Abstract
Ethyl butyrate is a typical flavor ester with pineapple-banana scents, but the poor yield from natural fruits limits its feasibility in food and fragrance industries. In this study, dendritic fibrous nano-silica (DFNS) was hydrophobically modified with octyl groups (DFNS-C8) to immobilize Candida antarctica lipase B (CALB) for solvent-free esterification of ethyl butyrate. The immobilized lipase CALB@DFNS-C8, with the enzyme loading of 354.6 mg/g and the enzyme activity of 0.064 U/mg protein, achieved 96.0% ethyl butyrate conversion under the optimum reaction conditions where the molar ratio of butyric acid to ethanol was 1:3, with a reaction temperature and time of 40 °C and 4 h. Under the solvent-free catalytic reactions, CALB@DFNS-C8 presented the maximum catalytic efficiency of 35.1 mmol/g/h and retained 89% initial activity after ten reuse cycles. In addition, the immobilized lipase can efficiently catalyze the synthesis of various flavor esters (such as butyl acetate, hexyl acetate, butyl butyrate, etc.) and exhibits excellent thermostability and solvent tolerance. A molecular docking simulation reveals that the hydrophobic cavity around the catalytic triad stabilizes the acyl intermediate and ensures the precise orientation of both acid and alcohol substrates. This work provides new insights into the sustainable production of flavor esters using highly active and recyclable immobilized lipases through rational carrier hydrophobization and structural confinement design.
1. Introduction
Flavor esters can exhibit pleasant fragrances such as pears, apples, bananas and pineapples, which are widely used as core ingredients in juices, beverages and baked products [1,2]. In addition, the antioxidant, antibacterial and anti-inflammatory effects highlight the broad applicability of flavor esters in cosmetics, pharmaceuticals and healthcare products [3,4]. For instance, ethyl butyrate is a typical short-chain ester with a strong sweet fruit aroma, similar to pineapple or other tropical fruits [5,6]. Due to its moderate polarity and high volatility, ethyl butyrate can significantly improve the aroma strength and recognition of fruit juice and baked products [7]. Ethyl butyrate is also an efficient oil-phase solvent in pharmaceutical formulations that enhances the solubility and sustained release of hydrophobic drugs [8].
Most esters have naturally existed in fruits and plants, but the poor content, low extraction yield and high processing cost largely limit the production of natural flavor esters [9,10]. In addition, chemical synthesis requires strong acid (such as concentrated sulfuric acid or p-toluenesulfonic acid) to catalyze the high-temperature reflux reactions, causing potential equipment corrosion, high energy consumption and side reactions [11,12]. In contrast, enzymatic esterification via certain lipases can catalyze the reaction between carboxylic acids and alcohols, allowing flavor esters to efficiently and selectively synthesize in a mild and sustainable way [13]. However, the free lipase is prone to conformational changes under organic solvents or high substrate concentration conditions, resulting in a decrease in activity. In addition, the difficulty in recollection and reuse of free lipase makes the enzymatic reactions far more expensive than chemical synthesis [14,15,16]. Hence, enzyme immobilization has been employed to anchor lipases onto solid supports such as silica nanoparticles, metal–organic frameworks (MOFs), covalent organic frameworks (COFs) through physical adsorption, covalent binding, entrapment and crosslinking, etc. [17,18,19,20]. Particularly, hydrophobic adsorption via hydrophobic interaction can immobilize the lipase throughout the carriers with little change to the enzyme’s structures, maintaining its initial catalytic activity [21]. Furthermore, the hydrophobic surface modification on carriers stimulates lipases to open the hydrophobic ‘lid’ structure and expose the active sites, a phenomenon known as interfacial activation [22,23]. Meanwhile, the hydrophobic microenvironment helps remove the by-product water during enzymatic synthesis of flavor esters, thereafter driving the esterification equilibrium toward ester formation [24]. For instance, Liu et al. [25] used octyl (C8) to hydrophobically functionalize the silicon carriers, for Candida antarctica lipase B immobilization, which achieved 98.0% of ethyl hexanoate in 30 min. Chen et al. [26] found that Candida sp. lipase immobilization on octyl-modified ordered mesoporous silica could maintain 60.1% of the lipase’s initial activity after a 10-cycle reuse. At present, catalytic synthesis of ethyl butyrate is generally involving the use of organic solvents including n-heptane, n-hexane and cyclohexane to improve the solubility of substrates, adjust the system polarity and lower the liquid viscosity for enhanced reaction efficiency [27,28,29,30]. However, the volatility, flammability and toxicity of these solvents pose serious safety and environmental risks, making them unsuitable for the production of food-grade products. Moreover, solvents separation and purification are a time-costing and labor-intensive process, which is associated with solvent residue issues [31,32,33]. Hence, enzymatic synthesis of flavor esters in solvent-free conditions are more favorable for the clean, safe and sustainable demands, especially in food and related fields.
In this study, dendritic fibrous nano-silica was prepared and hydrophobically modified to immobilize lipase CALB, which was used to catalyze the solvent-free synthesis of flavor esters. Immobilization conditions such as OTCS (n-Octyltrichlorosilane) modification amount, pH, enzyme concentration and ratio of carrier to enzyme solution were systematically studied based on the enzyme loading, enzyme activity and ethyl butyrate conversion. In solvent-free reaction conditions, enzymatic synthesis of ethyl butyrate was carried out by investigating the effects of the molar ratio of a substrate, the addition of water and immobilized enzyme, and the reaction temperature. In addition, the catalytic stability, reusability and wide adaptability of immobilized lipase were also evaluated, from the perspective of industrial application.
2. Materials and Methods
2.1. Materials
Lipases used in this study included Candida antarctica lipase B (CALB, freeze-dried powder; Amano Enzyme Inc., Nagoya, Japan), Candida rugosa lipase (CRL, freeze-dried powder; Sigma-Aldrich, St. Louis, MO, USA), Porcine pancreas lipase (PPL, freeze-dried powder; Sigma-Aldrich, St. Louis, MO, USA), Thermomyces lanuginosus lipase (NE-10, freeze-dried powder; Vland Biotech, Qingdao, China) and Novozym 435 (8000 U/g; Novozymes, Beijing, China). Tetraethyl orthosilicate (TEOS, 98%) and n-Octyltrichlorosilane (OTCS, 99%), Cetyltrimethylammonium bromide (CTAB), fluorescein isothiocyanate (FITC) and protein concentration detection kit (BCA) were purchased from Shanghai Yuanye Biotechnology Co., Ltd. (Shanghai, China). Butyric acid and n-hexanol were obtained from Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). Acetic acid, butanol, anhydrous ethanol, isopropanol and urea were purchased from Sinopharm Group Chemical Reagents Co., Ltd. (Shanghai, China). p-Nitrophenyl palmitate (p-NPP) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Unless otherwise specified, all reagents and solvents used in the experiment were analytical grade or chromatographic grade.
2.2. Preparation of DFNS-C8
The synthesis of dendritic fibrous nano-silica (DFNS) is based on the method of Xie et al. [34]. A total of 16.0 g of CTAB and 9.6 g of urea were dissolved in 480 mL deionized water at room temperature, followed by the addition of 480 mL cyclohexane and 3.68 mL isopropanol. Then, 40 mL of TEOS was slowly added into the mixture for 2 h stirring at room temperature and another 20 h reaction at 70 °C. After cooling at room temperature, the whole contents were filtered using a Büchner funnel, washed with pure ethanol and dried overnight at 60 °C. Finally, the obtained solids were calcined at 550 °C in a muffle furnace for 6 h.
The surface modification of DFNS was based on the method reported by Liu et al. [25]. In brief, 1.0 g of DFNS was dispersed in 10 mL n-hexane containing different amounts of OTCS. The mixture was pretreated by ultrasonic treatment (10 min), subsequently reacted (250 rpm, 25 °C, 2 h) and collected by a Büchner funnel. The collected contents were further washed with pure ethanol at least three times and dried in a 60 °C oven overnight to obtain the white solids, namely DFNS-C8.
2.3. Immobilization and Enzymatic Properties of Lipase CALB
The immobilization of lipase referred to the method of Liu et al. [35]. First, 0.02–0.12 g free lipase CALB was dissolved in 10 mL phosphate-buffer solution (PBS, 50 mM) to prepare lipase solution, 0.1 g DFNS-C8 was added and mixed (220 rpm, 30 °C, 40 min). Then, all the contents were centrifuged (7000× g, 15 °C, 15 min) to obtain the supernatant, which was used to determine the amount of residual protein by BCA protein assay. At the same time, the solid was collected and freeze-dried (−60 °C, 10 h) to obtain immobilized lipase CALB@DFNS-C8. The immobilized enzyme amount was determined by the following formula:
where C0 is the concentration of lipase before immobilization (g/L), C is the concentration of lipase in the supernatant after immobilization, V is the volume of the enzyme solution (mL), and m is the quality of the added carrier (g).
Enzyme activity was measured using an ultraviolet/visible spectrophotometer based on the hydrolysis of p-nitrophenyl palmitate (p-NPP) in 50 mM, pH 7.0 phosphate-buffer solution (PBS) [36]. Lipase activity was determined by the following formula [37]:
where A is the absorbance of p-NPP at 410 nm, V is the total volume after dilution (mL), ε410 is the molar absorption coefficient of p-NPP (M−1cm−1, 14.298 × 103), t is the hydrolysis time (min), n is the dilution factor and m is the mass of immobilized enzyme (g).
The Catalytic efficiency (CE) value was calculated according to the following formula:
where S is substrates amount (mmol), Y is the yield, T is the time of reaction (h) and L is the mass of immobilized enzyme (g).
2.4. Enzymatic Esterification of Ethyl Butyrate
Ethyl butyrate is enzymatically produced via the esterification between butyric acid and anhydrous ethanol catalyzed by immobilized enzyme CALB@DFNS-C8. Specifically, 0.48 g of butyric acid and 0.25–1.25 g of ethanol with molar ratios ranging from 1:1 to 1:5, followed by the addition of 0.062–0.246 g of ultrapure water and 0.019–0.045 g of lipase for the stirring reaction (300 rpm, 4 h, 30–50 °C). During the enzymatic reaction, 20 μL of reaction solution was sampled at regular intervals and diluted with n-hexane. The collected samples were filtered with 0.22 μm PVDF filter and analyzed by high performance gas chromatography. Finally, the immobilized lipase was collected by centrifugation (5000× g, 5 min, 15 °C), washed with n-hexane and dried by nitrogen blowing for subsequent reaction cycles.
The yield of ethyl butyrate in the reaction was analyzed using the method described by Bian et al. [38]. The samples were detected by Agilent 7890A gas chromatograph (Agilent Technologies, Santa Clara, CA, USA) equipped with DB-FFAP fused silica capillary column (30 m × 0.25 mm × 0.25 μm) and FID detector. N2 was the carrier gas, and the total gas flow rate was set to 21 mL/min. The sample (1 μL) was injected into a split-flow mode with a split ratio of 20:1. The inlet temperature and detector temperature were set at 275 °C and 250 °C, respectively. The specific temperature program is as follows: the column oven was kept at 80 °C for 1 min, then heated to 170 °C at 10 °C/min and then kept for 5 min.
2.5. Lipase Performance Evaluation
Temperature stability: The free lipase CALB and CALB@DFNS-C8 were incubated at 100 °C for 3 h to evaluate their thermal stability.
Organic solvent tolerance: The free lipase CALB and CALB@DFNS-C8 were immersed in n-hexane, isopropanol, acetonitrile, ethanol and acetic acid for 2 h, and the activity of the enzyme was determined after the solvent was dried by nitrogen gas flow.
Reusability: CALB@DFNS-C8 was subjected to repeated experiments. At the end of each experiment, lipase was collected by centrifugation and washed with n-hexane for subsequent reaction cycles.
Details of the characterization of CALB@DFNS-C8 are provided in Supplementary Method S1.
2.6. Molecular Docking Analysis
Molecular docking simulations were performed using AutoDock Tools 1.5.6 to investigate the binding interactions between lipase (CALB, PDB: 1TCA) and acids and alcohols [38]. The three-dimensional structures of acids and alcohols were retrieved from PubChem. Docking parameters, including energy evaluations and grid box dimensions, were set to encompass the catalytic pocket of the enzyme. The Lamarckian genetic algorithm implemented in AutoDock 4.2 was used to perform docking simulations. The lowest-energy conformation was selected as the representative binding mode, and PyMOL (version 2.4.0) and Ligplot (version 2.3) software were used to visually evaluate the docking results.
2.7. Statistical Analysis
Statistical significance was evaluated using one-way Analysis of Variance (ANOVA) with a significance level of p < 0.05. Data visualization was performed using OriginPro 2021 software (Origin Lab Corp, Waltham, MA, USA).
3. Results and Discussion
3.1. Lipase Screening
In this study, different lipases (i.e., PPL, NE-10, CRL and CALB) were investigated based on their catalytic ability to esterify butyric acid and ethanol in a solvent-free system. As shown in Figure 1, the free lipase CALB reached 88.9% of butyric acid conversion in 2 h, while the corresponding conversion rates from lipase PPL, NE-10 and CRL were less than 1%. Notably, Kaur et al. [39] reported that immobilized CRL on magnetic multiwalled carbon nanotubes (MWCNTs) yielded 89.7% ethyl butyrate with n-hexane used as the organic solvent. In contrast, in the solvent-free system in this study, lipase CRL showed limited catalytic performance while lipase CALB maintained higher catalytic efficiency, which could be attributed to its superior regio- and stereo-selectivity as well as strong tolerance and structural stability in acidic conditions [40,41,42]. Thus, lipase CALB was selected for the enzymatic synthesis of ethyl butyrate in this study.
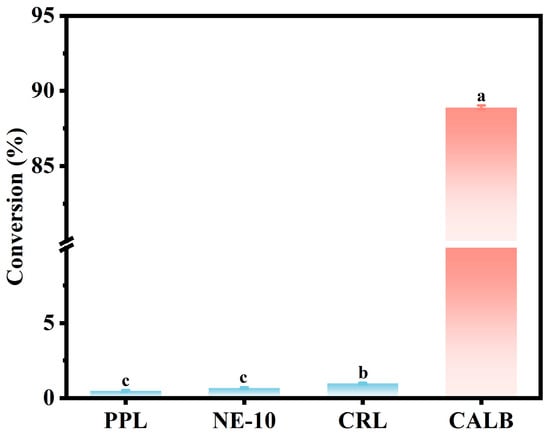
Figure 1.
Screening of different lipases based on enzymatic esterification of butyric acid and ethanol. The reaction was carried out in a water bath (40 °C, 300 rpm) for 2 h in an enzyme (3 wt%) with a molar ratio of butyric acid to ethanol of 1:4 and a water content of 10 wt%. A significant difference (p < 0.05) between groups is indicated by different lowercase letters.
3.2. Characterization of DFNS, DFNS-C8 and CALB@DFNS-C8
The prepared dendritic fibrous nano-silica (DFNS) carrier is a complete, uniform mesoporous sphere whose mean diameter is 260 nm (Figure 2a,b). The massive porous structures of DFNS provide sufficient specific surface area for lipase immobilization and subsequent enzyme-substrate contacts [43]. Energy-dispersive X-ray spectroscopy (EDX) shows that the content of C increased from 29.6% to 38.3% after alkyl modification and further increased to 46.6% with lipase CALB immobilization (Table 1; Figure 2c–e). Meanwhile, the alkyl modification and enzyme immobilization had little influence on the morphology of DFNS. XPS results further confirmed the enhancement of the C peak after modification and the emergence of a new N peak after immobilization, which proved the successful alkyl modification on DFNS and lipase immobilization (Figure 3a). FTIR analysis showed that the C-H symmetric and asymmetric stretching vibration peaks of DFNS-C8 appeared at 2921 cm−1 and 2854 cm−1, respectively, while the N-H bending vibration peaks related to the free lipase CALB appeared at 1537 cm−1 and 1654 cm−1 of CALB@DFNS-C8 (Figure 3b). Confocal laser scanning microscopy showed that FITC-labeled CALB was evenly distributed on the carrier DFNS-C8 (Figure S1).

Table 1.
Elemental contents and text parameters of sample characterization.
The internal structural changes in DFNS with surface modification and subsequent lipase immobilization were characterized based on the specific surface area and pore characteristics. As shown in Figure 3c, DFNS, DFNS-C8 and CALB@DFNS-C8 all showed type IV isotherms and H1 hysteresis loops, suggesting that the carrier has uniform and well-connected mesoporous channel structure, concentrated pore size distribution and regular pore shape [44]. With alkyl modification, the specific surface area of DFNS decreased from 449.6 m2/g to 309.1 m2/g, while the mesopores retained their initial size of 8.8 nm (Table 1). The decrease in specific surface area is due to the fact that the grafting of alkyl groups partially covers and occupies the surface of the material, so that nitrogen cannot fully contact the effective surface of mesoporous carrier [45]. Further immobilization of lipase greatly reduced the specific surface area to 48.1 m2/g, but the pore size increased to 11.8 nm because the entry of enzyme molecules into the internal cavity can partially block the smaller pores, thereby promoting the proportion of larger pores [46,47]. Thermogravimetric analysis in Figure 3d shows that DFNS had superior thermostability below 800 °C, while DFNS-C8 exhibited slight weight loss at 200–600 °C due to the decomposition of octyl chain. The weight loss of CALB@DFNS-C8 was about 27% in the same temperature range, corresponding to the thermal decomposition of the enzyme and organic components [48].
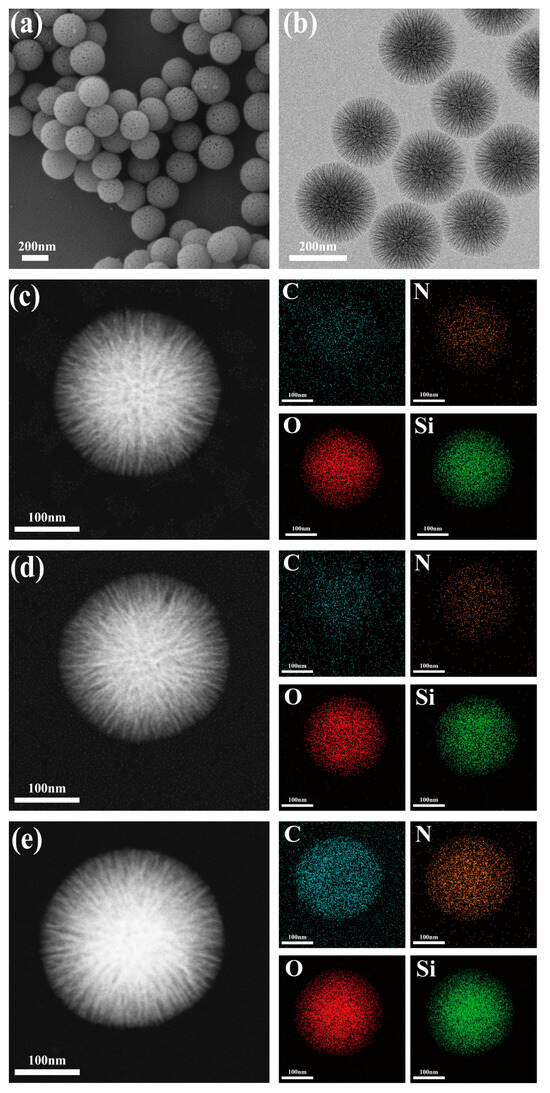
Figure 2.
SEM (a) and TEM (b) images of DFNS. TEM mapping images of DFNS (c), DFNS-C8 (d), CALB@DFNS-C8 (e).
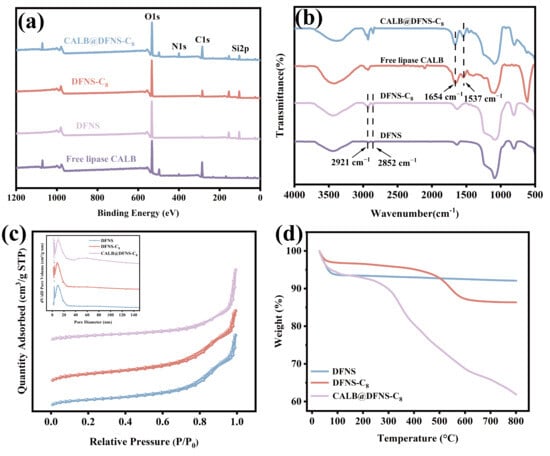
Figure 3.
(a) XPS images of free lipase CALB, DFNS, DFNS-C8 and CALB@DFNS-C8. (b) FT-IR analysis of DFNS, DFNS-C8, free lipase CALB and CALB@DFNS-C8. (c) N2 adsorption–desorption isotherms (adsorption capacity, cm3/g STP, relative pressure) and pore size distribution (pore volume, cm3/g, pore size, nm) of DFNS, DFNS-C8 and CALB@DFNS-C8. (d) Thermogravimetric analysis of DFNS, DFNS-C8 and CALB@DFNS-C8.
3.3. Lipase CALB Immobilization
Studies have shown that hydrophobic carriers are suitable for immobilization of lipases through interfacial adsorption, because there is a certain interaction between the open conformation of the enzyme and the hydrophobic surface [49]. On this basis, effects of the hydrophobic modification degree on carrier’s hydrophobicity and enzyme immobilization efficiency were studied. With the addition of OTCS increased from 0.25 mmol to 0.75 mmol, the enzyme loading and specific activity were increased from 336.0 mg/g to 341.5 mg/g and from 0.057 U/mg protein to 0.069 U/mg protein, respectively, and were associated with the increased ethyl butyrate conversion from 89.6% to 91.2% (Figure 4a and Figure S1). Further increase in OTCS reversely reduced the enzyme loading, enzyme activity and ethyl butyrate conversion to 315.4 mg/g, 0.065 U/mg protein and 90.8%, respectively. High concentration of OTCS means that more C8 molecules were located on the carrier, which inversely hinders the entry of enzymes into the mesopores and results in reduced enzyme immobilization efficiency [25]. Despite the slightly reduced immobilization amount, 0.75 mmol of OTCS induced more hydrophobic, thus enhancing interface activation of CALB@DFNS-C8 for higher specific activity and ethyl butyrate conversion [50].
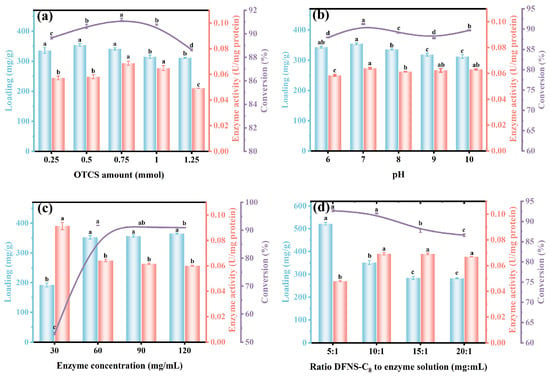
Figure 4.
Effects of (a) OTCS amount, (b) pH of lipase solution, (c) enzyme concentration, (d) ratio of DFNS-C8 to enzyme solution on the loading amount, enzyme activity of CALB@DFNS-C8 and ethyl butyrate conversion. The reaction was carried out in a water bath (40 °C, 300 rpm) for 2 h in an enzyme (3 wt%) with a butyric acid and ethanol molar ratio of 1:4. A significant difference (p < 0.05) between groups is indicated by different lowercase letters.
In addition, the effects of OTCS modification on the secondary structures of free CALB and CALB@DFNS-C8 were investigated. As shown in Figure S3, increasing the OTCS content of CALB@DFNS-C8 from 0 to 0.75 mmol promoted α-helix from 22% to 28% but reduced random coil from 21% to 0. Since higher α-helix content and reduced random coil could improve enzyme rigidity due to increased hydrogen bond, 0.75 mmol of OTCS is concluded to provide the hydrophobic environment needed to maintain the ordered conformation and stability of CALB@DFNS-C8. However, increasing the OTCS content to 1.0 mmol caused the proportion of β-sheet to decrease to 28%, accompanied by the conversion rate of ethyl butyrate slightly declining from 91.2% to 90.8%. Hence, 0.75 mmol of OTCS is an optimum value for DFNS hydrophobic modification to maximize the lipase loading and enzyme activity.
The effects of solution pH, enzyme concentration and the ratio of carrier to enzyme solution on lipase CALB immobilization, enzyme activity and catalytic performance were further investigated. As shown in Figure 4b, the maximum immobilization amount (354.6 mg/g), enzyme activity (0.064 U/mg protein) and conversion rate (91.2%) were identified with lipase solution reaching pH 7, whereas minor variations in enzyme loading amount and activity were observed at other pH levels, indicating that the immobilization process on DFNS-C8 was relatively insensitive to solution pH under the investigated conditions. Instead, with the increase in enzyme concentration from 30 mg/mL to 60 mg/mL, the immobilization amount increased from 192.1 mg/g to 352.5 mg/g, the enzyme activity decreased (from 0.091 U/mg protein to 0.064 U/mg protein), but the conversion rate increased from 53.0% to 91.7%, relatively (Figure 4c). Although continually increasing the enzyme concentration could slightly increase the lipase loading amount, the enzyme activity and corresponding ethyl butyrate conversion were decreased. The excessive accumulation of lipase CALB on DFNS-C8 caused enzyme molecules to be partially obscured or conformationally hindered, thereby reducing the effective activity [51].
Finally, the ratio of DFNS-C8 to lipase solution was also observed to affect performance of immobilized lipase. With the ratio increasing from 5:1 to 20:1, the loading amount of lipase CALB, enzyme activity and ethyl butyrate conversion changed from 521.9 mg/g to 282.3 mg/g, 0.048 U/mg protein to 0.067 U/mg protein, and 92.7% to 86.6%, respectively (Figure 4d). Although there were more adsorbed enzymes at 5:1, the accumulation between enzyme molecules would reduce the accessibility of some catalytic sites and cause waste of enzyme resources [52]. Therefore, considering the enzyme utilization rate and catalytic performance, the optimal condition of the ratio of the carrier to enzyme solution was determined to be 10:1.
3.4. Optimization of Reaction Conditions
Based on the above conditions, the optimized immobilized lipase CALB@DFNS-C8 was used to catalyze the esterification of butyric acid with ethanol for ethyl butyrate synthesis. As shown in Figure 5a, with the molar ratio of butyric acid to ethanol increased from 1:1 to 1:3, the conversion rate of ethyl butyrate also increased significantly from 69.2% to 96.9%, suggesting more ethanol promoted the contact-catalysis between substrates and immobilized lipase. Continually increasing the molar ratio to 1:5 slightly accelerated the reaction speed but had no further enhancement on the yield of ethyl butyrate. Considering the usage of substrates and corresponding product yield, the substrate molar ratio of 1:3 was selected for the following experiments. The effects of moisture content on enzymatic production were also identified and shown in Figure 5b. A 5 wt% ultrapure water induced 94.4% of butyric acid conversion at 3 h, although it was reduced to 93.3% at 4 h. An appropriate amount of water is conducive to maintaining the activity of lipase, but high water content (10–20 wt%) may cause the hydrolysis of ethyl butyrate [53]. In the initial stage, the conversion rate of the system with 5 wt% water was slightly higher than that of the anhydrous system, and reached the highest 94.4% at 3 h, while the anhydrous system was 93.4% at this time. Thus, the optimal water addition of 5 wt% was determined for the subsequent reactions.
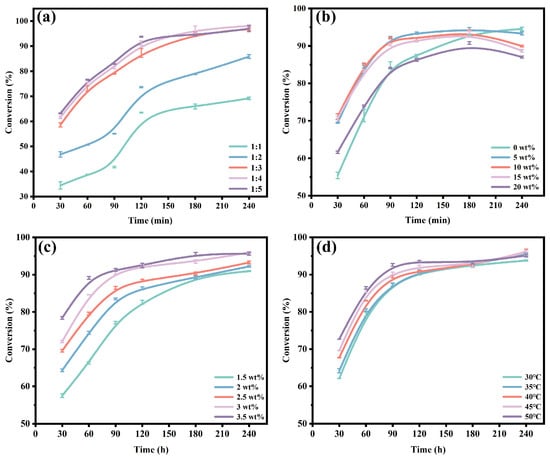
Figure 5.
Effects of (a) molar ratio of butyric acid to ethanol, (b) water addition, (c) enzyme addition and (d) reaction temperature on the enzymatic synthesis of ethyl butyrate by CALB@DFNS-C8.
The effects of enzyme addition and reaction temperature on the synthesis of ethyl butyrate catalyzed by CALB@DFNS-C8 were also studied. As shown in Figure 5c, as the amount of CALB@DFNS-C8 increased from 1.5 wt% to 3 wt%, the conversion rate of ethyl butyrate increased significantly from 91.0% to 96.0%, indicating that more enzyme molecules could provide sufficient catalytic sites to stimulate the substrate conversion rate [54]. Overloaded immobilized lipase had no intensified effects on butyric acid conversion, indicating that 3.5 wt% of CALB@DFNS-C8 had exceeded the threshold of enzyme saturation. Similarly, the highest conversion of ethyl butyrate was reached with the reaction temperature being 40 °C (Figure 5d). Increasing the temperature enhanced the catalytic activity of lipase and promoted the movement of substrate molecules and interfacial diffusion [26]. However, the conversion of ethyl butyrate remained constant, as the catalytic activity of the enzyme had reached a relatively stable state in the reaction temperature range of 40–50 °C. In addition, as shown in Figure S4, CALB@DFNS-C8 consistently exhibited higher conversion than Novozym435 throughout the reaction period, further confirming its superior catalytic performance.
3.5. Catalytic Stability and Applicability Evaluation
To assess industrial feasibility of enzymatic synthesis of ethyl butyrate, the thermostability, organic solvent tolerance and long-term reusability of CALB@DFNS-C8 were assessed. The relative activity of CALB@DFNS-C8 gradually decreased to 85.3%, 71.9% and 61.4% with 100 °C incubation for 1 h, 2 h and 3 h, respectively (Figure 6a). In comparison, free lipase CALB was observed to largely lose its catalytic capacity with the same thermal treatment. Enzyme immobilization provides multi-point interaction between the carrier and enzymes to prevent the conformational fluctuations and reduce heat-induced inactivation of lipases [55]. Since the enzymatic synthesis of flavor esters involves organic acids and alcohols, the organic solvent tolerance of immobilized lipase is a key indicator to affect its esterification application. As shown in Figure 6b, compared to free lipase, whose relative activity was drastically reduced after being incubated in n-hexane, isopropanol, acetonitrile, ethanol and acetic acid for 2 h, the corresponding relative activity for CALB@DFNS-C8 was maintained at 98.7%, 93.2%, 86.2%, 82.6% and 71.2%, respectively. Especially, the stable activity in high-ethanol environment validated the applicability of CALB@DFNS-C8 in catalytic synthesis of flavor esters.
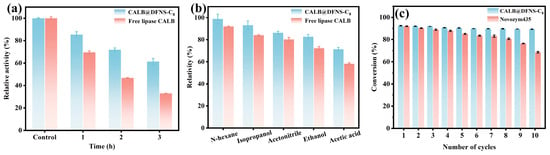
Figure 6.
(a) Thermostability with 100 °C incubation. (b) Organic solvent tolerance of free lipase CALB and CALB@DFNS-C8. (c) Reusability of CALB@DFNS-C8 and Novozym435.
The reusability of immobilized enzymes for the enzymatic synthesis of ethyl butyrate was determined and presented in Figure 6c. Initially, CALB@DFNS-C8 could reach 92.7% of butyrate acid conversion, which gradually declined to 89.2% after being consecutively collected and reused for 10 cycles. The biggest bioactivity reduction in immobilized lipase was noticed from the first-round reaction, and was mainly attributed to enzyme leakage from the carrier surface during the collection and separation washing process. During the multiple times of reuse, the performance degradation of immobilized lipase mainly resulted from the mild inactivation, increased mass transfer resistance or trace loss [56]. In contrast, the commercial immobilized lipase Novozym435 showed a more pronounced loss of activity during repeated reuse, with its conversion decreasing to only 68.5% after ten cycles (Figure 6c). Taken together, these results demonstrate that CALB@DFNS-C8 not only achieves higher initial catalytic efficiency but also maintains markedly superior operational stability over multiple reuse cycles.
Besides the enzymatic synthesis of ethyl butyrate, CALB@DFNS-C8 is also applicable to catalyze the synthesis of acetic acid-based flavor esters (i.e., ethyl acetate, propyl acetate, butyl acetate, pentyl acetate, hexyl acetate) and butyrate acid-based esters (i.e., propyl butyrate, butyl butyrate, pentyl butyrate, hexyl butyrate) with conversion rates of 94.5–98.1% (Table 2). The acetic acid-based esters mainly present sweet pear and banana aromas; butyrate-derived esters pose a more intense tropical fruity aroma, such as pineapple, peach and mango [57,58,59]. These substrates with different carbon-chain lengths all achieved high conversion rates, confirming the high catalytic activity and adaptivity of lipase CALB in catalyzing the production of flavor esters.

Table 2.
Enzymatic synthesis of various short-chain flavor esters.
Table 2.
Enzymatic synthesis of various short-chain flavor esters.
| Flavor Esters | Acyl Acceptor | Main Flavor Characteristics | Conversion (%) |
|---|---|---|---|
| Ethyl acetate | Ethanol | Sweet, Pear | 94. ± 0.4% |
| Propyl acetate | Propanol | Pear, Banana | 95.2 ± 0.1% |
| Butyl acetate | Butanol | Banana, Apple | 95.8 ± 0.4% |
| Pentyl acetate | Pentanol | Banana | 96.5 ± 0.3% |
| Hexyl acetate | Hexanol | Green apple, Pear | 97.9 ± 0.2% |
| Propyl butyrate | Propanol | Pineapple, Apple | 96.0 ± 0.3% |
| Butyl butyrate | Butanol | Pineapple, Mango | 96.1 ± 0.5% |
| Pentyl butyrate | Pentanol | Pineapple, Peach | 97.4 ± 0.2% |
| Hexyl butyrate | Hexanol | Pineapple, Banana | 98.1 ± 0.6% |
3.6. Molecular Docking Analysis
In this section, flavor esters synthesis is further explained through the binding mechanisms between lipase CALB and substrates using molecular docking simulation (Figure 7). The molecular docking results revealed that the acids could be stably embedded in the catalytic pocket of CALB, in which the carbonyl oxygen of the acidic substrates is integrated with Ser105 via a hydrogen bond to form the acyl-enzyme intermediates [60]. As an acyl acceptor, the alcohol molecule is located in the peripheral region of the catalytic pocket and assists the subsequent reactions by forming hydrogen bonds with adjacent residues [61,62]. Binding energy results (Table 3; acid: −3.1 to −4.1 kcal/mol; alcohol: −1.6 to −2.5 kcal/mol) indicate that lipase CALB has a stronger affinity for acyl donor (acid), which was consistent with its ping-pong disubstitution mechanism: in the first step, the acidic substrate was subjected to a Ser105 nucleophilic attack under the stability of an oxyanion hole (Thr40, etc.) to form a tetrahedral acyl-enzyme intermediate stabilized by Thr40 hydrogen bond; in the second step, the alcohol, as an acyl acceptor, initiates a nucleophilic attack on the acyl-enzyme intermediate to generate an ester product and regenerate the active site [63]. As the chain length increases, the interaction between the substrate and the hydrophobic residues is enhanced, thereby forming a more stable complex and maintaining a high conversion rate [64]. The disulfide bond of Cys216-Cys258 maintains the structural rigidity of the catalytic pocket and ensures the correct orientation of the substrate [65]. In summary, the high catalytic performance of lipase CALB is mainly attributed to its hydrophobic cavity and conformational rigidity to maintain the precise orientation of the substrate and allow the acid substrate to form a stable acyl-enzyme intermediate at the active center. At the same time, the alcohol substrate completes the deacylation step through the moderate combination of the peripheral hydrophobic pocket, thereby achieving efficient ester bond formation.
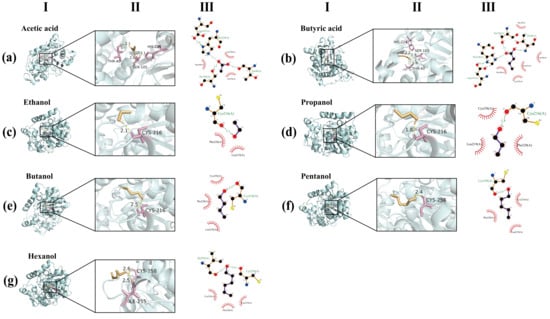
Figure 7.
A schematic diagram of the binding process of acetic acid (a), butyric acid (b), ethanol (c), propanol (d), butanol (e), pentanol (f), hexanol (g) with lipase CALB. The results include (I) and (II) 3D molecular docking, as well as the two-dimensional diagram of (III) interaction.

Table 3.
The binding energy of lipase CALB with different substances.
Table 3.
The binding energy of lipase CALB with different substances.
| Compounds | Binding Energy (kcal/mol) |
|---|---|
| Acetic acid | −3.1 |
| Butyric acid | −4.1 |
| Ethanol | −1.6 |
| Propanol | −1.9 |
| Butanol | −2.1 |
| Pentanol | −2.2 |
| Hexanol | −2.5 |
3.7. Comparison of Enzymatic Synthesis of Ethyl Butyrate
The solvent-free enzymatic synthesis of ethyl butyrate using CALB@DFNS-C8 was compared with organic solvents-involved studies from the previous literature (Table 4). For example, Monteiro et al. [66] immobilized Candida antarctica lipases A and B on magnetic nanoparticles to achieve 99.2% and 97.5% conversion in n-heptane after 6 h, but the whole reactions required a large amount of n-heptane as organic solvent. In the solvent-free system, Paludo et al. [67] achieved 90% ethyl butyrate conversion using 35 wt% Lipozyme TL-IM (Thermomyces lanuginosus lipase immobilized on acrylic resin) within 6 h of the reaction period. In contrast, the CALB@DFNS-C8 prepared in this work achieved 96.0% conversion of ethyl butyrate in 4 h, leading to a CE of 35.1 mmol·g−1·h−1 in solvent-free production of flavor esters.

Table 4.
Comparison of enzymatic synthesis of ethyl butyrate.
4. Conclusions
In this study, a hydrophobically modified DFNS-C8 was developed to immobilize Candida antarctica lipase B (CALB) for the solvent-free synthesis of ethyl butyrate. Maximum yield of 96% was achieved in 4 h by CALB@DFNS-C8, which could maintain 89% of its initial catalytic activity after ten times of reuse. In addition, the immobilized lipase also exhibited superior thermostability, organic solvent tolerance and broad applicability. Molecular docking simulation revealed that the hydrophobic microenvironment of the C8 modified carrier allowed the catalytic triad of lipase CALB to stabilize the acyl–enzyme intermediate at low water activity. Overall, this paper provides a green, efficient and recyclable biocatalytic route for flavor ester production based on the rational carrier preparation and hydrophobicity functionalization.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/foods14244272/s1. Method S1: Characterization of CALB@DFNS-C8. Figure S1: (a) FITC-labeled free lipase CALB. (b) DFNS-C8 carrier observed in CLSM bright field. (c) CALB@DFNS-C8 is shown by the superposition of green channel and bright field. Figure S2: The water contact angles of unmodified DFNS (a) and 0.25 mmol (b), 0.5 mmol (c), 0.75 mmol (d), 1 mmol (d) and 1.25 mmol octyl modified DFNS-C8 were measured. Figure S3: Changes in the secondary structure of free lipase CALB and different octyl modification amounts. Figure S4: Comparison of the catalytic performance of CALB@DFNS-C8 and Novozym435. Figure S5: Gas chromatographic diagram of butyric acid and reaction mixture.
Author Contributions
Conceptualization, investigation, writing—original draft, M.W.; writing—review and editing, supervision, Y.Z.; methodology, data curation, Y.G.; resources, supervision, H.Z.; formal analysis, funding acquisition, M.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Hubei Province Technology Innovation program (2025BBB072, 2024BBB040), Natural Science Foundation of Wuhan (202404081020313) and the Central Public-interest Scientific Institution Basal Research Fund (No. Y2025QC17, 1610172024002).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- SÁ, A.G.A.; de Meneses, A.C.; de Araújo, P.H.H.; de Oliveira, D. A review on enzymatic synthesis of aromatic esters used as flavor ingredients for food, cosmetics and pharmaceuticals industries. Trends Food Sci. Technol. 2017, 69, 95–105. [Google Scholar] [CrossRef]
- Bayramoglu, G.; Celikbicak, O.; Kilic, M.; Yakup Arica, M. Immobilization of Candida rugosa lipase on magnetic chitosan beads and application in flavor esters synthesis. Food Chem. 2022, 366, 130699. [Google Scholar] [CrossRef]
- Siva, S.; Meenatchi, V.; Bodkhe, G.A.; Kim, M. Exploring antibacterial ethyl cinnamate/cyclodextrin inclusion complex electrospun nanofibers. J. Mol. Liq. 2025, 426, 127311. [Google Scholar] [CrossRef]
- de Morais, M.C.; de Oliveira Lima, E.; Perez-Castillo, Y.; de Sousa, D.P. Synthetic Cinnamides and Cinnamates: Antimicrobial Activity, Mechanism of Action, and In Silico Study. Molecules 2023, 28, 1918. [Google Scholar] [CrossRef]
- Grosso, C.; Ferreira-Dias, S.; Pires-Cabral, P. Modelling and optimization of ethyl butyrate production catalysed by Rhizopus oryzae lipase. J. Food Eng. 2013, 115, 475–480. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, J.; Liu, X.; Zhang, C.; Zhao, Z.; Li, X.; Sun, B. Flavor mystery of Chinese traditional fermented baijiu: The great contribution of ester compounds. Food Chem. 2022, 369, 130920. [Google Scholar] [CrossRef]
- Queiroz, L.P.; Nogueira, I.B.R.; Ribeiro, A.M. Flavor Engineering: A comprehensive review of biological foundations, AI integration, industrial development, and socio-cultural dynamics. Food Res. Int. 2024, 196, 115100. [Google Scholar] [CrossRef]
- Li, C.-C.; Abrahamson, M.; Kapoor, Y.; Chauhan, A. Timolol transport from microemulsions trapped in HEMA gels. J. Colloid Interface Sci. 2007, 315, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Sousa, R.R.d.; dos Santos, M.M.; Medeiros, M.W.R.; Manoel, E.A.; Berenguer-Murcia, Á.; Freire, D.M.G.; Fernandez-Lafuente, R.; Ferreira-Leitão, V.S. Immobilized Lipases in the Synthesis of Short-Chain Esters: An Overview of Constraints and Perspectives. Catalysts 2025, 15, 375. [Google Scholar] [CrossRef]
- Xu, Y.; Minhazul, K.A.H.M.; Li, X. The occurrence, enzymatic production, and application of ethyl butanoate, an important flavor constituent. Flavour Fragr. J. 2020, 35, 601–615. [Google Scholar] [CrossRef]
- Jaiswal, K.S.; Rathod, V.K. Process Intensification of Enzymatic Synthesis of Flavor Esters: A Review. Chem. Rec. 2022, 22, e202100213. [Google Scholar] [CrossRef]
- Vilas Bôas, R.N.; de Castro, H.F. A review of synthesis of esters with aromatic, emulsifying, and lubricant properties by biotransformation using lipases. Biotechnol. Bioeng. 2022, 119, 725–742. [Google Scholar] [CrossRef]
- Zhong, L.; Feng, Y.; Wang, Z.; Tian, T.; Bi, Y.; Cui, J. Immobilized lipase on MIL-53(Al)-AM11 with regulatable hydrophobic surface for flavor ester synthesis. Int. J. Biol. Macromol. 2025, 305, 141322. [Google Scholar] [CrossRef]
- Ribeiro, E.S.; Machado, B.R.; Farias, B.S.d.; Han, L.H.; Santos, L.O.d.; Duarte, S.H.; Cadaval Junior, T.R.S.A.; Pinto, L.A.d.A.; Diaz, P.S. Bi-layer nanocapsules based on chitosan and xanthan gum for lipase immobilization. J. Mol. Liq. 2025, 434, 128031. [Google Scholar] [CrossRef]
- Thangaraj, B.; Solomon, P.R. Immobilization of Lipases—A Review. Part I: Enzyme Immobilization. ChemBioEng Rev. 2019, 6, 157–166. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Liu, R.; Liu, Z.; Goh, K.-L.; Zivkovic, V.; Zheng, M. Solvent-free synthesis of diacylglycerols via enzymatic glycerolysis between edible oils and glycerol catalyzed by self-made immobilized lipase PS@LXTE-1000. Oil Crop Sci. 2024, 9, 225–233. [Google Scholar] [CrossRef]
- Ismail, A.R.; Baek, K.-H. Lipase immobilization with support materials, preparation techniques, and applications: Present and future aspects. Int. J. Biol. Macromol. 2020, 163, 1624–1639. [Google Scholar] [CrossRef] [PubMed]
- Zieniuk, B.; Małajowicz, J.; Jasińska, K.; Wierzchowska, K.; Uğur, Ş.; Fabiszewska, A. Agri-Food and Food Waste Lignocellulosic Materials for Lipase Immobilization as a Sustainable Source of Enzyme Support—A Comparative Study. Foods 2024, 13, 3759. [Google Scholar] [CrossRef]
- An, H.; Gong, N.; Chen, H.; Xie, B.; Zhang, Y.; Luo, D. Metal-organic framework-based tunable platform for the immobilization of lipase with enhanced activity in non-aqueous systems. Int. J. Biol. Macromol. 2025, 300, 140272. [Google Scholar] [CrossRef]
- Yang, Y.; Xing, C.; Feng, M.; Su, Y.; Wang, Q.; Pang, D.; Feng, X.; Zhang, Y. Covalent Organic Framework Aerogels with Tailored Microenvironments for Optimizing Enzyme Immobilization and Catalytic Performance. Chin. J. Chem. 2025, 43, 3205–3212. [Google Scholar] [CrossRef]
- Hu, Y.; Dai, L.; Liu, D.; Du, W. Rationally designing hydrophobic UiO-66 support for the enhanced enzymatic performance of immobilized lipase. Green Chem. 2018, 20, 4500–4506. [Google Scholar] [CrossRef]
- Silva, A.R.d.M.; Gonçalves, L.R.B.; da Silva, I.J. Innovations in packed-bed reactors utilizing immobilized lipase catalysts: A comprehensive technical and scientific review. Mol. Catal. 2025, 573, 114814. [Google Scholar] [CrossRef]
- Manoel, E.A.; dos Santos, J.C.S.; Freire, D.M.G.; Rueda, N.; Fernandez-Lafuente, R. Immobilization of lipases on hydrophobic supports involves the open form of the enzyme. Enzyme Microb. Technol. 2015, 71, 53–57. [Google Scholar] [CrossRef]
- Hui, Y.; Wang, L.; Xiao, F.-S. Catalysis Enhanced by Catalyst Wettability. ACS Nano 2025, 19, 7617–7633. [Google Scholar] [CrossRef]
- Liu, R.; Zhou, Q.; Zhang, Y.; Xu, Y.; Liu, Z.; Goh, K.-L.; Zivkovic, V.; Zheng, M. Novel Immobilized Enzyme System Using Hydrophobic Dendritic Mesoporous Silica Nanospheres for Efficient Flavor Ester Production. J. Agric. Food Chem. 2025, 73, 12403–12417. [Google Scholar] [CrossRef]
- Chen, J.; Liu, T.; Zhang, Y.; Zheng, L.; Goh, K.-L.; Zivkovic, V.; Zheng, M. Ultrasound-assisted enzymatic synthesis of cinnamyl acetate by immobilized lipase on ordered mesoporous silicon with CFD simulation and molecular docking analysis. Food Chem. 2025, 464, 141843. [Google Scholar] [CrossRef]
- Souza, M.C.M.d.; Santos, K.P.d.; Freire, R.M.; Barreto, A.C.H.; Fechine, P.B.A.; Gonçalves, L.R.B. Production of flavor esters catalyzed by lipase B from Candida antarctica immobilized on magnetic nanoparticles. Braz. J. Chem. Eng. 2017, 34, 681–690. [Google Scholar] [CrossRef]
- Jiang, M.-Y.; Yu, Z.-T.; Zhang, M.-T.; Li, A.-Q.; Liu, W.; Zhong, H.-X.; Wu, M.-Y.; Cheng, K.-K. Central Composite Design Optimization for the Synthesis of Butyl Acetate Catalyzed by Liquid Lipase. Biotechnol. Appl. Biochem. 2025. [Google Scholar] [CrossRef]
- Suo, H.; Geng, H.; Zhang, L.; liu, G.; Yan, H.; Cao, R.; Zhu, J.; Hu, Y.; Xu, L. Covalent immobilization of lipase on an ionic liquid-functionalized magnetic Cu-based metal–organic framework with boosted catalytic performance in flavor ester synthesis. J. Mater. Chem. B 2023, 11, 1302–1311. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.-W.; Cai, X.; Dou, B.-J.; Qi, F.-Y.; Zhang, X.-J.; Liu, Z.-Q.; Zheng, Y.-G. Expression and characterization of a CALB-type lipase from Sporisorium reilianum SRZ2 and its potential in short-chain flavor ester synthesis. Front. Chem. Sci. Eng. 2020, 14, 868–879. [Google Scholar] [CrossRef]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Aboagye, E.A.; Chea, J.D.; Yenkie, K.M. Systems level roadmap for solvent recovery and reuse in industries. iScience 2021, 24, 103114. [Google Scholar] [CrossRef] [PubMed]
- Freitas, L.; Paula, A.V.; dos Santos, J.C.; Zanin, G.M.; de Castro, H.F. Enzymatic synthesis of monoglycerides by esterification reaction using Penicillium camembertii lipase immobilized on epoxy SiO2-PVA composite. J. Mol. Catal. B Enzym. 2010, 65, 87–90. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, J.; Wang, M.; Ge, X. Fabrication of fibrous amidoxime-functionalized mesoporous silica microsphere and its selectively adsorption property for Pb2+ in aqueous solution. J. Hazard. Mater. 2015, 297, 66–73. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, T.; Liu, R.; Zhou, Q.; Zhou, Y.; Zhang, Y.; Zheng, M. Enzymatic Deacidification and Aroma Characteristics Analysis of Rapeseed Oil Using Self-Made Immobilized Lipase CALB@MCM-41-C8. Foods 2024, 13, 2539. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Jin, J.; Wei, W.; Wu, G.; Wang, X.; Jin, Q. Hyperactivation of lipase by oil-water interface in interfacial immobilization on hierarchical porous hollow silica microsphere: Dynamics, mechanism and application. Food Biosci. 2024, 58, 103706. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, Y.; Xu, Y.; Liu, Z.; Chen, J.; Goh, K.L.; Zhang, Y.; Zheng, M. Molecular docking simulation reveals the lipase-substrate binding mechanism in the enzymatic synthesis of diacylglycerol-enriched vegetable oils. Food Chem. 2025, 474, 143236. [Google Scholar] [CrossRef]
- Bian, Y.; Zhang, Y.; Wang, T.; Yang, C.; Feng, Z.; Goh, K.-L.; Zhou, Y.; Zheng, M. Insights into the enzymatic synthesis of alcoholic flavor esters with molecular docking analysis. LWT 2024, 200, 116206. [Google Scholar] [CrossRef]
- Kaur, P.; Jana, A.K. Candida rugosa Lipase Immobilization on Fe3O4 Coated Carboxyl Functionalised Multiwalled Carbon Nanotubes for Production of Food Flavour Esters. Biotechnol. Bioprocess Eng. 2023, 28, 310–326. [Google Scholar] [CrossRef]
- Park, J.; Choi, Y.; Chang, P.-S. Interface-based kinetic model considering the integral stereoselectivity of lipases on tricapryloylglycerol in a reverse micelle system. Food Chem. 2025, 465, 141403. [Google Scholar] [CrossRef]
- Yu, D.; Zhang, X.; Wang, T.; Geng, H.; Wang, L.; Jiang, L.; Elfalleh, W. Immobilized Candida antarctica lipase B (CALB) on functionalized MCM-41: Stability and catalysis of transesterification of soybean oil and phytosterol. Food Biosci. 2021, 40, 100906. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, Y.; Deng, C.; Zhong, H.; Gu, T.; Goh, K.-L.; Han, Z.; Zheng, M.; Zhou, Y. Green and efficient synthesis of highly liposoluble and antioxidant L-ascorbyl esters by immobilized lipases. J. Clean. Prod. 2022, 379, 134772. [Google Scholar] [CrossRef]
- Saberi, S.; Zhiani, R.; Mehrzad, J.; Motavalizadehkakhky, A. Synthesis and characterization of a novel TEMPO@FeNi3/DFNS–laccase magnetic nanocomposite for the reduction of nitro compounds. RSC Adv. 2020, 10, 27297–27304. [Google Scholar] [CrossRef]
- Schlumberger, C.; Thommes, M. Characterization of Hierarchically Ordered Porous Materials by Physisorption and Mercury Porosimetry—A Tutorial Review. Adv. Mater. Interfaces 2021, 8, 2002181. [Google Scholar] [CrossRef]
- Das, T.; Singha, D.; Pal, A.; Nandi, M. Mesoporous silica based recyclable probe for colorimetric detection and separation of ppb level Hg2+ from aqueous medium. Sci. Rep. 2019, 9, 19378. [Google Scholar] [CrossRef]
- Bayne, L.; Ulijn, R.V.; Halling, P.J. Effect of pore size on the performance of immobilised enzymes. Chem. Soc. Rev. 2013, 42, 9000–9010. [Google Scholar] [CrossRef] [PubMed]
- Ghazi, I.; De Segura, A.G.; Fernández-Arrojo, L.; Alcalde, M.; Yates, M.; Rojas-Cervantes, M.L.; Plou, F.J.; Ballesteros, A. Immobilisation of fructosyltransferase from Aspergillus aculeatus on epoxy-activated Sepabeads EC for the synthesis of fructo-oligosaccharides. J. Mol. Catal. B Enzym. 2005, 35, 19–27. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, Y.; Xu, Y.; Zhou, Y.; Li, S.; Zheng, M. Broadly adapted and efficient enzymatic transesterification production of medium and long-chain triglycerides via coconut oil and long-chain triacylglycerols. Food Chem. 2025, 469, 142499. [Google Scholar] [CrossRef]
- Pota, G.; Bifulco, A.; Parida, D.; Zhao, S.; Rentsch, D.; Amendola, E.; Califano, V.; Costantini, A. Tailoring the hydrophobicity of wrinkled silica nanoparticles and of the adsorption medium as a strategy for immobilizing lipase: An efficient catalyst for biofuel production. Microporous Mesoporous Mater. 2021, 328, 111504. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Fernández-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef]
- Sabi, G.J.; de Souza, L.; Abellanas-Perez, P.; Tardioli, P.W.; Mendes, A.A.; Rocha-Martin, J.; Fernandez-Lafuente, R. Enzyme loading in the support and medium composition during immobilization alter activity, specificity and stability of octyl agarose-immobilized Eversa Transform. Int. J. Biol. Macromol. 2025, 295, 139667. [Google Scholar] [CrossRef]
- de Andrade Silva, T.; Keijok, W.J.; Guimarães, M.C.C.; Cassini, S.T.A.; de Oliveira, J.P. Impact of immobilization strategies on the activity and recyclability of lipases in nanomagnetic supports. Sci. Rep. 2022, 12, 6815. [Google Scholar] [CrossRef]
- Qin, Z.; Feng, N.; Li, Y.; Fei, X.; Tian, J.; Xu, L.; Wang, Y. Hydrogen-bonded lipase-hydrogel microspheres for esterification application. J. Colloid Interface Sci. 2022, 606, 1229–1238. [Google Scholar] [CrossRef] [PubMed]
- Dudu, A.I.; Lăcătuş, M.A.; Bencze, L.C.; Paizs, C.; Toşa, M.I. Green Process for the Enzymatic Synthesis of Aroma Compounds Mediated by Lipases Entrapped in Tailored Sol–Gel Matrices. ACS Sustain. Chem. Eng. 2021, 9, 5461–5469. [Google Scholar] [CrossRef]
- ACS Appl Mater InterfacesZhao, J.; Ma, M.; Yan, X.; Wan, D.; Zeng, Z.; Yu, P.; Gong, D. Immobilization of lipase on β-cyclodextrin grafted and aminopropyl-functionalized chitosan/Fe3O4 magnetic nanocomposites: An innovative approach to fruity flavor esters esterification. Food Chem. 2022, 366, 130616. [Google Scholar]
- Ren, Y.; Rivera, J.G.; He, L.; Kulkarni, H.; Lee, D.-K.; Messersmith, P.B. Facile, high efficiency immobilization of lipase enzyme on magnetic iron oxide nanoparticles via a biomimetic coating. BMC Biotech. 2011, 11, 63. [Google Scholar] [CrossRef]
- Carpena, M.; Fraga-Corral, M.; Otero, P.; Nogueira, R.A.; Garcia-Oliveira, P.; Prieto, M.A.; Simal-Gandara, J. Secondary Aroma: Influence of Wine Microorganisms in Their Aroma Profile. Foods 2021, 10, 51. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, S.; Sahney, R.; Dahiya, P. Green synthesis of iron-alginate nanoparticle for Taguchi-assisted immobilization of Candida rugosa lipase and its application in the synthesis of butyl butyrate ester. Process Biochem. 2024, 139, 81–92. [Google Scholar] [CrossRef]
- Ma, N.; Zhu, J.; Wang, H.; Qian, M.C.; Xiao, Z. Comparative Investigation of Aroma-Active Volatiles in (“Ruixue”, “Liangzhi”, “Crystal Fuji,” and “Guifei”) Apples by Application of Gas Chromatography–Mass Spectrometry–Olfactometry (GC–MS–O) and Two-Dimensional Gas Chromatography-Quadrupole Mass Spectrometry (GC × GC-qMS) Coupled with Sensory Molecular Science. J. Agric. Food Chem. 2024, 72, 25229–25250. [Google Scholar]
- Nazarian, Z.; Arab, S.S. Solvent-dependent activity of Candida antarctica lipase B and its correlation with a regioselective mono aza-Michael addition—experimental and molecular dynamics simulation studies. Heliyon 2022, 8, e10336. [Google Scholar] [CrossRef]
- Uppenberg, J.; Oehrner, N.; Norin, M.; Hult, K.; Kleywegt, G.J.; Patkar, S.; Waagen, V.; Anthonsen, T.; Jones, T.A. Crystallographic and molecular-modeling studies of lipase B from Candida antarctica reveal a stereospecificity pocket for secondary alcohols. Biochemistry 1995, 34, 16838–16851. [Google Scholar] [CrossRef]
- Wang, H.; Li, Z.; Yu, X.; Chen, R.; Chen, X.; Liu, L. Green synthesis of (R)-3-TBDMSO glutaric acid methyl monoester using Novozym 435 in non-aqueous media. RSC Adv. 2015, 5, 75160–75166. [Google Scholar] [CrossRef]
- Tjørnelund, H.D.; Vind, J.; Brask, J.; Woodley, J.M.; Peters, G.H.J. Candida antarctica lipase B performance in organic solvent at varying water activities studied by molecular dynamics simulations. Comput. Struct. Biotechnol. J. 2023, 21, 5451–5462. [Google Scholar] [CrossRef] [PubMed]
- Zisis, T.; Freddolino, L.; Turunen, P.; van Teeseling, M.C.F.; Rowan, A.E.; Blank, K.G. Interfacial Activation of Candida antarctica Lipase B: Combined Evidence from Experiment and Simulation. Biochemistry 2015, 54, 5969–5979. [Google Scholar] [CrossRef] [PubMed]
- Błaszczyk, J.; Kiełbasiński, P. Quarter of a Century after: A Glimpse at the Conformation and Mechanism of Candida antarctica Lipase B. Crystals 2020, 10, 404. [Google Scholar] [CrossRef]
- Monteiro, R.R.C.; Neto, D.M.A.; Fechine, P.B.A.; Lopes, A.A.S.; Gonçalves, L.R.B.; dos Santos, J.C.S.; de Souza, M.C.M.; Fernandez-Lafuente, R. Ethyl Butyrate Synthesis Catalyzed by Lipases A and B from Candida antarctica Immobilized onto Magnetic Nanoparticles. Improvement of Biocatalysts’ Performance under Ultrasonic Irradiation. Int. J. Mol. Sci. 2019, 20, 5807. [Google Scholar] [CrossRef]
- Paludo, N.; Alves, J.S.; Altmann, C.; Ayub, M.A.Z.; Fernandez-Lafuente, R.; Rodrigues, R.C. The combined use of ultrasound and molecular sieves improves the synthesis of ethyl butyrate catalyzed by immobilized Thermomyces lanuginosus lipase. Ultrason. Sonochem 2015, 22, 89–94. [Google Scholar] [CrossRef]
- Shu, C.; Cai, J.; Huang, L.; Zhu, X.; Xu, Z. Biocatalytic production of ethyl butyrate from butyric acid with immobilized Candida rugosa lipase on cotton cloth. J. Mol. Catal. B Enzym. 2011, 72, 139–144. [Google Scholar] [CrossRef]
- Asmat, S.; Anwer, A.H.; Husain, Q. Immobilization of lipase onto novel constructed polydopamine grafted multiwalled carbon nanotube impregnated with magnetic cobalt and its application in synthesis of fruit flavours. Int. J. Biol. Macromol. 2019, 140, 484–495. [Google Scholar] [CrossRef]
- de Oliveira, U.M.F.; Lima de Matos, L.J.B.; de Souza, M.C.M.; Pinheiro, B.B.; dos Santos, J.C.S.; Gonçalves, L.R.B. Efficient biotechnological synthesis of flavor esters using a low-cost biocatalyst with immobilized Rhizomucor miehei lipase. Mol. Biol. Rep. 2019, 46, 597–608. [Google Scholar] [CrossRef]
- Vrutika, P.; Datta, M. Lipase from Solvent-Tolerant Pseudomonas sp. DMVR46 Strain Adsorb on Multiwalled Carbon Nanotubes: Application for Enzymatic Biotransformation in Organic Solvents. Appl. Biochem. Biotechnol. 2015, 177, 1313–1326. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).