Spent Yeast Valorization for Food Applications: Effect of Different Extraction Methodologies
Abstract
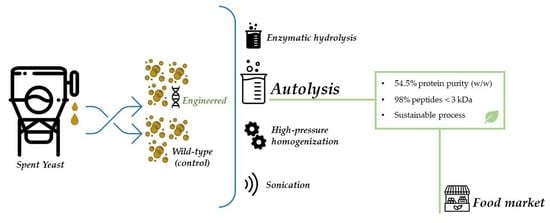
1. Introduction
2. Materials and Methods
2.1. Yeast Strains
2.2. Yeast Pre-Treatment
2.3. Extraction Methods
2.3.1. High Pressure Homogenization (HPH)
2.3.2. Sonication
2.3.3. Autolysis
2.3.4. Enzymatic Hydrolysis
2.4. Sustainable Metrics
2.5. Yeast Extract Characterization
2.5.1. Protein
2.5.2. Dry Weight
2.5.3. Protein and Peptides MW
2.5.4. Amino Acids
2.5.5. Neutral Sugars
2.5.6. Minerals
2.6. Statistical Analysis
3. Results and Discussion
3.1. Peptide-Rich Extracts Characterization
3.1.1. Protein Content (% w/w)
3.1.2. MW Distribution
3.2. Extraction Methodology Evaluation
3.2.1. Protein Recovery
3.2.2. Sustainable Metrics
3.3. Choice of Peptide-Rich Extract for Future Purification Process
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arentson-Lantz, E.; Clairmont, S.; Paddon-Jones, D.; Tremblay, A.; Elango, R. Protein: A nutrient in focus. Appl. Physiol. Nutr. Metab. 2015, 40, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Henchion, M.; Hayes, M.; Mullen, A.M.; Fenelon, M.; Tiwari, B. Future Protein Supply and Demand: Strategies and Factors Influencing a Sustainable Equilibrium. Foods 2017, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.S.; Ferreira, C.; Pereira, J.O.; Pintado, M.E.; Carvalho, A.P. Spent brewer’s yeast (Saccharomyces cerevisiae) as a potential source of bioactive peptides: An overview. Int. J. Biol. Macromol. 2022, 208, 1116–1126. [Google Scholar] [CrossRef] [PubMed]
- Transparency Market Research Peptide Therapeutics Market-Global Industry Analysis, Size, Share, Growth, Trends, and Forecast 2019–2027. Available online: https://www.transparencymarketresearch.com/peptide-therapeutics-market.html (accessed on 26 November 2021).
- Oliveira, A.S.; Ferreira, C.; Pereira, J.O.; Pintado, M.E.; Carvalho, A.P. Valorisation of protein-rich extracts from spent brewer’s yeast (Saccharomyces cerevisiae): An overview. Biomass Convers. Biorefin. 2022, 1–58. [Google Scholar] [CrossRef]
- Vieira, E.; Cunha, S.C.; Ferreira, I.M.P.L.V.O. Characterization of a Potential Bioactive Food Ingredient from Inner Cellular Content of Brewer’s Spent Yeast. Waste Biomass Valoriz. 2019, 10, 3235–3242. [Google Scholar] [CrossRef]
- Marson, G.V.; De Castro, R.J.S.; Belleville, M.-P.; Hubinger, M.D. Spent brewer’s yeast as a source of high added value molecules: A systematic review on its characteristics, processing and potential applications. World J. Microbiol. Biotechnol. 2020, 36, 95. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Pereira, J.O.; Ferreira, C.; Faustino, M.; Durão, J.; Pintado, M.E.; Carvalho, A.P. Peptide-rich extracts from spent yeast waste streams as a source of bioactive compounds for the nutraceutical market. Innov. Food Sci. Emerg. Technol. 2022, 81, 103148. [Google Scholar] [CrossRef]
- Meadows, A.L.; Hawkins, K.M.; Tsegaye, Y.; Antipov, E.; Kim, Y.; Raetz, L.; Dahl, R.H.; Tai, A.; Mahatdejkul-Meadows, T.; Xu, L.; et al. Rewriting yeast central carbon metabolism for industrial isoprenoid production. Nature 2016, 537, 694–697. [Google Scholar] [CrossRef]
- Nandy, S.K.; Srivastava, R. A review on sustainable yeast biotechnological processes and applications. Microbiol. Res. 2018, 207, 83–90. [Google Scholar] [CrossRef]
- Parapouli, M.; Vasileiadi, A.; Afendra, A.-S.; Hatziloukas, E. Saccharomyces cerevisiae and its industrial applications. AIMS Microbiol. 2020, 6, 1–31. [Google Scholar] [CrossRef]
- Ramos-Viana, V.; Møller-Hansen, I.; Kempen, P.; Borodina, I. Modulation of the cell wall protein Ecm33p in yeast Saccharomyces cerevisiae improves the production of small metabolites. FEMS Yeast Res. 2022, 22, foac037. [Google Scholar] [CrossRef] [PubMed]
- Ekpeni, L.E.; Benyounis, K.Y.; Stokes, J.; Olabi, A.G. Improving and optimizing protein concentration yield from homogenized baker’s yeast at different ratios of buffer solution. Int. J. Hydrog. Energy 2016, 41, 16415–16427. [Google Scholar] [CrossRef]
- Liu, D.; Zeng, X.-A.; Sun, D.-W.; Han, Z. Disruption and protein release by ultrasonication of yeast cells. Innov. Food Sci. Emerg. Technol. 2013, 18, 132–137. [Google Scholar] [CrossRef]
- Jacob, F.F.; Hutzler, M.; Methner, F.-J. Comparison of various industrially applicable disruption methods to produce yeast extract using spent yeast from top-fermenting beer production: Influence on amino acid and protein content. Eur. Food Res. Technol. 2019, 245, 95–109. [Google Scholar] [CrossRef]
- Chae, H.J.; Joo, H.; In, M.-J. Utilization of brewer’s yeast cells for the production of food-grade yeast extract. Part 1: Effects of different enzymatic treatments on solid and protein recovery and flavor characteristics. Bioresour. Technol. 2001, 76, 253–258. [Google Scholar] [CrossRef]
- Sheldon, R.A. Metrics of Green Chemistry and Sustainability: Past, Present, and Future. ACS Sustain. Chem. Eng. 2018, 6, 32–48. [Google Scholar] [CrossRef]
- Dumas, J.B.A. Procedes de l’analyse Organique. Ann. Chim. Phys. 1831, 247, 198–205. [Google Scholar]
- Marson, G.V.; de Castro, R.J.S.; Machado, M.T.D.C.; Zandonadi, F.D.S.; Barros, H.D.D.F.Q.; Júnior, M.R.M.; Sussulini, A.; Hubinger, M. Proteolytic enzymes positively modulated the physicochemical and antioxidant properties of spent yeast protein hydrolysates. Process Biochem. 2020, 91, 34–45. [Google Scholar] [CrossRef]
- Oliveira, C.M.; Horta, B.; Leal, T.; Pintado, M.; Oliveira, C.S.S. Valorization of Spent Sugarcane Fermentation Broth as a Source of Phenolic Compounds. Processes 2022, 10, 1339. [Google Scholar] [CrossRef]
- Long, W. Automated Amino Acid Analysis Using an Agilent Poroshell HPH-C18 Column; Application Note; Agilent Technologies Inc.: Santa Clara, CA. USA, 2017; pp. 1–10. [Google Scholar]
- Wang, L.; Yang, J.; Wang, Y.; Zhang, J.; Gao, Y.; Yuan, J.; Su, A.; Ju, X. Study on Antioxidant Activity and Amino Acid Analysis of Rapeseed Protein Hydrolysates. Int. J. Food Prop. 2016, 19, 1899–1911. [Google Scholar] [CrossRef]
- Pinto, M.; Coelho, E.; Nunes, A.; Brandão, T.; Coimbra, M.A. Valuation of brewers spent yeast polysaccharides: A structural characterization approach. Carbohydr. Polym. 2015, 116, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Selvendran, R.R.; March, J.F.; Ring, S.G. Determination of aldoses and uronic acid content of vegetable fiber. Anal. Biochem. 1979, 96, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Blakeney, A.B.; Harris, P.J.; Henry, R.J.; Stone, B.A. A simple and rapid preparation of alditol acetates for monosaccharide analysis. Carbohydr. Res. 1983, 113, 291–299. [Google Scholar] [CrossRef]
- Chatelain, P.G.; Pintado, M.E.; Vasconcelos, M.W. Evaluation of chitooligosaccharide application on mineral accumulation and plant growth in Phaseolus vulgaris. Plant Sci. 2014, 215–216, 134–140. [Google Scholar] [CrossRef]
- European Commission. Nutrition Claims. Available online: https://food.ec.europa.eu/safety/labelling-and-nutrition/nutrition-and-health-claims/nutrition-claims_en (accessed on 26 September 2022).
- Ganeva, V.; Galutzov, B. Electropulsation as an alternative method for protein extraction from yeast. FEMS Microbiol. Lett. 1999, 174, 279–284. [Google Scholar] [CrossRef][Green Version]
- Podpora, B.; Świderski, F.; Sadowska, A.; Rakowska, R.; Wasiak-Zys, G. Spent brewer’s yeast extracts as a new component of functional food. Czech J. Food Sci. 2016, 34, 554–563. [Google Scholar] [CrossRef]
- Ferreira, C.; Pereira, C.F.; Oliveira, A.S.; Faustino, M.; Pereira, A.M.; Durão, J.; Pereira, J.O.; Pintado, M.E.; Carvalho, A.P. A Step for the Valorization of Spent Yeast through Production of Iron–Peptide Complexes—A Process Optimization Study. Processes 2022, 10, 1464. [Google Scholar] [CrossRef]
- Amorim, M.; Pinheiro, H.; Pintado, M. Valorization of spent brewer’s yeast: Optimization of hydrolysis process towards the generation of stable ACE-inhibitory peptides. LWT 2019, 111, 77–84. [Google Scholar] [CrossRef]
- Xie, J.; Cui, C.; Ren, J.; Zhao, M.; Zhao, L.; Wang, W. High solid concentrations facilitate enzymatic hydrolysis of yeast cells. Food Bioprod. Process. 2017, 103, 114–121. [Google Scholar] [CrossRef]
- Mirzaei, M.; Mirdamadi, S.; Ehsani, M.R.; Aminlari, M.; Hosseini, E. Purification and identification of antioxidant and ACE-inhibitory peptide from Saccharomyces cerevisiae protein hydrolysate. J. Funct. Foods 2015, 19, 259–268. [Google Scholar] [CrossRef]
- Amorim, M.; Marques, C.; Pereira, J.; Guardão, L.; Martins, M.; Osório, H.; Moura, D.; Calhau, C.; Pinheiro, H.; Pintado, M. Antihypertensive effect of spent brewer yeast peptide. Process Biochem. 2019, 76, 213–218. [Google Scholar] [CrossRef]
- Podpora, B.; Swiderski, F.; Sadowska, A.; Piotrowska, A.; Rakowska, R. Spent Brewer’s Yeast Autolysates as a New and Valuable Component of Functional Food and Dietary Supplements. J. Food Process. Technol. 2015, 6, 12. [Google Scholar] [CrossRef]
- Halim, R.; Hill, D.R.A.; Hanssen, E.; Webley, P.A.; Blackburn, S.; Grossman, A.R.; Posten, C.; Martin, G.J.O. Towards sustainable microalgal biomass processing: Anaerobic induction of autolytic cell-wall self-ingestion in lipid-rich Nannochloropsis slurries. Green Chem. 2019, 21, 2967–2982. [Google Scholar] [CrossRef]
- Bertolo, A.P.; Biz, A.P.; Kempka, A.P.; Rigo, E.; Cavalheiro, D. Yeast (Saccharomyces cerevisiae): Evaluation of cellular disruption processes, chemical composition, functional properties and digestibility. J. Food Sci. Technol. 2019, 56, 3697–3706. [Google Scholar] [CrossRef]
- Jacob, F.F.; Striegel, L.; Rychlik, M.; Hutzler, M.; Methner, F.-J. Yeast extract production using spent yeast from beer manufacture: Influence of industrially applicable disruption methods on selected substance groups with biotechnological relevance. Eur. Food Res. Technol. 2019, 245, 1169–1182. [Google Scholar] [CrossRef]
- Amorim, M.; Pereira, J.O.; Gomes, D.; Pereira, C.D.; Pinheiro, H.; Pintado, M. Nutritional ingredients from spent brewer’s yeast obtained by hydrolysis and selective membrane filtration integrated in a pilot process. J. Food Eng. 2016, 185, 42–47. [Google Scholar] [CrossRef]
- Puligundla, P.; Mok, C.; Park, S. Advances in the valorization of spent brewer’s yeast. Innov. Food Sci. Emerg. Technol. 2020, 62, 102350. [Google Scholar] [CrossRef]
- World Health Organization. Protein and Amino Acid Requirements in Human Nutrition; Report of a Joint WHO/FAO/UNU Expert Consultation; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]


| CSY | ESY1 | ESY2 | |
|---|---|---|---|
| Raw yeast | 43.0 ± 1.1 | 38.4 ± 2.3 | 32.9 ± 0.4 |
| Autolysis | 64.8 ± 2.4 | 54.5 ± 1.0 | 48.3 ± 1.0 |
| Enzymatic hydrolysis | 34.6 ± 2.6 | 30.8 ± 0.4 | 25.3 ± 0.7 |
| HPH | 51.2 ± 3.3 | 45.6 ± 1.7 | 44.5 ± 1.8 |
| Sonication | 50.2 ± 14.6 | 50.1 ± 4.3 | 46.3 ± 0.9 |
| CSY | ESY1 | ESY2 | ||
|---|---|---|---|---|
| Autolysis | PMI | 22 | 27 | 26 |
| WI | 11 | 13 | 13 | |
| EIS | 5764 | 6891 | 6601 | |
| Hydrolysis | PMI | 35 | 36 | 35 |
| WI | 29 | 30 | 28 | |
| EIS | 2250 | 2330 | 2217 | |
| HPH | PMI | 27 | 66 | 29 |
| WI | 19 | 48 | 21 | |
| EIS | 44.2 | 110 | 47.2 | |
| Sonication | PMI | 1904 | 919 | 1509 |
| WI | 1882 | 909 | 1492 | |
| EIS | 2353 | 1137 | 1865 |
| Protein (%) | Sugars (%) | Minerals (ng/g) | ||||
|---|---|---|---|---|---|---|
| P | Mg | Ca | K | Total | ||
| 54.5 ± 1.0 | 14.8 ± 0.7 | 14.2 ± 1.0 | 4.34 ± 0.37 | 1.55 ± 0.13 | 16.5 ± 0.5 | 36.9 ± 1.9 |
| EAA | FAO/WHO Reference c | |
|---|---|---|
| Cys | 6.94 ± 0.41 | 6.0 |
| His | 47.1 ± 5.0 | 15.0 |
| Thr | 60.4 ± 2.4 | 11.0 |
| Arg | 59.1 ± 3.9 | NM |
| Val | 72.8 ± 4.4 | 15.0 |
| Met | 8.97 ± 1.89 | 16.0 |
| Phe | 43.7 ± 2.6 | 21.0 b |
| Tyr a | 41.3 ± 3.6 | |
| Ile | 56.9 ± 3.2 | 15.0 |
| Leu | 78.1 ± 4.8 | 21.0 |
| Lys | 69.9 ± 2.5 | 18.0 |
| Total | 545 ± 35 | 138 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, A.S.; Odila Pereira, J.; Ferreira, C.; Faustino, M.; Durão, J.; Pereira, A.M.; Oliveira, C.M.; Pintado, M.E.; Carvalho, A.P. Spent Yeast Valorization for Food Applications: Effect of Different Extraction Methodologies. Foods 2022, 11, 4002. https://doi.org/10.3390/foods11244002
Oliveira AS, Odila Pereira J, Ferreira C, Faustino M, Durão J, Pereira AM, Oliveira CM, Pintado ME, Carvalho AP. Spent Yeast Valorization for Food Applications: Effect of Different Extraction Methodologies. Foods. 2022; 11(24):4002. https://doi.org/10.3390/foods11244002
Chicago/Turabian StyleOliveira, Ana Sofia, Joana Odila Pereira, Carlos Ferreira, Margarida Faustino, Joana Durão, Ana Margarida Pereira, Carla Maria Oliveira, Manuela E. Pintado, and Ana P. Carvalho. 2022. "Spent Yeast Valorization for Food Applications: Effect of Different Extraction Methodologies" Foods 11, no. 24: 4002. https://doi.org/10.3390/foods11244002
APA StyleOliveira, A. S., Odila Pereira, J., Ferreira, C., Faustino, M., Durão, J., Pereira, A. M., Oliveira, C. M., Pintado, M. E., & Carvalho, A. P. (2022). Spent Yeast Valorization for Food Applications: Effect of Different Extraction Methodologies. Foods, 11(24), 4002. https://doi.org/10.3390/foods11244002