Abstract
This review addresses the recent advances made through various genetic engineering techniques to improve the properties of Lacticaseibacillus rhamnosus, not only for industrial applications, but also for the health-related benefits. However, due to the strict regulations on microorganisms intended for human consumption, concerning the insufficient characterization degree of the newly isolated strains and the lack of data regarding the safety of the genetically modified (GM) variants, the feasibility of bringing such L. rhamnosus strains to the market and their safety prospects were evaluated. Given their multiple in vivo functions in the contexts of synbiotic and symbiotic functionality, L. rhamnosus strains are more than classic probiotics and need furthermore attention. In the functional food context, this review highlights the impact of L. rhamnosus derived bioactives on the human gut–organ axis, pointing out recently demonstrated molecular mechanisms of action with the host’s gut microbiome to reduce the negative effects of obesity and its related metabolic disorders, as well as depression and Parkinson’s disease, as the major challenges confronting humans today. Beyond that, considering L. rhamnosus delivery and its postbiotics accessibility to consumers via functional foods, notable progress was made to enhance their stability by developing various encapsulation systems, which are also emphasized.
1. Introduction
Researchers from all over the world together with food industry experts show an increased interest in developing functional foods enriched with metabiotics (pre-, pro-, post-, and paraprobiotics) [1]. This trend derives from the growing recognition of the health-related benefits of probiotics and derivates, particularly in preventive health and wellness. In general, probiotics are exposed to multiple stress factors during gastrointestinal transit, which can compromise the cells’ functional efficacy. Thus, a critical point in probiotic formulation to benefit from its effectiveness is to maintain viable cells count higher than 106 CFU/g after ingestion [2]. In this context, Lacticaseibacillus rhamnosus (Lactobacillus rhamnosus) is one of the most studied probiotic species within the Lacticaseibacillus genus regarding fermentation processes carried out to obtain bioactive compounds with enhanced functionality. Several strains of this lactic acid bacterium (LAB) meet the necessary criteria to be qualified as probiotics, as they are able to withstand the stress conditions associated with the human gastrointestinal tract, to adhere to the gut’s epithelial cells and colonize it, and to prevent the pathogenic microorganisms’ proliferation due to their biofilm forming ability [3]. However, the functional benefits attributed to L. rhamnosus, as a probiotic species, are correlated with its proven biological activities within the host. Due to the recognized its benefits, it is estimated that L. rhamnosus market value (USD 1.2 billion in 2024) will double by the year 2033 [4].
In this regard, in the food engineering sector, different encapsulation techniques have been applied to L. rhamnosus to maintain a high viability in the human intestine, ensure its participation to the metabolic and immune processes and facilitate health benefits through microbiota–gut–brain axis. The choice of a suitable encapsulation method from a wide range of technologies (such as spray drying, freeze-drying, emulsification, coacervation, liposomal delivery, electrospraying) depends on several critical factors related to the size of capsule, the biocompatibility of encapsulating materials with the probiotic strain, and the intended food, pharmaceutical, or nutraceutical applications [5].
Commonly, the L. rhamnosus exceptional adaptability and persistence in the food matrix or human gastrointestinal tract are effects of its pan-genome structure expression. Adaptation to these conditions is possible due to the expression of different essential genes responsible for carbon sources utilization, cell envelope-related functions, or antimicrobial activities [6].
In order to explore the genomic and phenotypic diversity within the L. rhamnosus species, Douillard et al. [6] isolated 100 strains from various ecological niches, including human body sites (77 strains) and dairy products (23 strains). The whole-genome sequencing showed that gene content among the isolated strains varied significantly (with a median of 96.7%), when reads were aligned to the L. rhamnosus GG (LGG) reference strain. More precisely, the authors emphasized that genes content similarities or divergences relative to LGG expressed adaptation to specific ecological niches [6]. Furthermore, even though the complete set of LGG genes (3016) were reported in eleven strains of human origin and four strains isolated from dairy products, some particularities among strains regarding carbohydrate metabolism were observed. More precisely, the genes encoding phosphatase transfer systems (PTS) or various carbohydrates transporters, play a key role in strains adaptation to specific niche [6]. In a recent study, Huang et al. [7] explored the core and accessory gene content of 70 L. rhamnosus strains, isolated from infant and adult Chinese subjects. The main findings regarding carbohydrate utilization are related to the presence of genes encoding glycoside hydrolases (GHs), offering an integrative image about genetic diversity within species, and the possibility of using them as probiotic bioingredient [7].
This review aims to provide a comprehensive synthesis of the current knowledge and developments regarding the beneficial potential of L. rhamnosus strains, with a particular focus on their probiotic characteristics, which were enhanced by genetic engineering techniques, or preserved by encapsulation methods for both food industry applications and host functionality.
2. Improvement of L. rhamnosus Strains Through Genetic Engineering for Industrial Applications
Genetic engineering used to explore LAB’s functionalities, such as substrate metabolism, metabiotics’ production, and tolerance to stress, represents an ongoing research effort. To enhance L. rhamnosus beneficial characteristics, a variety of genome engineering tools have been employed (Figure 1). These techniques were both recombinant and non-recombinant. The recombinant techniques, such as homologous recombination, and CRISPR-Cas technology, can result in either genetically modified organisms (GMO), which contain DNA fragments originating from different species, or self-cloning organisms (SCO), which do not contain heterologous DNA or do not produce proteins other than those occurring naturally. The non-recombinant methods generate strains that are not considered as GMO by the legislation currently in force. These can, in turn, be untargeted non-recombinant strategies, including random mutagenesis and adaptive laboratory evolution (ALE), and targeted recombinant strategies, as is the case with bacterial conjugation [8].
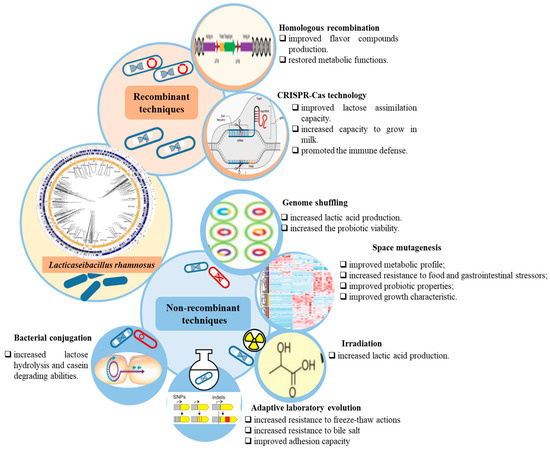
Figure 1.
Genetic engineering techniques to enhance L. rhamnosus metabolic functionalities along with their associated achievements.
2.1. Recombinant Strategies
2.1.1. Homologous Recombination Based on Exogenous Vectors
Homologous recombination based on exogenous vectors has been used to both delete and integrate genes of interest in the LAB’s genomes. Briefly, the strategy relies on an integrative plasmid that carries a construct containing two homologous regions through which the recombination process takes place and antibiotic resistance genes used as selection markers. The plasmid integrates into the host’s genome following a single crossover event via one of the homologous regions, being then eliminated after the second one. Depending on the region where the second crossover event occurred, either wild-type (the same homologous region) or mutant (the other homologous region) cells can be obtained [8]. This genome integration strategy allows permanent interventions, meaning that the recombinant genes remain stable without a selective pressure [9]. Plasmid-based homologous recombination allowed researchers to establish the role of some genes in L. rhamnosus probiotic traits. For instance, construction and analysis of a luxS knockout LGG mutant and its genetic complementation strain led to the conclusion that luxS gene plays a central role in the activated methyl cycle and sulfur amino acid metabolism, which are essential for growth and biofilm formation, rather than being involved in quorum sensing through production of AI-2 signaling molecules [10].
Besides assessing the specificity of gene knockout experiments, gene complementation can also be used as a strategy to restore some metabolic functions in L. rhamnosus strains. One L. rhamnosus strain unable to produce diacetyl and acetoin while growing in milk, as a consequence of a frameshift mutation in the als gene encoding for acetolactate synthase, became functional by transferring a wild-type als gene from a strain yielding high amounts of this flavor compound. The restored L. rhamnosus defective strain behaved similarly to the strains naturally producing elevated amounts of diacetyl and acetoin during cheese ripening [11].
2.1.2. CRISPR-Cas Technology
Clustered Regularly Interspaced Short Palindromic Repeats—associated protein (CRISPR-Cas) systems are adaptive immune mechanisms against mobile genetic elements encountered in prokaryotic organisms. These systems are used to intercept and destroy the invasive nucleic acids injected by bacteriophages or brought along with exogenous plasmids based on the memory of prior infections provided by the short DNA fragments (spacers) integrated in the CRISPR array, whose transcripts (single pre-CRISPR RNAs—crRNAs) guide the Cas nucleases to the specific cleavage sites (complementary protospacers) [12,13]. There are two classes of CRISPR-Cas systems, which are in turn divided into several types. Class 1 systems include type I, III, and IV systems together with their subtypes and class 2 systems comprise type II, V, and VI systems and their respective subtypes [14]. Class 1 systems use a multiple-protein effector complex such as Cascade (CRISPR-associated complex for antiviral defense), which is specific to the type 1 systems, to recognize and degrade the foreign DNA elements [15], while class 2 systems rely on single large proteins such as Cas9 to perform the effector function [16]. The valid CRISPR-Cas loci identified in L. rhamnosus strains have been reported to belong to the subtype II-A system (Lsal1 family) which encompasses Cas1, Cas2, Cas9, and Csn2 proteins [6,17]. This makes L. rhamnosus a suitable probiotic bacterium for industrial fermentation applications, as the high prevalence of the subtype II-A CRISPR-Cas systems confers great stability and efficiency in cases of phage contaminations [17]. The same is suggested by Pujato et al. [18] who conducted a comparative analysis of 8 strains of L. rhamnosus with other Lacticaseibacillus species regarding their spacers homology to foreign genetic elements and concluded that the subtype II-A system in this bacterium is more active in acquiring spacers following phage infections [18].
Moreover, it was shown that, similarly to Streptococcus pyogenes, L. rhamnosus possesses an RNA-based regulatory structure located upstream of the CRISPR array that promotes the immune defense against the most recent invaders. This works by crRNAs production from the newest spacers acquired. The underlying mechanism consists of a stable stem-loop structure formation between the pre-crRNA (precursor CRISPR RNA) leader and the first repeat bordering the newest spacer that enables tracrRNA (trans-activating CRISPR RNA) hybridization with the second repeat and speeds up crRNA processing by RNase III so that it can bind to the Cas9 nuclease [19].
The LGG derived Cas9 protein, termed LrCas9, was reported to be more efficient and specific in genome editing than LbCas12a, SpCas9-NG, or SpRY due to its preference for targeting A/T-rich PAM sequences (5′-NGAAA-3′). LrCas9 has been successfully used in the development of genetic engineering tools, such as vectors designed for targeting multiple genes simultaneously, CRISPRi (CRISPR interference) for genes repression, and CRISPRa (CRISPR activation) for genes overexpression [20]. More precisely, the authors proved that the LrCas9 enzyme maintains genome-editing activity even at lower temperatures, enabling precise genetic modifications in key crops such as rice, wheat, tomato, and larch [20]. By supporting the development of higher-yielding, and more nutritious crops, LrCas9 could accelerate innovation across the food supply chain, ensuring more sustainable food products. The high abundance of type II CRISPR-Cas systems in species belonging to the Lacticaseibacillus genus offers as well great potential for precise engineering of their genomes by repurposing them [12]. However, applying CRISPR-Cas technology for genome editing in these species might be challenging for several reasons, such as the following: limited homologous recombination ability which makes it difficult to edit multiple genes simultaneously, high cell death rate as a consequence of double-strand breaks, insufficient expression of sgRNA resulting in gene targeting failure, off-target effects leading to modification of non-specific DNA sequences, low rates of the systems delivery through cells transformation, and limited availability of PAM (Protospacer Adjacent Motif) sequences in the strains’ genomes [21]. In spite of these, Xie et al. [22] managed to correct the mutations at the level of the lacTEGF operon which were responsible for LGG inability to assimilate lactose by successfully reprogramming the bacterium’s native type II-A CRISPR-Cas9 system. The authors’ strategy consisted of constructing an editing plasmid containing the sgRNA placed under the control of a strong constitutive promotor and a repair template including the two homologous regions flanking the target sites. The spacers sequences of 30 bp length were selected so that the target sites were located upstream the PAM sequence recognized by the Cas9 enzyme and linked to the tracrRNA via an artificial loop to form a unique sgRNA molecule. Compared to the parental strain, the transformed cells were capable of inducing milk coagulation during incubation at 37 °C by decreasing the pH as a result of lactic acid production. Also, unlike LGG cells, the modified ones increased significantly (~1.21 log10 CFU/g growth) during yogurt fermentation, maintaining their viability at a higher rate under storage at low temperatures as well [22].
This important achievement gives rise to new research directions in the field of probiotics as CRISPR-Cas tools enable to engineer strains which not only exhibit greater stability, but also enhance the functional food properties, including shelf life, fermentation efficiency, or nutrients bioavailability.
2.2. Non-Recombinant Strategies
2.2.1. Random Mutagenesis as Untargeted Non-Recombinant Strategy
Genome shuffling is an evolutionary engineering method applied to increase the metabolites production of industrial microbial strains by improving their ability to metabolize specific substrates or withstand specific stressors present in the growth media [23]. Genome shuffling occurs through the non-homologous recombination of the multi-parental strains’ genomes with relevant phenotypes induced via random mutagenesis and selection [24]. This technique is often used together with protoplasts generation to increase the chances of the recombination process [25]. Genome shuffling strategy was applied to enhance L. rhamnosus ATCC 11443 strain tolerance towards glucose and acidity in order to increase its capacity to produce L-lactic acid. Both objectives were achieved by generating populations with the desirable properties via ultraviolet irradiation and nitrosoguanidine mutagenesis and recursive protoplast fusion application for several rounds. The improved L. rhamnosus strains were able to tolerate glucose concentrations of up to 200 g/L and pH levels of up to 3.8, demonstrating a significantly higher capacity regarding L-lactic acid production compared to the wild-type variant [26].
All these advancements offer significant advantages for food industry, particularly in the food fermentation industry. The new trait of L. rhamnosus, namely a more efficient glucose metabolism which increases lactic acid synthesis, enables rapid acidification which supports food safety and natural preservation. Moreover, it may contribute to a consistent development of the desirable sensory characteristics, improve the texture, or ensure the probiotic viability without compromising the product quality.
Space mutagenesis involves the exposure of organisms to the physical and chemical conditions of space, such as microgravity, high radiation, high vacuum, and extreme temperatures [27]. The changes in the organisms’ genetics, physiology, and metabolism, as a result of the stress induced by these extreme conditions, have the potential of generating new varieties with better traits [28]. However, the altered behavior of the evolved microbes must be comprehensively evaluated for potential harmful effects, like increased resistance to antibiotics, transfer of virulence factors between microorganisms, enhanced biofilm forming ability, or secondary metabolites production [29]. Several studies have reported that exposure of L. rhamnosus Probio-M9 strain to space conditions improved its growth characteristics, metabolism, probiotic properties and resistance towards stress factors that can be encountered in food matrices or in human gastrointestinal tract. The mutant cell line R7970 was able to form larger colonies on MRS agar compared to the Probio-M9 cells, this phenotype being associated with an increased capsular polysaccharides (CPSs) production related to the discovered mutation in the ywqD gene. The isolate had a better metabolic profile, particularly in glucose utilization, which led to higher lactic acid production, tryptophan metabolism, and bioactive compounds production, such as nicotinic and picolinic acids. The abundance of CPSs was also considered the reason for the mutant’s increased resistance to acidic, osmotic, heat, bile salt, and oxidative and fermentation stress [30]. Regarding their probiotic potential, CPSs not only provide an increased resistance to beneficial bacteria along the human gastrointestinal tract but also exert nutraceutical and bioactive effects [31]. Other L. rhamnosus Probio-M9 space mutants (HA-R7970-29, HG-R7970-18, HG-R7970-29), selected based on their superior growth characteristics, were evaluated regarding some probiotic properties, such as antibacterial activity against common pathogenic bacteria, including Escherichia coli, Salmonella enterica, Listeria monocytogenes, Staphylococcus aureus, Bacillus cereus, and Pseudomonas aeruginosa, auto-aggregation ability, cell surface hydrophobicity, and antioxidant activity [32]. Except for the HG-R7970-29 isolate, which, compared to the other strains, exhibited a stronger inhibition against P. aeruginosa, the mutants’ antibacterial activity against the six pathogens was comparable to that of the parental strain.
The auto-aggregation ability was significantly higher in Probio-M9 cells, while, regarding the hydrophobicity of the strains, only the HA-R7970-29 isolate reached a value similar to that of the wild-type strain [32]. The advantages regarding the mutants’ antioxidant capacity, in terms of 2,2-diphenyl-1-picrylhydrazyl (DDPH) free radicals, hydroxyl radicals, superoxide anions scavenging ability, differed based on the tested media (bacterial suspensions or fermentation supernatants) and the strain. Overall, the fermentation supernatants outperformed the cellular suspensions, likely due to the secreted metabolites. HG-R7970-18 strain showed the best DDPH and hydroxyl radicals scavenging ability, while the best superoxide anions scavenging ability was registered in the case of HA-R7970-29 strain [32].
Finally, the comparison between L. rhamnosus Probio-M9 strain and another isolate obtained following space mutagenesis, HG-R7970-41, concerning the metabolism of 190 single carbon sources, revealed a difference in the carbon metabolism pattern between these strains. The HG-R7970-41 mutant strain exhibited a significantly lower metabolic activity for certain carbon sources, including D-glucose, D-salicin, D-galactose, D-trehalose, N-acetyl-D-glucosamine, D-sorbitol, D-tagatose, D-fructose, D-mannose, and D-melezitose, which was linked to the changes in the phosphorylation and glycolysis pathways as a result of the related genes’ downregulation. From the industrial point of view, such a strain might contribute to the reduction of the costs associated with the substrate acquisition, given that it requires less energy to grow [33].
Irradiation is an uncontrolled genetic alteration method that has allowed the selection of lactic acid bacteria strains with improved traits to be used in the fermentative industry [34]. Lin [35] subjected L. rhamnosus 6013 strain to microwave radiation and the resulting mutated strains were screened for increased lactic acid production capacity. By applying a microwave radiation frequency of 2450 MHz for 3 min, it generated a mutant strain which, in the presence of glucose as the single carbon source, showed a 1.5-fold increased production of the metabolite compared to that of the wild-type strain. The amplified fragment length polymorphism analysis performed on the mutant L. rhamnosus 6013 cells indicated as possible causes for their enhanced lactic acid production the mutations occurred at the malate/lactate dehydrogenase and pyruvate kinase genes level [35].
Transposon mutagenesis uses mobile genetic elements, known as transposons or “jumping genes”, which induce random chromosomal mutations by shifting from one position in the genome to another [36]. The specialty literature concerning L. rhamnosus genetic modification by transposon mutagenesis is still scarce. Biswas et al. [37] attempted to create a L. rhamnosus LRB mutants’ library by using the ISS1 transposon delivered via the pGhos9::ISS1 thermosensitive plasmid, which would have allowed them to identify the genes involved in the bacterium’s response to acid stress. The ISS1 transposon failed to integrate into the L. rhamnosus LRB genome, the possible reasons of this advanced by the researchers being the presence of a high number of native insertion sequences (IS) and IS-like elements or host encoded factors like nucleoid associated proteins. Overall, it was concluded that ISS1 transposon is not an appropriate tool for genome modification in this species [37].
2.2.2. Bacterial Conjugation as Targeted Non-Recombinant Technique
Horizontal gene transfer through bacterial conjugation is a naturally occurring process that requires the presence of self-transmissible plasmids or integrated conjugative elements containing the genes involved in the DNA transmission [38]. The DNA transfer between the donor and the recipient cells takes place through specialized structures encoded by the tra operon of the conjugative plasmid that span the cell envelope [39]. Lactococcus lactis and Lactococcus lactis cremoris are common carriers of conjugative plasmids that encode useful traits in dairy fermentation, such as lactose, citrate, and casein metabolism, resistance to bacteriophages and heavy metals, and bacteriocin production [39].
Through bacterial conjugation, a new LGG strain was developed with lactose metabolism and proteolytic activity, enabling efficient growth in milk and generating a non-GMO strain suitable as starter culture for dairy industry applications [40]. More precisely, the lactose hydrolysis and casein degrading abilities were achieved by co-culturing the LGG with L. lactis subsp. cremoris NCDO 712, which carries the pLP712 plasmid containing the lacABCDFEGX operon responsible for the lactose metabolism and utilization and prtP gene encoding for a serine protease [40].
2.3. Adaptive Laboratory Evolution
ALE is an experimental technique used in the field of biotechnology for the purpose of generating evolved microbial strains with the desired traits by exposing a population to certain environmental conditions or selection pressures over the course of many generations. This method allows obtaining information regarding the molecular mechanisms’ evolution and the corresponding adaptive phenotypes that occur in the studied strains [41]. Experimental evolution under stress factors that mimic the conditions encountered in the human gastrointestinal tract may help understand how L. rhamnosus evolves genetically and how these changes impact its beneficial properties. Freezing and thawing stress may affect the viability of the starter cultures used in dairy industry [42]. A group of researchers attempted to obtain a stable LGG variant by employing the ALE technique. Subjecting this strain to freeze–thaw–growth for 150 cycles resulted in mutants with higher survival rate (up to 86.3%) compared to the parental strain, after 6 days of frozen storage in 0.3 M trehalose. Further, the evolved LGG mutants displayed faster exponential growth and a shorter lag phase, with a mean doubling time of 13 min shorter and a 29 min reduction in lag phase compared to their ancestor strain [43]. Genome sequencing of the evolved cells revealed genetic mutations primarily altering the cell wall and membrane physical properties [43]. Specifically, the improved resistance to freeze–thaw stress, and, consequently, the increased survival rate, was attributed to functional defects in the dacA, murQ, cls, and wze encoding genes. These alterations led to an incomplete cell wall or membrane structure, resulting in a more flexible cell envelope that enables cells to better withstand the mechanical forces generated by ice crystal formation compared to the parental strain [43].
Another study revealed that subjecting L. rhamnosus to bile salt stress for approximately 750 generations led to the activation of IS elements, resulting in the deletion of a large chromosol region located on the same genomic island, which includes the spaCBA-srtC1 pilus gene cluster, in all the adapted cells. Although the loss of piliation occurred to a lesser extent under shear stress, this also highlights a potential risk of mechanical stress on the strain’s genomic stability during industrial processing and handling [44].
However, evolution of bacteria under in vivo conditions such as superior organisms’ digestive tract represents a more complex approach that may result in strains with improved probiotic properties for dietary applications. This is the case of the study recently conducted by Mark et al. [45], whose purpose was to generate LGG variants with increased resistance to bile salt stress and improved adhesion to epithelial cells by repetitively administering the probiotic bacteria to mice lacking a functional immune system. Compared to the wild-type cells, the evolved isolates demonstrated a better retention in the mice gut and higher growth rates in the presence of bile salt, which were associated with the identified changes in the genes encoding for cell surface proteins and sortase enzymes, and ATP binding proteins and proton pumps, respectively. Surprisingly, the Caco-2 cell adhesion assay showed a slightly reduced binding capacity of the evolved variants compared to that of the wild-type strain, a fact that could be attributed to some putative mutations in the pili-related genes, as previously noticed by other studies [44].
2.4. Legislative Aspects on a Global Level
Genetic engineering of microorganisms intended for human applications is subjected to rules that may differ from one country to another. For instance, due to the difference in the risk perception, the European Union (E.U.) regulations on GMOs use in food and feed production [46] address the authorization procedure and its subsequent steps in a more comprehensive and rigorous manner compared to those in the United States [46,47]. While, in the process of the risk assessment, for precautionary reasons, the E.U. authorities consider GMO-based food and feed products fundamentally different from the conventional versions, in the U.S., these are treated as equivalents to the homologous non-GMO products as long as the scientific evidence does not indicate any possible hazard. Other major differences between the laws of these territories are related to the labelling of GMO-based food and feed products and their traceability, which, in the case of U.S. producers, are not mandatory [48]. However, an exception to the non-mandatory labelling of GMO-based products in the U.S. refers to the possible allergens that do not occur naturally in the non-GMO products [49]. Authorization of GMOs and GMO-based products for production and commercialization presents a different approach according to the country. While at the E.U. level, the decision is up to the political bodies, in the U.S., the process is depoliticized, with the state not participating directly [48]. In the EU, commercialization of GMOs as such or feed and food products containing GMOs falls under the jurisdiction of the Directive 2001/18/EC, which establishes the rules regarding deliberate GMOs release in the E.U. environment. According to the Regulation (EC) No. 1829/2003, before reaching the market, the applicants have to go through a multi-step procedure which starts with the notification of the national authorities and the subsequent request transmission to the European Food Safety Authority (EFSA) Based on the EFSA scientific opinion formulated following a rigorous evaluation of the GMOs risk to human and animal health or the environment from the genetic alteration process perspective, the Commission drafts a decision on the request approval or rejection. Then, the draft decision is subjected to the vote of the Standing Committee on Plants, Animals, Food, and Feed (PAFF Committee), which is formed of the Member State representatives. The PAFF Committee has to approve or reject the authorization by qualified majority. If the qualified majority is not reached, the Commission can summon an Appeal Committee or take responsibility for the decision [50]. In the US, the GMOs approval for placing on the market is up to three federal agencies, namely US Food and Drug Administration (F.D.A.), US Environmental Protection Agency (EPA), and US Department of Agriculture (USDA), which work together to ensure their safety for human, animal, and plant health and to monitor their impact on the environment. In this case, the main goal is to check whether the GMO-based products are safe according to their intended use [51]. In addition, in comparison to the E.U., the U.S. still lack a legal framework regulating GMOs, specifically GM probiotics, as a distinct product category, a situation that is encountered as well in other large countries, such as China [52]. These discrepancies can negatively affect the international trade of such GMO-based products and the advancement of genetic engineering for sustainable food production and food security ensuring [53].
To date, the acceptable genome engineering techniques for LAB modification in the E.U. are those which can occur naturally, such as bacterial conjugation, phage transduction, natural competence, and the random mutagenesis induced chemically or via irradiation. At the same time, the methods that are not currently approved are those aiming at chromosome modification or foreign DNA expression by the use of exogenous vectors, recombineering, and CRISPR-Cas derived systems [54]. Consumer perceptions of GMOs are multilateral, with factors such as cultural, social, and economic contexts, as well as awareness and trust in the regulatory bodies, playing a decisive role in their attitude towards GM food [55]. The degree of consumers’ acceptance with respect to foods derived from GMOs in the U.S. seems to surpass that of those in the E.U. [54].
2.5. Evaluation of L. rhamnosus and GM Probiotics Safety
Before obtaining probiotics with additional or improved functions for the benefit of human health, it is necessary that the parental strains be genetically modified and carefully evaluated in terms of safety and resilience [53]. L. rhamnosus strains isolated from different sources, including different geographical origins or food matrices, can present a high variability regarding the genetic characteristics [56]. Hence, sequencing the probiotic strains’ complete genomes to be precisely identified and classified, as well as reviewed for any genetic trait of concern, becomes mandatory to ensure their safety [57]. Also, clinical trials evaluating the efficacy and long-term effects of probiotic candidates need to be carried out under a standardized legal framework [58].
Some probiotics can naturally contain mobile genetic elements (MGEs) carrying antibiotic resistance genes (ARGs) [59]. Therefore, there is a high concern that the regular intake of probiotics for health benefits could lead to the dissemination of ARGs among the normal intestinal microbiota and occasionally occurring opportunistic species, rendering the future antibiotic therapies less effective [60]. The AR traits in L. rhamnosus strains were generally found to be of low frequency [59]. However, any microorganism intended for human consumption needs to be investigated for the possibility of ARGs transfer to other organisms, if present, and virulence factors [61]. It was shown that the vancomycin resistance factor of the frequently consumed probiotic LGG strain is not related to the van genes of the opportunistic pathogenic species Enterococcus faecalis and Enterococcus faecium, being stated that this AR trait is not a transferable one [62]. The erythromycin resistance in L. rhamnosus Oxy and Pen probiotic strains is determined by the erm chromosomal genes which, in the absence of carrying plasmids or other MGEs, are also less capable of horizontal transfer [63]. In addition to this, the decrease in the susceptibility of LGG to erythromycin and ciprofloxacin after repetitive exposure to these macrolides is more likely to be generated by phenotypical alterations or point mutations rather than the associated resistance mechanisms, meaning that the acquired resistance has a low potential of being transferable [64]. The failure in putting into evidence by molecular methods the AR genetic determinants in some L. rhamnosus strains identified as being resistant to either clindamycin, gentamicin, or tetracycline above the cut-off concentrations established by EFSA also indicates that these genes may be encoded at the chromosomal level [65]. Overall, according to the studies mentioned above, there is a low probability that AR traits from L. rhamnosus strains will be transmitted to other microorganisms mostly because of their intrinsic nature.
Toxigenicity, pathogenicity, allergenicity, immunological impact, and genetic stability over time are other important aspects to be considered in the case of probiotic candidates for human use. The toxicity studies performed on the potential probiotic strain L. rhamnosus MP108, including genetic and oral toxicity assays, did not put into evidence any safety issue [66]. However, although L. rhamnosus belongs to the normal human flora and is generally considered innocuous, this species can generate infections in certain circumstances. One study reported L. rhamnosus as the etiological agent of nosocomial bloodstream infections and, by corroboration of the results with other cases presented in the literature, it was concluded that, in the cases of hospitalized patients, the main associated risk factors were immunosuppression and catheters placement [67]. Other predisposing conditions leading to L. rhamnosus infections in humans could be gastrointestinal tract disruption, malignancy, complex surgeries or prior exposure to antibiotics [68]. On the other hand, many studies have reported significant benefits of diets supplemented with L. rhamnosus on food allergies and allergic diseases thorough modulation of the host intestinal microbiota and immune response [69,70,71]. Probiotics’ immunomodulatory activity is still questionable. Some strains were shown to exert positive effects, while others were shown to trigger a pro-inflammatory response [72]. The related studies conducted on L. rhamnosus generally indicated promising immunomodulatory potential of this bacterium, with limited side effects. However, the possibility of an exaggerated immune response or immune suppression in certain contexts needs to be further investigated [73,74].
The research area concerning the health impact of genetically modified (GM) probiotics in vivo is still narrow, with quite limited trials on animals and no clinical trials to date [52]. Although not including GM L. rhamnosus strains, there are a few studies conducted on animal models which demonstrated that some GM-LAB strains could be safely integrated into the food chain. The mice fed with LABs containing plasmids for nutraceuticals overproduction showed a similar behavior in terms of growth rate, food and water intake, hematological parameters, and organs’ relative weight, morphology, and histology, to that of those fed with the progenitor strain [75]. Another study analyzed the interaction between nattokinase producing GM-LABs and rats’ intestinal microflora, also evaluating organs, such as liver, spleen, and kidney, and blood for bacteria translocation. After feeding the rats for 4 weeks with both wild-type and GM strains, the concentration of the beneficial Bifidobacterium spp. in the rats’ feces increased significantly compared to that in the control group. At the same time, the harmful bacterium Clostridium perfringens remained undetectable in all the groups throughout the experiment. Neither non-GM-LAB nor GM-LAB strains negatively affected the rats’ overall health, and no bacterial translocation occurred [76].
On the other hand, there are scientists who claim that GM probiotics should be prohibited for therapeutic purposes because of their unpredictable behavior in relation to the resident bacteria within the human gut and host’s immune system [77]. Besides the intended effects, genetic modification in LABs may lead to unexpected side effects on consumers [34].
Among these, potential risks including the transfer of ARGs, multidrug resistance genes, and other MGEs to the gut microbiota, may compromise the host’s microbial resistance profile. Additionally, GM probiotics may produce harmful proteins or enzymes that trigger inflammation and disrupt gut microbiome balance, potentially inducing chronic disease [78].
Another main concerns regarding the safety of GM probiotics are their uncontrolled spreading and the transmission of the novel genetic traits to other organisms if accidentally released in the environment [79]. As universally applicable rules to avoid these events, it must be ensured that no antibiotic resistance gene is included in the GM bacteria’s genomes and that the exogenous DNA template used in the engineering process comes from GRAS (Generally Recognized as Safe) microorganisms, such as food grade vectors derived from LAB or bifidobacteria cryptic plasmids [80].
3. L. rhamnosus as an Active Health Promoter Included in Food Production
3.1. Beneficial Effects of L. rhamnosus Metabiotics
Metabiotics involve bioactive and functional complexes of the viable probiotics and inactivated intact or ruptured cells (paraprobiotics), their metabolites and signaling molecules which have been shown to specifically improve the host physiological functions and regulate the interconnection between host metabolic and behavioral functions and gut microbiome diversity, composition, and functionality [81]. The concept of metabiotics emerged following the need for a safer approach in biotherapy using live probiotics, due to their better documented composition and function. Some other advantages offered by metabiotics are as follows: extended shelf-life of food products, lack of side effects, and positive effects on human health [82]. Some representative beneficial postbiotics are short chain fatty acids (SCFAs), antimicrobial compounds, functional peptides, bacteriocins, vitamins, peptidoglycans, lipoteichoic acids, polysaccharides, or polar lipids [81,82]. Paraprobiotics are obtained from probiotics subjected to the inactivation process. Cell disruption can be achieved through different methods, which can be classified into thermal treatments [pasteurization (<100 °C), sterilization (≥100 °C)] and non-thermal treatments (ionizing radiation, ultraviolet rays, high pressure, sonication, dehydration, and pH modification). Other technological strategies are emerging methods, such as pulsed electric field technology, ohmic technology, and supercritical fluids–CO2 technology. When choosing an inactivation method, it is important to consider preserving or improving as well as possible the beneficial properties [83]. L. rhamnosus derived metabiotics showed various beneficial effects for human health. The cell wall (CW1505) and peptidoglycan (PG1505) of L. rhamnosus CRL1505 strain exhibited important immunoregulatory effects on co-cultured human intestinal epithelial cells and dendritic cells when challenged with TLR4 (toll-like receptor 4). MCW1505 had a stronger stimulatory effect, increasing the production of TNF-α, IL-1β, IL-6, and IL-8, while PG1505 was more efficient in improving the production of IL-10, which is closer to the effect of live and UV inactivated L. rhamnosus CRL1505 cells [84]. Metabiotic extract from the cell free supernatant obtained following the culture of L. rhamnosus MD 14, a strain isolated from infant feces, which consisted primarily of carboxylic acids, ethers, amides, and esters, showed high cytotoxicity against Caco-2 (86.8%) and HT-29 (86.3%) cells. It also slowed down the proliferation of the colon cancer cells by blocking the cell cycle in the G0/G1 phase [85]. Moreover, the metabiotic extract, later shown to contain SCFAs, such as butyrate, acetate, and propionate, acetamide, thiocyanic acid, and oxalic acid, showed promising results as antitumorigenic agent when administered in Sprague-Dawley rats with induced colon carcinogenesis. Administered daily in a medium dose (2 mL/kg) for 6 weeks, the metabiotic extract determined the reduction of fecal pro-carcinogenic enzymes, oxidants, and aberrant crypt foci, downregulation of oncogenes, such as K-ras, β-catenin, Cox-2, nuclear factor kappa B (NF-κB), and upregulation of tumor suppressor p53 gene [86].
The freeze-dried postbiotic extract obtained from L. rhamnosus LRH-B2 cells, containing bioactive compounds such as flavonoids, SCFAs, antioxidant enzymes, and organic acids, was shown to inhibit more than 50% of the populations of pathogenic bacteria L. monocytogenes ATCC 13932, E. coli ATCC 25922, S. enterica serovar Typhimurium ATCC 14028, and S. aureus ATCC 64542 after their co-incubation at 37 °C for 24 h. The extract exerted low cytotoxicity in MRC 5 and IPEC-J2 cell lines (10% at highest concentration tested of 150 mg/mL) and was not hemolytic to human erythrocyte cells [87].
3.2. Postbiotics and Their Functional Effects on Obesity and Related Metabolic Disorders
Obesity is a multifactorial disease characterized by hypertrophy and hyperplasia of adipocyte, being induced by consumption of high-fat diets (HFD), which are responsible for systemic metabolic disturbance, including insulin resistance, lipid metabolism imbalance, and gut microbiota dysbiosis. Once dysbiosis was initiated, the proteins from epithelial tight junction are degraded and the lipopolysaccharides (LPS) are translocated into liver via portal circulation, disrupting the gut–liver axis homeostasis and further activating inflammatory markers. To counteract these effects, Sun et al. [88] used the postbiotic compounds derived from L. rhamnosus HF01 strain (cell-free supernatant, CFS) to treat the obesity symptoms developed by mice fed for 12 weeks with HFD. Their results emphasized that, after CFS administration, beneficial bacteria able to synthesize SCFAs (acetic acid and propionate) or those having antagonistic activity against pathogenic bacteria were increased, leading to an equilibrium on gut microbiota diversity. Furthermore, the aryl hydrocarbon receptor pathway was activated through important synthesized metabolites, such as glutaric acid, tryptamine, indole-3-acetic acid, and 5-hydroxyindoleacetic acid, resulting in the formation of some specific compounds having the role to suppress the pro-inflammatory factors, inflammatory mediators, quorum sensing of pathogenic Proteobacteria, and to improve insulin sensitivity, restoring the gut–liver axis activity [88,89] (Table 1, Figure 2).
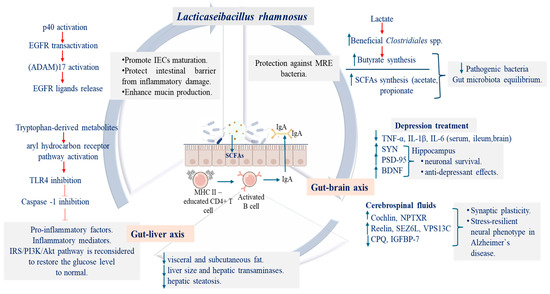
Figure 2.
Schematic representation of L. rhamnosus in vivo effects in protecting intestinal barrier and modulating the gut–liver and gut-brain axes. SCFAs-short chain fatty acids; MHC II-Major Histocompatibility Complex class II; IgA-immunoglobulin A; EGFR-Epidermal Growth Factor Receptor; (ADAM)17-A Disintegrin and Metalloproteinase 17; TLR4-toll-like receptor 4; NLRP3-Nucleotide-binding domain, Leucine-rich family, Pyrin domain containing 3; IRS/PI3K/Akt-Insulin receptor substrate/Phosphoinositide 3-kinase/Protein kinase B; TNF-tumor necrosis factor; SYN-synaptophysin PSD95-postsynaptic density protein-95 BDNF-brain-derived neurotrophic factor; NPTXR-Neuronal pentraxin receptor; SEZ6L-Seizure 6-Like Protein; VPS13C-Vacuolar Protein Sorting 13 Homolog C; CPQ-carboxypeptidase Q; IGFBP-7-Insulin-like Growth Factor-Binding Protein 7. The blue upward arrow symbol emphasizes increased synthesis or expression, while the downward decreased synthesis or expression.
In a meta-analysis study conducted by Nazarinejad et al. [90], it was highlighted that postbiotics (such as SCFAs, or heat-kill probiotics) derived from different other probiotics significantly reduced the body weight and fat, ameliorating the negative effects developed once obesity has set in, through some default mechanisms. In a similar way, LGG administrated (as probiotic or heat-inactivated) to rats fed with high-fat and high-fructose diets proved its efficacy in obesity management, decreasing the visceral and subcutaneous fat, the liver size and hepatic transaminases, while also preventing hepatic steatosis and attenuating the metabolic markers linked to the dysregulated glucose metabolism [91] (Table 1). Regarding the action mechanisms involved in obesity intervention, it seems that postbiotics administration induces downregulation of genes from adipogenesis and lipogenesis process along with an increase in expression of markers linked to lipolysis and β-oxidation. Additionally, it was observed that genes encoding key enzymes from de novo synthesis of fatty acid synthase (FAS) and acetyl-CoA carboxylase (ACC), have a decreased expression [92]. Even though, in the light of the recent findings, the therapeutic potential of L. rhamnosus derived postbiotics in managing metabolic disorders is well emphasized, future research is needed to identify which is the exact bacterial compound involved in the beneficial observed outcomes.
3.3. L. rhamnosus and Its Psychobiotic Potential
Major depressive disorder (MDD) is a mental health condition characterized by prolonged periods of sadness, dissenters, or lack of pleasure in daily activities; it is also closely related to suicide, particularly among young people. Unlike temporary emotional fluctuation, depression can deeply impact personal, social, and professional aspects of life. These conditions are more prevalent in woman than in man, with global estimates that 3.8% of the global population is affected (4% of men, 6% of women, 5.7% of older adults over the age of 60) [93,94]. Despite medication availability, there is no defined protocol for a definitive cure of depression. Also, for low- and middle-income countries, access to treatment is limited due to insufficient healthcare resources or persistent social stigma associated with mental disorders [93]. Recent studies highlighted a significant connection between gut microbiota and depression, actively mediated by the gut–brain axis. More precisely, individuals with depression exhibit distinctive alterations in gut microbiota composition, having elevated levels of certain microorganisms included into the families of Actinomycetaceae, Enterococcaceae, Leuconostocaceae, Porphyromonadaceae, and Streptococcaceae [66,95] and a higher abundance of Oscillibacter and Parabacteroides genera [96]. In the study of Bai et al. [95], the relation between gut microbiota and MDD has been explored using both liquid chromatography–mass spectrometry (LC-MS) for serum metabolites spectrum and 16S rRNA gene sequencing for gut microbial composition, on a total of 60 individuals diagnosed with MDD. The results were compared with those obtained from the health control group (HCs). The analysis revealed that of the 24 metabolites identified in the MDD group, 10 of them were linked to inflammatory processes being also involved in three inflammation-associated metabolic pathways. Further, gut microbiota analysis identified 17 genera with differential abundance, predominantly from the Firmicutes phylum, influencing four inflammation-related pathways. There were also highlighted five metabolites (LysoPC(16:0), deoxycholic acid, docosahexaenoic acid, taurocholic acid, and LysoPC(20:0)) as related inflammation biomarkers, showing a strong correlation with the Firmicutes phyla and having a high accuracy in distinguishing MDD from HCs individuals [95].
Since 2005, the use of probiotics as a complementary strategy in treating depression has gained interest due to their favorable profile, low incidence of side effects, or high tolerability [97]. From the Lactobacillus genus, L. rhamnosus has been the most extensively studied probiotic demonstrating in vivo effective actions in reducing depression symptoms (Table 1). For instance, Pan et al. [98] observed that after 3 consecutive weeks of LGG and antibiotics administration to mice chronically exposed to ethanol (CEE), the gut Lactobacillus spp. levels was restored, the interleukins and the tumor necrosis factor (TNF)-α concentrations decreased in the serum, ileum and brain, while the expression of the synaptophysin (SYN) and postsynaptic density protein-95 (PSD-95) together with the brain-derived neurotrophic factor (BDNF) increased in the hippocampus. All of these indicate protective or recovery effects of neural function [98] (Figure 3).
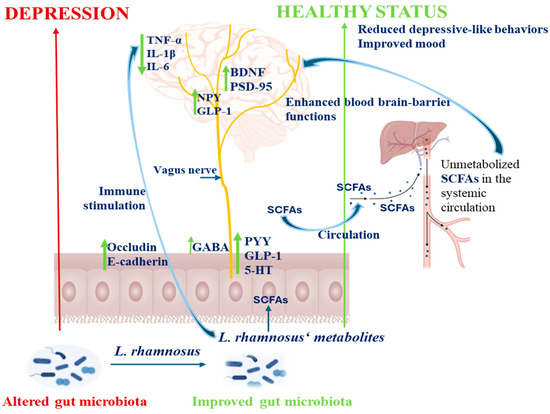
Figure 3.
Schematic representation of different molecular mechanisms (neural, endocrine, immune, and metabolic pathways) highlighting antidepressant effects of L. rhamnosus and its metabolites (SCFAs) by gut–brain axis modulation. SCFAs stimulate enteroendocrine cells to release GLP-1 and PYY, which act through vagal nerve signaling and modulate neurotransmitter systems, especially GABA serotonin pathways, leading to increase BDNF expression. SCFAs may cross the blood–brain barrier and enhance neuronal function, reduce neuroinflammation (by downregulation of TNF-α, IL-1β, IL-6) in specific brain regions, and increase the level of PSD-95, alleviating depressive behaviors, respectively. The green upward arrow symbol emphasizes increased synthesis or expression, while the downward decreased synthesis or expression.
The BDNF is an important neurotrophin family member having important roles in supporting neuronal growth, to maintain their structure and function, being expressed at high levels in hippocampus, cerebral cortex, and basal forebrain, areas which are vital for learning, memory, and thinking. In vivo studies with rats chronically subjected to stress, a significant decrease in hippocampal BDNF level was observed while following treatment with LGG, the BDNF levels were found to increase considerably [69,99] (Table 1, Figure 3). Similar results regarding upregulation of BDNF expression were obtained in depressive-like rodent models with the L. rhamnosus CCFM1228 strain [100]. Enhanced levels of brain BDNF contribute to neuronal survival and may enhance the upregulation of neurotransmitter receptors while promoting gamma aminobutyric acid (GABA) synthesis (Figure 3). This neurotrophic activity is likely to influence the regulation of receptors for serotonin, dopamine, and norepinephrine, which plays crucial roles in mitigating the symptoms of depression. Thus, the administration of LGG demonstrated antidepressant effects, and in some cases showed greater efficacy than conventional antidepressants, such as bupropion and venlafaxine [99].
In another study, Aktas et al. [101] examined the effects of L. rhamnosus E9 strain administration on dopaminergic markers and oxidative stress in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced mouse model of Parkinson’s disease (PD) (Table 1). It has been shown that after MPTP administration, the expression of dopamine receptor D1 (DR1) level significantly increased while the level of DR2 was unchanged.

Table 1.
Experimental in vivo framework detailing information of L. rhamnosus strains administration for therapeutic effects.
Table 1.
Experimental in vivo framework detailing information of L. rhamnosus strains administration for therapeutic effects.
| Experimental Information | Dosage/Administration Period | Symptoms | Results’ Summary | Reference |
|---|---|---|---|---|
| L. rhamnosus administration in obesity and related metabolic disorders | ||||
| Four-week-old male C57BL/6J mice fed with a high-fat diet (HF) | Postbiotics from L. rhamnosus HF01 strain were daily administered (0.1 mL/10 g body weight), for 12 weeks. | Obesity symptoms, such as increased body weight, high Lee index, and abdominal fat ratio, lower glucose tolerance, and alterations in the gut metabolic profile | Decreased level of lipopolysaccharides. The colon tissue integrity was preserved. The mRNA levels of NLRP3, Caspase-1, and TLR4 were inhibited. Protein expressions of Occludin, Claudin, and ZO-1 increased. The expression of genes encoding proteins involved in glucose metabolism was restored to normal levels. Liver insulin sensitivity was alleviated by remodeling the IRS/PI3K/Akt pathway. | [88] |
| 8–9-weeks-old Wistar rats | Rats fed for 6 weeks with high-fat high-fructose enriched with LGG or heat-inactivated LGG (postbiotics) (109 CFU/day). | Body fat deposition. Increase the liver size. Increase serum levels of hepatic transaminases. Lower concentration of species in the gut. | Firmicutes and Verrucomicrobia were the predominant taxa. Bacteroidetes were not found. Interaction between L. rhamnosus and Blautia glucerasea favorize the reduction of TG and TyG index, Hepatic steatosis was reduced. Inflammation in the liver decreased. Visceral fat deposition decreased. | [91] |
| L. rhamnosus administration in major depressive disorders (MDD) | ||||
| Chronic ethanol exposure (CEE) mouse model Male C57BL/6 mice (3-month-old); 12 weeks of ethanol exposure | Starting with week 11, mice received daily gavage of LGG (1 × 1010 CFU/mL, 10 μL/g) for three consecutive weeks. Then, for another three weeks, they received a cocktail of antibiotic (ampicillin, neomycin, metronidazole and vancomycin; 10 µL/g). | Anxiety, depression-like behavior with cognitive impairment | Antibiotic treatment reduced inflammation and behavioral symptoms in CEE mice. The gut microbiota in CEE mice contributed to inflammation and behavioral dysfunction. LGG treatment decreased inflammatory cytokine levels (TNF-α, IL-1β, IL-6) in both the peripheral systems and specific brain regions (striatum, amygdala, prefrontal cortex, hippocampus). LGG highlighted anti-inflammatory effects and neuroprotective potential. | [98] |
| 200 g of Wistar Albino male rats, 2-month-old. Chronic unpredictable mild stress (CUMS) protocol was applied for 8 weeks (such as, 45° cage tilt, wet sawdust for 21 h, leaving alone in dark cage for 1–2 h, immobilization at 4 °C for 3 h, food and water deprivation for 24 h, restricted access to food for 1 h, etc.) Also, moderate levels of anxiety were induced using dim light, and unfamiliar places. | Gavage with LGG in a concentration of 15 × 108 CFU/mL/day for 21 days. The effects of LGG administration were compared with those of bupropion (20 mg/kg/day) and venlafaxine (20 mg/kg/day). | Tendency to avoid luminous places and to discover new areas | LGG reduced weight loss during stress. Brain-derived neurotrophic factor level in the hippocampus significantly increased in the rat group treated with LGG compared with the control or with the stressed individuals, where its level decreased. Treatment with LGG and venlafaxine increased the gene expression of DRD1, enhanced the expression of both adrenergic receptor alpha-2A (ADRA2A) and serotonin receptor 5-HT1A. LGG was the only treatment effective in increasing the time of social interaction and locomotor activities in the stress-induced group. | [99] |
| Parkinsons’ disease | ||||
| Healthy male C57BL/6 mice of 8- to 10-week-old | 108 CFU/mL of L. rhamnosus E9 strain (isolated from healthy infant feces) by oral gavage for 15 days. For 5 consecutive days intraperitoneal injection of 30 mg/kg MPTP-HCl were administrated. | Motor dysfunction, dopaminergic damage in the mouse brain, chronic inflammatory state | MPTP injections affected the locomotor activity and exploration behavior even in the probiotic group. After, the E9 strain administration the muscle strength was preserved, and cataleptic symptoms were reduced. MPTP administration significantly decreased the tyrosine hydrolase expression and the dopamine level in the striatal tissue. The expression of dopamine transporter (DAT) also decreased in MPTP-treated mice, while D1-like receptor (DR1) expression increased. The E9 strain administration preserved DR1 and DAT expressions to the control levels, suggesting protective or stabilizing effects on dopaminergic signaling pathways. E9 may exert neuroprotective effects by attenuating the oxidative damages which were observed after MPTP administration. | [101] |
| Effects on the cerebrospinal fluid (CSF) | ||||
| Adult male Sprague Dawley® rats (250–265 g) | LGG cells concentrated in reverse osmosis water to 3.34 × 107 CFU/mL and administrated daily for 21 days. The goal was to administrate approximately 1.17 × 109 CFU of LGG per animal. | Healthy individuals | The levels of the extracellular matrix proteins from CSF (cochlin, NPTXR, reelin, Sez6l, and VPS13C) increased, while the levels of CPQ, and IGFBP-7 decreased. The relative expression of IL10 mRNA in the hippocampus also increased. The LGG administration reshapes the extracellular matrix proteins in order to support synaptic plasticity, influencing glutamatergic signaling pathway and promoting anti-inflammatory state within the CNS. | [102] |
Treatment with L. rhamnosus E9 strain maintained DR1, and dopamine transporter (DAT) expression at levels comparable with those of the healthy individuals, indicating a neuroprotective stabilization of dopaminergic pathways. Additionally, the MPTP administration determined elevated reactive oxygen species (ROS) levels, indicating major oxidative damages to proteins, membranes, and DNA, and α-synuclein misfolding in the brain, effects that were significantly attenuated after E9 strain administration [101,103]. These findings suggest that L. rhamnosus E9 administration alleviates oxidative stress and preserves dopaminergic function, highlighting its potential as therapeutic agent for PD.
Recent studies have shown that the extracellular vesicle of Lactobacillus spp., as part of their normal metabolic functioning, serve as mediators in cell–cell communication networks, having the capacity of crossing different biological barriers (blood, brain) and interacting directly with the neurons and glia cells [102]. In this context, Loupy et al. [102] highlighted that, after 21 days of LGG delivery to rats via drinking water, the levels of five proteins (cochlin, NPTXR, reelin, SEZ6L, and VPS13C) from the cerebrospinal fluids (CSF) increased while the abundance of CPQ, and IGFBP-7 decreased [102,104] (Table 1, Figure 2). These proteins are linked to synaptic plasticity and glutamatergic signaling and, together with the LGG immunoregulatory effects, may contribute to the development of a stress-resilient neural phenotype [105]. In addition, the LGG administration may modulate the main key pathways that are disrupted in Alzheimer’s disease (AD) [106]. Given the overlap with the MDD, these findings underscore the LGG strain potential as a therapeutic strategy targeting the common mechanisms shared in both disorders, MDD and AD.
4. Strategies and Systems for Efficient L. rhamnosus and Its Metabiotics Delivery for Functional Purposes
Functional foods are those that, beyond their nutritional properties, have been promoted as important providers of health benefits after consumption. Food products containing probiotics constitute a major category among functional foods, for which, due to the increasing interest in a healthy lifestyle and a high prevalence of gastrointestinal disorders, consumer demand is rapidly growing [107]. The main challenge in the development of such food products is to ensure sufficient levels of probiotic microorganisms at the end of the production process and during storage, so that they can exert the expected beneficial effects on consumers after consumption. To reach the human intestine in a concentration which exerts the probiotic effect, daily intake of probiotic microorganisms is generally recommended to be between 108 and 109 CFU, which means that the delivering food products should contain at least 106 CFU per mL or g [108].
Furthermore, in the manuscript of Guarner et al. [109], comprehensive information about the commonly used probiotic products, their constituent strains, and recommended daily intake dosage is also provided. More precisely, consumption of 50 g of probiotic cheese containing the LGG strain has been documented to treat oral candidiasis, or a dosage of 1010 CFU once per day obtained from a multi-strain probiotic product containing LGG, L. rhamnosus LC705, Propionibacterium freudenreichii ssp. shermanii JS DSM7067, and Bifidobacterium animalis ssp. lactis Bb12 DSM 15954 is recommended to improve irritable bowel syndrome symptoms, respectively.
Formulations combining live probiotics and selected prebiotics are advanced preparations defined as synbiotics which offer enhanced health benefits beyond what probiotics can achieve alone [110]. The new concept of tribiotics refers to a combined strategy that integrates the synbiotics and postbiotics into a single formula aiming to address synergistic effects on gut microbiome modulation, and therefore health benefits on the host [111]. Currently, the encapsulation of synbiotics is an emerging strategy which improves stability, viability, and targeted delivery of the two enclosed components.
L. rhamnosus has the capacity to adapt to different food matrices and to synthesize important metabolic compounds with impact on food functionality. Dairy products are, perhaps, the traditional vehicle of delivering probiotics to consumers. Fermented yogurt supplemented or not with prebiotics (yeast extract alone or in combination with inulin) and maintained under refrigeration conditions (4 °C) for 1 month, ensured a viability of L. rhamnosus GR-1 strain at a minimum of 1 × 107 CFU/mL [112]. Other studies aimed at extending the means of L. rhamnosus delivery to consumers have been focusing on obtaining functional cereal-based products. Buckwheat mashes proved to be a good substrate for facilitating LGG growth, even in co-culturing conditions with other LABs. The functional food was able to maintain a probiotic cells level higher than the minimum required throughout fermentation and storage [113]. Fermentation for 21 days of rice pudding supplemented with prebiotics, such as short and long chain inulin, or oat, resulted in a level of L. rhamnosus GR-1 cells higher than 1 × 108 CFU/mL. The addition of prebiotics, especially short chain inulin (4% w/w), increased the hedonic score of the newly developed functional product, improving its sensorial properties, such as flavor, sweetness, and texture, and overall acceptability [114]. Production of pasta and fresh noodles fortified with LGG resulted in levels of the probiotic bacteria of 7.5 log CFU/g, and 8.92 log CFU/g, respectively. Additionally, noodles boiling maintained the probiotic efficacy of the product, the concentration of LGG being reduced to 6.88 log CFU/g after 7 min of thermal treatment [115].
To limit the negative impact of certain stress factors encountered in the food matrices (fluctuating temperature, pH, relative humidity, or oxygen level) and the digestive tracts of consumers (electrolytes, gastric acid, bile salts, digestive enzymes, or antimicrobial peptides) on the level of the probiotic cells [116], different encapsulation systems have been explored (Table 2). Choosing the optimum delivery solution depends on several factors; the most important ones are the method of encapsulation, the afferent processing conditions, and the wall material composition. In turn, the encapsulation method has to be chosen according to different factors, such as capsules’ size, microparticles’ biocompatibility, and intention to be applied at a larger scale. Nonetheless, the encapsulation material must be suitable for the encapsulation technique and exhibit good film-forming ability, solubility, stability, digestibility, and releasing properties, as well as suitability for human consumption [5,117].

Table 2.
Encapsulation methods to enhance L. rhamnosus functionalities across gastrointestinal.
Among the currently available encapsulation techniques are spray drying, freeze-drying, emulsification, coacervation, liposomal delivery, and electrospraying [5].
The efficiency of the encapsulation methods applied to protect L. rhamnosus strain against the adverse conditions was reported to vary in terms of process parameters, percentage of cells entrapment, or physical properties of the capsules (moisture content, size, releasing efficiency). While freeze-drying led to a higher encapsulation percentage, electrospraying resulted in more compact and uniform microparticles, which ensured better protection of LGG against the acidity and cold temperature conditions related to yogurt storage. Moreover, the yogurt functionalized with the microcapsules obtained through electrospraying showed better sensory attributes, especially texture and taste, and higher consumer acceptability compared to the same product containing free L. rhamnosus cells or encapsulated ones by freeze-drying [125].
Co-encapsulation of L. rhamnosus with bioactive compounds as a way to simultaneously provide multiple health benefits to consumers, as well as improve the probiotic cells survivability when exposed to harsh conditions, has been also addressed. Freitas de Lima et al. [123] developed biphasic way based microparticles by spray drying LGG with β-carotene encapsulated in liposomes. The multifunctional capsules, which retained satisfactory levels of β-carotene (64.48%) and a high level of viable probiotic bacteria (>109 CFU/g) and had adequate physicochemical properties, exhibited great stability at room temperature for 90 days and important anti-inflammatory effects in Wistar rats with induced paw edema and pleurisy [123].
Co-encapsulation of probiotics with polyphenols is a promising strategy to maximize the potential of each of the microcapsules’ components. Polyphenols can protect probiotics against the unfavorable conditions met during digestion, while probiotics can contribute to the increase of the stability and bioavailability of the prebiotics [126]. Further, Camelo-Silva et al. [127] co-encapsulated L. rhamnosus with extract of beet residue (EB) for use in fortifying white chocolate. Although the multifunctional capsules showed a higher reduction in probiotics’ viability after being exposed to the simulated mouth, stomach, and ileum conditions compared to the free and encapsulated without EB ones, due to a softer texture of the chocolate, in the colon environment, after 48 h of fermentation, the cells level exceeded 11 log10 CFU/g. This spectacular proliferation of L. rhamnosus cells might be attributed to the prebiotic effect of EB, as an important fraction of the phenols is not absorbed in the small intestine and can reach the colon, where it becomes a valuable substrate for the resident microbiota [127].
Another approach to deliver L. rhamnosus simultaneously with other functional compounds is represented by the absorption of the bovine lactoferrin on the surface of the sodium alginate wall. This system was strengthened with calcium chloride and mineralized with disodium hydrogen phosphate proving an efficiency of ~95% due to the high porosity of the microcapsules and the availability of certain functional groups. The resulting capsules exhibited greater stability during simulated gastric digestion, demonstrating a lower swelling behavior than those made of sodium alginate alone or sodium alginate and calcium chloride and preserving better the viability of L. rhamnosus cells [128].
Combining the conventional wall materials with prebiotics at different proportions can increase the survivability of L. rhamnosus cells during the encapsulation process, storage, or passage through the upper gastrointestinal tract. Other used matrices for LGG encapsulation were represented by the whey protein isolate (WPI) combined with fructooligosaccharides (FOS) and galactooligosaccharides (GOS) [129], or inulin embedded in double emulsions (W/O/W), who can contribute to an extended preservation of the subcellular structures [130]. It was postulated that, under acidic conditions, inulin can form insoluble aggregates within the composite network and cover its pores, preventing the diffusion of the H+ ions within the core of the microcapsules [122].
Exclusive use of plant derived wall materials as alternatives to the frequently employed encapsulation agents, such as whey proteins or gelatin, has also led to the obtaining of promising L. rhamnosus delivery solutions. Santos et al. [124] obtained various wall material formulations by using pea protein, pectin, tapioca flour, and maltodextrin. The formulations which ensured the highest encapsulation efficiency and provided the greatest protection to LGG cells during storage and in vitro digestion consisted of different combinations and percents of maltodextrin, pea protein, tapioca flour, and pectin [124] or the carob flour with pectin [118] (Table 2).
However, limited information regarding the encapsulation of metabiotic products obtained from L. rhamnosus strains fermentation is currently presented in detail in scientific literature. In a study of particular interest, Lu et al. [131] incorporated the L. rhamnosus HN001 strain and its postbiotic products resulting from short-term fermentation into a composite film matrix composed of whey protein isolate, gum arabic, isomaltooligosaccharides, and glycerol. Their results emphasized significantly enhanced antioxidant and antimicrobial activities, along with maintaining the viability of the probiotic strain during storage at 4 °C.
When compared to each other, the encapsulation methods may present several advantages and disadvantages in terms of process duration, equipment complexity, costs, suitability for the materials to be encapsulated, and resulting capsules’ properties. Some advantages and disadvantages of the mainly employed techniques in probiotics encapsulation are illustrated in Table 3.

Table 3.
Advantages and disadvantages of current encapsulation techniques with relevance for industrial and therapeutic applications.
These findings offer new research directions for the incorporation of metabiotic products into edible films, as well as encapsulation, aiming to enhance not only the probiotics viability, but also the shelf life of foods.
5. Challenges and Limitations
Despite the researchers’ interest and effort in creating new genetically modified strains of L. rhamnosus, many limitations still block their use in the food industry sector. First of all, the literature has reported a limited number of recombinant L. rhamnosus strains due to the lack of efficient CRISPR-Cas systems, or the instability of plasmids after integration in the probiotic genome without maintaining selective pressure. On the other hand, even when the genetic modification was successful, no further research showing how these strains behave under human physiological conditions was made, including horizontal gene transfer risks, interactions with the host microbiota, or host immunity, due to the complexity of these types of experiments and the safety, regulatory, and ethical aspects. Moreover, in the process of food manufacturing, some important aspects regarding high lactose or glucose concentrations, the interaction with complex food matrices (proteins, fats, fibers), or storage under refrigeration conditions may compromise the GMO stability or minimize the fermentation rate, altering the organoleptic characteristics, which can further generate unpredictability with respect to product quality and consumer acceptance. Of course, these approaches are also necessary even for the unmodified strains when encapsulated for human consumption, to offer functional benefits. Many of the limitations about the releasing aspects of encapsulated probiotics were prior presented by [138], but additional challenges remain, particularly for L. rhamnosus strains. Precisely, long-term human studies may respond to the uncertainties regarding the colonization of encapsulated L. rhamnosus after prolonged consumption, or whether, following capsule degradation, these strains are able to remain stable under the microbiome inter-individual variability. Finally, to ensure the therapeutic effect, it is better to begin the intervention by determining the host’s exact microbiome composition, which can be assessed through next-generation sequencing (NGS), and then to stabilize an efficient and personalized probiotic/synbiotic formulation to enhance the health benefits.
6. Conclusions and Future Perspectives
By applying modern techniques like metagenomic sequencing, it is possible to select specific L. rhamnosus strains or probiotics consortia to use them in personalized formulations adapted to the specific needs of each person, considering their health profile. Beyond this, the use of genetic engineering, especially CRISPR-Cas technologies, to create new L. rhamnosus strains with enhanced traits has a high potential to revolutionize the food industry, contributing to the development of next-generation of functional foods.
These innovative gene editing technologies could also be able to design specific L. rhamnosus strains to target specific human diseases, including cancer, inflammatory disease, or neurodegenerative disorders [139,140].
On the other hand, the probiotic L. rhamnosus strains’ metabolites, as alternative promoters of health and wellbeing, require a deeper understanding of the underlying molecular mechanisms in order to prove that their safety and efficacy are comparable to those of the viable counterparts. Further, selection and metabolic activity coordination of L. rhamnosus strains for the synthesis of bioactive compounds with clinical implications, particularly on the gut–brain axis as new psychobiotic alternatives, are critical aspects to be considered in personalized health interventions.
Moreover, integrating synthetic biology approaches with encapsulation strategies will allow specific delivery of metabolites from micro- or nano-structured materials, enabling functional advantages for food products, such as improved safety, extended shelf life, or enhanced sensory and nutritional profiles. Furthermore, by combining prodrug nanomicelles with LGG, it was possible to improve probiotic survival and therapeutic efficacy in ulcerative colitis [141]. This approach opens a new direction focused on developing specialized nanocarriers for engineered probiotics to enable targeted therapies.
The bioactive compounds co-encapsulated with L. rhamnosus may modulate not only the gut microbiota composition and the immune responses, but also the interaction with the host metabolic pathways responsible for energy regulation, lipid metabolism, and inflammatory processes. Ultimately, future research should also focus on consumer perception, ethical considerations, and risk assessments under existing regulatory frameworks.
Author Contributions
Conceptualization, L.G.-G., F.I.L.-B. and G.-E.B.; Writing—original draft, L.G.-G. and F.I.L.-B.; Investigation, L.G.-G. and F.I.L.-B.; Data curation, L.G.-G. and F.I.L.-B.; Writing—review and editing, L.G.-G., F.I.L.-B. and G.-E.B.; Supervision, G.-E.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by “Dunărea de Jos” University of Galați, Romania, grant number GI 7960/2025.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
This work was supported by grant number GI 7960/2025 from “Dunărea de Jos” University of Galați, Romania. The Integrated Centre for Research, Expertise, and Technological Transfer in the Food Industry and the Microbial Resource Research Infrastructure (MIRRI) (www.mirri.org) is acknowledged for providing technical assistance, deposit, and development of probiotic cultures.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ACC | Acetyl-CoA Carboxylase |
| ALE | Adaptive Laboratory Evolution |
| ADRA2A | Adrenergic Receptor Alpha-2A |
| A/P/I | Alginate/Pectin/Inulin |
| AD | Alzheimer’s Disease |
| ADAM | A Disintegrin and Metalloproteinase |
| ARGs | Antibiotic Resistance Genes |
| BA | Bile Acids |
| BDM | Biphasic Dried Microparticles |
| BDNF | Brain-Derived Neurotrophic Factor |
| CEA | Carcinoembryonic Antigen |
| CPSs | Capsular polysaccharides |
| CXCL | Chemokine (C-X-C motif) ligand |
| CHI | Chitosan Solution |
| CEE | Chronic Ethanol Exposure |
| CUMS | Chronic Unpredictable Mild Stress |
| CFS | Cell-Free Supernatant |
| CSF | Cerebrospinal Fluids |
| CD8 | Cluster of Differentiation 8 |
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeats |
| cGAS | Cyclic GMP-AMP synthase |
| DR | Dopamine Receptor |
| DAT | Dopamine transporter |
| EGFR | Epidermal Growth Factor Receptor |
| FAS | Fatty Acid Synthase |
| FOS | Fructooligosaccharides |
| GABA | Gamma Aminobutyric Acid |
| GE | Gelatin Solution |
| HFD | High-Fat Diets |
| HB-EGF | Heparin-Binding Epidermal Growth Factor |
| ICB | Immune Checkpoint Blockade |
| IECs | Intestinal Epithelial Cells |
| IgA | Immunoglobulin A |
| IGFBP-7 | Insulin-like Growth Factor-Binding Protein 7 |
| IRS/PI3K/Akt | Insulin receptor substrate/Phosphoinositide 3-kinase/Protein kinase B |
| α-KG | α-Ketoglutarat |
| LGG | Lacticaseibacillusrhamnosus GG |
| LMVLB | Large Multivesicular Liposomes |
| LPS | Lipopolysaccharides |
| LC-MS | Liquid Chromatography-Mass Spectrometry |
| LysoPC | Lysophosphatidylcholine |
| MDD | Major Depressive Disorder |
| MGEs | Mobile Genetic Elements |
| MHC II | Major Histocompatibility Complex Class II |
| MPTP | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| MLN | Mesenteric Lymph Nodes |
| MRE | Multidrug- Resistant Enterobacteriaceae |
| NLs | Nanoliposomes |
| NPTXR | Neuronal Pentraxin Receptor |
| NLRP3 | Nucleotide-binding domain, Leucine-rich family, Pyrin domain containing 3 |
| PARP | Poly(ADP-ribose) Polymerase |
| PSD-95 | Postsynaptic Density protein-95 |
| PAM | Protospacer Adjacent Motif |
| ROS | Reactive Oxygen Species |
| SEZ6L | Seizure 6-Like Protein |
| 5-HT1A receptor | Serotonin 1A receptor |
| SBS | Short Bowel Syndrome |
| SCFAs | Short Chain Fatty Acids |
| SA | Sodium Alginate |
| SPF | Specific Pathogen-Free |
| STING | Stimulator of Interferon Genes |
| SYN | Synaptophysin |
| TyG | Triglyceride Glucose |
| TLR | Toll-Like Receptor |
| TNF | Tumor Necrosis Factor |
| VPS | Vacuolar Protein Sorting |
| W/O | Water in Oil Emulsification |
| ZO-1 | Zonula Occludens-1 |
| ZTFD | Zein/Tween-80/Fucoidan nanoparticles Dispersion |
References
- Wei, L.; Wang, B.; Bai, J.; Zhang, Y.; Liu, C.; Suo, H.; Wang, C. Postbiotics are a candidate for new functional foods. Food Chem. X 2024, 23, 101650. [Google Scholar] [CrossRef]
- Bustos, A.Y.; Taranto, M.P.; Gerez, C.L.; Agriopoulou, S.; Smaoui, S.; Varzakas, T.; Enshasy, H.A.E. Recent Advances in the Understanding of Stress Resistance Mechanisms in Probiotics: Relevance for the Design of Functional Food Systems. Probiotics Antimicrob. Prot. 2025, 17, 138–158. [Google Scholar] [CrossRef] [PubMed]
- Mathipa-Mdakane, M.G.; Thantsha, M.S. Lacticaseibacillus Rhamnosus: A Suitable Candidate for the Construction of Novel Bioengineered Probiotic Strains for Targeted Pathogen Control. Foods 2022, 11, 785. [Google Scholar] [CrossRef] [PubMed]
- Global Lactobacillus rhamnosus Market Size by Application (Probiotics Supplements, Dairy Products), by Formulation Type (Powdered Formulations, Liquid Formulations), by End-User (Hospitals, Clinics), by Distribution Channel (Supermarkets, Health Stores), by Product Type (Lactobacillus rhamnosus GG, Lactobacillus rhamnosus GR1), by Geographic Scope and Forecast. Lactobacillus rhamnosus Market Size, Consumer Behavior Insights & Forecast 2033. 2025, Report ID: 738928. Available online: https://www.verifiedmarketreports.com/product/lactobacillus-rhamnosus-market/ (accessed on 22 October 2025).
- De Deus, C.; Duque-Soto, C.; Rueda-Robles, A.; Martínez-Baena, D.; Borrás-Linares, I.; Quirantes-Piné, R.; Ragagnin De Menezes, C.; Lozano-Sánchez, J. Stability of Probiotics through Encapsulation: Comparative Analysis of Current Methods and Solutions. Food Res. Int. 2024, 197, 115183. [Google Scholar] [CrossRef]
- Douillard, F.P.; Ribbera, A.; Kant, R.; Pietilä, T.E.; Järvinen, H.M.; Messing, M.; Randazzo, C.L.; Paulin, L.; Laine, P.; Ritari, J.; et al. Comparative Genomic and Functional Analysis of 100 Lactobacillus rhamnosus Strains and Their Comparison with Strain GG. PLoS Genet. 2013, 9, e1003683. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Yang, B.; Chen, Y.; Stanton, C.; Ross, R.P.; Zhao, J.; Zhang, H.; Chen, W. Comparative Genomic Analyses of Lactobacillus rhamnosus Isolated from Chinese Subjects. Food Biosci. 2020, 36, 100659. [Google Scholar] [CrossRef]
- Xie, Z.; McAuliffe, O.; Jin, Y.-S.; Miller, M.J. Invited Review: Genomic Modifications of Lactic Acid Bacteria and Their Applications in Dairy Fermentation. J. Dairy Sci. 2024, 107, 8749–8764. [Google Scholar] [CrossRef]
- Ortiz-Velez, L.; Britton, R. Genetic Tools for the Enhancement of Probiotic Properties. Microbiol. Spectr. 2017, 5, 5.5.08. [Google Scholar] [CrossRef]
- Lebeer, S.; De Keersmaecker, S.C.J.; Verhoeven, T.L.A.; Fadda, A.A.; Marchal, K.; Vanderleyden, J. Functional Analysis of luxS in the Probiotic Strain Lactobacillus rhamnosus GG Reveals a Central Metabolic Role Important for Growth and Biofilm Formation. J. Bacteriol. 2007, 189, 860–871. [Google Scholar] [CrossRef]
- Lo, R.; Ho, V.T.T.; Bansal, N.; Turner, M.S. The Genetic Basis Underlying Variation in Production of the Flavour Compound Diacetyl by Lactobacillus rhamnosus Strains in Milk. Int. J. Food Microbiol. 2018, 265, 30–39. [Google Scholar] [CrossRef]
- Goh, Y.J.; Barrangou, R. Harnessing CRISPR-Cas Systems for Precision Engineering of Designer Probiotic Lactobacilli. Curr. Opin. Biotechnol. 2019, 56, 163–171. [Google Scholar] [CrossRef]
- Koonin, E.V.; Makarova, K.S. Origins and Evolution of CRISPR-Cas Systems. Philos. Trans. R. Soc. B 2019, 374, 20180087. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.; Barrangou, R. Applications of CRISPR-Cas Systems in Lactic Acid Bacteria. FEMS Microbiol. Rev. 2020, 44, 523–537. [Google Scholar] [CrossRef]
- Liu, T.Y.; Doudna, J.A. Chemistry of Class 1 CRISPR-Cas Effectors: Binding, Editing, and Regulation. J. Biol. Chem. 2020, 295, 14473–14487. [Google Scholar] [CrossRef]
- Shmakov, S.; Abudayyeh, O.O.; Makarova, K.S.; Wolf, Y.I.; Gootenberg, J.S.; Semenova, E.; Minakhin, L.; Joung, J.; Konermann, S.; Severinov, K.; et al. Discovery and Functional Characterization of Diverse Class 2 CRISPR-Cas Systems. Mol. Cell 2015, 60, 385–397. [Google Scholar] [CrossRef]
- Panahi, B.; Dehganzad, B.; Nami, Y. CRISPR-Cas Systems Feature and Targeting Phages Diversity in Lacticaseibacillus rhamnosus Strains. Front. Microbiol. 2023, 14, 1281307. [Google Scholar] [CrossRef]
- Pujato, S.; Galliani, V.; Irazoqui, J.M.; Amadío, A.; Quiberoni, A.; Mercanti, D. Analysis of CRISPR Systems of Types II-A, I-E and I-C in Strains of Lacticaseibacillus. Int. Dairy J. 2021, 118, 105027. [Google Scholar] [CrossRef]
- Liao, C.; Sharma, S.; Svensson, S.L.; Kibe, A.; Weinberg, Z.; Alkhnbashi, O.S.; Bischler, T.; Backofen, R.; Caliskan, N.; Sharma, C.M.; et al. Spacer Prioritization in CRISPR–Cas9 Immunity Is Enabled by the Leader RNA. Nat. Microbiol. 2022, 7, 530–541. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Liu, G.; Tang, Z.; Xiang, S.; Yang, L.; Huang, L.; He, Y.; Fan, T.; Liu, S.; Zheng, X.; et al. Efficient Plant Genome Engineering Using a Probiotic Sourced CRISPR-Cas9 System. Nat. Commun. 2023, 14, 6102. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Zhang, C.; Li, T.; Jin, F.-J.; Sung, Y.-J.; Oh, H.-M.; Lee, H.-G.; Jin, L. Development and Applications of CRISPR/Cas9-Based Genome Editing in Lactobacillus. Int. J. Mol. Sci. 2022, 23, 12852. [Google Scholar] [CrossRef]
- Xie, Z.; Jin, Y.-S.; Miller, M.J. Exploiting Endogenous CRISPR-Cas9 System for Functional Engineering of Probiotic Lacticaseibacillus rhamnosus GG (LGG). bioRxiv 2025. [Google Scholar] [CrossRef]
- Magocha, T.A.; Zabed, H.; Yang, M.; Yun, J.; Zhang, H.; Qi, X. Improvement of Industrially Important Microbial Strains by Genome Shuffling: Current Status and Future Prospects. Bioresour. Technol. 2018, 257, 281–289. [Google Scholar] [CrossRef]
- Zhang, Y.-X.; Perry, K.; Vinci, V.A.; Powell, K.; Stemmer, W.P.C.; Del Cardayré, S.B. Genome Shuffling Leads to Rapid Phenotypic Improvement in Bacteria. Nature 2002, 415, 644–646. [Google Scholar] [CrossRef] [PubMed]
- Leja, K.; Myszka, K.; Czaczyk, K. Review Paper Genome Shuffling:A Method to Improve Biotechnological Processes. BioTechnologia 2014, 92, 345–351. [Google Scholar] [CrossRef]
- Yu, L.; Pei, X.; Lei, T.; Wang, Y.; Feng, Y. Genome Shuffling Enhanced L-Lactic Acid Production by Improving Glucose Tolerance of Lactobacillus rhamnosus. J. Biotechnol. 2008, 134, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Horneck, G.; Rabbow, E. Mutagenesis by Outer Space Parameters Other than Cosmic Rays. Adv. Space Res. 2007, 40, 445–454. [Google Scholar] [CrossRef]
- Prasad, B.; Richter, P.; Vadakedath, N.; Haag, F.W.M.; Strauch, S.M.; Mancinelli, R.; Schwarzwälder, A.; Etcheparre, E.; Gaume, N.; Lebert, M. How the Space Environment Influences Organisms: An Astrobiological Perspective and Review. Int. J. Astrobiol. 2021, 20, 159–177. [Google Scholar] [CrossRef]
- Senatore, G.; Mastroleo, F.; Leys, N.; Mauriello, G. Effect of Microgravity & Space Radiation on Microbes. Future Microbiol. 2018, 13, 831–847. [Google Scholar] [CrossRef]
- Guo, S.; Sun, Y.; Wu, T.; Kwok, L.-Y.; Wang, J.; Zhang, H. Metabolic Profiling and Growth Characteristics of a Spaceflight-Induced Mutant of Lacticaseibacillus rhamnosus: Unveiling Enhanced Carbohydrate and Amino Acid Metabolism for Improved Probiotic Potential. Food Biosci. 2024, 58, 103758. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, S.; Yang, J.; Li, Y.; Sun, Z.; Kwok, L.-Y.; Sun, T.; Liu, W.; Liu, W. The Space Environment Activates Capsular Polysaccharide Production in Lacticaseibacillus rhamnosus Probio-M9 by Mutating the Wze (ywqD) Gene. Microbiol. Spectr. 2023, 11, e04677-22. [Google Scholar] [CrossRef]
- Yang, J.; Sun, Y.; Lei, X.; Zhao, L.; Luo, R.; Liu, W. Evaluation of Novel Isolates of Lacticaseibacillus rhamnosus Probio-M9 Derived through Space Mutagenesis. Food Biosci. 2023, 52, 102456. [Google Scholar] [CrossRef]
- Sun, Y.; Su, X.; Zhao, L.; Sun, T.; Liu, W. Carbon Metabolism of a Novel Isolate from Lacticaseibacillus rhamnosus Probio-M9 Derived through Space Mutant. J. Appl. Microbiol. 2024, 135, lxae205. [Google Scholar] [CrossRef]
- Sybesma, W.; Hugenholtz, J.; De Vos, W.M.; Smid, E.J. Safe Use of Genetically Modified Lactic Acid Bacteria in Food. Bridging the Gap between Consumers, Green Groups, and Industry. Electron. J. Biotechnol. 2006, 9, 424–448. [Google Scholar] [CrossRef]
- Lin, H. Screening of Lactobacillus rhamnosus Strains Mutated by Microwave Irradiation for Increased Lactic Acid Production. Afr. J. Microbiol. Res. 2012, 6, 6055–6065. [Google Scholar] [CrossRef]
- Choi, K.-H. Applications of Transposon-Based Gene Delivery System in Bacteria. J. Microbiol. Biotechnol 2009, 19, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Keightley, A.; Biswas, I. Characterization of a Stress Tolerance-defective Mutant of Lactobacillus rhamnosus LRB. Mol. Oral Microbiol. 2019, 34, 153–167. [Google Scholar] [CrossRef]
- Koraimann, G.; Wagner, M.A. Social Behavior and Decision Making in Bacterial Conjugation. Front. Cell. Infect. Microbiol. 2014, 4, 54. [Google Scholar] [CrossRef]
- Ortiz Charneco, G.; Kelleher, P.; Buivydas, A.; Streekstra, H.; Van Themaat, E.V.L.; De Waal, P.P.; Mahony, J.; Van Sinderen, D. Genetic Dissection of a Prevalent Plasmid-Encoded Conjugation System in Lactococcus lactis. Front. Microbiol. 2021, 12, 680920. [Google Scholar] [CrossRef]
- Hussain, N.; Li, R.; Takala, T.M.; Tariq, M.; Zaidi, A.H.; Saris, P.E.J. Generation of Lactose- and Protease-Positive Probiotic Lacticaseibacillus rhamnosus GG by Conjugation with Lactococcus lactis NCDO 712. Appl. Environ. Microbiol. 2021, 87, e02957-20. [Google Scholar] [CrossRef] [PubMed]
- Mavrommati, M.; Daskalaki, A.; Papanikolaou, S.; Aggelis, G. Adaptive Laboratory Evolution Principles and Applications in Industrial Biotechnology. Biotechnol. Adv. 2022, 54, 107795. [Google Scholar] [CrossRef]
- Hutkins, R.W. (Ed.) Starter Cultures. In Microbiology and Technology of Fermented Foods; Wiley: Hoboken, NJ, USA, 2006; pp. 67–106. ISBN 978-0-8138-0018-9. [Google Scholar]
- Kwon, Y.W.; Bae, J.-H.; Kim, S.-A.; Han, N.S. Development of Freeze-Thaw Tolerant Lactobacillus rhamnosus GG by Adaptive Laboratory Evolution. Front. Microbiol. 2018, 9, 2781. [Google Scholar] [CrossRef] [PubMed]
- Douillard, F.P.; Ribbera, A.; Xiao, K.; Ritari, J.; Rasinkangas, P.; Paulin, L.; Palva, A.; Hao, Y.; De Vos, W.M. Polymorphisms, Chromosomal Rearrangements, and Mutator Phenotype Development during Experimental Evolution of Lactobacillus rhamnosus GG. Appl. Env. Microbiol. 2016, 82, 3783–3792. [Google Scholar] [CrossRef]
- Mark, A.; Tosi, L.; Chkaiban, L.; Bento, R.; Parekkadan, B. In vivo Evolution of Lacticaseibacillus rhamnosus GG Leads to Prolonged Persistence in the Digestive Tract. Food Bioeng. 2025, 4, 39–52. [Google Scholar] [CrossRef]
- European Parliament and Council. Regulation (EC) No 1829/2003 of the European Parliament and of the Council of 22 September 2003 on genetically modified food and feed (Text with EEA revelance). Off. J. Eur. Union 2003, L 268, 1–23. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32003R1829 (accessed on 20 September 2025).
- Office of Science and Technology Policy. Coordinated framework for regulation of biotechnology. Fed. Regist. 1986, 51, 23302–23393. Available online: https://www.aphis.usda.gov/sites/default/files/coordinated_framework.pdf (accessed on 20 September 2025).
- Bühl, L.; Malarciuc, C.; Völlmecke, A. Transatlantic Differences in GMO Regulation. Marble 2016, 4, 147–184. [Google Scholar] [CrossRef]
- Yang, Y.T.; Chen, B. Governing GMOs in the USA: Science, Law and Public Health. J. Sci. Food Agric. 2016, 96, 1851–1855. [Google Scholar] [CrossRef]
- European Commission. GMO Authorisations for Food and Feed. European Commission–Food Safety. Available online: https://food.ec.europa.eu/plants/genetically-modified-organisms/gmo-authorisation/gmo-authorisations-food-and-feed_en (accessed on 20 December 2025).
- National Academies of Sciences, Engineering, and Medicine; Division on Earth and Life Studies; Board on Agriculture and Natural Resources. Committee on Genetically Engineered Crops: Past Experience and Future Prospects. 9 Regulation of Current and Future Genetically Engineered Crops. In Genetically Engineered Crops: Experiences and Prospects; National Academies Press: Washington, DC, USA, 2016. Available online: https://www.ncbi.nlm.nih.gov/books/NBK424533/ (accessed on 20 December 2025).
- Wei, Y.; Peng, J.; Wang, S.; Ding, Z.; Chen, G.; Sun, J. Probiotics and the Potential of Genetic Modification as a Possible Treatment for Food Allergy. Nutrients 2023, 15, 4159. [Google Scholar] [CrossRef]
- Mirsalami, S.M.; Mirsalami, M. Advances in Genetically Engineered Microorganisms: Transforming Food Production through Precision Fermentation and Synthetic Biology. Future Foods 2025, 11, 100601. [Google Scholar] [CrossRef]
- Plavec, T.V.; Berlec, A. Safety Aspects of Genetically Modified Lactic Acid Bacteria. Microorganisms 2020, 8, 297. [Google Scholar] [CrossRef]
- Del-Aguila-Arcentales, S.; Alvarez-Risco, A.; Rojas-Osorio, M.; Meza-Perez, H.; Simbaqueba-Uribe, J.; Talavera-Aguirre, R.; Mayo-Alvarez, L.; Espinoza-Ipanaque, P.; Davies, N.M.; Yáñez, J.A. Analysis of Genetically Modified Foods and Consumer: 25 Years of Research Indexed in Scopus. J. Agric. Food Res. 2025, 19, 101594. [Google Scholar] [CrossRef]
- Colautti, A.; Camprini, L.; Ginaldi, F.; Comi, G.; Reale, A.; Coppola, F.; Iacumin, L. Safety Traits, Genetic and Technological Characterization of Lacticaseibacillus rhamnosus Strains. LWT 2024, 207, 116578. [Google Scholar] [CrossRef]
- Merenstein, D.; Pot, B.; Leyer, G.; Ouwehand, A.C.; Preidis, G.A.; Elkins, C.A.; Hill, C.; Lewis, Z.T.; Shane, A.L.; Zmora, N.; et al. Emerging Issues in Probiotic Safety: 2023 Perspectives. Gut Microbes 2023, 15, 2185034. [Google Scholar] [CrossRef]
- Sahoo, D.; Rodriguez, E.; Nguyen, K.; Chintapula, U. Probiotic Bacteria as Therapeutics and Biohybrid Drug Carriers: Advances, Design Strategies, and Future Outlook. ACS Appl. Bio Mater. 2025, 8, 7513–7534. [Google Scholar] [CrossRef]
- Tóth, A.G.; Csabai, I.; Judge, M.F.; Maróti, G.; Becsei, Á.; Spisák, S.; Solymosi, N. Mobile Antimicrobial Resistance Genes in Probiotics. Antibiotics 2021, 10, 1287. [Google Scholar] [CrossRef]
- Daniali, M.; Nikfar, S.; Abdollahi, M. Antibiotic Resistance Propagation through Probiotics. Expert Opin. Drug Metab. Toxicol. 2020, 16, 1207–1215. [Google Scholar] [CrossRef]
- European Commission Health & Consumer Protection Directorate-General. Position Paper of the Scientific Committee on Animal Nutrition on Safety Assessment and Regulatory Aspects of Microorganisms in Feed and Food Applications. Available online: https://food.ec.europa.eu/system/files/2020-12/sci-com_scan-old_report_out85.pdf (accessed on 2 August 2025).
- Tynkkynen, S. Vancomycin Resistance Factor of Lactobacillus rhamnosus GG in Relation to Enterococcal Vancomycin Resistance (van) Genes. Int. J. Food Microbiol. 1998, 41, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Waśko, A.; Skrzypczak, K.; Polak-Berecka, M.; Kuzdraliński, A. Genetic Mechanisms of Variation in Erythromycin Resistance in Lactobacillus rhamnosus Strains. J. Antibiot. 2012, 65, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Drago, L.; Rodighiero, V.; Mattina, R.; Toscano, M.; De Vecchi, E. In Vitro Selection of Antibiotic Resistance in the Probiotic Strain Lactobacillus rhamnosus GG ATCC 53103. J. Chemother. 2011, 23, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Di Renzo, T.; Preziuso, M.; Zotta, T.; Iacumin, L.; Coppola, R.; Reale, A. Survey of Antibiotic Resistance Traits in Strains of Lactobacillus casei/paracasei/rhamnosus. Ann. Microbiol. 2015, 65, 1763–1769. [Google Scholar] [CrossRef]
- Zhang, B.; Lynch, B.; Zhao, J.; Guo, Y.; Mak, A. Lactobacillus rhamnosus MP108: Toxicological Evaluation. J. Food Sci. 2021, 86, 228–241. [Google Scholar] [CrossRef]
- Gouriet, F.; Million, M.; Henri, M.; Fournier, P.-E.; Raoult, D. Lactobacillus rhamnosus Bacteremia: An Emerging Clinical Entity. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 2469–2480. [Google Scholar] [CrossRef]
- Albarillo, F.; Shah, U.; Joyce, C.; Slade, D. Lactobacillus rhamnosus Infection: A Single-Center 4-Year Descriptive Analysis. J. Glob. Infect. Dis. 2020, 12, 119. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, X.; Hu, Y.; Zhang, B.; Zhang, Y.; Wang, S. Lactobacillus rhamnosus GG Alleviates β-Conglycinin-Induced Allergy by Regulating the T Cell Receptor Signaling Pathway. Food Funct. 2020, 11, 10554–10567. [Google Scholar] [CrossRef]
- Shi, J.; Dong, P.; Liu, C.; Xu, Y.; Zheng, M.; Cheng, L.; Wang, J.; Raghavan, V. Lactobacillus rhamnosus Probio-M9 Alleviates OVA-Sensitized Food Allergy through Modulating Gut Microbiota and Its Metabolism. Food Funct. 2023, 14, 10784–10795. [Google Scholar] [CrossRef]
- Smout, J.; Valentin, C.; Delbauve, S.; Pauwels, J.; Köhler, A.; Flamand, V. Maternal Lactobacillus rhamnosus Administration Impacts Neonatal CD4 T-Cell Activation and Prevents Murine T Helper 2-Type Allergic Airways Disease. Front. Immunol. 2023, 13, 1082648. [Google Scholar] [CrossRef]
- Evrard, B.; Coudeyras, S.; Dosgilbert, A.; Charbonnel, N.; Alamé, J.; Tridon, A.; Forestier, C. Dose-Dependent Immunomodulation of Human Dendritic Cells by the Probiotic Lactobacillus rhamnosus Lcr35. PLoS ONE 2011, 6, e18735. [Google Scholar] [CrossRef]
- Gao, K.; Wang, C.; Liu, L.; Dou, X.; Liu, J.; Yuan, L.; Zhang, W.; Wang, H. Immunomodulation and Signaling Mechanism of Lactobacillus rhamnosus GG and Its Components on Porcine Intestinal Epithelial Cells Stimulated by Lipopolysaccharide. J. Microbiol. Immunol. Infect. 2017, 50, 700–713. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Liu, Z.; Liu, F.; Chen, L.; Wang, W.; Ma, J.; Xu, C.; Jiang, Z.; Hou, J. Study of the Immunoregulatory Effect of Lactobacillus rhamnosus 1.0320 in Immunosuppressed Mice. J. Funct. Foods 2021, 79, 104423. [Google Scholar] [CrossRef]
- LeBlanc, J.G.; Van Sinderen, D.; Hugenholtz, J.; Piard, J.-C.; Sesma, F.; Savoy De Giori, G. Risk Assessment of Genetically Modified Lactic Acid Bacteria Using the Concept of Substantial Equivalence. Curr. Microbiol. 2010, 61, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-C.; Liu, C.-F.; Lin, T.-H.; Pan, T.-M. Safety and Risk Assessment of the Genetically Modified Lactococci on Rats Intestinal Bacterial Flora. Int. J. Food Microbiol. 2010, 142, 164–169. [Google Scholar] [CrossRef]
- Cummins, J.; Ho, M.-W. Genetically Modified Probiotics Should Be Banned. Microb. Ecol. Health Dis. 2005, 17, 66–68. [Google Scholar] [CrossRef]
- Lerner, A.; Benzvi, C.; Vojdani, A. The Potential Harmful Effects of Genetically Engineered Microorganisms (GEMs) on the Intestinal Microbiome and Public Health. Microorganisms 2024, 12, 238. [Google Scholar] [CrossRef]
- Mazhar, S.F.; Afzal, M.; Almatroudi, A.; Munir, S.; Ashfaq, U.A.; Rasool, M.; Raza, H.; Munir, H.M.W.; Rajoka, M.S.R.; Khurshid, M. The Prospects for the Therapeutic Implications of Genetically Engineered Probiotics. J. Food Qual. 2020, 2020, 9676452. [Google Scholar] [CrossRef]
- Bravo, D.; Landete, J.M. Genetic Engineering as a Powerful Tool to Improve Probiotic Strains. Biotechnol. Genet. Eng. Rev. 2017, 33, 173–189. [Google Scholar] [CrossRef]
- Jang, H.J.; Lee, N.-K.; Paik, H.-D. A Narrative Review on the Advance of Probiotics to Metabiotics. J. Microbiol. Biotechnol. 2024, 34, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Biswas, I.; Das Mohapatra, P.K. Recent Advancement in Metabiotics: A Consortium with Bioactive Molecules after Fermentation by Probiotic Bacteria with Multidisciplinary Application Potential and Future Solution in Health Sector. Bioresour. Technol. Rep. 2023, 23, 101583. [Google Scholar] [CrossRef]
- Vallejo-Cordoba, B.; Castro-López, C.; García, H.S.; González-Córdova, A.F.; Hernández-Mendoza, A. Postbiotics and Paraprobiotics: A Review of Current Evidence and Emerging Trends. Adv. Food Nutr. Res. 2020, 94, 1–34. [Google Scholar] [PubMed]
- Salva, S.; Tiscornia, I.; Gutiérrez, F.; Alvarez, S.; Bollati-Fogolín, M. Lactobacillus rhamnosus Postbiotic-Induced Immunomodulation as Safer Alternative to the Use of Live Bacteria. Cytokine 2021, 146, 155631. [Google Scholar] [CrossRef]
- Sharma, M.; Chandel, D.; Shukla, G. Antigenotoxicity and Cytotoxic Potentials of Metabiotics Extracted from Isolated Probiotic, Lactobacillus rhamnosus MD 14 on Caco-2 and HT-29 Human Colon Cancer Cells. Nutr. Cancer 2020, 72, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Shukla, G. Administration of Metabiotics Extracted from Probiotic Lactobacillus rhamnosus MD 14 Inhibit Experimental Colorectal Carcinogenesis by Targeting Wnt/β-Catenin Pathway. Front. Oncol. 2020, 10, 746. [Google Scholar] [CrossRef]
- Jalali, S.; Mojgani, N.; Sanjabi, M.R.; Saremnezhad, S.; Haghighat, S. Functional Properties and Safety Traits of L. rhamnosus and L. reuteri Postbiotic Extracts. AMB Expr. 2024, 14, 114. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, X.; Zhao, L.; Li, D.; Guan, K.; Ma, Y.; Wang, R.; Li, Q. Lacticaseibacillus rhamnosus HF01 Postbiotics Reprogram Gut Microbial Tryptophan Metabolism to Coordinate Enterohepatic Barrier-Insulin Signaling Axis. Curr. Res. Food Sci. 2025, 11, 101111. [Google Scholar] [CrossRef]
- Dang, G.; Wen, X.; Zhong, R.; Wu, W.; Tang, S.; Li, C.; Yi, B.; Chen, L.; Zhang, H.; Schroyen, M. Pectin Modulates Intestinal Immunity in a Pig Model via Regulating the Gut Microbiota-Derived Tryptophan Metabolite-AhR-IL22 Pathway. J. Anim. Sci. Biotechnol. 2023, 14, 38. [Google Scholar] [CrossRef]
- Nazarinejad, Z.; Molani-Gol, R.; Roozbeh Nasiraie, L.; Ebrahimzadeh-Attari, V. Postbiotics as a Novel Intervention for Obesity Management and Improving Metabolic Parameters: A Systematic Review and Meta-Analysis of Animal Studies. J. Transl. Med. 2025, 23, 913. [Google Scholar] [CrossRef]
- Caroline De Oliveira Melo, N.; Cuevas-Sierra, A.; Arellano-Garcia, L.; Portillo, M.P.; Milton-Laskibar, I.; Alfredo Martinez, J. Oral Administration of Viable or Heat-Inactivated Lacticaseibacillus rhamnosus GG Influences on Metabolic Outcomes and Gut Microbiota in Rodents Fed a High-Fat High-Fructose Diet. J. Funct. Foods 2023, 109, 105808. [Google Scholar] [CrossRef]
- Arellano-García, L.I.; Portillo, M.P.; Martínez, J.A.; Courtois, A.; Milton-Laskibar, I. Postbiotics for the Management of Obesity, Insulin Resistance/Type 2 Diabetes and NAFLD. Beyond Microbial Viability. Crit. Rev. Food Sci. Nutr. 2025, 65, 6209–6232. [Google Scholar] [CrossRef] [PubMed]
- Depressive Disorder (Depression). 2025. Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 20 September 2025).
- Marx, W.; Penninx, B.W.J.H.; Solmi, M.; Furukawa, T.A.; Firth, J.; Carvalho, A.F.; Berk, M. Major Depressive Disorder. Nat. Rev. Dis. Primers 2023, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Xie, J.; Bai, H.; Tian, T.; Zou, T.; Chen, J.-J. Gut Microbiota-Derived Inflammation-Related Serum Metabolites as Potential Biomarkers for Major Depressive Disorder. J. Inflamm. Res. 2021, 14, 3755–3766. [Google Scholar] [CrossRef]
- Gao, G.; Shen, S.; Zhang, T.; Zhang, J.; Huang, S.; Sun, Z.; Zhang, H. Lacticaseibacillus rhamnosus Probio-M9 Enhanced the Antitumor Response to Anti-PD-1 Therapy by Modulating Intestinal Metabolites. eBioMedicine 2023, 91, 104533. [Google Scholar] [CrossRef] [PubMed]
- Zagórska, A.; Marcinkowska, M.; Jamrozik, M.; Wiśniowska, B.; Paśko, P. From Probiotics to Psychobiotics—The Gut-Brain Axis in Psychiatric Disorders. Benef. Microbes 2020, 11, 717–732. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Guo, A.; Guan, K.; Chen, C.; Xu, S.; Tang, Y.; Li, X.; Huang, Z. Lactobacillus rhamnosus GG Attenuates Depression-like Behaviour and Cognitive Deficits in Chronic Ethanol Exposure Mice by Down-regulating Systemic Inflammatory Factors. Addict. Biol. 2024, 29, e13445. [Google Scholar] [CrossRef]
- Işık, M.; Köse, F.; Özbayer, C.; Budak, Ö.; Kaya, R.K.; Erdoğan, D.G.; Demirci, M.A.; Doğanay, S.; Bağcı, C. Promising Antidepressant Potential: The Role of Lactobacillus rhamnosus GG in Mental Health and Stress Response. Probiotics Antimicrob. Prot. 2025, 17, 5235–5265. [Google Scholar] [CrossRef]
- Xu, C.; Ma, J.; Liu, Z.; Wang, W.; Liu, X.; Qian, S.; Chen, L.; Gu, L.; Sun, C.; Hou, J.; et al. Preparation of Shell-Core Fiber-Encapsulated Lactobacillus rhamnosus 1.0320 Using Coaxial Electrospinning. Food Chem. 2023, 402, 134253. [Google Scholar] [CrossRef] [PubMed]
- Aktas, B.; Aslim, B.; Ozdemir, D.A. A Neurotherapeutic Approach with Lacticaseibacillus rhamnosus E9 on Gut Microbiota and Intestinal Barrier in MPTP-Induced Mouse Model of Parkinson’s Disease. Sci. Rep. 2024, 14, 15460. [Google Scholar] [CrossRef]
- Loupy, K.M.; Dawud, L.M.; Zambrano, C.A.; Lee, T.; Heinze, J.D.; Elsayed, A.I.; Hassell, J.E., Jr.; D’Angelo, H.M.; Frank, M.G.; Maier, S.F.; et al. Effects of Oral Administration of the Probiotic Lactobacillus rhamnosus GG on the Proteomic Profiles of Cerebrospinal Fluid and Immunoregulatory Signaling in the Hippocampus of Adult Male Rats. Neuroimmunomodulation 2025, 32, 94–109. [Google Scholar] [CrossRef]
- Jansen Van Rensburg, Z.; Abrahams, S.; Bardien, S.; Kenyon, C. Toxic Feedback Loop Involving Iron, Reactive Oxygen Species, α-Synuclein and Neuromelanin in Parkinson’s Disease and Intervention with Turmeric. Mol. Neurobiol. 2021, 58, 5920–5936. [Google Scholar] [CrossRef]
- Al Shweiki, M.R.; Oeckl, P.; Steinacker, P.; Barschke, P.; Dorner-Ciossek, C.; Hengerer, B.; Schönfeldt-Lecuona, C.; Otto, M. Proteomic Analysis Reveals a Biosignature of Decreased Synaptic Protein in Cerebrospinal Fluid of Major Depressive Disorder. Transl. Psychiatry 2020, 10, 144. [Google Scholar] [CrossRef]
- Guzman-Martinez, L.; Maccioni, R.B.; Andrade, V.; Navarrete, L.P.; Pastor, M.G.; Ramos-Escobar, N. Neuroinflammation as a Common Feature of Neurodegenerative Disorders. Front. Pharmacol. 2019, 10, 1008. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, T.; Xu, Y.; Gu, X.; Li, D.; Niu, K.; Wang, T.; Zhao, J.; Zhou, R.; Wang, H.-L. Long-Term Probiotic Intervention Mitigates Memory Dysfunction through a Novel H3K27me3-Based Mechanism in Lead-Exposed Rats. Transl. Psychiatry 2020, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Anwer, M.; Wei, M.Q. Harnessing the Power of Probiotic Strains in Functional Foods: Nutritive, Therapeutic, and next-Generation Challenges. Food Sci. Biotechnol. 2024, 33, 2081–2095. [Google Scholar] [CrossRef]
- Zommiti, M.; Feuilloley, M.G.J.; Connil, N. Update of Probiotics in Human World: A Nonstop Source of Benefactions till the End of Time. Microorganisms 2020, 8, 1907. [Google Scholar] [CrossRef] [PubMed]
- Guarner, F.; Sanders, M.E.; Szajewska, H.; Cohen, H.; Eliakim, R.; Herrera-deGuise, C.; Karakan, T.; Merenstein, D.; Piscoya, A.; Ramakrishna, B.; et al. World Gastroenterology Organisation Global Guidelines: Probiotics and Prebiotics. J. Clin. Gastroenterol. 2024, 58, 533–553. [Google Scholar] [CrossRef]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef]
- Smolinska, S.; Popescu, F.-D.; Zemelka-Wiacek, M. A Review of the Influence of Prebiotics, Probiotics, Synbiotics, and Postbiotics on the Human Gut Microbiome and Intestinal Integrity. J. Clin. Med. 2025, 14, 3673. [Google Scholar] [CrossRef]
- Hekmat, S.; Soltani, H.; Reid, G. Growth and Survival of Lactobacillus reuteri RC-14 and Lactobacillus rhamnosus GR-1 in Yogurt for Use as a Functional Food. Innov. Food Sci. Emerg. Technol. 2009, 10, 293–296. [Google Scholar] [CrossRef]
- Matejčeková, Z.; Liptáková, D.; Valík, Ľ. Functional Probiotic Products Based on Fermented Buckwheat with Lactobacillus rhamnosus. LWT—Food Sci. Technol. 2017, 81, 35–41. [Google Scholar] [CrossRef]
- Williams, M.; Hekmat, S. Lactobacillus Rhamnosus GR-1 in Fermented Rice Pudding Supplemented with Short Chain Inulin, Long Chain Inulin, and Oat as a Novel Functional Food. Fermentation 2017, 3, 55. [Google Scholar] [CrossRef]
- Wang, J.; Wu, P.; Chen, X.D.; Yu, A.; Dhital, S. Fortification of Cereal-Based Food with Lactobacillus rhamnosus GG and Bacillus coagulans GBI-30 and their Survival During Processing. Foods 2025, 14, 2250. [Google Scholar] [CrossRef]
- Castro-López, C.; Romero-Luna, H.E.; García, H.S.; Vallejo-Cordoba, B.; González-Córdova, A.F.; Hernández-Mendoza, A. Key Stress Response Mechanisms of Probiotics During Their Journey Through the Digestive System: A Review. Probiotics Antimicrob. Prot. 2023, 15, 1250–1270. [Google Scholar] [CrossRef]
- Yoha, K.S.; Nida, S.; Dutta, S.; Moses, J.A.; Anandharamakrishnan, C. Targeted Delivery of Probiotics: Perspectives on Research and Commercialization. Probiotics Antimicrob. Prot. 2022, 14, 15–48. [Google Scholar] [CrossRef]
- Tülek, E.; Karkar, B.; Şahin, S.; Yılmaz-Ersan, L.; Sucu, E. Effect of Encapsulation on the Viability of Lacticaseibacillus rhamnosus and Lacticaseibacillus paracasei During In Vitro Gastrointestinal Digestion and Storage Conditions. Food Sci. Nutr. 2025, 13, e70614. [Google Scholar] [CrossRef]
- Shoukat, L.; Javed, S.; Afzaal, M.; Akhter, N.; Shah, Y.A. Starch-Based Encapsulation to Enhance Probiotic Viability in Simulated Digestion Conditions. Int. J. Biol. Macromol. 2024, 283, 137606. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ma, Y.; Liu, Y.; Zhang, J.; Jiang, W.; Fang, X.; Wang, L. Preparation of a Lactobacillus rhamnosus ATCC 7469 Microencapsulated-Lactulose Synbiotic and Its Effect on Equol Production. Food Funct. 2024, 15, 9471–9487. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Xie, Z.; Zhao, A.; Colarelli, J.P.; Miller, M.J.; Lee, Y. Layer-by-Layer Coating of Lacticaseibacillus rhamnosus GG (LGG) Using Chitosan and Zein/Tween-80/Fucoidan Nanoparticles to Enhance LGG’s Survival under Adverse Conditions. Food Hydrocoll. 2024, 154, 110039. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, Y.; Han, Q.; Yang, H.; Cai, L.; Zhang, Y. Thermo-Kinetic Studies of in Vitro Digestion and Colonic Fermentation of Inulin/Lactobacillus rhamnosus GG Co-Encapsulated Composite Beads. Food Hydrocoll. 2024, 149, 109541. [Google Scholar] [CrossRef]
- Freitas De Lima, F.; Cesarim Mendonça, T.; Barreto Pinilla, C.M.; Dutra Alvim, I.; Alves Gragnani Vido, M.; De Paula, E.; Spadoti, L.M.; Torres Silva E Alves, A. Co-Encapsulation of Probiotic Bacteria L. rhamnosus GG and β-Carotene by a Novel Biphasic Encapsulation Technique: Stability and in vivo Anti-Inflammatory Properties. Food Biosci. 2024, 62, 105061. [Google Scholar] [CrossRef]
- Santos, N.C.; Almeida, R.L.J.; Monteiro, S.S.; De Lima, T.L.B.; Da Silva Lúcio, A.; Nogueira, L.P.D.S.; Paiva, Y.F.; Gregório, M.G.; Brito, A.C.D.O.; Da Silva, L.A.; et al. Microencapsulating Lacticaseibacillus rhamnosus GG by Spray Drying Using Pea Protein, Pectin, and Tapioca Flour: Probiotic Viability, Digestibility and Thermal Stability. Food Bioprod. Process. 2025, 150, 207–216. [Google Scholar] [CrossRef]
- Laina, K.T.; Drosou, C.; Frakolaki, G.; Krokida, M. Advancing Probiotic Delivery in Functional Yogurt: Encapsulation in Prebiotic-Based Matrices. Foods 2025, 14, 1423. [Google Scholar] [CrossRef]
- Ma, M.; Liu, Y.; Chen, Y.; Zhang, S.; Yuan, Y. Co-Encapsulation: An Effective Strategy to Enhance the Synergistic Effects of Probiotics and Polyphenols. Trends Food Sci. Technol. 2025, 158, 104927. [Google Scholar] [CrossRef]
- Camelo-Silva, C.; Mota E Souza, B.; Vicente, R.; Arend, G.D.; Sanches, M.A.R.; Barreto, P.L.M.; Ambrosi, A.; Verruck, S.; Di Luccio, M. Polyfunctional Sugar-Free White Chocolate Fortified with Lacticaseibacillus rhamnosus GG Co-Encapsulated with Beet Residue Extract (Beta vulgaris L.). Food Res. Int. 2024, 179, 114016. [Google Scholar] [CrossRef]
- Anwar, Y.; Ullah, I.; Kamal, T.; Ullah, M.W. Microencapsulation of Lacticaseibacillus rhamnosus GG for Oral Delivery of Bovine Lactoferrin: Study of Encapsulation Stability, Cell Viability, and Drug Release. Biomimetics 2022, 7, 152. [Google Scholar] [CrossRef]
- Izadi, M.; Niakousari, M.; Eskandari, M.H.; Shekarforoush, S.S.; Majdinasab, M. Microencapsulation of Lacticaseibacillus rhamnosus GG ATCC 53103 by Freeze-Drying: Evaluation of Storage Stability and Survival in Simulated Infant Gastrointestinal Digestion. Food Meas. 2024, 18, 5211–5221. [Google Scholar] [CrossRef]
- Yin, M.; Chen, L.; Chen, M.; Yuan, Y.; Liu, F.; Zhong, F. Encapsulation of Lactobacillus rhamnosus GG in Double Emulsions: Role of Prebiotics in Improving Probiotics Survival during Spray Drying and Storage. Food Hydrocoll. 2024, 151, 109792. [Google Scholar] [CrossRef]
- Lu, J.; Lu, Y.; Chang, C.; Wu, J. Novel Probiotic Fermented Edible Film Based on Gum Arabic/Whey Protein Isolate/Isomalt/Glycerol Incorporated with Lactobacillus Rhamnosus: Physical, Antibacterial, and Fresh Pork Preservation Properties. Food Hydrocoll. 2025, 161, 110875. [Google Scholar] [CrossRef]
- Padmanabhan, V.; Semwal, J.; Parvatam, G. Lyophilization and Spray-Drying of Probiotics for Oral Delivery: Advantages and Disadvantages. In Advances in Probiotic Delivery Systems; Academic Press: Cambridge, MA, USA, 2025; pp. 119–148. [Google Scholar]
- Agriopoulou, S.; Tarapoulouzi, M.; Varzakas, T.; Jafari, S.M. Application of Encapsulation Strategies for Probiotics: From Individual Loading to Co-Encapsulation. Microorganisms 2023, 11, 2896. [Google Scholar] [CrossRef]
- Camelo-Silva, C.; Verruck, S.; Ambrosi, A.; Di Luccio, M. Innovation and Trends in Probiotic Microencapsulation by Emulsification Techniques. Food Eng. Rev. 2022, 14, 462–490. [Google Scholar] [CrossRef]
- Bosnea, L.A.; Moschakis, T.; Biliaderis, C.G. Complex Coacervation as a Novel Microencapsulation Technique to Improve Viability of Probiotics Under Different Stresses. Food Bioprocess Technol. 2014, 7, 2767–2781. [Google Scholar] [CrossRef]
- Eratte, D.; Dowling, K.; Barrow, C.J.; Adhikari, B. Recent Advances in the Microencapsulation of Omega-3 Oil and Probiotic Bacteria through Complex Coacervation: A Review. Trends Food Sci. Technol. 2018, 71, 121–131. [Google Scholar] [CrossRef]
- Jia, H.; Li, Y.; Tian, Y.; Li, N.; Zheng, M.; Zhang, W.; Jiang, Y.; Zhao, Q.; Man, C. Recent Advances in Electrospray Encapsulation of Probiotics: Influencing Factors, Natural Polymers and Emerging Technologies. Crit. Rev. Food Sci. Nutr. 2025, 65, 6592–6609. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wei, S.; Wu, Y.; Fang, Y.; Deng, Z.; Xu, J.; Zhang, H. Encapsulation Techniques, Action Mechanisms, and Evaluation Models of Probiotics: Recent Advances and Future Prospects. Food Front. 2024, 5, 1212–1239. [Google Scholar] [CrossRef]
- Abouelela, M.E.; Helmy, Y.A. Next-Generation Probiotics as Novel Therapeutics for Improving Human Health: Current Trends and Future Perspectives. Microorganisms 2024, 12, 430. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, N.K.; Magdum, A.B.; Shinde, K.V.; Nimbalkar, M.S. Next-Generation Probiotics: From Traditional Strains to Personalized Therapeutics. Mol. Nutr. Food Res. 2025, 70, e70339. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, S.; Xin, R.; Hu, W.; Zhang, Q.; Lu, Q.; Han, L. Reactive Oxygen Species-Responsive Prodrug Nanomicelle-Functionalized Lactobacillus rhamnosus Probiotics for Amplified Therapy of Ulcerative Colitis. Mater. Horiz. 2025, 12, 5749–5761. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.