Abstract
The effect of pH is a key factor in biomineralization mediated by Acidithiobacillus ferrooxidans to promote the transformation of Fe into secondary iron minerals. This study aimed to investigate the effects of initial pH and carbonate rock dosage on bio-oxidation and secondary iron mineral synthesis. Variations in pH and the concentrations of Ca2+, Fe2+, and total Fe (TFe) in the growth medium of A. ferrooxidans were examined in the laboratory to determine how they affect the bio-oxidation process and secondary iron mineral synthesis. The results showed that in systems with an initial pH of 1.8, 2.3, and 2.8, the optimum dosages of carbonate rock were 30, 10, and 10 g, respectively, which significantly improved the removal rate of TFe and the amount of sediments. At an initial pH of 1.8 and a carbonate rock dosage of 30 g, the final removal rate of TFe reached 67.37%, which was 28.03% higher than that of the system without the addition of carbonate rock, and 36.9 g·L−1 of sediments were generated, which was higher than that of the system without the addition of carbonate rock (6.6 g·L−1). Meanwhile, the number of sediments generated by adding carbonate rock were significantly higher than those without the addition of carbonate rock. The secondary minerals were characterized by a progressive transition from low crystalline assemblages composed of calcium sulfate and subordinated jarosite, to well crystal-line assemblages composed of jarosite, calcium sulfate, and goethite. These results have important implications for comprehensively understanding the dosage of carbonate rock in mineral formation under different pH conditions. The findings help reveal the growth of secondary minerals during the treatment of AMD using carbonate rocks under low-pH conditions, which offers valuable information for combining the carbonate rocks with secondary minerals to treat AMD.
1. Introduction
Acidithiobacillus ferrooxidans is widely distributed in acid mine drainage (AMD). This Gram-negative bacterium can grow in a wide range of temperatures (4–37 °C) and acidic conditions (pH 1.0–4.5), but its optimal growth pH is about 2.0 [1]. Numerous studies have shown that the oxidation rate of Fe2+ can be increased by 105 to 106 times by A. ferrooxidans [2,3], and secondary minerals formed by hydrolysis of Fe3+, such as schwertmannite, jarosite, and goethite, with their relatively high availability, specific crystal structures, and large surface areas facilitates their use as adsorbents for remediating wastewater contaminated with heavy metals [4,5]. Acidophilic organisms contribute to the precipitation of secondary iron minerals in the modern sediments of the river [6,7]. It confirms that the presence of biological nucleation sites (cell walls of bacteria or fungi) can modify the expected mineral precipitation schemes offered by the bulk physicochemical conditions in which microorganisms grow. The extracellular polymeric substances (EPS) released by the microorganisms can serve as nucleation sites for biosynthesis of the secondary iron minerals process [8,9]. A. ferrooxidans plays an important role in the biosynthesis of secondary iron minerals, and particularly in improving the self-purification ability of the environment, thus enhancing environmental and economic benefits in AMD treatment [10].
The pH of a solution has a major influence on microbial activities and the oxidation rates of Fe2+ [11,12]. Unsuitable pH environments are harmful to the growth and metabolism of bacteria, resulting in a decline in bacterial oxidation [13]. A. ferrooxidans can substantially alter the pH conditions of the bacteria−mineral interface and its surrounding microenvironment, consequently enhancing the mineral dissolution process [14]. Under different pH and Eh conditions, microorganisms can induce the formation of secondary minerals with different morphologies, structures, and particle sizes [15,16]. The formation of secondary iron minerals depends on the content of hydroxyl complexes or the transformation of the monomers to polymers, which are greatly affected by the pH of a solution [17]. It is generally accepted that natural schwertmannite formation commonly occurs in Fe-rich sulfate solutions in the pH range of 2.50–4.50 with a lower or higher pH promoting either jarosite or ferrihydrite/goethite precipitation, respectively [18]. These secondary minerals will naturally passivate the heavy metals in water by absorption or co-precipitation, which effectively remove heavy metals from AMD [19]. It seemed that pH was a crucial factor in determining the formation of secondary minerals.
Globally, karst-dominated landscapes cover 10–15% of the land area and are typically characterized by rich carbonate rocks [20]. An increase in coal mining activities in karst areas produces considerable amounts of AMD, which is characterized by low pH, high concentrations of iron (mostly Fe2+), and other trace elements [21]. Considering the severe adverse effects of AMD on the fragile ecological environment of karst areas [22,23], the control and management of AMD have practical significance for the protection of ecological environments and sustainable development in karst watersheds. The AMD passive treatment technology was developed with cheap and easily available carbonate rocks as the reactive medium to effectively improve the pH of AMD, and it has had a good removal effect on Fe [24]. Additionally, the production of secondary minerals plays an important role in the removal of other heavy metals from AMD [25]. Our previous research has shown that the combined treatment of AMD, with biological oxidation and carbonate rocks under low-pH conditions, is a potential treatment technique [26]. The feasibility to pursue sequential and fractional secondary minerals is a driving factor that promotes this approach. However, it is still necessary to determine how the reaction processes differ based on pH, thereby impacting the combined mechanism of action of carbonate rocks and biological oxidation.
Therefore, the objectives of this study are to: (1) investigate effects of carbonate rock dosage and initial pH on the rates of pH rise; (2) explore the effect of pH regulation on the reaction processes of carbonate rock and biological oxidation in acidic environments; (3) select an optimal dosage of carbonate rocks based on initial pH to promote the synthesis of secondary iron minerals. This study will be beneficial for the rational selection of carbonate rocks, and it can be used to extend the application of low-cost and regenerable secondary iron minerals for AMD treatment in karst areas.
2. Materials and Methods
2.1. Preparation of A. ferrooxidans and Carbonate Rock Samples
The leachate from a coal mine was inoculated in a modified 9K liquid medium which consisted of FeSO4·7H2O (99.9%, Sinopharm Group Co., Ltd., Shanghai, China) 44.24 g·L−1, (NH4)2SO4 (≥99.0%, Sinopharm Group Co., Ltd., Shanghai, China) 3 g·L−1, KCl (≥99.0%, Sinopharm Group Co., Ltd., Shanghai, China) 0.1 g·L−1, K2HPO4 (≥98.0%, Tianjin Kemiou Chemical Reagent Co., Ltd., Tianjin, China) 0.5 g·L−1, Ca(NO3)2·4H2O (≥99.0%, Tianjin Kemiou Chemical Reagent Co., Ltd., Tianjin, China) 0.01 g·L−1, and MgSO4·7H2O (≥98.0%, Tianjin Kemiou Chemical Reagent Co., Ltd., Tianjin, China) 0.5 g·L−1 at 20% [27], and the mixture was then placed in a digital-display air thermostat oscillator (ZD-85A, China) at 30 °C and 160 r·min−1 for 3–4 d until the color of the mixture in the reaction system changed from green to red–brown. After multiple enrichments and cultures, an A. ferrooxidans bacterial solution was obtained. The purity of all reagents was analytical grade. The purified A. ferrooxidans bacterial solution was identified by metagenomic microbial classification, sequenced, and preserved at 4 °C.
The carbonate rocks used in this experiment were mainly comprised of calcite and dolomite (95.7%), and collected from Sandu County, Buyi and Miao Autonomous Prefecture of Qiannan, Guizhou Province (Figure 1) [28]. They were ground and screened to different particle sizes, washed, and dried.
Figure 1.
Sketch map of Sandu County, Buyi and Miao Autonomous Prefecture of Qiannan, Guizhou Province.
2.2. Experimental Methods
The carbonate rocks (Φ1.3–1.6 cm) were repeatedly washed with deionized water and dried in an oven at 50 °C for 8 h. A total of 15 mL of A. ferrooxidans LX5 inoculum was used to inoculate Erlenmeyer flasks with different amounts of carbonate rocks and 135 mL of a modified 9 K liquid medium stock solution. Treatment systems with different initial pH levels were set up, and three parallel experiments (treatments ①, ②, and ③) were established for each system (Table 1). The meaning of the labels in each figure in this study can be found in Table 1.

Table 1.
Different initial pH treatment systems.
2.3. Measurement Methods and Data Processing
The pH was determined using a pHS-3C digital pH-meter, Fe2+ was measured using the 1,10-phenanthroline method, and then TFe and Ca2+ contents were determined by atomic absorption spectrometry (TAS-990, Beijing Purkinje General Instrument Co., Ltd., Beijing, China). The mineral phases were analyzed with X-ray diffraction (XRD) using CuKα radiation (40 KV, 40 mA). The samples were scanned from 10° to 70° 2θ with steps of 0.02°. The characteristic reflection peaks of the minerals were compared with those in the data files of the Joint Committee on Powder Diffraction Standards (JCPDS). The surface morphologies of the carbonate rocks and sediments were directly observed using thermal field-emission scanning electron microscopy (SEM, JSM-7001F, JEOL Ltd., Japan).
Microsoft Excel (2019) was used for the statistical analysis of the data obtained in this experiment, and the XRD analysis was conducted using the Jade 6.5 software. All the experimental data were represented by the mean value and standard deviation, with related diagrams drawn using Origin 8.0.
In this study, the oxidation rate (OR) of Fe2+ and removal rate (RR) of TFe were calculated using Equations (1) and (2), where C(Fe2+) and C(TFe)0 denote the metal concentrations at the beginning and C(Fe2+)t and C(TFe)t denote the metal concentrations at different incubation times.
3. Results and Discussion
3.1. Variations of pH and Ca2+ Concentrations in Each System
The biomineralization process involving A. ferrooxidans included two chemical reactions: (1) Fe2+ biological oxidation, where the consumption of H+ increases pH (Equation (3)) [29], and (2) hydrolysis of Fe3+ to form secondary minerals, such as schwertmannite and jarosite, where the release of H+ reduces pH (Equations (4) and (5)) [30]. In karst areas, AMD is neutralized by the surrounding carbonate rocks in the early stages of AMD formation (Equation (6)) [28]:
When carbonate rocks were added to the systems, the pH of 2.8J decreased gradually at the beginning of the experiment (Figure 2), while the pH of the other systems rose quickly from 0–12 h, and then decreased. The results indicated that Fe3+ hydrolyzed more easily at a higher initial pH. Based on Equations (3) and (6), the pH values of the systems with carbonate rocks increased faster than those without carbonate rocks. Some scholars found that the active radicals (for example, •OH) were produced when the pH value was increased by carbonate rocks in the process of base release, which accelerated the oxidation of Fe2+ and hydrolytic precipitation of Fe [31,32]. pH = 2.50–4.50 is conducive for the efficient activity of A. ferrooxidans, and the formation of secondary iron minerals [33]. The pH values of the systems with carbonate rocks could be maintained at 2.50–4.50. Therefore, based on the initial pH value, the number of carbonate rocks added can promote the formation and transformation of secondary minerals. Additionally, the activity of A. ferrooxidans was inhibited in low-pH environments, which slowed the oxidation rate of Fe2+. For example, the pH of 1.8J was lower than that of 2.3J. According to Equations (4) and (5), during the synthesis of schwertmannite, 1 mol Fe3+ hydrolysis could release 2.75 mol H+, while during the synthesis of jarosite, 1 mol Fe3+ hydrolysis could release 2 mol H+ [34]. Compared with the other systems, the pH values of 1.8J30, 2.3J10, and 2.8J, at the end of experiment, were less than 2 because more H+ was being released.
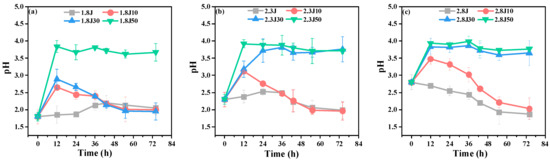
Figure 2.
Variation in the pH values in each system from an initial pH of (a) 1.8; (b) 2.3; and (c) 2.8.
High concentrations of Ca2+ slowed the transformation of ferrihydrite into other ferric minerals because of carbonate rock dissolution, as indicated by the Ca2+ concentration during the experimental process (Figure 3). For all dosages tested, the dissolution of carbonate rocks was rapid in the initial 12 h and fluctuated at later stages. The fluctuation in Ca2+ in each system was strong because of calcium sulfate production. The pH values of 1.8J50, 2.3J30, 2.3J50, 2.8J30, and 2.8J50 increased rapidly during 0–12 h, began to decrease slightly at 36 h, and then were maintained at 3.59–3.76 during 55–74 h. Slight fluctuations in pH lead to changes in the CaCO3-H2O system [35]. Practically, Ca in the solution plays a role in increasing the pH value of the wastewater. Masindi et al. found that the Ca in struvite matrices will react with AMD water to release hydroxyl ions (OH−) which then contribute to an increase in pH, and higher pH could result in absorption or co-precipitation of different contaminants from AMD, thus leading to the reduction of the levels in the product water [36].
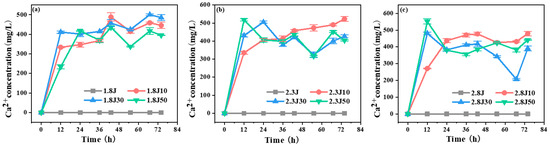
Figure 3.
Variation in Ca2+ concentrations in each system from an initial pH of (a) 1.8; (b) 2.3; and (c) 2.8.
3.2. Oxidation Rate of Fe2+ and Removal Rate of TFe in Each System
Excessive pollutants in AMD lead to continuous declines in water quality in karst areas. Improving the efficiency of Fe2+ oxidation and subsequent Fe3+ hydrolysis are key steps for AMD treatment. The oxidation rate of Fe2+ in each system (Figure 4a–c) displayed an approximately increasing linear trend from 0 to 12 h. The oxidation rates of Fe2+ in 1.8J10 and 1.8J30 reached 99% at 43 h, and were completely oxidized at 55 h in 1.8J, 2.3J, 2.3J10, 2.8J, and 2.8J10. The different patterns of Fe2+ oxidation can be primarily ascribed to variations in pH. An appropriate pH exists for the bio-oxidation of Fe2+ to promote the formation and phase transformation of secondary iron minerals. For example, when the initial pH was 1.8, 10 g or 30 g of carbonate rocks (2.18 < pH < 2.66) significantly accelerated the Fe2+ oxidation process. However, the degree of inhibition of Fe2+ oxidation caused by secondary iron minerals covering A. ferrooxidans was stronger than that of Fe2+ oxidation indirectly caused by the addition of carbonate rocks, which hindered the conversion of Fe2+ to Fe3+ [37]. The final oxidation rates of Fe2+ among the systems were relatively low with the additions of 30 g and 50 g of carbonate rocks. Zhou et al. found that the bio-oxidation of Fe2+ was slowed down by adding OH− into the system for pH-control in the later stage [9].
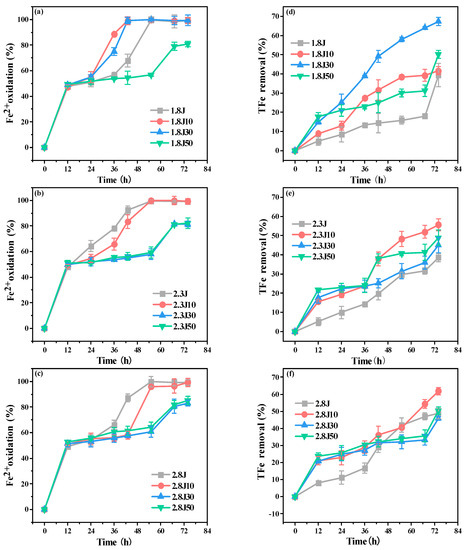
Figure 4.
Fe2+ oxidation rates (a–c) and total Fe precipitation (d–f) at pH 1.8, 2.3, and 2.8, respectively, under different dosages of carbonate rock.
The TFe removal rate in each treatment gradually increased (Figure 4d–f). The final removal rate of TFe in the 1.8J30 system was 67.37%, being 28.03% higher than that in 1.8J (39.34%), and the final removal rates of TFe in 2.3J10 and 2.8J10 were 55.63% and 61.82%, respectively, being 16.99% and 13.1% higher than those in 2.3J (38.64%) and 2.8J (48.72%), respectively. Therefore, the optimum dosages of carbonate rocks for the systems with initial pH values of 1.8, 2.3, and 2.8 were 30, 10, and 10 g, respectively. The addition of Fe3+ boosts the growth of A. ferrooxidans and eliminates the effect of low pH on its activity [38]. Thus, the addition of an appropriate amount of carbonate rocks accelerated the oxidation of Fe2+ and increased the content of Fe3+ in the solution, which was beneficial for increasing the activity of A. ferrooxidans and promoting the formation of secondary minerals (Equations (4) and (5)).
3.3. Characterization of the Sediments and Secondary Iron Mineral Phases for Each System
The oxidation of Fe2+ by A. ferrooxidans, and the combination of dissolved metals, resulted in the formation of a large quantity of precipitates [39].
The amounts of sediment produced in the different systems (Figure 5), with the addition of carbonate rocks, were significantly higher than those in the absence of carbonate rocks. The 1.8J30, 2.8J10, and 2.8J10 were the highest sediments produced in different initial pH systems, respectively. The treatment 1.8J30 generated 36.9 g·L−1 of sediment, which was higher than that in 1.8J (6.6 g·L−1). The reasons for the increase in mineral mass include: (1) the addition of carbonate rocks resulted in an optimal environment conducive to the growth of A. ferrooxidans, thereby promoting the synthesis of secondary iron minerals and the formation of new phases; (2) the existence of crystal seeds (including jarosite and CaSO4·2H2O) provided nucleation sites for mineral formation, thus eliminating the induction period, accelerating the initial precipitation of minerals, and increasing mineral production [40,41,42].
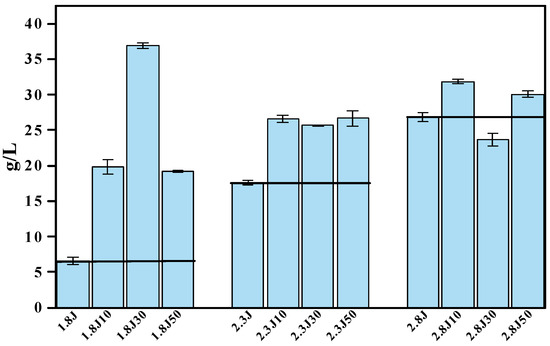
Figure 5.
Amount of sediment produced in the different systems at pH 1.8, 2.3, and 2.8 under different dosages of carbonate rock.
Under various pH conditions, different iron minerals, such as schwertmannite, jarosite, and goethite, were precipitated from AMD (Figure 6). The XRD patterns and SEM images of the sediments are shown in Figure 7 and Figure 8, respectively. According to the Joint Committee on Power Diffraction Standards (JCPDS) data files (jarosite [no. 22-0827], jarosite [no. 26-1014], calcium sulfate [CaSO4·2H2O] crystallization [no. 33-0311], and goethite [no. 76-2301]), the sediments were found to be composed of jarosite, calcium sulfate, and goethite. However, the weak XRD peaks of schwertmannite particles may be masked by the peaks of higher-strength crystalline minerals such as jarosite and goethite. Jarosite-rich sediments occurred as pseudocubic or polygonized crystals at pH values of 1.8–3.0 in 1.8J, 1.8J10, 1.8J30, 2.3J, 2.3J10, 2.8J, and 2.8J10 systems. The crystallization of calcium sulfate was observed in 1.8J30, 1.8J50, 2.3J10, 2.3J30, 2.3J50, 2.8J30, and 2.8J50 systems, and goethite formed in the 2.8J10 system.
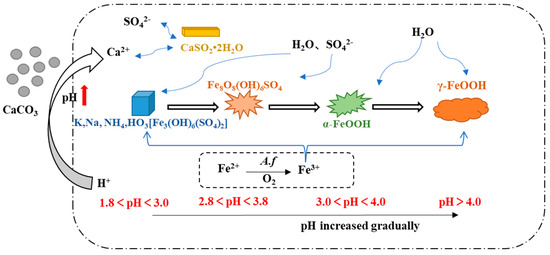
Figure 6.
Influence of pH changes on secondary mineral formation during the interaction between AMD and carbonate rock.
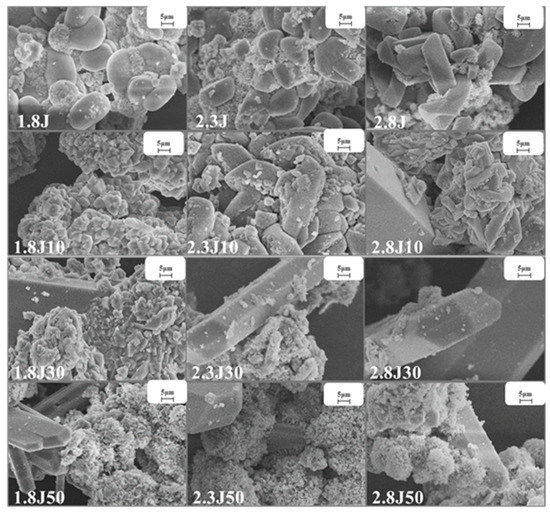
Figure 7.
SEM images of sediments collected from the different systems at 1.8, 2.3, and 2.8 under different dosages of carbonate rock.
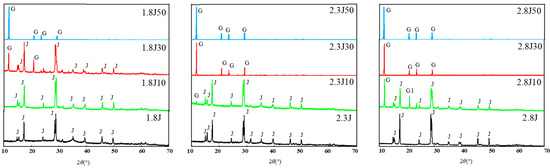
Figure 8.
XRD patterns of sediments in the different systems at pH 1.8, 2.3, and 2.8 under different dosages of carbonate rock: J: jarosite, G: calcium sulfate, G1: goethite.
The sediments were characterized by a progressive transition from low crystalline assemblages composed of calcium sulfate and subordinated jarosite, to well crystal-line assemblages composed of jarosite, calcium sulfate, and goethite. When the hydrolysis of Fe3+ releases H+ to reduce pH (pH < 3) and monovalent cations are present in the solution, schwertmannite transforms into jarosite [43]. Based on the XRD analysis of 2.8J10 and the range of pH variation, the spherulitic aggregate (Figure 7) was goethite, which may have retained the schwertmannite or mineral crystal form of schwertmannite during the transformation of jarosite or schwertmannite into goethite [44]. As per the XRD analysis, calcium sulfate in the 2.3J30, 2.3J50, 2.8J30, and 2.8J50 systems was thick, plate-like, and needle-like, while that on the rock surfaces of 2.8J30 and 2.8J50 (Figure 9) was mainly flaky and needle-like. The growth of calcium sulfate crystals is inhibited by Ca2+, Mg2+, and Fe3+ in the solution [45], which affects the morphology and size of gypsum crystals [46].

Figure 9.
SEM images of the carbonate rock surfaces of the different systems (magnified 10,000×).
The range of pH variation, oxidation rate of Fe2+, removal rate of TFe, and main secondary mineral categories of the different systems are shown in Table 2. In this study, significant chemical and mineralogical differences occurred due to variations in the initial pH and dosage of carbonate rock. Compared to other systems with different initial pH, in the systems of 1.8J30,2.3J10, and 2.8J10, the TFe removal significantly increased and the changes of pH were conducive to the formation of secondary minerals or to form the new secondary mineral phase. Liu et al. [47] also found that with the formation of secondary Fe minerals with a mixed schwertmannite and jarosite, total Fe precipitation efficiency increased. According to previous research, the pH value and pH gradient between the surface of the mineral and the surrounding aqueous solution are key factors affecting the formation and phase transformation of secondary minerals [48].

Table 2.
Range of pH variation, Fe2+ oxidation, TFe removal, sediment production, and main secondary minerals in the different systems.
4. Conclusions
The following conclusions could be drawn from this study.
- (1)
- In this study, variations in pH and Ca2+ concentrations were found to affect the formation and phase transformation of secondary iron minerals. In the systems with an initial pH value of 1.8, 2.3, and 2.8, the optimum dosage of carbonate rocks was 30, 10, and 10 g, respectively. The oxidation rates of Fe2+ all reached 99% and the final removal rate of TFe in the 1.8J30 system was 67.37%, being 28.03% higher than that in 1.8J (39.34%), and the final removal rates of TFe in 2.3J10 and 2.8J10 were 55.63% and 61.82%, respectively, being 16.99% and 13.1% higher than those in 2.3J (38.64%) and 2.8J (48.72%), respectively.
- (2)
- The number of sediments generated by adding carbonate rocks were significantly higher than those generated without carbonate rock addition. The 1.8J30, 2.8J10, and 2.8J30 were the highest sediments produced in different initial pH systems, respectively. In particular, 1.8J30, which generated 36.9 g·L−1 of sediment, was higher than that of 1.8J (6.6 g·L−1). The sediments were characterized by a progressive transition from low crystalline assemblages composed of calcium sulfate and subordinated jarosite, to well crystal-line assemblages composed of jarosite, calcium sulfate, and goethite.
- (3)
- As a consequence, based on the initial pH value, the number of carbonate rocks added can be reasonably selected to control the rates of pH rise. When the pH is raised to an appropriate value, the removal rate of TFe, the mineral production, and the types of secondary minerals can be significantly improved. The findings of this study are of interest for engineering applications that consider combined microbial and carbonate rock treatments for AMD in karst areas.
Author Contributions
Conceptualization, R.Z., N.W. and Y.F.; Data curation, Y.F. and N.W.; Funding acquisition, R.Z. and P.W.; Methodology, Y.F., N.W., Y.Z. (Yahui Zhang), L.A. and Y.Z.(Yuhao Zhang); Resources, N.W.; Writing—original draft, Y.F.; Writing—review & editing, R.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Key Research and Development Program of China (2019YFC1805300), the National Natural Science Foundation of China (No. U1612442), and High Level Talent Training Program in Guizhou (20165664).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors declare that [the/all other] data supporting the findings of this study are available within the article.
Acknowledgments
This research was supported by the National Key Research and Development Program of China (2019YFC1805300), the National Natural Science Foundation of China (No. U1612442), and High Level Talent Training Program in Guizhou (20165664). We are grateful to the anonymous reviewers and the editors for their valuable suggestions and comments on revising and improving this work.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper “Effects of initial pH and carbonate rock dosage on bio-oxidation and secondary iron mineral synthesis”.
References
- Jung, H.; Inaba, Y.; Banta, S. Genetic engineering of the acidophilic chemolithoautotroph Acidithiobacillus ferrooxidans. Trends Biotechnol. 2021, 40, 677–692. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Jo, C.; Ri, H. Immobilization of Acidithiobacillus ferrooxidans-1333 on the Waste Ore Particles for the Continuous Oxidation of Ferrous Iron. Iran. J. Biotechnol. 2020, 18, e2356. [Google Scholar] [PubMed]
- Wang, X.; Li, Q.; Liao, Q.; Yan, Y.; Xia, J.; Lin, Q.; Wang, Q.; Liang, Y. Arsenic(III) biotransformation to tooeleite associated with the oxidation of Fe(II) via Acidithiobacillus ferrooxidans. Chemosphere 2020, 248, 126080. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yan, L.; Xing, W.; Chen, P.; Zhang, Y.; Wang, W. Acidithiobacillus ferrooxidans and its potential application. Extremophiles 2018, 22, 563–579. [Google Scholar] [CrossRef] [PubMed]
- Gan, M.; Huang, D.; Chen, F.; Zhang, K.; Zhu, J. Enhanced Cr(VI) reduction and Cr(III) coprecipitation through the synergistic effect between sulfide minerals and chemoautotrophic decomposer. J. Environ. Chem. Eng. 2021, 9, 105942. [Google Scholar] [CrossRef]
- Ehrlich, H.; Bailey, E.; Wysokowski, M.; Jesionowski, T. Forced Biomineralization: A Review. Biomimetics 2021, 6, 46. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Faivre, D. Diversity of Microbial Metal Sulfide Biomineralization. ChemPlusChem 2022, 87, e202100457. [Google Scholar] [CrossRef]
- Luis, A.T.; Cordoba, F.; Antunes, C.; Loayza-Muro, R.; Grande, J.A.; Silva, B.; Diaz-Curiel, J.; Ferreira, D.S.E. Extremely Acidic Eukaryotic (Micro) Organisms: Life in Acid Mine Drainage Polluted Environments-Mini-Review. Int. J. Environ. Res. Public Health 2021, 19, 376. [Google Scholar] [CrossRef]
- Zhou, J.X.; Zhou, Y.J.; Zhang, J.; Dong, Y.; Liu, F.W.; Wu, Z.H.; Bi, W.L.; Qin, J.M. Effect of pH regulation on the formation of biogenic schwertmannite driven by Acidithiobacillus ferrooxidans and its arsenic removal ability. Envrion. Technol. 2022, 43, 3706–3718. [Google Scholar] [CrossRef]
- Wang, N.; Fang, D.; Zheng, G.; Liang, J.; Zhou, L. A novel approach coupling ferrous iron bio-oxidation and ferric iron chemo-reduction to promote biomineralization in simulated acidic mine drainage. RSC Adv. 2019, 9, 5083–5090. [Google Scholar] [CrossRef]
- Schoepfer, V.A.; Burton, E.D. Schwertmannite: A review of its occurrence, formation, structure, stability and interactions with oxyanions. Earth-Sci. Rev. 2021, 221, 103811. [Google Scholar] [CrossRef]
- Plumb, J.J.; Muddle, R.; Franzmann, P.D. Effect of pH on rates of iron and sulfur oxidation by bioleaching organisms. Miner. Eng. 2008, 21, 76–82. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, S.; Wang, B.; Wen, X.; Zeng, R.J. Effect of ferric ions on the anaerobic bio-dissolution of jarosites by Acidithiobacillus ferrooxidans. Sci. Total Environ. 2019, 710, 136334. [Google Scholar] [CrossRef] [PubMed]
- Yi, Q.; Wu, S.; Southam, G.; Robertson, L.; You, F.; Liu, Y.; Wang, S.; Saha, N.; Webb, R.; Wykes, J.; et al. Acidophilic Iron- and Sulfur-Oxidizing Bacteria, Acidithiobacillus ferrooxidans, Drives Alkaline pH Neutralization and Mineral Weathering in Fe Ore Tailings. Environ. Sci. Technol. 2021, 55, 8020–8034. [Google Scholar] [CrossRef] [PubMed]
- Richard, B.F.; Dennis, A.B. Biologically Induced Mineralization by Bacteria. Rev. Mineral. Geochem. 2003, 54, 95–114. [Google Scholar]
- Liao, Y.; Zhou, L.; Liang, J.; Xiong, H. Biosynthesis of schwertmannite by Acidithiobacillus ferrooxidans cell suspensions under different pH condition. Mater. Sci. Eng. C 2009, 29, 211–215. [Google Scholar] [CrossRef]
- Feng, K.; Wang, X.; Bo, Z.; Xu, M.; Liang, J.; Zhou, L. Hydroxyl, Fe2+, and Acidithiobacillus ferrooxidans Jointly Determined the Crystal Growth and Morphology of Schwertmannite in a Sulfate-Rich Acidic Environment. ACS Omega 2021, 6, 3194–3201. [Google Scholar] [CrossRef]
- Wang, X.; Wang, D.; Xu, J.; Fu, J.; Zheng, G.; Zhou, L. Modified chemical mineralization-alkali neutralization technology: Mineralization behavior at high iron concentrations and its application in sulfur acid spent pickling solution. Water Res. 2022, 218, 118513. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, R.; Wu, P.; Zhang, Y.; Fu, Y.; An, L.; Zhang, Y. Study on the precipitation of iron and the synchronous removal mechanisms of antimony and arsenic in the AMD under the induction of carbonate rocks. Environ. Sci. Pollut. Res. 2022, 29, 55161–55173. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, P.; Yuan, S.; Tong, M. Arsenic oxidation and immobilization in acid mine drainage in karst areas. Sci. Total Environ. 2020, 727, 138629. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, J.; Hu, Q.; Gao, X.; Li, C.; Luo, M.; Wang, Y. Effects of Fe-rich acid mine drainage on percolation features and pore structure in carbonate rocks. J. Hydrol. 2020, 591, 125571. [Google Scholar] [CrossRef]
- Chen, Q.; Lu, S.; Xiong, K.; Zhao, R. Coupling analysis on ecological environment fragility and poverty in South China Karst. Environ. Res. 2021, 201, 111650. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Yu, C.; Peng, B.; Xiao, H.; Zhang, W.; Zhou, Z.; Astrom, M. A re-assessment of metal pollution in the Dexing mining area in Jiangxi province, China: Current status, hydro-geochemical controls, and effectiveness of remediation practices. Int. J. Environ. Sci. Technol. 2022, 19, 10707–10722. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, R.; Wu, P.; Zhang, S.; Zhang, Y. Analysis of Bacterial Community Structure and Function in Acid Wastewater Treatment System of an Abandoned Coal Mine in Guizhou Province. Res. Environ. Sci. 2021, 34, 2154–2163. (In Chinese) [Google Scholar]
- Zhang, S.; Zhang, R.; Wu, P.; Wang, Y.; Wang, N.; Yang, X. Research progress on interactions between carbonate and acid mine drainage and its passive treatment technology. Environ. Eng. 2021, 39, 52–61. (In Chinese) [Google Scholar]
- Wang, N.; Zhang, R.; Wu, P.; Zhang, S.; An, L.; Zhang, Y.; Fu, Y. The influence of carbonate rock on Fe2+ biological oxidation and iron mineral syntheis. Acta Sci. Circumstantiae 2021, 41, 3555–3562. (In Chinese) [Google Scholar]
- Qiao, X.; Liu, L.; Shi, J.; Zhou, L.; Guo, Y.; Ge, Y.; Fan, W.; Liu, F. Heating Changes Bio-Schwertmannite Microstructure and Arsenic(III) Removal Efficiency. Minerals 2017, 7, 9. [Google Scholar] [CrossRef]
- Naidu, G.; Ryu, S.; Thiruvenkatachari, R.; Choi, Y.; Jeong, S.; Vigneswaran, S. A critical review on remediation, reuse, and resource recovery from acid mine drainage. Environ. Pollut. 2019, 247, 1110–1124. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, B.; Haderlein, S.; Gopalakrishnan, G.; Zhou, Y. Characteristics and environmental response of secondary minerals in AMD from Dabaoshan Mine, South China. Ecotoxicol. Environ. Saf. 2018, 155, 50–58. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, J.; Wang, H. Initial pH and K+ concentrations jointly determine the types of biogenic ferric hydroxysulfate minerals and their effect on adsorption removal of Cr(VI) in simulated acid mine drainage. Water Sci. Technol. 2018, 78, 2183–2192. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, P.; Yuan, S.; Liao, P.; Qian, A.; Liu, X.; Tong, M.; Li, L. Production of Hydroxyl radicals from oxygenation of simulated AMD due to CaCO(3)-induced pH increase. Water Res. 2017, 111, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Zheng, W.; Zhu, L.; Duan, W.; Wei, C.; Feng, C. One-Step Treatment of Phosphite-Laden Wastewater: A Single Electrochemical Reactor Integrating Superoxide Radical-Induced Oxidation and Electrocoagulation. Environ. Sci. Technol. 2019, 53, 5328–5336. [Google Scholar] [CrossRef]
- Song, Y.; Guo, Z.; Wang, R.; Yang, L.; Cao, Y.; Wang, H. A novel approach for treating acid mine drainage by forming schwertmannite driven by a combination of biooxidation and electroreduction before lime neutralization. Water Res. 2022, 221, 118748. [Google Scholar] [CrossRef]
- Zhou, L. Biomineralization: A Pivotal Process in Developing a Novel Passive Treatment System for Acid Mine Drainage. Acta Chim. Sin. 2017, 75, 552. [Google Scholar] [CrossRef]
- Labastida, I.; Armienta, M.A.; Lara, R.H.; Briones, R.; González, I.; Romero, F. Kinetic approach for the appropriate selection of indigenous limestones for acid mine drainage treatment with passive systems. Sci. Total Environ. 2019, 677, 404–417. [Google Scholar] [CrossRef]
- Masindi, V.; Fosso-Kankeu, E.; Mamakoa, E.; Nkambule, T.; Mamba, B.B.; Naushad, M.; Pandey, S. Emerging remediation potentiality of struvite developed from municipal wastewater for the treatment of acid mine drainage. Environ. Res. 2022, 210, 112944. [Google Scholar] [CrossRef]
- Mousavi, S.J.; Ahmadi, A. The influence of physicochemical parameters on the bioleaching of zinc sulfide concentrates using a mixed culture of moderately thermophilic microorganisms. Int. J. Miner. Process. 2015, 135, 32–39. [Google Scholar]
- He, Y.; Guo, J.; Song, Y.; Chen, Z.; Lu, C.; Han, Y.; Li, H.; Hou, Y.; Zhao, R. Acceleration mechanism of bioavailable Fe(III) on Te(IV) bioreduction of Shewanella oneidensis MR-1: Promotion of electron generation, electron transfer and energy level. J. Hazard. Mater. 2021, 403, 123728. [Google Scholar] [CrossRef]
- Lalinská-Voleková, B.; Majerová, H.; Kautmanová, I.; Brachtýr, O.; Szabóová, D.; Arendt, D.; Brčeková, J.; Šottník, P. Hydrous ferric oxides (HFO’s) precipitated from contaminated waters at several abandoned Sb deposits—Interdisciplinary assessment. Sci. Total Environ. 2022, 821, 153248. [Google Scholar] [CrossRef]
- Liu, F.; Zhou, J.; Jin, T.; Zhang, S.; Liu, L. Effect of calcium oxide on the efficiency of ferrous ion oxidation and total iron precipitation during ferrous ion oxidation in acid mine drainage treatment with inoculation of Acidithiobacillus ferrooxidans. Water Sci. Technol. 2015, 73, 1442–1453. [Google Scholar] [CrossRef]
- Wang, H.; Li, M.; Song, Y. Enhanced Monovalent Cation Biomineralization Ability by Quartz Sand for Effective Removal of Soluble Iron in Simulated Acid Mine Drainage. Water 2020, 12, 732. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, H.; Song, Y.; Wu, Y.; Guo, Z. The Synthesis of Secondary Iron Minerals Induced by Quartz Sand during the Bioleaching Process Improves the Dewaterability of Municipal Sewage Sludge. Minerals 2018, 8, 419. [Google Scholar] [CrossRef]
- Acero, P.; Ayora, C.; Torrentó, C.; Nieto, J. The behavior of trace elements during schwertmannite precipitation and subsequent transformation into goethite and jarosite. Geochim. Cosmochim. Acta 2006, 70, 4130–4139. [Google Scholar] [CrossRef]
- Bao, Y.; Guo, C.; Lu, G.; Yi, X.; Wang, H.; Dang, Z. Role of microbial activity in Fe(III) hydroxysulfate mineral transformations in an acid mine drainage-impacted site from the Dabaoshan Mine. Sci. Total Environ. 2018, 616–617, 647–657. [Google Scholar] [CrossRef]
- Pan, Z.; Lou, Y.; Yang, G.; Ni, X.; Chen, M.; Xu, H.; Miao, X.; Liu, J.; Hu, C.; Huang, Q. Preparation of calcium sulfate dihydrate and calcium sulfate hemihydrate with controllable crystal morphology by using ethanol additive. Ceram. Int. 2013, 39, 5495–5502. [Google Scholar] [CrossRef]
- Deng, L.; Zhang, Y.; Chen, F.; Cao, S.; You, S.; Liu, Y.; Zhang, Y. Reactive Crystallization of Calcium Sulfate Dihydrate from Acidic Wastewater and Lime. Chin. J. Chem. Eng. 2013, 21, 1303–1312. [Google Scholar] [CrossRef]
- Liu, F.; Gao, S.; Wang, M.; Yu, H.; Cui, C.; Zhou, L. Effect of KOH on the formation of biogenic secondary iron minerals in iron-and sulfate-rich acidic environment. Huanjing Kexue Xuebao/Acta Sci. Circumstantiae 2015, 35, 476–483. [Google Scholar]
- Ortiz-Castillo, J.; Mirazimi, M.; Mohammadi, M.; Dy, E.; Liu, W. The Role of Microorganisms in the Formation, Dissolution, and Transformation of Secondary Minerals in Mine Rock and Drainage: A Review. Minerals 2021, 11, 1349. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).