Abstract
The palynological and physicochemical analysis of 62 honey samples produced in different biogeographical areas of Algeria was conducted. Results showed high variety in the botanical origin of samples and their physicochemical profile. Twenty-six samples were polyfloral honey, 30 were unifloral honey from different botanical sources such as Eucalyptus, Citrus, Apiaceae, Punica, Erica, Rosmarinus, Eriobotrya, or Hedysarum, and 6 were characterized as honeydew honey. Pollen analysis allowed the identification of 104 pollen types belonging to 51 botanical families, whereas the physicochemical profile showed important variations between samples. Multivariate techniques were used to compare the characteristics of samples from different biogeographical areas, showing significant differences between humid-area samples, located in the northeast of the country, and samples taken in semiarid, subhumid, and arid zones. Principal-component analysis (PCA) extracted nine components explaining 72% of data variance, being 30%, the sum of Component 1 and Component 2. The plot of both components showed samples grouped upon botanical and geographical origin. The results of this paper highlighted the great variability in honey production of Algeria, evidencing the importance of honey characterization to guarantee authenticity and to valorize local production.
1. Introduction
Beekeeping in Algeria is part of agricultural life. It is practiced in mountainous regions such as the Aures Mountains, Kabylie, and Dahra, in the coastal plains, and the valleys of the big wadis, but it is more intensive in the northern part of the country where the flora provides resources for honey throughout most of the year [1]. Beekeeping plays an important role in the rural areas of the Mediterranean region due to the suitable climatic conditions and high biodiversity. Highly valued honey supplies are a welcome income for farmers and hobby beekeepers [2].
The composition of this food, obtained by bees from nectar, honeydew, or both sugary resources, is highly dependent on honeybee activity and the biogeography of the area in which it was produced. So, physicochemical properties such as color, pH, electrical conductivity, sugar content, organic acids, ash content, polyphenols, flavonoids, and other phytochemicals differ with different environmental conditions and plant communities visited by honeybees [3].
The Mediterranean region of Algeria is an outstanding biogeographical crossroads that resulted from a complex history and highly heterogeneous environmental factors [4]. Algeria has significant plant biodiversity, with about 3150 species [5], of which more than 500 species are endemic and characteristic of the Mediterranean basin, the Atlas Mountains, the high steppe plateau, the large Saharan plateau, and the mountainous massifs of the Algerian Sahara [6]. The melliferous area in Algeria is estimated at 797,122 hectares with a predominance of forests and maquis, natural meadows, orchards with orange fruits, and other crops.
The main honey sources in the area are trees like Eucalyptus and some wild herbaceous plants, mainly from the Apiaceae (Foeniculum, Daucus, Coriandrum, Eryngium, Pimpinella), Asteraceae (Galactites tomentosa, Centaurea, Echinops), Brassicaceae (Brassica and Sinapis), and Fabaceae (Hedysarum, Ononis natrix, Trifolium, Melilotus) families [7,8,9]. Some plants, such as Ziziphus lotus, or grown plants, such as Helianthus annuus or Citrus, were also named as important plants sources for honey production [10]. In this context, the main honey types produced in the country are Eucalyptus, Citrus, forest (honeydew), jujube, sunflower, rosemary, and wild mustard. However, the production of other rare or less known honey types was mentioned in some papers [11,12].
The country is considered a traditional consumer of honey, but national production does not achieve self-sufficiency, so to cover the relevant needs, large quantities of honey are imported every year from countries such as China, India, and Saudi Arabia. The lack of national legislation and unknowns about Algerian honey characteristics have a negative impact on the scarce development of Algerian beekeeping. In the absence of specific legislation, there are also no requirements to assess the quality or geographical and botanical origin of honey in the market. Therefore, consumers and local producers are not protected against frauds, and honey of good quality is not valorized. Improving knowledge in the characteristics of local production is the main course of action for their valorization and to link agricultural products with the territory in which they were produced. These studies facilitate the recognition of specific properties with botanical origin and allow differentiating local production from imported honey. Currently, there is no designation of origin for local honey that would contribute to valorizing Algeria honey production.
Some authors studied the characteristics of honey produced in Algeria and found differing results as to the pollen content [8,9,10,13], physicochemical attributes and pollen profile of samples from different areas [7,14,15,16], their botanical origin [9,11,17,18,19], antibacterial and antioxidant activity [20], or specific compounds such as sugars and phenolic or volatile compounds [21,22]. However, honey production in Algeria remains poorly studied. On the basis of these facts, this paper aimed to provide scientific information on the botanical origin and physicochemical profile of honey samples from different bioclimatic areas of Algeria.
2. Material and Methods
2.1. Study-Area Bioclimatology
The territory of Algeria is composed of four principal structural units. Near the coast, in the north, there is a narrow region with several hills and plains named Tell. This region is densely populated and constitutes the main agricultural land in the country. It has a Mediterranean climate with mild winters and moderate rainfall. Mean annual temperatures are close to 22 °C in summer and 10 °C in winter. Rainfall is abundant along this coastal area, increasing the amount of annual precipitation from 400 to 670 mm in the west to near 1000 mm in the east. These weather conditions determined a subhumid-humid climate tendency.
In the central area, several mountain chains were determined as the high plateau area that separates the Mediterranean area from the Saharan Atlas and the desert. This is a highly diverse area dominated in the plains by steppelike morphology. This region is characterized by a semiarid-to-arid climate with irregular and low precipitation (200–400 mm); its bowl profile explains the presence of many salt lakes (called “chotts”) collecting surface water. It continues to the south with the Saharan Atlas that delimits the Sahara desert. The Saharan Atlas is formed by three massifs that are the source of watercourses that supply the wells of oases along the northern edge of the desert; the massifs are Biskra, Laghouat, and Béchar. The Sahara is a windy and very arid area with a continental climate, high thermal amplitude, and extremely poor precipitation of no more than 130 mm.
2.2. Honey Samples
We collected 62 honey samples from different bioclimatic areas of Algeria (Table 1). Concretely, 34 samples were from the semiarid area, 15 were obtained in subhumid areas, 4 in humid areas, and 9 samples in the arid areas of the Sahara and Saharan Atlas (Figure 1). The samples were obtained directly from beekeepers during the 2015–2016 period and were stored refrigerated at 4 °C until analysis. All determinations were carried out after sample homogenization.

Table 1.
Geographical origin of samples, climate, and harvest period.
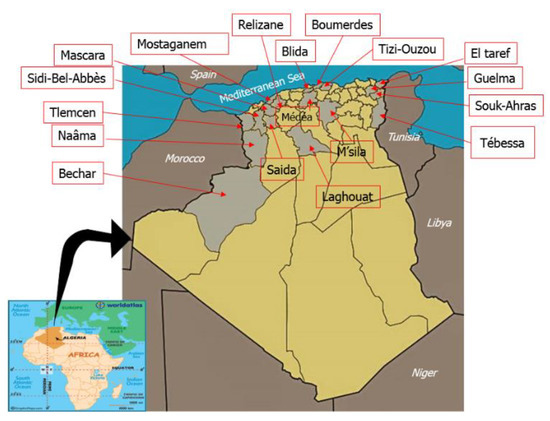
Figure 1.
Geographical areas where the samples were taken.
2.3. Melissopalynological Analysis
2.3.1. Quantitative Pollen Analysis
Ten grams of honey were weighed and dissolved in 20 mL of distilled water. The solution was centrifuged for 10 min at 4500 rpm, and the supernatant was removed until a final volume of 5 mL. The sediment was stirred, and then two aliquots of 10 µL were deposited over a slide to microscopically examine (Nikon UK Ltd., Surbiton, UK). All pollen in the aliquot was counted. The results are expressed as the number of pollen grains per gram of honey considering the average value of the two aliquots.
2.3.2. Qualitative Pollen Analysis
Ten grams of honey were weighed and fully dissolved in 20 mL of distilled water. The solution was centrifuged for 10 min at 4500 rpm, and the supernatant was drawn off. The sediment was again washed with distilled water, and another centrifugation in the same conditions was done. Then, two aliquots (100 µL) of the sediment were deposited into a slide over a heated surface until desiccation. Lastly, drops were covered with a 24 × 24 mm cover glass containing a drop of glicerogelatin with fuchsine.
Slide observation was carried out with a light microscope at 400× or 1000×, as appropriate to improve the identification of pollen types. For each honey sample, at least 500 pollen grains were counted. All pollen grains were classified as the level of pollen type due to difficulties in accessing information about the wild botanical species of apicultural interest in a large part of the territory.
The relative frequency, expressed as a percentage, of all identified pollen type was considered for the pollen spectra of the samples. The following frequency classes were used to establish the representation of pollen types: dominant pollen (≥45%), accompanying pollen (from ≥15% to <45%), important pollen (from ≥3% to <15%), minor pollen (from ≥1% to <3%) and present pollen (≤1%). However, for unifloral honeys, the percentage of the main pollen type was calculated excluding plants considered to be non-nectariferous species, namely, Acacia, Buxus sempervirens, Casuarina, Cannabis, Chamaerops, Cistus, Cyperus, Olea europaea, Papaver rhoeas, Paronychia argentea, Pinus, Pistacia, Poaceae, and Quercus.
2.3.3. Physicochemical Analysis
The quality parameters of moisture, electrical conductivity, pH, and hydroxymethylfurfural content were determined following the methodology of the International Honey Commission [23]. All determinations were made in duplicate. The results are expressed as the mean of the obtained values.
Moisture was determined with a Carl Zeiss Jena refractometer (Zeiss, Oberkochen, Germany) by measuring the refractive index at 20 °C. Moisture content was calculated using the Wedmore table, and results were expressed as percentages.
Electrical conductivity was measured at 20 °C in a 20% (w/v) honey solution (dry-matter basis) in CO2-free deionized distilled water with an EUTECH instrument conductivity meter (Thermo Fisher Scientific, Massachusetts, USA), and results were expressed as mS/cm; pH was measured by a pH meter (WTW in Lab pH 750) in a solution containing 10 g of honey in 75 mL of distilled water.
Hydroxymethyfurfural (HMF) content was determined using the White spectrophotometric method. Briefly, 5 g of honey was dissolved in 25 mL of distilled water and transferred to a volumetric flask of 50 mL; then, 0.5 mL of Carrez Solution I and 0.5 mL of Carrez Solution II were added. The final volume of 50 mL was set with distilled water. The honey solution was filtered, and the first 10 mL of the filtrate was rejected. Lastly, aliquots of 5 mL were pipetted into 2 tubes (reference and sample solution). Then, 5 mL of sodium bisulfite solution 0.2% was added to the reference, and 5 mL of water was added to the sample solution. The absorbance of the reference against the sample solution was determined at 284 and 336 nm with a UV-vis spectrophotometer (Fisher Scientific, Leicestershire, UK).
Diastase activity was determined through the amount of starch converted by a honey solution. The absorbance of yielded blue during the reactions was spectrophotometrically determined at 660 nm with a UV-vis spectrophotometer (Jenway 6305 UV-Visible Spectrophotometer, Staffordshire, UK) at different times until an endpoint of less than 0.235. Diastase activity was calculated as diastase index (DI) or grams of starch hydrolyzed at 40 °C each hour per 100 g of honey.
2.4. Color Determination
Prior to color determination, all samples were decrystallized and left for 20 min in an ultrasound bath to avoid bubbles. Sample color was determined using a HANNA Honey colorimeter (HANNA Instruments, Bedfordshire, UK). This is an instrument that gives the transmittance of honey using glycerol as a reference. Samples were introduced in square optical cuvettes of 10 mm sides, and the color value was taken directly; results are expressed in mm Pfund.
2.5. Determination of Total Polyphenol and Flavonoid Content
The Folin-Ciocalteu spectrophotometric method adapted to honey was used to the quantification of polyphenol content [24]. A UV-vis spectrophotometer (Jenway 6305, Staffordshire, UK) was used for this purpose. Absorbance at 765 nm of a honey solution (0.1 g/mL) that reacted with the Folin-Ciocalteu reactive was determined. Ethanolic solutions of gallic acid in different concentrations (0.01–0.50 mg/mL) were used as a standard to construct the calibration curve. The linearity of the curve was 0.997 (R2). The polyphenol content of the samples was expressed as gallic acid equivalents in mg/100 g.
Total flavonoid content was measured using a similar spectrophotometric method based on an adaptation of the Dowd method [25]. A solution of aluminum chloride reacted with the flavonoids of the samples, prepared in a concentration of 0.33 g/mL with methanol. Absorbance of yellow yielded by the reaction was measured at 425 nm. Different concentrations of the quercetin flavonoid (0.002–0.01 mg/mL) were employed to construct the calibration curve; linearity was 0.998 (R2). The flavonoid content of honey samples was expressed as mg equivalent of quercetin per 100 g.
2.6. Radical-Scavenging Activity
The method used to evaluate the antioxidant activity of honey is based on the discoloration of a 2,2-diphenyl-1-picrilhidrazil (DPPH) solution. The methodology measures the radical-scavenging activity of a honey solution against DPPH by spectrophotometry [26]. A solution (0.1 g/mL) of each honey sample in methanol was prepared. Then, 0.3 mL of the honey solution was mixed with 2.7 mL of the DPPH solution (6 × 10−5 M); a blank was also prepared. The honey sample solution and the blank were maintained in the dark at room temperature for 30 min. Then, absorbance at 517 nm was measured with a UV-vis spectrophotometer (Jenway 6305, Staffordshire, UK). Radical Scavenging Activity (RSA) percentage was calculated considering DPPH discoloration for each sample, tested as follows: RSA = [(AbsB − AbsS)/AbsB] × 100, where AbsB is the absorbance of the blank, and AbsS is the absorbance of the honey sample solution.
2.7. Sugar-Composition Analysis
Sugar composition was determined with an ion Dionex ICS-3000 chromatography system (Sunnyvale, CA, USA). The system separated sugars by using an analytical polyvinylidene/polyvinyl benzene CarboPac PA1 column (Dionex 3 × 250 mm) suitable for mono-, di-, and trisaccharides, and oligosaccharide analysis in general, and a pulsed amperometry detector with a gradient of two mobile phases (A and B). Phase A involved ultrapure water, while Phase B involved 200 mM NaOH (HPLC grade, Merck, Kenilworth, NJ, USA). The sugars of honey solutions (10 mg/L) were calculated using the calibration curves of the standard solution for each pure sugar (Sigma, Aldrich, St. Louis, MO, USA). The CHROMELEON Chromatography Management System was used for chromatogram acquisition. The concentrations of the identified sugars (fructose, glucose, sucrose, turanose, trehalose, and maltose) were expressed as g/100 g of honey.
2.8. Statistical Analysis
Multivariate statistical analysis was applied to identify differences and similarities between samples. IBM SPSS statistics software 23.0 (IBM, Armonk, NY, USA) and Statgraphics centurion V18 (The Plains, USA) were used. First, one-way ANOVA was carried out using the bioclimatic area as the factor, and the main pollen grains (those that presented values over 3% of the pollen spectra), physicochemical parameters, bioactive compounds, and sugars as variables. Differences between groups were tested through post hoc comparison using the Bonferroni test. Significance was calculated for p < 0.05. Lastly, an exploratory technique like principal-component analysis (PCA) was performed. This technique allows to reduce dimensionality in data, increasing the visibility of the relationship between introduced variables in analysis. The used variables were the same as those mentioned in ANOVA. Samples were considered as cases and marked according to their botanical origin.
3. Results and Discussion
3.1. Pollen Spectra and Content of Honey Samples
The samples were from a large area of northern Algeria (Table 1). We collected 33 samples in the northwest and west of the country, 13 in the north (central part), 14 in the east and northeast, and 2 from southern areas. Sampling covered different bioclimatic regions, the most common being the semiarid region that provided 34 samples, 15 were from Mediterranean subhumid areas, 4 from humid areas (with more than 1000 mm of annual rain), and 9 from arid areas with annual precipitation below 400 mm. Most samples were produced in Tell (42 samples), the most populated region, where agrosystems and forest areas are common. Fifteen were from the steppes of the high plateau, and 5 from the Sahara.
Palynological analysis of the samples showed high diversity in the plants represented in the pollen spectra. Most samples contained more than 20 different pollen grains, and some samples even more than 30.
A total of 104 pollen types belonging to 51 botanical families were identified in the 62 samples. Pollen types Eucalyptus, Olea europaea, Brassica napus, Echium, Ziziphus lotus, Papaver rhoeas, Genista, Foeniculum, Hedysarum, Tamarix, Eryngium campestre, and Pimpinella anisum were presented in more than 50% of the samples. Some of them reached maximal values in pollen spectra higher than 80%. These were Eucalyptus, O. europaea, Z. lotus, and Foeniculum. Other common dominant pollen types (≥45% of pollen spectra) were B. napus (maximum of 69.0%), Genista type (58.8%), Tamarix (58.1%), Eryngium campestre (52.1%), and Hedysarum (45.9%) (Table 2). Sporadically, other pollen types were dominant: Eriobotrya (75.4%), Eruca sativa (75.1%), Melilotus (68.7%), Punica granatum (56.2%), and Erica arborea (55.1%). The rest of the pollen types were always identified with percentages below 45% of the pollen spectra. Some samples stood out with secondary pollen grains from the Arecaceae family, such as Chamaerops (maximum value of 37.3%), Capparis (maximum of 36.7%), Asparagus (34.3%) or Paronychia argentea (maximum of 28.2%).

Table 2.
Main palynological characteristics regarding geographical origin.
Quantitative pollen analysis showed 3 samples (pressed honey) with pollen content higher than 100,000 grains of pollen per gram of honey. Two samples had a pollen content between 50,000 and 100,000 pollen grains/g honey (Class IV of Maurizio), 16 were from Class III (10,000–50,000 pollen grain/g honey), 30 had Class II pollen content (2000–10,000 pollen grain/g honey), and 11 a pollen content lower than 2000 pollen grain/g honey (Class I).
3.2. Sample Palynological Profile Regarding Bioclimatic Areas of Origin
Eucalyptus, Z. lotus and some Apiaceae such as Foeniculum or Eryngium pollen types were the dominant pollen in many samples from the semiarid and subhumid Tell regions. Other pollen types, such as Tamarix or Hedysarum were also common in these samples (Figure 2). Less common pollen types, such as P. granatum or Eriobotrya, were also found as dominant pollen in samples from this origin. The first appeared in three summer honey samples, and the second one in a single sample collected in winter. There stood out 11.1% of samples with high percentages of O. europaea pollen, sometimes over 80% of pollen spectra. This was mainly associated with pressed honey and samples that are extraordinarily rich in pollen grains, indicating that this procedure introduces high quantities of pollen grains in honey. Accompanying or important pollen types were found, pollen grains from herbaceous plants common in agrosystems like Hedysarum, Melilotus, Onobrychis, B. napus, Phacelia, Echium or Melilotus. Other well-represented pollen types were those from Apiaceae such as Thapsia or Pimpinella. Plants from semiarid and salted lands, named Tamarix or P. argentea, were frequent in many samples. One of the most representative pollen grains corresponded to Citrus, which determined the botanical origin of some samples. In addition to this, Asparagus pollen was found with high percentages in samples from Tizi Ouzou and Tlemcen, and Acacia in Mostaganem samples. The apicultural value of some Acacia species should be studied even though it is considered non-nectariferous. Nectar secretion was found in different Acacia species [27] and some Acacia spp. honey from different countries were studied [28,29].
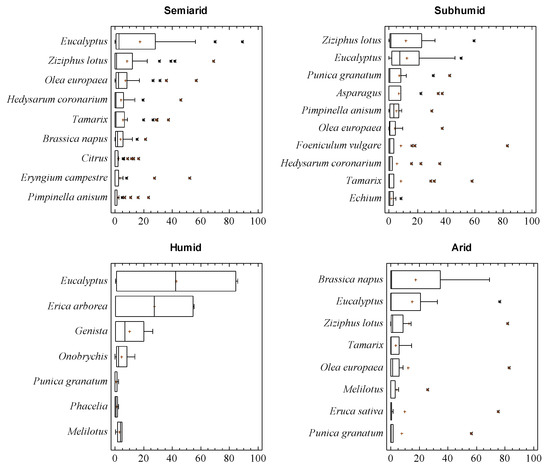
Figure 2.
Box and Whisker plot of the main pollen types in each area. * Atypical values, + Centroids.
Regarding honey samples from humid areas, two samples stood out from the El Taref region with Erica arborea as the dominant pollen. Samples from the high plateau contained as the dominant pollen Apiaceae-like Eryngium campestre type, Hedysarum or Ziziphus lotus, and, as accompanying or important pollen, a great diversity of Apiaceae, such as Foeniculum, Pimpinella, Thapsia, Asteraceae such as Carduus, Centaurea, or Cichorium type. The presence of Chamaerops was considered high. Samples from the M’Sila region had B. napus as dominant pollen.
Samples from the arid region had as the dominant pollen P. granatum (the sample collected in Laghouat) and Z. lotus or Eruca sativa (honey from the Naama region). The sample from Bechar was considered to be from Citrus.
3.3. Quality and Physicochemical Sample Properties
Samples showed important differences in the studied physicochemical parameters. Humidity varied from 14.4% to 22.5%. This maximal value corresponded to a sample collected in El Taref (the humid region). Electrical conductivity was from the 0.133 mS/cm of a sample collected in Sidi bel Abbés to the 1.460 mS/cm of a sample from Naama; pH was from 3.5 (El Taref sample) to 4.7 (Médea sample). Parameters related to sample freshness also had great variation. HMF was higher than 40 mg/kg in two samples, showing the bad quality of these samples. The rest of the samples presented lower values, frequently near 10 mg/kg, with the lowest being 1 mg/kg. Following this trend, diastase activity was low in samples with high HMF content. Excepting these samples, the lowest value was 8.9° and the highest was 40.6°. The high HMF content of some Algerian honey samples (over the limit of 40 mg/kg established in international quality schemes) was noted before [30]. Improvement of the management practices is necessary to avoid this situation. An important parameter for the first differentiation of samples is color, which varied from 13 (Tebessa sample) to 150 mm Pfund (Tizi Ouzou sample) (samples with higher HMF content were not considered for mean values of color, diastase content, and HMF content).
Most of the samples had a sugar content according to blossom honey, this being a sum of fructose and glucose higher than 60%. The lowest value corresponded to a honeydew sample (52.8%), while the highest value was 75.6%. Fructose content varied from 33.0% in a Tebessa sample to 44.4% in a Guelma sample. Glucose oscillated from 23.1% to 34.1%. The concentration of other sugars was really low. Maltose varied from 0.6% to 3.6%, turanose from 1.0% to 3.1%, and raffinose had a highest value of 3.2%. Lastly, sucrose had the highest value (3.3%) in a Citrus honey, but was only found in 20 samples.
Other important compounds, related to healthy properties of honey, were polyphenols and flavonoids. The first varied from 20.0 mg/100 g in a sample of E. japonica from Sidi bel Abbes to 182.3 mg/100 g of a honeydew sample from M’Sila. Flavonoid content varied from 1.0 mg/100 g to 12.9 mg/100 g in a sample from Bechar and a sample from Tlemcen, respectively. Moreover, RSA was greatly diverse, this variation being from 10% to 79.5%.
3.4. Influence of Area of Origin on Sample Characteristics
ANOVA was performed to mark differences and similarities between sample characteristics regarding their bioclimatic region. The highest differences were found between samples from humid areas, situated in the NE of the country, and samples collected in semiarid, subhumid, and arid areas. The most significant (p < 0.05) were higher electrical conductivity (mean value of 0.910 mS/cm), darker color (mean value of 122 mm Pfund), high water content (mean value of 21.4%), higher flavonoid content, and lower fructose content (mean value) in samples from humid areas in comparison with the others. In ANOVA results, the higher content in E. arborea stood out, together with Malus and Onobrychis types for honey of this origin. Furthermore, samples from semiarid, subhumid, and arid regions did not show significant differences (p < 0.05) between them in physicochemical variables except for lower sample humidity from those of the arid region (mean value of 15.7%). With regard to pollen spectra, samples from arid areas had higher content of B. napus and Rosmarinus than those from semiarid and subhumid regions (p < 0.05). However, due to the great variation between samples, more studies are needed.
3.5. Botanical Characterization of Honey Samples
The botanical origin of samples is the most important attribution to valorize local honey production. Sample typification was done with information provided by beekeepers, physicochemical data, and sample pollen profiles. To calculate the percentage of the main pollen type in the samples, nectarless plants were excluded. The sample classification and physicochemical data of each honey type are shown in Table 3.

Table 3.
Characteristics of the different honey types. Values are expressed as mean and standard deviation.
The main group was polyfloral honey with 26 samples. This type of honey is produced in all regions, presenting significant variations in the studied variables. The pollen spectra had as main pollen types Eucalyptus and Ziziphus, but Apiaceae (mainly Foeniculum, Eryngium, Pimpinella, and Thapsia pollen types), Hedysarum, Melilotus, Centaurea, B. napus, or Echium) were common. Pollen grains from plants growing in semiarid and arid areas, such as Tamarix or Capparis spinosa, were also present in relevant amounts. Five samples produced in Medea, Mostaganem, and Tlemcen had a pollen content higher than 30% of Tamarix pollen, but their physicochemical properties, and polyphenol and flavonoid content strongly varied between samples. The apicultural value of Tamarix is not well-known; while some authors considered this plant important for honey production [31], other authors indicated that the nectar concentration in flowers of Tamarix plants is marginal [32]. Regarding Capparis spinosa, their pollen grains reached percentages over 20% in one sample from Sidi bel Abbes and another from Medea (36.7% and 24.4%, respectively). Important nectar production for this plant was indicated in a few studies [33,34], but this honey type is little mentioned in the scientific literature [13,29,35]. Another interesting point regards two samples with a high quantity of Asparagus pollen type (22.1% and 34.3%); both were from the Tizi Ouzou region and presented similar patterns in the studied variables. The most significant was the low pollen content (less than 2000 pollen grains/g of honey), the color (value close to 90 mm Pfund), diastase content (more than 30° Gothe), and polyphenol (mean value of 100 mg/100 g) and flavonoid content (mean value of 7 mg/100 g). There is little information about the importance of Asparagaceae plants for honey production, but the pollination activity and pollen production in both female and male plants of Asparagus was constated [36]. All samples mentioned before were considered polyfloral, but further studies are needed to endorse possible monovarietal honey.
Regarding the number of pollen types, polyfloral samples had a high number with a mean value of 25, but frequently with more than 30 pollen types. The values on physicochemical parameters, sugar profile, and antioxidant components were like those of other polyfloral honey types studied in the country [8,13].
Seven samples were typified as Eucalyptus honey, with a mean value of this pollen of 76.7%. Two samples were pressed honey having a high amount of pollen. The rest had a mean value included in Class III of Maurizio. This is slightly high pollen content in comparison with that of E. globulus honey from the Atlantic area of Europe [37]. Some of these samples presented high humidity with a mean value of 19.4%. In comparison with Eucalyptus honey from other areas, the studied samples presented high electrical conductivity, color, and pH [3,37,38]. This sample type is mainly produced in forests of coastal areas during summer, so some honeydew supports can influence their physicochemical attributes. Eucalyptus samples are produced in the Mostaganem region and eastern areas of the country. In Algeria, Eucalyptus trees were introduced in 1850, with E. camaldulensis and E. globulus being the main introduced species. In the middle of S. XX, large areas of the southeast, center, and west, such as Mostaganem, were intensively planted with Eucalyptus for wood production. Currently, species such as E. gomphocephala, E. sideroxylon, E. robusta, or E. viminalis grow there [39].
Six samples from the regions of Tizi Ouzou, Tlemcen, M’Sila, and Naama were typified as honeydew honey, while beekeepers had named them forest honey. Samples had the highest electrical conductivity, polyphenol content, and dark color with the lowest sum of fructose and glucose, common features to other types of honeydew honey [40,41,42,43]. Little information is available about the honeydew production in Algeria, and knowledge about sources of this honey is scarce. However, it was the subject of a great number of recent studies in other territories [40,41,42,43,44,45,46]. Considering the interest of this honey type, and that differentiation between different types of honeydew honey or even other dark blossom honey is difficult [47], more studies regarding this honey type could be useful to valorize local honey production.
Another important source for honey production was Apiaceae plants. A total of five samples were typified within this group. Three samples had as the main pollen the F. vulgare type, one sample P. anisum, and another E. campestre type. Samples were included in a single group due to some of them sharing high percentages of the mentioned pollen types and other secondary pollen types, such as Thapsia or Apium showing the importance for the honey production of this botanical family in the area. In general, samples were characterized by electrical conductivity near 0.495 mS/cm, light amber color, and high fructose content; the sample from P. anisum had a darker color. These samples had high polyphenol content (among the highest) and with medium flavonoid content. Apiaceae honey from North African countries was mentioned in different papers, mainly from plants as Eryngium campestre, Ammi visnaga, Ridolfia segetum, or Bupleurum spinosum in Morocco [48,49]. Some Apiaceae from Algeria were studied regarding sugar composition or polyphenol content [12,21]. The studied fennel honey had electrical conductivity, diastase content, and sugar content similar to those from Tenerife samples [50]. A high amount of hydroxycinnamic acids, mainly caffeic acid, was mentioned for this honey type [51].
Citrus samples are produced mainly in the Tell region (Mostaganem, Mascara, and Relizane), where these fruit plants are common in agricultural lands, but also in oases of arid areas (Bechar). Five samples were considered from this botanical origin. The pollen of Citrus was identified with a mean value of 22.0% (nectarless pollen excluded), less than 15% of pollen spectra. It is common to find this pollen type with other pollen types such as O. europaea, Genista, or Tamarix. Samples had extralight amber color, mean electrical conductivity of 0.318 mS/cm, and high fructose and glucose content besides the highest mean sucrose content. These samples had similarities with other studied samples from Blida region, such as the presence of high percentages of O. europaea, and similar values in electrical conductivity, fructose, and glucose content [8,52].
Four samples were considered unifloral sedra honey. The most important species in the area to produce this type of honey is Z. lotus. The samples had light amber color, mean electrical conductivity of 0.535 mS/cm, high pH, and medium polyphenol and flavonoid contents. Sedra honey is more known than other monofloral honey types, as consumers appreciate them much due to healthy properties attributed to them [53]. The characteristics of the honey type are in accordance with the results of other publications [19,29].
The typification of three samples as pomegranate samples (P. granatum) stands out. The production of this honey type is not mentioned in the country, as it is rare. P. granatum pollen was presented in a mean value of 53.3%, and samples had medium-to-low pollen content (Class II of Maurizio). Water content was low (mean of 14.9%), color was extralight amber, the mean of electrical conductivity was of 0.580 mS/cm, and polyphenol and flavonoid contents were medium.
Two samples from El Taref were typified as heather honey, with E. arborea having a mean value of 55.7%. Northwestern evergreen forests and high shrubs growing in humid and warm climates facilitate the production of this honey type. Samples presented high humidity content (mean value 20%), high electrical conductivity, and the darkest color. Furthermore, polyphenol content was high, and samples presented major flavonoid content (mean of 11.1 mg/100 g). However, E. arborea honey remains produced in the area is poorly described [12].
Lastly, one sample was typified as unifloral from Retama sphaerocarpa (Genista pollen type was present in 79.9% of the pollen spectra), one sample as Rosmarinus (Rosmarinus pollen percentage was 17.0%), one sample as medlar honey (E. japonica pollen content was 75.4%), and one as sulla honey, with a pollen content of Hedysarum of 49.3%. Samples from Rosmarinus, Eriobotrya, and Hedysarum had the clearest color, the first being an extrawhite honey, and the last two white honey. The best-described honey type is Hedysarum honey [2,7,12].
As commented before, samples presented a wide variation in physicochemical parameters and pollen content. To reduce the dimensionality of the dataset and increase the interpretability of results, PCA was applied. The first nine components explained 72% of data variance, corresponding the sum of Components 1 and 2 to 30%. The plot of the scores of these two components is included in Figure 3A. Left is flavonoid content, RSA, and color close to electrical conductivity (negative quadrant) and polyphenol content (positive quadrant). Erica pollen is situated in the negative quadrant in the left with the electrical conductivity. Right shows Eriobotrya near glucose. Some pollen variables, such as Punica, Capparis, or Tamarix, were in the same direction as that of Fructose and Apiaceae. Ziziphus, Brassica, and pH are situated in the same quadrant as that of polyphenol content. On the opposite quadrant are Citrus and Rosmarinus pollen types, close to Maltose. Lastly, at the bottom, Fabaceae close to Eucalyptus, humidity, and raffinose sugar. PCA grouped samples from the same botanical origin (Figure 3B). Left are honeydew samples (Hd), Erica (Er), and Eucalyptus (Eu). This position corresponds to samples with the highest humidity content (Eucalyptus and Erica samples), major electrical conductivity, and darker color. The influence of polyphenol content determined the situation of B. napus in this area, as samples with a high content of this pollen type were considered honeydew honey due to their color, electrical conductivity, and the polyphenol content. In the top of the figure are Apiaceae (A) samples and Z. lotus (Z) honey, and in the center are P. granatum samples (Pu), close to some polyfloral samples. This sample group was separated from other, more disperse groups, situated in the left, which included types Citrus (C), Hedysarum (H), Rosmarinus (Ro), and R. sphaerocarpa honey. The Eriobotrya sample was clearly separated.
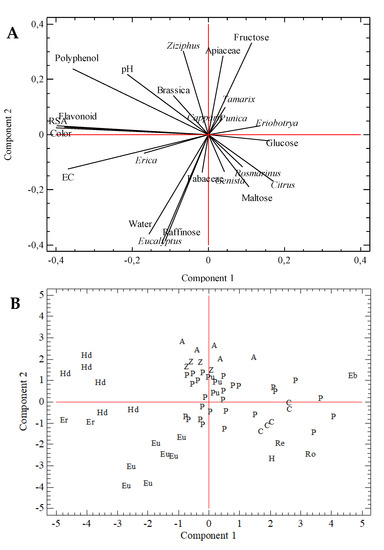
Figure 3.
Principal-component analysis. (A) Loading plot of variables; (B) Score plot of the first two extracted components and sample botanical origin. A, Apiaceae; C, Citrus; Eb, Eriobotrya; Er, Erica; Eu, Eucalyptus; Hd, honeydew; H, Hedysarum; P, polyfloral; Pu, Punica; Re, Retama; Ro, Rosmarinus; Z, Ziziphus.
PCA results showed sample similarities classified with the same botanical origin, but also variation in some of these sample groups. This was the case of samples from Eucalyptus, honeydew, and, to a lesser extent, Apiaceae or Citrus samples. On the other hand, common Mediterranean honey types (Citrus, Rosmarinus, Hedysarum, or Retama) were close, evidencing the similarities between them.
4. Conclusions
The results of this paper showed the great variability in local honey production of Algerian beekeeping, and the potentiality regarding different honey types that could be obtained. Some of the plant species mentioned in this work, such as Eucalyptus, Brassica napus, Hedysarum, and Citrus, are common honey plants in Mediterranean areas. However, others, such as Capparis spinosa, Asparagus, Tamarix, Ziziphus lotus, and some Apiaceae plants (Eryngium, Thapsia, Pimpinella), even Acacia, are representative of the honey of this country, useful as markers to guarantee their authenticity. In any case, there is little information on the apicultural value of some of them and of their honey; thus, more research is needed on these topics.
Author Contributions
Conceptualization, M.H., A.H. and D.F.; methodology, M.H., M.S.R.-F., B.M. and O.E.; formal analysis, M.H., D.F., M.S.R.-F. and O.E., data curation, M.H., M.C.S., M.S.R.-F.; writing—original draft preparation, M.H., M.C.S.; writing—review and editing, O.E., M.S.R.-F.; supervision, A.H., B.M., D.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We express our deepest gratitude to all the beekeepers for graciously helping us to obtain honey samples.
Conflicts of Interest
The authors declare no conflict of interest
References
- Hussein, M.H. A review of beekeeping in Arab countries. Bee World 2000, 81, 56–71. [Google Scholar] [CrossRef]
- Makhloufi, C.; Kerkvliet, J.D.; D’albore, G.R.; Choukri, A.; Samar, R. Characterization of Algerian honeys by palynological and physico-chemical methods. Apidologie 2010, 41, 509–521. [Google Scholar] [CrossRef]
- Escuredo, O.; Míguez, M.; Fernández-González, M.; Seijo, M.C. Nutritional value and antioxidant activity of honeys produced in a European Atlantic area. Food Chem. 2013, 138, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Migliore, J. Plant biogeography in the western mediterranean basin: New insights from phylogeographical studies. BMIB-Bollettino dei Musei e degli Istituti Biologici 2013, 75, 64–68. [Google Scholar]
- Médail, F.; Quézel, P. Hot-Spots Analysis for Conservation of Plant Biodiversity in the Mediterranean Basin. Ann. Mo. Bot. Gard. 1997, 84, 112–127. [Google Scholar] [CrossRef]
- Mediouni, K. Elaboration d’un Bilan et d’une Strategie Nationale de Developpement Durable de la Diversite Biologique; Projet ALG/97 G31; Tome IX. Editions du Ministere de la Amenagement du territoire et de Environnement; FEM/PNUD: New York, NY, USA, 2004; p. 69. [Google Scholar]
- Zerrouk, S.; Boughediri, L.; Seijo, M.C.; Fallico, B.; Arena, E.; Ballistreri, G. Pollen spectrum and physicochemical attributes of sulla (Hedysarum coronarium) honeys of Médéa region (Algeria). Albanian J. Agric. Sci. 2013, 12, 511–517. [Google Scholar]
- Makhloufi, C.; Kerkvliet, J.; Schweitzer, P. Characterisation of some monofloral Algerian honeys by pollen analysis. Grana 2015, 54, 156–166. [Google Scholar] [CrossRef]
- Mekious, S.; Houmani, Z.; Houmani, M. Étude des potentialités mellifères de deux régions du Nord de l’Algérie. Phytothérapie 2018, 1–6. [Google Scholar] [CrossRef]
- Zerrouk, S.; Seijo, M.C.; Boughediri, L.; Escuredo, O.; Rodríguez-Flores, M.S. Palynological characterisation of Algerian honeys according to their geographical and botanical origin. Grana 2014, 53, 147–158. [Google Scholar] [CrossRef]
- Latifa, H.; Mouna, B.; Arezki, M. Ziziphus lotus and Euphorbia bupleuroides Algerian honeys. World Appl. Sci. J. 2013, 24, 1536–1543. [Google Scholar]
- Ouchemoukh, S.; Amessis-Ouchemoukh, N.; Romero, M.G.; Aboud, F.; Giuseppe, A.; Gutierrez, A.F.; Segura-Carretero, A. Characterisation of phenolic compounds in Algerian honeys by RP-HPLC coupled to electrospray time-of-flight mass spectrometry. LWT-Food Sci. Technol. 2017, 85, 460–469. [Google Scholar] [CrossRef]
- Boutabia, L.; Telailia, S.; Chefrour, A. Spectre pollinique de miels d’abeille (Apis mellifera L.) de la région d’El Tarf (Nord-Est algérien). Livest. Res. Rural. Dev. 2016, 28, 1–8. [Google Scholar]
- Ouchemoukh, S.; Louaileche, H.; Schweitzer, P. Physicochemical characteristics and pollen spectrum of some Algerian honeys. Food Control 2007, 18, 52–58. [Google Scholar] [CrossRef]
- Azzedine, C.; Marie-José, B.; Yasmina, A.K.; Salima, B.; Ali, T. Melissopalynologic and physicochemical analysis of some north-east Algerian honeys. Eur. J. Sci. Res. 2005, 18, 389–401. [Google Scholar]
- Nair, S.; Maghraoui, N.B. Physicochemical Properties of Honeys Produced in North-West of Algeria. Adv. Food Sci. Eng. 2017, 1, 123–128. [Google Scholar] [CrossRef]
- Mesbahi, M.A.; Ouahrani, M.R.; Rebiai, A.; Amara, D.G.; Chouikh, A.; Mesbahi, M.A.; Ouahrani, M.R.; Rebial, A. Characterization of Zygophyllum album L Monofloral Honey from El-Oued, Algeria. Curr. Nutr. Food Sci. 2019, 15, 476–483. [Google Scholar] [CrossRef]
- Otmani, I.; Abdennour, C.; Dridi, A.; Kahalerras, L.; Halima-Salem, A. Characteristics of the bitter and sweet honey from Algeria Mediterranean coast. Vet. World 2019, 12, 551–557. [Google Scholar] [CrossRef]
- Zerrouk, S.; Seijo, M.C.; Escuredo, O.; Rodríguez-Flores, M.S. Characterization of Ziziphus lotus (jujube) honey produced in Algeria. J. Apic. Res. 2017, 57, 166–174. [Google Scholar] [CrossRef]
- Alzahrani, H.A.; Boukraa, L.; Bellik, Y.; Abdellah, F.; Bakhotmah, B.A.; Kolayli, S.; Sahin, H. Evaluation of the Antioxidant Activity of Three Varieties of Honey from Different Botanical and Geographical Origins. Glob. J. Health Sci. 2012, 4, 191–196. [Google Scholar] [CrossRef]
- Ouchemoukh, S.; Schweitzer, P.; Bey, M.B.; Djoudad-Kadji, H.; Louaileche, H. HPLC sugar profiles of Algerian honeys. Food Chem. 2010, 121, 561–568. [Google Scholar] [CrossRef]
- Neggad, A.; Benkaci-Ali, F.; Alsafra, Z.; Eppe, G. Headspace Solid Phase Microextraction Coupled to GC/MS for the Analysis of Volatiles of Honeys from Arid and Mediterranean Areas of Algeria. Chem. Biodivers. 2019, 16, e1900267. [Google Scholar] [CrossRef]
- Bogdanov, S.; Martin, P.; Lullmann, C. Harmonised Methods of the International Honey Commission; Swiss Bee Research Centre: Liebefeld, Switzerland, 2002. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Arvouet-Grand, A.; Vennat, B.; Pourrat, A.; Legret, P. Standardization of propolis extract and identification of principal constituents. J. Pharm. Belg. 1994, 49, 462–468. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Adgaba, N.; Al-Ghamdi, A.; Tadesse, Y.; Getachew, A.; Awad, A.M.; Rana, R.M.; Owayss, A.A.; Mohammed, S.E.A.; AlQarni, A.S. Nectar secretion dynamics and honey production potentials of some major honey plants in Saudi Arabia. Saudi J. Boil. Sci. 2016, 24, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Al-Khalifa, A.S.; Al-Arify, I. Physicochemical characteristics and pollen spectrum of some Saudi honeys. Food Chem. 1999, 67, 21–25. [Google Scholar] [CrossRef]
- Alqarni, A.S.; Owayss, A.A.; Mahmoud, A.A. Physicochemical characteristics, total phenols and pigments of national and international honeys in Saudi Arabia. Arab. J. Chem. 2016, 9, 114–120. [Google Scholar] [CrossRef]
- Achouri, M.; Selka, M.A.; Chenafa, A.; Brahim, S.; Messafeur, M.A.; Toumi, H. Teneur en 5-hydroxyméthylfurfural (HMF) dans les miels du Nord-Ouest de l’Algérie. Toxicol. Anal. Clin. 2019, 31, 100–105. [Google Scholar] [CrossRef]
- Ahmida, M.H.S.; Elwerfali, S.; Agha, A.; Elagori, M. Physicochemical, Heavy Metals and Phenolic Compounds Analysis of Libyan Honey Samples Collected from Benghazi during 2009–2010. Food Nutr. Sci. 2013, 4, 33–40. [Google Scholar] [CrossRef]
- Andersen, D.C.; Nelson, S. Floral ecology and insect visitation in riparian Tamarix sp. (saltcedar). J. Arid. Environ. 2013, 94, 105–112. [Google Scholar] [CrossRef]
- Eisikowitch, D.; Ivri, Y.; Dafni, A. Reward partitioning in Capparis spp. along ecological gradient. Oecologia 1986, 71, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Petanidou, T.; Van Laere, A.J.; Smets, E. Change in floral nectar components from fresh to senescent flowers of Capparis spinosa (Capparidaceae), a nocturnally flowering Mediterranean shrub. Plant. Syst. Evol. 1996, 199, 79–92. [Google Scholar] [CrossRef]
- El-Guendouz, S.; Al-Waili, N.; Aazza, S.; Elamine, Y.; Zizi, S.; Al-Waili, T.; Al-Waili, A.; Lyoussi, B. Antioxidant and diuretic activity of co-administration of Capparis spinosa honey and propolis in comparison to furosemide. Asian Pac. J. Trop. Med. 2017, 10, 974–980. [Google Scholar] [CrossRef] [PubMed]
- Greco, C.F.; Banks, P.; Kevan, P.G. Foraging behaviour of honeybees (Apis mellifera) on asparagus (Asparagus officinalis). Proc. Entomol. Soc. Ont. 1995, 126, 37–43. [Google Scholar]
- Flores, M.S.R.; Pérez, O.E.; Rodríguez-Flores, M.S. Characterization of Eucalyptus globulus honeys produced in the Eurosiberian Area of the Iberian Peninsula. Int. J. Food Prop. 2014, 17, 2177–2191. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Maia, M.; Karabagias, V.K.; Gatzias, I.; Badeka, A.V. Characterization of Eucalyptus, Chestnut and Heather Honeys from Portugal Using Multi-Parameter Analysis and Chemo-Calculus. Foods 2018, 7, 194. [Google Scholar] [CrossRef]
- Benayache, S.; Benayache, F.; Benyahia, S.; Chalchat, J.-C.; Garry, R.-P. Leaf Oils of some Eucalyptus Species Growing in Algeria. J. Essent. Oil Res. 2001, 13, 210–213. [Google Scholar] [CrossRef]
- Flores, M.S.R.; Escuredo, O.; Seijo, M.C. Assessment of physicochemical and antioxidant characteristics of Quercus pyrenaica honeydew honeys. Food Chem. 2015, 166, 101–106. [Google Scholar] [CrossRef]
- Seijo, M.C.; Escuredo, O.; Rodríguez-Flores, M.S. Physicochemical Properties and Pollen Profile of Oak Honeydew and Evergreen Oak Honeydew Honeys from Spain: A Comparative Study. Foods 2019, 8, 126. [Google Scholar] [CrossRef]
- Jara-Palacios, M.J.; Ávila, F.J.; Escudero-Gilete, M.L.; Pajuelo, A.G.; Heredia, F.J.; Hernanz, D.; Terrab, A. Physicochemical properties, colour, chemical composition, and antioxidant activity of Spanish Quercus honeydew honeys. Eur. Food Res. Technol. 2019, 245, 2017–2026. [Google Scholar] [CrossRef]
- Vasić, V.; Gašić, U.M.; Stanković, D.; Lušić, D.; Vukić-Lušić, D.; Milojković-Opsenica, D.; Tešić, Ž.; Trifković, J. Towards better quality criteria of European honeydew honey: Phenolic profile and antioxidant capacity. Food Chem. 2019, 274, 629–641. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Karabournioti, S.; Karabagias, V.K.; Badeka, A.V. Palynological, physico-chemical and bioactivity parameters determination, of a less common Greek honeydew honey: “dryomelo”. Food Control 2020, 109, 106940. [Google Scholar] [CrossRef]
- Nešović, M.; Gašić, U.M.; Tosti, T.; Trifković, J.; Baošić, R.; Blagojević, S.; Ignjatović, L.; Tešić, Ž. Physicochemical analysis and phenolic profile of polyfloral and honeydew honey from Montenegro. RSC Adv. 2020, 10, 2462–2471. [Google Scholar]
- Shaaban, B.; Seeburger, V.C.; Schroeder, A.; Lohaus, G. Sugar, amino acid and inorganic ion profiling of the honeydew from different hemipteran species feeding on Abies alba and Picea abies. PLoS ONE 2020, 15, e0228171. [Google Scholar] [CrossRef]
- Vasić, V.; Đurđić, S.; Tosti, T.; Radoičić, A.; Lušić, D.; Milojković-Opsenica, D.; Tešić, Ž.; Trifković, J. Two aspects of honeydew honey authenticity: Application of advance analytical methods and chemometrics. Food Chem. 2020, 305, 125457. [Google Scholar] [CrossRef] [PubMed]
- Terrab, A.; Díez, M.J.; Heredia, F.J. Characterisation of Moroccan unifloral honeys by their physicochemical characteristics. Food Chem. 2002, 79, 373–379. [Google Scholar] [CrossRef]
- Elamine, Y.; Aazza, S.; Lyoussi, B.; Antunes, M.D.; Estevinho, L.M.; Anjos, O.; Resende, M.; Faleiro, M.; Miguel, M.G. Preliminary characterization of a Moroccan honey with a predominance of Bupleurum spinosum pollen. J. Apic. Res. 2017, 57, 153–165. [Google Scholar] [CrossRef]
- Manzanares, A.B.; García, Z.H.; Galdón, B.R.; Rodríguez-Rodríguez, E.M.; Romero, C.D. Physicochemical characteristics and pollen spectrum of monofloral honeys from Tenerife, Spain. Food Chem. 2017, 228, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Vella, A.; Cammilleri, G.; Pulvirenti, A.; Galluzzo, F.; Randisi, B.; Giangrosso, G.; Macaluso, A.; Gennaro, S.; Ciaccio, G.; Cicero, N.; et al. High hydroxycinnamic acids contents in fennel honey produced in Southern Italy. Nat. Prod. Res. 2020, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zerrouk, S.; Boughediri, L.; Seijo, M.C.; Fallico, B.; Arena, E.; Ballistreri, G. Palynological and Physicochemical Properties of Citrus and Eucalyptus Honeys Produced in Blida Region (Algeria). Eur. J. Sci. Res. 2013, 104, 79–90. [Google Scholar]
- Masalha, M.; Abu-Lafi, S.; Abu-Farich, B.; Rayan, M.; Issa, N.; Zeidan, M.; Rayan, A. A New Approach for Indexing Honey for Its Heath/Medicinal Benefits: Visualization of the Concept by Indexing Based on Antioxidant and Antibacterial Activities. Medicines 2018, 5, 135. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).