Analysis of Naturally Occurring Phenolic Compounds in Aromatic Plants by RP-HPLC Coupled to Diode Array Detector (DAD) and GC-MS after Silylation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Standards
2.2. Solvents and Reagents
2.3. Samples
2.4. Sample Preparation and Derivatization
2.5. HPLC Analysis
2.6. GC-MS Analysis
3. Results and Discussion
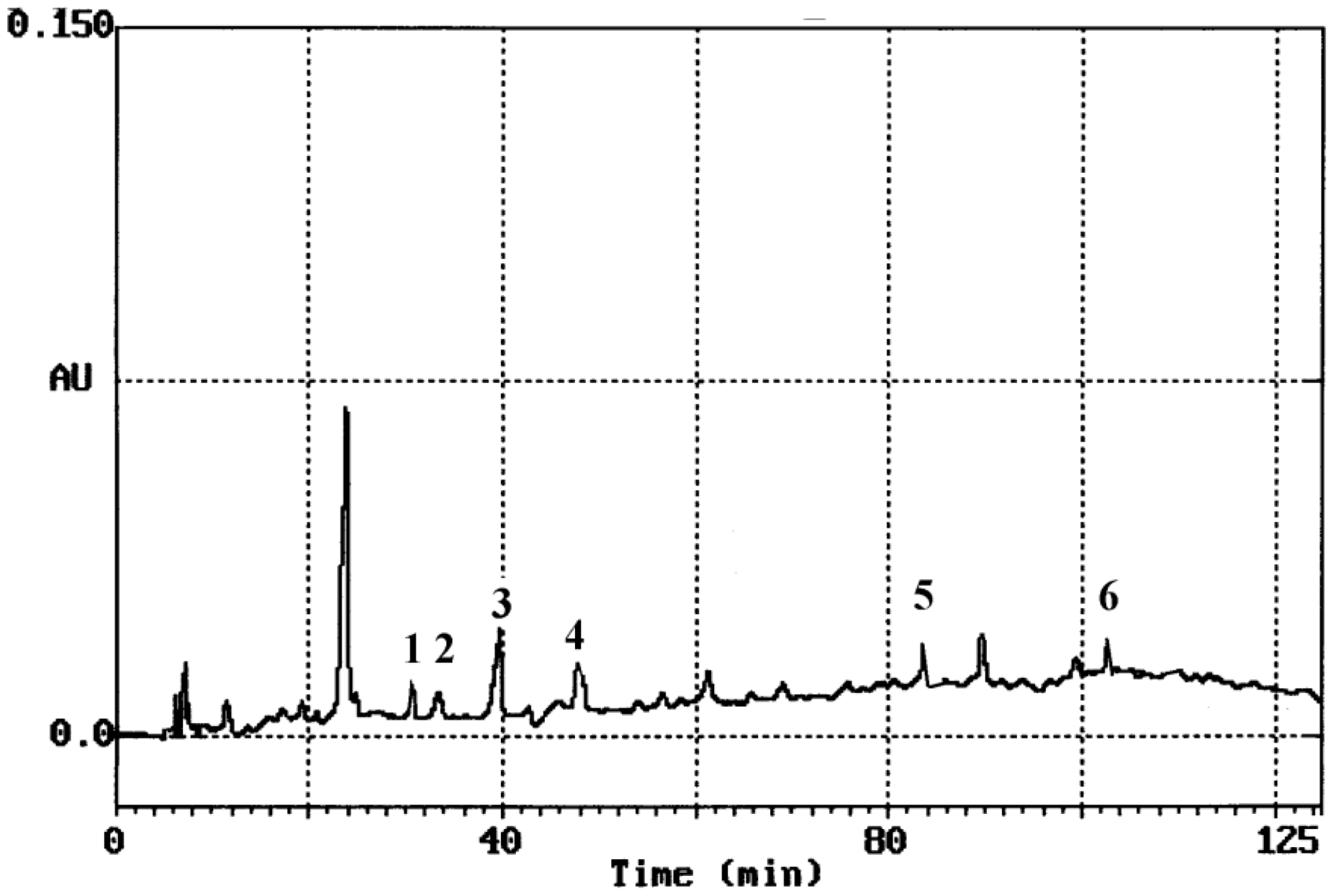
3.1. HPLC Analysis
| Content, mg/100 g dry sample a | ||||||||
|---|---|---|---|---|---|---|---|---|
| Plant | Gallic acid | Gentisic acid | Caffeic acid | p-Coumaric acid | Vanillic acid | Syringic acid | Ferulic acid | p-Hydroxybenzoic acid |
| O. dictamnus | ND | ND | ND | 13.9 ± 0.04 | ND | ND | 0.34 ± 0.01 | ND |
| E. globulus | 1.5 ± 0.03 | ND | ND | 6.6 ± 0.02 | ND | ND | ND | ND |
| O. vulgare L. | ND | ND | 1.0 ± 0.02 | ND | 1.0 ± 0.02 | ND | 3.2 ± 0.03 | ND |
| M. officinalis L. | ND | 2.1 ± 0.03 | 13.8 ± 0.1 | ND | ND | ND | 4.8 ± 0.03 | 2.3 ± 0.01 |
| S. cretica | 2.6 ± 0.02 | ND | ND | 4.1 ± 0.02 | ND | 1.1 ± 0.02 | 6.95 | ND |
| Plant | Quercetin | Apigenin | Luteolin | Naringenin | Eriodictyol | Rutin | (+)-Catechin hydrated | (−)-Epicatechin |
| O. dictamnus | ND | ND | ND | ND | ND | ND | 0.5 ± 0.01 | ND |
| E. globulus | 2.5 ± 0.02 | ND | ND | ND | ND | 1.8 ± 0.03 | ND | ND |
| O.vulgare L. | ND | ND | ND | ND | 0.7 ± 0.03 | 1.0 ± 0.02 | 17.7 ± 0.1 | 1.8 ± 0.05 |
| M. officinalis L. | ND | ND | ND | ND | 1.1 ± 0.05 | ND | 21.0 ± 0.15 | ND |
| S. cretica | 1.6 ± 0.04 | ND | 9.1 ± 0.02 | ND | ND | ND | 22.1 ± 0.17 | 6.9 ± 0.06 |
3.2. GC-MS Analysis
| Phenolic compound | MW (Silylated compounds) | Molecular ion [M]+ | Identified ions (m/z) |
|---|---|---|---|
| p-Hydroxybenzoic acid | 282 | 282 | 193 (79), 223 (82), 267 (100), 282 (21) |
| Vanillic acid | 312 | 312 | 149 (100), 165 (52), 223 (66), 312 (20) |
| Gentisic acid | 370 | 370 | 147 (90), 223 (24), 267 (36), 281 (21), 355 (100), 370 (19) |
| Gallic acid | 458 | 458 | 147 (94), 179 (48), 281 (100), 458 (32) |
| p-Coumaric acid | 308 | 308 | 219 (100), 249 (43), 293 (72), 308 (56) |
| Ferulic acid | 338 | 338 | 219 (22), 249 (77), 293 (16), 279 (13), 308 (56), 323 (60), 338 (100) |
| Caffeic acid | 396 | 396 | 179 (13), 191 (27), 219 (100), 396 (28) |
| Quercetin | 647 | 647 | 487 (30), 559 (12), 575 (100), 647 (24) |
| (+)-catechin | 650 | 650 | 179 (23), 267 (11), 355 (33), 368 (100), 650 (<1) |
| Hydroxytyrosol | 370 | 370 | 179 (33), 193 (43), 267 (100), 370 (23) |
| Syringic acid | 342 | 342 | 297 (71), 312 (70), 327 (100), 342 (69) |
| (−)-Epicatechin | 650 | 650 | 147 (19), 267 (15), 355 (30), 368 (100), 649 (3) |
| p-Hydroxyphenylacetic acid | 296 | 296 | 149 (59), 164 (71), 179 (100), 296 (16) |
| Protocatechuic acid | 370 | 370 | 193 (100), 223 (24), 267 (12), 370 (15) |
| Cinnamic acid | 220 | 220 | 103 (75), 131 (100), 161 (52), 205 (96), 220 (21) |

4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Singleton, V.L. Naturally occurring food toxicants: Phenolic substances of plant origin common in foods. Adv. Food Res. 1981, 27, 149–242. [Google Scholar] [CrossRef]
- Herrmann, H. On the occurrence of flavonol and flavone glycosides in vegetables. Z. Lebensm. Unters. Forsch. 1988, 186, 1–5. [Google Scholar]
- Porter, L.W. Methods in Plant Biochemistry, I: Plant Phenolics; Harborne, J.B., Ed.; Academic Press: London, UK, 1989; pp. 389–419. [Google Scholar]
- Peleg, H.; Naim, M.; Rouseff, R.L.; Zehavi, U. Distribution of bound and free phenolic acid in oranges (Citrus sinensis) and grapefruits (Citrus paradisi). J. Sci. Food Agric. 1991, 57, 417–426. [Google Scholar]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Jung, H.G. Forage lignins and their effects on fiber digestibility. Agron. J. 1989, 81, 33–38. [Google Scholar] [CrossRef]
- Wallace, G.; Chesson, A.; Lomax, J.A.; Jarvis, M.C. Lignin-carbohydrate complexes in graminaceous cell walls in relation to digestibility. Anim. Feed Sci. Tech. 1991, 32, 193–199. [Google Scholar]
- Lee, H.S.; Widmer, B.W. Handbook of Food Analysis; Nollet, L.M.L., Ed.; Marcel Dekker: New York, NY, USA, 1996; pp. 821–894. [Google Scholar]
- Harborne, J.B. Phytochemical Methods. A Guide to Modern Techniques of Plant Analysis; Chapman and Hall: London, UK, 1998; pp. 40–106. [Google Scholar]
- Hertog, M.G.L.; Hollman, P.C.H.; Venema, D.P. Optimization of a quantitative HPLC determination of potentially anticarcinogenic flavonoids in vegetables and fruits. J. Agric. Food Chem. 1992, 40, 1591–1598. [Google Scholar] [CrossRef]
- Justesen, U.; Knuthsen, P.; Leth, T. Quantitative analysis of flavonols, flavones, and flavanones in fruits, vegetables and beverages by high-performance liquid chromatography with photo-diode array and mass spectrometric detection. J. Chrom. A 1998, 799, 101–110. [Google Scholar]
- Merken, H.M.; Beecher, G.R. Measurement of food flavonoids by high performance liquid chromatography: A review. J. Agric. Food Chem. 2000, 48, 577–599. [Google Scholar] [CrossRef]
- Chen, H.; Zuo, Y.; Deng, Y. Separation and determination of flavonoids and other phenolic compounds in cranberry juice by high-performance liquid chromatography. J. Chrom. A 2001, 913, 387–395. [Google Scholar] [CrossRef]
- Suarez, B.; Picinelli, A.; Mangas, J.J. Solid-phase extraction and high-performance liquid chromatographic determination of polyphenols in apple musts and ciders. J. Chrom. A 1996, 727, 203–209. [Google Scholar] [CrossRef]
- Zuo, Y.; Wang, C.; Zhan, J. Separation, characterization and quantitation of benzoic and phenolic antioxidants in American cranberry fruit by GC-MS. J. Agric. Food Chem. 2002, 50, 3789–3794. [Google Scholar] [CrossRef]
- Soleas, G.J.; Diamandis, E.P.; Karumanchiri, A.; Goldberg, D.M. A multiresidue derivatization gas chromatographic assay for fifteen phenolic constituents with mass selective detection. Anal. Chem. 1997, 69, 4405–4409. [Google Scholar] [CrossRef]
- Proestos, C.; Chorianopoulos, N.; Nychas, G.J.E.; Komaitis, M. RP-HPLC analysis of the phenolic compounds of plant extracts. Investigation of their antioxidant capacity and antimicrobial activity. J. Agric. Food Chem. 2005, 53, 1190–1195. [Google Scholar] [CrossRef]
- Chu, T.Y.; Chang, C.H.; Liao, Y.C.; Chen, Y.C. Microwave-accelerated derivatization processes for the determination of phenolic acids by gas chromatography-mass spectrometry. Talanta 2001, 54, 1163–1171. [Google Scholar] [CrossRef]
- Deng, F.; Zito, S.W. Development and validation of a gas chromatographic-mass spectrometric method for simultaneous identification and quantification of marker compounds including bilobalide, gingolides and flavonoids in Ginkgo biloba L. extract and pharmaceutical preparations. J. Chrom. A 2003, 986, 121–127. [Google Scholar] [CrossRef]
- Betés-Saura, C.; Andrés-Lacueva, C.; Lamuela-Raventós, R.M. Phenolics in white free run juices and wines from Penedés by high-performance liquid chromatography: Changes during vinification. J. Agric. Food Chem. 1996, 44, 3040–3046. [Google Scholar] [CrossRef]
- Robbins, J.R. Phenolic acids in foods: An overview of analytical methodology. J. Agric. Food Chem. 2003, 51, 2866–2887. [Google Scholar]
- Halket, J.M. Handbook of Derivatives for Chromatography; Blau, K., Halket, J., Eds.; John Wiley & Sons: West Sussex, UK, 1993; pp. 297–326. [Google Scholar]
- Donovan, J.L.; Luthria, D.L.; Stremple, P.; Waterhouse, A.L. Analysis of (+)-catechin, (−)-epicatechin and their 3′- and 4′-O-methylated analogs: A comparison of sensitive methods. J. Chrom. B 1999, 726, 277–283. [Google Scholar]
- Angerosa, F.; D’Alessandro, N.; Konstantinou, P.; di Giacinto, L. GC-MS evaluation of phenolic compounds in virgin olive oil. J. Agric. Food Chem. 1995, 43, 1802–1807. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Proestos, C.; Komaitis, M. Analysis of Naturally Occurring Phenolic Compounds in Aromatic Plants by RP-HPLC Coupled to Diode Array Detector (DAD) and GC-MS after Silylation. Foods 2013, 2, 90-99. https://doi.org/10.3390/foods2010090
Proestos C, Komaitis M. Analysis of Naturally Occurring Phenolic Compounds in Aromatic Plants by RP-HPLC Coupled to Diode Array Detector (DAD) and GC-MS after Silylation. Foods. 2013; 2(1):90-99. https://doi.org/10.3390/foods2010090
Chicago/Turabian StyleProestos, Charalampos, and Michael Komaitis. 2013. "Analysis of Naturally Occurring Phenolic Compounds in Aromatic Plants by RP-HPLC Coupled to Diode Array Detector (DAD) and GC-MS after Silylation" Foods 2, no. 1: 90-99. https://doi.org/10.3390/foods2010090
APA StyleProestos, C., & Komaitis, M. (2013). Analysis of Naturally Occurring Phenolic Compounds in Aromatic Plants by RP-HPLC Coupled to Diode Array Detector (DAD) and GC-MS after Silylation. Foods, 2(1), 90-99. https://doi.org/10.3390/foods2010090






