Abstract
Fruits and vegetables are essential for a healthy diet, providing vital nutrients and contributing to global food security. Fungal pathogens that interact with fruits and vegetables reduce their quality and shelf life and lead to economic losses and risks to human health through the production of mycotoxins. Chemical fungicides, used to control postharvest pathogens, are posing serious environmental and health risks, driving interest in safer alternative strategies. Biocontrol methods using antagonistic microbes, such as yeasts, are eco-friendly, sustainable, and the most promising, but they often have limited efficacy and specificity in diverse produce. There is growing interest in the innovative enhancement of biocontrol strategies. The present review shows that inducing, enhancing, co-application, encapsulation, and post-application treatments are common enhancement techniques, while environmental, host, and pathogen characteristics, antagonistic microbial traits, and chemical inputs are the major gearing factors for the best application methods. These methods do not involve genetic modification, which is adequate to reduce the proliferation of GMOs (Genetically Modified Organisms) while optimizing antagonistic microbial performance by promoting growth, inducing host resistance, enhancing antifungal properties, improving adhesion, and boosting stress tolerance. Most enhancers fall under groups of nutritional additives, protective carriers, growth stimulants, and encapsulants. Integrating these enhancers and best methods promises reduced postharvest losses, supports sustainable agriculture, and addresses economic losses and food security challenges. This study highlights the role of organic and natural elicitors, their application methods, their mechanisms in improving BCAs (Biological Control Agents), and their overall efficiency. This review concisely compiles recent strategies, calling for further research to revolutionize fungal pathogen management, reduce food waste, and promote responsible farming practices.
1. Introduction
Fruits and vegetables are an essential part of a healthy diet. They provide vital nutrients for our health and contribute to global food security. However, this class of foods is affected by huge postharvest losses ranging from 28 to 55% of total production, which translates to an annual loss of approximately USD 750 billion [1,2]. It is estimated that up to 30–50% of harvested produce is lost globally due to postharvest diseases caused by fungal infection [3,4]. Significant research and innovation efforts have focused on reducing F&V postharvest losses [5,6]. The study explores the ways in which harvested fruits and vegetables are prone to decay and quality deterioration during storage. Although traditional packaging and chemical treatments are effective, they are harmful to the environment and human health. Hence, greater requirements for food preservation technology are increasingly proposed.
The primary factors contributing to these losses include suboptimal handling, physical damage, postharvest diseases (fungal and bacterial), insect pests, small animal attacks (rodents, birds, and other animals), harvesting at inappropriate stages of ripeness, insufficient storage and packaging methods, and poor transport conditions [7]. Furthermore, physiological processes, such as respiration, transpiration, and ethylene production, exacerbate the postharvest deterioration of fruits and vegetables [8]. Common diseases in fruits and vegetables arise from fungi, bacteria, viruses, and oomycetes (fungus-like organisms) [3,9], while fungal pathogens take the lion’s share. The most common of these fungal pathogens are Botrytis cinerea, Penicillium spp., Aspergillus spp., Colletotrichum spp., and Alternaria spp. [3,10]. These pathogens drastically drop the quality and shelf life of postharvest produce, affecting marketability and leading to economic setbacks for producers and processors. They also pose health risks to consumers [3,11].
Management of these fungal diseases takes different forms, with varying mechanisms. Mechanisms broadly encompass physical methods, chemical methods, and biological methods [12,13]. Some of the physical approaches have the benefits of maintaining nutritional quality and offering easy applicability, with an absence of residual effects. The potential for damage to fruits or vegetables, impacts on sensory and physicochemical quality, high energy costs, short-term effectiveness, and regulatory challenges are among the limitations this method [14]. Chemical fungicides are effective and widely used (>90%) in the postharvest disease management of fruits and vegetables. Commercial accessibility, ease of application, and effectiveness in broad product-based applications make this method preferable over others [15]. Despite its benefits, this method lacks the full approbation of the population due to its negative impacts on the environment and human health and its role in the development of resistance among pathogens. The biological strategy has gained attention mainly from a sustainability perspective [16,17]. Biological methods’ strengths are that they have little or no adverse effects on human health, they do not generate resistance in pathogens, they have long-lasting effects, and they affect the physicochemical quality of products as little as possible [18,19]. Other advantages include their environmental friendliness and compatibility with integrated pest management programs [11,20]. Antagonistic yeasts have a promising ability to kill and compete with pathogens without requiring the use of antibiotics that can promote the development of antibiotic-resistant strains in the environment. Saccharomyces cerevisiae, Wickerhamomyces anomalus, Debaryomyces hansenii, Meyerozyma spp., Candida spp., Pichia spp., and Metschnikowia are among the common antagonistic yeasts used in biocontrol methods for fruit and vegetable fungal pathogens. They mainly inhibit fungal pathogen growth through various mechanisms, including physical and nutrient competition, parasitism, rapid growth, efficient colonization of surface injuries, and physiological disruption through volatile organic compound (VOC) production [21].
Nevertheless, their effectiveness fluctuates most often under different environmental conditions, which limits their specification and market value, restricting their use worldwide. The overall impact of biological control on managing plant diseases in agricultural settings is currently minimal, accounting for approximately 1% of agricultural chemical usage, due to these limitations [22]. Similarly, one study shows that although the potential of antagonistic yeasts spans crop protection, food safety, and disease prevention, their commercial availability remains limited [23]. An important implication of these findings is that future research should prioritize the optimization and commercialization of yeast-based biocontrol products, effectively bridging the gap between scientific discovery and practical application in combating harmful pathogens.
There are different approaches to making BCAs more efficient, such as the use of mixed cultures, their physiological manipulation, and their combination with different types of substances [24]. Enhancement techniques involve the application of supplements, such as chitosan, phytic acid, ascorbic acid, trehalose, alginate oligosaccharide, beta-glucans, salicylic acid, essential oils, and plant extracts. These approaches improve the efficiency, cell viability and performance, adherence, and fungicidal properties of antagonistic yeasts. Each substance used can variably strengthen the cell walls of antagonistic yeasts, improve their colonization of fruit surfaces, and trigger the synthesis of antifungal compounds [25]. By fine-tuning these enhancement approaches, the full potential of antagonistic yeasts as dependable and sustainable solutions for managing postharvest diseases can be achieved. Many key factors shape the optimization of antagonistic yeasts’ mechanisms. These include environmental factors, such as temperature, relative humidity, UV intensity, pH levels, and the ecology and mechanisms of antagonism [26]; host plant characteristics, including inoculum density, virulence, and host plant genotype [27]; pathogen characteristics, such as its concentration, resistance to antagonistic microbes, and self-defense mechanisms [28]; biocontrol agent properties, like the mode of action, viability, stability, and application timing [18]; and chemical inputs, such as pesticides and fertilizers, which may harm antagonistic microbes or reduce their efficacy [29].
The present review focuses on three key areas; (i) highlighting the importance and necessity of improving antagonistic yeast by identifying and compiling reagents utilized in several studies to improve/boost their performance; (ii) highlighting possible application methods and exploring effective delivery techniques for enhancers along with antagonistic yeasts to mitigate target fungal pathogens; and (iii) compiling different mechanisms of actions by exposing the underlying mechanisms behind the various improvements in antagonistic yeasts against the development of pathogenic fungi. Over the past three decades, hundreds of studies have studied biological postharvest disease management in fruits and vegetables, particularly using antagonist yeasts. These studies confirm that fungal biocontrol is both effective and sustainable. However, existing findings also reveal limitations, including product specificity and low market accessibility due to challenges in mass production. Commercial development of a biocontrol product is a complex process involving several stages, including initial discovery and screening, pilot testing, upscale production and formulation, and patenting and registration, all of which require financial support from public grant agencies or a business partner [21]. Further constraints include the short shelf life and variable efficacy of biocontrol agents (BCAs), which depend on environmental conditions, host cell types, the substances applied, and other chemicals used on host cells.
A literature review was carried out by systematically searching Google Scholar, ScienceDirect, and Google to find peer-reviewed articles on antagonistic yeasts for biocontrol of fungal pathogens of fruits and vegetables. Search terms included “antagonistic yeast,” “biocontrol,” “postharvest disease,” and “fungal pathogen” in combination with specific hosts (e.g., apple, citrus, and tomato). Results were sorted by relevance, publication date (kept to very recent publications as much as possible), and availability of full-text articles. Curation was limited to research elucidating biocontrol mechanisms and efficacy, with the explicit exclusion of any AI-generated content to confirm that all synthesized information came from the published scientific literature.
In conclusion, there is no doubt that most antagonistic yeasts are effective in the biocontrol of specific pathogens in specific environments and under specific conditions, as demonstrated by numerous studies worldwide. However, without improving the viability, performance, and antagonistic efficiency of these microbial biocontrol agents, it will be impossible to meet market demand or compete with traditional chemical methods, which have already dominated the global market despite their numerous side effects.
2. Causes of Postharvest Loss in Fruits and Vegetables
The characteristics of fruits and vegetables, including their high water content and nutritional content, soft skin, and broad surface area, make them susceptible to various postharvest issues. Akanbi [7] categorizes postharvest losses in these forms of produce as stemming from physiological and technological origins, encompassing deterioration caused by biological/microbiological agents and mechanical damage. Additionally, postharvest losses in fruits and vegetables can come from socioeconomic factors, including a lack of policies promoting efficient use of human, economic, technical, and scientific resources to reduce losses [30,31]. This underscores the fact that despite its significance, the substantial postharvest waste of these commodities has not received sufficient emphasis in research and practice. Alegbeleye [32] reported that fruit and vegetable spoilage represents a significant food issue that has received substantially less research attention compared to produce-related foodborne illnesses and processing technologies, despite its considerable socioeconomic, food quality, and food security implications. Other contributing factors are insufficient resources for developing loss prevention programs, limited knowledge of preservation and distribution technologies, ineffective commercialization systems, inadequate government involvement in commodity postharvest loss management and marketing, and lack of appropriate credit policies tailored to national and stakeholder needs. About 95% of resources are invested into crop production, whereas as low as 5% of this investment is channeled into postharvest preservation [33,34].
Alegbeleye [32], in their study, found that infection and microbiological spoilage of fruits and vegetables may be initiated by various well-characterized microorganisms, including bacteria, fungi, yeasts, and molds. The authors further noted that spoilage microorganisms may be introduced through the farm environment, postharvest handling, processing operations, and various agronomic and environmental factors that predispose produce to microbial deterioration.
Postharvest fruits and vegetables can also face significant challenges due to their inherent properties, making them highly susceptible to microbial infections, pest infestations, physiological disorders, and physical or mechanical damage. These issues are exacerbated by a lack of coordinated effort among key stakeholders, such as producers, traders, processors, retailers, consumers, researchers, and policymakers, to address postharvest loss management effectively. Without collaborative action and shared consideration, the preservation and quality of these perishable commodities remain at risk, highlighting the need for a unified approach to overcome these challenges [3,35].
3. Common Postharvest Diseases in Fruits and Vegetables and Fungal Disease Management
Fruits and vegetables are susceptible to different postharvest diseases that can result in considerable losses during postharvest handling, such as harvesting, packaging, transportation, storage, and processing. Most fruits and vegetables have very high moisture content (approximately 70–95% water). They are usually large (0.5–5 kg) and exhibit a higher respiration rate, and they usually have a soft texture, all of which favor the growth and development of several diseases caused by microorganisms between the periods of harvest and consumption [8,9,36].
The most common diseases in postharvest fruits and vegetables are fungal diseases, such as gray mold (Botrytis cinerea), blue mold (Penicillium spp.), anthracnose (Colletotrichum spp.), alternaria rot (Alternaria spp.), sour rot (Geotrichum candidum), and Fusarium rot (Fusarium spp.); bacterial diseases, such as soft rot (Pectobacterium carotovorum, Dickeya spp.), bacterial spot/blight (Xanthomonas spp.), crown gall (Agrobacterium tumefaciens), and pseudomonas rot (Pseudomonas spp.); viral diseases, such as tomato spotted wilt virus (TSWV), cucumber mosaic virus (CMV), and potato virus y (PVY); and oomycete diseases (fungus-like organisms), such as late blight (Phytophthora infestans) and phytophthora rot (Phytophthora spp.) [3,9,16]. These pathogens can cause various types of rot and spoilage, making them unsuitable for consumption and potentially producing harmful mycotoxins [37,38].
It is widely recognized that fungi are primarily responsible for postharvest losses in these produce items [3,9,39]. Different mechanisms are used by pathogens in the infection of host cells. According to the literature [40,41,42], fungal pathogens in fruits and vegetables can be classified into different groups based on their characteristics during infection. These are neutrophilic fungi, toxigenic fungi, facultative saprophytes, opportunistic fungi, and latent fungi. Figure 1 shows common types of fungal pathogens along with major pathogens and targeted fruits and vegetables.
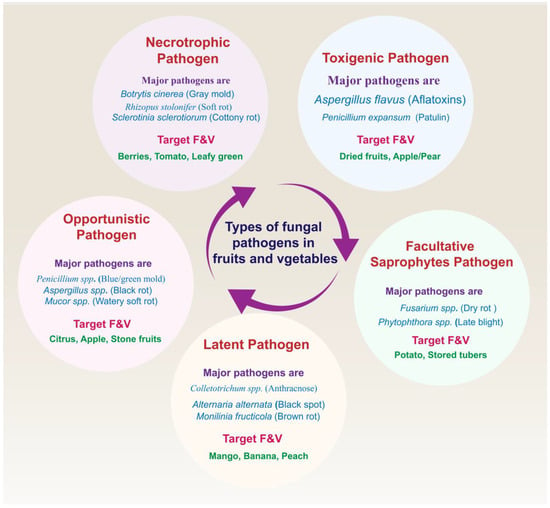
Figure 1.
Types of fungal pathogens along with major pathogens and targeted fruits and vegetables.
Proteomic studies reveal pathogen-induced changes in fruit proteomes, including the upregulation of defense-related proteins and the suppression of metabolic pathways crucial for pathogen survival [43,44,45]. After the survival of the pathogen, it then goes on to invade the host cell to cause disease, while some of them are responsible for the production of secondary by-products, such as patulin, citrinin, and penicillic acid, which are predominantly caused by Penicillium species, while patulin can also be produced by some Aspergillus species. These mycotoxins pose significant health concerns and economic threats, particularly in apples and apple-based products [46]. Fungal pathogens are responsible for the spoilage of various fruits and vegetables [47,48,49]. Table 1 indicates the common fungal pathogens identified in this study.

Table 1.
Common fungal pathogens in different fruits and vegetables.
The quality of fresh produce is influenced by biological processes that are also responsible for its degradation. These biological factors include respiratory rate, germination, root formation, alterations in color, taste, and texture, nutritional changes, ethylene generation, moisture loss, and pathological decay [51,52]. To address these diverse forms of spoilage, numerous researchers have explored multiple strategies for prevention, control, and elimination over prolonged durations [53]. Postharvest fungal pathogens often invade fruits and vegetables after harvesting, which leads to huge losses before reaching consumers [54,55]. Studies show that even after harvest, fruits and vegetables remain active in metabolic processes, which makes them susceptible to decay during postharvest operations [3,56]. One study delved into the various biological and environmental factors that reduce product quality, especially focusing on fungal infections. Nassarawa [57] classified the predominant and well-established approaches of fungal disease management for fruits and vegetables into three primary categories: physical, chemical, and biological methods.
3.1. Physical Methods
Various studies have shown that different physical methods for postharvest fruit and vegetable disease control have benefits over synthetic chemical fungicides in maintaining nutritional quality and sensory quality parameters, with an absence of residual effects and easy applicability [58,59].
De Chiara [60] reported that many traditional postharvest technologies can negatively impact the quality of fresh produce and/or affect the sustainability of processes, while a recent study spotlighted emerging innovative physical techniques like vacuum and hydrocooling, microwave heating, pulsed electric field, high hydrostatic pressure, and cold plasma application designed to reduce nutrient losses and enhance consumer acceptability. In that study, they also stated that each technique offers unique advantages and limitations. In contrast, there are some limitations to this approach due to varying efficacy, potential for damage, high costs, short-term effectiveness, and regulatory challenges [61].
3.2. Chemical Methods
Chemical methods for postharvest loss management of fruits and vegetables include other techniques that are used broadly in the world. This method has several advantages and limitations. According to Wu [62], synthetic fungicides have been proven to significantly reduce the incidence of postharvest diseases caused by various fungal pathogens. Studies have also demonstrated that synthetic fungicides can have negative effects on biodiversity [63]. According to that study, out of the 13 studied components of agricultural intensification, use of pesticides, especially insecticides and fungicides, had the most consistent negative effects, such as decline in species diversity of plants, impacts on carabids and ground-nesting farmland birds, and effects on the potential for biological pest control. Specifically, they stated that bird species’ diversity declined with increasing frequency of fungicide application. The misuse of artificial fungicides undermines the safety of farm crops because of their lasting effect on human health and the environment and susceptibility to pathogen resistance [64].
3.3. Biological Control Methods
The third and most recent method, which has received attention from producers, processors, consumers, and researchers over the past three decades, comprises biological disease control methods for postharvest fruit and vegetable decay. Biocontrol has numerous advantages over the other two methods in that it is more natural and has no residual effects and pathogens do not develop resistance to it. This approach utilizes various natural substances, including plant-derived extracts and essential oils, along with competitive bacterial and yeast strains, whereas actinomycetes have emerged as a novel focus in recent studies [65]
Different studies have tried to identify working principles of biocontrol methods against fungal pathogen infections in postharvest produce. As Ling [66] reported, endophytic fungi—living harmlessly inside of plant tissues—generate antifungal volatile organic compounds (VOCs). These airborne substances show significant effects in postharvest disease control due to their non-toxic nature and dispersive properties, which make them viable biological control alternatives.
Other studies have revealed that environmental yeasts exhibit significant biocontrol potential against pathogens [17,25]. These microorganisms apply multiple antagonistic strategies, including nutrient reduction, parasitic activity, production of antifungal metabolites, and stimulation of plant defense systems. This includes the role of reactive oxygen species (ROS) in mediating protective responses. Meaning they are highly reactive, oxygen-containing molecules that can damage important cellular components like DNA, proteins, and lipids
Similar findings by Li [67] showed that Bacillus spp., especially B. subtilis, serve as efficient biocontrol organisms. Their effectiveness is based on competitive hindering, synthesis of antimicrobial compounds, and activation of plants’ systemic resistance. Application can occur during either pre-harvest or postharvest stages, with endophytic alternatives providing greater protective benefits.
Common Biocontrol Agents for Postharvest Fruits and Vegetables
Recent advances in biotechnological approaches have positioned biological control as a widely researched, environmentally friendly solution, employing beneficial microbes to combat postharvest phytopathogens as an alternative to traditional chemical treatments [68]. These biocontrol agents are applied as postharvest treatments, either alone or in combination with other methods, to extend shelf life and reduce spoilage caused by fungal pathogens. Their use aligns with the growing demand for eco-friendly and safe food preservation strategies [69]. Table 2 shows a list of common biocontrol agents studied at different times to combat fruit and vegetable postharvest fungal pathogens.

Table 2.
List of common biocontrol agents used to combat postharvest fruit and vegetable fungal pathogens.
4. Factors Affecting the Efficacy of Antagonistic Microbes
4.1. Environmental Factors
Environmental factors, such as temperature and humidity, have significant effects on the growth and activity of antagonistic microbes [100]. The antagonistic efficacy of almost all yeast, bacteria, and fungi is highly dependent on the optimal temperature and humidity levels of the environment. pH can strongly affect the survival and functionality of antagonistic microbes. Chowdhury [101] reported that under acidic conditions, some bacteria, such as Bacillus amyloliquefaciens MBNC, can retain their antagonistic efficiency against fungal phytopathogens, although their metabolite production is altered. In addition, light exposure can influence the activity of these microbes. UV light harms microbes and reduces their viability. It was also reported that understanding ecological aspects and the mechanisms of antagonistic fungi is essential for developing effective and sustainable biocontrol strategies against fungal pathogens [26,102]. All of these environmental limitations of BCA can be overcome by enhancing antagonistic microbes. The study by Magan [26] supports this in reporting that extreme climatic events further impact the efficacy of BCAs and may require systems that can identify more resilient candidate BCAs for fungal pathogens and pest control in the near future.
4.2. Host Plant Characteristics
Plant species and variety determine the efficacy of antagonistic microbes. Pathosystem (conceptual framework that define and describe the entire system of interactions between host cell and pathogen in our case fungal pathogen within a given environment) components, such as inoculum density and virulence, and host plant genotype, are important factors influencing the efficacy of plant disease biocontrol [27]. Plant health influences these antagonistic microbes’ activity. Stressed or unhealthy plants may not effectively support microbial activity. Some antagonistic microbes trigger the plant’s own defense mechanisms, which can vary in effectiveness depending on the health and genetics of the plant. Therefore, understanding the complex interplay between plants, beneficial microbes, and pathogens is crucial for the development of effective and sustainable disease control strategies in agriculture [28,103,104]. Antagonistic microbes can suppress postharvest diseases in fruits and vegetables through various mechanisms, but their efficacy depends on factors like maturity, storage conditions, and combinations with other treatments [105]. Nonetheless, enhancing antagonistic yeast will inhibit these challenges.
4.3. Pathogen Characteristics
Fungal species and their strains can also determine the efficacy of antagonistic microbes, as some fungi are more resistant to antagonistic microbes than others and vary in their pathogen loads. High concentrations of fungal pathogens can overcome antagonistic microbes. Insight into the self-defense mechanisms used by pathogens to hinder antagonistic attack offers a novel strategy for improving the durability of biologically based disease control practices and is applicable to the introduction of transgenes [28]. Different studies have been working on better understanding pathogen–antagonist–host interactions and improving biocontrol activity to establish microbial antagonists as viable alternatives to synthetic fungicides in postharvest disease management [106,107,108]. By enhancing antagonistic microbes, it is possible to make it withstand resistance from the pathogen.
4.4. Chemical Inputs
Pre- and postharvest chemicals, such as pesticides and fertilizers, can harm antagonistic microbes or reduce their effectiveness. Supplemental organic inputs like compost and similar materials can stimulate beneficial microbial populations. Synthetic agricultural chemicals, such as pesticides and synthetic fertilizers, frequently inhibit the development, reproductive capacity, and viability of beneficial microorganisms used for biological control, consequently diminishing their efficacy [29].
4.5. Biocontrol Agents’ Characteristics
Strain selection of antagonistic microbes is also essential for their efficacy. Selecting the correct strain is critical [109]. The mode of action also affects efficacy, as some microbes produce antifungal compounds, while others compete for resources or induce plant resistance; as such, the mechanism matters [110,111]. The ability of microbes to survive storage and application and on the plant’s surface is crucial, and the application time of microbial antagonists affects its efficacy, while postharvest applications are more effective than pre-harvest applications.
The new paradigm shift proposed in biocontrol research includes the integration of low-risk chemical fungicides, natural antimicrobial substances, and physical means, such as hot water treatment, irradiation with ultraviolet light, microwave, and infrared treatment in the biocontrol process, as well as enhancement of the expression of crucial biocontrol genes and/or combining genes from different biocontrol agents in mass production, formulation, storage, or in response to exposure and contact with host plant tissues after application.
In general, different studies have been conducted to improve the efficiency of antagonistic yeasts by supplementing them with enhancers, such as trehalose, ascorbic acid, chitosan, alginate oligo saccharides, or certain elicitors, during yeast culture or shortly before their use [112,113]. The supplements enhance yeast cells’ efficacy against several stress factors, including temperature fluctuation, dehydration, ultraviolet irradiation, and infection by pathogens [113]. In some studies, it has also been observed that trehalose is especially good at safeguarding cell structure; it consolidates the membrane structure of antagonist strains and improve their properties to protect cell content at the time of application and against protein damage under stress [53,114,115].
5. Common Reagents Used as Enhancers of Antagonistic Yeast and Their Characteristics
Common reagents used can be classified into four major groups: nutrient supplements and molecules, complex structure polysaccharides, antioxidants, and pH modulators [116,117].
5.1. Nutrient Supplements and Molecules
Nutrients play a crucial role in enhancing the efficacy of biocontrol agents against plant pathogens. Several studies have demonstrated that supplementing biocontrol agents with nutrients improved the efficacy of biocontrol. Nutrients affect the interactions between microbial populations and the development of plant pathogens and may play a role in biocontrol [118]. For example, application of organic nitrogen enhanced biomass and antifungal compound production by Gliocladium virens and Trichoderma spp., antagonists of Sclerotium cepivorum. Another study by Lima [117] revealed that nutrient supplements, such as sugars and nitrogen sources, are required to supply the necessary energy and structural components for yeast growth and metabolic processes.
5.2. Complex Structure Polysaccharides
Defensive substances like chitosan and alginate also facilitate yeast’s attachment to production surfaces and induce protective biofilm formation, thereby prolonging their active duration [25,119]. It was reported that cell-free filtrate from Cryptococcus laurentii cultured in chitin-enriched medium exhibits high chitinase activity, which has direct antifungal activity against P. expansum in pear fruit wounds [120]. A study by Zhang [121] indicates that natural decay of strawberries induced by R. stolonifer and B. cinerea has been reported to be significantly reduced by using the yeast R. mucilaginosa grown in NYDB added with chitosan. In that study, two-dimensional electrophoresis coupled with identification through MALDI-TOF/TOF analysis indicated that certain proteins associated with fundamental cellular metabolism had higher levels in the yeast, which may be responsible for enhanced biocontrol efficacy. Pathways like MAPK signaling, peroxisome, glutathione metabolism, and oxidative phosphorylation could have been the cause of increased biocontrol activity of R. mucilaginosa induced by chitosan [122].
Other defensive compounds, like trehalose and glycerol, significantly enhance yeast survival under both temperature stress and dehydration stress. Meanwhile, fungal-cell wall-degrading enzymes (e.g., chitinase, glucanase) weaken the structure of the pathogen, rendering it more vulnerable to antagonism by the yeast. This combination of stress-resistant and lytic enzymes constitutes a complete strategy for the optimization of biocontrol yeast efficiency in postharvest application [25,112,122,123]. Chantrasri [124] developed a proteomic method to study how peach fruits gain improved resistance through Pichia membranefaciens and salicylic acid treatments. They report that yeast and salicylic acid treatments boosted PR-9 and PR-10 protein activities in peach fruit, which play a central role in the defense mechanism against a range of pathogens.
5.3. Antioxidants
Amino acids, such as glutamine, serve a two-way function by improving stress resistance and inducing antifungal compound biosynthesis [125]. It was reported that the expression of some proteins associated with stress response and regulation, e.g., serine/threonine protein kinase, was upregulated by elicitors. Moreover, antioxidants and food types like glutathione, ascorbic acid, amino acids, and phytic acids protect the yeast cells from oxidative damage and thus promote survival under the harsh conditions often experienced within postharvest environments [33]. Antioxidant molecules, such as trehalose and glutathione, are beneficial in boosting antioxidant activity in yeast [126,127]. Lu [128] discovered that antioxidant enzyme activity, that is, catalase (CAT) and superoxide dismutase (SOD), of the test group (yeast cultivated in NYDB with the supplementation of elicitor) was significantly greater than that of the control group (yeast cultivated in NYDB with no additives). Thus, they concluded that supplementation with the elicitor increased the antioxidant capacity of yeast.
5.4. pH Modulators
pH modulators like phytic acid are able to modify the microenvironment to favor yeast viability and, at the same time, suppress fungal growth [112]. All of these substances have a synergistic effect of improving the invasion and persistence of yeast and the suppression of pathogens. Further enhancement is provided by plant-derived materials, essential oils, and plant extracts, which not only improve yeast’s performance but also contribute direct antimicrobial activity. Meanwhile, encapsulation methods, such as alginate matrices or chitosan nanoparticles, have twin advantages, as they protect yeast cells from stress conditions and provide controlled delivery of active substances [129,130]. In general, such targeted advancements reduce the limitations of the application of yeast biocontrol, enhancing its efficiency for practical applications. Table 3 shows these enhancing substances and their corresponding biocontrol agent (BCA) subgroups. Difficulties using biocontrol approaches are largely related to specificity, as pathogens require specific modes of action instead of antagonistic microbes [25,112]. To overcome this gap, current research has been directed at increasing the effectiveness of microbial biocontrol agents (MBCAs) using various approaches [131].

Table 3.
List of common substances used as enhancers, along with their subgroups of BCA, to improve biocontrol effectiveness against pathogens.
6. Application Methods for Enhancers of Antagonistic Yeast Performance
Performance enhancers are a range of materials and environmental conditions that enhance the biocontrol efficacy of yeasts. There are different application methods for these enhancers, and selection of these application methods depends on the natural characteristics of antagonistic yeast, postharvest fungal pathogens in fruits and vegetables, the enhancing reagents to be used, environmental conditions, desired outcomes, and utilization purposes [116,142]. They consist of nutritional additives, protective carriers, growth stimulants, and encapsulants [143]. Using performance enhancers has recently gained interest. These strategies are extremely crucial to achieve maximum biocontrol efficacy by promoting improved microbial survival, improved metabolic activity, and improved functional capacity. Various studies show that antagonistic yeast enhancing substances can be applied through different techniques. These application techniques are classified based on the natural characteristics of the constituents and their effectiveness in improving antagonistic yeasts. There are five major categories of antagonistic yeast enhancers, eliciting different application approaches [112,113,123,144]. Figure 2 shows the common roles of enhancers and their interactions with pathogens, antagonistic yeasts, the environment, and host cells.
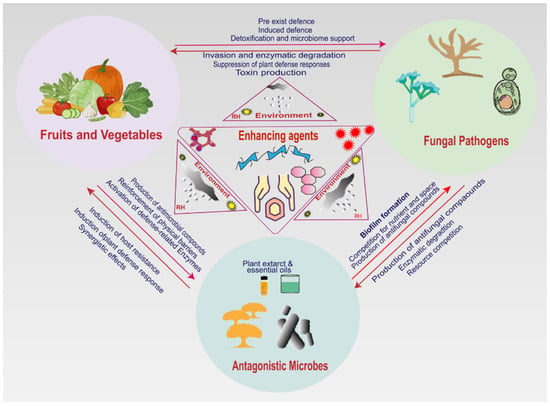
Figure 2.
The role of enhancers in interaction with pathogens, antagonistic yeasts, and host cells.
6.1. Pretreatment of Yeast Cells
Pretreatment/inducing involves adding performance-boosting substances to antagonistic yeast cultures before their application. This pre-enhancement approach essentially prepares the yeast cells by improving their stress tolerance and biocontrol activity in real-world conditions. While not traditional genetic modification, it modifies yeasts’ capabilities through physiological conditioning rather than DNA alteration. According to a study conducted by He [123], precultured or induced yeasts increase the induced systematic resistance and enhanced production of ROS in host cells, and they also compete for space and nutrients, play a role in the synthesis of antimicrobial compounds, such as enzymes, volatile compounds, and antibiotics, act through mycoparasitism, secrete lytic enzymes, contribute to biofilm formation, and provide quorum sensing against pathogens.
Ming [145] investigated how mannitol and sorbitol inducing/pretreatment affects stress tolerance in Debaryomyces hansenii. Their study revealed that antagonistic yeast cells induced/pretreated with polyol (MT or ST) maintained ~70% survival rates compared to untreated (NT) cells when exposed to oxidative stress (30 mM H2O2) or heat stress (40.5 °C) for 30 min. While NT cells under such conditions were ~50% viable, pretreated cells had significantly higher survival. Their study revealed that populations of yeasts show an improved ability to compete in ecological niches and thus limit the use of synthetic fungicides. These results establish that mannitol or sorbitol induction significantly improves yeast cell viability in stressful conditions. Chen [138] showed that Scheffersomyeces spartinae W9 cultures brought up in nutrient yeast dextrose broth (NYDB) supplemented with β-glucan had greater invasion rates in strawberry wound spots than control cultures developed from the usual NYDB medium. The population of the β-glucan-induced S. spartinae W9 increased from 6.1 log10 CFU wound−1 to 7.7 log10 CFU wound−1 at 24 h, which was significantly higher than that of S. spartinae W9 cultured in NYDB (p < 0.05). Liu’s [146] study shows that sublethal oxidative stress exposure increases Candida oleophila’s tolerance to subsequent stresses and improves its biocontrol performance against fruit pathogens. These enhancements are associated with the upregulation of antioxidant genes and reduced accumulation of reactive oxygen species. Similarly, a study by Zhang [116] shows that inducing improved yeast’s ability to compete with pathogens and enhanced its invading ability over host surfaces, making it an effective and biodegradable tool for replacing synthetic fungicides in postharvest loss management.
This inducing process works by creating selective pressure on yeast populations through nutrient and space competition [25]. Under these constrained conditions, less robust yeast cells struggle, while only the fittest survive. These stress-adapted cells subsequently demonstrate enhanced biocontrol performance, showing high resistance during pathogen interaction and more effective pathogen reduction [123,147].
Pretreatment primes yeast cells to resist and compete effectively against pathogens at the application site. The priming method attains two main benefits: it optimizes both improved cell viability and enhanced functional activity. Enhancing yeasts before exposure optimizes metabolism and resistance to stress by pathogens and is used for improving efficiency. In general, enhancer pretreatment presents a strategic approach to optimize the performance of antagonistic yeasts within agricultural biocontrol [112].
6.2. Co-Application of Yeast with Enhancers
Co-application mixing enhancer substances with yeast suspensions prior to treatment delivers both components (yeast and enhancers) simultaneously, providing immediate support for yeast viability and functionality [148]. The purpose of combining these substances with antagonistic yeast during application is to enhance the yeast’s viability and functionality, enabling it to endure reactions from both the host cell and the pathogen. Additionally, these elicitors serve as nutrients for the yeast, further supporting its mechanisms, such as nutrient and space competition, mycoparasitism, host resistance induction, and the production of volatile organic compounds (VOCs) and toxins to combat pathogens [116]. This method combines nutrients like phytic acid, ascorbic acid, amino acids, sodium bicarbonate, and calcium, defensive compounds, like chitosan or bioactive stimulants in some plant extracts, and plant hormones, like auxin indole acetic acid (IAA), Gibberellic acid (GA3), N6-benzyladenine (6-BA), harpin, and trehalose, with antagonistic yeasts to improve their biocontrol efficiency [6,33]. It was also reported that chitosan’s inclusion not only promotes yeast’s adhesion to the surfaces of plants but also its own antimicrobial ability [33,121]. Lima [117], in their study, reported that co-delivery of the elicitor proves to be an easy and effective method for the improvement of agricultural efficiency of biocontrol yeast. They reported that the utilization of food-grade additives could dramatically increase the activity of yeast-based formulation(s) and turn a moderate antagonist, such as the biocontrol isolate LS11, into a highly effective agent. The core benefits of such a process involve the ease of usage and the effect of fastening the action. With the provision of the required supporting compounds concurrently with the yeast, the cells instantly gain an edge in colonization, metabolic activities, and inhibitory ability toward pathogens [149,150]. In this method, combinations of yeast enhancers are applied directly to fruit and vegetable crops for postharvest disease management. Co-application simultaneously provides immediate microbial support, and this results in quicker and more effective control of the pathogens. Different studies have revealed that co-application significantly improves the efficiency and survival of yeast in many applications [108,123,151]. A key limitation of this method is the difficulty of clearly distinguishing whether the elicitors enhance the antagonistic yeast’s efficiency or whether they are involved in the action of directly combating pathogens.
6.3. Encapsulation or Immobilization
This method uses defensive coatings to improve the viability and performance of yeast. The physical barrier protects the cells from environmental harshness, including high and low temperatures, ultraviolet light, and drying. Along with providing protection, the medium supports the production of enhancers, thereby providing prolonged nutrition during storage and application stages. Bevilacqua [152] reported that alginate beads with sugars or plant extracts maintain yeast’s metabolic activity and improve antimicrobial activities. One of the first parameters reported in that study, encapsulation yield, was between 87.70 and 108% for different strains. Encapsulation approaches like these effectively improve the stability and efficiency of antagonistic yeasts’ biocontrol [153,154]. Encapsulation of the cells in such protective substances causes the survival rates to significantly improve even under harsh conditions [155,156,157].
Saccharomyces cerevisiae, which has an average diameter of about 5 μm, is now a popular option as an encapsulation carrier as whole cells, along with their derivatives, are used effectively as encapsulation agents [135]. It was also found to function as a carrier in the encapsulation process in the early 1970s. Some of the other strains that have also been utilized for microencapsulation are Torulopsis lipofera, Saccharomyces bayanus, Endomyces vernalis, and dairy yeasts, such as Candida utilis and Kluyveromyces fragilis. S. cerevisiae can be said to be an ideal, applicable, and familiar container in the encapsulation method that is utilized to microencapsulate a number of bioactive compounds. In a study carried out by [158], the non-plasmolyzed (NPYC) and plasmolyzed (PYC) S. cerevisiae yeast cells were utilized as microcarriers to encapsulate thymoquinone (black cumin seed oil). The study revealed that the bioactivity of thymoquinone was better preserved in PYC, whereas the PYC samples exhibited the lowest thymoquinone degradation ratio (52.63%) during storage. Dadkhodazade [159], in their research, analyzed the encapsulation efficiency of S. cerevisiae cells for cholecalciferol (vitamin D3). They reported the maximum EE (76.10 ± 6.92%) at the initial concentration of vitamin D3 of 2.5 mg/g of yeast cells for spray-dried and plasmolyzed yeast cells. The eukaryotic structure and plasma membrane of the cell wall make it distinctive as a physical barrier to environmental factors like oxygen or free radicals and radiation from light. This indicates that encapsulation is used to improve the viability of antagonistic yeasts by protecting them from environmental stresses.
In agricultural applications, microencapsulation systems based on biopolymers are increasingly being utilized to provide beneficial microbes with prolonged shelf life and timed-release properties [160]. This new method addresses major limitations of conventional formulations, with remarkable enhancement of microbial viability and activity under field conditions [155]. The key concerns raised by this approach are fear of chemical interactions between encapsulants, enhancers, and antagonistic yeasts, the simplicity and cost-effectiveness of the technique, and, most importantly, non-target effects on beneficial microbes or host organisms, which require rigorous assessment.
6.4. Post-Application Treatment
The post-application approach is a process in which enhancers are applied to the target environment after the initial introduction of yeast, which gives continuous support to maintain biocontrol activity [161]. This method utilizes multiple mechanisms, with enhancers prolonging yeast viability and metabolic activity, improving stress tolerance, indirectly suppressing pathogens, enabling delayed release of compounds, and fostering synergistic interactions for localized biocontrol effects. A practical application like foliar spraying of nutrient solutions (e.g., sugar or amino acid supplements) onto yeast-pre-treated crops is common under this method. Foliar spraying is a very common and straightforward method where nutrients or chemicals are dissolved in water and sprayed directly onto a plant’s leaves, and nutrient solutions are special supplements designed for quick energy. Sugar provides an immediate source of carbon and energy, while amino acids are the basic building blocks of proteins. Providing them directly saves the plant the energy needed to make them from scratch. These treatments keep yeast populations stable by stimulating metabolic function, colonization capacity, and pathogen suppression, which is particularly beneficial in environments with limited resources where indigenous nutrients are depleted [162].
Next to this treatment, interventions are unparalleled in their flexibility, allowing for tailored support based on environmental adjustments or different stages of yeast activity. A study by Sui [112] revealed that one benefit is enhanced yeast stress tolerance, a primary driver of commercial feasibility considering adverse abiotic conditions. The primary challenge of this approach lies in determining optimal application timing. Applying enhancers too early may compromise their supportive capacity for the antagonistic yeast, while delayed application becomes ineffective, as the yeast may have already lost its viability against pathogens, environmental stressors, and host defense responses.
7. Mechanisms Involved in Enhancement
As described in earlier studies, enhancers primarily function through mechanisms that include the stimulation of yeast growth, host resistance induction, the enhancement of antifungal activities, adhesion, and stress tolerance [25,116]. The working approach above includes groups of common substances used as antagonistic yeast boosting agents, which fall under five key mechanisms. The schematic diagram presented in Figure 3 illustrates some of these typical mechanisms through which enhancers function to enhance the efficacy of antagonistic yeast in fungal pathogen management.
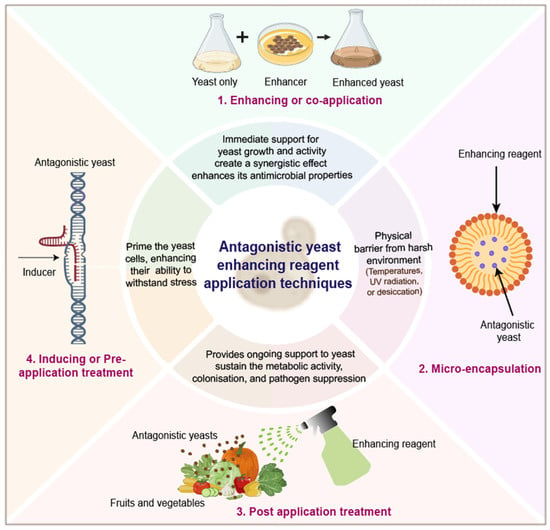
Figure 3.
Common action mechanisms of enhancers to improve the performance of antagonistic yeast in controlling fungal pathogens.
7.1. Stimulating Yeast Growth and Fitness Activity
Enhancers can supply nutrients or growth-stimulating substances that can enhance the growth and metabolic activity of antagonistic yeast in postharvest fruits and vegetables [117,163]. For instance, the inclusion of simple sugars (e.g., glucose) or nitrogen sources (e.g., amino acids) can stimulate yeast’s growth, allowing these microorganisms to compete better with fungal pathogens for resources on the surfaces of stored produce. Moreover, chitin and chitosan compounds are able to induce the formation of yeast enzymes (e.g., chitinases) that break down the cell walls of fungal pathogens, a property that is especially useful for inhibiting infection during storage. By stimulating the growth and activity of yeast, these substances keep the surface of fruit and vegetable crops healthy with a protective layer, thereby preventing spoilage and increasing their shelf life [164,165].
7.2. Improving the General Induction of Host Resistance
For fruits and vegetables, biocontrol enhancers can act as elicitors to activate or enhance the innate defense mechanisms of produce against fungal pathogens. Compounds like chitosan, salicylic acid, and β-glucans can be applied directly to harvested produce, triggering defensive responses, such as the production of phytoalexins, pathogenesis-related (PR) proteins, and reactive oxygen species (ROS) [166,167]. For example, a chitosan coating on strawberries or apples can initiate the natural defense of the fruit, making it more resistant to fungal infection when stored. This induced resistance increases the efficacy of the antagonistic yeast, giving a double-layered protection system. By enhancing the defense mechanisms of the host, these elicitors bring about the reduction of spoilage, extend the shelf life, and maintain the quality of postharvest vegetables and fruits [168,169].
7.3. Enhancing Antagonistic Properties
Enhancers can increase the production of antifungal metabolites by antagonistic yeast, such as killer toxins, antibiotics, or VOCs, in postharvest fruits and vegetables [66,82]. For example, small amounts of natural compounds, such as essential oils, or specific nutrients can act synergistically with yeasts’ action, thus enhancing their ability to inhibit fungal growth in stored agricultural commodities. These metabolites, according to reports, directly antagonize pathogens by disrupting their cell membranes, inhibiting spore germination, or suppressing hyphal growth. By enhancing the antagonistic activity of the yeast, these compounds make the biocontrol agent effective in inhibiting fungal infection in storage, reducing spoilage, and maintaining the quality of produce [82,116,117].
7.4. Improving Adhesion and Colonization
Enhancers can increase the production of antifungal metabolites by antagonist yeasts, such as killer toxins, antibiotics, or VOCs. For example, sub-inhibitory concentrations of certain fungicides or natural compounds (e.g., essential oils) can synergize with yeasts’ activity, augmenting their inhibitory action on fungal growth. By disrupting their cell membrane, inhibiting spore germination, or disrupting hyphal growth, these metabolites have a direct action on pathogens. These substances significantly increase the activity of biocontrol agents in mitigating fungal infections by amplifying the antagonistic character of the yeast [170,171].
7.5. Improving Stress Tolerance
Fruits and vegetables are exposed to environmental stresses like temperature fluctuations, low humidity, and ultraviolet radiation throughout storage and transportation during and after harvest. Enhancers can make antagonistic yeasts more resistant to such stresses and preserve their viability and functionality. For instance, osmoprotectants like trehalose or glycerol can make yeast cells more resilient to adverse storage conditions, enhancing their viability and functional efficacy [112,145]. Additionally, antioxidants have the ability to protect yeast against oxidative stress caused by UV exposure during transport. These compounds ensure yeast’s efficacy in controlling fungal pathogens along the postharvest chain, maintaining product quality and reducing losses by enhancing stress tolerance.
8. Conclusions and Predictions
Biocontrol methods aimed at fungal plant pathogen control have been attracting consumer interest due to their naturalness, harmlessness to human health, and environmental safety. Their extensive application is, nonetheless, hindered by reduced effectiveness, product specificity, variability in different environmental conditions, short shelf life, limited mass production, less accessibility for market demand, and lack of awareness of their action mechanisms. Overcoming these limitations is critical in order to harness the full potential of biological control in reducing global food wastage. Enhancing the efficacy of antagonistic microbes has emerged as a viable option for overcoming such limitations. An array of organic substances and natural constituents, such as nutrient supplements, antioxidants, and growth regulators, has proven successful in improving the efficacy of biocontrol techniques. This approach not only aligns with consumer preferences for environmentally friendly options but also addresses the immediate need to reduce economic losses and ensure food security. Future research should shift its focus from understanding the mechanisms behind yeast’s efficacy to addressing the practical challenges of product formulation and large-scale commercialization.
Author Contributions
G.S.E.: writing—original draft, conceptualization, visualization, data curation, software. E.A.G.: review and editing, data curation. G.L.N.N. and K.W.: review and editing. H.Z.: funding acquisition, resources, validation, supervision. Q.Y.: funding acquisition, resources, supervision, validation. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (32472804, 32472414, 32272383).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No data were used in this research.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (32472804, 32472414, 32272383).
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- Ali, M.; Ali, A.; Ali, S.; Chen, H.; Wu, W.; Liu, R.; Chen, H.; Ahmed, Z.F.R.; Gao, H. Global insights and advances in edible coatings or films toward quality maintenance and reduced postharvest losses of fruit and vegetables: An updated review. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70103. [Google Scholar] [CrossRef]
- Karoney, E.; Molelekoa, T.; Bill, M.; Siyoum, N.; Korsten, L. Global research network analysis of fresh produce postharvest technology: Innovative trends for loss reduction. Postharvest Biol. Technol. 2024, 208, 112642. [Google Scholar] [CrossRef]
- Rahul, S.N.; Khilari, K.; Sagar, S.; Chaudhary, S.; Kumar, S.; Vihan, N.; Tomar, A. Challenges in Postharvest Management of fungal Diseases in Fruits and Vegetables: A review. South Asian J. Food Technol. Environ. 2015, 1, 126–130. [Google Scholar] [CrossRef]
- Shanmugam, V.; Pothiraj, G.; Dauda, W.P. Endophytes for postharvest disease management in vegetables and fruits. In Postharvest Handling and Diseases of Horticultural Produce; CRC Press: Boca Raton, FL, USA, 2021; pp. 93–110. [Google Scholar]
- Araújo, A.S.; de Lima, G.S.; dos Santos Nunes, I.; de Oliveira Farias, J.C.R.; Navarro, D.M.d.A.F.; Melo, N.F.C.B.; Magalhães, N.S.S.; França, R.; Carvalho, R.d.S.F.; Stamford, T.C.M. Chitosan hydrochloride-gum Arabic-passion fruit seed oil nanoparticle edible coating to control fungal infection and maintain quality parameters of strawberries. Food Control 2024, 161, 110360. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, L.; Fan, K. Recent advances in polysaccharide-based edible coatings for preservation of fruits and vegetables: A review. Crit. Rev. Food Sci. Nutr. 2024, 64, 3823–3838. [Google Scholar] [CrossRef]
- Akanbi, T.A. Common Causes and Prevention of Postharvest Losses in Fruits and Vegetables. Quest J. J. Res. Agric. Anim. Sci. 2021, 8, 21–24. [Google Scholar]
- Ferdousi, J.; Hussain, M.; Saha, S.; Rob, M.; Afroz, T.; Pramanik, S.; Islam, M.; Nath, D. Postharvest physiology of fruits and vegetables and their management technology: A Review. J. Anim. Plant Sci. 2024, 34, 291–303. [Google Scholar] [CrossRef]
- Kamboj, M.; Chauhan, N.; Goswami, P. Postharvest Diseases in Fruits and Vegetables during Storage. In Packaging and Storage of Fruits and Vegetables; Apple Academic Press: New York, NY, USA, 2021; pp. 269–287. [Google Scholar]
- Fatimma, A.N. Efficacy of Allium sativum extract as post-harvest treatment of fruit rot of mango. Plant Pathol. Quar. 2018, 8, 144–152. [Google Scholar] [CrossRef]
- Singh, D.; Sharma, R.R.; Kesharwani, A.K. Postharvest losses of horticultural produce. In Postharvest Handling and Diseases of Horticultural Produce; CRC Press: Boca Raton, FL, USA, 2021; pp. 1–24. [Google Scholar]
- Sharma, R.; Yadav, A.; Lata, C.; Verma, A.; Singh, D.; Rajput, L.S.; Joshi, R.; Samota, M.K.; Kumar, S.; Kumar, K.; et al. Role of biotechnology for shelf-life extension and quality improvement of perishable fruits and vegetables: A comprehensive review. Food Sci. Biotechnol. 2025. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, X.; Huang, Z.; Zhao, X.; Qiao, L.; Wu, C.; Xue, Z.; Kou, X. Phenylpropanoids for the control of fungal diseases of postharvest fruit. Plant Mol. Biol. 2025, 115, 39. [Google Scholar] [CrossRef] [PubMed]
- James, A.; Zikankuba, V. Postharvest management of fruits and vegetable: A potential for reducing poverty, hidden hunger and malnutrition in sub-Sahara Africa. Cogent Food Agric. 2017, 3, 1312052. [Google Scholar] [CrossRef]
- Gahlot, D.; Meena, N.L.; Trivedi, A.; Kumar, S. In vitro efficacy of fungicides and phyto-extracts against Colletotrichum gloeosporioides causing Anthracnose of Mango. Ann. Plant Prot. Sci. 2021, 29, 217–221. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Xin, Y.; Godana, E.A.; Dhanasekaran, S.; Luo, R.; Li, J.; Zhao, L.; Zhang, H. Exploring the biocontrol performance of Bacillus velezensis against postharvest diseases of eggplants and the underlying action mechanisms in soft rot management. Postharvest Biol. Technol. 2025, 227, 113556. [Google Scholar] [CrossRef]
- Godana, E.A.; Yang, Q.; Zhang, X.; Zhao, L.; Wang, K.; Dhanasekaran, S.; Mehari, T.G.; Zhang, H. Biotechnological and biocontrol approaches for mitigating postharvest diseases caused by fungal pathogens and their mycotoxins in fruits: A review. J. Agric. Food Chem. 2023, 71, 17584–17596. [Google Scholar] [CrossRef]
- Abano, E.E.; Sam-Amoah, L.K. Application of antagonistic microorganisms for the control of postharvest decays in fruits and vegetables. Int. J. Adv. Biol. Res. 2012, 2, 1–8. [Google Scholar]
- Zhou, Y.; Zhao, L.; Chen, Y.; Dhanasekaran, S.; Chen, X.; Zhang, X.; Yang, X.; Wu, M.; Song, Y.; Zhang, H. Study on the control effect and physiological mechanism of Wickerhamomyces anomalus on primary postharvest diseases of peach fruit. Int. J. Food Microbiol. 2024, 413, 110575. [Google Scholar] [CrossRef]
- Wei, M.; Dhanasekaran, S.; Ji, Q.; Yang, Q.; Zhang, H. Sustainable and efficient method utilizing N-acetyl-L-cysteine for complete and enhanced ochratoxin A clearance by antagonistic yeast. J. Hazard. Mater. 2023, 448, 130975. [Google Scholar] [CrossRef] [PubMed]
- Droby, S.; Wisniewski, M.; El-Ghaouth, A.; Wilson, C. Biological Control of Postharvest Diseases of Fruit and Vegetables: Current Achievements and Future Challenges. In Proceedings of the XXVI International Horticultural Congress: Issues and Advances in Postharvest Horticulture, Toronto, ON, Canada, 11–17 August 2002; pp. 1–28. [Google Scholar] [CrossRef]
- Fravel, D.R. Commercialization and implementation of biocontrol. Annu. Rev. Phytopathol. 2005, 43, 337–359. [Google Scholar] [CrossRef]
- Dhillon, P.K.; Kaur, M.; Sharma, S.C.; Mahmood, A. Harnessing killer yeast system: From molecular insight to real world biocontrol solution. Arch. Microbiol. 2025, 207, 116. [Google Scholar] [CrossRef]
- Marín, A.; Atarés, L.; Chiralt, A. Improving function of biocontrol agents incorporated in antifungal fruit coatings: A review. Biocontrol Sci. Technol. 2017, 27, 1220–1241. [Google Scholar] [CrossRef]
- Liu, J.; Sui, Y.; Wisniewski, M.; Droby, S.; Liu, Y. Review: Utilization of antagonistic yeasts to manage postharvest fungal diseases of fruit. Int. J. Food Microbiol. 2013, 167, 153–160. [Google Scholar] [CrossRef]
- Magan, N. Importance of ecological windows for efficacy of biocontrol agents. In Progress in Biological Control; Springer Nature: London, UK, 2020; pp. 1–14. [Google Scholar]
- Bonaterra, A.; Badosa, E.; Daranas, N.; Francés, J.; Roselló, G.; Montesinos, E. Bacteria as biological control agents of plant diseases. Microorganisms 2022, 10, 1759. [Google Scholar] [CrossRef]
- Duffy, B.; Schouten, A.; Raaijmakers, J.M. Pathogen self-defense: Mechanisms to Counteract Microbial Antagonism. Annu. Rev. Phytopathol. 2003, 41, 501–538. [Google Scholar] [CrossRef]
- Patibanda, A.K.; Ranganathswamy, M. Effect of agrichemicals on biocontrol agents of plant disease control. In Microorganisms for Sustainability; Springer Nature: London, UK, 2018; pp. 1–21. [Google Scholar]
- Kumar, V.; Shankar, R.; Kumar, G. Strategies used for reducing postharvest losses in fruits and vegetables. Int. J. Eng. Res. 2015, 6, 130–137. [Google Scholar]
- Chhetri, R.T.; Magar, P.; Kandel, S.; Gnyawali, P. A review paper on post-harvest loss on fruits and vegetables. Food Agri Econ. Rev. 2023, 3, 1–4. [Google Scholar] [CrossRef]
- Alegbeleye, O.; Odeyemi, O.A.; Strateva, M.; Stratev, D. Microbial spoilage of vegetables, fruits and cereals. Appl. Food Res. 2022, 2, 100122. [Google Scholar] [CrossRef]
- Zhang, H.; Mahunu, G.K.; Castoria, R.; Yang, Q.; Apaliya, M.T. Recent developments in the enhancement of some postharvest biocontrol agents with unconventional chemicals compounds. Trends Food Sci. Technol. 2018, 78, 180–187. [Google Scholar] [CrossRef]
- Kader, A. Opportunities for International Collaboration in Postharvest Education and Extension Activities. Acta Hortic. 2013, 1012, 1363–1370. [Google Scholar] [CrossRef]
- De, S.; Banerjee, S.; Banerjee, S. Managing Postharvest losses of Vegetables and Fruits: A Methodological review. Recent Adv. Food Nutr. Agric. 2024, 15, 138–162. [Google Scholar] [CrossRef]
- Feng, P.; Zhang, X.; Godana, E.A.; Ngea, G.L.N.; Dhanasekaran, S.; Gao, L.; Li, J.; Zhao, L.; Zhang, H. Control of postharvest soft rot of green peppers by Bacillus subtilis through regulating ROS metabolism. Physiol. Mol. Plant Pathol. 2024, 131, 102280. [Google Scholar] [CrossRef]
- Nan, M.; Xue, H.; Bi, Y. Contamination, detection and control of mycotoxins in fruits and vegetables. Toxins 2022, 14, 309. [Google Scholar] [CrossRef]
- Lastochkina, O.; Seifikalhor, M.; Aliniaeifard, S.; Baymiev, A.; Pusenkova, L.; Garipova, S.; Kulabuhova, D.; Maksimov, I. Bacillus spp.: Efficient biotic strategy to control postharvest diseases of fruits and vegetables. Plants 2019, 8, 97. [Google Scholar] [CrossRef]
- Xu, M.; Godana, E.A.; Dhanasekaran, S.; Zhang, X.; Yang, Q.; Zhao, L.; Zhang, H. Comparative proteome and transcriptome analyses of the response of postharvest pears to Penicillium expansum infection. Postharvest Biol. Technol. 2022, 196, 112182. [Google Scholar] [CrossRef]
- Prusky, D.; Romanazzi, G. Induced resistance in fruit and vegetables: A host physiological response limiting postharvest disease development. Annu. Rev. Phytopathol. 2023, 61, 279–300. [Google Scholar] [CrossRef]
- Liu, J.; Sui, Y.; Chen, H.; Liu, Y.; Liu, Y. Proteomic Analysis of Kiwifruit in Response to the Postharvest Pathogen, Botrytis cinerea. Front. Plant Sci. 2018, 9, 158. [Google Scholar] [CrossRef]
- Ji, Q.; Yang, Q.; Dhanasekaran, S.; Ma, J.; Zhang, H. Hannaella sinensis, a promising biocontrol agent for combating postharvest pear fruit diseases and patulin degradation. Food Control 2024, 164, 110618. [Google Scholar] [CrossRef]
- Putra, A.I.; Khan, M.N.; Kamaruddin, N.; Khairuddin, R.F.R.; Al-Obaidi, J.R.; Flores, B.J.; Flores, L.F. Proteomic insights into fruit–pathogen interactions: Managing biotic stress in fruit. Plant Cell Rep. 2025, 44, 54. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Godana, E.A.; Wang, K.; Zhang, H. A proteomic analysis of Wickerhamomyces anomalus incubated with chitosan reveals dynamic changes in protein expression and metabolic pathways. Postharvest Biol. Technol. 2024, 211, 112806. [Google Scholar] [CrossRef]
- Zhang, H.; Apaliya, M.T.; Mahunu, G.K.; Chen, L.; Li, W. Control of ochratoxin A-producing fungi in grape berry by microbial antagonists: A review. Trends Food Sci. Technol. 2016, 51, 88–97. [Google Scholar] [CrossRef]
- Wei, M.; Dhanasekaran, S.; Godana, E.A.; Yang, Q.; Sui, Y.; Zhang, X.; Ngea, G.L.N.; Zhang, H. Whole-genome sequencing of Cryptococcus podzolicus Y3 and data-independent acquisition-based proteomic analysis during OTA degradation. Food Control 2022, 136, 108862. [Google Scholar] [CrossRef]
- Udoh, I.P.; Eleazar, C.I.; Ogeneh, B.O.; Ohanu, M.E. Studies on fungi responsible for the Spoilage/Deterioration of some edible fruits and vegetables. Adv. Microbiol. 2015, 5, 285–290. [Google Scholar] [CrossRef]
- Dhanasekaran, S.; Ackah, M.; Yang, Q.; Zhang, H. De novo transcriptome assembly and analysis unveil molecular insights into Cladosporidium rot development in harvested table grapes. Postharvest Biol. Technol. 2025, 225, 113497. [Google Scholar] [CrossRef]
- Wang, K.; Wang, H.; Xu, M.; Godana, E.A.; Lu, Y.; Zhang, H. Functional analysis of apple defense protein MdPL and screening of proteins interaction with Penicillium expansum. Postharvest Biol. Technol. 2025, 219, 113289. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kang, D.H. Microbial safety of pickled fruits and vegetables and hurdle technology. Internet J. Food Saf. 2004, 4, 21–32. [Google Scholar]
- Umeohia, U.E.; Olapade, A.A. Physiological processes affecting postharvest quality of fresh fruits and vegetables. Asian Food Sci. J. 2024, 23, 1–14. [Google Scholar] [CrossRef]
- Yang, Q.; Hu, Y.; Wang, Y.; Xu, B.; Zhou, C.; Adhikari, B.; Liu, J.; Xu, T.; Wang, B. Atmosphere-controlled high-voltage electrospray for improving conductivity, flexibility, and antibacterial properties of chitosan films. Food Res. Int. 2024, 200, 115450. [Google Scholar] [CrossRef]
- Jain, N.K.; Roy, I. Trehalose and protein stability. Curr. Protoc. Protein Sci. 2010, 59, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Romanazzi, G.; Tzortzakis, N.; Ippolito, A.; Allagui, M.B.; Spadaro, D.; Kinay-Teksur, P.; Pérezgago, M.; Kilic, M.; Montesinos, C.; Xylia, P.; et al. Innovative sustainable technologies to extend the shelf life of perishable mediterranean fresh fruit, vegetables, and aromatic plants and to reduce waste: The experience of prima STOPMEDWASTE project. In Proceedings of the 12th International Congress of Plant Pathology, Lyon, France, 20–25 August 2023; pp. 879–880. [Google Scholar]
- Zhao, Q.; Zhang, Y.; Solairaj, D.; Lu, Y.; Zhang, X.; Zhang, X.; Yang, Q.; Sui, Y.; Zhang, H. Unveiling the role of AcWRKY53: Bioinformatics and functional insights into kiwifruit postharvest resistance. Postharvest Biol. Technol. 2025, 222, 113431. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, Q.; Dhanasekaran, S.; Godana, E.A.; Zhang, X.; Yang, Q.; Zhao, L.; Zhang, H. The necrosis-inducing protein (NIP) gene contributes to Penicillium expansum virulence during postharvest pear infection. Food Res. Int. 2022, 158, 111562. [Google Scholar] [CrossRef]
- Nassarawa, S.S.; Xu, Y.; Luo, Z.; Abdelshafy, A.M.; Li, L. Effect of Light-Emitting Diodes (LEDs) on the Quality of Fruits and Vegetables During Postharvest Period: A Review. Food Bioprocess Technol. 2020, 14, 388–414. [Google Scholar] [CrossRef]
- Schirra, M.; D’Aquino, S.; Cabras, P.; Angioni, A. Control of postharvest diseases of fruit by heat and fungicides: Efficacy, residue levels, and residue persistence. A review. J. Agric. Food Chem. 2011, 59, 8531–8542. [Google Scholar] [CrossRef]
- Barkai-Golan, R. Postharvest Diseases of Fruits and Vegetables: Development and Control; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- de Chiara, M.L.V.; Castagnini, J.M.; Capozzi, V. Cutting-edge physical techniques in postharvest for fruits and vegetables: Unveiling their power, inclusion in ‘hurdle’approach, and latest applications. Trends Food Sci. Technol. 2024, 151, 104619. [Google Scholar] [CrossRef]
- Fan, X.; Wang, W. Quality of fresh and fresh-cut produce impacted by nonthermal physical technologies intended to enhance microbial safety. Crit. Rev. Food Sci. Nutr. 2020, 62, 362–382. [Google Scholar] [CrossRef]
- Wu, P.; Chang, H.; Shen, Y. Effects of synthetic and environmentally friendly fungicides on powdery mildew management and the phyllosphere microbiome of cucumber. PLoS ONE 2023, 18, e0282809. [Google Scholar] [CrossRef]
- Geiger, F.; Bengtsson, J.; Berendse, F.; Weisser, W.W.; Emmerson, M.; Morales, M.B.; Ceryngier, P.; Liira, J.; Tscharntke, T.; Winqvist, C.; et al. Persistent negative effects of pesticides on biodiversity and biological control potential on European farmland. Basic Appl. Ecol. 2010, 11, 97–105. [Google Scholar] [CrossRef]
- Droby, S.; Wisniewski, M.; Macarisin, D.; Wilson, C. Twenty years of postharvest biocontrol research: Is it time for a new paradigm? Postharvest Biol. Technol. 2009, 52, 137–145. [Google Scholar] [CrossRef]
- Fenta, L.; Mekonnen, H.; Kabtimer, N. The Exploitation of Microbial Antagonists against Postharvest Plant Pathogens. Microorganismes 2023, 11, 1044. [Google Scholar] [CrossRef]
- Ling, L.; Feng, L.; Li, Y.; Yue, R.; Wang, Y.; Zhou, Y. Endophytic Fungi Volatile Organic Compounds as Crucial Biocontrol Agents Used for Controlling Fruit and Vegetable Postharvest Diseases. J. Fungi 2024, 10, 332. [Google Scholar] [CrossRef]
- Li, X.; Zeng, S.; Wisniewski, M.; Droby, S.; Yu, L.; An, F.; Leng, Y.; Wang, C.; Li, X.; He, M.; et al. Current and future trends in the biocontrol of postharvest diseases. Crit. Rev. Food Sci. Nutr. 2022, 64, 5672–5684. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Montiel, L.G.; Droby, S.; Preciado-Rangel, P.; Rivas-García, T.; González-Estrada, R.R.; Gutiérrez-Martínez, P.; Ávila-Quezada, G.D. A sustainable alternative for postharvest disease management and phytopathogens biocontrol in fruit: Antagonistic yeasts. Plants 2021, 10, 2641. [Google Scholar] [CrossRef]
- Ribes, S.; Fuentes, A.; Talens, P.; Barat, J.M. Prevention of fungal spoilage in food products using natural compounds: A review. Crit. Rev. Food Sci. Nutr. 2017, 58, 2002–2016. [Google Scholar] [CrossRef]
- Kupper, K.C.; Cervantes, A.L.L.; Klein, M.N.; Da Silva, A.C. Avaliação De Microrganismos Antagônicos, Saccharomyces cerevisiae E Bacillus subtilis Para O Controle De Penicillium digitatum. Rev. Bras. Frutic. 2013, 35, 425–436. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, X.; Zhao, L.; Apaliya, M.T.; Yang, Q.; Sun, W.; Zhang, X.; Zhang, H. Screening of deoxynivalenol producing strains and elucidation of possible toxigenic molecular mechanism. Toxins 2017, 9, 184. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, X.; Solairaj, D.; Lin, R.; Ackah, M.; Ngea, G.L.N.; Zhang, H. Transcriptomic analyses reveal robust changes in the defense response of apples induced by Hannaella sinensis. Biol. Control 2023, 182, 105237. [Google Scholar] [CrossRef]
- Droby, S.; Vinokur, V.; Weiss, B.; Cohen, L.; Daus, A.; Goldschmidt, E.E.; Porat, R. Induction of Resistance to Penicillium digitatum in Grapefruit by the Yeast Biocontrol Agent Candida oleophila. Phytopathology 2002, 92, 393–399. [Google Scholar] [CrossRef]
- El-Ghaouth, A.; Wilson, C.L.; Wisniewski, M. Ultrastructural and Cytochemical Aspects of the Biological Control of Botrytis cinerea by Candida saitoana in Apple Fruit. Phytopathology 1998, 88, 282–291. [Google Scholar] [CrossRef]
- Haïssam, J.M. Pichia anomala in biocontrol for apples: 20 years of fundamental research and practical applications. Antonie Leeuwenhoek 2011, 99, 93–105. [Google Scholar] [CrossRef]
- Raynaldo, F.A.; Dhanasekaran, S.; Ngea, G.L.N.; Yang, Q.; Zhang, X.; Zhang, H. Investigating the biocontrol potentiality of Wickerhamomyces anomalus against postharvest gray mold decay in cherry tomatoes. Sci. Hortic. 2021, 285, 110137. [Google Scholar] [CrossRef]
- Zhu, M.; Yang, Q.; Godana, E.A.; Huo, Y.; Hu, S.; Zhang, H. Efficacy of Wickerhamomyces anomalus in the biocontrol of black spot decay in tomatoes and investigation of the mechanisms involved. Biol. Control 2023, 186, 105356. [Google Scholar] [CrossRef]
- Shi, Y.; Zhao, Q.; Xin, Y.; Yang, Q.; Dhanasekaran, S.; Zhang, X.; Zhang, H. Aureobasidium pullulans S2 controls tomato gray mold and produces volatile organic compounds and biofilms. Postharvest Biol. Technol. 2023, 204, 112450. [Google Scholar] [CrossRef]
- Nunes, C.; Usall, J.; Teixidó, N.; Abadias, M.; Viñas, I. Improved Control of Postharvest Decay of Pears by the Combination of Candida sake (CPA-1) and Ammonium Molybdate. Phytopathology 2002, 92, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Leng, J.; Dai, Y.; Qiu, D.; Zou, Y.; Wu, X. Utilization of the antagonistic yeast, Wickerhamomyces anomalus, combined with UV-C to manage postharvest rot of potato tubers caused by Alternaria tenuissima. Int. J. Food Microbiol. 2022, 377, 109782. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Verma, U.; Awasthi, P. A combination of the yeast Candida utilis and chitosan controls fruit rot in tomato caused by Alternaria alternata (Fr.) Keissler and Geotrichum candidum Link ex Pers. J. Hortic. Sci. Biotechnol. 2006, 81, 1043–1051. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, J.; Tian, R.; Liu, Y. Microbial volatile organic compounds: Antifungal mechanisms, applications, and challenges. Front. Microbiol. 2022, 13, 922450. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Y.; Zhang, B. Induced resistance in peach fruit as treated by Pichia guilliermondii and their possible mechanism. Int. J. Food Prop. 2020, 23, 34–51. [Google Scholar] [CrossRef]
- Qiu, J.E.; Zhao, L.; Jiang, S.; Godana, E.A.; Zhang, X.; Zhang, H. Efficacy of Meyerozyma caribbica in the biocontrol of blue mold in kiwifruit and mechanisms involved. Biol. Control 2022, 173, 105000. [Google Scholar] [CrossRef]
- Pan, H.; Zhong, C.; Wang, Z.; Deng, L.; Li, W.; Zhao, J.; Long, C.; Li, L. Biocontrol Ability and Action Mechanism of Meyerozyma guilliermondii 37 on Soft Rot Control of Postharvest Kiwifruit. Microorganismes 2022, 10, 2143. [Google Scholar] [CrossRef] [PubMed]
- Leibinger, W.; Breuker, B.; Hahn, M.; Mendgen, K. Control of postharvest pathogens and colonization of the apple surface by antagonistic microorganisms in the field. Phytopathology 1997, 87, 1103–1110. [Google Scholar] [CrossRef]
- Haggag, W.M.; Soud, M.A.E. Production and Optimization of Pseudomonas fluorescens Biomass and Metabolites for Biocontrol of Strawberry Grey Mould. Am. J. Plant Sci. 2012, 3, 836–845. [Google Scholar] [CrossRef]
- Tsalgatidou, P.C.; Papageorgiou, A.; Boutsika, A.; Chatzidimopoulos, M.; Delis, C.; Tsitsigiannis, D.I.; Paplomatas, E.; Zambounis, A. Insights into the Interaction between the Biocontrol Agent Bacillus amyloliquefaciens QST 713, the Pathogen Monilinia fructicola and Peach Fruit. Agronomy 2024, 14, 771. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, Y.; Wang, Y.; Li, B.; Gu, X.; Zhang, X.; Boateng, N.a.S.; Zhang, H. Effect of β-glucan on the biocontrol efficacy of Cryptococcus podzolicus against postharvest decay of pears and the possible mechanisms involved. Postharvest Biol. Technol. 2020, 160, 111057. [Google Scholar] [CrossRef]
- Zivkovic, S.; Stosic, S.; Ristic, D.; Vucurovic, I.; Stevanovic, M. Antagonistic potential of Lactobacillus plantarum against some postharvest pathogenic fungi. Zb. Matice Srp. Prir. Nauk. 2019, 136, 79–88. [Google Scholar] [CrossRef]
- Alijani, Z.; Amini, J.; Karimi, K.; Pertot, I. Characterization of the Mechanism of Action of Serratia rubidaea Mar61-01 against Botrytis cinerea in Strawberries. Plants 2022, 12, 154. [Google Scholar] [CrossRef]
- Allagui, M.B.; Moumni, M.; Romanazzi, G. Antifungal activity of thirty essential oils to control pathogenic fungi of postharvest decay. Antibiotics 2023, 13, 28. [Google Scholar] [CrossRef]
- Sefu, G.; Satheesh, N.; Berecha, G. Antifungal activity of ginger and cinnamon leaf essential oils on mango anthracnose disease causing fungi (C. gloeosporioides). Carpathian J. Food Sci. Technol. 2015, 7, 26–34. [Google Scholar]
- Carmello, C.R.; Magri, M.M.R.; Cardoso, J.C. Cinnamon and clove aqueous extracts promote in vitro and postharvest control of Alternaria alternate in tomato fruit. Eur. J. Plant Pathol. 2025, 172, 261–274. [Google Scholar] [CrossRef]
- Ali, J.; Hussain, A.; Siddique, M.; Rahman, Z.U.; Ikram, M.; Zahoor, M.; Ullah, R.; Gulfam, N.; Shah, A.B. Fungicidal effect of Azadirachta indica extracts against pathogenic fungi Rhizopus stolonifer and Monilinia fructicola in postharvest peaches. Discov. Plants 2025, 2, 126. [Google Scholar] [CrossRef]
- Chime, A.O.; Aiwansoba, R.O. Antifungal Activity of Neem (Azadirachta indica) Leaf Extract against Pathogens Associated with Tomato (Solanum lycopersicum L.) Fruit Spoilage. Afr. Sci. 2023, 24, 297–303. [Google Scholar] [CrossRef]
- Embaby, E.S.M.; Hazaa, M.M.; El-Dougdoug, K.A.; Abdel Monem, M.O.; Elwan, E.E. Control Apple Fruit Fungi Using a Green Biocide Neem (Azadirachta indica A. Juss). J. Basic Environ. Sci. 2017, 4, 253–261. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Cao, J.; Jiang, W. Antifungal activities of neem (Azadirachta indica) seed kernel extracts on postharvest diseases in fruits. Afr. J. Microbiol. Res. 2010, 4, 1100–1104. [Google Scholar]
- Sellamuthu, P.S.; Sivakumar, D.; Soundy, P. Antifungal Activity and Chemical Composition of Thyme, Peppermint and Citronella Oils in Vapor Phase against Avocado and Peach Postharvest Pathogens. J. Food Saf. 2013, 33, 86–93. [Google Scholar] [CrossRef]
- Jaronski, S.T. Ecological factors in the inundative use of fungal entomopathogens. Biocontrol 2009, 55, 159–185. [Google Scholar] [CrossRef]
- Chowdhury, N.; Hazarika, D.J.; Goswami, G.; Sarmah, U.; Borah, S.; Boro, R.C.; Barooah, M. Acid tolerant bacterium Bacillus amyloliquefaciens MBNC retains biocontrol efficiency against fungal phytopathogens in low pH. Arch. Microbiol. 2022, 204, 124. [Google Scholar] [CrossRef]
- Adnan, M.; Islam, W.; Shabbir, A.; Khan, K.A.; Ghramh, H.A.; Huang, Z.; Chen, H.Y.; Lu, G. Plant defense against fungal pathogens by antagonistic fungi with Trichoderma in focus. Microb. Pathog. 2019, 129, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Zehra, A.; Raytekar, N.A.; Meena, M.; Swapnil, P. Efficiency of microbial bio-agents as elicitors in plant defense mechanism under biotic stress: A review. Curr. Res. Microb. Sci. 2021, 2, 100054. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zheng, X.; Su, Y.; Lu, Y.; Yang, Q.; Shi, Y.; Lanhuang, B.; Zhang, X.; Zhao, L.; Godana, E.A.; et al. A glycoside hydrolase superfamily gene plays a major role in Penicillium expansum growth and pathogenicity in apples. Postharvest Biol. Technol. 2022, 198, 112228. [Google Scholar] [CrossRef]
- Sharma, R.R.; Reddy, S.V.R. Biological control of postharvest diseases. In II International Organic Fruit Symposium; Apple Academic Press: New York, NY, USA, 2018; pp. 167–212. [Google Scholar]
- Droby, S.; Wisniewski, M.; Teixidó, N.; Spadaro, D.; Jijakli, M.H. Biocontrol of postharvest diseases with antagonistic microorganisms. In Postharvest Pathology of Fresh Horticultural Produce; CRC Press: Boca Raton, FL, USA, 2019; pp. 463–498. [Google Scholar]
- Zhao, L.; Hu, Y.; Liang, L.; Dhanasekaran, S.; Zhang, X.; Yang, X.; Wu, M.; Song, Y.; Zhang, H. WSC1 Regulates the Growth, Development, Patulin Production, and Pathogenicity of Penicillium expansum Infecting Pear Fruits. J. Agric. Food Chem. 2024, 72, 1025–1034. [Google Scholar] [CrossRef]
- Zhang, X.; Xin, Y.; Yue, Q.; Godana, E.A.; Gao, L.; Dou, M.; Zhou, H.; Li, J.; Zhao, L.; Zhang, H. Insight into the mechanisms involved in the improved antagonistic efficacy of Pichia caribbica against postharvest black spot of tomato fruits by combined application with oligochitosan. Postharvest Biol. Technol. 2024, 213, 112968. [Google Scholar] [CrossRef]
- Köhl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of action of microbial biological control agents against plant diseases: Relevance beyond efficacy. Front. Plant Sci. 2019, 10, 845. [Google Scholar] [CrossRef]
- Hao, J.; Xu, H.; Yan, P.; Yang, M.; Mintah, B.K.; Dai, C.; Zhang, R.; Ma, H.; He, R. Application of fixed-frequency ultrasound in the cultivation of Saccharomyces cerevisiae for rice wine fermentation. J. Sci. Food Agric. 2024, 104, 6417–6430. [Google Scholar] [CrossRef]
- Sun, J.; Tan, X.; Liu, B.; Battino, M.; Meng, X.; Zhang, F. Blue light inhibits gray mold infection by inducing disease resistance in cherry tomato. Postharvest Biol. Technol. 2024, 215, 113006. [Google Scholar] [CrossRef]
- Sui, Y.; Wisniewski, M.; Droby, S.; Liu, J. Responses of yeast biocontrol agents to environmental stress. Appl. Environ. Microbiol. 2015, 81, 2968–2975. [Google Scholar] [CrossRef]
- Li, C.; Zhang, H.; Yang, Q.; Komla, M.G.; Zhang, X.; Zhu, S. Ascorbic Acid Enhances Oxidative Stress Tolerance and Biological Control Efficacy of Pichia caribbica against Postharvest Blue Mold Decay of Apples. J. Agric. Food Chem. 2014, 62, 7612–7621. [Google Scholar] [CrossRef]
- Crowe, J.H. Trehalose as a “Chemical chaperone”. Adv. Exp. Med. Biol. 2007, 594, 143–158. [Google Scholar]
- Kaushik, J.K.; Bhat, R. Why is trehalose an exceptional protein stabilizer? J. Biol. Chem. 2003, 278, 26458–26465. [Google Scholar] [CrossRef]
- Zhang, X.; Li, B.; Zhang, Z.; Chen, Y.; Tian, S. Antagonistic yeasts: A promising alternative to chemical fungicides for controlling postharvest decay of fruit. J. Fungi 2020, 6, 158. [Google Scholar] [CrossRef]
- Lima, G.; Castoria, R.; Spina, A.; De Curtis, F.; Caputo, L. Improvement of biocontrol yeast activity against postharvest pathogens: Recent experiences. Acta Hortic. 2005, 682, 2035–2040. [Google Scholar] [CrossRef]
- De Simone, N.; Capozzi, V.; Amodio, M.L.; Colelli, G.; Spano, G.; Russo, P. Microbial-based biocontrol solutions for fruits and vegetables: Recent insight, patents, and innovative trends. Recent Pat. Food Nutr. Agric. 2021, 12, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Dhanasekaran, S.; Liang, L.; Chen, Y.; Chen, J.; Guo, S.; Zhang, X.; Zhao, L.; Zhang, H. Alginate oligosaccharide induces resistance against Penicillium expansum in pears by priming defense responses. Plant Physiol. Biochem. 2025, 220, 109531. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Dawson, A.; Henderson, A.C.; Lockyer, E.J.; Read, E.; Sritharan, G.; Ryan, M.; Sgroi, M.; Ngou, P.M.; Woodruff, R.; et al. Nutrient supplements boost yeast transformation efficiency. Sci. Rep. 2016, 6, 35738. [Google Scholar] [CrossRef]
- Zhang, H.; Ge, L.; Chen, K.; Zhao, L.; Zhang, X. Enhanced biocontrol activity of Rhodotorula mucilaginosa cultured in media containing chitosan against postharvest diseases in strawberries: Possible mechanisms underlying the effect. J. Agric. Food Chem. 2014, 62, 4214–4224. [Google Scholar] [CrossRef]
- Gu, N.; Zhang, X.; Gu, X.; Zhao, L.; Dhanasekaran, S.; Qian, X.; Zhang, H. Proteomic analysis reveals the mechanisms involved in the enhanced biocontrol efficacy of Rhodotorula mucilaginosa induced by chitosan. Biol. Control 2020, 149, 104325. [Google Scholar] [CrossRef]
- He, Y.; Degraeve, P.; Oulahal, N. Bioprotective yeasts: Potential to limit postharvest spoilage and to extend shelf life or improve microbial safety of processed foods. Heliyon 2024, 10, e24929. [Google Scholar] [CrossRef]
- Chantrasri, P.; Sardsud, V.; Sangchote, S.; Sardsud, U. Combining yeasts and chitosan treatment to reduce anthracnose fruit rot in mangoes. Asian J. Biol. Educ. 2007, 3, 40–46. [Google Scholar]
- Zhimo, V.Y.; Bhutia, D.D.; Saha, J.; Panja, B. Exploitation of yeasts as an alternative strategy to control postharvest diseases of fruits-a review. World Appl. Sci. J. 2014, 31, 785–793. [Google Scholar]
- Fernando, I.D.N.S.; Abeysinghe, D.C.; Dharmadasa, R.M. Determination of phenolic contents and antioxidant capacity of different parts of Withania somnifera (L.) Dunal. from three different growth stages. Ind. Crops Prod. 2013, 50, 537–539. [Google Scholar] [CrossRef]
- Liu, H.; Cao, J.; Jiang, W. Evaluation and comparison of vitamin C, phenolic compounds, antioxidant properties and metal chelating activity of pulp and peel from selected peach cultivars. LWT-Food Sci. Technol. 2015, 63, 1042–1048. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, K.; Su, Y.; Dhanasekaran, S.; Yang, Q.; Zhang, H. Genome-wide investigation and analysis of C2H2 Zinc Finger Protein gene family in apple: Expression profiles during Penicillium expansum infection process. Physiol. Mol. Plant Pathol. 2023, 128, 102172. [Google Scholar] [CrossRef]
- Soto, E.R.; Rus, F.; Mirza, Z.; Ostroff, G.R. Yeast particles for encapsulation of terpenes and essential oils. Molecules 2023, 28, 2273. [Google Scholar] [CrossRef] [PubMed]
- Hasheminejad, N.; Khodaiyan, F.; Safari, M. Improving the antifungal activity of clove essential oil encapsulated by chitosan nanoparticles. Food Chem. 2018, 275, 113–122. [Google Scholar] [CrossRef]
- Abano, E.E.; Sam-Amoah, L.K.; Bart-Plange, A. Variation in ultrasonic frequency and time as pre-treatments to air-drying of carrot. J. Agric. Eng. 2012, 43, e23. [Google Scholar] [CrossRef]
- Godana, E.A.; Yang, Q.; Zhao, L.; Zhang, X.; Liu, J.; Zhang, H. Pichia anomala Induced With Chitosan Triggers Defense Response of Table Grapes Against Postharvest Blue Mold Disease. Front. Microbiol. 2021, 12, 704519. [Google Scholar] [CrossRef]
- Deng, Q.; Lei, X.; Zhang, H.; Deng, L.; Yi, L.; Zeng, K. Phenylalanine Promotes Biofilm Formation of Meyerozyma caribbica to Improve Biocontrol Efficacy against Jujube Black Spot Rot. J. Fungi 2022, 8, 1313. [Google Scholar] [CrossRef]
- Boateng, N.a.S.; Ackah, M.; Wang, K.; Dzah, C.S.; Zhang, H. Comparative physiological and transcriptomic analysis reveals an improved biological control efficacy of Sporidiobolus pararoseus Y16 enhanced with ascorbic acid against the oxidative stress tolerance caused by Penicillium expansum in pears. Plant Physiol. Biochem. 2024, 210, 108627. [Google Scholar] [CrossRef]
- Dhanasekaran, S.; Yang, Q.; Godana, E.A.; Liu, J.; Li, J.; Zhang, H. Trehalose supplementation enhanced the biocontrol efficiency of Sporidiobolus pararoseusY16 through increased oxidative stress tolerance and altered transcriptome. Pest Manag. Sci. 2021, 77, 4425–4436. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, H.; Li, J.; Cui, J.; Zhang, X.; Ren, X. Enhancement of Biocontrol Efficacy of Pichia carribbica to Postharvest Diseases of Strawberries by Addition of Trehalose to the Growth Medium. Int. J. Mol. Sci. 2012, 13, 3916–3932. [Google Scholar] [CrossRef]
- Xi, Y.; Yang, Q.; Godana, E.A.; Zhang, H. Study on the effect of Debaryomyces hansenii enhanced by alginate oligosaccharide against postharvest blue mold decay of apples and the physiological mechanisms involved. Biol. Control 2022, 176, 105081. [Google Scholar] [CrossRef]
- Chen, X.; Wei, Y.; Zou, X.; Zhao, Z.; Jiang, S.; Chen, Y.; Xu, F.; Shao, X. β-Glucan Enhances the Biocontrol Efficacy of Marine Yeast Scheffersomyeces spartinae W9 against Botrytis cinerea in Strawberries. J. Fungi 2023, 9, 474. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chan, Z.; Xu, Y.; Tian, S. Effect of Pichia membranaefaciens combined with salicylic acid on controlling brown rot in peach fruit and the mechanisms involved. J. Sci. Food Agric. 2008, 88, 1786–1793. [Google Scholar] [CrossRef]
- Gramisci, B.R.; Lutz, M.C.; Lopes, C.A.; Sangorrín, M.P. Enhancing the efficacy of yeast biocontrol agents against postharvest pathogens through nutrient profiling and the use of other additives. Biol. Control 2018, 121, 151–158. [Google Scholar] [CrossRef]
- Abd-Alla, M.A.; Nehal, S.E.-M.; Nadia, G.E.-G. Formulation of Essential Oils and Yeast for Controlling Postharvest Decay of Tomato Fruits. Plant Pathol. Bull. 2009, 18, 23–33. [Google Scholar]
- Wang, Y.; Luo, Y.; Sui, Y.; Xie, Z.; Liu, Y.; Jiang, M.; Liu, J. Exposure of Candida oleophila to sublethal salt stress induces an antioxidant response and improves biocontrol efficacy. Biol. Control 2018, 127, 109–115. [Google Scholar] [CrossRef]
- Oztekin, S.; Dikmetas, D.N.; Devecioglu, D.; Acar, E.G.; Karbancioglu-Guler, F. Recent Insights into the Use of Antagonistic Yeasts for Sustainable Biomanagement of Postharvest Pathogenic and Mycotoxigenic Fungi in Fruits with their Prevention Strategies against Mycotoxins. J. Agric. Food Chem. 2023, 71, 9923–9950. [Google Scholar] [CrossRef]
- Raynaldo, F.A.; Ackah, M.; Ngea, G.L.N.; Yolandani, N.; Rehman, S.A.; Yang, Q.; Wang, K.; Zhang, X.; Zhang, H. The potentiality of Wickerhamomyces anomalus against postharvest black spot disease in cherry tomatoes and insights into the defense mechanisms involved. Postharvest Biol. Technol. 2023, 209, 112699. [Google Scholar] [CrossRef]
- Ming, X.; Wang, Y.; Sui, Y. Pretreatment of the Antagonistic Yeast, Debaryomyces hansenii, With Mannitol and Sorbitol Improves Stress Tolerance and Biocontrol Efficacy. Front. Microbiol. 2020, 11, 601. [Google Scholar] [CrossRef]
- Liu, J.; Wisniewski, M.; Droby, S.; Norelli, J.; Hershkovitz, V.; Tian, S.; Farrell, R. Increase in antioxidant gene transcripts, stress tolerance and biocontrol efficacy of Candida oleophila following sublethal oxidative stress exposure. FEMS Microbiol. Ecol. 2012, 80, 578–590. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Yang, Q.; Xiao, J.; Solairaj, D.; Ngea, G.L.N.; Zhang, H. Study on the biocontrol effect and physiological mechanism of Hannaella sinensis on the blue mold decay of apples. Int. J. Food Microbiol. 2022, 382, 109931. [Google Scholar] [CrossRef]
- Benito, P.; Ligorio, D.; Bellón, J.; Yenush, L.; Mulet, J.M. A fast method to evaluate in a combinatorial manner the synergistic effect of different biostimulants for promoting growth or tolerance against abiotic stress. Plant Methods 2022, 18, 111. [Google Scholar] [CrossRef]
- González-Orozco, B.D.; Kosmerl, E.; Jiménez-Flores, R.; Alvarez, V.B. Enhanced probiotic potential of Lactobacillus kefiranofaciens OSU-BDGOA1 through co-culture with Kluyveromyces marxianus bdgo-ym6. Front. Microbiol. 2023, 14, 1236634. [Google Scholar] [CrossRef]
- Dou, Y.; Dhanasekaran, S.; Ngea, G.L.N.; Yang, Q.; Zhang, X.; Zhao, L.; Wang, K.; Zhang, H. Transcriptome analysis provides insights into potential mechanisms of epsilon-poly-L-lysine inhibiting Penicillium expansum invading apples. Postharvest Biol. Technol. 2023, 207, 112622. [Google Scholar] [CrossRef]
- Freimoser, F.M.; Rueda-Mejia, M.P.; Tilocca, B.; Migheli, Q. Biocontrol yeasts: Mechanisms and applications. World J. Microbiol. Biotechnol. 2019, 35, 154. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Campaniello, D.; Speranza, B.; Racioppo, A.; Altieri, C.; Sinigaglia, M.; Corbo, M.R. Microencapsulation of Saccharomyces cerevisiae into Alginate Beads: A Focus on Functional Properties of Released Cells. Foods 2020, 9, 1051. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wu, M.; Qin, X.; Dong, Q.; Li, Z. Antimicrobial function of yeast against pathogenic and spoilage microorganisms via either antagonism or encapsulation: A review. Food Microbiol. 2023, 112, 104242. [Google Scholar] [CrossRef] [PubMed]
- Gurusamy, S.; Dhanasekaran, S.; Liang, L.; Zhang, Y.; Yang, Q.; Li, Y.; Liu, X.; Zhang, H. Edible Fe2ZnO4 nanocomposite for extending shelf-life and preventing blue mold decay in apples. Food Control 2024, 171, 111111. [Google Scholar] [CrossRef]
- John, R.P.; Tyagi, R.D.; Brar, S.K.; Surampalli, R.Y.; Prévost, D. Bio-encapsulation of microbial cells for targeted agricultural delivery. Crit. Rev. Biotechnol. 2011, 31, 211–226. [Google Scholar] [CrossRef]
- Coradello, G.; Tirelli, N. Yeast cells in microencapsulation. General features and controlling factors of the encapsulation process. Molecules 2021, 26, 3123. [Google Scholar] [CrossRef]
- Dhanasekaran, S.; Liang, L.; Gurusamy, S.; Godana, E.A.; Yang, Q.; Zhang, H. Efficacy and mechanism of chitosan nanoparticles containing lemon essential oil against blue mold decay of apples. Int. J. Biol. Macromol. 2025, 308, 142633. [Google Scholar] [CrossRef]
- Karaman, K. Characterization of Saccharomyces cerevisiae based microcarriers for encapsulation of black cumin seed oil: Stability of thymoquinone and bioactive properties. Food Chem. 2020, 313, 126129. [Google Scholar] [CrossRef]
- Dadkhodazade, E.; Mohammadi, A.; Shojaee-Aliabadi, S.; Mortazavian, A.M.; Mirmoghtadaie, L.; Hosseini, S.M. Yeast cell microcapsules as a novel carrier for cholecalciferol encapsulation: Development, characterization and release properties. Food Biophys. 2018, 13, 404–411. [Google Scholar] [CrossRef]
- Saberi-Riseh, R.; Moradi-Pour, M.; Mohammadinejad, R.; Thakur, V.K. Biopolymers for biological control of plant pathogens: Advances in microencapsulation of beneficial microorganisms. Polymers 2021, 13, 1938. [Google Scholar] [CrossRef]
- Dinu, S. Biocontrol of Postharvest Fungal Diseases by Microbial Antagonists—Minireview. Rom. J. Plant Prot. 2022, 15, 1–14. [Google Scholar] [CrossRef]
- Gogoi, A.R.; Khiangte, L.; Thapa, S.; Baral, D.; Subba, A.B. Antagonistic yeast: Eco-friendly tool for management plant diseases. Int. J. Adv. Biochem. Res. 2024, 8, 906–914. [Google Scholar] [CrossRef]
- Agirman, B.; Carsanba, E.; Settanni, L.; Erten, H. Exploring yeast-based microbial interactions: The next frontier in postharvest biocontrol. Yeast 2023, 40, 457–475. [Google Scholar] [CrossRef]
- Romanazzi, G.; Feliziani, E.; Baños, S.B.; Sivakumar, D. Shelf-life extension of fresh fruit and vegetables by chitosan treatment. Crit. Rev. Food Sci. Nutr. 2015, 57, 579–601. [Google Scholar] [CrossRef] [PubMed]
- Rajestary, R.; Landi, L.; Romanazzi, G. Chitosan and postharvest decay of fresh fruit: Meta-analysis of disease control and antimicrobial and eliciting activities. Compr. Rev. Food Sci. Food Saf. 2020, 20, 563–582. [Google Scholar] [CrossRef]
- Herrera-González, J.A.; Bautista-Baños, S.; Serrano, M.; Romanazzi, G.; Gutiérrez-Martínez, P. Non-Chemical treatments for the pre- and Postharvest elicitation of defense mechanisms in the Fungi–Avocado pathosystem. Molecules 2021, 26, 6819. [Google Scholar] [CrossRef]
- Adss, I.; Hamza, H.; Hafez, E.; Heikal, H. Enhancing Tomato Fruits Post-Harvest Resistance by Salicylic Acid and Hydrogen Peroxide Elicitors Against Rot Caused by Alternaria solani. J. Agric. Chem. Biotechnol. 2017, 8, 1–8. [Google Scholar] [CrossRef]
- Bi, Y.; Xue, H.; Wang, J. Induced resistance in fruits and vegetables by elicitors to control postharvest diseases. In Postharvest Pathology of Fresh Horticultural Produce; CRC Press: Boca Raton, FL, UAS, 2019; pp. 793–816. [Google Scholar]
- Wang, B.; Bi, Y. The role of signal production and transduction in induced resistance of harvested fruits and vegetables. Food Qual. Saf. 2021, 5, 205–212. [Google Scholar] [CrossRef]
- Chen, P.; Chen, R.; Chou, J. Screening and Evaluation of Yeast Antagonists for Biological Control of Botrytis cinerea on Strawberry Fruits. Mycobiology 2018, 46, 33–46. [Google Scholar] [CrossRef]
- Choińska, R.; Piasecka-Jóźwiak, K.; Chabłowska, B.; Dumka, J.; Łukaszewicz, A. Biocontrol ability and volatile organic compounds production as a putative mode of action of yeast strains isolated from organic grapes and rye grains. Antonie Leeuwenhoek 2020, 113, 1135–1146. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).