Do Soil pH Levels Drive the Responses of Catalase Activity and Bacterial Communities to Microplastics? A Case Study in Mollisols
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Samples and MPs
2.2. Experimental Layout and Soil Incubation
2.3. Soil Physicochemical Properties
2.4. Soil CAT and Its Kinetic and Thermodynamic Analysis
2.5. DNA Extraction, Amplification, and Sequencing
2.6. Statistical Analysis
3. Results
3.1. Soil Physicochemical Properties
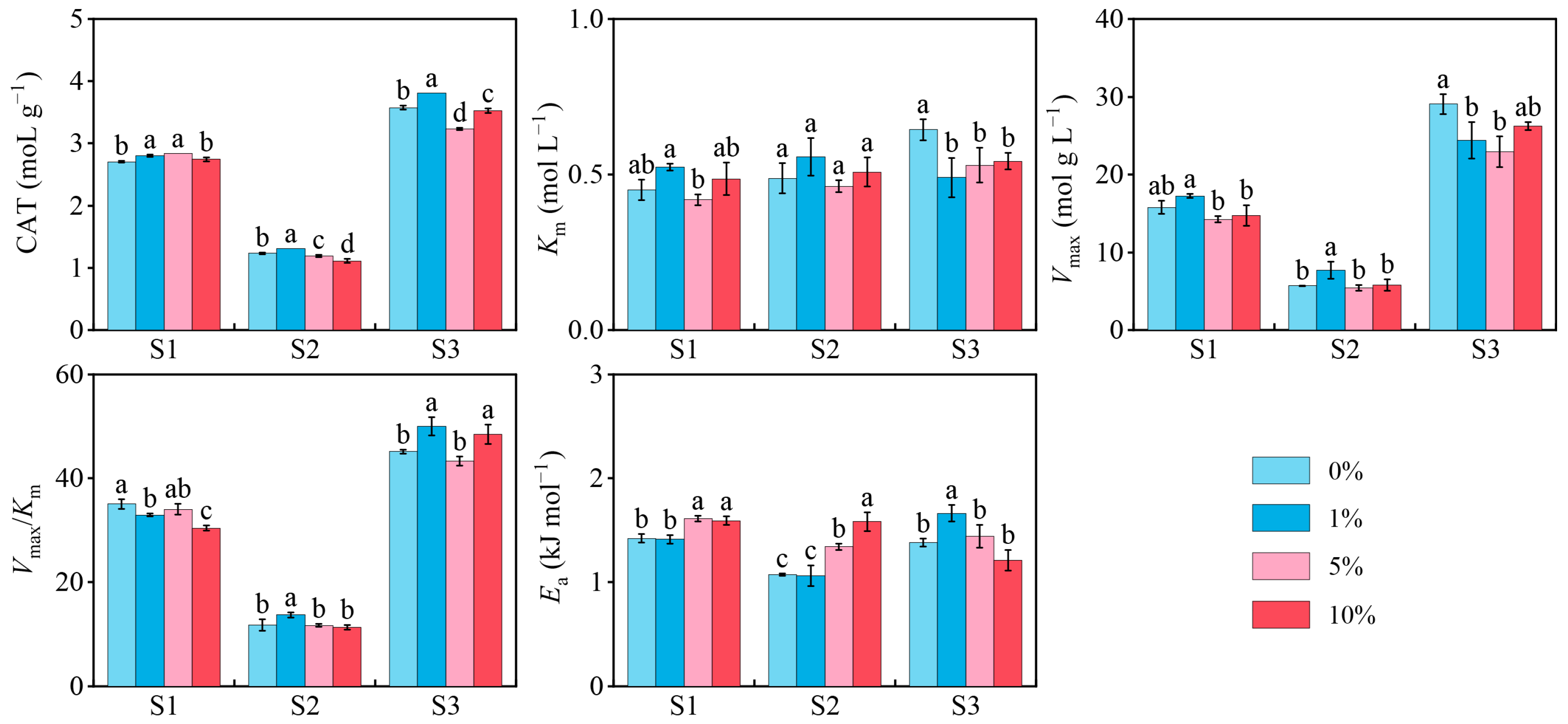
3.2. Soil CAT Activity
3.3. Soil CAT Kinetics and Thermodynamics
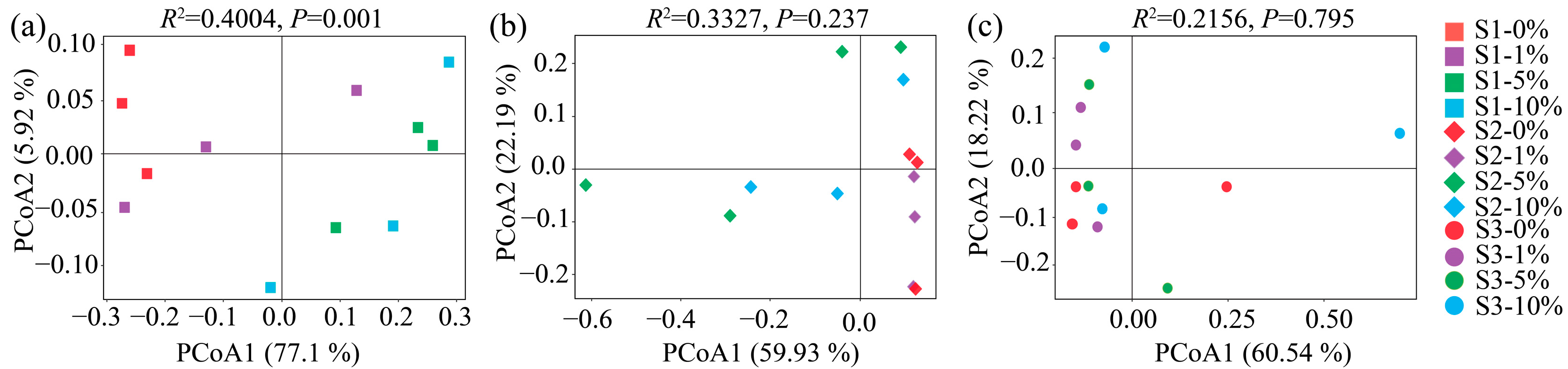
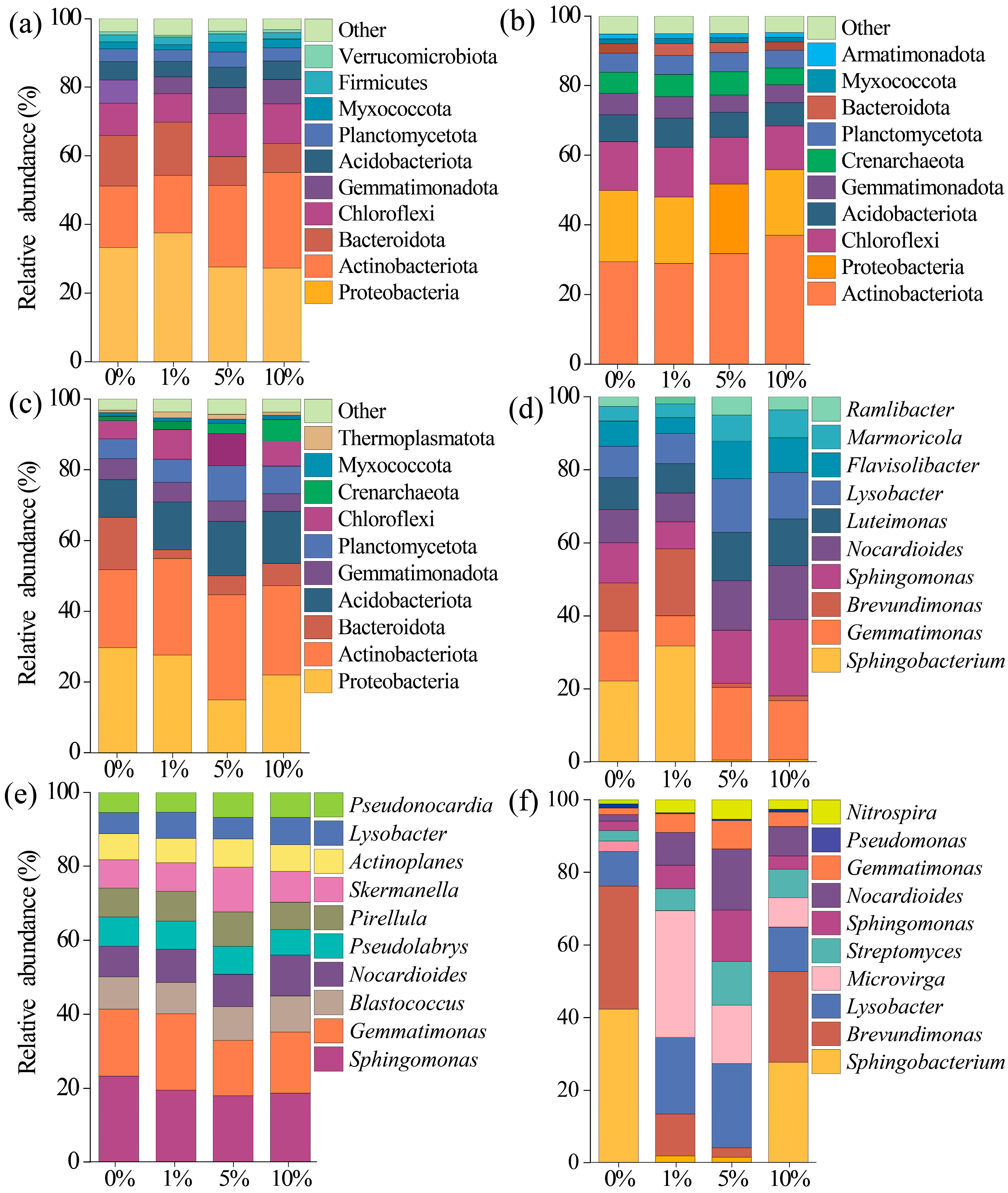
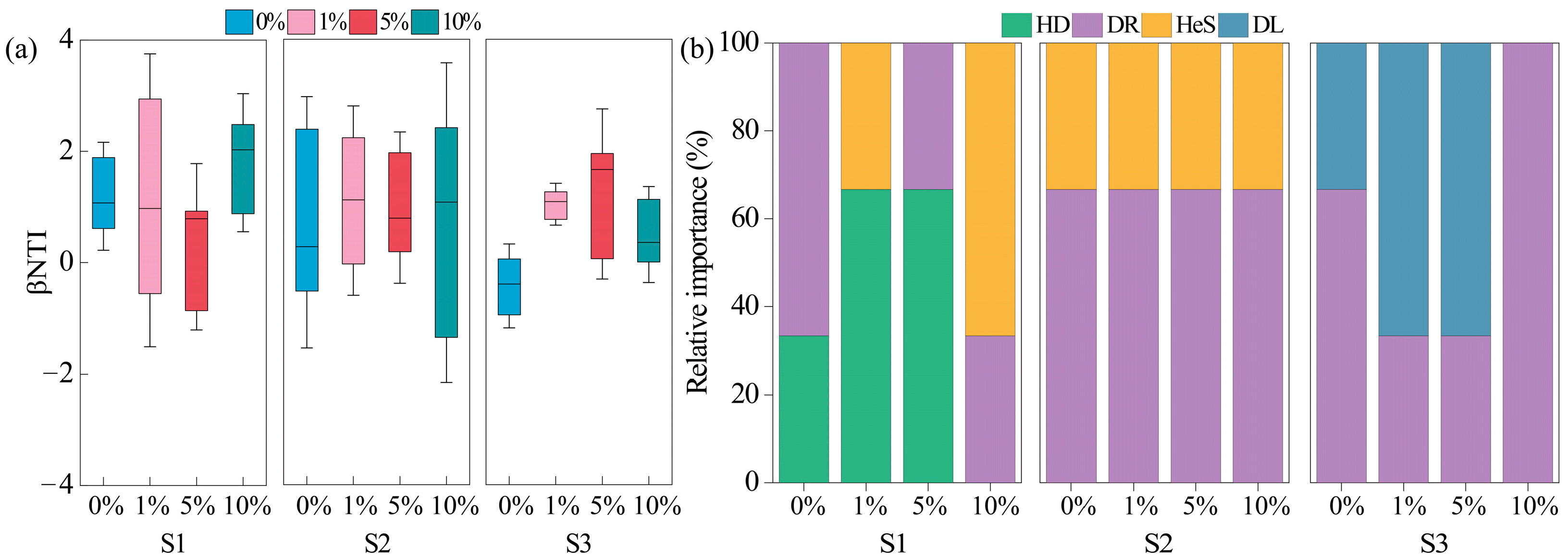
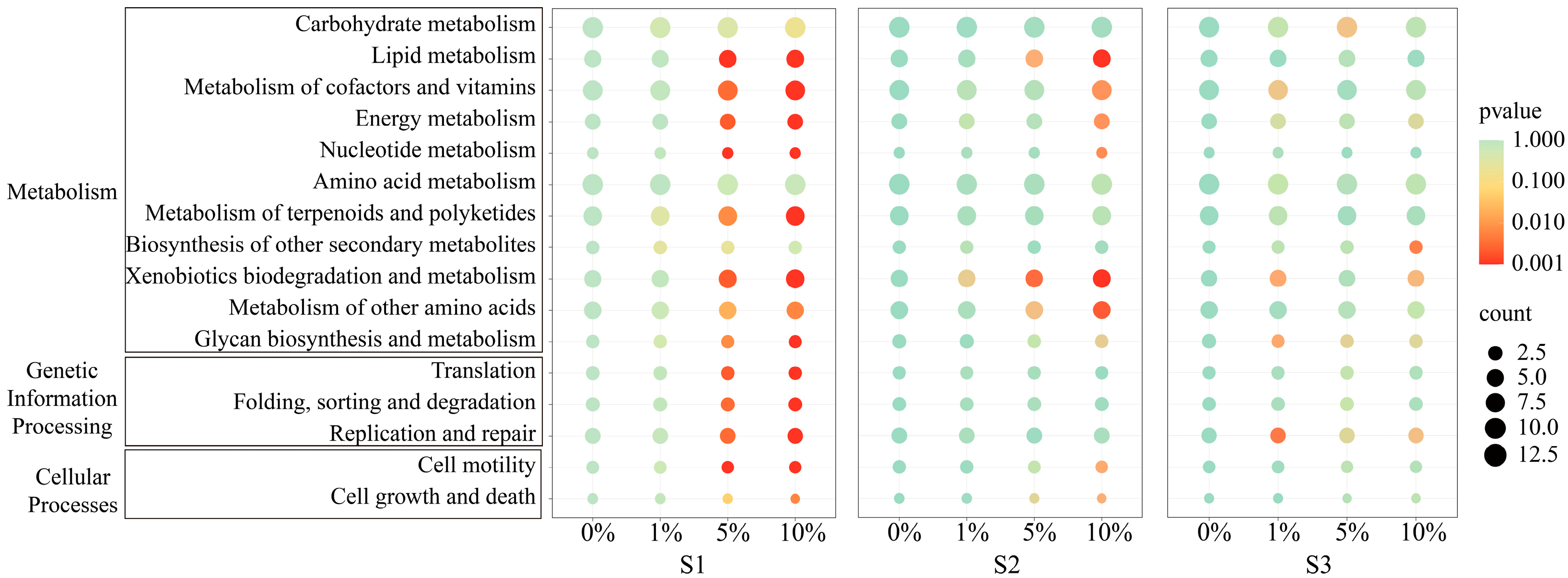
3.4. Soil Bacteria Diversity, Composition, and Assembly
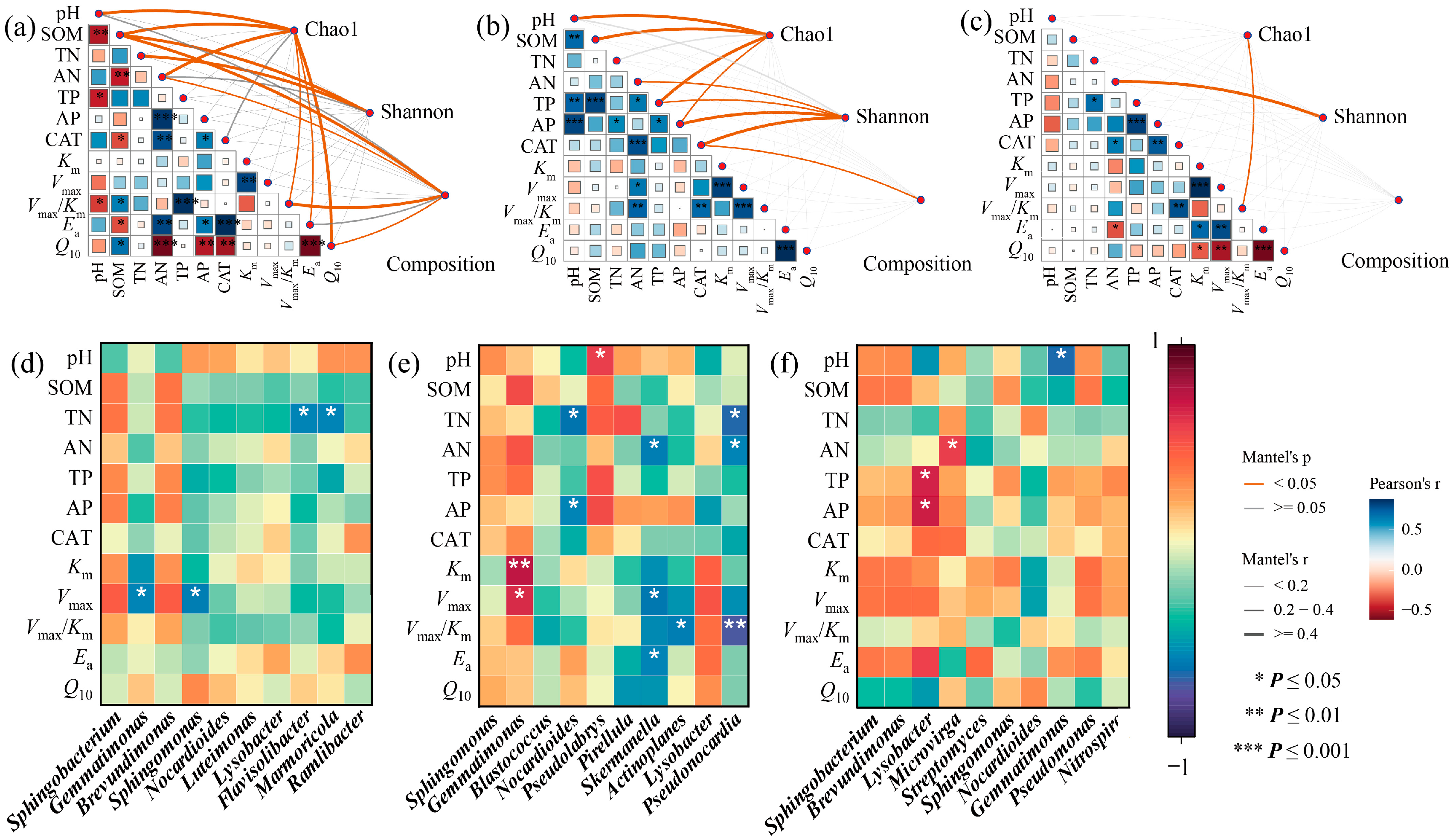
3.5. Connections Between Environmental Variables and Soil Bacterial Communities
4. Discussion
4.1. Response of CAT Activity and Enzymatic Reaction Characteristics in Three Soils to MPs
4.2. Response of Bacteria in Three Soils to MPs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, X.B.; Sun, M.X.; Zhang, L.X.; Xue, Y.H.; Li, C.; Ma, S.Y. Research Progress and Prospects on Soil Microplastic Pollution. J. Agric. Resour. Environ. 2021, 38, 1–9. [Google Scholar] [CrossRef]
- Zhou, B.Y.; Wang, J.Q.; Zhang, H.B.; Shi, H.H.; Fei, Y.F.; Huang, S.Y.; Tong, Y.Z.; Wen, D.S.; Luo, Y.M.; Barceló, D. Microplastics in Agricultural Soils on the Coastal Plain of Hangzhou Bay, East China: Multiple Sources Other Than Plastic Mulching Film. J. Hazard. Mater. 2020, 388, 121814. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.Q.; Chen, X.G.; Zhang, B.C.; Meng, H.W.; Jiang, P.; Peng, X.B. Current Status, Problems, and Countermeasures for Recycling and Resource Utilization of Plastic Mulch Residues in Xinjiang Cotton Fields. Trans. Chin. Soc. Agric. Eng. 2019, 35, 1–13. [Google Scholar]
- Chae, Y.; An, Y.J. Current Research Trends on Plastic Pollution and Ecological Impacts on the Soil Ecosystem: A Review. Environ. Pollut. 2018, 240, 387–395. [Google Scholar] [CrossRef]
- Demetres, B. Agricultural Plastics as a Potential Threat to Food Security, Health, and Environment through Soil Pollution by Microplastics: Problem Definition. Sci. Total Environ. 2023, 892, 164533. [Google Scholar] [CrossRef]
- Zhang, Y.; Wen, X.F.; Zhou, W.C.; Yang, Y.; Zhou, Z.L.; Chen, J.Y.; Wang, X.Y.; Wang, Y.; Tian, J.Y.; Yuan, Y.; et al. Retention and Migration of Microplastics in Stepped Paddy Fields: A Study on Microplastic Dynamics in the Special Irrigation System. Environ. Res. 2025, 269, 120909. [Google Scholar] [CrossRef]
- An, Q.; Zheng, N.; Chen, C.; Li, X.; Ji, Y.; Peng, L.; Xiu, Z.; Lin, Q. Regulation Strategies of Microplastics with Different Particle Sizes on Cadmium Migration Processes and Toxicity in Soil-pakchoi System. J. Hazard. Mater. 2025, 488, 137505. [Google Scholar] [CrossRef]
- Sander, M. Biodegradation of Polymeric Mulch Films in Agricultural Soils: Concepts, Knowledge Gaps, and Future Research Directions. Environ. Sci. Technol. 2019, 53, 2304–2315. [Google Scholar] [CrossRef]
- Li, S.T.; Ding, F.; Flury, M.; Wang, Z.; Xu, L.; Li, S.Y.; Jones, D.L.; Wang, J.K. Macro and Microplastic Accumulation in Soil after 32 Years of Plastic Film Mulching. Environ. Pollut. 2022, 300, 118945. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.F.; Jin, Y.Y. Effects of Microplastics Migration on Farmland Soil Physical and Chemical Properties under Leaching Conditions. Water Air Soil Pollut. 2024, 236, 40. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.Q.; Tian, H.X.; Megharaj, M.; Jia, H.Z.; He, W.X. Using Soil Enzyme Vmax as an Indicator to Evaluate the Ecotoxicity of Lower-ring Poly Cyclic Aromatic Hydrocarbons in Soil: Evidence from Fluorescein Diacetate Hydrolase Kinetics. Sci. Total Environ. 2023, 874, 162521. [Google Scholar] [CrossRef]
- Tourinho, P.S.; Loureiro, S.; Pavlaki, M.D.; Mocová Klará, A.; Ribeiro, F. A Systematic Review of Nano-and Microplastic (NMP) Influence on the Bioaccumulation of Environmental Contaminants: Part I—Soil Organisms. Toxics 2023, 11, 154. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Wen, J.H.; Liu, Z.X.; Wei, H.; Zhang, J.E. Polyethylene Microplastics Alter Soil Microbial Community Assembly and Ecosystem Multifunctionality. Environ. Int. 2024, 183, 108360. [Google Scholar] [CrossRef]
- Li, W.Y.; Luo, D.; Yan, N.; Miao, L.Z.; Adyel, T.M.; Kong, M.; Hou, J. Effects of Polyethylene Microplastics with Different Particle Sizes and Concentrations on the Community Structure and Function of Periphytic Biofilms. J. Environ. Chem. Eng. 2023, 11, 111287. [Google Scholar] [CrossRef]
- Josipa, P.Z.; Stefani, T.; Anamarija, P.; Katančić, Z.; Kovačić, M.; Kušić, H.; Zlata, H.M.; Ana, L.B. Effect of Aging on Physicochemical Properties and Size Distribution of PET Microplastic: Influence on Adsorption of Diclofenac and Toxicity Assessment. Toxics 2023, 11, 615. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, Y.R.; Wang, J.; Zhang, M.J.; Jia, W.Q.; Qin, X. LDPE Microplastic Films Alter Microbial Community Composition and Enzymatic Activities in Soil. Environ. Pollut. 2019, 254, 112983. [Google Scholar] [CrossRef]
- De Souza Machado, A.A.; Lau, C.W.; Till, J.; Kloas, W.; Lehmann, A.; Becker, R.; Rillig, M.C. Impacts of Microplastics on the Soil Biophysical Environment. Environ. Sci. Technol. 2018, 52, 9656–9665. [Google Scholar] [CrossRef]
- Zhu, F.X.; Yan, Y.Y.; Doyle, E.; Zhu, C.Y.; Jin, X.; Chen, Z.H.; Wang, C.; He, H.; Zhou, D.M.; Gu, C. Microplastics Altered Soil Microbiome and Nitrogen Cycling: The Role of Phthalate Plasticizer. J. Hazard. Mater. 2021, 427, 127944. [Google Scholar] [CrossRef]
- Wu, C.C.; Ma, Y.J.; Wang, D.; Shan, Y.P.; Song, X.P.; Hu, H.Y.; Ren, X.L.; Ma, X.Y.; Luo, J.Y.; Cui, J.J.; et al. Microbiology Combined with Metabonomics Revealing the Response of Soil Microorganisms and Their Metabolic Functions Exposed to Phthalic Acid Esters. Ecotox. Environ. Saf. 2022, 233, 113338. [Google Scholar] [CrossRef]
- Zettler, E.R.; Mincer, T.J.; Amaral-Zettler, L.A. Life in the “Plastisphere”: Microbial Communities on Plastic Marine Debris. Environ. Sci. Technol. 2013, 47, 7137–7146. [Google Scholar] [CrossRef]
- Ma, W.; Wang, J. Effect of Polypropylene Microplastics on Soil Enzyme Activity in Soil Microbiotic Systems. J. Zhejiang Univ. Technol. 2022, 50, 216–221. [Google Scholar]
- Awet, T.T.; Kohl, Y.; Meier, F.; Straskraba, S.; Grün, A.L.; Ruf, T.; Jost, C.; Drexel, R.; Tunc, E.; Emmerling, C. Effects of Polystyrene Nanoparticles on the Microbiota and Functional Diversity of Enzymes in Soil. Environ. Pollut. 2018, 30, 11. [Google Scholar] [CrossRef]
- Kaushal, J.; Singh, S.G.; Raina, A.; Shailendra, K.A. Catalase Enzyme: Application in Bioremediation and Food Industry. Biocatal. Agric. Biotech. 2018, 16, 192–199. [Google Scholar] [CrossRef]
- Pokharel, P.; Ma, Z.L.; Chang, S.X. Correction to: Biochar Increases Soil Microbial Biomass with Changes in Extra- and Intracellular Enzyme Activities: A Global Meta-analysis. Biochar 2020, 3, 715. [Google Scholar] [CrossRef]
- Nwaogu, L.A.; Ujowundu, C.O.; Iheme, C.I.; Ezejiofor, N.I.; Belonwu, D.C. Effect of Sublethal Concentration of Heavy Metal Contamination on Soil Physicochemical Properties, Catalase and Dehydrogenase Activities. Int. J. Biochem. Res. Rev. 2014, 4, 141–149. [Google Scholar] [CrossRef]
- Yu, L.; Cheng, J.M. Effect of Heavy Metals Cu, Cd, Pb and Zn on Enzyme Activity and Microbial Biomass Carbon in Brown Soil. Adv. Mater. Res. 2014, 1073–1076, 726–730. [Google Scholar] [CrossRef]
- Zheng, L.G.; Yang, L.; Shang, W.Q.; Dong, X.L.; Tang, Q.; Cheng, H. The Inhibitory Effect of Cadmium and/or Mercury on Soil Enzyme Activity, Basal Respiration, and Microbial Community Structure in Coal Mine–Affected Agricultural Soil. Ann. Microbiol. 2019, 69, 849–859. [Google Scholar] [CrossRef]
- Stone, M.M.; Plante, A.F. Changes in Phosphatase Kinetics with Soil Depth Across a Variable Tropical Landscape. Soil Biol. Biochem. 2014, 71, 61–67. [Google Scholar] [CrossRef]
- Allison, S.D.; Romero-Olivares, A.L.; Lu, Y.; Taylor, J.W.; Treseder, K.K. Temperature Sensitivities of Extracellular Enzyme Vmax and Km Across Thermal Environments. Glob. Change Biol. 2018, 24, 2884–2897. [Google Scholar] [CrossRef]
- Tao, K.L.; Tian, H.X.; Fan, J.; Li, D.X.; Liu, C.Y.; Megharaj, M.l.; Li, H.Y.; Hu, M.; Jia, H.Z.; He, W.X. Kinetics and Catalytic Efficiency of Soil Fluorescein Diacetate Hydrolase under the Pesticide Parathion Stress. Sci. Total Environ. 2021, 771, 144835. [Google Scholar] [CrossRef]
- Wu, J.Y.; Wu, Z.W.; Agathokleous, E.; Zhu, Y.L.; Fan, D.W.; Han, J.G. Unveiling a New Perspective on Cadmium-induced Hormesis in Soil Enzyme Activity: The Relative Importance of Enzymatic Reaction Kinetics and Microbial Communities. Agriculture 2024, 14, 904. [Google Scholar] [CrossRef]
- Liu, K.; Wei, M.H.; Dai, H.M.; Liu, G.D.; Jia, S.H.; Song, Y.H. Spatial and Temporal Changes in the Khickness of the Black Soil Layer in the Northeast Black Soil Region. Geol. Resour. 2022, 31, 434–442+394. [Google Scholar]
- Liu, X.T.; Yan, B.X. Soil erosion and food security in the black soil region of Northeast China. J. Soil Water Conser. 2019, 1, 17–19. [Google Scholar]
- Cui, M.; Zhang, X.D.; Cai, Q.G.; Wang, Y.; Fan, H.M.; Zhou, J.X. Relationships between Climate, Geomorphological Evolution, and Black Soil Development in the Typical Black SoilRegion of Northeast China. Geogr. Res. 2008, 27, 527–535. [Google Scholar]
- Hu, X.J.; Gu, H.D.; Wang, Y.B.; Liu, J.J.; Yu, Z.H.; Li, Y.S.; Jin, J.; Liu, X.B.; Dai, Q.W.; Wang, G.G. Succession of Soil Bacterial Communities and Network Patterns in Response to Conventional and Biodegradable Microplastics: A Microcosmic Study in Mollisol. J. Hazard. Mater. 2022, 436, 129218. [Google Scholar] [CrossRef]
- Fuller, S.; Gautam, A.A. Procedure for Measuring Microplastics Using Pressurized Fluid Extraction. Environ. Sci. Technol. 2016, 50, 5774–5780. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Kaur, M.; Yao, Z.S.; Chen, T.Z.; Xu, M. Phytotoxic Effects of Polyethylene Microplastics on the Growth of Food Crops Soybean (Glycine max) and Mung Bean (Vigna radiata). Int. J. Environ. Res. Public Health 2021, 18, 10629. [Google Scholar] [CrossRef]
- Liu, Y.H.; Xiao, M.L.; Shahbaz, M.; Hu, Z.E.; Zhu, Z.K.; Lu, S.B.; Yu, Y.X.; Yao, H.Y.; Chen, J.P.; Ge, T.D. Microplastics in Soil Can Increase Nutrient Uptake by Wheat. J. Hazard. Mater. 2022, 438, 129547. [Google Scholar] [CrossRef]
- Liu, S.; Huang, J.H.; He, W.J.; Shi, L.X.; Zhang, W.; Li, E.J.; Hu, J.Y.; Zhang, C.Y.; Pang, H.L. Insights into Effects of Drying-wetting Cycles on Dissolved Organic Matter and Cd Bioavailability in Riparian Sediments Amended with microplastics. Environ. Res. 2025, 271, 121087. [Google Scholar] [CrossRef]
- Bao, S.D. Analysis of Soil Agrochemistry, 3rd ed.; Chinese Agricultural Science and Technology Press: Beijing, China, 1981. [Google Scholar]
- Guan, S.Y.; Zhang, D.S.; Zhang, Z.M. Soil Enzymes and Its Methodology; Agricultural Press: Beijing, China, 1986. [Google Scholar]
- Gao, H.J.; Zhu, P.; Peng, C.; Zhang, X.Z.; Li, Q.; Zhang, W.J. Effects of Different Fertilization Methods on Water and Heat Characteristics of Soil in Spring Corn Fields in Northeast China. J. Soil Water Conserv. 2015, 29, 195–200. [Google Scholar]
- Guo, Y.X.; Zhao, J.Y.; Li, J.W.; Dong, Y.L.; Yue, Z.H.; Yin, Y.; Li, W.; Ren, Q.N.; Wu, X.Y. Straw, Biochar, and Nanocarbon Altered the Enzymatic Reaction Kinetics and Thermodynamic Process of Catalase in the Black Soil under Continuous Warming. Arch. Agron. Soil Sci. 2023, 69, 3637–3650. [Google Scholar] [CrossRef]
- Zhu, M.E. Soil Enzyme Kinetics and Thermodynamics, 1st ed.; Science Press: Beijing, China, 2011. [Google Scholar]
- Ma, Y.W.; Wang, H.Q.; Kang, Y.L.; Wen, T. Small Molecule Metabolites Drive Plant Rhizosphere Microbial Community Assembly Patterns. Front. Microbiol. 2025, 16, 1503537. [Google Scholar] [CrossRef]
- Ni, Y.Y.; Yang, T.; Ma, Y.Y.; Zhang, K.P.; Soltis, P.S.; Soltis, D.E.; Gilbert, J.A.; Zhao, Y.P.; Fu, C.X.; Chu, H.Y. Soil pH Determines Bacterial Distribution and Assembly Processes in Natural Mountain Forests of Eastern China. Glob. Ecol. Biogeogr. 2021, 30, 2164–2177. [Google Scholar] [CrossRef]
- Langille, M.G.I.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R.; et al. Predictive Functional Profiling of Microbial Communities Using 16s rRNA Marker Gene Sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef]
- Zhang, R.; Song, Z.; Fu, W.; Yun, L.; Gao, J.; Wang, R.; Wang, Z.; Zhang, G. Effects of Vegetation Restoration on the Structure and Function of the Rhizosphere Soil Bacterial Community of Solanum Rostratum. Chin. J. Environ. Sci. 2021, 42, 433–442. [Google Scholar] [CrossRef]
- Fan, P.; Tan, W.B.; Yu, H. Effects of Different Concentrations and Tpes of Microplastics on Bacteria and Fungi in Alkaline Soil. Ecotoxicol. Environ. Saf. 2022, 229, 113045. [Google Scholar] [CrossRef]
- Ma, X.; Tian, H.X.; Dai, Y.C.; Yang, Y.Z.; Megharaj, M.; He, W.X. Respecting Catalytic Efficiency of Soil Arylsulfatase as Soil Sb Contamination Bio-indicator by Enzyme Kinetic Strategy. Environ. Sci. Pollut. Res. Int. 2022, 30, 17644–17656. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Chen, L.J.; Sun, C.X.; Wu, Z.J.; Chen, Z.H.; Dong, G.H. Soil Hydrolase Activities and Kinetic Properties as Affected by Wheat Cropping Systems of Northeastern China. Plant Soil Environ. 2010, 56, 526–532. [Google Scholar] [CrossRef]
- German, D.P.; Weintraub, M.N.; Stuart, G.A.; Lauber, C.L.; Rinkes, Z.L.; Allison, S.D. Optimization of Hydrolytic and Oxidative Enzyme Methods for Ecosystem Studies. Soil Biol. Biochem. 2011, 43, 1387–1397. [Google Scholar] [CrossRef]
- Trasar-Cepeda, C.; Gil-Sotres, F.; Leirós, M.C. Thermodynamic Parameters of Enzymes in Grassland Soils from Galicia, NW Spain. Soil Biol. Biochem. 2006, 39, 311–319. [Google Scholar] [CrossRef]
- Kujur, M.; Patel, A.K. Kinetics of Soil Enzyme Activities under Different Ecosystems: An Index of Soil Quality. Chil. J. Agric. Res. 2014, 74, 96–104. [Google Scholar] [CrossRef]
- Liu, C.Y.; Tian, H.X.; Gu, X.Y.; Li, N.; Zhao, X.N.; Lei, M.; Alharbi, H.; Megharaj, M.; He, W.X.; Kuzyakov, Y. Catalytic Efficiency of Soil Enzymes Explains Temperature Sensitivity: Insights from Physiological Theory. Sci. Total Environ. 2022, 822, 153365. [Google Scholar] [CrossRef]
- Mwazembe, D.; Mortley, D.G.; Shange, R.; Ankumah, R.O.; Idehen, O.; Quansah, J.; Fall, S.; Santhosh-Mithra, V.S. Temperature and Cultivar Influence on Enzyme Activity and Composition of the Microbial Community in the Rhizosphere of Sweet Ptato during Early Growth Stage. Int. J. Environ. Clim. Change 2025, 15, 326–342. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, J.; Huang, L.; He, M.Z.; Yang, H.T.; Song, G.; Zhao, J.C.; Li, X.R. Microplastic Transport during Desertification in Drylands: Abundance and Characterization of Soil Microplastics in the Amu Darya-Aral Sea Basin, Central Asia. J. Environ. Manag. 2023, 348, 119353. [Google Scholar] [CrossRef]
- Feng, Q.; Chen, Z.; Huang, G.H.; An, C.J.; Yang, X.H.; Wang, Z. Prolonged Drying Impedes the Detachment of Microplastics in Unsaturated Substrate: Role of Flow Regimes. Water Res. 2024, 252, 121246. [Google Scholar] [CrossRef]
- Lozano, Y.M.; Aguilar-Trigueros, C.A.; Onandia, G.; Maaß, S.; Zhao, T.; Rillig, M.C. Effects of Microplastics and Drought on Soil Ecosystem Functions and Multifunctionality. J. Appl. Ecol. 2021, 58, 988–996. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, T.Y.; Zhang, Q.Q.; Xue, R.; Qu, Y.; Wang, Q.; Dong, Z.J.; Zhao, J.M. Different Patterns of Hypoxia Aggravate the Toxicity of Polystyrene Nanoplastics in the Mussels Mytilus galloprovincialis: Environmental Risk Assessment of Plastics under Global Climate Change. Sci. Total Environ. 2021, 818, 151818. [Google Scholar] [CrossRef]
- Li, Y.R.; Hou, F.W.; Sun, L.L.; Lan, J.; Han, Z.H.; Li, T.T.; Wang, Y.M.; Zhao, Z.S. Ecological Effect of Microplastics on Soil Microbe-driven Carbon Circulation and Greenhouse Gas Emission: A Review. J. Environ. Manag. 2024, 364, 121429. [Google Scholar] [CrossRef]
- Abraham, J.; Ghosh, E.; Mukherjee, P.; Gajendiran, A. Microbial degradation of low density polyethylene. Environ. Prog. Sustain. 2017, 36, 147–154. [Google Scholar] [CrossRef]
- Cesarano, G.; Filippis, F.D.; Storia, A.L.; Scala, F.; Bonanomi, G. Organic Amendment Type and Application Frequency Affect Crop Yields, Soil Fertility and Microbiome Composition. Appl. Soil Ecol. 2017, 120, 254–264. [Google Scholar] [CrossRef]
- De Souza Machado, A.A.; Lau, C.W.; Kloas, W.; Bergmann, J.; Bachelier, J.B.; Faltin, E.; Becker, R.; Görlich, A.S.; Rillig, M.C. Microplastics Can Change Soil Properties and Affect Plant Performance. Environ. Sci. Technol. 2019, 53, 6044–6052. [Google Scholar] [CrossRef]
- Gao, S.H.; Fu, Y.B.; Peng, X.Y.; Ma, S.L.; Liu, Y.R.; Chen, W.L.; Huang, Q.Y.; Hao, X.L. Microplastics Trigger Soil Dissolved Organic Carbon and Nutrient Turnover by Strengthening Microbial Network Connectivity and Cross-Trophic Interactions. Environ. Sci. Technol. 2025, 59, 5596–5606. [Google Scholar] [CrossRef]
- Meng, J.; Li, W.J.; Diao, C.M.; Li, Z.T.; Zhao, J.Y.; Haider, G.; Zhang, H.B.; Xu, J.; Hu, M.J.; Shan, S.D.; et al. Microplastics Drive Microbial Assembly, Their Interactions, and Metagenomic Functions in Two Soils with Distinct pH and Heavy Metal Availability. J. Hazard. Mater. 2023, 458, 131973. [Google Scholar] [CrossRef]
- Fei, Y.F.; Huang, S.Y.; Tong, D.S.; Zhang, H.B.; Tong, Y.Z.; Wen, D.S.; Xia, X.Y.; Wang, H.; Luo, Y.M.; Barceló, D. Response of Soil Enzyme Activities and Bacterial Communities to the Accumulation of Microplastics in an Acid Cropped soil. Sci. Total Environ. 2020, 707, 135634. [Google Scholar] [CrossRef]
- Fang, J.H.; Sheng, Z.H.; Liu, J.; Li, C.C.; L, T.; Wang, Z.Y.; Zhang, H.H. Interference of Microplastics on Autotrophic Microbiome in Paddy Soils: Shifts in Carbon Fixation Rate, Structure, Abundance, Co-occurrence, and Assembly Process. J. Hazard. Mater. 2024, 474, 134783. [Google Scholar] [CrossRef]
- Yan, J.F.; Xiang, L.; Zhang, B.Y.; Tang, C.; Xie, Y.Q.; Li, Y.W.; Feng, N.X.; Liu, B.L.; Li, H.; Cai, Q.Y.; et al. Mechanism and Association between Microbial Ntrogen Transformation in Rhizosphere and Accumulation of Ciprofloxacin in Choysum (Brassica parachinensis). Environ. Sci. Technol. 2023, 57, 16053–16064. [Google Scholar] [CrossRef]
- Sun, Y.Z.; Zhang, M.J.; Duan, C.X.; Cao, N.; Jia, W.Q.; Zhao, Z.L.; Ding, C.F.; Huang, Y.; Wang, J. Contribution of Stochastic Processes to the Microbial Community Assembly on Field-collected Microplastics. Environ. Microbiol. 2021, 23, 6707–6720. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 1999, 27, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Debroas, D.; Mone, A.; Halle, A.T. Plastics in the North Atlantic Garbagepatch: A Boat-microbe for Hitchhikers and Plastic Degraders. Sci. Total Environ. 2017, 599–600, 1222–1232. [Google Scholar] [CrossRef]
- Oh, M.; Yamada, T.; Hattori, M.; Goto, S.; Kanehisa, M. Systematic Analysis of Enzyme-catalyzed Reaction Patterns and Prediction of Microbial Biodegradation Pathways. J. Chem. Inf. Model. 2007, 47, 1702–1712. [Google Scholar] [CrossRef]
- Dijkstra, F.A.; ECarrillo, Y.; EPendall, E.; Morgan, J.A. Rhizosphere Priming: A Nutrient Perspective. Front. Microbiol. 2013, 4, 216. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C.; Leifheit, E.; Lehmann, J. Microplastic Effects on Carbon Cycling Processes in Soils. PLoS Biol. 2021, 19, e3001130. [Google Scholar] [CrossRef] [PubMed]
- Gai, X.; Xing, W.L.; Chen, G.C. Divergent Responses of Rhizosphere Soil Phosphorus Fractions and Biological Features of Salix Psammophila to Fertilization Strategies under Cadmium Contamination. Sci. Total Environ. 2024, 929, 172554. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.R.; Yang, L.; Xu, L.; Yang, J. Soil Microplastic Pollution under Different Land Uses in Tropics, Southwestern China. Chemosphere 2021, 289, 133176. [Google Scholar] [CrossRef]
| Soil Type | Treatment | pH | SOM (g kg−1) | TN (g kg−1) | AN (mg kg−1) | TP (g kg−1) | AP (mg kg−1) |
|---|---|---|---|---|---|---|---|
| S1 | 0% | 5.41 ± 0.05 c | 26.25 ± 0.99 a | 1.24 ± 0.02 a | 56.70 ± 0.70 d | 1.845 ± 0.02 a | 137.898 ± 0.45 d |
| 1% | 5.61 ± 0.10 b | 23.39 ± 0.00 b | 1.23 ± 0.04 a | 68.13 ± 1.07 a | 1.829 ± 0.01 b | 163.469 ± 0.68 a | |
| 5% | 5.80 ± 0.01 a | 22.24 ± 0.99 b | 1.22 ± 0.02 a | 66.03 ± 1.07 b | 1.819 ± 0.01 b | 148.688 ± 0.45 b | |
| 10% | 5.87 ± 0.02 a | 22.24 ± 0.99 b | 1.20 ± 0.02 a | 62.77 ± 1.07 c | 1.718 ± 0.01 c | 143.012 ± 0.35 c | |
| S2 | 0% | 6.64 ± 0.04 a | 12.50 ± 0.99 a | 1.10 ± 0.03 a | 34.77 ± 0.81 a | 1.115 ± 0.01 a | 104.909 ± 0.35 a |
| 1% | 6.50 ± 0.01 c | 10.20 ± 0.99 b | 1.09 ± 0.01 a | 35.93 ± 0.40 a | 1.050 ± 0.01 b | 103.054 ± 0.52 b | |
| 5% | 6.59 ± 0.01 b | 9.40 ± 0.40 b | 1.09 ± 0.01 a | 32.20 ± 0.70 b | 1.001 ± 0.01 c | 103.897 ± 0.35 b | |
| 10% | 6.53 ± 0.01 c | 9.86 ± 0.40 b | 1.07 ± 0.02 a | 32.67 ± 0.81 b | 1.003 ± 0.01 c | 101.368 ± 0.54 c | |
| S3 | 0% | 7.49 ± 0.01 a | 23.96 ± 0.99 a | 1.18 ± 0.02 c | 66.50 ± 0.70 b | 0.862 ± 0.04 a | 42.159 ± 0.74 a |
| 1% | 7.46 ± 0.01 a | 23.73 ± 2.26 a | 1.25 ± 0.02 b | 71.17 ± 1.07 a | 0.783 ± 0.01 b | 43.328 ± 0.21 a | |
| 5% | 7.52 ± 0.01 a | 23.39 ± 1.72 a | 1.24 ± 0.01 ab | 67.20 ± 0.70 b | 0.750 ± 0.02 c | 25.883 ± 0.27 b | |
| 10% | 7.56 ± 0.02 a | 22.81 ± 0.99 a | 1.21 ± 0.01 a | 66.03 ± 1.07 b | 0.685 ± 0.01 d | 23.815 ± 0.21 c |
| Index | Soil Type (S) | Concentration (C) | S × C |
|---|---|---|---|
| CAT | 34,300.695 ** | 165.831 ** | 120.605 ** |
| Km | 11.195 ** | 3.437 * | 4.903 * |
| Vmax | 870.534 ** | 9.374 ** | 6.482 ** |
| Vmax/Km | 3782.430 ** | 11.590 ** | 16.727 ** |
| Ea | 1982.409 ** | 32.682 ** | 212.702 ** |
| ΔG | 28,329.040 ** | 105.907 ** | 55.812 ** |
| Chao 1 | 53.634 ** | 12.386 ** | 17.119 ** |
| Shannon | 14.313 ** | 1.290 | 2.200 |
| Soil Type | Index | 0% | 1% | 5% | 10% |
|---|---|---|---|---|---|
| S1 | Q10-CAT | 0.49 ± 0.02 a | 0.45 ± 0.03 a | 0.48 ± 0.00 a | 0.48 ± 0.01 a |
| Q10-Km | 0.27 ± 0.03 a | 0.24 ± 0.01 a | 0.15 ± 0.01 b | 0.14 ± 0.03 b | |
| Q10-Vmax | 7.88 ± 0.86 a | 7.22 ± 0.23 a | 4.71 ± 0.23 b | 2.90 ± 0.96 c | |
| Q10-Vmax/Km | 10.71 ± 0.24 a | 7.59 ± 0.32 c | 6.99 ± 0.36 c | 8.54 ± 0.55 b | |
| S2 | Q10-CAT | 0.62 ± 0.02 a | 0.63 ± 0.01 a | 0.63 ± 0.00 a | 0.61 ± 0.10 a |
| Q10-Km | 0.23 ± 0.02 b | 0.28 ± 0.04 b | 0.46 ± 0.04 a | 0.49 ± 0.07 a | |
| Q10-Vmax | 2.94 ± 0.42 b | 3.38 ± 0.71 b | 5.66 ± 0.09 a | 6.43 ± 0.64 a | |
| Q10-Vmax/Km | 5.51 ± 0.48 a | 4.78 ± 0.28 a | 4.41 ± 0.26 a | 4.45 ± 0.68 a | |
| S3 | Q10-CAT | 0.62 ± 0.02 ab | 0.59 ± 0.04 b | 0.64 ± 0.01 a | 0.65 ± 0.01 a |
| Q10-Km | 0.13 ± 0.02 b | 0.14 ± 0.06 b | 0.27 ± 0.06 a | 0.09 ± 0.01 b | |
| Q10-Vmax | 7.32 ± 1.36 b | 6.73 ± 1.13 b | 11.16 ± 1.73 a | 7.28 ± 0.58 b | |
| Q10-Vmax/Km | 14.97 ± 0.44 a | 13.59 ± 0.37 b | 12.78 ± 0.40 b | 8.64 ± 1.01 c |
| Soil Type | Treatments | Chao1 | Shannon |
|---|---|---|---|
| S1 | 0% | 1829.4 ± 202.56 c | 8.935 ± 0.15 b |
| 1% | 3014.2 ± 344.24 b | 9.010 ± 0.09 a | |
| 5% | 3568.1 ± 10.86 ab | 9.330 ± 0.03 a | |
| 10% | 3777.6 ± 17.50 a | 9.260 ± 0.03 a | |
| S2 | 0% | 4089.4 ± 50.97 a | 9.530 ± 0.03 a |
| 1% | 3775.7 ± 18.83 b | 9.500 ± 0.01 ab | |
| 5% | 3825.7 ± 21.56 b | 9.403 ± 0.02 b | |
| 10% | 3762.8 ± 49.96 b | 9.250 ± 0.04 b | |
| S3 | 0% | 3945.5 ± 184.31 ab | 9.555 ± 0.00 a |
| 1% | 4063.4 ± 121.02 a | 9.353 ± 0.30 a | |
| 5% | 3634.0 ± 73.63 b | 9.647 ± 0.02 a | |
| 10% | 4218.3 ± 40.65 a | 9.600 ± 0.09 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, Y.; Wu, X.; Ren, Q.; Guo, Y.; Yue, Z.; Bai, X.; Xu, J.; Wang, P. Do Soil pH Levels Drive the Responses of Catalase Activity and Bacterial Communities to Microplastics? A Case Study in Mollisols. Toxics 2025, 13, 1005. https://doi.org/10.3390/toxics13121005
Yin Y, Wu X, Ren Q, Guo Y, Yue Z, Bai X, Xu J, Wang P. Do Soil pH Levels Drive the Responses of Catalase Activity and Bacterial Communities to Microplastics? A Case Study in Mollisols. Toxics. 2025; 13(12):1005. https://doi.org/10.3390/toxics13121005
Chicago/Turabian StyleYin, Yuan, Xiangyu Wu, Qina Ren, Yuxin Guo, Zhonghui Yue, Xin Bai, Jia Xu, and Pengwei Wang. 2025. "Do Soil pH Levels Drive the Responses of Catalase Activity and Bacterial Communities to Microplastics? A Case Study in Mollisols" Toxics 13, no. 12: 1005. https://doi.org/10.3390/toxics13121005
APA StyleYin, Y., Wu, X., Ren, Q., Guo, Y., Yue, Z., Bai, X., Xu, J., & Wang, P. (2025). Do Soil pH Levels Drive the Responses of Catalase Activity and Bacterial Communities to Microplastics? A Case Study in Mollisols. Toxics, 13(12), 1005. https://doi.org/10.3390/toxics13121005






