β-Glucosidase Activity of Lactiplantibacillus plantarum: A Key Player in Food Fermentation and Human Health
Abstract
1. Introduction
2. Roles of β-Glucosidases in Fermented Plant-Based Foods
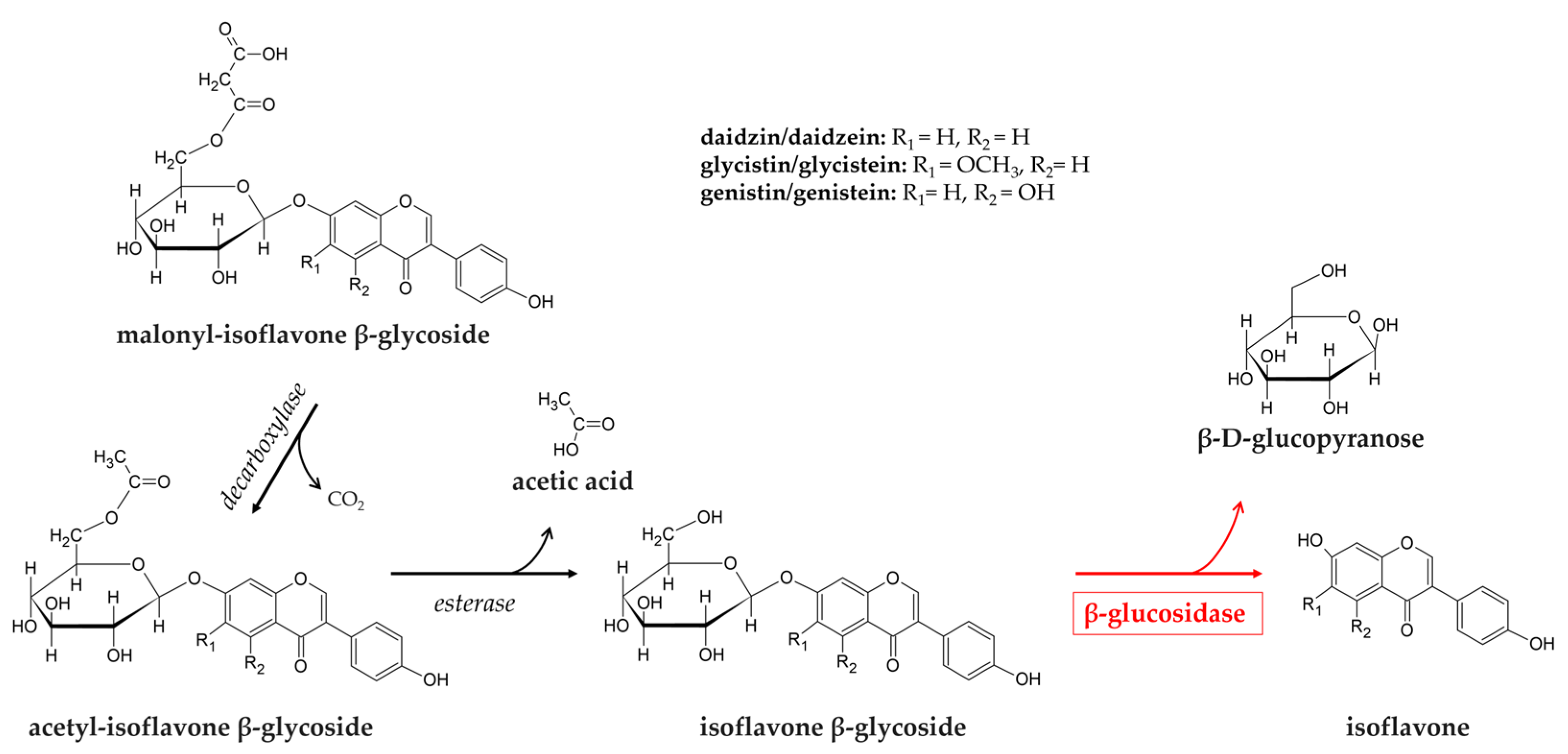
2.1. Soymilk and Soybean Products
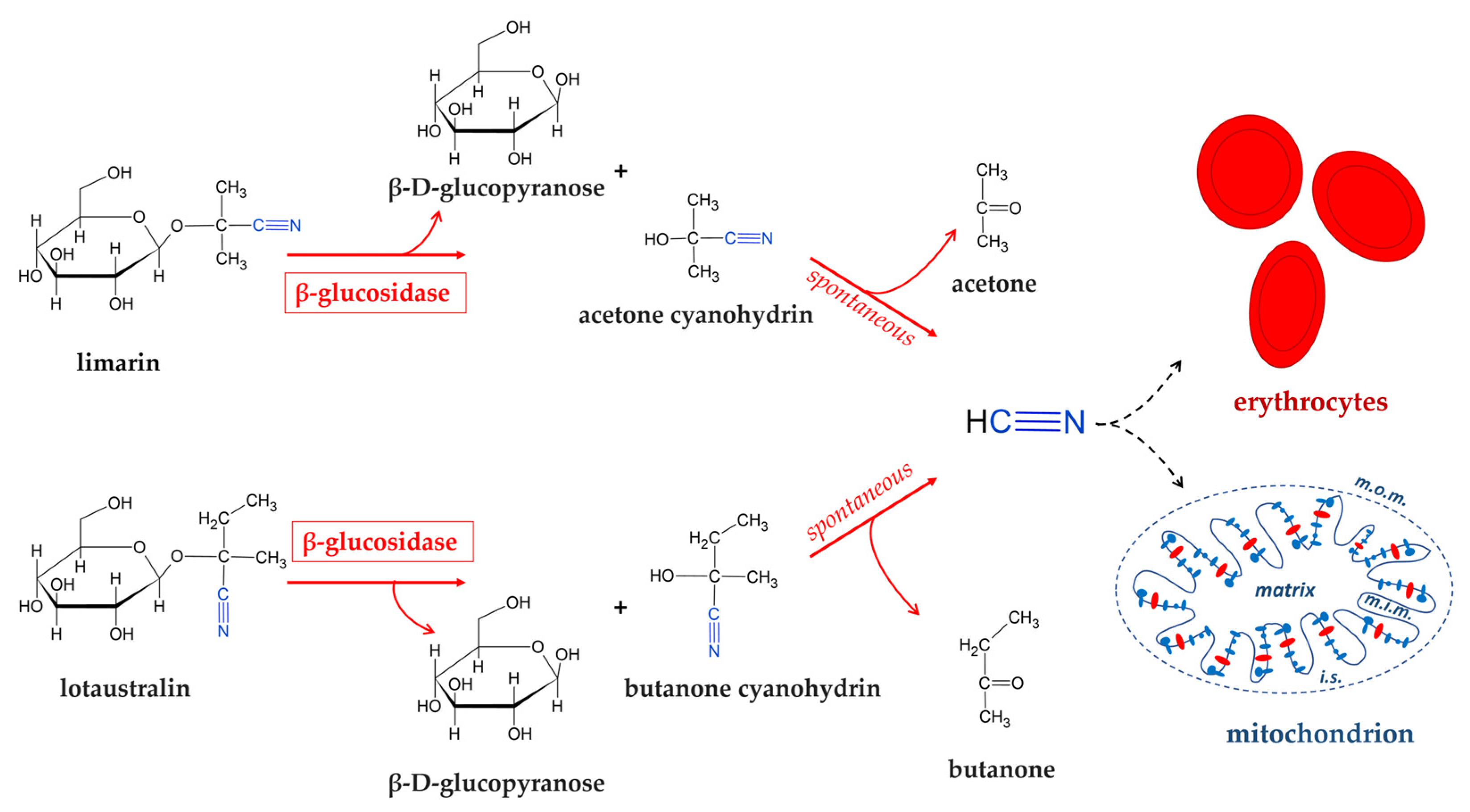
2.2. Cassava
2.3. Olive
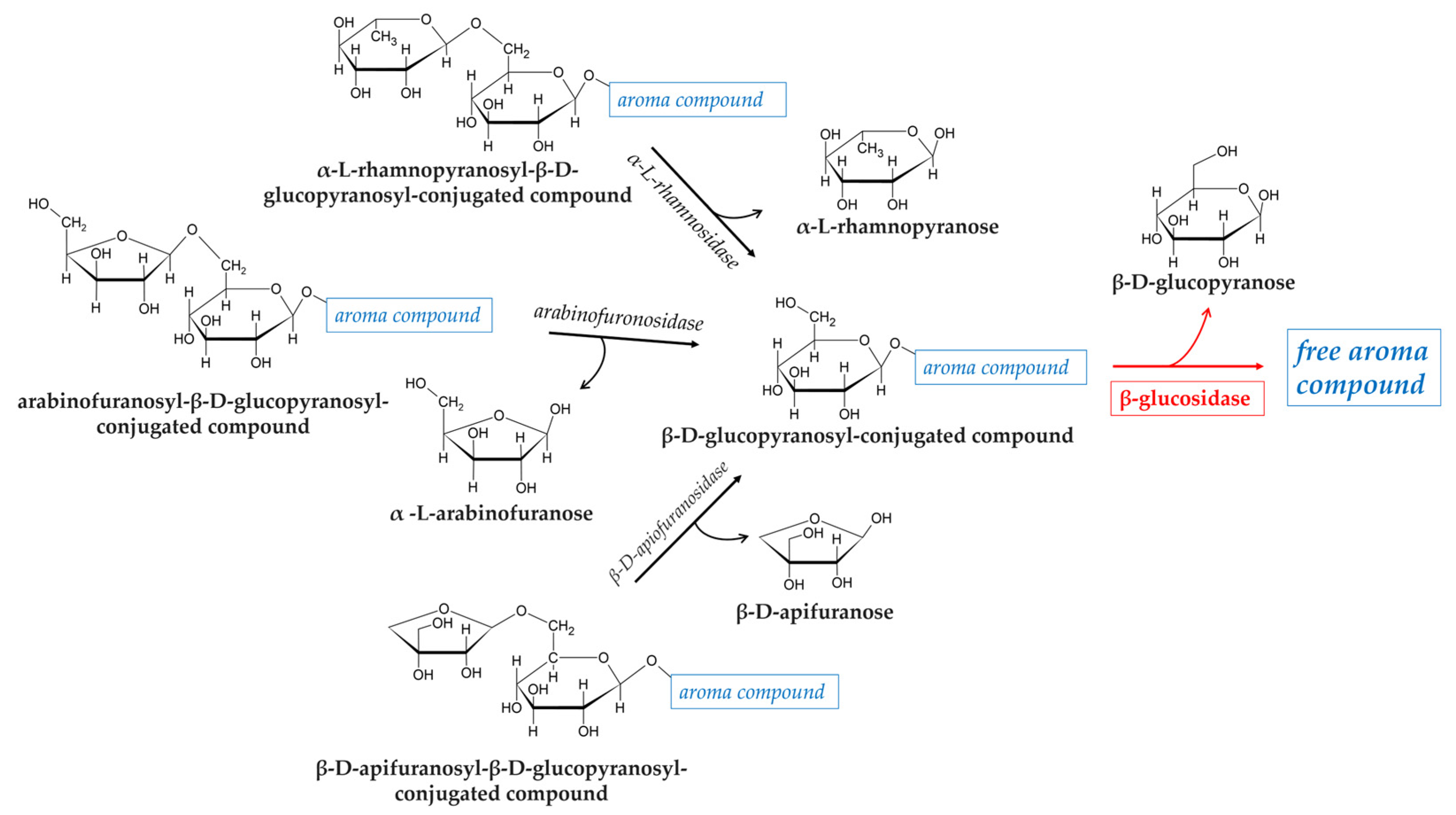
3. Fermented Beverages
3.1. Alcoholic Beverages
3.1.1. Wine
3.1.2. Beer
3.2. Non-Alcoholic Fermented Fruit Products
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sengupta, S.; Datta, M.; Datta, S. β-Glucosidase: Structure, Function and Industrial Applications. In Glycoside Hydrolases; Elsevier: Amsterdam, The Netherlands, 2023; pp. 97–120. [Google Scholar]
- Shen, H.; Byers, L.D. Thioglycoside Hydrolysis Catalyzed by β-Glucosidase. Biochem. Biophys. Res. Commun. 2007, 362, 717–720. [Google Scholar] [CrossRef] [PubMed]
- Henrissat, B. A Classification of Glycosyl Hydrolases Based on Amino Acid Sequence Similarities. Biochem. J. 1991, 280, 309–316. [Google Scholar] [CrossRef]
- Henrissat, B.; Davies, G. Structural and Sequence-Based Classification of Glycoside Hydrolases. Curr. Opin. Struct. Biol. 1997, 7, 637–644. [Google Scholar] [CrossRef]
- Ketudat Cairns, J.R.; Esen, A. β-Glucosidases. Cell. Mol. Life Sci. 2010, 67, 3389–3405. [Google Scholar] [CrossRef] [PubMed]
- Cantarel, B.L.; Coutinho, P.M.; Rancurel, C.; Bernard, T.; Lombard, V.; Henrissat, B. The Carbohydrate-Active EnZymes Database (CAZy): An Expert Resource for Glycogenomics. Nucleic Acids Res. 2009, 37, D233–D238. [Google Scholar] [CrossRef]
- Kannan, P.; Shafreen, M.M.; Achudhan, A.B.; Gupta, A.; Saleena, L.M. A Review on Applications of β-Glucosidase in Food, Brewery, Pharmaceutical and Cosmetic Industries. Carbohydr. Res. 2023, 530, 108855. [Google Scholar] [CrossRef]
- Magwaza, B.; Amobonye, A.; Pillai, S. Microbial β-Glucosidases: Recent Advances and Applications. Biochimie 2024, 225, 49–67. [Google Scholar] [CrossRef]
- Mól, P.C.G.; Júnior, J.C.Q.; Veríssimo, L.A.A.; Boscolo, M.; Gomes, E.; Minim, L.A.; Da Silva, R. β-Glucosidase: An Overview on Immobilization and Some Aspects of Structure, Function, Applications and Cost. Process Biochem. 2023, 130, 26–39. [Google Scholar] [CrossRef]
- Singh, G.; Verma, A.K.; Kumar, V. Catalytic Properties, Functional Attributes and Industrial Applications of β-Glucosidases. 3 Biotech. 2016, 6, 3. [Google Scholar] [CrossRef]
- Ouyang, B.; Wang, G.; Zhang, N.; Zuo, J.; Huang, Y.; Zhao, X. Recent Advances in β-Glucosidase Sequence and Structure Engineering: A Brief Review. Molecules 2023, 28, 4990. [Google Scholar] [CrossRef]
- Liu, C.; He, S.; Chen, J.; Wang, M.; Li, Z.; Wei, L.; Chen, Y.; Du, M.; Liu, D.; Li, C.; et al. A Dual-subcellular Localized Β-glucosidase Confers Pathogen and Insect Resistance without a Yield Penalty in Maize. Plant Biotechnol. J. 2024, 22, 1017–1032. [Google Scholar] [CrossRef]
- Kotik, M.; Kulik, N.; Valentová, K. Flavonoids as Aglycones in Retaining Glycosidase-Catalyzed Reactions: Prospects for Green Chemistry. J. Agric. Food Chem. 2023, 71, 14890–14910. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Wu, T.; Ali, A.; Wang, J.; Fang, Y.; Qiang, R.; Liu, Y.; Tian, Y.; Liu, S.; Zhang, H.; et al. Rice β-Glucosidase 4 (Os1βGlu4) Regulates the Hull Pigmentation via Accumulation of Salicylic Acid. Int. J. Mol. Sci. 2022, 23, 10646. [Google Scholar] [CrossRef]
- Lee, K.H.; Piao, H.L.; Kim, H.-Y.; Choi, S.M.; Jiang, F.; Hartung, W.; Hwang, I.; Kwak, J.M.; Lee, I.-J.; Hwang, I. Activation of Glucosidase via Stress-Induced Polymerization Rapidly Increases Active Pools of Abscisic Acid. Cell 2006, 126, 1109–1120. [Google Scholar] [CrossRef]
- Sakr, S.; Wang, M.; Dédaldéchamp, F.; Perez-Garcia, M.-D.; Ogé, L.; Hamama, L.; Atanassova, R. The Sugar-Signaling Hub: Overview of Regulators and Interaction with the Hormonal and Metabolic Network. Int. J. Mol. Sci. 2018, 19, 2506. [Google Scholar] [CrossRef] [PubMed]
- Boer, D.E.C.; van Smeden, J.; Bouwstra, J.A.; Aerts, J.M.F.G. Glucocerebrosidase: Functions in and Beyond the Lysosome. J. Clin. Med. 2020, 9, 736. [Google Scholar] [CrossRef] [PubMed]
- Day, A.J.; Cañada, F.J.; Díaz, J.C.; Kroon, P.A.; Mclauchlan, R.; Faulds, C.B.; Plumb, G.W.; Morgan, M.R.A.; Williamson, G. Dietary Flavonoid and Isoflavone Glycosides Are Hydrolysed by the Lactase Site of Lactase Phlorizin Hydrolase. FEBS Lett. 2000, 468, 166–170. [Google Scholar] [CrossRef]
- Elferink, H.; Bruekers, J.P.J.; Veeneman, G.H.; Boltje, T.J. A Comprehensive Overview of Substrate Specificity of Glycoside Hydrolases and Transporters in the Small Intestine. Cell. Mol. Life Sci. 2020, 77, 4799–4826. [Google Scholar] [CrossRef]
- He, S.; Jiang, B.; Chakraborty, A.; Yu, G. The Evolution of Glycoside Hydrolase Family 1 in Insects Related to Their Adaptation to Plant Utilization. Insects 2022, 13, 786. [Google Scholar] [CrossRef]
- Friedrichs, J.; Schweiger, R.; Müller, C. Unique Metabolism of Different Glucosinolates in Larvae and Adults of a Leaf Beetle Specialised on Brassicaceae. Sci. Rep. 2022, 12, 10905. [Google Scholar] [CrossRef]
- Bhatia, Y.; Mishra, S.; Bisaria, V.S. Microbial β-Glucosidases: Cloning, Properties, and Applications. Crit. Rev. Biotechnol. 2002, 22, 375–407. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Yin, Z.; Wu, L.; Yin, C. Diversity of Cultivable β-Glycosidase-Producing Micro-Organisms Isolated from the Soil of a Ginseng Field and Their Ginsenosides-Hydrolysing Activity. Lett. Appl. Microbiol. 2014, 58, 138–144. [Google Scholar] [CrossRef]
- Tiwari, R.; Kumar, K.; Singh, S.; Nain, L.; Shukla, P. Molecular Detection and Environment-Specific Diversity of Glycosyl Hydrolase Family 1 β-Glucosidase in Different Habitats. Front. Microbiol. 2016, 7, 1597. [Google Scholar] [CrossRef]
- Su, H.; Xiao, Z.; Yu, K.; Zhang, Q.; Lu, C.; Wang, G.; Wang, Y.; Liang, J.; Huang, W.; Huang, X.; et al. High Diversity of β-Glucosidase-Producing Bacteria and Their Genes Associated with Scleractinian Corals. Int. J. Mol. Sci. 2021, 22, 3523. [Google Scholar] [CrossRef] [PubMed]
- Oladoja, E.; Oyewole, O.; Adamu, B.; Balogun, A.; Musa, O. Microbial β-Glucosidase: Source, Production and Applications. Int. J. Biol. Sci. 2019, 1, 14–22. [Google Scholar] [CrossRef]
- Zang, X.; Liu, M.; Fan, Y.; Xu, J.; Xu, X.; Li, H. The Structural and Functional Contributions of β-Glucosidase-Producing Microbial Communities to Cellulose Degradation in Composting. Biotechnol. Biofuels 2018, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Nasim, F.; Batool, K.; Bibi, A. Microbial β-Glucosidase: Sources, Production and Applicationsc. J. Appl. Environ. Microbiol. 2017, 5, 31–46. [Google Scholar] [CrossRef]
- Yang, W.; Su, Y.; Wang, R.; Zhang, H.; Jing, H.; Meng, J.; Zhang, G.; Huang, L.; Guo, L.; Wang, J.; et al. Microbial Production and Applications of β-Glucosidase-A Review. Int. J. Biol. Macromol. 2024, 256, 127915. [Google Scholar] [CrossRef]
- Michlmayr, H.; Kneifel, W. β-Glucosidase Activities of Lactic Acid Bacteria: Mechanisms, Impact on Fermented Food and Human Health. FEMS Microbiol. Lett. 2014, 352, 1–10. [Google Scholar] [CrossRef]
- Gao, P.; Peng, S.; Sam, F.E.; Zhu, Y.; Liang, L.; Li, M.; Wang, J. Indigenous Non-Saccharomyces Yeasts with β-Glucosidase Activity in Sequential Fermentation with Saccharomyces Cerevisiae: A Strategy to Improve the Volatile Composition and Sensory Characteristics of Wines. Front. Microbiol. 2022, 13, 845837. [Google Scholar] [CrossRef]
- Testa, B.; Lombardi, S.J.; Iorizzo, M.; Letizia, F.; Di Martino, C.; Di Renzo, M.; Strollo, D.; Tremonte, P.; Pannella, G.; Ianiro, M.; et al. Use of Strain Hanseniaspora Guilliermondii BF1 for Winemaking Process of White Grapes Vitis vinifera Cv Fiano. Eur. Food Res. Technol. 2020, 246, 549–561. [Google Scholar] [CrossRef]
- Testa, B.; Coppola, F.; Iorizzo, M.; Di Renzo, M.; Coppola, R.; Succi, M. Preliminary Characterisation of Metschnikowia Pulcherrima to Be Used as a Starter Culture in Red Winemaking. Beverages 2024, 10, 88. [Google Scholar] [CrossRef]
- Colautti, A.; Camprini, L.; Ginaldi, F.; Comi, G.; Reale, A.; Coppola, F.; Iacumin, L. Safety Traits, Genetic and Technological Characterization of Lacticaseibacillus Rhamnosus Strains. LWT 2024, 207, 116578. [Google Scholar] [CrossRef]
- Coppola, F.; Abdalrazeq, M.; Fratianni, F.; Ombra, M.N.; Testa, B.; Zengin, G.; Ayala Zavala, J.F.; Nazzaro, F. Rosaceae Honey: Antimicrobial Activity and Prebiotic Properties. Antibiotics 2025, 14, 298. [Google Scholar] [CrossRef]
- Nazzaro, F.; Ombra, M.N.; Coppola, F.; De Giulio, B.; d’Acierno, A.; Coppola, R.; Fratianni, F. Antibacterial Activity and Prebiotic Properties of Six Types of Lamiaceae Honey. Antibiotics 2024, 13, 868. [Google Scholar] [CrossRef]
- Letizia, F.; Fusco, G.M.; Fratianni, A.; Gaeta, I.; Carillo, P.; Messia, M.C.; Iorizzo, M. Application of Lactiplantibacillus plantarum LP95 as a Functional Starter Culture in Fermented Tofu Production. Processes 2024, 12, 1093. [Google Scholar] [CrossRef]
- Berthiller, F.; Krska, R.; Domig, K.J.; Kneifel, W.; Juge, N.; Schuhmacher, R.; Adam, G. Hydrolytic Fate of Deoxynivalenol-3-Glucoside during Digestion. Toxicol. Lett. 2011, 206, 264–267. [Google Scholar] [CrossRef]
- Siezen, R.J.; Tzeneva, V.A.; Castioni, A.; Wels, M.; Phan, H.T.K.; Rademaker, J.L.W.; Starrenburg, M.J.C.; Kleerebezem, M.; Molenaar, D.; Van Hylckama Vlieg, J.E.T. Phenotypic and Genomic Diversity of Lactobacillus plantarum Strains Isolated from Various Environmental Niches. Environ. Microbiol. 2010, 12, 758–773. [Google Scholar] [CrossRef]
- Martino, M.E.; Bayjanov, J.R.; Caffrey, B.E.; Wels, M.; Joncour, P.; Hughes, S.; Gillet, B.; Kleerebezem, M.; van Hijum, S.A.F.T.; Leulier, F. Nomadic Lifestyle of Lactobacillus plantarum Revealed by Comparative Genomics of 54 Strains Isolated from Different Habitats. Environ. Microbiol. 2016, 18, 4974–4989. [Google Scholar] [CrossRef]
- Siezen, R.J.; van Hylckama Vlieg, J.E.T. Genomic Diversity and Versatility of Lactobacillus plantarum, a Natural Metabolic Engineer. Microb. Cell Fact. 2011, 10, S3. [Google Scholar] [CrossRef]
- Letizia, F.; Albanese, G.; Testa, B.; Vergalito, F.; Bagnoli, D.; Di Martino, C.; Carillo, P.; Verrillo, L.; Succi, M.; Sorrentino, E.; et al. In Vitro Assessment of Bio-Functional Properties from Lactiplantibacillus plantarum Strains. Curr. Issues Mol. Biol. 2022, 44, 2321–2334. [Google Scholar] [CrossRef]
- Filannino, P.; De Angelis, M.; Di Cagno, R.; Gozzi, G.; Riciputi, Y.; Gobbetti, M. How Lactobacillus plantarum Shapes Its Transcriptome in Response to Contrasting Habitats. Environ. Microbiol. 2018, 20, 3700–3716. [Google Scholar] [CrossRef]
- Testa, B.; Lombardi, S.J.; Tremonte, P.; Succi, M.; Tipaldi, L.; Pannella, G.; Sorrentino, E.; Iorizzo, M.; Coppola, R. Biodiversity of Lactobacillus plantarum from Traditional Italian Wines. World J. Microbiol. Biotechnol. 2014, 30, 2299–2305. [Google Scholar] [CrossRef] [PubMed]
- Iorizzo, M.; Lombardi, S.J.; Macciola, V.; Testa, B.; Lustrato, G.; Lopez, F.; De Leonardis, A. Technological Potential of Lactobacillus Strains Isolated from Fermented Green Olives: In Vitro Studies with Emphasis on Oleuropein-Degrading Capability. Sci. World J. 2016, 2016, 1917592. [Google Scholar] [CrossRef]
- Iorizzo, M.; Testa, B.; Ganassi, S.; Lombardi, S.J.; Ianiro, M.; Letizia, F.; Succi, M.; Tremonte, P.; Vergalito, F.; Cozzolino, A.; et al. Probiotic Properties and Potentiality of Lactiplantibacillus plantarum Strains for the Biological Control of Chalkbrood Disease. J. Fungi 2021, 7, 379. [Google Scholar] [CrossRef]
- Chundakkattumalayil, H.C.; Raghavan, K.T. Health Endorsing Potential of Lactobacillus plantarum MBTU-HK1 and MBTU-HT of Honey Bee Gut Origin. J. Appl. Biol. Biotechnol. 2021, 9, 63–68. [Google Scholar] [CrossRef]
- Iorizzo, M.; Pannella, G.; Lombardi, S.J.; Ganassi, S.; Testa, B.; Succi, M.; Sorrentino, E.; Petrarca, S.; De Cristofaro, A.; Coppola, R.; et al. Inter- and Intra-Species Diversity of Lactic Acid Bacteria in Apis mellifera ligustica Colonies. Microorganisms 2020, 8, 1578. [Google Scholar] [CrossRef]
- Iorizzo, M.; Albanese, G.; Testa, B.; Ianiro, M.; Letizia, F.; Succi, M.; Tremonte, P.; D’andrea, M.; Iaffaldano, N.; Coppola, R. Presence of Lactic Acid Bacteria in the Intestinal Tract of the Mediterranean Trout (Salmo macrostigma) in Its Natural Environment. Life 2021, 11, 667. [Google Scholar] [CrossRef]
- Park, S.-Y.; Lim, S.-D. Probiotic Characteristics of Lactobacillus plantarum FH185 Isolated from Human Feces. Korean J. Food Sci. Anim. Resour. 2015, 35, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Salvetti, E.; O’Toole, P.W. When Regulation Challenges Innovation: The Case of the Genus Lactobacillus. Trends Food Sci. Technol. 2017, 66, 187–194. [Google Scholar] [CrossRef]
- Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Hilbert, F.; Lindqvist, R.; et al. Update of the List of QPS-recommended Biological Agents Intentionally Added to Food or Feed as Notified to EFSA 12: Suitability of Taxonomic Units Notified to EFSA until March 2020. EFSA J. 2020, 18, e06174. [Google Scholar] [CrossRef] [PubMed]
- Iorizzo, M.; Paventi, G.; Di Martino, C. Biosynthesis of Gamma-Aminobutyric Acid (GABA) by Lactiplantibacillus plantarum in Fermented Food Production. Curr. Issues Mol. Biol. 2023, 46, 200–220. [Google Scholar] [CrossRef] [PubMed]
- Iorizzo, M.; Di Martino, C.; Letizia, F.; Crawford, T.W.; Paventi, G. Production of Conjugated Linoleic Acid (CLA) by Lactiplantibacillus plantarum: A Review with Emphasis on Fermented Foods. Foods 2024, 13, 975. [Google Scholar] [CrossRef]
- Paventi, G.; Di Martino, C.; Crawford, T.W., Jr.; Iorizzo, M. Enzymatic Activities of Lactiplantibacillus plantarum: Technological and Functional Role in Food Processing and Human Nutrition. Food Biosci. 2024, 61, 104944. [Google Scholar] [CrossRef]
- Behera, S.S.; Ray, R.C.; Zdolec, N. Lactobacillus plantarum with Functional Properties: An Approach to Increase Safety and Shelf-Life of Fermented Foods. BioMed Res. Int. 2018, 2018, 9361614. [Google Scholar] [CrossRef]
- Caffrey, A.J.; Lerno, L.A.; Zweigenbaum, J.; Ebeler, S.E. Direct Analysis of Glycosidic Aroma Precursors Containing Multiple Aglycone Classes in Vitis vinifera Berries. J. Agric. Food Chem. 2020, 68, 3817–3833. [Google Scholar] [CrossRef]
- Liang, Z.; Fang, Z.; Pai, A.; Luo, J.; Gan, R.; Gao, Y.; Lu, J.; Zhang, P. Glycosidically Bound Aroma Precursors in Fruits: A Comprehensive Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 215–243. [Google Scholar] [CrossRef]
- Zhu, N.; Wang, T.; Ge, L.; Li, Y.; Zhang, X.; Bao, H. γ-Amino Butyric Acid (GABA) Synthesis Enabled by Copper-Catalyzed Carboamination of Alkenes. Org. Lett. 2017, 19, 4718–4721. [Google Scholar] [CrossRef]
- González-Barreiro, C.; Rial-Otero, R.; Cancho-Grande, B.; Simal-Gándara, J. Wine Aroma Compounds in Grapes: A Critical Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 202–218. [Google Scholar] [CrossRef]
- Maicas, S.; Mateo, J.J. Hydrolysis of Terpenyl Glycosides in Grape Juice and Other Fruit Juices: A Review. Appl. Microbiol. Biotechnol. 2005, 67, 322–335. [Google Scholar] [CrossRef]
- Krammer, G.; Winterhalter, P.; Schwab, M.; Schreier, P. Glycosidically Bound Aroma Compounds in the Fruits of Prunus Species: Apricot (P. armeniaca, L.), Peach (P. persica, L.), Yellow Plum (P. domestica, L. ssp. syriaca). J. Agric. Food Chem. 1991, 39, 778–781. [Google Scholar] [CrossRef]
- de Morais Souto, B.; Florentino Barbosa, M.; Marinsek Sales, R.M.; Conessa Moura, S.; de Rezende Bastos Araújo, A.; Ferraz Quirino, B. The Potential of β-Glucosidases for Aroma and Flavor Improvement in the Food Industry. Microbe 2023, 1, 100004. [Google Scholar] [CrossRef]
- Fernández-Pacheco, P.; García-Béjar, B.; Briones Pérez, A.; Arévalo-Villena, M. Free and Immobilised β-Glucosidases in Oenology: Biotechnological Characterisation and Its Effect on Enhancement of Wine Aroma. Front. Microbiol. 2021, 12, 723815. [Google Scholar] [CrossRef] [PubMed]
- Sarry, J.; Gunata, Z. Plant and Microbial Glycoside Hydrolases: Volatile Release from Glycosidic Aroma Precursors. Food Chem. 2004, 87, 509–521. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, K.; Liu, C.; Ma, L.; Li, J. Effects of Glycosidase on Glycoside-Bound Aroma Compounds in Grape and Cherry Juice. J. Food Sci. Technol. 2023, 60, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Dziadas, M.; Jeleń, H.H. Comparison of Enzymatic and Acid Hydrolysis of Bound Flavor Compounds in Model System and Grapes. Food Chem. 2016, 190, 412–418. [Google Scholar] [CrossRef]
- Muradova, M.; Proskura, A.; Canon, F.; Aleksandrova, I.; Schwartz, M.; Heydel, J.-M.; Baranenko, D.; Nadtochii, L.; Neiers, F. Unlocking Flavor Potential Using Microbial β-Glucosidases in Food Processing. Foods 2023, 12, 4484. [Google Scholar] [CrossRef]
- Maicas, S.; Mateo, J. Microbial Glycosidases for Wine Production. Beverages 2016, 2, 20. [Google Scholar] [CrossRef]
- Hjelmeland, A.K.; Ebeler, S.E. Glycosidically Bound Volatile Aroma Compounds in Grapes and Wine: A Review. Am. J. Enol. Vitic. 2015, 66, 1–11. [Google Scholar] [CrossRef]
- Wilkowska, A.; Pogorzelski, E. Aroma Enhancement of Cherry Juice and Wine Using Exogenous Glycosidases from Mould, Yeast and Lactic Acid Bacteria. Food Chem. 2017, 237, 282–289. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.; Sang, S.; McClements, D.J.; Chen, L.; Long, J.; Jiao, A.; Jin, Z.; Qiu, C. Polyphenols as Plant-Based Nutraceuticals: Health Effects, Encapsulation, Nano-Delivery, and Application. Foods 2022, 11, 2189. [Google Scholar] [CrossRef]
- Sun, H.; Xue, Y.; Lin, Y. Enhanced Catalytic Efficiency in Quercetin-4′-Glucoside Hydrolysis of Thermotoga maritima β-Glucosidase A by Site-Directed Mutagenesis. J. Agric. Food Chem. 2014, 62, 6763–6770. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (Poly)Phenolics in Human Health: Structures, Bioavailability, and Evidence of Protective Effects against Chronic Diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Hong, J.; Wang, L.; Cai, C.; Mo, H.; Wang, J.; Fang, X.; Liao, Z. Effect of Lactic Acid Bacteria Fermentation on Plant-Based Products. Fermentation 2024, 10, 48. [Google Scholar] [CrossRef]
- Niamah, A.K.; Al-fekaiki, D.F.; Thyab Gddoa Al-Sahlany, S.; Verma, D.K.; Patel, A.R.; Singh, S. Investigating the Effect of Addition of Probiotic Microorganisms (Bacteria or Yeast) to Yoghurt on the Viability and Volatile Aromatic Profiles. J. Food Meas. Charact. 2023, 17, 5463–5473. [Google Scholar] [CrossRef]
- Gaya, P.; Peirotén, Á.; Landete, J.M. Expression of a β-Glucosidase in Bacteria with Biotechnological Interest Confers Them the Ability to Deglycosylate Lignans and Flavonoids in Vegetal Foods. Appl. Microbiol. Biotechnol. 2020, 104, 4903–4913. [Google Scholar] [CrossRef] [PubMed]
- Delgado, S.; Guadamuro, L.; Flórez, A.B.; Vázquez, L.; Mayo, B. Fermentation of Commercial Soy Beverages with Lactobacilli and Bifidobacteria Strains Featuring High β-Glucosidase Activity. Innov. Food Sci. Emerg. Technol. 2019, 51, 148–155. [Google Scholar] [CrossRef]
- Hati, S.; Vij, S.; Singh, B.P.; Mandal, S. β-Glucosidase Activity and Bioconversion of Isoflavones during Fermentation of Soymilk. J. Sci. Food Agric. 2015, 95, 216–220. [Google Scholar] [CrossRef]
- de Oliveira Galdino, I.K.C.P.; da Silva, M.O.M.; da Silva, A.P.A.; Santos, V.N.; Feitosa, R.L.P.; Ferreira, L.C.N.; Dantas, G.C.; dos Santos Pereira, E.V.; de Oliveira, T.A.; dos Santos, K.M.O.; et al. β-Glucosidase Activity and Antimicrobial Properties of Potentially Probiotic Autochthonous Lactic Cultures. PeerJ 2023, 11, e16094. [Google Scholar] [CrossRef]
- Letizia, F.; Fratianni, A.; Cofelice, M.; Testa, B.; Albanese, G.; Di Martino, C.; Panfili, G.; Lopez, F.; Iorizzo, M. Antioxidative Properties of Fermented Soymilk Using Lactiplantibacillus plantarum LP95. Antioxidants 2023, 12, 1442. [Google Scholar] [CrossRef]
- Son, S.-H.; Jeon, H.-L.; Yang, S.-J.; Sim, M.-H.; Kim, Y.-J.; Lee, N.-K.; Paik, H.-D. Probiotic Lactic Acid Bacteria Isolated from Traditional Korean Fermented Foods Based on β-Glucosidase Activity. Food Sci. Biotechnol. 2018, 27, 123–129. [Google Scholar] [CrossRef]
- Santos, M.M.; Piccirillo, C.; Castro, P.M.L.; Kalogerakis, N.; Pintado, M.E. Bioconversion of Oleuropein to Hydroxytyrosol by Lactic Acid Bacteria. World J. Microbiol. Biotechnol. 2012, 28, 2435–2440. [Google Scholar] [CrossRef]
- Landete, J.M.; Rodríguez, H.; Curiel, J.A.; de las Rivas, B.; de Felipe, F.L.; Muñoz, R. Degradation of Phenolic Compounds Found in Olive Products by Lactobacillus plantarum Strains. In Olives and Olive Oil in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2010; pp. 387–396. [Google Scholar]
- Pino, A.; Vaccalluzzo, A.; Solieri, L.; Romeo, F.V.; Todaro, A.; Caggia, C.; Arroyo-López, F.N.; Bautista-Gallego, J.; Randazzo, C.L. Effect of Sequential Inoculum of Beta-Glucosidase Positive and Probiotic Strains on Brine Fermentation to Obtain Low Salt Sicilian Table Olives. Front. Microbiol. 2019, 10, 174. [Google Scholar] [CrossRef]
- Servili, M.; Settanni, L.; Veneziani, G.; Esposto, S.; Massitti, O.; Taticchi, A.; Urbani, S.; Montedoro, G.F.; Corsetti, A. The Use of Lactobacillus pentosus 1MO To Shorten the Debittering Process Time of Black Table Olives (Cv. Itrana and Leccino): A Pilot-Scale Application. J. Agric. Food Chem. 2006, 54, 3869–3875. [Google Scholar] [CrossRef]
- De Leonardis, A.; Testa, B.; Macciola, V.; Lombardi, S.J.; Iorizzo, M. Exploring Enzyme and Microbial Technology for the Preparation of Green Table Olives. Eur. Food Res. Technol. 2016, 242, 363–370. [Google Scholar] [CrossRef]
- Grimaldi, A.; Bartowsky, E.; Jiranek, V. Screening of Lactobacillus Spp. and Pediococcus Spp. for Glycosidase Activities That Are Important in Oenology. J. Appl. Microbiol. 2005, 99, 1061–1069. [Google Scholar] [CrossRef]
- Grimaldi, A.; McLean, H.; Jiranek, V. Identification and Partial Characterization of Glycosidic Activities of Commercial Strains of the Lactic Acid Bacterium, Oenococcus oeni. Am. J. Enol. Vitic. 2000, 51, 362–369. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, N.; Xu, J.; Qi, Y.; Wei, X.; Fan, M. Homology Analysis of 35 β-Glucosidases in Oenococcus Oeni and Biochemical Characterization of a Novel β-Glucosidase BGL0224. Food Chem. 2021, 334, 127593. [Google Scholar] [CrossRef]
- Maturano, C.; Saguir, F.M. Influence of Glycosides on Behavior of Oenococcus Oeni in Wine Conditions: Growth, Substrates and Aroma Compounds. World J. Microbiol. Biotechnol. 2017, 33, 151. [Google Scholar] [CrossRef]
- Michlmayr, H.; Schümann, C.; Wurbs, P.; Barreira Braz da Silva, N.M.; Rogl, V.; Kulbe, K.D.; del Hierro, A.M. A β-Glucosidase from Oenococcus Oeni ATCC BAA-1163 with Potential for Aroma Release in Wine: Cloning and Expression in E. coli. World J. Microbiol. Biotechnol. 2010, 26, 1281–1289. [Google Scholar] [CrossRef]
- D’Incecco, N.; Bartowsky, E.; Kassara, S.; Lante, A.; Spettoli, P.; Henschke, P. Release of Glycosidically Bound Flavour Compounds of Chardonnay by Oenococcus Oeni during Malolactic Fermentation. Food Microbiol. 2004, 21, 257–265. [Google Scholar] [CrossRef]
- Iorizzo, M.; Testa, B.; Lombardi, S.J.; García-Ruiz, A.; Muñoz-González, C.; Bartolomé, B.; Moreno-Arribas, M.V. Selection and Technological Potential of Lactobacillus plantarum Bacteria Suitable for Wine Malolactic Fermentation and Grape Aroma Release. LWT 2016, 73, 557–566. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, C.; Zhang, H.; Qu, G.; Li, C.; Liu, L. Biotransformation of Polyphenols in Apple Pomace Fermented by β-Glucosidase-Producing Lactobacillus rhamnosus L08. Foods 2021, 10, 1343. [Google Scholar] [CrossRef]
- Modrackova, N.; Vlkova, E.; Tejnecky, V.; Schwab, C.; Neuzil-Bunesova, V. Bifidobacterium β-Glucosidase Activity and Fermentation of Dietary Plant Glucosides Is Species and Strain Specific. Microorganisms 2020, 8, 839. [Google Scholar] [CrossRef]
- Di Cagno, R.; Filannino, P.; Gobbetti, M. Lactic Acid Fermentation Drives the Optimal Volatile Flavor-Aroma Profile of Pomegranate Juice. Int. J. Food Microbiol. 2017, 248, 56–62. [Google Scholar] [CrossRef]
- Martorana, A.; Alfonzo, A.; Gaglio, R.; Settanni, L.; Corona, O.; La Croce, F.; Vagnoli, P.; Caruso, T.; Moschetti, G.; Francesca, N. Evaluation of Different Conditions to Enhance the Performances of Lactobacillus Pentosus OM13 during Industrial Production of Spanish-Style Table Olives. Food Microbiol. 2017, 61, 150–158. [Google Scholar] [CrossRef]
- Lei, V.; Amoa-Awua, W.K.A.; Brimer, L. Degradation of Cyanogenic Glycosides by Lactobacillus plantarum Strains from Spontaneous Cassava Fermentation and Other Microorganisms. Int. J. Food Microbiol. 1999, 53, 169–184. [Google Scholar] [CrossRef]
- Menon, R.; Munjal, N.; Sturino, J.M. Characterization of Amygdalin-Degrading Lactobacillus Species. J. Appl. Microbiol. 2015, 118, 443–453. [Google Scholar] [CrossRef]
- Gunawan, S.; Widjaja, T.; Zullaikah, S.; Ernawati, L.; Istianah, N.; Aparamarta, H.W.; Prasetyoko, D. Effect of Fermenting Cassava with Lactobacillus plantarum, Saccharomyces Cereviseae, and Rhizopus Oryzae on the Chemical Composition of Their Flour. Int. Food Res. J. 2015, 22, 1280–1287. [Google Scholar]
- Bustamante-Rangel, M.; Delgado-Zamarreño, M.M.; Pérez-Martín, L.; Rodríguez-Gonzalo, E.; Domínguez-Álvarez, J. Analysis of Isoflavones in Foods. Compr. Rev. Food Sci. Food Saf. 2018, 17, 391–411. [Google Scholar] [CrossRef] [PubMed]
- Atlante, A.; Bobba, A.; Paventi, G.; Pizzuto, R.; Passarella, S. Genistein and Daidzein Prevent Low Potassium-Dependent Apoptosis of Cerebellar Granule Cells. Biochem. Pharmacol. 2010, 79, 758–767. [Google Scholar] [CrossRef][Green Version]
- Sharma, D.; Singh, V.; Kumar, A.; Singh, T.G. Genistein: A Promising Ally in Combating Neurodegenerative Disorders. Eur. J. Pharmacol. 2025, 991, 177273. [Google Scholar] [CrossRef]
- Zaheer, K.; Humayoun Akhtar, M. An Updated Review of Dietary Isoflavones: Nutrition, Processing, Bioavailability and Impacts on Human Health. Crit. Rev. Food Sci. Nutr. 2017, 57, 1280–1293. [Google Scholar] [CrossRef]
- Jayachandran, M.; Xu, B. An Insight into the Health Benefits of Fermented Soy Products. Food Chem. 2019, 271, 362–371. [Google Scholar] [CrossRef]
- Hu, C.; Wong, W.T.; Wu, R.; Lai, W.F. Biochemistry and Use of Soybean Isoflavones in Functional Food Development. Crit. Rev. Food Sci. Nutr. 2020, 60, 2098–2112. [Google Scholar] [CrossRef]
- Jung, Y.S.; Rha, C.-S.; Baik, M.-Y.; Baek, N.-I.; Kim, D.-O. A Brief History and Spectroscopic Analysis of Soy Isoflavones. Food Sci. Biotechnol. 2020, 29, 1605–1617. [Google Scholar] [CrossRef]
- Izumi, T.; Osawa, S.; Obata, A.; Tobe, K.; Saito, M.; Kataoka, S.; Kikuchi, M.; Piskula, M.K.; Kubota, Y. Soy Isoflavone Aglycones Are Absorbed Faster and in Higher Amounts than Their Glucosides in Humans. J. Nutr. 2000, 130, 1695–1699. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.; Zhu, D.; Xu, J.; Xu, X.; Liu, J. Bioaccessibility and Application of Soybean Isoflavones: A Review. Food Rev. Int. 2022, 39, 5948–5967. [Google Scholar] [CrossRef]
- Setchell, K.D.; Brown, N.M.; Zimmer-Nechemias, L.; Brashear, W.T.; Wolfe, B.E.; Kirschner, A.S.; Heubi, J.E. Evidence for Lack of Absorption of Soy Isoflavone Glycosides in Humans, Supporting the Crucial Role of Intestinal Metabolism for Bioavailability. Am. J. Clin. Nutr. 2002, 76, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Chien, H.-L.; Huang, H.-Y.; Chou, C.-C. Transformation of Isoflavone Phytoestrogens during the Fermentation of Soymilk with Lactic Acid Bacteria and Bifidobacteria. Food Microbiol. 2006, 23, 772–778. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.; Kim, G.M.; Lee, K.W.; Choi, I.D.; Kwon, G.; Park, J.; Jeong, S.; Kim, J.; Kim, J.H. Conversion of Isoflavone Glucosides to Aglycones in Soymilk by Fermentation with Lactic Acid Bacteria. J. Food Sci. 2007, 72, M39–M44. [Google Scholar] [CrossRef]
- Pyo, Y.-H.; Lee, T.-C.; Lee, Y.-C. Enrichment of Bioactive Isoflavones in Soymilk Fermented with β-Glucosidase-Producing Lactic Acid Bacteria. Food Res. Int. 2005, 38, 551–559. [Google Scholar] [CrossRef]
- Lim, Y.J.; Lim, B.; Kim, H.Y.; Kwon, S.-J.; Eom, S.H. Deglycosylation Patterns of Isoflavones in Soybean Extracts Inoculated with Two Enzymatically Different Strains of Lactobacillus Species. Enzym. Microb. Technol. 2020, 132, 109394. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.L.; Shah, N.P.; Wilcox, G.; Walker, K.Z.; Stojanovska, L. Fermentation of Calcium-Fortified Soymilk with Lactobacillus: Effects on Calcium Solubility, Isoflavone Conversion, and Production of Organic Acids. J. Food Sci. 2007, 72, M431–M436. [Google Scholar] [CrossRef]
- Du, L.; Ro, K.-S.; Zhang, Y.; Tang, Y.-J.; Li, W.; Xie, J.; Wei, D. Effects of Lactiplantibacillus plantarum X7021 on Physicochemical Properties, Purines, Isoflavones and Volatile Compounds of Fermented Soymilk. Process Biochem. 2022, 113, 150–157. [Google Scholar] [CrossRef]
- Hidayati, D.; Soetjipto, S.; Catur Adi, A. Characteristic and Isoflavone Level of Soymilk Fermented by Single and Mixed Culture of Lactobacillus plantarum and Yoghurt Starter. J. Food Nutr. Res. 2021, 9, 55–60. [Google Scholar] [CrossRef]
- Izaguirre, J.K.; Barañano, L.; Castañón, S.; Alkorta, I.; Quirós, L.M.; Garbisu, C. Optimization of the Bioactivation of Isoflavones in Soymilk by Lactic Acid Bacteria. Processes 2021, 9, 963. [Google Scholar] [CrossRef]
- Bock, H.-J.; Lee, H.-W.; Lee, N.-K.; Paik, H.-D. Probiotic Lactiplantibacillus plantarum KU210152 and Its Fermented Soy Milk Attenuates Oxidative Stress in Neuroblastoma Cells. Food Res. Int. 2024, 177, 113868. [Google Scholar] [CrossRef]
- Choi, G.-H.; Bock, H.-J.; Lee, N.-K.; Paik, H.-D. Soy Yogurt Using Lactobacillus plantarum 200655 and Fructooligosaccharides: Neuroprotective Effects against Oxidative Stress. J. Food Sci. Technol. 2022, 59, 4870–4879. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Hou, K.; Mu, G.; Ma, C.; Tuo, Y. Antioxidative Effect of Soybean Milk Fermented by Lactobacillus plantarum Y16 on 2, 2-Azobis (2-Methylpropionamidine) Dihydrochloride (ABAP)-Damaged HepG2 Cells. Food Biosci. 2021, 44, 101120. [Google Scholar] [CrossRef]
- Peng, H.T.; Yang, C.Y.; Fang, T.J. Enhanced β-Glucosidase Activity of Lactobacillus plantarum by a Strategic Ultrasound Treatment for Biotransformation of Isoflavones in Okara. Food Sci. Technol. Res. 2018, 24, 777–784. [Google Scholar] [CrossRef]
- Wang, R.; Thakur, K.; Feng, J.-Y.; Zhu, Y.-Y.; Zhang, F.; Russo, P.; Spano, G.; Zhang, J.-G.; Wei, Z.-J. Functionalization of Soy Residue (Okara) by Enzymatic Hydrolysis and LAB Fermentation for B2 Bio-Enrichment and Improved in Vitro Digestion. Food Chem. 2022, 387, 132947. [Google Scholar] [CrossRef]
- Parmar, A.; Sturm, B.; Hensel, O. Crops That Feed the World: Production and Improvement of Cassava for Food, Feed, and Industrial Uses. Food Secur. 2017, 9, 907–927. [Google Scholar] [CrossRef]
- Malik, A.I.; Kongsil, P.; Nguyễn, V.A.; Ou, W.; Sholihin; Srean, P.; Sheela, M.; Becerra López-Lavalle, L.A.; Utsumi, Y.; Lu, C.; et al. Cassava Breeding and Agronomy in Asia: 50 Years of History and Future Directions. Breed. Sci. 2020, 70, 145–166. [Google Scholar] [CrossRef]
- Halake, N.H.; Chinthapalli, B. Fermentation of Traditional African Cassava Based Foods: Microorganisms Role in Nutritional and Safety Value. J. Exp. Agric. Int. 2020, 42, 56–65. [Google Scholar] [CrossRef]
- Lacerda, I.; Miranda, R.; Borelli, B.; Nunes, A.; Nardi, R.; Lachance, M.; Rosa, C. Lactic Acid Bacteria and Yeasts Associated with Spontaneous Fermentations during the Production of Sour Cassava Starch in Brazil. Int. J. Food Microbiol. 2005, 105, 213–219. [Google Scholar] [CrossRef]
- Howeler, R.; Lutaladio, N.; Thomas, G. Save and Grow: Cassava: A Guide to Sustainable Production Intensification; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013. [Google Scholar]
- Latif, S.; Müller, J. Potential of Cassava Leaves in Human Nutrition: A Review. Trends Food Sci. Technol. 2015, 44, 147–158. [Google Scholar] [CrossRef]
- Adebayo, W.G. Cassava Production in Africa: A Panel Analysis of the Drivers and Trends. Heliyon 2023, 9, e19939. [Google Scholar] [CrossRef]
- Kashala-Abotnes, E.; Okitundu, D.; Mumba, D.; Boivin, M.J.; Tylleskär, T.; Tshala-Katumbay, D. Konzo: A Distinct Neurological Disease Associated with Food (Cassava) Cyanogenic Poisoning. Brain Res. Bull. 2019, 145, 87–91. [Google Scholar] [CrossRef]
- Adamolekun, B. Neurological Disorders Associated with Cassava Diet: A Review of Putative Etiological Mechanisms. Metab. Brain Dis. 2011, 26, 79–85. [Google Scholar] [CrossRef]
- Fukushima, A.R.; Nicoletti, M.A.; Rodrigues, A.J.; Pressutti, C.; Almeida, J.; Brandão, T.; Kinue Ito, R.; Bafille Leoni, L.A.; Souza Spinosa, H. De Cassava Flour: Quantification of Cyanide Content. Food Nutr. Sci. 2016, 07, 592–599. [Google Scholar] [CrossRef]
- Cressey, P.; Reeve, J. Metabolism of Cyanogenic Glycosides: A Review. Food Chem. Toxicol. 2019, 125, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Renchinkhand, G.; Park, Y.W.; Cho, S.-H.; Song, G.-Y.; Bae, H.C.; Choi, S.-J.; Nam, M.S. Identification of β-Glucosidase Activity of Lactobacillus plantarum CRNB22 in Kimchi and Its Potential to Convert Ginsenoside Rb1 from Panax ginseng. J. Food Biochem. 2015, 39, 155–163. [Google Scholar] [CrossRef]
- National Research Council. Acute Exposure Guideline Levels for Selected Airborne Chemicals; National Academies Press: Washington, DC, USA, 2002; ISBN 978-0-309-08511-3. [Google Scholar]
- Oloya, B.; Adaku, C.; Andama, M. The Cyanogenic Potential of Certain Cassava Varieties in Uganda and Their Fermentation-Based Detoxification. In Cassava—Recent Updates on Food, Feed, and Industry; IntechOpen: London, UK, 2024. [Google Scholar]
- Brimer, L. Cassava Production and Processing and Impact on Biological Compounds; Academic Press: Cambridge, MA, USA, 2015; ISBN 9780124047099. [Google Scholar]
- Penido, F.C.L.; Piló, F.B.; Sandes, S.H.d.C.; Nunes, Á.C.; Colen, G.; Oliveira, E.D.S.; Rosa, C.A.; Lacerda, I.C.A. Selection of Starter Cultures for the Production of Sour Cassava Starch in a Pilot-Scale Fermentation Process. Braz. J. Microbiol. 2018, 49, 823–831. [Google Scholar] [CrossRef]
- Bouatenin, K.M.J.-P.; Theodore, D.N.; Alfred, K.K.; Hermann, C.W.; Marcellin, D.K. Excretion of β-Glucosidase and Pectinase by Microorganisms Isolated from Cassava Traditional Ferments Used for Attieke Production in Côte d’Ivoire. Biocatal. Agric. Biotechnol. 2019, 20, 101217. [Google Scholar] [CrossRef]
- Panghal, A.; Munezero, C.; Sharma, P.; Chhikara, N. Cassava Toxicity, Detoxification and Its Food Applications: A Review. Toxin Rev. 2021, 40, 1–16. [Google Scholar] [CrossRef]
- Ben Omar, N.; Ampe, F.; Raimbault, M.; Guyot, J.-P.; Tailliez, P. Molecular Diversity of Lactic Acid Bacteria from Cassava Sour Starch (Colombia). Syst. Appl. Microbiol. 2000, 23, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Bamigbade, G.B.; Sanusi, J.F.O.; Oyelami, O.I.; Daniel, O.M.; Alimi, B.O.; Ampofo, K.A.; Liu, S.-Q.; Shah, N.P.; Ayyash, M. Identification and Characterization of Lactic Acid Bacteria Isolated from Effluents Generated During Cassava Fermentation as Potential Candidates for Probiotics. Food Biotechnol. 2023, 37, 413–433. [Google Scholar] [CrossRef]
- Oyedeji, O.; Ogunbanwo, S.T.; Onilude, A.A. Predominant Lactic Acid Bacteria Involved in the Traditional Fermentation of Fufu and Ogi, Two Nigerian Fermented Food Products. Food Nutr. Sci. 2013, 04, 40–46. [Google Scholar] [CrossRef]
- Putri, W.D.R.; Haryadi; Marseno, D.W.; Cahyanto, M.N. Role of Lactic Acid Bacteria on Structural and Physicochemical Properties of Sour Cassava Starch. APCBEE Procedia 2012, 2, 104–109. [Google Scholar] [CrossRef]
- Crispim, S.M.; Nascimento, A.M.A.; Costa, P.S.; Moreira, J.L.S.; Nunes, A.C.; Nicoli, J.R.; Lima, F.L.; Mota, V.T.; Nardi, R.M.D. Molecular Identification of Lactobacillus Spp. Associated with Puba, a Brazilian Fermented Cassava Food. Braz. J. Microbiol. 2013, 44, 15–21. [Google Scholar] [CrossRef]
- Wilfrid Padonou, S.; Nielsen, D.S.; Hounhouigan, J.D.; Thorsen, L.; Nago, M.C.; Jakobsen, M. The Microbiota of Lafun, an African Traditional Cassava Food Product. Int. J. Food Microbiol. 2009, 133, 22–30. [Google Scholar] [CrossRef]
- Figueroa, C.; Davila, A.M.; Pourquié, J. Lactic Acid Bacteria of the Sour Cassava Starch Fermentation. Lett. Appl. Microbiol. 1995, 21, 126–130. [Google Scholar] [CrossRef]
- Kostinek, M.; Specht, I.; Edward, V.A.; Pinto, C.; Egounlety, M.; Sossa, C.; Mbugua, S.; Dortu, C.; Thonart, P.; Taljaard, L.; et al. Characterisation and Biochemical Properties of Predominant Lactic Acid Bacteria from Fermenting Cassava for Selection as Starter Cultures. Int. J. Food Microbiol. 2007, 114, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Wakil, S.M.; Benjamin, I.B. Starter Developed Pupuru, a Traditional Africa Fermented Food from Cassava (Manihot Esculenta). Int. Food Res. J. 2015, 22, 2565–2570. [Google Scholar]
- Kostinek, M.; Specht, I.; Edward, V.A.; Schillinger, U.; Hertel, C.; Holzapfel, W.H.; Franz, C.M.A.P. Diversity and Technological Properties of Predominant Lactic Acid Bacteria from Fermented Cassava Used for the Preparation of Gari, a Traditional African Food. Syst. Appl. Microbiol. 2005, 28, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Tefera, T.; Ameha, K.; Biruhtesfa, A. Cassava Based Foods: Microbial Fermentation by Single Starter Culture towards Cyanide Reduction, Protein Enhancement and Palatability. Int. Food Res. J. 2014, 21, 1751–1756. [Google Scholar]
- Kimaryo, V.M.; Massawe, G.A.; Olasupo, N.A.; Holzapfel, W.H. The Use of a Starter Culture in the Fermentation of Cassava for the Production of “Kivunde”, a Traditional Tanzanian Food Product. Int. J. Food Microbiol. 2000, 56, 179–190. [Google Scholar] [CrossRef]
- Damayanti, E.; Kurniadi, M.; Helmi, R.L.; Frediansyah, A. Single Starter Lactobacillus plantarum for Modified Cassava Flour (Mocaf) Fermentation. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Changsha, China, 18–20 September 2020; Volume 462. [Google Scholar]
- Charoenprasert, S.; Mitchell, A. Factors Influencing Phenolic Compounds in Table Olives (Olea Europaea). J. Agric. Food Chem. 2012, 60, 7081–7095. [Google Scholar] [CrossRef] [PubMed]
- Guggenheim, K.G.; Crawford, L.M.; Paradisi, F.; Wang, S.C.; Siegel, J.B. β-Glucosidase Discovery and Design for the Degradation of Oleuropein. ACS Omega 2018, 3, 15754–15762. [Google Scholar] [CrossRef]
- Rokni, Y.; Abouloifa, H.; Bellaouchi, R.; Gaamouche, S.; Mchiouer, K.; Hasnaoui, I.; Lamzira, Z.; Ghabbour, N.; Asehraou, A. Technological Process of Fermented Olive. Arab. J. Chem. Environ. Res. 2017, 07, 63–91. [Google Scholar]
- Habibi, M.; Golmakani, M.-T.; Farahnaky, A.; Mesbahi, G.; Majzoobi, M. NaOH-Free Debittering of Table Olives Using Power Ultrasound. Food Chem. 2016, 192, 775–781. [Google Scholar] [CrossRef]
- Corsetti, A.; Perpetuini, G.; Schirone, M.; Tofalo, R.; Suzzi, G. Application of Starter Cultures to Table Olive Fermentation: An Overview on the Experimental Studies. Front. Microbiol. 2012, 3, 248. [Google Scholar] [CrossRef] [PubMed]
- Ciafardini, G.; Marsilio, V.; Lanza, B.; Pozzi, N. Hydrolysis of Oleuropein by Lactobacillus plantarum Strains Associated with Olive Fermentation. Appl. Environ. Microbiol. 1994, 60, 4142–4147. [Google Scholar] [CrossRef]
- Rokni, Y.; Abouloifa, H.; Bellaouchi, R.; Hasnaoui, I.; Gaamouche, S.; Lamzira, Z.; Salah, R.B.E.N.; Saalaoui, E.; Ghabbour, N.; Asehraou, A. Characterization of β-Glucosidase of Lactobacillus plantarum FSO1 and Candida Pelliculosa L18 Isolated from Traditional Fermented Green Olive. J. Genet. Eng. Biotechnol. 2021, 19, 117. [Google Scholar] [CrossRef]
- Ghabbour, N.; Rokni, Y.; Abouloifa, H.; Bellaouchi, R.; Chihib, N.-E.; Ben Salah, R.; Lamzira, Z.; Saalaoui, E.; Asehraou, A. In Vitro Biodegradation of Oleuropein by Lactobacillus plantarum FSO175 in Stress Conditions (pH, NaCl and Glucose). J. Microbiol. Biotechnol. Food Sci. 2020, 9, 769–773. [Google Scholar] [CrossRef]
- Ghabbour, N.; Rokni, Y.; Lamzira, Z.; Thonart, P.; Chihib, N.E.; Peres, C.; Asehraou, A. Controlled Fermentation of Moroccan Picholine Green Olives by Oleuropein-Degrading Lactobacilli Strains. Grasas Aceites 2016, 67, e138. [Google Scholar] [CrossRef]
- Vaccalluzzo, A.; Pino, A.; De Angelis, M.; Bautista-Gallego, J.; Romeo, F.V.; Foti, P.; Caggia, C.; Randazzo, C.L. Effects of Different Stress Parameters on Growth and on Oleuropein-Degrading Abilities of Lactiplantibacillus plantarum Strains Selected as Tailored Starter Cultures for Naturally Table Olives. Microorganisms 2020, 8, 1607. [Google Scholar] [CrossRef]
- Zago, M.; Lanza, B.; Rossetti, L.; Muzzalupo, I.; Carminati, D.; Giraffa, G. Selection of Lactobacillus plantarum Strains to Use as Starters in Fermented Table Olives: Oleuropeinase Activity and Phage Sensitivity. Food Microbiol. 2013, 34, 81–87. [Google Scholar] [CrossRef]
- Vaccalluzzo, A.; Solieri, L.; Tagliazucchi, D.; Cattivelli, A.; Martini, S.; Pino, A.; Caggia, C.; Randazzo, C.L. Metabolomic and Transcriptional Profiling of Oleuropein Bioconversion into Hydroxytyrosol during Table Olive Fermentation by Lactiplantibacillus plantarum. Appl. Environ. Microbiol. 2022, 88, e0201921. [Google Scholar] [CrossRef]
- Romeo, F.V.; Granuzzo, G.; Foti, P.; Ballistreri, G.; Caggia, C.; Rapisarda, P. Microbial Application to Improve Olive Mill Wastewater Phenolic Extracts. Molecules 2021, 26, 1944. [Google Scholar] [CrossRef]
- Sciurba, L.; Indelicato, S.; Gaglio, R.; Barbera, M.; Marra, F.P.; Bongiorno, D.; Davino, S.; Piazzese, D.; Settanni, L.; Avellone, G. Analysis of Olive Oil Mill Wastewater from Conventionally Farmed Olives: Chemical and Microbiological Safety and Polyphenolic Profile for Possible Use in Food Product Functionalization. Foods 2025, 14, 449. [Google Scholar] [CrossRef]
- Cappello, M.S.; Zapparoli, G.; Logrieco, A.; Bartowsky, E.J. Linking Wine Lactic Acid Bacteria Diversity with Wine Aroma and Flavour. Int. J. Food Microbiol. 2017, 243, 16–27. [Google Scholar] [CrossRef]
- Virdis, C.; Sumby, K.; Bartowsky, E.; Jiranek, V. Lactic Acid Bacteria in Wine: Technological Advances and Evaluation of Their Functional Role. Front. Microbiol. 2021, 11, 612118. [Google Scholar] [CrossRef]
- Boido, E.; Lloret, A.; Medina, K.; Carrau, F.; Dellacassa, E. Effect of β-Glycosidase Activity of Oenococcus oeni on the Glycosylated Flavor Precursors of Tannat Wine during Malolactic Fermentation. J. Agric. Food Chem. 2002, 50, 2344–2349. [Google Scholar] [CrossRef]
- Swiegers, J.H.; Bartowsky, E.J.; Henschke, P.A.; Pretorius, I.S. Yeast and Bacterial Modulation of Wine Aroma and Flavour. Aust. J. Grape Wine Res. 2005, 11, 139–173. [Google Scholar] [CrossRef]
- Bartowsky, E.J.; Costello, P.J.; Chambers, P.J. Emerging Trends in the Application of Malolactic Fermentation. Aust. J. Grape Wine Res. 2015, 21, 663–669. [Google Scholar] [CrossRef]
- du Toit, M.; Engelbrecht, L.; Lerm, E.; Krieger-Weber, S. Lactobacillus: The Next Generation of Malolactic Fermentation Starter Cultures—An Overview. Food Bioprocess Techol. 2011, 4, 876–906. [Google Scholar] [CrossRef]
- Krieger-Weber, S.; Heras, J.M.; Suarez, C. Lactobacillus plantarum, a New Biological Tool to Control Malolactic Fermentation: A Review and an Outlook. Beverages 2020, 6, 23. [Google Scholar] [CrossRef]
- Berbegal, C.; Peña, N.; Russo, P.; Grieco, F.; Pardo, I.; Ferrer, S.; Spano, G.; Capozzi, V. Technological Properties of Lactobacillus plantarum Strains Isolated from Grape Must Fermentation. Food Microbiol. 2016, 57, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Succi, M.; Pannella, G.; Tremonte, P.; Tipaldi, L.; Coppola, R.; Iorizzo, M.; Lombardi, S.J.; Sorrentino, E. Sub-Optimal pH Preadaptation Improves the Survival of Lactobacillus plantarum Strains and the Malic Acid Consumption in Wine-like Medium. Front. Microbiol. 2017, 8, 470. [Google Scholar] [CrossRef]
- Tufariello, M.; Capozzi, V.; Spano, G.; Cantele, G.; Venerito, P.; Mita, G.; Grieco, F. Effect of Co-Inoculation of Candida zemplinina, Saccharomyces cerevisiae and Lactobacillus plantarum for the Industrial Production of Negroamaro Wine in Apulia (Southern Italy). Microorganisms 2020, 8, 726. [Google Scholar] [CrossRef]
- Pannella, G.; Lombardi, S.J.; Coppola, F.; Vergalito, F.; Iorizzo, M.; Succi, M.; Tremonte, P.; Iannini, C.; Sorrentino, E.; Coppola, R. Effect of Biofilm Formation by Lactobacillus plantarum on the Malolactic Fermentation in Model Wine. Foods 2020, 9, 797. [Google Scholar] [CrossRef]
- Bravo-Ferrada, B.M.; Hollmann, A.; Delfederico, L.; Valdés La Hens, D.; Caballero, A.; Semorile, L. Patagonian Red Wines: Selection of Lactobacillus plantarum Isolates as Potential Starter Cultures for Malolactic Fermentation. World J. Microbiol. Biotechnol. 2013, 29, 1537–1549. [Google Scholar] [CrossRef]
- Balmaseda, A.; Rozès, N.; Bordons, A.; Reguant, C. Characterization of Malolactic Fermentation by Lactiplantibacillus plantarum in Red Grape Must. LWT 2024, 199, 116070. [Google Scholar] [CrossRef]
- Lombardi, S.J.; Pannella, G.; Iorizzo, M.; Testa, B.; Succi, M.; Tremonte, P.; Sorrentino, E.; Di Renzo, M.; Strollo, D.; Coppola, R. Inoculum Strategies and Performances of Malolactic Starter Lactobacillus plantarum M10: Impact on Chemical and Sensorial Characteristics of Fiano Wine. Microorganisms 2020, 8, 516. [Google Scholar] [CrossRef]
- Devi, A.; Anu-Appaiah, K.A.; Lin, T.F. Timing of Inoculation of Oenococcus Oeni and Lactobacillus plantarum in Mixed Malo-Lactic Culture along with Compatible Native Yeast Influences the Polyphenolic, Volatile and Sensory Profile of the Shiraz Wines. LWT 2022, 158, 113130. [Google Scholar] [CrossRef]
- Brizuela, N.; Tymczyszyn, E.E.; Semorile, L.C.; Valdes La Hens, D.; Delfederico, L.; Hollmann, A.; Bravo-Ferrada, B. Lactobacillus plantarum as a Malolactic Starter Culture in Winemaking: A New (Old) Player? Electron. J. Biotechnol. 2019, 38, 10–18. [Google Scholar] [CrossRef]
- Brizuela, N.S.; Franco-Luesma, E.; Bravo-Ferrada, B.M.; Pérez-Jiménez, M.; Semorile, L.; Tymczyszyn, E.E.; Pozo-Bayon, M.A. Influence of Patagonian Lactiplantibacillus plantarum and Oenococcus oeni Strains on Sensory Perception of Pinot Noir Wine after Malolactic Fermentation. Aust. J. Grape Wine Res. 2021, 27, 118–127. [Google Scholar] [CrossRef]
- Sestelo, A.B.F.; Poza, M.; Villa, T.G. β-Glucosidase Activity in a Lactobacillus plantarum Wine Strain. World J. Microbiol. Biotechnol. 2004, 20, 633–637. [Google Scholar] [CrossRef]
- Brizuela, N.S.; Arnez-Arancibia, M.; Semorile, L.; Pozo-Bayón, M.Á.; Bravo-Ferrada, B.M.; Elizabeth Tymczyszyn, E. β-Glucosidase Activity of Lactiplantibacillus plantarum UNQLp 11 in Different Malolactic Fermentations Conditions: Effect of pH and Ethanol Content. Fermentation 2021, 7, 22. [Google Scholar] [CrossRef]
- Gouripur, G.; Kaliwal, B. Screening and Optimization of β-Glucosidase Producing Newly Isolated Lactobacillus plantarum Strain LSP-24 from Colostrum Milk. Biocatal. Agric. Biotechnol. 2017, 11, 89–96. [Google Scholar] [CrossRef]
- Barbagallo, R.N.; Spagna, G.; Palmeri, R.; Restuccia, C.; Giudici, P. Selection, Characterization and Comparison of β-Glucosidase from Mould and Yeasts Employable for Enological Applications. Enzym. Microb. Technol. 2004, 35, 58–66. [Google Scholar] [CrossRef]
- Olguín, N.; Alegret, J.O.; Bordons, A.; Reguant, C. β-Glucosidase Activity and Bgl Gene Expression of Oenococcus Oeni Strains in Model Media and Cabernet Sauvignon Wine. Am. J. Enol. Vitic. 2011, 62, 99–105. [Google Scholar] [CrossRef]
- Fia, G.; Millarini, V.; Granchi, L.; Bucalossi, G.; Guerrini, S.; Zanoni, B.; Rosi, I. Beta-Glucosidase and Esterase Activity from Oenococcus Oeni: Screening and Evaluation during Malolactic Fermentation in Harsh Conditions. LWT 2018, 89, 262–268. [Google Scholar] [CrossRef]
- Lorn, D.; Nguyen, T.-K.-C.; Ho, P.-H.; Tan, R.; Licandro, H.; Waché, Y. Screening of Lactic Acid Bacteria for Their Potential Use as Aromatic Starters in Fermented Vegetables. Int. J. Food Microbiol. 2021, 350, 109242. [Google Scholar] [CrossRef]
- Landete, J.M.; Curiel, J.A.; Rodríguez, H.; de las Rivas, B.; Muñoz, R. Aryl Glycosidases from Lactobacillus plantarum Increase Antioxidant Activity of Phenolic Compounds. J. Funct. Foods 2014, 7, 322–329. [Google Scholar] [CrossRef]
- Wang, S.-Y.; Zhu, H.-Z.; Lan, Y.-B.; Liu, R.-J.; Liu, Y.-R.; Zhang, B.-L.; Zhu, B.-Q. Modifications of Phenolic Compounds, Biogenic Amines, and Volatile Compounds in Cabernet Gernishct Wine through Malolactic Fermentation by Lactobacillus plantarum and Oenococcus oeni. Fermentation 2020, 6, 15. [Google Scholar] [CrossRef]
- Van Oevelen, D.; Spaepen, M.; Timmermans, P.; Verachtert, H. Microbiological Aspects of Spontaneous Wort Fermentation in the Production of Lambic and Gueuze. J. Inst. Brew. 1977, 83, 356–360. [Google Scholar] [CrossRef]
- Dysvik, A.; La Rosa, S.L.; De Rouck, G.; Rukke, E.-O.; Westereng, B.; Wicklund, T. Microbial Dynamics in Traditional and Modern Sour Beer Production. Appl. Environ. Microbiol. 2020, 86, e00566-20. [Google Scholar] [CrossRef]
- Testa, B.; Coppola, F.; Letizia, F.; Albanese, G.; Karaulli, J.; Ruci, M.; Pistillo, M.; Germinara, G.S.; Messia, M.C.; Succi, M.; et al. Versatility of Saccharomyces Cerevisiae 41CM in the Brewery Sector: Use as a Starter for “Ale” and “Lager” Craft Beer Production. Processes 2022, 10, 2495. [Google Scholar] [CrossRef]
- Karaulli, J.; Xhaferaj, N.; Coppola, F.; Testa, B.; Letizia, F.; Kyçyk, O.; Kongoli, R.; Ruci, M.; Lamçe, F.; Sulaj, K.; et al. Bioprospecting of Metschnikowia pulcherrima Strains, Isolated from a Vineyard Ecosystem, as Novel Starter Cultures for Craft Beer Production. Fermentation 2024, 10, 513. [Google Scholar] [CrossRef]
- Iorizzo, M.; Letizia, F.; Albanese, G.; Coppola, F.; Gambuti, A.; Testa, B.; Aversano, R.; Forino, M.; Coppola, R. Potential for Lager Beer Production from Saccharomyces Cerevisiae Strains Isolated from the Vineyard Environment. Processes 2021, 9, 1628. [Google Scholar] [CrossRef]
- Bossaert, S.; Crauwels, S.; Lievens, B.; De Rouck, G. The Power of Sour—A Review: Old Traditions, New Opportunities. BrewingScience 2019, 72, 78–88. [Google Scholar]
- Mahanta, S.; Sivakumar, P.S.; Parhi, P.; Mohapatra, R.K.; Dey, G.; Panda, S.H.; Sireswar, S.; Panda, S.K. Sour Beer Production in India Using a Coculture of Saccharomyces Pastorianus and Lactobacillus plantarum: Optimization, Microbiological, and Biochemical Profiling. Braz. J. Microbiol. 2022, 53, 947–958. [Google Scholar] [CrossRef]
- Cai, G.; Cao, Y.; Xiao, J.; Sheng, G.; Lu, J. The Mechanisms of Lactiplantibacillus plantarum J6-6 against Iso-α-Acid Stress and Its Application in Sour Beer Production. Syst. Microbiol. Biomanuf. 2024, 4, 1018–1027. [Google Scholar] [CrossRef]
- Nyhan, L.; Sahin, A.W.; Arendt, E.K. Co-Fermentation of Non-Saccharomyces Yeasts with Lactiplantibacillus plantarum FST 1.7 for the Production of Non-Alcoholic Beer. Eur. Food Res. Technol. 2023, 249, 167–181. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, T.; Fu, X.; Abbasi, A.M.; Zheng, B.; Liu, R.H. Phenolics Content, Antioxidant and Antiproliferative Activities of Dehulled Highland Barley (Hordeum vulgare L.). J. Funct. Foods 2015, 19, 439–450. [Google Scholar] [CrossRef]
- Aron, P.M.; Shellhammer, T.H. A Discussion of Polyphenols in Beer Physical and Flavour Stability. J. Inst. Brew. 2010, 116, 369–380. [Google Scholar] [CrossRef]
- De Francesco, G.; Bravi, E.; Sanarica, E.; Marconi, O.; Cappelletti, F.; Perretti, G. Effect of Addition of Different Phenolic-Rich Extracts on Beer Flavour Stability. Foods 2020, 9, 1638. [Google Scholar] [CrossRef] [PubMed]
- Humia, B.V.; Santos, K.S.; Barbosa, A.M.; Sawata, M.; Mendonça, M.d.C.; Padilha, F.F. Beer Molecules and Its Sensory and Biological Properties: A Review. Molecules 2019, 24, 1568. [Google Scholar] [CrossRef]
- Domínguez-Tornay, A.; Díaz, A.B.; Lasanta, C.; Durán-Guerrero, E.; Castro, R. Co-Fermentation of Lactic Acid Bacteria and S. Cerevisiae for the Production of a Probiotic Beer: Survival and Sensory and Analytical Characterization. Food Biosci. 2024, 57, 103482. [Google Scholar] [CrossRef]
- Das, A.J.; Seth, D.; Miyaji, T.; Deka, S.C. Fermentation Optimization for a Probiotic Local Northeastern Indian Rice Beer and Application to Local Cassava and Plantain Beer Production. J. Inst. Brew. 2015, 121, 273–282. [Google Scholar] [CrossRef]
- Tang, F.; Wei, B.; Qin, C.; Huang, L.; Xia, N.; Teng, J. Enhancing the Inhibitory Activities of Polyphenols in Passion Fruit Peel on α-Amylase and α-Glucosidase via β-Glucosidase-Producing Lactobacillus Fermentation. Food Biosci. 2024, 62, 105005. [Google Scholar] [CrossRef]
- Dasenaki, M.E.; Thomaidis, N.S. Quality and Authenticity Control of Fruit Juices—A Review. Molecules 2019, 24, 1014. [Google Scholar] [CrossRef]
- Plessas, S. Advancements in the Use of Fermented Fruit Juices by Lactic Acid Bacteria as Functional Foods: Prospects and Challenges of Lactiplantibacillus (Lpb.) plantarum subsp. plantarum Application. Fermentation 2021, 8, 6. [Google Scholar] [CrossRef]
- Montet, D.; Ray, R.C.; Zakhia-Rozis, N. Lactic Acid Fermentation of Vegetables and Fruits. Microorg. Ferment. Tradit. Foods 2014, 21, 108–140. [Google Scholar]
- Yuan, X.; Wang, T.; Sun, L.; Qiao, Z.; Pan, H.; Zhong, Y.; Zhuang, Y. Recent Advances of Fermented Fruits: A Review on Strains, Fermentation Strategies, and Functional Activities. Food Chem. X 2024, 22, 101482. [Google Scholar] [CrossRef]
- Septembre-Malaterre, A.; Remize, F.; Poucheret, P. Fruits and Vegetables, as a Source of Nutritional Compounds and Phytochemicals: Changes in Bioactive Compounds during Lactic Fermentation. Food Res. Int. 2018, 104, 86–99. [Google Scholar] [CrossRef]
- Gustaw, K.; Niedźwiedź, I.; Rachwał, K.; Polak-Berecka, M. New Insight into Bacterial Interaction with the Matrix of Plant-Based Fermented Foods. Foods 2021, 10, 1603. [Google Scholar] [CrossRef]
- Sevindik, O.; Guclu, G.; Agirman, B.; Selli, S.; Kadiroglu, P.; Bordiga, M.; Capanoglu, E.; Kelebek, H. Impacts of Selected Lactic Acid Bacteria Strains on the Aroma and Bioactive Compositions of Fermented Gilaburu (Viburnum opulus) Juices. Food Chem. 2022, 378, 132079. [Google Scholar] [CrossRef]
- Di Cagno, R.; Coda, R.; De Angelis, M.; Gobbetti, M. Exploitation of Vegetables and Fruits through Lactic Acid Fermentation. Food Microbiol. 2013, 33, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Filannino, P.; Di Cagno, R.; Gobbetti, M. Metabolic and Functional Paths of Lactic Acid Bacteria in Plant Foods: Get out of the Labyrinth. Curr. Opin. Biotechnol. 2018, 49, 64–72. [Google Scholar] [CrossRef]
- Tripathi, J.; Chatterjee, S.; Gamre, S.; Chattopadhyay, S.; Variyar, P.S.; Sharma, A. Analysis of Free and Bound Aroma Compounds of Pomegranate (Punica granatum L.). LWT-Food Sci. Technol. 2014, 59, 461–466. [Google Scholar] [CrossRef]
- Cele, N.P.; Akinola, S.A.; Manhivi, V.E.; Shoko, T.; Remize, F.; Sivakumar, D. Influence of Lactic Acid Bacterium Strains on Changes in Quality, Functional Compounds and Volatile Compounds of Mango Juice from Different Cultivars during Fermentation. Foods 2022, 11, 682. [Google Scholar] [CrossRef]
- Surendran, S.; Qassadi, F.; Surendran, G.; Lilley, D.; Heinrich, M. Myrcene—What Are the Potential Health Benefits of This Flavouring and Aroma Agent? Front. Nutr. 2021, 8, 699666. [Google Scholar] [CrossRef]
- Paventi, G.; de Acutis, L.; De Cristofaro, A.; Pistillo, M.; Germinara, G.S.; Rotundo, G. Biological Activity of Humulus lupulus (L.) Essential Oil and Its Main Components against Sitophilus granarius (L.). Biomolecules 2020, 10, 1108. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhao, C.; Pu, X.; Li, T.; Shi, X.; Wang, B.; Cheng, W. Flavor and Functional Analysis of Lactobacillus plantarum Fermented Apricot Juice. Fermentation 2022, 8, 533. [Google Scholar] [CrossRef]
- Mrabti, H.N.; Jaouadi, I.; Zeouk, I.; Ghchime, R.; El Menyiy, N.; El Omari, N.; Balahbib, A.; Al-Mijalli, S.H.; Abdallah, E.M.; El-Shazly, M.; et al. Biological and Pharmacological Properties of Myrtenol: A Review. Curr. Pharm. Des. 2023, 29, 407–414. [Google Scholar] [CrossRef]
- Gao, H.; Wen, J.-J.; Hu, J.-L.; Nie, Q.-X.; Chen, H.-H.; Nie, S.-P.; Xiong, T.; Xie, M.-Y. Momordica Charantia Juice with Lactobacillus plantarum Fermentation: Chemical Composition, Antioxidant Properties and Aroma Profile. Food Biosci. 2019, 29, 62–72. [Google Scholar] [CrossRef]
- Ricci, A.; Cirlini, M.; Levante, A.; Dall’Asta, C.; Galaverna, G.; Lazzi, C. Volatile Profile of Elderberry Juice: Effect of Lactic Acid Fermentation Using L. plantarum, L. rhamnosus and L. casei Strains. Food Res. Int. 2018, 105, 412–422. [Google Scholar] [CrossRef]
- Di Cagno, R.; Surico, R.F.; Paradiso, A.; De Angelis, M.; Salmon, J.-C.; Buchin, S.; De Gara, L.; Gobbetti, M. Effect of Autochthonous Lactic Acid Bacteria Starters on Health-Promoting and Sensory Properties of Tomato Juices. Int. J. Food Microbiol. 2009, 128, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Di Cagno, R.; Surico, R.F.; Minervini, G.; Rizzello, C.G.; Lovino, R.; Servili, M.; Taticchi, A.; Urbani, S.; Gobbetti, M. Exploitation of Sweet Cherry (Prunus avium L.) Puree Added of Stem Infusion through Fermentation by Selected Autochthonous Lactic Acid Bacteria. Food Microbiol. 2011, 28, 900–909. [Google Scholar] [CrossRef]
- Filannino, P.; Cardinali, G.; Rizzello, C.G.; Buchin, S.; De Angelis, M.; Gobbetti, M.; Di Cagno, R. Metabolic Responses of Lactobacillus plantarum Strains during Fermentation and Storage of Vegetable and Fruit Juices. Appl. Environ. Microbiol. 2014, 80, 2206–2215. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Mousavi Khaneghah, A.; Barba, F.J.; Nemati, Z.; Sohrabi Shokofti, S.; Alizadeh, F. Fermented Sweet Lemon Juice (Citrus Limetta) Using Lactobacillus plantarum LS5: Chemical Composition, Antioxidant and Antibacterial Activities. J. Funct. Foods 2017, 38, 409–414. [Google Scholar] [CrossRef]
- Wang, H.; He, X.; Li, J.; Wu, J.; Jiang, S.; Xue, H.; Zhang, J.; Jha, R.; Wang, R. Lactic Acid Bacteria Fermentation Improves Physicochemical Properties, Bioactivity, and Metabolic Profiles of Opuntia Ficus-Indica Fruit Juice. Food Chem. 2024, 453, 139646. [Google Scholar] [CrossRef]
- Mashitoa, F.M.; Manhivi, V.E.; Akinola, S.A.; Garcia, C.; Remize, F.; Shoko, T.; Sivakumar, D. Changes in Phenolics and Antioxidant Capacity during Fermentation and Simulated in Vitro Digestion of Mango Puree Fermented with Different Lactic Acid Bacteria. J. Food Process Preserv. 2021, 45, e15937. [Google Scholar] [CrossRef]
- Chen, L.; Cao, H.; Huang, Q.; Xiao, J.; Teng, H. Absorption, Metabolism and Bioavailability of Flavonoids: A Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 7730–7742. [Google Scholar] [CrossRef]
- Tang, S.; Cheng, Y.; Wu, T.; Hu, F.; Pan, S.; Xu, X. Effect of Lactobacillus plantarum-Fermented Mulberry Pomace on Antioxidant Properties and Fecal Microbial Community. LWT 2021, 147, 111651. [Google Scholar] [CrossRef]
- Hur, S.J.; Lee, S.Y.; Kim, Y.-C.; Choi, I.; Kim, G.-B. Effect of Fermentation on the Antioxidant Activity in Plant-Based Foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef]
- Slámová, K.; Kapešová, J.; Valentová, K. “Sweet Flavonoids”: Glycosidase-Catalyzed Modifications. Int. J. Mol. Sci. 2018, 19, 2126. [Google Scholar] [CrossRef]
- Gaur, G.; Gänzle, M.G. Conversion of (Poly)Phenolic Compounds in Food Fermentations by Lactic Acid Bacteria: Novel Insights into Metabolic Pathways and Functional Metabolites. Curr. Res. Food Sci. 2023, 6, 100448. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.-B.; Lei, Y.-T.; Li, Q.-Z.; Li, Y.-C.; Deng, Y.; Liu, D.-Y. Effect of Lactobacillus plantarum and Lactobacillus Acidophilus Fermentation on Antioxidant Activity and Metabolomic Profiles of Loquat Juice. LWT 2022, 171, 114104. [Google Scholar] [CrossRef]
- Arunachalam, S.; Nagoor Meeran, M.F.; Azimullah, S.; Sharma, C.; Goyal, S.N.; Ojha, S. Nerolidol Attenuates Oxidative Stress, Inflammation, and Apoptosis by Modulating Nrf2/MAPK Signaling Pathways in Doxorubicin-Induced Acute Cardiotoxicity in Rats. Antioxid 2021, 10, 984. [Google Scholar] [CrossRef] [PubMed]
- Iqubal, A.; Syed, M.A.; Ali, J.; Najmi, A.K.; Haque, M.M.; Haque, S.E. Nerolidol Protects the Liver against Cyclophosphamide-Induced Hepatic Inflammation, Apoptosis, and Fibrosis via Modulation of Nrf2, NF-ΚB P65, and Caspase-3 Signaling Molecules in Swiss Albino Mice. Biofactors 2020, 46, 963–973. [Google Scholar] [CrossRef]
- Tang, W.; Xing, Z.; Li, C.; Wang, J.; Wang, Y. Molecular Mechanisms and in Vitro Antioxidant Effects of Lactobacillus plantarum MA2. Food Chem. 2017, 221, 1642–1649. [Google Scholar] [CrossRef]
- Li, Z.; Teng, J.; Lyu, Y.; Hu, X.; Zhao, Y.; Wang, M. Enhanced Antioxidant Activity for Apple Juice Fermented with Lactobacillus plantarum ATCC14917. Molecules 2018, 24, 51. [Google Scholar] [CrossRef]
- Chen, C.; Lu, Y.; Yu, H.; Chen, Z.; Tian, H. Influence of 4 Lactic Acid Bacteria on the Flavor Profile of Fermented Apple Juice. Food Biosci. 2019, 27, 30–36. [Google Scholar] [CrossRef]
- Peng, W.; Meng, D.; Yue, T.; Wang, Z.; Gao, Z. Effect of the Apple Cultivar on Cloudy Apple Juice Fermented by a Mixture of Lactobacillus Acidophilus, Lactobacillus plantarum, and Lactobacillus Fermentum. Food Chem. 2021, 340, 127922. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Jafarpour, D. Fermentation of Bergamot Juice with Lactobacillus plantarum Strains in Pure and Mixed Fermentations: Chemical Composition, Antioxidant Activity and Sensorial Properties. LWT 2020, 131, 109803. [Google Scholar] [CrossRef]
- Tkacz, K.; Chmielewska, J.; Turkiewicz, I.P.; Nowicka, P.; Wojdyło, A. Dynamics of Changes in Organic Acids, Sugars and Phenolic Compounds and Antioxidant Activity of Sea Buckthorn and Sea Buckthorn-Apple Juices during Malolactic Fermentation. Food Chem. 2020, 332, 127382. [Google Scholar] [CrossRef] [PubMed]
- Filannino, P.; Cavoski, I.; Thlien, N.; Vincentini, O.; De Angelis, M.; Silano, M.; Gobbetti, M.; Di Cagno, R. Lactic Acid Fermentation of Cactus Cladodes (Opuntia ficus-indica L.) Generates Flavonoid Derivatives with Antioxidant and Anti-Inflammatory Properties. PLoS ONE 2016, 11, e0152575. [Google Scholar] [CrossRef]
- Wang, J.; Wei, B.-C.; Wang, X.; Zhang, Y.; Gong, Y.-J. Aroma Profiles of Sweet Cherry Juice Fermented by Different Lactic Acid Bacteria Determined through Integrated Analysis of Electronic Nose and Gas Chromatography–Ion Mobility Spectrometry. Front. Microbiol. 2023, 14, 1113594. [Google Scholar] [CrossRef]
- Kuerban, D.; Lu, J.; Huangfu, Z.; Wang, L.; Qin, Y.; Zhang, M. Optimization of Fermentation Conditions and Metabolite Profiling of Grape Juice Fermented with Lactic Acid Bacteria for Improved Flavor and Bioactivity. Foods 2023, 12, 2407. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, H.; Zhu, C. Effect of Lactic Acid Bacteria Fermentation on Antioxidation and Bioactivity of Hawthorn Pulp. IOP Conf. Ser. Earth Environ. Sci. 2019, 267, 062056. [Google Scholar] [CrossRef]
- Pan, X.; Zhang, S.; Xu, X.; Lao, F.; Wu, J. Volatile and Non-Volatile Profiles in Jujube Pulp Co-Fermented with Lactic Acid Bacteria. LWT 2022, 154, 112772. [Google Scholar] [CrossRef]
- Li, T.; Jiang, T.; Liu, N.; Wu, C.; Xu, H.; Lei, H. Biotransformation of Phenolic Profiles and Improvement of Antioxidant Capacities in Jujube Juice by Select Lactic Acid Bacteria. Food Chem. 2021, 339, 127859. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhang, F.; Zhang, L.; Liu, B.; Meng, X. Mixed Fermentation of Jujube Juice (Ziziphus jujuba Mill.) with L. rhamnosus GG and L. plantarum-1: Effects on the Quality and Stability. Int. J. Food Sci. Technol. 2019, 54, 2624–2631. [Google Scholar] [CrossRef]
- Wang, D.; Deng, Y.; Chen, X.; Wang, K.; Zhao, L.; Wang, Z.; Liu, X.; Hu, Z. Elucidating the Effects of Lactobacillus plantarum Fermentation on the Aroma Profiles of Pasteurized Litchi Juice Using Multi-Scale Molecular Sensory Science. Curr. Res. Food Sci. 2023, 6, 100481. [Google Scholar] [CrossRef]
- Liu, S.; Peng, Y.-J.; He, W.-W.; Song, X.-X.; He, Y.-X.; Hu, X.-Y.; Bian, S.-G.; Li, Y.-H.; Yin, J.-Y.; Nie, S.-P.; et al. Metabolomics-Based Mechanistic Insights into Antioxidant Enhancement in Mango Juice Fermented by Various Lactic Acid Bacteria. Food Chem. 2025, 466, 142078. [Google Scholar] [CrossRef]
- Park, J.B.; Lim, S.H.; Sim, H.S.; Park, J.H.; Kwon, H.J.; Nam, H.S.; Kim, M.D.; Baek, H.H.; Ha, S.J. Changes in Antioxidant Activities and Volatile Compounds of Mixed Berry Juice through Fermentation by Lactic Acid Bacteria. Food Sci. Biotechnol. 2017, 26, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Kwaw, E.; Ma, Y.; Tchabo, W.; Apaliya, M.T.; Wu, M.; Sackey, A.S.; Xiao, L.; Tahir, H.E. Effect of Lactobacillus Strains on Phenolic Profile, Color Attributes and Antioxidant Activities of Lactic-Acid-Fermented Mulberry Juice. Food Chem. 2018, 250, 148–154. [Google Scholar] [CrossRef]
- Yaqoob, S.; Imtiaz, A.; Awan, K.A.; Murtaza, M.S.; Mubeen, B.; Yinka, A.A.; Boasiako, T.A.; Alsulami, T.; Rehman, A.; Khalifa, I.; et al. Impact of Fermentation through Synergistic Effect of Different Lactic Acid Bacteria (Mono and Co-Cultures) on Metabolic and Sensorial Profile of Mulberry Juice. J. Food Meas. Charact. 2024, 18, 9364–9384. [Google Scholar] [CrossRef]
- de la Fuente, B.; Luz, C.; Puchol, C.; Meca, G.; Barba, F.J. Evaluation of Fermentation Assisted by Lactobacillus Brevis POM, and Lactobacillus plantarum (TR-7, TR-71, TR-14) on Antioxidant Compounds and Organic Acids of an Orange Juice-Milk Based Beverage. Food Chem. 2021, 343, 128414. [Google Scholar] [CrossRef]
- Dogan, K.; Akman, P.K.; Tornuk, F. Role of Non-thermal Treatments and Fermentation with Probiotic Lactobacillus plantarum on in Vitro Bioaccessibility of Bioactives from Vegetable Juice. J. Sci. Food Agric. 2021, 101, 4779–4788. [Google Scholar] [CrossRef]
- Fonseca, H.C.; Melo, D.d.S.; Ramos, C.L.; Dias, D.R.; Schwan, R.F. Lactiplantibacillus plantarum CCMA 0743 and Lacticaseibacillus Paracasei Subsp. Paracasei LBC-81 Metabolism during the Single and Mixed Fermentation of Tropical Fruit Juices. Braz. J. Microbiol. 2021, 52, 2307–2317. [Google Scholar] [CrossRef] [PubMed]
- Mantzourani, I.; Kazakos, S.; Terpou, A.; Alexopoulos, A.; Bezirtzoglou, E.; Bekatorou, A.; Plessas, S. Potential of the Probiotic Lactobacillus plantarum ATCC 14917 Strain to Produce Functional Fermented Pomegranate Juice. Foods 2019, 8, 4. [Google Scholar] [CrossRef]
- Vivek, K.; Mishra, S.; Pradhan, R.C.; Jayabalan, R. Effect of Probiotification with Lactobacillus plantarum MCC 2974 on Quality of Sohiong Juice. LWT 2019, 108, 55–60. [Google Scholar] [CrossRef]
- Liu, S.; He, Y.; He, W.; Song, X.; Peng, Y.; Hu, X.; Bian, S.; Li, Y.; Nie, S.; Yin, J.; et al. Exploring the Biogenic Transformation Mechanism of Polyphenols by Lactobacillus plantarum NCU137 Fermentation and Its Enhancement of Antioxidant Properties in Wolfberry Juice. J. Agric. Food Chem. 2024, 72, 12752–12761. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, Y.; Zhang, N.; Xiao, M.; Zhang, J.; Xing, X.; Zhang, Y.; Fan, Y.; Li, X.; Nan, B.; et al. Antioxidant Mechanism of Lactiplantibacillus plantarum KM1 Under H2O2 Stress by Proteomics Analysis. Front. Microbiol. 2022, 13, 897387. [Google Scholar] [CrossRef]
- Markkinen, N.; Laaksonen, O.; Nahku, R.; Kuldjärv, R.; Yang, B. Impact of Lactic Acid Fermentation on Acids, Sugars, and Phenolic Compounds in Black Chokeberry and Sea Buckthorn Juices. Food Chem. 2019, 286, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Wang, S.; Gu, P.; Ouyang, X.; Liu, S.; Li, Y.; Zhang, B.; Zhu, B. Comparison of Physicochemical Indexes, Amino Acids, Phenolic Compounds and Volatile Compounds in Bog Bilberry Juice Fermented by Lactobacillus plantarum under Different pH Conditions. J. Food Sci. Technol. 2018, 55, 2240–2250. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, B.; Bangar, S.P.; Echegaray, N.; Suri, S.; Tomasevic, I.; Lorenzo, J.M.; Melekoglu, E.; Rocha, J.M.; Ozogul, F. The Impacts of Lactiplantibacillus plantarum on the Functional Properties of Fermented Foods: A Review of Current Knowledge. Microorganisms 2022, 10, 826. [Google Scholar] [CrossRef] [PubMed]
| Food | LAB Species | β-Glucosidase Function | Refs. |
|---|---|---|---|
| soybean products | Lactococcus lactis Lacticaseibacillus casei Limosilactobacillus fermentum Limosilactobacillus mucosae Enterococcus faecalis L. plantarum Lacticaseibacillus rhamnosus Bifidobacterium pseudocatenulatum Bifidobacterium breve | deglycosylation of isoflavones (genistin, daidzin, and glycitin) | [77,78,79,80,81] |
| olive | L. plantarum L. casei Lacticaseibacillus paracasei Bifidobacterium lactis Enterococcus faecium Lactiplantibacillus pentosus | debittering (hydrolysis of the oleuropein) | [82,83,84,85,86,87] |
| alcoholic beverages (wine and beer) | Oenococcus oeni Pediococcus spp. L. plantarum | deglycosylation of flavor precursors with release of free volatile organic compounds | [88,89,90,91,92,93,94] |
| non-alcoholic fermented fruit | L. rhamnosus L. plantarum Leuconostoc mesenteroides Levilactobacillus brevis Bifidobacterium spp. | deglycosylation of flavor precursors with release of free volatile organic compounds | [82,95,96,97] |
| cassava | L. plantarum L. mesenteroides | hydrolysis of cyanogenic glycosides (linamarin) | [98,99,100,101] |
| Fruit Processed | Product Type | L. plantarum (Lp) Strains | Main Positive Effects | Ref. |
|---|---|---|---|---|
| Apple | Juice fermented at 37 °C for 72 h. | Lp ATCC14917 | Increased antioxidant activity and decreased total phenolics and flavonoid content. | [244] |
| Apple | Juice fermented at 37 °C for 80 h | Lp ST-III | Improved flavor profile | [245] |
| Apple | Single juices from nine apple cultivars fermented at 37 °C for 24 h | Lp CICC21805 | Increased terpenes D-limonene and eugenol in some apple cultivars | [246] |
| Apricot | Juice fermented at 37 °C for 12 h | Lp LP56 | Increased antioxidant activity and total phenolics; improved flavor profile | [225] |
| Bergamot (Citrus Bergamia Risso) | Juice fermented at 37 °C for 72 h | Single and mixed starter: Lp PTCC 1896 Lp AF1 Lp LP3 | Increased antioxidant activity | [247] |
| Buckthorn berries (Hippophaë rhamnoides L.) | Juice fermented at 30 °C for 72 h | Lp DSM 10492 Lp DSM 20174 Lp DSM 6872 | Increased antioxidant activity and flavonoids | [248] |
| Cactus (Opuntia ficus-indica L.) | Cladodes pulp fermented at 30 °C for 24 h | Single starters: Lp CIL6 Lp POM1 Lp 1MR20 | Increased antioxidant activity and flavonoids (kaemferol and isorhamnetin) | [249] |
| Cherries (Prunus avium L.) | Juice fermented at 37 °C for 48 h | Lp JYLP-375 | Improved flavor profile | [250] |
| Cranberrybush/Gilaburu (Viburnum opulus L.) | Juice fermented at 30 °C for 12 days | Lp-23 | Increased antioxidant activity and terpenes | [218] |
| Elderberry (Sambucus nigra L.) | Juice fermented at 37 °C for 48 h | Single starters: Lp POM1 Lp 1LE1 Lp C1 Lp 1486 Lp 285 | Increase in terpenes and norisoprenoids (limonene, β-linalool, β-damascenone, and eugenol) | [228] |
| Grapes | Juice fermented at 37 °C for 32 h | Single and mixed starter: Lp 90 L. casei | Increased total phenolics and improved flavor profile | [251] |
| Hawthorn (Crataegus pinnatifida) | Pulp fermented at 37 °C for 12 h | Mixed starter: Lp, Lactobacillus acidophilus and L. casei | Increased total phenolics and flavonoids | [252] |
| Jujube (Ziziphus jujuba Milll.) | Pulp fermented at 37 °C for 24 h | Lp CICC 20265 | Improved flavor profile | [253] |
| Jujube (Zizyphus jujuba Mill.) | Juice fermented at 37 °C for 48 h | Lp 90 | Increased antioxidant activity and flavor profile | [254] |
| Jujube (Ziziphus jujuba Milll.) | Juice fermented at 37 °C for 28 h | Single and mixed starter: L. rhamnosus GG Lp-1 Lp-2 L. paracasei 22709 Leuconostoc mesenteroides 22264 | Decreased total phenolics and increased total flavonoid content; improved flavor profile | [255] |
| Lemon (Citrus limetta) | Juice fermented at 37 °C for 48 h | Lp LS5 | Increased antioxidant activity | [232] |
| Litchi (Litchi chinensis Sonn. | Juice fermented at 37 °C for 40 h | Single starters: Lp LP28 Lp LP226 Lp LPC2W | Increased terpenes citronellol, linalool, geraniol, and prenol | [256] |
| Loquat (Eriobotrya japonica Lindl.) | Juice fermented at 36 °C for 48 h | Lp LZ 2-2 | Increased antioxidant activity, total phenolics, and total flavonoids | [240] |
| Mango (Mangifera indica L.) | Juice fermented at 37 °C for 48 h | Lp NCU116 | Increased antioxidant activity and total phenolics | [257] |
| Mango (Mangifera indica L.) | Juice fermented at 30 °C for 72 h | Single and mixed starter Lp L75 Leuconostoc pseudomesenteroides L 56 | Increased antioxidant activity and improved flavor profile | [222] |
| Mixed berry (acai berry, aronia, cranberry) | Juice fermented at 37 °C for 36 h | Lp LP-115 | Increased antioxidant activity | [258] |
| Momordica charantia L. | Juice fermented at 37 °C for 48 h | Lp NCU116 | Increased antioxidant activity, total phenolics, and total flavonoids | [227] |
| Mulberry (Morus nigra) | Juice fermented at 37 °C for 36 h | Lp ATCC SD5209 | Increased antioxidant activity and phenolics (phenolic acids, anthocyanins, and flavonols) | [259] |
| Mulberry (Morus nigra) | Juice fermented at 37 °C for 7 days | Lp CICC 20265 | Increased antioxidant activity | [236] |
| Mulberry (Morus nigra) | Juice fermented at 37 °C for 48 h | Lp (single colture and/or in co-colture with other LAB) | Improvement of both nutritional and aromatic profile | [260] |
| Orange | Juice-milk fermented at 37 °C for 72 h | Single starters: Lp TR-7 Lp TR-71 Lp TR-14 | Increased antioxidant activity and total phenolics | [261] |
| Orange, lemon, celery and carrot | Mixed vegetable juice fermented at 37 °C for 24 h | Lp HFC8 | Increased antioxidant activity and phenolics (flavonoids and anthocyanins) | [262] |
| Passion fruit (Passiflora edulis), acerola (Malpighia emarginata), and jelly palm (Butia capitata) | Juice fermented at 37 °C for 24 h | Lp CCMA 0743 | Increased flavonoids | [263] |
| Pomegranate (Punica granatum L.) | Juice fermented at 30 °C for 24 h | Lp ATCC 14917 | Increased antioxidant activity and total phenolics | [264] |
| Pomegranate (Punica granatum L.) | Juice fermented at 30 °C for 120 h | Single starter: Lp C2 Lp POM1 | Improved flavor profile | [97] |
| Sohiong (Prunus nepalensis) | Juice fermented at 37 °C for 72 h | Lp MCC 297 | Increased antioxidant activity, total phenolics, and anthocyanins | [265] |
| Wolfberry | Juice fermented at 37 °C for 48 h | Lp NCU137 | Increased antioxidant activity and free phenolics | [266] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paventi, G.; Di Martino, C.; Coppola, F.; Iorizzo, M. β-Glucosidase Activity of Lactiplantibacillus plantarum: A Key Player in Food Fermentation and Human Health. Foods 2025, 14, 1451. https://doi.org/10.3390/foods14091451
Paventi G, Di Martino C, Coppola F, Iorizzo M. β-Glucosidase Activity of Lactiplantibacillus plantarum: A Key Player in Food Fermentation and Human Health. Foods. 2025; 14(9):1451. https://doi.org/10.3390/foods14091451
Chicago/Turabian StylePaventi, Gianluca, Catello Di Martino, Francesca Coppola, and Massimo Iorizzo. 2025. "β-Glucosidase Activity of Lactiplantibacillus plantarum: A Key Player in Food Fermentation and Human Health" Foods 14, no. 9: 1451. https://doi.org/10.3390/foods14091451
APA StylePaventi, G., Di Martino, C., Coppola, F., & Iorizzo, M. (2025). β-Glucosidase Activity of Lactiplantibacillus plantarum: A Key Player in Food Fermentation and Human Health. Foods, 14(9), 1451. https://doi.org/10.3390/foods14091451









