1. Introduction
Chirality is the geometric property of an object that cannot be superimposed on its mirror image. As a fundamental feature of biological systems and nature, chiral materials and their mirror-image counterparts (enantiomers) lack inversion symmetry and exhibit distinctive physical and chemical behaviors [
1,
2]. They are being actively explored for circularly polarized photodetectors, circularly polarized light-emitting diodes (CP-LEDs), bioimaging, three-dimensional (3D) displays, quantum computing and communication, non-volatile memory, and spintronic devices [
3,
4,
5,
6,
7]. Although artificial optical elements such as metasurfaces and wave plates can reproduce certain chiroptical effects, their dependence on complex nanofabrication or bulky components elevates cost and can reduce efficiency. By contrast, intrinsically chiral materials offer a direct route to miniaturized, integrated devices with simplified processing [
8,
9,
10,
11]. Since the first report of a hydrogenated indanone derivative exhibiting circularly polarized luminescence (CPL) in 1967, the field has grown into an interdisciplinary frontier spanning molecular materials, nanotechnology, photochemistry, and optoelectronics [
12].
Recently, hybrid organic–inorganic metal halides (OIMHs)—including perovskite families across different dimensionalities—have drawn intense interest for optoelectronics owing to their strong optical absorption, efficient charge transport, long carrier diffusion lengths, and solution processability. Introducing enantiomerically pure organic molecules into the metal–halide lattice during crystallization enables low-cost, solution-grown chiral OIMHs. These materials exhibit pronounced chiroptical responses (circular dichroism, CPL) and spin-related phenomena such as strong spin–orbit coupling, Rashba splitting, and extended spin lifetimes, making them promising for spintronic applications. Billing and co-workers reported the first low-dimensional chiral OIMHs in 2003, followed by systematic structural analyses in 2006 and 2013 [
13,
14,
15]; in 2017, thin-film chiral OIMHs were comprehensively characterized for their chiroptical responses, marking a key milestone. Notably, even achiral organic cations can induce symmetry breaking through stereoselective arrangements of inorganic octahedra, yielding helical, chain-like architectures. Such structural engineering allows precise modulation of optical and electronic properties via rational selection of the chiral component (
Figure 1).
Extensive efforts now focus on incorporating diverse chiral spacers to enhance structural asymmetry and chiroptical strength in OIMHs [
13,
14,
17]. Remarkably, these materials display CPL and optical activity without external magnetic fields, coupling chirality directly with perovskites’ superior optoelectronic features. The hybrid lattice thus unites high carrier mobility and strong absorption from the inorganic framework with the functional flexibility of chiral organic moieties, enabling phenomena including circular dichroism, CPL, nonlinear optical effects, ferroelectricity, and the chirality-induced spin selectivity (CISS) effect. Dimensional control has revealed that two-dimensional (2D) and quasi-2D chiral OIMHs often show enhanced chiroptical responses and favorable exciton transport, supporting applications in light-emitting diodes and photodetectors. In addition, chiral organic cations can induce chiral phonons in the perovskite lattice, giving rise to strong Raman optical activity and opportunities in chiral phononics. OIMHs do not have a single emission origin. Depending on dimensionality and metal center, their PL typically falls into a few well-defined photophysical buckets (CPL is a polarization property layered on top of these same emissive states). Usually, the CPL emission in arises from (i) band-edge/free excitons, (ii) self-trapped excitons in low-dimensional soft lattices, (iii) ns
2-ion–centered (Sb
3+/Bi
3+/Sn
2+) s–p/CT states, (iv) transition-metal dopant d–d transitions (e.g., Mn
2+), and (v) cluster/charge-transfer states, with appropriate references [
18,
19,
20,
21].
Herein, we first introduce key concepts of chirality, including luminescence asymmetry factors and related evaluation metrics. We then summarize recent advances in chiral OIMHs, covering synthetic strategies, crystal structures, mechanisms of chirality transfer, and emerging applications in CPL emitters, circularly polarized photodetectors, and spintronic devices. Finally, we highlight major challenges and unresolved issues and outline a forward-looking perspective on designing next-generation chiral functional materials with improved stability and device performance.
2. Circular Dichroism (CD) Spectroscopy and CPL Spectroscopy
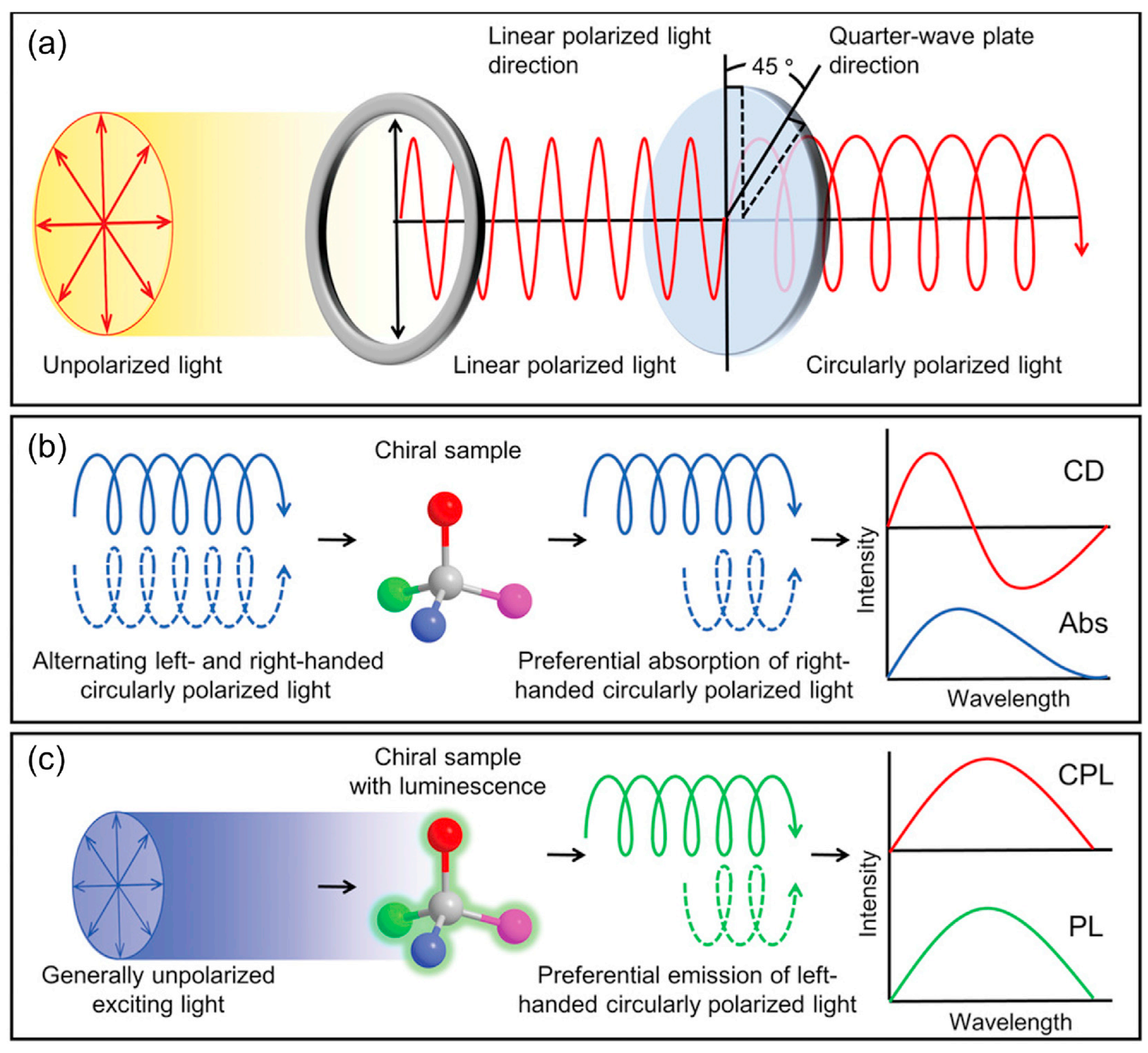
Among various spectroscopic tools for the characterization of chiral materials, the key tools for investigating the optical response of chiral materials are CD spectroscopy and CPL spectroscopy, which characterize the chiroptical properties in the ground state and excited state, respectively. CD spectroscopy is a spectral technique based on the differential absorption of left- and right-handed circularly polarized light by optically active substances. The CD phenomenon originates from the distinct absorption characteristics of chiral molecules towards left- and right-handed circularly polarized light. When plane-polarized light passes through an optically active medium, it can be decomposed into two circularly polarized beams with equal amplitude and phase but opposite rotation directions. During measurement, a xenon lamp source alternately generates circularly polarized light of both handedness. By comparing the intensity differences between incident and transmitted light through the chiral material, the CD spectrum is obtained. The Cotton effect typically appears at the characteristic absorption peaks of the material (
Figure 1a). If the medium exhibits different absorption coefficients (Δ
ε) for these circularly polarized lights, the emergent light becomes elliptically polarized, a phenomenon known as CD:
The magnitude of CD can be quantified using the absorption dissymmetry factor (
gabs, also called
gCD):
Experimentally,
gabs can be directly calculated from the ellipticity (
θ) measured by CD spectroscopy and the absorbance (
A) using the following formula:
where
θ is directly measured by CD spectropolarimeter in millidegrees (m
deg), representing the change in elliptical polarization state caused by differential absorption (Δ
A) of left- and right-circularly polarized light.
A is typically calculated from UV-Vis spectroscopy or the transmitted light intensity in CD spectra.
CPL spectroscopy serves as a crucial characterization tool for investigating the excited-state properties of chiral luminescent materials by detecting the differential emission intensity between left- and right-handed circularly polarized light. CPL measurements typically employ unpolarized excitation sources to avoid artificial polarization effects on the genuine CPL signal [
22,
23,
24]. Upon excitation, chiral emitters exhibit distinct emission intensities for left- (
IL) and right-handed (
IR) circularly polarized components. The CPL spectrum, which reflects this emission anisotropy, always accompanies the corresponding fluorescence spectrum (
Figure 1b). Notably, while chiral systems exhibiting CD signals may not necessarily demonstrate CPL activity, materials with CPL properties invariably display Cotton effects in their CD spectra [
16]. The CPL spectrum quantifies the intensity difference between emitted circularly polarized components:
In practice, direct measurement of absolute intensities faces technical challenges such as detector response variations and optical path losses. Thus, the luminescence dissymmetry factor (
glum), ranging from 0 (unpolarized) to ±2 (fully polarized), is adopted to quantify CPL asymmetry:
From a quantum mechanical perspective,
glum correlates with the scalar product of electric (
μ) and magnetic (
m) transition dipole moments:
For systems where |
m| ≪ |
μ| (i.e., electric-dipole-allowed transitions), Equation (6) simplifies to:
The sign of glum is determined by the relative phase between μ and m, which is positive when parallel (left-handed dominance) or negative when antiparallel (right-handed dominance). Pure electric-dipole (|m| → 0) or magnetic-dipole (|μ| → 0) transitions yield glum ≈ 0. The theoretical limit (±2) occurs when |μ| = |m| with a phase difference of ±π/2. In practical molecular systems, |m| is typically 1–2 orders of magnitude smaller than |μ|, resulting in glum values of 10−5–10−2 for most organic emitters.
Equation (7) reveals a critical trade-off: while a small |
μ| enhances
glum, achieving high quantum yield necessitates a large |
μ|. This inherent contradiction poses significant challenges in designing materials simultaneously exhibiting high
glum and quantum yield [
25].
Chiral purity denotes the relative abundance of one enantiomer over its counterpart within a chiral substance. A higher chiral purity indicates a greater proportion of chiral molecules or nanostructures with a specific configuration and a lower content of the opposing enantiomer. Achieving high chiral purity is crucial for enhancing the chiroptical properties of materials, particularly in applications involving CPL. To further amplify CPL of chiral OIMHs, two key challenges are being addressed: (1) Developing Structural Control Methods: Researchers are focusing on constructing highly uniform and ordered CPL-active materials. This involves precise engineering of the perovskite lattice to ensure consistent chirality throughout the material. (2) Designing Robust CPL Systems: Efforts are being made to design CPL systems with larger dissymmetry factors. This includes reinforcing the chiral structure to effectively amplify CPL, thereby enhancing the material’s chiroptical response. CD and CPL spectroscopy are pivotal in providing insights into the structural characteristics of chiral materials. Understanding the interacting mechanisms between chiral optics and chiral substances, as well as the structural selectivity toward polarized light, is essential. This knowledge aids in elucidating the structure–property relationships of chiral materials, offering strong support for designing novel chiral functional materials and advancing cutting-edge applications.
3. Crystal Structures of Chiral OIMHs
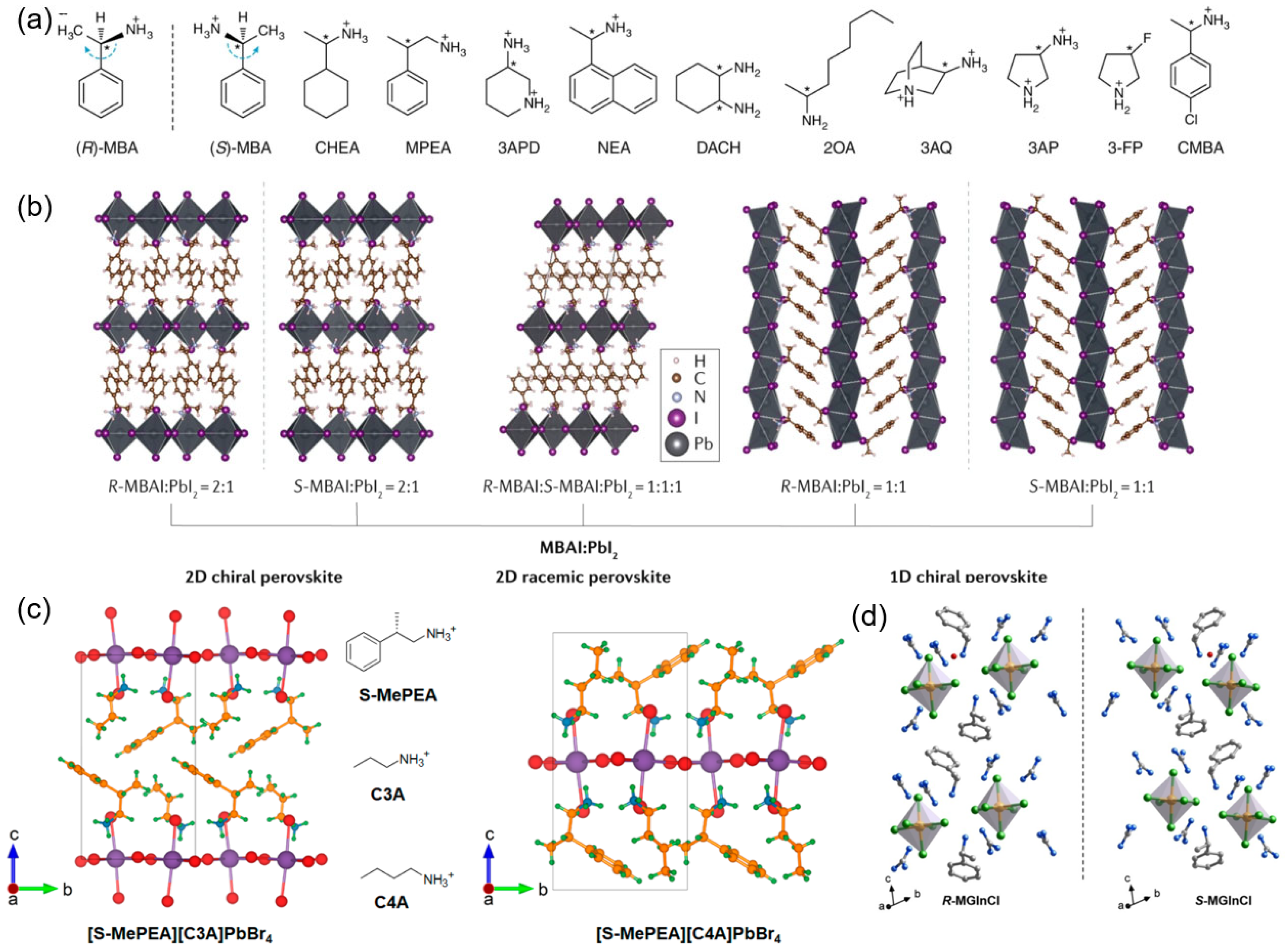
Current research on chiral OIMHs spans a wide range of dimensionalities at the molecular level, including three-dimensional (3D) networks, two-dimensional (2D) layers, one-dimensional (1D) chains, and zero-dimensional (0D) isolated structures [
26,
27,
28]. This dimensional hierarchy reflects differences in the separation of inorganic octahedral frameworks by organic molecules, which directly impact optical and electronic properties. Low-dimensional chiral OIMHs typically exhibit more pronounced chiral characteristics due to their higher content of chiral organic ligands (
Figure 2).
3.1. Three-Dimensional Chiral OIMHs
Three-dimensional chiral OIMHs, often referred to as three-dimensional perovskites, typically adopt centrosymmetric structures, inherently lacking chirality. This structural limitation prevents the manifestation of second-order nonlinear optical effects and CPL, thereby restricting their direct application in chirality-related functional devices. In contrast, low-dimensional OIMHs circumvent the restrictions imposed by the tolerance factor. By precisely tuning the composition of their organic and inorganic components, these materials enable exact control over both crystal structure and band characteristics [
31].
In 3D perovskites, with the general formula ABX3, the [MX
6] octahedra form a 3D framework through corner-sharing, with A-site cations occupying the interstitial voids. These structures typically exhibit high symmetry, such as cubic or tetragonal systems, often belonging to centrosymmetric point groups. This symmetry constraint makes it challenging to introduce chirality through simple structural design. To achieve a stable crystal structure, the chiral organic cations must be sufficiently small. However, most chiral organic cations are relatively large, complicating the direct synthesis of 3D chiral perovskites and often resulting in poor stability [
26,
27]. Furthermore, chirality must be effectively transferred from the organic components (e.g., chiral amines) to the inorganic framework (e.g., [PbX
6]
4− octahedra), but the rigidity of the 3D structure may weaken this transfer efficiency. Since most 3D perovskites lack intrinsic chirality, they rely on exogenous chiral molecules (e.g., chiral organic amines) for induction. However, such molecules are prone to racemization under high-temperature or solution-phase synthesis conditions [
32,
33].
Recent studies have demonstrated methods to induce chirality in 3D perovskites [
34]. For instance, Yang et al. reported a universal and controlled method for synthesizing chiral 3D lead halide perovskites using organic amines or alcohols as chiral templates. Introducing these templates to PbCl
2 in N,N-dimethylformamide (DMF) under acidic conditions induced the crystallization of R/S [DMA]PbCl
3, showcasing a potential approach to overcome the challenges associated with 3D chiral perovskite synthesis. Long et al. theoretically demonstrated that the introduction of chiral R or S-chlorofluoromethylammonium (R-/S-CFMA) cation can transfer the chirality of organic molecules to the internal structural framework and produce 3D OIMHs [
35].
Additionally, co-assembly of non-chiral OIMHs with chiral molecules has been employed to construct 3D chiral OIMHs. In such systems, chiral molecules can transfer their structural asymmetry to the metal halide lattice, thereby inducing CD and CPL signals. [
36,
37]. For example, Shi’s group co-assembled non-chiral CsPbX
3 (X = Cl, Br, I) perovskite nanocrystals with chiral lipid-based gelators featuring helical structures, achieving CPL across multiple wavelengths with a dissymmetry factor on the order of 10
−3. Notably, this CPL response was induced by the aggregated state, enabling reversible switching of the CPL signal via heating or cooling [
37,
38].
3.2. Two-Dimensional and Quasi-2D Chiral OIMHs
Two-dimensional OIMHs are characterized by planar inorganic octahedral frameworks and exhibit compositional and structural diversity, making them the most extensively studied configuration for chiral OIMHs systems [
39,
40]. Compared to 3D structures, 2D OIMHs demonstrate superior properties. Firstly, hydrophobic effects from long-chain organic molecules significantly improve moisture resistance and photo/thermal stability. Secondly, their photoelectronic properties can be precisely regulated by adjusting the inorganic layer number and chemical composition. In the realm of chiral 2D OIMHs, significant strides have been made in enhancing chiral purity to achieve stronger CPL signals. So far, advancements in the synthesis of chiral 2D OIMHs have led to materials exhibiting high degrees of CPL, with maximum g
lum reaching 17%.
In 2003, Billing et al. first reported chiral 2D OIMHs employing (S)-methylbenzylamine (S-MBA) as the chiral organic ligand (
Figure 2a) [
13,
14]. By 2006, the 2D single-crystal structures of these materials were resolved, demonstrating corner-sharing layered frameworks [(R/S-MBA)
2PbI
4]. After, Xiong et al. reported the 2D OIMHs ferroelectrics (R/S-CPEA)
2PbI
4 (R/S-CPEA = R/S-1-(4-chlorophenyl)-ethylammonium) with outstanding ferroelectric performance and robust CD and CPL signals [
27]. Meanwhile, (R/S-MBA)
2CuCl
4 with a 2D structure in which each [CuCl
4]
2− tetrahedron is isolated separately are reported, with a record g-factor as high as ~0.06. The corresponding CD spectra hardly changed after storage under ambient conditions for over a month [
41]. In 2019, Ma et al. found that chiral 2D (R-MBA)
2PbI
4 and (S-MBA)
2PbI
4 exhibit an average degree of circularly polarized PL of 9.6% and 10.1% at 77 K, respectively, and a maximum degree of the circularly polarized PL of 17.6% is achieved in (S-MBA)
2PbI
4 (
Figure 2b) [
42]. In 2020, mixed-cation 2D chiral perovskites were developed, combining chiral aromatic amines with non-chiral spacers to modulate CD properties without complex synthesis of new chiral cations. As shown in
Figure 2c, Wei’s group reported a 2D chiral OIMHs composed of chiral aromatic amines and non-chiral alkylammonium spacer cations (1:1 molar ratio) [
29]. These novel 2D chiral OIMHs integrate unprecedented chirality with alkyl-aryl functionality, governed by non-covalent intermolecular interactions (e.g., CH·π interactions) within their crystal structures. Unlike purely chiral cation-based analogs, the mixed-cation 2D chiral HOMH system exhibits distinct CD properties, offering a new strategy to modulate the chiral optical behavior of known chiral OIMHs without requiring complex synthesis of new chiral cations.
Two-dimensional OIMHs usually exhibit low PLQY values at room temperature. Recent work on chiral layered HOMHPs found that using chiral organic cations further decreased the PLQY, indicating strong losses from nonradiative recombination. However, Shangpu et al. found that 2D OIMHs (R/S)-3BrMBA
2PbI
4 show large PLQY values of 39% and a high degree of CPL of up to 52%. Chiral 3BrMBA molecules provide an excellent option to increase the perovskite crystal angle distortion degrees, resulting in outstanding chiral optical features, as well as structural configurations that strongly decrease the impact of nonradiative loss channels during recombination, resulting in fast radiative decay with large PLQY value [
43].
2D/quasi-2D OIMHs can be represented by the general formula (RNH
3)
2A
n−1MX
3n+1, where n denotes the number of inorganic layers between adjacent organic cation layers (n = 1 for pure 2D structures, n > 1 for quasi-2D structures), and RNH
3 represents the chiral ligand embedded between inorganic layers. The pure 2D OIMHs exhibit the strongest chirality. Differently, quasi-2D crystal structures (n = 2) endow OIMHs with chirality and PL properties simultaneously. Researchers incorporated a wider range of chiral organic spacers, enabling quasi-2D structures (n > 1) that combine chirality with tunable PL properties. In quasi-2D chiral OIMHs, the absorption dissymmetry factor demonstrates a decreasing trend with increasing n value between chiral ligands [
3]. Guan et al. achieved efficient blue CPL in corrugated quasi-2D R/S-(BPEA)FAPbBr
4·H
2O (BPEA = 4-bromophenylethylammonium). By incorporating the achiral FA
+ cation alongside BPEA, they achieved an 8-fold increase in g
lum (~8.4 × 10
−3). This enhancement was attributed to FA
+ reducing lattice distortion and altering the octahedral connectivity from edge-to-edge to corner-to-corner [
44]. These developments emphasized robust 2D and quasi-2D systems that maintain chiral optical activity under ambient conditions and offer tunable optoelectronic properties.
3.3. One-Dimensional Chiral OIMHs
In 1D chiral OIMHs, the inorganic metal halide units that share corner-, edge-, or face can form a chain-like inorganic frameworks surrounded by organic cations. In contrast to 2D and quasi-2D chiral OIMHs, 1D chiral OIMHs exhibit a characteristic chemical formula of RNH
3MX
3, requiring a 1:1 stoichiometric ratio between chiral organic alkylammonium cations and metal cations. The earliest reports introduced chiral organic ammonium cations into 1D halide frameworks, typically forming face-sharing [MX
6] chains or edge-sharing motifs. These studies established that 1D systems can accommodate relatively bulky chiral cations, unlike 3D OIMHs. However, research was mostly limited to crystallographic description, with little attention to optical chirality [
45].
After these initial studies, broader classes of aromatic and aliphatic chiral cations (e.g., R-/S-MBA, R-/S-PEA derivatives) were employed, yielding stable 1D chiral OIMHs. Tailored ligand design, co-assembly strategies, and enhanced exciton–phonon coupling in 1D lattices enabled stronger chirality transfer than in higher-dimensional counterparts. In 2003, Billing and Lemmerer pioneered to report the synthesis and crystal structure of 1D (S-MBA)PbBr
3 chiral OIMHs [
13]. Moreover, researchers achieved robust CD and CPL signals in 1D chiral OIMHs by optimizing cation–inorganic interactions and chain distortions. It has been reported that the (R-/S-MBA)PbI
3 exhibits a 1D crystal structure consisting of extended face-sharing [PbI
6]
4 octahedral chains, which are orientated along the a-axis and separated by isolated organic cations. It has been reported that 1D (R-/S-MBA)PbI
3 thin films exhibited significantly enhanced stability and almost the same spin transport properties within one month. One-Dimensional (R/S-α-PEA)PbI
3 (where α-PEA = α-phenylethylamine) chiral OIMHs with a unique crystal configuration: highly distorted face-sharing [MX
6] octahedral chains surrounded by chiral cations, forming a distinctive stacked framework structure [
46]. In addition, it is reported that (R/S-DMPZ)PbBr
4 (DMPZ = cis-2,5-dimethylpiperazine) can achieve a high g
lum of 2.3 × 10
−2, and moisture-stable 1D HOMH containing chiral R/S-3-aminopiperidine cations and water molecules that exhibit broadband yellowish-white CPL with a g
lum of 1.8 × 10
−3 [
47,
48]. Until now, a series of 1D chiral OIMHs emitting white light have been reported, representing a significant breakthrough in chiral symmetry breaking in OIMHs. These works highlighted the anisotropy and exciton confinement effects inherent to 1D structures, giving them distinct chiroptical properties compared to 2D systems. The chain-like inorganic frameworks of 1D chiral OIMHs not only tolerate large chiral cations but also facilitate efficient chirality transfer, making them promising candidates for CPL emitters, spintronic devices, and chiral optoelectronics.
3.4. 0D Chiral OIMHs
The octahedral units in 0D OIMHs are completely isolated by surrounding organic molecules, allowing for the generation of STEs emission characterized by a large Stokes shift and broadband. 0D chiral OIMHs exhibit outstanding PLQY due to their pronounced quantum confinement effects and large Stokes shifts [
49].
Additionally, 0D systems usually display enhanced environmental stability compared to 3D/2D perovskites because the isolated clusters reduce moisture sensitivity. The development of 0D chiral perovskites has progressed from early structural prototypes in the 2000s. First attempts at introducing chiral cations into isolated [MX6] octahedra or [MX4] tetrahedra produced 0D chiral OIMHs. These systems were structurally stable but initially received limited attention since their optical activity was weaker than 2D/1D counterparts. The focus was mainly on structural chirality rather than photophysical applications. Researchers demonstrated that chirality transfer can persist even in 0D systems, where inorganic clusters (e.g., [PbBr6]4− or [CuCl4]2−) are completely isolated. Key studies showed strong CD due to the rigid environment provided by bulky chiral cations.
With novel design strategies (e.g., chiral ammonium salts, aromatic chiral cations, and hydrogen-bonded supramolecular assemblies) introduced, robust CPL emission with g
lum reaching values is achieved comparable to those of 2D systems. Through the self-assembly of chiral
R/
S-(3-chloro-2-hydroxypropyl)trimethylammonium chloride with antimony(III) chloride, Xuan et al. synthesized 0D OIMHs (
R)-C
6H
15C
l2NO·SbCl
5 and (
S)-C
6H
15C
l2NO·SbCl
5 with CPL activity. These materials feature a 0D structure where SbCl
5 pyramidal units are isolated by chiral organic cations. Optical characterization revealed they have exceptionally high PLQY of 71.2%, originating from STEs emission. The presence of chiral organic cations endows this material with mirror-symmetric CD signals in the ground state and pronounced CPL activity in the excited state, exhibiting
glum of 2.5 × 10
−4 (
R-form) and −1.6 × 10
−4 (
S-form), respectively. This work represents the first report of 0D chiral OIMHs material demonstrating CPL activity, thereby providing a novel design strategy for developing environmentally friendly CPL materials with high emission efficiency [
49]. Moreover, Liu et al. found that chiral 0D OIMHs exhibit near-unity PLQY and large g
lum values up to around 1 × 10
−2. Du et al. found that partial substitution of InCl
3 with SbCl
3 in DMA
4In
1−xSb
xCl
7 (DMA=dimethylammonium) can result in strong CPL signals (g
lum~1.4 × 10
−2) and a high PLQY of 69.13% [
50].
It is found that the choice of different chiral ligands can modify the strength of hydrogen bonding interactions in these 0D OIMHs, to maximize their glum values.
Reports highlighted large Stokes shifts, reduced reabsorption losses, and minimized non-radiative recombination, making 0D chiral OIMHs efficient CPL emitters. Combined with their solution processability, diverse chemical architectures, and distinctive optoelectronic properties, chiral 0D OIMHs show great promise in developing efficient CPL-active materials. In addition, researchers also uncovered multifunctionality: some 0D chiral OIMHs exhibited ferroelectric, nonlinear optical, or CISS-related behaviors, broadening their potential in spin-optoelectronics.
4. Synthetics Strategies for Chiral OIMHs
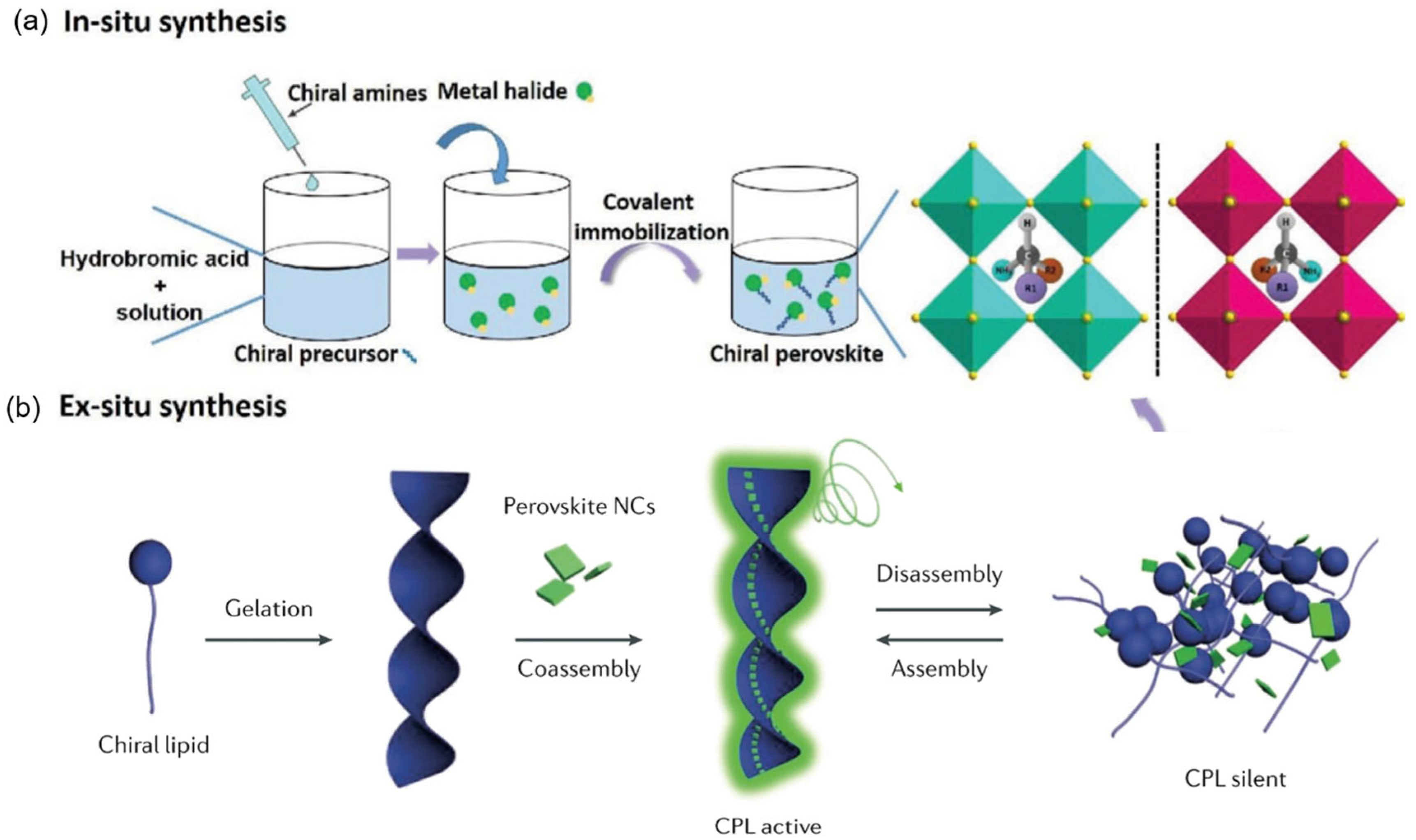
Considering the advanced applications of optoelectronic-active chiral materials, the construction of chiral OIMHs is of critical importance for structure analysis to meet the different demands. At present, a variety of synthetic strategies have been exploited to synthesize chiral OIMHs (
Figure 3). The synthesis methods of chiral OIMHs can be primarily categorized into two approaches: in situ synthesis and ex situ synthesis methods, according to the strategies employing distinct chemical pathways to introduce and modulate chirality within the metal halide lattice.
4.1. In Situ Synthesis
In situ synthesis is a well-established route to chiral OIMHs, as illustrated in
Figure 3a. In the presence of precursor salts and chiral amine monomers, long-chain chiral ammonium cations stabilize the lattice and enhance chiroptical responses—CD and CPL. This method enables the direct growth of chiral OIMHs with tunable sizes, varied dimensionalities, helical morphologies, and tailored chiral organic components [
13,
14,
52,
53]. It reliably affords phase-pure, highly oriented films, facilitating systematic studies of chirality transfer, and has been scaled to thin films and nanocrystals, making it directly compatible with device integration. In addition, in situ self-assembly with chiral templates, gels, or supramolecular frameworks allows fine tuning of both chirality strength and emission properties.
Common protocols include temperature-lowering crystallization, solvent-evaporation crystallization, antisolvent vapor–assisted crystallization (AVC), and aqueous syntheses, which improve film uniformity and strengthen chiral induction. For example, Dong et al. used AVC in which CHCl
3 gradually diffused into a DMF/DMSO ternary solvent, yielding a chiral HOMH of composition (MPEA)
1.5PbBr
3.5(DMSO)
0.5; the coordinating DMSO partially binds Pb
2+ within edge-shared octahedra, producing a distorted, edge-shared layered architecture [
54]. Chen’s group employed a chiral-template inducer, (R/S)-2-amino-1-propanol, in a PbCl
2/DMF system under acidic conditions to obtain chiral R/S-[DMA]PbCl
3 featuring helical [Pb
2Cl
9]
5− chains. The products’ optical rotation matched the template’s handedness and exhibited strong CD and circularly polarized absorption. Devices based on these chiral OIMHs showed excellent CPL detection, with a photocurrent anisotropy factor up to 0.296, demonstrating a general, controllable in situ strategy for chiral OIMHs [
28,
38].
4.2. Ex Situ Synthesis
Ex situ approaches impart chirality after OIMHs are formed by directly modifying pre-synthesized materials with chiral organic ligands or intermediates, so that synergistic ligand–HOMH interactions generate chiral responses [
55]. Chiral ligands are crucial not only for preparation and colloidal stabilization of inorganic nanocrystals in solution, but also for assembling them into ordered structures/superlattices; the transferred interfacial asymmetry induces CD and CPL. A key advantage is that ex situ modification preserves the intrinsic stability of the host perovskite while introducing chirality. Limitations arise because chirality is often surface-localized, which can lead to weaker chiroptical signals and reduced robustness under thermal or solvent stress. To address this, researchers co-assemble perovskite nanocrystals with chiral organic frameworks such as lipid-based gelators, helical polymers, or supramolecular scaffolds. For instance, Shi’s group achieved CPL from CsPbX
3 nanocrystals embedded in chiral lipid gels, with tunable dissymmetry factors (g
lum ≈ 10
−3).
Recently, ex situ strategies have expanded to post-synthetic ion exchange, layer-by-layer assembly, and chiral doping of perovskite films. Shilpa et al. used poly(lauryl methacrylate) (PLMA) as a matrix to encapsulate CsPbBr
3 nanocrystals and fabricate CsPbBr
3–PLMA films, enabling efficient chirality transfer from ligands to OIMHs [
56]. Likewise, electrospun PVA nanofibers loaded with MAPbX
3 nanocrystals produced long-axis alignment of HOMH nanocrystals along the fiber direction [
57].
A widely used starting point is surface-ligand exchange on pre-synthesized CsPbX
3 nanocrystals with chiral amines, lipids, or helicenes. For example, Shi et al. first passivated surfaces with OA/OAm to mitigate interference from highly polar chiral molecules (e.g., diamines), then introduced chiral ligands to achieve co-assembly in which configurational transfer of chiral groups induces lattice distortion and ultimately macroscopic chiral architectures (
Figure 3b) [
37]. In post-synthetic chiral ligand exchange, native ligands are partially or fully replaced by chiral ones, and the resulting chirality arises from surface chiral distortion, chiral packing of capping layers, and chiral field effects rather than bulk-phase reconstruction. Following this principle, He et al. replaced OAm with (1R,2R)- or (1S,2S)-diaminocyclohexane (R/S-DACH) to prepare chiral OAm-capped CsPb(I/Br)
3 nanocrystals, showing that chiroptical properties scale with capping-ligand loading and yielding well-defined chiral OIMHs [
32]. In parallel, mechanical rubbing during solvent evaporation offers a simple route to well-oriented CsPbBr
3 nanowires, increasing the degree of polarization from 0.148 to 0.5 [
58,
59,
60].
Overall, ex situ modification has delivered significant gains in CPL dissymmetry (often up to ~10−2) and ambient stability. Demonstrations in thin films, nanocrystal arrays, and heterostructures make this approach directly relevant to polarized emitters, detectors, and spintronic devices. Unlike in situ growth, ex situ methods flexibly integrate reversible, tunable, multifunctional chirality into pre-formed OIMHs; the remaining challenge is achieving deep, beyond-surface chirality transfer. Together with in situ routes, these strategies are paving the way toward scalable, device-compatible chiral OIMHs.
5. Properties of Chiral OIMHs
5.1. CD
CD refers to the differential absorption of left- and right-circularly polarized light by a chiral medium, is a widely used spectroscopic tool to measure the chiroptical responses (
Figure 4). To date, the CD properties of chiral OIMHs have been widely reported in various forms, including solutions, thin films, powders, and single crystals. Recent studies reveal that OIMHs materials, owing to their distinctive inorganic framework structures, can efficiently couple with various chiral components to form chiral crystals exhibiting pronounced CD signals. Zhang’s group proposed a ligand-exchange strategy: by modifying CsPbBr
3 nanocrystals with chiral ligands, they obtained chiral perovskite nanocrystals with tunable CD signals and a PLQY approaching 100% [
61]. Ahn’s group successfully synthesized chiral OIMHs single crystals (
S-MBA)
2PbI
4 and (
R-MBA)
2PbI
4 by incorporating
S-/
R-MBA molecules into layered lead iodide frameworks [
58]. As shown in
Figure 4a, their CD spectra demonstrate optical activity in the 350–800 nm wavelength range, primarily originating from the chirality transfer effect of organic cations. CD spectra can be employed as a powerful tool in material design techniques and growth monitoring for chiral OIMHs architectures.
5.2. CPL
CPL spectroscopy explicitly measures the differential spontaneous emission of left and right circularly polarized radiations by a luminescent system and thereby served as another key technique to act as chiral probes. At present, CPL studies based on halide OIMHs are mostly focused on films and powders. Liu et al. employed amine-containing lipid N,N′-dioctadecyl-d-glutamic diamide (DGAm) as building blocks to construct chiral spaces through molecular co-assembly. This co-gel system not only achieved high PLQY but also realized CPL (
Figure 4b,c). The prepared CPL-active CsPbX
3 nanocrystals exhibited broad-spectrum emission spanning blue to red wavelengths. The co-assembly CsPbX
3 with lipid molecules successfully induced chiral characteristics while achieving CPL signals with
glum up to 10
−3 [
37].
Compared with films and powders, the CPL signals of chiral OIMHs single crystals have been rarely reported, mainly due to the presence of linear polarized luminescence effects and crystal defects in single-crystal structures. Therefore, preparing single-crystal chiral OIMHs with high CPL activity remains a major challenge. In addition, the CPL activity of chiral OIMHs solutions has also been reported; however, the complicated preparation steps and the large number of organic solvents required have severely limited the development and application of these materials.
5.3. Nonlinear Optical Properties
Nonlinear optics investigates the nonlinear response of media under photoexcitation conditions with optical power as the variable. Chiral OIMHs, with their tunable chemical structures and electronic bandgaps, represent highly promising nonlinear optical materials for advanced techniques [
63,
64,
65,
66]. Due to their excellent nonlinear optical coefficients, current research primarily focuses on the third-order and higher-order nonlinear optical properties of these materials. However, for applications in optical information transmission, processing, and storage, the second-order nonlinear response characteristics of optical components are equally crucial. The inherent non-centrosymmetric nature of chiral molecules can induce non-zero electric dipole moments, thereby endowing chiral OIMHs with significant second-order nonlinear responses [
67].
Taking the typical chiral (
R-or
S-MPEA)
1.5PbBr
3.5(DMSO)
0.5 as an example (
Figure 4d), its structure consists of non-centrosymmetric chirality, DMSO intercalation, and partial edge-sharing [
54]. This chiral HOMH exhibits outstanding second-order nonlinear performance. Theoretical studies suggest that due to the magnetic effects arising from molecular structural asymmetry, chiral media can alter the polarization state of incident light [
68,
69]. Moreover, taking advantage of fluorine substitution on the reported molecular ferroelectric, (pyrrolidinium)MnCl
3, Yong et al. present enantiomeric perovskite ferroelectrics, namely, (R)and (S)-3-(fluoropyrrolidinium)MnCl
3 [
28]. The temperature-dependent second harmonic generation (SHG) signal is sensitive to the inversion symmetry breaking and the generation of ferroelectric polarization (
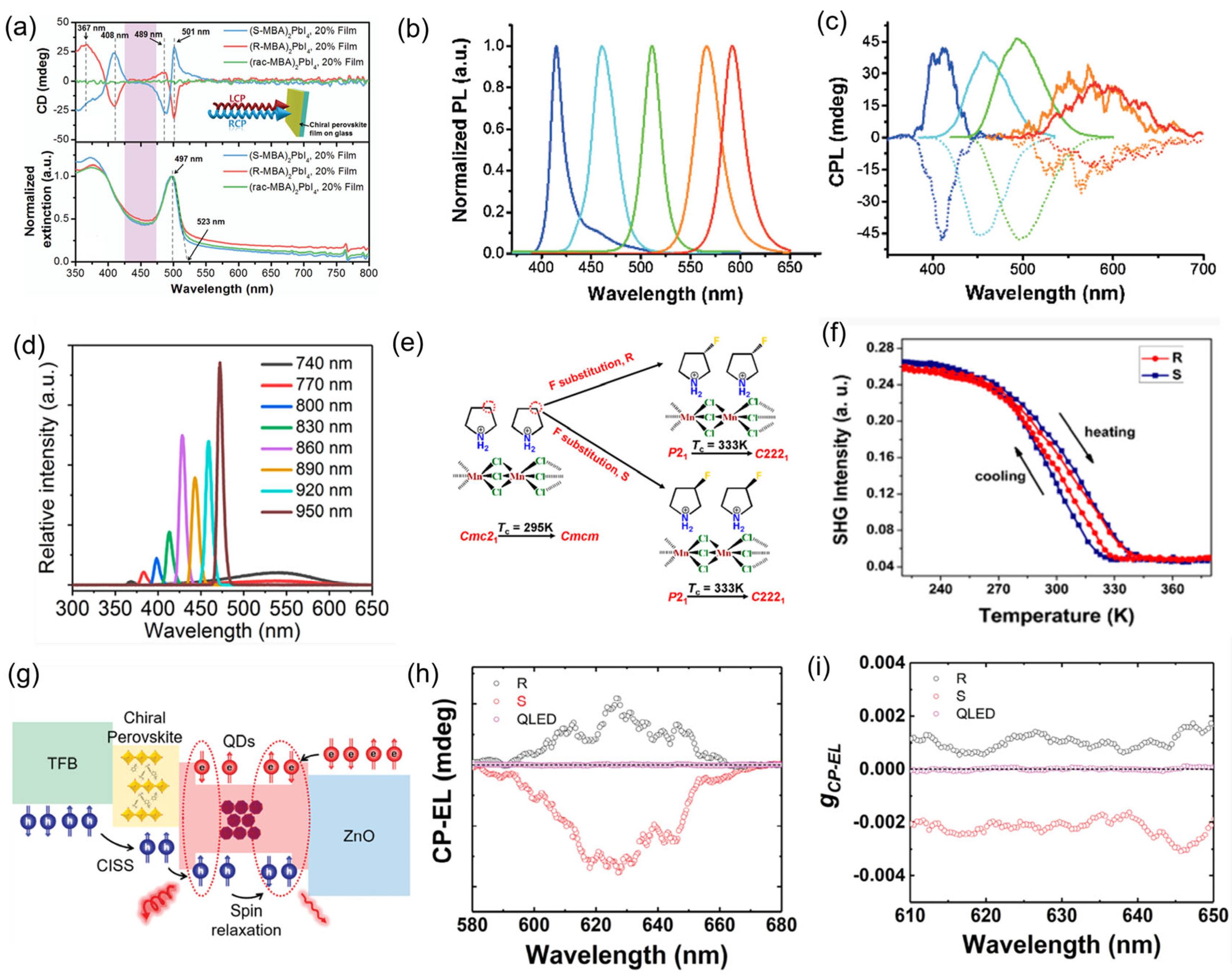
Figure 4e,f). Thus, by modulating the chiral configuration, the magnetic dipole moment can be optimized, thereby enhancing second-order nonlinear effects. Consequently, through further optimization of chiral synthesis and structural control, chiral OIMHs hold broad application prospects in the field of nonlinear optics.
5.4. Bulk Photovoltaic Effect (BPVE) in Chiral OIMHs
BPVE refers to the property of generating a steady-state photocurrent in a material even in the absence of an external bias voltage. The introduction of chirality can break spatial inversion symmetry, leading to the formation of asymmetry. The Miyasaka research group confirmed the BPVE in chiral perovskites by measuring the current–voltage (I–V) dependence of layered perovskite R/S-(MPA)2(MA)Pb2I7 single crystals. The results showed that, at zero bias, the sign of the photocurrent depends on the polarization direction (Pm) within the material. This indicates that the BPVE originates from the alignment of dipole moments induced by the chiral components. Therefore, by regulating polarity and chirality, perovskites offer new breakthroughs for BPVE materials.
5.5. Ferroelectric Properties
Ferroelectric materials have attracted significant attention due to their reversible spontaneous polarization controllable by external electric fields, which enables coupling with various electronic/optical properties [
70]. Recently, chiral OIMHs have gained prominence for their unique ferroelectric characteristics [
71,
72,
73]. The breaking of spatial inversion symmetry serves as a critical prerequisite for ferroelectric effects [
74]. According to the Neumann-Curie principle, when chiral OIMHs crystallize in special point groups (
C1,
C2,
C3,
C4,
C6), they can exhibit intrinsic ferroelectric properties [
75,
76]. Current research on ferroelectric behavior in chiral OIMHs spans diverse structural types, including 3D chiral OIMHs, 2D lead-based chiral metal halides, and 1D manganese-based chiral metal halides [
26,
27]. For instance, in the fluorine-modified (pyrrolidinium) MnCl
3 system, the derived chiral materials
R(
S)-3-FP-MnCl
3 (3-FP structure demonstrate a remarkably high Curie temperature of 333 K, which is a significant improvement over the parent compound pyrrolidinium manganese trichloride (295 K) [
28,
77]. This enhancement demonstrates that judicious selection of chiral organic cations can induce low-symmetry polar space groups, facilitating molecular rearrangement and establishing ferroelectric orientation ordering. The incorporation of chiral ligands not only provides tunability in composition and structure for metal halide systems but also opens new possibilities for developing novel ferroelectric functional materials in quantum communication and optical engineering applications.
5.6. Spintronics
Spintronics, combining the properties of spins with electronics, offers the opportunities for functionalizing conventional electronic devices by manipulating the degree of freedom of the electron’s spin. Chiral OIMHs demonstrate unique advantages for spintronic device applications due to their pronounced tunable spin–orbit coupling and extended spin lifetimes. Chirality-induced spin selectivity (CISS) refers to the ability of chiral materials to preferentially transport spin-polarized carriers without the need for an external magnetic field. This CISS effect effectively suppresses spin-flip elastic backscattering processes [
78,
79]. Studies indicate that electrons moving in a chiral electrostatic field generate an effective magnetic field along the propagation direction, which stabilizes electronic states with specific spin orientations. Moreover, chiral symmetry couples electron spin with linear momentum, enabling selective transmission of spin-polarized electrons and thereby achieving spin-dependent charge transport [
80].
Beard and co-workers reported a 2D (R/S-MBA)2SnI4, with strong octahedral distortion. Their study demonstrated that the vertical charge transport in (R/S-MBA) 2SnI4 films exhibits pronounced spin dependence, representing a concrete manifestation of the CISS effect in chiral OIMHs. Furthermore, they showed that the spin polarization in the current–voltage characteristics reached as high as 94%. This work highlights the tremendous potential of chiral hybrid semiconductors in controlling both spin and charge degrees of freedom.
Wang et al. have demonstrated a pronounced CISS effect in low-dimensional chiral OIMHs prepared by introducing chiral amine ligands. In this study, 2D chiral OIMHs were employed as the CISS spin injection layer, combined with a CdSe/ZnS quantum dot emitting layer, to successfully fabricate a novel spin light-emitting diode device [
62]. The research further revealed that the optical rotation properties and thickness modulation of the chiral OIMHs significantly influence the device’s circularly polarized electroluminescence performance. Notably, the spin injection polarization efficiency of this 2D chiral OIMHs exceeds 80%, with the highest achieved dissymmetry factor reaching 1.6 × 10
−2. This work establishes a groundbreaking approach for developing high-performance spin-LEDs. Chiral OIMHs offer a feasible way to optimize and control the electronic responses, providing much possibility for various electronic applications.
6. Optoelectronic Applications of Chiral OIMHs
In recent years, chiral OIMHs have garnered extensive interdisciplinary research interest in chemistry, physics, biology, and medicine, demonstrating promising optoelectronic applications in bio-monitoring, sensing, imaging, chiral photonics, and electronics. This section focuses on the advances in applications of chiral OIMHs, systematically discussing their unique properties and potential in various cutting-edge fields.
6.1. Circularly Polarized Spin Light-Emitting Diodes (Spin-LEDs)
In traditional optoelectronic approaches, control over spin, charge, and light requires the use of both electrical and magnetic fields. In a spin-LED, charges are injected, and circularly polarized light is emitted from spin-polarized carrier pairs. Circularly polarized spin-LED were first reported by Meijer et al. [
81]. Inspired by their structure, researchers introduced chiral OIMHs into the system, developing spin-LEDs capable of electrically driven circularly polarized emission [
82]. Under an electric field, electrons and holes are injected and transported into the chiral hybrid metal halide layer, where they form excitons or electron-hole pairs via Coulomb interactions. The energy of these excitons is subsequently transferred to the chiral OIMHs. Finally, the excitons in the excited state of the chiral emitter undergo radiative recombination back to the ground state. If the chiral component of the luminescent material exhibits selective asymmetry, circularly polarized emission is generated. Developing novel spin-LEDs that simultaneously achieve high device efficiency and strong CP-EL effects is significant. The breakthrough in this research direction, enabled by chiral emitters with superior device performance and pronounced CP-EL, will open new opportunities for advancing circularly polarized optoelectronic devices in quantum-based optical computing and information processing, three-dimensional (3D) displays, bioencoding, and tomography.
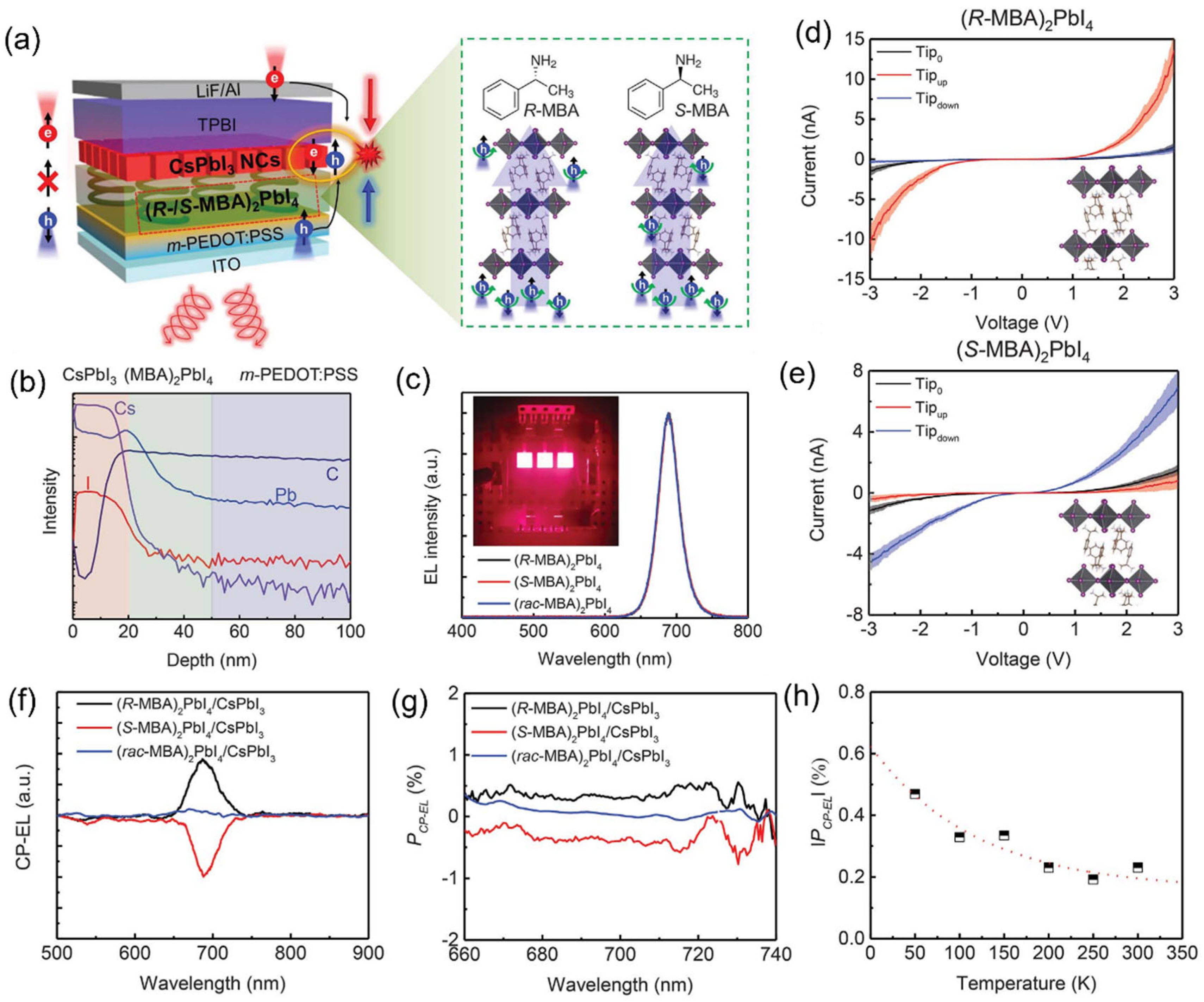
The performance of spin-LEDs is typically evaluated based on two key aspects: device performance and circularly polarized electroluminescence (CP-EL) characteristics. For device performance, the evaluation criteria resemble those of conventional LEDs. In terms of optical properties, the chiroptical performance of spin -LEDs is primarily characterized by the dissymmetry factor. Young et al. demonstrated spin-LEDs based on solution-processed HOMH heterostructures in which spin-polarized holes were injected into an adjacent layer of OIMHs emitters (
Figure 5a). The formation of a well-defined CISS/CsPbI
3 heterostructure was confirmed by time-of-flight secondary ion mass spectrometry (TOF-SIMS) depth profiling (
Figure 5b). The spin polarized hole injection layer is composed of a 30 to 60 nm thick chiral 2D layered R-/S methylbenzylammonium lead iodide [(R-/SMBA)
2PbI
4] polycrystalline bulk film. The spin-LEDs showed a turn-on voltage of 2.4 V and external quantum efficiencies (EQEs) 10.05%, 10.53%, and 11.05% for spin-LEDs based on (R-MBA)
2PbI
4, (S-MBA)
2PbI
4, and (rac-MBA)
2PbI
4 (
Figure 5c), respectively. Spin-LEDs based on CISS/CsPbI
3 NC heterostructure showed distinct CP-EL spectra centered at 688 nm with average PCP-EL = ±0.25% at 660 ≤ l ≤ 740 nm (
Figure 5f,g) and showed similar PCP-EL values with different CISS layer thicknesses (~30 and ~60 nm). By contrast, the (rac-MBA)
2PbI
4-based devices exhibited no CP-EL emission. The similar P
spin values suggested that spin-polarized injection from the CISS layer into the NC layer was high and that the spin-diffusion length in the NC layer was at least one to two NCs (
Figure 5d,e). In addition, they found that the prepared spin-LEDs control the orientation and intensity of CP-EL, depending on the spin polarization of the injected carriers, and the spin-LED achieves ±2.6% circularly polarized electroluminescence at room temperature without applying a magnetic field or using a ferromagnetic contact with a spin-polarized current of >80% (
Figure 5f,g). Ye et al. fabricated a multilayer spin-LED using core–shell structure luminescent layer comprising a 3D MAPbBr
3 core surrounded by a 2D perovskite layer with R/S-MBA, allowing spin-selective carrier injection via the shell while maintaining the optical and structural integrity of the luminescent core. The spin-LED exhibited a PLQY of 54% and a
of 4.0 × 10
−3, increasing to 6 × 10
−3 under electroluminescent operation [
83]. After, Jooho Moon used R/S-NEA to substitute the R/S-MBA, achieving an enhanced PLQY of 78% and a high CP-EL efficiency of 12%.
6.2. Circularly Polarized Light Detector
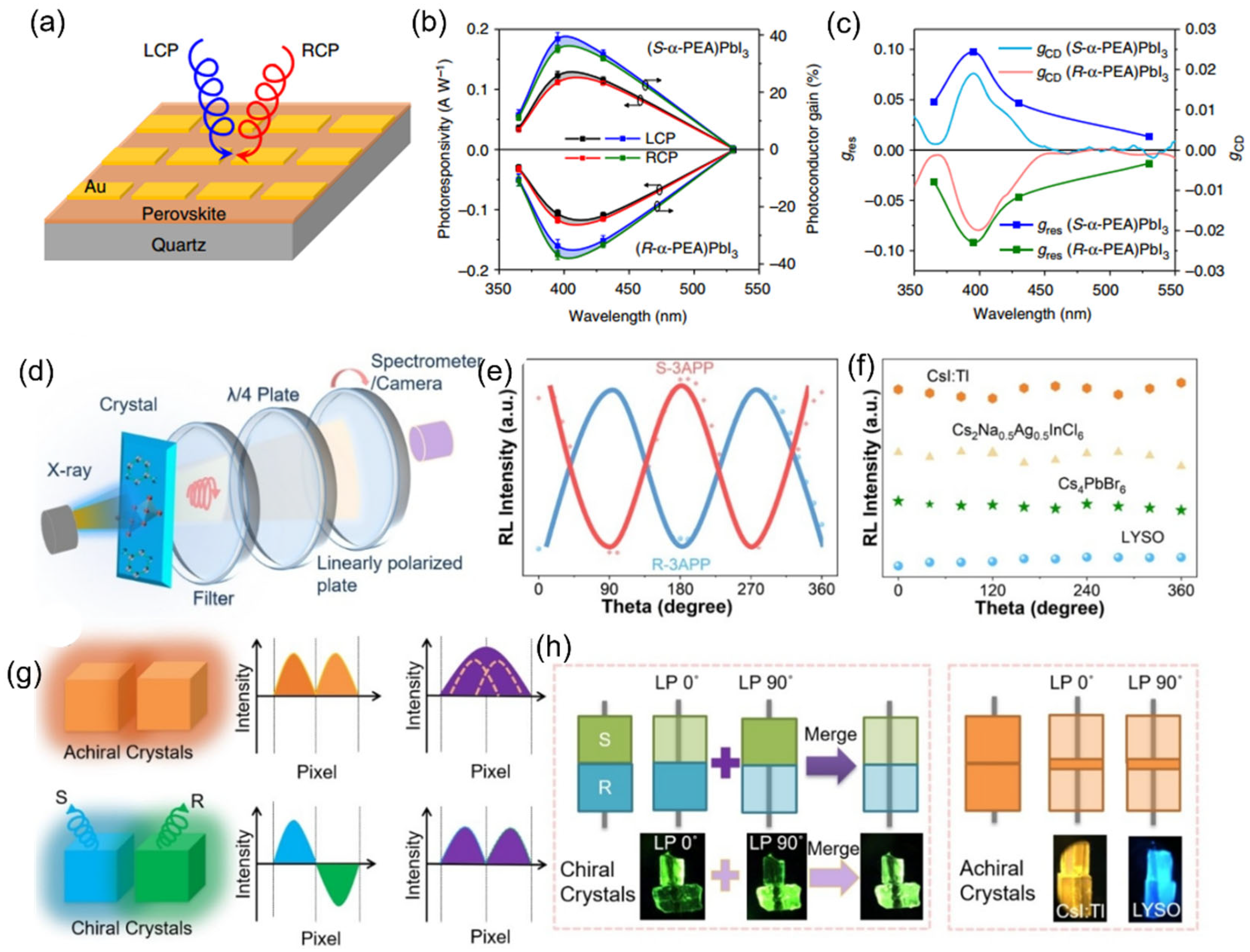
Conventional optical detectors require complex optical components, which severely limit device miniaturization and flexibility. Current optical-element-free detectors primarily employ chiral organic semiconductors or metallic metamaterials, yet these materials suffer from low responsivity. Chiral OIMHs, combining the circular dichroism effect of chiral ligands with the efficient charge transport properties of inorganic frameworks, offer a novel strategy for direct circularly polarized light detection. Tang et al. developed a groundbreaking circularly polarized photodetector based on chiral OIMHs (
Figure 6a–c), achieving remarkable performance metrics: a responsivity of 797 mA W
−1, detectivity of 7.1 × 10
11 Jones, and environmental stability exceeding one month [
46]. It has been reported that the average
gcurrent of the chiral circularly polarized photodetector based on chiral (R)-3BrMBA
2PbI
4 is ~0.5% under 490 nm excitations, which is slightly larger than expected from the CD results, likely because of additional effects from spin-related carrier generation and transport [
43]. These comprehensive parameters demonstrate significant competitive advantages of Chiral OIMHs in circularly polarized light detection.
6.3. Circularly Polarized Scintillator Imaging
Traditional X-ray detectors typically adopt a coupled scintillator array-photosensor design. However, the isotropic propagation of radioluminescence (RL) signals from scintillators induces optical crosstalk between adjacent pixels, substantially degrading spatial resolution and imaging quality [
84,
85,
86]. Scintillator materials exhibit centrosymmetric structures, where isotropic light emission is an intrinsic property of achiral systems, making physical modulation of RL behavior fundamentally challenging. Chiral OIMHs can be encoded into a polarized scintillator pair with the capacity to regulate RL propagation, providing a new opportunity to improve X-ray imaging quality, especially at the boundary region between adjacent scintillators.
Wang et al. pioneered chiral OIMHs scintillators (
R-3AP)PbBr
3Cl·H
2O (
R-3APP) and (
S-3AP)PbBr
3Cl·H
2O (
S-3APP), establishing the first material system with circularly polarized RL (
Figure 6) [
87]. These scintillators demonstrate exceptional performance, including a PLQY of 27.6%, dissymmetry factor of 4 × 10
−2, and outstanding X-ray absorption coefficients. They further explored their polarized RL properties as exhibited in
Figure 6d. The RL intensity of S-3APP and R-3APP displays a significant polarization dependence (
Figure 6e), but the RL intensity (
Figure 6f) of CsI:Tl, LYSO, Cs
2Na
0.5Ag
0.5InCl
6 bulk crystals and Cs
4PbBr
6 in polycrystalline crystals remains constant regardless of the rotation angle, consistent with isotropic propagation. The chiral
R-3APP and
S-3APP scintillators enable propagation control of RL through circular polarization selection, significantly enhancing signal contrast resolution (
Figure 6g). X-ray imaging experiments confirm superior image quality compared to conventional scintillators, as displayed in
Figure 6h. This work proposes an innovative paradigm for addressing persistent optical crosstalk that by precisely engineering the RL propagation direction, interference between adjacent pixels can be fundamentally suppressed. Therefore, the chiral scintillator concept opens new avenues for high-resolution radiation imaging technologies.
Figure 6.
(
a) Schematic diagram of circularly polarized photodetector. (
b) The responsivity and photoconductor gain of (R- and S-α-PEA)PbI
3 device under LCP and RCP light at the wavelengths of 365, 395, 430, and 530 nm. (
c) The wavelength dependent g
res spectra [
46]. (
d) Schematic illustration of a homemade polarized light collecting system under X-ray excitation. (
e) The polarization-dependent RL of chiral S-3APP and R-3APP under X-ray excitation. (
f) The RL of LYSO, Cs
2Na
0.5Ag
0.5InCl
6, Cs
4PbBr
6, and CsI:Tl under X-ray excitation. (
g) Illustration of the minimization of optical crosstalk by polarized RL. (
h) The X-ray imaging using chiral S-3APP and R-3APP achieve a high definition of image boundary quality [
87]. Reprinted with permission from Ref. [
46]. Copyright 2019, Springer Nature. Reprinted with permission from Ref. [
87]. Copyright 2022, Wiley.
Figure 6.
(
a) Schematic diagram of circularly polarized photodetector. (
b) The responsivity and photoconductor gain of (R- and S-α-PEA)PbI
3 device under LCP and RCP light at the wavelengths of 365, 395, 430, and 530 nm. (
c) The wavelength dependent g
res spectra [
46]. (
d) Schematic illustration of a homemade polarized light collecting system under X-ray excitation. (
e) The polarization-dependent RL of chiral S-3APP and R-3APP under X-ray excitation. (
f) The RL of LYSO, Cs
2Na
0.5Ag
0.5InCl
6, Cs
4PbBr
6, and CsI:Tl under X-ray excitation. (
g) Illustration of the minimization of optical crosstalk by polarized RL. (
h) The X-ray imaging using chiral S-3APP and R-3APP achieve a high definition of image boundary quality [
87]. Reprinted with permission from Ref. [
46]. Copyright 2019, Springer Nature. Reprinted with permission from Ref. [
87]. Copyright 2022, Wiley.
7. Conclusions
Chiral OIMHs have emerged as a novel class of functional materials, offering unprecedented opportunities for next-generation photonics and the development of advanced materials due to their unique organic-inorganic hybrid structure, intrinsic chirality, and outstanding optoelectronic properties. When it comes to practical uses, both high PLQYs and large glum values are crucial for the practical applications of CPL-active materials. Despite their remarkable advantages such as of high PLQYs over 80% or 90% in certain chiral OIMHs, several critical challenges remain to be addressed. Despite these advancements, challenges remain in the synthesis and stability of chiral OIMHs. Issues such as moisture sensitivity and scalability of fabrication processes need to be addressed to facilitate the practical application of these materials. Future research directions include the development of novel synthetic strategies, exploration of non-toxic and lead-free chiral perovskites, and integration of chiral OIMHs into functional devices for real-world applications.
Current studies predominantly rely on a narrow selection of chiral organic amines (e.g., 3-methylpiperidine, 3-hydroxypiperidine) as structure-directing agents, restricting versatility of chiral OIMHs. Thus, the development of novel chiral organic cations is urgently needed.
Improvements in g-factor and PLQY have been reported via molecular spacer design, nanoconfinement, host-guest doping, and compositional tuning. A fundamental compromise exists between high glum factor and PLQY. For instance, 0D indium-based materials achieve PLQY up to 83.44% but exhibit weak glum (~10−3), whereas 3D counterparts show enhanced glum (~10−1) at the expense of reduced PLQY. simultaneously achieving large room-temperature g-factors and high PLQY remain a major challenge.
Additionally, most reported chiral OIMHs suffer from instability, and lead-based variants pose toxicity risks, significantly impeding commercial viability. It has been found that humidity has been found to disrupt the structural integrity and chiroptical activity of chiral 2D OIMHs, highlighting the need for robust materials that can maintain their chiral properties under varying environmental conditions. The development of chiral HMOHs with long-term environmental stability under moisture, heat and light is still urgently needed to avoid obstacles to practical applications.
Enhancing chiral purity in chiral OIMHs is a critical step toward achieving strong CPL signals and developing advanced chiroptical devices. Precise control over chiral structure, crystal orientation, and optoelectronic properties requires deeper mechanistic studies on chirality-property relationships. Through innovative synthesis methods and a deeper understanding of structure–property relationships, significant progress is being made in this field, paving the way for the next generation of chiral functional materials. Further advancements in material processing, device architecture, and performance optimization are essential for practical applications.