Abstract
A popular non-alcoholic beverage worldwide, tea can regulate blood glucose levels, lipid levels, and blood pressure, and may even prevent type 2 diabetes mellitus (T2DM). Different tea fermentation levels impact these effects. Tea products with different fermentation degrees containing different functional ingredients can lower post-meal blood glucose levels and may prevent T2DM. There are seven critical factors that shed light on how teas with different fermentation levels affect blood glucose regulation in humans. These factors include the inhibition of digestive enzymes, enhancement of cellular glucose uptake, suppression of gluconeogenesis-related enzymes, reduction in the formation of advanced glycation end products (AGEs), inhibition of dipeptidyl peptidase-4 (DPP-4) activity, modulation of gut flora, and the alleviation of inflammation associated with oxidative stress. Fermented teas can be used to lower post-meal blood glucose levels and can help consumers make more informed tea selections.
1. Introduction
Tea is one of the most popular beverages in the world. According to different processing methods, tea can be divided into six categories: green tea (non-fermented tea), white tea and yellow tea (slightly fermented tea), oolong tea (moderately fermented tea), black tea (fully fermented tea), and dark tea (post-fermented tea) [1]. The primary basis for these classifications lies in the extent of fermentation, which plays a fundamental role in comprehending the vast realm of tea. Tea has been extensively and comprehensively investigated, particularly regarding its impact on the regulation of glucose and lipid metabolism. In recent years, the field of tea and health research has placed significant emphasis on the prominent aspect of tea’s health benefits. Similarly, Kombucha tea deserves attention, particularly in blood glucose regulation. It is produced through the co-cultivation of yeast, lactic acid bacteria, and acetic acid bacteria with tea of varying fermentation levels as the raw material [2,3,4]. Due to its rich content of tea polyphenols and organic acids [3], it can effectively reduce fasting blood glucose levels [4]. Tea is abundant in essential functional components such as tea polyphenols, tea polysaccharides, and tea pigments, which have garnered considerable attention. Extensive studies have consistently demonstrated the remarkable hypoglycemic effects of these components, particularly in mitigating postprandial blood glucose levels and their potential in the prevention and treatment of type 2 diabetes mellitus (T2DM).
Tea, derived from a range of tea varieties, geographical regions, and processing techniques, displays variations in its content, composition, and functional bioactive components. It is worth noting that the categorization of tea into six major classifications based on the degree of fermentation emphasizes these distinctions, resulting in a wide array of hypoglycemic effects and their underlying mechanisms. These variations serve to emphasize that different types of tea possess distinct profiles of bioactive compounds. Their effects on blood glucose regulation can vary significantly, providing an extensive scope for investigating tea’s multifaceted nature and potential health advantages. Presently, most review articles tend to focus on analyzing individual active components in connection with hypoglycemic effects. Nevertheless, there is a shortage of thorough investigations delving into blood glucose regulation from the standpoint of fermentation levels. This is an area where more extensive research and analysis could provide valuable insights into the various health advantages of tea.
Diabetes is a condition that arises from a disturbance in the body’s glucose metabolism. It can adversely affect crucial organs like the heart, blood vessels, and eyes, leading to significant health risks, and in severe instances, it can even become life-threatening [5]. Due to improved living standards and shifts in dietary habits, there has been a notable rise in diabetes cases. The three main types of diabetes are type 1 diabetes mellitus (T1DM), T2DM, and gestational diabetes mellitus (GDM). Of these, T2DM is the most common, representing around 90% of cases. This is a chronic condition that significantly impacts the quality of human life. In 2030, the International Diabetes Federation projects that the global diabetic population will reach an astounding 578 million individuals [6]. Insufficient insulin production by pancreatic β cells or the occurrence of insulin resistance (IR) can lead to the development of T2DM [7]. Lowering postprandial (after-meal) blood glucose levels is crucial in preventing T2DM. Managing diabetes is a lifelong commitment that includes dietary control, regular exercise, and medication to maintain blood glucose levels as close to normal as possible. Furthermore, it has been reported that stimulating the secretion of orexin in the hypothalamus can reduce the body’s metabolism and increase and maintain the body’s energy reserves [8]. In short, lowering postprandial blood glucose levels is beneficial in preventing diabetes and avoiding related complications.
Currently, numerous hypoglycemic medications on the market have potential side effects. Researchers are increasingly focused on finding natural dietary components to prevent diabetes and reduce complications. Tea is a natural, healthful beverage abundant in dietary active ingredients and serves as a significant source for effectively regulating human blood glucose levels. Research on the hypoglycemic effects and mechanisms of tea typically categorizes into several key aspects: First, enhancing insulin secretion and mitigating IR. Second, facilitating glucose absorption in peripheral tissues. Third, decreasing the activity of enzymes related to gluconeogenesis and digestive processes in the body.
In this paper, we comprehensively analyzed various fermented teas to investigate their potential in lowering blood glucose levels. We aimed to better understand how various fermented teas help maintain stable blood glucose levels in humans. The research progress on the effect and mechanism of tea products with different fermentation degrees in regulating T2DM are reviewed. The relationship among different fermentation levels of tea, functional ingredients of tea, and the prevention of T2DM is explored. We hope to provide insights into the optimal utilization of teas with various fermentation levels and their related products for blood glucose reduction, offering consumers informed choices in tea products. These findings advance the study of the hypoglycemic effects of six specific tea types and provide practical guidance for consumers selecting tea products.
To gain a better understanding of the impact of tea on T2DM, we conducted a literature search and collected high-quality articles from the Web of Science Core Collection, PubMed, and Scopus databases in the last twenty years. The search deadline was set for 25 October 2023. Those databases were searched to identify studies containing the keywords ‘tea’, ‘blood sugar’, ‘blood glucose’, and ‘hyperglycemia’.
2. The Main Factors of T2DM and Prevention Methods
2.1. The Main Factors of T2DM Mellitus
As shown in Figure 1, a diet high in both sugar and fat significantly contributes to the development of T2DM. The primary underlying causes of T2DM are IR and inadequate insulin secretion. IR denotes a diminished metabolic response in target cells, including the liver, muscle, and fat cells. Consequently, this results in the continuous overproduction of insulin by pancreatic β cells. Diminished tissue responsiveness to insulin, coupled with excessive insulin production, results in the development of hyperglycemia and hyperinsulinemia. These factors significantly contribute to the initiation of inflammation associated with T2DM [9,10]. Insulin deficiency denotes an inadequate production of insulin by the pancreas, leading to elevated blood glucose levels. The occurrence of insulin deficiency is closely linked to the apoptosis of pancreatic β cells. The escalation of β cell apoptosis is a pivotal factor in the progression of T2DM. While both IR and insulin deficiency have genetic and environmental factors at play, environmental influences exert a significant influence on the occurrence of IR and insulin deficiency, contributing significantly to their development [10,11].
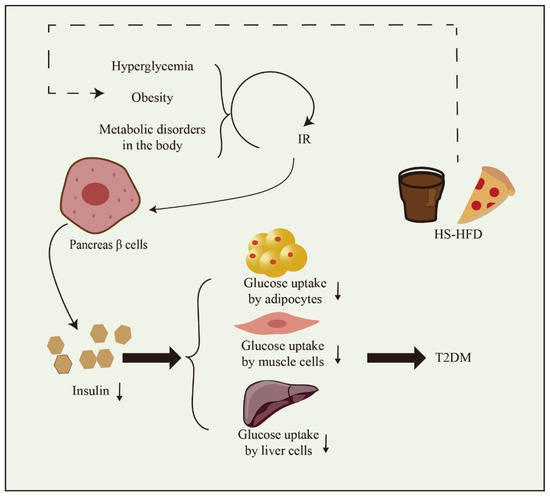
Figure 1.
The formation factors of T2DM. High-sugar and high-fat diet (HS-HFD). Downward arrow means down-regulation.
2.2. The Main Prevention and Treatment Methods of T2DM
2.2.1. Exercise Prevention
Exercise includes various forms such as stretching, aerobic activities, and resistance training, and the choice should be determined by factors like age, cognitive function, and physical activity level, especially in diabetic patients. Research has shown that imbalances in post-meal glucose, insulin, and triglyceride levels can lead to oxidative stress, which in turn leads to inflammation. Participating in short activities like walking and basic resistance exercises, rather than prolonged sitting, has shown the potential to reduce the post-meal rise in glucose, insulin, C-peptide (a marker of internal insulin production), and triglyceride levels in T2DM patients, providing relief from oxidative stress and inflammation [12,13]. Interestingly, in a randomized crossover experiment, researchers found that afternoon exercise was more effective in improving blood glucose levels in T2DM compared to morning exercise [14].
In addition, exercise can improve sleep quality and indirectly affect blood sugar level regulation. Healthy sleep is crucial for T2DM management [15]. Maintaining adequate sleep and limiting energy intake can effectively aid weight loss [16]. Another study demonstrated that extending the sleep duration of sleep-deprived individuals enhances insulin sensitivity [17].
2.2.2. Drug Therapy
Drug adjustment therapy is often more effective at reducing blood glucose levels and is categorized into the following groups [18]: (1) Insulin sensitizers, such as biguanides (e.g., metformin) and gliquidones (e.g., thiazolidinediones), enhance glucose uptake in peripheral tissues. (2) Insulin secretagogues, like sulfonylureas (e.g., glimepiride) and glibenclamides (e.g., repaglinide), stimulate insulin secretion by pancreatic β cells. (3) Digestive enzyme inhibitors, with acarbose as a prominent example, reduce glucose absorption in the small intestine by inhibiting the activity of pancreatic α-amylase and intestinal α-glucosidase. This mechanism effectively lowers blood glucose levels. (4) Sodium-glucose cotransporter-2 (SGLT2) inhibitors, such as caragliflozin, work by preventing the reabsorption of glucose in the kidneys, leading to glucose excretion through urine. (5) DPP-4 inhibitors, like sitagliptin, increase the levels of glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) by inhibiting DPP-4 activity. This, in turn, stimulates insulin secretion by pancreatic β cells. (6) GLP-1 receptor agonists, like liraglutide, activate pancreatic GLP-1 receptors, indirectly promoting insulin secretion. While drug therapy quickly and effectively lowers blood glucose levels with significant results, numerous studies have demonstrated that long-term use of hypoglycemic agents can result in numerous side effects, including flatulence, liver damage, weight imbalance, and hypoglycemia [15,19,20]. As a result, natural plant extracts have shown exceptional efficacy in regulating blood glucose.
2.2.3. Application of Natural Plant Extracts in Blood Glucose Regulation
Natural plant extracts, derived from nature’s abundance, are renowned for their healthful properties. Extensive scientific research consistently validates their potential in mitigating various chronic diseases, especially T2DM resulting from prolonged hyperglycemia. T2DM patients commonly exhibit an increased high-fat dietary intake alongside reduced physical activity, which exacerbates IR [21]. Digestive enzymes like α-amylase and α-glucosidase primarily break down carbohydrates into glucose, which is then absorbed and utilized by the small intestine. During digestion, different carbohydrates like galactose, maltose, and sucrose can stimulate the secretion of GLP-1, helping reduce postprandial blood glucose levels [22]. Moreover, increased protein intake can mitigate inflammation linked to T2DM. For example, glutamine can alleviate inflammation and enhance glucose uptake in skeletal muscle. Also, L-arginine stimulates GLP-1 release and supports in vivo glucose homeostasis [23,24]. Additionally, consuming fatty acids like omega-3, monounsaturated fats, α-linolenic acid, and docosahexaenoic acid has been observed to preserve muscle mass in elderly T2DM patients. Free fatty acids also play a crucial role in regulating GLP-1 secretion [22,25].
The recent literature emphasizes the increasing interest in using natural active ingredients to control postprandial hyperglycemia. These compounds, including polyphenols [26], polysaccharides [7], peptides [27], and dietary fiber [28], have shown significant research progress in managing postprandial hyperglycemia and preventing diabetes and its complications. Being the second most consumed beverage worldwide, tea contains numerous bioactive compounds such as tea polyphenols, tea polysaccharides, tea polypeptides, and tea dietary fibers. Exploring the functional components and activities of tea not only advances the field of tea processing but also aligns with green development principles. This effort has considerable research significance.
3. The Biochemical Characteristics and Hypoglycemic Effect of Tea with Different Fermentation Degrees
3.1. Biochemical Characteristics of Tea with Different Fermentation Degrees
Green tea, white tea, yellow tea, oolong tea, black tea, and dark tea are the six traditional categories of fermented tea, each with unique characteristics. When producing green tea, the chemical composition closely resembles that of fresh tea leaves because it is not fermented. A critical step in processing green tea is de-enzyming, which is accomplished by applying high-temperature treatment to deactivate polyphenol oxidase. This process stabilizes the chemical components in the tea and imparts the distinct green characteristics commonly associated with green tea [29].
White tea and yellow tea undergo oxidative fermentation levels between those of green tea and oolong tea. White tea production primarily comprises two essential steps: withering and drying. These processes help preserve a substantial amount of white tea polyphenols and amino acids. Particularly, the withering phase is crucial for preserving the aroma in the white tea manufacturing process [30,31]. Moreover, the drying process in white tea production can improve the overall aromatic profile, including ketones and aldehydes levels, while reducing alcohol formation. This process significantly contributes to shaping the aromatic qualities of the tea [31]. Yellowing is the central process in yellow tea production. During the 18 h yellowing stage in yellow tea processing, there is a significant rise in amino acids and sucrose levels. This increase is linked to the breakdown of epigallocatechin gallate (EGCG) and epicatechin gallate (ECG). This phenomenon’s primary mechanism is the oxidation of EGCG’s B-ring phenolic hydroxyl group by polyphenol oxidase, leading to o-quinone formation. In conditions of high heat and humidity, o-quinones tend to undergo further oxidation and polymerization, ultimately resulting in the formation of theaflavins [32,33].
In oolong tea processing, two critical techniques are used: shaking and stir-frying. Shaking involves the gentle collision of tea leaves, causing slight breakage, reducing moisture content, intensifying enzymatic catalysis and hydrolysis reactions, and resulting in the interaction of catechins with specific amino acids, leading to the release of aldehydes or oxidation into polyphenols [34]. Baking is the final stage in oolong tea production, playing a pivotal role in shaping the distinctive flavor profile of this tea variety. Research has shown that it effectively reduces the tea’s bitterness and astringency, enhances its sweet aftertaste, and contributes to the unique flavor that characterizes oolong tea [35].
Black tea, renowned for its full fermentation, is among the most widely consumed tea types. Fermentation is crucial in black tea processing. During fermentation, catechins undergo oxidation in the B-ring, involving coupling, condensation, hydroxylation, decarboxylation, and rearrangement [36]. Theaflavins and thearubigins, oxidation products of tea polyphenols, are vital indicators of the black tea infusion’s color and brightness. These compounds are intricately connected to the actions of polyphenol oxidase and peroxidase. During black tea fermentation, they give rise to the distinct color and flavor defining black tea [36,37].
Post-fermentation is a defining characteristic of dark tea. Pile-fermentation, a crucial step in dark tea production, is pivotal in creating its distinctive color and flavor. During microbial fermentation, catechins, flavonols, and flavonoids are reduced, while the levels of total theabrownins and phenolic acids increase due to enzymatic reactions or microbial metabolism [38]. In this process, pre-treated tea leaves are piled under controlled temperature and humidity, promoting the prolific growth of microorganisms. Enzymes and microorganisms working together facilitate the transformation and decomposition of active substances in the tea [38,39].
The bioactive components in tea, such as tea polyphenols, alkaloids, tea pigments, and amino acids, exhibit significant variation due to differences in processing technology, geographic environments, and the degree of tea leaf fermentation. These components typically constitute 18–36% of tea polyphenols, 3–5% of alkaloids, 0.3–2% of tea pigments, and 2–4% of the tea’s dry weight [40]. As indicated in Table 1, catechins in tea with varying degrees of fermentation predominantly consist of four phenotypic catechins and four non-phenotypic catechins. The phenotypic catechins comprise EGCG, ECG, epigallocatechin (EGC), and epicatechin (EC), while the non-phenotypic catechins encompass gallocatechin gallate (GCG), catechin gallate (CG), gallocatechin (GC), and catechin (C) [41].

Table 1.
The content of bioactive components in tea with different fermentation degrees (mg/g).
Alkaloids like caffeine and theobromine are byproducts of the extensive processing of tea. Their levels fluctuate depending on the fermentation degree, influencing the bitterness and astringency of the tea. Amino acids, such as L-theanine, glutamic acid, and arginine, are the key contributors to the umami taste of tea. Additionally, pigments like theaflavins, thearubigins, and theabrownins give rise to the characteristic color of the infusion in fermented teas like black tea and dark tea.
3.2. Effect of Tea Products with Different Fermentation Degrees on Blood Glucose Balance
Figure 2 illustrates that different processing techniques and tea varieties produce varying hypoglycemic effects. Recent research indicates that extracts from black tea have a stronger in vitro inhibitory effect on sucrase-isomaltase when compared to extracts from green tea and oolong tea [43]. Polysaccharides extracted from fully fermented black tea and post-fermented dark tea show superior in vitro antioxidant activity. In particular, black tea polysaccharides are more effective in inhibiting digestive enzymes. Moreover, administering black tea polysaccharides orally has led to a significant improvement in IR in diabetic mice [44]. In a recent study, researchers compared water extracts from stir-fried green tea and congou black tea. The study concluded that green tea was more effective than black tea in regulating lipid metabolism. On the other hand, black tea demonstrated the capacity to improve glucose uptake and metabolism, thereby reducing postprandial blood glucose levels. Furthermore, it had a positive impact on the composition of intestinal microorganisms by increasing the abundance of beneficial bacteria in the body [45]. This study revealed that two Japanese green tea varieties, Indo and Cha Chuukanbohon Nou 6, have potent DPP-4 inhibitory activity. This suggests that they have the potential to reduce the breakdown of insulin hormones by DPP-4 in the intestine, ultimately promoting insulin secretion and potentially aiding in managing elevated postprandial blood glucose levels [46]. Black tea and purple tea have the potential to provide benefits in diabetes management. A study conducted in Brazil discovered that both black tea and purple tea exhibited strong inhibition of α-amylase activity. Furthermore, black tea seemed to be more effective in enhancing glucose tolerance in mice, indicating its potential as a substance for postprandial anti-hyperglycemic use [47].
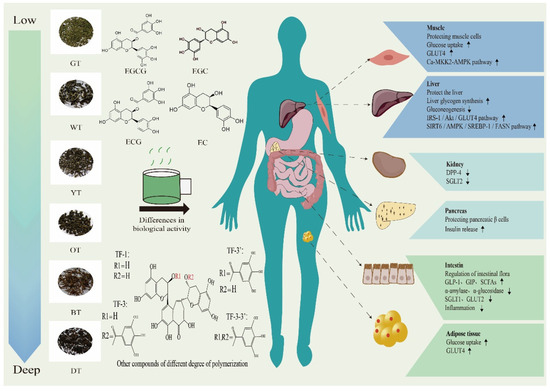
Figure 2.
The regulatory effect of different fermented teas on organs of patients with T2DM. Epigallocatechin gallate (EGCG), epigallocatechin (EGC), epicatechin gallate (ECG), epicatechin (EC); theaflavin (TF-1), theaflavin-3-gallate (TF-3), theaflavin-3′-gallate (TF-3′), theaflavin-3-3′-gallate (TF-3-3′); glucose transporters (GLUTs); calmodulin-dependent protein kinase kinase 2 (CaMKK2) -adenosine monophosphate activates protein kinases (AMPK); insulin receptor substrate 1/phosphoinositide 3-kinase/protein kinase B (IRS1/PI3K/AKT); sirtuin 6/adenosine monophosphate activated protein kinase/sterol regulatory element-binding protein-1/fatty acid synthase (SIRT6/AMPK/SREBP-1/FASN); dipeptidyl peptidase-4 (DPP-4); sodium-glucose cotransporter-2 (SGLT2); short-chain fatty acids (SCFAs). Downward arrow means down-regulation. Upward arrow means up-regulation.
3.2.1. Study on Hypoglycemic Activity of Tea with Different Fermentation Degrees in Cell Model
The use of cell models like 3T3-L1, Caco-2, C2C12, L6, HepG2, and similar cells is key in studying the impact of teas with different fermentation levels on blood glucose regulation. As shown in Table 2, among these cell lines, 3T3-L1 cells can differentiate into adipocytes, while C2C12 and L6 cells are classified as skeletal muscle cells. Caco-2 cells can differentiate and demonstrate a structure and function similar to that of intestinal epithelial cells, while HepG2 cells are derived from human liver tumors. When studying the blood glucose balance in vivo, Caco-2 and HepG2 cells are regarded as more mature cell models. Caco-2 cells, resembling the microvilli of the small intestine, are commonly used to evaluate the absorption, transport, and metabolism of bioactive substances from differently fermented teas in the intestinal mucosa. Additionally, HepG2 cells, a classical liver metabolism model, are employed to study the effects of different fermented teas on liver metabolism. The purpose of this study is to enhance our understanding of how bioactive components in different fermented teas affect blood glucose balance.

Table 2.
Study on hypoglycemic activity of different fermented tea and extracts in cell models.
The study aimed to assess the impact of water extracts from various teas, including green, oolong, and black tea, all grown in the same region, on glucose uptake in Caco-2 cells. The results showed that the water extract from green tea had the most pronounced inhibitory effect on glucose uptake in Caco-2 cells. The author attributed this effect to the high catechin content in green tea, including CG and ECG compounds. These catechins were found to decrease the expression of the SGLT1 gene. Therefore, the study proposes the possible use of catechins as additives in food or beverages to mitigate postprandial (after-meal) increases in blood glucose levels [48]. Additionally, their research report emphasized that black tea extract, particularly theaflavins, showed strong inhibition of sucrase-isomaltase activity, with an IC50 of 8.34 μg/mL. Meanwhile, green tea extract had the most significant inhibitory effect on glucose transporters, particularly SGLT1 and GLUT2 [43]. Furthermore, a Korean study also found that green tea extract can reduce the breakdown of sucrose and the absorption of glucose and fructose in Caco-2 cells [49]. Simultaneously, recent studies in Japan have emphasized the inhibitory effects of green tea extracts obtained through three different extraction methods: room temperature water, hot water, and organic reagents. These extracts have shown inhibitory effects on α-glucosidase in both rat and human Caco-2 cells. Specifically, in rat cells, EGCG, a prominent green tea catechin, is the primary contributor to this effect, while in human cells ECG plays a central role. The author contends that ECG demonstrates the most robust inhibition of intestinal glucose uptake and maintains greater stability in the intestinal and bloodstream environments compared to EGCG [50,61,62].
3.2.2. Study on Hypoglycemic Activity of Tea with Different Fermentation Degrees in Animal Models
Furthermore, animal models were also used to study the effects of bioactive compounds from teas with different fermentation levels on in vivo blood glucose regulation. The study primarily focused on four animal models: normal mice, KK-Ay mice, spontaneous diabetic Torri (SDT) rats, and the db/db mouse model. In a brief timeframe, hyperglycemia was induced in normal mice using a combination of a high-sugar and high-fat diet, as well as intraperitoneal injection of streptozotocin, thus establishing an animal model. Persistent hyperglycemia, hyperlipidemia, and hyperinsulinemia are prominent characteristics observed in db/db mice due to their inherent deficiency in leptin receptors [63]. KK-Ay mice are a common model for studying T2DM. These mice exhibit significant symptoms of obesity and diabetes, including elevated levels of random and fasting glucose, urinary protein, and glycated in the bloodstream [64]. SDT rats are a non-obese spontaneous diabetic model that develops insulin-deficient hyperglycemia after about 16 weeks. A high-sugar and high-fat diet can accelerate the formation of hyperglycemia symptoms, making them a spontaneous diabetic model [65,66]. This differs from KK-Ay and db/db mice, which are diabetic models but with a different onset and characteristics. Polyphenols, pigments, and polysaccharides found in green tea and black tea have been shown to have a positive impact on regulating glucose levels. The main methods used in these studies were free drinking and gavage. Table 3 summarizes the effects of various fermented teas and their extracts on blood glucose regulation.

Table 3.
Hypoglycemic effects of different fermented tea and extracts in animal models.
3.2.3. Epidemiological Investigation on Hypoglycemic Effect of Tea with Different Fermentation Degrees
Flourishing economies and advancements in social development have led to increased consumption of high-sugar and high-fat diets, thereby raising the risk of diabetes, as shown in Table 4. A recent 18.5-year prospective cohort study has notably revealed that regularly consuming tea (more than twice daily) or boiled water (more than five times daily) as alternatives to sugar-sweetened or artificially sweetened beverages can effectively reduce mortality rates and lower the incidence of cardiovascular diseases among patients with T2DM [81]. In a case-controlled study, researchers made an intriguing discovery: tea consumption showed a negative correlation with T2DM. The study involved 27,662 participants from eight European countries who, on average, consumed 152 ± 282 g of tea drinks daily. Encouragingly, supplementing tea drinks reduced the prevalence of T2DM by 22%, suggesting that tea consumption is beneficial for preventing T2DM [82]. Additionally, a meta-analysis has shown that interventions involving tea and its extracts can help maintain stable fasting insulin levels in T2DM patients, indicating a potential beneficial role of tea in aiding insulin control for T2DM management [83]. Meanwhile, Japanese researchers conducted a study with 17,413 subjects who daily consumed unfermented green tea, semi-fermented oolong tea, and fully fermented black tea (at least 6 cups per day). Their results revealed a significant 30% reduction in diabetes risk associated with green tea consumption. Conversely, the consumption of oolong tea and black tea showed no significant correlation with diabetes risk [84]. Furthermore, in a randomized, controlled, cross-over study, 16 obese men with IR received glucose followed by 100 mL of black tea. The results showed that black tea consumption reduced peripheral vascular resistance in both upper and lower limbs after glucose intake. This, in turn, improved postprandial blood glucose and insulin concentrations, effectively alleviating IR [85]. Generally, it is important to consider that the degree of tea fermentation, as well as factors like processing methods, dosage control, and the timing of measurements, can produce diverse results. This inconsistency emphasizes the necessity for additional research in this field to reach more conclusive findings.

Table 4.
The relationship between tea and diabetes in different countries.
4. The Potential Mechanism of Different Fermented Tea Regulating T2DM
The influence of tea on post-meal blood glucose regulation has been thoroughly investigated, with an emphasis on multiple mechanisms. In particular, the tea’s ability to lower blood glucose levels is demonstrated across varying levels of fermentation. This pertinent mechanism is visualized in Figure 3.
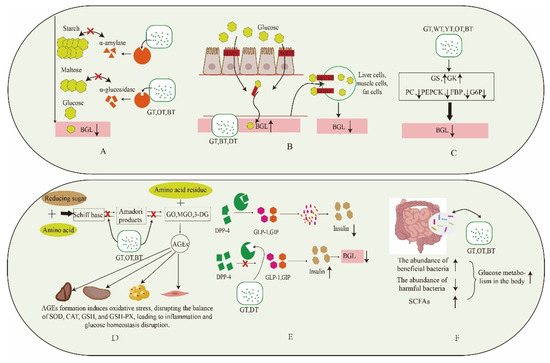
Figure 3.
Mechanism of different fermentation degree tea in balancing postprandial blood glucose. (A): Inhibition of digestive enzymes. (B): Effect on glucose transport. (C): Inhibition of gluconeogenesis pathway. (D): Inhibition of formation of AGEs. (E): Inhibition of DPP-4 activity. (F): Regulation of gut microbiota. Blood glucose levels (BGL); glucokinase (GK); glycogen synthase kinase (GSK); pyruvate carboxylase (PC); phosphoenolpyruvate carboxy kinase (PEPCK); gructose-1,6-bisphosphatase (FBP); glucose-6-phosphatase (G6P); glyoxal (GO), methylglyoxal (MGO),3-meoxyglucosone (3-DG); superoxide dismutase (SOD); catalase (CAT); glutathione (GSH); glutathione peroxidase (GSH-PX). Downward arrow means down-regulation. Upward arrow means up-regulation.
4.1. Inhibition of Digestive Enzymes
Carbohydrates are first consumed and enter the stomach and small intestine. Here, α-amylase breaks them down into oligosaccharides, such as maltodextrin. Then, α-glucosidase, secreted by intestinal epithelial cells, further converts these oligosaccharides into glucose. The small intestine absorbs and utilizes this glucose. Inhibiting the activity of α-amylase and α-glucosidase is an effective method for reducing postprandial hyperglycemia, as shown in Figure 3A. Hypoglycemic drugs, such as acarbose and voglibose, are commercially available and designed to selectively inhibit digestive enzymes, effectively lowering postprandial blood glucose levels in diabetic patients and aiding in maintaining glucose balance. A recent discovery by a Japanese researcher suggests that green tea extract can dose-dependently inhibit α-glucosidase secreted by human intestinal Caco-2 cells. Subsequent investigations into individual components showed that ECG (IC50 = 78.4 ± 15.2 µm) had a stronger inhibitory effect compared to EGCG (IC50 = 90.7 ± 15.8 µm). Interestingly, these results differed from those obtained with rat α-glucosidase [50]. Additionally, tea polysaccharides extracted from teas with different levels of fermentation display significant digestive enzyme inhibitory properties. It is important to note that the fermentation level of tea enhances the biological activity of tea polysaccharides. Smaller molecular weight variants show superior hypoglycemic activity [55,56]. Furthermore, fully fermented black tea is notably rich in theaflavins compared to green tea and oolong tea. It demonstrates a stronger inhibitory effect on the hydrolysis of sucrose, maltose, and isomaltose, with IC50 values of 8.34 μg/mL, 16.10 μg/mL, and 21.63 μg/mL, respectively, showing a clear dose-dependent relationship [43]. In general, black tea shows remarkable effectiveness in this context. Moreover, it is feasible to perform a thorough analysis of the structure–activity relationship of particular compounds present in black tea.
4.2. Effect on Glucose Transporters
The human glucose transporter family is categorized into two groups: GLUTs in the first category and SGLTs in the second [89]. Among these transporters, GLUT2 plays a crucial role as the primary glucose transporter in both liver cells and the intestines. In the intestines, GLUT2 aids in glucose absorption by translocating to the apical membrane of intestinal cells, helping to lower postprandial blood glucose levels in humans [90]. GLUT4 is predominantly located in human adipocytes and muscle cells. GLUT4 regulates insulin-mediated glucose uptake and primarily resides intracellularly. As postprandial blood glucose levels increase, insulin secretion triggers the relocation of GLUT4 from within the cell to the plasma membrane. This process assists in glucose absorption and the maintenance of the glucose balance [90,91], as shown in Figure 3.
SGLTs consist of two main types: SGLT1, located in the small intestine, aids in glucose absorption, and SGLT2 is mainly found in the kidney, where it plays a crucial role in reabsorbing glucose within renal tubules [92]. SGLT1 mainly facilitates the active transport of glucose and sodium from the intestinal cavity into cells, which then enter the bloodstream. Conversely, the primary role of SGLT2 is to absorb glucose from urine and reintroduce it into the bloodstream to maintain normal blood glucose levels. Inhibiting SGLT2 prevents the kidney from reabsorbing glucose, causing it to be excreted in urine and effectively reducing blood glucose levels. A study by Ni revealed that green tea extract (IC50 = 0.077 mg/mL) had the most potent inhibitory effect on glucose uptake by intestinal Caco-2 cells compared to oolong tea (IC50 = 0.136 mg/mL) and black tea (IC50 = 0.56 mg/mL) extracts. Furthermore, individual catechins suggested that ester catechins played a significant role in this inhibition. Furthermore, following 24 h of treatment, the gene and protein expression levels of the glucose transporter GLUT2 increased, indicating the potential utility of these compounds as dietary supplements to reduce postprandial blood glucose levels [48]. Another report emphasized that green tea extract demonstrated the most substantial inhibition of glucose transporters under both simulated fasting and simulated feeding conditions. The inhibition rates were 12.53% for SGLT1 and 32.62% for GLUT2. Notably, SGLT1 plays a more prominent role in glucose transport during low glucose states, while GLUT2 is dominant in high glucose conditions [43]. Both black tea thearubigins and dark tea theabrownins up-regulate GLUT4 gene and protein expression, effectively reducing postprandial blood glucose levels [57,75]. We can proceed to examine the effects of tea polyphenols, tea polysaccharides, and tea pigments on the inhibition of renal SGLT2. Furthermore, we can delve into the analysis of the structure–activity relationship.
4.3. Inhibition of Gluconeogenesis Pathway
The liver, being a primary glucose reservoir in the body, plays a crucial role in regulating blood glucose levels. It primarily regulates glucose levels through two processes: glycogen breakdown (glycogenolysis) and glucose synthesis (gluconeogenesis). Key enzymes for glycogenolysis include GK and GSK, while gluconeogenesis depends on enzymes like PC, PEPCK, FBP, and G6P [93]. As shown in Figure 3C, a rise in post-meal blood glucose prompts the release of insulin by pancreatic β cells, leading to a decrease in postprandial blood glucose levels. Cell and animal studies have shown that tea extracts enhance the expression of genes and proteins related to glycogen synthesis, leading to increased glycogen synthesis, and they also inhibit the activity of enzymes related to gluconeogenesis. For example, Zhou conducted a 5-week intervention using tea extracts on diabetic mice induced by a high-fat diet. The results indicated a positive correlation between the water extracts of stir-fried green tea and congou black tea with GSK expression levels, and a negative correlation with the gluconeogenesis-related enzyme G6P [45]. Another study demonstrated that intervention with white tea water extract in diabetic mice induced by a high-fat diet and streptozotocin activated the AMPK pathway. This activation resulted in the inhibition of G6P expression, effectively suppressing gluconeogenesis and improving IR [94]. Zhao conducted a 4-week intervention using large-leaf yellow tea extract on diabetic mice induced by a high-fat diet and streptozotocin. The intervention notably decreased the levels of thioredoxin-interacting protein (TXNIP), FBP protein, and FBP enzyme activity. This suppression effectively reduced gluconeogenesis and improved the glucose balance [95]. Tea and its extracts have been shown to decrease the expression of PEPCK, G6P, and FBP, thereby inhibiting gluconeogenesis. Additionally, they enhance GSK expression, promoting glycogen synthesis, which ultimately lowers blood glucose levels in both in vivo and in vitro experiments.
4.4. Inhibit the Formation of AGEs
AGEs are the outcome of non-enzymatic browning reactions, like protein glycosylation or the Maillard reaction, which occur when carbonyl groups in reducing sugars (such as glucose and fructose) react with free amino groups in proteins, lipids, or nucleic acids. They are regarded as oxidative derivatives linked to diabetic hyperglycemia [96]. Figure 3D illustrates how prolonged postprandial hyperglycemia produces AGEs, which contribute to diabetic hyperglycemia. The Maillard reaction comprises three stages: The first stage involves the reaction between reducing sugars and protein amino acids, resulting in the formation of an unstable initial glycosylation product called a Schiff base. In the second stage, this product undergoes rearrangement or cross-linking to form Amadori products. In the third stage, small carbonyl compounds derived from Amadori products isomerize with arginine and lysine residues of proteins, leading to the irreversible formation of AGEs [97]. In a recent in vitro experiment, it was shown that black tea polysaccharides effectively inhibit the formation of glycosylation products at each stage of non-enzymatic protein glycosylation. The degree of tea fermentation directly affects the molecular weight of tea polysaccharides, which, in turn, influences their biological activity [56]. Black tea, which is fully fermented, undergoes more extensive oxidative fermentation compared to green tea and oolong tea. Nuclear factor erythroid-2-related factor 2 (Nrf2) plays a crucial role as a transcription factor in regulating the cellular defense against oxidative stress and xenobiotics [98]. In a different in vivo experiment, EGCG reduced AGE levels in the plasma and liver of obese mice on a high-fat diet, suppressed AGE receptor expression, and activated the Nrf2 pathway to counteract AGE formation [68]. Catechins and tea polysaccharides play crucial roles in this process, but additional data are needed for further confirmation and a more comprehensive understanding of the structure–activity relationship.
4.5. Inhibition of DPP-4 Activity
DPP-4, being a serine protease, cleaves multiple substrates, including incretin, GLP-1, and GIP [99]. Figure 3E illustrates that natural hormone release stimulates pancreatic β cells, leading to insulin secretion and the regulation of postprandial blood glucose levels. Research suggests that tea extract can inhibit DPP-4 enzyme activity, resulting in increased insulin secretion, decreased glucagon secretion, and lower glucose levels. Zhao identified six protein peptides in dark tea extract. Among these, the peptide VVDLVFFAAAK exhibited the highest α-glucosidase inhibitory activity, with an IC50 of 0.04 ± 0.04 mg/mL, surpassing the efficacy of acarbose (IC50 = 1.51 ± 0.23 mg/mL). Additionally, the peptides MSLYPR and QGQELLPSDFK demonstrated significant inhibitory activities against the DPP-4 enzyme, with IC50 values of 1.35 ± 0.15 mg/mL and 3.89 ± 0.22 mg/mL, respectively [100]. Researchers have confirmed that water extracts from green tea exhibit DPP-4 enzyme inhibitory properties in various green tea varieties, primarily due to their high polyphenol content. Further analysis identified epigallocatechin-3-O-(3-O-methyl) gallate, kaempferol-3-O-rutinoside, myricetin-3-O-glucoside/galactoside, and theogallin as newly discovered DPP-4 inhibitors. These natural active compounds can be integrated into food products to mitigate the degradation of incretin hormones, consequently reducing postprandial blood glucose levels [46]. Furthermore, there is potential for research investigating various fermented teas and their components concerning DPP-4 activity. Such research could yield a deeper understanding of the mechanisms underlying their hypoglycemic effects.
4.6. Regulation of Gut Microbiota
Intestinal microbial flora are closely linked to human hyperglycemia and play a critical role in maintaining the body’s energy balance. They also play a key role in metabolic conditions such as diabetes. As shown in Figure 3F, a high-sugar and high-fat diet is a significant contributor to T2DM. Recent research showed that a 5-week intervention using water extracts of black tea and green tea in mice on a high-sugar and high-fat diet resulted in a significant increase in beneficial bacteria (Allobacteria, Lactobacillus, Turicibacter). Simultaneously, it led to a reduction in the levels of Clostridium and Bacteroides. Importantly, deeply fermented black tea exhibited a more significant effect in this context [45]. Additionally, administering extracts of both conventional black tea and selenium-enriched black tea to hyperglycemic mice can modify the composition of intestinal microorganisms. This, in turn, enhances the production of SCFAs and aids in alleviating hyperglycemia [69]. Furthermore, when green tea polysaccharides interacted with intestinal flora, they increased the levels of acetic acid, propionic acid, butyric acid, and total SCFAs in the intestinal environment. At the same time, they reduced the biosynthesis of gluconeogenesis-related amino acids, such as leucine, isoleucine, and valine, which in turn lowered endogenous glucose production. Furthermore, green tea polysaccharides increased lysine and tryptophan biosynthesis, promoted insulin secretion, and reduced postprandial blood glucose levels [77]. Polysaccharides derived from teas of varying fermentation levels show significant differences, especially in their molecular weights, monosaccharide composition, and glycosidic bond connections. Wuyi rock tea, categorized as a semi-fermented oolong tea, falls midway between green tea and black tea in terms of its fermentation level. Following a 40-day intervention in high-fat diet-fed mice, polysaccharides extracted from Wuyi rock tea notably elevated the levels of Lactobacillus and enriched Bifidobacterium, suggesting their ability to enhance glucose metabolism and improve the gut microbiota in vivo [78]. Sitagliptin is a well-established hypoglycemic drug that acts as a dipeptidyl peptide inhibitor. Recent research emphasizes that gut microbiota-produced DPP-4 (mDPP4), mainly originating from Bacteroides, can decrease GLP-1 levels due to intestinal leakage caused by a high-fat diet. Interestingly, sitagliptin exhibited limited effectiveness in inhibiting mDPP-4. Nevertheless, high-throughput screening identified daurisoline-d4 (Dau-d4) as a selective inhibitor of mDPP4. This compound holds the potential to improve glucose tolerance in diabetic mice with intestinal impairments [101]. We can explore how tea extracts with different fermentation levels and their active components impact mDPP4. This research can provide insights into the connection between these components and mDPP4, with the potential to develop a tailored tea product for individuals with high-fat diet-induced hyperglycemia (intestinal damage) to reduce blood glucose levels.
4.7. Reduce Oxidative Stress
Numerous studies have shown a robust connection between inflammation caused by oxidative stress and chronic metabolic conditions like T2DM. Oxidative stress represents an imbalance between the production of free radicals and the body’s antioxidant defenses. This imbalance can lower peripheral insulin sensitivity and initiate the development of T2DM [102]. The majority of cells in the human body have an intrinsic defense mechanism primarily dependent on various enzymes, including SOD, CAT, and GSH [103]. Teas of different fermentation levels contain unique bioactive components. In vivo experiments have confirmed that catechins, especially EGCG present in tea, act as a superb source of natural antioxidants. Experimental evidence from animal studies has shown that various fermented teas can effectively alleviate IR induced by oxidative stress, thus promoting and maintaining the glucose balance in the body. Sampath conducted an intervention in mice on a high-fat diet, administering green tea catechin (EGCG = 75 mg/kg). The results showed that, after 17 weeks, there was a significant rise in GSH levels in the liver, kidney, and adipose tissue of the mice. This intervention effectively activated the oxidative stress defense mechanism, maintaining a balanced equilibrium between free radicals and the antioxidant system [68]. Meanwhile, post-fermented dark tea, particularly ripened pu-erh tea, proved effective in alleviating dextran-induced colitis in mice. Following one week of treatment at a dosage of 10 mg/kg body weight per day, there was a decrease in the expression of NADPH oxidase 2 (NOX2) and NADPH oxidase 4 (NOX4), a reduction in myeloperoxidase (MPO) activity, and the restoration of GSH-PX and SOD activity. These positive effects can be primarily attributed to the presence of catechins and polysaccharide components in the tea [104].
5. Conclusions and Prospect
Teas of varying fermentation levels display differing biological activity because of their distinct processing methods. Extracts from tea and its derivatives, such as EGCG and tea polysaccharides, efficiently combat hyperglycemia while regulating glucose balance. In these studies, tea extracts were commonly employed in both in vivo and in vitro experiments, closely mirroring real tea consumption. Tea water extract promotes insulin release through the inhibition of digestive enzymes, enhancement of glucose uptake, suppression of gluconeogenesis-related enzymes, inhibition of AGE formation, reduction in DPP-4 enzyme activity, modulation of gut flora, and attenuation of inflammation caused by oxidative stress. This action assists in alleviating IR and reducing post-meal blood glucose levels. Notably, deeply fermented teas, such as black tea, seem to exhibit a more potent hypoglycemic effect. Exploring the potential of black tea fermentation in regulating post-meal blood glucose is warranted. Furthermore, a holistic approach that combines diet, exercise, and medication can assist in preventing or alleviating T2DM. In the future, research should explore the influence of room temperature and cold brewing on post-meal blood glucose. Tea offers health benefits, yet its daily intake among hyperglycemic patients remains controversial. Additional research in both experimental and clinical settings is required to clarify its impact on blood glucose regulation. Additionally, it is essential to investigate the effective and safe dosages of tea, along with its active ingredients of varying fermentation levels, to comprehend its impact on blood glucose regulation in vivo. The dose–effect relationship, structure–activity relationship, and interaction of different functional components in regulating blood glucose are also worthy of further study.
Finally, we should investigate the inclusion of tea derivatives into everyday staples such as tea rice and tea noodles to assess their effects on post-meal blood glucose. Furthermore, it is essential to develop products aimed at lowering post-meal blood glucose levels for hyperglycemic patients. Further exploration of the activities of different fermented teas and mDPP-4 is justified.
Author Contributions
Conceptualization, J.Z. and J.Y.; methodology, G.L.; software, G.L.; validation, G.L., J.Z. and H.C.; formal analysis, G.L.; investigation, G.L.; resources, J.Z. and J.Y.; data curation, G.L.; writing—original draft preparation, G.L. and J.Z; writing—review and editing, G.L, Z.F., Y.G., Y.W., J.C., Y.X. and D.N.; visualization, G.L. and J.Z.; supervision, J.Z., D.N. and J.Y.; project administration, J.Z. and J.Y.; funding acquisition, J.Z. and J.Y. All authors have read and agreed to the published version of the manuscript.
Funding
The authors thank the China National Key Research and Development Program (2021YFD1601105), the Hangzhou Science and Technology Commissioner Project (20231127), the Cooperation Project of Hangzhou City and Chinese Academy of Agricultural Sciences (202158-01), the China Hangzhou Agricultural and Social Development Scientific Research Key Project (202203A06), the China Agriculture Research System of MOF and MARA (CARS-19), and the Innovation Project for the Chinese Academy of Agricultural Sciences.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Li, H.; Guo, H.; Luo, Q.; Wu, D.-T.; Zou, L.; Liu, Y.; Li, H.-B.; Gan, R.-Y. Current Extraction, Purification, and Identification Techniques of Tea Polyphenols: An Updated Review. Crit. Rev. Food Sci. Nutr. 2023, 63, 3912–3930. [Google Scholar] [CrossRef]
- Greenwalt, C.J.; Steinkraus, K.H.; Ledford, R.A. Kombucha, the Fermented Tea: Microbiology, Composition, and Claimed Health Effects. J. Food Prot. 2000, 63, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wang, Y.; Wang, J.; Geng, W. Kombucha Reduces Hyperglycemia in Type 2 Diabetes of Mice by Regulating Gut Microbiota and Its Metabolites. Foods 2022, 11, 754. [Google Scholar] [CrossRef]
- Mendelson, C.; Sparkes, S.; Merenstein, D.J.; Christensen, C.; Sharma, V.; Desale, S.; Auchtung, J.M.; Kok, C.R.; Hallen-Adams, H.E.; Hutkins, R. Kombucha Tea as an Anti-Hyperglycemic Agent in Humans with Diabetes—A Randomized Controlled Pilot Investigation. Front. Nutr. 2023, 10, 1190248. [Google Scholar] [CrossRef]
- Constantino, M.I.; Molyneaux, L.; Limacher-Gisler, F.; Al-Saeed, A.; Luo, C.; Wu, T.; Twigg, S.M.; Yue, D.K.; Wong, J. Long-Term Complications and Mortality in Young-Onset Diabetes: Type 2 Diabetes Is More Hazardous and Lethal than Type 1 Diabetes. Diabetes Care 2013, 36, 3863–3869. [Google Scholar] [CrossRef]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and Regional Diabetes Prevalence Estimates for 2019 and Projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th Edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Guo, J.; Cao, T.; Zhang, T.; Liu, Y.; Yan, Y. Review on Mechanisms and Structure-Activity Relationship of Hypoglycemic Effects of Polysaccharides from Natural Resources. Food Sci. Hum. Wellness 2023, 12, 1969–1980. [Google Scholar] [CrossRef]
- Messina, A.; Monda, V. Role of the Orexin System on Arousal, Attention, Feeding Behaviour and Sleep Disorders. Acta Medica Mediterr. 2017, 33, 645–649. [Google Scholar] [CrossRef]
- Johnson, A.M.F.; Olefsky, J.M. The Origins and Drivers of Insulin Resistance. Cell 2013, 152, 673–684. [Google Scholar] [CrossRef]
- Czech, M.P. Insulin Action and Resistance in Obesity and Type 2 Diabetes. Nat. Med. 2017, 23, 804–814. [Google Scholar] [CrossRef]
- Rhodes, C.J. Type 2 Diabetes-a Matter of ß-Cell Life and Death? Science 2005, 307, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, P.C.; Larsen, R.N.; Sethi, P.; Sacre, J.W.; Straznicky, N.E.; Cohen, N.D.; Cerin, E.; Lambert, G.W.; Owen, N.; Kingwell, B.A.; et al. Benefits for Type 2 Diabetes of Interrupting Prolonged Sitting with Brief Bouts of Light Walking or Simple Resistance Activities. Diabetes Care 2016, 39, 964–972. [Google Scholar] [CrossRef] [PubMed]
- Henson, J.; Davies, M.J.; Bodicoat, D.H.; Edwardson, C.L.; Gill, J.M.R.; Stensel, D.J.; Tolfrey, K.; Dunstan, D.W.; Khunti, K.; Yates, T. Breaking Up Prolonged Sitting with Standing or Walking Attenuates the Postprandial Metabolic Response in Postmenopausal Women: A Randomized Acute Study. Diabetes Care 2015, 39, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Savikj, M.; Gabriel, B.M.; Alm, P.S.; Smith, J.; Caidahl, K.; Björnholm, M.; Fritz, T.; Krook, A.; Zierath, J.R.; Wallberg-Henriksson, H. Afternoon Exercise Is More Efficacious than Morning Exercise at Improving Blood Glucose Levels in Individuals with Type 2 Diabetes: A Randomised Crossover Trial. Diabetologia 2019, 62, 233–237. [Google Scholar] [CrossRef]
- Davies, M.J.; Aroda, V.R.; Collins, B.S.; Gabbay, R.A.; Green, J.; Maruthur, N.M.; Rosas, S.E.; Del Prato, S.; Mathieu, C.; Mingrone, G.; et al. Management of Hyperglycaemia in Type 2 Diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2022, 65, 1925–1966. [Google Scholar] [CrossRef]
- Tasali, E.; Wroblewski, K.; Kahn, E.; Kilkus, J.; Schoeller, D.A. Effect of Sleep Extension on Objectively Assessed Energy Intake Among Adults with Overweight in Real-Life Settings: A Randomized Clinical Trial. JAMA Intern. Med. 2022, 182, 365–374. [Google Scholar] [CrossRef]
- Sondrup, N.; Termannsen, A.-D.; Eriksen, J.N.; Hjorth, M.F.; Færch, K.; Klingenberg, L.; Quist, J.S. Effects of Sleep Manipulation on Markers of Insulin Sensitivity: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Sleep Med. Rev. 2022, 62, 101594. [Google Scholar] [CrossRef]
- Wan, C.; Ouyang, J.; Li, M.; Rengasamy, K.R.R.; Liu, Z. Effects of Green Tea Polyphenol Extract and Epigallocatechin-3-O-Gallate on Diabetes Mellitus and Diabetic Complications: Recent Advances. Crit. Rev. Food Sci. Nutr. 2022, 1–29. [Google Scholar] [CrossRef]
- Lorenzati, B.; Zucco, C.; Miglietta, S.; Lamberti, F.; Bruno, G. Oral Hypoglycemic Drugs: Pathophysiological Basis of Their Mechanism of ActionOral Hypoglycemic Drugs: Pathophysiological Basis of Their Mechanism of Action. Pharmaceuticals 2010, 3, 3005–3020. [Google Scholar] [CrossRef]
- Sanchez-Rangel, E.; Inzucchi, S.E. Metformin: Clinical Use in Type 2 Diabetes. Diabetologia 2017, 60, 1586–1593. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Takahashi, F.; Okamura, T.; Hamaguchi, M.; Fukui, M. Diet, Exercise, and Pharmacotherapy for Sarcopenia in People with Diabetes. Metabolism 2023, 144, 155585. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Eguchi, S.; Murayama, H.; Takahashi, Y.; Toda, M.; Imai, K.; Tsuda, K. Relationship between Diet/Exercise and Pharmacotherapy to Enhance the GLP-1 Levels in Type 2 Diabetes. Endocrinol. Diabetes Metab. 2019, 2, e00068. [Google Scholar] [CrossRef]
- Clemmensen, C.; Smajilovic, S.; Smith, E.P.; Woods, S.C.; Bräuner-Osborne, H.; Seeley, R.J.; D’Alessio, D.A.; Ryan, K.K. Oral L-Arginine Stimulates GLP-1 Secretion to Improve Glucose Tolerance in Male Mice. Endocrinology 2013, 154, 3978–3983. [Google Scholar] [CrossRef]
- Dollet, L.; Kuefner, M.; Caria, E.; Rizo-Roca, D.; Pendergrast, L.; Abdelmoez, A.M.; Karlsson, H.K.R.; Bjrnholm, M.; Dalbram, E.; Treebak, J.T.; et al. Glutamine Regulates Skeletal Muscle Immunometabolism in Type 2 Diabetes. Diabetes 2022, 71, 624–636. [Google Scholar] [CrossRef]
- He, Q.; Wang, X.; Yang, C.; Zhuang, X.; Yue, Y.; Jing, H.; Hu, J.; Sun, M.; Guo, L. Metabolic and Nutritional Characteristics in Middle-Aged and Elderly Sarcopenia Patients with Type 2 Diabetes. J. Diabetes Res. 2020, 2020, e6973469. [Google Scholar] [CrossRef]
- Serina, J.J.C.; Castilho, P.C.M.F. Using Polyphenols as a Relevant Therapy to Diabetes and Its Complications, a Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 8355–8387. [Google Scholar] [CrossRef]
- Chan-Zapata, I.; Sandoval-Castro, C.; Segura-Campos, M.R. Proteins and Peptides from Vegetable Food Sources as Therapeutic Adjuvants for the Type 2 Diabetes Mellitus. Crit. Rev. Food Sci. Nutr. 2022, 62, 2673–2682. [Google Scholar] [CrossRef]
- Waddell, I.S.; Orfila, C. Dietary Fiber in the Prevention of Obesity and Obesity-Related Chronic Diseases: From Epidemiological Evidence to Potential Molecular Mechanisms. Crit. Rev. Food Sci. Nutr. 2022, 63, 8752–8767. [Google Scholar] [CrossRef] [PubMed]
- Yi, T.; Zhu, L.; Peng, W.-L.; He, X.-C.; Chen, H.-L.; Li, J.; Yu, T.; Liang, Z.-T.; Zhao, Z.-Z.; Chen, H.-B. Comparison of Ten Major Constituents in Seven Types of Processed Tea Using HPLC-DAD-MS Followed by Principal Component and Hierarchical Cluster Analysis. LWT-Food Sci. Technol. 2015, 62, 194–201. [Google Scholar] [CrossRef]
- Zuo, H.; Si, X.; Li, P.; Li, J.; Chen, Z.; Li, P.; Chen, C.; Liu, Z.; Zhao, J. Dynamic Change of Tea (Camellia sinensis) Leaf Cuticular Wax in White Tea Processing for Contribution to Tea Flavor Formation. Food Res. Int. 2023, 163, 112182. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Zhang, J.; Ma, S.; Ou, C.; Feng, X.; Pan, Y.; Gong, S.; Fan, F.; Chen, P.; Chu, Q. Recent Advances on White Tea: Manufacturing, Compositions, Aging Characteristics and Bioactivities. Trends Food Sci. Technol. 2023, 134, 41–55. [Google Scholar] [CrossRef]
- Wei, Y.; Li, T.; Xu, S.; Ni, T.; Deng, W.-W.; Ning, J. The Profile of Dynamic Changes in Yellow Tea Quality and Chemical Composition during Yellowing Process. LWT 2021, 139, 110792. [Google Scholar] [CrossRef]
- Feng, X.; Yang, S.; Pan, Y.; Zhou, S.; Ma, S.; Ou, C.; Fan, F.; Gong, S.; Chen, P.; Chu, Q. Yellow Tea: More than Turning Green Leaves to Yellow. Crit. Rev. Food Sci. Nutr. 2023, 1–18. [Google Scholar] [CrossRef]
- Chen, Y.L.; Duan, J.; Jiang, Y.M.; Shi, J.; Peng, L.; Xue, S.; Kakuda, Y. Production, Quality, and Biological Effects of Oolong Tea (Camellia sinensis). Food Rev. Int. 2010, 27, 1–15. [Google Scholar] [CrossRef]
- Cao, Q.-Q.; Fu, Y.-Q.; Wang, J.-Q.; Zhang, L.; Wang, F.; Yin, J.-F.; Xu, Y.-Q. Sensory and Chemical Characteristics of Tieguanyin Oolong Tea after Roasting. Food Chem. X 2021, 12, 100178. [Google Scholar] [CrossRef]
- Long, P.; Rakariyatham, K.; Ho, C.-T.; Zhang, L. Thearubigins: Formation, Structure, Health Benefit and Sensory Property. Trends Food Sci. Technol. 2023, 133, 37–48. [Google Scholar] [CrossRef]
- Zhu, K.; Ouyang, J.; Huang, J.; Liu, Z. Research Progress of Black Tea Thearubigins: A Review. Crit. Rev. Food Sci. Nutr. 2021, 61, 1556–1566. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.-J.; Wei, X.-L.; Liu, H.-Y.; Li, H.; Xia, Y.; Wu, D.-T.; Zhang, P.-Z.; Gandhi, G.R.; Li, H.-B.; Gan, R.-Y. State-of-the-Art Review of Dark Tea: From Chemistry to Health Benefits. Trends Food Sci. Technol. 2021, 109, 126–138. [Google Scholar] [CrossRef]
- Zhu, M.; Li, N.; Zhou, F.; Ouyang, J.; Lu, D.; Xu, W.; Li, J.; Lin, H.; Zhang, Z.; Xiao, J.; et al. Microbial Bioconversion of the Chemical Components in Dark Tea. Food Chem. 2020, 312, 126043. [Google Scholar] [CrossRef]
- Jiang, H.; Yu, F.; Qin, L.; Zhang, N.; Cao, Q.; Schwab, W.; Li, D.; Song, C. Dynamic Change in Amino Acids, Catechins, Alkaloids, and Gallic Acid in Six Types of Tea Processed from the Same Batch of Fresh Tea (Camellia sinensis L.) Leaves. J. Food Compos. Anal. 2019, 77, 28–38. [Google Scholar] [CrossRef]
- Vuong, Q.V.; Golding, J.B.; Stathopoulos, C.E.; Nguyen, M.H.; Roach, P.D. Optimizing Conditions for the Extraction of Catechins from Green Tea Using Hot Water. J. Sep. Sci. 2011, 34, 3099–3106. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Yan, J.; Lu, A.; Kun, J.; Wang, B.; Song, C.; Tong, H.; Meng, Q. Characterizing Relationship between Chemicals and in Vitro Bioactivities of Teas Made by Six Typical Processing Methods Using a Single Camellia sinensis Cultivar, Meizhan. Bioengineered 2021, 12, 1251–1263. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Ai, Z.; Meng, Y.; Chen, Y.; Ni, D. Comparative Studies on the Physicochemical Profile and Potential Hypoglycemic Activity of Different Tea Extracts: Effect on Sucrase-Isomaltase Activity and Glucose Transport in Caco-2 Cells. Food Res. Int. 2021, 148, 110604. [Google Scholar] [CrossRef] [PubMed]
- Xiang, G.; Sun, H.; Chen, Y.; Guo, H.; Liu, Y.; Li, Y.; Lu, C.; Wang, X. Antioxidant and Hypoglycemic Activity of Tea Polysaccharides with Different Degrees of Fermentation. Int. J. Biol. Macromol. 2023, 228, 224–233. [Google Scholar] [CrossRef]
- Zhou, H.; Li, F.; Wu, M.; Zhu, J.; Wang, Y.; Wei, X. Regulation of Glucolipid Metabolism and Gut Microbiota by Green and Black Teas in Hyperglycemic Mice. Food Funct. 2023, 14, 4327–4338. [Google Scholar] [CrossRef]
- Fujimura, Y.; Watanabe, M.; Morikawa-Ichinose, T.; Fujino, K.; Yamamoto, M.; Nishioka, S.; Inoue, C.; Ogawa, F.; Yonekura, M.; Nakasone, A.; et al. Metabolic Profiling for Evaluating the Dipeptidyl Peptidase-IV Inhibitory Potency of Diverse Green Tea Cultivars and Determining Bioactivity-Related Ingredients and Combinations. J. Agric. Food Chem. 2022, 70, 6455–6466. [Google Scholar] [CrossRef]
- Da Silva, T.B.V.; Castilho, P.A.; de Sá-Nakanishi, A.B.; Seixas, F.A.V.; Dias, M.I.; Barros, L.; Ferreira, I.C.F.R.; Bracht, A.; Peralta, R.M. The Inhibitory Action of Purple Tea on in Vivo Starch Digestion Compared to Other Camellia sinensis Teas. Food Res. Int. 2021, 150, 110781. [Google Scholar] [CrossRef]
- Ni, D.; Ai, Z.; Munoz-Sandoval, D.; Suresh, R.; Ellis, P.R.; Yuqiong, C.; Sharp, P.A.; Butterworth, P.J.; Yu, Z.; Corpe, C.P. Inhibition of the Facilitative Sugar Transporters (GLUTs) by Tea Extracts and Catechins. FASEB J. 2020, 34, 9995–10010. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Oh, J.-H.; Chung, J.-O.; Rha, C.-S.; Park, M.-Y.; Hong, Y.-D.; Kim, W.-K.; Shim, S.-M. Effect of Whole Green Tea Products Including Catechins, Polysaccharides, and Flavonols on the Metabolism of Added Sugars. Food Biosci. 2021, 41, 100936. [Google Scholar] [CrossRef]
- Orita, T.; Chogahara, S.; Okuda, M.; Sakao, K.; Miyata, T.; Hou, D.-X. Extraction Efficiency and Alpha-Glucosidase Inhibitory Activities of Green Tea Catechins by Different Infusion Methods. Foods 2023, 12, 2611. [Google Scholar] [CrossRef]
- Li, S.; Zhang, W.; Wang, R.; Li, C.; Lin, X.; Wang, L. Screening and Identification of Natural α-Glucosidase and α-Amylase Inhibitors from Partridge Tea (Mallotus furetianus Muell-Arg) and in Silico Analysis. Food Chem. 2022, 388, 133004. [Google Scholar] [CrossRef]
- Ueda, M.; Furuyashiki, T.; Yamada, K.; Aoki, Y.; Sakane, I.; Fukuda, I.; Yoshida, K.; Ashida, H. Tea Catechins Modulate the Glucose Transport System in 3T3-L1 Adipocytes. Food Funct. 2010, 1, 167–173. [Google Scholar] [CrossRef]
- Meng, Q.; Qi, X.; Chao, Y.; Chen, Q.; Cheng, P.; Yu, X.; Kuai, M.; Wu, J.; Li, W.; Zhang, Q.; et al. IRS1/PI3K/AKT Pathway Signal Involved in the Regulation of Glycolipid Metabolic Abnormalities by Mulberry (Morus alba L.) Leaf Extracts in 3T3-L1 Adipocytes. Chin. Med. 2020, 15, 1. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Liu, A.; Liu, C.; Tang, Q.; Zhan, L.; Xiao, W.; Huang, J.; Liu, Z.; Zhang, S. Theaflavin Promotes Mitochondrial Abundance and Glucose Absorption in Myotubes by Activating the CaMKK2-AMPK Signal Axis via Calcium-Ion Influx. J. Agric. Food Chem. 2021, 69, 8144–8159. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Liu, Q.; Sun, D.; Wang, Y.; Wang, W.; Liu, D. Ultrasound-Assisted Deep Eutectic Solvent Extraction of Polysaccharides from Anji White Tea: Characterization and Comparison with the Conventional Method. Foods 2023, 12, 588. [Google Scholar] [CrossRef]
- Xu, L.; Chen, Y.; Chen, Z.; Gao, X.; Wang, C.; Panichayupakaranant, P.; Chen, H. Ultrafiltration Isolation, Physicochemical Characterization, and Antidiabetic Activities Analysis of Polysaccharides from Green Tea, Oolong Tea, and Black Tea. J. Food Sci. 2020, 85, 4025–4032. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.; Ren, N.; Soomi, P.; Wu, J.; Guo, N.; Kang, H.; Kim, E.; Wu, Y.; He, P.; Tu, Y.; et al. Theaflavins Improve Insulin Sensitivity through Regulating Mitochondrial Biosynthesis in Palmitic Acid-Induced HepG2 Cells. Molecules 2018, 23, 3382. [Google Scholar] [CrossRef]
- Zhou, H.; Wu, Y.; Kim, E.; Pan, H.; He, P.; Li, B.; Chen, Y.C.; Tu, Y. Simultaneous Tests of Theaflavin-3,3′-Digallate as an Anti-Diabetic Drug in Human Hepatoma G2 Cells and Zebrafish (Danio rerio). Nutrients 2021, 13, 4379. [Google Scholar] [CrossRef]
- Tenore, G.C.; Stiuso, P.; Campiglia, P.; Novellino, E. In Vitro Hypoglycaemic and Hypolipidemic Potential of White Tea Polyphenols. Food Chem. 2013, 141, 2379–2384. [Google Scholar] [CrossRef]
- Du, W.; Peng, S.-M.; Liu, Z.; Shi, L.; Tan, L.-F.; Zou, X.-Q. Hypoglycemic Effect of the Water Extract of Pu-Erh Tea. J. Agric. Food Chem. 2012, 60, 10126–10132. [Google Scholar] [CrossRef]
- Shimizu, M.; Kobayashi, Y.; Suzuki, M.; Satsu, H.; Miyamoto, Y. Regulation of Intestinal Glucose Transport by Tea Catechins. BioFactors 2000, 13, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.Y.; Zhang, A.; Tsang, D.; Huang, Y.; Chen, Z.-Y. Stability of Green Tea Catechins. J. Agric. Food Chem. 1997, 45, 4624–4628. [Google Scholar] [CrossRef]
- Park, C.W.; Kim, H.W.; Ko, S.H.; Lim, J.H.; Ryu, G.R.; Chung, H.W.; Han, S.W.; Shin, S.J.; Bang, B.K.; Breyer, M.D.; et al. Long-Term Treatment of Glucagon-Like Peptide-1 Analog Exendin-4 Ameliorates Diabetic Nephropathy through Improving Metabolic Anomalies in Db/Db Mice. J. Am. Soc. Nephrol. 2007, 18, 1227. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lv, Q.; Jia, S.; Chen, Y.; Sun, C.; Li, X.; Chen, K. Effects of Flavonoid-Rich Chinese Bayberry (Morella rubra Sieb. et Zucc.) Fruit Extract on Regulating Glucose and Lipid Metabolism in Diabetic KK-Ay Mice. Food Funct. 2016, 7, 3130–3140. [Google Scholar] [CrossRef] [PubMed]
- Ishii, Y.; Ohta, T.; Sasase, T.; Morinaga, H.; Hata, T.; Miyajima, K.; Katusda, Y.; Masuyama, T.; Shinohara, M.; Kakutani, M.; et al. A High-Fat Diet Inhibits the Progression of Diabetes Mellitus in Type 2 Diabetic Rats. Nutr. Res. 2010, 30, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Fu, L.; Kojima, R.; Yamamoto, A.; Ueno, T.; Matsui, T. Theaflavins Prevent the Onset of Diabetes through Ameliorating Glucose Tolerance Mediated by Promoted Incretin Secretion in Spontaneous Diabetic Torii Rats. J. Funct. Foods 2021, 86, 104702. [Google Scholar] [CrossRef]
- Ueda-Wakagi, M.; Nagayasu, H.; Yamashita, Y.; Ashida, H. Green Tea Ameliorates Hyperglycemia by Promoting the Translocation of Glucose Transporter 4 in the Skeletal Muscle of Diabetic Rodents. Int. J. Mol. Sci. 2019, 20, 2436. [Google Scholar] [CrossRef] [PubMed]
- Sampath, C.; Rashid, M.R.; Sang, S.; Ahmedna, M. Green Tea Epigallocatechin 3-Gallate Alleviates Hyperglycemia and Reduces Advanced Glycation End Products via Nrf2 Pathway in Mice with High Fat Diet-Induced Obesity. Biomed. Pharmacother. 2017, 87, 73–81. [Google Scholar] [CrossRef]
- Shang, L.; Li, F.; Zhu, J.; Sun, C.; Wang, Y. Selenium-Enriched and Ordinary Black Teas Regulate the Metabolism of Glucose and Lipid and Intestinal Flora of Hyperglycemic Mice. Plant Foods Hum. Nutr. 2023, 78, 61–67. [Google Scholar] [CrossRef]
- Gao, Y.; Xu, Y.; Ruan, J.; Yin, J. Selenium Affects the Activity of Black Tea in Preventing Metabolic Syndrome in High-Fat Diet-Fed Sprague–Dawley Rats. J. Sci. Food Agric. 2020, 100, 225–234. [Google Scholar] [CrossRef]
- Han, M.; Zhao, G.; Wang, Y.; Wang, D.; Sun, F.; Ning, J.; Wan, X.; Zhang, J. Safety and Anti-Hyperglycemic Efficacy of Various Tea Types in Mice. Sci. Rep. 2016, 6, 31703. [Google Scholar] [CrossRef] [PubMed]
- Imran, A.; Butt, M.S.; Arshad, M.S.; Arshad, M.U.; Saeed, F.; Sohaib, M.; Munir, R. Exploring the Potential of Black Tea Based Flavonoids against Hyperlipidemia Related Disorders. Lipids Health Dis. 2018, 17, 57. [Google Scholar] [CrossRef]
- Xu, W.; Zhou, Y.; Lin, L.; Yuan, D.; Peng, Y.; Li, L.; Xiao, W.; Gong, Z. Hypoglycemic Effects of Black Brick Tea with Fungal Growth in Hyperglycemic Mice Model. Food Sci. Hum. Wellness 2022, 11, 711–718. [Google Scholar] [CrossRef]
- Cai, X.; Liu, Z.; Dong, X.; Wang, Y.; Zhu, L.; Li, M.; Xu, Y. Hypoglycemic and Lipid Lowering Effects of Theaflavins in High-Fat Diet-Induced Obese Mice. Food Funct. 2021, 12, 9922–9931. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Zhang, N.; Wang, Q.; Huang, Y.; Huang, Y.; Lin, Y.; Huang, M.; Zheng, F.; Xiao, M.; Ye, J. Theabrownin of Raw and Ripened Pu-Erh Tea Varies in the Alleviation of HFD-Induced Obesity via the Regulation of Gut Microbiota. Eur. J. Nutr. 2023, 62, 2177–2194. [Google Scholar] [CrossRef]
- Su, K.; Mao, X.; Zhang, X. Glucose-Lowering Activity of Dark Tea Protein Extract by Modulating Spleen–Brain Axis of Diabetic Mice. Br. J. Nutr. 2021, 126, 961–969. [Google Scholar] [CrossRef]
- Li, H.; Fang, Q.; Nie, Q.; Hu, J.; Yang, C.; Huang, T.; Li, H.; Nie, S. Hypoglycemic and Hypolipidemic Mechanism of Tea Polysaccharides on Type 2 Diabetic Rats via Gut Microbiota and Metabolism Alteration. J. Agric. Food Chem. 2020, 68, 10015–10028. [Google Scholar] [CrossRef]
- Wu, Z.; Zeng, W.; Zhang, X.; Yang, J. Characterization of Acidic Tea Polysaccharides from Yellow Leaves of Wuyi Rock Tea and Their Hypoglycemic Activity via Intestinal Flora Regulation in Rats. Foods 2022, 11, 617. [Google Scholar] [CrossRef]
- Huang, H.; Chen, J.; Chen, Y.; Xie, J.; Xue, P.; Ao, T.; Chang, X.; Hu, X.; Yu, Q. Metabonomics Combined with 16S rRNA Sequencing to Elucidate the Hypoglycemic Effect of Dietary Fiber from Tea Residues. Food Res. Int. 2022, 155, 111122. [Google Scholar] [CrossRef]
- Hajizadeh Tekmeh, H.; Vanizor Kural, B.; Kör, S.; Arıkan Malkoç, M.; Yuluğ, E.; Kutlu, A.; Abidin, İ.; Orem, A. How Does L-Theanine Treatment Affect the Levels of Serum and Hippocampal BDNF, Insulin and Adipocytokines in Diabetic Rats? Biochem. Biophys. Res. Commun. 2023, 667, 95–103. [Google Scholar] [CrossRef]
- Ma, L.; Hu, Y.; Alperet, D.J.; Liu, G.; Malik, V.; Manson, J.E.; Rimm, E.B.; Hu, F.B.; Sun, Q. Beverage Consumption and Mortality among Adults with Type 2 Diabetes: Prospective Cohort Study. BMJ 2023, 381, e073406. [Google Scholar] [CrossRef] [PubMed]
- Imamura, F.; Schulze, M.B.; Sharp, S.J.; Guevara, M.; Romaguera, D.; Bendinelli, B.; Salamanca-Fernández, E.; Ardanaz, E.; Arriola, L.; Aune, D.; et al. Estimated Substitution of Tea or Coffee for Sugar-Sweetened Beverages Was Associated with Lower Type 2 Diabetes Incidence in Case–Cohort Analysis across 8 European Countries in the EPIC-InterAct Study. J. Nutr. 2019, 149, 1985–1993. [Google Scholar] [CrossRef]
- Li, Y.; Wang, C.; Huai, Q.; Guo, F.; Liu, L.; Feng, R.; Sun, C. Effects of Tea or Tea Extract on Metabolic Profiles in Patients with Type 2 Diabetes Mellitus: A Meta-Analysis of Ten Randomized Controlled Trials: Effects of Tea on T2DM Patients. Diabetes Metab. Res. Rev. 2016, 32, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Iso, H.; Date, C.; Wakai, K.; Fukui, M.; Tamakoshi, A. The Relationship between Green Tea and Total Caffeine Intake and Risk for Self-Reported Type 2 Diabetes among Japanese Adults. Ann. Intern. Med. 2006, 144, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, D.; Nyakayiru, J.; Draijer, R.; Mulder, T.P.J.; Hopman, M.T.E.; Eijsvogels, T.M.H.; Thijssen, D.H. Impact of Flavonoid-Rich Black Tea and Beetroot Juice on Postprandial Peripheral Vascular Resistance and Glucose Homeostasis in Obese, Insulin-Resistant Men: A Randomized Controlled Trial. Nutr. Metab. 2016, 13, 34. [Google Scholar] [CrossRef] [PubMed]
- Neyestani, T.R.; Shariatzade, N.; Kalayi, A.; Gharavi, A.; Khalaji, N.; Dadkhah, M.; Zowghi, T.; Haidari, H.; Shab-bidar, S. Regular Daily Intake of Black Tea Improves Oxidative Stress Biomarkers and Decreases Serum C-Reactive Protein Levels in Type 2 Diabetic Patients. Ann. Nutr. Metab. 2010, 57, 40–49. [Google Scholar] [CrossRef]
- Odegaard, A.O.; Pereira, M.A.; Koh, W.-P.; Arakawa, K.; Lee, H.-P.; Yu, M.C. Coffee, Tea, and Incident Type 2 Diabetes: The Singapore Chinese Health Study1. Am. J. Clin. Nutr. 2008, 88, 979–985. [Google Scholar] [CrossRef]
- Liu, J.; Liu, S.; Zhou, H.; Hanson, T.; Yang, L.; Chen, Z.; Zhou, M. Association of Green Tea Consumption with Mortality from All-Cause, Cardiovascular Disease and Cancer in a Chinese Cohort of 165,000 Adult Men. Eur. J. Epidemiol. 2016, 31, 853–865. [Google Scholar] [CrossRef]
- Deng, D.; Yan, N. GLUT, SGLT, and SWEET: Structural and Mechanistic Investigations of the Glucose Transporters. Protein Sci. 2016, 25, 546–558. [Google Scholar] [CrossRef]
- Mueckler, M.; Thorens, B. The SLC2 (GLUT) Family of Membrane Transporters. Mol. Asp. Med. 2013, 34, 121–138. [Google Scholar] [CrossRef]
- Huang, S.; Czech, M.P. The GLUT4 Glucose Transporter. Cell Metab. 2007, 5, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.M.; Loo, D.D.F.; Hirayama, B.A. Biology of Human Sodium Glucose Transporters. Physiol. Rev. 2011, 91, 733–794. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, S.; Chen, J.; Su, Z. Unraveling the Regulation of Hepatic Gluconeogenesis. Front. Endocrinol. 2019, 9, 802. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Xu, J.; Wang, X.; Wang, H.; Lin, Z.; Shao, K.; Fang, L.; Zhang, C.; Zhao, Y. Jiaogulan Tea (Gpostemma pentaphyllum) Potentiates the Antidiabetic Effect of White Tea via the AMPK and PI3K Pathways in C57BL/6 Mice. Food Funct. 2020, 11, 4339–4355. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Teng, J.; Dong, R.; Ban, Q.; Yang, L.; Du, K.; Wang, Y.; Pu, H.; Yang, C.S.; Ren, Z. Alleviating Effects and Mechanisms of Action of Large-Leaf Yellow Tea Drinking on Diabetes and Diabetic Nephropathy in Mice. Food Sci. Hum. Wellness 2023, 12, 1660–1673. [Google Scholar] [CrossRef]
- Dong, L.; Li, Y.; Chen, Q.; Liu, Y.; Wu, Z.; Pan, D.; Yan, N.; Liu, L. Cereal Polyphenols Inhibition Mechanisms on Advanced Glycation End Products and Regulation on Type 2 Diabetes. Crit. Rev. Food Sci. Nutr. 2023, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Hemmler, D.; Roullier-Gall, C.; Marshall, J.W.; Rychlik, M.; Taylor, A.J.; Schmitt-Kopplin, P. Evolution of Complex Maillard Chemical Reactions, Resolved in Time. Sci. Rep. 2017, 7, 3227. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal. 2018, 29, 1727–1745. [Google Scholar] [CrossRef]
- Röhrborn, D.; Wronkowitz, N.; Eckel, J. DPP4 in Diabetes. Front. Immunol. 2015, 6, 386. [Google Scholar] [CrossRef]
- Zhao, B.; Su, K.; Mao, X.; Zhang, X. Separation and Identification of Enzyme Inhibition Peptides from Dark Tea Protein. Bioorg. Chem. 2020, 99, 103772. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, Z.; Hang, J.; Liu, J.; Guo, F.; Ding, Y.; Li, M.; Nie, Q.; Lin, J.; Zhuo, Y.; et al. Microbial-Host-Isozyme Analyses Reveal Microbial DPP4 as a Potential Antidiabetic Target. Science 2023, 381, eadd5787. [Google Scholar] [CrossRef] [PubMed]
- Yaribeygi, H.; Sathyapalan, T.; Atkin, S.L.; Sahebkar, A. Molecular Mechanisms Linking Oxidative Stress and Diabetes Mellitus. Oxidative Med. Cell. Longev. 2020, 2020, e8609213. [Google Scholar] [CrossRef] [PubMed]
- Maritim, A.C.; Sanders, R.A.; Watkins, J.B. Diabetes, Oxidative Stress, and Antioxidants: A Review. J. Biochem. Mol. Toxicol. 2003, 17, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Li, S.; Liu, Y.; Sun, K.; Luo, L.; Zeng, L. Aged Ripe Pu-Erh Tea Reduced Oxidative Stress-Mediated Inflammation in Dextran Sulfate Sodium-Induced Colitis Mice by Regulating Intestinal Microbes. J. Agric. Food Chem. 2021, 69, 10592–10605. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).