Abstract
We herein report on the synthesis and structural characterization, as well as on the photophysical properties, of a series of isoleptic Pt(II) and Pd(II) complexes featuring tridentate N^N^N chelators as luminophores while bearing diverse ancillary co-ligands. Six new palladium complexes were synthesized using 2,6-bis(3-(tert-butyl/trifluoromethyl)-1H-1,2,4-triazol-5-yl)pyridine (tbu or CF3, respectively) in combination with four distinct ancillary ligands, namely: 4-amylpyridine (AmPy), 2,6-dimethylphenyl isonitrile (CNR), triphenylphosphane (PPh3), and 1,3,5-triaza-7-phosphaadamantane (PTA). Thus, two novel Pt(II) complexes incorporating the co-ligands CNR and PTA were explored. The remaining platinum-based complexes, namely CF3-Pt-AmPy, tbu-Pt-AmPy, CF3-Pt-PPh3, and tbu-Pt-PPh3, were re-synthesized according to our previous work for a systematic comparison with their Pd(II) homologues. Thus, photophysical studies were performed in different solvents and conditions. The Pt(II) complexes demonstrated comparable or superior photophysical characteristics in toluene when compared with their solutions in liquid dichloromethane at room temperature. In contrast, the Pd(II) complexes exhibited no significant photoluminescence in dichloromethane, but a surprisingly clear emission was observed for tbu-Pd-AmPy, tbu-Pd-CNR, and tbu-Pd-PPh3 in liquid toluene at room temperature. The significant differences regarding excited state lifetimes and photoluminescence quantum yields underscore the impact of solvent selection on photophysical characteristics, emphasizing the need to consider metal-ligand interactions, as well as the surrounding microenvironment, for a comprehensive interpretation of their photophysical properties. In addition, it is clear that AmPy and CNR render better luminescence efficiencies, whereas PTA is only suitable in toluene.
1. Introduction
The excited-state properties of heavy transition metal complexes render them useful for organic light-emitting diodes, molecular bioimaging, and photodynamic therapy, among other applications [1,2,3]. Luminescent complexes of late transition elements featuring d6, d8, and d10 electronic configurations present ultra-fast intersystem crossing rates, microsecond-range emission, and highly oxygen-sensitive phosphorescence in the visible part of the electromagnetic spectrum with up to 100% singlet oxygen quantum yields [4,5]. Among them, luminescent platinum complexes have gained widespread attention due to their applications in the broad fields of optoelectronics [6,7,8] and biology [9,10]. Notably, Pt(II) complexes have found use as antineoplastic drugs and as contrast agents in bioimaging [11,12].
Pt(II) ions possess a d8 electronic configuration and predominantly adopt square-planar coordination environments. Several studies have focused on the coordination of tridentate ligands and transition metals to form pincer-type complexes. This structure provides enhanced stability to coordination compounds against biochemical and ligand exchange reactions, while combining the properties of metal centers with organic scaffolds [13,14]. Highly luminescent coordination compounds have been achieved with the use of bidentate [15,16,17,18,19] and tridentate ligands [20,21,22], enhancing their applicability in diverse fields. As the rigidity and the chelate effect increase, the non-radiative deactivation is reduced, thus resulting in higher photoluminescence quantum yields and longer lifetimes [23,24]. Furthermore, neutral Pt(II) complexes featuring luminophoric N^N^N chelators can be used as triplet emitters, with potential for blue electroluminescent devices [25]. Their substitution pattern plays a crucial role in determining whether Pt(II) or Pd(II) complexes are able to support monomeric ligand-centered triplet states (3MP-LC). These emissive states are described as admixtures of 3LC (ligand-centered states, i.e., π-π* configurations) and 3MLCT (metal-to-ligand charge-transfer states, i.e., d-π* configurations), which has been extensively documented for Pt(II) derivatives [26,27]. Additionally, Pt(II) complexes are noteworthy for their tendency to aggregate both in solids and solutions, leading to a red-shifted phosphorescence [28]. The self-assembly of luminescent alkynylplatinum(II) terpyridyl complexes has been comprehensively documented by Yam et al. [29]. The formation of metallo-gels, characterized by a low critical gelation concentration, is attributed to both π-π and Pt···Pt interactions, resulting in highly efficient aggregation [30]. Lo and Yam, among others, have leveraged the self-assembling properties of luminescent Pt(II) complexes in biosensing applications [31]. In a similar approach, Ruiz et al. have elucidated the self-assembly mechanism of Pt(II) complexes for the specific recognition of AT-rich DNA sequences, showcasing the versatile applications of this approach in molecular recognition [32].
While luminescent Pt(II) complexes are extensively available and used, their Pd(II) counterparts have been rarely explored. Their sparce representation in bibliographic literature can be attributed to the lower ligand-field splitting associated with Pd(II), due to the less diffuse nature of 4d orbitals when compared with the 5d counterparts in Pt(II) [33]. Consequently, Pd(II) complexes allow for the thermal accessibility of ligand-to-metal charge-transfer or metal-centered states (LMCT or MC, i.e., π/d-d* configurations), leading to the population of the antibonding dx2-y2 orbitals. This often results in non-radiative deactivation pathways through excited state distortion and conical intersections with the ground state. As a result, Pd(II) complexes generally show inferior luminescence efficiencies when compared with their Pt(II) analogues [34].
Despite the structural similarities between Pt(II) and Pd(II) complexes, their ligand exchange rates exhibit significant differences. Pd(II) complexes are reported to be more reactive than Pt(II) complexes in analogous environments [35]. Besides the fact that both Pt(II) and Pd(II) complexes exhibit interesting photophysical and photochemical characteristics, these properties can be fine-tuned by modifying the chelating unit or the ancillary ligands [36]. The organic ligand’s rigidity, combined with the square planar coordination geometry around the metal center, hinders competitive radiationless decay pathways. The use of anionic heteroaromatic ligands in the design of luminescent Pt(II) and Pd(II) complexes has gained considerable attention in this regard [37,38]. These ligands are favoured for their ability to impart structural rigidity, to establish a strong ligand field splitting, and to provide luminescent excited states [39,40]. Additionally, the nature of the monodentate ancillary ligand occupying the remaining coordination site on the metal center assumes a pivotal role in influencing solid-state aggregation and the support of intermolecular interactions within these coordination compounds [41].
Our previous work focussed on neutral platinum(II) complexes with the N^N^N chromophoric ligand motif, as initially introduced by Strassert, De Cola et al. [25,42,43]. These photoactive chelators exhibit notable attributes, including a strong ligand field splitting that precludes a fast thermal population of dissociative metal-centered states, thereby minimizing radiationless deactivation processes. Building upon these prior findings, we herein employed two established luminescent chelators, namely 2,6-bis(3-(tert-butyl/trifluoromethyl)-1H-1,2,4-triazol-5-yl)pyridine, in combination with a broad range of ancillary ligands such as AmPy, CNR, PPh3, and PTA. This approach led to a series of isoleptic platinum(II) and palladium(II) complexes, with Pt(II) and Pd(II) centers serving as perturbative metal ions.
The main objective of this work is to assess how the choice of d8-coordinated metal centers influences the photophysical properties when exposed to two different solvents at room temperature (DCM and toluene) and at 77 K in frozen glassy matrices (DCM/MeOH at a 1:1 volume ratio). A comprehensive range of photophysical properties were examined, including photoluminescence quantum yields (ΦL), phosphorescence lifetimes (τ), as well as radiative and radiationless deactivation rate constants (kr and knr, respectively).
2. Results and Discussion
2.1. Design, Synthesis, Purification, and Structural Characterization
The synthesis and characterization of a series of platinum(II) complexes, including CF3-Pt-AmPy, tbu-Pt-AmPy, CF3-Pt-PPh3, and tbu-Pt-PPh3, were carried out by optimizing previously established methodologies [25]. In order to ensure reproducibility and internal consistency, these Pt(II) complexes were freshly prepared, thoroughly purified and comprehensive characterization studies were carried out. To close the coordination sphere, a variety of ancillary ligands were explored. Planar AmPy and CNR ligands possessing conjugated π-systems [44] were chosen. Since complexes with planar ligands tend to form aggregates, phosphane-based ligands such as PPh3 and PTA were utilized as bulky units. Notably, PTA has been frequently employed in the development of anticancer complexes in previous studies [11]. The incorporation of N^N^N ligands featuring 1,2,4-triazoles with trifluoromethyl (CF3) or tert-butyl (tbu) moieties at the C3 position of the triazolato unit allowed for the fine-tuning of emission and aggregation properties (Scheme 1).
Scheme 1.
Structural formulae and abbreviations of the complexes investigated herein.
Two novel platinum(II) complexes, namely tbu-Pt-CNR and tbu-Pt-PTA, were synthesized using a reaction mixture comprising the metal salt K2[PtCl4], the chelating N^N^N ligand precursor, and the respective monodentate ancillary units. The synthesis involved overnight reflux in a THF-H2O mixture for tbu-Pt-CNR and in an acetonitrile (MeCN)/H2O mixture for tbu-Pt-PTA. The corresponding isoleptic Pd(II) complexes CF3-Pd-AmPy, tbu-Pd-AmPy, tbu-Pd-CNR, CF3-Pd-PPh3, tbu-Pd-PPh3, and tbu-Pd-PTA were obtained by replacing the platinum(II) salt with palladium(II) acetate (Pd(OAc)2). The reactions were conducted in 2-ethoxyethanol at 70 °C for CF3-Pd-AmPy, tbu-Pd-AmPy, and tbu-Pd-CNR, whereas CF3-Pd-PPh3 and tbu-Pd-PPh3 required a THF-H2O mixture; finally, tbu-Pd-PTA was synthesized in MeCN-H2O.
A detailed description of the reaction conditions, purification methods, 1H, 13C, 19F, 31P-, and 195Pt NMR spectroscopy, as well as mass spectrometric characterization can be found in the Supplementary Materials (S02, S14, and S41).
2.2. Photophysical Characterization
All the complexes were characterized in terms of UV-visible absorption, steady-state and time-resolved photoluminescence (PL) spectroscopies, including absolute photoluminescence quantum yields (ΦL) and the estimation of average radiative and radiationless deactivation rate constants. The photophysical properties were measured in diluted (c ≈ 1 × 10−5 M) solutions in DCM or in toluene at RT, as well as in frozen glassy matrices at 77 K (DCM/MeOH, 1:1). Interestingly, the Pd(II) complexes were comparatively less soluble in toluene than their Pt(II) counterparts, which is attributed to the weaker polarizability of the Pd(II) center when compared to Pt(II) [45]. The photophysical data are summarized in Table 1 and Table 2.
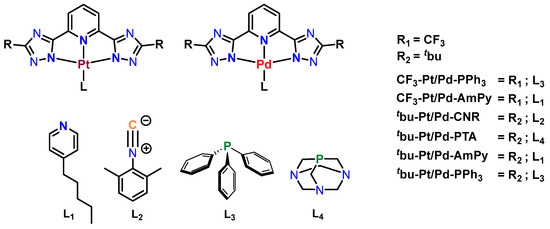
UV-vis absorption spectroscopy: The UV-visible absorption spectra of the Pt(II) and Pd(II) complexes show bands in the range from 230 nm to 450 nm (Figure 1 depicts the absorption spectra in DCM; the data in toluene are available in the Supplementary Materials, see Figure S78). According to bibliographic literature, the intense high-energy absorption bands can be assigned to spin-allowed transitions into 1LC states primarily involving the tridentate ligand [46]. In addition, the energetically lower bands between 350 nm and 450 nm are assigned to transitions involving 1MLCT and intra-ligand states [25]. However, it is noteworthy that the absorption bands are insensitive to the solvent’s polarity. This observation can be attributed to the primary LC character of the excited states, which have dipole moments resembling the ground state [47].
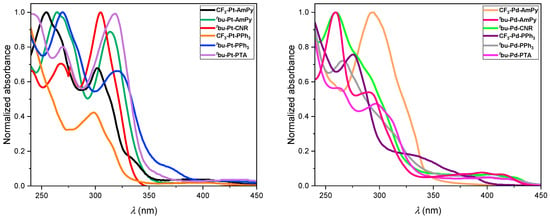
Figure 1.
Normalized absorption spectra of the Pt(II) complexes (left) and Pd(II) complexes (right) in liquid DCM at RT. The molar absorption coefficients can be found in Table 1.
Time-resolved and steady-state photoluminescence spectroscopy: All compounds were characterized as liquid solutions in DCM and in toluene at RT; the corresponding emission spectra are shown in Figure 2. In both solvents, all compounds exhibited a well-defined emission spectrum showing the characteristic vibrational progression of complexes emitting from mainly ligand-centered states [22,48]. In DCM, tbu-Pt-AmPy, tbu-Pt-CNR, tbu-Pt-PPh3, and tbu-Pt-PTA show emission maxima peaking between 510 nm–517 nm, with photoluminescence quantum yields of 79%, 69%, <2%, and 20%, respectively (Figure 2A). CF3-Pt-AmPy shows a hypsochromic shift of the emission when compared with the other Pt(II) complexes, along with a decreased efficiency. This is attributed to the introduction of an electron-withdrawing group, which stabilizes the highest occupied molecular orbital (HOMO), leading to a blue-shifted emission where the consequently high-lying excited state enables the thermal population of dark MC states [49]. Thus, this complex exhibits lower emission intensities, with a photoluminescence quantum yield that falls below the experimental uncertainty. No photoluminescence was detected for CF3-Pt-PPh3 and for the Pd(II) complexes in liquid DCM solutions at RT, however.
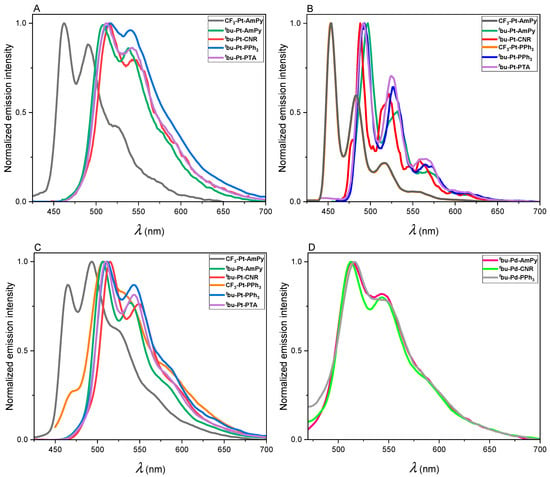
Figure 2.
Photoluminescence emission spectra: (A) CF3-Pt-AmPy, tbu-Pt-AmPy, tbu-Pt-CNR, tbu-Pt-PPh3, and tbu-Pt-PTA in liquid DCM at RT (λexc ranges between 320 nm and 350 nm); (B) CF3-Pt-AmPy, tbu-Pt-AmPy, tbu-Pt-CNR, CF3-Pt-PPh3, tbu-Pt-PPh3, and tbu-Pt-PTA at 77 K in frozen glassy matrices (DCM/MeOH 1:1, λexc ranges between 300 nm and 355 nm); (C) CF3-Pt-AmPy, tbu-Pt-AmPy, tbu-Pt-CNR, CF3-Pt-PPh3, tbu-Pt-PPh3, and tbu-Pt-PTA in liquid toluene at RT (λexc ranges between 320 nm and 355 nm); (D) tbu-Pd-AmPy, tbu-Pd-CNR, and tbu-Pd-PPh3 in liquid toluene at RT (λexc ranges between 320 nm and 350 nm). The spectra are normalized to the highest intensity (c ≈ 1 × 10−5 M).
In liquid toluene at RT, CF3-Pt-AmPy, tbu-Pt-AmPy, tbu-Pt-CNR, tbu-Pt-PPh3, and tbu-Pt-PTA show emission maxima peaking between 507 nm and 517 nm, comparable to those observed in DCM (see Figure 2C). The observed ΦL values in toluene were <2%, 89%, 77%, <2%, and 85%, respectively. In a comparative analysis, these ΦL either remained consistent, as in the case of CF3-Pt-AmPy and tbu-Pt-PPh3, or experienced substantial increases when compared with DCM. Even though the Pt(II) complexes exhibit a clear luminescence in both DCM and toluene at room temperature, a better resolved vibrational progression is discernible in toluene when compared with DCM. This phenomenon could be attributed to the weaker interaction of toluene with the excited state (as opposed to the more polar to DCM), which also diminishes the values of knr. As shown in Table 1 and Table 2, when toluene was used as the solvent instead of DCM, the kr values were nearly invariant, whereas the knr values dropped (especially when PTA is used). This solvent-related effect can also account for the fact that a sizeable emission in toluene is observed for CF3-Pt-PPh3 and for the Pd(II) complexes featuring tert-butyl substituents at the main luminophore (tbu-Pd-AmPy, tbu-Pd-CNR, and tbu-Pd-PPh3).

Table 1.
Selected photophysical data for the Pt(II) complexes in liquid DCM at RT and in frozen glassy matrices of DCM/MeOH 1:1.
Table 1.
Selected photophysical data for the Pt(II) complexes in liquid DCM at RT and in frozen glassy matrices of DCM/MeOH 1:1.
| Compound | λabs/nm | RT-DCM | 77 K-DCM/MeOH | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ε/L mol−1 cm−1 | λem/nm | τAr/μs a | τAir/μs a | ΦL (%) b | kr/104 s−1 c | knr/104 s−1 c | λem/nm | τ/μs | |
| CF3-Pt-AmPy | 302 (14,189) 254 (20,915) | 462 | 0.179 ± 0.004 e | 0.144 ± 0.006 e | <0.02 | kr < 30 | 485 < knr < 498 | 454 | 8.73 ± 0.02 e |
| tbu-Pt-AmPy | 314 (22,333) 265 (25,162) | 510 | 12.44 ± 0.02 d | 0.4069 ± 0.0005 d | 0.79 ± 0.04 | 6.3 ± 0.3 | 1.7 ± 0.3 | 496 | 6.64 ± 0.02 e |
| tbu-Pt-CNR | 309 (27,625) | 515 | 13.7 ± 0.3 d | 0.374 ± 0.003 d | 0.69 ± 0.03 | 5.0 ± 0.3 | 2.3 ± 0.2 | 487 | 16.75 ± 0.02 e |
| CF3-Pt-PPh3 | 298 (17,684) | - | - | - | - | - | - | 455 | 14.01 ± 0.02 d |
| tbu-Pt-PPh3 | 321 (13,569) 270 (20,523) | 517 | 4.88 ± 0.07 e | 0.23 ± 0.01 e | <0.02 | kr < 1 | 21 < knr < 22 | 495 | 13.94 ± 0.04 e |
| tbu-Pt-PTA | 318 (12,252) 270 (9893) | 512 | 3.819 ± 0.003 d | 0.408 ± 0.003 d | 0.20 ± 0.02 | 5.2 ± 0.9 | 21 ± 1 | 490 | 15.70 ± 0.02 d |
a For multi-exponential decays, the amplitude-weighted average lifetimes (τav_amp) are reported. b Quantum yields were measured in Ar-purged solutions. c The average rate constants were estimated according to kr = ΦL/τ and knr = (1 − ΦL)/τ employing amplitude-weighted average lifetimes, as recommended by Engelborghs et al. [50]. d Mono-exponential decays. e Bi-exponential decays.
These otherwise non-emissive complexes manifested a distinct emission spectrum with a well-defined vibrational progression in toluene (see Figure 2D). Conversely, the Pd(II) complexes bearing a CF3-substituent at the main ligand (CF3-Pd-AmPy, CF3-Pd-PPh3) do not exhibit any emission under any circumstance. Thus, it is clear that the electron-donating capacity of the tbu-moieties resulted in a marked improvement in emission efficiency, as opposed to those with the electron-withdrawing CF3-groups exhibiting a significantly faster nonradiative decay. This could be attributed to an elevation in the HOMO-LUMO gap with CF3, bringing the excited state in energetic proximity to dark MC states and rendering them thermally accessible with consequently increased knr values [25]. In addition, the radiative decay (kr) is diminished due to a weaker spin-orbit coupling for Pd(II). Thus, complexes with electron-withdrawing groups, particularly those containing a Pd(II) center, displayed lower emission efficiencies. Surprisingly, tbu-Pd-PTA does not show any significant emission, not even in toluene.
The emission spectra of CF3-Pt-AmPy, tbu-Pt-AmPy, tbu-Pt-CNR, CF3-Pt-PPh3, tbu-Pt-PPh3, and tbu-Pt-PTA at 77 K are shown in Figure 2B. The Pt(II) complexes exhibited a spectrum with well-defined vibrational progressions and excited-state lifetimes in the microsecond range (6.64–16.75 μs). The luminescence can be primarily attributed to triplet LC states with a perturbative MLCT character enabling the phosphorescence, which can be described as a metal-perturbed ligand-centered (3MP-LC) character.

Table 2.
Selected photophysical data for Pt(II) and Pd(II) complexes in liquid toluene at RT.
Table 2.
Selected photophysical data for Pt(II) and Pd(II) complexes in liquid toluene at RT.
| Compound | λabs/nm | RT-Toluene | |||||
|---|---|---|---|---|---|---|---|
| ε/L mol−1 cm−1 | λem/nm | τAr/μs a | τAir/μs a | ΦL (%) b | kr/104 s−1 c | knr/104 s−1 c | |
| CF3-Pt-AmPy | 303 (15,738) | 493 | 6.30 ± 0.05 e | 0.0181 ± 0.0005 e | <0.02 | kr < 0.33 | 14.8 < knr < 15.1 |
| tbu-Pt-AmPy | 313 (62,145) | 507 | 10.54 ± 0.01 d | 0.1843 ± 0.0001 d | 0.89 ± 0.04 | 8.4 ± 0.4 | 1.0 ± 0.4 |
| tbu-Pt-CNR | 307 (18,168) | 515 | 10.72 ± 0.02 d | 0.258 ± 0.001 e | 0.77 ± 0.04 | 7.2 ± 0.2 | 2.1 ± 0.2 |
| CF3-Pt-PPh3 | 300 (20,782) | 507 | 5.62 ± 0.07 f | 0.42 ± 0.01 e | <0.02 | kr < 0.39 | 17 < knr < 18 |
| tbu-Pt-PPh3 | 287 (20,664) 317 (18,213) | 511 | 2.77 ± 0.04 f | 1.11 ± 0.06 f | 0.11 ± 0.02 | 4.0 ± 0.9 | 32.1 ± 0.9 |
| tbu-Pt-PTA | 320 (11,146) | 510 | 12.21 ± 0.02 d | 0.170 ± 0.001 e | 0.85 ± 0.04 | 7.0 ± 0.2 | 1.2 ± 0.2 |
| tbu-Pd-AmPy | 295 (15,632) 399 (2041) | 513 | 3.59 ± 0.04 e | 0.00382 ± 0.00004 f | <0.02 | kr < 1.18 | 29 < knr < 30 |
| tbu-Pd-CNR | 282 (19,473) 412 (1863) | 512 | 7.85 ± 0.02 d | 0.0025 ± 0.0001 f | <0.02 | kr < 0.55 | 12 < knr < 13 |
| tbu-Pd-PPh3 | 289 (29,241) | 516 | 5.1 ± 0.2 e | 0.00228 ± 0.00004 f | <0.02 | kr < 0.43 | 19 < knr < 20 |
a For multi-exponential decays, the amplitude-weighted average lifetimes (τav_amp) are reported. b Quantum yields were measured in Ar-purged solutions. c The average rate constants were estimated according to kr = ΦL/τ and knr = (1 − ΦL)/τ employing amplitude-weighted average lifetimes, as recommended by Engelborghs et al. [50]. d Mono-exponential decays. e Bi-exponential decays. f Tri-exponential decays.
Furthermore, in a rigid glassy environment at low temperatures, all the complexes exhibited hypsochromic shifts of their emission bands with respect to their maxima at RT. This rigidochromic effect indicates a reduction in the MLCT character, as the reorientation of the solvent dipoles is restricted [51]. In frozen matrices at 77 K, the Pd(II) complexes showed no measurable emission, most likely due to the reduced MLCT character, which, along a weaker spin-orbit coupling (when compared with their Pt(II) counterparts), hampers the radiative deactivation. Thus, as the emission process mostly relies on ligand-cantered (LC) states, and given the inherently weaker spin-orbit coupling, the relaxation of the excited state predominantly occurs by non-radiative pathways.
3. Methods and Materials
Materials: All commercially available chemicals and solvents were used as purchased. For the purification of the ligand precursors and complexes, silica gel 60 from Merck was used as the stationary phase in column-chromatographic separations.
Synthesis: A detailed description of the synthesis (experimental procedures, reaction conditions, and purification methods) can be found in the Supplementary Materials.
Structural characterization: All compounds were characterized by 1D-NMR experiments (1H, 13C, 13C{1H} DEPT135, and 195Pt) and 2D-NMR experiments (HH-COSY, HC-HSQC, HC-HMBC). The NMR spectra were recorded at the Institut für Anorganische und Analytische Chemie (Universität Münster) using a Bruker Avance III 400, Bruker Avance Neo 400, or a Bruker Avance Neo 500. All NMR measurements were performed at 300 K. The chemical shifts (δ) of the spectra are given in parts per million (ppm). The signals are referenced to the residual proton signal in the corresponding deuterated solvents: DCM-d2 (1H = 5.32 ppm/13C = 54.00 ppm), methanol-d4 (1H = 3.31 ppm/13C = 49.00 ppm) and DMF-d7 (1H = 8.03 ppm, 2.92 ppm, 2.75 ppm/13C = 163.15 ppm, 34.89 ppm, 29.76 ppm). The signal multiplicities are given as s (singlet), d (doublet), t (triplet), and m (multiplet). The mass spectrometric analysis was carried out at the Institut für Anorganische und Analytische Chemie (Universität Münster) using a Bruker Impact II with electrospray ionization (ESI) to obtain exact mass (EM) spectra for all compounds. The samples were dissolved in MeOH and injected with an autosampler using a mixture of MeOH and MeCN as the solvent. The corresponding NMR spectra are presented in the Supplementary Materials, as well as the mass spectra for all compounds (see Figures S1–S77).
Photophysical characterization: All metal complexes were analyzed by steady-state and time-resolved photoluminescence spectroscopy. Absorption spectra were measured with a Shimadzu UV-1900i UV-VIS-NIR spectrophotometer. All the solvents used were of spectrometric grade (Uvasol®). Photoluminescence quantum yields were measured using a Hamamatsu Photonics absolute PL quantum yield measurement system (C9920-02) equipped with a L9799-01 CW Xe light source (150 W), a monochromator, a C7473 photonic multi-channel analyzer, and an integrating sphere while employing the U6039-05 software (Hamamatsu Photonics, Ltd., Shizuoka, Japan).
Steady-state excitation and emission spectra were recorded on a FluoTime 300 spectrometer from PicoQuant equipped with a 300 W ozone-free Xe lamp (250–900 nm), a 10 W Xe flashlamp (250–900 nm, pulse width ca. 1 µs) with repetition rates of 0.1–300 Hz, a double-grating excitation monochromator (Czerny-Turner type, grating with 1200 lines/mm, blaze wavelength: 300 nm), diode lasers (pulse width < 80 ps) operated by a computer-controlled laser driver PDL-828 “Sepia II” (repetition rate up to 80 MHz, burst mode for slow and weak decays), two double-grating emission monochromators (Czerny-Turner, selectable gratings blazed at 500 nm with 2.7 nm/mm dispersion and 1200 lines/mm, or blazed at 1200 nm with 5.4 nm/mm dispersion and 600 lines/mm) with adjustable slit width between 25 µm and 7 mm, and Glan-Thompson polarizers for excitation (after the Xe-lamps) and emission (after the sample). A sample holder with a Peltier-cooled mounting unit ranging from −15 to 110 °C, along with two detectors (namely a PMA Hybrid-07 from PicoQuant with transit time spread FWHM < 50 ps, 200–850 nm, or a H10330C-45-C3 NIR detector with transit time spread FWHM 0.4 ns, 950–1700 nm from Hamamatsu), were used. Steady-state spectra and photoluminescence lifetimes were recorded in the TCSPC mode using a PicoHarp 300 (minimum base resolution, 4 ps) or in the MCS mode with a TimeHarp 260 (where up to several ms can be traced). Emission and excitation spectra were corrected for source intensity (lamp and grating) by standard correction curves. Lifetime analysis was performed using the commercial EasyTau 2 software (PicoQuant). The quality of the fit was assessed by minimizing the reduced chi-squared function (χ2) and visual inspection of the weighted residuals and their autocorrelation.
4. Conclusions
In this study, we describe the synthesis and photophysical characterization of N^N^N-coordinated Pt(II) and Pd(II) complexes. A comparative approach was employed involving isoleptic Pt(II) and Pd(II) complexes with identical luminophoric ligands bearing either tbu- or CF3-substituents, and the auxiliary ligand was varied (including AmPy, CNR, PPh3, and PTA). All the complexes were characterized by NMR spectroscopy and by mass spectrometry.
In general, the photophysical properties are predominantly dictated by metal-perturbed ligand-centered states. The Pt(II) complexes exhibited either similar or superior photophysical behavior in toluene compared to DCM. For the Pd(II) complexes and despite their better solubility in DCM than in toluene (yet always lower than the corresponding Pt(II) analogues), only the tbu-substituted compounds exhibited a measurable photoluminescence when analyzed in toluene, while they remained dark in DCM. This is attributed to a reduced interaction of toluene with the excited state, leading to a decrease of the knr values. Additionally, tbu-moieties destabilize the HOMO and, thereby, the energy of the emissive triplet state, which hampers radiationless deactivation paths avoiding the thermal population of dissociative MC states. At 77 K, emission was only observed for the Pt(II) complexes due to the weaker spin-orbit coupling of encountered in Pd(II) complexes when the emission arises from almost purely LC states.
Based on these results, it is clear that CF3-groups impair the luminescence of monomeric species at RT (as previously described in the bibliographic literature by De Cola et al. [46,52,53]), whereas the best ancillary ligands in terms of efficiency are represented by AmPy, which is closely followed by CNR. It can be observed that phosphane-based ligands (PPh3 and PTA) do not significantly affect kr, but they do increase the knr values, resulting in lower emission efficiencies. On the other hand, it is observed that the knr value in the toluene of tbu-Pt-PTA is considerably lower than in DCM. This can be attributed to the dipole–dipole interactions between DCM molecules and the nitrogen atoms of the rigid PTA co-ligand, which is not the case in toluene. For PPh3 and in any solvent, the radiationless deactivation paths are rather fast, due to its bulky nature and high density of roto-vibrational degrees of freedom. Furthermore, it is interesting that toluene is able to switchon the emission of Pd(II) complexes at RT, most likely by reducing the coupling of solvent molecules with the emissive state.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/inorganics12020058/s1. Content: Synthesis and characterization—(S02): NMR spectroscopy—(S14): Mass spectrometry—(S41): UV-vis absorption and photoluminescence spectroscopies—(S49): References (S67).
Author Contributions
Conceptualization, S.P.S. and C.A.S.; validation, M.V.C., M.B.R.A., S.P.S. and C.A.S.; formal analysis, S.P.S., M.V.C. and M.B.R.A.; NMR measurements, A.H.; NMR structure analysis, A.H. and S.P.S.; photophysical investigation, M.V.C., M.B.R.A. and S.P.S.; resources, C.A.S.; data curation, S.P.S. and M.V.C.; writing—original draft preparation, S.P.S. and M.V.C.; writing—review and editing, S.P.S., M.V.C., M.B.R.A. and C.A.S.; visualization, S.P.S. and M.V.C.; supervision, C.A.S.; project administration, C.A.S.; funding acquisition, C.A.S. All authors have read and agreed to the published version of the manuscript.
Funding
C.A.S. gratefully acknowledges funding from the Deutsche Forschungsgemeinschaft (DFG—Collaborative Research Centre (CRC) 1450–431460824, Münster, Germany (project A02); project STR 1186/6-2 within the Priority Programme 2102 “Light-controlled reactivity of metal complexes”). C.A.S. gratefully acknowledges the generous financial support for the acquisition of an “Integrated Confocal Luminescence Spectrometer with Spatiotemporal Resolution and Multiphoton Excitation” (DFG/Land NRW: INST 211/915-1 FUGG; DFG EXC1003: “Berufungsmittel”).
Data Availability Statement
All the relevant data for this paper can be found in the article or in the Supplementary Material.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yam, V.W.-W.; Au, V.K.-M.; Leung, S.Y.-L. Light-Emitting Self-Assembled Materials Based on D8 and D10 Transition Metal Complexes. Chem. Rev. 2015, 115, 7589–7728. [Google Scholar] [CrossRef]
- Monro, S.; Colón, K.L.; Yin, H.; Roque, J.I.I.I.; Konda, P.; Gujar, S.; Thummel, R.P.; Lilge, L.; Cameron, C.G.; McFarland, S.A. Transition Metal Complexes and Photodynamic Therapy from a Tumor-Centered Approach: Challenges, Opportunities, and Highlights from the Development of TLD1433. Chem. Rev. 2019, 119, 797–828. [Google Scholar] [CrossRef]
- Imberti, C.; Zhang, P.; Huang, H.; Sadler, P.J. New Designs for Phototherapeutic Transition Metal Complexes. Angew. Chem. Int. Ed. 2020, 59, 61–73. [Google Scholar] [CrossRef]
- Zhao, Q.; Huang, C.; Li, F. Phosphorescent Heavy-Metal Complexes for Bioimaging. Chem. Soc. Rev. 2011, 40, 2508–2524. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Li, F.; Huang, C. Phosphorescent Chemosensors Based on Heavy-Metal Complexes. Chem. Soc. Rev. 2010, 39, 3007–3030. [Google Scholar] [CrossRef]
- Sajoto, T.; Djurovich, P.I.; Tamayo, A.B.; Oxgaard, J.; Goddard, W.A.I.I.I.; Thompson, M.E. Temperature Dependence of Blue Phosphorescent Cyclometalated Ir(III) Complexes. J. Am. Chem. Soc. 2009, 131, 9813–9822. [Google Scholar] [CrossRef] [PubMed]
- Omidyan, R.; Abbasi, M.; Azimi, G. Photophysical and Optoelectronic Properties of a Platinum(II) Complex and Its Derivatives, Designed as a Highly Efficient OLED Emitter: A Theoretical Study. Int. J. Quantum Chem. 2019, 119, e25793. [Google Scholar] [CrossRef]
- Lamansky, S.; Djurovich, P.; Murphy, D.; Abdel-Razzaq, F.; Lee, H.-E.; Adachi, C.; Burrows, P.E.; Forrest, S.R.; Thompson, M.E. Highly Phosphorescent Bis-Cyclometalated Iridium Complexes: Synthesis, Photophysical Characterization, and Use in Organic Light Emitting Diodes. J. Am. Chem. Soc. 2001, 123, 4304–4312. [Google Scholar] [CrossRef]
- Kobayashi, H.; Ogawa, M.; Alford, R.; Choyke, P.L.; Urano, Y. New Strategies for Fluorescent Probe Design in Medical Diagnostic Imaging. Chem. Rev. 2010, 110, 2620–2640. [Google Scholar] [CrossRef] [PubMed]
- Mauro, M.; Aliprandi, A.; Septiadi, D.; Kehr, N.S.; De Cola, L. When Self-Assembly Meets Biology: Luminescent Platinum Complexes for Imaging Applications. Chem. Soc. Rev. 2014, 43, 4144–4166. [Google Scholar] [CrossRef]
- Leal, J.; Durá, G.; Jalón, F.A.; Zafon, E.; Massaguer, A.; Cuevas, J.V.; Santos, L.; Rodríguez, A.M.; Manzano, B.R. Luminescent Cyclometalated Platinum Compounds with N, P, and O^O Ligands: Density-Functional Theory Studies and Analysis of the Anticancer Potential. Appl. Organomet. Chem. 2023, 37, e6983. [Google Scholar] [CrossRef]
- Septiadi, D.; Aliprandi, A.; Mauro, M.; De Cola, L. Bio-Imaging with Neutral Luminescent Pt(II) Complexes Showing Metal···metal Interactions. RSC Adv. 2014, 4, 25709. [Google Scholar] [CrossRef]
- Adams, R.D.; Captain, B. Bimetallic Cluster Complexes: Synthesis, Structures and Applications to Catalysis. J. Organomet. Chem. 2004, 689, 4521–4529. [Google Scholar] [CrossRef]
- Freeman, G.R.; Williams, J.A.G. Metal complexes of pincer ligands: Excited states, photochemistry, and luminescence. In Organometallic Pincer Chemistry; Springer: Berlin/Heidelberg, Germany, 2013; Volume 40. [Google Scholar]
- Williams, J.A.G. Photochemistry and Photophysics of Coordination Compounds: Platinum BT—Photochemistry and Photophysics of Coordination Compounds II; Balzani, V., Campagna, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 205–268. [Google Scholar] [CrossRef]
- Dehghanpour, S.; Mahmoudi, A.; Rostami, S. Platinum(II) Complexes with Bidentate Iminopyridine Ligands: Synthesis, Spectral Characterization, Properties and Structural Analysis. Polyhedron 2010, 29, 2190–2195. [Google Scholar] [CrossRef]
- Luo, Z.; Liu, Y.; Tong, K.-C.; Chang, X.-Y.; To, W.-P.; Che, C.-M. Luminescent Platinum(II) Complexes with Bidentate Diacetylide Ligands: Structures, Photophysical Properties and Application Studies. Chem.—An Asian J. 2021, 16, 2978–2992. [Google Scholar] [CrossRef] [PubMed]
- DePriest, J.; Zheng, G.Y.; Goswami, N.; Eichhorn, D.M.; Woods, C.; Rillema, D.P. Structure, Physical, and Photophysical Properties of Platinum(II) Complexes Containing Bidentate Aromatic and Bis(Diphenylphosphino)Methane as Ligands. Inorg. Chem. 2000, 39, 1955. [Google Scholar] [CrossRef] [PubMed]
- Danilov, E.O.; Pomestchenko, I.E.; Kinayyigit, S.; Gentili, P.L.; Hissler, M.; Ziessel, R.; Castellano, F.N. Ultrafast Energy Migration in Platinum(II) Diimine Complexes Bearing Pyrenylacetylide Chromophores. J. Phys. Chem. A 2005, 109, 2465–2471. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fei, Y.; Sun, H.; Yu, S.; Liu, J. Regulation of the Switchable Luminescence of Tridentate Platinum(II) Complexes by Photoisomerization. Front. Chem. 2021, 8, 2020. [Google Scholar] [CrossRef] [PubMed]
- Haque, A.; Xu, L.; Al-Balushi, R.A.; Al-Suti, M.K.; Ilmi, R.; Guo, Z.; Khan, M.S.; Wong, W.-Y.; Raithby, P.R. Cyclometallated Tridentate Platinum(Ii) Arylacetylide Complexes: Old Wine in New Bottles. Chem. Soc. Rev. 2019, 48, 5547–5563. [Google Scholar] [CrossRef]
- Hebenbrock, M.; González-Abradelo, D.; Hepp, A.; Meadowcroft, J.; Lefringhausen, N.; Strassert, C.A.; Müller, J. Influence of the Ancillary Ligands on the Luminescence of Platinum(II) Complexes with a Triazole-Based Tridentate C^N^N Luminophore. Inorganica Chim. Acta 2021, 516, 119988. [Google Scholar] [CrossRef]
- Shikhova, E.; Danilov, E.O.; Kinayyigit, S.; Pomestchenko, I.E.; Tregubov, A.D.; Camerel, F.; Retailleau, P.; Ziessel, R.; Castellano, F.N. Excited-State Absorption Properties of Platinum(II) Terpyridyl Acetylides. Inorg. Chem. 2007, 46, 3038–3048. [Google Scholar] [CrossRef] [PubMed]
- Rausch, A.F.; Murphy, L.; Williams, J.A.G.; Yersin, H. Improving the Performance of Pt(II) Complexes for Blue Light Emission by Enhancing the Molecular Rigidity. Inorg. Chem. 2012, 51, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Sanning, J.; Ewen, P.R.; Stegemann, L.; Schmidt, J.; Daniliuc, C.G.; Koch, T.; Doltsinis, N.L.; Wegner, D.; Strassert, C.A. Scanning-Tunneling-Spectroscopy-Directed Design of Tailored Deep-Blue Emitters. Angew. Chem. Int. Ed. 2015, 54, 786–791. [Google Scholar] [CrossRef]
- Hua, F.; Kinayyigit, S.; Cable, J.R.; Castellano, F.N. Platinum(II) Diimine Diacetylides: Metallacyclization Enhances Photophysical Properties. Inorg. Chem. 2006, 45, 4304–4306. [Google Scholar] [CrossRef][Green Version]
- Schwartz, G.; Reineke, S.; Rosenow, T.C.; Walzer, K.; Leo, K. Triplet Harvesting in Hybrid White Organic Light-Emitting Diodes. Adv. Funct. Mater. 2009, 19, 1319–1333. [Google Scholar] [CrossRef]
- Lü, Y.; Zhang, M.; Shang, Y.; Xu, H.; Wei, B.; Wang, Z. Platinum Complexes as Phosphorescent Emitters in Highly Efficient Organic Light-Emitting Diodes. J. Shanghai Univ. (Engl. Ed.) 2011, 15, 256–261. [Google Scholar] [CrossRef]
- Wong, K.M.-C.; Yam, V.W.-W. Self-Assembly of Luminescent Alkynylplatinum(II) Terpyridyl Complexes: Modulation of Photophysical Properties through Aggregation Behavior. Acc. Chem. Res. 2011, 44, 424. [Google Scholar] [CrossRef]
- Yam, V.W.-W.; Wong, K.M.-C. Luminescent Metal Complexes of D6, D8 and D10 Transition Metal Centres. Chem. Commun. 2011, 47, 11579. [Google Scholar] [CrossRef]
- Law, A.S.-Y.; Lee, L.C.-C.; Yeung, M.C.-L.; Lo, K.K.-W.; Yam, V.W.-W. Amyloid Protein-Induced Supramolecular Self-Assembly of Water-Soluble Platinum(II) Complexes: A Luminescence Assay for Amyloid Fibrillation Detection and Inhibitor Screening. J. Am. Chem. Soc. 2019, 141, 18570–18577. [Google Scholar] [CrossRef]
- Zamora, A.; Wachter, E.; Vera, M.; Heidary, D.K.; Rodríguez, V.; Ortega, E.; Fernández-Espín, V.; Janiak, C.; Glazer, E.C.; Barone, G.; et al. Organoplatinum(II) Complexes Self-Assemble and Recognize AT-Rich Duplex DNA Sequences. Inorg. Chem. 2021, 60, 2178–2187. [Google Scholar] [CrossRef]
- Onunga, D.O.; Bellam, R.; Mutua, G.K.; Sitati, M.; BalaKumaran, M.D.; Jaganyi, D.; Mambanda, A. Controlling the Reactivity of [Pd(II)(N^N^N)Cl]+ Complexes Using 2,6-Bis(Pyrazol-2-Yl)Pyridine Ligands for Biological Application: Substitution Reactivity, CT-DNA Interactions and in Vitro Cytotoxicity Study. J. Inorg. Biochem. 2020, 213, 111261. [Google Scholar] [CrossRef]
- Gutierrez Suburu, M.E.; Maisuls, I.; Kösters, J.; Strassert, C.A. Room-Temperature Luminescence from Pd(Ii) and Pt(Ii) Complexes: From Mechanochromic Crystals to Flexible Polymer Matrices. Dalton Trans. 2022, 51, 13342–13350. [Google Scholar] [CrossRef] [PubMed]
- Bugarčić, Ž.D.; Bogojeski, J.; van Eldik, R. Kinetics, Mechanism and Equilibrium Studies on the Substitution Reactions of Pd(II) in Reference to Pt(II) Complexes with Bio-Molecules. Coord. Chem. Rev. 2015, 292, 91–106. [Google Scholar] [CrossRef]
- Drouet, S.; Paul-Roth, C.O.; Fattori, V.; Cocchi, M.; Williams, J.A.G. Platinum and Palladium Complexes of Fluorenyl Porphyrins as Red Phosphors for Light-Emitting Devices. New J. Chem. 2011, 35, 438–444. [Google Scholar] [CrossRef]
- Zou, C.; Lin, J.; Suo, S.; Xie, M.; Chang, X.; Lu, W. Palladium(Ii) N-Heterocyclic Allenylidene Complexes with Extended Intercationic Pd···Pd Interactions and MMLCT Phosphorescence. Chem. Commun. 2018, 54, 5319–5322. [Google Scholar] [CrossRef]
- Lai, S.-W.; Cheung, T.-C.; Chan, M.C.W.; Cheung, K.-K.; Peng, S.-M.; Che, C.-M. Luminescent Mononuclear and Binuclear Cyclometalated Palladium(II) Complexes of 6-Phenyl-2,2′-Bipyridines: Spectroscopic and Structural Comparisons with Platinum(II) Analogues1,2. Inorg. Chem. 2000, 39, 255. [Google Scholar] [CrossRef] [PubMed]
- Dalmau, D.; Urriolabeitia, E.P. Luminescence and Palladium: The Odd Couple. Molecules 2023, 28, 2663. [Google Scholar] [CrossRef] [PubMed]
- Peris, E.; Crabtree, R.H. Key Factors in Pincer Ligand Design. Chem. Soc. Rev. 2018, 47, 1959–1968. [Google Scholar] [CrossRef]
- Feuerstein, W.; Breher, F. Synthetic Access to a Phosphorescent Non-Palindromic Pincer Complex of Palladium by a Double Oxidative Addition—Comproportionation Sequence. Chem. Commun. 2020, 56, 12589–12592. [Google Scholar] [CrossRef]
- Strassert, C.A.; Chien, C.-H.; Galvez Lopez, M.D.; Kourkoulos, D.; Hertel, D.; Meerholz, K.; De Cola, L. Lumineszenz Eines Platin(II)-Komplexes in Gelierenden Nanofasern Und Elektrolumineszierenden Filmen. Angew. Chem. 2011, 123, 976–980. [Google Scholar] [CrossRef]
- Mydlak, M.; Mauro, M.; Polo, F.; Felicetti, M.; Leonhardt, J.; Diener, G.; De Cola, L.; Strassert, C.A. Controlling Aggregation in Highly Emissive Pt(II) Complexes Bearing Tridentate Dianionic N^N^N Ligands. Synthesis, Photophysics, and Electroluminescence. Chem. Mater. 2011, 23, 3659. [Google Scholar] [CrossRef]
- Sanning, J.; Stegemann, L.; Ewen, P.R.; Schwermann, C.; Daniliuc, C.G.; Zhang, D.; Lin, N.; Duan, L.; Wegner, D.; Doltsinis, N.L.; et al. Colour-Tunable Asymmetric Cyclometalated Pt(II) Complexes and STM-Assisted Stability Assessment of Ancillary Ligands for OLEDs. J. Mater. Chem. C 2016, 4, 2560. [Google Scholar] [CrossRef]
- Henwood, A.F.; Lesieur, M.; Bansal, A.K.; Lemaur, V.; Beljonne, D.; Thompson, D.G.; Graham, D.; Slawin, A.M.Z.; Samuel, I.D.W.; Cazin, C.S.J.; et al. Palladium(0) NHC Complexes: A New Avenue to Highly Efficient Phosphorescence. Chem. Sci. 2015, 6, 3248–3261. [Google Scholar] [CrossRef]
- Mauro, M.; Aliprandi, A.; Cebrián, C.; Wang, D.; Kübel, C.; De Cola, L. Self-Assembly of a Neutral Platinum(II) Complex into Highly Emitting Microcrystalline Fibers through Metallophilic Interactions. Chem. Commun. 2014, 50, 7269. [Google Scholar] [CrossRef]
- Lakowicz, J.R. (Ed.) Principles of Fluorescence Spectroscopy; Springer: Boston, MA, USA, 2006. [Google Scholar] [CrossRef]
- Krause, M.; von der Stück, R.; Brünink, D.; Buss, S.; Doltsinis, N.L.; Strassert, C.A.; Klein, A. Platinum and Palladium Complexes of Tridentate −C^N^N (Phen-Ide)-Pyridine-Thiazol Ligands—A Case Study Involving Spectroelectrochemistry, Photoluminescence Spectroscopy and TD-DFT Calculations. Inorganica Chim. Acta 2021, 518, 120093. [Google Scholar] [CrossRef]
- Gangadharappa, S.C.; Maisuls, I.; Schwab, D.A.; Kösters, J.; Doltsinis, N.L.; Strassert, C.A. Compensation of Hybridization Defects in Phosphorescent Complexes with Pnictogen-Based Ligands—A Structural, Photophysical, and Theoretical Case-Study with Predictive Character. J. Am. Chem. Soc. 2020, 142, 21353–21367. [Google Scholar] [CrossRef] [PubMed]
- Sillen, A.; Engelborghs, Y. The Correct Use of “Average” Fluorescence Parameters. Photochem. Photobiol. 1998, 67, 475. [Google Scholar] [CrossRef]
- Lees, A.J. The Luminescence Rigidochromic Effect Exhibited by Organometallic Complexes: Rationale and Applications. Comments Inorg. Chem. 1995, 17, 319–346. [Google Scholar] [CrossRef]
- Carrara, S.; Aliprandi, A.; Hogan, C.F.; De Cola, L. Aggregation-Induced Electrochemiluminescence of Platinum(II) Complexes. J. Am. Chem. Soc. 2017, 139, 14605–14610. [Google Scholar] [CrossRef]
- Aliprandi, A.; Mauro, M.; De Cola, L. Controlling and Imaging Biomimetic Self-Assembly. Nat. Chem. 2016, 8, 10–15. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).