Abstract
Food adulteration refers to the alteration of food quality that takes place deliberately. It includes the addition of ingredients to modify different properties of food products for economic advantage. Color, appearance, taste, weight, volume, and shelf life are such food properties. Substitution of food or its nutritional content is also accomplished to spark the apparent quality. Substitution with species, protein content, fat content, or plant ingredients are major forms of food substitution. Origin misrepresentation of food is often practiced to increase the market demand of food. Organic and synthetic compounds are added to ensure a rapid effect on the human body. Adulterated food products are responsible for mild to severe health impacts as well as financial damage. Diarrhea, nausea, allergic reaction, diabetes, cardiovascular disease, etc., are frequently observed illnesses upon consumption of adulterated food. Some adulterants have shown carcinogenic, clastogenic, and genotoxic properties. This review article discusses different forms of food adulteration. The health impacts also have been documented in brief.
1. Introduction
Food are organic substances consumed for energy, growth, and nutritional purpose. Food adulteration refers to the process through which the quality of food is lowered [1,2]. Broadly, food adulteration is a category of food fraud which is accomplished deliberately by human beings for financial gain [1,2,3,4,5]. It is also termed as economically motivated adulteration (EMA) that sometimes gives rise to authenticity issues: brand, origin, manufacturing ingredients, and their composition are often misrepresented [6]. Protected Designation of Origin (PDO), Protected Geographical Indication (PGI), Certificate of Specific Character (CSC), and Traditional Specialties Guaranteed (TSG) are some of the familiar terms [7]. Adulterated food products pose several health hazards, including health diseases, and they weaken the immune system.
Adulteration of foodstuffs has been frequently observed for centuries due to the contribution of several common reasons. The perishable nature, heterogeneity, and huge production of certain food items have always been tempting for dishonest traders; the similarity and diversity of animal species, stock limitation, and market price pressure also encourage them to perform intentional adulteration [8]. Some food items have been severely prone to adulteration due to possessing high dietary value and vast popularity. Food with a narrow profit margin also have frequently appeared in fraud lists [9,10]. Beverages and other liquid foods have drawn special attraction, with a wide variation in chemical composition, high quality, long aging time, and high production cost [11,12]. The competitive nature of the food industry due to consumer’s extensive demand for variety and low-cost food products has stimulated this issue further. In addition, the limitation of raw materials, demand–supply gap, and the ever-present tendency to reduce cost and maximize profits have created more opportunities and interest for invidious traders [13,14]. Other reasons include degraded moral society, spoiled socio-economic structure, and low legal standards, and their improper enforcement may play a significant role [15,16].
Many food adulteration incidents were encountered in the past. The China gutter oil scandal is such an example that used illicit cooking oil from restaurant fryers, grease traps, and slaughterhouse waste, or extracted from discarded animal parts. Later, such contaminated food products were also found in Taiwan, Hong Kong, and Singapore. Addition of Sudan dye was reported in fats and oils, herbs and spices, food additives, and flavorings several times. In 2007, two cases of tetrodotoxin poisoning were found, which were caused by substitution of monkfish with pufferfish. In 2008, the contamination of powdered infant milk in China caused illness in 294,000 children, with 50,000 hospitalizations and 6 deaths. In 2013, a methanol poisoning affected 694 patients, with 8 deaths in Iran. In 2018, methanol toxication caused extensive hemorrhagic cerebral infarction or multiple organ failure that affected 90 individuals, with 64 fatalities in Malaysia [17]. Dioxins in pork in 2008, milk with detergent, fat, and urea in 2012, and processed beef products with horsemeat in 2013 are also some renown incidences [18]. It is reported that the global food industry faces an expense of approximately USD 10-15 billion per year due to health and financial damage associated to intentionally contaminated food products [19]. The demand for food increased with the emergence of COVID-19 and Brexit, which resulted in reduced industry inspections, weakened governance, audits, and ever-increasing pressure on the food industry [20].
Several incidents on food adulteration were reported in recent times. The Trello and European Commission food fraud databases reveal many such incidents. As part of Operation OPSON XI, coordinated by Europol for EU-wide action and which took place between December 2021 and May 2022, the authorities of 26 countries seized almost 27,000 tons of fake food and 15 million liters of alcoholic beverages. In October 2022, Food Safety and Halal Food Authority of Pakistan seized 4000 L of fake honey adulterated with sugars, chemicals, and wax. In December 2022, more than 12 people died and more than 15 people lost their eyesight in Bihar, India, after consuming adulterated alcohol. In April 2022, the Spanish Agency for Food Safety and Nutrition launched an alert regarding the production of fake olive oil and extra-virgin olive oil adulterated with other vegetable oils. In Turkey, it was reported in August 2022 that ground sumac was found to be adulterated with unauthorized color. In August 2022, curry powder from Cameroon was found adulterated with Orange II by authorities in Belgium. In August 2022, authorities in Pakistan seized tea made with textile dyes, sawdust, and other fillers. Cumin seeds mixed with 30 tons of adulterants were seized by authorities in India in July 2022. Between January 2022 and March 2022, the FDA collected and tested 144 samples of imported honey and found ten percent of the samples to be adulterated with undeclared added sweeteners. In Germany, sausage and poultry meat were alleged to contain undeclared “mechanically separated meat” ingredients (March 2022).
The technical progress for food adulteration research is quite prominent in modern times. Earlier, adulterated food products were identified on the basis of a few physical parameters, such as refractive index, viscosity, melting point, saponification, iodine value, etc. With the expansion of global markets and business competition, the frequency of food adulteration has increased exponentially, which gave rise to the necessity of highly efficient techniques. The food governing authorities around the world have also established the official methods for detection of food adulteration. Currently, chromatography and spectrometry are widely used analytical techniques. Protein and DNA-based techniques are also in practice. In addition, metabolomics, hyperspectral imaging, and chemometrics are some other techniques. The state-of-the-art techniques are highly contributing to combat food adulteration. Still, there remain some drawbacks, such as complexity, excessive use of toxic compounds, laborious sample preparation, etc. [21,22]. Lack of collaboration between scientists and food policy-makers might be an obstacle in identifying the need for proper research and development. In addition, there lies a gap between laboratory research and practical aspects [23]. Food adulteration is a complicated phenomenon and involves the availability of numerous fraudulent options discovered by dishonest people. It is important to understand different forms and the dynamic nature of food adulteration and their impacts on human health. Regular market studies and the development of laboratory techniques for the qualitative and quantitative assessment of new forms of food adulterants would be critical. Gathering and analyzing the possible forms of adulteration in a single frame would be highly useful to bridge the gap for research.
This article presents a cohesive review of the common forms of food adulteration and origin fraud of food. In this article, the health impacts associated with adulteration have also been addressed in brief. The scientific literature for the period of 1995 to 2022 was searched using the keywords “food adulteration”, “economically motivated adulteration”, “origin fraud”,” mislabeling”, and “health impact of food adulteration”. Approximately 400 scientific articles and reports were screened and reviewed. Of those articles, 174 were found more relevant and reviewed extensively. The Trello and European Commission food fraud databases along with relevant newspapers and magazine articles were reviewed for recent food adulteration incidents. The information covered in this article will help researchers, food engineers, scientists, and policy-makers to combat food adulteration and health hazard.
2. Overview of Food Adulteration
The main purpose of food adulteration is to alter the quality of food products for economic advantage. Such actions usually take place by substitution with inferior quality or less valued food and increasing the weight or volume by admixture of undeclared ingredients. Providing a more attractive appearance by injection of artificial chemicals and colorants is also included here [8,24].
The principal aim behind food substitution is to reduce raw material and manufacturing cost by incorporation of inferior compounds. The physical properties and taste of food items are modified in many ways. Artificial ripening of fruits is a common means of food adulteration. Adulteration with preservatives, colorants, and artificial sweeteners are also common food adulteration techniques. Among other adulterations, falsification of origin is also labeled as adulteration, as it includes false claim for superior origin.
Figure 1 presents the key forms of intentional food adulteration, which are briefly discussed in the following sections [3,4,5].
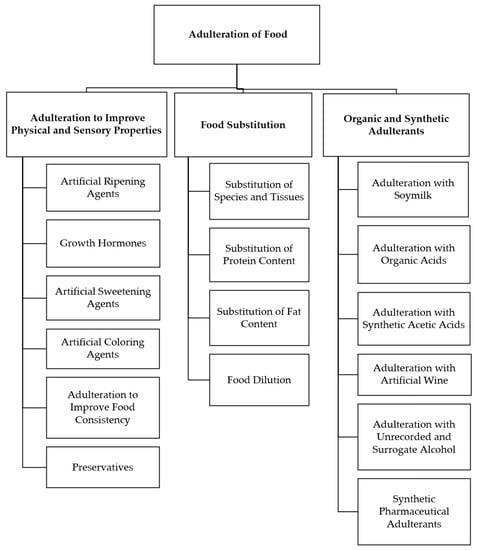
Figure 1.
Major forms of food adulteration.
3. Adulteration to Improve Physical and Sensory Properties
Taste and appearance have a high impact on the commercial value of food products. Increasing the shelf life of food items gives financial benefits. Artificial ripening and sweetening are used to increase food palatability. Similarly, artificial colorants are applied to improve the appearance of food. Preservatives are added to store food in a fresh condition for a long time. The following sections briefly describe different means of adulteration to improve physical and sensory properties.
3.1. Artificial Ripening Agents
To avoid the economic loss incurred by the spoilage of climacteric fruits during the harvest, processing, and transportation process, the fruit sellers pluck the fruits much before they attain proper maturity and use chemicals to ripen them artificially just before retailing. The necessity for artificial ripening also arises if the fruit sellers wish to sell fruits before their due season to make additional profit. Ethylene, ethanol, methanol, propylene, methyl jasmonate, ethylene glycol, ethephon, and calcium carbide are used to ripen fruits and vegetables artificially [25,26,27,28,29,30,31]. The ripening agents and their key features are documented in Table 1.

Table 1.
Ripening agents and their features.
3.2. Growth Hormones
Gibberellic acid, alpha naphthyl acetic acid, and oxytocin are growth hormones used on fruits and vegetables by farmers to trigger growth. Oxytocin, being a mammalian hormone and a veterinary drug, is not suitable for vegetable crops. However, it is widely used in bottle gourds, bitter gourds, pumpkins, and cucumbers to enhance size and color [29].
3.3. Artificial Sweetening Agents
Sweetness of food is an important criterion in terms of demand and marketability. So, there is always a tendency by the traders to increase the sweetness of selected food items by artificial means. Mainly, fruits and vegetables, beverages, sweeteners, and confectionary products fall into sweet food category.
Artificial sweeteners are injected by injector pumps on one side of fruit to alter the natural sweetness. To ensure uniform distribution, sellers inject sweeteners from several points in the fruit. Saccharine mixture was found to be injected into melons and watermelons to enhance sweetness artificially [29]. The addition of external sugar or sugar solution is a common form of adulteration of fruit juice. In the case of export purpose, the concentrated fruit juice is shipped; later, external water and sugar are added to the concentrated fruit juice to give natural properties similar to that of natural juice [11]. In addition, high-fructose corn syrup, partially hydrolyzed cane syrup, and beet medium invert sugar are also added to increase the Brix value and to improve composition quite similar to authentic juice [36]. The direct addition of sucrose, glucose, fructose, maltose, beet sugar, corn sugar, and cane sugar in honey is widely practiced [37]. Adulteration of honey and confectionary products with fructose or glucose changes the fructose-to-glucose ratio [38]. Feeding honeybees with syrups and industrial sugars after the broods have been naturally available is known as an indirect form of adulteration which is very difficult to detect. Feeding low quality honey to honeybees is also reported [38,39,40,41]. Different confectionary and bakery products are sweetened with Acesulfame-K and Aspartame [42,43]. Sugar, coloring agents, synthetic red dyes, aromatizing agents, and sweeter foreign wines are often added into wine for quality enhancement purpose [44,45]. Glycerol reduces the acidity and bitter taste, increases the sweetness, and stops fermentation, while diethylene glycol imparts relish to wines. The addition of root or cane sugar to tequila and the addition of cane or beet ethanol to whiskey have also been noted [46,47].
3.4. Artificial Coloring Agents
Color, texture, and appearance of food products are important criteria in the selection of desired food items by consumers. Foods with attractive color increase marketability and profit. Thus, following this trend, various natural as well as synthetic dyes are applied to different food items. Most of the colorful food items are at risk of such malpractice. Among those, fruits and vegetables, egg and egg-derived food, spices, sweeteners, and confectionary products are prime choices. Coloring agents are also added in processed meat and fish [48]. Paprika oleoresin is commonly used as a natural coloring agent in meat-based food products [49].
Natural colors, such as chlorophyll, annatto, and caramel, have been reported several times in fruits and vegetables. Synthetic dyes are more popular among the sellers, as those exceed natural ones in many aspects. These are chemically synthesized, more stable, shiny, and highly efficient. Their cheapness and easy availability are also considerable factors. Rhodamine B, auramine, Metanil yellow, Congo red, Orange II, malachite green, and other permitted and non-permitted colors are used in cut fruits and vegetables. Red dye is injected into watermelons to enhance the acceptability of the consumers. Malachite green is widely used to make green vegetables, such as green chili, green peas, bitter gourds, lady finger, and pointed gourdd, look greener, fresh, bright, and glowing. Another reagent frequently reported for bitter gourd and lady finger to be dipped into is copper sulfate solution, which is bright blue in hydrous form and pale green in anhydrous form. Mobile oil for coloring and carbofuran for a fresh purple appearance are injected into brinjals, tomatoes, cauliflowers, and cabbages. Phosphomidone, methyl parathion, monocrotophos, and formaldehyde are also injected for a fresh white appearance [29].
Eggs are dyed artificially by azo dyes. The addition of illegal synthetic dyes is inspiring among the traders, as eggs’ nutritional value and freshness are predicted by judging the egg yolk color. Sudan dyes are a type of synthetic azo dye that is used in industry or printing. Another azo dye, Para red, is chemically similar to Sudan dye I and is used for printing purposes. The yellow-orange-hue-colored eggs are preferable by consumers. So, the sellers often feed hens food mixed with dyes to enhance the color of egg yolk [50].
Sudan I, Sudan Ⅳ, Metanil Yellow, Sudan III, Oil Orange SS, Rhodamine B, Auramine O, coal tar red, Para Red, etc., are applied in red pepper chili powder. Sudan I, Sudan Ⅳ, Acid Black I, Annatto, etc., are mixed with paprika powder. Sudan I, Metanil Yellow, Lead Chromate, etc., are added with turmeric powder. Amaranth Red and Basic Red 46 are added into sumac. Auramine O and Chrysoidin are mixed with curry powder. Acid Orange II, Metanil Yellow, Ponceau 4R, Gardenia Yellow, dye extracted from the flowers of Buddleja officinalis Maxim, etc., are added with saffron flower. Crystal Violet is mixed into cayenne pepper. In addition to synthetic dyes, colored paper and wood are also used [9].
Dark honey or “forest honey” is richer in minerals and nutritional value than light honey and possesses a higher commercial value. That is why light honey is often tainted with sulfite ammonia caramel and presented as dark honey [40,41].
3.5. Adulteration to Improve Food Consistency
Detergents, along with oil and fat, have been used to improve consistency of dairy products. In addition, one of the reasons for the accidental presence of detergent in milk products is lack of hygiene, sanitation, and improper cleaning in the dairy industries [51,52,53]. Along with detergent, other compounds, such as salt, glucose, starch, neutralizers, pulverized soap, surfactant, and coloring agent, are also mixed to adjust the whiteness, viscosity, thickness, and solid-not-fat content. Addition of gelatin, stabilizers, enzymes, and external reagent to improve food consistency are also reported [15,51].
3.6. Preservatives
Preservatives are usually added to increase the shelf life of food items. Addition of preservatives is one of the most practiced forms of adulteration at present. Fruits and vegetables, fish and seafood, meat and processed meat, milk and dairy products, and beverages are the most tempting targets.
Formaldehyde is the most reported preservative used in fruits and vegetables. Formalin inhibits the growth of microbes by interacting with the amino groups of adenine, cytosine, and guanine and denaturing them. It also penetrates the interiors of bacterial spores, which makes it capable of preventing microbial contamination and prolonging the shelf life of food. Although there is no set standard for the daily intake of formaldehyde from food, the World Health Organization (WHO) estimated it to be in the range of 1.5–14 mg/d (mean 7.75 mg/d) for an average adult. According to the European Food Safety Authority (EFSA), the daily oral exposure to formaldehyde should not exceed 100 mg formaldehyde per day [54]. In a recent study, the formaldehyde level in fish and seafood were found to be higher than the recognized safety level of 5 mg/kg [55].
In addition to formalin, wax components, and different forms of esters, are also used to reduce the water loss and surface abrasion. The other purpose is to control internal gas composition and provide the shiny appearance of those fruits and vegetables that lost their natural wax during primary processing. Compared with other lipid and non-lipid coatings, wax coatings provide better resistance to moisture loss. Due to petroleum-based wax containing harmful wood rosins and solvent residues, beeswax, carnauba wax, and shellac are preferable. Fruits and vegetables are dried well before waxing and handling [29]. With the increasing demand and distant transportation of fish and seafood, the addition of low-cost preservatives has been a long-practiced issue. Fish, dry fish, and seafood are usually adulterated with preservatives, such as formalin, chlorofluorocarbon, and DDT powder, to tackle the spoilage and quality deterioration [56]. External parasites of fish egg are also treated with formalin [57]. Since a small quantity of formaldehyde is also naturally produced in fish as a byproduct of amine oxide degradation, artificially added formalin is quite difficult to detect [58]. Increment of shelf life by inhibiting microbial activities is a common form of milk and dairy product adulteration. It is accomplished by adding several preservative chemicals, such as formalin, urea, nitrate or pond water, borax acid, boric acid, cane sugar, sucrose, glucose, caustic soda, salicylic acid, hydrogen peroxide, benzoic acid, hypochlorite, and potassium dichromate [15,59,60]. Hydrogen peroxide acts as a preservative for pasteurized milk by activating the natural enzyme lactoperoxidase [60]. On the other hand, a significant reason for adding urea in dairy products is to elongate the shelf life. Preservatives, such as salicylic acid and benzoic acid, are added for preserving cheap wines prone to souring. Citric acid is added for pH adjustment. Beet sugar, cane sugar, concentrated rectified must, grape must, or grape wine are added to increase the natural content of ethanol and overall commercial value [51].
The key preservatives used in food items are listed in Table 2.

Table 2.
Preservatives and corresponding food items [61,62].
4. Food Substitution
Substitution is the most diverse form of intentional food adulteration, which includes the direct alteration of a part or whole food item or external addition of other inferior food products or fake nutritional compounds. The common forms of food substitution are discussed in the following section.
4.1. Substitution of Species and Tissue
4.1.1. Fishery Substitution:
Substitution of fish species is the most practiced form of fish fraud [63]. It refers to the replacement of a highly reputed fish species by a bad or inferior species [14]. A commercially valuable fish species is substituted with a low-value, non-declared, and non-specified species to make extra profit and to compensate for high tariffs paid for some species [14,19,64]. Crustaceans and high-quality shrimps are more prone to substitution due to their high market demand [65]. The traditional method of species identification is morphological analysis; however, in the case of seafood, it is quite inefficient as those are phenotypically similar and their external body parts are often removed during processing [63]. A risk of willful or unintentional substitution also prevails as visual specification becomes more difficult once fish has been processed into another form [66].
4.1.2. Substitution in Meat Products
In the case of inter-species substitution, meats with similar color, such as beef and horse meat, beef and mutton, or poultry and pork are visually quite difficult to distinguish when frozen or processed into another form and shape. Sausage is one such processed meat product highly relished worldwide that is traditionally made from intestine or obtained synthetically. Though it can be made from beef, chicken, or pork, fraudulent substitution of species is also prevalent here [67]. Minced meat, one of the most versatile meat products, used in hamburgers, patties, meatballs, sausages, and salami, is prone to frequent adulteration by substitution with other meat species [68]. In this case, identification of meat species is very difficult as the morphological structure gets removed during mincing, and the adulterated minced meat appears very similar to the authentic product [69]. The undeclared addition of animal tissue, such as collagen and offal, are also prevalent, which is profitable for the traders [70,71]. Due to pork being cheaper and more readily available than other meat species, frequent substitution of other meat with porcine meat is reported [67,72,73].
4.1.3. Substitution in Milk Products
The milk used to manufacture dairy products are usually derived from cow, sheep, goat, and buffalo. Cow milk is widely used both in developed and developing countries; however, there are incidents when cow milk is avoided due to allergenic reaction, religious restriction, ethical or cultural issues, personal preference, and impudence for certain food products [74,75]. Fraudulent substitution of other expensive milk with cow milk is very common. She-donkey milk, possessing high commercial value, is often substituted by cow or goat milk [60]. People with allergenic problems to cow milk prefer caprine milk due to its being easily digestible and containing low lactose content. Another example of malpractice is representing bovine milk as caprine milk [74,76]. Fraudulent replacement of sheep and goat milk with cheaper cow milk is also reported [77]. Replacement of fresh cow milk with reconstituted skim milk powder due to the popularity of cow milk is also reported [78].
Traditional cheese products, such as feta, manchego, and pecorino are manufactured from the mixture of ovine and caprine milk or solely ovine milk. However, the seasonal production and higher price of ovine and caprine milk make those cheese items prone to substitution by cheap bovine milk. Cheese products labelled as “pure buffalo mozzarella” are often found to contain cow milk. Cheese products made from one pure species and with protected designation of origin are rarely proved authentic and should be monitored from time to time [77].
4.1.4. Coffee Substitution
Admixture of costly coffee beans with comparatively cheap beans is a very common practice. The two commercially important species, Arabica coffee (Coffea arabica) and Robusta coffee (Coffea canephora) are different in quality and botanical characteristics. Arabica coffee is costlier and has greater acceptability than robusta coffee due to its organoleptic properties. As a blend of these two varieties is also available in the market, the valid representation and labelling as pure Arabica is often susceptible. It is possible to distinguish these two types of beans through visual inspection; however, for coffee in ground and roasted form, it is not possible to distinguish in such a way [79,80,81]. Similarly, other commercially valued high quality and rare coffee species, such as Kona coffee grown in Kona Island, Blue Mountain, Tanzanian Peaberry, and Indonesian palm civet coffee (Kopi luwak), are often substituted by coffee beans with cheap species. Foreign fillers and coffee byproducts are also admixed with pure coffee products [79].
4.2. Substitution of Protein Content
4.2.1. Protein Substitution in Meat
Animal protein, such as egg, gluten, and porcine gelatin are often added to meat products to increase the apparent protein content [67]. Soybean proteins and cereal flours are used in sausages to recover the desired all-meat properties, such as emulsifying capacity, emulsion stability, and water-binding capacity. Plasma protein is a complex mixture of serum albumin, globulins, and fibrinogen that possesses the ability to produce and stabilize foams and emulsions and to form heat-induced gel. It is utilized by the food industry to control the texture and desired structure of processed meat products [82,83]. Enzymes such as fibrinogen and thrombin are used as blood-based binding agents both in chilled and raw states to give a desired mass and shape to processed meat products [71,82]. Thrombin is used in conjunction with blood plasma as meat binders to give the desired shape in minced meat products. Collagen and its denatured form known as gelatin casing, is considered an important molecule. It offers excellent uniformity of appearance and strength and is usually used to fix the size and shape of processed meat products [84]. Gelatin solution is often injected into cured meats, which solidifies and increases water retention and resistance to cutting. Again, being a potential source of cheap and high-quality protein, chickpea flour added to comminuted meats increases cooking varieties and provides a softer texture that becomes commercially more valuable. Addition of organic compounds, such as melamine, milk, and urea, are also reported [85].
4.2.2. Protein Substitution in Milk
Milk protein, which is considered a precursor of many bioactive peptides with antimicrobial activity, is not only a good source of calcium, zinc, copper, and phosphate ions but also helps in the absorption of many other nutrients [86,87,88]. Compounds with high nitrogen content can mimic a high protein concentration [88]. As the non-protein nitrogen cannot be distinguished by Kjeldhal and Dumas methods used for the determination of total protein content, the addition of various nitrogenous compounds in dairy products to increase the apparent protein content is quite frequent. In this case, melamine, urea, and whey are the most reported agents [88,89].
Addition of urea has also been reported several times. A low concentration of urea is naturally present in milk that generally comes from the grass or feed given to dairy cattle [45,90]. Due to being comparatively cheaper than other alternatives, it is extensively used to increase the apparent protein content [60,76]. If the amount of urea is found greater than the permissible limit of 10–16 mg/dL, it simply indicates an external addition [90].
Whey, being produced in large volume as a cheap byproduct of cheese and caseinates manufacturing, is added to liquid milk as whey protein to increase both the protein content and the total volume [60,76]. The fraudulent addition of rennet whey solids has been reported several times. As cheese whey costs four to five times less than milk and does not affect the sensory properties, it is a lucrative option for dishonest traders [91,92].
Vegetable proteins, such as low-grade soya powder, pea, and wheat also contribute to increasing the protein content of milk and are a convenient option due to being cheap and easily available. Rice and almond proteins are sold as milk supplements for consumers possessing lactose intolerance [15,93].
4.2.3. Protein Substitution in Egg and Egg-Derived Foods
For eggs, melamine is a preferred compound to mimic proteins due to its nitrogen content of approximately 66.7%. Similarly, egg powder and some other egg-derived food products are also at risk of being contaminated with melamine to increase the apparent protein content for uplifting their market value [94].
4.2.4. Protein Substitution in Staple Foods
Staple foods undergo various types of substitution involving quality discrimination. One of the most well-known substitution cases is the admixture of gluten containing cereals in gluten-free products. Gluten induces allergenic reactions to many individuals for whom gluten-free food has gained much popularity. However, many traders violate the regulation of compulsory declaration of the presence of elements prone to create allergenic reaction through the fraudulent admixing of gluten-containing cereals in gluten-free diets [95,96,97]. Peanuts are also another allergen mixed into bulk cereals, legumes, oilseeds, and bulk samples [98]. Lupin flour is also used as a soybean protein substitute [99]. In addition, wheat flour, gluten, and soybeans are frequently adulterated with melamine to increase their apparent protein content [96].
4.3. Substitution of Fat Content
4.3.1. Fat Substitution in Milk
Being one of the major constituents of milk, the milk fat consisting of 97–98% triacylglycerols is present in all dairy products that contain milk and accounts for 3 to 5% in m/m of cow’s milk [60,100]. However, milk fat is costlier than most other edible fats; thus, production of milk derivatives is also an expensive process. Therefore, manufacturers often replace it with cheaper fats, such as vegetable fats or animal fats, not only for reducing manufacturing cost and to achieve an additional economic benefit but also to hide the effect of fraudulent dilution [15,76,101].
4.3.2. Substitution of Oil and Fat Content in Oil and Fat
Due to higher market value and limited production, some oils are more at risk of being adulterated. Extra virgin olive oil is high quality olive oil [102,103]. It is an important source of fatty acids and natural antioxidants [104]. It has a high market value for its nutritional property, excellent taste, and aroma. However, the market price difference between extra virgin olive oil and other edible oils encourages the dishonest traders to adulterate it with other cheaper oils with similar fatty acid and sterol profiles. Hazelnut oil, seed oils, esterified oils, refined olive oils, and olive–pomace oils are examples of such oils [101,105,106]. Virgin coconut oil is another type of high-value oil, which is often adulterated with palm kernel oil, palm oil, and lard. Cod liver oil possesses some therapeutic effects on human health due to the presence of fatty acids and other nutritional values. It is usually adulterated with animal fats, especially lard, chicken fat, mutton fat, and beef fat [103]. Pure sesame oil, having a unique flavor and high nutrition, is adulterated with various cheaper oils, such as soybean oil, corn oil, rapeseed oil, and palm oil [106]. In this category, the other possible adulterations are adulteration of soybean oil to groundnut and sunflower seed oil, adulteration of sunflower seed oil to safflower oil, adulteration of borage oil to evening promise oil [107], adulteration of rice bran oil to mustard oil [107], and adulteration of argemone Mexican seed oil to other edible oils. Mineral oils, such as sesame oil, linseed oil, cottonseed oil, and castor oil are found in high-value oils. Minimally processed oils or cod-pressed oils are often replaced by refined oils. Adulteration of vegetable oil with lard and beef tallow to minimize production cost of margarines and shortenings is also reported [107].
4.4. Food Dilution
Food dilution is the addition of a cheaper ingredient to a high-value food stuff without declaring it [20]. Dilution and overdilution are widely practiced adulterations. Not only liquid but also solid food items are prone to dilution with water or other liquids. This phenomenon is conventionally confirmed by refractometric Brix determination or density measurements. It is the most common form of adulteration in liquid milk [45]. It affects the density, refractive index of lactoserum, and freezing point of adulterated milk items [76]. Honey and other sweeteners are also often diluted with water, which causes honey to deteriorate faster during storage. Therefore, honey overdiluted with water lacks proper consistency and nutritional value [37]. Meat products are also prone to such activity. The lean meat acquires a high water-binding capacity after being chopped and, thus, absorbs a large quantity of water, which has been claimed to give necessary consistency for stuffing into thin cases. It was detected frequently in frankfurters, bologna, and pork sausages [85]. Technology is used to fraudulently increase the weight of fish and seafood in order to make extra economic benefit. Over-treating, which means over-breeding or over-glazing, soaking fish in brine solution, injecting chemicals to increase muscle water-holding capacity, injection of fish byproducts back into the fillet, and water addition are such instances [14,64].
Fruit juices in high demand are reported to be substituted and adulterated using cheap fruit juice [49]. Orange juice is adulterated with other citrus juices when their prices fall [108]. Adulteration of pomegranate juice with natural grape pigments to represent the actual color is practiced. Adulteration with poor quality juice and peel extract is another well-known practice [109]. Commercialization of reconstituted juice made from concentrate as fresh squeezed juice is also practiced [49,110]. The admixing of vinegar derived from a C3 or C4 plant is widely accomplished due to economic advantage and availability [111]. Admixing various proportions of wine vinegars and alcohol vinegars to reduce production cost and selling as pure wine vinegar is another frequently reported malpractice [112].
Edible wheat consists of common wheat and durum wheat [113]. The coarse flour obtained from durum wheat is the primary ingredient of pasta. Pasta made from 100% durum wheat is considered of the highest quality since it imparts unique and firmer dough. Therefore, there is a price difference between these two types of wheats that exists in the market and tempts the manufacturers to admix common wheat with durum wheat [95,96,114]. Due to the growing interest in organic food, organic wheat flour has a huge market demand and is often adulterated with common wheat flour, cassava flour, and corn flour. Their visual detection is quite difficult, as organic wheat flour is nearly the same in color as cassava flour and corn flour [115].
Another widespread adulteration is the admixing of high-quality rice with low quality rice. Rice of different varieties are cultivated which can be admixed fraudulently during the cultivation, harvesting, transporting, and processing [116]. As most varieties of rice are almost similar to look at, their visual discrimination is nearly impossible [117]. Basmati rice, grown in India and Pakistan, holds a prime position among the more than 5000 rice varieties all over the world for its high quality and fragrance. It is sold at 2–3 times higher in price than other varieties in the market [118]. These phenomena provide an incentive for dishonest merchants to adulterate Basmati rice with non-Basmati rice, such as Jasmine rice, long grain rice, etc. [119].
Spices and spices powders are frequently subject to substitution with low quality substances [120]. Table 3 presents spices and their adulterants.

Table 3.
Spices and their adulterants [9,120,121,122].
Dried bread, corn meal, potato starch, crackers, waste biscuit, and boiled rice are usually used as fillers in sausages [85]. Instead of the natural smoking of meat, smoke aromas are fraudulently used [71]. Other additives, such as natural bacon flavors, glycerin, and lecithin from animal fat, are also in use. Alcohol ingredients made from pork fat, such as lard, mono- and diglycerides, sodium stearoyl lactylate, and polysorbate 60 or 80, are also added. Grain or plant-based ingredients with pig-based carrier, such as Beta carotene, are also used [67]. Salt is used to increase the water holding capacity and weight of meat products artificially for economic gain, which causes changes in the secondary structure of the proteins [118].
The substitution adulteration with food items is summarized in Table 4.

Table 4.
Various substitution adulteration with food items.
5. Organic and Synthetic Adulterants
Illegal addition of organic acids, alcohols, and esters in certain food products are frequently reported. Synthetic pharmaceutical compounds and drugs are also added into food items to induce therapeutic effects. Dietary supplement is the most famous food category in this regard, making headlines in newspapers and food crime logs regularly.
5.1. Adulteration with Soymilk
Soymilk possesses similar properties to cow milk. Hence, soymilk is often fraudulently added to cow milk and buffalo milk in preparation of different dairy products [76,126]. The production cost of soymilk is 70% less than pure milk [15]. A combination of urea, vegetable oil, emulsifier, fat, and nitrogen content provide synthetic milk the same color, specific gravity, and consistency of pure buffalo milk and becomes undetectable. Its milky aroma turns it into a commercially valuable product; thus, 5–10% adulteration of dairy milk has been reported [76].
5.2. Adulteration with Organic Acids
Organic acids, such as malic acid, are added to apple juice concentrate to increase commercial value [45]. Unauthorized addition of organic acids, such as citric and tartaric acid, is beneficial because the sensory properties and commercial value of certain type of fruit juices rises with acidity level. Amino acids, such as glycine and glutamic acid or protein hydrolysates, are also added to food items to boost the total amino acid content. Addition of mixture of flavors, organic acids, and sugars is a commonly added chemical cocktail to fruit extract [11].
5.3. Adulteration with Synthetic Acetic Acids
The authenticity issues associated with vinegar is related to the raw material source and manufacturing processes [111]. Synthetic acetic acid is produced from non-biological origins obtained from either petroleum derivatives or by pyrolysis of wood. Synthetic acetic acid is reported to be sold as organic acetic acid or mixed with organic acetic acid to increase the volume [112,127,128].
5.4. Adulteration with Artificial Wine
Some adulterated wines are called artificial wine that consists of components organoleptically perceived as grape wine. Water, yeast, sugar, potassium tartarate, crystalline tartaric and citric acids, tannin, glycerol, ethanol, and ethyl esters of high fatty acids are the typical constituents of artificial wine [42]. Glycerol, diethylene glycol, citric acid, and semi-volatile additives, such as propylene glycol, sorbic acid, and benzoic acid, are also mixed for wine enhancement; other compounds, such as rectified alcohols, components of non-grape origin, and natural and synthetic flavor compounds, are also added [41,42,49].
5.5. Adulteration with Unrecorded and Surrogate Alcohol
Such alcohols represent alcoholic beverages that either do not possess an official registration in the jurisdiction, are manufactured illegally, or are consumed by cross-border trade. Alcohols that are not produced for human consumption, such as medicinal alcohol, disinfectant alcohol, denatured alcohol, synthetic alcohol, and other industrial alcohols, are often added to alcoholic beverages to increase their alcohol content [42].
5.6. Synthetic Pharmaceutical Adulterants
Addition of synthetic pharmaceutical ingredients in dietary supplements is a great concern of the present time. Dietary supplements marketed for various health benefits are fraudulently admixed with pharmaceutical compounds to boost the desired effect on the human body [129].
Various approved pharmaceutical drugs and their analogues, which are often very difficult to detect, have been found in food supplements advertised as a remedy for diseases [129]. The phosphodiesterase type 5 (PDE-5) inhibitors, such as sildenafil citrate, tadalafil hydrochloride, vardenafil hydrochloride, udenafil, mirodenafil hydrochloride, lodenafil carbonate, avanafil, and their unapproved designer analogues, are fraudulently added to herbal supplements. More exotic analogues synthesized by minor modifications to parent structures of approved PDE-5 inhibitors also have been added by traders to make their detection much more difficult [130,131]. In addition, adulteration with optical isomers of tadalafil has also been reported [131,132]. Up to the year of 2018, 80 synthetic PDE-5 inhibitors were found in herbal supplements among which, 62% of sildenafil, 26% of tadalafil, 9% of vardenafil, and 3% of others were reported [133]. Analgesics, such as paracetamol, antihistamines, theophylline, bromhexine, diazepam, chlordiazepoxide, glibenclamide, hydrochlorothiazide, aminopyrine, and phenytoin are frequently found in food supplements. Non-steroidal anti-inflammatory drugs, such as aspirin, mefenamic acid, and phenacetin, have also been reported in several dietary supplements [129,130,134,135,136,137].
To get rid of obesity and extra weight, people purchase several weight-reducing dietary supplements, which are often adulterated with synthetic drugs. Some are controlled by regulatory agencies and others are banned due to their adverse effect on human health [138,139]. Table 5 shows some examples of these adulterants.

Table 5.
Adulterant category for psychiatric issues, obesity, and other health problems with examples [138,139].
Plant food supplements prepared for body-building and athletic performance enhancement are often found adulterated with anabolic steroids or prohormones. Performance-enhancing drugs listed by the World Anti-Doping Agency [140] are defined as pharmacological substances by World Anti-Doping Code and are not allowed for human therapeutic use. Some of those drugs are presented in Table 6.

Table 6.
Adulterant category to improve body-building and athletic performance with examples [139,140,141,142,143,144].
Other related substances are growth hormone, erythropoietin, chorionic gonadotropin, β-2 agonists, hormones, and metabolic modulators, such as aromatase inhibitors and selective estrogen receptor modulators. Cannabinoids and glucocorticosteroids are also prohibited in athletic competition. New, modified, or “designer” steroids are of greatest concern because of the relatively little information available about their pharmacology and probable side effects. The presence of such adulterants poses a major risk for athletes since it results in a positive anti-doping control test, and the World Anti-Doping Agency does not justify whether it was deliberate or accidental doping [139,145].
6. Fraud and Mislabeling
Quality and origin of foodstuffs are of great concern to the consumers as food grown in certain regions and some special species have higher economic value due to their superior quality. Environmental pollution of the geographical origins is also another considerable factor in this regard [146]. Food from high quality batches, species, and cultures are not only often replaced with low quality products but also mislabeled deliberately by the traders for extra profit. Such malpractice violates customers’ rights, reduces the benefits of local cultivators, and creates unfair business competition [147]. Traceability is assured only by labelling and administrative documentation and, thus, is prone to frequent fraudulent practice. The evidence of such issues with fish, seafood, meat, processed meat, and staple foods is found mostly in scientific reports [63,71,117,148]. There are also reports on origin fraud and mislabeling of tea, fruit juice, vinegar, honey, and alcoholic beverages [41,45,47,149]. Expensive honey, such as pine, thyme, orange blossom, chestnut, heather, manuka, acacia, litchi, and linden, are frequently reported for mislabeling with respect to botanical origin [150,151]. The plant-based food “Fava Santorinis” having protected designation of origin (PDO) is often replaced with inferior yellow split peas [152]. ‘Eglouvis’ lentils cultivated in the Ionian Islands is often subject to origin fraud [153].
Misrepresentation of geographical and botanical origin is seen mostly in the case of olive oils and cocoa butter. The quality and constituents of olive oils vary with certain regional characteristics, surroundings, and manufacturing technique, holding different commercial values [104]. For example, some olive cultivars of Olea europaea L. are known to possess better quality because of breeding and selection strategies. Olive oils derived from those regions or cultivars are specified by labelling. Due to their high market value, they are often misrepresented [154,155,156]. Extra virgin olive oil has protected designation of origin (PDO) due to possessing a high content of monounsaturated fatty acids, vitamins, and antioxidants. Due to high commercial value, it is often mislabeled [157,158].
7. Health Impact
There is much historical evidence on the health hazard resulting from food adulteration. Some food adulterants do not participate in health degradation. Those only affect the nutritional parameter and reduce food quality. Species substitution of coffee products is such an example. Other adulterants are potential source of mild to severe illness. The financial loss associated with hospitalization and medication is also not negligible.
Consumption of fruits adulterated with ripening agents have been proved to be carcinogenic to the human body [25]. Other health issues, such as headache, dizziness, nausea, and kidney failure are also visible [32,34,159]. Similarly, artificial coloring agents have shown carcinogenic and genotoxic properties [9]. Artificial sweetening agents have been proved to be clastogenic and genotoxic [42]. Uterine cancer, exhaustion, and loss of energy have been found to be associated with the consumption of growth hormones [29]. Consumption of melamine with food products causes renal failure, kidney stones, and infection in urinary tract [15,88,160]. People consuming food preserved with formaldehyde have fallen victim to disturbances in the nervous system, kidney, liver, and lungs [54].
Substitution of food items can also be health hazardous. Fishery substitution may result into the consumption of illegal poisonous fish species that even cause death [14]. Substituted meat products may cause allergic reactions, diabetes, and cardiovascular diseases [72,161,162]. Evidence of massive death has been found from the consumption of Raki, a Turkish traditional aniseed-flavored distilled spirit [49]. Substitution of spices have resulted into intoxication, neurological, and gastrointestinal problems in children [163]; anaphylaxis, liver, and stomach problems have also been reported [9]. Species substitution in milk and dairy products may cause allergic reactions [164]. Oil substitution may result in gall bladder cancer, epidemic dropsy, glaucoma, loss of eyesight, paralysis, liver damage, and cardiac arrest [105,165].
Some notable food adulterants and their possible health effects are presented in Table 7.

Table 7.
Food adulterants and their possible health effects.
8. Conclusions
Food adulteration has been a major global concern due to its impact on health and economy. For years, food items have been decorated with artificial colorants to attract consumers. Protection of external freshness through the addition of several preservatives have been frequently accomplished by food traders. Deliberate substitution of high-quality food with inferior food products has been a regularly practiced issue. Alteration of nutritional parameters through fraudulent substitution has also been prominent. Food is directly related to health, and any form of alteration in its natural composition should be prohibited. The opportunities existing in favor of food contamination should be analyzed. Lack of proper legislations and their strict application is one of the root causes of rapidly increasing adulteration of food.
Though the food officials from around the world are trying to combat adulteration of food, there still exists some limitations. Adulterated food is usually not identified until it shows a health hazard. In addition, many developing countries still lag in terms of food adulteration analysis techniques. Proper law enforcement and regular inspection of food quality can bring about drastic changes. In the modern time, the contribution of scientists and researchers on food adulteration detection and quantification technologies is highly appreciable. The documentation of ever-increasing fraudulent ideas and practices with food must be kept up to date to cope up with food crimes. Different forms of food adulteration and associated health impacts should be well-documented and analyzed. Considering the detrimental health impacts, food safety and quality assurance is an urgent necessity. Considering the prospects of a rapidly expanding global food market, the regulation of food quality should be of prime importance. In a nutshell, food adulteration is a broad concept and cannot be managed only by the policymakers and executors. The food manufacturers and sellers, along with the customers, should contribute to making their country a safe place to live.
Author Contributions
M.M. contributed to the conceptualization of the study, carried out the literature review, and drafted and finalized the manuscript; S.Y.B. carried out the literature review; M.S.K. conceived the study, supervised the research project and manuscript preparation, contributed to the writing, and reviewed, edited, and finalized the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by BUET CASR Research Fund and ESTex Research Funding.
Data Availability Statement
Data is contained within the article.
Acknowledgments
The authors would like to express gratitude to the Department of Chemical Engineering, BUET and Bangladesh Agriculture Development Corporation (BADC) for technical assistance. The authors would also like to express gratitude to Tahiya Hossain, Islamic University of Technology, for assistance in organizing the manuscript. This article was supported by BUET CASR Research Fund and ESTex Research Fund.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rees, J. Food Adulteration and Food Fraud; Reaktion Books: Chicago, IL, USA, 2020. [Google Scholar]
- Choudhary, A.; Gupta, N.; Hameed, F.; Choton, S. An overview of food adulteration: Concept, sources, impact, challenges and detection. Int. J. Chem. Stud. 2020, 8, 2564–2573. [Google Scholar] [CrossRef]
- Ayza, A.; Belete, E. Food adulteration: Its challenges and impacts. Food Sci. Qual. Manag. 2015, 41, 50–56. [Google Scholar]
- Ayza, A.; Yilma, Z. Patterns of milk and milk products adulteration in Boditti town and its surrounding, South Ethiopia. J. Agric. Sci. 2014, 4, 512–516. [Google Scholar]
- El-Loly, M.M.; Mansour, A.; Ahmed, R. Evaluation of raw milk for common commercial additives and heat treatments. Internet J. Food Saf. 2013, 15, 7–10. [Google Scholar]
- Everstine, K.; Spink, J.; Kennedy, S. Economically motivated adulteration (EMA) of food: Common characteristics of EMA incidents. J. Food Protection. 2013, 76, 723–735. [Google Scholar] [CrossRef] [PubMed]
- Abbas, O.; Zadravec, M.; Baeten, V.; Mikuš, T.; Lešić, T.; Vulić, A.; Prpić, J.; Jemeršić, L.; Pleadin, J. Analytical methods used for the authentication of food of animal origin. Food Chem. 2018, 246, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Fiorino, G.M.; Garino, C.; Arlorio, M.; Logrieco, A.F.; Losito, I.; Monaci, L. Overview on untargeted methods to combat food frauds: A focus on fishery products. J. Food Qual. 2018, 2018, 1–13. [Google Scholar] [CrossRef]
- Galvin-King, P.; Haughey, S.; Elliott, C. Herb and spice fraud; the drivers, challenges and detection. Food Control. 2018, 88, 85–97. [Google Scholar] [CrossRef]
- Silvis, I.; van Ruth, S.; van der Fels-Klerx, H.; Luning, P. Assessment of food fraud vulnerability in the spices chain: An explorative study. Food Control. 2017, 81, 80–87. [Google Scholar] [CrossRef]
- Rinke, P. Tradition Meets High Tech for Authenticity Testing of Fruit Juices, in Advances in Food Authenticity Testing; Elsevier: Amsterdam, The Netherlands, 2016; pp. 625–665. [Google Scholar]
- Ríos-Reina, R.; Callejón, R.M.; Oliver-Pozo, C.; Amigo, J.M.; García-González, D.L. ATR-FTIR as a potential tool for controlling high quality vinegar categories. Food Control. 2017, 78, 230–237. [Google Scholar] [CrossRef]
- Elliott, C. Elliott Review into the Integrity and Assurance of Food Supply Networks–Final Report: A National Food Crime Prevention Framework. HM Government: London, UK, 2014. [Google Scholar]
- Fox, M.; Mitchell, M.; Dean, M.; Elliott, C.; Campbell, K. The seafood supply chain from a fraudulent perspective. Food Secur. 2018, 10, 939–963. [Google Scholar] [CrossRef]
- Azad, T.; Ahmed, S. Common milk adulteration and their detection techniques. Int. J. Food Contam. 2016, 3, 22. [Google Scholar] [CrossRef]
- Kamthania, M.; Saxena, J.; Saxena, K.; Sharma, D.K. Milk Adultration: Methods of Detection &Remedial Measures. Int. J. Eng. Tech. Res. 2014, 1, 15–20. [Google Scholar]
- Visciano, P.; Schirone, M. Food frauds: Global incidents and misleading situations. Trends Food Sci. Technol. 2021, 114, 424–442. [Google Scholar] [CrossRef]
- Kamruzzaman, M. Food Adulteration and Authenticity, in Food Safety; Springer: Berlin/Heidelberg, Germany, 2016; pp. 127–148. [Google Scholar]
- Johnson, R. Food Fraud and “Economically Motivated Adulteration" of Food and Food Ingredients; Congressional Research Service: Washington, DC, USA, 2018.
- Brooks, C.; Parr, L.; Smith, J.M.; Buchanan, D.; Snioch, D.; Hebishy, E. A review of food fraud and food authenticity across the food supply chain, with an examination of the impact of the COVID-19 pandemic and Brexit on food industry. Food Control. 2021, 130, 108171. [Google Scholar] [CrossRef]
- González-Pereira, A.; Otero, P.; Fraga-Corral, M.; Garcia-Oliveira, P.; Carpena, M.; Prieto, M.; Simal-Gandara, J. State-of-the-art of analytical techniques to determine food fraud in olive oils. Foods 2021, 10, 484. [Google Scholar] [CrossRef]
- McGrath, T.F.; Haughey, S.A.; Patterson, J.; Fauhl-Hassek, C.; Donarski, J.; Alewijn, M.; van Ruth, S.; Elliott, C.T. What are the scientific challenges in moving from targeted to non-targeted methods for food fraud testing and how can they be addressed?—Spectroscopy case study. Trends Food Sci. Technol. 2018, 76, 38–55. [Google Scholar] [CrossRef]
- He, Y.; Bai, X.; Xiao, Q.; Liu, F.; Zhou, L.; Zhang, C. Detection of adulteration in food based on nondestructive analysis techniques: A review. Crit. Rev. Food Sci. Nutr. 2021, 61, 2351–2371. [Google Scholar] [CrossRef]
- Pastor, K.; Ačanski, M.; Vujić, D.; Kojić, P. A rapid dicrimination of wheat, walnut and hazelnut flour samples using chemometric algorithms on GC/MS data. J. Food Meas. Charact. 2019, 13, 2961–2969. [Google Scholar] [CrossRef]
- Goonatilake, R. Effects of diluted ethylene glycol as a fruit-ripening agent. Glob. J. Biotechnol. Biochem. 2008, 3, 8–13. [Google Scholar]
- Islam, M.N.; Imtiaz, M.Y.; Alam, S.S.; Nowshad, F.; Shadman, S.A.; Khan, M.S. Artificial ripening on banana (Musa Spp.) samples: Analyzing ripening agents and change in nutritional parameters. Cogent Food Agric. 2018, 4, 1477232. [Google Scholar] [CrossRef]
- Maduwanthi, S.; Marapana, R. Induced ripening agents and their effect on fruit quality of banana. Int. J. Food Sci. 2019, 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Mursalat, M.; Rony, A.H.; Rahman, A.H.M.S.; Islam, M.N.; Khan, M.S. A critical analysis of artificial fruit ripening: Scientific, legislative and socio-economic aspects. Che Thoughts 2013, 3, 1–7. [Google Scholar]
- Panghal, A.; Yadav, D.; Khatkar, B.S.; Sharma, H.; Kumar, V.; Chhikara, N. Post-harvest malpractices in fresh fruits and vegetables: Food safety and health issues in India. Nutr. Food Sci. 2018, 48, 561–578. [Google Scholar] [CrossRef]
- Kathirvelan, J.; Vijayaraghavan, R. An infrared based sensor system for the detection of ethylene for the discrimination of fruit ripening. Infrared Phys. Technol. 2017, 85, 403–409. [Google Scholar] [CrossRef]
- Reyes-Díaz, M.; Lobos, T.; Cardemil, L.; Nunes-Nesi, A.; Retamales, J.; Jaakola, L.; Alberdi, M.; Ribera-Fonseca, A. Methyl jasmonate: An alternative for improving the quality and health properties of fresh fruits. Molecules 2016, 21, 567. [Google Scholar] [CrossRef]
- Ur-Rahman, A.; Chowdhury, F.; Alam, M. Artificial ripening: What we are eating. J. Med. 2008, 9, 42–44. [Google Scholar] [CrossRef]
- Janssen, S.; Schmitt, K.; Blanke, M.; Bauersfeld, M.L.; Wöllenstein, J.; Lang, W. Ethylene detection in fruit supply chains. Philos. Trans. R. Soc. A: Math. Phys. Eng. Sci. 2014, 372, 20130311. [Google Scholar] [CrossRef]
- Bhattarai, U.K.; Shrestha, K. Use of calcium carbide for artificial ripening of fruits: Its application and hazards. J. Food Sci. Technol. Nepal. 2005, 1, 3–6. [Google Scholar]
- Shaon, S.M.; Zaman, P.; Mahmud, S.M.N.; Shanzana, P.; Rajib, R.I.; Rahman, T.; Islam, M.-A.; Sardar, M.-R.; Kabir, M.-H.; Lira, S.A. Detection of many health hazardous chemicals used in tomato marketing in Bangladesh. Br. Biotechnol. J. 2016, 15, 1–12. [Google Scholar] [CrossRef]
- Silva, B.M.; Seabra, R.M.; Andrade, P.B.; Oliveira, M.B.; Ferreira, M.A. Adulteração por adição de açúcares a sumos de frutos: Uma revisão adulteration of fruit juice by addition of sugars: A review adulteración por adición de azúcares a zumos de frutas: Una revisión. CYTA-J. Food 1999, 2, 184–191. [Google Scholar] [CrossRef]
- Wu, L.; Du, B.; Heyden, Y.V.; Chen, L.; Zhao, L.; Wang, M.; Xue, X. Recent advancements in detecting sugar-based adulterants in honey—A challenge. TrAC Trends Anal. Chem. 2017, 86, 25–38. [Google Scholar] [CrossRef]
- Naila, A.; Flint, S.H.; Sulaiman, A.; Ajit, A.; Weeds, Z. Classical and novel approaches to the analysis of honey and detection of adulterants. Food Control. 2018, 90, 152–165. [Google Scholar] [CrossRef]
- Bogdanov, S.; Imdorf, A.; Charriere, J.; Fluri, P.; & Kilchenmann, V. The Contaminants of the Bee Colony; Swiss Bee Research Centre: Bern, Switzerland, 2003; p. 12. [Google Scholar]
- Zábrodská, B.; Vorlová, L. Adulteration of honey and available methods for detection—A review. Acta Vet. Brno. 2015, 83, 85–102. [Google Scholar] [CrossRef]
- Siddiqui, A.J.; Musharraf, S.; Choudhary, M. Application of analytical methods in authentication and adulteration of honey. Food Chem. 2017, 217, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Raychaudhuri, U.; Chakraborty, R. Artificial sweeteners—A review. J. Food Sci. Technol. 2014, 51, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Whitehouse, C.R.; Boullata, J.; McCauley, L. The potential toxicity of artificial sweeteners. Aaohn J. 2008, 56, 251–261. [Google Scholar] [CrossRef]
- Sagandykova, G.N.; Alimzhanova, M.B.; Nurzhanova, Y.T.; Kenessov, B. Determination of semi-volatile additives in wines using SPME and GC–MS. Food Chem. 2017, 220, 162–167. [Google Scholar] [CrossRef]
- Lachenmeier, D. Advances in the Detection of the Adulteration of Alcoholic Beverages including Unrecorded Alcohol, in Advances in Food Authenticity Testing; Elsevier: Amsterdam, The Netherlands, 2016; pp. 565–584. [Google Scholar]
- Lachenmeier, D.W.; Sohnius, E.M.; Attig, R.; López, M.G. Quantification of selected volatile constituents and anions in Mexican Agave spirits (Tequila, Mezcal, Sotol, Bacanora). J. Agric. Food Chem. 2006, 54, 3911–3915. [Google Scholar] [CrossRef]
- Kamiloglu, S. Authenticity and traceability in beverages. Food Chem. 2019, 277, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Sezgin, A.C.; Ayyıldız, S. Food additives: Colorants. In Science within Food: Up-to-Date Advances on Research and Educational Ideas; Formatex Research Center: Badajoz, Spain, 2017. [Google Scholar]
- Akramzadeh, N.; Hosseini, H.; Pilevar, Z.; Khosroshahi, N.K.; Khosravi-Darani, K.; Komeyli, R.; Barba, F.J.; Pugliese, A.; Poojary, M.M.; Khaneghah, A.M. Physicochemical properties of novel non-meat sausages containing natural colorants and preservatives. J. Food Process. Preserv. 2018, 42, e13660. [Google Scholar] [CrossRef]
- He, L.; Su, Y.; Shen, X.; Zeng, Z.; Liu, Y. Determination of Sudan dye residues in eggs by liquid chromatography and gas chromatography-mass spectrometry. Anal. Chim. Acta. 2007, 594, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Jha, S.N.; Jaiswal, P.; Grewal, M.K.; Gupta, M.; Bhardwaj, R. Detection of adulterants and contaminants in liquid foods—A review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1662–1684. [Google Scholar] [CrossRef] [PubMed]
- Barui, A.K.; Sharma, R.; Rajput, Y.S.; Singh, S. A rapid paper chromatographic method for detection of anionic detergent in milk. J. Food Sci. Technol. 2013, 50, 826–829. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lubis, H.N.; Mohd-Naim, N.F.; Alizul, N.N.; Ahmed, M.U. From market to food plate: Current trusted technology and innovations in halal food analysis. Trends Food Sci. Technol. 2016, 58, 55–68. [Google Scholar] [CrossRef]
- Nowshad, F.; Islam, M.; Khan, M. Concentration and formation behavior of naturally occurring formaldehyde in foods. Agric. Food Secur. 2018, 7, 17. [Google Scholar] [CrossRef]
- Jinadasa, B.; Elliott, C.; Jayasinghe, G. A review of the presence of formaldehyde in fish and seafood. Food Control. 2022, 1136, 108882. [Google Scholar] [CrossRef]
- Nasreen, S.; Ahmed, T. Food adulteration and consumer awareness in Dhaka City 1995-2011. J. Health Popul. Nutr. 2014, 32, 452. [Google Scholar]
- Rahman, M.M.; Ahmed, S.; Hosen, M.M.; Talukder, A.K. Detection of formalin and quality characteristics of selected fish from wet markets at Sylhet city in Bangladesh. Bangladesh Res. Publ. J. 2012, 7, 161–169. [Google Scholar]
- Mohanty, B.P.; Mahanty, A.; Parida, P.; Parija, S.; Das, B. Formaldehyde adulteration in fish as a public health concern and need for mass awarness. J. Inland Fish. Soc. India. 2018, 50, 71–74. [Google Scholar]
- Singh, P.; Gandhi, N. Milk preservatives and adulterants: Processing, regulatory and safety issues. Food Rev. Int. 2015, 31, 236–261. [Google Scholar] [CrossRef]
- Nascimento, C.F.; Santos, P.M.; Pereira-Filho, E.R.; Rocha, F.R. Recent advances on determination of milk adulterants. Food Chem. 2017, 221, 1232–1244. [Google Scholar] [CrossRef] [PubMed]
- Amit, S.K.; Uddin, M.; Rahman, R.; Islam, S.M.R.; Khan, M.S. A review on mechanisms and commercial aspects of food preservation and processing. Agric. Food Secur. 2017, 6, 1–22. [Google Scholar] [CrossRef]
- Panda, H. Aloe Vera Handbook Cultivation, Research Finding, Products, Formulations, Extraction & Processing; Niir Project Consultancy Services: Delhi, India, 2003. [Google Scholar]
- Ghidini, S.; Varrà, M.; Zanardi, E. Approaching authenticity issues in fish and seafood products by qualitative spectroscopy and chemometrics. Molecules 2019, 24, 1812. [Google Scholar] [CrossRef]
- Jennings, S.; Stentiford, G.D.; Leocadio, A.M.; Jeffery, K.R.; Metcalfe, J.D.; Katsiadaki, I.; Auchterlonie, N.A.; Mangi, S.C.; Pinnegar, J.K.; Ellis, T.; et al. Aquatic food security: Insights into challenges and solutions from an analysis of interactions between fisheries, aquaculture, food safety, human health, fish and human welfare, economy and environment. Fish and Fisheries. 2016, 17, 893–938. [Google Scholar] [CrossRef]
- Ortea, I.; Pascoal, A.; Cañas, B.; Gallardo, J.M.; Barros-Velázquez, J.; Calo-Mata, P. Food authentication of commercially-relevant shrimp and prawn species: From classical methods to foodomics. Electrophoresis 2012, 33, 2201–2211. [Google Scholar] [CrossRef] [PubMed]
- Gil, L.A. PCR-based methods for fish and fishery products authentication. Trends Food Sci. Technol. 2007, 18, 558–566. [Google Scholar]
- Nakyinsige, K.; Man, Y.; Sazili, A. Halal authenticity issues in meat and meat products. Meat Sci. 2012, 91, 207–214. [Google Scholar] [CrossRef]
- Park, B.; Yoon, S.-C.; Windham, W.R.; Lawrence, K.C.; Kim, M.S.; Chao, K. Line-scan hyperspectral imaging for real-time in-line poultry fecal detection. Sens. Instrum. Food Qual. Saf. 2011, 5, 25–32. [Google Scholar] [CrossRef]
- Kamruzzaman, M.; Makino, Y.; Oshita, S. Non-invasive analytical technology for the detection of contamination, adulteration, and authenticity of meat, poultry, and fish: A review. Anal. Chim. Acta. 2015, 853, 19–29. [Google Scholar] [CrossRef]
- Zhao, M.; Downey, G.; O’Donnell, C. Detection of adulteration in fresh and frozen beefburger products by beef offal using mid-infrared ATR spectroscopy and multivariate data analysis. Meat Sci. 2014, 96, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Ballin, N.Z. Authentication of meat and meat products. Meat Sci. 2010, 86, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Inbaraj, B.S.; Chen, B. Nanomaterial-based sensors for detection of foodborne bacterial pathogens and toxins as well as pork adulteration in meat products. J. Food Drug Anal. 2016, 24, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.E.; Hashim, U.; Mustafa, S.; Man, Y.B.C.; Islam, K.N. Gold nanoparticle sensor for the visual detection of pork adulteration in meatball formulation. J. Nanomater. 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- Sampson, H.A. 9. Food allergy. J. Allergy Clin. Immunol. 2003, 111, S540–S547. [Google Scholar] [CrossRef] [PubMed]
- Hurley, I.P.; Ireland, H.E.; Coleman, R.C.; Williams, J.H.H. Application of immunological methods for the detection of species adulteration in dairy products. Int. J. Food Sci. Technol. 2004, 39, 873–878. [Google Scholar] [CrossRef]
- Poonia, A.; Jha, A.; Sharma, R.; Singh, H.B.; Rai, A.K.; Sharma, N. Detection of adulteration in milk: A review. Int. J. Dairy Technol. 2017, 70, 23–42. [Google Scholar] [CrossRef]
- Zachar, P.; Šoltés, M.; Kasarda, R.; Novotný, J.; Novikmecová, M.; Marcinčáková, D. Analytical methods for the species identification of milk and milk products. Mljekarstvo Časopis Za Unaprjeđenje Proizv. I Prerade Mlijeka. 2011, 61, 199–207. [Google Scholar]
- Nikolaou, P.; Deskoulidis, E.; Topoglidis, E.; Kakoulidou, A.T.; Tsopelas, F. Application of chemometrics for detection and modeling of adulteration of fresh cow milk with reconstituted skim milk powder using voltammetric fingerpriting on a graphite/SiO2 hybrid electrode. Talanta 2020, 206, 120223. [Google Scholar] [CrossRef]
- Arbin, D.F.; de Souza Madureira Felicio, A.L.; Sun, D.-W.; Nixdorf, S.L.; Hirooka, E.Y. Application of infrared spectral techniques on quality and compositional attributes of coffee: An overview. Food Res. Int. 2014, 61, 23–32. [Google Scholar] [CrossRef]
- Burns, D.T.; Walker, M. Critical review of analytical and bioanalytical verification of the authenticity of coffee. J. AOAC Int. 2020, 103, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Toci, A.T.; Farah, A.; Pezza, H.R.; Pezza, L. Coffee adulteration: More than two decades of research. Crit. Rev. Anal. Chem. 2016, 46, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Herrero, A.; Cambero, M.; Ordóñez, J.; de la Hoz, L.; Carmona, P. Plasma powder as cold-set binding agent for meat system: Rheological and Raman spectroscopy study. Food Chem. 2009, 113, 493–499. [Google Scholar] [CrossRef]
- Saguer, E.; Dàvila, E.; Toldrà, M.; Fort, N.; Baixas, S.; Carretero, C.; Parés, D. Effectiveness of high pressure processing on the hygienic and technological quality of porcine plasma from biopreserved blood. Meat Sci. 2007, 76, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Guillén, M.; Giménez, B.; López-Caballero, M.; Montero, M. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef]
- Barai, B.; Nayak, R.; Singhal, R.; Kulkarni, P. Approaches to the detection of meat adulteration. Trends Food Sci. Technol. 1992, 3, 69–72. [Google Scholar] [CrossRef]
- Claeys, W.L.; Cardoen, S.; Daube, G.; De Block, J.; Dewettinck, K.; Dierick, K.; De Zutter, L.; Huyghebaert, A.; Imberechts, H.; Thiange, P.; et al. Raw or heated cow milk consumption: Review of risks and benefits. Food Control. 2013, 31, 251–262. [Google Scholar] [CrossRef]
- Ebringer, L.; Ferenčík, M.; Krajčovič, J. Beneficial health effects of milk and fermented dairy products. Folia Microbiol. 2008, 53, 378–394. [Google Scholar] [CrossRef]
- Domingo, E.; Tirelli, A.A.; Nunes, C.A.; Guerreiro, M.C.; Pinto, S.M. Melamine detection in milk using vibrational spectroscopy and chemometrics analysis: A review. Food Res. Int. 2014, 60, 131–139. [Google Scholar] [CrossRef]
- Hau, A.K.-C.; Kwan, T.; Li, P.-T. Melamine toxicity and the kidney. J. Am. Soc. Nephrol. 2009, 20, 245–250. [Google Scholar] [CrossRef]
- Abdallah, M.I.M.I. Determination of Urea in COW’S Milk Sold in Damietta GOVERNORATE. J. Egypt. Vet. Med. Assoc. 2008, 69. [Google Scholar]
- Martín-Hernández, C.; Muñoz, M.; Daury, C.; Weymuth, H.; Kemmers-Voncken, A.E.; Corbatón, V.; Toribio, T.; Bremer, M.G. Immunochromatographic lateral-flow test strip for the rapid detection of added bovine rennet whey in milk and milk powder. Int. Dairy J. 2009, 19, 205–208. [Google Scholar] [CrossRef]
- Sharma, R.; Rajput, Y.; Mann, B. Chemical and functional properties of glycomacropeptide (GMP) and its role in the detection of cheese whey adulteration in milk: A review. Dairy Sci. Technol. 2013, 93, 21–43. [Google Scholar]
- Scholl, P.F.; Farris, S.; Mossoba, M.M. Rapid turbidimetric detection of milk powder adulteration with plant proteins. J. Agric. Food Chem. 2014, 62, 1498–1505. [Google Scholar] [CrossRef] [PubMed]
- Mondal, A.M.R.; Desmarchelier, A.; Konings, E.; Acheson-Shalom, R.; Delatour, T. Liquid chromatography—Tandem mass spectrometry (LC-MS/MS) method extension to quantify simultaneously melamine and cyanuric acid in egg powder and soy protein in addition to milk products. J. Agric. Food Chem. 2010, 58, 11574–11579. [Google Scholar] [CrossRef] [PubMed]
- Pastor, K.; Ačanski, M.; Vujić, D. A review of adulteration versus authentication of flour. Flour Breads Fortif. Health Dis. Prev. 2019, 2019, 21–35. [Google Scholar]
- Delwiche, S. Advances in the Identification of Adulterated Cereals and Cereal Products. In Advances in Food Authenticity Testing; Elsevier: Amsterdam, The Netherlands, 2016; p. 491518. [Google Scholar]
- Colgrave, M.L.; Byrne, K.; Blundell, M.; Howitt, C. Identification of barley-specific peptide markers that persist in processed foods and are capable of detecting barley contamination by LC-MS/MS. J. Proteom. 2016, 147, 169–176. [Google Scholar] [CrossRef]
- Ghosh, S.; Mishra, P.; Mohamad, S.N.H.; de Santos, R.M.; Iglesias, B.D.; Elorza, P.B. Discrimination of peanuts from bulk cereals and nuts by near infrared reflectance spectroscopy. Biosyst. Eng. 2016, 151, 178–186. [Google Scholar] [CrossRef]
- Scarafoni, A.; Ronchi, A.; Duranti, M. A real-time PCR method for the detection and quantification of lupin flour in wheat flour-based matrices. Food Chem. 2009, 115, 1088–1093. [Google Scholar] [CrossRef]
- Yoshinaga, K.; Nagai, T.; Mizobe, H.; Kojima, K.; Gotoh, N. Simple method for the quantification of milk fat content in foods by LC-APCI-MS/MS using 1, 2-dipalmitoyl-3-butyroyl-glycerol as an indicator. J. Oleo Sci. 2013, 62, 115–121. [Google Scholar] [CrossRef][Green Version]
- Azadmard-Damirchi, S.; Torbati, M. Adulterations in some edible oils and fats and their detection methods. J. Food Qual. Hazards Control. 2015, 2, 38–44. [Google Scholar]
- Huang, C.L.; Sumpio, B. Olive oil, the mediterranean diet, and cardiovascular health. J. Am. Coll. Surg. 2008, 207, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Rohman, A.; Man, Y. Application of Fourier transform infrared spectroscopy for authentication of functional food oils. Appl. Spectrosc. Rev. 2012, 47, 1–13. [Google Scholar] [CrossRef]
- Dais, P.; Hatzakis, E. Quality assessment and authentication of virgin olive oil by NMR spectroscopy: A critical review. Anal. Chim. Acta. 2013, 765, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S. Edible oil adulterations: Current issues, detection techniques, and health hazards. IJCS 2018, 6, 1393–1397. [Google Scholar]
- Nunes, C.A. Vibrational spectroscopy and chemometrics to assess authenticity, adulteration and intrinsic quality parameters of edible oils and fats. Food Res. Int. 2014, 60, 255–261. [Google Scholar] [CrossRef]
- Shukla, A.; Dixit, A.; Singh, R. Detection of adulteration in edible oils. J. Oleo Sci. 2005, 54, 317–324. [Google Scholar] [CrossRef]
- Nuncio-Jáuregui, N.; Calín-Sánchez, Á.; Hernández, F.; Carbonell-Barrachina, Á.A. Pomegranate juice adulteration by addition of grape or peach juices. J. Sci. Food Agric. 2014, 94, 646–655. [Google Scholar] [CrossRef]
- Hong, E.; Lee, S.Y.; Jeong, J.Y.; Park, J.M.; Kim, B.H.; Kwon, K.; Chun, H.S. Modern analytical methods for the detection of food fraud and adulteration by food category. J. Sci. Food Agric. 2017, 97, 3877–3896. [Google Scholar] [CrossRef]
- Włodarska, K.; Khmelinskii, I.; Sikorska, E. Authentication of apple juice categories based on multivariate analysis of the synchronous fluorescence spectra. Food Control. 2018, 86, 42–49. [Google Scholar] [CrossRef]
- Boffo, E.F.; Tavares, L.A.; Ferreira, M.M.; Ferreira, A.G. Classification of Brazilian vinegars according to their 1H NMR spectra by pattern recognition analysis. LWT-Food Sci. Technol. 2009, 42, 1455–1460. [Google Scholar] [CrossRef]
- Mazáč, A.G.H.Č.J.; Voldřich, M. Authenticity and quality of spirit vinegar: Methods for detection of synthetic acetic acid addition. J. Food Nutr. Res. 2012, 51, 123–131. [Google Scholar]
- Zapotoczny, P. Discrimination of wheat grain varieties using image analysis and neural networks. Part I. Single Kernel Texture. J. Cereal Sci. 2011, 54, 60–68. [Google Scholar]
- Ambrose, A.; Cho, B.-K. A review of technologies for detection and measurement of adulterants in cereals and cereal products. J. Biosyst. Eng. 2014, 39, 357–365. [Google Scholar] [CrossRef]
- Su, W.-H.; Sun, D.-W. Evaluation of spectral imaging for inspection of adulterants in terms of common wheat flour, cassava flour and corn flour in organic Avatar wheat (Triticum spp.) flour. J. Food Eng. 2017, 200, 59–69. [Google Scholar] [CrossRef]
- Kuo, T.-Y.; Chung, C.-L.; Chen, S.-Y.; Lin, H.-A.; Kuo, Y.-F. Identifying rice grains using image analysis and sparse-representation-based classification. Comput. Electron. Agric. 2016, 127, 716–725. [Google Scholar] [CrossRef]
- Vemireddy, L.R.; Satyavathi, V.V.; Siddiq, E.A.; Nagaraju, J. Review of methods for the detection and quantification of adulteration of rice: Basmati as a case study. J. Food Sci. Technol. 2015, 52, 3187–3202. [Google Scholar] [CrossRef]
- Valdés, A.; Beltrán, A.; Mellinas, C.; Jiménez, A.; Garrigós, M.C. Analytical methods combined with multivariate analysis for authentication of animal and vegetable food products with high fat content. Trends Food Sci. Technol. 2018, 77, 120–130. [Google Scholar] [CrossRef]
- Melini, V.; Melini, F. Asian grain-based food products and the European scheme for food protected designations of origin: A critical analysis. Trends Food Sci. Technol. 2019, 91, 83–94. [Google Scholar] [CrossRef]
- Dhanya, K.; Sasikumar, B. Molecular marker based adulteration detection in traded food and agricultural commodities of plant origin with special reference to spices. Curr. Trends Biotechnol. Pharm. 2010, 4, 454–489. [Google Scholar]
- Kuballa, T.; Brunner, T.S.; Thongpanchang, T.; Walch, S.G.; Lachenmeier, D.W. Application of NMR for authentication of honey, beer and spices. Curr. Opin. Food Sci. 2018, 19, 57–62. [Google Scholar] [CrossRef]
- Ravindran, P.N.; Kallupurackal, J.A. Black Pepper, in Handbook of Herbs and Spices; Elsevier: Amsterdam, The Netherlands, 2012; pp. 86–115. [Google Scholar]
- Moatsou, G.; Anifantakis, E. Recent developments in antibody-based analytical methods for the differentiation of milk from different species. Int. J. Dairy Technol. 2003, 56, 133–138. [Google Scholar] [CrossRef]
- Rani, A.; Sharma, V.; Arora, S.; Lal, D.; Kumar, A. A rapid reversed-phase thin layer chromatographic protocol for detection of adulteration in ghee (clarified milk fat) with vegetable oils. J. Food Sci. Technol. 2015, 52, 2434–2439. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, N.; Kumar, A.; Rathod, G.; Goyal, A.; Lal, D. Development of a method employing reversed-phase thin-layer chromatography for establishing milk fat purity with respect to adulteration with vegetable oils. Int. J. Dairy Technol. 2015, 68, 207–217. [Google Scholar] [CrossRef]
- Sharma, R.; Rajput, Y.S.; Poonam; Dogra, G.; Tomar, S.K. Estimation of sugars in milk by HPLC and its application in detection of adulteration of milk with soymilk. Int. J. Dairy Technol. 2009, 62, 514–519. [Google Scholar] [CrossRef]
- Hsieh, C.; Li, P.-H.; Cheng, J.-Y.; Ma, J.-T. Using SNIF-NMR method to identify the adulteration of molasses spirit vinegar by synthetic acetic acid in rice vinegar. Ind. Crops Prod. 2013, 50, 904–908. [Google Scholar] [CrossRef]
- Thomas, F.; Jamin, E. 2H NMR and 13C-IRMS analyses of acetic acid from vinegar, 18O-IRMS analysis of water in vinegar: International collaborative study report. Anal. Chim. Acta. 2009, 649, 98–105. [Google Scholar] [CrossRef]
- Haneef, J.; Shaharyar, M.; Husain, A.; Rashid, M.; Mishra, R.; Siddique, N.A.; Pal, M. Analytical methods for the detection of undeclared synthetic drugs in traditional herbal medicines as adulterants. Drug Test. Anal. 2013, 5, 607–613. [Google Scholar] [CrossRef]
- Vaclavik, L.; Krynitsky, A.; Rader, J. Mass spectrometric analysis of pharmaceutical adulterants in products labeled as botanical dietary supplements or herbal remedies: A review. Anal. Bioanal. Chem. 2014, 406, 6767–6790. [Google Scholar] [CrossRef]
- Patel, D.N.; Li, L.; Kee, C.L.; Ge, X.; Low, M.Y.; Koh, H.L. Screening of synthetic PDE-5 inhibitors and their analogues as adulterants: Analytical techniques and challenges. J. Pharm. Biomed. Anal. 2014, 87, 176–190. [Google Scholar] [CrossRef]
- Venhuis, B.J.; Zomer, G.; Vredenbregt, M.J.; De Kaste, D. The identification of (−)-trans-tadalafil, tadalafil, and sildenafil in counterfeit Cialis® and the optical purity of tadalafil stereoisomers. J. Pharm. Biomed. Anal. 2010, 51, 723–727. [Google Scholar] [CrossRef]
- Kee, C.L.; Ge, X.; Gilard, V.; Malet-Martino, M.; Low, M.Y. A review of synthetic phosphodiesterase type 5 inhibitors (PDE-5i) found as adulterants in dietary supplements. J. Pharm. Biomed. Anal. 2018, 147, 250–277. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, J.H.; Park, H.J.; Kim, J.-Y.; Cho, S.; Kim, W.S. Determination of non-opioid analgesics in adulterated food and dietary supplements by LC-MS/MS. Food Addit. Contam. Part A. 2014, 31, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Calahan, J.; Howard, D.; Almalki, A.J.; Gupta, M.P.; Calderón, A.I. Chemical adulterants in herbal medicinal products: A review. Planta Medica. 2016, 82, 505–515. [Google Scholar] [CrossRef]
- Byard, R.W. A review of the potential forensic significance of traditional herbal medicines. J. Forensic Sci. 2010, 55, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Venhuis, B.; De Kaste, D. Towards a decade of detecting new analogues of sildenafil, tadalafil and vardenafil in food supplements: A history, analytical aspects and health risks. J. Pharm. Biomed. Anal. 2012, 69, 196–208. [Google Scholar] [CrossRef]
- de Carvalho, L.M.; Martini, M.; Moreira, A.P.L.; de Lima, A.P.S.; Correia, D.; Falcão, T.; Garcia, S.C.; de Bairros, A.V.; Nascimento, P.C.D.; Bohrer, D. Presence of synthetic pharmaceuticals as adulterants in slimming phytotherapeutic formulations and their analytical determination. Forensic Sci. Int. 2011, 204, 6–12. [Google Scholar] [CrossRef]
- Rocha, T.; Amaral, J.; Oliveira, M.B. Adulteration of dietary supplements by the illegal addition of synthetic drugs: A review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 43–62. [Google Scholar] [CrossRef]
- Hallur, V.; Atharga, B.; Hosur, A.; Binjawadagi, B.; Bhat, K. Design and development of a portable instrument for the detection of artificial ripening of banana fruit. In Proceedings of the International Conference on Circuits, Communication, Control and Computing, Bangalore, India, 21–22 November 2014; IEEE: New York, NY, USA, 2014. [Google Scholar]
- Geyer, H.; Parr, M.K.; Koehler, K.; Mareck, U.; Schänzer, W.; Thevis, M. Nutritional supplements cross-contaminated and faked with doping substances. J. Mass Spectrom. 2008, 43, 892–902. [Google Scholar] [CrossRef]
- Becue, I.; Poucke, C.; Peteghem, C. An LC-MS screening method with library identification for the detection of steroidsin dietary supplements. J. Mass Spectrom. 2011, 46, 327–335. [Google Scholar] [CrossRef]
- Aqai, P.; Cevik, E.; Gerssen, A.; Haasnoot, W.; Nielen, M.W.F. High-throughput bioaffinity mass spectrometry for screening and identification of designer anabolic steroids in dietary supplements. Anal. Chem. 2013, 85, 3255–3262. [Google Scholar] [CrossRef]
- Odoardi, S.; Castrignanò, E.; Martello, S.; Chiarotti, M.; Strano-Rossi, S. Determination of anabolic agents in dietary supplements by liquid chromatography-high-resolution mass spectrometry. Food Addit. Contam. Part A 2015, 32, 635–647. [Google Scholar] [CrossRef]
- Hughes, D. The world anti-doping code in sport: Update for 2015. Aust. Prescr. 2015, 38, 167. [Google Scholar] [CrossRef]
- Gu, X.; Zhang, L.; Li, L.; Ma, N.; Tu, K.; Song, L.; Pan, L. Multisource fingerprinting for region identification of walnuts in Xinjiang combined with chemometrics. J. Food Process Eng. 2018, 41, e12687. [Google Scholar] [CrossRef]
- Zhang, J.; Nie, J.; Kuang, L.; Shen, Y.; Zheng, H.; Zhang, H.; Farooq, S.; Asim, S.; Shah, B.S.A. Geographical origin of Chinese apples based on multiple element analysis. J. Sci. Food Agric. 2019, 99, 6182–6190. [Google Scholar] [CrossRef]
- Singh, C.B.; Paliwal, J.; Jayas, D.S.; White, N. Near-infrared spectroscopy: Applications in the grain industry. In Proceedings of the 2006 ASAE Annual Meeting, Boston, MA, USA, 19–22 August 2006; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2006. [Google Scholar]
- Bertelli, D.; Lolli, M.; Papotti, G.; Bortolotti, L.; Serra, G.; Plessi, M. Detection of honey adulteration by sugar syrups using one-dimensional and two-dimensional high-resolution nuclear magnetic resonance. J. Agric. Food Chem. 2010, 58, 8495–8501. [Google Scholar] [CrossRef]
- Wu, X.; Xu, B.; Ma, R.; Gao, S.; Niu, Y.; Zhang, X.; Du, Z.; Liu, H.; Zhang, Y. Botanical origin identification and adulteration quantification of honey based on Raman spectroscopy combined with convolutional neural network. Vib. Spectrosc. 2022, 123, 103439. [Google Scholar] [CrossRef]
- Hegazi, N.M.; Elghani, G.; Farag, M. The super-food Manuka honey, a comprehensive review of its analysis and authenticity approaches. J. Food Sci. Technol. 2021, 59, 2527–2534. [Google Scholar] [CrossRef]
- Drivelos, S.A.; Danezis, G.; Haroutounian, S.A.; Georgiou, C.A. Rare earth elements minimal harvest year variation facilitates robust geographical origin discrimination: The case of PDO “Fava Santorinis”. Food Chem. 2016, 213, 238–245. [Google Scholar] [CrossRef]
- Tsanakas, G.F.; Mylona, P.V.; Koura, K.; Gleridou, A.; Polidoros, A.N. Genetic diversity analysis of the Greek lentil (Lens culinaris) landrace ‘Eglouvis’ using morphological and molecular markers. Plant Genet. Resour. 2018, 16, 469–477. [Google Scholar] [CrossRef]
- Ganopoulos, I.; Bazakos, C.; Madesis, P.; Kalaitzis, P.; Tsaftaris, A. Barcode DNA high-resolution melting (Bar-HRM) analysis as a novel close-tubed and accurate tool for olive oil forensic use. J. Sci. Food Agric. 2013, 93, 2281–2286. [Google Scholar] [CrossRef]
- Raieta, K.; Muccillo, L.; Colantuoni, V. A novel reliable method of DNA extraction from olive oil suitable for molecular traceability. Food Chem. 2015, 172, 596–602. [Google Scholar] [CrossRef]
- Pereira, L.; Gomes, S.; Barrias, S.; Fernandes, J.R.; Martins-Lopes, P. Applying high-resolution melting (HRM) technology to olive oil and wine authenticity. Food Res. Int. 2018, 103, 170–181. [Google Scholar] [CrossRef]
- Tsopelas, F.; Konstantopoulos, D.; Kakoulidou, A. Voltammetric fingerprinting of oils and its combination with chemometrics for the detection of extra virgin olive oil adulteration. Anal. Chim. Acta. 2018, 1015, 8–19. [Google Scholar] [CrossRef]
- Song, W.; Song, Z.; Vincent, J.; Wang, H.; Wang, Z. Quantification of extra virgin olive oil adulteration using smartphone videos. Talanta 2020, 216, 120920. [Google Scholar] [CrossRef]
- Fattah, S.; Ali, M. Carbide ripened fruits-a recent health hazard. Faridpur Med. Coll. J. 2010, 5, 37. [Google Scholar] [CrossRef][Green Version]
- Haughey, S.A.; Graham, S.F.; Cancouët, E.; Elliott, C.T. The application of near-infrared reflectance spectroscopy (NIRS) to detect melamine adulteration of soya bean meal. Food Chem. 2013, 136, 1557–1561. [Google Scholar] [CrossRef]
- Karabasanavar, N.S.; Singh, S.P.; Kumar, D.; Shebannavar, S.N. Detection of pork adulteration by highly-specific PCR assay of mitochondrial D-looFood chemistry. Food Chem. 2014, 145, 530–534. [Google Scholar] [CrossRef]
- Ali, M.; Hashim, U.; Mustafa, S.; Man, Y.C.; Adam, T.; Humayun, Q. Nanobiosensor for the detection and quantification of pork adulteration in meatball formulation. J. Exp. Nanosci. 2014, 9, 152–160. [Google Scholar] [CrossRef]
- Perret, C.; Tabin, R.; Marcoz, J.P.; Llor, J.; Cheseaux, J.J. Apparent life-threatening event in infants: Think about star anise intoxication! Arch. De Pediatr. Organe Off. De La Soc. Fr. De Pediatr. 2011, 18, 750–753. [Google Scholar] [CrossRef]
- Pesic, M.; Barac, M.; Vrvic, M.; Ristic, N.; Macej, O.; Stanojevic, S. Qualitative and quantitative analysis of bovine milk adulteration in caprine and ovine milks using native-PAGE. Food Chem. 2011, 125, 1443–1449. [Google Scholar] [CrossRef]
- Mishra, V.; Mishra, M.; Ansari, K.M.; Chaudhari, B.P.; Khanna, R.; Das, M. Edible oil adulterants, argemone oil and butter yellow, as aetiological factors for gall bladder cancer. Eur. J. Cancer 2012, 48, 2075–2085. [Google Scholar] [CrossRef]
- Shan, W.C.; Xi, J.Z.; Sun, J.; Zhang, Y.J.; Wang, J.P. Production of the monoclonal antibody against Sudan 4 for multi-immunoassay of Sudan dyes in egg. Food Control. 2012, 27, 146–152. [Google Scholar] [CrossRef]
- Chang, X.C.; Hu, X.Z.; Li, Y.Q.; Shang, Y.J.; Liu, Y.Z.; Feng, G.; Wang, J.P. Multi-determination of Para red and Sudan dyes in egg by a broad specific antibody based enzyme linked immunosorbent assay. Food Control. 2011, 22, 1770–1775. [Google Scholar] [CrossRef]
- Ghosh, D.; Singha, P.S.; Firdaus, S.B.; Ghosh, S. Metanil yellow: The toxic food colorant. Asian Pac. J. Health Sci. 2017, 4, 65–66. [Google Scholar] [CrossRef]
- Abd Elhalem, S.; EL-Atrash, A.; Osman, A.; Sherif, A.; Salim, E. Short term toxicity of food additive azo dye, sunset yellow (E110), at low doses, in male Sprague-Dawley rats. Egypt. J. ExBiol. Zool. 2016, 12, 13–21. [Google Scholar]
- Dwivedi, K.; Kumar, G. Genetic damage induced by a food coloring dye (sunset yellow) on meristematic cells of Brassica campestris L. J. Environ. Public Health 2015, 2015, 319727. [Google Scholar] [CrossRef]
- Sarhan, M.A.; Shati, A.; Elsaid, F. Biochemical and molecular studies on the possible influence of the Brassica oleracea and Beta vulgaris extracts to mitigate the effect of food preservatives and food chemical colorants on albino rats. Saudi J. Biol. Sci. 2014, 21, 342–354. [Google Scholar] [CrossRef]
- Song, S.; Lin, F.; Liu, L.; Kuang, H.; Wang, L.; Xu, C. Immunoaffinity removal and immunoassay for rhodamine B in chilli powder. Int. J. Food Sci. Technol. 2010, 45, 2589–2595. [Google Scholar] [CrossRef]
- Xu, H.; Heinze, T.M.; Paine, D.D.; Cerniglia, C.E.; Chen, H. Sudan azo dyes and Para Red degradation By prevalent bacteria of the human gastrointestinal tract. Anaerobe 2010, 16, 114–119. [Google Scholar] [CrossRef]
- Xu, H.; Heinze, T.M.; Chen, S.; Cerniglia, C.E.; Chen, H. Anaerobic metabolism of 1-amino-2-naphthol-based azo dyes (Sudan dyes) by human intestinal microflora. Appl. Environ. Microbiol. 2007, 73, 7759–7762. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).