Understanding the Metabolism and Dissipation Kinetics of Flutriafol in Vegetables under Laboratory and Greenhouse Scenarios
Abstract
1. Introduction
2. Materials and Methods
2.1. Material and Reagents
2.2. UHPLC-Q-ORBITRAP-MS Equipment
2.3. Laboratory Trials
2.4. Greenhouse Experiment
2.5. Sample Extraction
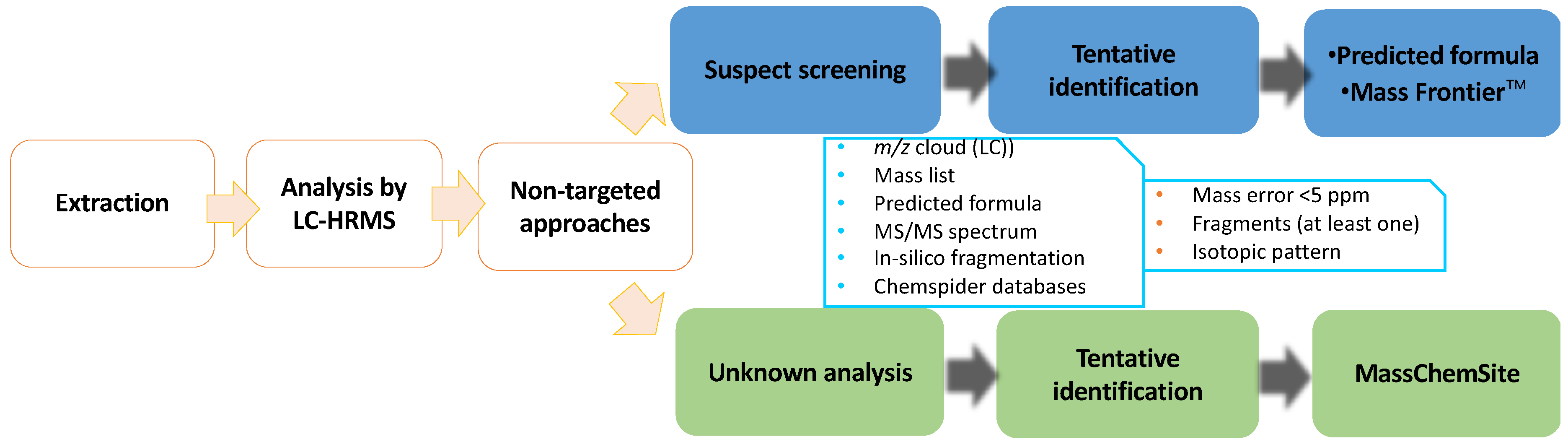
2.6. Data Calculation and Analysis
3. Results and Discussion
3.1. Dissipation Study of Flutriafol
3.1.1. Dissipation under Laboratory Conditions
3.1.2. Dissipation under Greenhouse Conditions
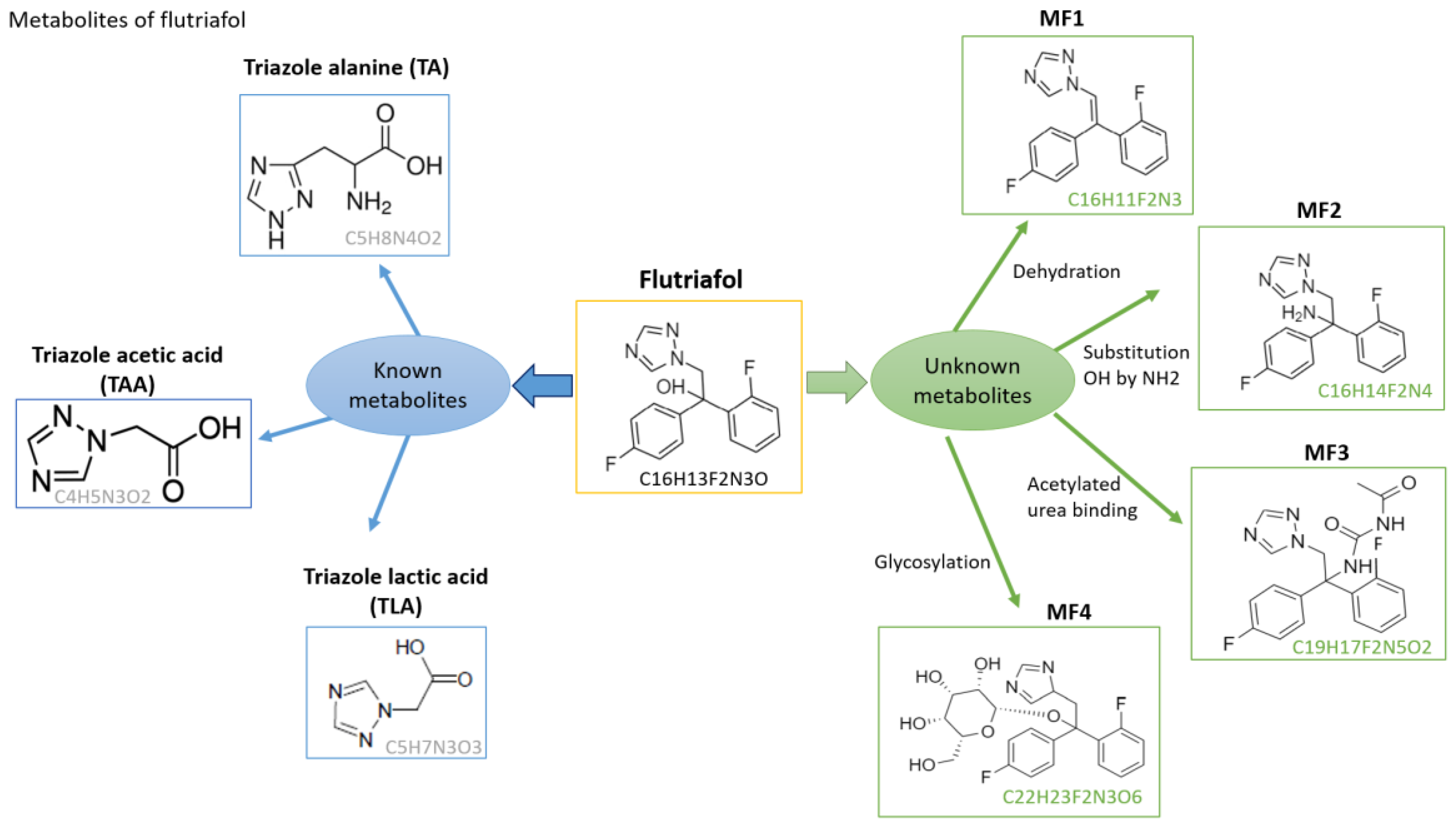
3.2. Detection of Known Flutriafol Metabolites
3.3. Tentative Identification of Unknown Flutriafol Metabolites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Honoré, M.N.; Belmonte-Ureña, L.J.; Navarro-Velasco, A.; Camacho-Ferre, F. Profit Analysis of Papaya Crops under Greenhouses as an Alternative to Traditional Intensive Horticulture in Southeast Spain. Int. J. Environ. Res. Public Health 2019, 16, 2908–2930. [Google Scholar] [CrossRef] [PubMed]
- Ávalos-Sánchez, E.; López-Martínez, A.; Molina-Aiz, F.D.; Reca, J.; Marín-Membrive, P.; Valera-Martínez, D.L. Effect of Different Substrates, and Irrigation with Water with Different Saline Concentrations, on the Development of Tomato Fungal Diseases in an Almería-Type Greenhouse. Agronomy 2022, 12, 1050–1067. [Google Scholar] [CrossRef]
- Duque-Acevedo, M.; Belmonte-Ureña, L.J.; Plaza-Úbeda, J.A.; Camacho-Ferre, F. The Management of Agricultural Waste Biomass in the Framework of Circular Economy and Bioeconomy: An Opportunity for Greenhouse Agriculture in Southeast Spain. Agronomy 2020, 10, 489–511. [Google Scholar] [CrossRef]
- Li, R.; Chen, Z.; Dong, F.; Xu, J.; Liu, X.; Wu, X.; Pan, X.; Tao, Y.; Zheng, Y. Supercritical Fluid Chromatographic-Tandem Mass Spectrometry Method for Monitoring Dissipation of Thiacloprid in Greenhouse Vegetables and Soil under Different Application Modes. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1081–1082, 25–32. [Google Scholar] [CrossRef]
- Pogǎcean, M.O.; Gavrilescu, M. Plant Protection Products and Their Sustainable and Environmentally Friendly Use. Environ. Eng. Manag. J. 2009, 8, 607–627. [Google Scholar] [CrossRef]
- European Commission Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 Concerning the Placing of Plant Protection Products on the Market and Repealing Council Directives 79/117/EEC and 91/414/EEC. Off. J. Eur. Union 2021, L309, 1–84.
- European Food Safety Authority; Anastassiadou, M.; Bernasconi, G.; Brancato, A.; Carrasco Cabrera, L.; Ferreira, L.; Greco, L.; Jarrah, S.; Kazocina, A.; Leuschner, R.; et al. Evaluation of Confirmatory Data Following the Article 12 MRL Review and Setting of an Import Tolerance for Flutriafol in Cucurbits (Inedible Peel). EFSA J. 2020, 18, 6315. [Google Scholar] [CrossRef]
- Tao, Y.; Zheng, Z.; Yu, Y.; Xu, J.; Liu, X.; Wu, X.; Dong, F.; Zheng, Y. Supercritical Fluid Chromatography–Tandem Mass Spectrometry-Assisted Methodology for Rapid Enantiomeric Analysis of Fenbuconazole and Its Chiral Metabolites in Fruits, Vegetables, Cereals, and Soil. Food Chem. 2018, 241, 32–39. [Google Scholar] [CrossRef]
- Kwon, H.C.; Sohn, H.; Kim, D.H.; Jeong, C.H.; Kim, D.W.; Han, S.G. Effects of Flutriafol Fungicide on the Lipid Accumulation in Human Liver Cells and Rat Liver. Foods 2021, 10, 1346. [Google Scholar] [CrossRef]
- University of Hertfordshire. PPDB: Pesticide Properties DataBase. Available online: http://sitem.herts.ac.uk/aeru/ppdb/en/atoz_fung.htm (accessed on 20 October 2022).
- Zhang, Q.; Wang, M.; Tian, M.; Wang, M.; Shi, H. Simultaneous Enantioselective Determination of Triazole Fungicide Flutriafol in Vegetables, Fruits, Wheat, Soil, and Water by Reversed-Phase High-Performance Liquid Chromatography. J. Agric. Food Chem. 2014, 62, 2809–2815. [Google Scholar] [CrossRef]
- Tao, Y.; Dong, F.; Xu, J.; Liu, X.; Cheng, Y.; Liu, N.; Chen, Z.; Zheng, Y. Green and Sensitive Supercritical Fluid Chromatographic-Tandem Mass Spectrometric Method for the Separation and Determination of Flutriafol Enantiomers in Vegetables, Fruits, and Soil. J. Agric. Food Chem. 2014, 62, 11457–11464. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.; Wei, K.; Zhang, Y.; Han, W.; Li, J.; Sun, X.; Shen, J.; Wang, L. A Facile-Operation Tubular Electro-Fenton System Combined with Oxygen Evolution Reaction for Flutriafol Degradation: Modeling and Parameters Optimizing. Electrochim. Acta 2017, 246, 1200–1209. [Google Scholar] [CrossRef]
- Zhang, Q.; Hua, X.; Yang, Y.; Yin, W.; Tian, M.; Shi, H.; Wang, M. Stereoselective Degradation of Flutriafol and Tebuconazole in Grape. Environ. Sci. Pollut. Res. 2015, 22, 4350–4358. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Jia, C.; Song, W.; Liu, F. Dissipation and Residues of Flutriafol in Wheat and Soil under Field Conditions. Bull. Environ. Contam. Toxicol. 2012, 89, 1040–1045. [Google Scholar] [CrossRef]
- Ströher Kolberg, I.D.; Zechmann, S.; Wildgrube, C.; Sigalov, I.; Scherbaum, E.; Anastassiades, M. Determination of Triazole Derivative Metabolites (TDMs) in Fruit and Vegetables Using the QuPPe Method and Differential Mobility Spectrometry (DMS) and Survey of the Residue Situation in Organic and Conventional Produce. EURL-SRM Stuttg. 2016, 1–31. Available online: https://www.eurl-pesticides.eu/userfiles/file/EurlSRM/EurlSrm_meth_TriazoleDerivativeMetabolites.pdf (accessed on 20 October 2022).
- European Commission COMMISSION REGULATION (EU) 2017/626 of 31 March 2017 Amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as Regards Maximum Residue Levels for Acetamiprid, Cyantraniliprole, Cypermethrin, Cyprodinil, Di. Off. J. Eur. Union 2017, L96, 1–43.
- European Food Safety Authority (EFSA); Brancato, A.; Brocca, D.; Carrasco Cabrera, L.; Chiusolo, A.; Civitella, C.; Court Marques, D.; Crivellente, F.; De Lentdecker, C.; Erdös, Z.; et al. Peer Review of the Pesticide Risk Assessment for the Triazole Derivative Metabolites in Light of Confirmatory Data Submitted. EFSA J. 2018, 16, 5376. [Google Scholar] [CrossRef]
- Hergueta-Castillo, M.E.; López-Rodríguez, E.; López-Ruiz, R.; Romero-González, R.; Garrido Frenich, A. Targeted and Untargeted Analysis of Triazole Fungicides and Their Metabolites in Fruits and Vegetables by UHPLC-Orbitrap-MS2. Food Chem. 2022, 368, 130860–130869. [Google Scholar] [CrossRef]
- Prata, R.; López-Ruiz, R.; Petrarca, M.H.; Teixeira Godoy, H.; Garrido Frenich, A.; Romero-González, R. Targeted and Non-Targeted Analysis of Pesticides and Aflatoxins in Baby Foods by Liquid Chromatography Coupled to Quadrupole Orbitrap Mass Spectrometry. Food Control 2022, 139, 109072. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency Toxicity Estimation Software Tool (TEST). Available online: https://www.epa.gov/chemical-research/toxicity-estimation-software-tool-test (accessed on 20 October 2022).
- Dong, F.; Li, J.; Chankvetadze, B.; Cheng, Y.; Xu, J.; Liu, X.; Li, Y.; Chen, X.; Bertucci, C.; Tedesco, D.; et al. Chiral Triazole Fungicide Difenoconazole: Absolute Stereochemistry, Stereoselective Bioactivity, Aquatic Toxicity, and Environmental Behavior in Vegetables and Soil. Environ. Sci. Technol. 2013, 47, 3386–3394. [Google Scholar] [CrossRef]
- López-Ruiz, R.; Romero-González, R.; Serra, B.; Garrido Frenich, A. Dissipation Kinetic of Fenamidone and Propamocarb in Vegetables under Greenhouse Conditions Using Liquid and Gas Chromatography Coupled to High-Resolution Mass Spectrometry. Chemosphere 2019, 226, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Marín-Sáez, J.; López-Ruiz, R.; Romero-Gonzalez, R.; Garrido Frenich, A.; Zamora Rico, I. Looking beyond the Active Substance: Comprehensive Dissipation Study of Myclobutanil-Based Plant Protection Products in Tomatoes and Grapes Using Chromatographic Techniques Coupled to High-Resolution Mass Spectrometry. J. Agric. Food Chem. 2022, 70, 6385–6396. [Google Scholar] [CrossRef] [PubMed]
- Giweta, M. Role of Litter Production and Its Decomposition, and Factors Affecting the Processes in a Tropical Forest Ecosystem: A Review. J. Ecol. Environ. 2020, 44, 11. [Google Scholar] [CrossRef]
- López-Ruiz, R.; Maldonado-Reina, A.J.; Marín-Sáez, J.; Romero-Gonzalez, R.; Martínez-Vidal, J.L.; Garrido Frenich, A.I. Unravelling Plant Protection Product Analysis: Use of Chromatography Techniques (GC and LC) and High Resolution Mass Spectrometry. Trends Environ. Anal. Chem. 2023, 37, e00191. [Google Scholar] [CrossRef]
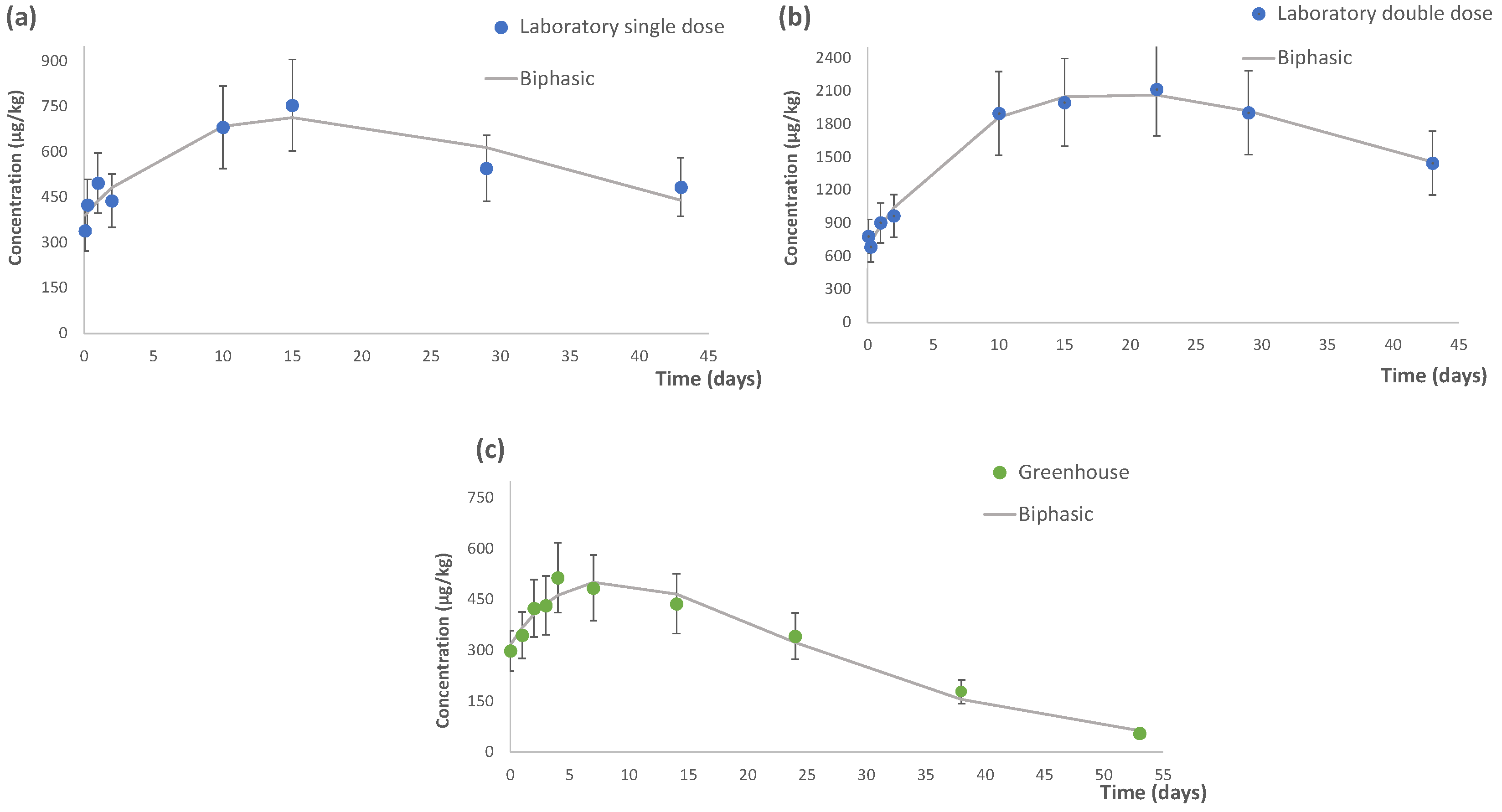

| Kinetic Parameters | Laboratory Trials | Greenhouse Study (0.09%) | |
|---|---|---|---|
| Single Dose (0.09%) | Double Dose (0.18%) | ||
| C0 (µg/kg) | 385.9 | 689.1 | 315.6 |
| k1 (days−1) | 0.05 | 0.04 | 0.08 |
| k2 (days−1) | 0.06 | 0.07 | 0.09 |
| t1/2(k1) (days) | 14.8 | 16.3 | 8.9 |
| t1/2 (k2) (days) | 14.0 | 14.9 | 8.2 |
| DT90 (days) | 49.2 | 54.1 | 29.5 |
| R2 | 0.986 | 0.995 | 0.966 |
| Compound | RTW (min) | Molecular Formula | Theoretical Mass (m/z) | Mass Error (ppm) | Fragment ions | Ionization Mode | ||
|---|---|---|---|---|---|---|---|---|
| Molecular Formula | Theoretical Mass (m/z) | Mass Error (ppm) | ||||||
| Triazole alanine (TA) | 1.52–1.68 | C5H8N4O2 | 157.07202 | −0.18 | C2H4N3 | 70.03997 | −1.42 | ESI (+) |
| C5H7N4O | 139.06144 | −1.96 | ||||||
| Triazole acetic acid (TAA) CGA 142856 | 1.23–1.30 | C4H5N3O2 | 128.04545 | 0.54 | C2H4N3 | 70.03997 | 0.25 | ESI (+) |
| Triazole lactic acid (TLA) CGA 205369 | 1.70–1.80 | C5H7N3O3 | 158.05601 | −4.09 | C4H7N2O3 | 131.04512 | 2.28 | ESI (+) |
| C5H5N3O2 | 139.03763 | 1.41 | ||||||
| Compound | RTW (min) | Molecular Formula | Theoretical Mass (m/z) | Mass Error (ppm) | Fragment Ions | Ionization Mode | ||
|---|---|---|---|---|---|---|---|---|
| Molecular Formula | Theoretical Mass (m/z) | Mass Error (ppm) | ||||||
| MF1 | 2.26–2.38 | C6H11F2N3 | 284.09938 | −1.12 | C9H11N2F3 | 185.09114 | 4.37 | ESI (+) |
| C4H10O5N | 152.05535 | 3.55 | ||||||
| MF2 | 1.57–1.65 | C16H14F2N4 | 301.12593 | −0.99 | C10H3NF2 | 175.02281 | 4.45 | ESI (+) |
| C14H11NF2 | 231.08541 | 2.26 | ||||||
| MF3 | 3.37–3.54 | C19H17F2N5O2 | 386.14231 | 1.45 | C8H10N | 120.08078 | 3.41 | ESI (+) |
| C7H10ON4 | 166.08491 | 2.82 | ||||||
| MF4 | 6.74–6.97 | C22H23F2N3O6 | 464.16170 | 0.87 | C6H8N | 94.06513 | 3.40 | ESI (+) |
| C12H20NO | 194.15394 | −3.86 | ||||||
| Metabolite | Sampling Period (Days) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 7 | 14 | 24 | 38 | 55 | |
| MF2 | <LOQf | -- | -- | -- | <LOQf | -- | -- | -- | -- |
| MF3 | -- | -- | -- | -- | -- | 4.1 | 3.9 | -- | -- |
| MF4 | -- | -- | <LOQf | <LOQf | <LOQf | <LOQf | <LOQf | 2.4 | 4.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hergueta-Castillo, M.E.; López-Ruiz, R.; Garrido Frenich, A.; Romero-González, R. Understanding the Metabolism and Dissipation Kinetics of Flutriafol in Vegetables under Laboratory and Greenhouse Scenarios. Foods 2023, 12, 201. https://doi.org/10.3390/foods12010201
Hergueta-Castillo ME, López-Ruiz R, Garrido Frenich A, Romero-González R. Understanding the Metabolism and Dissipation Kinetics of Flutriafol in Vegetables under Laboratory and Greenhouse Scenarios. Foods. 2023; 12(1):201. https://doi.org/10.3390/foods12010201
Chicago/Turabian StyleHergueta-Castillo, María Elena, Rosalía López-Ruiz, Antonia Garrido Frenich, and Roberto Romero-González. 2023. "Understanding the Metabolism and Dissipation Kinetics of Flutriafol in Vegetables under Laboratory and Greenhouse Scenarios" Foods 12, no. 1: 201. https://doi.org/10.3390/foods12010201
APA StyleHergueta-Castillo, M. E., López-Ruiz, R., Garrido Frenich, A., & Romero-González, R. (2023). Understanding the Metabolism and Dissipation Kinetics of Flutriafol in Vegetables under Laboratory and Greenhouse Scenarios. Foods, 12(1), 201. https://doi.org/10.3390/foods12010201







