Abstract
Background/Objectives: Certain components of natural products help maintain the oral microbiota balance, thereby promoting oral health. This study aimed to identify natural components with anticariogenic properties by analyzing evidence from in vivo studies. Methods: A systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The literature search was performed across multiple databases and included English-language studies published between 2013 and 2025. The review included intervention and comparative studies that examined the effects of dietary habits involving natural components in participants of any age, with or without dental caries. Results: A total of 77 studies were included in the review, most of which were clinical trials conducted in pediatric populations. To assess the impact of the interventions, most studies measured outcomes such as Streptococcus mutans levels, dental caries incidence, and salivary pH, among other parameters. The most frequently studied components included probiotics, plant extracts, sugar substitutes, propolis, arginine, dairy products, among others. Significant effects were most reported on biological risk factors (72.8%). In addition, 16.9% of the studies reported a statistically significant reduction in clinically diagnosed dental caries. Conclusions: This review identified preliminary evidence suggesting that certain natural compounds may play a role in modulating cariogenic factors. However, further high-quality studies are needed to strengthen the evidence base and confirm these findings. The protocol for this review was registered on the Open Science Framework platform.
1. Introduction
Over 700 bacterial species have been identified in the oral cavity, along with various species of fungi, viruses, and protozoa [,,,,]. The presence and interactions of these microorganisms are influenced by factors such as the host’s immune response, systemic conditions, hygiene habits, diet, and antimicrobial use []. In response to these changing factors, the microbiota demonstrates both resistance and resilience. These properties can be strengthened by timely interventions that address early signs of disease [,].
When the microbiota is exposed to factors that disrupt the oral ecosystem, dysbiosis occurs. This condition is characterized by an imbalance favoring microorganisms with virulence factors promoting disease [,,]. For example, dental caries—mainly triggered by excessive intake of fermentable carbohydrates—encourage the proliferation of cariogenic bacteria. These bacteria produce organic acids that demineralize tooth enamel, tolerate acidic environments, and synthesize extracellular polysaccharides from sucrose, facilitating colonization and dental biofilm formation [,,].
Naturally occurring components with anticariogenic properties may contribute to rebalancing the oral microbiota through various mechanisms [,]. These include stimulating salivary flow, enhancing buffering capacity, and activating innate immune responses. Other effects involve inhibiting dental biofilm formation and cariogenic microorganisms (antimicrobial activity), promoting beneficial microbial species (prebiotic effect), and modulating the adaptive immune response [,].
However, most of these components—including plant extracts (e.g., leaves, seeds, flowers, and fruits) [], dairy products [], vitamins [], probiotics [], and sugar substitutes []—have primarily been studied under in vitro conditions or in animal models. Understanding their effectiveness in humans—specifically in reducing dental caries and modulating biological risk factors—is crucial. This knowledge will help identify which components could be incorporated into preventive strategies, such as nutritional guidance or other non-pharmacological interventions aimed at improving oral health.
This systematic review aims to address these gaps by focusing exclusively on in vivo human studies of natural anticariogenic components. It evaluates both clinical caries outcomes and relevant biological risk markers to better identify which agents may be effective under physiologically real conditions in humans.
2. Materials and Methods
2.1. Search Strategy
A systematic review was conducted and reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [] to ensure a transparent and reproducible search strategy for clinical studies evaluating naturally derived components for the prevention of dental caries and associated risk factors. Additionally, the Peer Review of Electronic Search Strategies (PRESS) framework was employed to validate the completeness and accuracy of the search strategy (conducted by Y.Y.-N and M.d.P.A.-D). The review protocol was registered on the Open Science Framework platform (DOI: 10.17605/OSF.IO/KF94A). No amendments were made to the registered protocol.
2.2. Eligibility Criteria
English-language studies published between 2013 and 2025 were included if they involved human participants of any age and assessed the effects of natural components on clinically diagnosed dental caries or related biological risk factors. Eligible designs included randomized and non-randomized clinical trials, longitudinal interventions studies, and observational studies. Exclusion criteria comprised studies conducted in vitro, in silico, or in animal models; grey literature; reviews; editorials; letters; unpublished studies, or expert opinions. Based on the PICO format, the following criteria were established: Population: Systematically healthy individuals of any age, not undergoing antibiotic treatment, with either caries-free or with measurable dental caries indices, with or without risk factors such as S. mutans quantification or dental biofilm index. Interventions: Naturally derived components with anticariogenic properties that can be ingested. Comparators: Placebo, other types of components, or no intervention. Outcome: Variables that assess the anticariogenic capacity (statistically significant effect). The included studies were grouped based on the type of natural components used in the interventions, and the study design.
2.3. Data Sources and Search Strategy
The databases consulted were PubMed, Scopus, Ovid, J-Stage, BVS, and Google Scholar. The review was conducted in November 2023, and updated in October 2025 using Medical Subject Headings (MeSH) keywords, including “dental caries,” “food,” “beverages,” “meals,” and “snacks,” as well as non-MeSH terms such as “anticariogenic,” “caries-free,” “antimicrobial,” and “cariostatic”. These keywords were combined with Boolean operators such as AND and OR (Appendix A). A supplementary manual search (conducted by M.d.P.A.-D) was also performed based on the components identified in the initial search.
2.4. Study Selection Process and Quality Assessment
Study Selection
The screening phase was conducted using the Rayyan Artificial Intelligence system, where the selection process was performed independently through the “Blind On” tool (Y.Y.-N, K.G.-P., L.C.-G, and H.G.-R.). In the event of discrepancies among the four reviewers, a fifth peer reviewer (M.d.P.A.-D adjudicated the final decision. In the initial phase, articles were selected based on their titles, abstracts, and full texts. In the second phase, data from studies that met the eligibility criteria (author, year, participants, components, frequency, duration, follow-up variables, and outcomes) were compiled in an Excel spreadsheet (Y.Y.-N, K.G.-P., L.C.-G, and H.G.-R.) and verified by M.d.P.A.-D and LB. Due to clinical and methodological heterogeneity among the studies (type of components, population, sample size, follow up duration), a meta-analysis was not appropriate. The findings were summarized after organizing key study characteristics for descriptive synthesis. To determine study eligibility for each synthesis, we tabulated the characteristics of all included studies, such as intervention type, frequency, follow up duration, comparator, and outcome measures. For studies reporting multiple interventions or outcomes, only data relevant to the predefined synthesis criteria were included. Additionally, the mechanisms of action of each component and their effectiveness, as reported in the literature, were investigated.
2.5. Methodological Quality
The methodological quality of the studies was first assessed using the Joanna Briggs Institute critical appraisal tool (JBI) [], where the level of bias was evaluated based on four possible responses: compliant, non-compliant, unclear, and not applicable. Subsequently, to classify the grade of recommendation for each selected study, the Grading of Recommendations Assessment, Development, and Evaluation system [] was used, categorizing the evidence as high, moderate, low, or very low quality. This process was independently performed by M.d.P.A.-D, L.J.B.-C., and J.S.-O. (Appendix B).
3. Results
3.1. Characteristics of the Included Studies
After removing duplicates, a total of 3310 records were screened for eligibility (Figure 1). Of these studies, 51 that met the inclusion criteria were selected, and 26 additional studies identified through the manual search were included (Figure 1). The majority of the studies were clinical trials (80.5%) (Table 1). The most frequently evaluated components were probiotics (24.7%) (Table 1), particularly strains from the genus Lactobacillus species, followed by Bifidobacterium, Limosilactobacillus, and Streptococcus (Table 2). The second most studied component was plant extracts (22.1%), sugar substitutes (9.1%), followed by propolis (6.5%), arginine (6.5%), dairy products (5.2%), among others (Table 1). The variables used to assess anticariogenic effects included the clinically diagnosed dental caries (15%) and biological risk factors (85%) (Table 1). Furthermore, several studies have assessed the adverse effects and acceptability of the products.
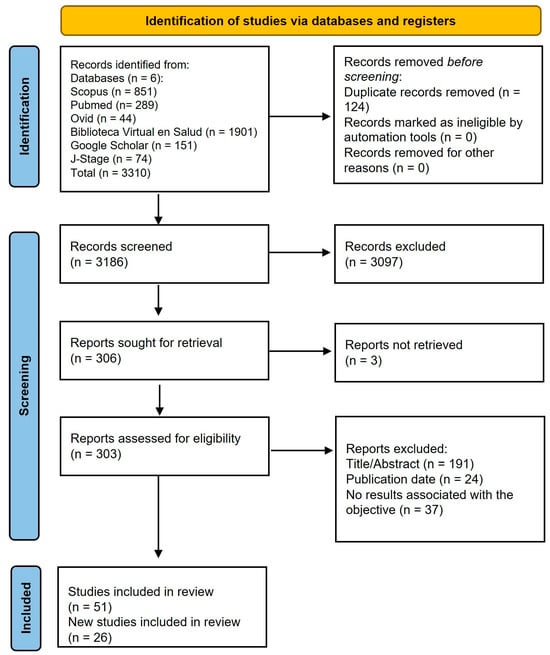
Figure 1.
Flow diagram of literature search process.

Table 1.
Frequency of key characteristics in included studies.

Table 2.
In vivo studies evaluating anticaries compounds of natural origin.
The effects of the components were evaluated through various routes of administration, doses, and concentrations. The most common route included gum (15.6%), mouthwash (7.8%), tablet (6.5%) milk (5.2%) and varnish (5.2%) (Table 1). In total, 12,548 participants were included, with the majority falling within the age range of 6–12 years (childhood), followed by those between 12 and 18 years (adolescence), and 14–26 years (youth) (Table 2). Given the substantial heterogeneity in intervention components, dosing regimens, treatment durations, study populations, and outcome measures precluded the conduct of a meta-analysis.
3.2. Effects of the Anticariogenic Compounds
72.8% of the studies demonstrated significant effects on biological risk factors, in addition, 16.9% reported significant effects on clinically diagnosed dental caries (Table 1). Among the components demonstrating significant effects on clinically diagnosed dental caries were probiotics such as L. rhamnosus SP1 L. reuteri ATCC55730, L. paracasei SD1; synbiotic (probiotic and arginine); xylitol; toothpaste with arginine combined with dicalcium phosphate or with calcium carbonate; propolis; vitamin D; milk and vegetables (Table 2).
In biological risk factors, we identified components (probiotics, arginine, propolis, plant extracts, sugar substitutes, cherry or cranberry juice) with a significant effect on the decrease in S. mutans in saliva. Regarding salivary pH, the majority of the studies assessing this variable reported favorable outcomes, with probiotics, propolis, arginine, vegetable extracts, and dairy products being the most notable components. As for the dental biofilm index, most studies found a significant reduction, particularly with probiotics, propolis, vegetable extracts, apple chewing, and sugar substitutes like xylitol. Other beneficial effects, although less frequently reported, included increases in salivary flow, enhanced buffering capacity, and the production of nitrate, ammonium, and urea, as well as the arginolytic capacity of the microbiota. Regarding the adverse effects, a few studies (conducted with yogurt, milk, and sugar substitutes) reported mild adverse events, such as abdominal discomfort, headache, and allergic reactions (Table 2).
3.3. Quality of the Studies
The majority of the randomized, quasi-experimental, cross-sectional analytic, and cohort clinical studies (76.6%) met all or most of the criteria for the level of bias according to the tool used (Appendix B). Regarding the degree of recommendation, most studies were classified as having a moderate level (45.5%), followed by low (26%) and high (23.4%) levels (Table 1, Appendix B).
4. Discussion
Probiotics are the most extensively studied components; however, few studies have included long-term follow up and caries index assessment. Nevertheless, it is important to highlight that, depending on the strain of the probiotic used, a favorable effect on clinically diagnosed dental caries has been observed. In this review, the bacterial strains found to have favorable effect on caries indices included L. rhamnosus SP1 [], L. reuteri ATCC 55730 [], L. paracasei SD1 []. These findings support the potential of probiotics as a promising therapeutic strategy, particularly in children, as demonstrated in meta-analysis [,]. In contrast, other bacteria, such as those from the Bifidobacterium genus [], do not exhibit the same effect, emphasizing the importance of selecting the correct strains, as well as determining the appropriate dosage, treatment duration, application vehicle, and considering interactions with other microorganisms and/or the host [].
Some mechanisms of action of these beneficial microorganisms include the production of active metabolites (such as bacteriocins, hydrogen peroxide, and enzymes), inhibition of cariogenic microbial biofilm or adhesion, and competitive colonization against pathogens, competition for nutrients, and regulation of the immune system []. The other bacteria studied for dental caries control include Streptococcus strains, such as S. dentisani. Beyond its antimicrobial activity and prevalence in the oral cavity, this bacterium metabolizes arginine to produce ammonia, thereby contributing to the stabilization of dental biofilm pH [].
Another component identified as impacting caries reduction is the amino acid arginine, which is considered a prebiotic compound in the oral cavity due to its ability to stimulate alkaline-producing bacteria []. This review identified L-arginine in reducing caries indices as reported in a two-year follow up study [].
Tea is another component with favorable effects on dental caries-related indices, primarily due to its polyphenols, such as catechins, epigallocatechin-3-gallate, and epicatechin-3-gallate. These compounds possess antimicrobial properties, interfere with dental biofilm formation, and inhibit the glucosyltransferase activity of S. mutans []. In addition, tea contains amino acids, caffeine, and minerals such as fluoride, calcium, and phosphorus. However, the current evidence is insufficient to support the use of tea as a first-line treatment for caries []. In our review, the primary form of tea administration was through mouthwashes; however, chewing gum was also used for up to 2 years [], showing a significantly favorable effect on caries reduction with no reported adverse effects. The effect of routine tea consumption remains controversial due to its potential erosive impact on enamel [], although there is no conclusive evidence to support this claim [].
Polyphenols are also found in other agents with a favorable impact on dental caries, such as licorice (Glycyrrhiza glabra), which contains flavonoids and isoflavonoids, as well as other phytocompounds like the triterpenoid glycyrrhizin, known to inhibit S. mutans glucosyltransferase. It also provides essential minerals (such as calcium) and vitamins (including biotin and niacin) []. Studies have demonstrated the stable antimicrobial capacity of licorice, with no adverse effects reported []. In this review, licorice extract was primarily used, delivered through mouthwashes and food-based products such as lollipops, offering an appealing route of administration for children and adolescents.
Sugar substitutes such as xylitol, sorbitol, erythritol, stevia, and maltitol [] have been incorporated into food products like chewing gum, candies, milk, and cookies. Xylitol, a naturally occurring 5-carbon polyol, has been the most studied sugar substitute for its effect on the incidence of dental caries. It demonstrates significant favorable effects, particularly when used in chewing gum, as chewing stimulates saliva production, enhancing enamel remineralization [,]. Additionally, xylitol is non-fermentable by oral microbiota, thereby reducing acidogenic potential. However, some studies have reported adverse effects in participants with gastrointestinal issues. It is therefore recommended to limit the dosage to a maximum of 5 g, taken three times daily, or 7.5 g once daily [].
Other components significantly affecting caries incidence include vitamin D, cranberry rinse, milk, and vegetables. Children and adolescents with insufficient vitamin D levels (<50 nmol/L) have a significantly higher probability of developing dental caries (odds ratio [OR]: 1.13–2.57). In contrast, individuals with sufficient levels (≥50 nmol/L) demonstrate a protective effect (OR in children: 0.80 and OR in adolescents: 0.59). This finding underscores the importance of improving vitamin D levels. Vitamin D is primarily obtained through exposure to sunlight, although it can also be sourced from certain foods, such as some fish []. Its mechanism of action in oral health is primarily linked to bone metabolism [], mineralization of hard tissues, odontogenesis, and the immune system [].
Milk has been associated with a lower risk of caries across various age groups []—either alone or in combination with fluoride, probiotics, and sugar substitutes—as well as its derivatives, such as yogurt and cheese, with preliminary results. Vegetables are another component of interest due to their association with caries-free individuals, as reported by other studies []. These foods are rich in vitamins, antioxidants, and fiber []. Moreover, vegetables, particularly leafy greens and root vegetables such as beetroot, are rich in inorganic nitrates that are absorbed into the bloodstream and various organs, including the oral cavity, after ingestion. Once in the oral cavity, these nitrates are converted to nitrite [,] and, in some cases, to nitric oxide. This process has a beneficial effect on the oral microbiota by increasing the abundance of nitrate-reducing bacteria, such as Neisseria and Rothia species []. Additionally, vegetables help stabilize salivary pH and reduce lactate production following a sugar rinse, as lactate is utilized by nitrate-reducing bacteria [].
Our review also identified interventions that significantly and favorably impact the biological risk factors associated with dental caries. However, although the interventions demonstrated antimicrobial activity by inhibiting S. mutans, it is not the only cariogenic bacterium [] and is not always present in patients with caries. Furthermore, not all studies conducted adequate microbiological analyses to quantify this bacterium. Another frequently studied variable was salivary pH, with some interventions (probiotics, arginine, tea, licorice, dairy products such as cheese, and fennel seeds) significantly increasing the pH. This result is attributed to the reduction in cariogenic bacteria, thereby decreasing the production of organic acids that induce enamel demineralization [,]. High pH levels, along with elevated salivary flow rate and buffering capacity, help prevent dental caries by neutralizing the acids produced by cariogenic bacteria []. Finally, the dental biofilm index was significantly reduced with various interventions (probiotics, synbiotic, propolis, licorice, apple, and cocoa bean husk extract), contributing to a reduction in the presence of cariogenic bacteria and their organic acids in the dental biofilm.
This review highlights the identification of ingredients categorized as biotics, which include probiotics (various bacterial strains), prebiotics (arginine, nitrate, sugar substitutes, vegetable extracts), and a combination of both, known as synbiotics (probiotics + arginine). Biotics are gaining popularity as they are increasingly preferred by populations, and emerging evidence supports their role in oral health management [].
To better identify the natural components that can effectively improve oral health, further high quality randomized controlled trials with adequate sample sizes, longer follow-up periods, and standardized protocols are needed. Although this review included a substantial number of studies, most exhibited moderate to low methodological quality, relied on indirect measures such as S. mutans quantification rather than clinically diagnosed dental caries, had small samples sizes, short durations, and potential risks of bias such as inadequate randomization and lack of blinding. Moreover, significant heterogeneity in interventions, and outcome measures limits the generalizability of the findings and prevented the performance of a meta-analysis.
Clinically, natural anticariogenic agents show emerging potential for integration into preventive oral health programs; however, their effective and safe application requires careful evaluation of optimal formulations, dosages, and delivery methods, supported by robust regulatory oversight. Future research should focus on addressing these gaps by conducting rigorously designed trials that evaluate both efficacy and safety, as well as exploring the development of functional foods incorporating these components. Such efforts will be essential to translate preliminary findings into evidence-based clinical practice and public health strategies.
5. Conclusions
Probiotics, arginine, propolis, plant-derived compounds such as tea and licorice, and sugar substitutes like xylitol, vitamin D, milk, and vegetables have shown preliminary protective effects against dental caries through various mechanisms of action. However, the clinical efficacy of these agents remains to be confirmed, as most studies included in this review were of low or moderate methodological quality. Adverse events, such as stomach aches, headaches, or allergies, were infrequently reported, mainly in studies involving dairy products or sugar substitutes. Therefore, further high-quality clinical trials are necessary to substantiate these initial findings and better evaluate safety profiles.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/dj13110518/s1, Table S1: PRISMA 2020 Checklist.
Author Contributions
Conceptualization, M.d.P.A.-D.; methodology, Y.Y.-N., K.G.-P., H.G.-R. and L.C.-G.; investigation, M.d.P.A.-D., L.J.B.-C. and J.M.S.-O.; data curation, M.d.P.A.-D.; analysis, M.d.P.A.-D.; writing—original draft preparation, M.d.P.A.-D.; writing—review and editing, L.J.B.-C. and J.M.S.-O.; visualization, Y.Y.-N.; project administration, M.d.P.A.-D.; funding acquisition, M.d.P.A.-D. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been funded by the Universidad Cooperativa de Colombia (INV3580) (M.D.P.A.-D, Y.Y.-N, K.G.-P., L.C.-G, and H.G.-R.), Universidad Nacional (L.J.B.-C.), Universidad Católica de Cuenca (EXT-IC-035) (J.S.-O.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its Supplementary Information files.
Acknowledgments
We would like to thank Richard German Florez and Katherine Castañeda Enciso for their contribution in explaining the search process strategy.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Appendix A

Table A1.
Search strategy in various scientific databases.
Table A1.
Search strategy in various scientific databases.
| Scientific Databases | Search Strategy |
|---|---|
| Pubmed | ((“Anticariogenic” [Title/Abstract] OR “Caries-free” [Title/Abstract] OR “Dental Caries Susceptibility” [MeSH Terms] OR “Cariogenic Agents” [MeSH Terms]) AND (“Food” [MeSH Terms] OR “Foods” [Title/Abstract] OR (“Beverages” [MeSH Terms] OR “Beverage” [Title/Abstract]) OR (“Meals” [MeSH Terms] OR “meal *” [Title/Abstract] OR “dinner *” [Title/Abstract] OR “supper *” [Title/Abstract]) OR “Lunch”[MeSH Terms] OR (“Snacks”[MeSH Terms] OR “snack *” [Title/Abstract]))) AND (y_10 [Filter]) |
| Scopus | (TITLE-ABS-KEY (food OR foods OR beverages OR beverage OR lunch OR dinner OR extract) AND TITLE-ABS-KEY (anticariogenic OR “cariostatic agent” OR “Cariogenic Agents”) AND NOT TITLE-ABS-KEY (animals OR cancer OR vitro)) AND PUBYEAR > 2012 AND PUBYEAR < 2024 |
| Ovid | (Food.sh. or Foods.ab,kf,ti. or (Beverages.sh. or Beverage.ab,kf,ti.) or (Meals.sh. or Dinner.ab,kf,ti.) or (Snacks.sh. or Snack.ab,kf,ti.)) and (Anticariogenic.ab,kf,ti. or Cariostatic Agent.sh,ab,kf,ti. or Cariogenic Agent.sh,ab,kf,ti.) |
| J-Stage | (title:(Food * OR Beverage * OR Dinner * OR Lunch * OR Snack *) OR abstracttext:(Food * OR Beverage * OR Dinner * OR Lunch * OR Snack * OR Extract *) OR keyword:(Food * OR Beverage * OR Dinner * OR Lunch * OR Snack *)) AND (title:(Anticariogenic OR Cariostatic OR Cariogenic OR Antimicrobial OR Bactericide OR Bacteriostatic) OR abstracttext:(Anticariogenic OR Cariostatic OR Cariogenic OR Antimicrobial OR Bactericide OR Bacteriostatic) OR keyword:(Anticariogenic OR Cariostatic OR Cariogenic OR Antimicrobial OR Bactericide OR Bacteriostatic)) NOT (title:(vitro OR Animals OR Situ OR Fertilizer OR Cancer OR Pesticide OR Electric OR Foodborne) OR abstracttext:(Animals OR Situ OR Fertilizer OR Cancer OR Pesticide OR Electric OR Foodborne)) |
| BVS | (mh:(food)) OR (foods) OR (mh:(beverages)) OR (lunch) OR (dinner) OR (mh:(snacks)) OR (snack) AND (anticariogenic) OR (mh:(“Cariostatic Agent”)) OR (mh:(“Cariogenic Agent”)) AND NOT (mh:(animals)) AND NOT (ti:(vitro)) AND NOT (situ) AND NOT (rats) AND (fulltext:(“1” OR “1”) AND type_of_study:(“observational_studies” OR “clinical_trials” OR “incidence_studies” OR “evaluation_studies” OR “systematic_reviews” OR “screening_studies” OR “sysrev_observational_studies”) AND la:(“en” OR “es” OR “pt” OR “fr”)) AND (year_cluster: [2013 TO 2023]) |
| Google Scholar | Anticariogenic OR Cariostatic Food OR Foods OR Meals OR Beverages OR Beverage OR Lunch OR Dinner OR Snack-animals-“in vitro”-“vitro”-“in situ”-electromagnetic-ultrasound-nano-preservative-electric-peroxide-pesticide-fertilizer -foodborne–irradiation |
* In some keywords signals truncation to capture both singular and plural (and other variant) forms.
Appendix B. Quality of Included Studies

Table A2.
Randomized controlled trials.
Table A2.
Randomized controlled trials.
| Authors | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Q12 | Q13 | JBI | GRADE |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Poureslami et al., 2013 [] | 12/13 | MODERATE | |||||||||||||
| Taipale et al., 2013 [] | 13/13 | HIGH | |||||||||||||
| Chinnappa et al., 2013 [] | 4/13 | VERY LOW | |||||||||||||
| Pinto et al., 2014 [] | 13/13 | HIGH | |||||||||||||
| Nishihara et al., 2014 [] | 8/13 | MODERATE | |||||||||||||
| Stensson et al., 2014 [] | 13/13 | HIGH | |||||||||||||
| Bhalla et al., 2015 [] | 9/13 | LOW | |||||||||||||
| Cortés et al., 2015 [] | 9/13 | VERY LOW | |||||||||||||
| Srivastava et al., 2016 [] | 10/13 | MODERATE | |||||||||||||
| Koopaie et al., 2019 [] | 10/13 | LOW | |||||||||||||
| Piwat et al., 2020 [] | 13/13 | HIGH | |||||||||||||
| Ferrer et al., 2020 [] | 13/13 | HIGH | |||||||||||||
| Sakhare et al., 2021 [] | 10/13 | LOW | |||||||||||||
| Sandoval et al., 2021 [] | 8/13 | MODERATE | |||||||||||||
| Janiani et al., 2022 [] | 10/13 | MODERATE | |||||||||||||
| Hasslöf et al., 2022 [] | 13/13 | MODERATE | |||||||||||||
| Staszczyk et al., 2022 [] | 10/13 | MODERATE | |||||||||||||
| Pørksen et al., 2023 [] | 13/13 | HIGH | |||||||||||||
| Pørksen et al., 2023 [] | 13/13 | HIGH | |||||||||||||
| Campus et al., 2013 [] | 13/13 | HIGH | |||||||||||||
| Runnel et al., 2013 [] | 12/13 | HIGH | |||||||||||||
| Chi et al., 2017 [] | 13/13 | HIGH | |||||||||||||
| Aluckal et al., 2018 [] | 13/13 | MODERATE | |||||||||||||
| Abdelwahab et al., 2018 [] | 11/13 | MODERATE | |||||||||||||
| Cocco et al., 2019 [] | 13/13 | MODERATE | |||||||||||||
| Akgül et al., 2020 [] | 10/13 | MODERATE | |||||||||||||
| Neturi et al., 2014 [] | 10/13 | MODERATE | |||||||||||||
| Thomas et al., 2016 [] | 11/13 | MODERATE | |||||||||||||
| Prihastari and Putri, 2022 [] | 9/13 | LOW | |||||||||||||
| Tao et al., 2013 [] | 12/13 | HIGH | |||||||||||||
| Hedge and Kamath, 2017 [] | 10/13 | LOW | |||||||||||||
| Kamath et al., 2021 [] | 10/13 | LOW | |||||||||||||
| Jain et al., 2013 [] | 10/13 | LOW | |||||||||||||
| Almaz et al., 2017 [] | 12/13 | MODERATE | |||||||||||||
| Kim and Nam, 2021 [] | 13/13 | HIGH | |||||||||||||
| Helmy et al., 2021 [] | 13/13 | MODERATE | |||||||||||||
| Kamal et al., 2021 [] | 13/13 | MODERATE | |||||||||||||
| Kibriya et al., 2023 [] | 7/13 | LOW | |||||||||||||
| Bansal et al., 2024 [] | 13/13 | HIGH | |||||||||||||
| Salem et al., 2025 [] | 9/13 | MODERATE | |||||||||||||
| Vuletic et al., 2013 [] | 12/13 | MODERATE | |||||||||||||
| Nascimiento, et al., 2013 [] | 10/13 | HIGH | |||||||||||||
| Li et al., 2015 [] | 10/13 | HIGH | |||||||||||||
| Xue et al., 2017 [] | 13/13 | HIGH | |||||||||||||
| Razeghian-Jahromi et al., 2022 [] | 13/13 | HIGH | |||||||||||||
| Tulsani et al., 2014 [] | 11/13 | MODERATE | |||||||||||||
| Rodrigues et al., 2020 [] | 8/13 | VERY LOW | |||||||||||||
| El-Allaky et al., 2020 [] | 10/13 | MODERATE | |||||||||||||
| Rodrigues et al., 2021 [] | 10/13 | MODERATE | |||||||||||||
| Bapat et al., 2021 [] | 10/13 | LOW | |||||||||||||
| Rubido et al., 2018 [] | 8/13 | MODERATE | |||||||||||||
| Mojarad et al., 2021 [] | 7/13 | LOW | |||||||||||||
| Padminee et al., 2018 [] | 13/13 | MODERATE | |||||||||||||
| Philip et al., 2019 [] | 13/13 | HIGH | |||||||||||||
| Mishra et al., 2019 [] | 12/13 | MODERATE | |||||||||||||
| Somaraj et al., 2018 [] | 9/13 | MODERATE | |||||||||||||
| Gyll et al., 2018 [] | 13/13 | MODERATE | |||||||||||||
| Arponen et al., 2022 [] | 13/13 | HIGH | |||||||||||||
| Kamalaksharappa et al., 2018 [] | 6/13 | LOW | |||||||||||||
| Manikandan et al., 2020 [] | 7/13 | LOW | |||||||||||||
| Shetty et al., 2021 [] | 6/13 | LOW | |||||||||||||
| Talreja et al., 2018 [] | 6/13 | LOW |
JBI Critical appraisal checklist for randomized controlled trials. Q1. Was true randomization used for assignment of participants to treatment groups? Q2. Was allocation to treatment groups concealed? Q3. Were treatment groups similar at the baseline? Q4. Were participants blind to treatment assignment? Q5. Were those delivering treatment blind to treatment assignment? Q6. Were outcomes assessors blind to treatment assignment? Q7. Were treatment groups treated identically other than the intervention of interest? Q8. Was follow up complete and if not, were differences between groups in terms of their follow up adequately described and analyzed? Q9. Were participants analyzed in the groups to which they were randomized? Q10. Were outcomes measured in the same way for treatment groups? Q11. Were outcomes measured in a reliable way? Q12. Was appropriate statistical analysis used? Q13. Was the trial design appropriate, and any deviations from the standard RCT design (individual randomization, parallel groups) accounted for in the conduct and analysis of the trial? Green: compliant, Red: non-compliant, Yellow: unclear.

Table A3.
Quasi-experimental studies.
Table A3.
Quasi-experimental studies.
| Authors | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | JBI | GRADE |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Natassa et al., 2019 [] | 7/9 | MODERATE | |||||||||
| Patil et al., 2021 [] | 2/9 | VERY LOW | |||||||||
| Ferrazzano et al., 2016 [] | 8/9 | MODERATE | |||||||||
| Manohar et al., 2020 [] | 4/9 | LOW | |||||||||
| Lorenzini, et al., 2022 [] | 5/9 | MODERATE | |||||||||
| Silva et al., 2025 [] | 7/9 | LOW | |||||||||
| Homoki et al., 2018 [] | 8/9 | MODERATE | |||||||||
| Chen et al., 2019 [] | 5/9 | MODERATE | |||||||||
| Sterzenbach et al., 2023[] | 5/9 | LOW | |||||||||
| Pärnänen et al., 2023 [] | 5/9 | LOW | |||||||||
| Gul et al., 2018 [] | 6/9 | LOW |
JBI checklist for quasi-experimental studies. Q1. Is it clear in the study what is the “cause” and what is the “effect” (i.e., there is no confusion about which variable comes first)? Q2. Was there a control group? Q3. Were participants included in any comparisons similar? Q4. Were the participants included in any comparisons receiving similar treatment/care, other than the exposure or intervention of interest? Q5. Were there multiple measurements of the outcome, both pre and post the intervention/exposure? Q6. Were the outcomes of participants included in any comparisons measured in the same way? Q7. Were outcomes measured in a reliable way? Q8. Was follow-up complete and if not, were differences between groups in terms of their follow-up adequately described and analyzed? Q9. Was appropriate statistical analysis used? Green: compliant, Red: non-compliant, Yellow: unclear.

Table A4.
Analytical cross-sectional studies.
Table A4.
Analytical cross-sectional studies.
| Authors | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | JBI | GRADE |
|---|---|---|---|---|---|---|---|---|---|---|
| Wang et al., 2022 [] | 7/8 | LOW |
JBI critical appraisal checklist for analytical cross-sectional studies. Q1. Were the criteria for inclusion in the sample clearly defined? Q2. Were the study subjects and the setting described in detail? Q3. Was the exposure measured in a valid and reliable way? Q4. Were objective, standard criteria used for measurement of the condition? Q5. Were confounding factors identified? Q6. Were strategies to deal with confounding factors stated? Q7. Were the outcomes measured in a valid and reliable way? Q8. Was appropriate statistical analysis used? Green: compliant, Yellow: unclear.

Table A5.
Cohort studies.
Table A5.
Cohort studies.
| Authors | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | JBI | GRADE |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Suárez-Calleja et al., 2021 [] | 11/11 | MODERATE | |||||||||||
| Chankanka et al., 2015 [] | 11/11 | MODERATE | |||||||||||
| Lempert et al., 2015 [] | 11/11 | MODERATE |
JBI critical appraisal checklist for cohort studies. Q1. Were the two groups similar and recruited from the same population? Q2. Were the exposures measured similarly to assign people to both exposed and unexposed groups? Q3. Was the exposure measured in a valid and reliable way? Q4. We’re confounding factors identified? Q5. Were strategies to deal with confounding factors stated? Q6. Were the groups/participants free of the outcome at the start of the study (or at the moment of exposure)? Q7. Were the outcomes measured in a valid and reliable way? Q8. Was the follow up time reported and sufficient to be long enough for outcomes to occur? Q9. Was follow up complete, and if not, were the reasons to loss to follow up described and explored? Q10. Were strategies to address incomplete follow up utilized? Q11. Was appropriate statistical analysis used? Green: compliant.
References
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.; Yu, W.H.; Lakshmanan, A.; Wade, W.G. The human oral microbiome. J. Bacteriol. 2010, 192, 5002–5017. [Google Scholar] [CrossRef]
- Peters, B.A.; Wu, J.; Hayes, R.B.; Ahn, J. The oral fungal mycobiome: Characteristics and relation to periodontitis in a pilot study. BMC Microbiol. 2017, 17, 157. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.L.; Mark Welch, J.L.; Kauffman, K.M.; McLean, J.S.; He, X. The oral microbiome: Diversity, biogeography and human health. Nat. Rev. Microbiol. 2024, 22, 89–104. [Google Scholar] [CrossRef]
- Thakkar, P.; Banks, J.M.; Rahat, R.; Brandini, D.A.; Naqvi, A.R. Viruses of the oral cavity: Prevalence, pathobiology and association with oral diseases. Rev. Med. Virol. 2022, 32, e2311. [Google Scholar] [CrossRef]
- Gichki, A.S.; Mooen, M.; Hasni, M.T.; Ghilzi, D. Oral protozoa Entamoeba gingivalis and Trichomonas tenax among periodontitis and gingivitis patients in periodontology department dental section Quetta. J. Ayub Med. Coll. Abbottabad 2023, 35, 732–739. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Yang, X.; Li, C.; Song, Z. The Oral microbiota: Community composition, influencing factors, pathogenesis, and interventions. Front. Microbiol. 2022, 13, 895537. [Google Scholar] [CrossRef]
- Rosier, B.T.; Marsh, P.D.; Mira, A. Resilience of the oral microbiota in health: Mechanisms that prevent dysbiosis. J. Dent. Res. 2018, 97, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Wade, W.G. Resilience of the oral microbiome. Periodontol. 2000 2021, 86, 113–122. [Google Scholar] [CrossRef]
- Abisado, R.G.; Benomar, S.; Klaus, J.R.; Dandekar, A.A.; Chandler, J.R. Bacterial quorum sensing and microbial community interactions. mBio 2018, 9, e02331-17. [Google Scholar] [CrossRef]
- Xiao, E.; Mattos, M.; Vieira, G.H.A.; Chen, S.; Corrêa, J.D.; Wu, Y.; Albiero, M.L.; Bittinger, K.; Graves, D.T. Diabetes enhances IL-17 expression and alters the oral microbiome to increase its pathogenicity. Cell Host Microbe 2017, 22, 120–128.e4. [Google Scholar] [CrossRef] [PubMed]
- Kahharova, D.; Pappalardo, V.; Buijs, M.; de Menezes, R.; Peters, M.; Jackson, R.; Hara, A.; Eckert, G.; Katz, B.; Keels, M.; et al. Microbial indicators of dental health, dysbiosis, and early childhood caries. J. Dent. Res. 2023, 102, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Bowen, W.H.; Koo, H. Biology of Streptococcus mutans-derived glucosyltransferases: Role in extracellular matrix formation of cariogenic biofilms. Caries Res. 2011, 45, 69–86. [Google Scholar] [CrossRef]
- Krzyściak, W.; Jurczak, A.; Kościelniak, D.; Bystrowska, B.; Skalniak, A. The virulence of Streptococcus mutans and the ability to form biofilms. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 499–515. [Google Scholar] [CrossRef]
- Bojanich, M.A.; Orlietti, M.D. Virulence factors of Streptococcus mutans related to dental caries. In Staphylococcus and Streptococcus; IntechOpen: London, UK, 2020; Available online: https://www.intechopen.com/chapters/66603 (accessed on 15 June 2025).
- Tzimas, K.; Antoniadou, M.; Varzakas, T.; Voidarou, C.C. Plant-derived compounds: A promising tool for dental caries prevention. Curr. Issues Mol. Biol. 2024, 46, 5257–5290. [Google Scholar] [CrossRef]
- Jeon, J.G.; Rosalen, P.L.; Falsetta, M.L.; Koo, H. Natural products in caries research: Current (limited) knowledge, challenges and future perspective. Caries Res. 2011, 45, 243–263. [Google Scholar] [CrossRef]
- Anwar, M.A.; Sayed, G.A.; Hal, D.M.; El Hafeez, M.S.A.; Shatat, A.-A.S.; Salman, A.; Eisa, N.M.; Ramadan, A.; El-Shiekh, R.A.; Hatem, S.; et al. Herbal remedies for oral and dental health: A comprehensive review of their multifaceted mechanisms including antimicrobial, anti-inflammatory, and antioxidant pathways. Inflammopharmacology 2025, 33, 1085–1160. [Google Scholar] [CrossRef]
- Li, A.; Ma, Y.; Cui, N.; Zhang, X.; Zheng, Q.; Du, P.; Sun, M. Research progress of milk and dairy products to prevent caries. J. Funct. Foods 2023, 110, 105837. [Google Scholar] [CrossRef]
- Malin, A.J.; Wang, Z.; Khan, D.; McKune, S.L. The potential systemic role of diet in dental caries development and arrest: A narrative review. Nutrients 2024, 16, 1463. [Google Scholar] [CrossRef]
- Meng, N.; Liu, Q.; Dong, Q.; Gu, J.; Yang, Y. Effects of probiotics on preventing caries in preschool children: A systematic review and meta-analysis. J. Clin. Pediatr. Dent. 2023, 47, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.W.; Liang, N.L.; Townsend, J.A.; Lo, E.C.M.; Chu, C.H.; Duangthip, D. Sugar substitutes on caries prevention in permanent teeth among children and adolescents: A systematic review and meta-analysis. J. Dent. 2024, 146, 105069. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
- Joanna Briggs Institute. Critical Appraisal Tools. Available online: https://jbi.global/critical-appraisal-tools (accessed on 20 January 2025).
- Prasad, M. Introduction to the GRADE tool for rating certainty in evidence and recommendations. Clin. Epidemiol. Glob. Health 2024, 25, 101484. [Google Scholar] [CrossRef]
- Poureslami, H.; Pishbin, L.; Eslaminejad, Z.; Jahani Moqadam, F.; Rashid Farokhi, M. The effects of a dairy probiotic product, espar, on salivary calcium and mutans streptococci. J. Dent. Res. Dent. Clin. Dent. Prospects 2013, 7, 147–151. [Google Scholar] [CrossRef]
- Taipale, T.; Pienihäkkinen, K.; Alanen, P.; Jokela, J.; Söderling, E. Administration of Bifidobacterium animalis subsp. lactis BB-12 in early childhood: A post-trial effect on caries occurrence at four years of age. Caries Res. 2013, 47, 364–372. [Google Scholar] [CrossRef]
- Chinnappa, A.; Konde, H.; Konde, S.; Raj, S.; Beena, J.P. Probiotics for future caries control: A short-term clinical study. Indian J. Dent. Res. 2013, 24, 547–549. [Google Scholar] [CrossRef]
- Pinto, G.S.; Cenci, M.S.; Azevedo, M.S.; Epifanio, M.; Jones, M.H. Effect of yogurt containing Bifidobacterium animalis subsp lactis DN-173010 probiotic on dental plaque and saliva in orthodontic patients. Caries Res. 2014, 48, 63–68. [Google Scholar] [CrossRef]
- Nishihara, T.; Suzuki, N.; Yoneda, M.; Hirofuji, T. Effects of Lactobacillus salivarius-containing tablets on caries risk factors: A randomized open-label clinical trial. BMC Oral Health 2014, 14, 110. [Google Scholar] [CrossRef] [PubMed]
- Stensson, M.; Koch, G.; Coric, S.; Abrahamsson, T.R.; Jenmalm, M.C.; Birkhed, D.; Wendt, L.-K. Oral administration of Lactobacillus reuteri during the first year of life reduces caries prevalence in the primary dentition at 9 years of age. Caries Res. 2014, 48, 111–117. [Google Scholar] [CrossRef]
- Bhalla, M.; Ingle, N.A.; Kaur, N.; Yadav, P. Mutans streptococci estimation in saliva before and after consumption of probiotic curd among school children. J. Int. Soc. Prev. Community Dent. 2015, 5, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Dorantes, N.; Ruiz-Rodríguez, M.S.; Karakowsky-Kleiman, L.; Garrocho-Rangel, J.A.; Sánchez-Vargas, L.O.; Pozos-Guillén, A.J. Probiotics and their effect on oral bacteria count in children: A pilot study. Eur. J. Paediatr. Dent. 2015, 16, 56–60. [Google Scholar]
- Srivastava, S.; Saha, S.; Kumari, M.; Mohd, S. Effect of probiotic curd on salivary pH and Streptococcus mutans: A double blind parallel randomized controlled trial. J. Clin. Diagn. Res. 2016, 10, ZC13–ZC16. [Google Scholar] [CrossRef] [PubMed]
- Koopaie, M.; Fatahzadeh, M.; Jahangir, S.; Bakhtiari, R. Comparison of the effect of regular and probiotic cake (Bacillus coagulans) on salivary pH and Streptococcus mutans count. Dent. Med. Probl. 2019, 56, 33–38. [Google Scholar] [CrossRef]
- Piwat, S.; Teanpaisan, R.; Manmontri, C.; Wattanarat, O.; Pahumunto, N.; Makeudom, A.; Krisanaprakornkit, S.; Nirunsittirat, A. Efficacy of probiotic milk for caries regression in preschool children: A multicenter randomized controlled trial. Caries Res. 2020, 54, 491–501. [Google Scholar] [CrossRef]
- Ferrer, M.D.; López-López, A.; Nicolescu, T.; Perez-Vilaplana, S.; Boix-Amorós, A.; Dzidic, M.; Garcia, S.; Artacho, A.; Llena, C.; Mira, A. Topic application of the probiotic Streptococcus dentisani improves clinical and microbiological parameters associated with oral health. Front. Cell. Infect. Microbiol. 2020, 10, 465. [Google Scholar] [CrossRef]
- Sakhare, S.; Shantanu, C.; Mopagar, V.; Hadpe, H.S.; Choughule, K.; Dahapute, S.; Shetty, S.; Joshi, S. A comparative evaluation of probiotic formulations in prevention of dental caries: A clinical study. J. Indian Soc. Pedod. Prev. Dent. 2021, 39, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, F.; Faleiros, S.; Cabello, R.; Díaz-Dosque, M.; Rodríguez, G.; Escobar, A. The consumption of milk supplemented with probiotics decreases the occurrence of caries and the salivary concentration of hβD-3 in children. Clin. Oral Investig. 2021, 25, 3823–3830. [Google Scholar] [CrossRef]
- Janiani, P.; Ravindran, V. Comparative evaluation of the antimicrobial effects of probiotic milk and probiotic powder on the salivary Streptococcus mutans counts and the plaque scores in children aged 3–6 years: A randomized controlled trial. Dent. Med. Probl. 2022, 59, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Hasslöf, P.; Granqvist, L.; Stecksén-blicks, C.; Twetman, S. Prevention of recurrent childhood caries with probiotic supplements: A randomized controlled trial with a 12-month follow-up. Probiotics Antimicrob. Proteins 2022, 14, 384–390. [Google Scholar] [CrossRef]
- Staszczyk, M.; Jamka-Kasprzyk, M.; Kościelniak, D.; Cienkosz-Stepańczak, B.; Krzyściak, W.; Jurczak, A. Effect of a short-term intervention with lactobacillus salivarius probiotic on early childhood caries-an open label randomized controlled trial. Int. J. Environ. Res. Public Health 2022, 19, 12447. [Google Scholar] [CrossRef]
- Pørksen, C.J.; Keller, M.K.; Damholt, A.; Frederiksen, A.K.S.; Ekstrand, K.R.; Markvart, M.; Larsen, T.; Bakhshandeh, A. The effect of a lozenge combining prebiotic arginine and probiotics on caries increment in children during 10–12 months, a randomized clinical trial. J. Dent. 2023, 135, 104599. [Google Scholar] [CrossRef]
- Pørksen, C.J.; Ekstrand, K.R.; Markvart, M.; Larsen, T.; Garrido, L.E.; Bakhshandeh, A. The efficacy of combined arginine and probiotics as an add-on to 1450 ppm fluoride toothpaste to prevent and control dental caries in children—A randomized controlled trial. J. Dent. 2023, 137, 104670. [Google Scholar] [CrossRef]
- Campus, G.; Cagetti, M.G.; Sale, S.; Petruzzi, M.; Solinas, G.; Strohmenger, L.; Lingström, P. Six months of high-dose xylitol in high-risk caries subjects—A 2-year randomised, clinical trial. Clin. Oral Investig. 2013, 17, 785–791. [Google Scholar] [CrossRef]
- Runnel, R.; Mäkinen, K.K.; Honkala, S.; Olak, J.; Mäkinen, P.-L.; Nõmmela, R.; Vahlberg, T.; Honkala, E.; Saag, M. Effect of three-year consumption of erythritol, xylitol and sorbitol candies on various plaque and salivary caries-related variables. J. Dent. 2013, 41, 1236–1244. [Google Scholar] [CrossRef] [PubMed]
- Chi, D.L.; Zegarra, G.; Huerta, E.C.V.; Castillo, J.L.; Milgrom, P.; Roberts, M.C.; Cabrera-Matta, A.R.; Merino, A.P. Milk sweetened with xylitol: A proof-of-principle caries prevention randomized clinical trial. J. Dent. Child. 2016, 83, 152–160. [Google Scholar]
- Aluckal, E.; Ankola, A.V. Effectiveness of xylitol and polyol chewing gum on salivary Streptococcus mutans in children: A randomized controlled trial. Indian J. Dent. Res. 2018, 29, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahab, D.H.; Allam, G.G.; Abdel-Aziz, A.M. Effect of xylitol and sugar-free chewing gums on salivary bacterial count of Streptococcus mutans and Lactobacilli in a group of Egyptian school children of different ages: A randomized clinical trial. Futur. Dent. J. 2018, 4, 216–220. [Google Scholar] [CrossRef]
- Cocco, F.; Cagetti, M.G.; Livesu, R.; Camoni, N.; Pinna, R.; Lingstrom, P.; Campus, G. Effect of a daily dose of snacks containing maltitol or Stevia rebaudiana as sweeteners in high caries risk schoolchildren. A double-blind rct study. Oral Health Prev. Dent. 2019, 17, 515–522. [Google Scholar] [CrossRef]
- Akgül, Ö.; Topaloğlu Ak, A.; Zorlu, S.; Öner Özdaş, D.; Uslu, M.; Çayirgan, D. Effects of short-term xylitol chewing gum on pro-inflammatory cytokines and Streptococcus mutans: A randomised, placebo-controlled trial. Int. J. Clin. Pract. 2020, 74, e13623. [Google Scholar] [CrossRef]
- Neturi, R.S.; Srinivas, R.; Simha, V.; Sree, S.; Shekar, C.; Kumar, S. Effects of Green Tea on Streptococcus mutans Counts—A Randomised Control Trail. J. Clin. Diagn. Res. 2014, 8, ZC128–ZC130. [Google Scholar] [CrossRef]
- Thomas, A.; Thakur, S.R.; Shetty, S.B. Anti-microbial efficacy of green tea and chlorhexidine mouth rinses against Streptococcus mutans, Lactobacilli spp. and Candida albicans in children with severe early childhood caries: A randomized clinical study. J. Indian Soc. Pedod. Prev. Dent. 2016, 34, 65–70. [Google Scholar] [CrossRef]
- Prihastari, L.; Putri, M.A. The changes in salivary ph by chewing black tea (Camellia sinensis) candy containing the sorbitol. Dentino 2022, 7, 210–214. [Google Scholar] [CrossRef]
- Tao, D.Y.; Shu, C.B.; Lo, E.C.; Lu, H.X.; Feng, X.P. A randomized trial on the inhibitory effect of chewing gum containing tea polyphenol on caries. J. Clin. Pediatr. Dent. 2013, 38, 67–70. [Google Scholar] [CrossRef]
- Hegde, R.J.; Kamath, S. Comparison of the Streptococcus mutans and Lactobacillus colony count changes in saliva following chlorhexidine (0.12%) mouth rinse, combination mouth rinse, and green tea extract (0.5%) mouth rinse in children. J. Indian Soc. Pedod. Prev. Dent. 2017, 35, 150–155. [Google Scholar] [CrossRef]
- Kamath, S.; Hegde, R.; Kamath, N. Comparison of the Streptococcus mutans colony count changes in plaque following chlorhexidine (0.12%) mouth rinse and green tea extract (0.5%) mouth rinse in 8–12-year-old children. J. Indian Soc. Pedod. Prev. Dent. 2021, 39, 310–315. [Google Scholar] [CrossRef]
- Jain, E.; Pandey, R.K.; Khanna, R. Liquorice root extracts as potent cariostatic agents in pediatric practice. J. Indian Soc. Pedod. Prev. Dent. 2013, 31, 146–152. [Google Scholar] [CrossRef]
- Almaz, M.E.; Sönmez, I.Ş.; Ökte, Z.; Oba, A.A. Efficacy of a sugar-free herbal lollipop for reducing salivary Streptococcus mutans levels: A randomized controlled trial. Clin. Oral Investig. 2017, 21, 839–845. [Google Scholar] [CrossRef]
- Kim, Y.R.; Nam, S.H. A randomized, double-blind, placebo-controlled clinical trial of a mouthwash containing Glycyrrhiza uralensis extract for preventing dental caries. Int. J. Environ. Res. Public Health 2021, 19, 242. [Google Scholar] [CrossRef]
- Helmy, N.; Hafez, S.; Farid, A. Efficacy of licorice on salivary Streptococcus mutans levels vs chlorhexidine mouthwash in high caries risk patients: A randomized clinical trial. J. Contemp. Dent. Pract. 2021, 22, 914–921. [Google Scholar] [CrossRef]
- Kamal, D.; Hassanein, H.; Akah, M.; Abdelkawy, M.A.; Hamza, H. Caries preventive and antibacterial effects of two natural mouthwashes vs chlorhexidine in high caries-risk patients: A randomized clinical trial. J. Contemp. Dent. Pract. 2020, 21, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- Kibriya, S.; Srinivasan, I.; Setty, J.V.; Anu, S.; Khan, B.S. Characterization of cocoa bean husk extract particles and its comparison as a mouthrinse with different vehicles in children aged 7–12 years. Int. J. Clin. Pediatr. Dent. 2023, 16, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Bansal, K.; Shamoo, A.; Mohapatra, S.; Kalaivani, M.; Batra, P.; Mathur, V.P.; Srivastava, A.; Chaudhry, R. Comparative evaluation of cranberry extract and sodium fluoride as mouth rinses on S. mutans counts in children: A double-blind randomized controlled trial. Eur. Arch. Paediatr. Dent. 2024, 25, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Salem, G.; ElMansy, M.; Allam, E.; Anter, A.; Abdelmonem, R.; Rashed, M. Anticariogenic efficacy of Indian Costus mouthwash in children. J. Herb. Med. 2025, 50, 100997. [Google Scholar] [CrossRef]
- Vuletic, L.; Spalj, S.; Rogic, D.; Ruzic, L.; Alajbeg, I. Effect of L-arginine dietary supplementation on salivary urea concentration and pH in physically active individuals. Aust. Dent. J. 2013, 58, 491–497. [Google Scholar] [CrossRef]
- Nascimento, M.M.; Browngardt, C.; Xu, X.; Klepac-Ceraj, V.; Paster, B.J.; Burne, R.A. The effect of arginine on oral biofilm communities. Mol. Oral Microbiol. 2014, 29, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhong, Y.; Jiang, X.; Mateo, L.R.; Morrison, B.M., Jr.; Zhang, Y.-P. Randomized clinical trial of the efficacy of dentifrices containing 1.5% arginine, an insoluble calcium compound and 1450 ppm fluoride over two years. J. Clin. Dent. 2015, 26, 7–12. [Google Scholar]
- Xue, Y.; Lu, Q.; Tian, Y.; Zhou, X.; Cheng, L.; Ren, B. Effect of toothpaste containing arginine on dental plaque—A randomized controlled in situ study. J. Dent. 2017, 67, 88–93. [Google Scholar] [CrossRef]
- Razeghian-Jahromi, I.; Babanouri, N.; Ebrahimi, Z.; Najafi, H.Z.; Sarbaz, M.; Montazeri-Najafabady, N. Effect of 8% arginine toothpaste on Streptococcus mutans in patients undergoing fixed orthodontic treatment: Randomized controlled trial. Dent. Press J. Orthod. 2022, 27, e2220322. [Google Scholar] [CrossRef]
- Tulsani, S.G.; Chikkanarasaiah, N.; Siddaiah, S.B.; Krishnamurthy, N.H. The effect of Propolis and Xylitol chewing gums on salivary Streptococcus mutans count: A clinical trial. Indian J. Dent. Res. 2014, 25, 737–741. [Google Scholar] [CrossRef]
- Neto, E.M.R.; Valadas, L.A.R.; Lobo, P.L.D.; Fernandes, A.M.B.; Fonseca, S.G.d.C.; Fechine, F.V.; Júnior, F.J.G.; Bandeira, M.A.M.; Fonteles, M.M.d.F. Dose-response evaluation of propolis dental varnish in children: A randomized control study. Recent Pat. Biotechnol. 2020, 14, 41–48. [Google Scholar] [CrossRef]
- El-Allaky, H.S.; Wahba, N.A.; Talaat, D.M.; Zakaria, A.S. Antimicrobial effect of propolis administered through two different vehicles in high caries risk children: A randomized clinical trial. J. Clin. Pediatr. Dent. 2020, 44, 289–295. [Google Scholar] [CrossRef]
- Neto, E.M.R.; Valadas, L.A.R.; Lobo, P.L.D.; Fonseca, S.G.d.C.; Fechine, F.V.; Lotif, M.A.L.; Bandeira, M.A.M.; Mendonça, J.F.; de Mendonça, K.M.; Fonteles, M.M.d.F. Antimicrobial efficacy of propolis-containing varnish in children: A randomized and double-blind clinical trial. Evid. Based Complement. Altern. Med. 2021, 2021, 5547081. [Google Scholar] [CrossRef]
- Bapat, S.; Nagarajappa, R.; Ramesh, G.; Bapat, K. Effect of propolis mouth rinse on oral microorganisms—A randomized controlled trial. Clin. Oral Investig. 2021, 25, 6139–6146. [Google Scholar] [CrossRef] [PubMed]
- Rubido, S.; García-Caballero, L.; Abeleira, M.T.; Limeres, J.; García, M.; Diz, P. Effect of chewing an apple on dental plaque removal and on salivary bacterial viability. PLoS ONE 2018, 13, e0199812. [Google Scholar] [CrossRef] [PubMed]
- Mojarad, F.; Moghaddam, N.E.; Farhadian, M.; Ahmadi, B.; Torkzaban, P. Plaque removal efficacy of chewing apples and tooth-brushing: A comparative cross over clinical study. Avicenna J. Dent. Res. 2021, 13, 86–91. [Google Scholar] [CrossRef]
- Padminee, K.; Poorni, S.; Diana, D.; Duraivel, D.; Srinivasan, M.R. Effectiveness of casein phosphopeptide-amorphous calcium phosphate and xylitol chewing gums on salivary pH, buffer capacity, and Streptococcus mutans levels: An interventional study. Indian J. Dent. Res. 2018, 29, 616–621. [Google Scholar] [CrossRef]
- Philip, N.; Leishman, S.J.; Bandara, H.M.H.N.; Healey, D.L.; Walsh, L.J. Randomized controlled study to evaluate microbial ecological effects of CPP-AP and cranberry on dental plaque. JDR Clin. Trans. Res. 2020, 5, 118–126. [Google Scholar] [CrossRef]
- Mishra, P.; Marwah, N.; Agarwal, N.; Chaturvedi, Y.; Suohu, T. Comparison of Punica granatum, Terminalia chebula, Vitis vinifera seed extracts used as mouthrinse on salivary Streptococcus mutans levels in children. J. Contemp. Dent. Pract. 2019, 20, 920–927. [Google Scholar]
- Somaraj, V.; Shenoy, R.P.; Panchmal, G.S.; Jodalli, P.S.; Sonde, L.; Nagaraj, K. Effect of paneer and cheese consumption on salivary acidogenicity and calcium concentration: A comparative study. Oral Health Prev. Dent. 2018, 16, 169–174. [Google Scholar] [CrossRef]
- Gyll, J.; Ridell, K.; Öhlund, I.; Åkeson, P.K.; Johansson, I.; Holgerson, P.L. Vitamin D status and dental caries in healthy Swedish children. Nutr. J. 2018, 17, 11. [Google Scholar] [CrossRef]
- Arponen, H.; Waltimo-Sirén, J.; Hauta-Alus, H.H.; Tuhkiainen, M.; Sorsa, T.; Tervahartiala, T.; Andersson, S.; Mäkitie, O.; Holmlund-Suila, E. Effects of a 2-year early childhood vitamin d3 intervention on tooth enamel and oral health at age 6–7 years. Horm. Res. Paediatr. 2023, 96, 385–394. [Google Scholar] [CrossRef]
- Kamalaksharappa, S.K.; Rai, R.; Babaji, P.; Pradeep, M.C. Efficacy of probiotic and green tea mouthrinse on salivary pH. J. Indian Soc. Pedod. Prev. Dent. 2018, 36, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Talreja, N.; Devendrappa, S.; Singla, S.; Agrawal, N.; Mali, S. An in vivo comparison of plaque ph changes in children aged 8–12 years after consumption of milk and green tea with sugar. J. Int. Oral Health 2018, 10, 10–15. [Google Scholar] [CrossRef]
- Manikandan, S.; Behera, S.; Karthikeyan, R.; Niranjana, A.; Bharathan, R.; Mohammed, O.F.B. Effect of green tea extract mouthrinse and probiotic mouthrinse on salivary ph in a group of schoolchildren: An in vivo study. J. Pharm. Bioallied Sci. 2020, 12, S404–S409. [Google Scholar] [CrossRef] [PubMed]
- Shetty Naik, S.; Shetty, A.; Kodical, S.; Thakur, K.; Choudhury, S.; Balasubramanian, N. Efficacy of probiotic and green tea mouth rinse on salivary pH after a chocolate challenge. J. Cardiovasc. Dis. Res. 2021, 12, 3504–3511. [Google Scholar]
- Natassa, S.E.; Pintauli, S.; Ilyas, S. Effectivity of probiotic and non-probiotic milk consumption on salivary pH and Streptococcus mutans count. IOSR-JDMS 2019, 18, 67–72. [Google Scholar]
- Patil, R.U.; Nachan, V.P.; Patil, S.S.; Mhaske, R.V. A clinical trial on topical effect of probiotics on oral Streptococcus mutans counts in children. J. Indian Soc. Pedod. Prev. Dent. 2021, 39, 279–283. [Google Scholar] [CrossRef]
- Ferrazzano, G.F.; Cantile, T.; Coda, M.; Alcidi, B.; Sangianantoni, G.; Ingenito, A.; Di Stasio, M.; Volpe, M.G. In vivo release kinetics and antibacterial activity of novel polyphenols-enriched chewing gums. Molecules 2016, 21, 1008. [Google Scholar] [CrossRef] [PubMed]
- Manohar, R.; Ganesh, A.; Abbyramy, N.; Abinaya, R.; Balaji, S.K.; Priya, S.B. The effect of fennel seeds on pH of saliva—A clinical study. Indian J. Dent. Res. 2020, 31, 921–923. [Google Scholar] [CrossRef]
- Lorenzini, E.C.; Lazzari, B.; Tartaglia, G.M.; Farronato, G.; Lanteri, V.; Botti, S.; Biscarini, F.; Cozzi, P.; Stella, A. Oral ecological environment modifications by hard-cheese: From pH to microbiome: A prospective cohort study based on 16S rRNA metabarcoding approach. J. Transl. Med. 2022, 20, 312. [Google Scholar] [CrossRef]
- Silva, J.R.; Villas-Bôas, J.; Biz, G.; Couto-Almeida, R.S.; Spinosa, W.; Prudencio, S.H. Impact of organic, conventional, and stingless bee honeys on the antibacterial activity of gummy candies against oral bacteria. J. Oral Biosci. 2025, 67, 100589. [Google Scholar] [CrossRef]
- Homoki, J.; Gyémánt, G.; Balogh, P.; Stündl, L.; Bíró-Molnár, P.; Paholcsek, M.; Váradi, J.; Ferenc, F.; Kelentey, B.; Nemes, J.; et al. Sour cherry extract inhibits human salivary α-amylase and growth of Streptococcus mutans (a pilot clinical study). Food Funct. 2018, 9, 4008–4016. [Google Scholar] [CrossRef]
- Chen, Y.; Agnello, M.; Dinis, M.; Chien, K.C.; Wang, J.; Hu, W.; Shi, W.; He, X.; Zou, J. Lollipop containing Glycyrrhiza uralensis extract reduces Streptococcus mutans colonization and maintains oral microbial diversity in Chinese preschool children. PLoS ONE 2019, 14, e0221756. [Google Scholar] [CrossRef] [PubMed]
- Sterzenbach, T.; Hannig, C.; Hertel, S. Influence of consumption of nitrate-rich beetroot juice on lactate production in saliva and oral biofilm—A clinical trial. Oral Health Prev. Dent. 2023, 21, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Pärnänen, P.; Lomu, S.; Räisänen, I.T.; Tervahartiala, T.; Sorsa, T. Antimicrobial and anti-inflammatory oral effects of fermented lingonberry juice—A one-year prospective human intervention study. Eur. J. Dent. 2023, 17, 1235–1240. [Google Scholar] [CrossRef]
- Gul, P.; Akgul, N.; Seven, N. Anticariogenic potential of white cheese, xylitol chewing gum, and black tea. Eur. J. Dent. 2018, 12, 199–203. [Google Scholar] [CrossRef]
- Wang, X.; Ma, Z.; Lei, M.; Zhao, C.; Lin, X.; Cao, F.; Shi, H. Association between early childhood caries and diet quality among Chinese children aged 2–5 years. Front. Public Health 2022, 10, 974419. [Google Scholar] [CrossRef]
- Suárez-Calleja, C.; Aza-Morera, J.; Iglesias-Cabo, T.; Tardón, A. Vitamin D, pregnancy and caries in children in the INMA-Asturias birth cohort. BMC Pediatr. 2021, 21, 380. [Google Scholar] [CrossRef] [PubMed]
- Chankanka, O.; Levy, S.M.; Marshall, T.A.; Cavanaugh, J.E.; Warren, J.J.; Broffitt, B.; Kolker, J.L. The associations between dietary intakes from 36 to 60 months of age and primary dentition non-cavitated caries and cavitated caries. J. Public Health Dent. 2015, 75, 265–273. [Google Scholar] [CrossRef]
- Lempert, S.M.; Christensen, L.B.; Froberg, K.; Raymond, K.; Heitmann, B.L. Association between dairy intake and caries among children and adolescents. results from the Danish EYHS follow-up study. Caries Res. 2015, 49, 251–258. [Google Scholar] [CrossRef]
- Shi, J.; Wang, Q.; Ruan, G.; Chen, Y.; Zhao, M.; Shi, D.; Pan, B.; Xu, Z.; Zhang, T.; Wang, F.; et al. Efficacy of probiotics against dental caries in children: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2023, 63, 9977–9994. [Google Scholar] [CrossRef]
- Hao, S.; Wang, J.; Wang, Y. Effectiveness and safety of Bifidobacterium in preventing dental caries: A systematic review and meta-analysis. Acta Odontol. Scand. 2021, 79, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.-C.; Wei, S.-M.; Luo, X.-T.; Yang, Q.-Q.; Wong, K.-H.; Cheung, P.C.K.; Zhang, B.-B. How probiotics, prebiotics, synbiotics, and postbiotics prevent dental caries: An oral microbiota perspective. NPJ Biofilms Microbiomes 2024, 10, 14. [Google Scholar] [CrossRef]
- Camelo-Castillo, A.; Benítez-Páez, A.; Belda-Ferre, P.; Cabrera-Rubio, R.; Mira, A. Streptococcus dentisani sp. nov., a novel member of the mitis group. Int. J. Syst. Evol. Microbiol. 2014, 64 Pt 1, 60–65, Erratum in Int. J. Syst. Evol. Microbiol. 2014, 64 Pt 3, 1073. [Google Scholar] [CrossRef]
- Bijle, M.N.; Ekambaram, M.; Yiu, C.K.Y. A Scoping review on arginine in caries prevention. J. Evid. Based Dent. Pract. 2020, 20, 101470. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.-Y.; Meng, X.; Gan, R.-Y.; Zhao, C.-N.; Liu, Q.; Feng, Y.-B.; Li, S.; Wei, X.-L.; Atanasov, A.G.; Corke, H.; et al. Health functions and related molecular mechanisms of tea components: An update review. Int. J. Mol. Sci. 2019, 20, 6196. [Google Scholar] [CrossRef]
- Mazur, M.; Ndokaj, A.; Jedlinski, M.; Ardan, R.; Bietolini, S.; Ottolenghi, L. Impact of green tea (Camellia Sinensis) on periodontitis and caries. Systematic review and meta-analysis. Jpn. Dent. Sci. Rev. 2021, 57, 1–11. [Google Scholar] [CrossRef]
- Brunton, P.A.; Hussain, A. The erosive effect of herbal tea on dental enamel. J. Dent. 2001, 29, 517–520. [Google Scholar] [CrossRef]
- Jin, B.; Chen, H.; Liu, P.; Wang, Y.; Guo, Y.; Wang, C.; Jia, Y.; Zou, R.; Niu, L. Assessing the association between tea intake and risk of dental caries and periodontitis: A two-sample Mendelian randomization study. Sci. Rep. 2024, 14, 4728. [Google Scholar] [CrossRef]
- AlDehlawi, H.; Jazzar, A. The power of licorice (Radix glycyrrhizae) to improve oral health: A comprehensive review of its pharmacological properties and clinical implications. Healthcare 2023, 11, 2887. [Google Scholar] [CrossRef]
- Nuvvula, S.; Nunna, M.; Almaz, M.E.; Mallineni, S.K. Efficacy of licorice lollipops in reducing dental caries in a paediatric population: A systematic review. Oral Health Prev. Dent. 2020, 18, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Ramasubbu, D.; Duane, B. Do chewing gums and sweets containing xylitol prevent caries in children? Evid. Based Dent. 2024, 25, 89–90. [Google Scholar] [CrossRef]
- ALHumaid, J.; Bamashmous, M. Meta-analysis on the effectiveness of xylitol in caries prevention. J. Int. Soc. Prev. Community Dent. 2022, 12, 133–138. [Google Scholar] [CrossRef]
- Vernacchio, L.; Vezina, R.M.; Mitchell, A.A. Tolerability of oral xylitol solution in young children: Implications for otitis media prophylaxis. Int. J. Pediatr. Otorhinolaryngol. 2007, 71, 89–94. [Google Scholar] [CrossRef]
- Botelho, J.; Machado, V.; Proença, L.; Delgado, A.S.; Mendes, J.J. Vitamin D deficiency and oral health: A comprehensive review. Nutrients 2020, 12, 1471. [Google Scholar] [CrossRef] [PubMed]
- Tenelanda-López, D.; Valdivia-Moral, P.; Castro-Sánchez, M. Eating habits and their relationship to oral health. Nutrients 2020, 12, 2619. [Google Scholar] [CrossRef]
- Brennan, D.S.; Singh, K.A.; Liu, P.; Spencer, A. Fruit and vegetable consumption among older adults by tooth loss and socio-economic status. Aust. Dent. J. 2010, 55, 143–149. [Google Scholar] [CrossRef]
- Alhulaefi, S.S.; Watson, A.W.; Ramsay, S.E.; Jakubovics, N.S.; Matu, J.; Griffiths, A.; Kimble, R.; Siervo, M.; Brandt, K.; Shannon, O.M. Effects of dietary nitrate supplementation on oral health and associated markers of systemic health: A systematic review. Crit. Rev. Food Sci. Nutr. 2024, 11, 2813–2828. [Google Scholar] [CrossRef]
- Rosier, B.T.; Johnston, W.; Carda-Diéguez, M.; Simpson, A.; Cabello-Yeves, E.; Piela, K.; Reilly, R.; Artacho, A.; Easton, C.; Burleigh, M.; et al. Nitrate reduction capacity of the oral microbiota is impaired in periodontitis: Potential implications for systemic nitric oxide availability. Int. J. Oral Sci. 2024, 16, 1, Erratum in Int. J. Oral Sci. 2024, 16, 8. [Google Scholar] [CrossRef]
- Rosier, B.T.; Palazón, C.; García-Esteban, S.; Artacho, A.; Galiana, A.; Mira, A. A Single dose of nitrate increases resilience against acidification derived from sugar fermentation by the oral microbiome. Front. Cell. Infect. Microbiol. 2021, 11, 692883. [Google Scholar] [CrossRef] [PubMed]
- Simón-Soro, A.; Mira, A. Solving the etiology of dental caries. Trends Microbiol. 2015, 23, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, R.; Massei, V.; Ferrari, E.; Gallo, M.; Pertinhez, T.A.; Vescovi, P.; Pizzi, S.; Meleti, M. Salivary diagnosis of dental caries: A systematic review. Curr. Issues Mol. Biol. 2024, 46, 4234–4250. [Google Scholar] [CrossRef] [PubMed]
- Attia, D.; ElKashlan, M.K.; Saleh, S.M. Early childhood caries risk indicators among preschool children in rural Egypt: A case control study. BMC Oral Health 2024, 24, 10. [Google Scholar] [CrossRef] [PubMed]
- Chavan, S.; Chetani, H.; Yumnam, G.; Kumari, D.; Hari, K.; Vidyadharan, M. Evaluation of early childhood caries and its association with risk factors among school children: A cross-sectional study. J. Contemp. Dent. Pract. 2024, 25, 758–761. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).