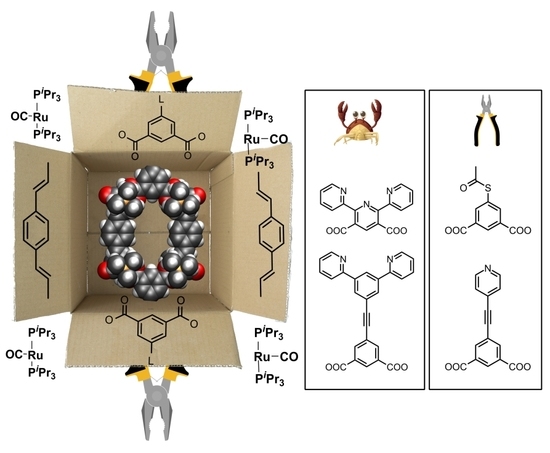
Tetraruthenium Metallamacrocycles with Potentially Coordinating Appended Functionalities
Abstract
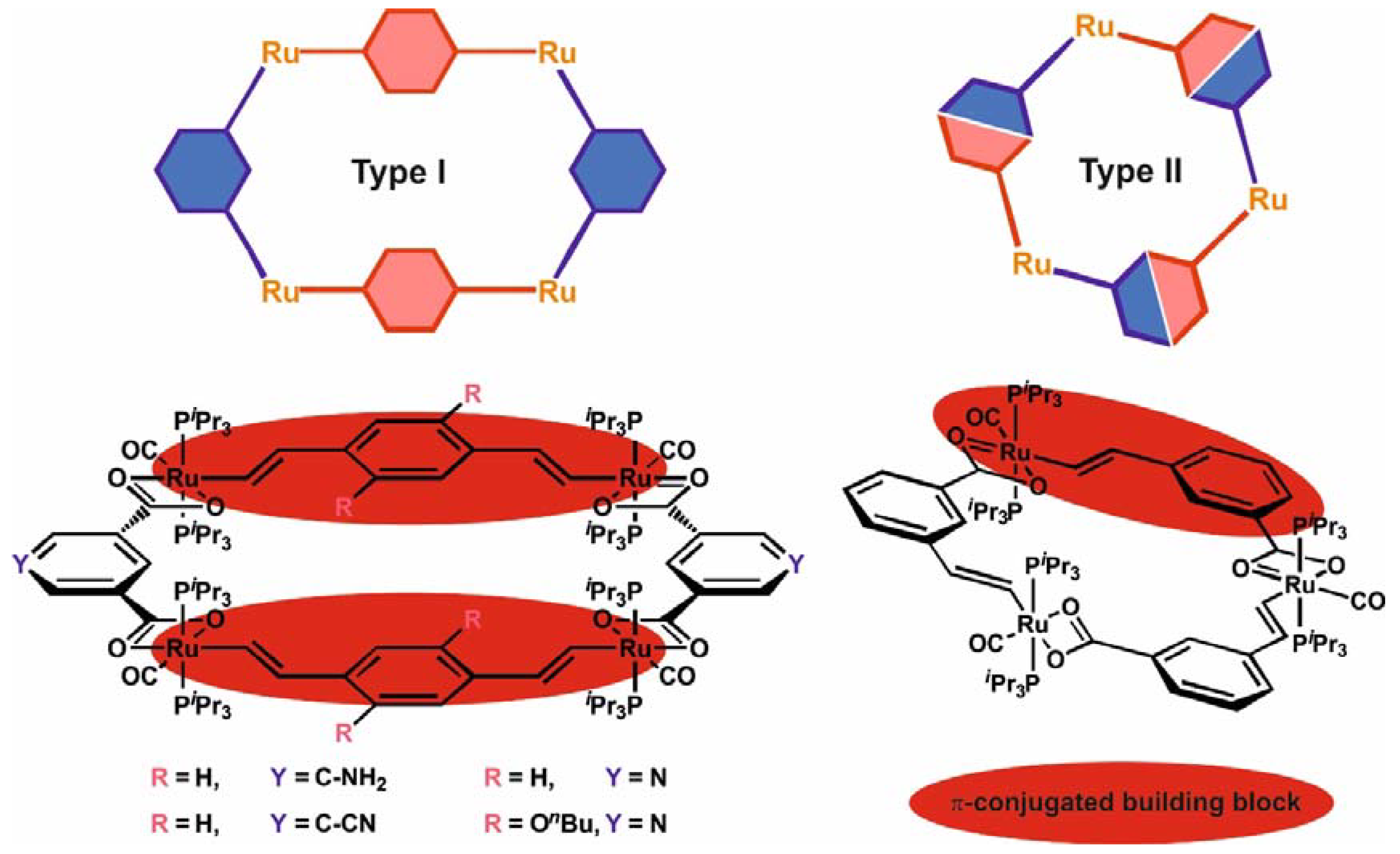
1. Introduction
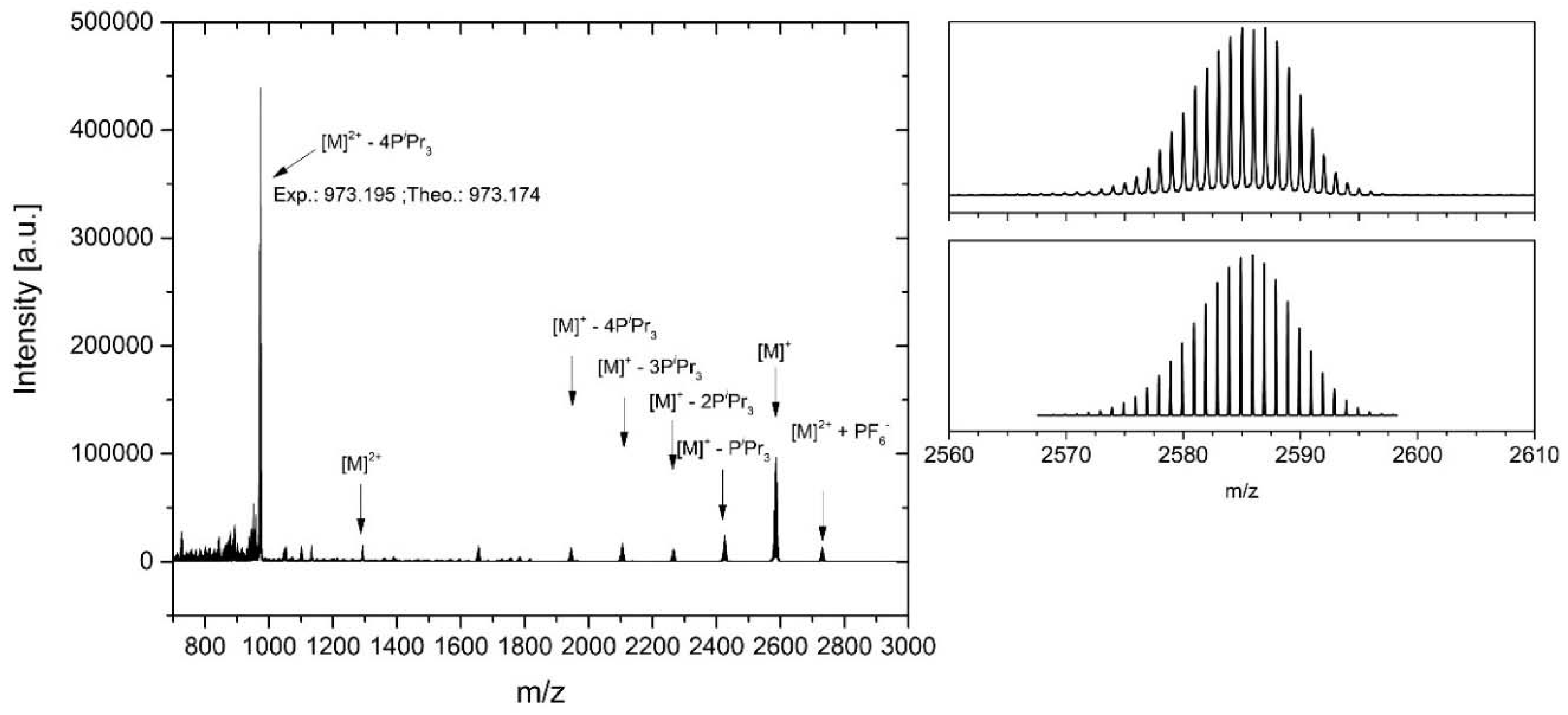
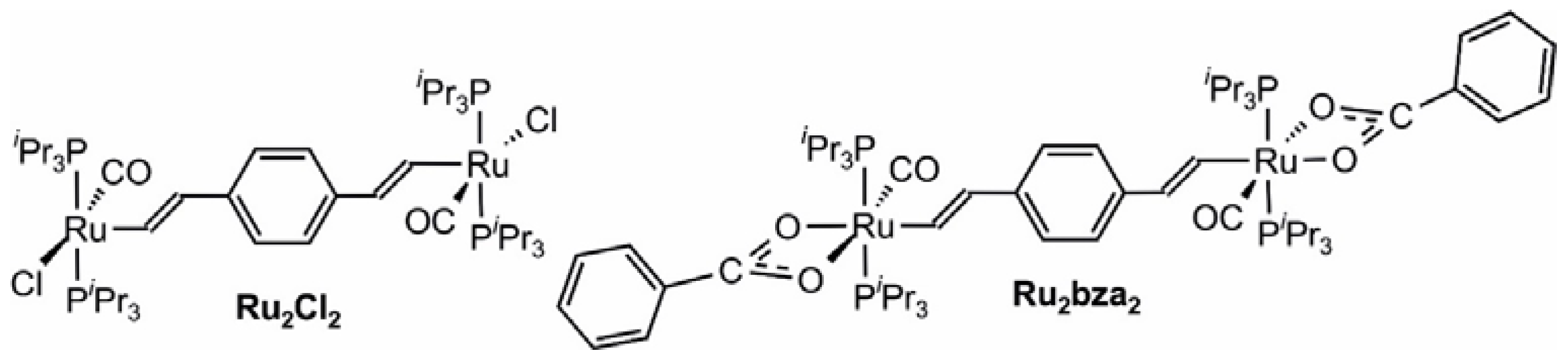
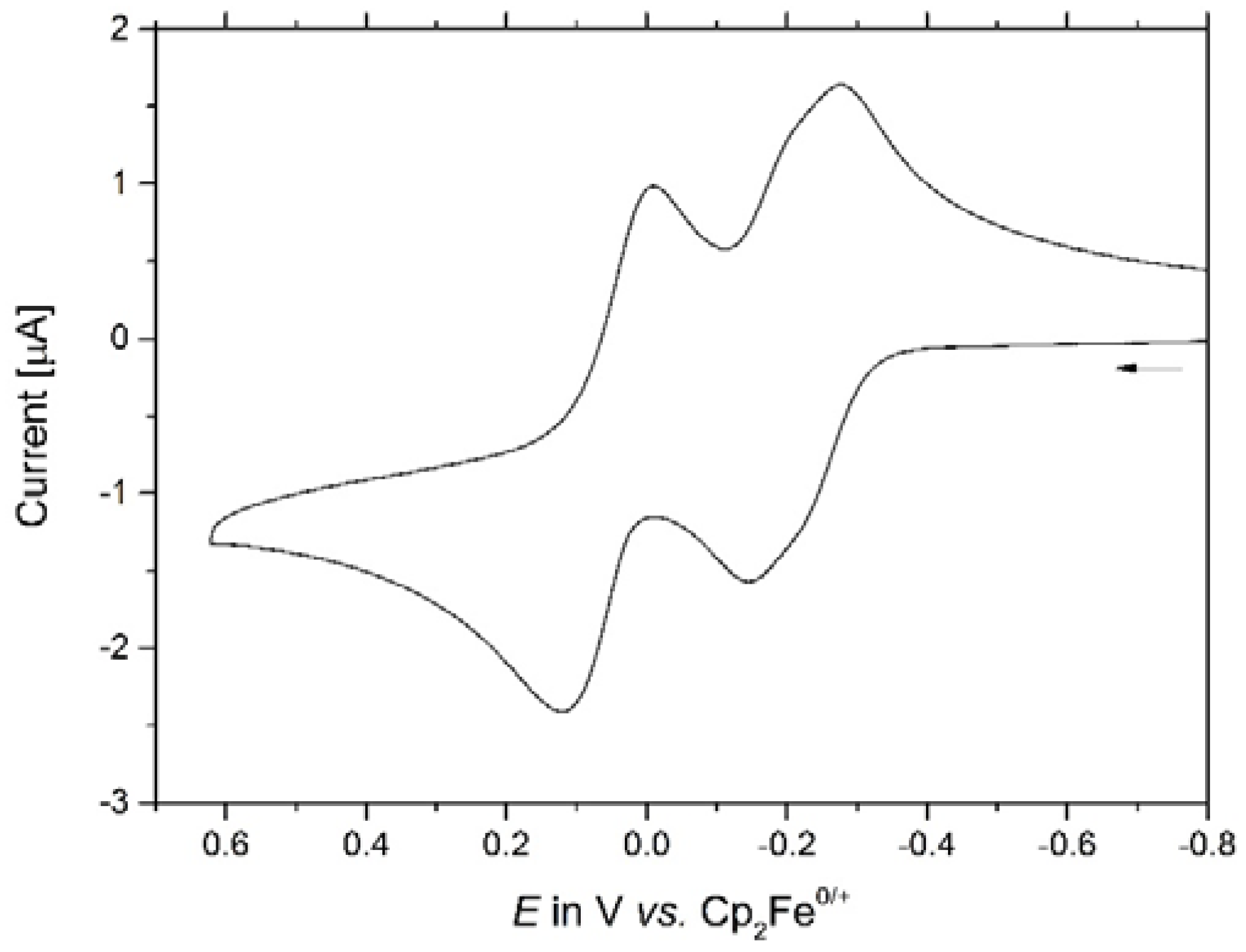
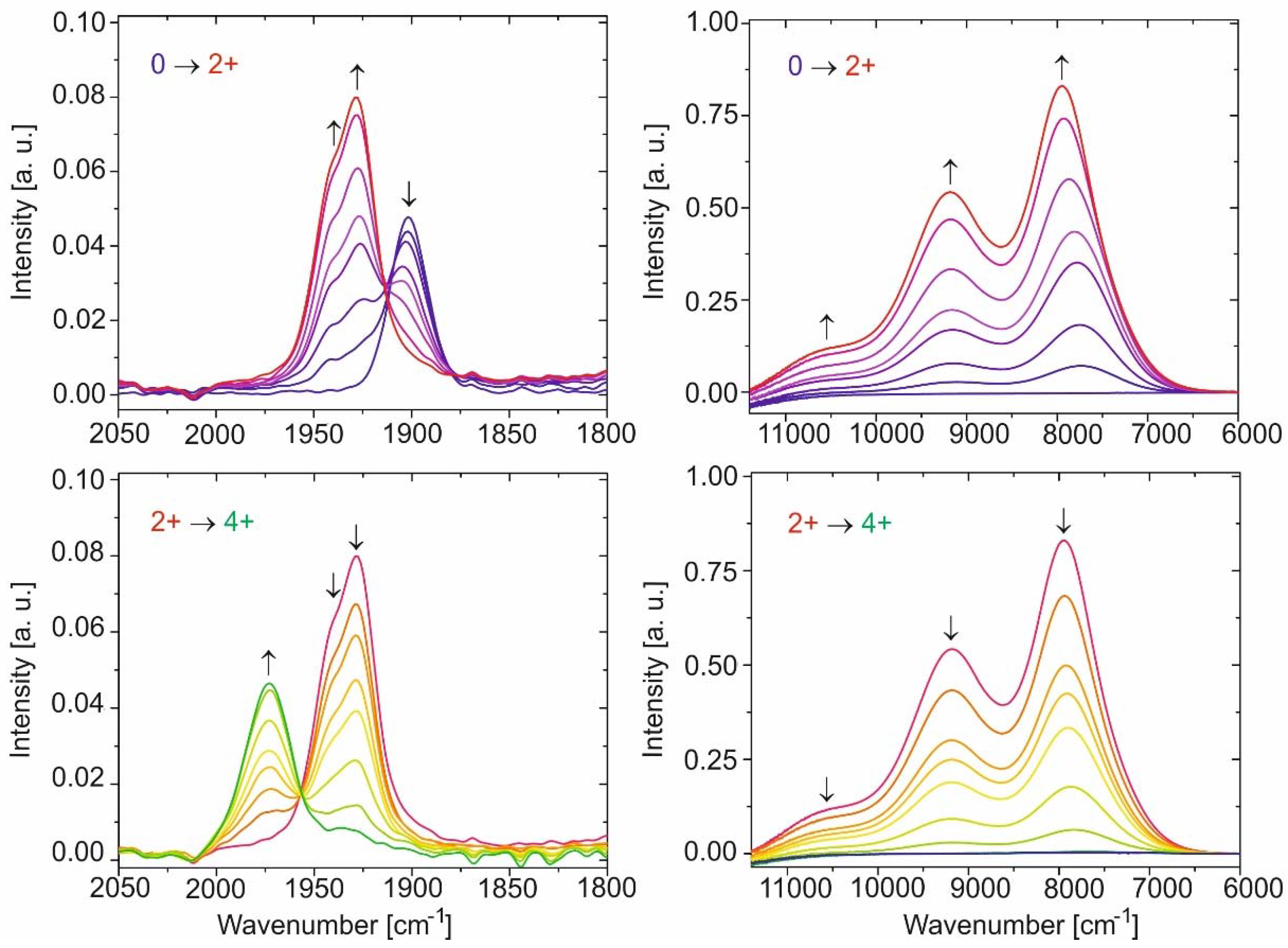
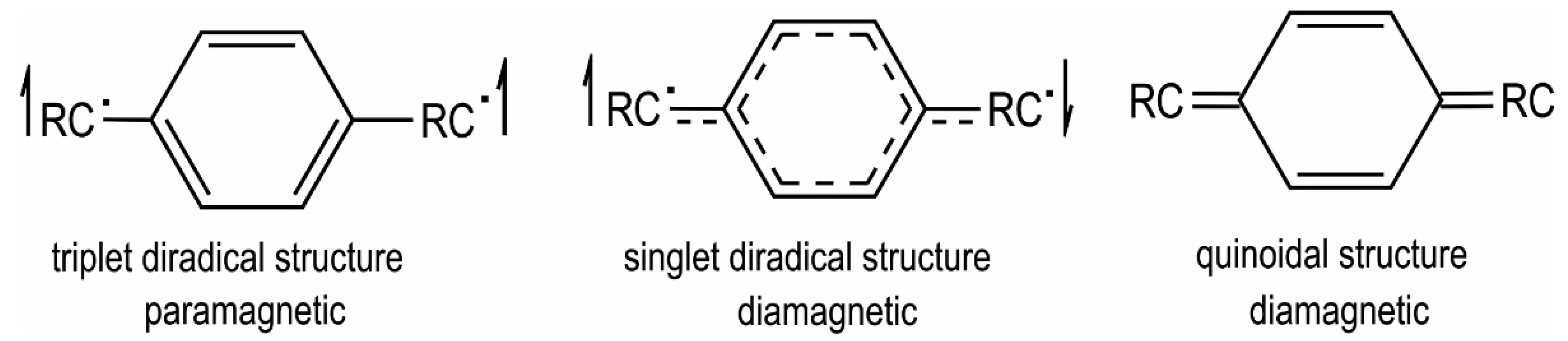
2. Results and Discussion
3. Materials, Methods, Syntheses and Characterization Data
3.1. Materials and General Methods
3.2. Electrochemical and Spectroelectrochemical Measurements
3.3. Syntheses and Characterization
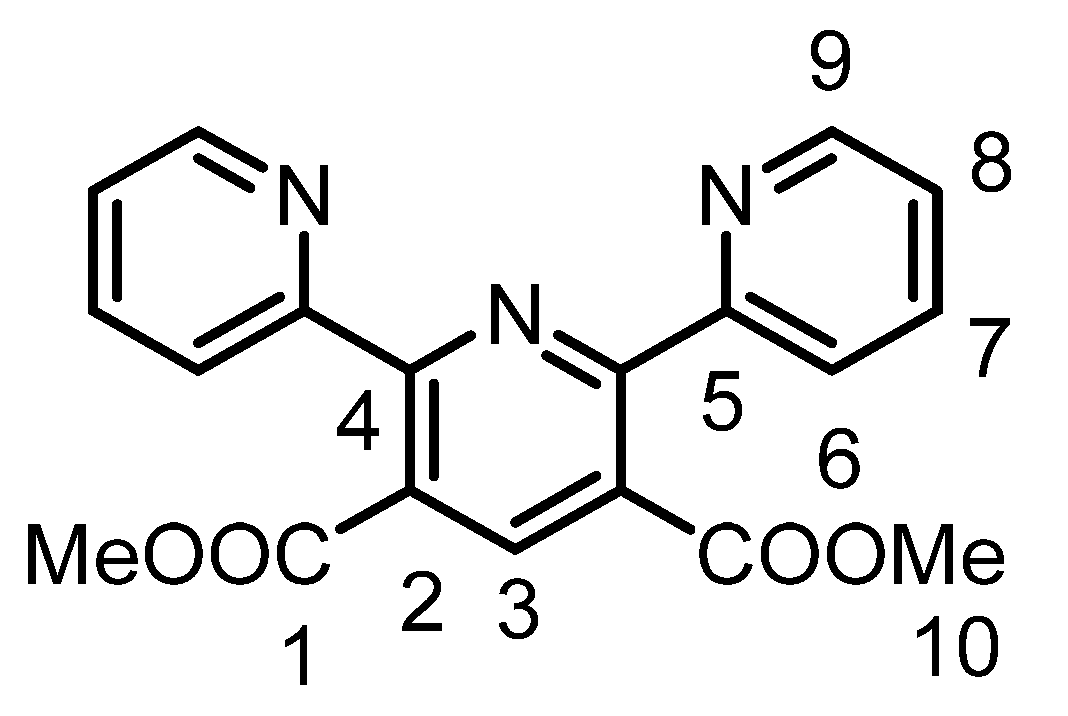
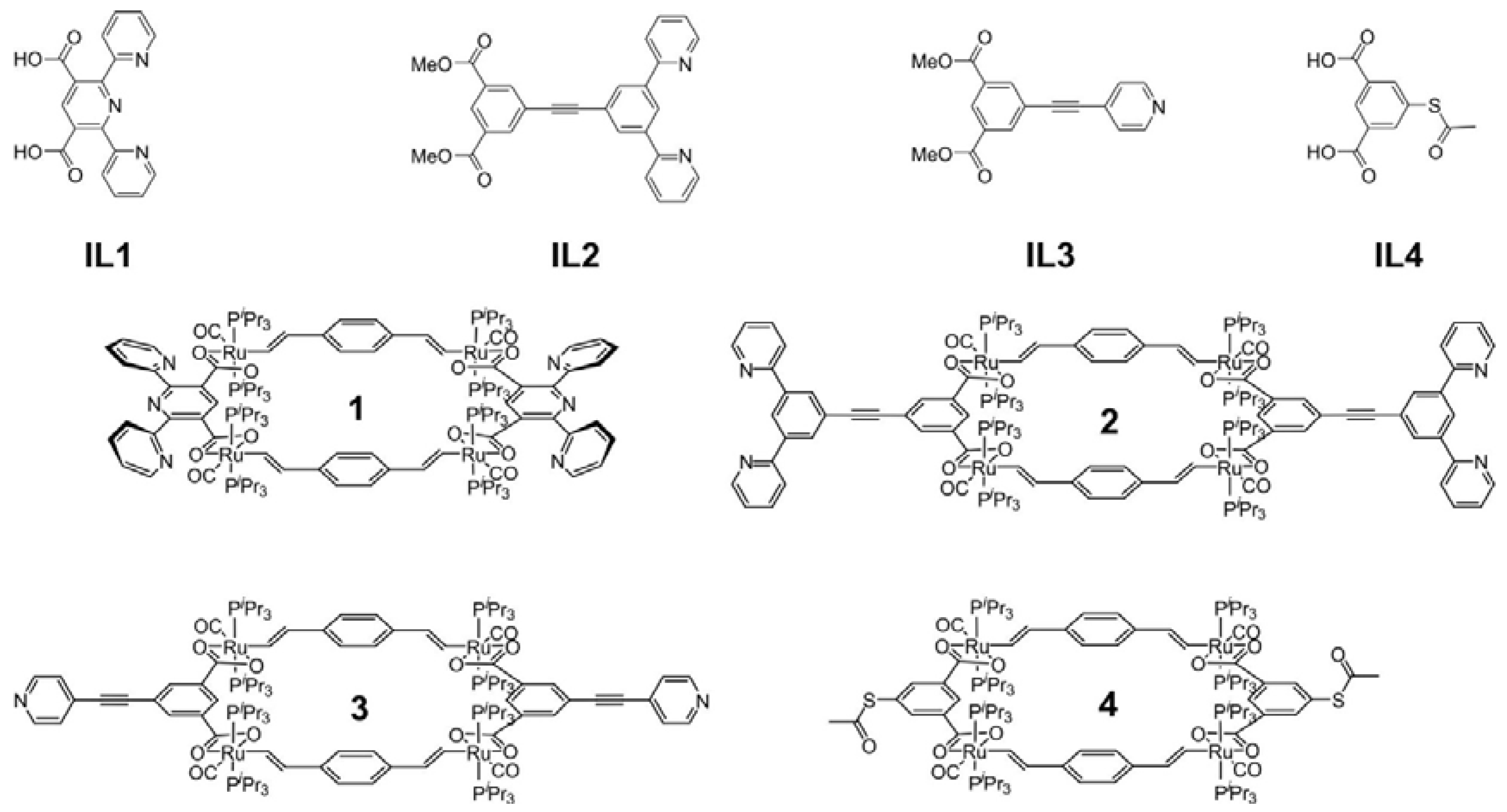
3.3.1. Syntheses and Characterization of 2,6-Di(pyrid-2-yl)-pyridine-3,5-dicarboxylic acid dimethyl ester (IL1)
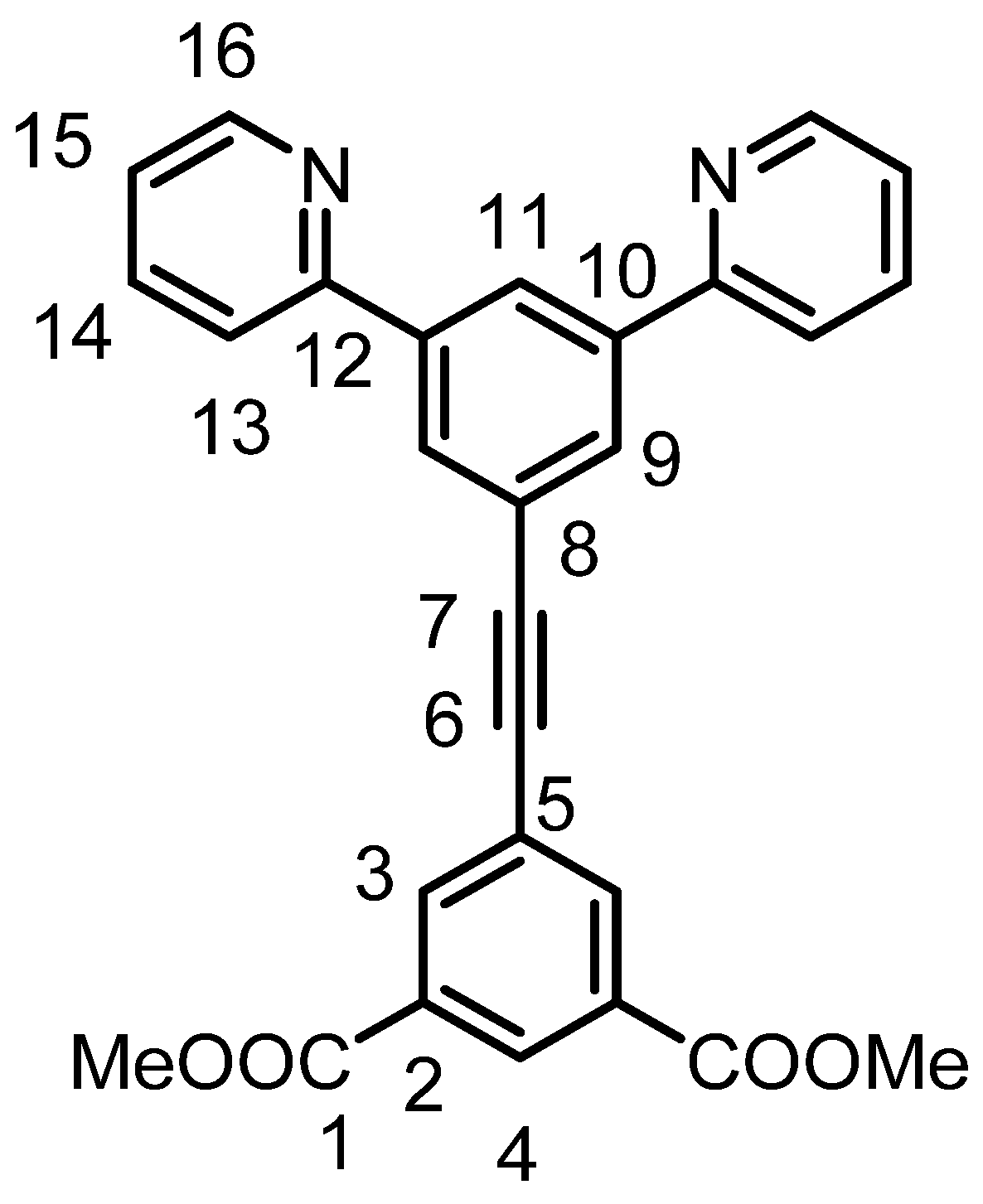
3.3.2. Syntheses and Characterization of 3,5-Bis(pyrid-2-yl)-phenylethynyl isophthalic acid dimethyl ester (IL2)
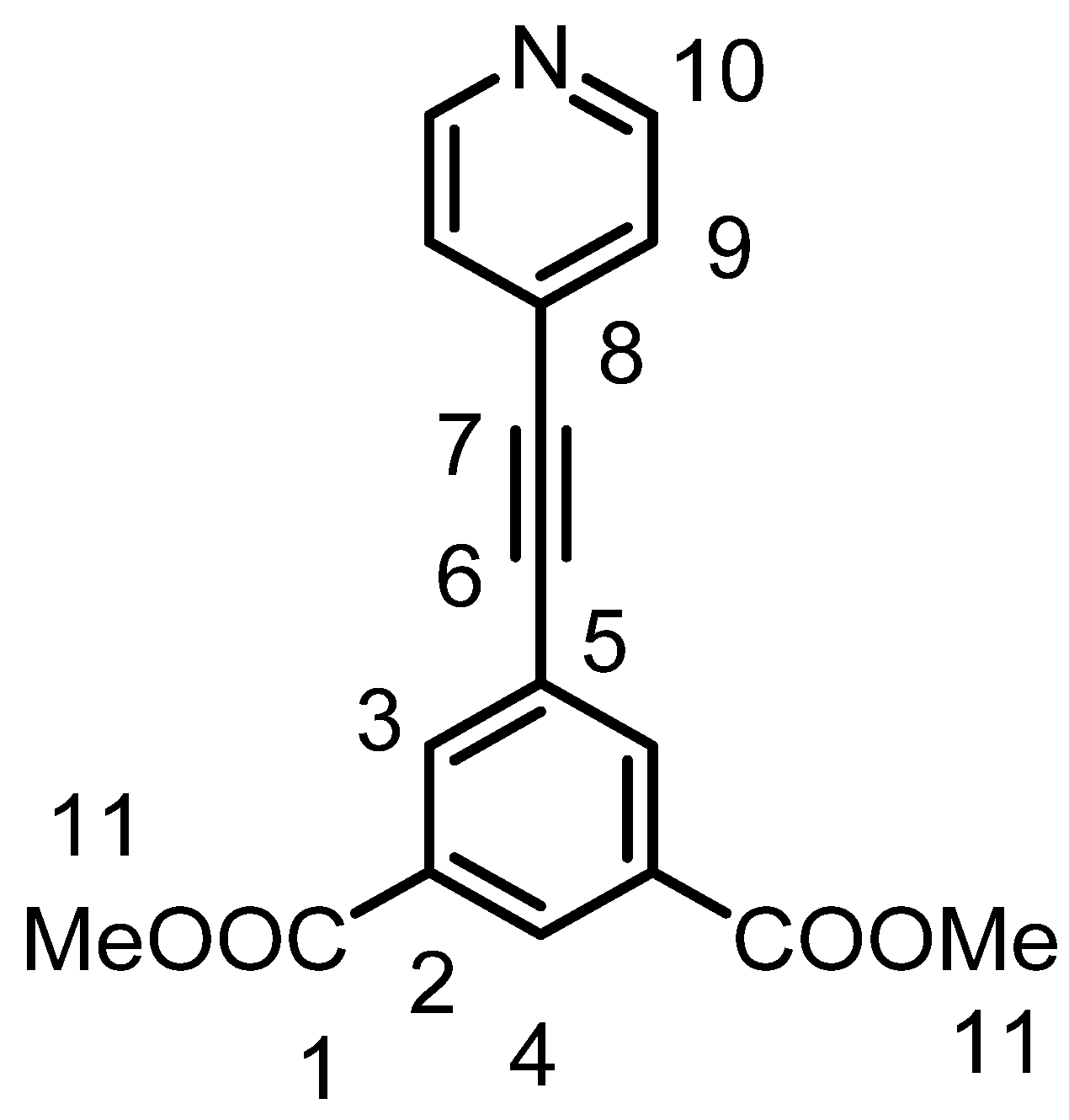
3.3.3. Syntheses and Characterization of 1,3-Dimethyl-5-(ethynyl-4-pyridyl)isophthalate(IL3)
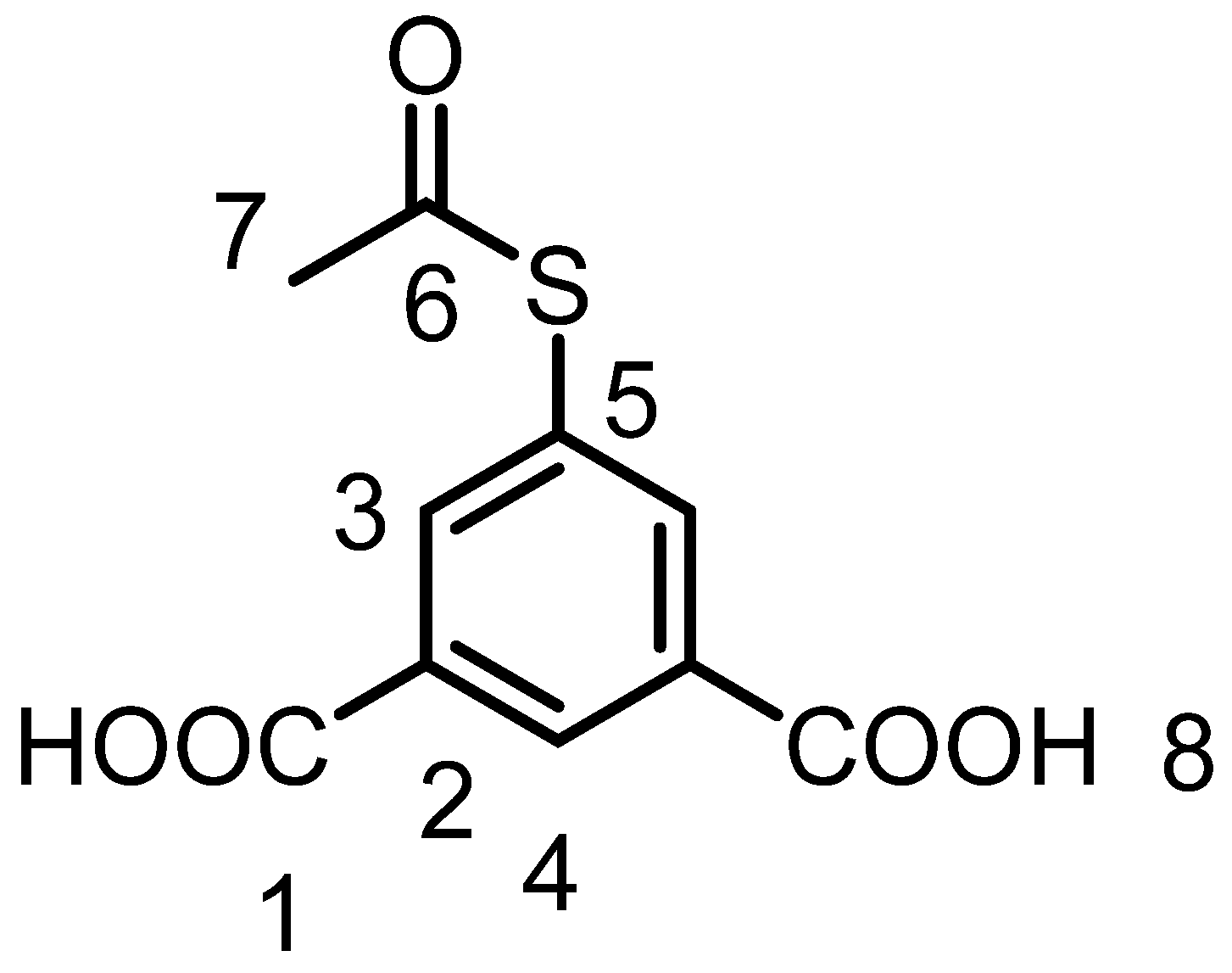
3.3.4. Syntheses and Characterization of 5-Acetylthioisophthalic acid (IL4)
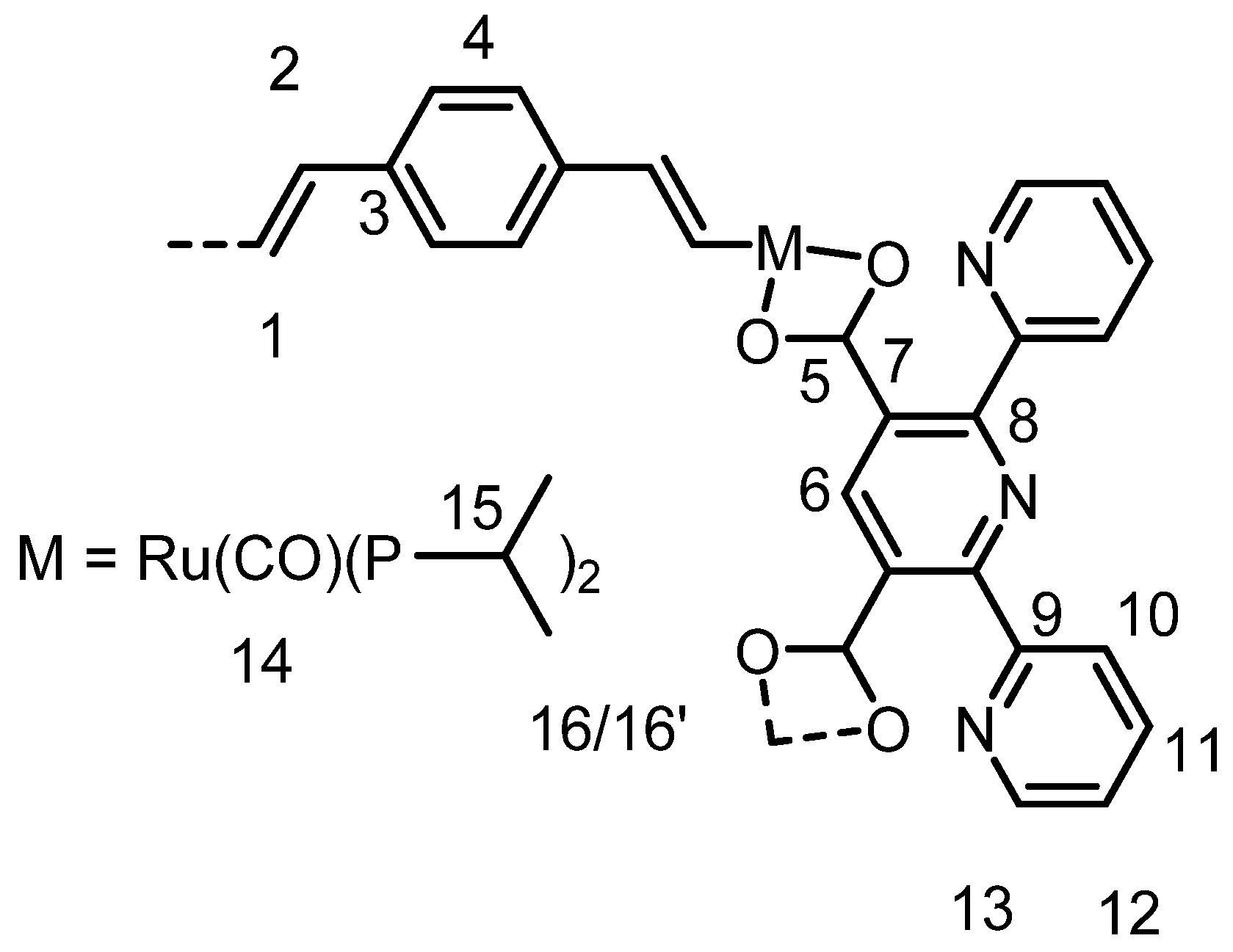
3.3.5. Syntheses and Characterization of Macrocycle 1
3.3.6. Syntheses and Characterization of Macrocycle 2

3.3.7. Syntheses and Characterization of Macrocycle 3

3.3.8. Syntheses and Characterization of Macrocycle 4

Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Scheerer, S.; Linseis, M.; Wuttke, E.; Weickert, S.; Drescher, M.; Tröppner, O.; Ivanović-Burmazović, I.; Irmler, A.; Pauly, F.; Winter, R.F. Redox-Active Tetraruthenium Macrocycles Built from 1,4-Divinylphenylene-Bridged Diruthenium Complexes. Chem. Eur. J. 2016, 22, 9574–9590. [Google Scholar] [CrossRef] [PubMed]
- Fink, D.; Weibert, B.; Winter, R.F. Redox-active tetraruthenium metallacycles: Reversible release of up to eight electrons resulting in strong electrochromism. Chem. Commun. 2016, 52, 6103–6106. [Google Scholar] [CrossRef] [PubMed]
- Fink, D.; Bodensteiner, M.; Linseis, M.; Winter, R.F. Macrocyclic Triruthenium Complexes Having Electronically Coupled Mixed-Valent States. Chem. Eur. J. 2018, 24, 992–996. [Google Scholar] [CrossRef] [PubMed]
- Fink, D.; Linseis, M.; Winter, R.F. Constitutional Isomers of Macrocyclic Tetraruthenium Complexes with Vastly Different Spectroscopic and Electrochemical Properties. Organometallics 2018, 37, 1817–1820. [Google Scholar] [CrossRef]
- Sadhukhan, N.; Patra, S.K.; Sana, K.; Bera, J.K. Novel Heterobimetallic Metallamacrocycles Based on the 1,1′-Bis(1,8-naphthyrid-2-yl)ferrocene (FcNP2) Ligand: Structural Characterization of the Complexes [{M(FcNP2)}2]2+ (M = CuI, AgI) and {MCl2(FcNP2)}4 (M = ZnII, CoII). Organometallics 2006, 25, 2914–2916. [Google Scholar] [CrossRef]
- Croue, V.; Goeb, S.; Salle, M. Metal-driven self-assembly: The case of redox-active discrete architectures. Chem. Commun. 2015, 51, 7275–7289. [Google Scholar] [CrossRef] [PubMed]
- Winter, R.F. The molecular electrochemistry of metal–organic metallamacrocycles. Curr. Opin. Electrochem. 2018, 8, 14–23. [Google Scholar] [CrossRef]
- Maurer, J.; Sarkar, B.; Schwederski, B.; Kaim, W.; Winter, R.F.; Záliš, S. Divinylphenylene bridged diruthenium complexes bearing Ru(CO)Cl(PiPr3)2 entities. Organometallics 2006, 25, 3701–3712. [Google Scholar] [CrossRef]
- Linseis, M.; Winter, R.F.; Sarkar, B.; Kaim, W.; Záliš, S. Multistep Electrochromic Behaviour from an Organometallic Tetranuclear Complex of a Tetradonor-Substituted Olefin. Organometallics 2008, 27, 3321–3324. [Google Scholar] [CrossRef]
- Linseis, M.; Záliš, S.; Zabel, M.; Winter, R.F. Ruthenium Stilbenyl and Diruthenium Distyrylethene Complexes: Aspects of Electron Delocalization and Electrocatalyzed Isomerization of the Z-Isomer. J. Am. Chem. Soc. 2012, 134, 16671–16692. [Google Scholar] [CrossRef] [PubMed]
- Wuttke, E.; Hervault, Y.-M.; Polit, W.; Linseis, M.; Erler, P.; Rigaut, S.; Winter, R.F. Divinylphenylene-and Ethynylvinylphenylene-Bridged Mono-, Di-, and Triruthenium Complexes for Covalent Binding to Gold Electrodes. Organometallics 2014, 33, 4672–4686. [Google Scholar] [CrossRef]
- Pfaff, U.; Hildebrandt, A.; Korb, M.; Oßwald, S.; Linseis, M.; Schreiter, K.; Spange, S.; Winter, R.F.; Lang, H. Electronically Strongly Coupled Divinylheterocyclic-Bridged Diruthenium Complexes. Chem. Eur. J. 2016, 22, 783–801. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.D. Near-infrared electrochromic materials for optical attenuation based on transition-metal coordination complexes. J. Solid State Electrochem. 2005, 9, 778–787. [Google Scholar] [CrossRef]
- Mortimer, R.J.; Dyer, A.L.; Reynolds, J.R. Electrochromic organic and polymeric materials for display applications. Org. Disp. 2006, 27, 2–18. [Google Scholar] [CrossRef]
- Sreejith, S.; Carol, P.; Chithra, P.; Ajayaghosh, A. Squaraine dyes: A mine of molecular materials. J. Mater. Chem. 2008, 18, 264–274. [Google Scholar] [CrossRef]
- Astruc, D.; Ornelas, C.; Ruiz, J. Dendritic Molecular Electrochromic Batteries Based on Redox-Robust Metallocenes. Chem. Eur. J. 2009, 15, 8936–8944. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, R.J. Electrochromic Materials. Annu. Rev. Mater. Res. 2011, 41, 241–268. [Google Scholar] [CrossRef]
- D’Alessandro, D.M. Exploiting redox activity in metal-organic frameworks: Concepts, trends and perspectives. Chem. Commun. 2016. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Chen, S.; Lu, B.; Xu, J. Hybrid π-conjugated polymers from dibenzo pentacyclic centers: Precursor design, electrosynthesis and electrochromics. Sci. China Chem. 2017, 60, 38–53. [Google Scholar] [CrossRef]
- Dai, F.; Dou, J.; He, H.; Zhao, X.; Sun, D. Self-Assembly of Metal—Organic Supramolecules: From a Metallamacrocycle and a Metal—Organic Coordination Cage to 1D or 2D Coordination Polymers Based on Flexible Dicarboxylate Ligands. Inorg. Chem. 2010, 49, 4117–4124. [Google Scholar] [CrossRef] [PubMed]
- Case, F.H.; Butte, W.A. Further Preparation of Substituted 2,6-Bis(2′-pyridyl)pyridines. J. Org. Chem. 1961, 26, 4415–4418. [Google Scholar] [CrossRef]
- Wang, H.-B.; Mudraboyina, B.P.; Li, J.; Wisner, J.A. Minimal complementary hydrogen-bonded double helices. Chem. Commun. 2010, 46, 7343–7345. [Google Scholar] [CrossRef] [PubMed]
- Kazuki, O.; Daisuke, S.; Tomoya, Y.; Katsuhiro, I.; Ryota, Y.; Toshio, T.; Hirofumi, S.; Tetsuya, O.; Hiroki, K.; Nobuhiro, Y.; et al. Synthesis and Self-Assembly of NCN-Pincer Pd-Complex-Bound Norvalines. Chem. Eur. J. 2013, 19, 12356–12375. [Google Scholar]
- Evans, B.J.; Doi, J.T.; Musker, W.K. The aqueous periodate oxidation of aromatic and carboxylic acid disufides. Phosphorus Sulfur Silicon Relat. Elem. 1992, 73, 5–13. [Google Scholar] [CrossRef]
- Lawrence, D.S.; Jiang, T.; Levett, M. Self-Assembling Supramolecular Complexes. Chem. Rev. 1995, 95, 2229–2260. [Google Scholar] [CrossRef]
- Swiegers, G.F.; Malefetse, T.J. New Self-Assembled Structural Motifs in Coordination Chemistry. Chem. Rev. 2000, 100, 3483–3538. [Google Scholar] [CrossRef] [PubMed]
- Oliveri, C.G.; Ulmann, P.A.; Wiester, M.J.; Mirkin, C.A. Heteroligated Supramolecular Coordination Complexes Formed via the Halide-Induced Ligand Rearrangement Reaction. Acc. Chem. Res. 2008, 41, 1618–1629. [Google Scholar] [CrossRef] [PubMed]
- Psomas, G.; Stemmler, A.J.; Dendrinou-Samara, C.; Bodwin, J.J.; Schneider, M.; Alexiou, M.; Kampf, J.W.; Kessissoglou, D.P.; Pecoraro, V.L. Preparation of Site-Differentiated Mixed Ligand and Mixed Ligand/Mixed Metal Metallacrowns. Inorg. Chem. 2001, 40, 1562–1570. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.V.; Raymond, K.N. The Big Squeeze: Guest Exchange in an M4L6 Supramolecular Host. J. Am. Chem. Soc. 2005, 127, 7912–7919. [Google Scholar] [CrossRef] [PubMed]
- Garci, A.; Marti, S.; Schurch, S.; Therrien, B. Insight into the dynamic ligand exchange process involved in bipyridyl linked arene ruthenium metalla-rectangles. RSC Adv. 2014, 4, 8597–8604. [Google Scholar] [CrossRef]
- Garci, A.; Gupta, G.; Dalvit, C.; Therrien, B. Investigating the Formation Mechanism of Arene Ruthenium Metallacycles by NMR Spectroscopy. Eur. J. Inorg. Chem. 2014, 2014, 5651–5661. [Google Scholar] [CrossRef]
- Hiraoka, S.; Sakata, Y.; Shionoya, M. Ti(IV)-Centered Dynamic Interconversion between Pd(II), Ti(IV)-Containing Ring and Cage Molecules. J. Am. Chem. Soc. 2008, 130, 10058–10059. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.-R.; Stang, P.J. Direct and Quantitative Characterization of Dynamic Ligand Exchange between Coordination-Driven Self-Assembled Supramolecular Polygons. J. Am. Chem. Soc. 2009, 131, 3487–3489. [Google Scholar] [CrossRef] [PubMed]
- Pevny, F.; Winter, R.F.; Sarkar, B.; Záliš, S. How to elucidate and control the redox sequence in vinylbenzoate and vinylpyridine bridged diruthenium complexes. Dalton Trans. 2010, 8000–8011. [Google Scholar] [CrossRef] [PubMed]
- Záliš, S.; Winter, R.F.; Kaim, W. Quantum chemical interpretation of redox properties of ruthenium complexes with vinyl and TCNX type non-innocent ligands. Coord. Chem. Rev. 2010, 254, 1383–1396. [Google Scholar] [CrossRef]
- Richardson, D.E.; Taube, H. Mixed-valence molecules: Electronic delocalization and stabilization. Coord. Chem. Rev. 1984, 60, 107–129. [Google Scholar] [CrossRef]
- De la Rosa, R.; Chang, P.J.; Salaymeh, F.; Curtis, J.C. Redox asymmetry and metal–metal coupling in pyrazine-bridged ruthenium dimers. Inorg. Chem. 1985, 24, 4229–4231. [Google Scholar] [CrossRef]
- Crutchley, R.J. Intervalence Charge Transfer and Electron Exchange Studies of Dinuclear Ruthenium Complexes. In Advances in Inorganic Chemistry; Sykes, A.G., Ed.; Academic Press: Cambridge, MA, USA, 1994; Volume 41, pp. 273–325. [Google Scholar]
- Winter, R.F. Half-Wave Potential Splittings ΔE1/2 as a Measure of Electronic Coupling in Mixed-Valent Systems: Triumphs and Defeats. Organometallics 2014, 33, 4517–4536. [Google Scholar] [CrossRef]
- Hildebrandt, A.; Lang, H. (Multi)ferrocenyl Five-Membered Heterocycles: Excellent Connecting Units for Electron Transfer Studies. Organometallics 2013, 32, 5640–5653. [Google Scholar] [CrossRef]
- Connelly, N.G.; Geiger, W.E. Chemical Redox Agents for Organometallic Chemistry. Chem. Rev. 1996, 96, 877–910. [Google Scholar] [CrossRef] [PubMed]
- Ovchinnikov, A.A. Multiplicity of the ground state of large alternant organic molecules with conjugated bonds (do organic ferromagnets exist?). Theor. Chim. Acta 1978, 47, 297–304. [Google Scholar] [CrossRef]
- Yoshizawa, K.; Hoffmann, R. Potential Linear-Chain Organic Ferromagnets. Chem. Eur. J. 1995, 1, 403–413. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, H.; Wang, L.; Wang, R.; Wang, L. Effect of configuration and conformation on the spin multiplicity in xylylene type biradicals. Sci. China Ser. B Chem. 2000, 43, 524–530. [Google Scholar] [CrossRef]
- Baumgarten, M. High Spin Molecules Directed Towards Molecular Magnets. In EPR of Free Radicals in Solids II; Lund, A., Shiotani, M., Eds.; Springer: Dordrecht, The Netherlands, 2012; Volume 25, pp. 205–244. [Google Scholar]
- Abe, M. Diradicals. Chem. Rev. 2013, 113, 7011–7088. [Google Scholar] [CrossRef] [PubMed]
- Barlow, S.; Risko, C.; Chung, S.-J.; Tucker, N.M.; Coropceanu, V.; Jones, S.C.; Levi, Z.; Brédas, J.-L.; Marder, S.R. Intervalence Transitions in the Mixed-Valence Monocations of Bis(triarylamines) Linked with Vinylene and Phenylene—Vinylene Bridges. J. Am. Chem. Soc. 2005, 127, 16900–16911. [Google Scholar] [CrossRef] [PubMed]
- Barlow, S.; Risko, C.; Odom, S.A.; Zheng, S.; Coropceanu, V.; Beverina, L.; Brédas, J.-L.; Marder, S.R. Tuning Delocalization in the Radical Cations of 1,4-Bis[4-(diarylamino)styryl]benzenes, 2,5-Bis[4-(diarylamino)styryl]thiophenes, and 2,5-Bis[4-(diarylamino)styryl]pyrroles through Substituent Effects. J. Am. Chem. Soc. 2012, 134, 10146–10155. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.-J.; Yao, C.-J.; Shao, J.-Y.; Yao, J.; Zhong, Y.-W. Oligotriarylamines with a Pyrene Core: A Multicenter Strategy for Enhancing Radical Cation and Dication Stability and Tuning Spin Distribution. Chem. Eur. J. 2014, 20, 17454–17465. [Google Scholar] [CrossRef] [PubMed]
- Hassenrück, C.; Winter, R.F. Manipulation and Assessment of Charge and Spin Delocalization in Mixed-Valent Triarylamine–Vinylruthenium Conjugates. Inorg. Chem. 2017, 56, 13517–13529. [Google Scholar] [CrossRef] [PubMed]
- Garnovskii, A.D.; Kharisov, B.I.; Blanco, L.M.; Sadimenko, A.P.; Uraev, A.I.; Vasilchenko, I.S.; Garnovskii, D.A. Metal Complexes as Ligands. J. Coord. Chem. 2002, 55, 1119–1134. [Google Scholar] [CrossRef]
- Serroni, S.; Campagna, S.; Puntoriero, F.; Di Pietro, C.; McClenaghan, N.D.; Loiseau, F. Dendrimers based on ruthenium(II) and osmium(II) polypyridine complexes and the approach of using complexes as ligands and complexes as metals. Chem. Soc. Rev. 2001, 30, 367–375. [Google Scholar] [CrossRef]
- Herrendorf, W.; Bärnighausen, W. HABITUS. Program for the Optimization of the Crystal Shape for Numerical Absorption Correction in X-SHAPE, version 1.06; Fa. STOE: Darmstadt, Germany, 1999. [Google Scholar]
- Sheldrick, G.M. SHELXL-97, Program for Crystal Structure Solution and Refinement; Universität Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; Van De Streek, J.; Wood, P.A. Mercury CSD 2.0—New features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Krejcik, M.; Danicek, M.; Hartl, F. Simple construction of an infrared optically transparent thin-layer cell: Applications to the redox reactions of ferrocene, Mn2(CO)10 and Mn(CO)3(3,5-di-t-butylcatcholate)−. J. Eletcroanal. Chem. 1991, 317, 179–187. [Google Scholar] [CrossRef]

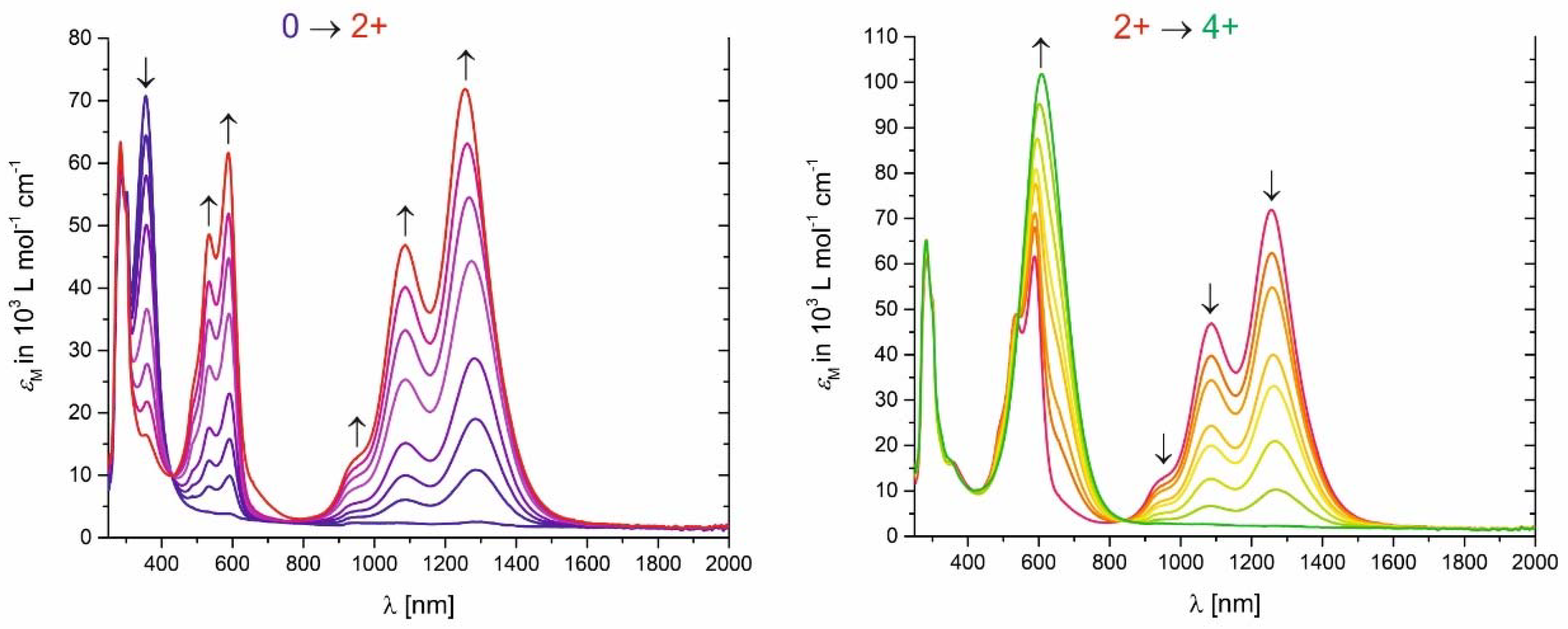
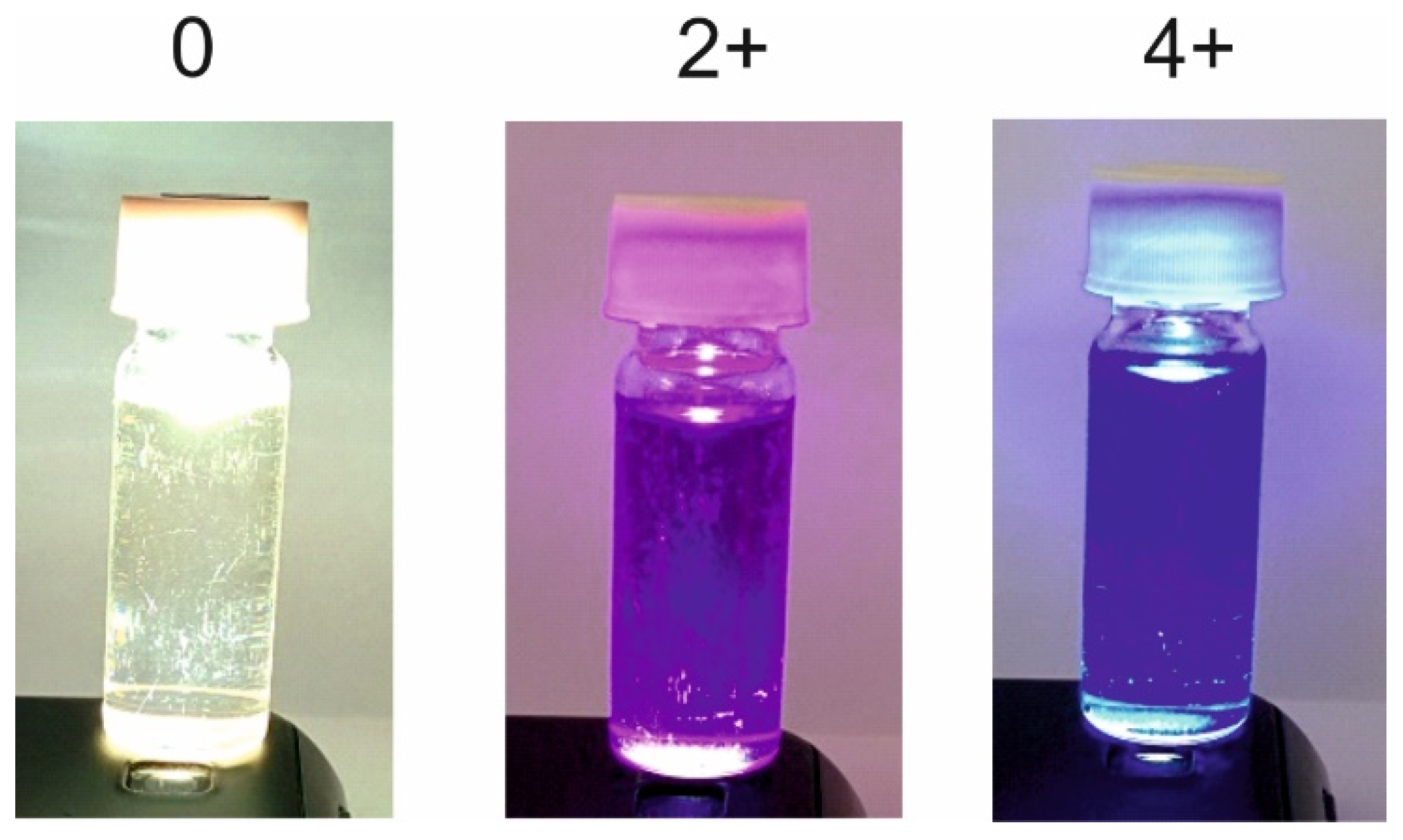

| Complex | E1/20/+ (mV) | E1/21+/2+ (mV) | E1/2+2/4+ (mV) | ΔE1/2+1/2+ (mV) | ΔE1/22+/4+ (mV) |
|---|---|---|---|---|---|
| 12 | −244 | −147 | 82 | 97 | 229 |
| 13 | −330 | −241 | 74/125 4 | 89 | 315 |
| 22 | −256 | −173 | 59 | 83 | 232 |
| 32 | −256 | −175 | 73 | 81 | 248 |
| 42 | −220 | −150 | 45 | 70 | 195 |
| 43 | −330 | −255 | 57/114 4 | 75 | 312 |
| Ru2bza2 | −250 | - | 20 5 | - | 270 |
| Ru2Cl2 | −75 | - | 175 5 | - | 250 |
| Complex | υ(CO) 1 | λmax(εmax) 1 |
|---|---|---|
| 1 | 1901 | 354(85,000) |
| 12+ | 1927, 1944 | 279(59,300), 492(35,700), 534(69,000), 587(83,000), 963(15,900), 1089(62,700), 1259(95,000) |
| 14+ | 1973 | 279(79,200), 602(126,000) |
| 2 | 1901 | 354(85,000) |
| 22+ | 1927, 1943 | 279(59,500), 495(37,000), 535(69,000), 587(83,000), 963(15,600), 1088(62,700), 1257(95,000) |
| 24+ | 1973 | 279(79,600), 602(127,000) |
| 3 | 1902 | 354(70,700) |
| 32+ | 1928, 1944 | 283(63,300), (495(25,900), 535(48,600), 587(61,600) 936(13,400), 1085(46,900), 1256(72,000) |
| 34+ | 1972 | 282(65,300), 608 (102,000) |
| 4 | 1901 | 356(60,600) |
| 42+ | 1928, 1944 | 279(46,400), 537(49,700), 589(61,000), 963(12,100), 1086(45,100), 1258(70,000) |
| 44+ | 1973 | 283(116,000), 364(42,700), 592(109,000) |
| Ru2bza2 | 1904 | 362(19,000) |
| Ru2bza2+ | 1924, 1944 | 357(19300), 487(6600), 536(6800), 587(8000), 1016(9200), 1084(1900), 1272 (5800) |
| Ru2bza22+ | 1971 | 375 (9200), 427(9000), 606(13000) |
| Complex | Dication | Tetracation | ||
|---|---|---|---|---|
| r.t. | 123 K | r.t. | 123 K | |
| 1 | 2.019 | 2.019 | 2.030 | 2.036 |
| 2 | 2.019 | 2.018 | 2.031 | 2.028 |
| 3 | 2.019 | 2.018 | 2.031 | 2.028 |
| 4 | 2.019 | 2.018 | 2.032 | 2.029 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anders, P.; Rapp, M.R.; Linseis, M.; Winter, R.F. Tetraruthenium Metallamacrocycles with Potentially Coordinating Appended Functionalities. Inorganics 2018, 6, 73. https://doi.org/10.3390/inorganics6030073
Anders P, Rapp MR, Linseis M, Winter RF. Tetraruthenium Metallamacrocycles with Potentially Coordinating Appended Functionalities. Inorganics. 2018; 6(3):73. https://doi.org/10.3390/inorganics6030073
Chicago/Turabian StyleAnders, Patrick, Mario Robin Rapp, Michael Linseis, and Rainer F. Winter. 2018. "Tetraruthenium Metallamacrocycles with Potentially Coordinating Appended Functionalities" Inorganics 6, no. 3: 73. https://doi.org/10.3390/inorganics6030073
APA StyleAnders, P., Rapp, M. R., Linseis, M., & Winter, R. F. (2018). Tetraruthenium Metallamacrocycles with Potentially Coordinating Appended Functionalities. Inorganics, 6(3), 73. https://doi.org/10.3390/inorganics6030073