Emulsion-Coated Active Papers Extend the Storage Life of Tomato Fruit
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Mixture Screening
2.2.2. Pure Essential Oil Composition by GC-MS
2.2.3. Antifungal Activity Tests
Fungal Strain
Pure EOs
Mixture of EOs
2.2.4. Emulsion Preparation
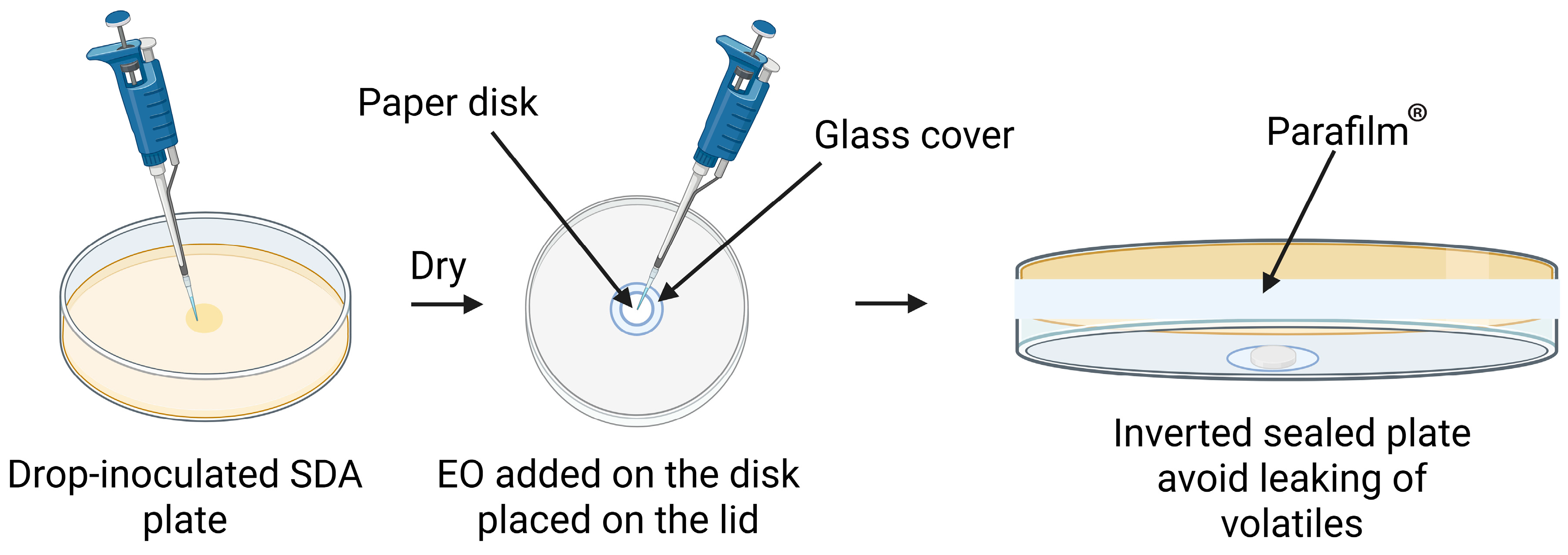
2.2.5. Paper Coating and Storage
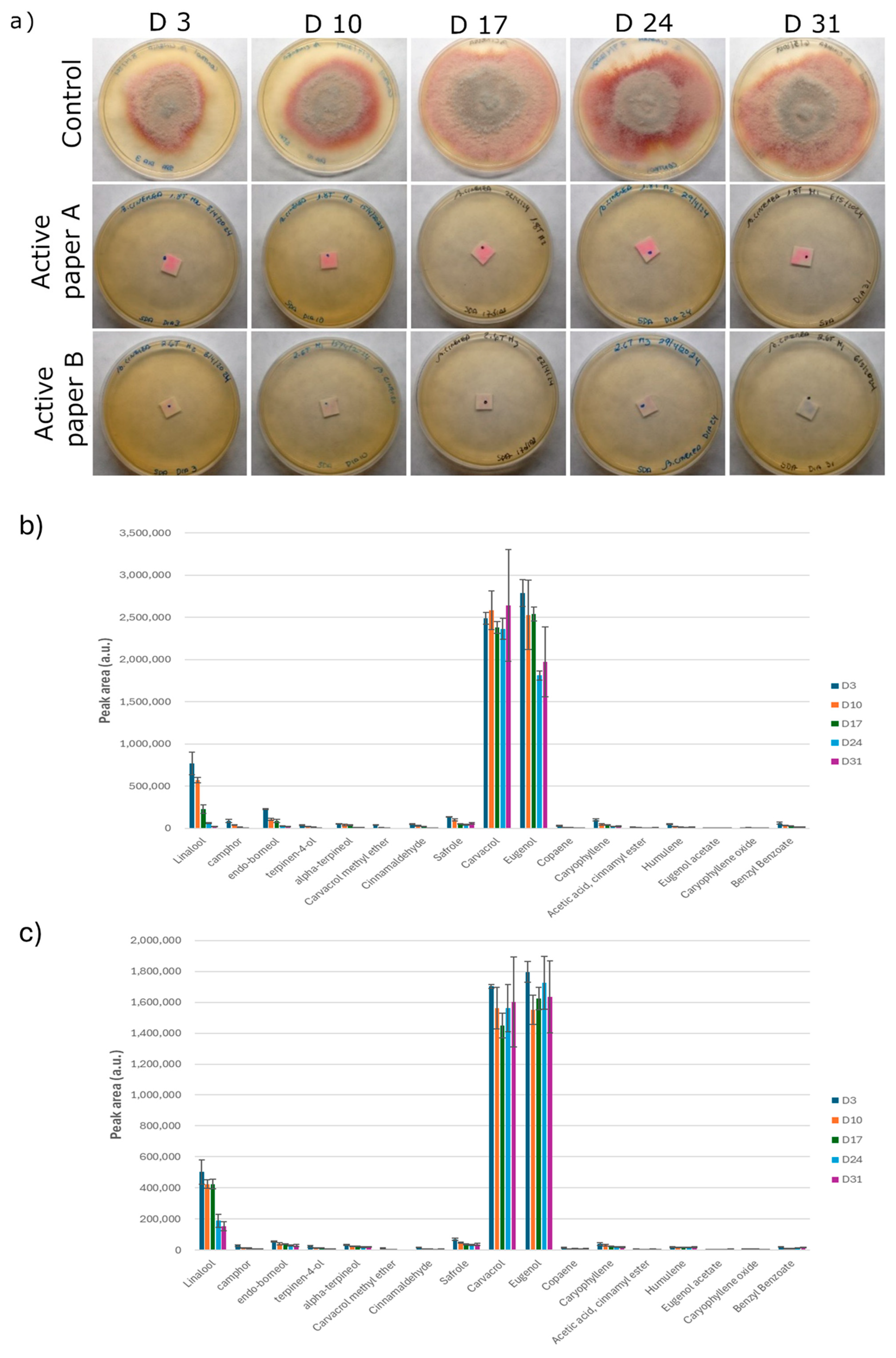
2.2.6. Assessment of Active Paper Stability
2.2.7. Evaluation of Active Papers on Fresh Tomatoes
Initial Fruit Quality
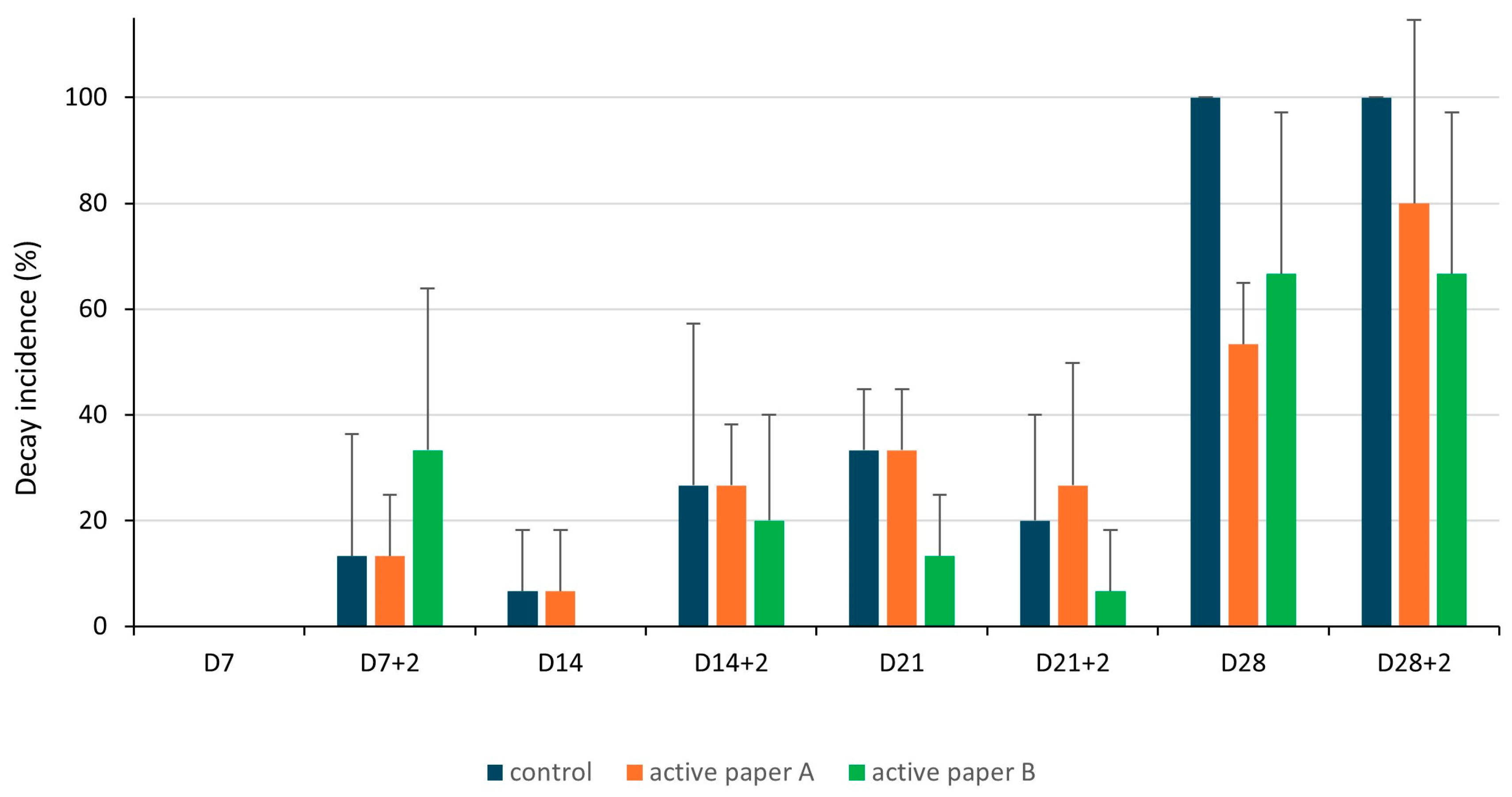
Decay Incidence
Decay Index
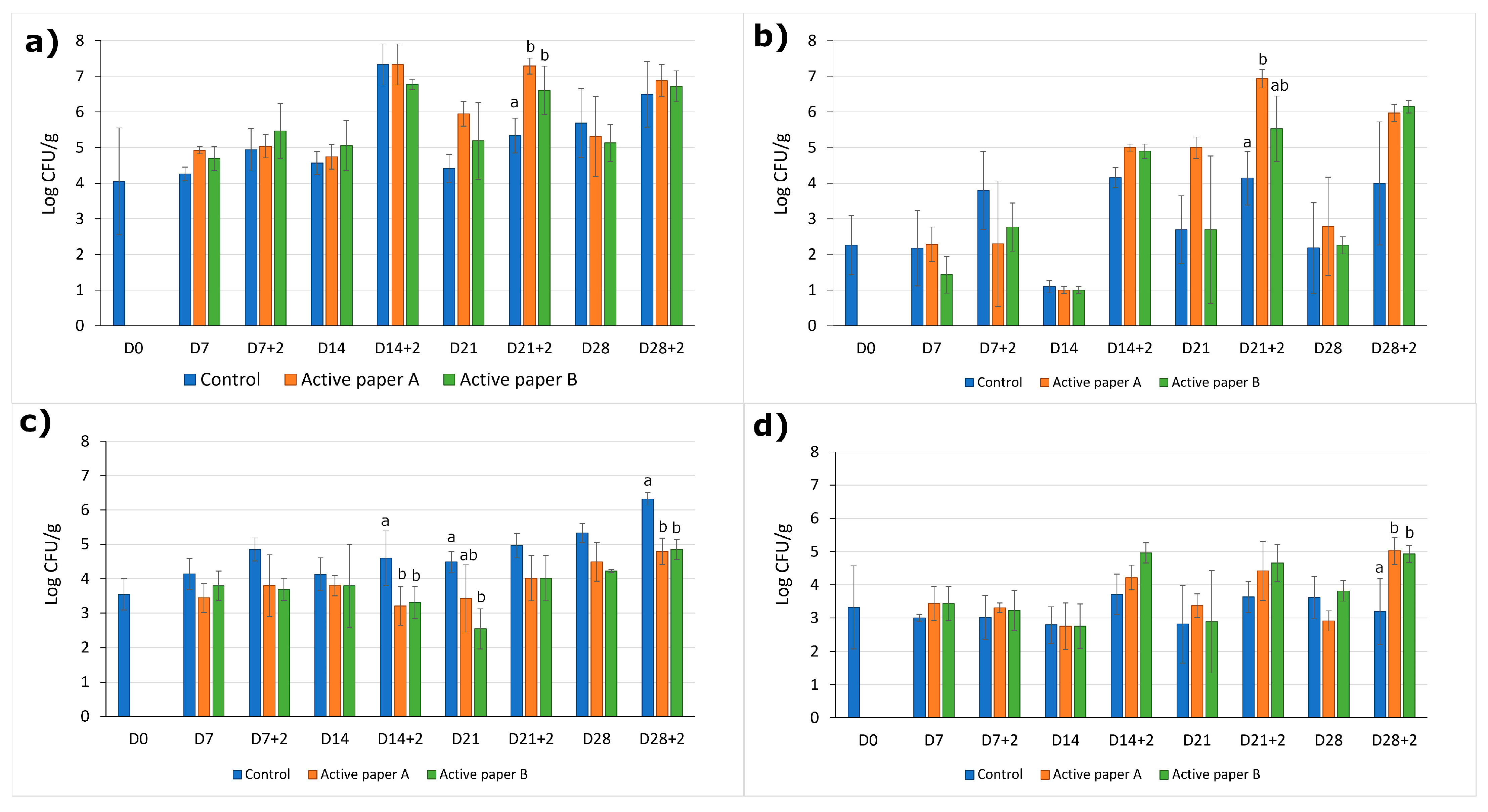
Microbial Growth
Consumer Acceptance
2.2.8. Statistics
3. Results and Discussion
3.1. Antifungal Activity of Pure EOs
3.2. Screening of Mixtures and Optimal Combination
3.3. Paper Stability Through Time (Volatile Composition and Culture-Media Assays)
3.4. Evaluation of the Active Packaging on Tomatoes
3.4.1. Decay Incidence
3.4.2. Decay Index
3.4.3. Microbial Growth
3.4.4. Consumer Perception
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EO | Essential oil |
| RT | Red thyme essential oil |
| CL | Cinnamon leaf essential oil |
| OR | Oregano essential oil |
| SH | Summer savory essential oil |
| EOmix | Mixture of CL and OR (2:1 v/v) |
| MIC | Minimal Inhibitory Concentration |
| SDA | Sabouraud Dextrose Agar |
| DI | Decay Index |
References
- Food and Agriculture Organization of the United Nations. Food Wastage Footprint: Impacts on Natural Resources; Food and Agriculture Organization: Rome, Italy, 2013. [Google Scholar]
- Food and Agriculture Organization of the United Nations. Mitigation of Food Wastage Societal Costs and Benefits; Food and Agriculture Organization: Rome, Italy, 2014. [Google Scholar]
- European Commission. Eurostat Statistics Explained—Food Waste and Food Waste Prevention—Estimates. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Food_waste_and_food_waste_prevention_-_estimates (accessed on 11 June 2025).
- Porat, R.; Lichter, A.; Terry, L.A.; Harker, R.; Buzby, J. Postharvest Losses of Fruit and Vegetables during Retail and in Consumers’ Homes: Quantifications, Causes, and Means of Prevention. Postharvest Biol. Technol. 2018, 139, 135–149. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Global Food Losses and Food Waste—Extent, Causes and Prevention; Food and Agriculture Organization: Rome, Italy, 2011. [Google Scholar]
- Food and Agriculture Organization of the United Nations. The State of Food and Agriculture. Moving Forward on Food Loss and Waste Reduction; Food and Agriculture Organization: Rome, Italy, 2019. [Google Scholar]
- Alegbeleye, O.; Odeyemi, O.A.; Strateva, M.; Stratev, D. Microbial Spoilage of Vegetables, Fruits and Cereals. Appl. Food Res. 2022, 2, 100122. [Google Scholar] [CrossRef]
- Barth, M.; Zhuang, H.; Breidt, F.; Hankinson, T.R. Microbiological Spoilage of Fruits and Vegetables. In Compendium of the Microbiological Spoilage of Foods and Beverages; Sperber, W.H., Doyle, M.P., Eds.; Food Microbiology and Food Safety; Springer Science: New York, NY, USA, 2009; pp. 135–183. ISBN 978-1-4419-0825-4. [Google Scholar]
- Pitt, J.I.; Hocking, A.D. Fungi and Food Spoilage; Springer: Boston, MA, USA, 2009; ISBN 978-0-387-92206-5. [Google Scholar]
- Spadaro, D.; Droby, S. Development of Biocontrol Products for Postharvest Diseases of Fruit: The Importance of Elucidating the Mechanisms of Action of Yeast Antagonists. Trends Food Sci. Technol. 2016, 47, 39–49. [Google Scholar] [CrossRef]
- Milicevic, D.; Nesic, K.; Jaksic, S. Mycotoxin Contamination of the Food Supply Chain—Implications for One Health Programme. Procedia Food Sci. 2015, 5, 187–190. [Google Scholar] [CrossRef]
- Stoev, S.D. Foodborne Mycotoxicoses, Risk Assessment and Underestimated Hazard of Masked Mycotoxins and Joint Mycotoxin Effects or Interaction. Environ. Toxicol. Pharmacol. 2015, 39, 794–809. [Google Scholar] [CrossRef]
- World Health Organization. Mycotoxins. Available online: https://www.who.int/news-room/fact-sheets/detail/mycotoxins (accessed on 11 July 2024).
- Punja, Z.K.; Rodriguez, G.; Tirajoh, A.; Formby, S. Role of Fruit Surface Mycoflora, Wounding and Storage Conditions on Post-Harvest Disease Development on Greenhouse Tomatoes. Can. J. Plant Pathol. 2016, 38, 448–459. [Google Scholar] [CrossRef]
- Hahn, F. Rhizopus Stolonifer Detection by Sensing the Tomato Peduncle Scar. Biosyst. Eng. 2006, 95, 171–179. [Google Scholar] [CrossRef]
- Doehlemann, G.; Ökmen, B.; Zhu, W.; Sharon, A. Plant Pathogenic Fungi. Microbiol. Spectr. 2017, 5, 5.1.14. [Google Scholar] [CrossRef]
- Charles, M.T.; Makhlouf, J.; Arul, J. Physiological Basis of UV-C Induced Resistance to Botrytis Cinerea in Tomato Fruit. Postharvest Biol. Technol. 2008, 47, 21–26. [Google Scholar] [CrossRef]
- Obande, M.A.; Tucker, G.A.; Shama, G. Effect of Preharvest UV-C Treatment of Tomatoes (Solanum lycopersicon Mill.) on Ripening and Pathogen Resistance. Postharvest Biol. Technol. 2011, 62, 188–192. [Google Scholar] [CrossRef]
- Stevens, C.; Liu, J.; Khan, V.A.; Lu, J.Y.; Kabwe, M.K.; Wilson, C.L.; Igwegbe, E.C.K.; Chalutz, E.; Droby, S. The Effects of Low-Dose Ultraviolet Light-C Treatment on Polygalacturonase Activity, Delay Ripening and Rhizopus Soft Rot Development of Tomatoes. Crop Prot. 2004, 23, 551–554. [Google Scholar] [CrossRef]
- Hua, L.; Yong, C.; Zhanquan, Z.; Boqiang, L.; Guozheng, Q.; Shiping, T. Pathogenic Mechanisms and Control Strategies of Botrytis cinerea Causing Post-Harvest Decay in Fruits and Vegetables. Food Qual. Saf. 2018, 2, 111–119. [Google Scholar] [CrossRef]
- Wu, F.; Lin, Y.; Zheng, B.; Wang, H.; Qu, Z.; Zhang, X.; Cai, H.; Li, X.; Feng, S. 2-Phenylethanol Biocontrol Postharvest Tomato Gray Mold and Its Effect on Tomato Quality. Sci. Hortic. 2024, 337, 113550. [Google Scholar] [CrossRef]
- Elad, Y.; Williamson, B.; Tudzynski, P.; Delen, N. Botrytis: Biology, Pathology and Control; Springer Netherlands: Dordrecht, The Netherlands, 2007; ISBN 978-1-4020-2624-9. [Google Scholar]
- Elad, Y.; Pertot, I.; Cotes Prado, A.M.; Stewart, A. Plant Hosts of Botrytis spp. In Botrytis—The Fungus, the Pathogen and Its Management in Agricultural Systems; Fillinger, S., Elad, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 413–486. ISBN 978-3-319-23370-3. [Google Scholar]
- Duda-Chodak, A.; Tarko, T.; Petka-Poniatowska, K. Antimicrobial Compounds in Food Packaging. Int. J. Mol. Sci. 2023, 24, 2457. [Google Scholar] [CrossRef] [PubMed]
- Jahani, M.; Pira, M.; Aminifard, M.H. Antifungal Effects of Essential Oils against Aspergillus niger in Vitro and in Vivo on Pomegranate (Punica granatum) Fruits. Sci. Hortic. 2020, 264, 109188. [Google Scholar] [CrossRef]
- Leyva Salas, M.; Mounier, J.; Valence, F.; Coton, M.; Thierry, A.; Coton, E. Antifungal Microbial Agents for Food Biopreservation—A Review. Microorganisms 2017, 5, 37. [Google Scholar] [CrossRef]
- Reyes-Jurado, F.; Franco-Vega, A.; Ramírez-Corona, N.; Palou, E.; López-Malo, A. Essential Oils: Antimicrobial Activities, Extraction Methods, and Their Modeling. Food Eng. Rev. 2015, 7, 275–297. [Google Scholar] [CrossRef]
- Türkmenoğlu, A.; Özmen, D. Allergenic Components, Biocides, and Analysis Techniques of Some Essential Oils Used in Food Products. J. Food Sci. 2021, 86, 2225–2241. [Google Scholar] [CrossRef]
- Arraiza, M.P.; González-Coloma, A.; Andres, M.F.; Berrocal-Lobo, M.; Domínguez-Núñez, J.A.; Da Costa, A.C., Jr.; Navarro-Rocha, J.; Calderón-Guerrero, C. Antifungal Effect of Essential Oils. In Potential of Essential Oils; El-Shemy, H.A., Ed.; InTech: London, UK, 2018; ISBN 978-1-78923-779-5. [Google Scholar]
- De Souza, E.L.; Lundgren, G.A.; De Oliveira, K.Á.R.; Berger, L.R.R.; Magnani, M. An Analysis of the Published Literature on the Effects of Edible Coatings Formed by Polysaccharides and Essential Oils on Postharvest Microbial Control and Overall Quality of Fruit. Comp. Rev. Food Sci. Food Saf. 2019, 18, 1947–1967. [Google Scholar] [CrossRef] [PubMed]
- Lucas-González, R.; Yilmaz, B.; Mousavi Khaneghah, A.; Hano, C.; Shariati, M.A.; Bangar, S.P.; Goksen, G.; Dhama, K.; Lorenzo, J.M. Cinnamon: An Antimicrobial Ingredient for Active Packaging. Food Packag. Shelf Life 2023, 35, 101026. [Google Scholar] [CrossRef]
- Manso, S.; Becerril, R.; Nerín, C.; Gómez-Lus, R. Influence of pH and Temperature Variations on Vapor Phase Action of an Antifungal Food Packaging against Five Mold Strains. Food Control 2015, 47, 20–26. [Google Scholar] [CrossRef]
- Echegoyen, Y.; Nerín, C. Performance of an Active Paper Based on Cinnamon Essential Oil in Mushrooms Quality. Food Chem. 2015, 170, 30–36. [Google Scholar] [CrossRef]
- Stoleru, E.; Brebu, M. Stabilization Techniques of Essential Oils by Incorporation into Biodegradable Polymeric Materials for Food Packaging. Molecules 2021, 26, 6307. [Google Scholar] [CrossRef]
- Mendes, J.F.; Norcino, L.B.; Martins, H.H.A.; Manrich, A.; Otoni, C.G.; Carvalho, E.E.N.; Piccoli, R.H.; Oliveira, J.E.; Pinheiro, A.C.M.; Mattoso, L.H.C. Correlating Emulsion Characteristics with the Properties of Active Starch Films Loaded with Lemongrass Essential Oil. Food Hydrocoll. 2020, 100, 105428. [Google Scholar] [CrossRef]
- Tomić, A.; Šovljanski, O.; Erceg, T. Insight on Incorporation of Essential Oils as Antimicrobial Substances in Biopolymer-Based Active Packaging. Antibiotics 2023, 12, 1473. [Google Scholar] [CrossRef]
- Turek, C.; Stintzing, F.C. Stability of Essential Oils: A Review. Comp. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Xue, F.; Gu, Y.; Wang, Y.; Li, C.; Adhikari, B. Encapsulation of Essential Oil in Emulsion Based Edible Films Prepared by Soy Protein Isolate-Gum Acacia Conjugates. Food Hydrocoll. 2019, 96, 178–189. [Google Scholar] [CrossRef]
- Yammine, J.; Chihib, N.E.; Gharsallaoui, A.; Ismail, A.; Karam, L. Advances in Essential Oils Encapsulation: Development, Characterization and Release Mechanisms. Polym. Bull. 2024, 81, 3837–3882. [Google Scholar] [CrossRef]
- Shao, P.; Yu, J.; Chen, H.; Gao, H. Development of Microcapsule Bioactive Paper Loaded with Cinnamon Essential Oil to Improve the Quality of Edible Fungi. Food Packag. Shelf Life 2021, 27, 100617. [Google Scholar] [CrossRef]
- Aguado, R.; Ferreira, A.C.S.; Gramacho, S.; Murtinho, D.; Valente, A.J. Crosslinking of Surface-sizing Starch with Cyclodextrin Units Enhances the Performance of Paper as Essential Oil Carrier. Nord. Pulp Pap. Res. J. 2022, 37, 413–421. [Google Scholar] [CrossRef]
- Oyom, W.; Zhang, Z.; Bi, Y.; Tahergorabi, R. Application of Starch-based Coatings Incorporated with Antimicrobial Agents for Preservation of Fruits and Vegetables: A Review. Prog. Org. Coat. 2022, 166, 106800. [Google Scholar] [CrossRef]
- The European Parliament. European Commission Regulation (EC) No 1935/2004 of the European Parliament and of the Council of 27 October 2004 on Materials and Articles Intended to Come into Contact with Food and Repealing Directives 80/590/EEC and 89/109/EEC; The European Parliament: Strasbourg, France, 2004.
- European Committee on Antimicrobial Susceptibility Testing (EUCAST). Method for the Determination of Broth Dilution Minimum Inhibitory Concentrations of Antifungal Agents for Conidia Forming Moulds.E.Def. 9.4; EUCAST: Växjö, Sweden, 2022. [Google Scholar]
- Tullio, V.; Nostro, A.; Mandras, N.; Dugo, P.; Banche, G.; Cannatelli, M.A.; Cuffini, A.M.; Alonzo, V.; Carlone, N.A. Antifungal Activity of Essential Oils against Filamentous Fungi Determined by Broth Microdilution and Vapour Contact Methods. J. Appl. Microbiol. 2007, 102, 1544–1550. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; Rabea, E.I. Potential of the Biopolymer Chitosan with Different Molecular Weights to Control Postharvest Gray Mold of Tomato Fruit. Postharvest Biol. Technol. 2009, 51, 110–117. [Google Scholar] [CrossRef]
- Perdones, A.; Sánchez-González, L.; Chiralt, A.; Vargas, M. Effect of Chitosan-Lemon Essential Oil Coatings on Storage-Keeping Quality of Strawberry. Postharvest Biol. Technol. 2012, 70, 32–41. [Google Scholar] [CrossRef]
- ISO 6887-1:2017; Microbiology of the Food Chain—Preparation of Test Samples, Initial Suspension and Decimal Dilutions for Microbiological Examination—Part 1: General Rules for the Preparation of the Initial Suspension and Decimal Dilutions. International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 4833-2:2013; Microbiology of the Food Chain. International Organization for Standardization: Geneva, Switzerland, 2013.
- ISO 21528-2:2017; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Enterobacteriaceae. Part 2: Colony-Count Technique. International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 21527-1:2008; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Yeasts and Moulds—Part 1: Colony Count Technique in Products with Water Activity Greater than 0.95. International Organization for Standardization: Geneva, Switzerland, 2008.
- ISO 11035:1994; Sensory Analysis—Identification and Selection of Descriptors for Establishing a Sensory Profile by a Multidimensional Approach. International Organization for Standardization: Geneva, Switzerland, 1994.
- Bland, M. An Introduction to Medical Statistics, 4th ed.; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Moussa, H.; El Omari, B.; Chefchaou, H.; Tanghort, M.; Mzabi, A.; Chami, N.; Remmal, A. Action of Thymol, Carvacrol and Eugenol on Penicillium and Geotrichum Isolates Resistant to Commercial Fungicides and Causing Postharvest Citrus Decay. Can. J. Plant Pathol. 2021, 43, 26–34. [Google Scholar] [CrossRef]
- Rao, A.; Zhang, Y.; Muend, S.; Rao, R. Mechanism of Antifungal Activity of Terpenoid Phenols Resembles Calcium Stress and Inhibition of the TOR Pathway. Antimicrob. Agents Chemother. 2010, 54, 5062–5069. [Google Scholar] [CrossRef] [PubMed]
- Xiang, F.; Zhao, Q.; Zhao, K.; Pei, H.; Tao, F. The Efficacy of Composite Essential Oils against Aflatoxigenic Fungus Aspergillus flavus in Maize. Toxins 2020, 12, 562. [Google Scholar] [CrossRef] [PubMed]
- Abdolahi, A.; Hassani, A.; Ghuosta, Y.; Bernousi, I.; Meshkatalsadat, M.H. In Vitro Efficacy of Four Plant Essential Oils against Botrytis cinerea Pers.:Fr. and Mucor piriformis A. Fischer. J. Essent. Oil Bear. Plants 2010, 13, 97–107. [Google Scholar] [CrossRef]
- Álvarez-García, S.; Moumni, M.; Romanazzi, G. Antifungal Activity of Volatile Organic Compounds from Essential Oils against the Postharvest Pathogens Botrytis cinerea, Monilinia fructicola, Monilinia fructigena, and Monilinia laxa. Front. Plant Sci. 2023, 14, 1274770. [Google Scholar] [CrossRef]
- Burt, S. Essential Oils: Their Antibacterial Properties and Potential Applications in Foods—A Review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Clemente, I.; Aznar, M.; Nerín, C. Synergistic Properties of Mustard and Cinnamon Essential Oils for the Inactivation of Foodborne Moulds in Vitro and on Spanish Bread. Int. J. Food Microbiol. 2019, 298, 44–50. [Google Scholar] [CrossRef]
- Nikkhah, M.; Hashemi, M.; Habibi Najafi, M.B.; Farhoosh, R. Synergistic Effects of Some Essential Oils against Fungal Spoilage on Pear Fruit. Int. J. Food Microbiol. 2017, 257, 285–294. [Google Scholar] [CrossRef]
- Lambert, R.J.; Skandamis, P.N.; Coote, P.J.; Nychas, G.J. A Study of the Minimum Inhibitory Concentration and Mode of Action of Oregano Essential Oil, Thymol and Carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Sonboli, A.; Babakhani, B.; Mehrabian, A.R. Antimicrobial Activity of Six Constituents of Essential Oil from Salvia. Z. Naturforschung C 2006, 61, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Khanoonkon, N.; Rugthaworn, P.; Kongsin, K.; Sukyai, P.; Harnkarnsujarit, N.; Sothornvit, R.; Chollakup, R.; Sukatta, U. Enhanced Antimicrobial Effectiveness of Synergistic Mixtures of Rambutan Peel Extract and Cinnamon Essential Oil on Food Spoilage Bacteria and Bio-based Food Packaging. J. Food Saf. 2022, 42, e12976. [Google Scholar] [CrossRef]
- El-Zehery, H.R.A.; Ashry, N.M.; Faiesal, A.A.; Attia, M.S.; Abdel-Maksoud, M.A.; El-Tayeb, M.A.; Aufy, M.; El-Dougdoug, N.K. Antibacterial and Anticancer Potential of Bioactive Compounds and Secondary Metabolites of Endophytic Fungi Isolated from Anethum Graveolens. Front. Microbiol. 2024, 15, 1448191. [Google Scholar] [CrossRef]
- Buendía−Moreno, L.; Sánchez−Martínez, M.J.; Antolinos, V.; Ros−Chumillas, M.; Navarro−Segura, L.; Soto−Jover, S.; Martínez−Hernández, G.B.; López−Gómez, A. Active Cardboard Box with a Coating Including Essential Oils Entrapped within Cyclodextrins and/or Halloysite Nanotubes. A Case Study for Fresh Tomato Storage. Food Control 2020, 107, 106763. [Google Scholar] [CrossRef]
- Buendía-Moreno, L.; Soto-Jover, S.; Ros-Chumillas, M.; Antolinos, V.; Navarro-Segura, L.; Sánchez-Martínez, M.J.; Martínez-Hernández, G.B.; López-Gómez, A. Innovative Cardboard Active Packaging with a Coating Including Encapsulated Essential Oils to Extend Cherry Tomato Shelf Life. LWT 2019, 116, 108584. [Google Scholar] [CrossRef]
- Campos, A.D.; Sena Neto, A.R.D.; Rodrigues, V.B.; Luchesi, B.R.; Moreira, F.K.V.; Correa, A.C.; Mattoso, L.H.C.; Marconcini, J.M. Bionanocomposites Produced from Cassava Starch and Oil Palm Mesocarp Cellulose Nanowhiskers. Carbohydr. Polym. 2017, 175, 330–336. [Google Scholar] [CrossRef]
- Buendía-Moreno, L.; Soto-Jover, S.; Ros-Chumillas, M.; Antolinos-López, V.; Navarro-Segura, L.; Sánchez-Martínez, M.J.; Martínez-Hernández, G.B.; López-Gómez, A. An Innovative Active Cardboard Box for Bulk Packaging of Fresh Bell Pepper. Postharvest Biol. Technol. 2020, 164, 111171. [Google Scholar] [CrossRef]
- Becerril, R.; Nerín, C.; Silva, F. Encapsulation Systems for Antimicrobial Food Packaging Components: An Update. Molecules 2020, 25, 1134. [Google Scholar] [CrossRef] [PubMed]
- Chaidech, P.; Matan, N. Cardamom Oil-Infused Paper Box: Enhancing Rambutan Fruit Post-Harvest Disease Control with Reusable Packaging. LWT 2023, 189, 115539. [Google Scholar] [CrossRef]
| Cationic starch | EVO or HI-CAT |
| Starch solution | 80–98% |
| Tween 80 | 0% or 10% |
| EOmix | 2–10% |
| EO | SH | OR | CL | RT |
|---|---|---|---|---|
| MIC (µL EO/L air) | 65.8 | 32.9 | 32.9 | 65.8 |
| Added volume (µL) | 2.50 | 1.25 | 1.25 | 2.50 |
| Assay | SH (%) | OR (%) | CL (%) | RT (%) | MIC (µL EO/L air) | Added Volume of Mixture (µL) |
|---|---|---|---|---|---|---|
| 1 | 0.125 | 0.125 | 0.625 | 0.125 | 65.8 | 2.50 |
| 2 | - | - | - | 1 | 65.8 | 2.50 |
| 3 | 0.333 | 0.333 | 0.333 | 32.9 | 1.25 | |
| 4 | 0.333 | - | 0.333 | 0.333 | 65.8 | 2.50 |
| 5 | - | - | 0.333 | 0.667 | 65.8 | 2.50 |
| 6 | 0.333 | - | - | 0.667 | 65.8 | 2.50 |
| 7 | - | 0.667 | 0.333 | - | 32.9 | 1.25 |
| 8 | 1 | - | - | - | 65.8 | 2.50 |
| 9 | - | 1 | - | - | 32.9 | 1.25 |
| 10 | 0.125 | 0.625 | 0.125 | 0.125 | 32.9 | 1.25 |
| 11 | - | - | 1 | - | 32.9 | 1.25 |
| 12 | 0.333 | 0.667 | - | - | 32.9 | 1.25 |
| 13 | - | 0.333 | - | 0.667 | 32.9 | 1.25 |
| 14 | - | - | - | 1 | 65.8 | 2.50 |
| 15 | 0.333 | - | 0.667 | - | 32.9 | 1.25 |
| 16 | 0.333 | 0.333 | - | 0.333 | 32.9 | 1.25 |
| 17 | - | 0.667 | - | 0.333 | 32.9 | 1.25 |
| 18 | - | 0.333 | 0.667 | - | 16.4 | 0.625 |
| 19 | 0.625 | 0.125 | 0.125 | 0.125 | 32.9 | 1.25 |
| 20 | 0.333 | 0.333 | 0.333 | - | 32.9 | 1.25 |
| 21 | 1 | - | - | - | 65.8 | 2.50 |
| 22 | 0.125 | 0.125 | 0.125 | 0.625 | 32.9 | 1.25 |
| 23 | - | - | 0.667 | 0.333 | 32.9 | 1.25 |
| 24 | - | 1 | - | - | 32.9 | 1.25 |
| 25 | 0.667 | - | 0.333 | - | 65.8 | 2.50 |
| 26 | 0.333 | 0.333 | 0.333 | - | 32.9 | 1.25 |
| 27 | 0.25 | 0.25 | 0.25 | 0.25 | 32.9 | 1.25 |
| 28 | - | - | 1 | - | 32.9 | 1.25 |
| 29 | 0.667 | - | - | 0.333 | 65.8 | 2.50 |
| 30 | 0.667 | 0.333 | - | - | 32.9 | 1.25 |
| Treatment | D7 | D7+2 | D14 | D14+2 | D21 | D21+2 | D28 | D28+2 |
|---|---|---|---|---|---|---|---|---|
| Control | 1.0 ± 0.0 | 1.1 ± 0.1 | 1.1 ± 0.1 | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.1 ± 0.2 | 1.3 ± 0.2 | 2.5 ± 0.2 a |
| Active paper A | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.1 ± 0.1 | 1.1 ± 0.1 | 1.1 ± 0.1 | 1.3 ± 0.3 | 1.2 ± 0.2 | 1.7 ± 0.5 b |
| Active paper B | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.1 ± 0.2 | 1.1 ± 0.2 | 1.1 ± 0.1 | 1.1 ± 0.2 | 1.5 ± 0.1 b |
| Treatment | Appearance | Firmness | Juiciness | Mealiness | Tomato Flavor | Off-Flavors | Global Acceptance |
|---|---|---|---|---|---|---|---|
| D7+2 | |||||||
| Control | 7.3 a | 7.9 a | 7.6 a | 3.9 a | 6.8 a | 2.9 b | 7.3 a |
| Active paper A | 7.9 a | 7.1 a | 7.3 a | 4.3 a | 6.5 a | 4.9 a | 6.3 a |
| Active paper B | 7.6 a | 7.2 a | 7.2 a | 4.6 a | 6.4 a | 4.7 a | 6.5 a |
| D14+2 | |||||||
| Control | 7.6 a | 7.3 a | 6.9 a | 4.9 a | 6.5 a | 2.9 b | 6.8 a |
| Active paper A | 7.2 a | 6.2 a | 6.8 a | 5.2 a | 6.5 a | 3.5 b | 6.7 a |
| Active paper B | 6.9 a | 6.1 a | 7.1 a | 4.9 a | 6.0 a | 4.5 a | 6.1 a |
| D21+2 | |||||||
| Control | 7.2 a | 6.1 a | 6.6 a | 4.6 a | 5.9 a | 2.6 b | 7.0 a |
| Active paper A | 6.6 a | 6.2 a | 7.2 a | 3.8 a | 6.4 a | 4.5 a | 6.8 a |
| Active paper B | 6.5 a | 5.9 a | 7.0 a | 4.5 a | 5.9 a | 4.2 a | 6.7 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguerri, L.; Cantín, C.M.; Quintero, M.; Lóbez, S.; Marco, P.; Silva, F. Emulsion-Coated Active Papers Extend the Storage Life of Tomato Fruit. Foods 2025, 14, 2774. https://doi.org/10.3390/foods14162774
Aguerri L, Cantín CM, Quintero M, Lóbez S, Marco P, Silva F. Emulsion-Coated Active Papers Extend the Storage Life of Tomato Fruit. Foods. 2025; 14(16):2774. https://doi.org/10.3390/foods14162774
Chicago/Turabian StyleAguerri, Laura, Celia M. Cantín, Marinelly Quintero, Silvia Lóbez, Pedro Marco, and Filomena Silva. 2025. "Emulsion-Coated Active Papers Extend the Storage Life of Tomato Fruit" Foods 14, no. 16: 2774. https://doi.org/10.3390/foods14162774
APA StyleAguerri, L., Cantín, C. M., Quintero, M., Lóbez, S., Marco, P., & Silva, F. (2025). Emulsion-Coated Active Papers Extend the Storage Life of Tomato Fruit. Foods, 14(16), 2774. https://doi.org/10.3390/foods14162774






