Abstract
Cultured meat has progressed from early in vitro cell culture concepts to regulatory approvals and preliminary commercialization, with recent advancements propelled by interdisciplinary innovations in cell line engineering, serum-free media, bioreactor design, and three-dimensional (3D) assembly technologies. This review synthesizes recent developments from 2023 to 2025, utilizing peer-reviewed publications, patent analyses, regulatory frameworks, and media reports to assess global preparedness for large-scale production. Asia has emerged as a leading hub, with China, Japan, South Korea, and Singapore focusing on scaffold-based 3D cultures, bioinks, and serum-free strategies, complemented by national centers and pilot facilities. The United States leverages its technological advancements and established regulatory framework, as evidenced by recent Food and Drug Administration and United States Department of Agriculture approvals. However, potential complications related to political regional bans and legislation may arise. Europe and the UK prioritize defined media, cell optimization, and structured novel-food regulations, with early commercialization primarily in pet food. Looking ahead, the industrialization of cultured meat is anticipated to be driven by process engineering and hybrid product strategies, with initial pilot-to-demonstration facilities established in countries open to alternative food products. Premium and hybrid cultured meat products are expected to enter the market first, while whole-cut cultured meat is likely to remain a premium offering into the early 2030s.
1. Introduction
As global population and income levels rise, so does the consumption of animal-based foods. Concurrently, negative perceptions of the livestock industry are increasing. These factors have heightened consumer and industry interest in alternative protein sources, such as cultured meat, insect-derived foods, and mycoprotein. Cultured meat, in particular, is predicted to enter the market soon, though it has yet to achieve full-scale industrialization, generating significant interest among consumers and industry stakeholders regarding its timing.
The technological foundation of cultured meat continues to evolve incrementally [1]. Recent breakthroughs represent substantial progress toward producing thick, three-dimensional (3D) cuts rather than solely minced products [2]. Nonetheless, high production costs remain a critical challenge, primarily owing to expensive culture media and the nascent stage of scalable bioreactor infrastructure. Researchers worldwide are intensifying efforts to mitigate costs by enhancing cell proliferation, developing serum-free alternatives, and optimizing production systems.
As of mid-2025, regulatory approvals have been granted in Singapore, the United States (US), and Australia for cultivated products such as chicken, quail, salmon, and pork fat by companies including GOOD Meat, UPSIDE Foods, Wildtype, Mission Barns, and Vow [3]. However, market-wide pricing remains unattainable, and numerous jurisdictions continue to impose legislative bans or regulatory obstacles reflecting concerns regarding traditional livestock industries and policies. Collectively, these developments indicate that while commercialization remains nascent, scientific innovation and strategic investment show promise.
The status of cultured meat technology and its commercialization necessitates continuous updates owing to the rapid advancements at the intersection of science, regulation, and market adoption. Breakthroughs in cell line development, serum-free media, and bioreactor design occur alongside regulatory milestones. This fragmented and rapidly evolving landscape means that assessments of technological readiness, consumer acceptance, and investment flows can quickly become outdated. Therefore, regularly updated global overviews are essential not only for researchers and policymakers monitoring food security and sustainability goals but also for investors, startups, and consumers seeking clarity on the integration of cultured meat into the food system. Consequently, this study aims to provide an update on recent developments within the cultured meat landscape to predict the timeline for full-scale industrialization by analyzing recent publications, patents, regulatory breakthroughs, and media reports worldwide.
2. Status of Technologies Relevant to Cultured Meat Industrialization
2.1. Status of Cutting-Edge Technologies for Cultured Meat
The development of cultured meat technology can be traced back to early ideas proposed by Winston Churchill, evolving through the gradual discovery and development of in vitro cell culture (Figure 1). A significant milestone occurred in 2001 when biologist Willem van Eelen patented methods for producing meat through cell culture [4]. This work laid the groundwork for further academic and commercial interest, culminating in the first public demonstration of a lab-grown hamburger by Mark Post at Maastricht University in 2013, underscoring the feasibility of scaling muscle cell cultivation into edible products. Since then, advances in stem cell biology, bioreactor design, and serum-free media formulations have accelerated progress, reducing costs and enhancing product quality. The field has evolved into a multidisciplinary endeavor, driven by academic research and industry collaborations, aimed at providing a sustainable and ethical alternative to conventional animal agriculture.
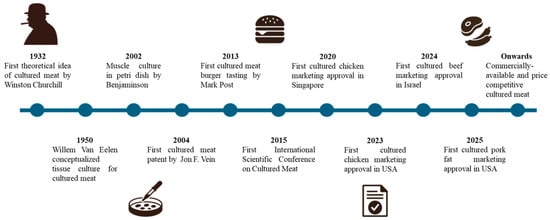
Figure 1.
Timeline of cultured meat technological development and progress.
The commercialization of cultured meat is deeply rooted in academic research, which provides the essential scientific foundation for this emerging field. Universities and research institutes have pioneered key breakthroughs in stem cell biology, tissue engineering, and bioprocess design, enabling the development of reliable methods for cultivating animal cells into edible products. Supplementary Table S1 details global research from 2023 to 2025 focusing on cultured meat development.
2.1.1. Asia
Asian countries are emerging as significant contributors to cultured meat development owing to their rapidly growing populations, high meat consumption, and robust technological infrastructure. Countries such as China, Japan, Singapore, and South Korea are investing heavily in research and development (R&D) to address food security, environmental sustainability, and ethical concerns associated with conventional livestock production (Supplementary Table S1) [5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149]. China leads in research publications, with the majority focusing on 3D culture utilizing scaffolds and hydrogels [5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35]. Other countries with substantial publication outputs include Israel, South Korea, and Japan, with research topics predominantly involving scaffolds, microcarriers, and bioinks, emphasizing the importance of 3D culture in cultured meat production. Additionally, Japan, Korea, and Singapore have published numerous studies on culture media development, ranging from the use of natural materials (e.g., Anabaena sp., Chlorella vulgaris, and Okara) to engineered cells (e.g., RL24 cells, E. coli ribosome-incorporated chick muscle cells, Lactococcus lactis) capable of producing growth factors to achieve serum-free media conditions. Research showcasing 3D assembled cultured meat has emerged from countries including China, India, Israel, Japan, South Korea, and Singapore, reflecting the technological preparedness of these nations for developing cultured meat products for human consumption [36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93].
High scientific research publication rates among Asian countries may indicate both public demand and governmental support for alternative food production research. These publications also suggest the preparedness of these countries for cultured meat commercialization. In China, reports on 3D hydrogel chicken fibroblasts for creating whole cut meat constructs, the large-scale expansion of myogenic cells using porous microcarriers, and cultivated fish fillets that closely resemble commercial products demonstrate significant technological advancements in cultured meat production [10,12,18]. Meanwhile, Israel has reported the successful production of steak-like cuts via 3D printing technology and the scaffold-free cultured beef production [40,44]. Japan’s use of hollow fiber bioreactors for whole cut meat production and serum-free media formulations for cultured quail indicates a commitment to achieving meat-like characteristics while alleviating production costs [47,51]. South Korea has also showcased its ability to produce 3D cultured meat cuts using bioinks and enhance the umami-related metabolites of chicken cells for cultured meat [58,69,78]. A keyword analysis of cultured meat-related research papers in Korea reveals a focus on alternative protein sources, such as edible insects and plant-based proteins (Figure 2), emphasizing the importance of exploring alternative protein sources for hybrid cultured meat products [150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181]. Hybrid cultured meat products are food that contain animal-based cultured meat in combination with other types of alternative proteins. Singapore, the first and only Asian country to commercialize cultured meat, is optimizing spent media recycling using Lactococcus lactis for FGF2-G3 production and developing a flavor extraction method to achieve high sensory similarity to conventional meat [91,92].

Figure 2.
Resent research keyword trends in Korea regarding cultured meat and meat alternatives from 2023 to 2025. Word cloud analysis was conducted exclusively using the top 50 keywords. Furthermore, databases (Lens.org, PubMed, Web of Science, and Scopus) for these journals’ publications from 2023 to 2025 were utilized.
2.1.2. America
Among the countries listed in Supplementary Table S1, the US primarily focuses on cultured meat media development and formulation [103,104,105,106,107,108,109,110,111,112,113]. Notably, two studies concentrate on ammonia reduction strategies that could enhance media recycling. One study highlights the effectiveness of Chlorella sorokiniana, which can assimilate ammonia without affecting glucose levels [103]. On the other hand, an alkalization-stripping method shows promising results, achieving at least an 82% reduction in ammonia in cultured media for lamb muscle cell growth [104]. In addition to ammonia reduction efforts, artificial intelligence (AI)-driven cultured meat media formulations have been reported, revealing the potential role of AI in optimizing cultured meat production [109]. Another common theme in American research is 3D cultures, which encompass scaffolds and bioprinting [114,115,116,117,118,119,120]. These scaffolds are often derived from plant-based materials, which contribute to their non-cytotoxicity and edibility [114,115,118,119]. Brazil has introduced innovations such as fetal bovine serum (FBS) substitutes made from soy and peanut processing by-products, canola oil-containing microcarriers for culturing chicken cells, and cellulose acetate-based nanofibers as scaffolds for 3D cultured meat [121,122,123]. Chile has developed marine biopolymer-based edible scaffolds that promote the alignment of myotubes and enhance myogenic gene expression [125].
2.1.3. Europe and the United Kingdom (UK)
As pioneers in the alternative food movement, European countries have published extensive research on cultured meat production [126,127,128,129,130,131,132,133,134,135,136,137]. Denmark has developed a non-invasive monitoring system for the proliferation and differentiation of cultured meat precursor cells, a crucial technology for optimizing culture [128]. Germany has successfully produced spheroids using bovine adipose-derived stem cells, which remain viable when mixed with edible gellan gum for 3D bioprinting [131]. Meanwhile, the Netherlands, noted for hosting the first public cultured meat tasting, has focused on cell culture optimization. A particularly notable report indicates that a defined medium targeting mitogen-activated protein kinase (MEK)/extracellular signal-regulated kinase (ERK), NOTCH, and retinoid X receptor (RXR) pathways can achieve nearly 100% myogenic fusion while enhancing bovine muscle cell myotube formation [133]. Furthermore, the characterization of cellular heterogeneity in muscle cultures through single-cell analysis could aid in the selection of desirable cultured meat progenitor cells [134].
Beyond Europe, the UK has also published significant research related to cultured meat production. Among these publications, a report on the spontaneous immortalization of a porcine stem cell line achieving up to 100% adipogenic efficiency shows promise for efficient cultured fat production [138]. Furthermore, a serum-free medium formulation that can enhance the proliferation and differentiation of bovine muscle cells in both 2D and 3D cultures has been reported [141].
2.1.4. Others
Australia is making substantial contributions to cultured meat development by combining agricultural expertise with advanced biotechnology [142,143,144,145,146,147]. A notable report describes a co-culture system utilizing glucose-sparing Chlorella BDH-1 algae, which sustains oxidative metabolism and pH control in mammalian cell cultures, resulting in extended culture longevity [144]. This technology optimizes culture media use, potentially mitigating cultured meat production costs. Additionally, the use of microfluidic platforms for large-scale stem cell production has been reported [146], highlighting the potential of microfluidics to achieve cost-effective, energy-efficient, and automated culture technology for both cultured meat production and regenerative medicine. In Russia, the integration of 3D printing technology with an AI model for real-time monitoring and quality control of cell cultures may facilitate the commercialization of cultured meat production in Russia [148]. Finally, Türkiye has developed a postbiotic, Biftek-1, which serves as a growth promoter for bovine satellite cells [149].
2.2. Patents
The patent landscape for cultured meat technology is becoming increasingly important as countries vie for leadership in this emerging bioeconomy. Given the complexity of innovations in cell line development, growth media formulation, bioreactor design, and scalable production methods, patents serve to protect intellectual property and incentivize further investment in research and commercialization. Nations that establish robust patent portfolios not only provide competitive advantages for their domestic companies in the global market but also attract foreign investment and partnerships. Moreover, patents help define standards and ownership in a rapidly evolving field where regulatory frameworks are still developing. Resultantly, strategic patenting in cultured meat encompasses not only the safeguarding of technological advancements but also the shaping of future trade dynamics, thereby ensuring food security and strengthening national positions in the pursuit of sustainable protein production. Supplementary Table S2 presents patents related to cultured meat from 2023 to 2025.
2.2.1. Asia
China, the leading jurisdiction globally for cultivated meat filings, notes that Chinese universities and public entities have filed more cultured meat patents than their US and European counterparts combined, reflecting considerable state-backed momentum (Supplementary Table S2) [182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198]. Most of these patents originate from academic institutions, including Zhejiang University, Nanjing Agricultural University, and Jiangnan University [182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198]. Meanwhile, companies in Israel remain among the most active filers globally, with Aleph Farms and other cultured meat startups maintaining the country’s prominence in cultured meat-related patents [199,200,201,202,203,204]. Japan’s patent landscape is diverse, with contributions from both universities (e.g., Kyoto University, Tokyo University, Osaka University, and Tokyo University of Agriculture and Technology) and private companies (e.g., Nippon Ham Food, Ltd., Ajinomoto, IntegriCulture, Inc.) yielding a limited number of cultured meat-related patents [205,206,207,208,209,210,211,212,213,214]. South Korea has experienced a notable increase in patenting activity, with universities and firms such as Seoul National University, Yonsei University, Chung-Ang University, and Hanwha Solutions filing multiple patents related to scaffold technology and culture media development [215,216,217,218,219,220,221,222,223,224,225,226,227].
2.2.2. America
Between 2023 and 2025, the US patent landscape for cultured meat technologies has been influenced by regulatory milestones and intensified competition among startups and established food companies. Following Food and Drug Administration (FDA) and United States Department of Agriculture (USDA) approvals in 2023 that authorized Upside Foods and Good Meat to commercially sell cultivated chicken, US firms accelerated their patent filings across critical domains, including serum-free media formulations, immortalized cell lines, edible scaffolds, and large-scale bioreactor systems [199,228,229,230,231,232,233,234,235,236,237,238,239,240,241,242,243,244,245,246,247,248,249]. These patents serve not only as tools for technological protection but also as strategic assets to attract investment in a newly legitimized market. Overall, the US patent landscape during this period reflects both the opportunities unlocked by federal regulatory clarity and the ongoing necessity to secure exclusive rights in a field where technical differentiation is vital for survival.
2.2.3. Europe and the UK
The European patent landscape for cultured meat technologies is characterized by continuous innovation, cautious regulation, and significant institutional involvement [250,251,252,253,254,255,256,257,258,259,260,261]. In contrast to the US, where market approvals have expedited private filings, Europe’s more stringent regulatory environment implies that patents frequently precede commercialization, serving as early markers in the competition for future market access. Companies, primarily based in the Netherlands, have been particularly proactive in protecting innovations related to cell differentiation protocols, plant-based scaffolds, and cost-effective serum-free media [253,254,255,256,257,258]. In the UK, however, patents are relatively scarce, with Cellular Agriculture, Ltd., and Ivy Farms being the most notable players in the cultured meat landscape [262,263,264]. However, in Australia, a patent has been granted for developed methods for aseptically processing and packaging cultured biomass meat [265].
2.3. Regulatory Approval
A clear and well-structured regulatory framework is essential for the advancement of cultured meat technology, as it establishes the foundation for both consumer trust and industry growth. Cultured meat involves innovative scientific processes, such as animal cell cultivation, bioreactor production, and new food safety protocols, which fall outside the purview of traditional meat regulation, rendering tailored oversight critical. Without appropriate regulations, companies face uncertainties that may hinder commercialization and deter investment. Conversely, well-defined approval pathways ensure that products satisfy rigorous safety and quality standards, thereby enhancing public confidence in this novel food category. Moreover, harmonized regulations across countries can reduce trade barriers, foster global collaboration, and accelerate innovation. Overall, a comprehensive regulatory framework not only safeguards public health but also balances innovation with responsibility, enabling cultured meat to fulfill its potential for sustainable and ethical protein production. Supplementary Table S3 presents the status of cultured meat regulation in countries actively engaged in commercialization efforts.
2.3.1. Asia
Globally, regulatory frameworks for cultured meat are evolving, with countries adopting varied yet converging approaches to ensure safety and oversight. In India, the Food Safety and Standards Authority established rules for innovative and unspecified foods in 2017, requiring prior approval for any cell-based products [266]. Similarly, Israel permits commercialization only after a thorough review of documentation concerning safety, nutrition, and intake levels [267,268]. In Japan, while no specific legislation addresses cultured meat, it is classified as food under the Food Sanitation Act, with ongoing evaluations of standards and complementary assessments under the Slaughterhouse Act and Feed Safety Law, alongside efforts to draft new guidelines [269]. The Republic of Korea updated its Food Sanitation Act Enforcement Rules in 2023 to formally recognize cell- and microbe-derived raw materials, followed by guidance from the Ministry of Food and Drug Safety (MFDS) in 2024 to aid submissions for provisional standards [270,271]. Meanwhile, Singapore’s Food Agency (SFA) mandates that any food or ingredient not consumed in the past 20 years must undergo evaluation and approval prior to market entry [271]. Taken together, these examples illustrate a trend toward structured pre-market approval processes that prioritize safety while also reflecting regional differences in the speed and scope of regulatory adaptation for cultured meat.
2.3.2. America
The US has emerged as one of the earliest major markets to formally approve cultured meat for human consumption. In November 2022, the US FDA issued its first “no questions” letter confirming the safety of cultivated chicken produced by UPSIDE Foods, followed by a similar approval for GOOD Meat in 2023. After the FDA reviews safety data, the USDA oversees facility inspections and product labeling. This dual-agency regulatory system ensures both food safety and consumer protection. In June 2023, the USDA granted final approval for UPSIDE Foods and GOOD Meat to sell cultivated chicken to consumers, positioning the US among the few countries with an operational regulatory pathway [272]. Recently, Mission Barn was also granted approval for its cultured pork fat cells [273,274]. In contrast, Canada currently lacks a dedicated regulatory pathway for cultured meat. Oversight falls under Health Canada’s Food and Drug Regulations, which necessitate comprehensive safety assessments by Health Canada, the Canadian Food Inspection Agency, and Environment and Climate Change Canada [275]. To date, no company has received full approval, despite several engagements with regulators to establish safety dossiers. Brazil has demonstrated early openness to regulating cultured meat; in 2020, the Brazilian National Health Surveillance Agency (ANVISA) and the Ministry of Agriculture, Livestock, and Supply (MAPA) announced their intention to share regulatory responsibilities, with ANVISA focusing on food safety and MAPA on production oversight and labeling [276].
2.3.3. Europe and the UK
In Europe, cultured meat is regulated under Regulation (European Union, EU) 2015/2283 on novel foods, which classifies products derived from animal, plant, microbial, fungal, or algal cell or tissue cultures as novel foods. Each product must undergo a safety assessment by the European Food Safety Authority (EFSA) and receive final authorization from the European Commission prior to commercialization [277]. Following Brexit, the UK Food Standards Agency (FSA) assumed responsibility for regulating cultured meat, maintaining an approach aligned with the EU by treating it as a novel food subject to equivalent risk assessment and approval procedures. This harmonized framework underscores both the EU’s and the UK’s emphasis on rigorous safety evaluations as prerequisites for market entry of cell-cultured products. Since leaving the EU, the UK has established its own framework for approving novel foods. Cultured meat is regulated by the FSA in England, Wales, and Northern Ireland, and by Food Standards Scotland in Scotland. The UK mandates a pre-market authorization process akin to that of the EU, including safety and production assessments [277]. As of 2025, Meatly managed to launch its cultured pet food in the UK and EU [278]. However, no cultured meat product for human consumption has yet received recognition.
2.3.4. Other Regions
Australia and New Zealand operate under a unified regulatory framework through Food Standards Australia New Zealand (FSANZ). Cultivated meat is classified as a novel food, necessitating pre-market approval via a comprehensive safety and production dossier [279,280]. Once FSANZ grants approval, the authorization is automatically applicable in both countries, ensuring regulatory consistency. Vow in Australia recently secured approval for its cultured quail as a novel food [281]. This marks the first instance of a cultured meat product receiving approval from this joint regulatory system.
2.4. Media Reports
Media coverage plays an indispensable role in the commercialization of cultured meat technology, influencing public perception, consumer acceptance, and market readiness for this novel food. Considering that cultured meat challenges traditional notions of meat production, clear and accessible media communication helps demystify the science, highlighting benefits—such as sustainability, animal welfare, and food security—and addressing potential safety and ethical concerns. Positive media coverage also attracts the attention of investors, policymakers, and industry stakeholders, generating momentum for regulatory approvals and infrastructure development. Conversely, misinformation or negative framing can obstruct adoption, underscoring the necessity for accurate, transparent, and engaging media narratives. Ultimately, strategic media coverage not only raises awareness but also fosters the trust and enthusiasm required to transition cultured meat from laboratory innovation to mainstream food product. Supplementary Table S4 presents recent media activities related to cultured meat worldwide.
2.4.1. Asia
South Korea is emerging as a significant player in the cultivated meat sector, bolstered by substantial public investment and innovative research [282,283,284,285,286]. The government has established the National Cell Culture Food Tech Research Support Center (Uiseong, South Korea), with approximately $10 million allocated to enhance R&D, regulatory frameworks, and commercialization. This center features facilities such as 1000 L bioreactors and comprehensive startup support [282]. Academic contributions are notable as well; for instance, Gyeongsang National University’s startup “Orange CAU” has introduced the world’s first hybrid cultured meat with marbling resembling that of real beef, while researchers at Sogang University have developed a self-healing scaffold that aligns muscle and fat cells to replicate marbling, enhancing scalability for cost-effective production [283,284]. Despite these advancements, industry surveys indicate that several Korean startups prefer to launch abroad owing to exorbitant domestic regulatory application fees (₩45 million, approximately $35,000–40,000), in stark contrast to minimal or no fees in markets such as Singapore, the US, and Europe [285]. MFDS officials argue that the fee reflects necessary review costs, yet it underscores a regulatory obstacle that may impede Korea’s commercialization efforts, even as the nation seeks to position itself as a global hub for food-tech innovation [286].
Japan is making steady strides in cultivated meat research, innovation, and public engagement, with contributions from both academia and industry [287,288,289,290,291]. At the University of Tokyo, Professor Shoji Takeuchi and his team successfully produced and tasted a 1 cm3 piece of lab-grown beef, which exhibited umami flavor and chewiness, although it did not entirely replicate conventional beef [287]. Additionally, the same university has developed a hollow fiber-based technique that enables the production of 1 cm thick cultured chicken meat, improving texture and amino acid content by overcoming nutrient diffusion limitations in thicker tissues [287]. Broader national collaboration is evident through the Cultivated Meat Future Creation Consortium—which includes Osaka University, Shimadzu, Itoham Yonekyu, Toppan, SigmaX, and Zacros—hosting the “Cultivated Meat Journey 2025” at the Osaka–Kansai Expo, featuring 3D-bioprinted meat, grilling aroma demonstrations, and discussions on societal adoption [289]. Regulatory progress is also underway, with the Japan Association for Cellular Agriculture submitting proposed risk assessment guidelines for cell-based foods to the Consumer Affairs Agency [290]. Complementing these efforts, Professor Noriyoshi Matsuzaki from Osaka University showcased advancements in 3D-bioprinting of muscle, fat, and vasculature at the Shimadzu 4th Global Food Summit, emphasizing Japan’s commitment to both technological breakthroughs and public engagement initiatives that bring cultivated meat closer to realistic, widely accepted applications [291].
China is rapidly advancing its cultivated meat sector through coordinated government, academic, and private initiatives that combine infrastructure investment, technological advancements, and regulatory planning [292,293,294,295]. In Beijing, a national alternative protein center has been launched, featuring a 200 L bioreactor for cultured meat and plans for two 2000 L units, reflecting ambitions for large-scale production [292]. The China Meat Research Center has pioneered hybrid products such as lab-grown chicken and pork rice, designed to integrate muscle, fat, and grain nutrients for a balanced diet [293]. Concurrently, Zhouzi Future Foods, in collaboration with Nanjing Agricultural University, has made significant progress by developing serum-free culture media and completing pilot-scale pork production, which currently yields approximately 10 kg per month, with plans to scale up to 20 t annually [295]. Regulatory oversight remains critical, warranting approval from the National Health Commission and risk assessments from the China National Center for Food Safety Risk Assessment. Alongside these technical and regulatory measures, startups are strategically targeting premium products such as eel, caviar, and foie gras to offset high costs, a commercialization strategy supported by international media [296]. With milestones including successful 5 kg pilot production in 2023 and expert endorsements of its technological leadership, China’s pathway toward the industrialization of cultivated meat is becoming in creasingly evident, aligning sustainability goals with global competitiveness [297].
Singapore has established itself as a global leader in the commercialization of cultivated meat, despite experiencing both breakthroughs and setbacks [298,299,300,301,302]. Esco Aster has announced plans for a large-scale facility in Changi by 2025, projected to produce 400–500 t annually, reinforcing Singapore’s role as a manufacturing hub [298]. Regulatory progress has been pivotal; the SFA authorized Eat Just’s GOOD Meat division to utilize serum-free culture media, enhancing safety and scalability, while also granting Vow approval to market cell-cultured quail, thereby expanding the diversity of cultivated products available [299,300,301]. However, challenges persist, as evidenced by the closure of GOOD Meat’s planned Bedok facility in 2023, reflecting financial and operational difficulties within the sector [301]. Looking ahead, international collaboration continues to grow, highlighted by Meatable’s partnership with local entity TruMeat to establish Singapore’s first pilot-scale facility designed to supply cultivated meat at cost-effective levels for commercial partners, emphasizing the city-state’s ongoing role as a regulatory pioneer and innovation hub for alternative proteins [302].
2.4.2. America
In the US, recent regulatory approvals signify a turning point, as the USDA has cleared Upside Foods and GOOD Meat to produce and sell cultivated chicken, with GOOD Meat commencing immediate production and Upside preparing for a restaurant debut [303,304]. Additionally, Mission Barns has secured an FDA “no questions” letter for its cultivated pork fat products, which will soon be available to consumers via Fiorella restaurants and Sprouts Farmers Market, thereby expanding beyond poultry to pork [305]. However, this progress encounters resistance; Senate Bill 261 will prohibit lab-grown meat sales in Texas starting September 2025, prompting lawsuits from Upside and Wildtype [306]. Similar bans or restrictions are emerging across Florida, Alabama, Indiana, Iowa, Nebraska, and South Dakota, reflecting political pushback from traditional meat sectors, even as cultivated meat continues to gain regulatory traction and industrial investment globally [307,308].
Media reports from Brazil regarding cultivated meat reflect both strong industrial investment and increasing political resistance [309,310]. JBS, the world’s largest animal protein producer, is committing over USD 60 million to its Biotech Innovation Center in Santa Catarina, aiming to establish itself at the forefront of cultivated meat development [303]. Concurrently, Embrapa’s Swine and Poultry Division has achieved a scientific milestone by conducting pioneering research on lab-grown chicken meat, indicating public-sector involvement in advancing foundational R&D [310]. However, these initiatives face challenges from a proposed law (PL 4.616/23) seeking to ban research and commercialization of cultivated meat entirely, underscoring the tension between Brazil’s industrial and scientific innovation and institutional resistance [310].
2.4.3. Europe and the UK
Europe’s cultivated meat landscape reflects both momentum and divergence, with certain countries embracing innovation while others impose restrictions [311,312,313,314,315,316,317,318,319]. In the Netherlands, Mosa Meat raised €40 million and organized the first formal tasting of its cultivated beef, while Meatable hosted Europe’s inaugural lab-grown pork sausage tasting, rendered possible by a pioneering national code of practice that permits controlled tastings [316]. In contrast, Italy has adopted a restrictive approach: its 2024 law bans the commercialization of cultivated meat, with critics, including Professor Conti, warning that such bans hinder ethical and societal progress. The political debate is further complicated by accusations that Italy’s technical panel is dominated by Coldiretti appointees, prompting calls for reform; nevertheless, Italy may ultimately be required to comply with EU-level approvals [317,318]. Elsewhere in Europe, new players are emerging; for instance, Poland’s LabFarm, founded in 2021, secured a €2 million government grant to scale its production of antibiotic-free, cell-based chicken, positioning itself as a sustainable alternative to conventional poultry [319]. Collectively, these developments illustrate Europe’s fragmented yet advancing journey toward cultivated meat commercialization.
The UK is emerging as a significant hub for innovation in cultivated meat, effectively balancing early product launches with regulatory preparations. Fortnum & Mason, in collaboration with Ivy Farms, recently unveiled a high-profile scotch egg made from lab-grown Aberdeen Angus beef. This product has received endorsement from Prime Minister Rishi Sunak, although it still awaits FSA approval [313]. Meanwhile, Meatly has achieved a European first by launching cultivated chicken dog treats, branded as “Chick Bites,” in Pets at Home stores following the approval of pet food containing cultivated meat in 2024 [312]. On the regulatory front, the FSA has received applications for cultivated steak, chicken, and foie gras, bolstered by £1.6 million in government funding aimed at expediting safety assessments, with a goal of completing evaluations within 2 years [313,314]. Public attitudes towards cultivated meat show promise yet also reveal challenges; an Ipsos study indicates that 47% of Generation Z are open to consuming cultivated meat, in contrast to lower acceptance rates among older demographics, while 58% of British adults report limited knowledge regarding the technology [315]. Notably, the regulatory pathway for cultured meat is likely to accelerate as the FSA endeavors to shorten safety evaluations to under 2 years [314]. Taken together, these developments underline both the advancements and obstacles as the UK positions itself at the forefront of sustainable protein commercialization.
2.4.4. Others
Australia and Africa are marking significant milestones in the commercialization and innovation of cultured meat [320,321,322,323]. In 2025, Vow, an Australian startup, became the first to secure FSANZ approval for its cultured Japanese quail foie gras, with plans to debut the product in high-end restaurants in Sydney and Melbourne, followed by expansion into gourmet supermarkets [279,280]. Alongside Vow, Magic Valley is developing cultivated lamb mince, targeting supermarket availability by 2026 at competitive prices, signaling a broader push into mainstream markets. In Africa, pioneering efforts by Mzansi Meat Company and Mogale Meat have become visible, with Mzansi producing Africa’s first lab-grown burger patty [322]. While Mzansi projects that cultivated meat could reach supermarket shelves within 2 years, challenges such as regulatory hurdles and labeling requirements remain significant barriers [323].
3. The Future of Global Cultured Meat Production
Based on the information collected in this study, the future of cultured meat commercialization appears predictable. From 2025 to 2030, industrialization is likely to be driven by deeper process engineering rather than groundbreaking discoveries. Numerous publications and patents suggest that the combination of immortal or engineered progenitor lines, fully serum-free media with recycled components (e.g., ammonia control and media refurbishment), and perfused or hollow-fiber bioreactors for thicker tissues could lead to cost-effective cultured meat production. Additionally, further development of edible scaffolds and composite structures that mimic traditional meat characteristics can be achieved by leveraging advances in 3D printing/bioprinting, as well as self-healing or alignment-guiding matrices and flavor/aroma precursors. AI-assisted media design and inline sensing may effectively compress experimental cycles and mitigate media costs, while hybrid products serve as a bridge to more affordable options in the near term.
Pilot-to-demonstration plants (200–2000 L) are expected to proliferate in regions with established public funding and clear regulatory intent, such as Singapore, selected EU states like the Netherlands, Australia, the US, and China. If sustained or developed, several facilities could achieve an annual production threshold of approximately 5000–10,000 t for ingredient-grade cultured fat or mince, with considerable reliance on fermentation capacity for growth factors and media components, supported by national centers equipped with shared bioprocess utilities and regulatory liaison.
The commercialization of cultured meat will begin with tastings and limited service in premium restaurants, followed by retail hybrids and processed products (e.g., dumplings, sausages, and patties), ultimately culminating in whole-cut meat. Premium products (e.g., foie gras, eel, quail, and wagyu-style marbled meat cuts) are expected to serve as introductory offerings in restaurants and markets, while mainstream volume will derive from hybrid products containing cultured components.
Based on current regulatory approvals, Singapore, Australia, Israel, and the US are likely to continue to maintain leadership in regulatory approvals under established pathways, while the EU may remain cautious yet incrementally receptive as dossiers mature and member-state pilots demonstrate safety. Parts of Asia, specifically China, Korea, and Japan, will gain momentum as stable regulatory frameworks are developed. Cultured meat companies are anticipated to strategically launch in favorable jurisdictions first, subsequently establishing a safety and traceability network to gradually penetrate stricter regions such as Europe and certain areas of the Americas.
Media narratives and transparent labeling will significantly influence public perception and consumer demand. Initial demand is likely to be strongest among younger, urban consumers and sustainability-oriented diners; widespread adoption will depend on achieving parity in taste and convenience at a modest premium. Realistically, hybrid products may reach foodservice price points first within the decade, while fully cultivated whole cuts are expected to remain premium offerings through the early 2030s.
4. Conclusions
Cultured meat has evolved from a speculative concept to a maturing, multidisciplinary technology grounded in advances in stem-cell biology, 3D tissue engineering (scaffolds, hydrogels, microcarriers, and bioinks), bioreactor architectures, and serum-free, recyclable media—much of this progress driven by academia and rapidly translated into industry partnerships. Asia (particularly China, Japan, Singapore, Korea, and Israel) and the US now anchor global R&D as well as prototyping, while Europe and the UK contribute high-fidelity cell culture protocols and non-invasive monitoring tools. Parallel growth in strategic intellectual property (IP), led by China’s universities and reinforced by active portfolios in Israel, the US, Japan, Korea, and the Netherlands, signals a race to secure differentiation in cell lines, media formulation, edible scaffolds, and scale-up systems. These developments indicate technical readiness for narrow, premium launches and hybrid formats that bridge current cost constraints and sensory targets. Clearer pathways in Singapore, the US, Israel, and the FSANZ system exemplify how structured pre-market reviews can facilitate pilots and early sales, while the EU and UK’s rigorous novel-food frameworks and political resistance (e.g., state-level bans, Italy’s restrictive stance, etc.) underscore the necessity for robust safety dossiers, labeling clarity, and stakeholder engagement. The alignment of regulatory standards among countries could lead to intensified global collaborations, which could hasten cultured meat commercialization. Media narratives that communicate both breakthroughs and drawbacks can significantly influence consumer adoption and investment. Sustainable and efficient production will depend on validated serum-free processes, ammonia- and waste-minimizing media cycles, quality assurance and monitoring at scale, and credible nutrition and safety evidence. Regions that effectively align R&D depth, IP strategy, regulation, and public communication are best positioned to transition cultured meat from pilot runs to mainstream markets.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/foods14244222/s1, Table S1. Global status of cutting-edge technologies for cultured meat (last 3 years). Table S2. Global status of patents for cultured meat. Table S3. Global status of approval for cultured meat. Table S4. Global status of media reports on cultured meat.
Author Contributions
Conceptualization, Y.-H.H. and S.-T.J.; investigation, S.K. (Swati Kumari) and C.K.; resources, C.K. and S.A.; writing—original draft preparation, Y.-H.H.; writing—review and editing, S.K. (SoHee Kim); visualization, S.K. (Swati Kumari) and S.A.; supervision, S.-T.J.; project administration, Y.-H.H.; funding acquisition, Y.-H.H. and S.-T.J. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Research Resurgence under the Glocal University 30 Project at Gyeongsang National University in 2025. Following are results of a study on the "Gyeongsangnam-do Regional Innovation System & Education(RISE)" Project, supported by the Ministry of Education and Gyeongsangnam-do.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lee, S.Y.; Lee, D.Y.; Yun, S.H.; Lee, J.; Mariano, E.; Park, J.; Choi, Y.; Han, D.; Kim, J.S.; Hur, S.J. Current technology and industrialization status of cell-cultivated meat. J. Anim. Sci. Technol. 2023, 66, 1–30. [Google Scholar] [CrossRef]
- Barbosa, W.; Correia, P.; Vieira, J.; Leal, I.; Rodrigues, L.; Nery, T.; Barbosa, J.; Soares, M. Trends and technological challenges of 3D bioprinting in cultured meat: Technological prospection. Appl. Sci. 2023, 13, 12158. [Google Scholar] [CrossRef]
- Swartz, E. The Science of Cultivated Meat; The Good Food Institute: Washington, DC, USA, 2025; Available online: https://gfi.org/science/the-science-of-cultivated-meat/ (accessed on 8 October 2025).
- Kumar, P.; Sharma, N.; Sharma, S.; Mehta, N.; Verma, A.K.; Chemmalar, S.; Sazili, A.Q. In-vitro meat: A promising solution for sustainability of meat sector. J. Anim. Sci. Technol. 2021, 63, 693–724. [Google Scholar] [CrossRef]
- Xue, T.; Zheng, H.; Zhao, Y.; Zhao, Z.; Wang, J.; Zhang, Y.; Guo, H. A spontaneously immortalized muscle stem cell line (EfMS) from brown-marbled grouper for cell-cultured fish meat production. Commun. Biol. 2024, 7, 1697. [Google Scholar] [CrossRef]
- Yang, R.; Fei, Z.; Wang, L.; Tang, H.; Sun, W.; Li, M.; Lei, Q.; Chen, J.; Guan, X. Highly efficient isolation and 3D printing of fibroblasts for cultured meat production. Front. Sustain. Food Syst. 2024, 8, 1358862. [Google Scholar] [CrossRef]
- Dai, W.; Chen, Y.; Xiong, W.; Li, S.; Tan, W.S.; Zhou, Y. Development of a serum-free medium for myoblasts long-term expansion and 3D culture for cell-based meat. J. Food Sci. 2024, 89, 851–865. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Lou, H.; Lu, H.; Xu, E.; Liu, D.; Chen, Q. Characterization of proliferation medium and its effect on differentiation of muscle satellite cells from Larimichthys crocea in cultured fish meat production. Fishes 2023, 8, 429. [Google Scholar] [CrossRef]
- Zheng, Y.Y.; Hu, Z.N.; Liu, Z.; Jiang, Y.C.; Guo, R.P.; Ding, S.J.; Zhou, G.H. The effect of long-term passage on porcine SMCs’ function and the improvement of TGF-β1 on porcine SMCs’ secretory function in late passage. Foods 2023, 12, 2682. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Ren, R.; Lv, J.; Yang, R.; Zheng, X.; Hu, Y.; Zhu, G.; Wang, H.; Wang, H. Transdifferentiation of fibroblasts into muscle cells to constitute cultured meat with tunable int ramuscular fat deposition. Elife 2024, 13, RP93220. [Google Scholar] [CrossRef]
- Wang, Y.; Zhuang, D.; Munawar, N.; Zan, L.; Zhu, J. A rich-nutritious cultured meat via bovine myocytes and adipocytes co-culture: Novel prospect for cultured meat production techniques. Food Chem. 2024, 460, 140696. [Google Scholar] [CrossRef]
- Yin, H.; Wang, L.; Hur, S.J.; Liu, Y.; Cong, P.; Liu, H.; Jiang, X.; Zheng, H.; Xue, C. Cell-cultured fish meat via scale-up expansion of Carassius auratus skeletal muscle cells using edible porous microcarriers and quality evaluation. J. Agric. Food Chem. 2024, 72, 16475–16483. [Google Scholar] [CrossRef]
- Du, Z.; Lao, J.; Jiang, Y.; Liu, J.; Liu, S.; Zheng, J.; Li, F.; Jia, Y.; Gu, Z.; Chen, J.; et al. Fin cells as a promising seed cell source for sustainable fish meat cultivation. Foods 2025, 14, 2075. [Google Scholar] [CrossRef]
- Cao, M.; Liao, L.; Zhang, X.; Chen, X.; Peng, S.; Zou, L.; Liang, R.; Liu, W. Electric field-driven fabrication of anisotropic hydrogels from plant proteins: Microstructure, gel performance and formation mechanism. Food Hydrocoll. 2023, 136, 108297. [Google Scholar] [CrossRef]
- Gu, X.; Hua, S.; Huang, Y.; Liu, S.; Wang, Y.; Zhou, M.; Shan, T. κ-Carrageenan/konjac glucomannan composite hydrogel-based 3D porcine cultured meat production. Food Hydrocoll. 2024, 151, 109765. [Google Scholar] [CrossRef]
- Chen, Y.; Bassey, A.P.; Zhu, H.; Zhou, G. Fabrication of cell cultured meat by hydrogel with topographic microstructures. Food Biosci. 2023, 55, 102910. [Google Scholar] [CrossRef]
- Niu, R.; Xin, Q.; Xu, E.; Yao, S.; Chen, M.; Liu, D. Nanostarch-stimulated cell adhesion in 3D bioprinted hydrogel scaffolds for cell cultured meat. ACS Appl. Mater. 2024, 16, 23015–23026. [Google Scholar] [CrossRef]
- Lou, H.; Lu, H.; Zhang, S.; Shi, Y.; Xu, E.; Liu, D.; Chen, Q. Highly aligned myotubes formation of piscine satellite cells in 3D fibrin hydrogels of cultured meat. Int. J. Biol. Macromol. 2024, 282, 136879. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ding, X.; Yang, Z.; Wang, J.; Li, C.; Zhou, G. Emerging microfluidic building blocks for cultured meat construction. ACS Appl. Mater. Interfaces 2025, 17, 8771–8793. [Google Scholar] [CrossRef] [PubMed]
- You, K.; Xie, L.; Li, J.; Liu, Q.; Zhuang, L.; Chen, W. Versatile platforms of mussel-inspired agarose scaffold for cell cultured meat. J. Adv. Res. 2025, 77, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xiong, W.; Guo, Y.; Jin, X.; Wang, L.; Ge, C.; Tan, W.; Zhou, Y. Three-dimensional pore structure of the decellularized parsley scaffold regulates myogenic differentiation for cell cultured meat. J. Food Sci. 2024, 89, 5646–5658. [Google Scholar] [CrossRef]
- Wang, J.; Dai, S.; Xiang, N.; Zhang, L.; Zhong, W.; Shao, P.; Feng, S. Cell-based meat scaffold based on a 3D-printed starch-based gel. J. Agric. Food Chem. 2024, 72, 19143–19154. [Google Scholar] [CrossRef]
- Feng, S.; Dai, S.; Wei, Z.; Wang, J.; Xiang, N.; Shao, P. Soy conglycinin amyloid fibril and chitosan complex scaffold for cultivated meat application. Food Hydrocoll. 2024, 153, 110017. [Google Scholar] [CrossRef]
- Wang, X.; Wang, M.; Xu, Y.; Yin, J.; Hu, J. A 3D-printable gelatin/alginate/ε-poly-l-lysine hydrogel scaffold to enable porcine muscle stem cells expansion and differentiation for cultured meat development. Int. J. Biol. Macromol. 2024, 271, 131980. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.M.; Han, W.M.; Hou, L.Y.; Lin, D.D.; Li, J.Y.; Lin, S.T.; Yang, J.P.; Liao, L.; Zeng, X.A. Glutenin-chitosan 3D porous scaffolds with tunable stiffness and systematized microstructure for cultured meat model. Int. J. Biol. Macromol. 2024, 267, 131438. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gao, A.; Wang, T.; Zhang, Y.; Zhu, G.; Ling, S.; Wu, Z.; Jin, Y.; Chen, H.; Lai, Y.; et al. Growing meat on autoclaved vegetables with biomimetic stiffness and micro-patterns. Nat. Commun. 2025, 16, 161. [Google Scholar] [CrossRef]
- Wang, Y.; Zhong, Z.; Wang, R.; Munawar, N.; Zan, L.; Zhu, J. Effects of proanthocyanidins and dialdehyde chitosan on the proliferation and differentiation of bovine myoblast for cultured meat production. Int. J. Biol. Macromol. 2023, 246, 125618. [Google Scholar] [CrossRef]
- Wang, Y.; Zhong, Z.; Munawar, N.; Wang, R.; Zan, L.; Zhu, J. Production of green-natural and “authentic” cultured meat based on proanthocyanidins-dialdehyde chitosan-collagen ternary hybrid edible scaffolds. Food Res. Int. 2024, 175, 113757. [Google Scholar] [CrossRef]
- Su, L.; Jing, L.; Zeng, X.; Chen, T.; Liu, H.; Kong, Y.; Wang, X.; Yang, X.; Fu, C.; Sun, J.; et al. 3D-printed prolamin scaffolds for cell-based meat culture. Adv. Mater. 2023, 35, 2207397. [Google Scholar] [CrossRef]
- Wei, Z.; Dai, S.; Huang, J.; Hu, X.; Ge, C.; Zhang, X.; Yang, K.; Shao, P.; Sun, P.; Xiang, N. Soy protein amyloid fibril scaffold for cultivated meat application. ACS Appl. Mater. Interfaces 2023, 15, 15108–15119. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, W.; Ding, X.; Ding, S.; Tang, C.; Zeng, X.; Wang, J.; Zhou, G. Programmable scaffolds with aligned porous structures for cell cultured meat. Food Chem. 2024, 430, 137098. [Google Scholar] [CrossRef]
- Cheng, Y.M.; Hong, P.C.; Song, M.M.; Zhu, H.N.; Qin, J.; Zhang, Z.D.; Chen, H.; Ma, X.Z.; Tian, M.Y.; Zhu, W.Y.; et al. An immortal porcine preadipocyte cell strain for efficient production of cell-cultured fat. Commun. Biol. 2023, 6, 1202. [Google Scholar] [CrossRef]
- Huo, Y.; Hu, J.; Yin, Y.; Liu, P.; Cai, K.; Ji, W. Self-assembling peptide-based functional biomaterials. ChemBioChem 2023, 24, e202200582. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Ruan, H.; Ye, C.; Jiang, W.; Wang, X.; Chen, S.; Chen, Z.; Li, D. Plant-derived leaf vein scaffolds for the sustainable production of dog cell-cultured meat. Food Chem. X 2024, 23, 101603. [Google Scholar] [CrossRef]
- Zhu, G.; Gao, D.; Li, L.; Yao, Y.; Wang, Y.; Zhi, M.; Zhang, J.; Chen, X.; Zhu, Q.; Gao, J.; et al. Generation of three-dimensional meat-like tissue from stable pig epiblast stem cells. Nat. Commun. 2023, 14, 8163. [Google Scholar] [CrossRef]
- Mithra, S.; Abdul Majeed, S.; Eisa Abdullah, S.A.; Ajay Pathra, G.; Taju, G.; Bright Singh, I.S.; Santhanam, P.; Sahul Hameed, A.S. Production of small-scale laboratory-grown cell-based fish meat from Asian seabass muscle and fin cell lines. In Vitro Cellular Developmental Biology-Animal; Springer: Berlin/Heidelberg, Germany, 2025. [Google Scholar] [CrossRef]
- Singh, A.; Singh, S.K.; Kumar, V.; Gupta, J.; Kumar, M.; Sarma, D.K.; Singh, S.; Kumawat, M.; Verma, V. Derivation and characterization of novel cyto compatible decellularized tissue scaffold for myoblast growth and differentiation. Cells 2023, 13, 41. [Google Scholar] [CrossRef] [PubMed]
- Gome, G.; Chak, B.; Tawil, S.; Shpatz, D.; Giron, J.; Brajzblat, I.; Weizman, C.; Grishko, A.; Schlesinger, S.; Shoseyov, O. Cultivation of bovine mesenchymal stem cells on plant-based scaffolds in a macrofluidic single-use bioreactor for cultured meat. Foods 2024, 13, 1361. [Google Scholar] [CrossRef]
- Yakir, I.; Cohen, E.; Schlesinger, S.; Hayouka, Z. Random antimicrobial peptide mixtures asnon-antibiotic antimicrobial agents for cultured meat industry. Food Chem. Mol. Sci. 2025, 10, 100240. [Google Scholar] [CrossRef]
- Yen, F.C.; Glusac, J.; Levi, S.; Zernov, A.; Baruch, L.; Davidovich-Pinhas, M.; Fishman, A.; Machluf, M. Cultured meat platform developed through the structuring of edible microcarrier-derived microtissues with oleo gel-based fat substitute. Nat. Commun. 2023, 14, 2942. [Google Scholar] [CrossRef]
- Zehorai, E.; Maor-Shoshani, A.; Molotski, N.; Dorojkin, A.; Marelly, N.; Dvash, T.; Lavon, N. From fertilised oocyte to cultivated meat–harnessing bovine embryonic stem cells in the cultivated meat industry. Reprod. Fertil. Dev. 2023, 36, 124–132. [Google Scholar] [CrossRef]
- Levi, S.; Zernov, A.; Martin, P.; Baruch, L.; Zussman, E.; Machluf, M. Not just a protein source: Chickpea protein-based scaffolds for cultured meat. Food Hydrocolloids 2025, 172 Pt 1, 111847. [Google Scholar] [CrossRef]
- David, S.; Ianovici, I.; Guterman Ram, G.; Shaulov Dvir, Y.; Lavon, N.; Levenberg, S. Pea protein-rich scaffolds support 3D bovine skeletal muscle formation for cultivated meat application. Adv. Sustain. Syst. 2024, 8, 2300499. [Google Scholar] [CrossRef]
- Ianovici, I.; Zagury, Y.; Afik, N.; Hendel, M.; Lavon, N.; Levenberg, S. Embedded three-dimensional printing of thick pea-protein-enriched constructs for large, customized structured cell-based meat production. Biofabrication 2024, 16, 045023. [Google Scholar] [CrossRef] [PubMed]
- Moslemy, N.; Sharifi, E.; Asadi-Eydivand, M.; Abolfathi, N. Review in edible materials for sustainable cultured meat: Scaffolds and microcarriers production. Int. J. Food Sci. Technol. 2023, 58, 6182–6191. [Google Scholar] [CrossRef]
- Ikeda, D.; Otsuka, Y.; Kan-No, N. Development of a novel Japanese eel myoblast cell line for application in cultured meat production. Biochem. Biophys. Res. Commun. 2024, 734, 150784. [Google Scholar] [CrossRef]
- Nie, M.; Shima, A.; Yamamoto, M.; Takeuchi, S. Scalable tissue biofabrication via perfusable hollow fiber arrays for cultured meat applications. Trends Biotechnol. 2025, 43, 1938–1960. [Google Scholar] [CrossRef]
- Chu, S.; Haraguchi, Y.; Asahi, T.; Kato, Y.; Kondo, A.; Hasunuma, T.; Shimizu, T. A serum-free culture medium production system by co-culture combining growth factor-secreting cells and L-lactate-assimilating cyanobacteria for sustainable cultured meat production. Sci. Rep. 2024, 14, 19578. [Google Scholar] [CrossRef]
- Morikura, T.; Sakaguchi, K.; Tanaka, R.I.; Yoshida, A.; Takahashi, H.; Iwasaki, K.; Shimizu, T. Conditioned serum-free culture medium accomplishes adhesion and proliferation of bovine myogenic cells on uncoated dishes. NPJ Sci. Food 2024, 8, 108. [Google Scholar] [CrossRef]
- Yamanaka, K.; Haraguchi, Y.; Takahashi, H.; Kawashima, I.; Shimizu, T. Development of serum-free and grain-derived-nutrient-free medium using microalga-derived nutrients and mammalian cell-secreted growth factors for sustainable cultured meat production. Sci. Rep. 2023, 13, 498. [Google Scholar] [CrossRef]
- Ghosh, J.; Haraguchi, Y.; Asahi, T.; Nakao, Y.; Shimizu, T. Muscle cell proliferation using water-soluble extract from nitrogen-fixing cyanobacteria Anabaena sp. PCC 7120 for sustainable cultured meat production. Biochem. Biophys. Res. Commun. 2023, 682, 316–324. [Google Scholar] [CrossRef]
- Ghosh, J.; Akiyama, Y.; Haraguchi, Y.; Yamanaka, K.; Asahi, T.; Nakao, Y.; Shimizu, T. Proliferation of mammalian cells with Chlorococcum littorale algal compounds without serum support. Biotechnol. Prog. 2024, 40, e3402. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Istiaq, A.; Datta, A.; Lu, M.; Nakayama, S.; Takashi, K.; Nakajo, N.; Tamara, S.; Kawashima, I.; Ohta, K. Ribosome incorporation transdifferentiates Chick Primary Cells and Induces Their Proliferation by Secreting Growth Factors. J. Dev. Biol. 2025, 13, 19. [Google Scholar] [CrossRef]
- Tanaka, R.I.; Sakaguchi, K.; Yoshida, A.; Takahashi, H.; Shimizu, T. Efficient expansion culture of bovine myogenic cells with differentiation capacity using muscle extract-supplemented medium. Food Biosci. 2024, 61, 104610. [Google Scholar] [CrossRef]
- Kakehi, R.; Yoshida, A.; Takahashi, H.; Shimizu, T. Repeated and long-term cryopreservation of primary bovine myogenic cells to maintain quality in biomimetic cultured meat. Front. Sustain. Food Syst. 2023, 7, 1023057. [Google Scholar] [CrossRef]
- Naraoka, Y.; Mabuchi, Y.; Kiuchi, M.; Kumagai, K.; Hisamatsu, D.; Yoneyama, Y.; Takebe, T.; Akazawa, C. Quality control of stem cell-based cultured meat according to specific differentiation abilities. Cells 2024, 13, 135. [Google Scholar] [CrossRef]
- Louis, F.; Furuhashi, M.; Yoshinuma, H.; Takeuchi, S.; Matsusaki, M. Mimicking Wagyu beef fat in cultured meat: Progress in edible bovine adipose tissue production with controllable fatty acid composition. Mater. Today Bio 2023, 21, 100720. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Hong, Y.; Park, S.; Kim, W.; Gwon, Y.; Jang, K.J.; Kim, J. Designing highly aligned cultured meat with nanopatterns-assisted bio-printed fat scaffolds. J. Biosyst. Eng. 2023, 48, 503–511. [Google Scholar] [CrossRef]
- Seo, J.W.; Jung, W.K.; Park, Y.H.; Bae, H. Development of cultivable alginate fibers for an ideal cell-cultivated meat scaffold and production of hybrid cultured meat. Carbohydr. Polym. 2023, 321, 121287. [Google Scholar] [CrossRef]
- Iram, S.; Akash, A.; Kathera, C.S.; Park, K.W.; Cho, Y.S.; Kim, J. Serum markers for beef meat quality: Potential media supplement for cell-cultured meat production. Curr. Res. Food Sci. 2025, 10, 100943. [Google Scholar] [CrossRef]
- Yun, S.H.; Lee, S.Y.; Lee, J.; Mariano, E.J.; Joo, S.T.; Choi, I.; Choi, J.S.; Kim, G.D.; Lee, J.H.; Choi, S.H.; et al. Analysis of commercial fetal bovine serum (FBS) and its substitutes in the development of cultured meat. Food Res. Int. 2023, 174, 113617. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Shaikh, S.; Lee, J.H.; Hur, S.J.; Choi, I. Glycyrrhiza uralensis crude water extract and licochalcone A and B to enhance chicken muscle satellite cell differentiation for cultured meat production. Sci. Rep. 2024, 15, 14350. [Google Scholar] [CrossRef]
- Lee, D.Y.; Majó, M.P.; Han, D.; Choi, Y.; Kim, J.S.; Park, J.; Yun, S.H.; Mariano, E.J.; Lee, J.H.; Hur, S.J. Development of fetal bovine serum substitute derived from egg for muscle satellite cell culture: A preliminary study. Future Foods 2024, 10, 100396. [Google Scholar] [CrossRef]
- Yu, I.S.; Choi, Y.R.; Choi, J.; Kim, M.K.; Jung, C.H.; Um, M.Y.; Kim, M.J. Discovery of novel stimulators of Pax7 and/or MyoD: Enhancing the efficacy of cultured meat production through culture media enrichment. Biosensors 2023, 14, 24. [Google Scholar] [CrossRef]
- Jang, S.W.; Han, J.H.; Kim, Y.R.; Jang, H.; Shim, K.S.; Choi, H.W. Eggshell membrane as a natural food-grade scaffold for cultured meat. Innov. Food Sci. Emerg. Technol. 2024, 95, 103734. [Google Scholar] [CrossRef]
- Kim, M.; Jung, H.Y.; Jo, C. Fundamental study on structural formation, amino acids and nucleotide-related compounds of cultivated meat from 3D-cultured pig muscle stem cells. Food Sci. Biotechnol. 2025, 34, 457–469. [Google Scholar] [CrossRef]
- Park, S.; Park, G.; Oh, S.; Park, Y.; Kim, Y.; Kim, J.; Choi, J. Investigating proliferation and differentiation capacities of Hanwoo steer myosatellite cells at different passages for developing cell-cultured meat. Sci. Rep. 2023, 13, 15614. [Google Scholar] [CrossRef]
- Kim, Y.A.; Oh, S.; Park, G.; Park, S.; Park, Y.; Choi, H.; Kim, M.; Choi, J. Characteristics of bovine muscle satellite cell from different breeds for efficient production of cultured meat. J. Anim. Sci. Technol. 2024, 66, 1257. [Google Scholar] [CrossRef] [PubMed]
- Eom, K.H.; Jeong, D.; Choi, J.Y.; Gim, G.M.; Yum, S.Y.; Jin, S.; Bae, H.; Jang, G. MSTN knockout enhances the production of MYOD1-mediated steak-type cultivated meat. J. Anim. Sci. Biotechnol. 2025, 16, 41. [Google Scholar] [CrossRef] [PubMed]
- Jeong, I.; Hong, S.; Kim, D.Y.; Song, Y.J.; Seo, B.J.; Hwang, H.; Hong, H.S.; Yoo, K.H. Production of alternative fat from adipose-derived stem cell from bovine in 3D culture. Appl. Sci. 2025, 15, 7333. [Google Scholar] [CrossRef]
- Jung, D.Y.; Lee, H.J.; Lee, Y.S.; Kim, M.; Lee, C.K.; Jo, C. Dynamic shifts in metabolic demand during myogenic progression in porcine skeletal muscle stem cells. NPJ Sci. Food 2025, 9, 115. [Google Scholar] [CrossRef]
- Kim, M.; Jung, H.Y.; Kim, B.; Jo, C. Laminin as a key extracellular matrix for proliferation, differentiation, and maturation of porcine muscle stem cell cultivation. Food Sci. Anim. Resour. 2024, 44, 710. [Google Scholar] [CrossRef]
- Oh, S.; Park, S.; Park, Y.; Kim, Y.A.; Park, G.; Cui, X.; Kim, K.; Joo, S.T.; Hur, S.; Kim, G.D.; et al. Culturing characteristics of Hanwoo myosatellite cells and C2C12 cells incubated at 37 °C and 39 °C for cultured meat. J. Anim. Sci. Technol. 2023, 65, 664. [Google Scholar] [CrossRef]
- Kim, C.J.; Kim, S.H.; Lee, E.Y.; Son, Y.M.; Bakhsh, A.; Hwang, Y.H.; Joo, S.T. Optimal temperature for culturing chicken satellite cells to enhance production yield and umami intensity of cultured meat. Food Chem. 2023, 2, 100307. [Google Scholar] [CrossRef]
- Yun, S.H.; Lee, S.Y.; Lee, J.; Mariano, E.J.; Joo, S.T.; Choi, I.; Kim, G.D.; Hur, S.J. Improved culture procedure for bovine muscle satellite cells for cultured meat. Food Res. Int. 2023, 174, 113660. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.; Park, S.; Lee, M.; Jung, S.; Lee, H.; Bang, G.; Kim, J.; Hwang, H.; Yoo, K.H.; Han, D.; et al. High protein-containing new food by cell powder meat. NPJ Sci. Food 2023, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.S.; Lim, J.H.; Ahmad, K.; Chun, H.J.; Hur, S.J.; Lee, E.J.; Choi, I. Targeting myostatin using quercetin as a media supplement to improve myogenesis for cultured meat production: An in silico and in vitro study. Curr. Res. Food Sci. 2024, 8, 100678. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.T.; Lee, S.; Hwang, D.S.; Jeon, H.; Park, J.; Kim, H.J.; Oh, D.X. Self-healing scaffolding technology with strong, reversible interactions under physiological conditions for engineering marbled cultured meat. ACS Appl. Mater. Interfaces 2025, 17, 31881–31897. [Google Scholar] [CrossRef]
- Lee, H.; Kim, D.; Choi, K.H.; Lee, S.; Jo, M.; Chun, S.Y.; Son, Y.; Lee, J.H.; Kim, K.; Lee, T.B.; et al. Animal-free scaffold from brown algae provides a three-dimensional cell growth and differentiation environment for steak-like cultivated meat. Food Hydrocoll. 2024, 152, 109944. [Google Scholar] [CrossRef]
- Mariano, E.J.; Lee, D.Y.; Choi, Y.; Park, J.; Han, D.; Kim, J.S.; Park, J.W.; Namkyung, S.; Joo, S.T.; Choi, I.; et al. Crusting-fabricated soy protein-based scaffolds yield three-dimensional muscle tissues for cultured chicken meat production. J. Food Sci. 2025, 90, e70139. [Google Scholar] [CrossRef]
- Zo, S.M.; Sood, A.; Won, S.Y.; Choi, S.M.; Han, S.S. Structuring the future of cultured meat: Hybrid gel-based scaffolds for edibility and functionality. Gels 2025, 11, 610. [Google Scholar] [CrossRef]
- Lee, M.; Park, S.; Choi, B.; Choi, W.; Lee, H.; Lee, J.M.; Lee, T.S.; Yoo, K.H.; Han, D.G.; Bang, G.; et al. Cultured meat with enriched organoleptic properties by regulating cell differentiation. Nat. Commun. 2024, 15, 77. [Google Scholar] [CrossRef]
- Kim, M.; Kim, W.; Lee, C.; Kim, D.; Jang, H.; Park, J.H. Sustainable aligned gelatin-chitosan cryogel scaffolds as a cost-effective platform for steak-like cultured meat. Food Hydrocoll. 2025, 163, 111149. [Google Scholar] [CrossRef]
- Hong, S.J.; Kim, D.H.; Ryoo, J.H.; Park, S.M.; Kwon, H.C.; Keum, D.H.; Shin, D.M.; Han, S.G. Influence of gelatin on adhesion, proliferation, and adipogenic differentiation of adipose tissue-derived stem cells cultured on soy protein–agarose scaffolds. Foods 2024, 13, 2247. [Google Scholar] [CrossRef]
- Lee, M.; Choi, W.; Lee, J.M.; Lee, S.T.; Koh, W.G.; Hong, J. Flavor-switchable scaffold for cultured meat with enhanced aromatic properties. Nat. Commun. 2024, 15, 5450. [Google Scholar] [CrossRef]
- Sood, A.; Singhmar, R.; Son, Y.; Jo, C.H.; Choi, S.; Kumar, A.; Han, S.S. Tuning the efficacy of decellularized apple by coating with alginate/gelatin to behave as a bioscaffold for cultured meat production. Food Res. Int. 2024, 177, 113907. [Google Scholar] [CrossRef]
- Mariano, E., Jr.; Yun, S.H.; Lee, J.; Choi, Y.W.; Park, J.; Han, D.; Kim, J.S.; Choi, I.; Hur, S.J. Crusting-fabricated three-dimensional soy-based scaffolds for cultured meat production: A preliminary study. Food Chem. 2024, 452, 139511. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.; Jang, G.; Jung, W.K.; Park, Y.H.; Bae, H. Stretchable zein-coated alginate fiber for aligning muscle cells to artificially produce cultivated meat. NPJ Sci. Food 2024, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Toh, N.P.; Wu, Y.; Huang, D. Trypsin-treated chickpea protein hydrolysate enhances the cytoaffinity of microbeads for cultured meat application. Food Res. Int. 2023, 173, 113299. [Google Scholar] [CrossRef] [PubMed]
- Teng, T.S.; Lee, J.J.L.; Chen, W.N. Ultrafiltrated extracts of fermented okara as a possible serum alternative for cell culturing: Potential in cultivated meat production. ACS Food Sci. Technol. 2023, 3, 699–709. [Google Scholar] [CrossRef]
- Rizal, J.; Mainali, P.; Quek, J.P.; Tan, L.L.; Bi, J.; Chan, A.J.; Gaffoor, A.A.; Chew, L.J.H.; Sugii, S.; Ng, S.K.; et al. Valorisation of spent cultivated meat media for recombinant FGF2 production in Lactococcus lactis. Syst. Microbiol. Biomanuf. 2025, 5, 1328–1334. [Google Scholar] [CrossRef]
- Zhou, H.; Loo, L.S.W.; Ong, F.Y.T.; Lou, X.; Wang, J.; Myint, M.K.; Thong, A.; Seow, D.C.S.; Wibowo, M.; Ng, S.; et al. Cost-effective production of meaty aroma from porcine cells for hybrid cultivated meat. Food Chem. 2025, 473, 142946. [Google Scholar] [CrossRef]
- Kong, Y.; Huang, D. Pumpkin seed proteins rival animal gelatin in increasing the cytoaffinity of edible microbeads for cell-based meat culture. Food Res. Int. 2023, 168, 112750. [Google Scholar] [CrossRef]
- Su, L.; Jing, L.; Zeng, S.; Fu, C.; Huang, D. Sorghum prolamin scaffolds-based hybrid cultured meat with enriched sensory properties. J. Agric. Food Chem. 2024, 72, 23355–23365. [Google Scholar] [CrossRef]
- Murugan, P.; Yap, W.S.; Ezhilarasu, H.; Suntornnond, R.; Le, Q.B.; Singh, S.; Seah, J.S.H.; Tan, P.L.; Zhou, W.; Tan, L.P.; et al. Decellularised plant scaffolds facilitate porcine skeletal muscle tissue engineering for cultivated meat biomanufacturing. NPJ Sci. Food 2024, 8, 25. [Google Scholar] [CrossRef]
- Yawut, N.; Leksakul, K.; Vichiansan, N.; Boonyawan, D.; Mekwilai, T. Approach to enhancing myoblast cell proliferation through plasma jet stimulation for the design of lab-grown meat. J. Agric. Food Res. 2025, 19, 101718. [Google Scholar] [CrossRef]
- Goswami, M.; Pinto, N.; Yashwanth, B.S.; Sathiyanarayanan, A.; Ovissipour, R. Development of a cell line from skeletal trunk muscle of the fish Labeo rohita. Cytotechnology 2023, 75, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Stout, A.J.; Zhang, X.; Letcher, S.M.; Rittenberg, M.L.; Shub, M.; Chai, K.M.; Kaul, M.; Kaplan, D.L. Engineered autocrine signaling eliminates muscle cell FGF2 requirements for cultured meat production. Cell Rep. Sustain. 2024, 1, 100009. [Google Scholar] [CrossRef]
- Letcher, S.M.; Calkins, O.P.; Clausi, H.J.; McCreary, A.; Trimmer, B.A.; Kaplan, D.L. Establishment & characterization of a non-adherent insect cell line for cultivated meat. Sci. Rep. 2025, 15, 7850. [Google Scholar] [CrossRef] [PubMed]
- Stout, A.J.; Arnett, M.J.; Chai, K.; Guo, T.; Liao, L.; Mirliani, A.B.; Mirian, L.R.; Shub, M.; White, E.C.; Yuen, J.S.K.; et al. Immortalized bovine satellite cells for cultured meat applications. ACS Synth. Biol. 2023, 12, 1567–1573. [Google Scholar] [CrossRef]
- Yuen, J.S.K., Jr.; Saad, M.K.; Xiang, N.; Barrick, B.M.; DiCindio, H.; Li, C.; Kaplan, D.L. Aggregating in vitro-grown adipocytes to produce macroscale cell-cultured fat tissue with tunable lipid compositions for food applications. eLife 2023, 12, e82120. [Google Scholar] [CrossRef]
- Lew, E.T.; Yuen, J.S., Jr.; Zhang, K.L.; Fuller, K.; Frost, S.C.; Kaplan, D.L. Chemical and sensory analyses of cultivated pork fat tissue as a flavor enhancer for meat alternatives. Sci. Rep. 2024, 14, 17643. [Google Scholar] [CrossRef]
- Thyden, R.; Dominko, T.; Weathers, P.; Freitas dos Santos, A.C.; Perreault, L.; Reddig, D.; Kloster, J.; Gaudette, G. Recycling spent animal cell culture media using the thermally resistant microalga Chlorella sorokiniana. Syst. Microbiol. Biomanuf. 2025, 5, 371–384. [Google Scholar] [CrossRef]
- Pakbin, B.; Amanipour, A.; Amirvaresi, A.; Shahsavari, A.; Ovissipour, R. Development and optimization of the ammonia removal strategy for sustainable recycling of cell culture spent media in cultivated meat production: From concept to implementation. Front. Bioeng. Biotechnol. 2025, 13, 1617115. [Google Scholar] [CrossRef] [PubMed]
- Timoneda, A.; Amirvaresi, A.; Ovissipour, R. Black soldier fly bioconversion to cultivated meat media components using blue catfish gut microbiome. Bioresour. Technol. Rep. 2024, 26, 101834. [Google Scholar] [CrossRef]
- Yuen, J.S., Jr.; Barrick, B.M.; Di Cindio, H.; Pietropinto, J.A.; Kaplan, D.L. Optimization of culture media and cell ratios for 3D in vitro skeletal muscle tissues with endothelial cells. ACS Biomater. Sci. Eng. 2023, 9, 4558–4566. [Google Scholar] [CrossRef]
- Amirvaresi, A.; Sarkarat, R.; Jones, C.; Shahsavari, A.; Ovissipour, R. Sustainable alternatives to fetal bovine serum: Evaluating the role of plant and insect protein isolates in serum-free media for bovine satellite cell proliferation in cultivated meat production. ACS Food Sci. Technol. 2025, 5, 1614–1624. [Google Scholar] [CrossRef]
- Lim, T.; Chang, H.; Saad, M.K.; Joyce, C.M.; Park, B.; O’Beirne, S.X.; Cohen, M.A.; Kaplan, D.L. Development of serum-reduced medium for mackerel muscle cell line cultivation. ACS Sustain. Chem. Eng. 2024, 12, 11683–11691. [Google Scholar] [CrossRef]
- Nikkhah, A.; Rohani, A.; Zarei, M.; Kulkarni, A.; Batarseh, F.A.; Blackstone, N.T.; Ovissipour, R. Toward sustainable culture media: Using artificial intelligence to optimize reduced-serum formulations for cultivated meat. Sci. Total Environ. 2023, 894, 164988. [Google Scholar] [CrossRef]
- Amirvaresi, A.; Ovissipour, R. Assessment of plant-and microbial-derived protein hydrolysates as sustainable alternatives to fetal bovine serum in seafood cell culture media. Future Foods 2024, 10, 100443. [Google Scholar] [CrossRef]
- Stout, A.J.; Rittenberg, M.L.; Shub, M.; Saad, M.K.; Mirliani, A.B.; Dolgin, J.; Kaplan, D.L. A beefy-R culture medium: Replacing albumin with rapeseed protein isolates. Biomaterials 2023, 296, 122092. [Google Scholar] [CrossRef]
- Lee, K.; Jackson, A.; John, N.; Zhang, R.; Ozhava, D.; Bhatia, M.; Mao, Y. Bovine fibroblast-derived extracellular matrix promotes the growth and preserves the stemness of bovine stromal cells during in vitro expansion. J. Funct. Biomater. 2023, 14, 218. [Google Scholar] [CrossRef]
- O’Neill, E.N.; Neffling, M.; Randall, N.; Kwong, G.; Ansel, J.; Baar, K.; Block, D.E. The effect of serum-free media on the metabolic yields and growth rates of C2C12 cells in the context of cultivated meat production. Future Foods 2023, 7, 100226. [Google Scholar] [CrossRef]
- Ogawa, M.; Kermani, A.S.; Huynh, M.J.; Baar, K.; Leach, J.K.; Block, D.E. Edible mycelium as proliferation and differentiation support for anchorage-dependent animal cells in cultivated meat production. NPJ Sci. Food 2024, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Perreault, L.R.; Thyden, R.; Kloster, J.; Jones, J.D.; Nunes, J.; Patmanidis, A.A.; Reddig, D.; Dominko, T.; Gaudette, G.R. Repurposing agricultural waste as low-cost cultured meat scaffolds. Front. Food Sci. Technol. 2023, 3, 1208298. [Google Scholar] [CrossRef]
- Kawecki, N.S.; Norris, S.C.; Xu, Y.; Wu, Y.; Davis, A.R.; Fridman, E.; Chen, K.K.; Crosbie, R.H.; Garmyn, A.J.; Li, S.; et al. Engineering multicomponent tissue by spontaneous adhesion of myogenic and adipogenic microtissues cultured with customized scaffolds. Food Res. Int. 2023, 172, 113080. [Google Scholar] [CrossRef]
- Wu, C.S.; Wang, S.S.; Wu, D.Y. Three-dimensionally printed scaffolds of crab shell-derived calcium hydroxide, beef bone-derived hydroxyapatite, and poly (butylene succinate) composites. ACS Appl. Polym. Mater. 2023, 5, 3416–3426. [Google Scholar] [CrossRef]
- Ikuse, M.; Marchus, C.R.; Schiele, N.R.; Ganjyal, G.M. Extruded plant protein two-dimensional scaffold structures support myoblast cell growth for potential applications in cultured meat. Food Res. Int. 2024, 195, 114981. [Google Scholar] [CrossRef]
- Yao, Y.; Yuen, J.S., Jr.; Sylvia, R.; Fennelly, C.; Cera, L.; Zhang, K.L.; Li, C.; Kaplan, D.L. Cultivated meat from aligned muscle layers and adipose layers formed from glutenin films. ACS Biomater. Sci. Eng. 2024, 10, 814–824. [Google Scholar] [CrossRef]
- Tahir, I.; Foley, C.; Floreani, R. Whey protein isolate and β-lactoglobulin-modified alginate hydrogel scaffolds enhance cell proliferation for cultivated meat applications. Foods 2025, 14, 2534. [Google Scholar] [CrossRef]
- Flaibam, B.; Meira, C.S.; Nery, T.B.R.; Galland, F.; Pacheco, M.T.B.; Goldbeck, R. Low-cost protein extracts and hydrolysates from plant-based agro-industrial waste: Inputs of interest for cultured meat. Innov. Food Sci. Emerg. Technol. 2024, 93, 103644. [Google Scholar] [CrossRef]
- Santos, A.E.A.D.; Guadalupe, J.L.; Albergaria, J.D.S.; Almeida, I.A.; Moreira, A.M.S.; Copola, A.G.L.; Araújo, I.P.D.; Paula, A.M.D.; Neves, B.R.A.; Santos, J.P.F.; et al. Random cellulose acetate nanofibers: A breakthrough for cultivated meat production. Front. Nutr. 2024, 10, 1297926. [Google Scholar] [CrossRef] [PubMed]
- Albergaria, J.D.S.; dos Santos, A.E.A.; Guadalupe, J.L.M.; de Araújo, I.P.; Copola, A.G.L.; Santos, J.P.F.; Jorge, E.C.; de Oliveira Andrade, L.; da Silva, A.B. Edible microcapsules containing canola oil for cultivated meat production. Appl. Food Res. 2025, 5, 101158. [Google Scholar] [CrossRef]
- Bektas, C.; Lee, K.; Jackson, A.; Bhatia, M.; Mao, Y. Bovine placentome-derived extracellular matrix: A sustainable 3D scaffold for cultivated meat. Bioengineering 2024, 11, 854. [Google Scholar] [CrossRef]
- Bezjak, D.; Orellana, N.; Valdés, J.H.; Corrales, T.; Acevedo, C.A. Towards understanding the role of microstructured edible scaffolds for cultured meat production. Food Bioprocess Technol. 2024, 17, 767–779. [Google Scholar] [CrossRef]
- Recchia, K.; Wathikthinnakon, M.; Bressan, F.F.; Freude, K. Generation of bovine iPSCs from fetal fibroblasts for in vitro myogenesis and cultured meat. Front. Nutr. 2025, 12, 1562981. [Google Scholar] [CrossRef]
- Skrivergaard, S.; Rasmussen, M.K.; Sahebekhtiari, N.; Young, J.F.; Therkildsen, M. Satellite cells sourced from bull calves and dairy cows differ in proliferative and myogenic capacity–Implications for cultivated meat. Food Res. Int. 2023, 173, 113217. [Google Scholar] [CrossRef]
- Skrivergaard, S.; Rasmussen, M.K.; Therkildsen, M.; Young, J.F. High-Throughput Label-Free continuous quantification of muscle stem cell proliferation and myogenic differentiation. Stem Cell Rev. Rep. 2025, 21, 2103–2120. [Google Scholar] [CrossRef] [PubMed]
- Neuhäusler, A.; Rogg, K.; Schröder, S.; Spiehl, D.; Zora, H.; Arefaine, E.; Blaeser, A. Electrospun microfibers to enhance nutrient supply in bioinks and 3D-bioprinted tissue precursors. Biofabrication 2024, 17, 015038. [Google Scholar] [CrossRef] [PubMed]
- Wali, M.E.; Karinen, H.; Rønning, S.B.; Skrivergaard, S.; Dorca-Preda, T.; Rasmussen, M.K.; Young, J.F.; Therkildsen, M.; Mogensen, L.; Ryynänen, T.; et al. Life cycle assessment of culture media with alternative compositions for cultured meat production. Int. J. Life Cycle Assess. 2024, 29, 2077–2093. [Google Scholar] [CrossRef]
- Klatt, A.; Wollschlaeger, J.O.; Albrecht, F.B.; Rühle, S.; Holzwarth, L.B.; Hrenn, H.; Melzer, T.; Heine, S.; Kluger, P.J. Dynamically cultured, differentiated bovine adipose-derived stem cell spheroids as building blocks for biofabricating cultured fat. Nat. Commun. 2024, 15, 9107. [Google Scholar] [CrossRef]
- Miretti, S.; Manenti, I.; Toschi, P.; Macchi, E.; Martignani, E.; Accornero, P.; Baratta, M. Bovine skeletal muscle satellite cells: Isolation, growth, and differentiation. In Epithelial Cell Culture: Methods and Protocols; Springer: New York, NY, USA, 2023; pp. 165–174. [Google Scholar] [CrossRef]
- Melzener, L.; Schaeken, L.; Fros, M.; Messmer, T.; Raina, D.; Kiessling, A.; Haaften, T.V.; Spaans, S.; Doǧan, A.; Mark, J.P.; et al. Optimisation of cell fate determination for cultivated muscle differentiation. Commun. Biol. 2024, 7, 1493. [Google Scholar] [CrossRef] [PubMed]
- Messmer, T.; Dohmen, R.G.; Schaeken, L.; Melzener, L.; Hueber, R.; Godec, M.; Didoss, C.; Post, M.J.; Flack, J.E. Single-cell analysis of bovine muscle-derived cell types for cultured meat production. Front. Nutr. 2023, 10, 1212196. [Google Scholar] [CrossRef] [PubMed]
- Bodiou, V.; Cristini, N.; De Cristofaro, L.; Pareek, T.; Rajagopal, V.; Verrougstraete, L.; Heinrich, J.M.; Post, M.J.; Moutsatsou, P. Process intensification of cultivated meat production through microcarrier addition strategy optimisation. Sci. Rep. 2025, 15, 14080. [Google Scholar] [CrossRef]
- Sibinčić, N.; Krstić Ristivojević, M.; Gligorijević, N.; Veličković, L.; Ćulafić, K.; Jovanović, Z.; Ivanov, A.; Tubić, L.; Vialleix, C.; Michel, T.; et al. Screening algal and cyanobacterial extracts to identify potential substitutes for fetal bovine serum in cellular meat cultivation. Foods 2024, 13, 3741. [Google Scholar] [CrossRef]
- Thrower, T.; Riley, S.E.; Lee, S.; Esteves, C.L.; Xavier Donadeu, F. A unique spontaneously immortalized cell line from pig with enhanced adipogenic capacity. NPJ Sci. Food 2025, 9, 52. [Google Scholar] [CrossRef]
- Trautmann, C.L.; Ghosh, A.; Kalkan, A.K.; Noé, F.; Bar-Nur, O. Enhanced Media Optimize Bovine Myogenesis in 2D and 3D Models for Cultivated Meat Applications. Adv. Sci. 2025, 12, e13998. [Google Scholar] [CrossRef]
- Sundaram, T.S.; Giromini, C.; Rebucci, R.; Lanzoni, D.; Petrosillo, E.; Baldi, A.; Cheli, F. Milk whey as a sustainable alternative growth supplement to fetal bovine serum in muscle cell culture. J. Dairy Sci. 2025, 108, 4749–4760. [Google Scholar] [CrossRef]
- Dan-Jumbo, S.O.; Riley, S.E.; Cortes-Araya, Y.; Ho, W.; Lee, S.; Thrower, T.; Donadeu, F.X. Derivation and long-term maintenance of porcine skeletal muscle progenitor cells. Sci. Rep. 2024, 14, 9370. [Google Scholar] [CrossRef]
- Dagès, B. Novel Edible Microcarriers for the Scalable Production of Cultivated Meat. Ph.D. Thesis, Aston University, Birmingham, UK, 2024. [Google Scholar] [CrossRef]
- Musgrove, L.; Bhojwani, A.; Hyde, C.; Glendinning, S.; Nocillado, J.; Russell, F.D.; Ventura, T. Transcriptomic analysis across crayfish (Cherax quadricarinatus) claw regeneration reveals potential stem cell sources for cultivated crustacean meat. Int. J. Mol. Sci. 2024, 25, 8623. [Google Scholar] [CrossRef]
- Charlesworth, J.C.; Jenner, A.; Le Coutre, J. Plant-based hydrolysates as building blocks for cellular agriculture. Food Chem. 2024, 460, 140621. [Google Scholar] [CrossRef] [PubMed]
- Oey, M.; Marx, U.; Schirra, H.J.; Ross, I.L.; Parton, R.G.; Hankamer, B.; Lo, H.P. Co-cultivation with new glucose-sparing Chlorella algae boosts tissue culture efficiency by reducing cell waste. Biotechnol. J. 2025, 20, e70067. [Google Scholar] [CrossRef] [PubMed]
- Cox, T.R.; Lesmana, D.; O’Keeffe, C.J.; Lam, A.; Zou, W.; Lin, Z.; Ding, L. Maximising adherent cell production via customisable and dissolvable bio-polymer microcarriers. Biomed. Mater. 2025, 20, 055002. [Google Scholar] [CrossRef]
- Ding, L.; Oh, S.; Shrestha, J.; Lam, A.; Wang, Y.; Radfar, P.; Warkiani, M.E. Scaling up stem cell production: Harnessing the potential of microfluidic devices. Biotechnol. Adv. 2023, 69, 108271. [Google Scholar] [CrossRef]
- Goswami, A.B.; Biazik, J.M.; Le Coutre, J. Fat forward: Cultivating bovine adipocytes on bioscaffolds. Curr. Res. Food Sci. 2025, 10, 101047. [Google Scholar] [CrossRef] [PubMed]
- Nabiullina, R.; Golovin, S.; Kirichenko, E.; Petrushan, M.; Logvinov, A.; Kaplya, M.; Rodkin, S. 3D bioprinting of cultivated meat followed by the development of a fine-tuned YOLO model for the detection and counting of lipoblasts, fibroblasts, and myogenic cells. Front. Biosci.–Landmark 2025, 30, 36266. [Google Scholar] [CrossRef] [PubMed]
- Celebi-Birand, D.; Genc, K.; Agun, I.; Erikci, E.; Akcali, K.C.; Kiran, F. Microbiota-derived postbiotics enhance the proliferative effects of growth factors on satellite cells in cultivated meat applications. Sustainability 2023, 15, 16164. [Google Scholar] [CrossRef]
- Guo, H.; Fan, L.; Ding, L.; Yang, W.; Zang, C.; Guan, H. Separation and purification of antioxidant peptide from fermented whey protein by Lactobacillus rhamnosus B2-1. Food Sci. Anim. Resour. 2023, 43, 10. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, T.K.; Park, S.Y.; Kang, M.C.; Cha, J.Y.; Lim, M.C.; Choi, Y.S. Effects of blanching methods on nutritional properties and physicochemical characteristics of hot-air dried edible insect larvae. Food Sci. Anim. Resour. 2023, 43, 428. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Jeon, Y.B.; Yoo, D.G.; Kim, K.H.; Jeong, H.J.; Kim, B.K.; Park, M.H.; Kim, K.H.; Hwang, J.H.; Cho, G.H.; et al. Fermented whey protein supplementation improves muscular strength, muscle parameters, and physical performance in middle-aged Korean adults: An 8-week double blind randomized controlled trial. Food Sci. Anim. Resour. 2023, 43, 512. [Google Scholar] [CrossRef]
- Seo, C.W.; Oh, N.S. Rheological, physicochemical, microbiological, and aroma characteristics of sour creams supplemented with milk protein concentrate. Food Sci. Anim. Resour. 2023, 43, 540. [Google Scholar] [CrossRef]
- Uyen, N.T.; Van Cuong, D.; Thuy, P.D.; Son, L.H.; Ngan, N.T.; Quang, N.H.; Tuan, N.D.; Hwang, I.H. A comparative study on the adipogenic and myogenic capacity of muscle satellite cells, and meat quality characteristics between Hanwoo and Vietnamese yellow steers. Food Sci. Anim. Resour. 2023, 43, 563. [Google Scholar] [CrossRef]
- Yang, J.; Lee, S.K.; Kim, Y.S.; Suh, H.J.; Ahn, Y. Preparation of hypoallergenic whey protein hydrolysate by a mixture of alcalase and prozyme and evaluation of its digestibility and immunoregulatory properties. Food Sci. Anim. Resour. 2023, 43, 594. [Google Scholar] [CrossRef]
- Jin, E.S.; Kim, J.Y.; Min, J.; Jeon, S.R.; Choi, K.H.; Khan, S.A.; Moon, G.S.; Jeong, J.H. Preliminary study on effect of Lactiplantibacillus plantarum on osteoporosis in the ovariectomized rat. Food Sci. Anim. Resour. 2023, 43, 712. [Google Scholar] [CrossRef]
- Lee, S.; Jo, K.; Choi, Y.S.; Jung, S. Level optimization of beet powder and caramel color for beef color simulation in meat analogs before and after cooking. Food Sci. Anim. Resour. 2023, 43, 889. [Google Scholar] [CrossRef]
- Santacroce, L.; Topi, S.; Bottalico, L.; Charitos, I.A.; Jirillo, E. Current knowledge about gastric microbiota with special emphasis on Helicobacter pylori-related gastric conditions. Curr. Issues Mol. Biol. 2024, 46, 4991–5009. [Google Scholar] [CrossRef]
- Yu, I.S.; Choi, J.; Kim, M.K.; Kim, M.J. The comparison of commercial serum-free media for Hanwoo satellite cell proliferation and the role of fibroblast growth factor 2. Food Sci. Anim. Resour. 2023, 43, 1017. [Google Scholar] [CrossRef]
- Choi, J.S.; Kim, G.H.; Kim, H.E.; Kim, M.J.; Chin, K.B. Evaluation of gelation properties of salt-soluble proteins extracted from Protaetia brevitarsis larvae and Tenebrio molitor larvae and application to pork myofibrillar protein gel system. Food Sci. Anim. Resour. 2023, 43, 1031. [Google Scholar] [CrossRef] [PubMed]
- Mariano, E.J.; Yun, S.H.; Lee, J.; Lee, S.Y.; Hur, S.J. Check meat: A review on the applicability of conventional meat authentication techniques to cultured meat. Food Sci. Anim. Resour. 2023, 43, 1055. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Yun, S.H.; Lee, J.; Mariano, E., Jr.; Park, J.; Choi, Y.; Han, D.; Kim, S.J.; Hur, S.J. Current technologies and future perspective in meat analogs made from plant, insect, and mycoprotein materials: A review. Food Sci. Anim. Resour. 2024, 44, 1. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.N.; Kim, C.J.; Kim, S.H.; Kumari, S.; Lee, S.Y.; Hwang, Y.H.; Joo, S.T. Trends in hybrid cultured meat manufacturing technology to improve sensory characteristics. Food Sci. Anim. Resour. 2024, 44, 39. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.D.; Lee, K.; Han, T.; Jhun, H.; Han, A.R.; Hwang, Y.; Hong, S. Antihypertensive effect of milk fermented by Lactiplantibacillus plantarum K79 on spontaneously hypertensive rats. Food Sci. Anim. Resour. 2024, 44, 178. [Google Scholar] [CrossRef]
- Kim, K.H.; Hwang, Y.; Kang, S.S. Regulatory effect of spray-dried Lactiplantibacillus plantarum K79 on the activation of vasodilatory factors and inflammatory responses. Food Sci. Anim. Resour. 2024, 44, 216. [Google Scholar] [CrossRef]
- Lee, S.H.; Choi, J. The need for research on the comparison of sensory characteristics between cultured meat produced using scaffolds and meat. Food Sci. Anim. Resour. 2024, 44, 269. [Google Scholar] [CrossRef] [PubMed]
- Rackerby, B.; Le, H.N.M.; Haymowicz, A.; Dallas, D.C.; Park, S.H. Potential prebiotic properties of whey protein and glycol macropeptide in gut microbiome. Food Sci. Anim. Resour. 2024, 44, 299. [Google Scholar] [CrossRef]
- Yun, S.H.; Lee, J.; Mariano, E., Jr.; Choi, Y.; Park, J.; Han, D.; Kim, S.J.; Hur, S.J. Current research, industrialization status, and future perspective of cultured meat. Food Sci. Anim. Resour. 2024, 44, 326. [Google Scholar] [CrossRef]
- Kang, K.M.; Lee, D.B.; Kim, H.Y. Industrial research and development on the production process and quality of cultured meat hold significant value: A review. Food Sci. Anim. Resour. 2024, 44, 499. [Google Scholar] [CrossRef]
- Kim, G.H.; Chin, K.B. Effect of faba bean isolate and microbial transglutaminase on rheological properties of pork myofibrillar protein gel and physicochemical and textural properties of reduced-salt, low-fat pork model sausages. Food Sci. Anim. Resour. 2024, 44, 586. [Google Scholar] [CrossRef]
- Choi, J.K.; Kwon, O.Y.; Lee, S.H. Oral administration of Bifidobacterium lactis ameliorates cognitive deficits in mice intracerebroventricularly administered amyloid beta via regulation of the activation of mitogen-activated protein kinases. Food Sci. Anim. Resour. 2024, 44, 607. [Google Scholar] [CrossRef] [PubMed]
- Min, J.H.; Lee, Y.J.; Kang, H.J.; Moon, N.R.; Park, Y.K.; Joo, S.T.; Jung, Y.H. Characterization of yeast protein hydrolysate for potential application as a feed additive. Food Sci. Anim. Resour. 2024, 44, 723. [Google Scholar] [CrossRef] [PubMed]
- Choi, N.; Park, S.; Park, Y.; Park, G.; Oh, S.; Kim, Y.A.; Lim, Y.; Jang, S.Y.; Kim, Y.J.; Ahn, K.S.; et al. Effects of edible insect powders as meat partial substitute on physicochemical properties and storage stability of pork patties. Food Sci. Anim. Resour. 2024, 44, 817. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; So, Y.J.; Jung, W.H.; Kim, T.R.; Sohn, M.; Jeong, Y.J.; Imm, J.Y. Lactiplantibacillus plantarum LM 1001 improves digestibility of branched-chain amino acids in whey proteins and promotes myogenesis in C2C12 myotubes. Food Sci. Anim. Resour. 2024, 44, 951. [Google Scholar] [CrossRef]
- Chantakun, K.; Petcharat, T.; Wattanachant, S.; Ab Karim, M.S.B.; Kaewthong, P. Fatty acid profile and thermal behavior of fat-rich edible insect oils compared to commonly consumed animal and plant oils. Food Sci. Anim. Resour. 2024, 44, 790. [Google Scholar] [CrossRef]
- Bakhsh, A.; Lee, E.Y.; Ncho, C.M.; Kim, C.J.; Son, Y.M.; Hwang, Y.H.; Joo, S.T. Quality characteristics of meat analogs through the incorporation of textured vegetable protein: A systematic review. Foods 2022, 11, 1242. [Google Scholar] [CrossRef] [PubMed]
- Han, S.G.; Lim, S.J.; Hong, S.J.; Kwon, H.C.; Jung, H.S.; Han, S.G. Comparison of soy and pea protein for cultured meat scaffolds: Evaluating gelation, physical properties, and cell adhesion. Food Sci. Anim. Resour. 2024, 44, 1108. [Google Scholar] [CrossRef]
- Kim, C.J.; Kim, S.H.; Lee, E.Y.; Hwang, Y.H.; Lee, S.Y.; Joo, S.T. Effect of chicken age on proliferation and differentiation abilities of muscle stem cells and nutritional characteristics of cultured meat tissue. Food Sci. Anim. Resour. 2024, 44, 1167. [Google Scholar] [CrossRef]
- Venter, C.; Choi, Y.; Park, J.M.; Han, D.; Kim, J.S.; Park, J.W.; Kung, S.H.; Mariano, E.J.; Lee, J.H.; Park, K.H.; et al. Market status of meat analogs and their impact on livestock industries. Food Sci. Anim. Resour. 2024, 44, 1213. [Google Scholar] [CrossRef]
- Baek, J.; Lee, S.; Lee, J.; Park, J.; Choi, E.; Kang, S.S. Utilization of probiotic-derived extracellular vesicles as postbiotics and their role in mental health therapeutics. Food Sci. Anim. Resour. 2024, 44, 1252. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kumari, S.; Kim, C.J.; Lee, E.Y.; Alam, A.N.; Chung, Y.S.; Hwang, Y.H.; Joo, S.T. Effect of adding cultured meat tissue on physicochemical and taste characteristics of hybrid cultured meat manufactured using wet-spinning. Food Sci. Anim. Resour. 2024, 44, 1440. [Google Scholar] [CrossRef]
- Wang, S.; Hu, H.; Li, Y.; Li, S.; Yang, F. Edible 3D Printing Biological Ink, Preparation Method Thereof and Application Thereof in Meat Cultivation. CN Patent No. 115747197A, 6 August 2025. [Google Scholar]
- Shan, Y.; Liu, Z.; You, W.; Gu, X.; Tu, A.; Wang, Y. Construction Method and Application of Pig FAPs Immortalized Cells. CN Patent No.115386541A, 7 September 2022. [Google Scholar]
- Wang, F.; Huang, B.; Chen, J. Isolation and Extraction of Muscle Stem Cells and a New Hydrolyzate Culture System and Its Application. CN Patent No. 114703126A, 5 July 2022. [Google Scholar]
- Liu, H.; Wei, Y.; Zhou, Z. Cell Culture Meat Culture Apparatus. CN Patent No. 217297879U, 26 August 2022. [Google Scholar]
- Zhou, G.; Wang, J.; Ding, X.; Ding, S. Cell-Cultured Meat Production Device Based on Microfluidic 3D Printing Technology and Process of Preparing Cell-Cultured Meat by Use Thereof. US Patent No. 20250151771A1, 15 May 2023. [Google Scholar]
- Shan, T.; Zhou, Y.; Liu, S. Composition Capable of Optimizing Composition of Cell Lipid in Cultured meat, Culture Method and Application. CN Patent No. 118562724A, 30 August 2024. [Google Scholar]
- Guan, Y.; Zhou, R.; Lei, M.; Chen, J.; Tang, G. Method for Preparing Cell Culture Snowflake Meat. CN Patent No. 117625518A, 3 January 2025. [Google Scholar]
- Tang, C.; Da, C.; Ding, J.; Zhou, G.; Wang, S.; Deng, Y.; Li, W. A Method for Screening High-Quality Porcine Fibroblasts for Cell-Cultured Meat. CN Patent No. 118374440A, 23 July 2024. [Google Scholar]
- Peng, D.; Jin, Y. An Optimized Method for Promoting Adipogenic Differentiation of Bovine Skeletal Muscle Cells. CN Patent No. 118530938A, 22 October 2024. [Google Scholar]
- Zhou, G.; Ding, S.; Zhu, H.; Wang, Z.; Zhuang, H. A Safe and Efficient Method for Inducing Cell Adipogenic Differentiation by Hypoxia and Its Application. CN Patent No. 120210108A, 17 October 2025. [Google Scholar]
- Guan, X.; Zhou, J.; Lei, Q.; Chen, J.; Du, G. A Recombinant Cerevisiae Yeast Producing four Cytokines and Its Construction Method and Application. CN Patent No. 118325746A, 12 July 2023. [Google Scholar]
- Chan, K.Y.C.; Chin, P.S.M.; Poon, C.H. Methods of Meat Production by In Vitro Cell Cultivation. CA Patent No. 3163651A1, 10 June 2021. [Google Scholar]
- Tang, C.; Guo, Y.; Zhou, G.; Ding, S.; Zhu, H.; Ding, X.; Xin, S.; Wang, J. Muscle Stem Cell Proliferation Medium, Differentiation Medium and Application Thereof. CN Patent No. 114958730A, 30 August 2022. [Google Scholar]
- Xu, C.; Zhou, L.; Zheng, H.; Feng, M.; Wei, Y.; Hong, M. Serum-Free Induced Adipogenic Differentiation Medium for Fish Stem Cells and Application Thereof. CN Patent No. 119842599A, 22 July 2025. [Google Scholar]
- Xu, C.; Zheng, H.; Wei, Y.; Xu, W.; Liu, H.; Feng, M. Marine Fish Cell Culture Fish Oil and Preparation Method Thereof. CN Patent No. 119020275A, 18 March 2024. [Google Scholar]
- Xiang, N.; Li, Y. Method for Continuously Preparing Edible Fiber Scaffold for Cell Culture Meat. CN Patent No. 114622296A, 25 June 2022. [Google Scholar]
- Wang, J.; Zhou, G.; Ding, X. Microfluidic Bionic Fiber for Cell Culture Meat Production and Preparation Method and Application Thereof. CN Patent No. 114854677A, 5 August 2022. [Google Scholar]
- Hauser, B.G.; Moravec, D.B.; Nelson, J.A.; Wansapura, P.T. System and Method for Cultured Cell Meat Production. WO Patent No. 2025122851 A1, 12 June 2025. [Google Scholar]
- Savyon, G.; Nachman, I.; Hauser, M.; Avni, L. Bovine Pluripotent Stem Cell-Based Muscle Organoids, Method of Preparing the Same and Uses Thereof. US Patent Application No. 20240392247A1, 28 November 2024. [Google Scholar]
- Lavon, N.; Maor-Shoshani, A.; Lapidoth, G.; Zimmerman, L.; Alon, N.; Levin, I.; Baran, D. Growth Factor Substituents. WO Patent No. 2024231922A1, 9 November 2025. [Google Scholar]
- Ben-Arye, T.; Levenberg, S. Cultured Meat Compositions. U.S. Patent No. 12123025B2, 22 October 2024. [Google Scholar]
- Savir, I.; Friedman, S.; Barak, K. Cultured Meat-Containing Hybrid Food. WO Patent No. 2018189738A1, 18 October 2018. [Google Scholar]
- Hamami, R.; Harel, O. Edible Hydrogel Microcarriers Comprising Cells, Compositions Comprising Same and Methods of Producing and Using Same. WO Patent Application No. 2024241315A1, 28 November 2024. [Google Scholar]
- Segawa, M.; Furusawa, Y.; Nishiyama, Y. Cell Proliferation Medium for Producing Cultured Meat. WO Patent No. 2023127970A1, 6 July 2023. [Google Scholar]
- Toyama, K.; Hakuba, H.; Yoneda, K.; Tabata, Y. Activator, Medium, Culture Method, and Method for Producing Cultured Meat. JP Patent No. 2024172204A, 12 December 2024. [Google Scholar]
- Iwasa, T.; Kojima, R. Cell Culture Substrate and Method for Producing Same. JP Patent No. 2024154690A, 30 October 2023. [Google Scholar]
- Shimizu, M.; Saito, A.; Nakai, T. Media for Cell Growth in Cultured Meat Production. JP Patent No. 7692122B2, 12 June 2024. [Google Scholar]
- Tsuyoshi, N.; Mannen, T.; Matsuda, Y.; Ogawa, S.; Koseki, Y.; Kado, T. Method for Producing Cultured Meat. WO Patent No. 2023200008A1, 19 October 2023. [Google Scholar]
- Toshiro, O.; Ibuki, K. Cell Culture System, Cultured Meat, and Culture Supernatant. EP Patent No. 4582532A1, 9 July 2025. [Google Scholar]
- Takeuchi, S.; Morimoto, Y.; Furuhashi, M. Cell Culture Gel, Method for Producing Cell Culture Gel with Cells, Method for Producing Cells, Three-Dimensional Muscle Tissue Produced by Same, and Cultured Meat. WO Patent No. 2023047831A1, 30 March 2023. [Google Scholar]
- Sekiguchi, Y.; Sudo, T.; Hishiki, T.; Konishi, T.; Kawasaki, Y. Adhesion Improver Containing Edible and Non-Lethal Animal-Derived Component. US Patent No. 20240191182A1, 13 June 2024. [Google Scholar]
- Liu, L.; Sawa, J.; Miyagawa, S.; Ito, E. Multi-Layered Heavy Structure, Its Manufacturing Method and Its Use. JP Patent No. 2024092786A, 8 June 2024. [Google Scholar]
- Nakazawa, Y.; Akioka, S.; Sato, M. Fiber Structure, Composite Material, Three-Dimensional Cell Aggregate, Cultured Meat, Method for Producing a Fiber Structure, and Method for Producing a Composite Material. JP Patent No. 2024172165A, 12 December 2024. [Google Scholar]
- Kim, G.; Song, S.; Park, J. Method for Isolating Muscle Stem Cells from Livestock with High Purity for Manufacturing Cultured Meat. KR Patent No. 1020250098441, 1 July 2025. [Google Scholar]
- Hong, J.; Choi, B. Cell Powder Type Cultured Meat and Its Manufacturing Method. US Patent No. 20240373887A1, 1 July 2025. [Google Scholar]
- Kim, H.; Lee, E. Medium Additives for Culturing Cultured Meat, Cell Culture Medium for Culturing Cultured Meat and Culturing Method of Cultured Meat. KR Patent No. 1020250084331, 11 June 2025. [Google Scholar]
- Kim, H.T.; Park, J.H.; Lee, E.; Lee, S. Culture Medium Composition for Transdifferentiation of Muscle Stem Cells into Adipocytes, and Method for Producing Cultured Meat by Using Same. WO Patent No. 2025136060A1, 26 June 2025. [Google Scholar]
- Hur, S.J.; Lee, D.Y.; Lee, S.Y.; Jeong, J.W.; Kim, J.H.; Kim, H.W. Method for Producing Cultured Meat Using FBS(Fetal Bovine Serum) Substitute Derived from Slaughter By-Products. KR Patent No. 1020230077644, 1 June 2023. [Google Scholar]
- Choi, I.H.; Lee, E.J. Medium Composition for Promoting Differentiation of Chicken Muscle Stem Cells Comprising Glycyrrhiza Uralensis Fischer Extract or Compound Derived Therefrom as Active Ingredient. WO Patent No. 2024242256A1, 28 December 2024. [Google Scholar]
- Ok, S.; Byun, S. Method for Manufacturing Cultured Chicken Meat Using SPF-Fertilized Eggs. KR Patent No. 1020240164155, 19 November 2024. [Google Scholar]
- Jo, C.; Kim, D.; Jeon, M. Methods for Manufacturing Cultured Meat by Differentiation of Muscle and Fat, and the Cultured Meat Thereby. KR Patent Application No. 1020230125719, 29 August 2023. [Google Scholar]
- Han, I.; Kim, G. Method for Producing Cultured Meat and Device for Performing the Same. KR Patent No. 1020250084084, 10 June 2025. [Google Scholar]
- Park, G.; Kim, J. Scaffold for Manufacturing Cultured Meat and Manufacturing Method Thereof. KR Patent No. 1020250021276, 12 February 2025. [Google Scholar]
- Rho, S.C. Cultivated Meat Scaffold, Method for Producing Same, and Cultivated Meat Containing Same. EP Patent No. 4549545A1, 7 May 2025. [Google Scholar]
- Lee, M.H. Compositions and Methods for Production of Edible Cell-Infused Scaffolds. WO Patent No. 2025126155A1, 19 June 2025. [Google Scholar]
- Hong, J.; Choi, W.; Lee, M. Scaffold for Cultured Meat that Enhances the Meat Flavor of Cultured Meat. US Patent No. 20250040578A1, 6 February 2025. [Google Scholar]
- Dariani, M.; Bhatia, M. Nonhuman Stem Cells and Their Use for Production of Cultured Meat. WO Patent No. 2023129418A1, 6 July 2023. [Google Scholar]
- Leung, M.; Carswell, K.; Warner, M.; Vanderpol, R.E.; Hsiu, T.P. Apparatuses and Systems for Preparing a Meat Product. CA Patent No. 3160109C, 4 July 2023. [Google Scholar]
- Ranger, B.; Gaudette, G.R.; Thyden, R.; Perreault, L.R.; Freitas Dos Santos, A.C. Bioreactor System for Production of Cell-Cultured Meat and Related Method. U.S. Patent No. 20240417668A1, 19 December 2024. [Google Scholar]
- Budoff, S.; Kravitz, A.; Wang, Y. Compositions and Methods Relating to Adipocyte Production. WO Patent No. 2024086370A1, 25 April 2024. [Google Scholar]
- Rease, M.L.; Wicke, A. Comestible Cell-Based Meat Products Comprising Dry Cell Powder and Methods of Making such Products. US Patent No. 20250221433A1, 10 July 2025. [Google Scholar]
- Antony John WICKE. Method for Shaping a Cell-Mass Mixture by Vacuum Sealing. WO Patent No. 2023204868A1, 26 October 2023.
- Rease, M.; Wicke, A. Comestible Cell-Based Meat Products Comprising Dry Cell Powder and Methods of Making Such Products. WO Patent No. 2022261647A1, 15 December 2022. [Google Scholar]
- Hosseini, S. Adipogenic Cell Culture Media. WO Patent No. 2025059173A1, 11 September 2024. [Google Scholar]
- Santo, V.E.; Bou-Ghannam, S.S.; Lee, J. Cultivated Animal Cells Adapted for Growth in Low Amounts, and/or the Absence of, Direct Growth Factors, Indirect Growth Factors, Animal Serum, and/or Animal Components, and Methods of use Thereof. WO Patent No. 2024007033A1, 4 January 2024. [Google Scholar]
- Kaplan, D.L.; Stout, A. Supplemented Serum-Free Media Including Unhydrolyzed Plant Protein for Cultured Meat Production. WO Patent No. 2023225686A1, 23 November 2023. [Google Scholar]
- Kaplan, D.L.; Stout, A. Supplemented Serum-Free Media Including Unhydrolyzed Plant Protein for Cultured Meat Production. EP Patent No. 4526431A1, 26 March 2025. [Google Scholar]
- Elfenbein, A.; Kolbeck, J.L.; Larson, B. Synthetic Food Compositions. WO Patent No. 2020123876A1, 12 December 2019. [Google Scholar]
- González, B.J.; Forgacs, G.; Gupta, N. Compositions and Methods for Cell Engineering for Meat Production. WO Patent No. 2023178084A2, 21 September 2023. [Google Scholar]
- Gora, K.; Goranov, A.; Katz, D.; Lamb, A. Engineered Bovine Cell Lines for Suspension Culture. WO Patent No. 2023205677A1, 26 October 2023. [Google Scholar]
- Pattillo, A. Enhanced Aerobic Fermentation Methods for Producing Edible Fungal Mycelium Blended Meats and Meat Analogue Compositions. WO Patent No. 2020061502A1, 26 March 2020. [Google Scholar]
- Lavon, N.; Tamari, Z. System and Processes for Culturing Non-Human-Animal Cells Under Variable Gravity Conditions. EN Patent No. 20250215392A1, 3 July 2025. [Google Scholar]
- Liu, Y.; Emerson, S.B.; Walker, M.J. Chickpea Microcarriers. U.S. Patent Application No. 20240400979A1, 5 December 2024. [Google Scholar]
- Hosseini, S. Egg Based Microcarriers and Scaffolds for Cellular Agriculture and Other Applications. WO Patent No. 2024211384A1, 10 October 2024. [Google Scholar]
- Jones, J.K.; Oost, T. Edible Scaffolds for Cultured Meat Production. KR Patent No. 1020250061719, 18 January 2024. [Google Scholar]
- Floreani, R.; Charron, P. Polymer/Milk-Protein Hydrogels, Cell Scaffolds and Cultured Meat Products Made Therewith, and Associated Methods. WO Patent No. 2023205490A1, 22 April 2023. [Google Scholar]
- Johnson, J.K.; Ohst, D. Electrospun Polymer Fibers for Cultured Meat Production. WO Patent No. 2020160533A1, 6 August 2020. [Google Scholar]
- Moore, B.W.; Alkhaldi, F.; Rebello, A.S.; Sochacki, D.; Murphy, W.; Jones, J.; Gaudette, G. Plant-Derived Scaffolds for Generation of Synthetic Animal Tissue. WO Patent No. 2020214964A1, 22 October 2020. [Google Scholar]
- Banerjee, A.R.; Martens, M.-E.; Liira, R.; Neivelt, K.I.; Mölder, L.; Howlader, M.M.; Loos, M.; Enn, R. Plant-Based Microfibrous Scaffolds for Culturing Meat. EP Patent No. 4527920A1, 9 August 2024. [Google Scholar]
- Nakano, R.F.J. Growth Factor Composition for Cell-Cultured Meat. JP Patent Application No. 2023528510A, 4 July 2023. [Google Scholar]
- Maria Biressi, S.A.; Conti, L.; Fioravanti, G.; Lattanzi, S. Temperature-Sensitive Induction of the Myogenic and/or Adipogenic Program in Cells Producing Muscle and/or Adipose Tissue. IT Patent No. 202200000788A1, 19 July 2023. [Google Scholar]
- Breemhaar, J.; Post, M. Apparatus and Process for Production of Tissue from Cells. U.S. Patent No. 11512273B2, 29 November 2022. [Google Scholar]
- Mitic, R.; Cantoni, F.; Hubalek, S.C.; Jackisch, L.; Mei, A.; Melke, J. Serum-Free Media for Producing Adipocytes for Animal Consumption. NL Patent No. 2028813B1, 267 January 2023. [Google Scholar]
- Mitic, R.; Cantoni, F.; Hubalek, S.C.; Jackisch, L.; Mei, A.; Melke, J. Serum-Free Media for Producing Adipocytes for Animal Consumption. U.S. Patent No. 20240344028A1, 26 January 2023. [Google Scholar]
- Vander Wal, E.; Quinney, K.B.; Out, R. Maturation of Skeletal Muscle Cells. WO Patent No. 2024170696A1, 15 February 2024. [Google Scholar]
- Flack, J.E.; Meßmer, T.; Huber, R.H.; Schaeken, L.M.L.; Martins, B.S.L.; Dohmen, R.G.J. Method of Generating a Homocellular Progenitor Cell Culture from a Heterocellular Tissue Sample. NL Patent No. 2032514B1, 25 January 2022. [Google Scholar]
- Garcia, E.S.; Vandenberg, C. Microbial Cell Extract. WO Patent No. 2023175150A1, 21 September 2023. [Google Scholar]
- Guembe Lapuente, A.; Zúñiga Arrarás, T.; Recalde Irurzun, J.I.; Izco Zaratiegui, J.M. Collagen Hydrogels are Useful as Cell Carriers. IL Patent No. 307765A, 1 December 2022. [Google Scholar]
- Morel, A.; Eglauf, J.S.; Razza, N.; Das, S.K. Edible Microcarrier for the Preparation of Cultured Meat and Method of Producing Same. WO Patent No. 2023104768A1, 15 June 2023. [Google Scholar]
- Martin, R.J.; Ingram, H.R. Hollow Fibres. WO Patent No. 2024209200A1, 2 April 2024. [Google Scholar]
- Ellis, M.; Dunsford, I.L. Method of Culturing Muscle Cells with Perfusion Bioreactor. WO Patent No. 2024038281A1, 28 March 2024. [Google Scholar]
- Hahn, F.; Doran, C.M.; Shelford, J.; Wilkinson, L.; Rimington, R. Genetically Manipulated Cells. WO Patent No. 2024141754A1, 4 July 2024. [Google Scholar]
- Partridge, S.W.; Tan-Mulligan, A.; Lawton, K.; Nyland, R. Substrate Assembly, Cell Culture Method, Bioreactor and Cultured Meat Product. WO Patent No. 2023152493A1, 17 Agust 2023. [Google Scholar]
- Beaumont Nicholas, J. Processing Technology. WO Patent No. 2025050181A1, 13 March 2025. [Google Scholar]
- Food Safety and Standards Authority of India (FSSAI). Food Safety and Standards (Approval of Non-Specified Food and Food Ingredients) Regulations. 2017. Available online: https://fssai.gov.in/upload/uploadfiles/files/Gazette_Notification_NonSpecified_Food_Ingredients_15_09_2017.pdf (accessed on 22 July 2025).
- Government of Israel Ministry of Health (MOH). Novel Food. 2024. Available online: https://fssai.gov.in/cms/non-specified-food.php (accessed on 22 July 2025).
- US Department of Agriculture, Foreign Agricultural Service (USDA FAS). Israel: Israeli Government Finds Cultivated Beef to be Safe for Human Consumption. 2024. Available online: https://www.fas.usda.gov/data/israel-israeli-government-finds-cultivated-beef-be-safe-human-consumption (accessed on 22 July 2025).
- Ministry of Health, Labour and Welfare (MHLW). Overview of Opinions to Date and Future Directions (draft) on So-Called “Cultured Meat” by the New Food Investigation Subcommittee, Food Standards Examination Division. 2024. Available online: https://www.law.go.kr/%EB%B2%95%EB%A0%B9/%EC%8B%9D%ED%92%88%EC%9C%84%EC%83%9D%EB%B2%95%20%EC%8B%9C%ED%96%89%EA%B7%9C%EC%B9%99 (accessed on 21 July 2025).
- Ministry of Food and Drug Safety (MFDS). Partial Amendment Notification to the Standards and Specifications for Provisional Recognition of Food Raw Materials. 2024. Available online: https://www.sfa.gov.sg/regulatory-standards-frameworks-guidelines/novel-food-framework/guidelines-on-novel-food (accessed on 21 July 2025).
- Singapore Food Agency (SFA). Safety of Alternative Protein. 2023. Available online: https://www.sfa.gov.sg/food-safety-tips/food-risk-concerns/risk-at-a-glance/safety-of-alternative-protein?utm (accessed on 23 July 2025).
- Good Food Institute (GFI). Good Meat and UPSIDE Foods Approved to Sell Cultivated Chicken Following Landmark USDA Action. 2023. Available online: https://gfi.org/press/good-meat-and-upside-foods-approved-to-sell-cultivated-chicken-following-landmark-usda-action/?utm (accessed on 22 July 2025).
- Mridul, A.; Mission Barns Gets FDA Approval to Sell Cultivated Pork in US Supermarkets Restaurants. Green Queen. 2025. Available online: https://www.greenqueen.com.hk/mission-barns-lab-grown-pork-fat-fda-approved-cultivated-meat-sprouts-fiorella/ (accessed on 21 July 2025).
- US Food and Drug Administration (FDA). FDA Completes Pre-Market Consultation for Human Food Made with Cultured Pork Fat Cells. 2025. Available online: https://www.fda.gov/food/hfp-constituent-updates/fda-completes-pre-market-consultation-human-food-made-with-cultured-pork-fat-cell (accessed on 22 July 2025).
- Government of Canada. Health Canada. Cellular Agriculture. 2024. Available online: https://www.canada.ca/en/health-canada/services/food-nutrition/cellular-agriculture.html?utm (accessed on 22 July 2025).
- National Health Surveillance Agency (ANVISA). Resolution–RDC No. 839, of December 14, 2023. 2023. Available online: https://www.fda.gov/food/human-food-made-cultured-animal-cells/formal-agreement-between-fda-and-usda-regarding-oversight-human-food-produced-using-animal-cell (accessed on 21 July 2025).
- European Union. Regulation (EU) 2015/2283 of the European Parliament and of the Council of 25 November 2015 on Novel Foods. 2015. Available online: https://eur-lex.europa.eu/eli/reg/2015/2283/oj (accessed on 21 July 2025).
- Meatly. Meatly launches world’s first cultivated pet food! 2025. Available online: https://meatly.pet/meatly-launches-worlds-first-cultivated-pet-food/ (accessed on 22 July 2025).
- Food Standards Australia New Zealand (FSANZ). Novel Foods. 2022. Available online: https://www.foodstandards.gov.au/business/novel?utm (accessed on 22 July 2025).
- Food Standards Australia New Zealand (FSANZ). Approval report—Application A1269: Cultured Quail as a Novel Food. 2025. Available online: https://www.foodstandards.gov.au/food-standards-code/applications/A1269-Cultured-Quail-as-a-Novel-Food (accessed on 22 July 2025).
- Federal Register of Legislation (Australian Government). Australia New Zealand Food Standards Code—Standard 1.5.4—Cell cultured foods (Legislative Instrument F2025L00686). 2025. Available online: https://www.legislation.gov.au/F2025L00686/latest/text (accessed on 22 July 2025).
- Sungduk, W. 2,600 Square Meters of Total Floor Area in Uiseong Bio Valley Investment of 14.5 Billion Won and Support Production of Equipment and Prototypes, etc. Last Year, Pohang Was Selected as a Research Centre in the Field of ‘Food Robots’. North Gyeongsang Province is Expected to Take the Lead in Food Tech Industry Technology. Maeil Business Newspaper. 2025. Available online: https://www.mk.co.kr/en/society/11255984 (accessed on 21 July 2025).
- Jeongheon, P. Gyeongsang National University Startup Develops Hybrid Cultivated Meat Resembling Real Steak. Yonhap News. 2025. Available online: https://www.yna.co.kr/view/AKR20250526092600052 (accessed on 21 July 2025).
- Areum, H. Marbling in Cultured Meat Was Achieved Using a LEGO-Inspired Modular Scaffold System. Chosun Biz. 2025. Available online: https://biz.chosun.com/science-chosun/science/2025/06/10/MZ5SOYR2GNHZJOTPTQP444ZLFQ/ (accessed on 21 July 2025).
- Kim, M. “Leaping Forward as a Global Hub for the Cultured Meat Industry”… Korean Food Tech Officially Spotlights Anuga 2025. Food Icon. 2025. Available online: https://www.foodicon.co.kr/news/articleView.html?idxno=30270 (accessed on 12 September 2025).
- Lee, S. Half of Cultured Meat Companies Prefer Overseas Markets over Korea, with the 45 Million Won Application Fee Cited as the Reason. 2025. Health Chosun. Available online: https://m.health.chosun.com/svc/news_view.html?contid=2025031401592 (accessed on 12 September 2025).
- Jiji. New Method Developed to Make Cultured Meat Thicker. The Japan Times. 2025. Available online: https://www.japantimes.co.jp/news/2025/04/21/japan/new-method-cultured-meat-thicker/ (accessed on 21 July 2025).
- Otake, T. Japanese Researcher Pushes the Boundaries of Lab-Grown ‘Real’ Meat. The Japan Times. 2023. Available online: https://www.japantimes.co.jp/news/2023/02/02/national/science-health/cultured-meat-research/ (accessed on 21 July 2025).
- Shimadzu. The Cultured Meat Future Creation Consortium Will Exhibit Actual Cultured Meat and a Meat Maker at the Osaka Healthcare Pavilion at the Osaka-Kansai Expo, Showcasing “Marbled Meat Made at Home.” 2025. Shimadzu. Available online: https://www.shimadzu.co.jp/news/2025/919bo89eid_c2qqy.html (accessed on 12 September 2025).
- Ayumi, S. JACA Submits Risk Assessment Proposal for Cell-Based Foods Such as Cultured Meat to Consumer Affairs Agency|Promotes the Formation of “Shared Expert Knowledge” as Administrative Deliberations Progress. 2025. Foovo. Available online: https://foodtech-japan.com/2025/06/10/jaca-2/ (accessed on 12 September 2025).
- Shimadzu Today. Shimadzu 4th Global Food Summit 2025: Thinking About Sustainability Through Food. 2025. Shimadzu Today. Available online: https://www.shimadzu.co.jp/today/20250908-1.html (accessed on 12 September 2025).
- Green Queen Heyi Subdistrict Office. China’s First New Protein Food Technology Innovation Base is Established in Fengtai District, Beijing. 2025. Green Queen Fengtai District People’s Government of Beijing Municipality. Available online: https://www.bjft.gov.cn/ftq/jxdt/202501/4e0178f8786040f9bb75dfa8a3af87cf.shtml (accessed on 12 September 2025).
- SCMP (South China Media Production). China Food Scientists Combine Pork, Chicken with Rice in Lab-Grown Meat Advance. 2025. SCMP (South China Media Production). Available online: https://www.scmp.com/news/china/science/article/3268458/china-food-scientists-combine-pork-chicken-rice-lab-grown-meat-advance (accessed on 12 September 2025).
- The Paper. Will We Be Able to Eat Pork in the Future Without Raising Pigs? Talk to Researchers About the Development and Prospects of Cell-Cultured Meat in My Country. 2025. The Pape. Available online: https://www.thepaper.cn/newsDetail_forward_23523492 (accessed on 12 September 2025).
- Nanjing Municipal Bureau of Agriculture and Rural Affairs. Dual-Core Drive Leads the RapidDevelopment of Urban Modern Agriculture: A Visit to Nanjing’s Two National Agricultural Science and Technology Innovation Platforms. 2025. Nanjing Municipal Bureau of Agriculture and Rural Affairs. Available online: https://nyncj.nanjing.gov.cn/njsnywyh/202504/t20250428_5137190.html (accessed on 12 September 2025).
- Science and Technology Daily. Cell-Cultured Meat Targets the High-End Food Market by Promoting Commercialization with High-Premium Dishes. 2025. Science and Technology Daily. Available online: https://www.stdaily.com/web/gdxw/2025-05/13/content_338294.html (accessed on 12 September 2025).
- Xinhuanet. Cell-Cultured Meat: Growing Meat in the Lab. Xinhuanet. 2024. Available online: http://www.news.cn/tech/20240613/2768a8d80ead48c1abb7221797f761d8/c.html (accessed on 12 September 2025).
- Zhaki, A.; Klein, S.-I. New Programme Aims to Make Lab-Grown Meat Safe from Contamination. The Straits Times. 2023. Available online: https://www.straitstimes.com/singapore/environment/new-programme-aims-to-make-lab-grown-meat-safe-from-contamination (accessed on 12 September 2025).
- Cheryl, T. Eat Just Receives Approval from SFA to Produce Serum-Free Cultivated Meat. The Straits Times. 2023. Available online: https://www.straitstimes.com/singapore/eat-just-receives-approval-from-sfa-to-produce-serum-free-cultivated-meat (accessed on 12 September 2025).
- Chim, H.S.; Shabana, B. Cultivated Meat Producer Eat Just Pauses Operations in Singapore. The Straits Times. 2024. Available online: https://www.straitstimes.com/singapore/cultivated-meat-producer-eat-just-pauses-operations-in-singapore (accessed on 12 September 2025).
- Cheryl, T. Singapore Approves Lab-Grown Quail for Consumption. The Straits Times. 2024. Available online: https://www.straitstimes.com/singapore/s-pore-approves-lab-grown-quail-for-consumption-here (accessed on 12 September 2025).
- Elaine, W. Meatable Aims to Start Construction of Pilot-Scale Cultivated Meat Facility in Singapore Later This Year, Ag Funder News. 2025. Available online: https://agfundernews.com/meatable-aims-to-start-construction-of-pilot-scale-cultivated-meat-facility-in-singapore-later-this-year (accessed on 12 September 2025).
- Hanacek, A. JBS Subsidiary BioTech Foods Begins Construction on Massive Cultivated-Meat Plant. Food Processing. 2023. Available online: https://www.foodprocessing.com/ingredients/alternative-protein/news/33006472/jbs-subsidiary-biotech-foods-begins-construction-on-massive-cultivated-meat-plant (accessed on 21 July 2025).
- Wiener-Bronner, D. Lab-Grown Meat Is Cleared for Sale in the United States. CNN. 2023. Available online: https://edition.cnn.com/2023/06/21/business/cultivated-meat-us-approval (accessed on 21 July 2025).
- Mario, C. Lab-Grown Pork Is Coming to These Bay Area Restaurants and Stores. San Francisco Chronicle. 2025. Available online: https://www.sfchronicle.com/food/article/mission-barns-lab-grown-pork-20207276.php (accessed on 21 July 2025).
- Shuran, Y.J. Texas Sued over Its Lab-Grown Meat Ban. The Texas Tribune. 2025. Available online: https://www.texastribune.org/2025/09/03/texas-cultivated-meat-lab-grown-ban-lawsuit/ (accessed on 21 July 2025).
- Margery, A.B. Proposal to Ban Lab-Grown Meat in Nebraska Gets Pushback from Ranchers and Farm Groups. AP News. 2025. Available online: https://apnews.com/article/nebraska-lab-grown-meat-ban-f897a369dfa4f84235c9aae33cf1712a (accessed on 21 July 2025).
- Leis, B. Though Not Yet on Grocery Shelves, Lab-Grown Meat Is Focus of New Laws and Legislation. CSG Midwest. 2025. Available online: https://csgmidwest.org/2025/04/25/though-not-yet-on-grocery-shelves-lab-grown-meat-is-focus-of-new-laws-and-legislation/ (accessed on 21 July 2025).
- Cesar, D.L.S. Lab-Grown Meat? Brazil’s Agenda for Regulating Food Technology. Lex Latin. 2023. Available online: https://lexlatin.com/reportajes/carne-laboratorio-foodtech-regulacion-brasil (accessed on 21 July 2025).
- Lucas, S. Brazil Is at the Forefront of the Development of Cultured Meat. Embrapa. 2023. Available online: https://www.embrapa.br/en/busca-de-noticias/-/noticia/77704192/brazil-is-at-the-forefront-of-the-development-of-cultured-meat (accessed on 21 July 2025).
- Horton, H. A Very Cultured Snack: Trying Fortnum and Mason’s Scotch Egg with Lab-Grown Meat. The Guardian. 2024. Available online: https://www.theguardian.com/food/2024/feb/03/a-very-cultured-snack-trying-fortnum-and-masons-scotch-egg-with-lab-grown-meat (accessed on 21 July 2025).
- Grierson, H. Dog Treat Made from Lab-Grown Meat on Sale in UK as Retailer Claims a ‘World First’. The Guardian. 2025. Available online: https://www.theguardian.com/environment/2025/feb/06/uk-pets-at-home-world-first-lab-grown-meat-dog-treats (accessed on 21 July 2025).
- Carrington, D. Lab-Grown Meat Could Be Sold in UK in Next Few Years, Says Food Regulator. The Guardian. 2024. Available online: https://www.theguardian.com/environment/2024/oct/08/cell-cultivated-lab-grown-meat-sold-uk-regulator (accessed on 21 July 2025).
- Daly, P. UK Food Regulator Looks into Legalising Lab-Grown Meat. Experts Explain How It Works and Why Now. Northeastern Global News. 2025. Available online: https://news.northeastern.edu/2025/03/14/lab-grown-meat-uk/ (accessed on 21 July 2025).
- Cooper, P. Nearly Half of Gen Z Say They Would Eat Lab-Grown Meat Products, Much Higher Than Older Generations. Ipsos. 2025. Available online: https://www.ipsos.com/en-uk/nearly-half-gen-z-say-they-would-eat-lab-grown-meat-products-much-higher-older-generations (accessed on 21 July 2025).
- Dutch News. Lab-Grown Meat Firms Hope for the Taste of Success. Dutch News. 2024. Available online: https://www.dutchnews.nl/2024/04/lab-grown-meat-firms-hope-for-the-taste-of-success/ (accessed on 21 July 2025).
- Gaita, L. Cultured Meat, Everything Is Blocked in Italy. Accusations at the Technical Committee: “Only People Appointed by Coldiretti”. Il Fatto Quotidiano. 2025. Available online: https://www.ilfattoquotidiano.it/2025/03/25/carne-coltivata-legge-bloccata-agricoltori-tavolo-tecnico-coldiretti-proteste/7923029/ (accessed on 21 July 2025).
- Arosio, P. Cultured Meat, the Great Puzzle: Pros and Cons. La Repubblica. 2025. Available online: https://www.repubblica.it/green-and-blue/dossier/festival-greenandblue-2025/2025/06/02/news/carne_coltivata-424643779/ (accessed on 21 July 2025).
- Pyka, A. Poland’s First Lab-Grown Meat Firm Gets State Grant. Notes from Poland. 2024. Available online: https://notesfrompoland.com/2024/08/07/polands-first-lab-grown-meat-firm-gets-state-grant/ (accessed on 21 July 2025).
- DalyN Lab-Grown Meat Has Just Been Approved for Consumption in Australia. What Is It and How Is It Made? ABC. 2025. Available online: https://www.abc.net.au/news/2025-06-24/lab-grown-meat-approved-for-sale-australia/105448604 (accessed on 21 July 2025).
- The Australian. Meat the Hi-Tech, Cost-Friendly New Food Culture. The Australian. 2025. Available online: https://www.foodformzansi.co.za/food-that-never-mooed-the-cultivated-meat-revolution/ (accessed on 21 July 2025).
- Christa Grobler. Food that never mooed – the cultivated meat revolution. Available online: https://www.foodformzansi.co.za/food-that-never-mooed-the-cultivated-meat-revolution/ (accessed on 21 July 2025).
- In 24 Months You Will Be Able to Buy Lab-Grown Meat. Mail Guardian. 2023. Available online: https://mg.co.za/business/2023-02-20-in-24-months-you-will-be-able-to-buy-lab-grown-meat/?utm_source=chatgpt.com (accessed on 21 July 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).