Abstract
Oxidative stress arises from an imbalance between reactive oxygen species (ROS) and antioxidant defense mechanisms and disrupts the structural integrity of macromolecules such as lipids, proteins, and DNA. This biochemical imbalance triggers the pathogenesis of cardiovascular and neurodegenerative diseases and leads to lipid oxidation and quality degradation in food systems. Plant-derived bioactive compounds (BACs) such as polyphenols and terpenes develop versatile molecular strategies to mitigate this oxidative damage. In addition to their direct radical scavenging effects, polyphenols stimulate the synthesis of endogenous antioxidant enzymes such as superoxide dismutase (SOD) and catalase (CAT) by activating the Nrf2–Keap1 signaling pathway. Terpenes, on the other hand, create a specialized protective shield in lipid-based matrices through “chain-breaking” reactions and a “slingshot” mechanism that externally halts the oxidation of γ-terpinene. In food engineering applications, these compounds meet the demand for “clean-label” products by providing alternatives to synthetic antioxidants such as BHA and BHT. Specific terpenes, such as carnosic acid, demonstrate higher performance in inhibiting lipid oxidation compared to their synthetic counterparts. Although BAC use extends the shelf life of products while maintaining color and flavor stability, potential interactions with protein digestibility necessitate dosage management. From a clinical perspective, these compounds suppress inflammatory responses by inhibiting the NF-κB pathway and contribute to the prevention of chronic diseases by modulating the gut microbiota. This review evaluates the capacity of BACs to manage oxidative stress in food preservation technologies and human health through a mechanistic and application-based approach.
1. Introduction
Oxidative stress results from the disruption of the balance between reactive oxygen species (ROS) and antioxidant defense mechanisms, which damages the structural integrity of lipids, proteins, and DNA. Such cellular damage has played an important role in the development of chronic diseases such as cardiovascular and neurodegenerative diseases, cancer, and diabetes.
In redox metabolic processes, reactive free radical species such as singlet oxygen (1O2), superoxide anion (O2−·), hydrogen peroxide (H2O2), or hydroxyl radical (OH·) are generated. Biomolecules from cells react strongly with these ROS, transforming into free radicals that trigger a chain reaction. As a result, the biomolecules involved in the organization and functioning of the cell are degraded. The acute and long-term action of free radicals in living tissues leads to the disruption of many metabolic pathways and modifies the properties of cellular structures with pathological consequences.
Cell membranes, which contain easily oxidizable polyunsaturated fatty acids, are the most exposed to the action of free radicals. The structural modifications of these fatty acids have the effect of changing the characteristics of the membranes, mainly their fluidity and semipermeability. Some possible consequences of tissue lipid peroxidation include changes in low-density lipoproteins (LDLs), increased risk of atheroma formation, altered platelet function, an altered arachidonic acid cascade, protein polymerization, and DNA mutations. The free radical and peroxide neutralization reactions, in which antioxidants play an important role, represent a general protective mechanism against increased lipoperoxidation, which is considered to have repercussions on health by favoring numerous pathologies.
The human body has a wide range of means to control peroxidation and rapidly inhibit, eliminate, and/or inactivate the free radical generators (Table 1). Scavenging enzymes, namely, primary and secondary antioxidants, are able to provide protection against free radical-induced degradation.

Table 1.
Antioxidant protection mechanisms existing in the human body.
The great effectiveness of antioxidants lies in their synergism, each functioning according to different mechanisms and at various levels of the chain of evolution of the free radicals in the body. Coming from food, the non-enzymatic antioxidants comprise a large group of compounds, including plant-derived bioactive compounds.
Bioactive compounds of plant origin, especially phenolic compounds (polyphenols including flavonoids) and carotenoids (terpenoid pigments), can neutralize the harmful effects of ROS and reduce the level of oxidative damage. Thus, these compounds have been reported to have a protective function in reducing the likelihood of oxidative stress-related diseases [1].
Oxidative processes pose a serious problem not only for human health but also for food quality. Lipid oxidation in foods leads to spoilage of products, loss of flavor and aroma, reduction in nutritional value, and the emergence of toxic compounds. Therefore, in the field of food engineering, it is of great importance to keep oxidation under control in order to preserve the flavor, nutritional content, and safety of products. Today, increasing consumer demand for “clean-labeled” foods strengthens the interest in the substitution of synthetic antioxidants such as BHA and BHT with natural alternatives. Plant extracts rich in polyphenols, flavonoids, and terpenoids show high antioxidant capacity in various food systems. These compounds prevent oxidative degradation of lipids and other macromolecules by neutralizing free radicals [2].
In the current review, we discuss oxidative stress, types of antioxidants, and some representatives of plant-derived bioactive compounds (BACs), namely polyphenols, flavonoids, and terpenes. For healthcare professionals, it is important to understand their mechanisms of action. For the food industry, it is important to identify sources of phytochemicals that could replace the synthetic antioxidants in order to develop new food products and understand how BACs could be effectively extracted and integrated in the food matrix. Finally, for consumers, it is important to distinguish between the scientific evidence and the commercial hype when they are choosing food based on their antioxidant content.
2. Materials and Methods
A review of the relevant literature was conducted to achieve the objectives of this study. The Boolean search strategy was used for searching the literature in the prominent databases Web of Science (WoS), Scopus, and Google Scholar. We focused on specific keywords from different groups: “plant-derived bioactive compounds”, “polyphenols”, “flavonoids”, and “terpenes” in combination with other keywords such as “antioxidants”, “mechanism of action”, “encapsulation”, and ‘’extraction’’. The Boolean operators “AND” and “OR” were employed. The interval of publication and type of publication were used as filters. All the references included in this paper were selected and reviewed in agreement with the proposed objectives.
3. The Issue of Oxidative Stress
Oxidative stress is defined as a condition that arises from the disruption of the balance between the production of reactive oxygen species (ROS) and reactive nitrogen species (RNS) within the cell and the antioxidant defense systems responsible for eliminating them, ultimately leading to cellular damage [3]. The disturbance of the equilibrium between the production of ROS and endogenous antioxidant defense systems constitutes the primary triggering mechanism that results in the loss of redox homeostasis in cells and initiates oxidative stress [4,5]. When the rate of ROS production increases due to factors such as inflammation, hyperglycemia, hypoxia, toxic agents, or mitochondrial dysfunction or when the expression and activity of antioxidant systems decrease owing to genetic, environmental, or age-related causes, this balance is disrupted, and the antioxidant capacity is exceeded [6,7]. As a consequence of this disequilibrium, cellular redox signaling is impaired, ROS levels rise beyond the physiological signaling range, and the cell becomes subjected to a pathological oxidant burden defined as oxidative stress [4,8].
When oxidative stress develops, ROS and RNS induce cumulative damage to nucleic acids, proteins, and lipids, thereby disrupting cellular integrity in multifaceted ways [9,10]. At the DNA level, oxidative modification of certain bases, single- and double-strand breaks, and replication errors emerge, increasing mutational load and predisposing to tumor development and degenerative processes [11,12]. Thiol groups in proteins undergo oxidation, carbonylation increases, and structural and functional impairments occur, leading to reduced catalytic efficiency of enzymes and altered intracellular signaling pathways [3,9]. Polyunsaturated fatty acids in cell membranes undergo lipid peroxidation, membrane fluidity and permeability are disturbed, ion balance is altered, and consequently calcium homeostasis becomes compromised, triggering cell death pathways toward necrosis or apoptosis [12,13].
Oxidative stress also plays a role in the pathophysiology of chronic diseases. In cardiovascular diseases, this process disrupts the proper functioning of vascular walls (endothelial dysfunction) [14,15], accelerates the formation of atherosclerotic plaques (arterial hardening) through the oxidation of low-density lipoproteins (LDLs) [4], and reduces nitric oxide bioavailability, thereby predisposing to hypertension and heart failure [16,17]. Similarly, in neurodegenerative disorders such as Alzheimer’s and Parkinson’s disease, oxidative stress damages vulnerable neuronal populations [16,18], enhances abnormal protein accumulations (such as amyloid and tau) [19], accelerates neuronal loss [15], and intensifies neuroinflammation (the inflammatory response within the brain) [20,21]. Thus, oxidative stress serves as a key mechanism in the onset and progression of these diseases [15,17,21].
Because oxidative stress has different intensities and manifestations in different cell types, it is crucial to study the biomarkers of oxidative stress. Five types of biomarkers related to oxidative stress have been defined, and it is useful to measure more than one biomarker to understand their clinical relevance [22].
4. Antioxidants
Modern lifestyles, including the industrialized lifestyle, expose people to a variety of exogenous harmful factors, such as air pollution, chronic stress, and unhealthy dietary habits. These factors can damage cells and lead to advancement of various maladies [23,24]. ROS generation is higher in ultra-processed food consumers and individuals living in contaminated areas [5,23]. Western diets, high-calorie diets, and low consumption of plant-based fiber cause oxidative stress and are associated with diseases [5].
Antioxidant systems are present in cells in order to protect them against ROS [24]. Antioxidants encompass a wide range of compounds, extremely diverse in biological and chemical terms [25]. Biological antioxidants avoid oxidative stress based on their capability to delay or to prevent the oxidation of a substrate.
Antioxidants or ‘’scavengers’’ exhibit activities to decrease oxidants by donating or accepting electrons to radicals, transforming themselves into less reactive radicals [5]. The scavenging ability of antioxidants against free radicals to prevent cellular damage differs from one compound to another. The antioxidant activity depends by the number of active groups (−OH or NH2) and the position of these functional groups.
Antioxidants can be classified based on different criteria. If their nature is discussed, we are talking about natural and synthetic antioxidants. The last ones include BHA, BHT, tert-butylhydroquinone (TBHQ), propyl gallate, and 4-hexylresorcinol. The natural antioxidants are classified as enzymatic and non-enzymatic antioxidants. Superoxide dismutase (SOD), catalase, glutathione reductase, and glutathione peroxidase belong to the group of enzymatic antioxidants. Glutathione and coenzyme Q are known as endogenous non-enzymatic antioxidants. Both enzymatic and non-enzymatic endogenous antioxidants are products of cellular metabolism and act to enhance cellular antioxidant defenses to reduce ROS [5].
Polyphenols, vitamins, and carotenoids are dietary/exogenous non-enzymatic antioxidants. Flavonoids, phenolic acids, stilbenes, and lignans constitute the primary classes of polyphenols [26,27]. The consumption of fruits, vegetables, and their derived products represents the main source of intake of exogenous antioxidants. Along with endogenous antioxidants, dietary antioxidants can maintain redox homeostasis, helping to maintain low ROS concentrations [5,28].
The possible action of exogenous dietary antioxidants and their positive effects on human health are still disputable due to several factors, such as an insufficient knowledge regarding the mechanisms underlying the interaction of the dietary antioxidants in the body. To achieve a precise conclusion regarding the relationship between antioxidant-containing foods and their health benefits, additional investigations and observations are still needed, while numerous challenges must be overcome [25,26,27,29]. The therapeutic effects of antioxidants depend not only on their chemical structures, which are responsible for their mechanisms of action and biochemical pathways, but also on the optimal dose and the local environment factors (i.e., pH, ROS concentration, and presence of other bioactive compounds) [25]. The source of antioxidants has an impact on their efficacy, and other nutrients from the food matrix often act synergistically with these antioxidants.
5. Plant-Derived Bioactive Compounds (BACs)
Bioactive molecules are found in high amounts in natural products and exhibit a range of potential applications. Almost all types of living beings produce BACs, including various microorganisms and animals [24].
Polyphenols, vitamins, bioactive peptides, dietary fibers, biogenic amines, and carotenoids are included in the group of plant-derived bioactive compounds. Originating from metabolism, they are generally known as secondary metabolites and recognized for their promising therapeutic properties [30].
In recent years, people have shown considerable interest in phytochemical compounds in foods, which are promoted as antioxidants and healthful agents in the popular literature, often in contrast to harmful oxidants [26]. There have been many research articles and reviews focused on various aspects of antioxidants from different dietary sources, such as antioxidant assays and in vitro and in vivo mechanisms of action.
The antioxidant activity depends on the chemical structure of the plant-based phytochemicals, which are measured in vitro using antioxidants assays sensitive to the metadata associated to the sample. Cupric ion reducing antioxidant capacity (CUPRAC), ferric reducing antioxidant power (FRAP), oxygen radical absorbance capacity (ORAC), DPPH scavenging activity, Folin–Ciocalteu reducing capacity, Trolox equivalent antioxidant capacity (TEAC), glutathione peroxidase (GSHPx) estimation, and superoxide dismutase (SOD)-based methods are examples of assays with advantages but also with some limitations if their specificity and equivalence are discussed. Advanced analytical techniques (i.e., spectroscopy and chromatography) can provide an accurate evaluation of phytochemicals in terms of content and efficacy [31].
The bioaccessibility and bioavailability of antioxidants must be taken into account to evaluate the biological activity of phytochemicals. BACs must be released from the food matrix in an absorbable form into the stomach/intestine, with the matrix interaction influencing the bioaccessibility. The bioavailability of BACs, specifically their absorption into the blood stream, can be affected by biological membranes and the biological environment inside the intestine [26].
5.1. Polyphenols as Antioxidants
Polyphenols constitute a large group of secondary plant metabolites recognized for their high antioxidant capacity and include subgroups such as phenolic acids, flavonoids, stilbenes, and tannins. These compounds contribute to the direct control of oxidative stress by neutralizing reactive oxygen species (ROS) such as hydroxyl radicals and superoxide anions before they damage cells [32].
The protective effect of polyphenols on oxidative stress is mediated through multifaceted and interconnected biochemical mechanisms. First, they show strong antioxidant properties thanks to their capacity to directly remove reactive oxygen species and nitrogen species (RNS) [33,34]. Multiple hydroxyl groups in their chemical structure play an important role in electron donation that facilitates the neutralization of free radicals [35]. In addition, polyphenols contribute to the reduction of free radical formation by chelating metal ions [34]. Their effects are not only limited to direct scavenging processes, but they also reduce ROS production by suppressing the activity of oxidative enzymes such as xanthine oxidoreductase [36]. These biochemical interactions help maintain the balance of general oxidoreductase enzyme systems [37].
Polyphenols can be classified as flavonoids and nonflavonoids [38] as shown in Figure 1.
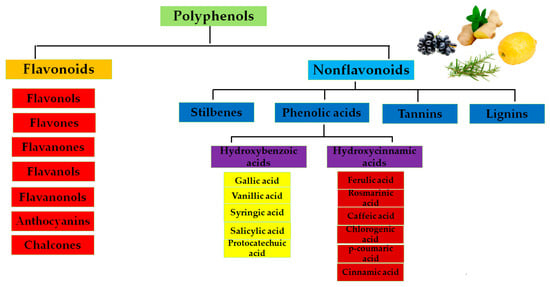
Figure 1.
Classification of polyphenols.
The processes by which antioxidants neutralize free radicals are explained through multiple reaction pathways. In hydrogen atom transfer (HAT), at certain temperatures, a storage atom is transferred in the radical direction, and the radical reactivity between them is extinguished. This pathway proceeds in a single step, and its kinetic and thermodynamic structure is mostly discussed in terms of the O–H breaking energy (BDE). The proton-coupled electron transfer (PCET) approach is based on the premise that protons and electrons appear in their modern form as a quantum-level coupled structure. In this mechanism, proton and electron motion proceed within a single coordinated framework. In sequential proton loss electron transfer (SPLET), at certain temperatures, an initial tunable, humidifying proton loss occurs, and an intermediate species is formed. This subsequently transfers an electron to the radical, strengthening the radical characteristics. This pathway is more suitable in polar solvents like water, as the solvent stabilizes the anion through solvation. In single electron transfer—proton transfer (SET-PT) pathways, electron distribution occurs before the radical transforms into a cation. Then, a more stable radical form is formed through proton loss. This pathway involves high ionization potential, and researches favoring broad polarity tend to pursue this route less frequently. This reciprocal HAT progresses with a direct product policy. SPLET is becoming more dominant in polar solvents. SET-PT is processed in a more limited number of applications due to its energy units. PCET, which is the simultaneous configuration of proton and electron transport, is given as a model [39].
Polyphenols not only exert direct antioxidant effects, but also strongly activate the natural defense mechanisms of the organism. These compounds act on intracellular signaling pathways by increasing the activity of proteins that regulate gene expression, such as Nrf2 (nuclear factor erythroid 2-related factor 2) [35,36]. Activation of the Nrf2–Keap1 pathway increases the synthesis and function of many antioxidant enzymes such as superoxide dismutase and catalase [33,40]. In particular, stimulation of the Nrf2/GPx4 pathway plays a fundamental role in cellular protection processes [41]. The glutathione peroxidase 4 (GPx4) enzyme targets lipid peroxides formed in cell membranes and ensures the reduction of these compounds. Thus, the structural integrity of the cell membrane is maintained, and the iron-dependent cell death process called ferroptosis is prevented [41].
The protective effects of polyphenols are closely related to anti-inflammatory mechanisms. These plant compounds control cellular communication pathways that initiate inflammation [35]. In particular, they inhibit the activation of proteins (transcription factors) that activate genes such as NF-κB [34]. This inhibition reduces the synthesis and release of cytokines that increase inflammation [34,36]. Polyphenols interact with key proteins involved in stress response such as PI3K–Akt and IL-17 [42]. Polyphenols exhibit anti-inflammatory effects by regulating intracellular signaling and gene expression profile [43].
Polyphenols contribute to the maintenance of mitochondrial health and function at the cellular level. They support energy production by repairing or revitalizing mitochondrial functions and reduce the levels of mitochondria-derived reactive oxygen species in this process [37,44]. Polyphenols stimulate autophagy mechanisms, enabling the clearance and recycling of damaged cellular components [44]. These integrated functions protect fats, proteins and DNA from stress-induced damage [33]. In addition, polyphenols improve nitric oxide homeostasis and contribute to the regulation of eNOS enzyme activity [36,43]. The correlation between the mechanism of action of polyphenols and their effects is shown in Table 2.

Table 2.
Different mechanisms of action of polyphenols in relation to their antioxidant properties.
5.2. Flavonoids and Oxidative Stress
Flavonoids constitute an important subgroup of polyphenols, which are highly abundant in fruits, vegetables, tea, and cocoa. Flavonols such as quercetin and flavanols such as catechins and anthocyanins are included in this group. These compounds exhibit strong antioxidant properties both directly and indirectly. Flavonoids donate electrons or hydrogen atoms to neutralize reactive oxygen species (ROS). Thus, flavonoids ensure the interruption of free radical chain reactions in cells. They also contribute to the reduction of oxidative stress by regulating the antioxidant enzyme systems of the organism [32].
Flavonoids, which are widely found in the plant kingdom and constitute one of the largest classes of phenylpropanoid derivative secondary metabolites, are defined as polyphenolic compounds with a C6-C3-C6 carbon skeleton [45]. This broad chemical family, with approximately 10,000 identified members, is divided into various subclasses such as flavones, flavonols, flavanones, flavanonols, flavanols (catechins), anthocyanins, and chalcones, depending on their structural properties, oxidation levels, and substitution patterns [46,47]. While most flavonoid classes exhibit a 2-phenylchroma structure, chalcones differ structurally from other groups because they lack a central heterocyclic ring and exhibit an open-chain structure [48]. The spectrum of biological activity exhibited by these compounds is directly related to the specific properties in their molecular structure. In particular, the hydroxyl group at the third carbon position, the double bond between the second and third carbons, and the carbonyl group at the fourth position are among the key elements that determine antioxidant capacity and free radical scavenging effects [49]. From the perspective of plant physiology, flavonoids not only attract pollinators by providing pigmentation in flowers, but also activate defense mechanisms that protect plants against various biotic and abiotic stress factors such as UV radiation, drought, salinity, and pathogen attacks [47]. When their effects on human health are examined, it is seen that dietary flavonoids reduce the risk of atherosclerosis and thrombosis by inhibiting low-density lipoprotein (LDL) oxidation and platelet aggregation, thus exhibiting a strong cardioprotective function [50]. It has been reported that phenolic compounds, which are concentrated especially in fruits and nuts of the Rosaceae family, contribute to the prevention of chronic metabolic diseases such as type 2 diabetes by regulating insulin resistance and glucose intolerance [51]. In addition, the literature highlights that flavonoids have anti-inflammatory, antiviral, and neuroprotective properties and exhibit anticancer effects through mechanisms such as cell cycle arrest or apoptosis induction [52,53]. Pharmacological research has shown that chalcone derivatives and some synthetic flavonoids, in particular, exhibit much higher antibacterial activity compared to standard antibiotics and can be effective even against bacterial strains that have developed multidrug resistance [54]. However, the variability of flavonoid concentrations in plant matrices depending on environmental factors and the diversity of their chemical structures make the isolation of these compounds difficult, and the use of optimized methods such as ultrasound-assisted solvent extraction has become a necessity for obtaining high-purity and high-yield substances [45,53].
Flavonoids are effective in keeping oxidative stress under control, mainly through their ability to directly capture free radicals [55,56]. These compounds neutralize reactive oxygen species that can damage cell structure [57]. This neutralization power of flavonoids is based on their unique chemical structure [58]. They show a pronounced binding tendency especially against hydroxyl radicals with high reactivity [59]. This feature is due to structural elements such as catechol group, conjugated double bonds between certain pairs of atoms and hydroxyl groups in their molecular structure [58,59]. In addition, some additional structures, such as a gallate group, further strengthen the free radical scavenging capacity of flavonoids when added to the molecular structure [59].
The protective effect of flavonoids against oxidative stress is not limited to the direct elimination of harmful molecules. These compounds also contribute to the regulation of endogenous and enzyme-based defense systems of the organism [56]. Flavonoids increase the activity of essential antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) [57,60]. In studies, it has been determined that certain flavonoid species, such as diosmin and diosmetin, contribute to the normal functioning of SOD and CAT enzymes that lose their function after exposure to hydrogen peroxide (H2O2), even if they do not show direct radical scavenging function [61]. This effect strengthens the defense system responsible for detoxification of reactive oxygen species [56].
This regulatory effect of flavonoids on the body’s enzymatic defense is mediated by intracellular signal transduction pathways. These compounds initiate the antioxidant response by stimulating the key transcription factor Nrf2, which controls the activation of protective genes [60,62]. Activation of the Nrf2 pathway enables the organism to synthesize more antioxidant enzymes [60]. The dual effect of flavonoids in both direct radical scavenging function and strengthening cellular defense systems helps to maintain the redox balance of cells [57]. As a result of this process, there is a decrease in the level of intracellular reactive oxygen species [63,64] and the amount of malondialdehyde (MDA), an indicator of cell membrane damage [61]. These mechanisms increase the resistance of cells to oxidative damage [55] and contribute to restore the integrity of mitochondrial membranes [64]. The action of flavonoids as antioxidants is outlined in Table 3.

Table 3.
Different mechanisms of action of flavonoids in relation to their antioxidant properties.
5.3. Terpenes and Oxidative Stress
Oxidation of lipophilic components in food reduces the quality of food and shortens its shelf life [65,66]. This process leads to the formation of peroxides in the first stage, followed by the formation of secondary harmful compounds such as malondialdehyde (MDA) [67]. Terpenes, which are considered as a natural alternative to synthetic antioxidants, have an important potential to increase the resistance of foods against oxidative deterioration [67,68]. Terpenes have the capacity to neutralize reactive oxygen species formed in oily environments through different mechanisms. In particular, essential oils and extracts obtained from plants such as thyme, hops, and rosemary significantly slow down the oxidative degradation of oils thanks to the protective effects of these components [67,69].
Terpenes are characterized as hydrocarbons with the general chemical formula (C5H8)n, which consists of repeating five-carbon (C5) isoprene units, and their oxygenated derivatives (Figure 2). One of the main mechanisms of action of terpenes is based on the “chain-breaking” process [69]. Terpene components such as carnosic acid and carnosol in rosemary extract act as free radical neutralizers in oil-based systems [67]. These natural compounds stop the chain reactions leading to oxidative degradation of oils and thus prevent the formation of harmful oxidation products [67]. In some studies, carnosic acid has shown a stronger protective effect compared to synthetic antioxidants such as BHA and BHT [68]. Another mechanism utilized by terpenes is the “singlet oxygen quenching” process. In this process, the terpene compound absorbs the excess energy of high-energy reactive oxygen and converts it into a lower-energy, stable form. Thus, the progression of oxidative degradation is prevented [65].
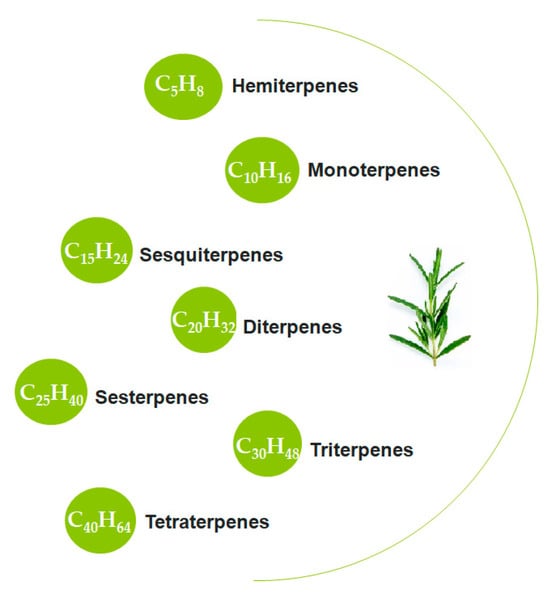
Figure 2.
Classification of terpenes.
The antioxidant defense mechanism of terpenes occurs via the Nrf2–Keap1–ARE pathway. Under normal conditions, the Nrf2 protein is retained in the cytoplasm by Keap1 and degraded via the proteasome. Terpenes cause chemical changes in the critical cysteine regions of Keap1, preventing the degradation of Nrf2. As a result of this process, Nrf2 accumulates and is transported to the nucleus. Upon reaching the nucleus, Nrf2 binds to ARE sequences and increases the transcription of antioxidant enzymes such as HO-1, NQO1, SOD, catalase, and glutathione peroxidase. Thus, cellular redox balance is maintained, oxidative stress is reduced, and inflammatory processes are suppressed [70].
Among the mechanisms of action of terpenes, more specific processes are also involved. Some terpenes terminate the degradation reaction through a method called “termination enhancing” [71]. For example, the terpene γ-terpinene shows a highly unique mode of action known as the “slingshot” mechanism [72,73]. In this process, γ-terpinene is transformed into an unstable molecule by losing a hydrogen atom in the first step [72]. This unstable molecule then undergoes a structural rearrangement into a p-cymene form and releases a small, water-soluble radical called HOO- into the environment [72,73]. The HOO- radical moves rapidly from the depths of the oil phase to the external environment and stops the progression of the reaction by interfering with the oxidative degradation chain from the outside [72]. The most remarkable aspect of this “slingshot” mechanism is that, unlike vitamin E, γ-terpinene does not acquire pro-oxidant properties even when used at high concentrations [73].
These protection mechanisms have been found to be directly applicable in food production. For example, when rosemary extract (RLE) was added to products such as salmon paste, the formation of harmful MDA and cholesterol-derived degradation products was prevented, and the natural color of the product was preserved [67]. Similarly, the addition of thyme and hops oils to peanut oil leads to a decrease in peroxide values and increases the oxidative resistance of the oil [69].
Due to the lipophilic character of terpenes, they sometimes need to be stabilized in “carrier systems” in order to function more effectively in foods. In these carrier systems, thick protective layers of biopolymers are formed around the oil droplets [66,74]. These biopolymer layers physically limit the access of oxygen and other degradation-accelerating agents to the oil, thus enhancing the protective activity of terpenes and extending the shelf life of the product [74,75]. The role of terpenes as antioxidants is shown in Table 4.

Table 4.
Different mechanisms of action of terpenes in relation to their antioxidant properties.
6. Health Benefits of Plant-Derived Bioactive Compounds
The observed antioxidant and anti-inflammatory effects of plant-derived bioactive compounds in relation to meaningful health benefits as well as their mechanism of action are illustrated in Table 5.

Table 5.
Selected health benefits of plant-derived bioactive compounds.
7. Encapsulation and Delivery Systems of Plant-Derived Bioactive Compounds
Encapsulation and delivery systems of plant-derived bioactive compounds are important advancements in improving the antioxidant effectiveness by preventing degradation throughout processing, storage, and digestion [93]. Encapsulation of polyphenols in nanocarriers such as liposomes and nanoemulsions contributes not only to their stability and bioavailability, but also to their use in food applications as natural preservatives, satisfying consumers’ demand for safer and clean-label products. Meat and dairy products as well as beverages can constitute suitable food matrices for integrating BACs as antioxidants.
In brief, encapsulation refers to entrapping one material within another, and the resulting particles are characterized by sizes ranging from a few nanometers to a few millimeters [94]. Several approaches for the encapsulation of BACs have been developed to overcome some limitations regarding their bioavailability and food application. The choice of a suitable coating material is critical in encapsulation. The material must meet certain requirements, such as mechanical resistance, protection of the encapsulated material from degradation, and enabling the regulated release of the core substance. Recent studies on polyphenol encapsulation techniques, including their related advantages and disadvantages, are synthesized in Table 6.

Table 6.
Selected studies on the encapsulation of some plant-derived bioactive compounds.
Food-grade encapsulated polyphenols have gained great attention as novel food additives, both in food production and storage. They can act as self-life enhancer type additives. Some examples are follows: anthocyanins in desserts, milk and yogurt; flavonoids in sunflower oil and beef; resveratrol in fish oil; and catechin in bacon. Encapsulated polyphenols are applied as novel enhancers of physicochemical properties based on some major properties such as emulsifying (anthocyanin in pastry cream), water retention (anthocyanin in jelly candy), gelling capacity (flavonoids in meat batter and jelly), and thickening properties (polyphenols in ice cream and yogurt). A variety of sensory enhancers is used by food producers to enhancing consumers’ perception and acceptance of foods. Encapsulated polyphenols are gaining attention in recent years as types of additives that enhance sensory properties. In practical terms, they are used as colorants (anthocyanin in pastry cream, dairy products, cookie dough, and gummy candy), flavoring agents in baking food systems, and as colorant and flavoring agents (flavonoids in cookie production). In addition, encapsulated polyphenols can act as novel nutrition/function enhancers. For example, polyphenols have been recognized as nutrition fortifiers in dairy products, snacks, and fish burgers [110].
8. Applications in Food Engineering and Health
Flavonoids and terpenes are among the main phytochemical groups that enhance both technological performance and biofunctional properties in food engineering. Thanks to their antioxidant and antimicrobial properties, these compounds slow down lipid oxidation, contribute to the preservation of color and aroma, and extend the shelf life of products. These effects are particularly pronounced in meat, fish, and oil-based food matrices, where oxidative degradation is inhibited [2]. Polyphenols and flavonoids play an important role in reducing oxidative stress-induced cellular damage, regulating the immune response, and promoting human health, partly through antihypertensive mechanisms. Current research suggests that these compounds enhance product safety through their bioprotective properties and offer potential for functional food development strategies [111,112]. It has been determined that terpenes and polyphenols constitute an alternative to synthetic additives as natural preservative components and can be used to develop a preservation approach in line with consumer expectations [113].
A significant part of the technological value of these compounds comes from the rational choice of processing and extraction strategies (Figure 3). Modern technologies such as high hydrostatic pressure, pulsed electric fields, ultrasound, and ohmic heating increase cell wall permeability and facilitate the liberation of bound phenolic compounds. This process also contributes to the preservation of the total amount of phenolic compounds [114]. The adoption of optimization-oriented process designs with the use of green solvents helps to reduce the environmental burden and increases the yield [31,115]. Enzymatic hydrolysis and fermentation applications reduce bioaccessibility limitations and transform phenolic compounds from a bound form into more easily absorbable structures [116]. In addition, encapsulation methods and biopolymer-based carrier systems maintain the stability of compounds under gastrointestinal conditions and offer controlled release to the target site [116]. Engineering-based design of polyphenol–protein complexes increases the stability and target transportability of these compounds, thus strengthening the effectiveness of their functional effects [117].
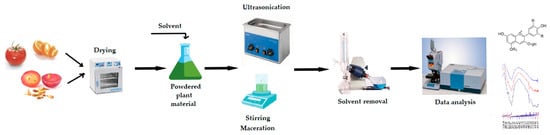
Figure 3.
Extraction of plant-derived bioactive compounds. The direction of the arrows indicates the order of the various operations needed for the extraction and analysis of bioactive compounds. In brief, the plant material is subjected to drying under conditions that protect the compounds of interest, which are heat-sensitive (e.g., by lyophilization). Liquid solvents (water, alcohol, glycerin, etc.) are used and the extraction methods applied include classical techniques (maceration) or/and modern techniques (e.g., ultrasonication). The extracts are subjected to solvent removal stages, such as (micro)filtration, centrifugation or solvent vacuum evaporation. Various techniques ranging from spectroscopic methods to novel chromatographic techniques are employed further, for the identification and quantification of the bioactive compounds by interest.
Polyphenol–protein interactions at the formulation level influence not only bioavailability but also sensory and structural parameters. Polyphenols pre-aggregated with proteins prolong the half-life of foam drainage, increase structural strength, and contribute to a reduced perception of shrinkage. This also positively affects the flavor acceptance of the product, and this complexation mechanism may even lead to the masking of certain allergenic epitopes, leading to a decrease in allergenicity indicators [118]. However, in some matrices, polyphenols can have binding effects on protein digestibility and mineral absorption, so dosage and carrier system design should be carefully managed [119]. It has been reported that polyphenol–protein conjugates maintain their functionality through controlled release and resistance to oxidative degradation mechanisms, thus providing a balanced optimization between shelf life and biofunctional activity [117,120]. These approaches are considered as important formulation strategies that support the sustainability of the biological effect while maintaining the targeted sensory profile in food products [118].
In terms of health-oriented contributions, these compounds have a wide range of effects. Polyphenols and flavonols contribute to the strengthening of the antioxidant defense system and maintenance of mucosal immune balance by regulating the composition and metabolic activities of the gut microbiota [121]. Biofortification applications and genomics-based agricultural strategies enable the development of polyphenol-rich food raw materials and provide a scientific basis for nutritional solutions, especially for the reduction of intestinal inflammatory processes [122]. In vitro studies and early clinical data have shown that polyphenols have antimicrobial effects as well as inhibition potential on specific viral targets, providing dual benefits in terms of both food safety and consumer health [111]. The sustainable production of terpenes using yeast-based metabolic engineering approaches increases the supply of natural preservative resources and provides a higher level of flexibility to formulation processes [113]. This holistic picture (Figure 4) suggests that the combined evaluation of process and carrier system designs may contribute to the improvement of bioaccessibility, thereby reducing clinical effect differences and strengthening the scientific basis of functional foods [116,123].
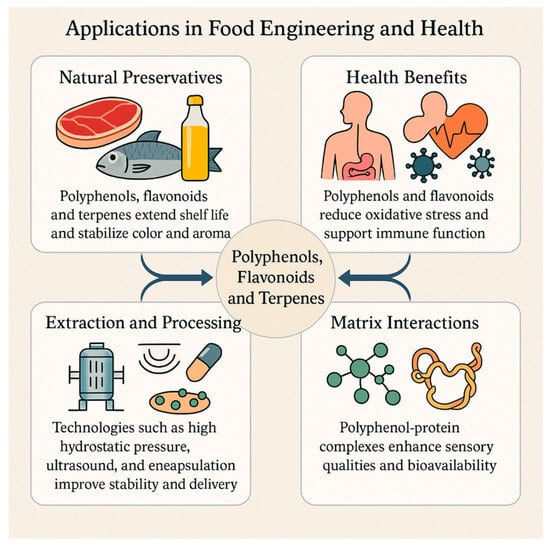
Figure 4.
Applications of polyphenols, flavonoids and terpenes in food engineering and health.
In conclusion, polyphenols, flavonoids, and terpenes have important functions in delaying oxidative and microbial degradation in food systems, preserving tissue integrity and sensory properties, as well as favorable effects on gut microbiota, inflammation processes, and cardiometabolic indicators. Process innovations and green extraction approaches enable efficient transport and controlled release of these compounds by maintaining their stability throughout the product life cycle [31,114]. Encapsulation systems with protein-based complexes contribute to the management of matrix interactions and thus offer strategic advantages in terms of both technological efficiency and biofunctional performance [118,120]. Rational management of dosage, safety parameters, and possible anti-nutritional effects is critical for the sustainability of applications and the continuity of consumer benefit [117,119]. The scaling up of natural preservative production through biotechnological methods and the efficient utilization of agricultural by-products strengthen industrial viability and demonstrate a structural fit with circular economy principles [112,113].
9. Conclusions
Oxidative stress is a fundamental mechanism that threatens both human health and food quality as an imbalance between reactive oxygen species (ROS) and antioxidant capacity. In human physiology, it leads to the damage of cellular macromolecules (lipids, proteins, DNA) and paves the way for chronic conditions such as neurodegenerative and cardiovascular diseases. In the food industry, oxidative processes, especially through lipid oxidation, cause a reduction in the shelf life of products, unwanted taste and odor development, and the loss of nutritional value. Plant-derived bioactive compounds such as polyphenols, flavonoids, and terpenes play important roles in both food preservation and physiological health protection by providing powerful natural defense mechanisms against this bidirectional oxidative damage.
The activity of these bioactive compounds is based on multiple and interrelated biochemical pathways. Polyphenols and flavonoids have the capacity to directly scavenge free radicals and donate electrons due to their chemical structure (e.g., catechol groups). However, their activity is further enhanced by indirect mechanisms: They enhance the body’s own antioxidant enzymes (SOD, CAT, GPx4) by activating the Nrf2–Keap1 signaling pathway and suppress inflammatory responses through NF-kB inhibition. Terpenes, especially in lipophilic (oily) food matrices, effectively stop oxidative degradation through specialized methods such as chain-breaking reactions mediated by compounds such as carnosic acid and the “slingshot” mechanism of γ-terpinene. Specifically, while polyphenols and flavonoids primarily utilize enzymatic modulation and direct scavenging, terpenes employ distinct physical and chemical shielding strategies in lipid matrices, demonstrating how molecular mechanisms differ across bioactive classes.
From a food engineering perspective, these plant-derived bioactive compounds (BPCs) represent a powerful alternative to synthetic antioxidants (BHA and BHT), meeting consumer demands for “clean-labeled” products. In terms of effectiveness and safety, natural compounds such as carnosic acid compare favorably to their synthetic counterparts, offering potent oxidative protection without the potential health risks associated with synthetic antioxidants. They retard lipid peroxidation and the formation of secondary spoilage products such as malondialdehyde (MDA) in meat, fish, and oil-based products, preserving the flavor, color, and nutrient profile of foods. The activity of these compounds in food matrices is enhanced by modern processing and formulation techniques such as encapsulation, biopolymer carriers, and the formation of polyphenol–protein complexes. These approaches optimize the stability, bioaccessibility, and sensory acceptance of BACs. However, while encapsulation and carrier systems enhance functional stability and sensory properties, they may present disadvantages such as interactions with protein digestibility, necessitating careful dosage management.
In conclusion, flavonoids and terpenes are versatile compounds that offer protective effects on human health while enhancing stability in food systems. Beyond their technological applications, these compounds also have the potential to modulate gut microbiota and support immune balance. To translate preclinical findings into meaningful health benefits for human populations, particularly in neurodegenerative diseases, strategies to overcome variations in bioaccessibility and enhance absorption in the gastrointestinal tract must be prioritized. Future research should focus on the optimization of these delivery systems to improve bioaccessibility and thus clinical efficacy. Furthermore, the development of green extraction methods such as high hydrostatic pressure or ultrasound and the utilization of agricultural by-products (e.g., food processing wastes) as a source for these valuable compounds will secure the sustainable use of BACs in the food and health fields. These sustainable production and extraction methods align with circular economy principles by valorizing waste streams, thereby significantly reducing the environmental impact of food production.
Author Contributions
Conceptualization, A.T. and C.L.B.; methodology, A.T.; software, A.T.; validation, C.L.B.; formal analysis, C.L.B.; investigation, A.T.; data curation, A.T.; writing—original draft preparation, A.T.; writing—review and editing, A.T.; visualization, A.T. and C.L.B.; supervision, C.L.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chandimali, N.; Bak, S.G.; Park, E.H.; Lim, H.-J.; Won, Y.-S.; Kim, E.-K.; Prak, S.-Y.; Lee, S.J. Free radicals and their impact on health and antioxidant defences: A review. Cell Death Discov. 2025, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-del-Río, I.; López-Ibáñez, S.; Magadán-Corpas, P.; Fernández-Calleja, L.; Pérez-Valero, Á.; Tuñón-Granda, M.; Miguelez, E.M.; Villar, C.J.; Lombó, F. Terpenoids and polyphenols as natural antioxidant agents in food preservation. Antioxidants 2021, 10, 1264. [Google Scholar] [CrossRef] [PubMed]
- Pruchniak, M.P.; Araźna, M.; Demkow, U. Biochemistry of oxidative stress. In Advances in Clinical Science; Springer: Cham, Switzerland, 2015; pp. 9–19. [Google Scholar]
- Garcia-Llorens, G.; El Ouardi, M.; Valls-Belles, V. Oxidative Stress Fundamentals: Unraveling the Pathophysiological Role of Redox Imbalance in Non-Communicable Diseases. Appl. Sci. 2025, 15, 10191. [Google Scholar] [CrossRef]
- Aranda-Rivera, A.K.; Cruz-Gregorio, A.; Arancibia-Hernández, Y.L.; Hernández-Cruz, E.Y.; Pedraza-Chaverri, J. RONS and oxidative stress: An overview of basic concepts. Oxygen 2022, 2, 437–478. [Google Scholar] [CrossRef]
- Afzal, S.; Abdul Manap, A.S.; Attiq, A.; Albokhadaim, I.; Kandeel, M.; Alhojaily, S.M. From imbalance to impairment: The central role of reactive oxygen species in oxidative stress-induced disorders and therapeutic exploration. Front. Pharmacol. 2023, 14, 1269581. [Google Scholar] [CrossRef]
- Anwar, H.; Nawaz, L.; Saddiqa, A.; Shaheen, S.; Muzaffar, H.; Ijaz, M.U.; Mukhtaar, I. Oxidative Stress as a Triggering Mechanism of Various Diseases. In The Role of Natural Antioxidants in Brain Disorders; Springer International Publishing: Cham, Switzerland, 2023; pp. 71–87. [Google Scholar]
- Poljšak, B.; Jamnik, P.; Milisav, I. The importance of multifaceted approach for accurate and comprehensive evaluation of oxidative stress status in biological systems. Antioxidants 2025, 14, 1083. [Google Scholar] [CrossRef]
- Silva, J.P.; Coutinho, O.P. Free radicals in the regulation of damage and cell death—Basic mechanisms and prevention. Drug Discov. Ther. 2010, 4, 144–167. [Google Scholar]
- Skoryk, O.D.; Horila, M.V. Oxidative stress and disruption of the antioxidant defense system as triggers of diseases. Regul. Mech. Biosyst. 2023, 14, 665–672. [Google Scholar] [CrossRef]
- Corrales, L.C.; Muñoz Ariza, M.M. Oxidative Stress: Origin, evolution and consequences of oxygen toxicity. Nova 2012, 10, 213–225. [Google Scholar] [CrossRef]
- Camila Alcalde, M.; Edmo Atique, G.; de Mello, M.A.J. Oxidative Stress: Cause Mechanisms and Defense Agents. Cardiol. Vasc. Res. 2019, 3, 1–4. [Google Scholar] [CrossRef]
- Berakdar, N.O.U.R.A.; Alahmad, A. Review of oxidative stress and antioxidative. J. Clin. Diagn. Res. 2022, 16, BE01–BE06. [Google Scholar] [CrossRef]
- García-Sánchez, A.; Miranda-Díaz, A.G.; Cardona-Muñoz, E.G. The role of oxidative stress in physiopathology and pharmacological treatment with pro-and antioxidant properties in chronic diseases. Oxidative Med. Cell. Longev. 2020, 1, 2082145. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, J.; Wang, Y.; Deng, F.; Deng, Z. Oxidative Stress: Signaling Pathways, Biological Functions, and Disease. MedComm 2025, 6, e70268. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, oxidative stress, and antioxidants: Back and forth in the pathophysiology of chronic diseases. Front. Physiol. 2020, 11, 552535. [Google Scholar] [CrossRef] [PubMed]
- Al-Kufaishi, A.M.; Al-Musawi, N.J. Oxidative Stress and Related Diseases: A Comprehensive Review. JPDTSM 2025, 4, 65–70. [Google Scholar] [CrossRef]
- Chong, Z.Z.; Souayah, N. Oxidative Stress: Pathological Driver in Chronic Neurodegenerative Diseases. Antioxidants 2025, 14, 696. [Google Scholar] [CrossRef]
- Reddy, V.P. Oxidative stress in health and disease. Biomedicines 2023, 11, 2925. [Google Scholar] [CrossRef]
- Altanam, S.Y.; Darwish, N.; Bakillah, A. Exploring the Interplay of Antioxidants, Inflammation, and Oxidative Stress: Mechanisms, Therapeutic Potential, and Clinical Implications. Diseases 2025, 13, 309. [Google Scholar] [CrossRef]
- Francati, S.; Fiore, M.; Ferraguti, G. The janus face of oxidative stress in health and disease: The cause or the cure? Biomed. Rev. 2023, 34, 13–26. [Google Scholar] [CrossRef]
- Sies, H. Oxidative Stress: Concept and Some Practical Aspects. Antioxidants 2020, 9, 852. [Google Scholar] [CrossRef]
- Jawhara, S. How Do Polyphenol-Rich Foods Prevent Oxidative Stress and Maintain Gut Health? Microorganisms 2024, 12, 1570. [Google Scholar] [CrossRef] [PubMed]
- Chandra, P.; Sharma, R.K.; Arora, D.S. Antioxidant compounds from microbial sources: A review. Food Res. Int. 2020, 129, 108849. [Google Scholar] [CrossRef] [PubMed]
- Hyży, A.; Rozenek, H.; Gondek, E.; Jaworski, M. Effect of Antioxidants on the Gut Microbiome Profile and Brain Functions: A Review of Randomized Controlled Trial Studies. Foods 2025, 14, 176. [Google Scholar] [CrossRef] [PubMed]
- Kotha, R.R.; Tareq, F.S.; Yildiz, E.; Luthria, D.L. Oxidative Stress and Antioxidants—A Critical Review on In Vitro Antioxidant Assays. Antioxidants 2022, 11, 2388. [Google Scholar] [CrossRef]
- Mohammadi, N.; Farrell, M.; O’Sullivan, L.; Langan, A.; Franchin, M.; Azevedo, L.; Granato, D. Effectiveness of anthocyanin-containing foods and nutraceuticals in mitigating oxidative stress, inflammation, and cardiovascular health-related biomarkers: A systematic review of animal and human interventions. Food Funct. 2024, 15, 3274. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Poljsak, B.; Kovac, V.; Milisav, I. Antioxidants, Food Processing and Health. Antioxidants 2021, 10, 433. [Google Scholar] [CrossRef]
- Samtiya, M.; Aluko, R.E.; Dhewa, T.; Moreno-Rojas, J.M. Potential Health Benefits of Plant Food-Derived Bioactive Components: An Overview. Foods 2021, 10, 839. [Google Scholar] [CrossRef]
- Sun, S.; Liu, Z.; Lin, M.; Gao, N.; Wang, X. Polyphenols in health and food processing: Antibacterial, anti-inflammatory, and antioxidant insights. Front. Nutr. 2024, 11, 1456730. [Google Scholar] [CrossRef]
- Bińkowska, W.; Szpicer, A.; Stelmasiak, A.; Wojtasik-Kalinowska, I.; Półtorak, A. Microencapsulation of Polyphenols and Their Application in Food Technology. Appl. Sci. 2024, 14, 11954. [Google Scholar] [CrossRef]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary polyphenols and their role in oxidative stress-induced human diseases: Insights into protective effects, antioxidant potentials and mechanism(s) of action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative stress and inflammation: What polyphenols can do for us? Oxidative Med. Cell. Longev. 2016, 1, 7432797. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Kerimi, A.; Williamson, G. At the interface of antioxidant signalling and cellular function: Key polyphenol effects. Mol. Nutr. Food Res. 2016, 60, 1770–1788. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Luo, M.; Wei, S. The bioprotective effects of polyphenols on metabolic syndrome against oxidative stress: Evidences and perspectives. Oxidative Med. Cell. Longev. 2019, 2019, 6713194. [Google Scholar] [CrossRef]
- Villalobos-Delgado, L.H.; Nevárez-Moorillon, G.V.; Caro, I.; Quinto, E.J.; Mateo, J. Natural antimicrobial agents to improve foods shelf-life. In Food Quality and Shelf-Life; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 125–157. [Google Scholar]
- Markovic, Z.; Jeremic, S.; Dimitric Markovic, J.; Stanojevic Pirkovic, M.; Amic, D. Influence of structural characteristics of substituents on the antioxidant activity of some anthraquinone derivatives. Comput. Theor. Chem. 2016, 1077, 25–31. [Google Scholar] [CrossRef]
- Andriantsitohaina, R.; Auger, C.; Chataigneau, T.; Étienne-Selloum, N.; Li, H.; Martínez, M.C.; Laher, I. Molecular mechanisms of the cardiovascular protective effects of polyphenols. Br. J. Nutr. 2012, 108, 1532–1549. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, J.; Yu, Y.; Liu, X.; Shang, X.; Du, Z.; Xu, M.; Zhang, T. Recent advances in the inhibition of membrane lipid peroxidation by food-borne plant polyphenols via the nrf2/gpx4 pathway. J. Agric. Food Chem. 2024, 72, 12340–12355. [Google Scholar] [CrossRef]
- Wu, F.; Lin, B.; Chen, J.; Zheng, F.; Yang, Y.; Rasheed, U.; Chen, G. Mechanistic Insights into the Antioxidant Potential of Sugarcane Vinegar Polyphenols: A Combined Approach of DPPH-UPLC-MS, Network Pharmacology and Molecular Docking. Foods 2024, 13, 3379. [Google Scholar] [CrossRef]
- Gormaz, J.G.; Valls, N.; Sotomayor, C.; Turner, T.; Rodrigo, R. Potential role of polyphenols in the prevention of cardiovascular diseases: Molecular bases. Curr. Med. Chem. 2016, 23, 115–128. [Google Scholar] [CrossRef]
- García-Aguilar, A.; Palomino, O.; Benito, M.; Guillén, C. Dietary polyphenols in metabolic and neurodegenerative diseases: Molecular targets in autophagy and biological effects. Antioxidants 2021, 10, 142. [Google Scholar] [CrossRef] [PubMed]
- Santos-Buelga, C.; San Feliciano, A. Flavonoids: From Structure to Health Issues. Molecules 2017, 22, 477. [Google Scholar] [CrossRef]
- Dixon, R.A.; Pasinetti, G.M. Flavonoids and Isoflavonoids: From Plant Biology to Agriculture and Neuroscience. Plant Physiol. 2010, 154, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, W.-B.; Li, Y.-H.; Shu, X.-C.; Pu, Y.-T.; Wang, X.-J.; Wang, T.; Wang, Z. The Classification, Molecular Structure and Biological Biosynthesis of Flavonoids, and Their Roles in Biotic and Abiotic Stresses. Molecules 2023, 28, 3599. [Google Scholar] [CrossRef] [PubMed]
- Iwashina, T. The Structure and Distribution of the Flavonoids in Plants. J. Plant Res. 2000, 113, 287–299. [Google Scholar] [CrossRef]
- Cook, N.C.; Samman, S. Flavonoids—Chemistry, metabolism, cardioprotective effects, and dietary sources. J. Nutr. Biochem. 1996, 7, 66–76. [Google Scholar]
- Khan, J.; Deb, P.K.; Priya, S.; Medina, K.D.; Devi, R.; Walode, S.G.; Rudrapal, M. Dietary Flavonoids: Cardioprotective Potential with Antioxidant Effects and Their Pharmacokinetic, Toxicological and Therapeutic Concerns. Molecules 2021, 26, 4021. [Google Scholar] [CrossRef]
- Ogah, O.; Watkins, C.S.; Ubi, B.E.; Oraguzie, N.C. Phenolic Compounds in Rosaceae Fruit and Nut Crops. J. Agric. Food Chem. 2014, 62, 9369–9386. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef]
- Liga, S.; Paul, C.; Peter, F. Flavonoids: Overview of Biosynthesis, Biological Activity, and Current Extraction Techniques. Plants 2023, 12, 2732. [Google Scholar] [CrossRef]
- Farhadi, F.; Khameneh, B.; Iranshahi, M.; Iranshahy, M. Antibacterial activity of flavonoids and their structure-activity relationship: An update review. Phytother. Res. 2019, 33, 13–40. [Google Scholar] [CrossRef]
- Assalve, G.; Lunetti, P.; Zara, V.; Ferramosca, A. In vivo antioxidant activity of common dietary flavonoids: Insights from the yeast model Saccharomyces cerevisiae. Antioxidants 2024, 13, 1103. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.; Janmeda, P.; Docea, A.O.; Yeskaliyeva, B.; Abdull Razis, A.F.; Modu, B.; Calina, D.; Sharifi-Rad, J. Oxidative stress, free radicals and antioxidants: Potential crosstalk in the pathophysiology of human diseases. Front. Chem. 2023, 11, 1158198. [Google Scholar] [CrossRef] [PubMed]
- Ahmadinejad, F.; Geir Møller, S.; Hashemzadeh-Chaleshtori, M.; Bidkhori, G.; Jami, M.S. Molecular mechanisms behind free radical scavengers function against oxidative stress. Antioxidants 2017, 6, 51. [Google Scholar] [CrossRef] [PubMed]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Treml, J.; Šmejkal, K. Flavonoids as potent scavengers of hydroxyl radicals. CRFSFS 2016, 15, 720–738. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several lines of antioxidant defence against oxidative stress: Antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef]
- Wójciak, M.; Feldo, M.; Borowski, G.; Kubrak, T.; Płachno, B.J.; Sowa, I. Antioxidant potential of diosmin and diosmetin against oxidative stress in endothelial cells. Molecules 2022, 27, 8232. [Google Scholar] [CrossRef]
- Kim, T.Y.; Leem, E.; Lee, J.M.; Kim, S.R. Control of reactive oxygen species for the prevention of Parkinson’s disease: The possible application of flavonoids. Antioxidants 2020, 9, 583. [Google Scholar] [CrossRef]
- Delgado, L.; González-Paramás, A.M.; González-Manzano, S.; Ayuda-Durán, B.; Santos-Buelga, C. Influence of flavonoids in ROS production and oxidative DNA damage in Caenorhabditis elegans submitted to thermal stress. Planta Med. 2014, 80, P2O5. [Google Scholar] [CrossRef]
- Zheng, Q.; Feng, K.; Zhong, W.; Tan, W.; Rengaowa, S.; Hu, W. Investigating the hepatoprotective properties of mulberry leaf flavonoids against oxidative stress in HepG2 cells. Molecules 2024, 29, 2597. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Zhong, Y. Lipid oxidation and improving the oxidative stability. Chem. Soc. Rev. 2010, 39, 4067–4079. [Google Scholar] [CrossRef] [PubMed]
- Keramat, M.; Ehsandoost, E.; Golmakani, M.T. Recent trends in improving the oxidative stability of oil-based food products by inhibiting oxidation at the interfacial region. Foods 2023, 12, 1191. [Google Scholar] [CrossRef] [PubMed]
- Cedeño-Pinos, C.; Martínez-Tomé, M.; Mercatante, D.; Rodríguez-Estrada, M.T.; Bañón, S. Assessment of a diterpene-rich rosemary (Rosmarinus officinalis L.) extract as a natural antioxidant for salmon pate formulated with linseed. Antioxidants 2022, 11, 1057. [Google Scholar] [CrossRef]
- Qi, Y.; Huang, Y.; Dong, Y.; Zhang, W.; Xia, F.; Bai, H.; Stevanovic, Z.D.; Li, H.; Shi, L. Effective improvement of the oxidative stability of Acer truncatum bunge seed oil, a new woody oil food resource, by rosemary extract. Antioxidants 2023, 12, 889. [Google Scholar] [CrossRef]
- López, P.L.; Enemark, G.K.G.; Grosso, N.R.; Olmedo, R.H. Antioxidant effectiveness between mechanisms of “Chain breaking antioxidant” and “Termination enhancing antioxidant” in a lipid model with essential oils. Food Biosci. 2024, 57, 103498. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, M.; Zhang, W.H.; Zhu, Q.M.; Ning, J.; Huo, X.K.; Xiao, H.T.; Sun, C.P. Total terpenoids of Inula japonica activated the Nrf2 receptor to alleviate the inflammation and oxidative stress in LPS-induced acute lung injury. Phytomedicine 2022, 104, 154307. [Google Scholar] [CrossRef]
- Juncos, N.S.; Ponso, C.F.C.; Grosso, N.R.; Olmedo, R.H. Oxidation protection efficiency of the combination of Minthostachys mollis K. and Origanum vulgare L. essential oils with “chain-breaking” and “termination- enhancing” antioxidant mechanisms. J. Food Sci. 2024, 89, 9166–9178. [Google Scholar] [CrossRef]
- Jin, Z.; Mollica, F.; Huang, Y.; Guernelli, S.; Baschieri, A.; Diquigiovanni, C.; Amorati, R. Pro-aromatic Natural Terpenes as Unusual “Slingshot” Antioxidants with Promising Ferroptosis Inhibition Activity. Chem. Eur. J. 2024, 30, e202403320. [Google Scholar] [CrossRef]
- Foti, M.C.; Ingold, K.U. Mechanism of inhibition of lipid peroxidation by γ-terpinene, an unusual and potentially useful hydrocarbon antioxidant. J. Agric. Food Chem. 2003, 51, 2758–2765. [Google Scholar] [CrossRef]
- Lee, M.H.; Do Kim, H.; Jang, Y.J. Delivery systems designed to enhance stability and suitability of lipophilic bioactive compounds in food processing: A review. Food Chem. 2024, 437, 137910. [Google Scholar] [CrossRef]
- Bravo-Díaz, C. Advances in the control of lipid peroxidation in oil-in-water emulsions: Kinetic approaches. Crit. Rev. Food Sci. Nutr. 2023, 63, 6252–6284. [Google Scholar] [CrossRef] [PubMed]
- Mirossay, L.; Varinska, L.; Mojzis, J. Antiangiogenic effect of flavonoids and chalcones: An update. Int. J. Mol. Sci. 2018, 19, 27. [Google Scholar] [CrossRef] [PubMed]
- Targhi, R.G.; Saba, V. Grape seed extract alleviates radiation-induced damages in human blood lymphocytes. Avicenna J. Phytomed. 2020, 10, 398–406. [Google Scholar]
- Ganesan, K.; Mickymaray, S.; Al Aboody, M.S.; Alfaiz, F.A.; Thatchinamoorthi, R.; Xu, B. Immunomodulatory and antineoplastic efficacy of common spices and their connection with phenolic antioxidants. BCHD 2020, 3, 15–31. [Google Scholar]
- Seidel, C.; Schnekenburger, M.; Dicato, M.; Diederich, M. Antiproliferative and proapoptotic activities of 4-hydroxybenzoic acid-based inhibitors of histone deacetylases. Cancer Lett. 2014, 343, 134–146. [Google Scholar] [CrossRef]
- Wang, L.C.; Chu, K.H.; Liang, Y.C.; Lin, Y.L.; Chiang, B.L. Caffeic acid phenethyl ester inhibits nuclear factor-kB and protein kinase B signalling pathways and induces caspase-3 expression in primary human CD4+ T cells. Clin. Exp. Immunol. 2010, 160, 223–232. [Google Scholar] [CrossRef]
- Fukuda, M.; Kobayashi, K.; Hirono, Y.; Miyagawa, M.; Ishida, T.; Ejiogu, E.C.; Sawai, M.; Pinkerton, K.E.; Takeuchi, M. Jungle honey enhances immune function and antitumor activity. Evid. Based Complement. Altern. Med. 2011, 8, 908743. [Google Scholar] [CrossRef]
- Yu, H.M.; Wang, B.S.; Chu, H.L.; Chang, L.W.; Yen, W.J.; Lin, C.J.; Duh, P.D. Napiergrass (Pennisetum purpureum S.) protects oxidative damage of biomolecules and modulates antioxidant enzyme activity. Food Chem. 2007, 105, 1364–1374. [Google Scholar] [CrossRef]
- Cheng, D.M.; Pogrebnyak, N.; Kuhn, P.; Poulev, A.; Waterman, C.; Rojas-Silva, P.; Johnson, W.D.; Raskin, I. Polyphenol-rich Rutgers Scarlet Lettuce improves glucose metabolism and liver lipid accumulation in diet-induced obese C57BL/6 mice. Nutrients 2014, 30, S52–S58. [Google Scholar] [CrossRef]
- Chacar, S.; Itani, T.; Hajal, J.; Saliba, Y.; Louka, N.; Faivre, J.-F.; Maroun, R.; Fares, N. The Impact of Long-Term Intake of Phenolic Compounds-Rich Grape Pomace on Rat Gut Microbiota. J. Food Sci. 2018, 83, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Anhê, F.F.; Roy, D.; Pilon, G.; Dudonné, S.; Matamoros, S.; Varin, T.V.; Garofalo, C.; Moine, Q.; Desjardins, Y.; Levy, E.; et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 2015, 64, 872–883. [Google Scholar] [CrossRef] [PubMed]
- Nagata, R.; Echizen, M.; Yamaguchi, Y.; Kyu-Ho Han Shimada, K.; Ohba, K.; Kitano-Okada, T.; Nagura, T.; Uchino, H.; Fukushima, M. Effect of a combination of inulin and polyphenol-containing adzuki bean extract on intestinal fermentation in vitro and in vivo. Biosci. Biotechnol. Biochem. 2018, 82, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, S.; Presannakumar, G.; Vijayalakshmi, N.R. Investigations on the Effect of Flavonoids from Banana, Musa Paradisiaca L. on Lipid Metabolism in Rats. J. Diet. Suppl. 2009, 6, 111–123. [Google Scholar] [CrossRef]
- Li, N.; Sun, Y.-R.; He, L.-B.; Huang, L.; Li, T.-T.; Wang, T.-Y.; Tang, L. Amelioration by Idesia polycarpa Maxim. var. vestita Diels. of Oleic Acid-Induced Nonalcoholic Fatty Liver in HepG2 Cells through Antioxidant and Modulation of Lipid Metabolism. Oxidative Med. Cell. Longev. 2020, 2020, 120872. [Google Scholar] [CrossRef]
- Barona, J.; Aristizabal, J.C.; Blesso, C.N.; Volek, J.S.; Fernandez, M.L. Grape Polyphenols Reduce Blood Pressure and Increase Flow-Mediated Vasodilation in Men with Metabolic Syndrome. J. Nutr. 2012, 142, 1626–1632. [Google Scholar] [CrossRef]
- Sakthivel, R.; Devi, K.P. Antioxidant, anti-inflammatory and anticancer potential of natural bioactive compounds from seaweeds. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2019; Chapter 5; Volume 63, pp. 113–160. ISBN 1572-5995. [Google Scholar]
- Yang, D.; Jiang, Y.; Wang, Y.; Lei, Q.; Zhao, X.; Yi, R.; Zhang, X. Improvement of Flavonoids in Lemon Seeds on Oxidative Damage of Human Embryonic Kidney 293T Cells Induced by H2O2. Oxidative Med. Cell. Longev. 2020, 2020, 3483519. [Google Scholar] [CrossRef]
- Regitz, C.; Fitzenberger, E.; Mahn, F.L.; Dußling, L.M.; Wenzel, U. Resveratrol reduces amyloid-beta (Aβ1–42)-induced paralysis through targeting proteostasis in an Alzheimer model of Caenorhabditis elegans. Eur. J. Nutr. 2016, 55, 741–747. [Google Scholar] [CrossRef]
- Parveen, B.; Rajinikanth, V.; Narayanan, M. Natural plant antioxidants for food preservation and emerging trends in nutraceutical applications. Discov. Appl. Sci. 2025, 7, 845. [Google Scholar] [CrossRef]
- Zhang, D.; Ivane, N.M.A.; Haruna, S.A.; Zekrumah, M.; Elysé, F.K.R.; Tahir, H.E.; Wang, G.; Wang, C.; Zou, X. Recent trends in the micro-encapsulation of plant-derived compounds and their specific application in meat as antioxidants and antimicrobials. Meat Sci. 2022, 191, 108842. [Google Scholar] [CrossRef]
- Lourenco, S.C.; Torres, C.A.; Nunes, D.; Duarte, P.; Freitas, F.; Reis, M.A.; Alves, V.D. Using a bacterial fucose-rich polysaccharide as encapsulation material of bioactive compounds. Int. J. Biol. Macromol. 2017, 104, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Vladić, J.; Ambrus, R.; Szabó-Révész, P.; Vasić, A.; Cvejin, A.; Pavlić, B.; Vidović, S. Recycling of filter tea industry by-products: Production of A. millefolium powder using spray drying technique. Ind. Crop Prod. 2015, 80, 197–206. [Google Scholar] [CrossRef]
- Weber, F.; Boch, K.; Schieber, A. Influence of copigmentation on the stability of spray dried anthocyanins from blackberry. LWT—Food Sci. Technol. 2017, 75, 72–77. [Google Scholar] [CrossRef]
- Ramakrishnan, Y.; Mohd, N.; Aniza, Y.; Muhammad, K. Effect of wall materials on the spray drying efficiency, powder properties and stability of bioactive compounds in tamarillo juice microencapsulation. Powder Technol. 2018, 328, 406–414. [Google Scholar] [CrossRef]
- Lavelli, V.; Harsha, P.S.; Spigno, G. Modelling the stability of maltodextrin-encapsulated grape skin phenolics used as a new ingredient in apple puree. Food Chem. 2016, 209, 323–331. [Google Scholar] [CrossRef]
- Hoskin, R.T.; Xiong, J.; Esposito, D.A.; Lila, M.A. Blueberry polyphenol-protein food ingredients: The impact of spray drying on the in vitro antioxidant activity, anti-inflammatory markers, glucose metabolism and fibroblast migration. Food Chem. 2019, 280, 187–194. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Ramirez, M.J.; Orrego, C.E.; Teixeira, J.A.; Mussatto, S.I. Encapsulation of antioxidant phenolic compounds extracted from spent coffee grounds by freeze-drying and spray-drying using different coating materials. Food Chem. 2017, 237, 623–631. [Google Scholar] [CrossRef]
- Rocha-Parra, D.F.; Lanari, M.C.; Zamora, M.C.; Chirife, J. Influence of storage conditions on phenolic compounds stability, antioxidant capacity and colour of freeze-dried encapsulated red wine. LWT- Food Sci. Technol. 2016, 70, 162–170. [Google Scholar] [CrossRef]
- Pudziuvelyte, L.; Marksa, M.; Sosnowska, K.; Winnicka, K.; Morkuniene, R.; Bernatoniene, J. Freeze-drying technique for microencapsulation of Elsholtzia ciliata ethanolic extract using different coating materials. Molecules 2020, 25, 2237. [Google Scholar] [CrossRef]
- Kelemen, V.; Pichler, A.; Ivic, I.; Buljeta, I.; Šimunovi’c, J.; Kopjar, M. Brown rice proteins as delivery system of phenolic and volatile compounds of raspberry juice. Int. J. Food Sci. Technol. 2022, 57, 1866–1874. [Google Scholar] [CrossRef]
- de Moura, S.; Schettini, G.N.; Garcia, A.O.; Gallina, D.A.; Alvim, I.D.; Hubinger, M.D. Stability of hibiscus extract encapsulated by ionic gelation incorporated in yogurt. Food Bioprocess Technol. 2019, 12, 1500–1515. [Google Scholar] [CrossRef]
- Mendoza-Wilson, A.M.; Balandrán-Quintana, R.R.; Valdés-Covarrubias, M.Á.; Cabellos, J.L. Potential of quercetin in combination with antioxidants of different polarity incorporated in oil-in-water nanoemulsions to control enzymatic browning of apples. J. Mol. Struct. 2022, 1254, 132372. [Google Scholar] [CrossRef]
- Hong, S.-C.; Park, K.-M.; Hong, C.R.; Kim, J.-C.; Yang, S.-H.; Yu, H.-S.; Paik, H.-D.; Pan, C.-H.; Chang, P.-S. Microfluidic assembly of liposomes dual-loaded with catechin and curcumin for enhancing bioavailability. Colloids Surf. A Physicochem. Eng. Asp. 2020, 594, 124670. [Google Scholar] [CrossRef]
- Ha, E.-S.; Park, H.; Lee, S.-K.; Sim, W.-Y.; Jeong, J.-S.; Baek, I.-H.; Kim, M.-S. Pure Trans-resveratrol nanoparticles prepared by a supercritical antisolvent process using alcohol and dichloromethane mixtures: Effect of particle size on dissolution and bioavailability in rats. Antioxidants 2020, 9, 342. [Google Scholar] [CrossRef]
- Lopez de Dicastillo, C.; Lopez-Carballo, G.; Gavara, R.; Galet, V.M.; Guarda, A.; Galotto, M.J. Improving polyphenolic thermal stability of Aristotelia Chilensis fruit extract by encapsulation within electrospun cyclodextrin capsules. J. Food Process Preserv. 2019, 43, 14044. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, D.; Liu, D.; Zhu, B. Food-grade encapsulated polyphenols: Recent advances as novel additives in foodstuffs. Crit. Rev. Food Sci. Nutr. 2023, 63, 11545–11560. [Google Scholar] [CrossRef]
- Rathod, N.B.; Elabed, N.; Punia, S.; Ozogul, F.; Kim, S.K.; Rocha, J.M. Recent developments in polyphenol applications on human health: A review with current knowledge. Plants 2023, 12, 1217. [Google Scholar] [CrossRef]
- Coşkun, N.; Sarıtaş, S.; Bechelany, M.; Karav, S. Polyphenols in Foods and Their Use in the Food Industry: Enhancing the Quality and Nutritional Value of Functional Foods. Int. J. Mol. Sci. 2025, 26, 5803. [Google Scholar] [CrossRef]
- Lyu, X.; Lee, J.; Chen, W.N. Potential natural food preservatives and their sustainable production in yeast: Terpenoids and polyphenols. J. Agric. Food Chem. 2019, 67, 4397–4417. [Google Scholar] [CrossRef]
- Khan, M.K.; Ahmad, K.; Hassan, S.; Imran, M.; Ahmad, N.; Xu, C. Effect of novel technologies on polyphenols during food processing. Innov. Food Sci. Emerg. Technol. 2018, 45, 361–381. [Google Scholar] [CrossRef]
- Câmara, J.S.; Albuquerque, B.R.; Aguiar, J.; Corrêa, R.C.; Gonçalves, J.L.; Granato, D.; Pereira, J.A.; Barros, L.; Ferreira, I.C. Food bioactive compounds and emerging techniques for their extraction: Polyphenols as a case study. Foods 2020, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Pop, O.L.; Suharoschi, R.; Socaci, S.A.; Berger Ceresino, E.; Weber, A.; Gruber-Traub, C.; Vodnar, D.C.; Fărcaș, A.C.; Johansson, E. Polyphenols-ensured accessibility from food to the human metabolism by chemical and biotechnological treatments. Antioxidants 2023, 12, 865. [Google Scholar] [CrossRef] [PubMed]
- El-Saadony, M.T.; Yang, T.; Saad, A.M.; Alkafaas, S.S.; Elkafas, S.S.; Eldeeb, G.S.; Mohammed, D.M.; Salem, H.M.; Korma, S.A.; Loutfy, S.A.; et al. Polyphenols: Chemistry, bioavailability, bioactivity, nutritional aspects and human health benefits: A review. Int. J. Biol. Macromol. 2024, 277, 134223. [Google Scholar] [CrossRef] [PubMed]
- Lila, M.A.; Schneider, M.; Devlin, A.; Plundrich, N.; Laster, S.; Foegeding, E.A. Polyphenol-enriched berry extracts naturally modulate reactive proteins in model foods. Food Funct. 2017, 8, 4760–4767. [Google Scholar] [CrossRef]
- Zhang, K.; Huang, J.; Wang, D.; Wan, X.; Wang, Y. Covalent polyphenols-proteins interactions in food processing: Formation mechanisms, quantification methods, bioactive effects, and applications. Front. Nutr. 2024, 11, 1371401. [Google Scholar] [CrossRef]
- Li, Y.; He, D.; Li, B.; Lund, M.N.; Xing, Y.; Wang, Y.; Li, F.; Cao, X.; Liu, Y.; Chen, X.; et al. Engineering polyphenols with biological functions via polyphenol-protein interactions as additives for functional foods. Trends Food Sci. Technol. 2021, 110, 470–482. [Google Scholar] [CrossRef]
- Gervasi, T.; Calderaro, A.; Barreca, D.; Tellone, E.; Trombetta, D.; Ficarra, S.; Smeriglio, A.; Mandalari, G.; Gattuso, G. Biotechnological applications and health-promoting properties of flavonols: An updated view. Int. J. Mol. Sci. 2022, 23, 1710. [Google Scholar] [CrossRef]
- Scarano, A.; Chieppa, M.; Santino, A. Plant polyphenols-biofortified foods as a novel tool for the prevention of human gut diseases. Antioxidants 2020, 9, 1225. [Google Scholar] [CrossRef]
- Polia, F.; Pastor-Belda, M.; Martínez-Blázquez, A.; Horcajada, M.N.; Tomás-Barberán, F.A.; García-Villalba, R. Technological and biotechnological processes to enhance the bioavailability of dietary (poly)phenols in humans. J. Agric. Food Chem. 2022, 70, 2092–2107. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.