Fishery Anesthetics in Aquaculture Products: Safety Concerns and Analytical Methods
Abstract
1. Introduction
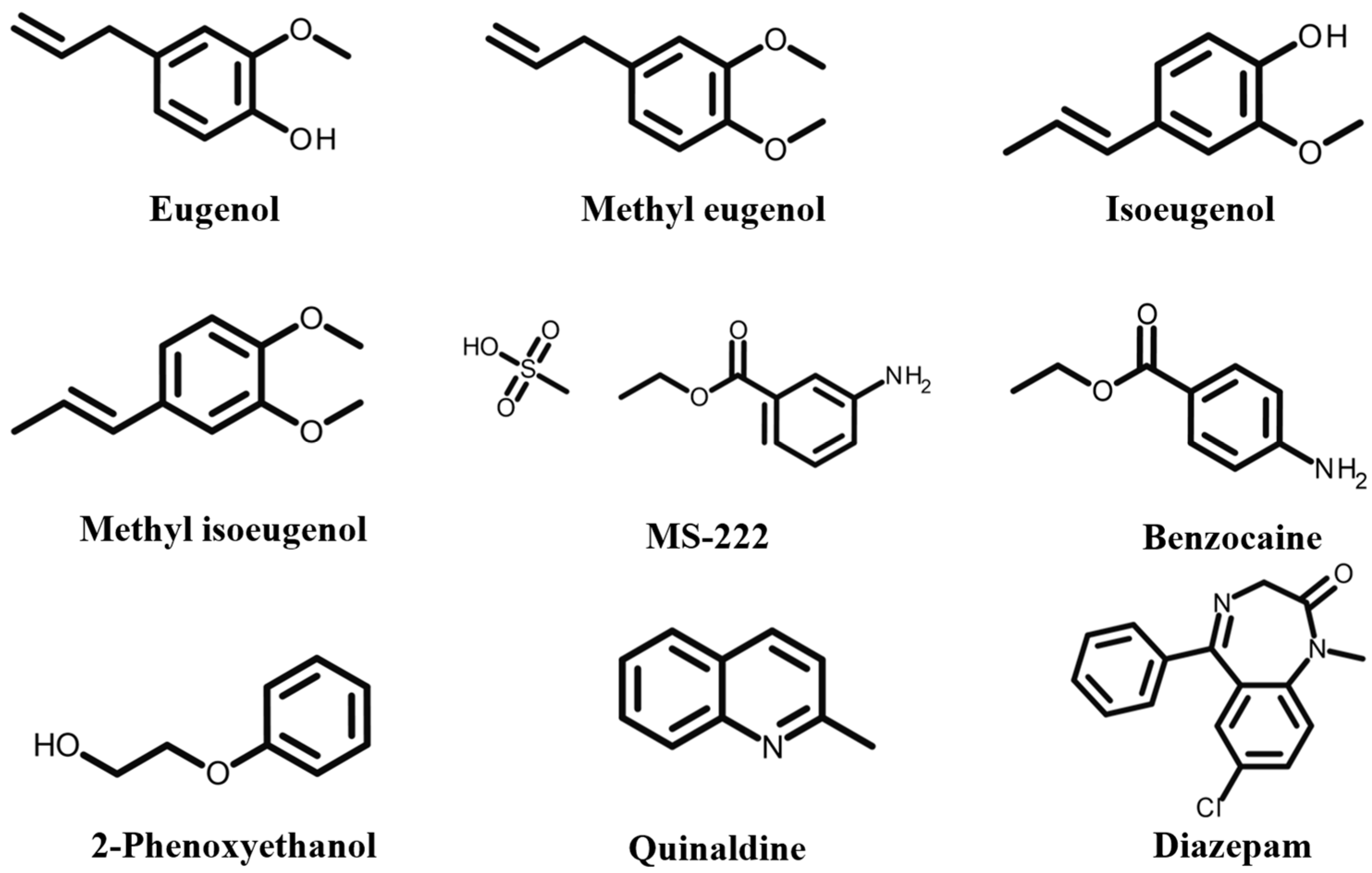
2. Overview of Fishery Anesthetics
3. Pharmacological Actions and Safety Assessment
3.1. Eugenol
3.2. MS-222
3.3. Benzocaine
3.4. 2-Phenoxyethanol
3.5. Diazepam
3.6. Quinaldine
4. Considerations on the Applicability of Commonly Used Anesthetics in Aquaculture
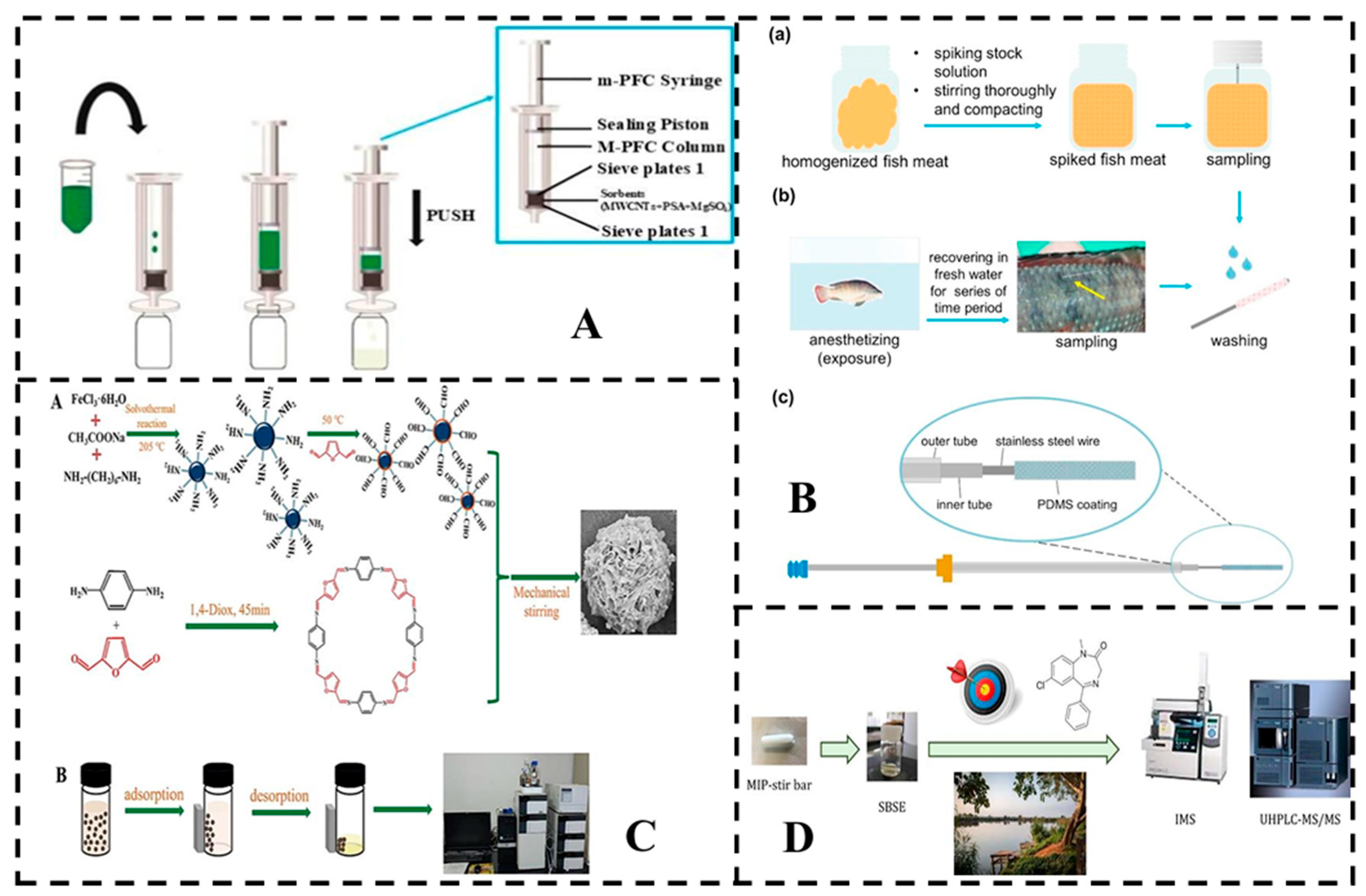
5. Analytical Methods for Residue Detection
5.1. Instrument Detection
5.1.1. GAS Chromatography (GC, GC-MS)
5.1.2. Liquid Chromatograph (LC or HPLC)
5.2. Rapid Detection
5.2.1. Immunoassays
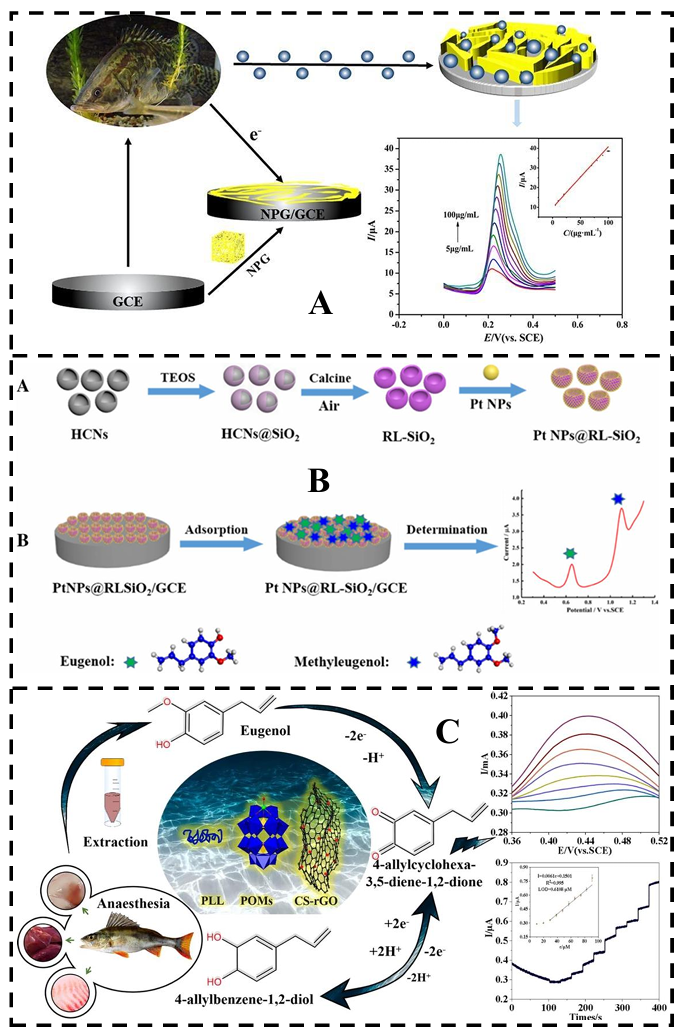
5.2.2. Electrochemical Sensor
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zapata-Guerra, N.A.; Rueda-Gomez, D.S.; Lozano-Villegas, K.J.; Herrera-Sanchez, M.P.; Uribe-Garcia, H.F.; Rondon-Barragan, I.S. Menthol as anaesthetic for red-bellied pacu (Piaractus brachypomus) and its effect onHIF1aandGlucoRgene expression. Aquac. Res. 2020, 51, 4421–4429. [Google Scholar] [CrossRef]
- Park, I.-S. The Anesthetic Effects of Clove Oil and MS-222 on Far Eastern Catfish, Silurus asotus. Dev. Reprod. 2019, 23, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Suski, C.D. Development of Carbon Dioxide Barriers to Deter Invasive Fishes: Insights and Lessons Learned from Bigheaded Carp. Fishes 2020, 5, 25. [Google Scholar] [CrossRef]
- Atsumi, T.; Fujisawa, S.; Tonosaki, K. A comparative study of the antioxidant/prooxidant activities of eugenol and isoeugenol with various concentrations and oxidation conditions. Toxicol. Vitr. 2005, 19, 1025–1033. [Google Scholar] [CrossRef]
- Gressler, L.T.; Riffel, A.P.K.; Parodi, T.V.; Saccol, E.M.H.; Koakoski, G.; da Costa, S.T.; Pavanato, M.A.; Heinzmann, B.M.; Caron, B.; Schmidt, D.; et al. Silver catfish Rhamdia quelen immersion anaesthesia with essential oil of Aloysia triphylla (L’Herit) Britton or tricaine methanesulfonate: Effect on stress response and antioxidant status. Aquac. Res. 2014, 45, 1061–1072. [Google Scholar] [CrossRef]
- Ghosh, D. Food safety regulations in Australia and New Zealand Food Standards. J. Sci. Food Agric. 2014, 94, 1970–1973. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Liu, Y. Optimization of solid-phase-extraction cleanup and validation of quantitative determination of eugenol in fish samples by gas chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2015, 407, 6563–6568. [Google Scholar] [CrossRef] [PubMed]
- Kaartinen, L. Scientific Advisory Group on Antimicrobials (SAGAM) of the Committee for Medicinal Products for Veterinary Use—Mandate and work plan. Int. J. Med. Microbiol. 2006, 296, 9–10. [Google Scholar] [CrossRef]
- Raymond, C.A.; Davies, N.W.; Larkman, T. GC-MS method validation and levels of methyl eugenol in a diverse range of tea tree (Melaleuca alternifolia) oils. Anal. Bioanal. Chem. 2017, 409, 1779–1787. [Google Scholar] [CrossRef]
- Wagner, E.; Arndt, R.; Hilton, B. Physiological stress responses, egg survival and sperm motility for rainbow trout broodstock anesthetized with clove oil, tricaine methanesulfonate or carbon dioxide. Aquaculture 2002, 211, 353–366. [Google Scholar] [CrossRef]
- Zou, N.; Chen, R.; Qin, Y.; Song, S.; Tang, X.; Pan, C. Comparison of pulse glow discharge-ion mobility spectrometry and liquid chromatography with tandem mass spectrometry based on multiplug filtration cleanup for the analysis of tricaine mesylate residues in fish and water. J. Sep. Sci. 2016, 39, 3638–3646. [Google Scholar] [CrossRef]
- Priborsky, J.; Velisek, J. A Review of Three Commonly Used Fish Anesthetics. Rev. Fish. Sci. Aquac. 2018, 26, 417–442. [Google Scholar] [CrossRef]
- da Paz, C.A.; da Costa, B.M.P.A.; Hamoy, M.K.O.; dos Santos, M.F.; da Rocha, L.L.; da Silva Deiga, Y.; de Sousa Barbosa, A.; do Amaral, A.L.G.; Câmara, T.M.; Barbosa, G.B.; et al. Establishing a safe anesthesia concentration window for Nile tilapia (Oreochromis niloticus) (Linnaeus 1758) by monitoring cardiac activity in eugenol immersion baths. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2024, 278, 109839. [Google Scholar] [CrossRef]
- Rucinque, D.S.; Ferreira, P.F.; Leme, P.R.P.; Lapa-Guimarães, J.; Viegas, E.M.M. Ocimum americanum and Lippia alba essential oils as anaesthetics for Nile tilapia: Induction, recovery of apparent unconsciousness and sensory analysis of fillets. Aquaculture 2021, 531, 735902. [Google Scholar] [CrossRef]
- Gladden, J.N.; Brainard, B.M.; Shelton, J.L.; Camus, A.C.; Divers, S.J. Evaluation of isoeugenol for anesthesia in koi carp (Cyprinus carpio). Am. J. Vet. Res. 2010, 71, 859–866. [Google Scholar] [CrossRef]
- Speare, R.; Speare, B.; Muller, R.; Bishop, P. Anesthesia of Tadpoles of the Southern Brown Tree Frog (Litoria ewingii) with Isoeugenol (AQUI-S). J. Zoo Wildl. Med. 2014, 45, 492–496. [Google Scholar] [CrossRef]
- Pattanasiri, T.; Taparhudee, W.; Suppakul, P. Acute toxicity and anaesthetic effect of clove oil and eugenol on Siamese fighting fish, Betta splendens. Aquac. Int. 2017, 25, 163–175. [Google Scholar] [CrossRef]
- Hobani, Y.H.; Mohan, S.; Shaheen, E.; Abdelhaleem, A.; Faruque Ahmad, M.; Bhatia, S.; Abou-Elhamd, A.S. Gastroprotective effect of low dose Eugenol in experimental rats against ethanol induced toxicity: Involvement of antiinflammatory and antioxidant mechanism. J. Ethnopharmacol. 2022, 289, 115055. [Google Scholar] [CrossRef]
- Tago, A.; Yokoyama, S.; Ishikawa, M.; Koshio, S. Pharmacokinetics of Eugenol in Japanese Flounder, Paralichthys olivaceus. J. World Aquac. Soc. 2018, 49, 780–787. [Google Scholar] [CrossRef]
- Kiessling, A.; Johansson, D.; Zahl, I.H.; Samuelsen, O.B. Pharmacokinetics, plasma cortisol and effectiveness of benzocaine, MS-222 and isoeugenol measured in individual dorsal aorta-cannulated Atlantic salmon (Salmo salar) following bath administration. Aquaculture 2009, 286, 301–308. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, H.; Yang, G.; Fang, C.; Kong, C.; Tian, L.; Huang, X. Pharmacokinetics studies of eugenol in Pacific white shrimp (Litopenaeus vannamei) after immersion bath. BMC Vet. Res. 2022, 18, 122. [Google Scholar] [CrossRef] [PubMed]
- Ke, C.; Liu, Q.; Huang, K.; Mo, M.; Chen, H.; Cheng, B. Effect of Water Temperature on the Depletion of Eugenol in Sea Bass under the Simulated Settings in Handling and Transport. Appl. Sci. 2021, 11, 10882. [Google Scholar] [CrossRef]
- Priya, P.S.; Guru, A.; Meenatchi, R.; Haridevamuthu, B.; Velayutham, M.; Seenivasan, B.; Pachaiappan, R.; Rajagopal, R.; Kuppusamy, P.; Juliet, A.; et al. Syringol, a wildfire residual methoxyphenol causes cytotoxicity and teratogenicity in zebrafish model. Sci. Total Environ. 2023, 864, 160968. [Google Scholar] [CrossRef]
- Tao, Y.; Du, C.; Duan, B.; Wang, W.; Guo, H.; Feng, J.; Xu, H.; Li, Y. Eugenol exposure inhibits embryonic development and swim bladder formation in zebrafish. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2023, 268, 109602. [Google Scholar] [CrossRef]
- Hume, W.R. Basic Biological Sciences Effect of Eugenol on Respiration and Division in Human Pulp, Mouse Fibroblasts, and Liver Cells in vitro. J. Dent. Res. 1984, 63, 1262–1265. [Google Scholar] [CrossRef]
- Jeng, J.H.; Hahn, L.J.; Lu, E.; Wang, Y.; Kuo, M.Y.P. Eugenol Triggers Different Pathobiological Effects on Human Oral Mucosal Fibroblasts 1. J. Dent. Res. 1994, 73, 1050–1055. [Google Scholar] [CrossRef]
- Thompson, D.C.; Constantin-Teodosiu, D.; Moldéus, P. Metabolism and cytotoxicity of eugenol in isolated rat hepatocytes. Chem. Biol. Interact. 1991, 77, 137–147. [Google Scholar] [CrossRef]
- Escobar-Garcia, M.; Rodriguez-Contreras, K.; Ruiz-Rodriguez, S.; Pierdant-Perez, M.; Cerda-Cristerna, B.; Pozos-Guillen, A. Eugenol Toxicity in Human Dental Pulp Fibroblasts of Primary Teeth. J. Clin. Pediatr. Dent. 2016, 40, 312–318. [Google Scholar] [CrossRef]
- Houdkova, M.; Rondevaldova, J.; Doskocil, I.; Kokoska, L. Evaluation of antibacterial potential and toxicity of plant volatile compounds using new broth microdilution volatilization method and modified MTT assay. Fitoterapia 2017, 118, 56–62. [Google Scholar] [CrossRef]
- Kasugai, S.; Hasegawa, N.; Ogura, H. Application of the MTT Colorimetric Assay to Measure Cytotoxic Effects of Phenolic Compounds on Established Rat Dental Pulp Cells. J. Dent. Res. 1991, 70, 127–130. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Tai, K.-W.; Huang, F.-M.; Huang, M.-F. Cytotoxic and Nongenotoxic Effects of Phenolic Compounds in Human Pulp Cell Cultures. J. Endod. 2000, 26, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Kozam, G.; Mantell, G.M. The Effect of Eugenol on Oral Mucous Membranes. J. Dent. Res. 1978, 57, 954–957. [Google Scholar] [CrossRef]
- Ke, C.; Liu, Q.; Li, L.; Chen, J.; Wang, X.; Huang, K. Simultaneous determination of eugenol, isoeugenol and methyleugenol in fish fillet using gas chromatography coupled to tandem mass spectrometry. J. Chromatogr. B—Anal. Technol. Biomed. Life Sci. 2016, 1031, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.-H.; Ke, C.-L.; Liu, Q.; Wang, X.-F.; Wang, Q.; Li, L.-D. Elimination kinetics of eugenol in grass carp in a simulated transportation setting. Bmc Vet. Res. 2017, 13, 346. [Google Scholar] [CrossRef]
- Mohammadi Nejad, S.; Ozgunes, H.; Basaran, N. Pharmacological and Toxicological Properties of Eugenol. Turk. J. Pharm. Sci. 2017, 14, 201–206. [Google Scholar] [CrossRef]
- Hu, Q.; Zhou, M.F.; Wei, S.Y. Progress on the Antimicrobial Activity Research of Clove Oil and Eugenol in the Food Antisepsis Field. J. Food Sci. 2018, 83, 1476–1483. [Google Scholar] [CrossRef]
- Carter, K.M.; Woodley, C.M.; Brown, R.S. A review of tricaine methanesulfonate for anesthesia of fish. Rev. Fish Biol. Fish. 2011, 21, 51–59. [Google Scholar] [CrossRef]
- Matthews, M.; Varga, Z.M. Anesthesia and Euthanasia in Zebrafish. ILAR J. 2012, 53, 192–204. [Google Scholar] [CrossRef]
- Matsche, M.A. Evaluation of tricaine methanesulfonate (MS-222) as a surgical anesthetic for Atlantic Sturgeon Acipenser oxyrinchus oxyrinchus. J. Appl. Ichthyol. 2011, 27, 600–610. [Google Scholar] [CrossRef]
- Fraser, T.W.K.; Mayer, I.; Skjaeraasen, J.E.; Hansen, T.; Fjelldal, P.G. The effect of triploidy on the efficacy and physiological response to anesthesia with MS 222 and isoeugenol in Atlantic salmon post-smolts. Aquac. Int. 2014, 22, 1347–1359. [Google Scholar] [CrossRef]
- Park, G.D.; Mitchel, J.T. Working with the US Food and Drug Administration to obtain approval of products under the Animal Rule. In Countermeasures Against Chemical Threats; Laskin, J.D., Ed.; Annals of the New York Academy of Sciences: New York, NY, USA, 2016; Volume 1374, pp. 10–16. [Google Scholar]
- Kolanczyk, R.C.; Fitzsimmons, P.N.; McKim, J.M.; Erickson, R.J.; Schmieder, P.K. Effects of anesthesia (tricaine methanesulfonate, MS222) on liver biotransformation in rainbow trout (Oncorhynchus mykiss). Aquat. Toxicol. 2003, 64, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Popovic, N.T.; Strunjak-Perovic, I.; Coz-Rakovac, R.; Barisic, J.; Jadan, M.; Berakovic, A.P.; Klobucar, R.S. Tricaine methane-sulfonate (MS-222) application in fish anaesthesia. J. Appl. Ichthyol. 2012, 28, 553–564. [Google Scholar] [CrossRef]
- Bernstein, P.S.; Digre, K.B.; Creel, D.J. Retinal toxicity associated with occupational exposure to the fish anesthetic MS-222. Am. J. Ophthalmol. 1997, 124, 843–844. [Google Scholar] [CrossRef] [PubMed]
- Rairat, T.; Chi, Y.; Hsieh, C.Y.; Liu, Y.K.; Chuchird, N.; Chou, C.C. Determination of Optimal Doses and Minimum Effective Concentrations of Tricaine Methanesulfonate, 2-Phenoxyethanol and Eugenol for Laboratory Managements in Nile Tilapia (Oreochromis niloticus). Animals 2021, 11, 1521. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.L.; de Souza e Silva, W.; Neves, L.d.C.; Ferreira, N.S.; Takata, R.; Luz, R.K. Benzocaine and menthol as anesthetics for the African cichlid Aulonocara nyassae. Aquac. Int. 2020, 28, 1837–1846. [Google Scholar] [CrossRef]
- Gomes, L.C.; Chippari-Gomes, A.R.; Lopes, N.P.; Roubach, R.; Araujo-Lima, C.A.R.M. Efficacy of benzocaine as an anesthetic in juvenile tambaqui Colossoma macropomum. J. World Aquac. Soc. 2001, 32, 426–431. [Google Scholar] [CrossRef]
- Stehly, G.R.; Meinertz, J.R.; Gingerich, W.H. Effects of temperature on the elimination of benzocaine and acetylated benzocaine residues from the edible fillet of rainbow trout (Oncorhynchus mykiss). Food Addit. Contam. 2000, 17, 387–392. [Google Scholar] [CrossRef]
- Meinertz, J.R.; Gingerich, W.H.; Allen, J.L. Metabolism and elimination of benzocaine by rainbow trout, Oncorhynchus mykiss. Xenobiotica 1991, 21, 525–533. [Google Scholar] [CrossRef]
- Jiwa, N.; Ibe, U.; Beri, R. Benzocaine Spray-Induced Methemoglobinemia. Am. J. Respir. Crit. Care Med. 2018, 197, A6587. [Google Scholar]
- Khair-ul-Bariyah, S.; Arshad, M.; Ali, M.; Din, M.I.; Sharif, A.; Ahmed, E. Benzocaine: Review on a Drug with Unfold Potential. Mini Rev. Med. Chem. 2020, 20, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Brock, W.J.; Bell, T.A. The In Vitro and In Vivo Genotoxicity of Benzocaine: A Brief Communication. Int. J. Toxicol. 2012, 31, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Food Standards Australia, New Zealand. Maximum Residue Limits–Benzocaine (Local Anaesthetic). Available online: https://www.foodstandards.gov.au/sites/default/files/food-standards-code/applications/Documents/A538_Benzocaine_FAR.pdf (accessed on 12 October 2025).
- Svacina, P.; Priborsky, J.; Blecha, M.; Policar, T.; Velisek, J. Haematological and biochemical response of burbot (Lota lota L.) exposed to four different anaesthetics. Czech J. Anim. Sci. 2016, 61, 414–420. [Google Scholar] [CrossRef]
- Imamura-Kojima, H.; Takashima, F.; Yoshida, T. Absorption, distribution and excretion of 2-phenoxyetanol in rainbow trout. Nippon Suisan Gakkaishi 1987, 53, 1339–1342. [Google Scholar] [CrossRef]
- Burka, J.F.; Hammell, K.L.; Horsberg, T.E.; Johnson, G.R.; Rainnie, D.J.; Speare, D.J. Drugs in salmonid aquaculture—A review. J. Vet. Pharmacol. Ther. 1997, 20, 333–349. [Google Scholar] [CrossRef] [PubMed]
- Öğretmen, F.; Gökçek, C. Comparative Efficacy of Three Anesthetic Agents on Juvenile African Catfish, Clarias gariepinus (Burchell, 1822). Turk. J. Fish. Aquat. Sci. 2013, 13, 51–56. [Google Scholar] [CrossRef]
- Coyle, S.D.; Durborow, R.M.; Tidwell, J.H. Anesthetics in Aquaculture; Southern Regional Aquaculture Center Texas: Stoneville, MS, USA, 2004; Volume 3900. [Google Scholar]
- Zahl, I.H.; Samuelsen, O.; Kiessling, A. Anaesthesia of farmed fish: Implications for welfare. Fish Physiol. Biochem. 2012, 38, 201–218. [Google Scholar] [CrossRef]
- Uçar, A.; Atamanalp, M. The Effects of Natural (Clove Oil) and Synthetical (2-phenoxyethanol) Anesthesia Substances on Hematology Parameters of Rainbow Trout (Oncorhynchus mykiss) and Brown Trout (Salmo trutta fario). J. Anim. Vet. Adv. 2010, 9, 1925–1933. [Google Scholar] [CrossRef]
- Api, A.M.; Belsito, D.; Botelho, D.; Bruze, M.; Burton, G.A., Jr.; Buschmann, J.; Dagli, M.L.; Date, M.; Dekant, W.; Deodhar, C.; et al. RIFM fragrance ingredient safety assessment, 2-phenoxyethanol, CAS Registry Number 122-99-6. Food Chem. Toxicol. 2019, 130 (Suppl. S1), 110629. [Google Scholar] [CrossRef]
- Hwang, J.-H.; Jeong, H.; Jung, Y.-O.; Nam, K.T.; Lim, K.-M. Skin irritation and inhalation toxicity of biocides evaluated with reconstructed human epidermis and airway models. Food Chem. Toxicol. 2021, 150, 112064. [Google Scholar] [CrossRef] [PubMed]
- Troutman, J.A.; Rick, D.L.; Stuard, S.B.; Fisher, J.; Bartels, M.J. Development of a physiologically-based pharmacokinetic model of 2-phenoxyethanol and its metabolite phenoxyacetic acid in rats and humans to address toxicokinetic uncertainty in risk assessment. Regul. Toxicol. Pharmacol. 2015, 73, 530–543. [Google Scholar] [CrossRef] [PubMed]
- Velíšek, J.; Svobodova, Z. Anaesthesia of common carp (Cyprinus carpio L.) with 2-phenoxyethanol: Acute toxicity and effects on biochemical blood profile. Acta Vet. Brno 2004, 73, 247–252. [Google Scholar] [CrossRef]
- Crestani, F.; Löw, K.; Keist, R.; Mandelli, M.-J.; Möhler, H.; Rudolph, U. Molecular Targets for the Myorelaxant Action of Diazepam. Mol. Pharmacol. 2001, 59, 442. [Google Scholar] [CrossRef]
- Zhou, H.; Xu, F.; Wei, L.; Lin, J.; Zhao, C.; Mei, H.; Shan, Q.; Wang, Q.; Mu, Y.; Yin, Y. Determination of diazepam and its active metabolites in aquatic products and aquaculture environments using modified QuEChERS-based UPLC-MS/MS. Anal. Methods 2025, 17, 2806–2816. [Google Scholar] [CrossRef]
- Mandelli, M.; Tognoni, G.; Garattini, S. Clinical Pharmacokinetics of Diazepam. Clin. Pharmacokinet. 1978, 3, 72–91. [Google Scholar] [CrossRef] [PubMed]
- Seddon, T.; Michelle, I.; Chenery, R.J. Comparative drug metabolism of diazepam in hepatocytes isolated from man, rat, monkey and dog. Biochem. Pharmacol. 1989, 38, 1657–1665. [Google Scholar] [CrossRef]
- Hunan Provincial Department of Agriculture and Rural Affairs. The Notice on the Results of the Second Supervision and Sampling Inspection of Key Agricultural Product Quality and Safety. Available online: https://agri.hunan.gov.cn/agri/ztzl/c102414/c102416/202408/t20240806_33425197.html (accessed on 12 October 2025).
- Li, J.; Zhang, J.; Liu, H.; Wu, L. A comparative study of primary secondary amino (PSA) and multi-walled carbon nanotubes (MWCNTs) as QuEChERS absorbents for the rapid determination of diazepam and its major metabolites in fish samples by high-performance liquid chromatography–electrospray ionisation–tandem mass spectrometry. J. Sci. Food Agric. 2016, 96, 555–560. [Google Scholar] [CrossRef]
- Kamble, A.; Kennady, C.J.; Badiye, A.; Kapoor, N. Detection of diazepam in spiked drink using thin-layer chromatography. JPC J. Planar Chromatogr. Mod. TLC 2022, 35, 543–546. [Google Scholar] [CrossRef]
- Ministry of Agriculture of the People’s Republic of China. Announcement No. 193: List of Banned Veter-inary Drugs and other Chemical Substances for Food Animals. Ministry of Agriculture of the People’s Republic of China. 2002. Available online: https://www.moa.gov.cn/govpublic/SYJ/201006/t20100606_1535262.htm (accessed on 21 October 2025).
- National Standardization Management Committee. GB 31650-2019: National Food Safety Standard—Maximum Residue Limits for Veterinary Drugs in Foods. China Standard. 2019. Available online: https://www.chinesestandard.net/PDF-EN/GB31650-2019EN-P15P-H12580H-566717.pdf (accessed on 21 October 2025).
- European Union. Regulation (EC) No 470/2009: Food and Feed Safety Law and Residue Regulation. Official Journal of the European Union. 2009. Available online: http://data.europa.eu/eli/reg/2009/470/oj (accessed on 21 October 2025).
- Carmona Araújo, A.; Casal, R.J.; Goulão, J.; Martins, A.P. Misuse of psychoactive medicines and its consequences in the European Union—A scoping review. J. Subst. Use 2024, 29, 629–640. [Google Scholar] [CrossRef]
- Muench, B. Quinaldine, a new anesthetic for fish. Progress. Fish Cult. 1958, 20, 42–44. [Google Scholar] [CrossRef]
- Hasan, M.; Bart, A.N. Improved survival of rohu, Labeo rohita (Hamilton-Buchanan) and silver carp, Hypophthalmichthys molitrix (Valenciennes) fingerlings using low-dose quinaldine and benzocaine during transport. Aquac. Res. 2007, 38, 50–58. [Google Scholar] [CrossRef]
- Bircan-Yildirim, Y.; Genc, E.; Turan, F.; Cek, S.; Yanar, M. The anaesthetic effects of quinaldine sulphate, muscle relaxant diazepam and their combination on convict cichlid, Cichlasoma nigrofasciatum (Günther, 1867) juveniles. J. Anim. Veter. Adv. 2010, 9, 547–550. [Google Scholar]
- Miethling, R.; Hecht, V.; Deckwer, W.D. Microbial degradation of quinoline: Kinetic studies with Comamonas acidovorans DSM 6426. Biotechnol. Bioeng. 1993, 42, 589–595. [Google Scholar] [CrossRef]
- Gomes, D.P.; Chaves, B.W.; Becker, A.G.; Baldisserotto, B. Water parameters affect anaesthesia induced by eugenol in silver catfish, Rhamdia quelen. Aquac. Res. 2011, 42, 878–886. [Google Scholar] [CrossRef]
- Santos, S.; Ghanawi, J.; Saoud, I.P. Effects of water temperature and body weight on anaesthetic efficiency in marbled rabbitfish (Siganus rivulatus). Aquac. Res. 2015, 46, 928–936. [Google Scholar] [CrossRef]
- Li, Y.; She, Q.; Han, Z.; Sun, N.; Liu, X.; Li, X. Anaesthetic Effects of Eugenol on Grass Shrimp (Palaemonetes sinensis) of Different Sizes at Different Concentrations and Temperatures. Sci. Rep. 2018, 8, 11007. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Dong, S.; Wang, F.; Wu, L. The effects of temperature changes on the oxygen consumption of juvenile Chinese shrimp Fenneropenaeus chinensis Osbeck. J. Exp. Mar. Biol. Ecol. 2004, 310, 59–72. [Google Scholar] [CrossRef]
- Zhang, C.; Li, F.; Xiang, J. Effect of temperature on the standard metabolic rates of juvenile and adult Exopalaemon carinicauda. Chin. J. Oceanol. Limnol. 2015, 33, 381–388. [Google Scholar] [CrossRef]
- Zhang, C.; Li, F. Anesthetic effects of eugenol on adult ridgetail white prawn (Exopalaemon carinicauda) and its protective effects under simulated transportation. Aquaculture 2024, 588, 740852. [Google Scholar] [CrossRef]
- Waterstrat, P.R.; Plnkham, L. Evaluation of Eugenol as an Anesthetic for the American Lobster Homerus americanus. J. World Aquac. Soc. 2005, 36, 420–424. [Google Scholar] [CrossRef]
- He, Y.; Fu, Z.; Dai, S.; Yu, G.; Guo, Y.; Ma, Z. Effects of Eugenol on Water Quality and the Metabolism and Antioxidant Capacity of Juvenile Greater Amberjack (Seriola dumerili) under Simulated Transport Conditions. Animals 2022, 12, 2880. [Google Scholar] [CrossRef]
- Hoseini, S.M.; Ghelichpour, M. Efficacy of clove solution on blood sampling and hematological study in Beluga, Huso huso (L.). Fish Physiol. Biochem. 2012, 38, 493–498. [Google Scholar] [CrossRef]
- Jia, Y.; Xie, T.; Gao, Y.; Qin, H.; Guan, C. Anesthetics efficacy and physiological response of MS222 and clove oil in spotted knifejaw Oplegnathus punctatus. Aquac. Rep. 2022, 25, 101201. [Google Scholar] [CrossRef]
- Coyle, S.D.; Dasgupta, S.; Tidwell, J.H.; Beavers, T.; Bright, L.A.; Yasharian, D.K. Comparative Efficacy of Anesthetics for the Freshwater Prawn Macrobrachiurn rosenbergii. J. World Aquac. Soc. 2005, 36, 282–290. [Google Scholar] [CrossRef]
- de Souza Valente, C. Anaesthesia of decapod crustaceans. Vet. Anim. Sci. 2022, 16, 100252. [Google Scholar] [CrossRef] [PubMed]
- Ghanawi, J.; Monzer, S.; Saoud, I.P. Anaesthetic efficacy of clove oil, benzocaine, 2-phenoxyethanol and tricaine methanesulfonate in juvenile marbled spinefoot (Siganus rivulatus). Aquac. Res. 2013, 44, 359–366. [Google Scholar] [CrossRef]
- Moon, H.; Nam, A.-j.; Sim, W.; Oh, J.-E. Risk assessment and monitoring of tranquilizers in live seafood: Analysis of natural essential oil and 2-phenoxyethanol by GC–MS/MS. Sci. Total Environ. 2025, 959, 178338. [Google Scholar] [CrossRef]
- Tsantilas, H.; Galatos, A.D.; Athanassopoulou, F.; Prassinos, N.N.; Kousoulaki, K. Efficacy of 2-phenoxyethanol as an anaesthetic for two size classes of white sea bream, Diplodus sargus L., and sharp snout sea bream, Diplodus puntazzo C. Aquaculture 2006, 253, 64–70. [Google Scholar] [CrossRef]
- Munday, P.L.; Wilson, S.K. Comparative efficacy of clove oil and other chemicals in anaesthetization of Pomacentrus amboinensis, a coral reef fish. J. Fish Biol. 1997, 51, 931–938. [Google Scholar] [CrossRef]
- Belal, I.; Assem, H. Pharmacological mechanisms of diazepam in fish: Effect on growth. Journal of Environmental Science and Engineering 2011, 5, 1363–1369. [Google Scholar]
- Ogueji, O.; Iheanacho, S.; Nwani, C.; Mbah, C.; Chiamah, O.; Usman, B. Toxicity of diazepam on lipid peroxidation, biochemical and oxidative stress indicators on liver and gill tissues of African catfish Clarias gariepinus (Burchell, 1822). Int. J. Fish. Aquat. Stud. 2017, 114, 114–123. [Google Scholar]
- Shan, Q.; Huang, X.; Ye, S.; Zhou, H.; Xu, F.; Li, J.; Lin, J.; Li, L.; Yin, Y. Residue Behavior and Risk Assessment of Diazepam and Its Metabolites in Crucian Carp (Carassius auratus) After Oral Administration. J. Vet. Pharmacol. Ther. 2025, 48, 212–220. [Google Scholar] [CrossRef]
- Kildea, M.A.; Allan, G.L.; Kearney, R.E. Accumulation and clearance of the anaesthetics clove oil and AQUI-S™ from the edible tissue of silver perch (Bidyanus bidyanus). Aquaculture 2004, 232, 265–277. [Google Scholar] [CrossRef]
- Ye, L. Development and validation of a LC-MS/MS method for the determination of isoeugenol in finfish. Food Chem. 2017, 228, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Ouyang, L.; Meng, P.; He, M.; Lin, Q.; Chen, Y.; Liu, W.; Su, X.; Dai, M. Determination of 18 caine anesthetics in animal meat using solid phase extraction combined with ultra-performance liquid chromatography-tandem mass spectrometry. Chin. J. Chromatogr. 2023, 41, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Abreu, D.C.P.; Botrel, B.M.C.; Bazana, M.J.F.; e Rosa, P.V.; Sales, P.F.; Marques, M.d.S.; Saczk, A.A. Development and comparative analysis of single-drop and solid-phase microextraction techniques in the residual determination of 2-phenoxyethanol in fish. Food Chem. 2019, 270, 487–493. [Google Scholar] [CrossRef]
- Wang, M.; Qiao, Y.; Luo, Z.; Guo, E.; Ma, W.; Wang, K.; Guo, A.; Lian, K. Development of a QuEChERS combined with LC-MS/MS method for determining 24 sedatives and anesthetics in animal-derived foods. J. Food Compos. Anal. 2024, 127, 106000. [Google Scholar] [CrossRef]
- Sun, H.; Lai, J.-P.; Chen, F.; Zhu, D.-R. Molecularly imprinted microspheres synthesized by a simple, fast, and universal suspension polymerization for selective extraction of the topical anesthetic benzocaine in human serum and fish tissues. Anal. Bioanal. Chem. 2015, 407, 1745–1752. [Google Scholar] [CrossRef]
- Liang, X.; Feng, T.T.; Wu, J.H.; Du, M.; Qin, L.; Wang, Z.Y.; Xu, X.B. Vortex-Assisted Liquid-Liquid Micro-extraction Followed by Head Space Solid Phase Micro-extraction for the Determination of Eugenol in Fish Using GC-MS. Food Anal. Methods 2018, 11, 790–796. [Google Scholar] [CrossRef]
- Rafson, J.P.; Turnipseed, S.B.; Casey, C.; De Bono, A.; Madson, M.R. Analysis and Stability Study of Isoeugenol in Aquaculture Products by Headspace Solid-Phase Microextraction Coupled to Gas Chromatography–Mass Spectrometry. J. Agric. Food Chem. 2024, 72, 14411–14418. [Google Scholar] [CrossRef] [PubMed]
- Haji Abdolrasouli, M.; Roostaie, A.; Abedi, H.; Mohammadiazar, S. Determination of Lorazepam and Diazepam Using Modified Nanocrystalline Cellulose for Ultrasonic-Assisted Dispersive Solid Phase Microextraction (UA-DSPME) and Gas Chromatography-Mass Spectrometry (GC-MS). Anal. Lett. 2024, 57, 2085–2102. [Google Scholar] [CrossRef]
- Klimánková, E.; Riddellová, K.; Hajšlová, J.; Poustka, J.; Kolářová, J.; Kocourek, V. Development of an SPME–GC–MS/MS procedure for the monitoring of 2-phenoxyethanol in anaesthetised fish. Talanta 2008, 75, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ai, X.; Li, L.; Li, J.; Yang, H. A fast and accurate isotope dilution GC-IT-MS/MS method for determination of eugenol in different tissues of fish: Application to a depletion study in mandarin fish. Biomed. Chromatogr. 2018, 32, e4163. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.X.; Li, Q.; Zhang, Y.L.; Meng, Z.J.; Yuan, X.X.; Fan, S.F.; Zhang, Y. Determination of Six Eugenol Residues in Aquatic Products by Gas Chromatography-Orbitrap Mass Spectrometry. J. Food Qual. 2021, 2021, 438853. [Google Scholar] [CrossRef]
- Li, J.; Liu, H.; Yu, M.; Wu, L.; Wang, Q.; Lv, H.; Ma, B.; Song, Y. Rapid determination of tricaine mesylate residues in fish samples using modified QuEChERS and high-performance liquid chromatography-tandem mass spectrometry. Anal. Methods 2014, 6, 9124–9128. [Google Scholar] [CrossRef]
- Zhao, D.-H.; Wang, Q.; Wang, X.-F.; Li, Z.-G.; Li, Y.-X.; Huang, K.; Li, L.-D. Determination of MS-222 in Water Samples by Solid-phase Extraction Coupled with Liquid Chromatography/Tandem Mass Spectrometry. J. Chromatogr. Sci. 2017, 55, 813–817. [Google Scholar] [CrossRef]
- Xie, C.N.; Li, Q.; Han, G.; Liu, H.; Yang, J.; Li, J.C. Stable isotope dilution assay for the accurate determination of tricaine in fish samples by HPLC-MS-MS. Biomed. Chromatogr. 2019, 33, e4512. [Google Scholar] [CrossRef]
- Shen, K.; Zou, X.; Wang, J. Simultaneous determination of the four key fluoroquinolones and two antipsychotics in fish and shrimp by LC-MS/MS. Food Addit. Contam. Part A 2022, 39, 678–686. [Google Scholar] [CrossRef]
- Meinertz, J.R.; Hess, K.R. Evaluation of analytical techniques to determine AQUI-S (R) 20E (eugenol) concentrations in water. Aquaculture 2014, 418, 62–66. [Google Scholar] [CrossRef]
- Mu, S.; Wang, C.; Liu, H.; Han, G.; Wu, L.; Li, J. Development and evaluation of a novelty single-step cleanup followed by HPLC-QTRAP-MS/MS for rapid analysis of tricaine, tetracaine, and bupivacaine in fish samples. Biomed. Chromatogr. 2021, 35, 5176. [Google Scholar] [CrossRef]
- Huang, Q.; Zhou, H.; Wu, X.; Song, C.; Zheng, J.; Lei, M.; Mu, P.; Wu, P. Simultaneous determination of the residues of anesthetics and sedatives in fish using LC-QLIT-MS/MS combined with DSPE. Food Chem. 2023, 403, 134407. [Google Scholar] [CrossRef] [PubMed]
- Abad, M.O.K.; Masrournia, M.; Javid, A. Synthesis of novel MOF-on-MOF composite as a magnetic sorbent to dispersive micro solid phase extraction of benzodiazepine drugs prior to determination with HPLC-UV. Microchem. J. 2024, 197, 109797. [Google Scholar] [CrossRef]
- Li, J.C.; Liu, H.; Wang, C.Y.; Wu, L.D.; Liu, D. Determination of eugenol in fish and shrimp muscle tissue by stable isotope dilution assay and solid-phase extraction coupled gas chromatography-triple quadrupole mass spectrometry. Anal. Bioanal. Chem. 2016, 408, 6537–6544. [Google Scholar] [CrossRef]
- Huang, S.Y.; Xu, J.Q.; Wu, J.Y.; Hong, H.J.; Chen, G.S.; Jiang, R.F.; Zhu, F.; Liu, Y.; Ouyang, G.F. Rapid detection of five anesthetics in tilapias by in vivo solid phase microextraction coupling with gas chromatography-mass spectrometry. Talanta 2017, 168, 263–268. [Google Scholar] [CrossRef]
- Xia, G.; Ruan, G.; Huang, Y.; Hu, H.; Yu, S.; Lai, B.; Li, Z.; Zhang, Y.; Tang, N. Highly efficient enrichment of eugenol anesthetics in aquatic products using magnetic nanospheres decorated covalent organic framework microflowers. Microchem. J. 2023, 195, 109362. [Google Scholar] [CrossRef]
- Sorribes-Soriano, A.; Albert Esteve-Turrillas, F.; Armenta, S.; Manuel Herrero-Martínez, J. Molecularly imprinted polymer –stir bar sorptive extraction of diazepam from natural water. Microchem. J. 2023, 186, 108354. [Google Scholar] [CrossRef]
- Cheng, X.H.; Hochlowski, J. Current application of mass spectrometry to combinatorial chemistry. Anal. Chem. 2002, 74, 2679–2690. [Google Scholar] [CrossRef]
- Scherpenisse, P.; Bergwerff, A.A. Determination of residues of tricaine in fish using liquid chromatography tandem mass spectrometry. Anal. Chim. Acta 2007, 586, 407–410. [Google Scholar] [CrossRef]
- Liang, H.-W.; Jia, B.-Z.; Zhang, W.-F.; Wang, X.-X.; Zhou, K.; Lei, H.-T.; Xu, Z.-L.; Luo, L. Ratiometric Fluorescence Immunoassay Based on MnO2 Nanoflakes for Sensitive and Accurate Detection of Tricaine. J. Agric. Food Chem. 2023, 71, 7575–7583. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Luo, S.-Z.; Jia, B.-Z.; Zhang, W.-F.; Wang, H.; Wei, X.-Q.; Shen, Y.-D.; Lei, H.-T.; Xu, Z.-L.; Yang, J.-Y. A high-resolution colorimetric immunoassay for tyramine detection based on enzyme-enabled growth of gold nanostar coupled with smartphone readout. Food Chem. 2022, 396, 133729. [Google Scholar] [CrossRef]
- Lin, L.; Wu, X.; Cui, G.; Song, S.; Kuang, H.; Xu, C. Colloidal Gold Immunochromatographic Strip Assay for the Detection of Azaperone in Pork and Pork Liver. ACS Omega 2020, 5, 1346–1351. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, Y.; Eremin, S.A.; Yakup, O.; Yao, G.; Zhang, X. Detection of kanamycin and gentamicin residues in animal-derived food using IgY antibody based ic-ELISA and FPIA. Food Chem. 2017, 227, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Can-Herrera, L.A.; Oliva, A.I.; Dzul-Cervantes, M.A.A.; Pacheco-Salazar, O.F.; Cervantes-Uc, J.M. Morphological and Mechanical Properties of Electrospun Polycaprolactone Scaffolds: Effect of Applied Voltage. Polymers 2021, 13, 662. [Google Scholar] [CrossRef]
- Shen, X.; Wu, X.; Liu, L.; Kuang, H. Development of a colloidal gold immunoassay for the detection of four eugenol compounds in water. Food Agric. Immunol. 2019, 30, 1318–1331. [Google Scholar] [CrossRef]
- Ahmed, S.; Ning, J.N.; Peng, D.P.; Chen, T.; Ahmad, I.; Ali, A.; Lei, Z.X.; Shabbir, M.A.; Cheng, G.Y.; Yuan, Z.H. Current advances in immunoassays for the detection of antibiotics residues: A review. Food Agric. Immunol. 2020, 31, 268–290. [Google Scholar] [CrossRef]
- Maragos, C. Fluorescence Polarization Immunoassay of Mycotoxins: A Review. Toxins 2009, 1, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Rivas, L.; de la Escosura-Muñiz, A.; Serrano, L.; Altet, L.; Francino, O.; Sánchez, A.; Merkoçi, A. Triple lines gold nanoparticle-based lateral flow assay for enhanced and simultaneous detection of Leishmania DNA and endogenous control. Nano Res. 2015, 8, 3704–3714. [Google Scholar] [CrossRef]
- Guo, L.; Wu, X.; Liu, L.; Kuang, H.; Xu, C. Gold Nanoparticle-Based Paper Sensor for Simultaneous Detection of 11 Benzimidazoles by One Monoclonal Antibody. Small 2018, 14, 1701782. [Google Scholar] [CrossRef]
- Lei, X.; Xu, X.; Liu, L.; Kuang, H.; Xu, L.; Hao, C. Immunochromatographic test strip for the rapid detection of tricaine in fish samples. Food Agric. Immunol. 2020, 31, 687–699. [Google Scholar] [CrossRef]
- Lei, X.; Xu, X.; Wang, L.; Zhou, W.; Liu, L.; Xu, L.; Kuang, H.; Xu, C. A quadruplex immunochromatographic assay for the ultrasensitive detection of 11 anesthetics. Nano Res. 2023, 16, 11269–11277. [Google Scholar] [CrossRef]
- Lin, S.-Q.; Jia, B.-Z.; Luo, W.; Wang, H.; Lei, H.-T.; Zhang, W.-F.; Xu, Z.-L.; Luo, L. Controllable formation of polydopamine on carbon dots for ultrasensitive detection of alkaline phosphatase and ratiometric fluorescence immunoassay of benzocaine. Food Chem. 2023, 426, 136582. [Google Scholar] [CrossRef]
- Luo, L.; He, Z.-X.; Jia, B.-Z.; Kang, R.-Y.; Zhang, W.-F.; Huang, R.-M.; Xu, Z.-L. Gold nanocluster-based ratiometric fluorescence immunoassay for broad-spectrum screening of five eugenols. Anal. Chim. Acta 2024, 1310, 342723. [Google Scholar] [CrossRef]
- Luo, L.; Lei, H.-T.; Yang, J.-Y.; Liu, G.-L.; Sun, Y.-M.; Bai, W.-D.; Wang, H.; Shen, Y.-D.; Chen, S.; Xu, Z.-L. Development of an indirect ELISA for the determination of ethyl carbamate in Chinese rice wine. Anal. Chim. Acta 2017, 950, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Jia, B.-Z.; Wei, X.-Q.; Xiao, Z.-L.; Wang, H.; Sun, Y.-M.; Shen, Y.-D.; Lei, H.-T.; Xu, Z.-L. Development of an inner filter effect-based fluorescence immunoassay for the detection of acrylamide using 9-xanthydrol derivatization. Sens. Actuators B Chem. 2021, 332, 129561. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, X.; Peng, H.; Arabi, M.; Li, J.; Xiong, H.; Choo, J.; Chen, L. Ratiometric fluorescence and colorimetry dual-mode assay based on manganese dioxide nanosheets for visual detection of alkaline phosphatase activity. Sens. Actuators B Chem. 2020, 302, 127176. [Google Scholar] [CrossRef]
- Huang, X.; Song, J.; Yung, B.C.; Huang, X.; Xiong, Y.; Chen, X. Ratiometric optical nanoprobes enable accurate molecular detection and imaging. Chem. Soc. Rev. 2018, 47, 2873–2920. [Google Scholar] [CrossRef]
- Wang, J.; Xu, X.; Li, Z.; Li, M.; Qiu, B. Sensitive electrochemical detection of benzocaine based on hollow carbon nanobowl modified electrode. J. Electroanal. Chem. 2024, 952, 117893. [Google Scholar] [CrossRef]
- Mahdi, M.A.; Yousefi, S.R.; Jasim, L.S.; Salavati-Niasari, M. Green synthesis of DyBa2Fe3O7.988/DyFeO3 nanocomposites using almond extract with dual eco-friendly applications: Photocatalytic and antibacterial activities. Int. J. Hydrog. Energy 2022, 47, 14319–14330. [Google Scholar] [CrossRef]
- Felix de Lima, R.M.; de Oliveira Silva, M.D.; Felix, F.S.; Angnes, L.; Pio dos Santos, W.T.; Saczk, A.A. Determination of Benzocaine and Tricaine in Fish Fillets Using BIA with Amperometric Detection. Electroanalysis 2018, 30, 283–287. [Google Scholar] [CrossRef]
- Cai, S.; Chen, X.; Liu, J.; Wang, L.; Liu, G.; Gu, Y. Highly efficient detection of Tricaine methanesulfonate based on the nanoporous gold electrochemical sensor. Mater. Lett. 2021, 301, 130286. [Google Scholar] [CrossRef]
- Shi, Z.; Xia, L.; Li, G.; Hu, Y. Platinum nanoparticles-embedded raspberry-liked SiO2 for the simultaneous electrochemical determination of eugenol and methyleugenol. Microchim. Acta 2021, 188, 241. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wei, J.; Li, J.; Jiao, T.; Wang, L.; Chen, Q. Rapid detection of eugenol in perch utilizing electrochemical method by transition metal substituted polyoxometalates. Food Chem. 2023, 426, 136584. [Google Scholar] [CrossRef]
- Pysarevska, S.; Dubenska, L.; Plotycya, S.; Švorc, Ľ. A state-of-the-art approach for facile and reliable determination of benzocaine in pharmaceuticals and biological samples based on the use of miniaturized boron-doped diamond electrochemical sensor. Sens. Actuators B Chem. 2018, 270, 9–17. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, L.; Huang, Y.; Chen, Y.; Cao, H. Highly sensitive determination of anesthetic drug benzocaine based on hydroxypropyl-β-cyclodextrin–carbon black nanohybrids. Anal. Methods 2022, 14, 900–906. [Google Scholar] [CrossRef]

| Anesthetic | MRL | Withdrawal Period | Reference | |
|---|---|---|---|---|
| Eugenol | New Zealand | 100 ng mL−1 | — | [6] |
| Japan | 50 ng mL−1 | 7 d | [7] | |
| Isoeugenol | European Conformity (CE) | 6 mg kg−1 | — | [8] |
| AQUI-S | Australia and Chile | — | 0d | [9] |
| MS-222 | FDA | 1 µg mL−1 | 21 d | [10] |
| Canada | — | 7 d | [11] | |
| Methods | Anesthetic | Sample | Pretreatment | Linearity | Repeatability/Reproducibility | Detection Limits | Reference |
|---|---|---|---|---|---|---|---|
| GC-MS | eugenol | fish back, fish belly, and fish tail | VALLME/HS-SPME | 15.0–750.0 μg kg−1 | RSD < 20%, n = 3 | 0.5 μg kg−1 | [105] |
| eugenol | carp muscle tissues | SPE | 5.0–500.0 μg L−1 | RSD < 12%, n = 6 | 2.5 μg kg−1 | [7] | |
| Isoeugenol | shrimp, tilapia, and salmon | Headspace solid-phase microextraction | 0–160 ng g−1 | RSD 5–13%, n = 9 | below 15ng g−1 | [106] | |
| GC-MS/MS | diazepam | water samples | dispersive solid-phase microextraction | 10–1000 ng mL−1 | RSD = 6%, n = 5 | 3 ng mL−1 | [107] |
| eugenol, isoeugenol‚ and methyleugenol | groupers | SPE | 5–500 μg L−1 | RSD 2.18–15.5%, n = 4 | eugenol 0.4 μg kg−1, isoeugenol 1.2 μg kg−1 ‚ and methyleugenol 0.2 μg kg−1 | [33] | |
| 2-Phenoxyethanol | rainbow trout | SPME | 0.1–250 mg kg−1 | RSD 3–11%, n = 5 | 0.03mg kg−1 | [108] | |
| GC-IT-MS/MS | eugenol | mandarin | QuEChERS | 5–1000 μg L−1 | RSD 1.82–9.74%, n = 6 | 5.0 μg/kg | [109] |
| Orbitrap GC-MS | Eugenol, methyleugenol, isoeugenol, methyl isoeugenol, eugenol acetate, and acetyl isoeugenol | prawns | m-PFC column | 0.001–0.1 μg mL−1 | RSD 1.2–7.5%, n = 6 | 2–10 μg kg−1 | [110] |
| HPLC-MS/MS | MS-222 | carp and eel | QuEChERS | 2–1000 μg L−1 | RSD < 6%, n = 3 | 2.5 μg kg−1 | [111] |
| / | SPE | 0.05–10 μg L−1 | RSD < 9.36%, n = 5 | 0.01 μg/L | [112] | ||
| finfish | extracted with acetone using a tissue homogenizer, followed by derivatization with dansyl chloride | 2.5–40.0 ng g−1 | RSD 2.6–8.0%, n = 6 | 0.2–1 μg kg−1 | [100] | ||
| marine fish and freshwater fish | isotope dilution assay | 2.0–200.0 μg L−1 | inter- and intra-assay relative standard deviations (RSD values) were 0.39–3.01 and 0.85–2.77% | 1 μg kg−1 | [113] | ||
| LC-MS/MS | diazepam | fish and shrimp tissue | C18 cartridge solid-phase extraction | 0.05–5ng mL−1 | RSD < 4.9%, n = 3 | 0.01 μg kg−1 | [114] |
| LC | AQUI-S® 20E (eugenol) | standard water containing fish feed | SPE | 5–500mg L−1 | RSD < 0.7%, n = 3 | 0.0011 mg L−1 | [115] |
| HPLC-QTRAP-MS/MS | tricaine, tetracaine, and bupivacaine | fish samples | QuEChERS | 1.0–50.0 μg L−1 | RSD < 15%, n = 3 | 2.0 μg kg−1 | [116] |
| PGD-IMS/LC-MS/MS | MS-222 | fish-raising water samples | m-PFC | 0.005–0.2 mg L−1 | PGD-IMS RSD 6.9–10.3%, n = 5 LC-MS/MS RSD 1.3–3.4%, n = 5 | 6 μg kg−1/0.6 μg kg−1 | [11] |
| LC-QLIT-MS/MS | eugenol | fish samples | dispersive solid-phase extraction (DSPE) | 1–100 μg kg−1 | RSD 1.9–8.9%, n = 6 | 0.0–0.4 μg kg−1 | [117] |
| HPLC-UV | Diazepam | water samples | Dispersive micro solid phase extraction | 0.3–450 ng mL−1 | RSD 3.42–3.75%, n = 3 | 0.09 ng mL−1 | [118] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, B.-Z.; Rui, X.-Y.; Wang, Y.; Zeng, X.; Sheng, S.-J.; Zeng, B.-J.; Xu, Z.-L.; Luo, L. Fishery Anesthetics in Aquaculture Products: Safety Concerns and Analytical Methods. Foods 2025, 14, 3928. https://doi.org/10.3390/foods14223928
Jia B-Z, Rui X-Y, Wang Y, Zeng X, Sheng S-J, Zeng B-J, Xu Z-L, Luo L. Fishery Anesthetics in Aquaculture Products: Safety Concerns and Analytical Methods. Foods. 2025; 14(22):3928. https://doi.org/10.3390/foods14223928
Chicago/Turabian StyleJia, Bao-Zhu, Xue-Ying Rui, Yu Wang, Xi Zeng, Shu-Jing Sheng, Bi-Jian Zeng, Zhen-Lin Xu, and Lin Luo. 2025. "Fishery Anesthetics in Aquaculture Products: Safety Concerns and Analytical Methods" Foods 14, no. 22: 3928. https://doi.org/10.3390/foods14223928
APA StyleJia, B.-Z., Rui, X.-Y., Wang, Y., Zeng, X., Sheng, S.-J., Zeng, B.-J., Xu, Z.-L., & Luo, L. (2025). Fishery Anesthetics in Aquaculture Products: Safety Concerns and Analytical Methods. Foods, 14(22), 3928. https://doi.org/10.3390/foods14223928






