Abstract
Exercise and physical training induce diverse physiological responses that can be modulated by functional foods. This article examines how different exercise models—from moderate exercise to intense training—can be applied to evaluate food functionality in preventing inflammation, oxidative stress, and enhancing athletic performance. We discuss the paradoxical nature of exercise, where moderate physical activity promotes health through anti-inflammatory and antioxidant effects, while intense exercise can induce muscle damage, inflammation, and immunosuppression. Through analysis of recent research, including studies on polyphenols, amino acid derivatives, and novel delivery systems, we highlight the importance of appropriate exercise model selection, timing, and dosage of nutritional interventions. Emerging approaches such as nano-processed compounds, gut microbiota modulation, and synergistic combinations offer promising strategies. This review provides guidance for researchers and practitioners in selecting suitable exercise models to evaluate functional foods, emphasizing the need for personalized approaches that balance performance enhancement with health protection.
1. Introduction
Moderate exercise is useful for improving health and preventing disease, but intense exercise and training can cause muscle pain, fatigue, and other health problems that can deteriorate conditions and lead to poor performance [,,]. In addition, the range of exercise is wide, from moderate exercise at the level of exercise prescription to intense exercise such as running a marathon, as well as various types of training such as those performed by athletes. The use of foods in each situation is also wide-ranging. The type, dosage, and timing of food intake need to be considered according to the characteristics of the athletic events, such as sprint, endurance, and power, the season of competition, and the conditions before and after training and/or competition. To combine these various conditions and evaluate biological effects, various exercise models have been used in research, including walking or cycling [,,] as a moderate exercise and voluntary running exercise [,,] in animal experiments. Intense exercise, such as the maximal exercise stress test [,,,], long-distance running [,,,], and, in animal experiments, exhaustive exercise [,,,], is frequently used. Since there are various types of exercise and training, it is necessary to use different foods for different purposes, and to be careful to consume the appropriate amount of food that expresses functionality and does not constitute doping. In this paper, we introduce specific exercise models and biomarkers that are useful for evaluation of prevention, early recovery, and performance evaluation of muscle injury, visceral injury, inflammation, oxidative stress, fatigue, and immunodepression caused by intense exercise and training [,], along with light exercise effectiveness on anti-inflammatory and antioxidant actions [].
2. Moderate Exercise
Exercise for health promotion typically involves sessions of 20–60 min at moderate intensity, where safety considerations often take precedence over performance outcomes and the workloads are relatively low [,]. This exercise domain provides an ideal platform for evaluating the long-term health benefits of functional foods, as inflammation and oxidative stress responses are generally mild and manageable.
Recent investigations have revealed interesting temporal dynamics in the immunological effects of moderate exercise []. A study examining 30 min of treadmill walking (6 km/h) in healthy but sedentary undergraduate students found no acute immunological changes following single exercise sessions []. However, when this protocol was extended to 6 days per week for 3 weeks, significant immuno-potentiating adaptations emerged, including decreased blood levels of interleukin (IL)-12p40, a receptor antagonist of the cellular immunostimulatory cytokine IL-12 [,].
Gender-specific responses to moderate exercise interventions reveal important considerations for functional food applications. Recent evidence demonstrates that females exhibit distinct inflammatory and antioxidant responses during the menstrual cycle phases, with the follicular phase showing enhanced anti-inflammatory effects from polyphenol supplementation compared to the luteal phase []. Recent evidence shows that females exhibit distinct inflammatory and antioxidant responses during moderate exercise. A randomized controlled trial in healthy female endurance athletes reported that nitrate-rich beetroot juice supplementation significantly enhanced aerobic capacity and improved oxidative stress markers, supporting the concept that sex-specific physiological characteristics may create unique windows of responsiveness to nutritional interventions []. Furthermore, sex differences in substrate utilization during moderate exercise indicate that females may benefit more from lipid-based functional foods, while males show greater responsiveness to carbohydrate-amino acid combinations []. These findings emphasize the need for sex-stratified approach in evaluating functional food efficacy during moderate exercise protocols.
The elderly population presents unique considerations for exercise and nutritional interventions due to age-related increases in chronic inflammation and oxidative stress []. A 12-week walking intervention (100 min/week) in previously sedentary elderly subjects demonstrated remarkable improvements in antioxidant status, including increased blood glutathione peroxidase activity, elevated thioredoxin levels, and enhanced total antioxidant capacity, accompanied by reduced oxidative stress markers and neutrophil-related inflammation []. Notably, these benefits occurred despite exercise volume below current guidelines (150 min/week), suggesting heightened sensitivity to exercise-induced adaptations in inactive elderly individuals [].
Animal models provide valuable mechanistic insights into the synergistic effects of moderate exercise and functional foods. An 8-week study combining voluntary wheel running with black ginger (Kaempferia parviflora) administration in mice revealed enhanced endurance capacity and increased spontaneous running distance []. Molecular analysis showed activation of the Nrf2 (Nuclear factor erythroid 2-related factor 2)/ARE (Antioxidant Response Element) pathway in skeletal muscle, with upregulated gene expression of superoxide dismutase (SOD) 1 and 3, and components of the thioredoxin system. These changes resulted in increased antioxidant capacity in both blood and skeletal muscle []. Another study showed that combining exercise with grape polyphenol supplementation produced a synergistic effect, enhancing endurance by promoting muscle lipid oxidation and reducing glycogen utilization []. Such findings demonstrate how functional foods can amplify the beneficial effects of moderate exercise through targeted molecular mechanisms. Figure 1 summarizes the synergistic effects of moderate exercise combined with functional food intake.
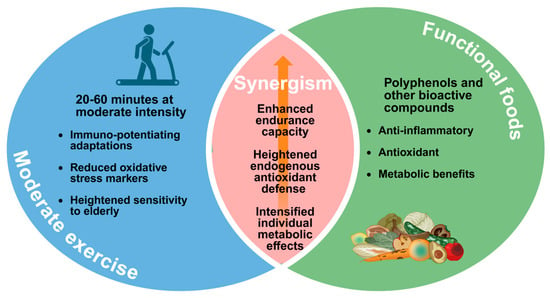
Figure 1.
Synergistic benefits of combining moderate exercise with functional foods. Moderate exercise and functional foods each confer immunological, antioxidant, and metabolic advantages; when combined, they synergistically enhance endurance capacity, elevate endogenous antioxidant defenses, and intensify beneficial metabolic adaptations. Created in BioRender. Ma, S. (2025) https://BioRender.com/hlzqo3a (accessed on 20 November 2025).
Emerging biomarkers provide unprecedented sensitivity for detecting subtle effects of functional foods during moderate exercise. Circulating cell-free DNA (cfDNA), released from cells undergoing stress or death, serves as a real-time indicator of cellular turnover even during low-intensity exercise. Recent studies demonstrate that 30 min of walking at 50–60% VO2 max increases cfDNA by 1.5-2-fold []. This suggests protective effects at the cellular level not detectable through traditional markers.
Extracellular vehicles (EVs) and exosomes represent novel communication pathways between tissues during exercise. Moderate exercise induces a distinct exosome signature characterized by increased miR-21 and miR-146a content, which mediate anti-inflammatory signaling []. Functional foods, particularly omega-3 fatty acids and curcumin, have been shown to modify exosome cargo, enhancing their anti-inflammatory potential. A proteomics analysis revealed that 8 weeks of moderate exercise combined with quercetin supplementation altered exosomal proteins involved in muscle adaptation and mitochondrial biogenesis [].
Continuous glucose monitoring (CGM) technology now enables real-time metabolic assessment during moderate exercise interventions. Recent data indicate that even non-diabetic individuals show glycemic variability during exercise that correlates with fatigue perception and recovery quality []. Functional foods that stabilize glucose excursions, such as resistant starch or chromium picolinate, demonstrate improved exercise enjoyment and adherence rates in sedentary populations initiating moderate exercise programs [].
3. Intense Exercise
Table 1 summarizes key advantages, limitations and typical biomarkers associated with common exercise models used to assess food functionality in humans and rodents.

Table 1.
Key advantages, limitations and typical biomarkers associated with common exercise models used to assess food functionality in humans and rodents.
Intense exercise, exemplified by marathons, triathlons, and exhaustive training sessions, creates substantial physiological stress characterized by muscle damage, organ dysfunction, inflammation, and oxidative stress [,,,]. This exercise domain has traditionally been the focus of antioxidant supplementation strategies, though recent research has revealed unexpected complexities in these interventions [,].
The “antioxidant paradox” represents a critical consideration in sports nutrition. High-dose antioxidant supplementation, including vitamin C, has been shown to potentially impair training adaptations and, in some cases, exacerbate exercise-induced muscle damage [,,,]. Moreover, supplementation with vitamins C and E has been reported to attenuate key skeletal muscle adaptations to training, such as SOD and mitochondrial transcription factor A []. This paradoxical effect highlights the importance of reactive oxygen species (ROS) as signaling molecules essential for exercise-induced adaptations, challenging simplistic approaches to antioxidant supplementation [,].
3.1. Polyphenols and Other Bioactive Compounds
Polyphenols offer multi-targeted effects beyond simple antioxidant activity, including anti-inflammatory and metabolic benefits. Research with taheebo polyphenol demonstrated improved endurance through enhanced muscle glycogen metabolism in exhaustive exercise mouse models [,]. Furthermore, the beneficial effects of polyphenols have also been supported by clinical studies, reinforcing their potential as promising nutritional interventions for exercise performance and recovery [,,,,]. However, the effects of polyphenols can be highly compound-specific and dose-dependent []. A cautionary example comes from studies with genistein, an isoflavonoid abundant in soybeans []. Despite its high antioxidant capacity [,,], genistein administration in mice subjected to exhaustive exercise resulted in unexpected hepatotoxicity, with increased liver oxidative stress markers and suppressed skeletal muscle antioxidant gene expression, ultimately impairing exercise performance []. Similarly, (−)-Epigallocatechin-3-gallate (EGCG), the principal bioactive compound in green tea, demonstrates dual behavior as either an antioxidant or a prooxidant depending on the dose and the biological environment. At high doses, EGCG has also been reported to induce hepatotoxicity [].
These contrasting findings emphasize the importance of careful compound selection and dose optimization. The development of highly bioavailable formulations has helped address some of these challenges. Nano-processed curcumin, achieving 185-fold increased absorption compared to standard formulations, has shown promise in preventing exercise-induced oxidative stress without interfering with adaptations []. Studies demonstrated that this enhanced curcumin formulation prevented oxidative stress during submaximal exercise (1 h run at 65% of maximal oxygen uptake (VO2 max) without causing muscle damage [], and eccentric-exercise-induced muscle damage as well []. When mice were administered after downhill running, it reduced next-day oxidative stress while suppressing inflammatory cell infiltration and ROS production in the skeletal muscle [].
Emerging research has identified novel mechanisms through which functional foods support intense exercise performance. The intestinal metabolite 3-(4-hydroxy-3-methoxyphenyl)propionic acid (HMPA), derived from polyphenol metabolism, not only increased antioxidant capacity and reduced oxidative stress but also enhanced muscle strength by promoting fast-twitch fiber formation via the insulin-like growth factor-1 (IGF-1) pathway []. Similarly, sulforaphane from broccoli sprouts has demonstrated protective effects against exhaustive exercise-induced inflammation and organ damage through Nrf2-mediated induction of antioxidant enzymes [,]. Another study reported that polyphenol extracts from Lonicera caerulea berries alleviate exercise-induced fatigue and improve performance in mice by reducing oxidative stress via the PKCα-Nox2/Nox4 pathway and enhancing mitochondrial biogenesis through the AMPK-PGC1α-NRF1-TFAM axis []. Figure 2 summarizes the evidence of polyphenols’ function in intense exercise regimens.
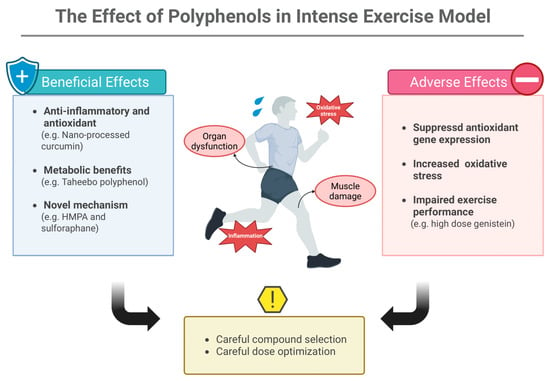
Figure 2.
The Effect of Polyphenols in Intense Exercise Model. Polyphenols exert both protective and harmful actions during intense exercise: while certain compounds support anti-inflammatory, antioxidant, and metabolic adaptations, others may suppress antioxidant gene expression, increase oxidative stress, or impair performance at high doses. Appropriate compound selection and dose optimization are essential to maximize benefits and avoid adverse effects. HMPA: 3-(4-hydroxy-3-methoxyphenyl)propionic acid. Created in BioRender. Ma, S. (2025) https://BioRender.com/vx3cxfq (accessed on 20 November 2025).
Metabolic approaches offer additional strategies for managing intense exercise stress. Ketogenic diets and medium-chain fatty acids have shown anti-inflammatory and antioxidant effects while improving endurance in animal models []. These interventions appear to work by improving energy metabolism efficiency, thereby reducing metabolic stress and associated ROS production. Both exercise and dietary compounds activate or inhibit conserved cellular signaling networks. Table 2 provides examples of compounds, their molecular targets and observed effects. Figure 3 integrates recent insights into key pathways (Nrf2–Keap1, NF-κB, AMPK, IGF-1/mTOR, SIRT1/PGC 1α) and their modulation by exercise and nutrition.

Table 2.
Examples of functional compounds, their molecular targets and observed effects.
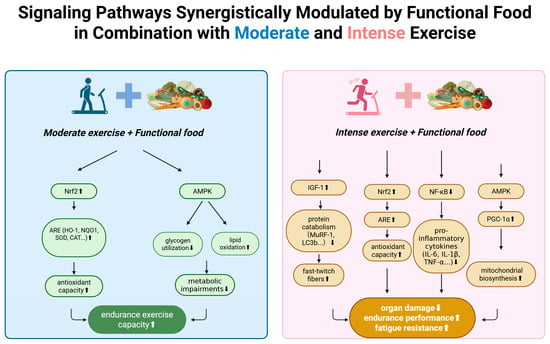
Figure 3.
Synergistic modulation of signaling pathways by functional food and exercise. Functional foods enhance key molecular responses to moderate and intense exercise, including Nrf2-ARE antioxidant signaling, AMPK-mediated metabolic regulation, IGF-1–related muscle remodeling, and NF-κB suppression, collectively supporting improved endurance and reduced exercise-induced stress. Nrf2: Nuclear factor erythroid 2–related factor 2. ARE: Antioxidant Response Element. HO-1: Heme oxygenase-1. NQO1: NAD(P)H quinone dehydrogenase 1. SOD: Superoxide dismutase. CAT: Catalase. AMPK: AMP-activated protein kinase. IGF-1: Insulin-like growth factor-1. MuRF-1: Muscle RING-finger protein-1. LC3b: Microtubule-associated protein 1 light chain 3 beta. NF-κB: Nuclear factor kappa B. IL-6: Interleukin-6. IL-1β: Interleukin-1 beta. TNF-α: Tumor necrosis factor-alpha. PGC-1α: Peroxisome proliferator-activated receptor gamma coactivator-1 alpha. ↑: up regulation. ↓: down regulation. Created in BioRender. Ma, S. (2025) https://BioRender.com/7i6b8pw (accessed on 20 November 2025).
3.2. Timing Strategies for Intense Exercise
The temporal dynamics of nutrient availability critically influence functional food efficacy during intense exercise. Recent pharmacokinetic studies using isotope labeling reveal distinct absorption windows that optimize performance benefits. Pre-exercise loading (2–4 h) allows for complete gastric emptying and peak plasma concentrations coinciding with exercise onset []. Nitrate supplementation shows optimal effects at 2–3 h pre-exercise, achieving peak plasma nitrite levels []. Polyphenols require longer lead times at muscle, with quercetin showing maximal at 1–3 h post-ingestion in circulation [].
During-exercise supplementation feasibility depends on exercise intensity and gastrointestinal tolerance. Above 75% VO2 max, splanchnic blood flow reduces by 60–80%, severely limiting absorption []. However, novel delivery systems including hydrogels and nanoencapsulation maintain nutrient release at lower intensities. Branched-chain amino acids (leucine, isoleucine, valine) consumed during exercise below lactate threshold reduces central fatigue without gastrointestinal distress [].
The post-exercise window represents a critical period for recovery optimization. The traditional “anabolic window” concept has evolved to recognize multiple phases: immediate (0–30 min) for glycogen replenishment, early (30 min–4 h) for protein synthesis, and extended (4–24 h) for inflammation resolution [].
3.3. Environmental Stressors and Intense Exercise Models
The combination of environmental stressors with intense exercise creates unique models for evaluating functional food efficacy under extreme conditions. Heat stress (35–40 °C, 50–60% humidity) combined with exhaustive exercise amplifies oxidative stress by 3–4-fold compared to thermoneutral conditions []. Recent investigations demonstrate that pre-loading with specific polyphenols, particularly methylated catechins from green tea, enhances heat shock protein expression and improves thermal tolerance. Athletes supplemented with 600-mg EGCG daily for 7 days before heat-stress exercise showed 15% lower core temperature rise and 25% improved time to exhaustion [].
Cold exposure exercise models (5–10 °C) reveal distinct nutritional requirements, with brown adipose tissue activation increasing metabolic demands []. Capsaicin and other thermogenic compounds demonstrate synergistic effects with cold-induced metabolism, enhancing fat oxidation during submaximal exercise []. Interestingly, antioxidant requirements paradoxically decrease in cold conditions due to reduced mitochondrial ROS production, suggesting environment-specific supplementation strategies [].
Simulated altitude (2500–4000 m equivalent) combined with intense exercise provides insights into hypoxic stress management. Recent studies using normobaric hypoxic chambers demonstrate that nitrate supplementation (6–8 mmol/day) partially compensates for reduced oxygen availability, improving VO2 max at altitude []. Iron-vitamin C co-supplementation shows enhanced efficacy at altitude, with greater improvements in hemoglobin mass compared to sea level training [,,]. These models are particularly relevant for athletes preparing for high-altitude competitions or training camps.
4. Training Models: Optimizing Adaptation and Recovery
Training represents a unique challenge in sports nutrition, as interventions must support both acute recovery and long-term adaptations. Different training modalities create distinct nutritional demands, requiring targeted approaches for optimal outcomes.
4.1. Resistance Training and Muscle Adaptation
Leucine and its metabolites play crucial roles in muscle protein synthesis and hypertrophy. β-Hydroxy-β-methylbutyrate (HMB), a leucine derivative, has evolved from a compound with limited efficacy due to poor absorption to a promising intervention through the development of HMB free acid (HMB-FA) [,,]. A 6-week double-blind, randomized controlled trial demonstrated that 3-g HMB-FA daily, consumed immediately after resistance training, significantly increased muscle strength compared to placebo. This timing-specific effect appears related to exercise-induced secretion of growth hormone and IGF-1, creating an optimal anabolic environment [].
Age-Specific Training Adaptations: Youth athletes (<18 years) present unique considerations due to ongoing growth and development. Peak height velocity, occurring around age 12 in females and 14 in males, creates windows of heightened nutritional demands []. During this period, protein requirements increase to 1.2–2.0 g/kg/day of protein, depending on the type of sport and training intensity []. Calcium and vitamin D become critical, with supplementation (1200 mg Ca + 1000 IU D3) reducing stress fracture risk in adolescent runners []. Dietary creatine intake among U.S. children and adolescents (2–19 years) averages 1.07 ± 1.07 g/day, with higher intake (≥1.5 g/day) associated with greater height, weight, and BMI, and each additional 0.1 g/day predicting ~0.3–0.6 cm taller stature, suggesting a potential role in growth regulation [].
Sarcopenia is driven in part by age-related anabolic resistance—characterized by blunted muscle protein synthesis due to impaired signaling, reduced amino acid delivery, and increased splanchnic retention—exacerbated by obesity and inactivity, with evidence suggesting that older adults require higher protein/leucine doses to overcome this deficit []. Endurance exercise activates PGC-1α–mediated mitochondrial biogenesis, and emerging evidence suggests that PQQ (≈20 mg/day), through redox and energy metabolism pathways, may synergize with exercise to enhance mitochondrial density and function, though human data remain scarce []. Long-term collagen peptide supplementation (10–20 g/day, 6–9 months) improves activities of daily living, pain, and mental health scores in middle-aged active adults, with additional physical health benefits observed in females [].
Plyometric training, characterized by explosive movements that enhance power and athletic performance [], induces substantial muscle damage and oxidative stress. HMB-FA supplementation has proven effective in mitigating these negative effects while maintaining performance benefits []. Additionally, capsaicin (12 mg) has emerged as an interesting intervention for plyometric training, preventing delayed-onset muscle soreness while enhancing jump performance through transient receptor potential vanilloid 1(TRPV1) channel-mediated anti-inflammatory effects and enhanced energy production [].
Gut–Muscle–Brain Axis in Training: The bidirectional communication between gut, muscle, and brain profoundly influences training adaptations and performance []. Gut-derived metabolites, particularly short-chain fatty acids (SCFAs), cross the blood–brain barrier and modulate central fatigue mechanisms []. Athletes exhibit greater gut microbial diversity and higher fecal SCFAs (butyrate, propionate), with taxa such as Akkermansia and Veillonella enriched, supporting enhanced energy metabolism, recovery, and immune regulation compared to sedentary individuals [].
Mental fatigue, induced by prolonged cognitive tasks, reduces subsequent physical performance by 15–25% through altered perception of effort. The gut–brain–muscle axis links microbiota, neurotransmitters, and metabolism, with optimal gut health enhancing nutrient absorption, energy availability, immune function, and mental resilience to support peak athletic performance []. In elite wrestlers, combining L-theanine (≈3 mg/kg) with caffeine (≈3 mg/kg) enhanced strength, endurance, and Stroop test performance while lowering anxiety and side effects compared to caffeine alone, suggesting this blend more reliably preserves both cognitive and physical performance []. Recent fMRI studies reveal this combination maintains prefrontal cortex activation during combined mental-physical challenges []. A study in collegiate female swimmers found that 6-week supplementation with Bifidobacterium longum 35,624 (1 billion CFU/day) improved sport recovery scores and cognitive outlook without altering performance or immune markers []. The gut–brain axis also influences exercise-induced neuroplasticity, with certain probiotics enhancing BDNF production and potentially accelerating motor learning [].
4.2. Endurance Training and Gut–Immune Interactions
Intense endurance training can compromise intestinal barrier function, leading to increased permeability “leaky gut”, systemic inflammation, and performance decrements []. Novel interventions targeting this gut–immune axis have shown promising results. Hyperimmune milk-derived immune proteins, obtained from cows vaccinated against specific pathogens, demonstrated protective effects in young runners []. Following 8 weeks of supplementation, runners maintained renal urinary concentrating ability after 3000 m time trials, indicating better hydration status. More importantly, exercise-induced elevations in intestinal injury markers (I-FABP), IL-1β, and TNF-α were significantly attenuated, suggesting preservation of intestinal barrier function []. Emerging evidence suggests that these effects may be mediated through beneficial modulation of the gut microbiota.
Direct manipulation of the gut microbiome through probiotic and prebiotic combinations has yielded sport-specific benefits. Sport-specific interventions have shown efficacy in other disciplines: carob polyphenols in taekwondo athletes increased antioxidant enzyme activities (SOD and catalase), reduced lipid peroxidation, and improved kick frequency after 6 weeks of supplementation []. However, not all interventions translate to performance benefits—beetroot juice supplementation in volleyball players prevented muscle soreness but failed to enhance jumping ability [], highlighting the importance of selecting appropriate outcome measures.
5. Future Perspectives: Toward Personalized and Synergistic Approaches
The field of exercise nutrition is evolving toward more sophisticated, personalized approaches. Several key trends are shaping future directions:
5.1. Technological Advances in Bioavailability
Beyond traditional biomarkers, multi-omics integration provides systems-level understanding of exercise–nutrition interactions. Metabolomics profiling identifies over 500 exercise-responsive metabolites, with functional foods modulating specific pathways []. Real-time mass spectrometry, now portable for field testing, enables immediate metabolic phenotyping. Recent developments in sweat analysis using wearable sensors continuously monitor electrolytes, lactate, glucose, and cortisol, allowing dynamic supplementation adjustments []. Artificial intelligence algorithms analyzing these multi-dimensional datasets predict individual responses with 83% accuracy, enabling truly personalized nutrition prescriptions []. Digital twins—computational models of individual physiology—simulate supplement responses before implementation, reducing trial-and-error approaches [].
5.2. Microbiome-Targeted Interventions
Recognition of the gut microbiota’s role in exercise adaptation, immune function, and metabolism is driving development of next-generation probiotics, prebiotics, and symbiotic specifically designed for athletic populations. Recent metagenomic studies reveal that elite athletes harbor distinct microbial signatures, with increased abundance of Veillonella atypical, which metabolizes lactate to propionate, potentially enhancing endurance []. This discovery has led to development of performance-specific probiotic formulations targeting metabolic pathways unique to exercise.
Strain-specific effects are now well-documented, moving beyond generic probiotic recommendations. Lactobacillus plantarum PS128 specifically increases dopamine and serotonin production, improving exercise motivation and reducing perceived exertion []. Bifidobacterium breve BR03 combined with Streptococcus thermophilus FP4 reduces exercise-induced IL-6 elevation by 43% [].
Prebiotic strategies have evolved beyond simple fiber supplementation to targeted microbiome modulation. Microbial fermentation metabolites such as SCFAs exert wide-ranging effects on gut and systemic physiology, whereas protein-derived metabolites occur at non-toxic levels; however, current evidence is insufficient to use fecal metabolite concentrations as reliable markers of prebiotic efficacy, highlighting the need for integrated metabolomics–metagenomics approaches and flux-based analyses []. Polyphenol-rich prebiotics, particularly pomegranate ellagitannins and green tea catechins, create a selective advantage for Akkermansia muciniphila, associated with improved metabolic efficiency and better running economy [].
Postbiotic applications represent the newest frontier, offering standardized bioactivity without viability concerns. Heat-killed Lactobacillus paracasei MCC1849 maintains anti-inflammatory effects while eliminating refrigeration requirements, crucial for traveling athletes []. Extracellular vesicles from certain probiotics can deliver bioactive compounds to intestinal cells and enhance gut barrier integrity more effectively than whole probiotic cells. For example, vesicles from Limosilactobacillus reuteri reduced pathogen-induced gut leakage by up to 65% in vitro, demonstrating a potent barrier-protective effect []. Taking SCFAs directly (such as tributyrin at 3–6 g/day) can bypass the need for microbial fermentation and provide performance benefits. SCFAs are readily absorbed and used in muscle; studies show they enhance lipid oxidation and spare glycogen during exercise [].
Filtered, cell-free supernatants containing probiotic metabolites (postbiotics like bacteriocins) can confer immune benefits without needing live colonization. In athletes, probiotic supplementation is linked to reduced upper respiratory tract infection incidence by ~27–50% []. For example, over a winter training period only 35% of athletes on probiotics reported any cold symptoms vs. 79% on placebo []. This immediate immune protection suggests that even non-living probiotic products could similarly lower infection risk (around one-third reduction) in high-stress periods for athletes.
Exercise training itself modulates gut microbial composition. Regular vigorous exercise (e.g., 6 weeks of interval training) is associated with increased gut microbe diversity []. Athletes show a significantly wider range of gut microbiota than non-athletes. One study found elite athletes had much higher microbial diversity than BMI-matched controls []. Prolonged extreme exercise can temporarily reduce gut diversity and beneficial bacteria. For instance, after a 217 km ultramarathon, an obese runner’s gut α-diversity dropped and symbiotic microbes decreased, while harmful bacteria spiked []. This acute dysbiosis post-ultra supports reports of 20–30% diversity reductions transiently after ultra-endurance events. Targeted restoration (through diet or probiotics) is often needed to rebound the microbiome to pre-race status []. Emerging research uses machine learning to analyze athletes’ microbiomes for performance insights. Early studies in animal models show gut profiles can predict endurance capacity. In humans, high-throughput “-omics” data are being used to predict traits like substrate utilization or fatigue []. In short, the exercise–microbiome relationship is bidirectional: training alters the microbiome, and analyzing the microbiome can potentially guide individualized training and nutrition in the near future.
5.3. Combination Strategies—Interactions of Functional Foods and Drugs
Large doses of Vitamins C (>1 g) and E (>400 IU) can impair training gains by blunting exercise-induced oxidative signaling. Research shows antioxidant-supplemented athletes had significantly smaller increases in mitochondrial proteins (e.g., COX4 up +59% in placebo vs. −13% with vitamins) and PGC-1α (no rise with vitamins) after training []. In short, excessive antioxidants reduced markers of mitochondrial biogenesis by ~25–30%, corroborating the caution that high-dose C/E dampen endurance training benefits [].
Iron supplements can inhibit zinc absorption when co-administered, due to competitive uptake. Clinical studies show high iron intakes negatively affect zinc absorption in adults []. To avoid this, athletes are advised to separate iron and zinc dosing to prevent one micronutrient impairing the other’s uptake.
Calcium (from dairy or supplements) interferes with non-heme iron absorption []. Even a single glass of milk or calcium tablet can significantly reduce iron uptake []. Caffeine alone can boost performance but mixing it with ephedrine (a stimulant once in some weight-loss supplements) amplifies cardiovascular strain. This combo has been associated with elevated heart rate, blood pressure, and even arrhythmia or stroke in case reports []. Due to such risks, ephedrine-containing supplements are banned in sports. Athletes are warned that caffeine’s effects on the heart and blood pressure are markedly potentiated by ephedrine.
5.4. Precision Nutrition
Gilbert’s syndrome, present in ~5–10% of Caucasians, is a mild genetic bilirubin metabolism disorder (low UGT1A1 enzyme) that slows glucuronidation in the liver. Athletes with Gilbert’s have reduced capacity to conjugate compounds—meaning they may clear some supplements more slowly []. They tend to be more sensitive to substances requiring glucuronidation (e.g., curcumin, quercetin). For instance, large doses of curcumin or other polyphenols might lead to higher plasma levels in Gilbert’s patients because the usual glucuronidation pathway is only ~30% as active []. Such individuals may benefit from lower doses or spacing out doses of glucuronidated supplements, and focusing on liver-supportive foods (cruciferous veggies, fermented foods, etc.).
A common polymorphism in the MTHFR gene (C677T) reduces the conversion of folic acid to active folate []. About 40% of people are heterozygous for an MTHFR variant that can lead to elevated homocysteine and lower B-vitamin levels []. Those with MTHFR mutations often do better with methylated B-vitamins (e.g., L-5-methylfolate, methylcobalamin) instead of standard folic acid or B12. Indeed, studies show supplementing with 5-MTHF (methyl-folate) improves folate status more effectively than folic acid in MTHFR mutation carriers. Athletes with known MTHFR issues use activated forms of B9/B12, ensure adequate B6 and riboflavin (which support the pathway), and monitor homocysteine—this can optimize their energy and recovery, given B-vitamin roles in methylation and endurance performance.
Ultimately, individual responses vary based on genetics, health status, and environment. Personal tolerance must be established gradually. Sport nutritionists emphasize slow dose escalation and close monitoring when introducing any potent functional food or supplement. For example, with caffeine or beta-alanine or bicarbonate, some athletes respond strongly or get GI upset, while others do not []. Starting with a low dose in training and tracking performance and side effects is crucial to find one’s safe and effective range. Additionally, new wearable biosensors (glucose monitors, etc.) can help tailor nutrition in real-time (e.g., adjusting fuel intake to one’s blood sugar trends) [,]. The overarching principle is personalization: what works for one athlete might need modification for another. Gradual self-experimentation under professional guidance ensures safety while optimizing efficacy.
6. Conclusions
The selection of appropriate exercise models is crucial for evaluating food functionality. Moderate exercise reveals long-term anti-inflammatory and antioxidant benefits of functional foods, though effects may require weeks to manifest. Intense exercise models expose the complexity of nutritional interventions, exemplified by the antioxidant paradox, while highlighting successful strategies like nano-processed compounds and metabolic modulators. Training models demonstrate the importance of matching interventions to specific athletic demands, with emerging evidence supporting gut–immune axis modulation as a promising approach.
Future translational research should prioritize three key areas: (1) development of point-of-care biomarker testing enabling real-time personalization of exercise–nutrition interventions based on individual responses; (2) clinical validation of microbiome-targeted approaches, particularly sport-specific probiotic formulations and their integration into training periodization; (3) implementation of digital health technologies combining wearable sensors with AI algorithms to optimize supplement timing and dosing.
For clinical applications, immediate opportunities exist in sports medicine clinics for nano-formulated supplements with enhanced bioavailability, in geriatric care for combined exercise–polyphenol interventions targeting sarcopenia, and in post-COVID-19 rehabilitation programs integrating graduated exercise with targeted nutritional support. The convergence of precision medicine with exercise science promises to transform functional foods from general supplements into precisely prescribed interventions, ultimately achieving optimal balance between performance enhancement and health protection through evidence-based, personalized nutritional strategies.
Author Contributions
Writing—original draft preparation, K.S. and S.M.; writing—review and editing, K.S., C.W. and S.M.; All authors have read and agreed to the published version of the manuscript.
Funding
S.M. received a grant-aid of department of science and technology of Jilin province, No. 20210402019GH.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
We thank for the cooperation of the related researchers and laboratory staff members of Waseda University.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Suzuki, K. Recent progress in applicability of exercise immunology and inflammation research to sports nutrition. Nutrients 2021, 13, 4299. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K. Chronic inflammation as an immunological abnormality and effectiveness of exercise. Biomolecules 2019, 9, 223. [Google Scholar] [CrossRef]
- La Gerche, A.; Rakhit, D.J.; Claessen, G. Exercise and the right ventricle: A potential Achilles’ heel. Cardiovasc Res. 2017, 113, 1499–1508. [Google Scholar] [CrossRef]
- Terada, O.; Suzuki, K.; Kurihara, Y.; Moriguchi, S. Effects of low-intensity brief exercise and training on cell-mediated immunity. Jpn. J. Complement. Altern. Med. 2007, 4, 71–77. [Google Scholar] [CrossRef]
- Matsuda, T.; Ogata, H.; Kanno, M.; Ishikawa, A.; Yamada, M.; Sakamaki-Sunaga, M. Effects of the menstrual cycle on oxidative stress and antioxidant response to high-intensity intermittent exercise until exhaustion in healthy women. J. Sports Med. Phys. Fit. 2020, 60, 1335–1341. [Google Scholar] [CrossRef]
- Neteca, J.; Veseta, U.; Liepina, I.; Volgemute, K.; Dzintare, M.; Babarykin, D. Effect of Beetroot Juice Supplementation on Aerobic Capacity in Female Athletes: A Randomized Controlled Study. Nutrients 2024, 17, 63. [Google Scholar] [CrossRef]
- Henderson, G.C. Sexual dimorphism in the effects of exercise on metabolism of lipids to support resting metabolism. Front. Endocrinol. 2014, 5, 162. [Google Scholar]
- Takahashi, M.; Miyashita, M.; Kawanishi, N.; Park, J.H.; Hayashida, H.; Kim, H.S.; Nakamura, Y.; Sakamoto, S.; Suzuki, K. Low-volume exercise training attenuates oxidative stress and neutrophils activation in older adults. Eur. J. Appl. Physiol. 2013, 113, 1117–1126. [Google Scholar] [CrossRef]
- Huang, J.; Tong, Y.; Wang, S.; Tagawa, T.; Seki, Y.; Ma, S.; Zhang, Z.; Cao, T.; Kobori, H.; Suzuki, K. 8-week Kaempferia parviflora extract administration improves submaximal exercise capacity in mice by enhancing skeletal muscle antioxidant gene expression and plasma antioxidant capacity. Antioxidants 2024, 13, 1147. [Google Scholar] [CrossRef] [PubMed]
- Lambert, K.; Hokayem, M.; Thomas, C.; Fabre, O.; Cassan, C.; Bourret, A.; Bernex, F.; Feuillet-Coudray, C.; Notarnicola, C.; Mercier, J.; et al. Combination of nutritional polyphenols supplementation with exercise training counteracts insulin resistance and improves endurance in high-fat diet-induced obese rats. Sci. Rep. 2018, 8, 2885. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, A.; Neuberger, E.; Esch-Heisser, L.; Haller, N.; Jorgensen, M.M.; Baek, R.; Möbius, W.; Simon, P.; Krämer-Albers, E.-M. Platelets, endothelial cells and leukocytes contribute to the exercise-triggered release of extracellular vesicles into the circulation. J. Extracell. Vesicles 2019, 8, 1615820. [Google Scholar] [CrossRef]
- D’Souza, R.F.; Woodhead, J.S.; Zeng, N.; Blenkiron, C.; Merry, T.L.; Cameron-Smith, D.; Mitchell, C.J. Circulatory exosomal miRNA following intense exercise is unrelated to muscle and plasma miRNA abundances. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E723–E733. [Google Scholar] [CrossRef]
- Rojano-Ortega, D.; Peña-Amaro, J.; Berral-Aguilar, A.; Berral-de la Rosa, F. Quercetin supplementation promotes recovery after exercise-induced muscle damage: A systematic review and meta-analysis of randomized controlled trials. Biol. Sport 2023, 40, 813–825. [Google Scholar] [CrossRef]
- Solomon, T.P. Sources of inter-individual variability in the therapeutic response of blood glucose control to exercise in type 2 diabetes: Going beyond exercise dose. Front. Physiol. 2018, 9, 896. [Google Scholar] [CrossRef] [PubMed]
- Venkatakrishnan, K.; Wang, C.K. Functional Foods: Role in Endurance Sports. In Extreme and Rare Sports: Performance Demands, Drivers, Functional Foods, and Nutrition; CRC Press: Boca Raton, FL, USA, 2019; pp. 73–113. [Google Scholar]
- Sugama, K.; Suzuki, K.; Yoshitani, K.; Shiraishi, K.; Miura, S.; Yoshioka, H.; Mori, Y.; Kometani, T. Changes of thioredoxin, oxidative stress markers, inflammation and muscle/renal damage following intensive endurance exercise. Exerc. Immunol. Rev. 2015, 21, 130–142. [Google Scholar]
- Tiller, N.B.; Millet, G.Y. Decoding ultramarathon: Muscle damage as the main impediment to performance. Sports Med. 2025, 55, 535–543. [Google Scholar] [CrossRef]
- Niemelä, M.; Kangastupa, P.; Niemelä, O.; Bloigu, R.; Juvonen, T. Acute changes in inflammatory biomarker levels in recreational runners participating in a marathon or half-marathon. Sports Med. Open 2016, 2, 21. [Google Scholar] [CrossRef]
- Flockhart, M.; Nilsson, L.C.; Tais, S.; Ekblom, B.; Apró, W.; Larsen, F.J. Excessive exercise training causes mitochondrial functional impairment and decreases glucose tolerance in healthy volunteers. Cell Metab. 2021, 33, 957–970.e6. [Google Scholar] [CrossRef]
- Larsen, S. Acute antioxidant supplementation and performance—Should this be considered. Free Radic. Biol. Med. 2024, 224, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Peake, J.M.; Suzuki, K.; Coombes, J.S. The influence of antioxidant supplementation on markers of inflammation and the relationship to oxidative stress after exercise. J. Nutr. Biochem. 2007, 18, 357–371. [Google Scholar] [CrossRef]
- Morrison, D.; Hughes, J.; Della Gatta, P.A.; Mason, S.; Lamon, S.; Russell, A.P.; Wadley, G.D. Vitamin C and E supplementation prevents some of the cellular adaptations to endurance-training in humans. Free Radic. Biol. Med. 2015, 89, 852–862. [Google Scholar] [CrossRef]
- Merry, T.L.; Ristow, M. Do antioxidant supplements interfere with skeletal muscle adaptation to exercise training? J. Physiol. 2016, 594, 5135–5147. [Google Scholar] [CrossRef]
- Suzuki, K.; Hayashida, H. Effect of Exercise Intensity on Cell-Mediated Immunity. Sports 2021, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Yada, K.; Roberts, L.A.; Oginome, N.; Suzuki, K. Effect of acacia polyphenol supplementation on exercise-induced oxidative stress in mice liver and skeletal muscle. Antioxidants 2020, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Suzuki, K. The effects of flavonoids on skeletal muscle mass, muscle function, and physical performance in individuals with sarcopenia: A systematic review of randomized controlled trials. Nutrients 2023, 15, 3897. [Google Scholar] [CrossRef]
- Rahman Mazumder, M.A.; Hongsprabhas, P. Genistein as antioxidant and antibrowning agents in in vivo and in vitro: A review. Biomed. Pharmacother. 2016, 82, 379–392. [Google Scholar] [CrossRef]
- Zhang, X.; Zhong, Y.; Rajabi, S. Polyphenols and post-exercise muscle damage: A comprehensive review of literature. Eur. J. Med. Res. 2025, 30, 260. [Google Scholar] [CrossRef]
- Yada, K.; Suzuki, K.; Oginome, N.; Ma, S.; Fukuda, Y.; Iida, A.; Radak, Z. Single dose administration of taheebo polyphenol enhances endurance capacity in mice. Sci. Rep. 2018, 8, 14625. [Google Scholar] [CrossRef]
- Roberts, J.D.; Lillis, J.B.; Pinto, J.M.; Chichger, H.; López-Samanes, Á.; Coso, J.D.; Zacca, R.; Willmott, A.G.B. The effect of a hydroxytyrosol-rich, olive-derived phytocomplex on aerobic exercise and acute recovery. Nutrients 2023, 15, 421. [Google Scholar] [CrossRef]
- Howatson, G.; Snaith, G.C.; Kimble, R.; Cowper, G.; Keane, K.M. Improved endurance running performance following Haskap berry (Lonicera caerulea L.) ingestion. Nutrients 2022, 14, 780. [Google Scholar] [CrossRef]
- Gelabert-Rebato, M.; Wiebe, J.C.; Martin-Rincon, M.; Galvan-Alvarez, V.; Curtelin, D.; Perez-Valera, M.; Habib, J.J.; Pérez-López, A.; Vega, T.; Morales-Alamo, D.; et al. Enhancement of exercise performance by 48 hours, and 15-day supplementation with mangiferin and luteolin in men. Nutrients 2019, 11, 344. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Noguera, F.J.; Marín-Pagán, C.; Carlos-Vivas, J.; Alcaraz, P.E. 8-week supplementation of 2S-hesperidin modulates antioxidant and inflammatory status after exercise until exhaustion in amateur cyclists. Antioxidants 2021, 10, 432. [Google Scholar] [CrossRef]
- Deley, G.; Guillemet, D.; Allaert, F.A.; Babault, N. An acute dose of specific grape and apple polyphenols improves endurance performance: A randomized, crossover, double-blind versus placebo controlled study. Nutrients 2017, 9, 917. [Google Scholar] [CrossRef]
- Wu, C.; Zhou, S.; Ma, S.; Suzuki, K. Effect of genistein supplementation on exercise-induced inflammation and oxidative stress in mice liver and skeletal muscle. Medicina 2021, 57, 1028. [Google Scholar] [CrossRef] [PubMed]
- Shete, V.; Mahajan, N.M.; Shivhare, R.; Akkewar, A.; Gupta, A.; Gurav, S. Genistein: A promising phytoconstituent with reference to its bioactivities. Phytother. Res. 2024, 38, 3935–3953. [Google Scholar] [CrossRef]
- Yang, L.; Jia, L.; Li, X.; Zhang, K.; Wang, X.; He, Y.; Hao, M.; Rayman, M.P.; Zhang, J. Prooxidant activity-based guideline for a beneficial combination of (−)-epigallocatechin-3-gallate and chlorogenic acid. Food Chem. 2022, 386, 132812. [Google Scholar] [CrossRef] [PubMed]
- Ashtary-Larky, D.; Rezaei Kelishadi, M.; Bagheri, R.; Moosavian, S.P.; Wong, A.; Davoodi, S.H.; Khalili, P.; Dutheil, F.; Suzuki, K.; Asbaghi, O. The effects of nano-curcumin supplementation on risk factors for cardiovascular disease: A GRADE-assessed systematic review and meta-analysis of clinical trials. Antioxidants 2021, 10, 1015. [Google Scholar] [CrossRef]
- Tanabe, Y.; Fujii, N.; Suzuki, K. Dietary supplementation for attenuating exercise-induced muscle damage and delayed-onset muscle soreness in humans. Nutrients 2022, 14, 70. [Google Scholar] [CrossRef]
- Takahashi, M.; Suzuki, K.; Kim, H.K.; Otsuka, Y.; Imaizumi, A.; Miyashita, M.; Sakamoto, S. Effects of curcumin supplementation on exercise-induced oxidative stress in humans. Int. J. Sports Med. 2014, 35, 469–475. [Google Scholar] [CrossRef]
- Kawanishi, N.; Kato, K.; Takahashi, M.; Mizokami, T.; Otsuka, Y.; Imaizumi, A.; Shiva, D.; Yano, H.; Suzuki, K. Curcumin attenuates oxidative stress following downhill running-induced muscle damage. Biochem. Biophys. Res. Commun. 2013, 441, 573–578. [Google Scholar] [CrossRef]
- Tong, Y.; Ma, S.; Awa, R.; Tagawa, T.; Seki, Y.; Cao, T.; Kobori, H.; Suzuki, K. Effects of 3-(4-hydroxy-3-methoxyphenyl)propionic acid on regulating oxidative stress and muscle fiber composition. Nutrients 2025, 17, 668. [Google Scholar] [CrossRef]
- Ruhee, R.T.; Roberts, L.A.; Ma, S.; Suzuki, K. Organosulfur compounds: A review of their anti-inflammatory effects in human health. Front. Nutr. 2020, 7, 64. [Google Scholar] [CrossRef]
- Ruhee, R.T.; Ma, S.; Suzuki, K. Effects of sulforaphane treatment on skeletal muscle from exhaustive exercise-induced inflammation and oxidative stress through the Nrf2/HO-1 signaling pathway. Antioxidants 2025, 14, 210. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Meng, F.; Zhang, D.; Shi, D.; Zhou, J.; Guo, S.; Chang, X. Lonicera caerulea berry polyphenols extract alleviates exercise fatigue in mice by reducing oxidative stress, inflammation, skeletal muscle cell apoptosis, and by increasing cell proliferation. Front. Nutr. 2022, 9, 853225. [Google Scholar] [CrossRef] [PubMed]
- Fukazawa, A.; Koike, A.; Karasawa, T.; Tsutsui, M.; Kondo, S.; Terada, S. Effects of a ketogenic diet containing medium-chain triglycerides and endurance training on metabolic enzyme adaptations in rat skeletal muscle. Nutrients 2020, 12, 1269. [Google Scholar] [CrossRef]
- Wiciński, M.; Leis, K.; Szyperski, P.; Węclewicz, M.M.; Mazur, E.; Pawlak-Osińska, K. Impact of resveratrol on exercise performance: A review. Sci. Sports 2018, 33, 207–212. [Google Scholar] [CrossRef]
- Butler, C.A. The Effects of Acute Omega-3 Fatty Acid Supplementation on Delayed-Onset Muscle Soreness and Recovery. Master’s Thesis, Georgia Southern University, Statesboro, GA, USA, 2019. [Google Scholar]
- Lansley, K.E.; Winyard, P.G.; Bailey, S.J.; Vanhatalo, A.; Wilkerson, D.P.; Blackwell, J.R.; Gilchrist, M.; Benjamin, N.; Jones, A.M. Acute dietary nitrate supplementation improves cycling time trial performance. Med. Sci. Sports Exerc. 2011, 43, 1125–1131. [Google Scholar] [CrossRef]
- Boorsma, R.K.; Whitfield, J.; Spriet, L.L. Beetroot juice supplementation does not improve performance of elite 1500-m runners. Med. Sci. Sports Exerc. 2014, 46, 2326–2334. [Google Scholar] [CrossRef]
- Wilson, J.M.; Lowery, R.P.; Joy, J.M.; Walters, J.A.; Baier, S.M.; Fuller, J.C., Jr.; Stout, J.R.; Norton, L.E.; Sikorski, E.M.; Wilson, S.M.C.; et al. β-Hydroxy-β-methylbutyrate free acid reduces markers of exercise-induced muscle damage and improves recovery in resistance-trained men. Br. J. Nutr. 2013, 110, 538–544. [Google Scholar] [CrossRef]
- Kerksick, C.M.; Arent, S.; Schoenfeld, B.J.; Stout, J.R.; Campbell, B.; Wilborn, C.D.; Taylor, L.; Kalman, D.; Smith-Ryan, A.E.; Kreider, R.B.; et al. International society of sports nutrition position stand: Nutrient timing. J. Int. Soc. Sports Nutr. 2017, 14, 33. [Google Scholar] [CrossRef]
- Macuh, M.; Knap, B. Effects of nitrate supplementation on exercise performance in humans: A narrative review. Nutrients 2021, 13, 3183. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, J.A.; Vandusseldorp, T.A.; Uken, B.; Otis, J. Quercetin in sports and exercise: A review. Int. J. Exerc. Sci. 2023, 16, 1334. [Google Scholar] [CrossRef]
- Rangsimahariwong, W.; Kulaputana, O.; Tabtieang, T.; Sanguanrungsirikul, S.; Chuaypen, N. The effect of fluid replacement during 60 minutes of moderate-intensity running on splanchnic blood flow. Chulalongkorn Med. J. 2024, 68, 5. [Google Scholar] [CrossRef]
- Luan, C.; Wang, Y.; Li, J.; Zhou, N.; Song, G.; Ni, Z.; Xu, C.; Tang, C.; Fu, P.; Wang, X.; et al. Branched-chain amino acid supplementation enhances substrate metabolism, exercise efficiency and reduces post-exercise fatigue in active young males. Nutrients 2025, 17, 1290. [Google Scholar] [CrossRef]
- Aragon, A.A.; Schoenfeld, B.J. Nutrient timing revisited: Is there a post-exercise anabolic window? J. Int. Soc. Sports Nutr. 2013, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Périard, J.D.; Eijsvogels, T.M.; Daanen, H.A. Exercise under heat stress: Thermoregulation, hydration, performance implications, and mitigation strategies. Physiol. Rev. 2021, 101, 1873–1979. [Google Scholar] [CrossRef]
- Peel, J.S.; McNarry, M.A.; Heffernan, S.M.; Nevola, V.R.; Kilduff, L.P.; Waldron, M. The effect of dietary supplements on core temperature and sweating responses in hot environmental conditions: A meta-analysis and meta-regression. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2025, 328, R515–R555. [Google Scholar] [CrossRef]
- Martin, A.R.; Chung, S.; Koehler, K. Is exercise a match for cold exposure? Common molecular framework for adipose tissue browning. Int. J. Sports Med. 2020, 41, 427–442. [Google Scholar] [CrossRef]
- Saito, M. Capsaicin and related food ingredients reducing body fat through the activation of TRP and brown fat thermogenesis. Adv. Food Nutr. Res. 2015, 76, 1–28. [Google Scholar]
- Dreyer, A.; Dietz, K.J. Reactive oxygen species and the redox-regulatory network in cold stress acclimation. Antioxidants 2018, 7, 169. [Google Scholar] [CrossRef]
- Carriker, C. Effect of Acute Dietary Nitrate Consumption on Submaximal Oxygen Consumption and Oxidative Stress in Hypoxia. Master’s Thesis, University of New Mexico, Albuquerque, NM, USA, 2014. [Google Scholar]
- Verdon, F.; Burnand, B.; Fallab-Stubi, C.-L.; Bonard, C.; Graff, M.; Michaud, A.; Bischoff, T.; De Vevey, M.; Studer, J.-P.; Herzig, L.; et al. Iron supplementation for unexplained fatigue in non-anaemic women: Double blind randomised placebo controlled trial. BMJ 2003, 326, 1124. [Google Scholar] [CrossRef] [PubMed]
- Houston, B.L.; Hurrie, D.; Graham, J.; Perija, B.; Rimmer, E.; Rabbani, R.; Bernstein, C.N.; Turgeon, A.F.; Fergusson, D.A.; Houston, D.S.; et al. Efficacy of iron supplementation on fatigue and physical capacity in non-anaemic iron-deficient adults: Systematic review. BMJ Open 2018, 8, e019240. [Google Scholar] [CrossRef]
- Rathmacher, J.A.; Pitchford, L.M.; Stout, J.R.; Townsend, J.R.; Jäger, R.; Kreider, R.B.; Campbell, B.I.; Kerksick, C.M.; Harty, P.S.; Candow, D.G.; et al. International society of sports nutrition position stand: β-hydroxy-β-methylbutyrate (HMB). J. Int. Soc. Sports Nutr. 2025, 22, 2434734. [Google Scholar] [CrossRef] [PubMed]
- Bideshki, M.V.; Behzadi, M.; Jamali, M.; Jamilian, P.; Zarezadeh, M.; Gargari, B.P. Ergogenic Benefits of β-Hydroxy-β-Methyl Butyrate (HMB) Supplementation on Body Composition and Muscle Strength: An Umbrella Review of Meta-Analyses. J. Cachexia Sarcopenia Muscle 2025, 16, e13671. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.R.; Belozo, F.L.; Micheletti, T.O.; Conrado, M.; Stout, J.R.; Pimentel, G.D.; Gonzalez, A.M. β-Hydroxy-β-methylbutyrate free acid supplementation may improve recovery and muscle adaptations after resistance training: A systematic review. Nutr. Res. 2017, 45, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Asadi, A.; Arazi, H.; Suzuki, K. Effects of β-hydroxy-β-methylbutyrate-free acid supplementation on strength, power and hormonal adaptations following resistance training. Nutrients 2017, 9, 1316. [Google Scholar] [CrossRef]
- Retzepis, N.O.; Avloniti, A.; Kokkotis, C.; Stampoulis, T.; Balampanos, D.; Gkachtsou, A.; Aggelakis, P.; Kelaraki, D.; Protopapa, M.; Pantazis, D.; et al. The effect of peak height velocity on strength and power development of young athletes: A scoping review. J. Funct. Morphol. Kinesiol. 2025, 10, 168. [Google Scholar] [CrossRef]
- Colecchia, F.P.; Di Padova, M.; Mancini, S.; Polito, R.; Basta, A.; Grosu, V.T.; Limone, P.; Messina, G.; Monda, M.; Monda, A.; et al. Protein intake in adolescent athletes: Nutritional requirements and performance implications. J. Phys. Educ. Sport 2025, 25, 773–784. [Google Scholar]
- Wentz, L.M. Calcium Intake and the Incidence of Stress Fractures. Ph.D. Thesis, Florida State University, Tallahassee, FL, USA, 2011. [Google Scholar]
- Korovljev, D.; Stajer, V.; Ostojic, S.M. Relationship between dietary creatine and growth indicators in children and adolescents aged 2–19 years: A cross-sectional study. Nutrients 2021, 13, 1027. [Google Scholar] [CrossRef]
- Aragon, A.A.; Tipton, K.D.; Schoenfeld, B.J. Age-related muscle anabolic resistance: Inevitable or preventable? Nutr. Rev. 2023, 81, 441–454. [Google Scholar] [CrossRef] [PubMed]
- Hwang, P.; Willoughby, D.S. Mechanisms behind pyrroloquinoline quinone supplementation on skeletal muscle mitochondrial biogenesis: Possible synergistic effects with exercise. J. Am. Coll. Nutr. 2018, 37, 738–748. [Google Scholar] [CrossRef]
- Kviatkovsky, S.A.; Hickner, R.C.; Cabre, H.E.; Small, S.D.; Ormsbee, M.J. Collagen peptides supplementation improves function, pain, and physical and mental outcomes in active adults. J. Int. Soc. Sports Nutr. 2023, 20, 2243252. [Google Scholar] [CrossRef]
- Ramírez-Campillo, R.; Andrade, D.C.; Izquierdo, M. Effects of plyometric training volume and training surface on explosive strength. J. Strength Cond. Res. 2013, 27, 2714–2722. [Google Scholar] [CrossRef] [PubMed]
- Holland, B.M.; Roberts, B.M.; Krieger, J.W.; Schoenfeld, B.J. Does HMB enhance body composition in athletes? A systematic review and meta-analysis. J. Strength Cond. Res. 2022, 36, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Rashki, M.; Hemmatinafar, M.; Safari, K.; Imanian, B.; Rezaei, R.; Koushkie Jahromi, M.; Suzuki, K. Capsaicin’s role in mitigating muscle soreness and enhancing futsal players’ recovery after exercise-induced muscle damage. Nutrients 2025, 17, 813. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, B.; Surendran, S.; Khaitan, J.; Patil, M.; Shathi, M.A. The relationship between gut health and sports performance. In Evaluating the Effectiveness of Functional Ingredients in Sports Nutrition; IGI Global Scientific Publishing: Hershey, PA, USA, 2025; pp. 307–342. [Google Scholar]
- Li, Q.; Wen, X.; Wang, G. Gut microbiota and exercise-induced fatigue: Unraveling the connections. Food Sci. Anim. Prod. 2024, 2, 9240061. [Google Scholar] [CrossRef]
- Mohr, A.E.; Jäger, R.; Carpenter, K.C.; Kerksick, C.M.; Purpura, M.; Townsend, J.R.; West, N.P.; Black, K.; Gleeson, M.; Pyne, D.B.; et al. The athletic gut microbiota. J. Int. Soc. Sports Nutr. 2020, 17, 24. [Google Scholar] [CrossRef]
- Razazan, R.; Hemmatinafar, M.; Imanian, B.; Jahaniboushehri, N.; Rezaei, R.; Nazemzadegan, G. Performance-enhancing effects of caffeine and L-theanine among Iranian elite wrestlers: A focus on cognitive and specific physical performance. J. Int. Soc. Sports Nutr. 2025, 22, 2564238. [Google Scholar] [CrossRef]
- Gajewski, P.D.; Falkenstein, M. Physical activity and neurocognitive functioning in aging—A condensed updated review. Eur. Rev. Aging Phys. Act. 2016, 13, 1. [Google Scholar] [CrossRef]
- Carbuhn, A.F.; Reynolds, S.M.; Campbell, C.W.; Bradford, L.A.; Deckert, J.A.; Kreutzer, A.; Fry, A.C. Effects of probiotic (Bifidobacterium longum 35624) supplementation on exercise performance, immune modulation, and cognitive outlook in Division I female swimmers. Sports 2018, 6, 116. [Google Scholar] [CrossRef]
- Dehghani, F.; Abdollahi, S.; Shidfar, F.; Clark, C.C.; Soltani, S. Probiotics supplementation and brain-derived neurotrophic factor (BDNF): A systematic review and meta-analysis of randomized controlled trials. Nutr. Neurosci. 2023, 26, 942–952. [Google Scholar] [CrossRef]
- Ma, S.; Tominaga, T.; Kanda, K.; Sugama, K.; Omae, C.; Hashimoto, S.; Aoyama, K.; Yoshikai, Y.; Suzuki, K. Effects of an 8-week protein supplementation regimen with hyperimmunized cow milk on exercise-induced organ damage and inflammation in male runners: A randomized, placebo-controlled, cross-over study. Biomedicines 2020, 8, 51. [Google Scholar] [CrossRef]
- Gaamouri, N.; Zouhal, H.; Suzuki, K.; Hammami, M.; Ghaith, A.; El Mouhab, E.H.; Hackney, A.C.; Laher, I.; Ben Ounis, O. Effects of carob rich-polyphenols on oxidative stress markers and physical performance in taekwondo athletes. Biol. Sport 2024, 41, 277–284. [Google Scholar] [CrossRef]
- Hemmatinafar, M.; Zaremoayedi, L.; Koushkie Jahromi, M.; Alvarez-Alvarado, S.; Wong, A.; Niknam, A.; Suzuki, K.; Imanian, B.; Bagheri, R. Effect of beetroot juice supplementation on muscle soreness and performance recovery after exercise-induced muscle damage in female volleyball players. Nutrients 2023, 15, 3763. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.B.; Chow, L.S.; Stagg, D.B.; Gillingham, J.R.; Evans, M.D.; Pan, M.; Hughey, C.C.; Myers, C.L.; Han, X.; Crawford, P.A.; et al. Acute aerobic exercise reveals that FAHFAs distinguish the metabolomes of overweight and normal-weight runners. JCI Insight 2022, 7, e158037. [Google Scholar] [CrossRef]
- Yang, G.; Hong, J.; Park, S.B. Wearable device for continuous sweat lactate monitoring in sports: A narrative review. Front. Physiol. 2024, 15, 1376801. [Google Scholar] [CrossRef]
- Huang, C.; Clayton, E.A.; Matyunina, L.V.; McDonald, L.D.; Benigno, B.B.; Vannberg, F.; McDonald, J.F. Machine learning predicts individual cancer patient responses to therapeutic drugs with high accuracy. Sci. Rep. 2018, 8, 16444. [Google Scholar] [CrossRef]
- Subasi, A.; Subasi, M.E. Digital twins in healthcare and biomedicine. In Artificial Intelligence, Big Data, Blockchain and 5G for the Digital Transformation of the Healthcare Industry; Academic Press: Cambridge, MA, USA, 2024; pp. 365–401. [Google Scholar]
- Scheiman, J.; Luber, J.M.; Chavkin, T.A.; MacDonald, T.; Tung, A.; Pham, L.D.; Wibowo, M.C.; Wurth, R.C.; Punthambaker, S.; Tierney, B.T.; et al. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nat. Med. 2019, 25, 1104–1109. [Google Scholar] [CrossRef]
- Yu, C.H.; Lai, C.C.; Chen, J.H.; Chen, I.C.; Tai, H.L.; Fu, S.K. Effect of Lactobacillus plantarum PS128 on neuromuscular efficiency after a half-marathon. Front. Physiol. 2023, 14, 1254985. [Google Scholar] [CrossRef] [PubMed]
- Jäger, R.; Purpura, M.; Stone, J.D.; Turner, S.M.; Anzalone, A.J.; Eimerbrink, M.J.; Pane, M.; Amoruso, A.; Rowlands, D.S.; Oliver, J.M. Probiotic Streptococcus thermophilus FP4 and Bifidobacterium breve BR03 supplementation attenuates performance and range-of-motion decrements following muscle damaging exercise. Nutrients 2016, 8, 642. [Google Scholar] [CrossRef]
- Verbeke, K.A.; Boobis, A.R.; Chiodini, A.; Edwards, C.A.; Franck, A.; Kleerebezem, M.; Nauta, A.; Raes, J.; Van Tol, E.A.F.; Tuohy, K.M. Towards microbial fermentation metabolites as markers for health benefits of prebiotics. Nutr. Res. Rev. 2015, 28, 42–66. [Google Scholar] [CrossRef]
- Luo, C.; Wei, X.; Song, J.; Xu, X.; Huang, H.; Fan, S.; Zhang, D.; Han, L.; Lin, J. Interactions between gut microbiota and polyphenols: New insights into the treatment of fatigue. Molecules 2022, 27, 7377. [Google Scholar] [CrossRef]
- Lee, M.C.; Ho, C.S.; Hsu, Y.J.; Huang, C.C. Live and heat-killed probiotic Lactobacillus paracasei PS23 accelerated the improvement and recovery of strength and damage biomarkers after exercise-induced muscle damage. Nutrients 2022, 14, 4563. [Google Scholar] [CrossRef]
- Pang, Y.; Ermann Lundberg, L.; Mata Forsberg, M.; Ahl, D.; Bysell, H.; Pallin, A.; Sverremark-Ekström, E.; Karlsson, R.; Jonsson, H.; Roos, S. Extracellular membrane vesicles from Limosilactobacillus reuteri strengthen the intestinal epithelial integrity, modulate cytokine responses and antagonize activation of TRPV1. Front. Microbiol. 2022, 13, 1032202. [Google Scholar] [CrossRef] [PubMed]
- Sales, K.M.; Reimer, R.A. Unlocking a novel determinant of athletic performance: The role of the gut microbiota, short-chain fatty acids, and “biotics” in exercise. J. Sport Health Sci. 2023, 12, 36–44. [Google Scholar] [CrossRef]
- Batatinha, H.; Tavares-Silva, E.; Leite, G.S.; Resende, A.S.; Albuquerque, J.A.; Arslanian, C.; Fock, R.A.; Lancha, A.H.; Lira, F.S.; Krüger, K.; et al. Probiotic supplementation in marathonists and its impact on lymphocyte population and function after a marathon: A randomized placebo-controlled double-blind study. Sci. Rep. 2020, 10, 18777. [Google Scholar] [CrossRef]
- Wang, Y.; Bai, S.; Yang, T.; Guo, J.; Zhu, X.; Dong, Y. Impact of exercise-induced alterations on gut microbiota diversity and composition: Comparing effects of different training modalities. Cell Regen. 2025, 14, 28. [Google Scholar] [CrossRef]
- Clarke, S.F.; Murphy, E.F.; O’Sullivan, O.; Lucey, A.J.; Humphreys, M.; Hogan, A.; Hayes, P.; O’Reilly, M.; Jeffery, I.B.; Wood-Martin, R.; et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut 2014, 63, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Saragiotto, G.K.; de Oliveira, L.F.V.; Scharlack, N.K.; de Oliveira, M.M.; Freire, F.C.; Simabuco, F.M.; Sivieri, K.; Sartoratto, A.; Belli, T.; Antunes, A.E.C. Effects of a 217-km mountain ultramarathon on the gut microbiota of an obese runner: A case report. Physiol. Rep. 2024, 12, e70017. [Google Scholar] [CrossRef]
- O’Brien, M.T.; O’Sullivan, O.; Claesson, M.J.; Cotter, P.D. The athlete gut microbiome and its relevance to health and performance: A review. Sports Med. 2022, 52 (Suppl. S1), 119–128. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, G.; Cumming, K.T.; Holden, G.; Hallén, J.; Rønnestad, B.R.; Sveen, O.; Skaug, A.; Paur, I.; Bastani, N.E.; Østgaard, H.N.; et al. Vitamin C and E supplementation hampers cellular adaptation to endurance training in humans: A double-blind, randomised, controlled trial. J. Physiol. 2014, 592, 1887–1901. [Google Scholar] [CrossRef]
- Whittaker, P. Iron and zinc interactions in humans. Am. J. Clin. Nutr. 1998, 68, 442S–446S. [Google Scholar] [CrossRef]
- Koleini, N.; Shapiro, J.S.; Geier, J.; Ardehali, H. Ironing out mechanisms of iron homeostasis and disorders of iron deficiency. J. Clin. Investig. 2021, 131, e148201. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, Y.; Tang, N. Unraveling the intricate interplay: Iron and zinc’s regulatory mechanisms on calcium bioavailability during intestinal absorption. Food Rev. Int. 2025, 41, 345–372. [Google Scholar] [CrossRef]
- Hallberg, L.; Brune, M.; Erlandsson, M.; Sandberg, A.S.; Rossander-Hultén, L. Calcium and iron absorption. Am. J. Clin. Nutr. 1991, 53, 112–119. [Google Scholar] [CrossRef]
- Haller, C.A.; Benowitz, N.L. Enhanced stimulant and metabolic effects of combined ephedrine and caffeine. Clin. Pharmacol. Ther. 2005, 77, 312–321. [Google Scholar] [CrossRef]
- McCarty, M.F. “Iatrogenic Gilbert syndrome”—A strategy for reducing vascular and cancer risk by increasing plasma unconjugated bilirubin. Med. Hypotheses 2007, 69, 974–994. [Google Scholar] [CrossRef] [PubMed]
- Bulmer, A.C.; Blanchfield, J.T.; Toth, I.; Fassett, R.G.; Coombes, J.S. Improved resistance to serum oxidation in Gilbert’s syndrome: A mechanism for cardiovascular protection. Atherosclerosis 2008, 199, 390–396. [Google Scholar] [CrossRef]
- Ledowsky, C.J.; Schloss, J.; Steel, A. Variations in folate prescriptions for patients with the MTHFR genetic polymorphisms: A case series study. J. Australas. Coll. Nutr. Environ. Med. 2025, 44, 40–55. [Google Scholar] [CrossRef] [PubMed]
- Margulis, D. Interactions Between Food Supplements in Sport: A Systematic Review. Master’s Thesis, Lithuanian University of Health Sciences, Kaunas, Lithuania, 2019. [Google Scholar]
- BaHammam, A.S.; Pirzada, A. Timing matters: The interplay between early mealtime, circadian rhythms, gene expression, circadian hormones, and metabolism—A narrative review. Clocks Sleep 2023, 5, 507–535. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).