Abstract
Numerous reports confirm that microplastics exposure could induce reproductive toxicity in mammals. However, the effects of microplastics exposure during juveniles on ovarian apoptosis through oxidative and endoplasmic reticulum (ER) stresses remains unclear, which is the focus of our study. In the present study, female rats (4 weeks old) were exposed to polystyrene microplastics (PS-MPs, 1 μm) at different dosages (0, 0.5, and 2.0 mg/kg) for 28 days. Findings revealed that 2.0 mg/kg of PS-MPs distinctly increased the atretic follicle ratio in the ovary and dramatically reduced the serum levels of estrogen and progesterone. Additionally, the oxidative stress indicators declined, including the activity of superoxide dismutase and catalase, whereas the malondialdehyde content in the ovary was considerably enhanced in the 2.0 mg/kg PS-MPs group. Furthermore, the expressions of genes related to ER stress (PERK, eIF2α, ATF4, and CHOP) and apoptosis were remarkably elevated in the 2.0 mg/kg PS-MPs group compared with those in the control group. We found that PS-MPs induced oxidative stress and activated the PERK-eIF2α-ATF4-CHOP signaling pathway in juvenile rats. Moreover, with the oxidative stress inhibitor N-acetyl-cysteine and eIF2α dephosphorylation blocker Salubrinal treatment, ovarian damage induced by PS-MPs was repaired and associated enzyme activities were improved. Overall, our results indicated that PS-MPs exposure induced ovarian injury associated with oxidative stress and activation of the PERK-eIF2α-ATF4-CHOP signaling pathway in juvenile rats, providing new prospects for assessing the health risks of children exposed to microplastics.
1. Introduction
Microplastics (MPs) are smaller than 5 mm in size and are developed from plastic debris through the long-term effects of water, ultraviolet radiation, and biological degradation [1]. According to estimates, the accumulation of MPs in the soil is 4 to 23 times greater than in the ocean, posing a major threat to terrestrial ecosystems [2]. MPs have become typical environmental contaminants and can be found in a wide variety of ecological settings, biological species, human living habitats, and even ordinary necessities, such as shampoos, toothpaste, and cosmetics [3,4]. Moreover, emerging evidence indicates that humans are ubiquitously exposed to MPs [5] mainly through oral ingestion, inhalation, and skin contact. Previous research has established that MPs may build in the digestive organs [6], the blood [7], and the placenta [8], posing a health concern owing to their complicated chemical characteristics. MPs have been linked to a high risk of metabolic disruption, neurotoxicity, and cancer in humans [9]. Current research suggests that MPs could cause dose-dependent microstructural and functional ovarian damage, which is mediated by pro-oxidant and pro-inflammatory pathways. These pathways affect genes, molecular effectors, and hormones that regulate folliculogenesis [10]. Thus, MPs may affect human reproduction, requiring a full assessment of the harm caused by MPs to the human reproductive system. Pregnancy, infancy, and puberty are highly vulnerable periods in which the sensitivity to environmental pollution is high [11,12]. Nevertheless, knowledge about the reproductive toxicity induced by MPs exposure at different stages of human growth, especially during childhood, is limited.
Children’s special behaviors, such as crawling, hand–mouth activity, playing with toys, and touching personal products, increase their exposure to MPs [13]. Researchers recently found that the exposure of infants and children to MPs is indeed greater than that of adults [14]. The effects on reproduction are one of the main concerns when considering the effects of MPs on children’s health [15]. Children’s reproductive systems are immature; thus, juvenile reproductive toxicity can eventually lead to infertility [16]. A previous study demonstrated that MPs exposure (five weeks) in a hypothetical scenario may pose an irreversible endocrine disturbance with particular indicators of reproductive toxicity in mammals [17]. The ovaries’ hormone secretion contributes to maintaining pubertal development and growth. Mice exposed to MPs developed inflammation of the ovaries and experienced reduced-quality oocytes [18]. MPs trigger reproductive damage in mice by the peroxidation and amplification of the P38 MAPK signal transduction [19]. These investigations prove that MPs can cause oxidative stress, which ultimately leads to reproductive damage [20]. It is postulated that oxidative stress is one of the key processes causing female infertility [21]. Of note, Haddadi et al. recently published that 5 μm MPs exposure by oral gavage was detected in the duodenum and ovary in rats, leading to defective ovarian function via oxidative stress [22]. The abnormal production of free radicals (ROS) might contribute to the buildup of unfolded or misfolded peptides in the endoplasmic reticulum lumen [23]. However, if the system is overwhelmed with the accumulation of misfolded proteins, the unfolded protein response (UPR) is provoked to trigger ER stress. Chronic ER stress mediates apoptotic signaling. Under pathological conditions, the protein kinase R-like ER kinase (PERK) signaling pathway was activated and dissociated from the ER chaperone immunoglobulin protein (BIP) which is the initiator of ER stress, exacerbating cellular apoptosis [24]. PERK is activated and directly phosphorylates the eukaryotic initiation factor (eIF2α), which in turn activates the transcription factor the C/EBP homologous protein (CHOP) mainly through the activated transcription factor 4 (ATF4) [25]. Extensive studies have demonstrated that ER stress exerts a pivotal role in the development of the ovary and involves multiple ovarian diseases [26]. Nevertheless, the mechanism related to ER stress-activated by MPs is rarely articulated. The Female Pubertal Assay serves as an in vivo screening assay to help identify chemicals or mixtures that have the potential to interact with the endocrine system, by identifying effects on pubertal development and thyroid function in the intact juvenile/peripubertal female rat. This assay is capable of detecting anti-thyroid, estrogenic, or anti-estrogenic chemicals, or agents which alter pubertal development [27]. Consequently, the following contents will elucidate the ovarian apoptosis in detail induced by MPs exposure through the oxidative and ER stresses in juvenile rats.
Polystyrene MPs (PS-MPs) were widely used to study toxic effects in mammals. Therefore, our experiment aimed to assess the ovarian poisoning from PS-MPs in juvenile female rats and identify the inherent risks of a 28-day exposure to PS-MPs. We assumed that MPs could induce ovary injury in juvenile rats via oxidation and ER stress stimulation. Accordingly, the peroxidation inhibitor N-acetyl-cysteine (NAC) and the eIF2α dephosphorylation blocker Salubrinal (Sal) were utilized to verify the toxic mechanism of PS-MPs. Our study could provide a better mechanical understanding of the health risks of early life exposure to MPs.
2. Materials and Methods
2.1. Materials
PS-MPs were obtained from the Tianjin Baseline ChromTech Research Centre (2.5 percent w/v, 10 mL) (Tianjin, China). The PS-MPs employed in the investigation have a diameter of 1 μm. Scanning electron microscopy (SEM) was performed to characterize the morphology of the PS-MPs (Regulus 8100, Hitachi, Japan). Spectroscopy dispersion was used to determine the diameter of the PS-MPs suspended in ultra-pure water (Zetasizer Nano ZS90, Malvern Instruments Ltd., Malvern, UK) and chemical components were analyzed by Fourier transform infrared spectroscopy (FTIR) (Fourier Transform Infrared Spectrometer, Nicolet iS50, Shanghai, China). Beyotime Biotechnology Co., Ltd. (Shanghai, China) and Macklin Reagents, Ltd. (Shanghai, China) provided the NAC and Sal, respectively.
2.2. Animals and Experiment Design
Twenty-four female Sprague Dawley (SD) rats (3 weeks old, 52 ± 3 g) were supplied by Nanchang University’s experimental animal center (Nanchang, China). Food and water were available to all the rats in the animal laboratory room ad libitum, which was kept at a consistent temperature (25 °C) and light (12 h dark/12 h light cycles). Four rats were kept per cage. All animal experiments followed the standards of the institutional animal care committee and were authorized by the Animal Care Review Panel (approval number 0064257; Nanchang University, Nanchang, China). After a week of acclimatization, all rats (4 weeks old, 70 ± 5 g) were freely categorized into three groups (n = 8): 0 (control), 0.5, and 2.0 mg/kg. The PS-MPs treatment groups received 0.5 and 2.0 mg/kg by oral gavage, once daily for 28 consecutive days, respectively, whereas the control group received the same volume of deionized water. The high dose employed here was determined by an earlier article that claimed PS-MPs significantly cause intestinal injury and intestinal barrier dysregulation in vivo at 2.0 mg/kg [28], which may promote PS-MPs to pass through the gut to distant organs. Moreover, the exposure levels employed here are based on the precautionary principle, which states that identifying the target machine is a prerequisite to evaluating the risk at environmentally relevant concentrations, according to a previous study [29]. An earlier study showed that oral gavage of 0.1 mg/d (about 1.5 × 106 particles/day) [22] PS-MPs caused significantly impaired ovarian function in rats. In our experiment, rats weigh 200 g on average, thus a dose of 0.5 mg/kg/d corresponds to a daily intake of PS-MPs of 0.1 mg. Therefore, we believe that the dose of 0.5 mg/kg/d PS-MPs can cause reproductive toxicity. Additionally, 0.1–0.5 g of MPs may be consumed by humans per week on average in the world [30]. The average rat weighs 200 g in our experiment, with a daily intake of PSMPs of 0.2 mg and 0.5 mg, then a weekly intake of 2.8 mg and 1.4 mg MPs, respectively, both of which are within the acceptable range for human ingestion. Prior to usage, MP particles were disseminated in deionized water and subjected to 20 min of supersonic wave vibration to thoroughly suspend them. During the trial, the rats’ weight gain and associated presentations were monitored daily. All rats were sacrificed by cervical dislocation at PND 59, which already have full sexual maturity, and their organs were quantified. Blood was collected by eyeball extraction. The serum was isolated at 4000 rpm for 10 min and stored at −20 °C for 24 h. For further analysis, the serum and tissue specimens were frozen at −80 °C.
2.3. NAC and Sal Supplementation
After one week of acclimatization, thirty-two female rats were split into four categories: the control (Group C), 2.0 mg/kg PS-MPs (Group I), 2.0 mg/kg PS-MPs with NAC (Group II), and 2.0 mg/kg PS-MPs with Sal (Group III) treatment groups (n = 8). The rats in Group C were treated with deionized water, and the rats in the other three groups were administered 2.0 mg/kg through oral gavage. Once every two days, Groups II and III received 100 mg/kg NAC and 1.5 mg/kg Sal by intraperitoneal injection, respectively [25]. Half an hour following the end of the gavage operation, the intraperitoneal injection procedure was carried out. NAC and Sal exposure times were 14 days due to the 28-day length of the overall experimental period. Beyotime Biotechnology Co., Ltd. (Shanghai, China) and Macklin Reagents, Ltd. (Shanghai, China) provided the NAC and Sal, respectively. The rats in Groups C and I were intraperitoneally injected with the same volume of sterile saline. The weights of the rats were measured daily for 28 days. All rats were sacrificed by cervical dislocation at PND 59, which already have full sexual maturity. The procedures of blood sample collection and sample storage were the same as described above.
2.4. Staining with Hematoxylin and Eosin (H&E) and Histomorphometric Analysis
The right ovaries were fixed in Bouin’s solution for 24 h and then dehydrated in graded ethanol before being embedded in paraffin. The paraffin-embedded ovaries were sectioned at 5 μm thickness and flattened on a slide before being stained with H&E. Five ovarian samples from each group were used to produce H&E slices and one section per animal. The sections were sealed with a cover glass and filmed using an optical microscope (Nikon T-U optical microscope, Tokyo, Japan). The percentage of atretic oocytes in each area was recorded, and the ovarian eggs were categorized according to the findings of a previous study [31]. The oocyte in the primordial follicle is surrounded by a layer of three to six flattened pre-granulosa cells; the oocyte in the primary follicle is surrounded by a layer of expanded cells; the secondary follicles have one to two layers of granulosa cells that are slightly larger in diameter around the oocytes; the preantral follicles have a variety of layers of granulosa cells that are larger. The primary follicles, secondary follicles, and preantral follicles were classified as growing follicles. To count the atretic follicles, 5 non-overlapping visual areas were randomly chosen from each slice under the microscope and the sum represented the total number of atretic follicles in one ovarian sample. The present “atretic follicle ratio (%)” per animal = the total number of atretic follicles seen in all five visual areas/total number of follicles (atretic follicles plus non-atretic follicles) seen in the same visual fields.
The quantitative histomorphometric analyses were performed on rats’ ovaries according to Tassinari et al. [32]. Briefly, using one of the largest sections in a central position of the ovary, primary and secondary follicles, preantral follicles, and atretic follicles were counted in the whole ovarian section.
2.5. Analysis of Sex Hormones and Oxidative Stress
The serum levels of estradiol and progesterone were determined using ELISA kits (Shanghai YSRIBIO Industrial Co., LTD., Shanghai, China), and the tiers of the ROS stress indicators superoxide dismutase (SOD), catalase (CAT), and malondialdehyde (MDA) in the ovaries were determined using commercial kits (Jiancheng, Nanjing, China). Fresh ovarian samples weighed approximately 0.1 g and saline was added following the standard of adding 1 g of tissue to 9 mL of homogenate. After homogenizing tissues in an ice bath, 10% tissue homogenates were obtained. The supernatant was centrifuged at 5000 rpm for 15 min and stored at −80 °C for protein measurement. A commercial BCA Protein Assay Kit (Applygen Technologies Inc., Beijing, China) was used to quantify the tissue protein concentration. The manufacturer’s instructions were followed for all parameters.
2.6. Quantitative Polymerase Chain Reaction in Real-Time (RT-qPCR)
Liu et al. found that the relative fluorescent intensity of GSH in mouse oocytes was significantly decreased after PS-MPs exposure [18]. Therefore, we chose the gene GSH as one of the oxidative stress indicators in this study. We explored the ovarian injury caused by PS-MPs exposure via the PERK-ATF4-eIF2α-CHOP signaling pathway. The PERK-ATF4 pathway is critical for regulating the CHOP expression, with increased CHOP expression leading to cellular apoptosis [33]. CHOP regulates ER stress-induced apoptosis by boosting the transcription of the pro-apoptosis gene Bax and suppressing the production of anti-apoptosis Bcl-2 in the mitochondrial membrane [34]. Caspase-9, which can be activated by the imbalance of Bcl-2 and Bax, engages in the subsequent activation of Caspase-3 [35]. The total RNA was isolated from the ovaries using kits (TSINKE Biotechnology Co., Ltd., Beijing, China). Then, using a reverse transcriptase mixture (Takara PrimeScript RT reagent package (Cat#RR047A, Lot#AK2802), the RNA of each subject was retro-converted into cDNA. Primer Express Software (Applied Biosystems, Foster City, CA, USA) and Oligo Primer Analysis Software version 6.0 (Molecular Biology Insights, Inc.; DBA Oligo, Inc. Colorado Springs, CO, USA) were used to construct the primers (Table 1). General Biosystems Co., Ltd. was commissioned to manufacture the primers (Chuzhou, China). According to prior studies [36,37], 40 cycles of 5 s at 95 °C, 5 s at 95 °C, and 1 min at 60 °C were programmed into the AriaMx Real-time qPCR system (New York, NY, USA, Agilent, MY 19435252). Relative quantification of messenger RNA (mRNA) was determined using the 2-∆∆Ct method, with GAPDH as the housekeeping gene.

Table 1.
Primers used for a quantitative real-time polymerase chain reaction.
2.7. Immunohistochemical (IHC) Analysis
The ovarian tissue embedded in paraffin is sectioned, dewaxed with xylene, and hydrated. Sections were then repaired with antigen and immersed in 3% hydrogen peroxide for the elimination of endogenous peroxidase activity. Subsequently, the sections were blocked with 3% BSA for 30 min at room temperature, and interacted with the following primary antibodies: BIP (1:100, Proteintech, Wuhan, China, 11587-1-AP) and PERK (1:200, Proteintech, Wuhan, China, 24390-1-AP) at 4 °C overnight. These slices interacted with IgG (1:200), an HRP-labeled rabbit secondary antibody, for 50 min following 3 PBS washes. Light-microscopy examination of the sections is performed after staining with a fresh 3,3′-diaminobenzidine solution and hematoxylin counterstain at room temperature. Positive cells are dark brown. For immunohistochemistry quantification, the average optical density of six high-magnification areas in three rat ovaries was determined using ImageJ 6.0.
2.8. Assay for Immunofluorescence
The abundance of target proteins eIF2α and Caspase-3 in the ovaries was measured by immunofluorescence. Previously embedded ovarian tissue blocks in paraffin in this work were deparaffinized with xylene and rehydrated with gradient ethanol. The slides were immersed in EDTA antigen buffer (pH 8.0) to retrieve the antigens. Then, 3% BSA was added to the slides to block the non-specific binding. The sections were incubated overnight at 4 °C with proportionally prepared primary antibodies eIF2α (1:500, GB13572) or Caspase-3 (1:500, GB11532) and rinsed, then incubated with fluorescent secondary antibody (1:300, GB21303) for 50 min. After the sections were dried, DAPI (Servicebio, G1012 Wuhan, China) was applied to counterstain the nuclei. This was incubated for 10 min, followed by the addition of a tissue autofluorescence quencher for 5 min. The sections were then washed with water and then blocked. The DAPI-stained nuclei turn blue, and the matching fluorescein-labeled green light indicates a positive expression (DAPI UV excitation wavelength 330–380 nm, emission wavelength 420 nm, blue light; FITC excitation wavelength 465–495 nm, emission wavelength 515–555 nm, green light). Finally, three slides per animal were analyzed and the sections were observed under a microscope, and images were collected using fluorescent microscopy (Nikon-Ti-U fluorescence inverted microscope, Tokyo, Japan). ImageJ 6.0 was used to examine the average image quantum yield with background subtraction (National Institutes of Health).
2.9. Analytical Statistics
The experimental data are reported as mean ± SD, and the results were statistically analyzed using SPSS (Version 26.0). One-way and two-way analysis of variance (ANOVA) combined with Tukey’s multiple comparison tests were used to determine the significance of differences between groups. Differences were considered significant at p < 0.05.
3. Results
3.1. Characterization of PS-MPs
All particles of PS-MPs approximated a spherical shape and were well-dispersed, as shown in the SEM image (Figure 1A). To measure the hydrodynamic size of PS-MPs, the solution of PS-MPs was diluted with deionized water to 0.1 mg/mL. PS-MPs presented a mean size of 1.5 μm (Figure 1B). Characteristics of PS-MPs displayed by FTIR confirmed that the chemical composition was polystyrene (Figure 1C), according to previous studies [38,39].
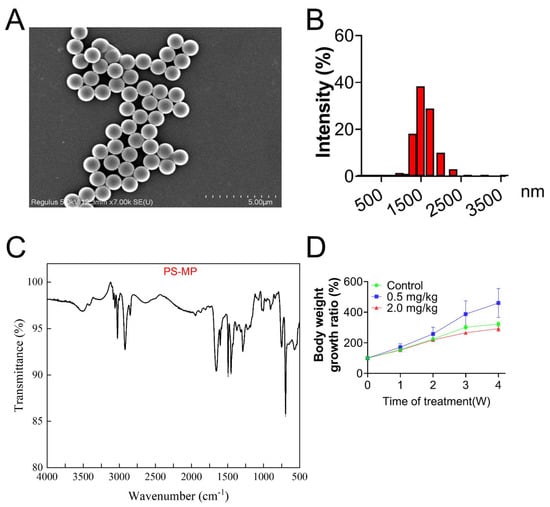
Figure 1.
Characterization of PS-MPs by SEM, DLS, and FTIR. (A) PS-MP morphology (scale bar = 5 μm). (B) Hydrodynamic size distribution of PS-MPs. (C) FTIR image of PS-MP. (D) Body weight growth ratio.
3.2. PS-MPs Exposure Has Negative Impacts on the Development of Follicles
As shown in Figure 2A, no obvious structural differences in follicles existed between the control and 0.5 mg/kg PS-MPs groups. However, the percentage of atretic follicles in the 2.0 mg/kg PS-MPs group was higher (p < 0.05, Figure 2B) than that in the other groups. Histomorphometric data were summarized in Table S1. The number of growing follicles significantly decreased at a dose of 2.0 mg/kg PS-MPs level. Furthermore, the 2.0 mg/kg PS-MPs group presented notably reduced serum levels of estrogen and progesterone compared with the control group (Figure 2C,D). No difference in findings was observed between the 0.5 mg/kg PS-MPs and control groups. Moreover, the PS-MPs group exhibited considerably lower levels of estrogen controller cytochrome P450 family 19 subfamilies A member 1 (CYP19A1) and steroidogenic acute regulatory protein (StAR) (Figure 2E,F). Nevertheless, the bodyweight growth ratio during the experiment showed no significant difference between the control and 2.0 mg/kg PS-MPs group except for a decreased trend in the 2.0 mg/kg PS-MPs group from the second week (Figure 1D). Likewise, the ovarian index in PS-MPs groups was lower than that in the control group, but had no significance (Figure S1A). In addition, we observed that the ovarian weight significantly declined after PS-MPs exposure compared with the control (Figure S2A).
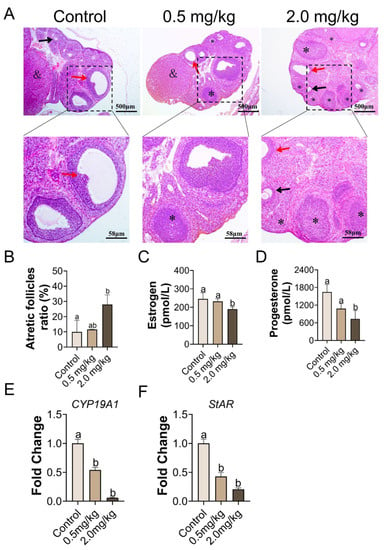
Figure 2.
Histopathological alterations, serum hormone concentration, and gene expression relevant to hormonogenesis in response to PS-MPs. (A) The representative images of H&E stained ovary (black arrow: primary follicles, red arrow: cystic follicles, asterisk: atretic follicles, and: corpus luteum), n = 5 per group. (B) Atretic follicles ratio, n = 5 per group. (C,D) ELISA was used to assess the amount of estradiol and progesterone in serum. (E,F) qPCR detection the comparative mRNA expression of CYP19A1 and StAR as in ovary of rats, standardized to GAPDH, n = 3 per group. The information was presented as the mean ± SD. Different letters indicate significance.
3.3. Exposure to PS-MPs Culminated in Oxidative Response in the Ovary
The MDA levels were higher in the 2.0 mg/kg PS-MPs treatment group than in the control group (p < 0.05, Figure 3A). Compared with the control group, the antioxidant catalytic abilities of SOD and CAT were drastically reduced in the 2.0 mg/kg PS-MPs group (p < 0.05, Figure 3B,C). However, the results in the 0.5 mg/kg PS-MPs group were equivocal and contradictory on the activity of SOD and CAT (Figure 3B,C). The expression level of the antioxidative gene glutathione (GSH) decreased substantially in the 2.0 mg/kg group, as per RT-qPCR testing of the ovarian tissues (Figure 3D).
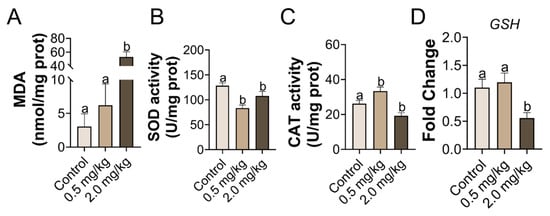
Figure 3.
PS-MPs cause oxidative stress in the ovary. (A) The concentration of MDA, the antioxidant activity within SOD, and CAT (B,C). (D) The comparative mRNA expression of GSH in the ovary was analyzed. The information was presented as the mean ± SD. Different letters indicate significance. n = 5 per group.
3.4. PS-MPs Consumption Led to ER Stress and Apoptosis in the Ovary
Gene transcription related to ER stress and apoptosis was investigated in the ovary. In the group treated with 2.0 mg/kg of PS-MPs, the ER stress genes PERK and eIF2α markedly increased. CHOP and ATF4 were augmented. In addition, the induced apoptosis genes, such as Bax, Caspase-9, and Caspase-3, were strongly amplified in the 2.0 mg/kg PS-MPs group, but Bcl-2 was greatly dysregulated (Figure 4). Relative expression of ATF4, Bax and Bcl-2 in the 0.5 mg/kg PS-MPs group showed significance compared with the control group. No significant differences were found among the extra genes in the 0.5 mg/kg PS-MPs group.
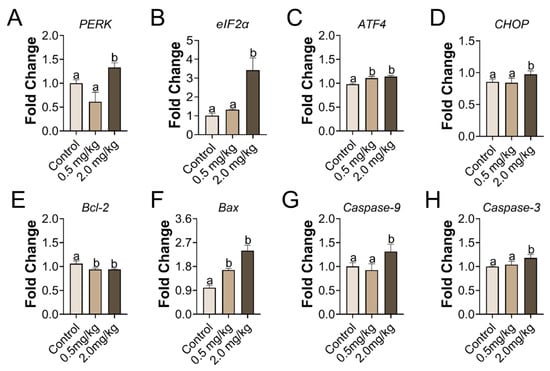
Figure 4.
The mRNA expression of genes involved in ER stress and apoptosis in the ovary upon PS-MPs stimulation. (A–D) Relative expression levels of genes associated with ER stress, including PERK, eIF2α, ATF4, and CHOP. (E–H) Relative expression levels of genes associated with apoptosis, including Bcl-2, Bax, Caspase-9, and Caspase-3. The information was presented as the mean ± SD. Different letters indicate significance. n = 3 per group.
3.5. PS-MPs Exposure Induced Ovarian Apoptosis Closely Associated with Oxidative Stress and ER Stress
Based on the above results, 2.0 mg/kg of PS-MPs caused obvious ovarian damage, likely through oxidative stress and the PERK-eIF2α-ATF4 signaling pathway of ER stress. To further test the mechanism induced by PS-MPs, oxygen strain antagonist NAC and the eIF2α dephosphorylation blocker Sal were used. To evaluate the biological alterations after PS-MPs exposure, the ovarian index was recorded. Additionally, 2.0 mg/kg PS-MPs oral exposure induced a significant decrease in the ovarian index compared with Group C. There was an upward trend but no significance of the ovarian index with NAC and Sal treatment compared with the PS-MPs group (Figure S1B). Similarly, results of the absolute ovarian weight showed that the ER stress inhibitor Sal but not the NAC could effectively alleviate the loss of ovary weight induced by PS-MPs exposure (Figure S2B). As depicted in Figure 5B and histomorphometric data (Table S2), results showed that the number of ovarian atretic follicles increased and the number of growing follicles decreased in the PS-MPs group compared with the control. The proportion of atretic follicles in Group I was much lower than that in Group C. Following the NAC and Sal treatment, the percentage of atretic follicles decreased significantly compared to that in Group I (Figure 5C). The estrogen and progesterone levels in the serum were increased because of the effects of the NAC and Sal treatment (Figure 5D,E). The mRNA levels of CYP19A1 and StAR were elevated in Group I (Figure 6D).
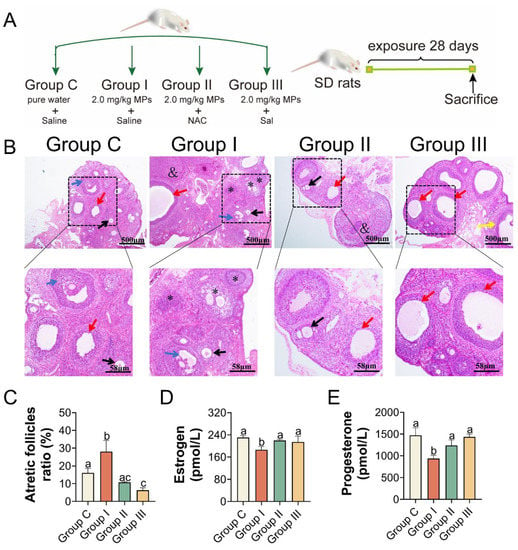
Figure 5.
The effects of NAC and Sal treatment on atretic follicles ratio, serum hormone levels. (A) Format of the trial, groups, and interventions. (B) H&E staining in ovarian slices (black arrow: primary follicles, red arrow: cystic follicles, blue arrow: secondary follicles, yellow arrow: antral follicles, asterisk: atretic follicles, and: corpus luteum), n = 5 per group. (C) Atretic follicles ratio, n = 5 per group. (D,E) ELISA was used to assess the amount of estradiol and progesterone in serum. The information was presented as the mean ± SD. Different letters indicate significance.
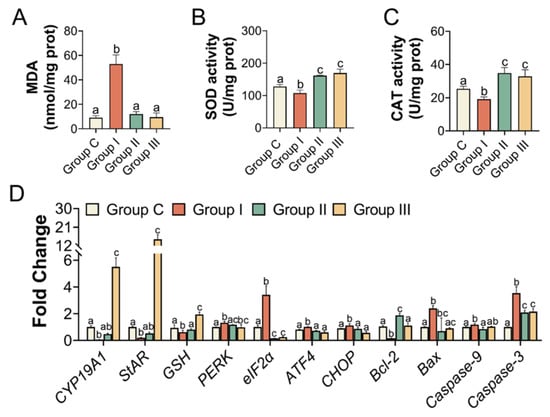
Figure 6.
Oxidative stress indicators and relative expression of genes in the ovary. (A) The concentration of MDA, the antioxidant activity of SOD, and CAT (B,C), n = 5 per group. (D) The relative mRNA levels of oxidative stress, ER stress, apoptosis, and hormones in the ovary, n = 3 per group. Different letters indicate significance.
In addition, the levels of antioxidant enzymes, such as SOD and CAT, in the ovaries were significantly elevated in Groups II and III compared to those in Group I. MDA levels were lower in Groups II and III than in Group I (Figure 6A–C). We also examined the transcriptional activity of the gene variants in ER stress and apoptosis. The mRNA expression levels in the NAC- and Sal-treated groups were similar to those in Group C (Figure 6D). IHC and immunofluorescence assays were performed to quantify the protein levels of BIP, PERK as well as eIF2α, and Caspase-3, separately, to further clarify the mechanism. According to the IHC staining, the IHC scores of BIP and PERK showed the same results that the levels of BIP and PERK were dramatically increased in Group I compared to Group C, which were both lowered (p < 0.05) with the NAC and Sal treatment (Figure 7A,C,D). The median intensity of eIF2α in the ovary in Group I exhibited an upward tendency but was not significantly different from that in Group C. Immunofluorescence analysis of Caspase-3 in Group I showed a marked increase compared to that in Group C. It presented a significantly lower mean intensity of eIF2α and Caspase-3 after NAC and Sal treatment than that in Group I (Figure 7B,E,F). Figure 8 illustrates the process of PS-MP-induced ovarian toxicity.
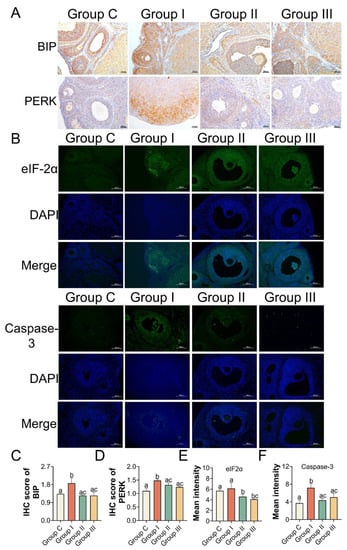
Figure 7.
Protein activity of BIP, PERK, eIF2α, and Caspase-3 in ovaries after NAC and Sal treatment. (A) IHC images of BIP and PERK (scale bar = 100 μm). (B) eIF2α and Caspase-3. Blue: DAPI, green: eIF2α, and Caspase-3 (scale bar =100 μm). (C,D) IHC scores of BIP and PERK. (E,F) Mean intensity results. Different letters indicate significance. n = 3 per group.
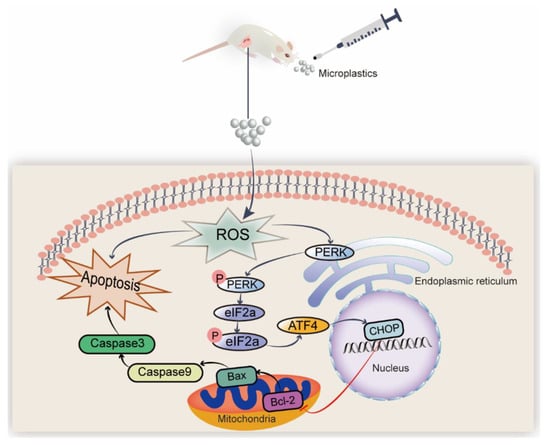
Figure 8.
A proposed route of ovarian injury induced by PS-MPs administration. PS-MPs consumption enhanced the buildup in oxidative stress and then caused mitochondrial apoptosis by activating PERK-ATF4-eIF2α-CHOP molecular cascade. “↑” activation, “⊥” inactivation, and “P” phosphorylation.
4. Discussion
Humans are ubiquitously in contact with MPs. The daily usage of plastic feeding bottles, water bottles, and medical syringes is the primary source of MPs for infants and children, causing harm to their bodies [40]. Moreover, the exposure of children to certain MPs components can affect their immune system components and functions, and even induce cancers, including ovarian cancer [41]. According to research, the health hazards of MPs to individuals, particularly children, should be taken seriously. In this study, a juvenile rat model was established to explore juvenile ovarian toxicity following MPs exposure.
The H&E sections showed that the number of ovarian atretic follicles increased and the number of preantral follicles decreased in the PS-MPs group compared with the control, indicating a change in ovarian structure. The ovary has two functions: endocrine and ovulation. The primary component of the ovary is the ovarian follicle, which provides the oocyte with a suitable environment for growth and development in preparation for ovulation and fertilization. Our findings showed that PS-MPs intake impaired follicle maturation. Estrogen and progesterone levels can affect follicle development by affecting granulosa cell (GC) growth and follicular fluid formation [42,43]. Therefore, in this investigation, the overall estrogen and progesterone concentrations in the 2.0 mg/kg PS-MPs group were generally lower than those in the control group. Our findings are consistent with those of previous research, showing that PS-MPs triggered fibrosis and death in the ovarian granulosa cells in adult rats [44]. Sex hormones, mainly estradiol and progesterone, are produced by the ovarian granulosa cells, which maintain oocyte development and follicle maturation [45]. Consequently, GC apoptosis is the main mechanism underlying follicular atresia and a decline in sex hormone levels. According to the literature, all the stages of follicular atresia are connected to the apoptosis of the GC and affect female fertility in adulthood by affecting ovulation [46]. Hou et al. discovered that 0.5 μm of PS-MPs may permeate the ovarian granulosa cells and trigger pyroptosis and apoptosis via the NOD-like receptor thermal protein domain associated protein 3-Caspase-1 signaling pathway in rats [47]. Previous studies demonstrated that 5 μm fluorescent PS-MPs accumulated in ovarian tissue after adult Wister rats exposure for 4 estrous cycles (24–26 days). Accumulation of corpus luteum and atretic follicles were higher than other types of follicles and stroma [22]. Fluorescence microscopy revealed the accumulation of microplastics (5–5.9 μm) in ovarian tissue [48]. MPs with a particle size of even 5 μm larger could accumulate in the ovaries. In the current work, we speculated that PS-MPs with diameter of 1 μm built up in the ovaries and induced ovarian damage. Microplastics were more likely to be internalized by cells when exposed to the environment. Additionally, internalization of cells was a crucial avenue for the transfer of microplastic particles into tissues, where they could have toxicological consequences that exert an impact on both human and environmental health [49]. Therefore, our observations suggested that PS-MPs exposure impaired follicle development and ovarian function through the increase in follicular atresia.
To further prove that the hormone synthesis was affected by PS-MPs exposure, we also analyzed the gene level. Steroid hormones play a crucial role in follicular atresia. Aromatase is the crucial enzyme facilitating the critical stage of estrogen production, and CYP19A1 is a member of the cyp450 gene superfamily that encodes it. Estrogen release deficits, GC condition aberrations, ovulation insufficiency, or even female fertility are mainly characterized by CYP19A1 disruption [50]. Furthermore, StAR is implicated in the regulation of the rate-limiting phase in hormone secretion and thus precipitates a permitting process in progesterone generation [51]. PS-MPs intake drastically reduced the CYP19A1 and the StAR expression compared with the control group, implying that PS-MPs consumption was deleterious toward hormonogenesis. PS-MPs also effectively diminished the StAR expression in the testicular tissue of rats [52]. Treatment with NAC and Sal reversed these effects. These results indicated that the mechanisms of MPs-induced ovarian toxicity may be associated with oxidative stress and ER stress.
Understanding the ovarian toxicity of PS-MPs may help extrapolate the risks to mammals. The ovary is vulnerable to oxidative damage due to the richness of unsaturated lipids. Studies have demonstrated that oxidative stress activation has negative effects on follicle development, oocyte maturation, and ovulation [53]. The predominant mechanism of PS-MPs poisoning is oxidative stress. PS-MPs produced apoptosis and fibrosis in the follicle oocytes of rats due to oxidative stress [44]. PS-MPs exposure caused ovarian inflammation in rodents by lowering the ER calcium ([Ca2+] ER) levels and increasing the ROS in oocytes [18]. MDA is a lipid peroxidation cumulative measure that implicitly represents the degree of overall tissue lipid peroxidation damage. In this study, the activity of antioxidant proteins SOD and GSH in the 2.0 mg/kg PS-MPs group was less than that of the control group, while the MDA content was notably higher. Nevertheless, Liu et al. obtained the opposite results of the level of MDA to our results, which might be caused by the decline of fatty acids in PS-MPs treated group in the previous study [18]. However, the results for the 0.5 mg/kg PS-MPs group could be attributed to body defenses. Previous research shows that 1.5 mg/d of PS-MPs causes ovarian damage, which is linked to oxidative stress, as measured by the spike in MDA and the decline in antioxidant enzyme activity [44]. Furthermore, the mRNA expression level of GSH declined. PS-MPs-induced ovarian damage is linked to oxidative stress, which is consistent with our findings. The outcomes of peroxidation antagonist NAC administration, which could appreciably lessen ovarian peroxidation, corroborate this hypothesis. Interestingly, ER stress inhibitor Sal also blocks the elevated oxidative stress levels caused by PS-MPs. Early studies provided evidence that NAC and Sal are bidirectional inhibitors [25,54]. Hence, our findings implied that oxidative stress and endoplasmic reticulum stress were linked.
Oxidative stress could induce ER stress. Liu et al. found that oxidative stress mediates the ER stress induced by microcystin-leucine arginine in the ovary of mice [55]. The high levels of ROS stimulated by external materials can induce oxidative stress, leading to ER dysfunction that activates unfolded protein response (UPR). We found that 2.0 mg/kg/d of PS-MPs induced the significant up-regulation of the PERK signaling pathway-related genes, PERK, eIF2α, ATF4, and CHOP, compared to the control. A recent study elucidated that ER stress activation affects follicular and oocyte health, which consequently plays a role in the etiology of a number of ovarian disorders following exposure to environmental pollution [26]. In this study, we speculated that PS-MPs activated the PERK-ATF4-CHOP signaling pathway of ER stress. The UPR activation by ER stress has three branches: inositol-requiring enzyme 1 (IRE1), PERK, and ATF6. PERK-eIF2α-ATF4 is one of the most important and earliest pathways. The oral delivery of PS-MPs enhances ER stress in the liver via the PERK signaling cascade [56]. On the basis of previous work, we proposed that MPs induced ovarian apoptosis through the activation of the PERK-eIF2α-ATF4-CHOP signaling pathway and oxidative stress.
ER stress is involved in ovarian GC apoptosis. In the 2.0 mg/kg/d condition, the PERK signaling pathway and the apoptotic genes were dramatically engaged. Therefore, our observations revealed that PS-MPs induced ovarian apoptosis was probably associated with the oxidative stress and activation of the PERK--eIF2α-ATF4-CHOP in juvenile rats.
To further verify the mechanism, rats from Groups II and III were intraperitoneally injected with cellular redox blocker NAC and eIF2α dephosphorylation inhibitor Sal after 2.0 mg/kg/d PS-MPs gavage. With inhibitor treatment, the atretic follicles ratio, levels of estrogen and progesterone in the serum, and the oxidative stress indicators of MDA, SOD, and CAT in the ovary tissues were corrected accordingly compared with the PS-MPs group. Sal can specifically inactivate eIF2a phosphatase complexes, boost eIF2a phosphorylation levels, lower translation rates, and restore ER homeostasis. The ovulatory dysfunction of obese mice treated with Sal was remedied, and their oocyte developmental competency improved [57]. Thus, reducing ER stress in the ovary could be a potential treatment method to replace hormone therapy. Treatment with NAC downregulated the expressions of PERK, eIF2α, ATF4, and CHOP compared to that in Group I, as supported by the IHC results of BIP and PERK, and immunofluorescence results of eIF2α and Caspase-3. Consequently, our results confirmed that PS-MPs consumption promoted ovarian apoptosis, which is likely attributed to oxidative stress-induced PERK-eIF2α-ATF4-CHOP transcriptional activation.
This study provides basic toxicological data to elucidate the impact of MPs on the reproductive system of young mammals. However, direct evidence of PS-MP being present in ovarian tissue was absent, and this study did not follow the impact of young mices’ exposure to MPs on fertility in adulthood. More evidence suggesting the PERK-eIF2α-ATF4-CHOP signaling pathway involving oxidative stress and apoptosis is lacking in our study, particularly in Western blot data. Thus, the impact of MPs on children’s health requires further investigation. Furthermore, appropriate and proportionate measures should be taken to reduce children’s exposure to MPs.
5. Conclusions
Summarily, our study indicates that PS-MPs could induce ovary apoptosis in juvenile rats through oxidative stress and activation of the PERK-eIF2α-ATF4 signaling pathway, resulting in the defective development of follicles, decreased production of estrogen and progesterone, decreased activity of SOD and CAT, and increased MDA in juvenile rats. Furthermore, a ROS scavenger NAC and eIF2α dephosphorylation inhibitor (Salubrinal) effectively attenuated the quality of follicles, improving estrogen and progesterone production and associated enzyme activity. Our findings highlight the negative impacts and mechanisms of PS-MPs on female juvenile reproduction, presenting new avenues for analyzing the health hazards of exposure to MPs for children. We urge more academics to study juvenile toxicity exposure to chemicals and to take additional steps to shield children from environmental pollution in light of our results.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/toxics11030225/s1, Figure S1. Alterations of the ovarian index induced by PS-MPs exposure. Figure S2. Absolute ovaries’ weight of female rats treated with PS-MPs. Table S1. Histomorphometric data of female rats treated with PS-MPs at a dose level of 0, 0.5, and 2.0 mg/kg per day during the juvenile period (PND 28-56). Table S2. Histomorphometric data of female rats treated with deionized water (Group C), PS-MPs (Group I), PS-MPs + NAC (Group II), and PS-MPs + Sal (Group III) during the juvenile period (PND 28-56).
Author Contributions
Conceptualization: W.W.; Methodology: W.W., J.G.; Formal analysis and investigation: W.W., Y.F., Y.X.; Writing original draft preparation: W.W.; Writing review and editing: S.L., Y.Z., H.X.; Resources: F.F., H.X.; Supervision: F.F., H.X. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Research Foundation from Academic and Technical Leaders of Major Disciplines in Jiangxi Province, China, Grant Number: 20194BCJ22004.
Institutional Review Board Statement
All animal experiments followed the standards of the institutional animal care committee and were authorized by the Animal Care Review Panel (approval number 0064257; Nanchang University, Jiangxi, China).
Data Availability Statement
The data presented in this study are available in this article.
Acknowledgments
The authors thank Fen Fu for her generosity in this experiment and Hengyi Xu for his financial support and technical guidance.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Xu, D.; Ma, Y.; Han, X.; Chen, Y. Systematic toxicity evaluation of polystyrene nanoplastics on mice and molecular mechanism investigation about their internalization into Caco-2 cells. J. Hazard. Mater. 2021, 417, 126092. [Google Scholar] [CrossRef]
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total. Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef]
- Fendall, L.S.; Sewell, M.A. Contributing to marine pollution by washing your face: Microplastics in facial cleansers. Mar. Pollut. Bull. 2009, 58, 1225–1228. [Google Scholar] [CrossRef]
- Jing, J. Polystyrene micro-/nanoplastics induced hematopoietic damages via the crosstalk of gut microbiota, metabolites, and cytokines. Environ. Int. 2022, 161, 107131. [Google Scholar] [CrossRef] [PubMed]
- Koelmans, B. A scientific perspective on microplastics in nature and society. SAPEA 2019. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Trasande, L.; Kannan, K. Occurrence of polyethylene terephthalate and polycarbonate microplastics in infant and adult feces. Environ. Sci. Technol. Lett. 2021, 8, 989–994. [Google Scholar] [CrossRef]
- Leslie, H.A.; van Velzen, M.J.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First evidence of microplastics in human placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Sarkar, A.; Yadav, O.P.; Achari, G.; Slobodnik, J. Potential human health risks due to environmental exposure to nano- and microplastics and knowledge gaps: A scoping review. Sci. Total. Environ. 2021, 757, 143872. [Google Scholar] [CrossRef]
- Marcelino, R.C.; Cardoso, R.M.; Domingues, E.L.; Gonçalves, R.V.; Lima, G.D.; Novaes, R.D. The emerging risk of microplastics and nanoplastics on the microstructure and function of reproductive organs in mammals: A systematic review of preclinical evidence. Life Sci. 2022, 295, 120404. [Google Scholar] [CrossRef]
- Barouki, R.; Gluckman, P.D.; Grandjean, P.; Hanson, M.; Heindel, J.J. Developmental origins of non-communicable disease: Implications for research and public health. Environ. Health 2012, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- Sales, V.M.; Ferguson-Smith, A.C.; Patti, M.-E. Epigenetic mechanisms of transmission of metabolic disease across generations. Cell Metab. 2017, 25, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Sripada, K.; Wierzbicka, A.; Abass, K.; Grimalt, J.O.; Erbe, A.; Röllin, H.B.; Weihe, P.; Díaz, G.J.; Singh, R.R.; Visnes, T.; et al. A children’s health perspective on nano- and microplastics. Environ. Health Perspect. 2022, 130, 9086. [Google Scholar] [CrossRef]
- Nor, N.H.M.; Kooi, M.; Diepens, N.J.; Koelmans, A.A. Lifetime accumulation of microplastic in children and adults. Environ. Sci. Technol. 2021, 55, 5084–5096. [Google Scholar] [CrossRef]
- Park, E.-J.; Han, J.-S.; Seong, E.; Lee, G.-H.; Kim, D.-W.; Son, H.-Y.; Han, H.-Y.; Lee, B.-S. Repeated-oral dose toxicity of polyethylene microplastics and the possible implications on reproduction and development of the next generation. Toxicol. Lett. 2020, 324, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Della Seta, D.; Minder, I.; Belloni, V.; Aloisi, A.M.; Dessì-Fulgheri, F.; Farabollini, F. Pubertal exposure to estrogenic chemicals affects behavior in juvenile and adult male rats. Horm. Behav. 2006, 50, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Amereh, F.; Babaei, M.; Eslami, A.; Fazelipour, S.; Rafiee, M. The emerging risk of exposure to nano(micro)plastics on endocrine disturbance and reproductive toxicity: From a hypothetical scenario to a global public health challenge. Environ. Pollut. 2020, 261, 114158. [Google Scholar] [CrossRef]
- Liu, Z.; Zhuan, Q.; Zhang, L.; Meng, L.; Fu, X.; Hou, Y. Polystyrene microplastics induced female reproductive toxicity in mice. J. Hazard. Mater. 2022, 424, 127629. [Google Scholar] [CrossRef]
- Xie, X.; Deng, T.; Duan, J.; Xie, J.; Yuan, J.; Chen, M. Exposure to polystyrene microplastics causes reproductive toxicity through oxidative stress and activation of the p38 MAPK signaling pathway. Ecotoxicol. Environ. Saf. 2020, 190, 110133. [Google Scholar] [CrossRef]
- Yang, S.; Zhou, F.; Dong, Y.; Ren, F. α-mangostin induces apoptosis in human osteosarcoma cells through ROS-mediated endoplasmic reticulum stress via the WNT pathway. Cell Transplant. 2021, 30, 9636897211035080. [Google Scholar] [CrossRef]
- Gupta, S.; Ghulmiyyah, J.; Sharma, R.; Halabi, J.; Agarwal, A. Power of proteomics in linking oxidative stress and female infertility. Bio. Med. Res. Int. 2014, 2014, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Haddadi, A. Exposure to microplastics leads to a defective ovarian function and change in cytoskeleton protein expression in rat. Environ. Sci. Pollut. Res. Int. 2022, 29, 34594–34606. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, H.; Nishitoh, H. Endoplasmic reticulum quality control by garbage disposal. FEBS J. 2019, 286, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Persaud, I.; Shannahan, J.H.; Raghavendra, A.J.; Alsaleh, N.; Podila, R.; Brown, J.M. Biocorona formation contributes to silver nanoparticle induced endoplasmic reticulum stress. Ecotoxicol. Environ. Saf. 2019, 170, 77–86. [Google Scholar] [CrossRef]
- Chen, B.; Hong, W.; Tang, Y.; Zhao, Y.; Aguilar, Z.P.; Xu, H. Protective effect of the NAC and Sal on zinc oxide nanoparticles-induced reproductive and development toxicity in pregnant mice. Food Chem. Toxicol. 2020, 143, 111552. [Google Scholar] [CrossRef] [PubMed]
- Harada, M.; Takahashi, N.; Azhary, J.M.K.; Kunitomi, C.; Fujii, T.; Osuga, Y. Endoplasmic reticulum stress: A key regulator of the follicular microenvironment in the ovary. Mol. Hum. Reprod. 2021, 27, 88. [Google Scholar] [CrossRef]
- Stoker, T.E.; Gibson, E.K.; Zorrilla, L.M. Triclosan exposure modulates estrogen-dependent responses in the female Wistar rat. Toxicol. Sci. 2010, 117, 45–53. [Google Scholar] [CrossRef]
- Qiao, J.; Chen, R.; Wang, M.; Bai, R.; Cui, X.; Liu, Y.; Wu, C.; Chen, C. Perturbation of gut microbiota plays an important role in micro/nanoplastics-induced gut barrier dysfunction. Nanoscale 2021, 13, 8806–8816. [Google Scholar] [CrossRef]
- Babaei, A.A.; Rafiee, M.; Khodagholi, F.; Ahmadpour, E.; Amereh, F. Nanoplastics-induced oxidative stress, antioxidant defense, and physiological response in exposed Wistar albino rats. Environ. Sci. Pollut. Res. 2022, 29, 11332–11344. [Google Scholar] [CrossRef]
- Senathirajah, K. Estimation of the mass of microplastics ingested—A pivotal first step towards human health risk assessment. J. Hazard. Mater. 2021, 404, 124004. [Google Scholar] [CrossRef]
- Nestorović, N.; Lovren, M.; Sekulić, M.; Negić, N.; Šošić-Jurjević, B.; Filipović, B.; Milošević, V. Chronic somatostatin treatment affects pituitary gonadotrophs, ovaries and onset of puberty in rats. Life Sci. 2004, 74, 1359–1373. [Google Scholar] [CrossRef] [PubMed]
- Tassinari, R.; Cubadda, F.; Moracci, G.; Aureli, F.; D’Amato, M.; Valeri, M.; De Berardis, B.; Raggi, A.; Mantovani, A.; Passeri, D.; et al. Oral, short-term exposure to titanium dioxide nanoparticles in Sprague-Dawley rat: Focus on reproductive and endocrine systems and spleen. Nanotoxicology 2014, 8, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Rozpedek, W. The role of the PERK/eIF2α/ATF4/CHOP signaling pathway in tumor progression during endoplasmic reticulum stress. Curr. Mol. Med. 2016, 16, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Tian, M.; Ding, C.; Yu, S. The C/EBP Homologous Protein (CHOP) transcription factor functions in endoplasmic reticulum stress-induced apoptosis and microbial infection. Front. Immunol. 2019, 9, 3083. [Google Scholar] [CrossRef]
- Chen, M.; Guerrero, A.D.; Huang, L.; Shabier, Z.; Pan, M.; Tan, T.-H.; Wang, J. Caspase-9-induced mitochondrial disruption through cleavage of anti-apoptotic BCL-2 family members. J. Biol. Chem. 2007, 282, 33888–33895. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, Y.; Liu, Y.; Tang, Y.; Xu, X.; Wang, M.; Tao, X.; Xu, H. Pre-exposure to TiO2-NPs aggravates alcohol-related liver injury by inducing intestinal barrier damage in mice. Toxicol. Sci. 2021, 185, 28–37. [Google Scholar] [CrossRef]
- Tang, Y.; Chen, B.; Hong, W.; Chen, L.; Yao, L.; Zhao, Y.; Aguilar, Z.P.; Xu, H. ZnO nanoparticles induced male reproductive toxicity based on the effects on the endoplasmic reticulum stress signaling pathway. Int. J. Nanomed. 2019, 14, 9563–9576. [Google Scholar] [CrossRef]
- Jiang, X.; Chang, Y.; Zhang, T.; Qiao, Y.; Klobučar, G.; Li, M. Toxicological effects of polystyrene microplastics on earthworm (Eisenia fetida). Environ. Pollut. 2019, 259, 113896. [Google Scholar] [CrossRef]
- Wu, B.; Wu, X.; Liu, S.; Wang, Z.; Chen, L. Size-dependent effects of polystyrene microplastics on cytotoxicity and efflux pump inhibition in human Caco-2 cells. Chemosphere 2019, 221, 333–341. [Google Scholar] [CrossRef]
- Song, K. Microparticles and microplastics released from daily use of plastic feeding and water bottles and plastic injectors: Potential risks to infants and children in China. Environ. Sci. Pollut. Res. 2021, 28, 59813–59820. [Google Scholar] [CrossRef]
- Segovia-Mendoza, M.; Nava-Castro, K.E.; Palacios-Arreola, M.I.; Garay-Canales, C.; Morales-Montor, J. How microplastic components influence the immune system and impact on children health: Focus on cancer. Birth Defects Res. 2020, 112, 1341–1361. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, S.C.; Korach, K.S. Progesterone action and responses in the αERKO mouse. Steroids 2000, 65, 551–557. [Google Scholar] [CrossRef]
- Kezele, P.; Skinner, M.K. Regulation of ovarian primordial follicle assembly and development by estrogen and progesterone: Endocrine model of follicle assembly. Endocrinology 2003, 144, 3329–3337. [Google Scholar] [CrossRef] [PubMed]
- An, R.; Wang, X.; Yang, L.; Zhang, J.; Wang, N.; Xu, F.; Hou, Y.; Zhang, H.; Zhang, L. Polystyrene microplastics cause granulosa cells apoptosis and fibrosis in ovary through oxidative stress in rats. Toxicology 2021, 449, 152665. [Google Scholar] [CrossRef] [PubMed]
- Hua, D.; Zhou, Y.; Lu, Y.; Zhao, C.; Qiu, W.; Chen, J.; Ju, R. Lipotoxicity impairs granulosa cell function through activated endoplasmic reticulum stress pathway. Reprod. Sci. 2020, 27, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Kunitomi, C.; Harada, M.; Takahashi, N.; Azhary, J.M.K.; Kusamoto, A.; Nose, E.; Oi, N.; Takeuchi, A.; Wada-Hiraike, O.; Hirata, T.; et al. Activation of endoplasmic reticulum stress mediates oxidative stress–induced apoptosis of granulosa cells in ovaries affected by endometrioma. Mol. Hum. Reprod. 2020, 26, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Hou, J. Polystyrene microplastics lead to pyroptosis and apoptosis of ovarian granulosa cells via NLRP3/Caspase-1 signaling pathway in rats. Ecotoxicol. Environ. Saf. 2021, 212, 112012. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, Y.; Wang, S.; Xie, J.; Han, Q.; Chen, M. Comparing the effects of polystyrene microplastics exposure on reproduction and fertility in male and female mice. Toxicology 2022, 465, 153059. [Google Scholar] [CrossRef]
- Ramsperger, A.F.R.M.; Narayana, V.K.B.; Gross, W.; Mohanraj, J.; Thelakkat, M.; Greiner, A.; Schmalz, H.; Kress, H.; Laforsch, C. Environmental exposure enhances the internalization of microplastic particles into cells. Sci. Adv. 2020, 6, eabd1211. [Google Scholar] [CrossRef]
- Li, H.; Guo, S.; Cai, L.; Ma, W.; Shi, Z. Lipopolysaccharide and heat stress impair the estradiol biosynthesis in granulosa cells via increase of HSP70 and inhibition of smad3 phosphorylation and nuclear translocation. Cell Signal. 2017, 30, 130–141. [Google Scholar] [CrossRef]
- Zhou, R.; Miao, Y.; Li, Y.; Li, X.; Xi, J.; Zhang, Z. MicroRNA-150 promote apoptosis of ovine ovarian granulosa cells by targeting STAR gene. Theriogenology 2019, 127, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, M.U.; Shahzadi, S.; Samad, A.; Ehsan, N.; Ahmed, H.; Tahir, A.; Rehman, H.; Anwar, H. Dose-dependent effect of polystyrene microplastics on the testicular tissues of the male sprague dawley rats. Dose-Response 2021, 19, 15593258211019882. [Google Scholar] [CrossRef] [PubMed]
- Da Broi, M.G.; Jordão-Jr, A.A.; Ferriani, R.A.; Navarro, P.A. Oocyte oxidative DNA damage may be involved in minimal/mild endometriosis-related infertility. Mol. Reprod. Dev. 2018, 85, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Hong, W.; Yang, P.; Tang, Y.; Zhao, Y.; Aguilar, Z.P.; Xu, H. Nano zinc oxide induced fetal mice growth restriction, based on oxide stress and endoplasmic reticulum stress. Nanomaterials 2020, 10, 259. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, X.; Zhang, S.; Huang, H.; Wu, J.; Wang, Y.; Yuan, L.; Liu, C.; Zeng, X.; Cheng, X.; et al. Oxidative stress mediates microcystin-LR-induced endoplasmic reticulum stress and autophagy in KK-1 cells and C57BL/6 mice ovaries. Front. Physiol. 2018, 9, 1058. [Google Scholar] [CrossRef]
- Pan, L.; Yu, D.; Zhang, Y.; Zhu, C.; Yin, Q.; Hu, Y.; Zhang, X.; Yue, R.; Xiong, X. Polystyrene microplastics-triggered mitophagy and oxidative burst via activation of PERK pathway. Sci. Total. Environ. 2021, 781, 146753. [Google Scholar] [CrossRef]
- Wu, L.L. Mitochondrial dysfunction in oocytes of obese mothers: Transmission to offspring and reversal by pharmacological endoplasmic reticulum stress inhibitors. Development 2015, 142, 681–691. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).