Bioassay-Guide Preparative Separation of Hypoglycemic Components from Gynura divaricata (L.) DC by Conventional and pH-Zone Refining Countercurrent Chromatography
Abstract
1. Introduction
2. Materials and Methods
2.1. Apparatus
2.2. Reagents and Materials
2.3. Extraction of Gynura divaricata
2.4. Bioassay of Extracts
2.4.1. α-Glucosidase Inhibition Assay
2.4.2. Glucose Uptake Assay
2.5. CCC Separation Procedure
2.6. HPLC Analysis and Elucidation of Isolated Compounds
2.7. Molecular Docking
3. Results and Discussion
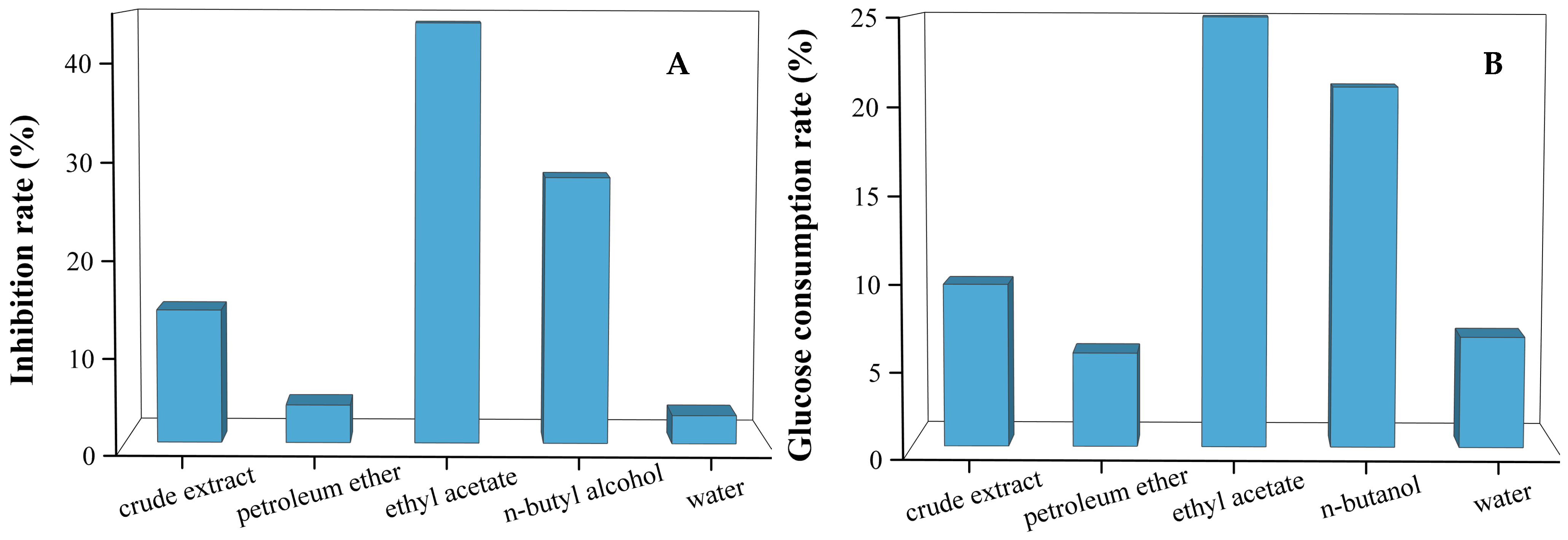
3.1. The Hypoglycemic Effect of Different Solvent Fractions
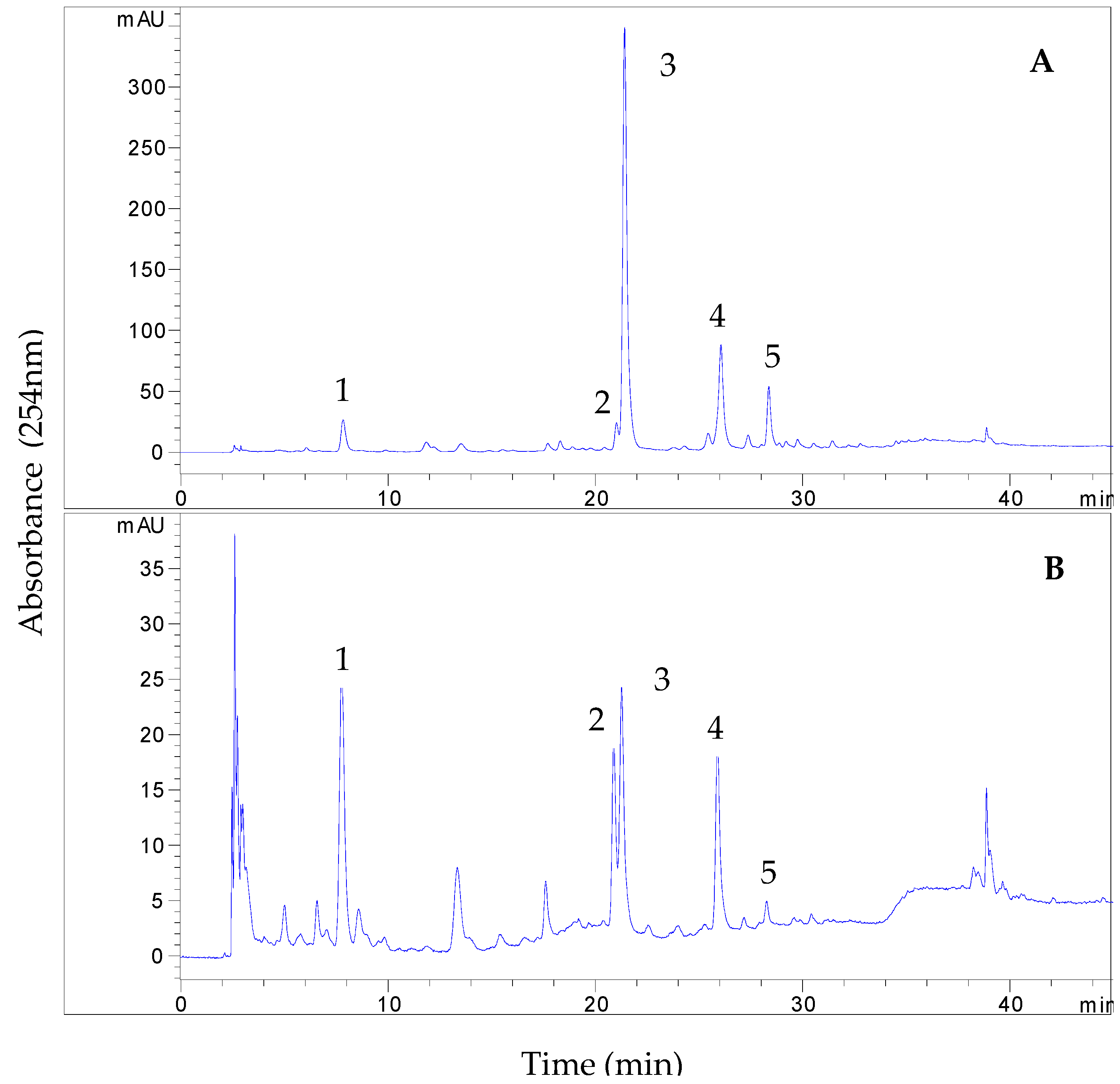
3.2. HPLC Analysis of EtOAc and BuOH Fractions
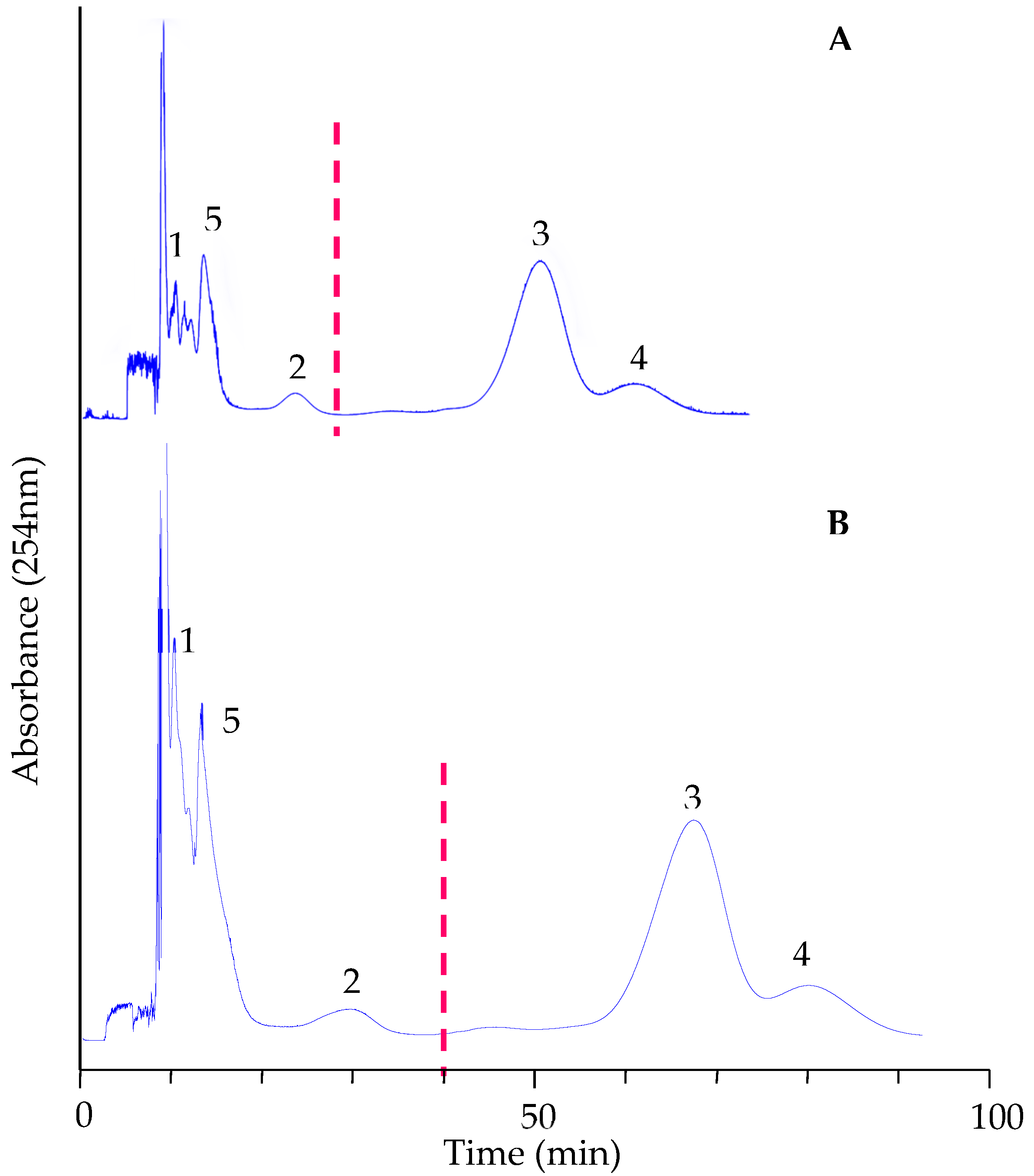
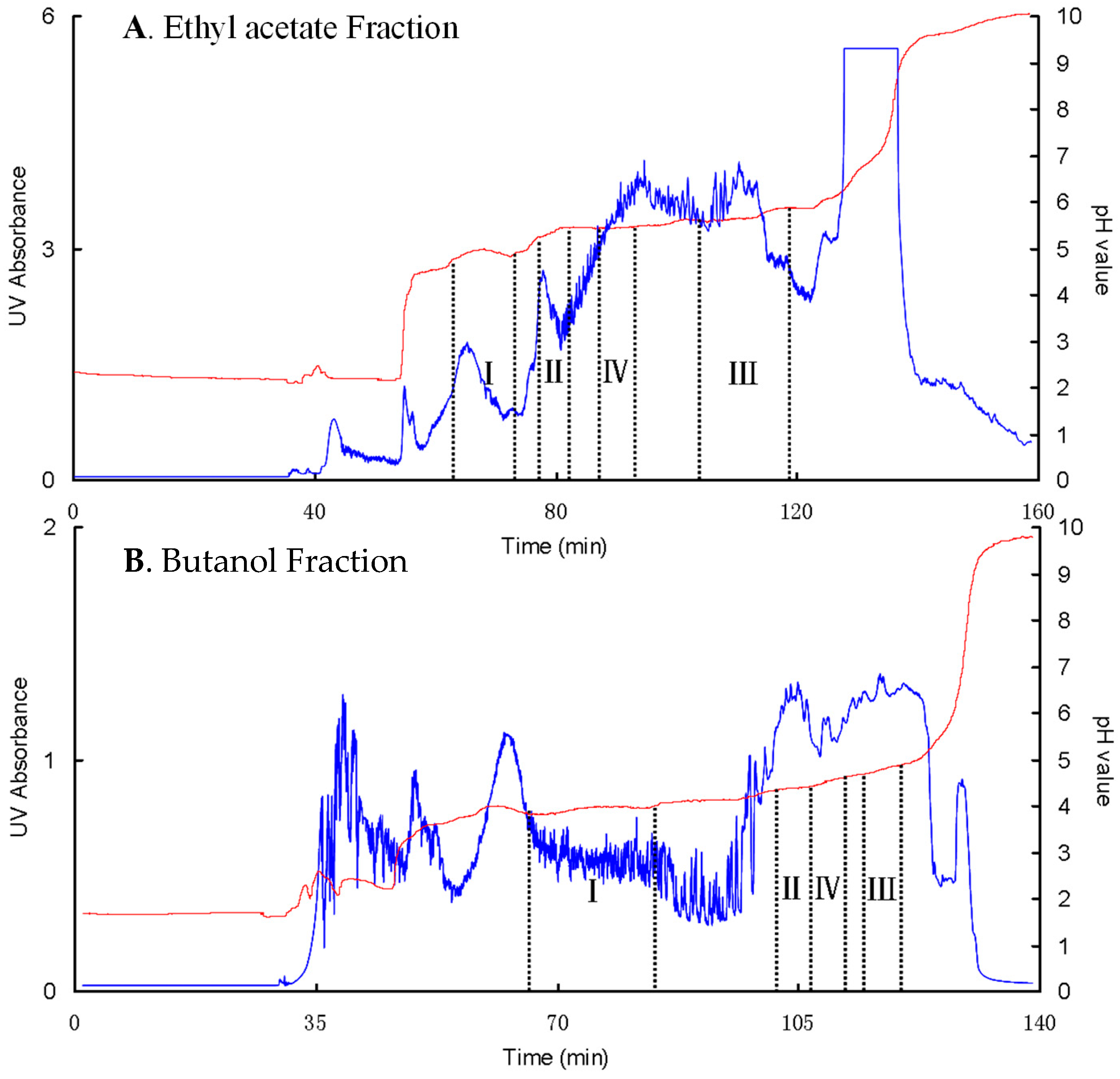
3.3. Conventional CCC Separation of the EtOAc Fraction
3.4. Structure Identification of Pure Compounds
3.5. PZRCCC Separation of Four Phenolic Acids in Large-Scale
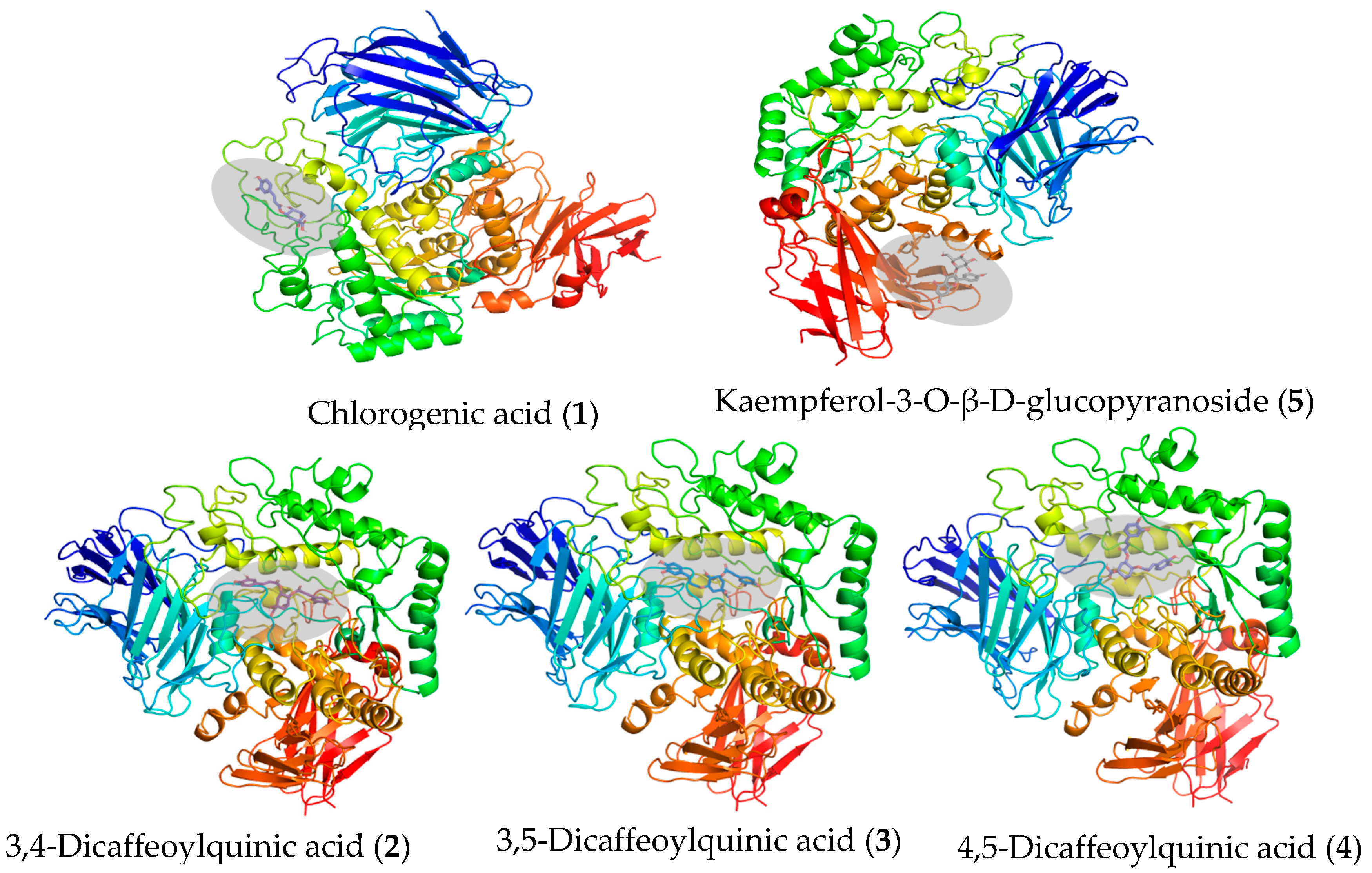
3.6. Molecular Interaction Between the Isolated Compounds and α-Glucosidase
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, L.; Xu, X.; Liu, A.; Sun, J.; Xue, Y. Advances in studies on Gynura divarica (L.) DC. J. Med. Pharm. Chin. Minor. 2014, 10, 61–65. [Google Scholar]
- Xu, B.; Zhang, Y. Bioactive components of gynura divaricata and its potential use in health, food and medicine: A mini-review. Afr. J. Tradit. Complement. Altern. Med. AJTCAM. 2017, 14, 113–127. [Google Scholar] [CrossRef]
- Hong, M.H.; Jin, X.J.; Yoon, J.J.; Lee, Y.J.; Oh, H.C.; Lee, H.S.; Kim, H.Y.; Kang, D.G. Antihypertensive effects of Gynura divaricata (L.) DC in rats with renovascular hypertension. Nutrients 2020, 12, 3321. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.H.; Lai, C.C.; Shi, T.H.; Chen, M.; Yu, H.C.; Liu, Y.P.; Chang, F.R. Gynura divaricata attenuates tumor growth and tumor relapse after cisplatin therapy in HCC xenograft model through suppression of cancer stem cell growth and Wnt/β-catenin signalling. J. Ethnopharmacol. 2018, 213, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Li, W.L.; Ren, B.R.; Zhuo, M.; Hu, Y.; Lu, C.G.; Wu, J.L.; Chen, J.; Sun, S. The anti-hyperglycemic effect of plants in genus gynura cass. Am. J. Chin. Med. 2009, 37, 961–966. [Google Scholar] [CrossRef]
- Wu, T.T.; Zhou, X.T.; Deng, Y.F.; Jing, Q.; Li, M.; Yuan, L.J. In vitro studies of gynura divaricata (L.) DC extracts as inhibitors of key enzymes relevant for type 2 diabetes and hypertension. J. Ethnopharmacol. 2011, 136, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.Q.; Yang, P.; Zhang, Y.Q. Hypoglycemic activities of lyophilized powder of gynura divaricata by improving antioxidant potential and insulin signaling in type 2 diabetic mice. Food Nutr. Res. 2015, 59, 29652. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, J.N.; Feng, J.L.; Wei, H.; Liu, Q.Y.; Yang, T.; Hou, S.P.; Zhao, Y.; Zhang, B.; Yang, C. The aqueous extract of gynura divaricata (L.) DC. Improves glucose and lipid metabolism and ameliorates type 2 diabetes mellitus. Evid.-Based Complement. Altern. Med. 2018, 2018, 8686297. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.M.; Hu, L.X.; Zeng, J.; Wu, A.G.; Deng, S.L.; Zhao, Z.J.; Geng, K.; Luo, J.S.; Wang, L.; Zhou, X.G.; et al. Gynura divaricata (L.) DC. promotes diabetic wound healing by activating Nrf2 signaling in diabetic rats. J. Ethnopharmacol. 2024, 323, 117638. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, D.; Zhou, S.; Zhou, T. Hypoglycemic effects of Gynura divaricata (L.) DC polysaccharide and action mechanisms via modulation of gut microbiota in diabetic mice. J. Agric. Food Chem. 2024, 72, 9893–9905. [Google Scholar] [CrossRef]
- Ye, X.Y.; Xiong, L.; Fu, Q.F.; Wang, B.Y.; Wang, Y.W.; Zhang, K.L.; Yang, J.; Kantawong, F.; Kumsaiyai, W.; Zhou, J.; et al. Chemical characterization and DPP-IV inhibitory activity evaluation of tripeptides from Gynura divaricata (L.) DC. J. Ethnopharmacol. 2022, 292, 115203. [Google Scholar] [CrossRef]
- Chen, J.; Mangelinckx, S.; Ma, L.; Wang, Z.T.; Li, W.L.; De Kimpe, N. Caffeoylquinic acid derivatives isolated from the aerial parts of Gynura divaricata and their yeast α-glucosidase and PTP1B inhibitory activity. Fitoterapia 2014, 99, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.L.; Xu, B.Q.; Zhang, Y.Q. Gynura divaricata rich in 3, 5−/4, 5-dicaffeoylquinic acid and chlorogenic acid reduces islet cell apoptosis and improves pancreatic function in type 2 diabetic mice. Nutr. Metab. 2018, 15, 73. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.P.; Yu, Y.Y.; Zhou, S.R.; Tian, S.G.; Cao, S.W. Isolation and identification of phenolic compounds from Gynura divaricata leaves. Pharmacogn. Mag. 2011, 7, 101–108. [Google Scholar]
- Ito, Y. Golden rules and pitfalls in selecting optimum conditions for high-speed counter-current chromatography. J. Chromatogr. A 2005, 1065, 145–168. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y. PH-zone-refining counter-current chromatography: Origin, mechanism, procedure and applications. J. Chromatogr. A 2013, 1271, 71–85. [Google Scholar] [CrossRef]
- Shia, S.Y.; Huang, K.L.; Zhang, Y.P.; Zhao, Y.; Du, Q.Z. Purification and identification of antiviral components from laggera pterodonta by high-speed counter-current chromatography. J. Chromatogr. B Analyt. Technol. Biomed Life Sci. 2007, 859, 119–124. [Google Scholar] [CrossRef]
- Tong, S.Q.; Yan, J.Z.; Guan, Y.X. Preparative separation of isomeric caffeoylquinic acids from Flos Lonicerae by pH-zone-refining counter-current chromatography. J. Chromatogr. A 2008, 1212, 48–53. [Google Scholar] [CrossRef]
- Romero-González, R.R.; Verpoorte, R. Salting-out gradients in centrifugal partition chromatography for the isolation of chlorogenic acids from green coffee beans. J. Chromatogr. A 2009, 1216, 4245–4251. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Liu, C.M.; Yu, M.; Zhang, Z.K.; Qi, Y.J.; Wang, J.; Wu, G.M.; Li, S.A.; Yu, J.; Hu, Y. Application of accelerated solvent extraction coupled with high-performance counter-current chromatography to extraction and online isolation of chemical constituents from Hypericum perforatum L. J. Chromatogr. A 2011, 1218, 2827–2834. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.J.; Du, N.; Wen, L.; Zhu, H.; Liu, F.; Wang, X.; Du, J.H.; Li, S.B. An efficient method for the preparative Isolation and purification of flavonoid glycosides and caffeoylquinic acid derivatives from leaves of lonicera japonica thunb. Using high speed counter-current chromatography (HSCCC) and prep-HPLC guided by DPPH-HPLC experiments. Molecules 2017, 22, 229. [Google Scholar] [CrossRef]
- Ma, T.Y.; Dong, H.J.; Lu, H.; Zhao, H.Q.; Guo, L.P.; Wang, X. Preparative separation of caffeoylquinic acid isomers from lonicerae japonicae Flos by pH-zone-refining counter-current chromatography and a strategy for selection of solvent systems with high sample loading capacities. J. Chromatogr. A 2018, 1578, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.J.; Wang, X.; Liu, P.L.; Li, M.; Dong, H.J.; Qiao, X.G. Semi-preparative separation of 10 caffeoylquinic acid derivatives using high speed counter-current chromatogaphy combined with semi-preparative HPLC from the roots of burdock (Arctium lappa L.). Molecules 2018, 23, 429. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.T.; Gao, M.Z.; Yang, Q.; Cui, Q.; Jian, Y.; Fan, X.H.; Yao, L.P.; Fu, Y.J. An efficient strategy based on liquid-liquid extraction with acid condition and HSCCC for rapid enrichment and preparative separation of three caffeoylquinic acid isomers from mulberry leaves. J. Chromatogr. Sci. 2019, 57, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Liu, X.X.; Zhang, X.Z. Preparative isolation of caffeoylquinic acid isomers from kuding tea by salt-containing aqueous two-phase extraction and purification by high-speed countercurrent chromatography. Sep. Sci. Plus 2019, 2, 170–177. [Google Scholar] [CrossRef]
- Sun, S.W.; Wang, R.R.; Sun, X.Y.; Fan, J.H.; Qi, H.; Liu, Y.; Qin, G.Q.; Wang, W. Identification of transient receptor potential vanilloid 3 antagonists from achillea alpina L. and separation by liquid-liquid-refining extraction and high-speed counter-current chromatography. Molecules 2020, 25, 2025. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.Y.; Hu, J.W.; Gu, Z.; Xiong, W.; Wu, L.; Xu, J.G.; Wu, L.Y. Purification of four caffeoylquinic acid derivatives from the flowers of gynura procumbens by HSCCC. J. Chromatogr. Sci. 2021, 59, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Tagami, T.; Yamashita, K.; Okuyama, M.; Mori, H.; Yao, M.; Kimura, A. Molecular Basis for the Recognition of Long-chain Substrates by Plant α-Glucosidases. J. Biol. Chem. 2013, 288, 19296–19303. [Google Scholar] [CrossRef]
- Wu, H.; Dushenkov, S.; Ho, C.-T.; Sang, S. Novel acetylated flavonoid glycosides from the leaves of Allium ursinum. Food Chem. 2009, 115, 592–595. [Google Scholar] [CrossRef]
- Friesen, J.B.; Pauli, G.F. Rational development of solvent system families in counter-current chromatography. J. Chromatogr. A 2007, 1151, 51–59. [Google Scholar] [CrossRef]



| Solvent Systems (v/v) | Compound (1) | Compound (2) | Compound (3) | Compound (4) | ||||
|---|---|---|---|---|---|---|---|---|
| Kacid | Kbase | Kacid | Kbase | Kacid | Kbase | Kacid | Kbase | |
| MtBE: n-butanol: acetonitrile: water (2:2:1:5, v/v) | 1.37 | 0.01 | 3.15 | 0.03 | 3.27 | 0.03 | 6.64 | 0.03 |
| MtBE: n-butanol: acetonitrile: water (1:3:1:5, v/v) | 4.71 | 0.01 | 4.60 | 0.05 | 15.14 | 0.08 | 20.74 | 0.03 |
| Compounds | Hydrogen Bonds | Binding Affinity (kcal/mol) |
|---|---|---|
| Chlorogenic acid | ASP 423, GLU 424, ASN 417, ASP 440 | −7.6 |
| Kaempferol-3-O-β-D-glucopyranoside | ASP 684, THR 681, ARG 699 | −8.5 |
| 3,4-Dicaffeoylquinic acid | HIS 626, ALA 234, SER 497 | −9.7 |
| 3,5-Dicaffeoylquinic acid | HIS 626, ALA 234, SER 497 ASP 357, ASP 232, ASN 496, SER 505 | −9.2 |
| 4,5-Dicaffeoylquinic acid | ALA 234, ASP 357 | −8.9 |
| Acarbose * | HIS 626, ASP 357, ASP 568, ARG 522, ASP 232, ASN 237, SER 497 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Z.; Xu, J.; Wen, L.; Yin, L.; Cao, X.; Pei, H.; Zhao, X. Bioassay-Guide Preparative Separation of Hypoglycemic Components from Gynura divaricata (L.) DC by Conventional and pH-Zone Refining Countercurrent Chromatography. Foods 2025, 14, 578. https://doi.org/10.3390/foods14040578
Shen Z, Xu J, Wen L, Yin L, Cao X, Pei H, Zhao X. Bioassay-Guide Preparative Separation of Hypoglycemic Components from Gynura divaricata (L.) DC by Conventional and pH-Zone Refining Countercurrent Chromatography. Foods. 2025; 14(4):578. https://doi.org/10.3390/foods14040578
Chicago/Turabian StyleShen, Zetao, Jing Xu, Lijiao Wen, Lu Yin, Xueli Cao, Hairun Pei, and Xi Zhao. 2025. "Bioassay-Guide Preparative Separation of Hypoglycemic Components from Gynura divaricata (L.) DC by Conventional and pH-Zone Refining Countercurrent Chromatography" Foods 14, no. 4: 578. https://doi.org/10.3390/foods14040578
APA StyleShen, Z., Xu, J., Wen, L., Yin, L., Cao, X., Pei, H., & Zhao, X. (2025). Bioassay-Guide Preparative Separation of Hypoglycemic Components from Gynura divaricata (L.) DC by Conventional and pH-Zone Refining Countercurrent Chromatography. Foods, 14(4), 578. https://doi.org/10.3390/foods14040578






