rhizome oil concentrations, with DNCB included as the positive control at a final concentration of 8 µg/mL. Controls for the medium (FBS in RPMI 1640; 10%, v/v) and solvent (DMSO at 0.2%, v/v) were included in both the dose range finding and CD86/CD54 assays. Incubations were conducted for 24 h at 37 °C in a 5% CO2 atmosphere.
After incubation, cells were washed with a staining buffer PBS containing BSA (0.1%, w/v), followed by the FcR blocking, followed by staining with anti-human CD86-FITC and anti-human CD54-FITC and mouse IgG1 antibodies. After washing, the cells were stained with propidium iodide (0.625 µg/mL) to distinguish between live and dead cells. The samples were analysed using flow cytometry (FACS LyricTM, BD, Franklin Lakes, NJ, USA) and the relative fluorescence intensity (RFI) of CD86 and CD54 was calculated. The A. calamus rhizome oil was classified as a skin sensitiser if the RFI exceeded the established thresholds (RFI ≥ 150 for CD86 and RFI ≥ 200 for CD54). An increase in these markers indicates the activation of dendritic cell-like responses, suggesting potential skin sensitisation.
3. Results
3.1. The A. calamus Rhizome Oil Is Dominated by Beta-Asarone
The α-asarone, β-asarone, and γ-asarone were baseline separated with the expected retention times. Peak morphology demonstrated optimal symmetry (tailing factor < 1.1), and column performance was confirmed by theoretical plate counts exceeding 5800. Analysis of the A. calamus rhizome oil produced distinct peaks corresponding to α-asarone and β-asarone, while γ-asarone was not detected under these conditions (Figure 1). LC-MS/MS analysis verified molecular ions for all three isomers within the anticipated mass range (m/z 270–300), confirming clear identification of each asarone isomer.
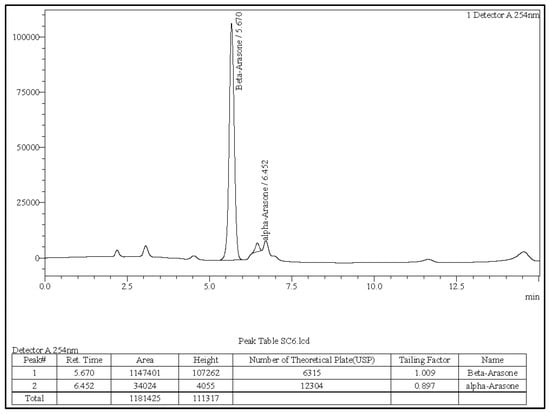
Figure 1.
A. calamus rhizome oil sample solution.
Retention times of the detected analytes matched those of the reference standards and LC-MS/MS spectra confirmed their molecular signatures. Representative chromatograms for the α, β, and γ reference standards are provided in Figures S3–S5.
3.2. Quantitative Determination of Asarone Isomers
Quantification was based on peak area ratios of the sample to the corresponding standards. Calculated concentrations of each analyte in the A. calamus rhizome oil are summarised in Table 1.

Table 1.
Quantification of α-, β-, and γ-asarone in the A. calamus rhizome oil.
The rhizome oil contained a predominant proportion of β-asarone (40.75%) and a moderate amount of α-asarone (4.16%). γ-asarone was below the detection limit of the method. Concordance of retention times and LC–MS/MS spectra between standards and sample peaks ensured the accuracy of analyte identification and quantification.
3.3. The Skin Irritation Test Results Show That A. calamus Rhizome Oil Is an Irritant
The tissue viability for A. calamus rhizome oil and the positive control was measured as a percentage of the negative control and NSMTT was calculated (Table 2). Tissues treated with DPBS (negative control) resulted in high viability across replicates. The mean viability of tissues treated with A. calamus rhizome oil was 23.5% (standard deviation (SD) = 1.50%, coefficient of variation (CV) = 6.39%) compared to the negative control. Tissues treated with positive control (5% SDS) showed a mean viability of 1.5% (SD = 0.058%, CV = 3.87%), indicative of suitable assay performance. The NSMTT values for the negative control were negligible, confirming no interference with the MTT assay. The NSMTT values for the A. calamus rhizome oil were negative, with a mean value of -11.2%, which demonstrated that the rhizome oil did not cause a non-specific reduction in MTT. The individual tissue replicates’ viability for negative, positive, and A. calamus-treated rhizome oil is provided in Figure S6.

Table 2.
Viability and non-specific MTT reduction (NSMTT) for A. calamus rhizome oil in the skin irritation test.
3.4. The Skin Corrosion Test Identified A. calamus Rhizome Oil as Non-Corrosive
The tissue viability of A. calamus rhizome oil and the positive control was measured as a percentage of the negative control, with NSMTT assessed to confirm the accuracy of the results. After 3 min of exposure (Table 3), A. calamus rhizome oil-treated tissues showed a mean viability of 92.72% (SD = 2.13%, CV = 2.30%), compared to the negative control. These results showed minimal impact on the tissue viability within the 3 min exposure period. The NSMTT for A. calamus rhizome oil was negligible, with a mean value of −0.7%, confirming that the A. calamus rhizome oil itself did not interfere with MTT. After 60 min of exposure (Table 3), A. calamus rhizome oil-treated tissues retained a mean viability of 91.17% (SD = 1.49%, CV = 1.63%). The positive control (8N KOH) reduced tissue viability to 0.17% (SD = 0.03%, CV = 17.65%), confirming the assay’s performance. NSMTT for A. calamus rhizome oil during the 60 min exposure was also negligible, with a mean value of 0.1%. The negative control (distilled water) showed high tissue viability for both exposure times, while the positive control reduced viability to nearly zero, confirming the assay’s performance. The skin corrosion test identified A. calamus rhizome oil as non-corrosive under the test conditions. Tissue viability remained well above the critical threshold of 50% for both short (3 min) and prolonged (60 min) exposure times. Negligible NSMTT values further confirmed the reliability of the viability readings. The individual tissue replicates’ viability for negative, positive, and A. calamus-treated rhizome oil treated with 3 min of exposure and 60 min of exposure is provided in Figures S7 and S8.

Table 3.
Viability and non-specific MTT reduction (NSMTT) for A. calamus rhizome oil in the skin corrosion test.
3.5. A. calamus Rhizome Oil Is Assigned to GHS Category 2, i.e., Skin Irritant
Since the skin corrosion test showed that A. calamus rhizome oil was non-corrosive, and the skin irritation test showed that A. calamus rhizome oil was an irritant, A. calamus rhizome oil is assigned to a GHS Category 2, i.e., skin irritant.
As the A. calamus rhizome oil was not corrosive, testing for skin sensitisation was performed to address the (1) molecular interaction with skin proteins, (2) inflammatory responses in keratinocytes, and (3) activation of dendritic cells. This method provides information about only one mechanistic event. A combination of the methods needs to be used in the DA to assign the skin sensitisation hazard potential.
3.6. A. calamus Rhizome Oil Showed Moderate Reactivity in the DPRA
The DPRA test was performed following the cysteine 1:10/lysine 1:50 prediction model (Table S1). A linear standard curve was obtained for both cysteine and lysine peptides (R2 > 0.99, Figure S9). All reference controls and peptide stability checks met the OECD acceptance criteria (Table 4). A mean peptide depletion threshold of 6.38% was used to discriminate sensitisers from non-sensitisers. A. calamus rhizome oil produced a mean cysteine peptide depletion of 46%, indicating moderate reactivity, while lysine peptide depletion was <1% (Table 5). The positive control, cinnamaldehyde, showed the expected high reactivity (81% cysteine, 67% lysine), confirming assay validity. Based on the combined mean depletion (23%), the test item was classified as a moderate sensitiser under the DPRA prediction model due to selective cysteine reactivity.

Table 4.
Acceptance criteria and observed results for the DPRA using cinnamic aldehyde as the positive control. All parameters met OECD TG No. 442C requirements, confirming assay validity.

Table 5.
Cysteine and lysine depletion for A. calamus rhizome oil in the DPRA.
3.7. The KeratinoSens™ Assay Identified A. calamus Rhizome Oil as a Sensitiser
The KeratinoSens™ assay was conducted to evaluate the skin sensitisation potential of the A. calamus rhizome oil. The results are summarised in Table 6. Key parameters, including IC50, Imax, and EC1.5, were calculated to assess luciferase gene expression associated with the Keap1-Nrf2-ARE pathway. The IC50, Imax, and EC1.5 for A. calamus rhizome oil and trans-cinnamaldehyde are presented in Table 6. The IC50 value, representing the concentration of the A. calamus rhizome oil causing 50% cell viability, was determined to be 28.51 µg/mL, and the observed IC30 was 21.48 µg/mL. As shown in Figure 2, cell viability remained above 75% at concentrations ≤25 µg/mL but dropped sharply at concentrations ≥50 µg/mL, indicating significant cytotoxicity. Concentration wise luciferase induction activity and cell viability is provided in Table S2.

Table 6.
Cytotoxicity (IC50) and luciferase induction (Imax and EC1.5) for A. calamus rhizome oil and trans-cinnamaldehyde (positive control) in the KeratinoSens™ assay.
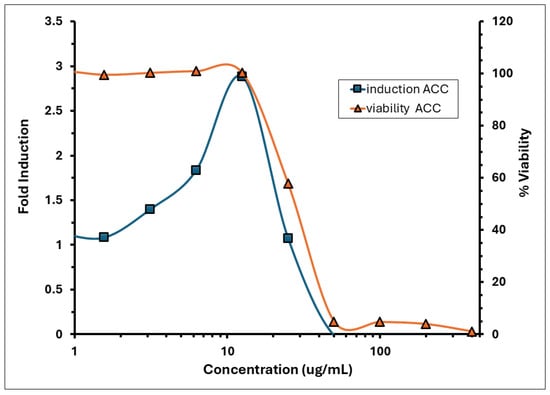
Figure 2.
Induction and viability of A. calamus rhizome oil in the KeratinoSens™ assay.
The induction of luciferase activity was concentration-dependent, with the maximum induction (Imax) observed at 12.5 µg/mL, averaging 2.88 across replicates. The concentration required to achieve a 1.5-fold increase in luciferase activity (EC1.5) was 3.84 µg/mL. The average fold induction of luciferase activity (Imax) for the test item exceeded 1.5-fold, reaching 1.86 and 3.17 at concentrations of 6.25 and 12.5 µg/mL, respectively, in experiment 1; and 1.82 and 2.60 at the same concentrations in experiment 2 (Figure 2). A decline in induction and an increase in cytotoxicity was observed from >12.5 µg/mL. The % CV observed for the negative control (DMSO) during experiments 1 and 2 was 11.09% and 14.03%, respectively, which was below 20%. The positive control demonstrated strong luciferase induction, with an Imax of 11.52 (Figure 3). The EC1.5 value was calculated as 8.74 µg/mL, confirming that the assay was performing correctly and confirming the positive control as a strong sensitiser.
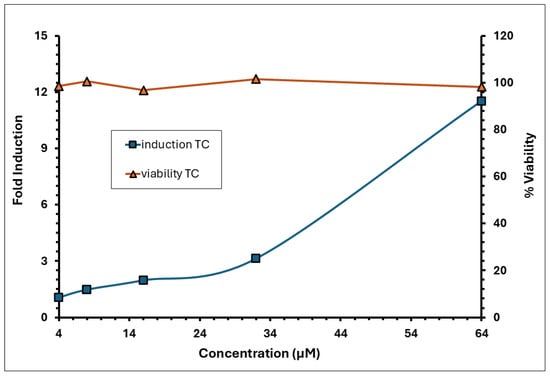
Figure 3.
Induction and viability of trans-cinnamaldehyde (positive control–TC) in the KeratinoSens™ assay.
The KeratinoSens™ assay identified A. calamus rhizome oil as a sensitiser, characterised by moderate cytotoxicity (IC50 = 28.5 µg/mL) and luciferase induction (Imax = 2.88, EC1.5 = 3.84 µg/mL).
3.8. A. calamus Rhizome Oil Met the Positive Response Criteria of RFI ≥ 150% for CD86 and RFI ≥ 200% for CD54, Indicating Its Sensitising Potential
Two independent dose range-finding assays were conducted to determine the CV75 value (i.e., the concentration resulting in 75% cell viability) of the A. calamus rhizome oil. This value was subsequently used to calculate doses for the CD86 and CD54 expression measurement experiments. Cytotoxicity (cell viability < 75%) was observed at test concentrations between 62.5 and 1000 µg/mL in DRF 1 and 125–1000 µg/mL in DRF 2 (Figure 4). The CD86 and CD54 expression measurements were performed using the CV75 value of 51 µg/mL to determine the RFI. Based on the CV75 values, the CD86/CD54 expression measurement assays were performed with concentrations ranging from 17 to 61 µg/mL.
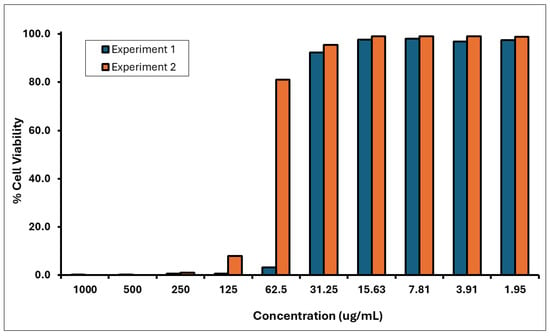
Figure 4.
Cell viability of THP-1 Cells treated with A. calamus rhizome oil from concentrations between 1.95 and 1000 µg/mL.
The medium, solvent, and positive control met the assay acceptance criteria for a valid test in both experiments 1 and 2 (Table 7). The A. calamus rhizome oil met the positive response criteria of RFI ≥ 150% for CD86 and RFI ≥ 200% for CD54, indicating a sensitising potential (Figure 5). The calculated EC150 was 27 µg/mL, while the EC200 values could not be calculated as the RFI values for CD54 did not show a dose-dependent increase; however, all values exceeded the positive criteria. Here, we used 17 µg/mL as a surrogate minimum induction threshold (MIT) value. Results of the hCLAT assay are summarised in Table 8.

Table 7.
Summary of CD86/CD54 expression, cell viability, and relative fluorescence intensity (RFI) in h-CLAT assay controls across two experiments, demonstrating assay validity and sensitisation thresholds.
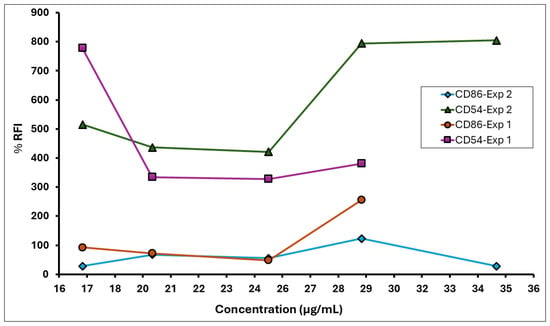
Figure 5.
% RFI for CD86 and CD54 expression on THP-1 cells following exposure to A. calamus rhizome oil in the hCLAT assay.

Table 8.
Summary of the human Cell Line Activation Test (h-CLAT) results for A. calamus rhizome oil, including individual values for CD86 and CD54 induction in experiments 1 and 2.
3.9. A. calamus Rhizome Oil Was Predicted to Be a GHS Category 1B Sensitiser
Based on the “2-out-of-3” criterion outlined in OECD TG No. 497, the A. calamus rhizome oil was predicted to be a skin sensitiser. The “2-out-of-3” DA can be used to make a prediction as to whether the substance is a skin sensitiser (Category 1) or not; however, it does not provide information on the skin sensitisation potency (Sub-category 1A versus 1B). Therefore, if the substance is predicted to be a skin sensitiser based on the “2-out-of-3” DA, for REACH information requirements, further information needs to be generated to conclude the skin sensitisation potency. Subsequently, the KE3/1 Sequential Testing Strategy [,], endorsed by the US EPA [,], was also applied. This approach addresses KE1 and KE3 in the AOP for skin sensitisation via the DPRA and h-CLAT, respectively, and provides both hazard identification and GHS potency classification (1A, 1B, or Not Classified). A test item with an h-CLAT minimum induction threshold (MIT) of ≤10 µg/mL is classified as “Strong” (GHS 1A), whereas an MIT of >10 µg/mL indicates “Weak” (GHS 1B), where MIT indicates the lowest value eliciting a positive outcome. The DASS app utilises the DA to predict skin sensitisation hazards, classifying substances as sensitisers or non-sensitisers and their potency based on UN GHS categories. These predictions integrate data from in vitro assays representing key events in the skin sensitisation adverse outcome pathway, along with in silico hazard assessments []. Using coupled data from the DPRA (mean %Cys and %Lys depletion of 23%) and hCLAT (using EC150 of 27 µg/mL), using the DASS app, A. calamus rhizome oil was predicted to be a GHS Category 1B sensitiser. The CD54 RFI exceeded the 200% threshold across the tested range without a clear dose response. We therefore avoided using EC200 as the primary potency anchor and used finite EC150 as MIT. While the KE 3/1 DA provides a biologically meaningful categorical potency for hazard classification, quantitative risk assessment is better anchored to a probabilistic point-of-departure (PoD) from SARA-ICE; accordingly, we report the KE3/1 Category 1B as the hazard/potency classification and use the SARA-ICE ED01 (subsequently combined with QRA2 exposure/SAF workflows) to derive product-use concentration limits.
Using the Scientific Committee on Consumer Safety (SCCS) default usage parameters (daily amount, frequency, and treated skin surface area), data were calculated as applied mass per application (M) and derived product-specific conservative safe concentrations (Cmax) from the SARA-ICE ED01 values [,]. The results from the SARA-ICE model are provided in Table 9. The SCCS-derived per application applied masses used here were as follows: face cream: 1.27 mg/cm2 (derived from daily amount of product category that is applied/received (qx) = 1.54 g/day, frequency = 2.14 use/day, and face SSA = 565 cm2); body lotion: 0.219 mg/cm2 (qx = 7.82 g/day, frequency = 2.28, and SSA = 15,670 cm2); hand cream: 1.26 mg/cm2 (qx = 2.16 g/day, frequency = 2, and SSA = 860 cm2); shower gel (rinse-off whole body): 0.0076 mg/cm2 (qx = 0.19 g/day, frequency = 1.43, and SSA = 17,500 cm2). Applying the point-of-departure (PoD) and QRA2 product SAFs (SAF = 100 for leave-on face/body/hand; SAF = 300 for rinse-off), the calculated provisional maxima are as follows: face cream Cmax = 0.13% (w/w), 1300 ppm, body lotion Cmax = 0.78%, (w/w), 7800 ppm, hand cream Cmax = 0.13%, (w/w), 1300 ppm, and shower gel (rinse-off) Cmax = 7.46%, (w/w) 74,600 ppm using the formula given below
where % Cmax is the Provisional Maxima, PoD is the geometric mean of the ED01 predicated on being a sensitiser, SAF is the sensitisation assessment factor, and M is the applied mass/cm2. The amount applied mass/unit area (M, mg/cm2) was computed as (qx/f × 1000)/SSA where qx is the SCCS effective daily amount for each product category(g/day), f is the application frequency (uses/day), and SSA is the treated skin surface area (SSA, cm2).

Table 9.
SARA-ICE model results.
The calculated safe maximum concentrations (Cmax) expressed as % (w/w) for representative consumer product categories using SCCS default applied-mass inputs are provided in Table 10.

Table 10.
Calculated safe maximum concentrations (Cmax) expressed as % (w/w) (and ppm in parentheses) for representative consumer product categories using SCCS default applied-mass inputs using the OECD SARA-ICE DA PoD (170 µg/cm2).
4. Discussion
The use of NAMs to identify chemical hazards has accelerated in recent years [,]. In the crop protection industry, a battery of in vitro NAMs was used to identify the skin irritation, skin sensitisation, ocular irritation and lung irritation hazards for captan and folpet; the in vitro NAM data correlated well with existing in vivo data except for an under prediction for ocular irritation [].
In the current DPRA, selective modification of cysteine over lysine indicates that the reactive species in the A. calamus rhizome oil could behave as soft Michael acceptors that favour nucleophilic thiolate attacks []. β-asarone, an allylbenzene in the oil’s composition, possesses an α, β-unsaturated propenyl side chain capable of 1,4-conjugate addition, a reaction mechanism entirely consistent with the observed thiol specificity [,]. Such cysteine-biassed depletion profiles are recurrent among botanical sensitisers of moderate human potency, lending mechanistic plausibility to the classification [,,].
For the KeratinoSens™ assay, mechanistically, the modest yet clear induction pattern suggested the electrophilic modification of Keap1 cysteines by constituents present in the oil, liberating Nrf2 to translocate and drive ARE transcription. The sharp fall-off in signal at cytotoxic doses further supports that luciferase increases stem from pathway activation rather than generalised stress, since induction collapses once viability drops below 60%. For the hCLAT assay, the requirement that both CD86 and CD54 exceed their respective cut-off points at sub-toxic concentrations demonstrates that the oil can trigger the maturation signals necessary for T-cell priming [,]. The convergent activation of these two markers at the same concentration is a characteristic of genuine skin sensitisers, where CD54 alone may rise secondary to reactive oxygen species [].
For the skin irritation test, the A. calamus rhizome oil decreased tissue viability to a mean of 23.5% (SD = 1.5%), far beneath the 50% cut-off that operationally defines an irritant response in this model. Because viability remained well above the <5% range that typifies corrosive destruction, the result is diagnostic of reversible irritation rather than irreversible corrosion. The viability loss also aligns with previous findings for essential-oil irritants whose primary constituents disrupt intercellular lipid lamellae without penetrating to the basal layer [,]. The chemical profile of the oil, such as β-asarone and allied phenylpropenoids, possesses surfactant-like amphiphilicity and can fluidise the stratum corneum lipids, precipitating local cytokine release and erythema but not the protein denaturation characteristic of strong bases or oxidisers [,,]. The short recovery interval in the skin irritation test does not permit visual scoring of erythema; nevertheless, the substantial decrease in viability might be due to mitochondrial compromise sufficient to predict clinical redness and oedema [,,].
The high viability of tissue treated with A. calamus rhizome oil in the skin corrosion test suggests a lack of irreversible protein-denaturing effects, with any membrane disruption being transient. This aligns with the amphiphilic nature of its major phenylpropenoids, which lack the extreme pK or redox potential typical of corrosives [,]. Despite its limitations, the RhE model’s >90% predictive accuracy supports the non-corrosive classification [,].
The present investigation utilised the OECD “3-Key-Event” battery, KE 3/1 Sequential Testing Strategy and the RhE irritation and corrosion assays to define the dermal hazard profile of A. calamus rhizome oil. For skin sensitisation, the data revealed a coherent mechanistic progression from selective cysteine conjugation in the DPRA, through Nrf2-ARE activation in keratinocytes, to the upregulation of co-stimulatory markers on THP-1 dendritic surrogates. Using the OECD TG No. 497 DA for skin sensitisation, A. calamus rhizome oil is defined as a skin sensitiser. Additionally, based on the KE 3/1 Sequential Testing Strategy, the rhizome oil is further categorised as a Category 1B sensitiser, i.e., moderate potency. The five-assay battery delivers a strong narrative that A. calamus rhizome oil fulfils every mechanistic checkpoint of the skin sensitisation AOP while remaining non-corrosive yet clearly irritant. Additionally, A. calamus rhizomes contain a diverse array of chemical constituents in addition to phenylpropanoids, monoterpenes, and sesquiterpenes, each contributing to the oil’s biological and dermal effects [,,,]. While its bioactive compounds contribute to its therapeutic efficacy, they also raise concerns regarding dermal toxicity. These findings align with previous studies highlighting the allergenic and irritant properties of plant-derived essential oils [,,]. For example, clove oil, dominated by eugenol, shares similar electrophilic properties and sassafras oil, rich in safrole, demonstrates comparable irritation potential but poses additional systemic toxicity risks, including carcinogenicity [,]. Isoeugenol’s skin sensitisation potential could be due to hydroxy quinone methide formation, while quinone methide and an ortho-quinone mediate eugenol’s sensitisation potential []. Similarly, the skin sensitisation potential of eugenol and isoeugenol was also evaluated []. Enhanced luciferase induction was found in the presence of Aroclor-induced rat liver S9 in the KeratinoSens™ reporter assay for methyl-isoeugenol and eugenol. However, methyl-isoeugenol gave a weak (Imax 2.4) and eugenol gave no gene induction (Imax 1.7) in the absence of S9, suggesting their pro-hapten nature []. α- and β-Pinene were found to be positive (i.e., sensitiser) in the GPMT at a concentration of 4% [] while in the LLNA, they both gave a weak response []. Eucalyptus and camphor oils are primarily irritant due to 1,8-cineole and camphor which act through physical disruption of the skin barrier rather than chemical sensitisation []. Oils like lavender and rosemary, rich in linalool and carnosic acid, respectively, demonstrate protective effects due to their antioxidant and anti-inflammatory properties []. Phenylpropanoids present in the oils may exacerbate irritation by inducing oxidative stress in keratinocytes, driving the inflammatory response []. At the concentrations tested in the skin irritation test, the oils’ components induce sufficient inflammatory responses to qualify as irritants without causing the extensive tissue damage associated with corrosion. Corrosive agents typically cause full-thickness necrosis by penetrating beyond the epidermis to the dermis, denaturing proteins and destroying cellular membranes []. Some minor constituents, such as linalool and caryophyllene, in the oil might provide partial anti-inflammatory and antioxidant effects, mitigating the overall dermal reactivity of the oil [,]. The results emphasise the importance of using A. calamus rhizome oil in appropriately diluted concentrations and formulating it with agents that may mitigate its irritant effects and the need for regulatory oversight. The concentration of compounds, along with the frequency, duration of exposure, and skin condition, could be considered important factors. The SARA-ICE DA Model predicted an ED01 (50th percentile) of 180 µg/cm2, and a range spanning from 2.0 µg/cm2 (5th percentile) to 13,000 µg/cm2 (95th percentile) with PoD being 170 µg/cm2.
The probabilistic ED01 distribution already accounts for inter-individual and model variability in human dose–response data. The QRA2 SAF, in contrast, aggregates additional uncertainties not captured by potency estimations, namely (1) matrix and formulation effects (vehicle influence, volatility, and occlusion); (2) exposure modelling variability (frequency, surface area, and product amount); (3) inter- and intra-individual physiological variability; and (4) database and read-across limitations. By choosing the OECD DA PoD + SAF as the primary scenario, we separate statistical uncertainty (captured in PoD derivation) from extrapolation uncertainty (captured in SAF). This layered yet non-redundant strategy aligns with contemporary risk-assessment principles [,].
Furthermore, the SCCS-based applied masses show that consumer leave-on facial and hand creams deliver 1.25 mg/cm2 per application, for example, producing face cream Cmax of 0.13% (w/w). On the other hand, rinse-off products distribute small, retained masses over large surface areas, yielding much higher permissible concentrations. For example, the shower gel Cmax is 7.46% (w/w), but rinse-off classification also implies different retention and absorption dynamics, so interpretation must be cautious. The higher permissible levels for rinse-off products reflect transient skin contact and limited retention; however, final concentrations should also consider dermal absorption kinetics and potential cumulative exposure across products. Where aggregate exposure is relevant, the per-product Cmax must be adjusted downward or some categories removed to achieve an acceptable margin.
The increasing regulatory acceptance of DAs and NAM batteries makes the present integration of DPRA, KeratinoSens™, and h-CLAT with SARA-ICE potency modelling particularly timely for cosmetics safety assessments. OECD TG No. 497 formally recognises that DAs can be used to replace animal tests for hazard and potency decision-making when implemented with a fixed data interpretation procedure, and the SARA-ICE workflow is explicitly described in the supporting OECD materials as an implementation route for deriving ED01-based PoDs. Historically, the fragrance industry established the QRA concept and QRA2 refined it into a transparent framework (NESIL to AEL and to CEL) with composite sensitisation assessment factors (SAFs) that can be used to translate pos probabilistic or Bayesian PoD derivation, continuous PoDs, percentile selection [], as well as practical NGRA case studies, and weight of evidence approach methods for skin sensitisation potency categorisation [], which supports that NAM data can be combined with SAF-based extrapolation to produce both a recommended regulatory limit and precautionary sensitivity bounds for exposure modelling to fragrance and botanical constituents for vulnerable subpopulations. With this said, applying this approach to botanicals requires additional safeguards. Natural oils are chemically complex and chemotypes can vary geographically and by extraction method; consequently, compositional standardisation, specification of analytical marker content (for instance β-asarone in A. calamus), and batch control are prerequisites before a single NAM-derived PoD can be reliably applied across commercial supplies. Taken together, the regulatory potential of the NAM-anchored, DA-driven potency estimates can be integrated into the QRA2 workflow to yield product-specific safe concentrations, but only when supported by robust exposure characterisation, compositional specification, and, where possible, targeted empirical data (dermal absorption or limited human tolerance studies) to reduce residual uncertainty while emphasising that its defensible application to botanicals rests on compositional control. Robust exposure characterisation (or conservative SCCS defaults) and transparent presentation of both primary (OECD-DA PoD + QRA2 SAF) and precautionary scenarios can also provide regulators with transparent options that balance precaution with practicability and align with current NGRA best practice and the evolving SCCS/OECD expectations for cosmetic safety assessments.
5. Conclusions
This study delivers a comprehensive in vitro toxicological evaluation of A. calamus rhizome oil, leveraging a full suite of OECD-compliant NAMs. The oil was classified as a GHS Category 1B skin sensitiser and a Category 2 irritant, while demonstrating a non-corrosive profile. These findings were supported by mechanistic evidence across all three key events of the skin sensitisation AOP: protein reactivity (DPRA), keratinocyte activation (KeratinoSens™), and dendritic cell activation (h-CLAT). The study highlights the utility of NAMs to replace traditional animal-based assays with mechanistically informed, regulatory-accepted tests. This approach not only improves ethical and scientific standards but also enables hazard identification and potency categorisation. Chemical analysis revealed high β-asarone content (40.75%), reinforcing the need for compositional control due to its known toxicological risks. Risk modelling using the SARA-ICE Model and SCCS parameters established conservative safe concentration limits for topical applications, ranging from 0.13% to 0.78% (w/w) for leave-on products and up to 7.46% (w/w) for rinse-off formulations. These data fill a critical gap in the safety assessment of A. calamus oil and support its regulated use in consumer products. While the study integrates NAM-based in vitro and potency modelling, a few limitations should be acknowledged. The probabilistic point of departure values are model-derived and not yet supported by human patch-test validation; dermal absorption kinetics were assumed rather than measured; and the compositional variability inherent to natural oils may influence sensitisation potency. These uncertainties, although mitigated through structured assessment factors (SAFs), highlight the need for future empirical work on human exposure, bioavailability, and formulation effects. Overall, this work exemplifies the application of NAMs in botanical safety evaluation and provides a robust framework for future assessments of complex natural ingredients. This study also forms a base to demonstrate how NAM-derived probabilistic potency data (SARA-ICE) can be integrated quantitatively with SCCS QRA2 frameworks, enabling reproducible, evidence-based safety limits for complex natural mixtures. Continued research should focus on formulation-level testing, chemotype standardisation, and strategies to mitigate dermal reactivity.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/toxics13121006/s1: Figure S1: Acorus calamus rhizome (Left) and Acorus calamus rhizome powder (right); Figure S2: Hydro distillation by the Clevenger apparatus (left), Acorus calamus rhizome powder in an oil bath (middle), and extracted mixture (right); Figure S3: Reference standard solution of α-asarone; Figure S4: Reference standard solution of β-asarone; Figure S5: Reference standard solution of γ-asarone; Figure S6: Tissue viability for A. calamus rhizome oil (ACC) and negative controls in the skin irritation test; Figure S7: Tissue viability for A. calamus rhizome oil (ACC) and negative control in the skin corrosion test (3 min of exposure); Figure S8: Tissue viability for A. calamus rhizome oil (ACC) and controls in the skin corrosion test (60 min of exposure); Figure S9: Cysteine and lysine standard curve; Table S1: Prediction model as per OECD 442c for the DPRA prediction; Table S2: Luciferase induction activity and cell viability for A. calamus rhizome oil in the KeratinoSens™ assay; Text S1: Skin Irritation; Text S2: Skin Corrosion; Text S3:The Direct Peptide Reactivity Assay.
Author Contributions
Conceptualization, K.R.D., J.R.R. and R.M.N.; methodology, K.R.D., J.R.R. and R.M.N.; validation, K.R.D., J.R.R. and C.S.R.; formal analysis, K.R.D. and J.R.R.; investigation, K.R.D. and J.R.R.; resources, R.M.N., M.V.P. and A.D.D.; data curation, K.R.D. and J.R.R.; writing—original draft preparation, K.R.D. and J.R.R.; writing—review and editing, K.R.D., J.R.R., R.M.N., M.V.P., A.D.D., C.S.R. and G.B.K.; visualisation, J.R.R.; supervision, R.M.N., M.V.P., A.D.D. and G.B.K.; project administration, K.R.D., R.M.N., M.V.P. and G.B.K.; funding acquisition, K.R.D., R.M.N., M.V.P. and A.D.D. All authors have read and agreed to the published version of the manuscript.
Funding
The intramural research programme of the Jai Research Foundation supported this research.
Institutional Review Board Statement
Prior to conducting skin irritation and skin corrosion assays, ethics approval was obtained from the Institutional Human Ethics Committee (IHEC) [JRF/IHEC/2024/SIT/1 and JRF/IHEC/2024/SCT/1 (approval date 12 April 2024)] and the Institutional Biosafety Committee (IBSC), JRF [JRF/IBSC/2023/49 (for SIT-approval date 9 November 2023) and JRF/IBSC/2024/14 (for SCT-approval date 24 February 2024)].
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
Author Clive S. Roper is the founder of Roper Toxicology Consulting Ltd. He acts as one of the consultants at JRF. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Balakumbahan, R.; Rajamani, K.; Kumanan, K. Acorus calamus: An overview. J. Med. Plants Res. 2010, 4, 2740–2745. [Google Scholar]
- Sharma, V.; Singh, I.; Chaudhary, P. Acorus calamus (The Healing Plant): A review on its medicinal potential, micropropagation and conservation. Nat. Prod. Res. 2014, 28, 1454–1466. [Google Scholar] [CrossRef]
- Sharma, P.K.; Chauhan, N.S.; Lal, B. Observations on the traditional phytotherapy among the inhabitants of Parvati valley in western Himalaya, India. J. Ethnopharmacol. 2004, 92, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Kirtikar, K.R.; Basu, B.D. Indian Medicinal Plants; Mahendra Pal Sing Publishers: Dehradun, India, 1987; Volume IV, pp. 1229–1230. [Google Scholar]
- Raj, R.K. Screening of some indigenous plants for anthelmintic action against human Ascaris lumbricoides. Indian J. Physiol. Pharmacol. 1974, 18, 129–131. [Google Scholar] [PubMed]
- Kapur, S.K. Ethno-medico plants of Kangra valley (Himachal Pradesh). J. Econ. Tax. Bot. 1993, 17, 395–408. [Google Scholar]
- Mukherjee, P.K.; Kumar, V.; Mal, M.; Houghton, P.J. Acorus calamus: Scientific Validation of Ayurvedic Tradition from Natural Resources. Pharm. Biol. 2007, 45, 651–666. [Google Scholar] [CrossRef]
- Du, Z.; Clery, R.A.; Hammond, C.J. Volatiles from leaves and rhizomes of fragrant Acorus spp. (Acoraceae). Chem. Biodivers. 2008, 5, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Bains, J.S.; Dhuna, V.; Singh, J.; Kamboj, S.S.; Nijjar, K.K.; Agrewala, J.N. Novel lectins from rhizomes of two Acorus species with mitogenic activity and inhibitory potential towards murine cancer cell lines. Int. Immunopharmacol. 2005, 5, 1470–1478. [Google Scholar] [CrossRef]
- Arseculeratne, S.N.; Gunatilaka, A.A.L.; Panabokke, R.G. Studies on medicinal plants of Sri Lanka. Part 14: Toxicity of some traditional medicinal herbs. J. Ethnopharmacol. 1985, 13, 323–335. [Google Scholar] [CrossRef]
- Tariq, R.M.; Naqvi, N.H.; Choudhary, M.I.; Abbas, A. Importance and implementation of essential oil of Pakistanian Acorus calamus Linn., as a biopesticide. Pak. J. Bot. 2010, 42, 2043–2050. [Google Scholar]
- Gilani, A.U.H.; Shah, A.J.; Ahmad, M.; Shaheen, F. Antispasmodic effect of Acorus calamus Linn. is mediated through calcium channel blockade. Phytother. Res. 2006, 20, 1080–1084. [Google Scholar] [CrossRef]
- Glara, D.A.; Sivaranjani, D.K.; Ashmi, R.; Shymala, D.K. Vasambu kaapu in prevention of common paediatric ailments—An overview. World J. Pharm. Pharm. Sci. 2017, 360–367. [Google Scholar] [CrossRef]
- Antignac, E.; Nohynek, G.J.; Re, T.; Clouzeau, J.; Toutain, H. Safety of botanical ingredients in personal care products/cosmetics. Food Chem. Toxicol. 2011, 49, 324–341. [Google Scholar] [CrossRef]
- Gilissen, L.; Huygens, S.; Goossens, A. Allergic contact dermatitis caused by topical herbal remedies: Importance of patch testing with the patients’ own products. Contact Dermat. 2018, 78, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Panico, A.; Serio, F.; Bagordo, F.; Grassi, T.; Idolo, A.; De Giorgi, M.; Guido, M.; Congedo, M.; De Donno, A. Skin safety and health prevention: An overview of chemicals in cosmetic products. J. Prev. Med. Hyg. 2019, 60, E50–E57. [Google Scholar] [CrossRef] [PubMed]
- Andrade, R.-J.; Robles, M.; Fernández-Castañer, A.; López-Ortega, S.; López-Vega, M.-C.; Lucena, M.-I. Assessment of drug-induced hepatotoxicity in clinical practice: A challenge for gastroenterologists. World J. Gastroenterol. 2007, 13, 329–340. [Google Scholar] [CrossRef]
- Andrade, T.; Aguiar, A.; Guedes, F.; Leite, M.; Caetano, G.; Coelho, E.; Das, P.; Frade, M. Ex vivo model of human skin (hOSEC) as alternative to animal use for cosmetic tests. Procedia Eng. 2015, 110, 67–73. [Google Scholar] [CrossRef]
- Maffè, S.; Paffoni, P.; Colombo, M.L.; Davanzo, F.; Dellavesa, P.; Cucchi, L.; Zenone, F.; Paino, A.M.; Pardo, N.F.; Bergamasco, L.; et al. Cardiotossicità da erbe selvatiche. G. Ital. Cardiol. 2013, 14, 445–455. [Google Scholar]
- Parez, B.E.; Rodriquez, O.R.; Sanchez, V.M.C. Toxic plants: Brugmansia (floripondio) neurotoxicity. Arch. Med. Urg. Mex. 2012, 4, 119–124. [Google Scholar]
- Strickland, J.A.; Maldonado, H.; Farley-Dawson, E.A.; LaPratt, T.; To, K.T.; Truax, J.F.; Reinke, E.; Allen, D.G.; Germolec, D.; Kleinstreuer, N. Evaluation of Substances of Regulatory Interest Using Non-Animal Skin Sensitization Test Methods; NICEATM Report 05; Division of Translational Toxicology: Durham, NC, USA, 2025. [Google Scholar] [CrossRef]
- Miroddi, M.; Calapai, G.; Isola, S.; Minciullo, P.L.; Gangemi, S. Rosmarinus officinalis L. as cause of contact dermatitis. Allergol. Immunopathol. 2014, 42, 616–619. [Google Scholar] [CrossRef]
- Monroe, J. Toxicodendron Contact Dermatitis: A Case Report and Brief Review. J. Clin. Aesthet. Dermatol. 2020, 13 (Suppl. S1), S29–S34. [Google Scholar]
- Jack, A.R.; Norris, P.L.; Storrs, F.J. Allergic contact dermatitis to plant extracts in cosmetics. Semin. Cutan. Med. Surg. 2013, 32, 140–146. [Google Scholar] [CrossRef]
- Collett, M.G. Photosensitisation diseases of animals: Classification and a weight of evidence approach to primary causes. Toxicon X 2019, 3, 100012. [Google Scholar] [CrossRef]
- Walling, A.L.; Walling, H.W. Phytophotodermatitis induced by wild parsnip. Dermatol. Online J. 2018, 24, 19. [Google Scholar] [CrossRef]
- Sugimoto, N.; Kiuchi, F.; Mikage, M.; Mori, M.; Mizukami, H.; Tsuda, Y. DNA profiling of Acorus calamus chemotypes differing in essential oil composition. Biol. Pharm. Bull. 1999, 22, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Deshmukh, P.; Joshi, S.; Ghag, M.; Kulkarni, Y.; Vyas, B.; Shah, D. Toxicity study of ethanolic extract of Acorus calamus rhizome. Int. J. Green Pharm. 2012, 6, 29–35. [Google Scholar] [CrossRef]
- Uebel, T.; Hermes, L.; Haupenthal, S.; Müller, L.; Esselen, M. α-Asarone, β-asarone, and γ-asarone: Current status of toxicological evaluation. J. Appl. Toxicol. 2021, 41, 1166–1179. [Google Scholar] [CrossRef]
- Calamus and Its Derivatives. 21 CFR 189.110. Available online: https://www.ecfr.gov/current/title-21/section-189.110 (accessed on 12 June 2025).
- Sharma, V.; Sharma, R.; Gautam, D.S.; Kuca, K.; Nepovimova, E.; Martins, N. Role of Vacha (Acorus calamus Linn.) in Neurological and Metabolic Disorders: Evidence from Ethnopharmacology, Phytochemistry, Pharmacology and Clinical Study. J. Clin. Med. 2020, 9, 1176. [Google Scholar] [CrossRef]
- International Fragrance Association (IFRA). IFRA Standard: Cis- and Trans-Asarone (Amendment 40); IFRA: Geneva, Switzerland, 2006. [Google Scholar]
- Singh, C.; Jamwal, U.S.P. Acorus calamus (sweet flag), an overview of oil decomposition, biological activity and usage. Int. J. Med. Arom Plants 2001, 23, 687–708. [Google Scholar]
- Maibach, H.I. Principles and methods of toxicology. J. Am. Acad. Dermatol. 1984, 10, 152. [Google Scholar] [CrossRef]
- Talalay, P.; Talalay, P. The importance of using scientific principles in the development of medicinal agents from plants. Acad. Med. 2001, 76, 238–247. [Google Scholar] [CrossRef]
- Barceloux, D.G. Medical Toxicology of Natural Substances: Foods, Fungi, Medicinal Herbs, Plants, and Venomous Animals; John Wiley & Sons: Hoboken, NJ, USA, 2008; pp. 1–1157. [Google Scholar]
- Kingston, C.; Jeeva, S.; Jeeva, G.M.; Kiruba, S.; Mishra, B.P.; Kannan, D. Indigenous knowledge of using medicinal plants in treating skin diseases in Kanyakumari district, Southern India. Indian J. Tradit. Knowl. 2009, 8, 196–200. [Google Scholar]
- Haupenthal, S.; Berg, K.; Gründken, M.; Vallicotti, S.; Hemgesberg, M.; Sak, K.; Schrenk, D.; Esselen, M. In vitro genotoxicity of carcinogenic asarone isomers. Food Funct. 2017, 8, 1227–1234. [Google Scholar] [CrossRef]
- Rajput, S.B.; Tonge, M.B.; Karuppayil, S.M. An overview on traditional uses and pharmacological profile of Acorus calamus Linn. (Sweet flag) and other Acorus species. Phytomedicine 2014, 21, 268–276. [Google Scholar] [CrossRef] [PubMed]
- OECD. Guideline No. 497: Defined Approaches on Skin Sensitisation, OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2025. [Google Scholar] [CrossRef]
- OECD. Test No. 442C: In Chemico Skin Sensitisation: Assays Addressing the Adverse Outcome Pathway Key Event on Covalent Binding to Proteins, OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2025. [Google Scholar] [CrossRef]
- OECD. Test No. 442D: In Vitro Skin Sensitisation: Assays Addressing the Adverse Outcome Pathway Key Event on Keratinocyte Activation, OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2024. [Google Scholar] [CrossRef]
- OECD. Test No. 442E: In Vitro Skin Sensitisation: In Vitro Skin Sensitisation Assays Addressing the Key Event on Activation of Dendritic Cells on the Adverse Outcome Pathway for Skin Sensitisation, OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2024. [Google Scholar] [CrossRef]
- OECD. Test No. 439: In Vitro Skin Irritation: Reconstructed Human Epidermis Test Method, OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2025. [Google Scholar] [CrossRef]
- OECD. Test No. 431: In Vitro Skin Corrosion: Reconstructed Human Epidermis (RHE) Test Method, OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2025. [Google Scholar] [CrossRef]
- Quintans-Júnior, L.J.; Souza, T.T.; Leite, B.S.; Lessa, N.M.; Bonjardim, L.R.; Santos, M.R.; Alves, P.B.; Blank, A.F.; Antoniolli, A.R. Phythochemical screening and anticonvulsant activity of Cymbopogon winterianus Jowitt (Poaceae) leaf essential oil in rodents. Phytomedicine 2008, 15, 619–624. [Google Scholar] [CrossRef]
- Nukada, Y.; Miyazawa, M.; Kazutoshi, S.; Sakaguchi, H.; Nishiyama, N. Data integration of non-animal tests for the development of a test battery to predict the skin sensitizing potential and potency of chemicals. Toxicol. Vitr. 2013, 27, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Takenouchi, O.; Fukui, S.; Okamoto, K.; Kurotani, S.; Imai, N.; Fujishiro, M.; Kyotani, D.; Kato, Y.; Kasahara, T.; Fujita, M.; et al. Test battery with the human cell line activation test, direct peptide reactivity assay and DEREK based on a 139 chemical data set for predicting skin sensitizing potential and potency of chemicals. J. Appl. Toxicol. 2015, 35, 1318–1332. [Google Scholar] [CrossRef]
- Strickland, J.; Truax, J.; Corvaro, M.; Settivari, R.; Henriquez, J.; McFadden, J.; Gulledge, T.; Johnson, V.; Gehen, S.; Germolec, D.; et al. Application of Defined Approaches for Skin Sensitization to Agrochemical Products. Front.Toxicol. 2022, 4, 852856. [Google Scholar] [CrossRef] [PubMed]
- Mohoric, T.; Wilm, A.; Onken, S.; Milovich, A.; Logavoch, A.; Ankli, P.; Tagorti, G.; Kirchmair, J.; Schepky, A.; Kühnl, J.; et al. Increasing Accessibility of Bayesian Network-Based Defined Approaches for Skin Sensitisation Potency Assessment. Toxics 2024, 12, 666. [Google Scholar] [CrossRef]
- To, K.T.; Strickland, J.; Reinke, E.; Borrel, A.; Truax, J.; Maldonado, H.; Allen, D.; Kleinstreuer, N. Computational application of internationally harmonized defined approaches to skin sensitization: DASS App. BMC Bioinform. 2024, 25, 4. [Google Scholar] [CrossRef]
- Bernauer, U.; Bodin, L.; Chaudhry, Q.; Coenraads, P.J.; Dusinska, M.; Ezendam, J.; Gaffet, E.; Galli, C.L.; Panteri, E.; Rogiers, V.; et al. The SCCS Notes of Guidance for the Testing of Cosmetic Ingredients and Their Safety Evaluation: 12th Revision; Publications Office of the European Union: Luxembourg, 2024; Available online: https://hal.science/hal-04100663/ (accessed on 12 June 2025).
- Reinke, E.N.; Reynolds, J.; Gilmour, N.; Reynolds, G.; Strickland, J.; Germolec, D.; Allen, D.G.; Maxwell, G.; Kleinstreuer, N.C. The skin allergy risk assessment-integrated chemical environment (SARA-ICE) defined approach to derive points of departure for skin sensitization. Curr. Res. Toxicol. 2025, 8, 100205. [Google Scholar] [CrossRef]
- Stucki, A.O.; Barton-Maclaren, T.S.; Bhuller, Y.; Henriquez, J.E.; Henry, T.R.; Hirn, C.; Miller-Holt, J.; Nagy, E.G.; Perron, M.M.; Ratzlaff, D.E.; et al. Use of new approach methodologies (NAMs) to meet regulatory requirements for the assessment of industrial chemicals and pesticides for effects on human health. Front. Toxicol. 2022, 4, 964553. [Google Scholar] [CrossRef]
- Haber, L.T.; Bradley, M.A.; Buerger, A.N.; Behrsing, H.; Burla, S.; Clapp, P.W.; Dotson, S.; Fisher, C.; Genco, K.R.; Kruszewski, F.H.; et al. New approach methodologies (NAMs) for the in vitro assessment of cleaning products for respiratory irritation: Workshop report. Front. Toxicol. 2024, 6, 1431790. [Google Scholar] [CrossRef]
- Kluxen, F.M.; Roper, C.S.; Jensen, S.M.; Koenig, C.M. Characterizing local acute irritation properties of captan and folpet with new approach methods. Appl. Vitr. Toxicol. 2022, 8, 83–101. [Google Scholar] [CrossRef]
- Roseli, R.B.; Keto, A.B.; Krenske, E.H. Mechanistic Aspects of Thiol Additions to Michael Acceptors: Insights from Computations. WIREs Comput. Mol. Sci. 2023, 13, e1636. [Google Scholar] [CrossRef]
- Gao, S.; Tzeng, T.; Sastry, M.; Chu, C.-M.; Liu, J.-T.; Lin, C.; Yao, C.-F. Iodine catalyzed conjugate addition of mercaptans to α,β-unsaturated carboxylic acids under solvent-free condition. Tetrahedron Lett. 2006, 47, 1889–1893. [Google Scholar] [CrossRef]
- Lutolf, M.P.; Tirelli, N.; Cerritelli, S.; Cavalli, L.; Hubbell, J.A. Systematic modulation of Michael-type reactivity of thiols through the use of charged amino acids. Bioconjug. Chem. 2001, 12, 1051–1056. [Google Scholar] [CrossRef]
- Little, R.D.; Masjedizadeh, M.R.; Wallquist, O.; McLoughlin, J.I. The Intramolecular Michael Reaction. Org. React. 1995, 47, 315–552. [Google Scholar] [CrossRef]
- Omeragic, E.; Dedic, M.; Elezovic, A.; Becic, E.; Imamovic, B.; Kladar, N.; Niksic, H. Application of direct peptide reactivity assay for assessing the skin sensitization potential of essential oils. Sci. Rep. 2022, 12, 7470. [Google Scholar] [CrossRef] [PubMed]
- Kern, P.S.; Ellingson, K.; Gao, Y.; Krutz, N.L.; Krivos, K.; Quijano, M.; Xu, Y.; Ryan, C.A. Development of a peptide reactivity assay for screening botanicals and natural substances: Proof of concept studies. Toxicol. Vitr. 2023, 90, 105591. [Google Scholar] [CrossRef]
- Deckers, J.; Hammad, H.; Hoste, E. Langerhans Cells: Sensing the Environment in Health and Disease. Front. Immunol. 2018, 9, 93. [Google Scholar] [CrossRef]
- van Endert, P. Editorial: Insights in antigen presenting cell biology: 2021. Front. Immunol. 2022, 13, 1079913. [Google Scholar] [CrossRef]
- Mitachi, T.; Kouzui, M.; Maruyama, R.; Yamashita, K.; Ogata, S.; Kojima, H.; Itagaki, H. Some non-sensitizers upregulate CD54 expression by activation of the NLRP3 inflammasome in THP-1 cells. J. Toxicol. Sci. 2019, 44, 213–224. [Google Scholar] [CrossRef]
- Correa, M.C.M.; Mao, G.; Saad, P.; Flach, C.R.; Mendelsohn, R.; Walters, R.M. Molecular interactions of plant oil components with stratum corneum lipids correlate with clinical measures of skin barrier function. Exp. Dermatol. 2014, 23, 39–44. [Google Scholar] [CrossRef]
- Darmstadt, G.L.; Mao-Qiang, M.; Chi, E.; Saha, S.K.; Ziboh, V.A.; Black, R.E.; Santosham, M.; Elias, P.M. Impact of topical oils on the skin barrier: Possible implications for neonatal health in developing countries. Acta Paediatr. 2002, 91, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Lodén, M.; Andersson, A.C. Effect of topically applied lipids on surfactant-irritated skin. Br. J. Dermatol. 1996, 134, 215–220. [Google Scholar] [CrossRef]
- Som, I.; Bhatia, K.; Yasir, M. Status of surfactants as penetration enhancers in transdermal drug delivery. J. Pharm. Bioallied Sci. 2012, 4, 2–9. [Google Scholar] [CrossRef]
- Leanpolchareanchai, J.; Teeranachaideekul, V. Topical Microemulsions: Skin Irritation Potential and Anti-Inflammatory Effects of Herbal Substances. Pharmaceuticals 2023, 16, 999. [Google Scholar] [CrossRef]
- Robinson, M.K.; Cohen, C.; de Fraissinette Ade, B.; Ponec, M.; Whittle, E.; Fentem, J.H. Non-animal testing strategies for assessment of the skin corrosion and skin irritation potential of ingredients and finished products. Food Chem. Toxicol. 2002, 40, 573–592. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Toh, P.Z.; Tan, J.Y.; Zin, M.T.; Lee, C.-Y.; Li, B.; Leolukman, M.; Bao, H.; Kang, L. Selected Biomarkers Revealed Potential Skin Toxicity Caused by Certain Copper Compounds. Sci. Rep. 2016, 6, 37664. [Google Scholar] [CrossRef] [PubMed]
- Murthy, P.B.; Kishore, A.S.; Surekha, P. Assessment of in vitro skin irritation potential of nanoparticles: RHE model. Methods Mol. Biol. 2012, 926, 219–234. [Google Scholar] [CrossRef]
- Gugleva, V.; Ivanova, N.; Sotirova, Y.; Andonova, V. Dermal Drug Delivery of Phytochemicals with Phenolic Structure via Lipid-Based Nanotechnologies. Pharmaceuticals 2021, 14, 837. [Google Scholar] [CrossRef]
- Korkina, L.G. Phenylpropanoids as naturally occurring antioxidants: From plant defense to human health. Cell. Mol. Biol. 2007, 53, 15–25. [Google Scholar] [PubMed]
- Danilenko, D.M.; Phillips, G.D.; Diaz, D. In Vitro Skin Models and Their Predictability in Defining Normal and Disease Biology, Pharmacology, and Toxicity. Toxicol. Pathol. 2016, 44, 555–563. [Google Scholar] [CrossRef]
- do Nascimento Pedrosa, T.; Catarino, C.M.; Pennacchi, P.C.; de Moraes Barros, S.B.; Maria-Engler, S.S. Skin Equivalent Models: Protocols for In Vitro Reconstruction for Dermal Toxicity Evaluation. In Toxicity Assessment. Methods in Molecular Biology; Palmeira, C.M.M., de Oliveira, D.P., Dorta, D.J., Eds.; Humana: New York, NY, USA, 2021; Volume 2240. [Google Scholar] [CrossRef]
- Liu, X.C.; Zhou, L.G.; Liu, Z.L.; Du, S.S. Identification of insecticidal constituents of the essential oil of Acorus calamus rhizomes against Liposcelis bostrychophila Badonnel. Molecules 2013, 18, 5684–5696. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.S.; Padalia, R.C.; Chauhan, A. Chemical Composition of Root Essential Oil of Acorus calamus L. Natl. Acad. Sci. Lett. 2015, 38, 121–125. [Google Scholar] [CrossRef]
- Chandra, D.; Prasad, K. Phytochemicals of Acorus calamus (Sweet flag). J. Med. Plants Stud. 2017, 5, 277–281. [Google Scholar]
- Timilsina, R.; Tandukar, P.; Pathak, I. Biological and Chemical Studies of Essential Oil and Extracts of Rhizome of Acorus calamus Linn. J. Nepal Chem. Soc. 2022, 43, 35–42. [Google Scholar] [CrossRef]
- Sindle, A.; Martin, K. Art of Prevention: Essential Oils—Natural Products Not Necessarily Safe. Int. J. Women’s Dermatol. 2020, 7, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Yap, P.S.X.; Yusoff, K.; Lim, S.-H.E.; Chong, C.-M.; Lai, K.-S. Membrane Disruption Properties of Essential Oils—A Double-Edged Sword? Processes 2021, 9, 595. [Google Scholar] [CrossRef]
- Nejad, S.M.; Özgüneş, H.; Başaran, N. Pharmacological and Toxicological Properties of Eugenol. Turk. J. Pharm. Sci. 2017, 14, 201–206. [Google Scholar] [CrossRef]
- Chiang, S.Y.; Lee, P.Y.; Lai, M.T.; Shen, L.C.; Chung, W.S.; Huang, H.F.; Wu, K.Y.; Wu, H.C. Safrole-2′,3′-oxide induces cytotoxic and genotoxic effects in HepG2 cells and in mice. Mutat. Res. 2011, 726, 234–241. [Google Scholar] [CrossRef]
- Hu, L.; Wu, F.; He, J.; Zhong, L.; Song, Y.; Shao, H. Cytotoxicity of safrole in HepaRG cells: Studies on the role of CYP1A2-mediated ortho-quinone metabolic activation. Xenobiotica 2019, 49, 1504–1515. [Google Scholar] [CrossRef]
- Ahn, J.; Avonto, C.; Pandey, P.; Khan, S.I.; Khan, I.A.; Roberts, D.W.; Chittiboyina, A.G. Chemistry of Isoeugenol and Its Oxidation Products: Mechanism and Kinetics of Isoeugenol as a Skin Sensitizer. Chem. Res. Toxicol. 2023, 36, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Port-Lougarre, Y.; Gourlaouen, C.; Vileno, B.; Giménez-Arnau, E. Antioxidant Activity and Skin. Sensitization of Eugenol and Isoeugenol: Two Sides of the Same Coin? Chem. Res. Toxicol. 2023, 36, 1804–1813. [Google Scholar] [CrossRef] [PubMed]
- Natsch, A.; Haupt, T. Utility of rat liver S9 fractions to study skin-sensitizing prohaptens in a modified KeratinoSens assay. Toxicol. Sci. 2013, 135, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Harada, K.; Ohmori, S.; Minamoto, K.; Wei, C.; Ueda, A. Toxicity study of the volatile constituents of Myoga utilizing acute dermal irritation assays and the Guinea-pig Maximization test. J. Occup. Health 2006, 48, 480–486. [Google Scholar] [CrossRef]
- Wei, Q.J.; Wei, C.N.; Harada, K.; Minamoto, K.; Okamoto, Y.; Otsuka, M.; Ueda, A. Evaluation of allergenicity of constituents of myoga using the murine local lymph node assay. Int. J. Immunopathol. Pharmacol. 2010, 23, 463–470. [Google Scholar] [CrossRef]
- Lan, Y.; Wang, J.Y.; Liu, Y.; Ru, Q.G.; Wang, Y.F.; Yu, J.X.; Wu, Q. Effect of terpene penetration enhancer and its mechanisms on membrane fluidity and potential of HaCaT keratinocytes. China J. Chin. Mater. Med. 2015, 40, 643–648. (In Chinese) [Google Scholar]
- Nieto, G.; Ros, G.; Castillo, J. Antioxidant and Antimicrobial Properties of Rosemary (Rosmarinus officinalis, L.): A Review. Medicines 2018, 5, 98. [Google Scholar] [CrossRef]
- Lee, C.L.; Lo, P.T.; Jhan, Y.L.; Chen, C.J.; Chang, Y.S. New ent-kauran diterpene and antioxidant components from the seed of Ipomoea nil. Nat. Prod. Res. 2021, 35, 2551–2557. [Google Scholar] [CrossRef] [PubMed]
- Raabe, H.A.; Costin, G.E.; Allen, D.G.; Lowit, A.; Corvaro, M.; O’Dell, L.; Sullivan, K. Human relevance of in vivo and in vitro skin irritation tests for hazard classification of pesticides. Cutan. Ocul. Toxicol. 2024, 44, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Liu, S.; Deng, W. Dual responsive linalool capsules with high loading ratio for excellent antioxidant and antibacterial efficiency. Colloids Surf. B Biointerfaces 2020, 190, 110978. [Google Scholar] [CrossRef] [PubMed]
- Haro-González, J.N.; Castillo-Herrera, G.A.; Martínez-Velázquez, M.; Espinosa-Andrews, H. Clove Essential Oil (Syzygium aromaticum L. Myrtaceae): Extraction, Chemical Composition, Food Applications, and Essential Bioactivity for Human Health. Molecules 2021, 26, 6387. [Google Scholar] [CrossRef]
- Api, A.M.; Vey, M. Implementation of the dermal sensitization Quantitative Risk Assessment (QRA) for fragrance ingredients. Regul. Toxicol. Pharmacol. 2008, 52, 53–61. [Google Scholar] [CrossRef]
- Api, A.M.; Basketter, D.; Bridges, J.; Cadby, P.; Ellis, G.; Gilmour, N.; Greim, H.; Griem, P.; Kern, P.; Khaiat, A.; et al. Updating exposure assessment for skin sensitization quantitative risk assessment for fragrance materials. Regul. Toxicol. Pharmacol. 2020, 118, 104805. [Google Scholar] [CrossRef]
- Tourneix, F.; Carron, L.; Jouffe, L.; Hoffmann, S.; Alépée, N. Deriving a Continuous Point of Departure for Skin Sensitization Risk Assessment Using a Bayesian Network Model. Toxics 2024, 12, 536. [Google Scholar] [CrossRef]
- Ebmeyer, J.; Najjar, A.; Lange, D.; Boettcher, M.; Voß, S.; Brandmair, K.; Meinhardt, J.; Kuehnl, J.; Hewitt, N.J.; Krueger, C.T.; et al. Next generation risk assessment: An ab initio case study to assess the systemic safety of the cosmetic ingredient, benzyl salicylate, after dermal exposure. Front. Pharmacol. 2024, 15, 1345992. [Google Scholar] [CrossRef]
- Na, M.; O’Brien, D.; Lavelle, M.; Lee, I.; Gerberick, G.F.; Api, A.M. Weight of Evidence Approach for Skin Sensitization Potency Categorization of Fragrance Ingredients. Dermatitis 2022, 33, 161–175. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).