Abstract
Ulcerative colitis (UC) is associated with gut microbiota imbalance, and probiotics may restore gut flora and improve intestinal health. Sea buckthorn, which is rich in antioxidants and anti-inflammatory compounds, may enhance these effects when combined with probiotics. In this study, we incorporated our screened strain Lactiplantibacillus plantarum YHG-87 (L. plantarum YHG-87) into sea buckthorn fermented milk to examine its additional benefits in a dextran sulfate sodium (DSS)-induced UC mouse model. Five groups (n = 6) were included: healthy control, DSS-induced colitis, regular fermented milk, sea buckthorn fermented milk, and sea buckthorn probiotic fermented milk (SC group). Results indicated that SC group significantly alleviated UC symptoms, such as weight loss, bloody stools, and colon shortening (p < 0.05), and these improvements were more pronounced than those observed in the sea buckthorn fermented milk group. Moreover, the SC group exhibited stronger anti-inflammatory and antioxidant effects, including reduced IL-6, increased IL-4 and IL-10, elevated glutathione, and reduced myeloperoxidase and malondialdehyde (p < 0.05). Additionally, the SC intervention led to notable shifts in gut microbial community composition. In conclusion, the addition of L. plantarum YHG-87 to sea buckthorn fermented milk provided enhanced protective effects against UC, suggesting that the combination of bioactive plant components with selected probiotics offers promising potential for UC prevention and therapy.
1. Introduction
Ulcerative colitis (UC) is a major type of inflammatory bowel disease (IBD), commonly characterized by diarrhea, bloody stools, and colon shortening [1]. Studies indicate that the development of UC is closely linked to abnormal intestinal epithelial cell function and a compromised intestinal mucosal barrier [2]. Without proper treatment, UC tends to recur, and ongoing intestinal damage raises the risk of colorectal cancer [3]. Current UC treatments mainly involve anti-inflammatory drugs and immunosuppressants, such as aminosalicylates, sulfasalazine, and corticosteroids [4]. While these drugs can relieve symptoms, they rarely cure the disease completely and may cause side effects or drug resistance with prolonged use. Therefore, the focus of current research is on exploring safe, effective, and long-term preventive and adjuvant therapies.
Recently, the role of probiotics in promoting health has gained significant attention, with research spanning IBD, irritable bowel syndrome, obesity, and allergies [5]. Probiotic fermented milk has been shown to benefit intestinal inflammation by improving digestion, lowering cholesterol [6], and modulating immune cells like T cells, dendritic cells, and macrophages [7]. Lactiplantibacillus plantarum (L. plantarum), in particular, has shown promise in alleviating UC by modulating immune responses, reducing oxidative stress, enhancing tight junction protein secretion, improving gut microbiota, and increasing short-chain fatty acid levels [7,8].
Sea buckthorn (Hippophae rhamnoides L.) is a medicinal and edible plant widely distributed in Asia and Europe, rich in vitamins (0.48–2.87%FW), polysaccharides (0.04–0.39%FW), organic acids (8.56 (g/kg), and polyphenols (28.97 mg GAE/g dw) [9], Its bioactive components, including polysaccharides, seed oil, and proanthocyanidins, have demonstrated anti-inflammatory, antioxidant, and immunomodulatory effects, and have been reported to alleviate UC symptoms [10]. In addition, sea buckthorn contains polysaccharides and polyphenols that may act as prebiotic substrates, selectively stimulating the growth and activity of beneficial bacteria, thus exerting a prebiotic-like effect. This suggests that sea buckthorn not only provides bioactive compounds but also may enhance the growth and function of probiotics, supporting gut health and UC prevention. Therefore, the abundant nutrients and health-promoting functions of sea buckthorn provide a strong rationale for its selection in this study. However, its high organic acid content contributes to a sour and astringent taste, limiting consumer acceptance. To overcome this limitation, we combined sea buckthorn with probiotic fermented milk, hypothesizing that this combination could not only improve palatability but also enhance synergistic protective effects against UC.
Previous studies have shown that sea buckthorn fermented milk, made by fermenting sea buckthorn puree and milk with L. plantarum YHG-87, contains significantly higher total phenol content than regular fermented milk. This not only improves flavor but also enhances in vitro antioxidant activity and lactic acid bacteria(LAB) count [11]. Relevant studies indicate that LAB can convert bound phenols in plants into free phenols, enhancing in vitro antioxidant activity [12]. Additionally, LAB produce substances such as short-chain fatty acids and amino acids through metabolism, which promote the expression of tight junction proteins in intestinal epithelial cells, thereby improving intestinal barrier function [13]. Therefore, combining bioactive substances from sea buckthorn with L. plantarum YHG-87 in fermented milk may positively affect UC prevention.
This research aims to assess the potential protective effects of sea buckthorn fermented milk with L. plantarum YHG-87 in a dextran sulfate sodium (DSS)-induced mouse model of UC. Key parameters, including disease activity index (DAI), colon length, body weight changes, cellular inflammatory factors, oxidative stress markers, and gut microbiota diversity and abundance, will be evaluated to further explore the fermented milk’s therapeutic effects and underlying mechanisms. This study provides evidence supporting the use of functional foods based on natural products and probiotics for UC prevention and adjunctive treatment, offering new insights for the development of safe and effective interventions.
2. Materials and Methods
2.1. Animals
Male specific pathogen-free (SPF) KM (Kunming mice) mice (age 8 weeks, weight 20 ± 2 g) were purchased from Chengdu dossy experimental animals Co., Ltd. (Chengdu, Sichuan, China). All mice were housed in a standard SPF environment in the animal house of the Sichuan Lilaisino Biotechnology Co., Ltd. (Chengdu, Sichuan, China). (granted the laboratory animal use permit, SYXK 2021-246). Six mice were maintained in each individually ventilated cage (temperature, 23 ± 3 °C; relatively humidity, 50 ± 10%; standard 12 h/12 h light/dark cycle). All mice were acclimatized one week before the formal experiment with free access to food and water ad libitum, and basic dietary feed by Chengdu dossy Laboratory Animal Co. (Chengdu, Sichuan, China). (Production License No. SCXK(Chuan)2019-028). All animal experimental protocols were strictly performed in accordance with the provisions of the National Institutes of Health of the United States, approved by the Ethics Committee of Sichuan Lilaisinuo Biotechnology Co. Ltd. (Chengdu, Sichuan, China) (LLSN-2023017).
One mouse in the DSS group died during modeling and was considered an adverse event, leading to missing end-point data. Therefore, the DSS group included five mice, while the other groups included six mice. All surviving animals were included in the analysis (Figure S1).
2.2. Preparation of Sea Buckthorn Fermented Milk with L. plantarum YHG-87, Sea Buckthorn Fermented Milk and Regular Fermented Milk
In this study, L. plantarum YHG-87, isolated from the traditionally fermented food “Jiangshui” in the Ningxia Hui Autonomous Region, was combined with a commercial fermented milk starter in a 2:1 ratio (3.48 × 109 CFU/mL: 1.74 × 109 CFU/mL, respectively). Pasteurized milk, sea buckthorn pulp (hereinafter referred to as “sea buckthorn,” Hippophae rhamnoides L., Ningxia Huaxinda Health Technology Co., LT, Yinchuan, Ningxia), and white sugar were mixed in approximate proportions of 87%, 6%, and 7% (w/w, Unit: g), respectively, and pasteurized at 70 °C for 30 min. After cooling, the mixture was reserved for later use. The activated bacterial L. plantarum YHG-87 culture was inoculated into the cooled mixture, and fermentation was carried out at 42 °C for 6 h to achieve a target pH of 4.5. Following fermentation, the product was refrigerated at 4 °C for overnight ripening, and fermented milk was prepared weekly during gavage. The regular and sea buckthorn fermented milk preparations followed the same procedure as that of the sea buckthorn fermented milk with L. plantarum YHG-87.
2.3. Trial Design
After a 7-day acclimatization period, the mice were randomly assigned to five treatment groups, each containing six mice: the healthy control group (CON), the model group (DSS), the regular fermented milk group (PS), the sea buckthorn fermented milk group (SS), and the sea buckthorn fermented milk with L. plantarum YHG-87 group (SC). The experiment lasted for 30 days. The CON and DSS groups were gavaged with 9% (w/v) saline, and the SC group were given a daily dose of 10 μL/g body weight, respectively. The modeling process began after 23 days of continuous gavage. A 3% (w/v) DSS solution was prepared in sterile water for modeling. The CON group continued to drink sterile water, while the other four groups received DSS solution. Simultaneously, the PS, SS, and SC groups continued their gavage treatments with the respective samples. The experiment concluded after 7 days of continuous gavage in all experimental groups (Figure 1A).
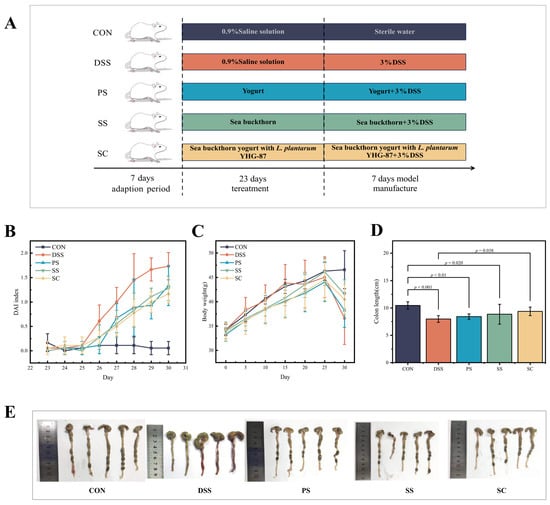
Figure 1.
Sea buckthorn probiotic fermented milk administration attenuated the symptoms of DSS-induced colitis. (A) Schematic illustration of the experimental design. The “CON”, “DSS”, “PS”, “SS” and “SC” groups represented the healthy control, model, regular fermented milk, sea buckthorn fermented milk, and sea buckthorn fermented milk with L. plantarum YHG-87, respectively. (B) Disease activity index (DAI) scores at the end of the animal trial. (C) Body weight change in mice in the animal trial. (D) Colon length at the end of the animal trial. (E) Representative pictures of colon morphology of mice from the five treatment groups. Error bars represent standard deviation of the mean. Significant differences are represented by asterisks.
2.4. Disease Activity Index (DAI)
Mice were weighed daily, and their general condition was monitored. Fecal characterization and occult blood were scored, along with disease activity scoring. The DAI was determined daily based on body weight loss, stool consistency, and fecal occult blood (Determination by o-toluidine method). The DAI was calculated as follows: (Weight loss score + Stool consistency score + fecal occult blood score)/3, with higher values indicating more severe intestinal inflammation in mice (Table 1).

Table 1.
Criteria for scoring disease activity index.
2.5. Colon Length Measurement
A section of the mouse colon was treated with cold physiological saline. Excess moisture was then removed with clean filter paper. The colon tissue was then spread flat, and its length was measured using a ruler.
2.6. Histopathological Analysis
A 0.5 cm colon tissue sample was processed using an automatic dehydrator, followed by embedding and sectioning. The sections were subsequently stained with hematoxylin and eosin (H&E) and imaged with a digital slide scanner. The histological score was determined according to criteria in Table 2.

Table 2.
Histological grading of colitis.
2.7. Changes in Inflammatory Cytokines in the Serum
After collecting blood from the mice, the samples were centrifuged at 3500 rpm for 10 min at 4 °C, and the supernatant was isolated. Inflammatory cytokine levels, including Interleukin-4 (IL-4), Interleukin-10 (IL-10), Interleukin-6 (IL-6), and Tumor Necrosis Factor-α (TNF-α), were quantified using an ELISA kit (Shanghai Zhuocai Biotechnology Co., Ltd., Shanghai, China). An automatic plate washer(PW-480, Shenzhen Huisong Technology Development Co., Ltd., Shenzhen, Guangdong, China) was used for washing, and readings were taken using a SpectraMAX Plus384 (Meigu Molecular Instruments Co., Ltd., Shanghai, China).
2.8. Tissue Oxidative Damage Assessment
After obtaining the serum from the mice, malondialdehyde (MDA) levels were measured using an MDA assay kit (Nanjing Jianjian Bioengineering Research Institute, Nanjing, Jiangsu, China). Glutathione (GSH) and myeloperoxidase (MPO) levels were measured using the same methods as those used for inflammatory cytokines (Shanghai Zhuocai Biotechnology Co., Ltd., Shanghai, China).
2.9. Fecal DNA Extraction, Sequencing and Analysis
150 mg of mouse feces was transferred into a sterile centrifuge tube. Then, 1 mL of lysis buffer (PBS) was added, and the mixture was vortexed. 20 µL of proteinase K (final concentration ~20 mg/mL) was added, followed by another vortexing. The sample was incubated at 55 °C in a water bath for 30–60 min, vortexing every 10 min to ensure thorough mixing. Afterward, an equal volume of phenol-chloroform-isoamyl alcohol (25:24:1) was added, and the sample was mixed thoroughly before centrifugation at 12,000× g for 10 min. Twice the volume of cold anhydrous ethanol and one-tenth the volume of sodium acetate (3M, pH 5.2) were added. The sample was mixed thoroughly and incubated at −20 °C for 30 min. The DNA precipitate was collected by centrifugation at 12,000× g for 10 min. The precipitate was washed with 70% ethanol, dried, and dissolved in TE buffer or sterile water. Low-speed centrifugation (500–1000× g for 1–2 min) was performed to remove large particles, leaving the supernatant. DNA integrity and size were assessed using 1.2% agarose gel electrophoresis.
The 16S rRNA gene was amplified using primers 341F (5′-CCTACGGGNGGCWGCAG-3′) and 806R (5′-GGACTACHVGGGTATCTAAT-3′), targeting the V3-V4 region. The DNA libraries were prepared using the TruSeq Nano DNA LT Library Prep Kit (Illumina, San Diego, CA, USA), and sequencing was performed on an Illumina platform, with paired-end 2 × 300 bp reads. Negative controls (NTC) were included by substituting sterile water for the sample DNA. After sequencing, the raw data were initially screened based on sequence quality. Problematic samples were re-sequenced or supplemented as necessary to ensure data integrity. Following quality control, the raw sequences were processed based on their index and barcode information, and barcode sequences were removed. Sequence denoising and clustering were performed using the QIIME2 dada2 pipeline [14,15], which generates Amplicon Sequence Variants (ASVs), instead of traditional Operational Taxonomic Units (OTUs), for a more accurate microbial community profile. The use of ASVs provides a finer-resolution classification of microbial sequences, enhancing the accuracy of diversity analysis. The species composition of each sample was analyzed at different taxonomic levels, providing a comprehensive overview of the gut microbiome. Alpha diversity indices, such as Chao1, Shannon, and Simpson, were calculated based on the distribution of ASVs across samples. Rarefaction curves were generated to confirm sufficient sequencing depth, ensuring that the results were not influenced by sequencing bias. To assess beta diversity, a distance matrix was calculated at the ASV level, and various unsupervised methods, such as ordination (PCoA) and clustering, were applied to evaluate differences in microbial communities across the samples. Appropriate statistical tests, including PERMANOVA, were performed to determine the significance of these differences. At the species classification level, both unsupervised and supervised methods were used to analyze the abundance composition of species across different samples. We then constructed a network based on the distribution of species in each sample, calculating topological indices to identify key species that may play critical roles in the microbiome. All experiments were conducted in triplicate to ensure the robustness and reliability of the data. The results were further validated using multiple statistical analyses, including Kruskal–Wallis tests for alpha diversity, PERMANOVA for beta diversity, and differential abundance analyses (LEfSe and Wilcoxon rank-sum tests), to ensure the consistency and robustness of the findings.
2.10. Statistical Analyses
Statistical analysis was conducted using IBM SPSS Statistics 22.0. For repeated measures, appropriate methods such as repeated-measures analysis of variance (RM-ANOVA) and linear mixed-effects models (LMMs) were used to account for within-subject correlation and unequal sample sizes. For single-time-point analyses, one-way ANOVA was applied. Post hoc multiple comparisons were performed using the LSD test when variance homogeneity was assumed, and Tamhane’s T2 test when it was not. Data were expressed as mean ± standard deviation. p < 0.05 was considered statistically significant.
3. Results
3.1. Effects of Sea Buckthorn Fermented Milk with L. plantarum YHG-87 on DAI Score and Colon Length in Mice
The DAI scores were plotted based on changes in rectal bleeding, stool consistency, and weight loss in mice (Figure 1B, Tables S1–S5). At the end of the intervention, DAI scores in the DSS group were significantly higher than those in the CON group (p < 0.001). Meanwhile, in the DAI score, the PS group, SS group and the SC group showed significant differences from the DSS group (p < 0.05). Thus, fermented milk administration helps alleviate the underlying symptoms reflected in the DAI score. Regarding body weight, the DSS group showed a significant decrease in body weight change compared to the CON group (p < 0.01) (Figure 1C, Table S6). The body weight changes in the PS, SS, and SC groups were significantly higher than those in the DSS group (p < 0.05). However, no significant difference was observed among the fermented milk groups (p > 0.05). In this experiment, the average colon length in the DSS group was significantly shorter than in the CON group (p < 0.01) (Figure 1D,E). The colon length in the SC groups significantly increased compared to the DSS group.
3.2. Histopathological Analysis of Colon Tissue
DSS-induced UC is characterized by intestinal mucosal damage, colon epithelial cell injury, and inflammatory cell infiltration, representing typical morphological and pathological changes [16,17]. Therefore, we used colon tissue pathology as an evaluation criterion in this experiment. H&E staining (Figure 2) showed that the CON group’s colon tissue structure was intact with normal epithelial cell morphology, no significant degeneration, necrosis, or detachment, and a normal number of goblet cells. In contrast, the DSS group’s mucosal layer exhibited varying degrees of degeneration and necrosis, with tissue hyperplasia and some inflammatory cell infiltration in necrotic areas. Some SS and PS group samples showed minimal inflammatory cell infiltration and mild fibrous tissue hyperplasia. Following sea buckthorn probiotic fermented milk intervention, the mucosal, submucosal, muscular, and serosal layers of the colon tissues remained intact, with no significant degeneration, necrosis, or detachment, indicating a protective effect from prior consumption.
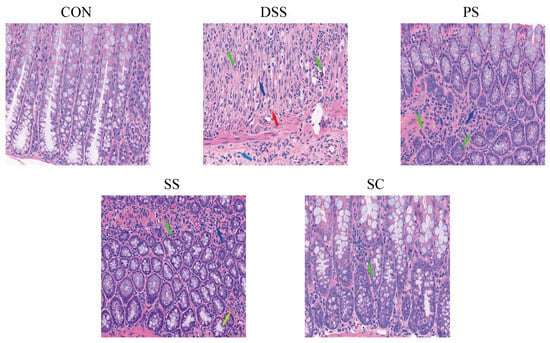
Figure 2.
Sea buckthorn probiotic fermented milk administration protected against DSS-induced colon tissue damage (Hematoxylin and eosin staining microscopic images). Green, light blue, blue, red, and yellow arrows denote. neutrophils, lymphocytes, fibroblasts, new capillaries, and vascular congestion, respectively. (CON: the healthy control group, DSS: the model group, PS: the regularly fermented milk group, SS: the sea buckthorn fermented milk group, SC: the sea buckthorn with L. plantarum YHG-87 fermented milk group).
3.3. Changes in Serum Cytokines Levels
This study investigated the effects of sea buckthorn fermented milk with L. plantarum YHG-87 on inflammatory markers in DSS-induced UC. We measured pro-inflammatory factors (IL-6 and TNF-α) and anti-inflammatory factors (IL-4 and IL-10) to evaluate the immune response. Compared with the CON group, IL-4 (Figure 3A, Table S7) and IL-10 (Figure 3B, Table S8) levels were significantly reduced in the DSS group (p < 0.05), while IL-6 (Figure 3C, Table S9) and TNF-α (Figure 3D, Table S10) levels were significantly increased (p < 0.05). After intervention, IL-4 and IL-10 levels were significantly higher and IL-6 levels significantly lower in the SC group compared with the DSS group (p < 0.05). In contrast, TNF-αshowed only a downward trend in the SC group but did not reach statistical significance (p > 0.05). No significant changes in IL-10 were observed among the fermented milk groups compared with the CON group, suggesting that fermented milk intervention may help maintain baseline IL-10 levels. These results indicate that sea buckthorn fermented milk with L. plantarum YHG-87 exerts a stronger modulatory effect on cytokines, particularly by enhancing IL-4 and IL-10 and suppressing IL-6.
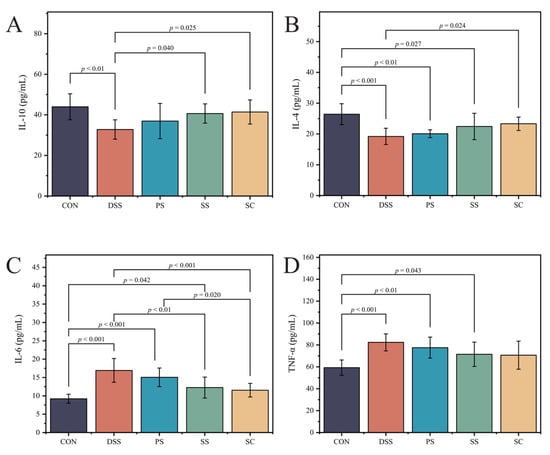
Figure 3.
The fermented milk intake alleviated DSS-induced changes in serumPro-/anti-inflammatory cytokine levels (A) Interleukin-10 (IL-10), (B) Interleukin-4 (IL-4), (C) Interleukin-6 (IL-6), (D) Tumor necrosis factor-α (TNF-α). Error bars represent standard deviation of the mean. Significant differences are represented by asterisks. (CON: the healthy control group, DSS: the model group, PS: the regular fermented milk group, SS: the sea buckthorn fermented milk group, SC: the sea buckthorn fermented milk with L. plantarum YHG-87 group).
3.4. Changes in Oxidative Stress Levels
Glutathione (GSH), myeloperoxidase (MPO), and malondialdehyde (MDA) were used as indicators of tissue oxidative damage (Figure 4). The results showed that compared to CON group, the DSS group had significantly lower GSH(Figure 4A, Table S11) levels and significantly higher MPO(Figure 4B, Table S12) and MDA(Figure 4C, Table S13) levels (p < 0.05). Additionally, MDA levels were significantly reduced in all three fermented milk intervention groups compared to the DSS group (p < 0.05). Although GSH levels increased in the SC group compared to the DSS group, the difference was not statistically significant (p > 0.05). The SC group had significantly lower MPO levels compared to the DSS group (p < 0.05). No significant differences were observed between the fermented milk groups for the other two indicators, except for MPO. However, the SC group displayed some advantages (p > 0.05). These results indicate that sea buckthorn fermented milk with L. plantarum YHG-87 has the strongest antioxidative and anti-inflammatory effects.
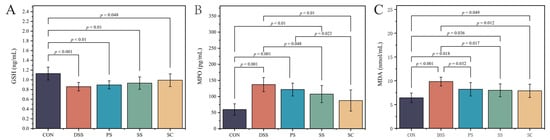
Figure 4.
Effect of sea buckthorn probiotic fermented milk supplementation on oxidative stress marker. (A) Glutathione (GSH), (B) Myeloperoxidase (MPO) and (C) Malondialdehyde (MDA). Error bars represent standard deviation of the mean. Significant differences are represented by asterisks. (CON: the healthy control group, DSS: the model group, PS: the regular fermented milk group, SS: the sea buckthorn fermented milk group, SC: the sea buckthorn fermented milk with L. plantarum YHG-87 group).
3.5. Gut Microbiota Composition
To visually represent OTU similarity and overlap among samples, Venn diagrams were used for statistical analysis of each experimental group’s OTUs. The results showed that the five experimental groups shared 67 OTUs. The SC group had 472 unique OTUs, followed by 459 in the SS group, 445 in the PS group, 351 in the CON group, and 418 in the DSS group (Figure 5A). Further taxonomic analysis at the genus level revealed compositional variations among groups (Figure 5B). Genera such as Bacteroides, Adlercreutzia, Akkermansia, and Ligilactobacillus showed different relative abundances across treatments. Compared with the DSS group, fermented milk interventions—particularly the SC group—resulted in clear shifts in microbial community structure. PCoA demonstrated distinct separation between the DSS and SC groups, while the three fermented milk groups showed partial overlap, suggesting similar yet differentiated community patterns (Figure 5C, Table S14). Overall, these results indicate that supplementation with L. plantarum YHG-87 in sea buckthorn fermented milk altered the gut microbial composition and may contribute to maintaining intestinal homeostasis.
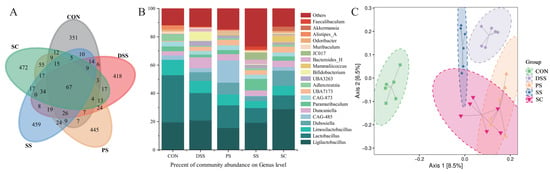
Figure 5.
Effect of sea buckthorn probiotic fermented milk on the gut Microbiota composition. (A) The Venn network plot illustrates the common and unique microorganisms identified in comparisons among different groups. (B) Genus-level gut microbiota profile. (C) Principal coordinate analysis (PCoA; Bray–Curtis distance) score plot of mouse gut microbiota. (CON: the healthy control group, DSS: the model group, PS: the regular fermented milk group, SS: the sea buckthorn fermented milk group, SC: the sea buckthorn fermented milk with L. plantarum YHG-87 group).
4. Discussion
Intestinal microecological imbalance is considered a key pathogenic factor in the pathophysiology of UC [18,19]. In UC patients, beneficial bacteria decrease while pathogenic bacteria increase, leading to impaired intestinal barrier function and excessive immune responses. Probiotics, as biotherapeutics that balance gut microbiota and improve the intestinal environment, have gained significant attention in recent years [20]. Sea buckthorn is a medicinal and edible plant rich in vitamins, flavonoids, and active compounds [9], possessing antioxidant, anti-inflammatory, and immunomodulatory properties [21]. Studies show that sea buckthorn polysaccharides and proanthocyanidins benefit gut health by inhibiting inflammatory factor expression and alleviating intestinal inflammation [10]. Thus, combining sea buckthorn with L. plantarum YHG-87 may synergistically modulate gut microbiota, enhance mucosal barrier function, and alleviate inflammatory responses, providing a protective effect against UC. This study combined sea buckthorn with L. plantarum YHG-87 to evaluate the therapeutic effects of sea buckthorn fermented milk with L. plantarum YHG-87 in a DSS-induced UC model.
In preliminary experiments, we investigated the mixed fermentation process of sea buckthorn fermented milk with L. plantarum YHG-87. Results showed that adding L. plantarum YHG-87significantly increased key flavor compounds in fermented milk, such as cyclohexanone, 2-heptanol, 2,3-butanedione, and trans-2-hexenal. Incorporating sea buckthorn introduced 11 volatile compounds, including acids, esters, ketones, alcohols, α-pinene, 2,5-dimethylfuran, and 2,3,5-trimethylpyrazine, enhancing the fermented milk’s flavor profile. Furthermore, adding sea buckthorn not only enhanced flavor but also significantly increased L. plantarum content. The experimental results showed that sea buckthorn fermented milk with L. plantarum YHG-87 not only significantly enhanced L. plantarum YHG-87 growth but also exhibited the highest levels of total phenolic content and in vitro antioxidant activities, including DPPH radical scavenging, ABTS radical scavenging, and ferric ion reducing power, among the tested products [11].
Based on previous research, this study further explored the effects of sea buckthorn fermented milk with L. plantarum YHG-87 on DSS-induced UC. DSS disrupts the intestinal barrier, making the gut susceptible to harmful microorganisms, which leads to symptoms like weight loss, shortened colon length, and bloody stool [22]. Results indicated that, compared to the DSS group, SC group mice treated with sea buckthorn fermented milk with L. plantarum YHG-87 showed significant improvements in weight, bleeding scores, and colon length, particularly in alleviating weight loss, demonstrating its preventive and alleviating effects. Similar studies have shown that probiotic preparations protect against UC, probiotics had been shown to alleviate symptoms of UC, including weight loss, diarrhea, blood in the stool, and a shortened colon length, while also restoring intestinal microecological homeostasis, improving gut barrier function, modulating the intestinal immune response, and attenuating intestinal inflammation [23]. For example, Georgios et al. reported that Lactobacillus rhamnosus reduce UC symptoms by modulating gut microbiota and enhancing intestinal barrier function [24]. Regarding colon length, the PS, SS, and SC groups all showed some beneficial effects compared with the DSS group, but only the SC group showed a significant increase in colon length, indicating that sea buckthorn fermented milk with L. plantarum YHG-87 positively affected colon shortening. Colon shortening was a hallmark of UC. The experiments demonstrated that several treatment groups had positive effects on body weight relative to the model group. In terms of colon length, the SC group showed a more significant effect, although other groups showed positive effects, albeit without significant changes. Similarly, DAI scores yielded comparable results. Mantel et al. [25] fermented whole milk with the probiotic Propionibacterium freudenreichii, demonstrating its significant reduction in UC clinical signs in a mouse model of colitis. This study further confirms that adding sea buckthorn components can enhance this protective effect. This effect may be attributed to the polyphenols and antioxidants present in sea buckthorn, which enhance the integrity of the intestinal mucosal barrier and immune function, thereby combating intestinal inflammation.
During intestinal inflammation, the balance between pro-inflammatory and anti-inflammatory factors is crucial for disease progression and recovery. Increased levels of pro-inflammatory factors like IL-6 and TNF-α [26] and decreased levels of anti-inflammatory factors like IL-4 and IL-10 are key markers of disease progression. IL-4 regulates the expression of pro-inflammatory factors in immune responses and alleviates UC by increasing intestinal mucus thickness through goblet cell modulation [27]. IL-10 exerts anti-inflammatory effects by inhibiting the secretion of pro-inflammatory factors. This study found that after sea buckthorn fermented milk with L. plantarum YHG-87 intervention, levels of anti-inflammatory factors IL-10 and IL-4 in mice significantly increased, while the level of pro-inflammatory factor IL-6 significantly decreased. Although TNF-α did not show a significant change, it exhibited a downward trend. These findings suggest that sea buckthorn fermented milk with L. plantarum YHG-87 may regulate the balance of inflammatory factors. Previous studies have shown that probiotics can balance immune responses by modulating inflammatory factor expression, thereby alleviating intestinal inflammation [28]. Moreover, studies using anti-TNF-α and anti-IL-6 monoclonal antibodies have demonstrated that reducing TNF-α and IL-6 levels significantly alleviates colonic damage in colitis [26]. These findings further support the potential of sea buckthorn fermented milk with L. plantarum YHG-87 for preventing and treating UC. Additional studies support this finding. For example, Ladda et al. [29] demonstrated that probiotics can reduce IL-6 and TNF-α expression by modulating the NF-κB signaling pathway, thereby alleviating intestinal inflammation. Consistent with these findings, sea buckthorn fermented milk with L. plantarum YHG-87 seems to better maintain immune balance in the gut and reduce abnormal elevations in inflammatory factors. Additionally, UC is often characterized by intestinal mucosal damage, epithelial cell injury, and inflammatory cell infiltration. In this study, H&E staining revealed significant structural damage in the colonic tissues of DSS-treated mice. In contrast, mice treated with sea buckthorn fermented milk with L. plantarum YHG-87 showed intact intestinal structures and significantly reduced inflammatory cell infiltration. This suggests that sea buckthorn fermented milk with L. plantarum YHG-87 may repair intestinal mucosa and enhance gut barrier function, likely due to the anti-inflammatory properties of L. plantarum YHG-87. Previous studies have shown that certain probiotics, such as Lactobacillus and Bifidobacterium, maintain gut barrier function by increasing tight junction protein expression, thereby reducing inflammatory responses [30]. L. plantarum YHG-87 combined with the antioxidant components in fermented milk and sea buckthorn showed a strong synergistic effect in this respect.
Oxidative stress is a crucial mechanism in the occurrence and development of UC [31]. In this study, antioxidant GSH levels in DSS group mice significantly decreased, while MPO and MDA levels significantly increased, indicating severe tissue damage and oxidative stress response. After intervention with sea buckthorn fermented milk containing L. plantarum YHG-87, GSH levels in the SC group showed a slight increase compared to the DSS group, but the difference was not statistically significant, while MPO and MDA levels decreased significantly. Although the SC group showed a smaller advantage over the PS and SS groups at the MDA level, it demonstrated an advantage at both the MPO levels, with significantly higher MPO levels than the SS group, indicating a positive antioxidant effect. Previous studies have shown that probiotics effectively reduce oxidative stress by promoting antioxidant enzyme expression and reducing free radical production, thereby alleviating tissue damage. Similarly, Nithya et al. [32] discussed probiotic fermented foods, such as Axone and Chathur, which are fermented with Lactobacillus and had been shown to inhibit oxidative stress and modulated the immune response to reduce intestinal damage. This study further suggests that incorporating sea buckthorn components may enhance the antioxidant effects of L. plantarum YHG-87. Previous studies, including Ge et al., had demonstrated that the addition of sea buckthorn to fermented milk might enhance the activity of LAB by providing bioactive compounds such as polysaccharides and polyphenols, which served as fermentable substrates for LAB [33]. In our study, L. plantarum YHG-87, a LAB that metabolizes carbohydrated to produce lactic acid, was combined with sea buckthorn to ferment fermented milk. We hypothesized that the polysaccharides and polyphenols in sea buckthorn might further promote the growth and metabolic activity of L. plantarum YHG-87, leading to enhanced lactic acid production and increased health benefits, including improvements in gut microbiota, oxidative stress, and inflammation, which were observed in our DSS-induced UC model.
IBD is closely related to intestinal microbiota composition [34], and changes in microbial diversity and abundance can affect colitis occurrence and progression [35,36]. In this study, distinct shifts in the gut microbial community structure were observed after the administration of sea buckthorn fermented milk with L. plantarum YHG-87. Compared with the DSS group, the SC group showed compositional differences at the genus level, involving taxa such as Akkermansia, Ligilactobacillus, Adlercreutzia, Dubosiella, and Bacteroides. Previous studies have shown that certain Akkermansia species modulate mucosal immunity and promote short-chain fatty acid production [37,38]. Ligilactobacillus contributes to intestinal barrier integrity and the regulation of inflammatory cytokines [39,40]. Adlercreutzia has been reported to metabolize polyphenols such as resveratrol and exert antioxidant effects [41,42]. Dubosiella has been suggested to alleviate oxidative stress and improve mucosal barrier function through the production of propionate and L-lysine [43,44]. In addition, Bacteroides species are commonly associated with polysaccharide degradation and maintenance of gut metabolic balance [43,44]. However, because 16S rRNA sequencing provides genus-level rather than species-level resolution, the functional implications of these community changes cannot be definitively determined. Therefore, the observed alterations should be interpreted cautiously, and further studies using higher-resolution techniques (e.g., metagenomic sequencing or strain-specific PCR) are warranted to verify whether probiotic supplementation truly enhances beneficial bacterial populations and to clarify the precise roles of these genera in gut health. In summary, supplementation with L. plantarum YHG-87 in sea buckthorn fermented milk effectively alleviated UC-related symptoms, enhanced antioxidant and anti-inflammatory responses, and modulated gut microbial composition. These results suggest that combining bioactive plant compounds with probiotics may provide synergistic benefits for maintaining intestinal homeostasis and preventing colitis. Nevertheless, although distinct microbial shifts were observed, further studies are needed to confirm whether these changes correspond to actual increases in beneficial bacteria and to elucidate the underlying mechanisms linking microbial modulation with host protection.
Although this study shows that sea buckthorn fermented milk with L. plantarum YHG-87 has a significant effect in alleviating DSS-induced UC, there are some limitations. First, the study primarily examined the effects of sea buckthorn fermented milk with L. plantarum YHG-87 in a mouse model; further research is needed to assess its efficacy in humans. Second, the study did not comprehensively analyze the mechanisms behind changes in gut microbiota, such as the links between specific microbiota and inflammatory responses. Therefore, future research should investigate the regulatory mechanisms of sea buckthorn fermented milk with L. plantarum YHG-87 on specific microbiota and explore its action pathways using multi-omics approaches. Additionally, the study did not explore the specific mechanisms of action and interactions of the various active components in sea buckthorn fermented milk with L. plantarum YHG-87. Future research should concentrate on elucidating the molecular mechanisms, especially the signaling pathways involving the combined effects of sea buckthorn components and L. plantarum YHG-87. Sea buckthorn fermented milk with L. plantarum YHG-87 showed potential in alleviating UC symptoms by reducing inflammation, improving oxidative stress, and promoting gut health. This combination offered a promising approach for UC treatment.
5. Conclusions
In conclusion, sea buckthorn fermented milk with L. plantarum YHG-87 demonstrates significant potential in the prevention and treatment of UC by alleviating clinical symptoms, modulating inflammatory and antioxidant markers, repairing colon tissue damage, and promoting beneficial gut microbiota. These effects suggest a synergistic benefit from combining the antioxidant and anti-inflammatory properties of sea buckthorn with L. plantarum YHG-87, offering a promising therapeutic strategy for UC management.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/foods14213791/s1, Figure S1: Schematic diagram of a simple experimental design.; Table S1 DAI scores after modeling (CON); Table S2: Changes in body weight of mice in each group after modeling (±SD,DSS); Table S3: Changes in body weight of mice in each group after modeling (±SD,PS); Table S4: Changes in body weight of mice in each group after modeling (±SD,SS); Table S5: Changes in body weight of mice in each group after modeling (±SD,SC); Table S6: Length of colon (±SD); Table S7: Changes in IL-4 concentration in serum of mice in each group (±SD); Table S8: Changes in IL-10 concentration in serum of mice in each group (±SD); Table S9: Changes in serum concentrations of IL-6 in mice in each group (±SD); Table S10: Changes in TNF-α concentration in serum of mice in each group (±SD); Table S11: Changes in GSH concentration in serum of mice in each group (±SD); Table S12: Changes in MPO concentration in serum of mice in each group (±SD); Table S13: Changes in MDA content in serum of mice in each group (±SD); Table S14: Permutational multivariate analysis of variance.
Author Contributions
N.J.: writing—review and editing, resources and methodology. X.G.: conceptualization, data curation, formal analysis and writing—original draft. Y.D.: conceptualization and methodology. Y.L.: formal analysis, investigation and validation. S.G.: formal analysis, supervision and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Natural Science Foundation Project of Ningxia Hui Autonomous Region (2025AAC030221), National Natural Science Foundation of China (32160593), Research and Development Program Project of Ningxia Hui Autonomous Region for the Year 2021 (2021BEF02022).
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Review Board (or Ethics Committee) of Sichuan Lilaisinuo Biotechnology Co. LTD (LLSN-2023017, 12 June 2023).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| UC | Ulcerative colitis |
| L. plantarum | Lactiplantibacillus plantarum |
| DSS | dextran sulfate sodium |
| IBD | inflammatory bowel disease |
| DAI | disease activity index |
| SPF | specific pathogen-free |
| KM | Kunming mice |
| IL-4 | Interleukin-4 |
| IL-10 | Interleukin-10 |
| IL-6 | Interleukin-6 |
| TNF-α | Tumor Necrosis Factor-α |
| MDA | malondialdehyde |
| GSH | glutathione |
| MPO | myeloperoxidase |
| OTUs | operational taxonomic units |
References
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef]
- Ramos, L.; Teo-Loy, J.; Barreiro-de Acosta, M. Disease clearance in ulcerative colitis: Setting the therapeutic goals for future in the treatment of ulcerative colitis. Front. Med. 2022, 9, 1102420. [Google Scholar] [CrossRef]
- Świrkosz, G.; Szczygieł, A.; Logoń, K.; Wrześniewska, M.; Gomułka, K. The Role of the Microbiome in the Pathogenesis and Treatment of Ulcerative Colitis—A Literature Review. Biomedicines 2023, 11, 3144. [Google Scholar] [CrossRef]
- Guo, F.; Tsao, R.; Li, C.; Wang, X.; Zhang, H.; Jiang, L.; Sun, Y.; Xiong, H. Green Pea (Pisum sativum L.) Hull Polyphenol Extracts Ameliorate DSS-Induced Colitis through Keap1/Nrf2 Pathway and Gut Microbiota Modulation. Foods 2021, 10, 2765. [Google Scholar] [CrossRef] [PubMed]
- Cremon, C.; Barbaro, M.R.; Ventura, M.; Barbara, G. Pre- and probiotic overview. Curr. Opin. Pharmacol. 2018, 43, 87–92. [Google Scholar] [CrossRef]
- Burton, K.J.; Rosikiewicz, M.; Pimentel, G.; Bütikofer, U.; von Ah, U.; Voirol, M.J.; Croxatto, A.; Aeby, S.; Drai, J.; McTernan, P.G.; et al. Probiotic yogurt and acidified milk similarly reduce postprandial inflammation and both alter the gut microbiota of healthy, young men. Br. J. Nutr. 2017, 117, 1312–1322. [Google Scholar] [CrossRef]
- Guo, N.; Lv, L.L. Mechanistic insights into the role of probiotics in modulating immune cells in ulcerative colitis. Immun. Inflamm. Dis. 2023, 11, e1045. [Google Scholar] [CrossRef]
- Wu, Y.; Jha, R.; Li, A.; Liu, H.; Zhang, Z.; Zhang, C.; Zhai, Q.; Zhang, J. Probiotics (Lactobacillus plantarum HNU082) Supplementation Relieves Ulcerative Colitis by Affecting Intestinal Barrier Functions, Immunity-Related Gene Expression, Gut Microbiota, and Metabolic Pathways in Mice. Microbiol. Spectr. 2022, 10, e0165122. [Google Scholar] [CrossRef]
- Chen, Y.; Cai, Y.; Wang, K.; Wang, Y. Bioactive Compounds in Sea Buckthorn and their Efficacy in Preventing and Treating Metabolic Syndrome. Foods 2023, 12, 1985. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Q.; Li, X.; Liang, Y.; Liu, R. Sea Buckthorn Polysaccharide Ameliorates Colitis. Nutrients 2024, 16, 1280. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y. Development of Sea-Buckthorn Probiotic Yogurt and its Antioxidant Activity In Vitro; Ningxia University: Yinchuan, China, 2023. [Google Scholar]
- Liu, L.; Zhang, C.; Zhang, H.; Qu, G.; Li, C.; Liu, L. Biotransformation of Polyphenols in Apple Pomace Fermented by β-Glucosidase-Producing Lactobacillus rhamnosus L08. Foods 2021, 10, 1343. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, G.; Fang, J. Progress on the mechanisms of Lactobacillus plantarum to improve intestinal barrier function in ulcerative colitis. J. Nutr. Biochem. 2024, 124, 109505. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Meng, Y.; Xu, Y.; Chang, C.; Qiu, Z.; Hu, J.; Wu, Y.; Zhang, B.; Zheng, G. Extraction, characterization and anti-inflammatory activities of an inulin-type fructan from Codonopsis pilosula. Int. J. Biol. Macromol. 2020, 163, 1677–1686. [Google Scholar] [CrossRef]
- Wiese, J.J.; Manna, S.; Kühl, A.A.; Fascì, A.; Elezkurtaj, S.; Sonnenberg, E.; Bubeck, M.; Atreya, R.; Becker, C.; Weixler, B.; et al. Myenteric Plexus Immune Cell Infiltrations and Neurotransmitter Expression in Crohn’s Disease and Ulcerative Colitis. J. Crohn’s Colitis 2024, 18, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Li, D.; Sun, H. Herba Origani alleviated DSS-induced ulcerative colitis in mice through remolding gut microbiota to regulate bile acid and short-chain fatty acid metabolisms. Biomed. Pharmacother. 2023, 161, 114409. [Google Scholar] [CrossRef] [PubMed]
- Törüner, M.; Ünal, N.G. Epigenetics of Inflammatory Bowel Diseases. Turk. J. Gastroenterol. 2023, 34, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Shen, X.; Shi, X.; Sakandar, H.A.; Quan, K.; Li, Y.; Jin, H.; Kwok, L.-Y.; Zhang, H.; Sun, Z. Targeting gut microbiota and metabolism as the major probiotic mechanism—An evidence-based review. Trends Food Sci. Technol. 2023, 138, 178–198. [Google Scholar] [CrossRef]
- Suryakumar, G.; Gupta, A. Medicinal and therapeutic potential of Sea buckthorn (Hippophae rhamnoides L.). J. Ethnopharmacol. 2011, 138, 268–278. [Google Scholar] [CrossRef]
- Poaty Ditengou, J.I.C.; Ahn, S.I.; Chae, B.; Choi, N.J. Are heat-killed probiotics more effective than live ones on colon length shortness, disease activity index, and the histological score of an inflammatory bowel disease-induced murine model? A meta-analysis. J. Appl. Microbiol. 2023, 134, lxad008. [Google Scholar] [CrossRef]
- Huang, C.; Hao, W.; Wang, X.; Zhou, R.; Lin, Q. Probiotics for the treatment of ulcerative colitis: A review of experimental research from 2018 to 2022. Front. Microbiol. 2023, 14, 1211271. [Google Scholar] [CrossRef] [PubMed]
- Vakadaris, G.; Stefanis, C.; Giorgi, E.; Brouvalis, M.; Voidarou, C.C.; Kourkoutas, Y.; Tsigalou, C.; Bezirtzoglou, E. The Role of Probiotics in Inducing and Maintaining Remission in Crohn’s Disease and Ulcerative Colitis: A Systematic Review of the Literature. Biomedicines 2023, 11, 494. [Google Scholar] [CrossRef]
- Mantel, M.; da Silva, T.F.; Gloria, R.; Vassaux, D.; Vital, K.D.; Cardoso, V.N.; Fernandes, S.O.A.; Guédon, É.; Le Loir, Y.; Faria, A.M.C.; et al. Fat matters: Fermented whole milk potentiates the anti-colitis effect of Propionibacterium freudenreichii. J. Funct. Foods 2023, 106, 105614. [Google Scholar] [CrossRef]
- Xiao, Y.T.; Yan, W.H.; Cao, Y.; Yan, J.K.; Cai, W. Neutralization of IL-6 and TNF-α ameliorates intestinal permeability in DSS-induced colitis. Cytokine 2016, 83, 189–192. [Google Scholar] [CrossRef]
- Kelly-Welch, A.E.; Hanson, E.M.; Boothby, M.R.; Keegan, A.D. Interleukin-4 and interleukin-13 signaling connections maps. Science 2003, 300, 1527–1528. [Google Scholar] [CrossRef]
- Zhao, H.M.; Huang, X.Y.; Zuo, Z.Q.; Pan, Q.H.; Ao, M.Y.; Zhou, F.; Liu, H.N.; Liu, Z.Y.; Liu, D.Y. Probiotics increase T regulatory cells and reduce severity of experimental colitis in mice. World J. Gastroenterol. 2013, 19, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Ladda, B.; Jantararussamee, C.; Pradidarcheep, W.; Kasorn, A.; Matsathit, U.; Taweechotipatr, M. Anti-Inflammatory and Gut Microbiota Modulating Effects of Probiotic Lactobacillus paracasei MSMC39-1 on Dextran Sulfate Sodium-Induced Colitis in Rats. Nutrients 2023, 15, 1388. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, B.; Delgado, S.; Blanco-Míguez, A.; Lourenço, A.; Gueimonde, M.; Margolles, A. Probiotics, gut microbiota, and their influence on host health and disease. Mol. Nutr. Food Res. 2017, 61, 1600240. [Google Scholar] [CrossRef]
- Higashi, Y. Roles of Oxidative Stress and Inflammation in Vascular Endothelial Dysfunction-Related Disease. Antioxidants 2022, 11, 1958. [Google Scholar] [CrossRef]
- Nithya, A.; Misra, S.; Panigrahi, C.; Dalbhagat, C.G.; Mishra, H.N. Probiotic potential of fermented foods and their role in non-communicable diseases management: An understanding through recent clinical evidences. Food Chem. Adv. 2023, 3, 100381. [Google Scholar] [CrossRef]
- Ge, X.; Tang, N.; Huang, Y.; Chen, X.; Dong, M.; Rui, X.; Zhang, Q.; Li, W. Fermentative and physicochemical properties of fermented milk supplemented with sea buckthorn (Hippophae eleagnaceae L.). LWT 2022, 153, 112484. [Google Scholar] [CrossRef]
- Ni, J.; Wu, G.D.; Albenberg, L.; Tomov, V.T. Gut microbiota and IBD: Causation or correlation? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Nishida, A.; Inoue, R.; Inatomi, O.; Bamba, S.; Naito, Y.; Andoh, A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 2018, 11, 1–10. [Google Scholar] [CrossRef]
- Xu, H.M.; Zhao, H.L.; Guo, G.J.; Xu, J.; Zhou, Y.L.; Huang, H.L.; Nie, Y.Q. Characterization of short-chain fatty acids in patients with ulcerative colitis: A meta-analysis. BMC Gastroenterol. 2022, 22, 117. [Google Scholar] [CrossRef]
- Zheng, M.; Han, R.; Yuan, Y.; Xing, Y.; Zhang, W.; Sun, Z.; Liu, Y.; Li, J.; Mao, T. The role of Akkermansia muciniphila in inflammatory bowel disease: Current knowledge and perspectives. Front. Immunol. 2022, 13, 1089600. [Google Scholar] [CrossRef]
- Shin, Y.; Han, S.; Kwon, J.; Ju, S.; Choi, T.G.; Kang, I.; Kim, S.S. Roles of Short-Chain Fatty Acids in Inflammatory Bowel Disease. Nutrients 2023, 15, 4466. [Google Scholar] [CrossRef]
- Gámez-Macías, P.E.; Félix-Soriano, E.; Samblas, M.; Sáinz, N.; Moreno-Aliaga, M.J.; González-Muniesa, P. Intestinal Permeability, Gut Inflammation, and Gut Immune System Response Are Linked to Aging-Related Changes in Gut Microbiota Composition: A Study in Female Mice. J. Gerontol. A Biol. Sci. Med. Sci. 2024, 79, glae045. [Google Scholar] [CrossRef]
- He, J.; Jin, Y.; He, C.; Li, Z.; Yu, W.; Zhou, J.; Luo, R.; Chen, Q.; Wu, Y.; Wang, S.; et al. Danggui Shaoyao San: Comprehensive modulation of the microbiota-gut-brain axis for attenuating Alzheimer’s disease-related pathology. Front. Pharmacol. 2023, 14, 1338804. [Google Scholar] [CrossRef] [PubMed]
- Gorbatsova, J.; Lõugas, T.; Vokk, R.; Kaljurand, M. Comparison of the contents of various antioxidants of sea buckthorn berries using CE. Electrophoresis 2007, 28, 4136–4142. [Google Scholar] [CrossRef]
- Yin, D.; Zhao, L.; Deng, S.; Xie, Y.; Ro, K.S.; Yang, Z.; Du, L.; Xie, J.; Wei, D. Lactiplantibacillus plantarum X7022 Plays Roles on Aging Mice with Memory Impairment Induced by D-Galactose Through Restoring Neuronal Damage, Relieving Inflammation and Oxidative Stress. Probiotics Antimicrob. Proteins 2024, 17, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tu, S.; Ji, X.; Wu, J.; Meng, J.; Gao, J.; Shao, X.; Shi, S.; Wang, G.; Qiu, J.; et al. Dubosiella newyorkensis modulates immune tolerance in colitis via the L-lysine-activated AhR-IDO1-Kyn pathway. Nat. Commun. 2024, 15, 1333. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.H.; Wang, J.; Zhang, C.Y.; Zhao, L.; Sheng, Y.Y.; Tao, G.S.; Xue, Y.Z. Gut microbial characteristical comparison reveals potential anti-aging function of Dubosiella newyorkensis in mice. Front. Endocrinol. 2023, 14, 1133167. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).