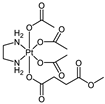
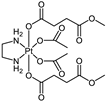
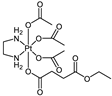
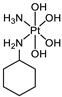
Abstract
Lipophilicity is a crucial parameter for drug discovery, usually determined by the logarithmic partition coefficient (Log P) between octanol and water. However, the available detection methods have restricted the widespread use of the partition coefficient in inorganic medicinal chemistry, and recent investigations have shifted towards chromatographic lipophilicity parameters, frequently without a conversion to derive Log P. As high-performance liquid chromatography (HPLC) instruments are readily available to research groups, a HPLC-based method is presented and validated to derive the partition coefficient of a set of 19 structurally diverse and cytotoxic platinum(IV) complexes exhibiting a dynamic range of at least four orders of magnitude. The chromatographic lipophilicity parameters φ0 and Log kw were experimentally determined for the same set of compounds, and a correlation was obtained that allows interconversion between the two lipophilicity scales, which was applied to an additional set of 34 platinum(IV) drug candidates. Thereby, a φ0 = 58 corresponds to Log P = 0. The same approaches were successfully evaluated to determine the distribution coefficient (Log D) of five ionisable platinum(IV) compounds to sample pH-dependent effects on the lipophilicity. This study provides straight-forward HPLC-based methods to determine the lipophilicity of cytotoxic platinum(IV) complexes in the form of Log P and φ0 that can be interconverted and easily expanded to other metal-based compound classes.
1. Introduction
Optimising lipophilicity is a crucial process in drug discovery [1,2], because it significantly affects the diffusion of drugs through cell membranes and thus, plays a role in pharmacokinetic processes, including absorption, distribution, metabolism, and excretion [2,3]. Some authors have also suggested that an optimal lipophilicity might increase the chances of success during drug development [1].
Lipophilicity is typically determined by means of a compound’s partition coefficient between octanol and water on a logarithmic scale, commonly abbreviated as Log Po/w, henceforth called Log P [4,5,6]. The Organisation for Economic Cooperation and Development (OECD) has outlined the standard method for experimentally obtaining partition coefficients of investigational compounds by the shake flask method [7], which is followed by an appropriate detection technique such as photometry, gas chromatography, or high-performance liquid chromatography (HPLC) [7,8,9]. The partition coefficient obtained by the shake flask method is typically found between −2 < Log P < 4 [7]. Hydrophilic compounds are characterised by negative values and lipophilic compounds by positive values.
Purely chromatographic alternatives to the partition coefficient between octanol and water have emerged as well because of the potential for automation, higher throughput, and minimising sample preparation efforts [10,11]. In particular, reversed phase-HPLC (RP-HPLC) has been suggested to provide a suitable means to directly assess the lipophilic property of an investigational compound. As these methods do not involve the shake flask procedure, they have the additional advantage of being independent of the concentration effects. In this setup, RP-HPLC is performed by using a C8- or C18-bonded stationary phase and a polar mobile phase, the latter being a mixture of water and methanol or water and acetonitrile. Chromatographic retention results from the partition of analytes between the two phases and can thus directly relate to the lipophilicity of an analyte [10]. For example, high capacity factors are indicative of a strong interaction with the lipophilic stationary phase and, thus, the strong lipophilic character of an analyte.
The corresponding chromatographic parameters Log kw and φ0 are independent of the flow rate and column length and can indeed provide relevant information about the lipophilic property of an analyte featuring its own scale even without conversion into Log P values [11]. On the one hand, Log kw is defined as the logarithmic capacity factor of a compound in a mobile phase containing pure water. It is usually obtained by extrapolation using the linear Soczewinski–Snyder relationship [12,13], defined as Log k = Log kw − Sφ, where k is the capacity factor of a compound in a specific mobile phase composition, φ is the volume percentage of the organic modifier in the eluent, and S is a constant for a given analyte and HPLC system. On the other hand, φ0 corresponds to the volume percentage of the organic modifier in the mobile phase at which the analyte is equally distributed in the mobile and the stationary phase [14].
The estimation of lipophilicity has been mostly implemented in the discovery process of organic drugs. However, lipophilicity determinations have also become of growing importance for the optimisation of metal-based anticancer drugs, among which is the class of platinum compounds [15,16,17,18,19,20,21], as they must accumulate efficiently in cells to execute their DNA-binding properties [22]. The lipophilicity of platinum anticancer agents was most commonly assessed by the shake flask method, followed by the detection of a platinum isotope by atomic absorption spectroscopy or inductively coupled plasma mass spectrometry (ICP-MS) [16,17,18,21]. Hyphenation of microemulsion electrokinetic chromatography to ICP-MS is a promising alternative but is experimentally demanding [23]. Due to restrictions in accessing such instruments, the chromatographically determined lipophilicity parameter Log kw has recently become more popular [24,25,26]. In addition, quantitative structure property relationships have been developed for modelling the lipophilicity of platinum complexes, and a free access database for the prediction of their Log P values is available [20,25,27,28,29,30]. However, an in depth analysis of representatives of the platinum(IV) class has not been reported to date. Also, a direct conversion of the chromatographic lipophilicity parameter φ0 into partition coefficients has not been established, which would allow the interconversion between the two lipophilicity scales of φ0 and Log P.
In this study, a set of 19 structurally diverse cytotoxic platinum(IV) complexes was used for evaluating the shake flask method by chromatographic detection and deriving lipophilicity parameters directly by chromatographic approaches (Table 1). The findings were validated by ICP-MS detection and the approach was extended to the distribution coefficient that accounts for ionisable compounds (e.g., containing carboxylic acids). Finally, we provide for the first time an empirical equation to convert the chromatography-based lipophilicity parameter φ0 (and Log kw) of platinum(IV) complexes into Log P. This equation was then used to calculate Log P values for an additional set of 34 cytotoxic platinum(IV) complexes from experimentally determined φ0.

Table 1.
List of 19 platinum(IV) complexes included in the standard set. The lipophilicity of each compound was assessed by the shake flask method using chromatographic (the logarithmic partition coefficient (Log P, HPLC) and element specific (Log P, inductively coupled plasma mass spectrometry, ICP-MS) detection. The chromatographic lipophilicity parameters Log kw and φ0 were determined using potassium iodide (KI) as a dead time marker. The compounds were sorted according to increasing Log P (HPLC).
2. Results and Discussion
Typically, lipophilicity is determined by the shake flask method as the logarithmic partition coefficient (Log P) of the concentration of an analyte between 1-octanol and water [7]. The concentration of each phase is derived by spectroscopic or spectrometric detection techniques [7,8,9]. Alternatively, lipophilicity can be directly obtained from chromatographic experiments, such as RP-HPLC, where the retention time of the analyte depends on the partition between the lipophilic stationary and the hydrophilic mobile phase. Here, the analyte is detected online by either spectroscopic or mass spectrometric techniques. The chromatographic lipophilicity parameters are calculated from capacity factors that are independent of the void volume, flow rate, and column length.
Several studies have found correlations between the lipophilicity of metal-based anticancer agents, usually determined by the shake flask method with element-specific detection, and their cytotoxic activity [21,30,31,32,33,34,35]. However, a restricted access to such instrumentation may have favoured the recent emergence of chromatographic lipophilicity parameters for metallodrugs [24,26,31,32]. So far, correlations between Log P and chromatographic parameters have been derived only for relatively homogenous series [20,25,35]. Thus, such approaches are presented and validated here to directly obtain Log P from shake flask experiments and indirectly by converting the chromatographic lipophilicity parameter φ0 into Log P (Scheme 1). The current investigation was performed with a set of 19 structurally diverse cytotoxic platinum(IV) complexes (Table 1). An additional set of 34 platinum(IV) complexes was used to test the conversion of the chromatographic lipophilicity parameter φ0 into Log P. A detailed list of the additionally employed compounds can be found in Tables S1 and S2.
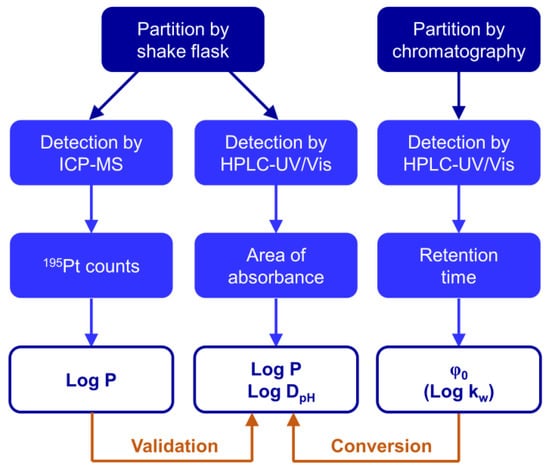
Scheme 1.
The lipophilicity of platinum(IV) complexes was evaluated by chromatographic workflows and validated by ICP-MS detection.
2.1. Determining Partition Coefficients by the Shake Flask Method
The platinum complexes were individually dissolved in water, saturated with 1-octanol, and the classical shake flask method was performed according to the OECD guidelines (Scheme 1) [7]. A stock solution of 0.5 mmol·L−1 was prepared of each compound. Then, equal volumes of the stock solution and 1-octanol pre-saturated with water were mixed for 60 min, and after phase separation, the individual phases were analysed. It must be noted that the metal-based anticancer agents must be inert with respect to ligand exchange reactions during this time period in order to be amenable to the shake flask procedure.
Of each sample, the stock solution and the resulting aqueous and 1-octanol phases were analysed by RP-HPLC using isocratic methods. The Log P values of the platinum compounds were calculated based on the areas of absorbance determined in the aqueous and 1-octanol phases that are directly proportional to the concentration according to the Lambert–Beer law. The sum of the area values of the aqueous and 1-octanol phase was compared with the stock solution and considered valid if the two values matched. After each run of a 1-octanol sample, the column was washed with 95% methanol before equilibrating to the original mobile phase composition. This was necessary to remove any remaining 1-octanol bound to the stationary phase of the column, which would increase the back pressure of the system, alter the properties of the stationary phase, and thus, influence the partition of the analytes between the mobile and the stationary phase.
Under these conditions, the lipophilicity of the representative platinum(IV) complexes was found between −2.0 < Log P < 2.4, spanning at least four orders of magnitude. In general, the complexes featuring equatorial acetates 1–3 showed the lowest lipophilicity, while the examples containing carboplatin- (11, 13, 15, 18, and 19) or cisplatin/satraplatin-derived cores (12, 14, 16, and 17) were the most tuneable and lipophilic compounds in our series (Table 1).
Aliquots of the stock solution and the aqueous phase of the same samples were serially diluted for ICP-MS analysis by acquiring the 195Pt isotope signal. Because the injection of the organic phase containing 1-octanol into the ICP-MS is problematic, the Log P was calculated by assuming coctanol = ctotal − cwater. The lipophilicity of the same compounds was then found between −2.2 < Log P < 1.3, spanning roughly three orders of magnitude.
The Log P values obtained by the two methods correlated well with a regression coefficient of R2 = 0.957 (Figure 1). Importantly, the slope of the fitting line was close to one and the intercept close to zero, thus validating the chromatographic approach to determine Log P of the cytotoxic platinum(IV) complexes. Some variation was observed at the hydrophilic and hydrophobic ends, which probably stems from the uncertainty of the assumption of coctanol = ctotal − cwater to calculate Log P by ICP-MS. A linear relationship seemed to exist over three orders of magnitudes between −1.5 < Log P < 1.8 (R2 = 0.991) between the two approaches.
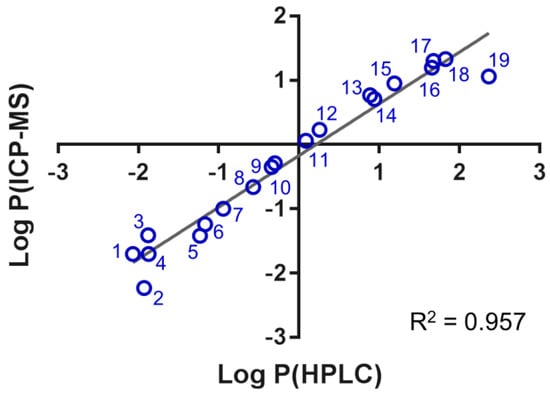
Figure 1.
The correlation of the Log P values obtained by the shake flask method using HPLC–UV/Vis and ICP-MS detection was linear according to Log P (ICP-MS) = 0.805·Log P (HPLC) − 0.177.
2.2. Extending the Approach to Determine Distribution Coefficients
For ionisable compounds, the partition between an aqueous and 1-octanol phase is a function of the pH value and is described as the logarithmic distribution coefficient (Log DpH, abbreviated as Log D) [36]. The distribution coefficient includes all species of an analyte in each of the two phases (e.g., both the ionised and neutral species) and also the potential hydrolysis products. Lipophilic properties at different pH values are essential for orally administered platinum(IV) anticancer agents that must additionally permeate several cellular membranes [37].
In order to extend the previous experiments to Log D values, five acidic platinum(IV) compounds (20–24, Table S1) featuring free carboxylic groups were analysed at different pH values (i.e., pH = 1.7, 2.5, 3.7, 4.2, 4.7, 5.6, 6.2, and 7.4 (Table 2)). Again, the shake flask method was used, followed by RP-HPLC–UV/Vis detection. Compound 10 was used as a non-ionisable control, as its lipophilicity should be independent of the pH value of the solution. Indeed, it showed an average Log P = −0.27 ± 0.02 over the entire tested pH range. Compound 20 did not yield a clear UV/Vis signal during Log D determination and, thus, was not analysed. In general, the acidic platinum(IV) representatives showed a clear dependence of the Log D on the pH value that is indicative of their titration curves. Increasing the pH above the pKa of the acidic groups significantly increased the hydrophilicity by deprotonation. Thus, the distribution coefficient can also be obtained by the shake flask method using chromatography-based methods with UV/Vis detection and can provide important insight into the lipophilicity of different species of an analyte in solution depending on the pH.

Table 2.
The distribution coefficient (Log D) values of the platinum(IV) compounds at different pH values, determined by the shake flask method using RP-HPLC–UV/Vis detection. The colour code is determined by blue = +0.5, white = 0, red = −3. n.d. = not detected.
2.3. Determination of the Chromatographic Lipophilicity Parameter φ0
The chromatographic lipophilicity parameter φ0 is intuitive, as it represents the percentage of the organic phase in the eluent at which the partition of an analyte between the mobile and stationary phase is equal. Higher values indicate a stronger lipophilic character of the analyte, and φ0 ranges between 0–100. The parameter is obtained by determining the capacity factors of an analyte with at least three different mobile phase compositions and then solving the linear Soczewinski–Snyder relationship for Log k = 0 (see Section 4).
In a first step, uracil and potassium iodide (KI) were investigated as system dead time (t0) markers, and their retention times were determined in the range of 5–90% organic modifier (Figure S1). These were used to calculate φ0 values for the same set of 19 platinum(IV) complexes. The φ0 values derived from the two different dead time markers correlated linearly with R² = 0.999 and covered the range of φ = 20–80. The retention time of uracil increased with increasing aqueous phase from 70–95% (Figure S1) and consequently, KI may be more appropriate for hydrophilic compounds, as its retention time remained relatively constant over the entire range of mobile phase compositions. As metal-based anticancer agents may undergo ligand exchange reactions, it is advised to use potassium iodide as an external marker if necessary. Of the platinum(IV) compounds, 4 displayed the lowest φ0 = 22.5, being the most hydrophilic, and 19 was the most lipophilic, with the highest φ0 = 76.5.
The correlation of φ0 and Log kw for the same set of compounds appeared to be a quadratic polynomial, with an acceptable fit of R² = 0.982 for potassium iodide as the dead time marker (Figure S2). The quadratic dependence of these parameters was already observed by Schoenmakers [38]. In a more recent publication, it was noted that the dependence of φ0 and Log kw was linear for narrow ranges of φ0 and generally quadratic when considering wide ranges of φ0. Also, the choice of organic modifier has a considerable impact on the Log kw value of purely organic molecules with respect to the regression model used, and methanol was suggested as the most suitable [39].
2.4. Converting Chromatographic Lipophilicity to Shake Flask Lipophilicity
According to the OECD guidelines, the Log kw values of compounds with known Log P can be used to create a calibration curve to evaluate partition coefficients from chromatographic data [7]. In this study, the φ0 values were plotted against the chromatographically detected Log P values of the 19 platinum(IV) complexes. The resulting correlation curve was a quadratic polynomial with an R² = 0.951 (Figure 2). Thus, the calculated Log P values (cLog P) of platinum(IV) complexes can be obtained from φ0-values according to Equation (1):
cLog P = 0.001φ02 (KI) − 0.027φ0 (KI) − 1.758.
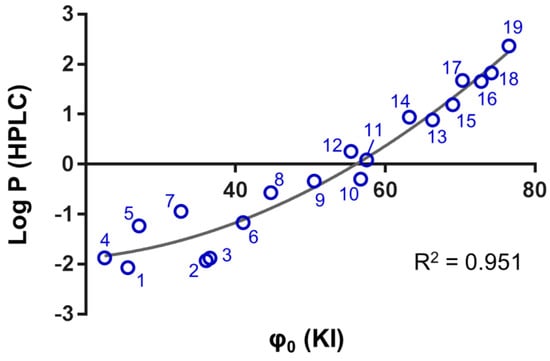
Figure 2.
The overall correlation of the chromatographically determined φ0 (using potassium iodide as a dead time marker) and Log P by the shake flask method (using HPLC–UV/Vis) is shown.
The corresponding linear correlation between Log kw and Log P featured R² = 0.918 (Figure S2). However, the hydrophilic region showed increased variance with both chromatographic approaches reflecting the uncertainty of the experimental determination of Log P < −1 directly by HPLC. As a rule of thumb, a φ0 = 58 yields a Log P = 0, and consequently, lipophilic platinum(IV) compounds are characterised by φ0 > 58, while hydrophilic platinum(IV) compounds display φ0 < 58. The calibration curve was applied to the set of 34 platinum(IV) complexes, and their cLog P values were calculated based on their experimentally determined φ0 (Table S2). The conversion worked well for the lipophilic representatives. For example, 54 featured a cLog P = 0.6, which was very similar to the reported Log P = 0.7 [21]. The quadratic approximation implied that φ0-values < 25 would yield constant cLog P values of −1.9. In fact, the uncertainty in the hydrophilic region is also reflected by the OECD guidelines that restrain the range of experimental Log P values determined by the shake flask method to a lower end of −2 [7]. The increased uncertainty of the correlation in the hydrophilic region is further exemplified by compound 34, which displayed a cLog P = −1.5, while its reported value was Log P = −0.8 [21].
As the antiproliferative activity is known for most of the complexes reported herein, we tried to directly correlate the concentration to inhibit 50% of cell proliferation (IC50) of the set of 19 platinum(IV) complexes (except 4–6) in a particular cell line with their lipophilicity parameters (Figure S4). As expected, the two parameters did not directly correlate, and the regression coefficients were uniformly below 0.1. This was indicative that active cellular accumulation and stability towards reduction may have strongly contributed to the cytotoxic property of these platinum(IV) complexes in addition to the lipophilicity [33,40,41].
3. Materials and Methods
Ultrapure water (18.2 MΩ cm, Milli-Q Advantage, Darmstadt, Germany) was used for the RP-HPLC experiments, as well as for all the dilutions in the ICP-MS measurements. Nitric acid (TraceSELECT®, ≥65%, p.a., Fluka, Buchs, Switzerland) was used without further purification. The platinum and rhenium standards for the ICP-MS measurements were obtained from CPI International (Amsterdam, The Netherlands). All the other reagents and solvents were obtained from standard commercial sources and were used without further purification.
NMR spectra were recorded with a Bruker Avance III 500 MHz NMR spectrometer (Bruker Daltonics, Bremen, Germany) at 500.32 MHz (1H). Mass spectra were measured with a Bruker maXis electrospray-ionisation-quadrupole-time-of-flight mass spectrometer (Bruker Daltonics, Bremen, Germany) using ACN/MeOH + 1% H2O as the solvent or a Bruker Amazon SL ion trap using water/MeOH. The elemental analysis was performed at the Microanalytical Laboratory of the University of Vienna with a Eurovector EA3000 elemental analyser (Eurovector Srl, Pavia, Italy) for CHNS analysis.
3.1. Platinum(IV) Complexes
The platinum(IV) compounds used in this investigation were prepared at the Institute of Inorganic Chemistry, University of Vienna. A complete list of the complexes, together with their structures and determined lipophilicity parameters can be found in Table 1, Tables S1 and S2. The complexes 1–15, 17–19 [24,26,30,33,35,40,41,42], the ionisable complexes 20–24 [24,43], and the extended set of platinum(IV) complexes 25–26, 28, 30, and 32–58 [24,26,32,33,35,40,41,42,44,45,46,47] were synthesised according to procedures in the literature.
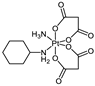
3.2. (OC-6-43)-Amminedichlorido(cyclohexaneamine)bis[(4-ethoxy)-4-oxobutanoato]platinum(IV) (16)
Complex 16 was obtained upon the reaction of (OC-6-43)-amminedichlorido(cyclohexaneamine)dihydroxidoplatinum(IV) with 3-(ethoxycarbonyl)propanoic anhydride (3 eq) in N,N-dimethylformamide (DMF) under anhydrous conditions and light protection, as described for analogous compounds in reference [41]. Yield = 45% (55 mg). Elemental analysis: C18H34Cl2N2O8Pt (672.46); calcd. C 32.15, H 5.10, N 4.17; found C 32.17, H 5.12, N 3.97. 1H-NMR (DMF-d7): δ= 7.49 (bs, 2H, NH2), 6.96 (m, 3H, NH3), 4.10 (m, 4H, H9), 3.01 (bs, 1H, H1), 2.58 (m, 4H, H6), 2.50 (m, 4H, H7), 2.16 (m, 2H, H2), 1.72 (m, 2H, H3), 1.60 (m, 1H, H4), 1.36 (m, 4H, H2 and H3), 1.22 (t, 3JH,H = 7.1 Hz, 6H, H10), 1.17 (m, 1H, H4) ppm. ESI-MS (positive): m/z 673.1397 (mtheor 673.1411) [M + H]+, 695.1215 (mtheor 695.1230) [M + Na]+. ESI-MS (negative): m/z 671.1245 (671.1265) [M − H]−.
3.3. (OC-6-54)-Dichlorido(N,N-dimethyl-ethane-1,2-diamine)hydroxido[4-(2-propyn-1-ylamino)-4-oxo-butanoato]platinum(IV) (27)
Complex 27 was synthesised by dissolving the precursor (0.20 g, published in [46]), 0.41 mmol) in 4 mL of DMF under an argon atmosphere and heating it to 50 °C. Then, 1,1-carbonyldiimidazol (0.07 g, 0.45 mmol) dissolved in 2 mL of DMF was added dropwise. After stirring for 10 min, the reaction mixture was cooled to room temperature, and the remaining CO2 was removed by a flush of argon stream through the solution. Propargylamine (29 µL) was added, and the reaction was stirred overnight. DMF was removed under reduced pressure, and the crude mixture was purified by column chromatography (mobile phase EtOAc/MeOH/triethylamine = 3:1:0.1) to yield a white powder. Yield = 33% (70 mg). Elemental analysis: C11H21Cl2N3O4Pt (525.29); calcd. C 25.12, H 4.03, N 8.00; found C 25.06, H 4.26, N 7.65. 1H-NMR (d6-DMSO): δ = 9.45 (bs, 1H, NH5a); 8.25 (t, 1H, NH10, J = 5.3 Hz); 7.10 (bs, 1H, NH5b); 3.81 (dd, 2H, H11, J = 2.5, 5.4 Hz); 3.07 (t, 1H, H13, J = 2.5 Hz); 2.86–2.75 (bm, 4H, H3/H4); 2.68 (bs (s+d), 3H, H2a or H2b); 2.59 (bs (s+d), 3H, H2a or H2b); 2.50–2.25 (bm, 4H, H7/H8); 1.48 (bs, 1H, OH1) ppm. ESI-MS (positive): m/z 547.6 [M + Na+]+, 563.6 [M + K+]+. ESI-MS (negative): m/z 523.5 [M − H+]+.
3.4. (OC-6-33)-Dichlorido(ethane-1,2-diamine)bis[(4-(2-propyn-1-ylamino)-4-oxobutanoato]platinum(IV) (29)
Complex 29 was synthesised by dissolving 20 (0.40 g, 1.11 mmol) in 8 mL DMF under an argon atmosphere and heating it to 60 °C. Then, 1,1-carbonyldiimidazol (0.24 g, 1.48 mmol) dissolved in 16 mL of DMF was added dropwise. After stirring for 10 min, the reaction mixture was cooled to room temperature and the remaining CO2 was removed by a flush of argon stream through the solution. Propargylamine (110 µL) dissolved in 24 mL of DMF was added, and the reaction was stirred overnight. DMF was removed under reduced pressure, and the crude mixture was purified by column chromatography (mobile phase EtOAc/MeOH = 7:1) to yield a white powder. Yield = 23% (110 mg). Elemental analysis: C16H24Cl2N4O6Pt (634.40); calcd. C16H24Cl2N4O6Pt·0.5H2O (644.39) C 29.87, H 3.92, N 8.71; found C 29.56, H 3.58, N 8.61. 1H-NMR (d6-DMSO): δ = 8.42 (bs, 4H, NH22), 8.27 (t, 2H, NH7, J = 5.4 Hz), 3.84 (dd, 4H, H8, J = 2.5, 5.4 Hz), 3.09 (t, 2H, H10, J = 2.5 Hz), 2.67 (bs, 4H, H1), 2.48 (t, 4H, H4 or H5, J = 7.1 Hz), 2.31 (t, 4H, H4 or H5, J = 7.1 Hz) ppm. ESI-MS (positive): m/z 657.1 [M + Na+]+.
3.5. (OC-6-33)-Dichlorido(ethane-1,2-diamine)bis[(4-dimethylamino)-4-oxobutanoato]platinum(IV) (31)
Complex 31 was synthesised by dissolving 1-(dimethylcarbamoyl)-3-methyl-1H-imidazol-3-ium iodide (0.41 g, 2.95 mmol) and 20 (0.40 g, 1.11 mmol) in 8 mL of DMF under an argon atmosphere. Trimethylamine (0.15 g) was added, and the clear brown reaction mixture was stirred at room temperature for 24 h. DMF was removed under reduced pressure, and the crude mixture was purified by column chromatography (mobile phase EtOAc/MeOH = 1:1). A white powder was obtained after crystallisation in methanol. Yield = 35% (150 mg). Elemental analysis: C14H28Cl2N4O6Pt (614.4); calcd. C14H28Cl2N4O6Pt·H2O (632.4) C 26.59, H 4.78, N 8.86; found C 26.47, H 4.64, N 8.61. 1H-NMR (d6-DMSO): δ = 8.44 (bs, 4H, 2NH2), 2.95 (s, 6H, H7 or H7’), 2.79 (s, 6H, H7 or H7’), 2.68 (bs, 4H, H1), 2.47 (bs, 8H, H4/H5) ppm. ESI-MS (positive): m/z 637.1 [M + Na+]+. ESI-MS (negative): m/z 613.0 [M − H+]−.
3.6. Instrumentation
HPLC. The RP-HPLC experiments were carried out on a Dionex Ultimate 3000 RS system (Dionex, Germering, Germany), controlled by the Dionex Chromeleon 6.80 software. The following chromatographic conditions were used: Agilent Poroshell 120 SB-C18 column (2.1 × 150 mm, 2.7 µm); injection volume: 1 µL; flow rate: 0.2 mL·min−1; collection rate: 5–100 Hz; temperature of the column and autosampler: 25°C; UV/Vis detection set up at 210–256 nm. Mobile phases consisted of (A) water with 0.1% FA and (B) methanol with 0.1% FA.
ICP-MS. The ICP-MS measurements were carried out on an ICP-quadrupole MS instrument Agilent 7500ce (Agilent Technologies, Waldbronn, Germany) equipped with a CETAC ASX-520 autosampler (Omaha, NE, USA) and a MicroMist nebuliser at a sample uptake rate of approx. 0.25 mL min−1. The instrument was tuned on a daily basis in order to achieve the maximum sensitivity. Rhenium (185Re) served as internal standard for platinum to account for instrumental fluctuations, and the platinum isotopes 194Pt and 195Pt were acquired. The ICP-MS was equipped with nickel cones and operated at an RF power of 1500 W. Argon was used as the plasma gas (15 L·min−1), carrier gas (~1 L·min−1), and make up gas (~0.2 L·min−1). The dwell time was set to 0.1 s, and the measurement was performed in 10 replicates. The Agilent MassHunter software package (Workstation Software, Version B.01.01, 2012, Agilent, Santa Clara, CA, USA) was used for data processing.
3.7. Shake Flask Procedure
The logarithmic partition coefficient Log P between 1-octanol and water phases was determined for platinum complexes using the shake flask method according to the OECD guidelines with slight modifications [7]. The platinum compounds were dissolved in water (Milli-Q, pre-saturated with 1-octanol) to yield stock solutions of 0.5 mmol·L−1, which were filtered through a 0.45-µm filter (Minisart RC 25, Sartorius AG, Göttingen, Germany). An equal volume of 1-octanol (pre-saturated with ultrapure water) was added to the platinum-containing aqueous solutions, and the mixtures were shaken mechanically for 60 min at room temperature. The samples were centrifuged to assist with bilayer formation. The experiments were performed at least in duplicates.
3.8. Analysis of Samples Obtained from the Shake Flask Procedure
RP-HPLC. The two phases obtained by the shake flask method were analysed by RP-HPLC for each complex, using an isocratic mode in a suitable water/methanol ratio ranging from 95:5 to 10:90. However, after elution of each sample containing 1-octanol, the methanol percentage was increased to 95% for 20 min before returning to the initial isocratic conditions in order to remove any remaining 1-octanol from the column. Additionally, the total platinum concentration was determined by analysing an aliquot of each stock solution. The concentration of the platinum compounds was detected by UV/Vis spectroscopy. According to the Lambert–Beer law, the area of absorbance (A) of an eluting analyte is directly proportional to its concentration (c). The Log P was calculated according to Equation (2):
ICP-MS. The total platinum content in the initial stock solution and in the final aqueous phase obtained by the shake flask method was determined by ICP-MS. Each aliquot was diluted gravimetrically with 1% HNO3 to match the linear range of the ICP-MS system. By assuming coctanol = ctotal − cwater, the Log P was calculated according to Equation (3):
Log P(HPLC) = Log (coctanol/cwater) = Log (Aoctanol/Awater).
Log P(ICP-MS) = Log [(ctotal − cwater)/cwater)]
3.9. Determining the Distribution Coefficient by the Shake Flask Method with HPLC–UV/Vis Detection
Compared with the partition coefficient (Log P), the distribution coefficient (Log DpH, henceforth called Log D) takes into account all the species of an analyte at a given pH and is suitable for ionisable compounds. Thus, the Log D of 5 platinum(IV) compounds (20–24) featuring an acidic moiety was determined at different pH values. The platinum(IV) complex 10, bearing non-ionisable functional groups, was evaluated as a control.
In this case, the aqueous phase consisted of phosphate or acetic acid buffer solutions at 10 mmol·L−1, saturated with octanol. The phosphate buffers were prepared at pH = 1.7, 2.5, 6.2, and 7.4, and the acetic acid buffers were prepared at pH = 3.7, 4.2, 4.7, and 5.6. Thus, the platinum complexes were dissolved in those buffer solutions, and the shake flask method was performed as described above using HPLC–UV/Vis detection.
3.10. Determining the Lipophilicity from Chromatographic Parameters
The platinum complexes (0.5 mmol·L−1) were dissolved in 1 mL of water/methanol (1:1) and the solutions were filtered through a 0.45-µm filter (Minisart RC 25, Sartorius AG, Göttingen, Germany). Uracil was employed as an internal standard (1 µmol·L−1) and potassium iodide (KI) as an external standard for determining the system dead time (t0). The obtained samples were directly analysed by RP-HPLC. The RP-HPLC experiments were performed in isocratic modes at least in duplicates, employing at least 3 different water/methanol compositions per complex. The capacity factors k = (tr − t0)/t0, where tr is the retention time of the analyte, were determined for all the investigated eluent compositions. From these, the capacity factor at 100% aqueous mobile phase (Log kw) was extrapolated using the linear Soczewinski–Snyder relationship [12,13], Log k = Log kw − Sφ, where Log k is the capacity factor in a specific mobile phase composition, φ is the volume fraction of the methanol in the eluent, and S is a constant for a given analyte and HPLC system (slope). Then, the chromatographic hydrophobicity parameter φ0 was derived, defined as the volume percentage of the organic modifier required to achieve an equal distribution of a compound between the mobile and stationary phase, corresponding to Log k = 0 [10,14].
4. Conclusions
Lipophilicity is a crucial parameter in drug discovery and also for metal-based drug candidates. However, experimentally determining lipophilicity by the shake flask method may have been somewhat impeded by restricted access to suitable detection methods besides element-specific spectroscopy or spectrometry. Because high-performance liquid chromatography (HPLC) instruments are more widely available to research groups, chromatographic lipophilicity parameters have recently become more popular but are in different scales compared with the classically obtained partition coefficient. Here, a straight-forward HPLC-based method was validated to derive the partition coefficient (Log P) of cytotoxic platinum(IV) complexes from shake flask experiments, which exhibited a dynamic range of at least four orders of magnitude from −2 < Log P < 2.4. In addition, chromatographic partition by means of the parameter φ0 was assessed and calibrated against the corresponding Log P values so that the chromatographic lipophilicity of cytotoxic platinum(IV) complexes may be directly converted into Log P, which is valid for φ0 > 25. The same methods were extended to determine the distribution coefficient (Log D) of ionisable platinum(IV) representatives, which showed pH-dependent lipophilicity changes. These approaches can be applied to other classes of metal complexes that may ultimately enable the estimation of lipophilicity for metal-based anticancer agents in general.
Supplementary Materials
The following are available online at https://www.mdpi.com/2304-6740/6/4/130/s1: Table S1: List of five platinum(IV) complexes bearing ionisable groups for determining distribution coefficients; Table S2: List of 35 platinum(IV) complexes for converting φ0 into calculated Log P (cLog P) based on the calibration curve described in the main text; Figure S1: Comparison of the retention times of uracil (detection at 256 nm) and potassium iodide (KI, detection at 230 nm) at different percentages of methanol (φ) in the eluent; Figure S2: The correlation between the chromatographic lipophilicity parameters φ0 and Log kw is a quadratic polynomial (using potassium iodide as a dead time marker) with R² = 0.982 for the standard set of 19 platinum compounds; Figure S3: The correlation between Log P by the shake flask method and the chromatographic Log kw is linear (using potassium iodide as a dead time marker) with R² = 0.918 for the standard set of 19 platinum compounds. Log P (HPLC) = 1.250·Log kw − 3.000.
Author Contributions
Conceptualisation, M.H.M.K., S.T., S.M.M.-M., and B.K.K.; data curation, M.H.M.K. and S.T.; formal analysis, S.M.M.-M.; investigation, M.H.M.K. and S.T.; resources, H.P.V., D.H., V.P., M.S.G., and B.K.K.; validation, M.H.M.K. and S.T.; visualisation, S.M.M.-M.; original draft preparation, M.H.M.K. and S.M.M.-M.; and editing of manuscript, S.T., H.P.V., D.H., V.P., M.S.G., S.M.M.-M., and B.K.K.
Funding
M.H.M.K. is grateful to the “Stipendium der Monatshefte für Chemie” for financial support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Waring, M.J. Lipophilicity in Drug Discovery. Expert Opin. Drug Discov. 2010, 5, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Varma, M.V.S.; Radi, Z.A.; Rotter, C.J.; Litchfield, J.; El-Kattan, A.F.; Lyubimov, A.V. Pharmacokinetics and Toxicokinetics in Drug Discovery and Development. In Encyclopedia of Drug Metabolism and Interactions; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011. [Google Scholar]
- Mälkiä, A.; Murtomäki, L.; Urtti, A.; Kontturi, K. Drug Permeation in Biomembranes: In Vitro and In Silico Prediction and Influence of Physicochemical Properties. Eur. J. Pharm. Sci. 2004, 23, 13–47. [Google Scholar] [CrossRef] [PubMed]
- Hansch, C.; Maloney, P.P.; Fujita, T. Correlation of Biological Activity of Phenoxyacetic Acids with Hammett Substituent Constants and Partition Coefficients. Nature 1962, 194, 178–180. [Google Scholar] [CrossRef]
- Hansch, C.; Fujita, T. p-s-p Analysis. A Method for the Correlation of Biological Activity and Chemical Structure. J. Am. Chem. Soc. 1964, 86, 1616–1626. [Google Scholar] [CrossRef]
- Leo, A.; Hansch, C.; Elkins, D. Partition Coefficients and Their Uses. Chem. Rev. 1971, 71, 525–616. [Google Scholar] [CrossRef]
- OECD. Test No. 107: Partition Coefficient (n-Octanol/Water): Shake Flask Method; OECD Publishing: Paris, France, 1995. [Google Scholar]
- Albert, A. Selective Toxicity: The Physicochemical Basis of Therapy; Chapman and Hall: London, UK, 1979. [Google Scholar]
- Kerns, E.H. High Throughput Physicochemical Profiling for Drug Discovery. J. Pharm. Sci. 2001, 90, 1838–1858. [Google Scholar] [CrossRef] [PubMed]
- Nasal, A.; Siluk, D.; Kaliszan, R. Chromatographic Retention Parameters in Medicinal Chemistry and Molecular Pharmacology. Curr. Med. Chem. 2003, 10, 381–426. [Google Scholar] [CrossRef] [PubMed]
- Valkó, K. Application of High-Performance Liquid Chromatography Based Measurements of Lipophilicity to Model Biological Distribution. J. Chromatogr. A 2004, 1037, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Soczewinski, E.; Wachtmeister, C.A. The Relation between the Composition of Certain Ternary Two-Phase Solvent Systems and RM Values. J. Chromatogr. A 1962, 7, 311–320. [Google Scholar] [CrossRef]
- Snyder, L.R.; Dolan, J.W.; Gant, J.R. Gradient Elution in High-Performance Liquid Chromatography: I. Theoretical Basis for Reversed-Phase Systems. J. Chromatogr. A 1979, 165, 3–30. [Google Scholar] [CrossRef]
- Valkó, K.; Slégel, P. New Chromatographic Hydrophobicity Index (φ0) Based on the Slope and the Intercept of the log k versus Organic Phase Concentration Plot. J. Chromatogr. A 1993, 631, 49–61. [Google Scholar] [CrossRef]
- Souchard, J.P.; Ha, T.T.; Cros, S.; Johnson, N.P. Hydrophobicity Parameters for Platinum Complexes. J. Med. Chem. 1991, 34, 863–864. [Google Scholar] [CrossRef] [PubMed]
- Screnci, D.; McKeage, M.J.; Galettis, P.; Hambley, T.W.; Palmer, B.D.; Baguley, B.C. Relationships Between Hydrophobicity, Reactivity, Accumulation and Peripheral Nerve Toxicity of a Series of Platinum Drugs. Br. J. Cancer 2000, 82, 966–972. [Google Scholar] [CrossRef] [PubMed]
- Robillard, M.S.; Galanski, M.; Zimmermann, W.; Keppler, B.K.; Reedijk, J. (Aminoethanol)dichloroplatinum(II) Complexes: Influence of the Hydroxyethyl Moiety on 5′-GMP and DNA Binding, Intramolecular Stability, the Partition Coefficient and Anticancer Activity. J. Inorg. Biochem. 2002, 88, 254–259. [Google Scholar] [CrossRef]
- Hall, M.D.; Amjadi, S.; Zhang, M.; Beale, P.J.; Hambley, T.W. The Mechanism of Action of Platinum(IV) Complexes in Ovarian Cancer Cell Lines. J. Inorg. Biochem. 2004, 98, 1614–1624. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, A.; Aceto, M.; Cassino, C.; Gabano, E.; Osella, D. Uptake of Antitumor Platinum(II)-Complexes by Cancer Cells, Assayed by Inductively Coupled Plasma Mass Spectrometry (ICP-MS). J. Inorg. Biochem. 2004, 98, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Platts, J.A.; Oldfield, S.P.; Reif, M.M.; Palmucci, A.; Gabano, E.; Osella, D. The RP-HPLC Measurement and QSPR Analysis of Log Po/w Values of Several Pt(II) Complexes. J. Inorg. Biochem. 2006, 100, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Reithofer, M.R.; Bytzek, A.K.; Valiahdi, S.M.; Kowol, C.R.; Groessl, M.; Hartinger, C.G.; Jakupec, M.A.; Galanski, M.; Keppler, B.K. Tuning of Lipophilicity and Cytotoxic Potency by Structural Variation of Anticancer Platinum(IV) Complexes. J. Inorg. Biochem. 2011, 105, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Gibson, D. The Mechanism of Action of Platinum Anticancer Agents—What Do We Really Know About it? Dalton Trans. 2009, 10681–10689. [Google Scholar] [CrossRef]
- Bytzek, A.K.; Reithofer, M.R.; Galanski, M.; Groessl, M.; Keppler, B.K.; Hartinger, C.G. The First Example of MEEKC-ICP-MS Coupling and its Application for the Analysis of Anticancer Platinum Complexes. Electrophoresis 2010, 31, 1144–1150. [Google Scholar] [CrossRef]
- Varbanov, H.P.; Valiahdi, S.M.; Kowol, C.R.; Jakupec, M.A.; Galanski, M.; Keppler, B.K. Novel Tetracarboxylatoplatinum(IV) Complexes as Carboplatin Prodrugs. Dalton Trans. 2012, 41, 14404–14415. [Google Scholar] [CrossRef] [PubMed]
- Ermondi, G.; Caron, G.; Ravera, M.; Gabano, E.; Bianco, S.; Platts, J.A.; Osella, D. Molecular interaction fields vs. quantum-mechanical-based descriptors in the modelling of lipophilicity of platinum(IV) complexes. Dalton Trans. 2013, 42, 3482–3489. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Varbanov, H.P.; Göschl, S.; Heffeter, P.; Theiner, S.; Roller, A.; Jensen, F.; Jakupec, M.A.; Berger, W.; Galanski, M.; Keppler, B.K. A Novel Class of Bis- and Tris-Chelate Diam(m)inebis(dicarboxylato)platinum(IV) Complexes as Potential Anticancer Prodrugs. J. Med. Chem. 2014, 57, 6751–6764. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, S.P.; Hall, M.D.; Platts, J.A. Calculation of Lipophilicity of a Large, Diverse Dataset of Anticancer Platinum Complexes and the Relation to Cellular Uptake. J. Med. Chem. 2007, 50, 5227–5237. [Google Scholar] [CrossRef] [PubMed]
- Tetko, I.V.; Jaroszewicz, I.; Platts, J.A.; Kuduk-Jaworska, J. Calculation of Lipophilicity for Pt(II) Complexes: Experimental Comparison of Several Methods. J. Inorg. Biochem. 2008, 102, 1424–1437. [Google Scholar] [CrossRef] [PubMed]
- Platts, J.A.; Ermondi, G.; Caron, G.; Ravera, M.; Gabano, E.; Gaviglio, L.; Pelosi, G.; Osella, D. Molecular and Statistical Modeling of Reduction Peak Potential and Lipophilicity of Platinum(IV) Complexes. J. Biol. Inorg. Chem. 2011, 16, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Tetko, I.V.; Varbanov, H.P.; Galanski, M.; Talmaciu, M.; Platts, J.A.; Ravera, M.; Gabano, E. Prediction of LogP for Pt(II) and Pt(IV) Complexes: Comparison of Statistical and Quantum-Chemistry Based Approaches. J. Inorg. Biochem. 2016, 156, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Meier, S.M.; Hanif, M.; Adhireksan, Z.; Pichler, V.; Novak, M.; Jirkovsky, E.; Jakupec, M.A.; Arion, V.B.; Davey, C.A.; Keppler, B.K.; et al. Novel Metal(II) Arene 2-Pyridinecarbothioamides: A Rationale to Orally Active Organometallic Anticancer Agents. Chem. Sci. 2013, 4, 1837–1846. [Google Scholar] [CrossRef]
- Pichler, V.; Goschl, S.; Meier, S.M.; Roller, A.; Jakupec, M.A.; Galanski, M.; Keppler, B.K. Bulky (N,N)-(Di)alkylethane-1,2-diamineplatinum(II) Compounds as Precursors for Generating Unsymmetrically Substituted Platinum(IV) Complexes. Inorg. Chem. 2013, 52, 8151–8162. [Google Scholar] [CrossRef]
- Varbanov, H.P.; Jakupec, M.A.; Roller, A.; Jensen, F.; Galanski, M.; Keppler, B.K. Theoretical Investigations and Density Functional Theory Based Quantitative Structure-Activity Relationships Model for Novel Cytotoxic Platinum(IV) Complexes. J. Med. Chem. 2013, 56, 330–344. [Google Scholar] [CrossRef]
- Meier-Menches, S.M.; Gerner, C.; Berger, W.; Hartinger, C.G.; Keppler, B.K. Structure-Activity Relationships for Ruthenium and Osmium Anticancer Agents—Towards Clinical Development. Chem. Soc. Rev. 2018, 47, 909–928. [Google Scholar] [CrossRef] [PubMed]
- Varbanov, H.; Valiahdi, S.M.; Legin, A.A.; Jakupec, M.A.; Roller, A.; Galanski, M.; Keppler, B.K. Synthesis and Characterization of Novel Bis(carboxylato)dichloridobis(ethylamine)platinum(IV) Complexes with Higher Cytotoxicity than Cisplatin. Eur. J. Med. Chem. 2011, 46, 5456–5464. [Google Scholar] [CrossRef] [PubMed]
- Low, Y.W.; Blasco, F.; Vachaspati, P. Optimised Method to Estimate Octanol Water Distribution Coefficient (Log D) in a High Throughput Format. Eur. J. Pharm. Sci. 2016, 92, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.D.; Mellor, H.R.; Callaghan, R.; Hambley, T.W. Basis for Design and Development of Platinum(IV) Anticancer Complexes. J. Med. Chem. 2007, 50, 3403–3411. [Google Scholar] [CrossRef]
- Schoenmakers, P.J.; Billiet, H.A.H.; Tussen, R.; De Galan, L. Gradient Selection in Reversed-Phase Liquid Chromatography. J. Chromatogr. A 1978, 149, 519–537. [Google Scholar] [CrossRef]
- Baczek, T.; Markuszewski, M.; Kaliszan, R.; van Straten, M.A.; Claessens, H.A. Linear and Quadratic Relationships between Retention and Organic Modifier Content in Eluent in Reversed Phase High-Performance Liquid Chromatography: A Systematic Comparative Statistical Study. J. High Resolut. Chromatogr. 2000, 23, 667–676. [Google Scholar] [CrossRef]
- Goschl, S.; Varbanov, H.P.; Theiner, S.; Jakupec, M.A.; Galanski, M.; Keppler, B.K. The Role of the Equatorial Ligands for the Redox Behavior, Mode of Cellular Accumulation and Cytotoxicity of Platinum(IV) Prodrugs. J. Inorg. Biochem. 2016, 160, 264–274. [Google Scholar] [CrossRef]
- Hofer, D.; Varbanov, H.P.; Hejl, M.; Jakupec, M.A.; Roller, A.; Galanski, M.; Keppler, B.K. Impact of the Equatorial Coordination Sphere on the Rate of Reduction, Lipophilicity and Cytotoxic Activity of Platinum(IV) Complexes. J. Inorg. Biochem. 2017, 174, 119–129. [Google Scholar] [CrossRef]
- Hofer, D.; Varbanov, H.P.; Legin, A.; Jakupec, M.A.; Roller, A.; Galanski, M.; Keppler, B.K. Tetracarboxylatoplatinum(IV) Complexes Featuring Monodentate Leaving Groups—A Rational Approach Towards Exploiting the Platinum(IV) Prodrug Strategy. J. Inorg. Biochem. 2015, 153, 259–271. [Google Scholar] [CrossRef]
- Reithofer, M.; Galanski, M.; Roller, A.; Keppler, B.K. An Entry to Novel Platinum Complexes: Carboxylation of Dihydroxoplatinum(IV) Complexes with Succinic Anhydride and Subsequent Derivatization. Eur. J. Inorg. Chem. 2006, 2006, 2612–2617. [Google Scholar] [CrossRef]
- Reithofer, M.R.; Valiahdi, S.M.; Jakupec, M.A.; Arion, V.B.; Egger, A.; Galanski, M.; Keppler, B.K. Novel Di- and Tetracarboxylatoplatinum(IV) Complexes. Synthesis, Characterization, Cytotoxic Activity and DNA Platination. J. Med. Chem. 2007, 50, 6692–6699. [Google Scholar] [CrossRef] [PubMed]
- Reithofer, M.R.; Schwarzinger, A.; Valiahdi, S.M.; Galanski, M.; Jakupec, M.A.; Keppler, B.K. Novel Bis(carboxylato)dichlorido(ethane-1,2-diamine)platinum(IV) Complexes with Exceptionally High Cytotoxicity. J. Inorg. Biochem. 2008, 102, 2072–2077. [Google Scholar] [CrossRef] [PubMed]
- Pichler, V.; Valiahdi, S.M.; Jakupec, M.A.; Arion, V.B.; Galanski, M.; Keppler, B.K. Mono-Carboxylated Diaminedichloridoplatinum(IV) Complexes—Selective Synthesis, Characterization and Cytotoxicity. Dalton Trans. 2011, 40, 8187–8192. [Google Scholar] [CrossRef] [PubMed]
- Pichler, V.; Heffeter, P.; Valiandi, S.M.; Kowol, C.R.; Egger, A.; Berger, W.; Jakupec, M.A.; Galanski, M.; Keppler, B.K. Unsymmetric Mono- and Dinuclear Platinum(IV) Complexes Featuring an Ethylene Glycol Moiety: Synthesis, Characterization, and Biological Activity. J. Med. Chem. 2012, 55, 11052–11061. [Google Scholar] [CrossRef] [PubMed][Green Version]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).