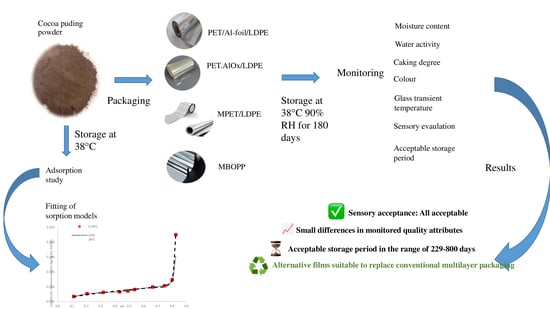
Sustainable Alternatives in Multilayer Packaging: Storage Stability of Pudding Powder Under Accelerated Storage Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fitting of Sorption Models to Experimental Data
2.3. Setting Up Accelerated Storage Conditions of Packaged Pudding Powder and Investigation of Quality Parameters
2.3.1. Sample Preparation
2.3.2. Accelerated Storage
2.3.3. Water Vapor Transmission Rate
2.3.4. Oxygen Transmission Rate
2.3.5. Moisture Content
2.3.6. Water Activity
2.3.7. Caking Degree
2.3.8. Color
2.3.9. Glass Transition Temperature
2.3.10. Sensory Evaluation
2.3.11. Acceptable Storage Period
2.3.12. Statistical Analysis
3. Results
3.1. Fitting of Sorption Models to Experimental Data
3.2. Analysis of Quality Changes in Packaged Pudding Powder Under Temperature-Dependent Accelerated Storage Conditions
3.2.1. Permeability Properties of the Packaging Materials
3.2.2. Moisture Content and Water Activity
3.2.3. Caking Degree
3.2.4. Glass Transition Temperature
3.2.5. Color Analysis
3.2.6. Sensory Analysis
3.3. Acceptable Storage Period of the Pudding Powder
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barlow, C.Y.; Morgan, D.C. Polymer film packaging for food: An environmental assessment. Resour. Conserv. Recycl. 2013, 78, 74–80. [Google Scholar] [CrossRef]
- European Organisation for Packaging and the Environment (EUROPEN). Packaging Industry Manifesto: For the 2024–2029 EU Political Term. 2024. Available online: https://www.europen-packaging.eu/wp-content/uploads/2024/10/Packaging-Manifesto-Final-EUROPEN-Identity.pdf (accessed on 17 September 2025).
- European Parliament and Council. Regulation (EU) 2025/40 on packaging and packaging waste, amending Regulation (EU) 2019/1020 and Directive (EU) 2019/904, and repealing Directive 94/62/EC. Off. J. Eur. Union 2025. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=OJ:L_202500040 (accessed on 17 September 2025).
- Environmental Protection Agency. National Strategy to Prevent Plastic Pollution—Part Three of a Series on Building a Circular Economy for All; EPA: Washington, DC, USA, 2024. Available online: https://www.epa.gov/system/files/documents/2024-11/final_national_strategy_to_prevent_plastic_pollution.pdf (accessed on 17 September 2025).
- Sustainable Packaging Coalition. Definition of Sustainable Packaging; Sustainable Packaging Coalition: Charlottesville, VA, USA, 2023; Available online: https://sustainablepackaging.org/wp-content/uploads/2024/01/SPC_Definition-of-Sust-Packaging_Landscape.pdf (accessed on 17 September 2025).
- European Commission. Proposal for a Regulation of the European Parliament and of the Council on Packaging and Packaging Waste. 2022. Available online: https://eur-lex.europa.eu/legal-content/EN/HIS/?uri=CELEX%3A52022PC0677 (accessed on 17 September 2025).
- World Packaging Organization. Packaging Design for Recycling: A Global Recommendation for Packaging Design; WPO: Tokyo, Japan, 2020; Available online: https://worldpackaging.org/Uploads/2021-10/ResourcePDF37_1635406572.pdf (accessed on 17 September 2025).
- Australian Packaging Covenant Organization. Sustainable Packaging Guidelines; APCO: Sydney, Australia, 2020; Available online: http://documents.packagingcovenant.org.au/public-documents/Sustainable%20Packaging%20Guidelines%20(SPGs) (accessed on 29 October 2025).
- Robertson, G.L. Sustainable food packaging. In Handbook of Waste Management and Co-Product Recovery in Food Processing; Woodhead Publishing: Cambridge, UK, 2009; pp. 221–254. [Google Scholar] [CrossRef]
- Plastics Europe. Plastics—The Facts 2022; Plastics Europe: Brussels, Belgium, 2022; Available online: https://plasticseurope.org/wp-content/uploads/2022/10/PE-PLASTICS-THE-FACTS_V7-Tue_19-10-1.pdf (accessed on 17 September 2025).
- Fayshal, M.A. Current practices of plastic waste management, environmental impacts, and potential alternatives for reducing pollution and improving management. Heliyon 2024, 10, e40838. [Google Scholar] [CrossRef]
- Alias, A.; Wan, M.K.; Sarbon, N. Emerging materials and technologies of multi-layer film for food packaging application: A review. Food Control 2022, 136, 108875. [Google Scholar] [CrossRef]
- Bauer, A.S.; Tacker, M.; Uysal-Unalan, I.; Cruz, R.M.; Varzakas, T.; Krauter, V. Recyclability and redesign challenges in multilayer flexible food packaging—A review. Foods 2021, 10, 2702. [Google Scholar] [CrossRef] [PubMed]
- Tamizhdurai, P.; Mangesh, V.; Santhosh, S.; Vedavalli, R.; Kavitha, C.; Bhutto, J.K.; Alreshidi, M.A.; Yadav, K.K.; Kumaran, R. A state-of-the-art review of multilayer packaging recycling: Challenges, alternatives, and outlook. J. Clean. Prod. 2024, 447, 141403. [Google Scholar] [CrossRef]
- Horodytska, O.; Valdés, F.J.; Fullana, A. Plastic flexible films waste management—A state of art review. Waste Manag. 2018, 77, 413–425. [Google Scholar] [CrossRef]
- Bayus, J.; Ge, C.; Thorn, B. A preliminary environmental assessment of foil and metallized film centered laminates. Resour. Conserv. Recycl. 2016, 115, 31–41. [Google Scholar] [CrossRef]
- Ciawi, Y.; Tonyes, S.G.; Dwipayanti, N.M.U. Multilayer packaging recycling: Challenges, current practices, and future prospects. Acad. Environ. Sci. Sustain. 2025, 2, 1–12. [Google Scholar] [CrossRef]
- Lamberti, M.; Escher, F. Aluminium foil as a food packaging material in comparison with other materials. Food Rev. Int. 2007, 23, 407–433. [Google Scholar] [CrossRef]
- Decker, W.; Roy, D.; Voght, C.; Roy, C.; Dabbert, P. Metallized polymer films as replacement for aluminum foil in packaging applications. In Proceedings of the 47th Annual Technical Conference, Dallas, TX, USA, 24–29 April 2004; Society of Vacuum Coaters: Albuquerque, NM, USA, 2004; pp. 594–599. Available online: https://www.svc.org/clientuploads/directory/resource_library/04_594.pdf (accessed on 29 October 2025).
- Üçüncü, M. Gıda Ambalajlama Teknolojisi; Ambalaj Sanayicileri Derneği: İstanbul, Türkiye, 2011; 877p. [Google Scholar]
- Copeland, N.J.; Astbury, R. Evaporated aluminium on polyester: Optical, electrical, and barrier properties as a function of thickness and time (Part I). In Proceedings of the AIMCAL Technical Conference, Myrtle Beach, SC, USA, 17–20 October 2010; Volume 14. [Google Scholar]
- Struller, C.F.; Kelly, P.J.; Copeland, N.J. Aluminium oxide barrier coatings on polymer films for food packaging applications. Surf. Coat. Technol. 2014, 241, 130–137. [Google Scholar] [CrossRef]
- Struller, C.; Kelly, P.; Copeland, N. Conversion of aluminium oxide coated films for food packaging applications—From a single layer material to a complete pouch. Food Packag. Shelf Life 2019, 20, 100309. [Google Scholar] [CrossRef]
- Sharbafian, F.; Tosic, K.; Schmiedt, R.; Novak, M.; Krainz, M.; Rainer, B.; Apprich, S. Investigation and comparison of alternative oxygen barrier coatings for flexible PP films as food packaging material. Coatings 2024, 14, 1086. [Google Scholar] [CrossRef]
- Struller, C.F.; Kelly, P.J.; Copeland, N.J.; Tobin, V.; Assender, H.E.; Holliday, C.W.; Read, S.J. Aluminium oxide barrier films on polymeric web and their conversion for packaging applications. Thin Solid Films 2014, 553, 153–156. [Google Scholar] [CrossRef]
- Hirvikorpi, T.; Laine, R.; Vähä-Nissi, M.; Kilpi, V.; Salo, E.; Li, W.M.; Lindfors, S.; Vartiainen, J.; Kenttä, E.; Nikkola, J.; et al. Barrier properties of plastic films coated with an Al2O3 layer by roll-to-roll atomic layer deposition. Thin Solid Films 2014, 550, 164–169. [Google Scholar] [CrossRef]
- Patel, J.; Sonar, C.R.; Al-Ghamdi, S.; Tang, Z.; Yang, T.; Tang, J.; Sablani, S.S. Influence of ultra-high barrier packaging on the shelf-life of microwave-assisted thermally sterilized chicken pasta. LWT Food Sci. Technol. 2021, 136, 110287. [Google Scholar] [CrossRef]
- Parhi, A.; Tang, J.; Sablani, S.S. Functionality of ultra-high barrier metal oxide-coated polymer films for in-package, thermally sterilized food products. Food Packag. Shelf Life 2020, 25, 100514. [Google Scholar] [CrossRef]
- CEFLEX. Designing for a Circular Economy: Recyclability of Polyolefin-Based Flexible Packaging, Phase 1; CEFLEX: Brussels, Belgium, 2020; Available online: https://swissrecycle.ch/fileadmin/user_upload/pdfs/Firmen/Drehscheibe_KLW/CEFLEX-D4ACE-Phase-1-Guidelines-Technical-report-SPREAD-VIEW-low-res-June-2020.pdf (accessed on 29 October 2025).
- Soares, C.T.; Ek, M.; Östmark, E.; Gällstedt, M.; Karlsson, S. Recycling of multi-material multilayer plastic packaging: Current trends and future scenarios. Resour. Conserv. Recycl. 2022, 176, 105905. [Google Scholar] [CrossRef]
- Ueda, J.M.; Morales, P.; Fernández-Ruiz, V.; Ferreira, A.; Barros, L.; Carocho, M.; Heleno, S.A. Powdered foods: Structure, processing, and challenges—A review. Appl. Sci. 2023, 13, 12496. [Google Scholar] [CrossRef]
- Intipunya, P.; Bhandari, B. Chemical deterioration and physical instability of food powders. In Food Powders: Physical Properties, Processing, and Functionality; Elsevier eBooks: Amsterdam, The Netherlands, 2010; pp. 663–700. [Google Scholar] [CrossRef]
- Robertson, G.L. Food Packaging: Principles and Practice, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar] [CrossRef]
- Chandan, R.C.; Kilara, A. Dairy Ingredients for Food Processing; Wiley-Blackwell: Hoboken, NJ, USA, 2011. [Google Scholar] [CrossRef]
- Chandan, R.C.; Kilara, A.; Shah, N.P. Dairy Processing and Quality Assurance, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2015. [Google Scholar] [CrossRef]
- Packaging Requirements and Quality Assurance Provisions for Dessert Powder Pudding. 28 September 2007. Available online: https://www.dla.mil/Portals/104/Documents/TroopSupport/Subsistence/Rations/cids/qapkg/Q-20344.pdf (accessed on 17 September 2025).
- Hedegaard, R.S.V.; Skibsted, L.H. Shelf-life of food powders. In Handbook of Food Powders: Processes and Properties; Bhandari, B., Bansal, N., Zhang, M., Schuck, P., Eds.; Woodhead Publishing: Cambridge, MA, USA, 2013; pp. 409–434. [Google Scholar]
- Gökmen, V.; Öztan, A. Gıdaların raf ömrünü etkileyen faktörler ve raf ömrünün belirlenmesi. Gıda 1995, 20, 265–271. Available online: https://dergipark.org.tr/tr/download/article-file/79510 (accessed on 17 September 2025).
- Wang, H.J.; Lee, D.S. Packaging and the shelf life of milk powder products. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Wang, R.; Hartel, R.W. Understanding stickiness in sugar-rich food systems: A review of mechanisms, analyses, and solutions of adhesion. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5901–5937. [Google Scholar] [CrossRef]
- Hernandez, R.J.; Giacin, J.R. Factors affecting permeation, sorption and migration processes in package-product systems. In Food Storage Stability; Taub, I.A., Singh, R.P., Eds.; CRC Press: Boca Raton, FL, USA, 1998; pp. 269–330. [Google Scholar]
- Deshmukh, G.P.; Ravindra, M.R.; Jose, N.; Wasnik, P.G.; Dhotre, A.V. Moisture sorption behaviour and thermodynamic properties of dry-crystallized Palada payasam (rice flakes milk pudding) mix determined using the dynamic vapour sorption method. J. Food Process. Preserv. 2020, 44, e14819. [Google Scholar] [CrossRef]
- Ocieczek, A.; Ruszkowska, M. Influence of water activity on the compressibility and mechanical properties of cocoa products. LWT Food Sci. Technol. 2014, 60, 1054–1060. [Google Scholar] [CrossRef]
- Potter, N.N. Food Science, 3rd ed.; The AVI Publishing Company: Westport, CT, USA, 1978; pp. 79–81. [Google Scholar]
- Muzaffar, K.; Kumar, P. Quality assessment and shelf life prediction of spray dried tamarind pulp powder in accelerated environment using two different packaging materials. J. Food Meas. Charact. 2017, 11, 265–271. [Google Scholar] [CrossRef]
- Devi, K.D.; Paul, S.K.; Sahu, J.K. Study of sorption behavior, shelf life and colour kinetics of vacuum puffed honey powder at accelerated storage conditions. J. Food Sci. Technol. 2016, 53, 2334–2341. [Google Scholar] [CrossRef] [PubMed]
- Henríquez, C.; Córdova, A.; Lutz, M.; Saavedra, J. Storage stability test of apple peel powder using two packaging materials: High-density polyethylene and metallized films of high barrier. Ind. Crops Prod. 2013, 45, 121–127. [Google Scholar] [CrossRef]
- Ramachandra, C.T.; Rao, P.S. Shelf life and colour change kinetics of Aloe vera gel powder under accelerated storage in three different packaging materials. J. Food Sci. Technol. 2013, 50, 747–754. [Google Scholar] [CrossRef]
- Jena, S.H.; Das, H. Shelf life prediction of aluminium foil laminated polyethylene packed vacuum dried coconut milk powder. J. Food Eng. 2012, 108, 135–142. [Google Scholar] [CrossRef]
- Kumar, P.; Mishra, H.N. Storage stability of mango soy fortified yoghurt powder in two different packaging materials: HDPE and ALP. J. Food Eng. 2004, 65, 569–576. [Google Scholar] [CrossRef]
- Jaya, S.; Das, H. Accelerated storage, shelf life and color of mango powder. J. Food Process. Preserv. 2005, 29, 45–62. [Google Scholar] [CrossRef]
- Spiess, W.E.L.; Wolf, W.R. Results of the COST 90 project on water activity. In Physical Properties of Foods; Applied Science Publishers: London, UK, 1983; pp. 65–91. [Google Scholar]
- Labuza, T.P. Moisture Sorption: Practical Aspects of Isotherm Measurement and Use; American Association of Cereal Chemists: St. Paul, MN, USA, 1984. [Google Scholar]
- Wolf, W.; Spiess, W.E.L.; Jung, G. Standardization of isotherm measurements (COST Project 90 and 90 bis). In Properties of Water in Foods: In Relation to Quality and Stability; Springer: Dordrecht, The Netherlands, 1985; pp. 661–679. [Google Scholar] [CrossRef]
- Greenspan, L. Humidity fixed points of binary saturated aqueous solutions. J. Res. Natl. Bur. Stand. Sect. A Phys. Chem. 1977, 81, 89–96. [Google Scholar] [CrossRef]
- Lomauro, C.J.; Bakshi, A.S.; Labuza, T.P. Evaluation of food moisture sorption isotherm equations. Part I: Fruit, vegetable and meat products. Lebensm. Wiss. Und Technol. 1985, 18, 111–117. [Google Scholar]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Van den Berg, C.; Bruin, S. Water activity and its estimation in food systems: Theoretical aspects. In Water Activity: Influences on Food Quality, 1st ed.; Rockland, L.B., Stewart, G.E., Eds.; Academic Press: New York, NY, USA, 1981; pp. 45–58. [Google Scholar]
- Halsey, G. Physical adsorption on non-uniform surfaces. J. Chem. Phys. 1948, 16, 931–937. [Google Scholar] [CrossRef]
- Iglesias, H.A.; Chirife, J. An empirical equation for fitting water sorption isotherms of fruits and related products. Can. Inst. Food Sci. Technol. J. 1978, 11, 12–15. [Google Scholar] [CrossRef]
- Henderson, S.M. A basic concept of equilibrium moisture. Agric. Eng. 1952, 33, 29–32. [Google Scholar]
- Peleg, M. An empirical model for the description of moisture sorption curves. J. Food Sci. 1988, 53, 1216–1217. [Google Scholar] [CrossRef]
- ASTM F1249-1; Standard Test Method for Water Vapor Transmission Rate Through Plastic Film and Sheeting Using a Modulated Infrared Sensor. ASTM International: West Conshohocken, PA, USA, 2013.
- ASTM D3985-17; Standard Test Method for Oxygen Gas Transmission Rate Through Plastic Film and Sheeting Using a Coulometric Sensor. ASTM International: West Conshohocken, PA, USA, 2017. [CrossRef]
- AOAC International. Official Methods of Analysis of AOAC International, 21st ed.; AOAC International: Gaithersburg, MD, USA, 2019. [Google Scholar]
- Goula, A.M.; Adamopoulos, K.G. Effect of maltodextrin addition during spray drying of tomato pulp in dehumidified air: II. Powder properties. Dry. Technol. 2008, 26, 726–737. [Google Scholar] [CrossRef]
- Li, D.; Chen, R.; Liu, J.; Liu, C.; Deng, L.; Chen, J. Characterizing and alleviating the browning of Choerospondias axillaris fruit cake during drying. Food Control 2022, 132, 108522. [Google Scholar] [CrossRef]
- Altuğ-Onoğur, T.; Elmacı, Y. Gıdalarda Duyusal Değerlendirme; Sidas Medya Yayıncılık: İzmir, Türkiye, 2015. [Google Scholar]
- Nelson, K.A.; Labuza, T.P. Water activity and food polymer science: Implications of state on Arrhenius and WLF models in predicting shelf life. In Water in Foods; Elsevier: Amsterdam, The Netherlands, 1994; pp. 271–289. [Google Scholar]
- Koc, B.; Yilmazer, M.S.; Balkır, P.; Ertekin, F.K. Moisture sorption isotherms and storage stability of spray-dried yoghurt powder. Dry. Technol. 2010, 28, 816–822. [Google Scholar] [CrossRef]
- Souza, R.M.; Moreira, C.Q.; Vieira, R.P.; Coltro, L.; Alves, R.M.V. Alternative flexible plastic packaging for instant coffees. Food Res. Int. 2023, 172, 113165. [Google Scholar] [CrossRef]
- Kaymak-Ertekin, F.; Gedik, A. Sorption isotherms and isosteric heat of sorption for grapes, apricots, apples and potatoes. LWT Food Sci. Technol. 2004, 37, 429–438. [Google Scholar] [CrossRef]
- Katz, E.E.; Labuza, T.P. Effect of water activity on the sensory crispness and mechanical deformation of snack food products. J. Food Sci. 1981, 46, 403–409. [Google Scholar] [CrossRef]
- Bejar, A.K.; Mihoubi, N.B.; Kechaou, N. Moisture sorption isotherms—Experimental and mathematical investigations of orange (Citrus sinensis) peel and leaves. Food Chem. 2012, 132, 1728–1735. [Google Scholar] [CrossRef]
- Quirijns, E.J.; Van Boxtel, A.J.; Van Loon, W.K.; Van Straten, G. Sorption isotherms, GAB parameters and isosteric heat of sorption. J. Sci. Food Agric. 2005, 85, 1805–1814. [Google Scholar] [CrossRef]
- Atalar, İ. Effect of agglomeration process on the moisture sorption isotherms and thermodynamics properties of yoghurt powder. GIDA J. Food 2019, 44, 837–848. [Google Scholar] [CrossRef]
- Lewicki, P.P. The applicability of the GAB model to food water sorption isotherms. Int. J. Food Sci. Technol. 1997, 32, 553–557. [Google Scholar] [CrossRef]
- Taitano, L.Z.; Singh, R.P. Moisture adsorption and thermodynamic properties of California-grown almonds (varieties: Nonpareil and Monterey). Int. J. Food Stud. 2012, 1, 33–47. [Google Scholar] [CrossRef]
- Chirife, J.; Iglesias, H.A. Equations for fitting water sorption isotherms of foods: Part 1—A review. Int. J. Food Sci. Technol. 1978, 13, 159–174. [Google Scholar] [CrossRef]
- Labuza, T.P.; Kaanane, A.; Chen, J.Y. Effect of temperature on the moisture sorption isotherms and water activity shift of two dehydrated foods. J. Food Sci. 1985, 50, 385–392. [Google Scholar] [CrossRef]
- Lazić, V.; Budinski-Simendić, J.; Gvozdenović, J.; Simendić, B. Barrier properties of coated and laminated polyolefin films for food packaging. Acta Phys. Pol. A 2010, 117, 855–858. [Google Scholar] [CrossRef]
- Johansson, P.; Lahti, J.; Kuusipalo, J. Influence of pinholes on water vapour and oxygen permeation of packaging foil and films. J. Appl. Packag. Res. 2021, 13, 4. [Google Scholar]
- Ryabova, A.; Semipyatny, V.; Galstyan, A. Effects of storage conditions on milk powder properties. J. Dairy Sci. 2023, 106, 6741–6758. [Google Scholar] [CrossRef]
- Chávez Montes, E.; Ardila Santamaría, N.; Gumy, J.-C.; Marchal, P. Moisture-induced caking of beverage powders. J. Sci. Food Agric. 2011, 91, 2582–2586. [Google Scholar] [CrossRef] [PubMed]
- Decker, W.; Henry, B. Basic Principles of Thin Film Barrier Coatings. Proc. Annu. Tech. Conf. Soc. Vac. Coaters 2002, 45, 492–502. [Google Scholar]
- Tobin, V.R.; Suttle, H.; Assender, H.E. Nanodefect-Controlled Permeation in AlOx/Polymer Gas Barrier Films. Thin Solid Films 2017, 642, 142–150. [Google Scholar] [CrossRef]
- Murray, L. The Impact of Foil Pinholes and Flex Cracks on the Moisture and Oxygen Barrier of Flexible Packaging. In Proceedings of the TAPPI PLACE Conference, Las Vegas, NV, USA, 25–29 September 2005. [Google Scholar]
- Codex Alimentarius Commission. Report of the Eighteenth Session of the Codex Committee on Cocoa Products and Chocolate; FAO: Fribourg, Switzerland, 2000; Available online: https://openknowledge.fao.org/server/api/core/bitstreams/4b7c73b3-2338-450e-ba21-93e5d49ea94c/content (accessed on 29 October 2025).
- Forsido, S.F.; Welelaw, E.; Belachew, T.; Hensel, O. Effects of storage temperature and packaging material on physico-chemical, microbial and sensory properties and shelf life of extruded composite baby food flour. Heliyon 2021, 7, e06821. [Google Scholar] [CrossRef]
- Yian, L.Y.; Phing, P.L. Storage stability of kuini powder in two packaging materials: Aluminum laminated polyethylene and polyethylene terephthalate. Malays. J. Anal. Sci. 2020, 24, 657–669. [Google Scholar]
- Wong, C.W.; Lim, W.T. Storage stability of spray-dried papaya (Carica papaya L.) powder packaged in aluminium laminated polyethylene (ALP) and polyethylene terephthalate (PET). Int. Food Res. J. 2016, 23, 1887–1894. [Google Scholar]
- Shiby, V.K.; Karthik, S.; Priyamvada, T.; Pandey, M.C. Water vapour sorption thermodynamic properties and stability of spray-dried avocado milkshake powder. Indian J. Dairy Sci. 2021, 74, 161–169. [Google Scholar] [CrossRef]
- Jose, N.; Ravindra, M.R.; Deshmukh, G.P.; Rao, K.J. Sorption and thermodynamic properties of dry crystallized Palada payasam mix prepared using manual and mechanical stirring. J. Food Sci. Technol. 2022, 59, 1075–1086. [Google Scholar] [CrossRef]
- Petit, J.; Michaux, F.; Jacquot, C.; Montes, E.C.; Dupas, J.; Girard, V.; Gianfrancesco, A.; Scher, J.; Gaiani, C. Storage-induced caking of cocoa powder. J. Food Eng. 2017, 199, 42–53. [Google Scholar] [CrossRef]
- Jacquot, C.; Petit, J.; Michaux, F.; Montes, E.C.; Dupas, J.; Girard, V.; Gianfrancesco, A.; Scher, J.; Gaiani, C. Cocoa powder surface composition during aging: A focus on fat. Powder Technol. 2016, 292, 195–202. [Google Scholar] [CrossRef]
- Ho, T.M.; Truong, T.; Bhandari, B.R. Methods to characterize the structure of food powders—A review. Biosci. Biotechnol. Biochem. 2017, 81, 651–671. [Google Scholar] [CrossRef] [PubMed]
- Petronilho, S.; Oliveira, A.; Domingues, M.R.; Nunes, F.M.; Coimbra, M.A.; Gonçalves, I. Hydrophobic starch-based films using potato washing slurries and spent frying oil. Foods 2021, 10, 2897. [Google Scholar] [CrossRef]
- Aguilera, J.; Del Valle, J.; Karel, M. Caking phenomena in amorphous food powders. Trends Food Sci. Technol. 1995, 6, 149–155. [Google Scholar] [CrossRef]
- GEA Process Engineering A/S. A 15 a—Degree of Caking. GEA NIRO® Method No. A 15 a. Revised: January 2024. Available online: https://www.gea.com/en/assets/170114/ (accessed on 18 October 2025).
- Calva-Estrada, S.; Utrilla-Vázquez, M.; Vallejo-Cardona, A.; Roblero-Pérez, D.; Lugo-Cervantes, E. Thermal properties and volatile compounds profile of commercial dark chocolates from different genotypes of cocoa beans (Theobroma cacao L.) from Latin America. Food Res. Int. 2020, 136, 109594. [Google Scholar] [CrossRef]
- Dey, A.; Dutta, D.; Singh, A.; Sit, N. Physicochemical and functional properties of starches isolated from different millets. Meas. Food 2024, 15, 100188. [Google Scholar] [CrossRef]
- Alcázar-Alay, S.C.; Meireles, M.A.A. Physicochemical properties, modifications and applications of starches from different botanical sources. Food Sci. Technol. 2015, 35, 215–236. [Google Scholar] [CrossRef]
- Zeleznak, K.J.; Hoseney, R.C. The glass transition in starch. Cereal Chem. 1987, 64, 121–124. [Google Scholar]
- Pérez, A.; De Sousa, A.; López, J.V.; Laredo, E.; Newman, D.; Sandoval, A.J.; Müller, A.J. Thermal and mechanical characteristics of a glassy food model based on cassava starch. Appl. Food Res. 2022, 2, 100231. [Google Scholar] [CrossRef]
- Chung, H.J.; Lee, E.J.; Lim, S.T. Comparison in glass transition and enthalpy relaxation between native and gelatinized rice starches. Carbohydr. Polym. 2002, 48, 287–298. [Google Scholar] [CrossRef]
- Figueroa, Y.; Guevara, M.; Pérez, A.; Cova, A.; Sandoval, A.J.; Müller, A.J. Effect of sugar addition on glass transition temperatures of cassava starch with low to intermediate moisture contents. Carbohydr. Polym. 2016, 146, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Yu, L.; Liu, H.; Chen, L.; Li, L. Glass transition temperature of starch studied by a high-speed DSC. Carbohydr. Polym. 2009, 77, 250–253. [Google Scholar] [CrossRef]
- Kristanti, D.; Herminiati, A. Physicochemical and microbiological properties of pudding powder as a complementary food during storage. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1011, 012034. [Google Scholar] [CrossRef]
- Gvozdenović, J. Investigation of characteristic colour stability of powdered orange. Food Chem. 2000, 70, 291–301. [Google Scholar] [CrossRef]
- Irwandi, J.; Man, Y.B.C.; Yusof, S.; Jinap, S.; Sugisawa, H. Effects of type of packaging materials on physicochemical, microbiological and sensory characteristics of durian fruit leather during storage. J. Sci. Food Agric. 1998, 76, 427–434. [Google Scholar] [CrossRef]







| Film Type | Explanation | OTR (cc/m2·Day·Atm) | WVTR (g/m2·Day) | Acceptable Storage Period (Days) |
|---|---|---|---|---|
| PET/Al-foil/LDPE (Control) | 12 µm PET (Polyethylene terephthalate)/ 9 µm Al-Foil (Aluminum foil/60 µm LDPE (Low density polyethylene) | 0.026 ± 0.002 | 0.049 ± 0.053 | 800.32 |
| PET.AlOx/LDPE | 12 µm PET.AlOx (AlOx-coated PET)/ 50 µm LDPE | 0.130 ± 0.000 | 0.190 ± 0.020 | 577.92 |
| MPET/LDPE | 12 µm MPET (Metallized polyethylene terephthalate)/50 µm LDPE | 0.335 ± 0.134 | 0.356 ± 0.035 | 407.58 |
| MBOPP | 30 µm MBOPP (Metallized biaxially oriented polypropylene) | 12.190 ± 1.965 | 0.650 ± 0.050 | 229.26 |
| Model | Parameters | ||
|---|---|---|---|
| Name | Equation | ||
| BET * [57] | M0 | 0.0164 | |
| C | 13.150 | ||
| P (%) | 5.09 | ||
| RMSE (%) | 6.55 | ||
| R2 | 0.977 | ||
| GAB [58] | M0 | 0.0106 | |
| C | 1282 | ||
| K | 1.163 | ||
| P (%) | 3.66 | ||
| RMSE (%) | 0.045 | ||
| R2 | 0.9953 | ||
| Halsey [59] | k | 0.111 | |
| n | 0.353 | ||
| P (%) | 10.89 | ||
| RMSE (%) | 0.123 | ||
| R2 | 0.972 | ||
| Iglesias-Chirife [60] | k | 36.798 | |
| c | −1.260 | ||
| M0.5 | 0.0311 | ||
| P (%) | 1.524 | ||
| RMSE (%) | 0.027 | ||
| R2 | 0.856 | ||
| Henderson [61] | k | 2.316 | |
| n | 0.184 | ||
| P (%) | 11.95 | ||
| RMSE (%) | 0.004 | ||
| R2 | 0.964 | ||
| Peleg [62] | k1 | 0.0514 | |
| n1 | 0.7392 | ||
| k2 | 18.598 | ||
| n2 | 24.407 | ||
| P (%) | 4.277 | ||
| RMSE (%) | 0.0015 | ||
| R2 | 0.999 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Türksever, C.; Koç, B.; Kizilirmak Esmer, O. Sustainable Alternatives in Multilayer Packaging: Storage Stability of Pudding Powder Under Accelerated Storage Conditions. Foods 2025, 14, 3806. https://doi.org/10.3390/foods14223806
Türksever C, Koç B, Kizilirmak Esmer O. Sustainable Alternatives in Multilayer Packaging: Storage Stability of Pudding Powder Under Accelerated Storage Conditions. Foods. 2025; 14(22):3806. https://doi.org/10.3390/foods14223806
Chicago/Turabian StyleTürksever, Can, Banu Koç, and Ozlem Kizilirmak Esmer. 2025. "Sustainable Alternatives in Multilayer Packaging: Storage Stability of Pudding Powder Under Accelerated Storage Conditions" Foods 14, no. 22: 3806. https://doi.org/10.3390/foods14223806
APA StyleTürksever, C., Koç, B., & Kizilirmak Esmer, O. (2025). Sustainable Alternatives in Multilayer Packaging: Storage Stability of Pudding Powder Under Accelerated Storage Conditions. Foods, 14(22), 3806. https://doi.org/10.3390/foods14223806