Abstract
The reactivity of the open-shell Gd@C2v-C82 with different charge states towards benzyl bromide was investigated. [Gd@C2v-C82]3− exhibited enhanced activity relative to Gd@C2v-C82 and [Gd@C2v-C82]−. The structural characterizations, including MALDI-TOF MS, UV-vis-NIR, and single crystal X-ray diffraction, indicate the formation of isomeric benzyl monoadducts of Gd@C2v-C82. All three monoadducts contain 1:1 mirror-symmetric enantiomers. Additionally, the addition of the benzyl group and its specific position result in distinct electrochemical behavior of the products compared to the parent Gd@C2v-C82. Theoretical studies demonstrate that only [Gd@C2v-C82]3− has a HOMO energy level that matches well with the LUMO energy level of the PhCH2 radical, providing a rationalization for the observed significantly different reactivity.
1. Introduction
Endohedral metallofullerenes (EMFs) have garnered substantial attention from scientists due to their exceptional chemical and physical properties [1,2,3,4,5,6,7,8,9]. Since the discovery of the first solvent-extractable EMF, La@C82 [10], there has been a boom in interest in EMFs. According to the electronic structure, EMFs are categorized into two main groups: closed-shell EMFs and open-shell EMFs. Closed-shell EMFs possess completely filled electron shells, meaning that all the molecular orbitals are paired with electrons. Consequently, they exhibit a stable and relatively non-reactive structure. In contrast, open-shell EMFs have partially filled electron shells, resulting in the presence of unpaired electrons in their molecular orbitals. This characteristic makes them more reactive and prone to chemical reactions [11,12,13]. Nevertheless, compared with closed-shell EMFs, the reaction of open-shell EMFs is relatively less studied.
The physical and chemical properties of open-shell EMFs can be altered by functionalization [11,14,15,16,17]. By introducing trifluoromethyl, phenyl, or dichlorophenyl groups into the Er@C82 cages, the derived products exhibit distinct photoluminescent properties [18]. Additionally, multi-hydroxylated Pr@C82 [19], Gd@C82 [20,21,22,23], and Hox@C82 [24] show enhanced solubility, rendering them suitable for use in biomedicine as novel biological radical scavengers, radiographic agents, magnetic resonance imaging agents, and “missile” drugs [20].
Gd@C2v-C82, with the inner Gd atom capable of transferring three electrons to the C82 cages, represents a typical open-shell molecule. The electronic states of this system can be described as Gd3+@C823− [25,26,27]. Gd@C2v-C82 has magnetic properties stemming from the interaction between the spin of the metal atom and the electron spin of the cage [28]. Gd@C2v-C82 has found widespread use in various biomedical applications, including MRI contrast [20,29,30], antitumor therapeutics [31,32,33], adjuvant therapeutic agents [34,35], metabolic disease modulation [22,36,37], and so forth. Indeed, the diverse range of applications for Gd@C2v-C82 in biomedicine can be attributed to appropriate derivatization. The process of modifying its chemical structure through derivatization allows for tailoring its properties to suit specific biomedical applications, thereby enhancing its effectiveness and versatility in various medical contexts. In addition, the carbon cage encasing the Gd ion serves as a protective barrier, preventing the release of Gd3+ and thereby eliminating potential toxicity concerns. Therefore, expanding the variety of Gd@C2v-C82 derivatives holds great significance as it allows for a more comprehensive exploration of their potential properties and applications.
In this work, we utilized the electrochemical method to produce Gd@C2v-C82 anions, which were then subjected to the benzylization reaction. Interestingly, this reaction has been demonstrated to be ineffective for the neutral form of the molecule. The reduction of Gd@C2v-C82 to its trianions resulted in a notable increase in reactivity towards the benzylization reaction. Three benzylated Gd@C2v-C82 isomers (2a, 2b, and 2d) are isolated and characterized. The structures are confirmed through X-ray single crystal diffraction, revealing that they are monoadducts with a benzyl group attached to the [5,6,6] junction of the C82 cage. In addition, each isomer exists in a mirror-symmetric form. The electrochemical properties of the benzylated Gd@C2v-C82 isomers show distinct characteristics compared to the pristine Gd@C2v-C82. Through density functional theory (DFT) calculations, the reactivity of the Gd@C2v-C82 trianion towards the benzyl group was unveiled.
2. Results and Discussion
Despite Gd@C2v-C82 having an unpaired electron, it does not exhibit reactivity towards benzyl bromide (PhCH2Br) as shown in Scheme 1. As previously known, Gd@C82 demonstrates its capability as an excellent electron acceptor, with the capacity to accept up to seven electrons [38,39]. Electrochemical reduction can be used to selectively synthesize fullerene anions by controlling the applied potential. To enhance the reactivity, Gd@C2v-C82 was subjected to electrochemical reduction, specifically to its monoanion, using controlled potential electrolysis (CPE). The resulting Gd@C2v-C82 monoanion was then subjected to the benzylation reaction. However, it was observed that the monoanion also shows no reactivity toward PhCH2Br (Scheme 1), indicating that the nucleophilicity of the Gd@C2v-C82 monoanion is not sufficiently strong to attack the carbon cation (C+) of PhCH2Br. Therefore, additional electrons were added to the Gd@C2v-C82 cage through the CPE process. According to the cyclic voltammogram of Gd@C2v-C82, the second reduction is a simultaneous two-electron transfer process [27,39]; therefore, it is rational to consider the anions obtained via CPE to be trianions. Once the electrogeneration of [Gd@C2v-C82]3− was completed, PhCH2Br was added to the solution in one portion and stirred for 3h. High performance liquid chromatography (HPLC) observation confirms the occurrence of the reaction between [Gd@C2v-C82]3− and PhCH2Br (Scheme 1). It is evident that [Gd@C2v-C82]3− shows enhanced reactivity toward PhCH2Br.
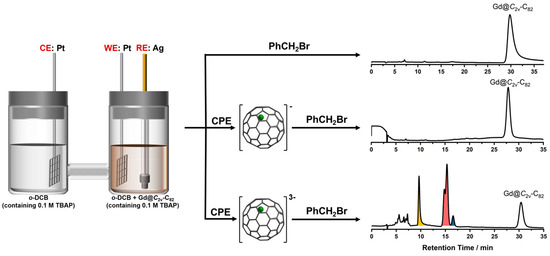
Scheme 1.
Benzylation reactions of Gd@C2v−C82, [Gd@C2v−C82]−, and [Gd@C2v−C82] 3−, and the corresponding HPLC profiles of the reaction crude. Conditions: Buckyprep (φ 4.6 × 250 mm), toluene, 1 mL/min. (colors indicate the areas of each fraction).
As observed in Figure 1a, the HPLC results show the synthesis of multiple products through the reaction of [Gd@C2v-C82]3− with PhCH2Br. To separate and purify the products from the crude mixture, a three-stage HPLC procedure was performed. Initially, the reaction mixtures in toluene were separated using a Buckyprep column to isolate the main fractions 1, 2, and 3 (Figure 1a). Subsequent chromatography employing a Buckyprep column (Figure 1b) and a 5PBB column (Figure 1c) revealed that fraction 2 comprises four compounds: 2a, 2b, 2c, and 2d. (Figure 1c). Notably, as we only succeeded in getting single crystal structures of 2a, 2b, and 2d, the subsequent discussion in this study was only focused on these three products. Electrosynthesis has been commonly recognized as an effective strategy for the highly selective synthesis of fullerene derivations. The production of multiple adducts is likely ascribed to the delocalization of charges caused by the low symmetry of the C82 cage and the high reactivity of trianionic Gd@C2v-C82.
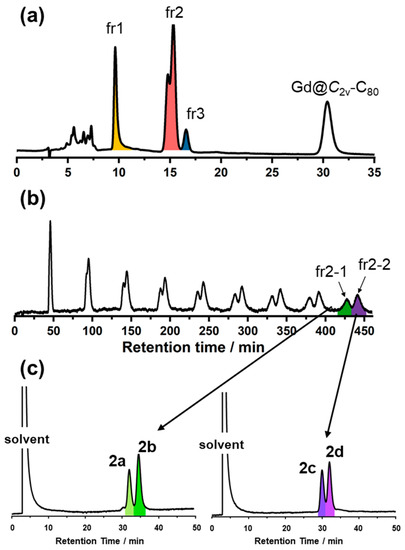
Figure 1.
(a) HPLC profile of the reaction mixture (colors indicate the areas of each fraction). Conditions: Buckyprep (φ 4.6 × 250 mm), toluene, 1 mL/min. (b) Recycling HPLC profile of fraction 2. Conditions: Buckyprep (φ 20 × 250 mm), toluene, 7 mL/min. (c) HPLC profiles of fractions 2-1 and 2-2. Conditions: 5PBB (φ 4.6 × 250 mm), toluene, 1 mL/min.
The matrix-assisted laser desorption/ionization time-of-flight (MALDI–TOF) mass spectrometry gave preliminary speculation on the structure of the three products (Figure 2). The mass spectra of 2a, 2b, and 2d reveal very similar molecular ion peaks, positioned around m/z = 1233. This observation indicates that they are isomers, each featuring a single benzyl group (CH2C6H5) attached to the Gd@C2v-C82 cage, i.e., Gd@C2v-C82(CH2C6H5) (theoretical m/z = 1232.979). The addition pattern is consistent with that of the reported open-shell EMFs derivatives [12,40,41,42,43,44]. This can be attributed to the paramagnetic behavior of Gd@C2v-C82, which leads to specific addition patterns of functional groups in its derivatives.
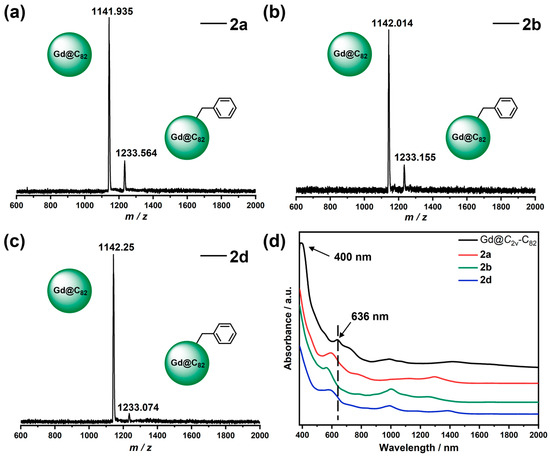
Figure 2.
MALDI-TOF mass spectra of 2a (a), 2b (b), and 2d (c) with TPB as the matrix. (d) UV-vis-NIR spectra of 2a, 2b, 2d, and Gd@C2v-C82 in CS2 solution.
The optical properties that can provide crucial insights into the electronic structures were studied through the analysis of the UV-vis-NIR absorption spectra. As shown in Figure 2, the UV-vis-NIR spectra of 2a, 2b, and 2d show remarkably different behavior compared to that of the parent Gd@C2v-C82, which is attributed to the changes in the electronic structure of the cage caused by the addition of the benzyl groups [45]. The characteristic absorption of Gd@C2v-C82 at λ = 636 nm undergoes a blue shift upon the addition of the benzyl groups, which can be explained by the reduction of the π-conjugated region in the molecule [44]. The characteristic band of Gd@C82 at 400 nm disappears in all benzyl adducts, which is ascribed to the quenching of unpaired electrons by the odd number of benzyl groups on the Gd@C82 cage. A similar phenomenon was observed in the absorption of Gd@C82 morpholine derivatives [44].
Single crystal X-ray diffraction measurement is widely accepted as the most reliable technique for analyzing the structure of isomers [46,47,48,49]. It provides solid evidence for the structure confirmation of 2a, 2b, and 2d. We made efforts to obtain single crystals for all the products, unfortunately, only products 2a, 2b, and 2d were successfully crystallized. Figure 3 displays the single crystal structures of the three isomers, namely, 2a, 2b, and 2d. Notably, 2a and 2b were co-crystallized with decapyrrylcorannulene (DPC) molecules [50]. The DPC molecules were found to form a V-shaped structure when combined with 2a and 2b, with the angle between the DPC molecules being 64.9° and 64.8°, respectively. The arrangement of these two molecules is very similar to other reported structures [51,52,53,54]. In 2a·2DPC, the distances between the centroid of the C82 cage and the central five-membered rings of DPC molecules are 7.361 Å and 7.394 Å, while they are 7.439 Å and 7.474 Å in 2b·2DPC. From there, it can be deduced that the distance between the carbon cage and the DPC is approximately 2.8–3.2 Å, indicating the presence of π-π interactions between the carbon cage and DPC molecules. These π-π interactions play a significant role in stabilizing the co-crystallized structure of 2a and 2b with DPC molecules. All three crystals feature a monoclinic P21/c space group. The carbon cages and embedded Gd atoms (in cyan color) display varying degrees of disorder. An interesting observation is that, irrespective of the location of the benzyl addition, the Gd atoms with major occupancy are consistently found underneath a hexagonal ring along the C2 axis of the C2v-C82 cage (marked in orange in Figure 3). And, the distances between Gd and the adjacent carbon atoms, ranging from 1.9 Å to 2.4 Å, indicate a strong interaction between the endohedral Gd and the outer C2v-C82 cage.
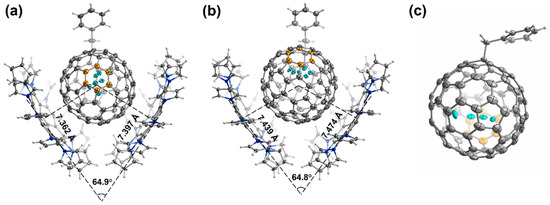
Figure 3.
Single crystal structures of 2a·2DPC (a), 2b·2DPC (b), and 2d (c) with thermal ellipsoids set at the 20% probability level. The solvent molecules are omitted and only one cage orientation is shown.
As depicted in Figure 3, the benzyl group was added in the form of a single bond at the [5,6,6]-ring junction of the C2v-C82 cage. Despite the addition, the C2v symmetry of the cage is preserved, indicating that the attachment of the benzyl group did not disrupt the overall symmetry of the molecule (Figure 4). Interestingly, all three crystal structures of 2a, 2b, and 2d contain mirror-symmetric enantiomers, suggesting that each compound exists in a pair of structures that are mirror images of each other. To gain a better understanding of the addition sites and the arrangement of the benzyl groups in each enantiomer, a Schlegel diagram of the C2v-C82 cage was mapped. In this diagram, the addition sites of the benzyl groups in each enantiomer are marked (1–2 for 2a, 3–4 for 2b, and 5–6 for 2d) (Figure 4a), providing valuable insights into the spatial orientation and symmetry of the derivatives. It can be observed that the addition sites in structures that are mutually enantiomeric are mirror-symmetric. The denoted sites 1 and 2 refer to cage 1 and cage 2 of 2a, sites 3 and 4 refer to cage 1 and cage 2 of 2b, and sites 5 and 6 refer to cage 1 and cage 2 of 2d. Figure 4b displays the mirror-symmetric enantiomers for 2a, 2b, and 2d. For every isomer, there is an identical structure that is a mirror image of the original isomer. Owing to the existence of the C2 axis, the addition sites labeled as 1′ to 6′ on the cage are equivalent to the corresponding sites of 1 to 6.
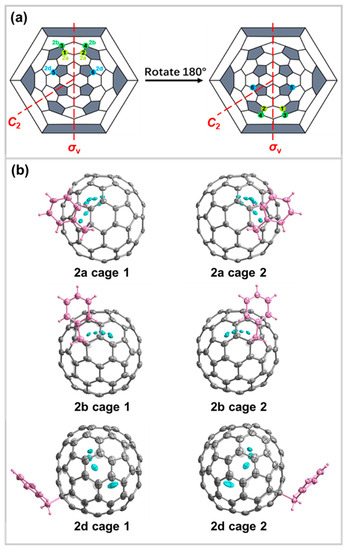
Figure 4.
(a) Schlegel diagram showing the addition sites of 2a, 2b, and 2d. (b) Structures of enantiomers of 2a, 2b, and 2d with 20% thermal probability. Internal disordered sites of gadolinium atoms were also shown. Benzyl groups are labeled in pink. The co-crystallization and solvent molecules were omitted for clarity.
The crystal packing and molecular organization for each isomer were studied. As shown in Figure 5, the smallest asymmetric unit for the three products consists of four fullerene cages. Furthermore, 2a and 2b are arranged in a head-to-tail mode with DPC molecules along the b-axis. Additionally, the DPC molecules adopt an S-shape configuration along the c-axis. The distances between the two back-to-back DPC molecules are in the range of 3.4–3.8 Å, indicating the existence of π-π interactions. These interactions lead to favorable π-electron overlap and stabilize the crystal packing. The crystal structure of 2d without DPC co-crystallization reveals the formation of a one-dimensional zigzag supramolecular chain along the b-axis. The detailed crystallographic data for these compounds can be found in the experimental section.
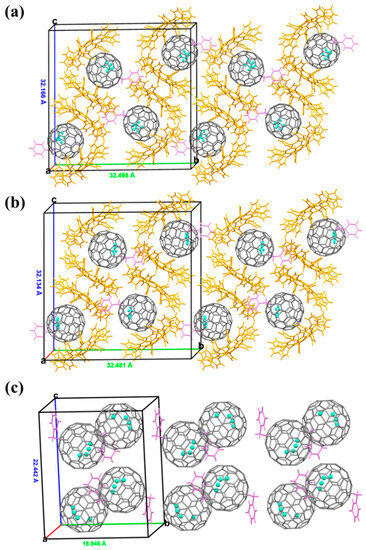
Figure 5.
The packing structures of (a) 2a·2DPC, (b) 2b·2DPC, and (c) 2d. The solvent molecules are omitted and only one cage orientation is shown.
The addition of functional groups on the fullerene cages can greatly alter the electrochemical properties of the compounds. Cyclic voltammetry (CV) measurements were conducted for each isomer and compared with the CV curves of the pristine Gd@C2v-C82 [27]. As reported in the literature, the pristine Gd@C2v-C82 shows three pairs of reversible redox peaks and two irreversible redox processes in its CV curves, and the second and the fourth reduction are two-electron processes [27]. However, the addition of the benzyl group to form isomers 2a, 2b, and 2d has led to changes in the electrochemical characteristics of the compounds. Moreover, the addition position of the benzyl group was found to have effects on the electrochemical behavior [46,55]. As illustrated in Figure 6, the CV curves of 2a, 2b, and 2d exhibit more redox signals compared with the pristine Gd@C2v-C82, suggesting the presence of distinct electron transfer processes, which is attributed to the addition of the benzyl group and its influence on the electronic structure of Gd@C2v-C82. In the case of 2a, the CV curves display irreversible cathodic electrochemical behavior, with peak potential (Epc) values observed at −0.97, −1.51, −1.66, −1.93, and −2.39 V vs. Fc/Fc+ (Table 1). On the other hand, 2a also shows one quasi-reversible oxidation peak with an Epa value of +0.14 V vs. Fc/Fc+ and two irreversible oxidations with Epa values of +0.74 and +1.03 V vs. Fc/Fc+. For compound 2b, there are three quasi-reversible reductions with Epc values of −1.06, −1.89, and −2.11 V vs. Fc/Fc+, while the second reduction is found to be irreversible, with an Epc value of −1.58 V vs. Fc/Fc+. The electrochemically anodic behavior of 2b exhibits irreversible characteristics. CV measurements for 2d show two irreversible reductions with Epc values of −0.86 and −1.52 V vs. Fc/Fc+ and two quasi-reversible reductions with Epc values of −1.77 and −2.04 V vs. Fc/Fc+. Moreover, compound 2d exhibits electrochemically irreversible anodic behavior, which is similar to the behavior observed for 2d. The distinct electrochemical characteristics of 2a, 2b, and 2d may be attributed to the specific addition position of the benzyl group and its influence on the electronic structure of the Gd@C2v-C82 cage. In addition, compared to the redox behavior of Gd@C2v-C82, the first reduction potentials of 2a, 2b, and 2d show negative shifts, and the first oxidation potentials show positive shifts, which indicates a decrease in electron-donating/accepting properties after modification with the benzyl group. The enlarged electrochemical gaps in the derivatives 2a, 2b, and 2d demonstrate the enhanced stability of the derivatives relative to the parent Gd@C2v-C82. The redox potentials for Gd@C2v-C82 and its benzylated products are summarized in Table 1.
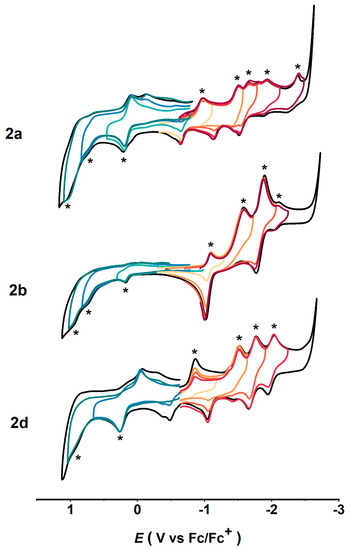
Figure 6.
Cyclic voltammograms of 2a, 2b, and 2d recorded in a 0.05 M solution of TBAPF6 in o-DCB at a scan rate of 100 mV s−1. (The redox processes are labeled with a star and each process is indicated in different colors).

Table 1.
Redox potentials (V vs. Fc/Fc+) of Gd@C2v-C82 and 2a, 2b, and 2d.
Density functional theory calculations at the M06-2X/6-31G*~SDD level were then carried out to disclose the underlying reactivity of Gd@C2v-C82 and its anions. Figure 7 illustrates the optimized structures of 2a, 2b, and 2d, which are consistent with the corresponding single crystal structures. For pristine Gd@C2v-C82, it is well known that it has an unpaired electron distributed on the outer cage due to the 3e− transfer from the internal metal to the cage. However, our calculations with different spin multiplicities reveal that all three monoadducts have the octet ground state completely stemming from the Gd-4f7 electron configuration of their internal Gd3+ cation (Table 2). Clearly, the unpaired electron (spin) on the outer cage of Gd@C2v-C82 has been effectively quenched as a result of the formation of a single bond with the benzyl group, which thus leads to the excellent stability of the adducts.
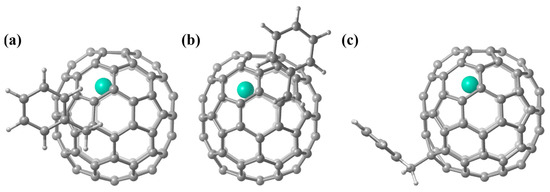
Figure 7.
Optimized structures of (a) 2a, (b) 2b, and (c) 2d.

Table 2.
Relative energies (kcal/mol) of 2a, 2b, and 2d as well as [Gd@C2v-C82]− and [Gd@C2v-C82]3− with different spin multiplicities (M).
In addition, the reactivity of different charged forms of Gd@C2v-C82 was elaborated through DFT simulations. Figure 8 shows the calculated energy levels of frontier molecular orbitals of Gd@C2v-C82 with various charge states (0, −1, −3). Consistent with our experimental findings, only [Gd@C2v-C82]3− demonstrates HOMO (highest occupied molecular orbital) energy levels that are comparable to the LUMO (lowest unoccupied molecular orbital) of PhCH2 radical, thus enhancing the orbital interactions and charge transfer during the addition reaction. Through the process of electrochemical reduction, the orbital energy levels of Gd@C2v-C82 are greatly elevated, resulting in a significant improvement in its reactivity.
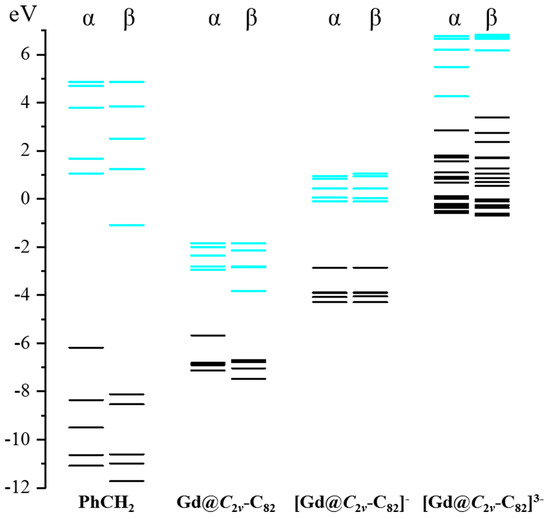
Figure 8.
Frontier molecular orbital energy levels of PhCH2 radical and Gd@C2v-C82 in different charge states (occupied: black; unoccupied: cyan).
3. Materials and Methods
Gd@C2v-C82 was purchased from Xiamen Funano New Material Technology Company LTD, Xiamen, China. PhCH2Br, anhydrous o-DCB, 1,1,4,4-Tetraphenyl-1,3-butadiene (TPB), toluene, CDCl3, and CS2 were purchased from Aladdin and used as received. Tetrabutylammonium perchlorate (TBAP) and Tetra-n-butylammonium hexauorophosphate (TBAPF6) were recrystallized from absolute ethanol and dried under vacuum before use. The purity of the products was verified by HPLC (LaboACE LC-5060, Japan Analytical Industry Co., Ltd., Tokyo, Japan) equipped with Buckyprep and 5PBB columns, with toluene used as the eluent. UV-vis-NIR spectra were recorded using a SHIMADZU UV-3600 spectrophotometer (Shimadzu Corporation, Kyoto, Japan). MALDI-TOF MS measurements were conducted using a Bruker autoex speed mass spectrometer. CV studies were conducted in a solution of o-DCB containing 0.05 M TBAPF6 in a one-compartment cell connected to a CHI 760E workstation, Shanghai, China. A 2 mm diameter glassy carbon disk, a Pt wire, and a Ag wire were used as the working, counter, and pseudoreference electrodes, respectively. Ferrocene (Fc) was introduced into the solution at the end of each experiment as an internal standard. All reactions were carried out inside a glove box under Ar protection.
3.1. Synthesis of 2a, 2b, and 2d
A 2 mg (1.75 mmol) sample of Gd@C2v-C82 was electrochemically reduced at −1.36 V versus a silver wire reference electrode in 8 mL of o-DCB containing 0.1 M TBAP as the supporting electrolyte under an argon atmosphere. The potentiostat was turned off once the electrogeneration of [Gd@C2v-C82]3− was completed. Subsequently, 100 equivalents of PhCH2Br (21.5 μL) were added into the solution in one portion under stirring. The reaction was allowed to proceed for 2 h with stirring, and, finally, it was electrochemically oxidized at a potential of 0 V. The solvent was evaporated under a vacuum, and the crude was washed with plenty of methanol to remove the unreacted PhCH2Br and the supporting electrolyte. The slurry was separated by filtration. The residue was dried at 70 °C under a vacuum and dissolved in toluene. The soluble fraction was then purified by HPLC using Buckyprep and 5PBB columns.
3.2. Single Crystal X-ray Crystallography
The single crystals of 2a and 2b were obtained by co-crystallization with DPC molecules. In a 5.0 mL centrifuge tube, 0.5 mg of 2a was dissolved in 0.5 mL of carbon disulfide. Then, a toluene solution containing DPC was added to the tube, with a molar ratio of DPC to fullerene in the mixed solution being 2:1. After around three weeks, black sheet-like crystals were formed. A crystal of 0.4 mm × 0.25 mm × 0.03 mm dimension was mounted in the 100 K nitrogen cold stream on an XtaLAB PRO MM007HF diffractometer with Cu-Kα radiation (λ = 1.5406 Å). The CrystalClear software package (Rigaku) was used for data collection, cell refinement, and data reduction. The crystal structures were solved by direct methods and refined by the full-matrix method based on F2 using the SHELXLTL software package. All the non-hydrogen atoms were refined anisotropically, and the positions of the hydrogen atoms were generated geometrically.
Crystal data of 2a·2DPC: C216H95Gd0.88N20, Mw = 3108.89 amu, monoclinic, P21/C, a = 14.6998 (1) Å, b = 32.4984 (3) Å, c = 32.1664 (3) Å, α = 90°, β = 102.071 (1)°, γ = 90°, V = 15,026.8 (2) Å3, T = 100 K, Z = 4, R indices (all data) R1 = 0.0896 (23,666), wR2 = 0.2647 (29,671), GOF = 1.054.
Black sheet-like crystals of 2b were obtained through the same procedures as for 2a. Crystallographic characterization of a piece of co-crystal (0.12 mm × 0.1 mm × 0.08 mm) was performed at 100 K by using synchrotron radiation (0.65250 Å) with a MarCCD detector at the beamline BL17B station of Shanghai Synchrotron Radiation Facility.
Crystal data of 2b·2DPC: C216H95Gd0.87N20, Mw = 3106.92 amu, monoclinic, P21/C, a = 14.6857 (4) Å, b = 32.4812 (11) Å, c = 32.1342 (9) Å, α = 90°, β = 101.791 (1)°, γ = 90°, V = 15,004.9 (8) Å3, T = 100 K, Z = 4, R indices (all data) R1 = 0.1054 (20,309), wR2 = 0.3207 (29,611), GOF = 1.052.
The crystal of 2d was grown through solvent diffusion in which a near-saturated carbon disulfide solution was injected into the glass tube followed by slow injections of hexane, and the fullerene solution was layered at a height of about 5 cm. At this point, a clear delamination between hexane and carbon disulfide solution appears, creating a diffusion interface that provides favorable conditions for crystal growth. The tube is closed with a cap and left at a low temperature for several weeks until the solvent diffusion is complete and several crystals are attached to the tube walls. The crystal of 2d (0.1 mm × 0.08 mm × 0.06 mm) was tested using the same instruments and methods as 2b.
Crystal data of 2d: C87H7Gd0.91, Mw = 1233.20 amu, monoclinic, P21/C, a = 10.891 (2) Å, b = 18.946 (4) Å, c = 22.442 (5) Å, α = 90°, β = 96.45 (3)°, γ = 90°, V = 4601.4 (16) Å3, T = 100 K, Z = 4, R indices (all data) R1 = 0.1409 (6211), wR2 = 0.3370 (8277), GOF = 1.069.
The supplementary crystallographic data for this paper can be obtained free of charge from the Cambridge Crystallographic Data Centre with CCDC numbers 2281483, 2283118, and 2283119 via www.ccdc.cam.ac.uk/data_request/cif (accessed on 1 July 2023).
3.3. Computational Details
The calculations were carried out at the DFT level with the M06-2X [56] function, the 6-31G* all-electron basis set of C and H atoms, the SDD basis set, and the corresponding effective core potentials of Gd [57,58,59]. All the above DFT calculations were performed using the Gaussian 09 software package [60]. The results were visualized with CYLview [61] and VMD [62] software.
4. Conclusions
In summary, this study presents the first successful synthesis of open-shell Gd@C2v-C82 benzyl monoadducts using the electrochemical synthesis method. Three Gd@C2v-C82(PhCH2) isomers (2a, 2b, and 2d) were synthesized, isolated, and characterized. The crystal structures revealed that the benzyl group is attached to the [5,6,6] junction of the cage through a single bond and each isomer contains a mirror-symmetric enantiomer. The addition of the benzyl group and its specific position have notable effects on the electrochemical behavior. DFT calculations reveal that [Gd@C2v-C82]3− has a HOMO energy level that aligns effectively with the LUMO energy levels of the PhCH2 radical, rationalizing the higher reactivity towards benzyl. The electrochemical method shows great potential in regulating the chemical reactivity of fullerenes, opening up possibilities for creating a wide range of new fullerene products.
Author Contributions
Conceptualization, F.-F.L.; data curation, X.Z. and Y.-R.Y.; formal analysis, X.Z., Y.-R.Y. and Y.H.; funding acquisition, Q.Z., P.P., P.J. and F.-F.L.; investigation, X.Z., Y.H., S.Y. and P.P.; methodology, Y.H., L.Y., Y.Z., Q.Z. and P.J.; project administration, P.P. and F.-F.L.; software, Y.-R.Y. and P.J.; supervision, P.P. and F.-F.L.; validation, Y.-R.Y.; writing—original draft, X.Z. and L.Y.; Writing—review and editing, P.J. and F.-F.L. All authors have read and agreed to the published version of the manuscript.
Funding
The financial support from the National Natural Science Foundation of China (Grant No. 22071070, 21925104, 22171068, and 22271238) and the Fundamental Research Funds for the Central Universities (WK2060000051) is gratefully acknowledged.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Popov, A.A.; Yang, S.; Dunsch, L. Endohedral fullerenes. Chem. Rev. 2013, 113, 5989–6113. [Google Scholar] [CrossRef] [PubMed]
- Dunsch, L.; Yang, S. Metal nitride cluster fullerenes: Their current state and future prospects. Small 2007, 3, 1298–1320. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wei, T.; Jin, F. When metal clusters meet carbon cages: Endohedral clusterfullerenes. Chem. Soc. Rev. 2017, 46, 5005–5058. [Google Scholar] [CrossRef]
- Li, F.-F.; Rodríguez-Fortea, A.; Poblet, J.M.; Echegoyen, L. Reactivity of Metallic Nitride Endohedral Metallofullerene Anions: Electrochemical Synthesis of a Lu3N@Ih-C80 derivative. J. Am. Chem. Soc. 2011, 133, 2760–2765. [Google Scholar] [CrossRef]
- Li, F.-F.; Rodríguez-Fortea, A.; Peng, P.; Campos Chavez, G.A.; Poblet, J.M.; Echegoyen, L. Electrosynthesis of Sc3N@Ih-C80 methano derivative from trianionic Sc3N@Ih-C80. J. Am. Chem. Soc. 2012, 134, 7480–7487. [Google Scholar] [CrossRef] [PubMed]
- Duong, H.M.; Myint, S.M.; Tran, T.Q.; Le, D.K. Chapter 6—Post-spinning treatments to carbon nanotube fibers. In Carbon Nanotube Fibers and Yarns; Miao, M., Ed.; Woodhead Publishing: Soston, UK, 2020; pp. 103–134. [Google Scholar] [CrossRef]
- Paukov, M.; Kramberger, C.; Begichev, I.; Kharlamova, M.; Burdanova, M. Functionalized Fullerenes and Their Applications in Electrochemistry, Solar Cells, and Nanoelectronics. Materials 2023, 16, 1276. [Google Scholar]
- Serda, M.; Korzuch, J.; Dreszer, D.; Krzykawska-Serda, M.; Musioł, R. Interactions between modified fullerenes and proteins in cancer nanotechnology. Drug Discov. Today 2023, 28, 103704. [Google Scholar] [CrossRef]
- Grebowski, J.; Litwinienko, G. Metallofullerenols in biomedical applications. Eur. J. Med. Chem. 2022, 238, 114481. [Google Scholar] [CrossRef]
- Chai, Y.; Guo, T.; Jin, C.; Haufler, R.E.; Chibante, L.P.; Fure, J.; Wang, L.V.; Alford, J.M.; Smalley, R.E. Fullerenes with Metals Inside. J. Phys. Chem. 1991, 95, 7564–7568. [Google Scholar]
- Maeda, Y.; Tsuchiya, T.; Lu, X.; Takano, Y.; Akasaka, T.; Nagase, S. Current progress on the chemical functionalization and supramolecular chemistry of M@C82. Nanoscale 2011, 3, 2421–2429. [Google Scholar]
- Fang, H.; Cong, H.; Suzuki, M.; Bao, L.; Yu, B.; Xie, Y.; Mizorogi, N.; Olmstead, M.M.; Balch, A.L.; Nagase, S.; et al. Regioselective Benzyl Radical Addition to an Open-Shell Cluster Metallofullerene. Crystallographic Studies of Cocrystallized Sc3C2@Ih-C80 and Its Singly Bonded Derivative. J. Am. Chem. Soc. 2014, 136, 10534–10540. [Google Scholar] [CrossRef]
- Bao, L.; Chen, M.; Pan, C.; Yamaguchi, T.; Kato, T.; Olmstead, M.M.; Balch, A.L.; Akasaka, T.; Lu, X. Crystallographic Evidence for Direct Metal–Metal Bonding in a Stable Open-Shell La2@Ih-C80 Derivative. Angew. Chem. Int. Ed. 2016, 55, 4242–4246. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, L.; Gao, X.J.; Guo, X.; Cui, R.; Xu, B.; Dong, J.; Li, Y.; Gan, L.; Chang, F. Regioselective Polyamination of Gd@C2v(9)-C82 and non-high performance liquid chromatography rapid separation of Gd@C82(morpholine)7. Chem. Mater. 2018, 30, 64–68. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, H.; Wang, Y.-X.; Dong, B.-W.; Sun, B.-Y.; Jiang, S.-D.; Gao, S. Amination of the Gd@C82 endohedral fullerene: Tunable substitution effect on quantum coherence behaviors. Chem. Sci. 2020, 11, 10737–10743. [Google Scholar] [CrossRef]
- Kandrashkin, Y.E.; Zaripov, R.B.; Liu, F.; Büchner, B.; Kataev, V.; Popov, A.A. Temperature-dependent dynamics of endohedral fullerene Sc2@C80(CH2Ph) studied by EPR spectroscopy. Phys. Chem. Chem. Phys. 2021, 23, 18206–18220. [Google Scholar] [CrossRef]
- Kandrashkin, Y.E.; Zaripov, R.B. Scandium dimetallofullerene with a single-electron metal–metal bond as a spectroscopic ruler for EPR measurements. Phys. Chem. Chem. Phys. 2022, 24, 19743–19752. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Jiang, Y.; Wang, Y.; Zhou, T.; Shi, Z.; Omachi, H.; Shinohara, H.; Sun, B.; Wang, Z. Turning on the Near-Infrared Photoluminescence of Erbium Metallofullerenes by Covalent Modification. Inorg. Chem. 2019, 58, 14325–14330. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Huang, H.; Yang, S.; Liu, Z.; Liu, S. Synthesis and characterization of a water-soluble endohedral metallofullerol. Chem. Mater. 1999, 11, 1003–1006. [Google Scholar] [CrossRef]
- Li, T.; Dorn, H.C. Biomedical applications of metal-encapsulated fullerene nanoparticles. Small 2017, 13, 1603152. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhen, M.; Wang, H.; Sun, Z.; Jia, W.; Zhao, Z.; Zhou, C.; Liu, S.; Wang, C.; Bai, C. Functional Gadofullerene Nanoparticles Trigger Robust Cancer Immunotherapy Based on Rebuilding an Immunosuppressive Tumor Microenvironment. Nano Lett. 2020, 20, 4487–4496. [Google Scholar] [CrossRef]
- Jia, W.; Li, X.; Zhang, T.; Wang, C.; Zhen, M. Efficiently normalizing leukopoiesis by gadofullerene nanoparticles to ameliorate radiation-triggered myelosuppression. J. Mater. Chem. B 2023, 11, 7401–7409. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Zhen, M.; Yu, M.; Li, X.; Yu, T.; Liu, J.; Jia, W.; Liu, S.; Li, L.; Li, J.; et al. Gadofullerene inhibits the degradation of apolipoprotein B100 and boosts triglyceride transport for reversing hepatic steatosis. Sci. Adv. 2020, 6, eabc1586. [Google Scholar] [CrossRef] [PubMed]
- Cagle, D.W.; Kennel, S.J.; Mirzadeh, S.; Alford, J.M.; Wilson, L.J. In vivo studies of fullerene-based materials using endohedral metallofullerene radiotracers. Proc. Natl. Acad. Sci. USA 1999, 96, 5182–5187. [Google Scholar] [CrossRef] [PubMed]
- Hino, S.; Umishita, K.; Iwasaki, K.; Miyazaki, T.; Miyamae, T.; Kikuchi, K.; Achiba, Y. Photoelectron spectra of metallofullerenes, Gd@C82 and La2@C80: Electron transfer from the metal to the cage. Chem. Phys. Lett. 1997, 281, 115–122. [Google Scholar] [CrossRef]
- Hino, S.; Iwasaki, K.; Umishita, K.; Miyazaki, T.; Kikuchi, K.; Achiba, Y. Photoelectron spectra of metal fullerenes: Are metal fullerenes molecular crystals or not? J. Electron. Spectrosc. Relat. Phenom. 1996, 78, 493–496. [Google Scholar] [CrossRef]
- Suzuki, T.; Kikuchi, K.; Oguri, F.; Nakao, Y.; Suzuki, S.; Achiba, Y.; Yamamoto, K.; Funasaka, H.; Takahashi, T. Electrochemical properties of fullerenolanthanides. Tetrahedron 1996, 52, 4973–4982. [Google Scholar] [CrossRef]
- Takano, Y.; Aoyagi, M.; Yamada, M.; Nikawa, H.; Slanina, Z.; Mizorogi, N.; Ishitsuka, M.O.; Tsuchiya, T.; Maeda, Y.; Akasaka, T. Anisotropic magnetic behavior of anionic Ce@C82 carbene adducts. J. Am. Chem. Soc. 2009, 131, 9340–9346. [Google Scholar] [CrossRef]
- Li, J.; Wang, T.; Feng, Y.; Zhang, Y.; Zhen, M.; Shu, C.; Jiang, L.; Wang, Y.; Wang, C. A water-soluble gadolinium metallofullerenol: Facile preparation, magnetic properties and magnetic resonance imaging application. Dalton Trans. 2016, 45, 8696–8699. [Google Scholar] [CrossRef]
- Kato, H.; Kanazawa, Y.; Okumura, M.; Taninaka, A.; Yokawa, T.; Shinohara, H. Lanthanoid endohedral metallofullerenols for MRI contrast agents. J. Am. Chem. Soc. 2003, 125, 4391–4397. [Google Scholar] [CrossRef]
- Kang, S.-G.; Araya-Secchi, R.; Wang, D.; Wang, B.; Huynh, T.; Zhou, R. Dual inhibitory pathways of metallofullerenol Gd@C82(OH)22 on matrix metalloproteinase-2: Molecular insight into drug-like nanomedicine. Sci. Rep. 2014, 4, 4775. [Google Scholar] [CrossRef]
- Chen, S.H.; Kang, S.-g.; Luo, J.; Zhou, R. Charging nanoparticles: Increased binding of Gd@C82(OH)22 derivatives to human MMP-9. Nanoscale 2018, 10, 5667–5677. [Google Scholar] [CrossRef] [PubMed]
- Deng, R.; Wang, Y.; Zhen, M.; Li, X.; Zou, T.; Li, J.; Yu, T.; Zhou, Y.; Lu, Z.; Xu, H.; et al. Real-time monitoring of tumor vascular disruption induced by radiofrequency assisted gadofullerene. Sci. China Mater. 2018, 61, 1101–1111. [Google Scholar] [CrossRef]
- Zhang, Y.; Shu, C.; Zhen, M.; Li, J.; Yu, T.; Jia, W.; Li, X.; Deng, R.; Zhou, Y.; Wang, C. A novel bone marrow targeted gadofullerene agent protect against oxidative injury in chemotherapy. Sci. China Mater. 2017, 60, 866–880. [Google Scholar] [CrossRef]
- Lucas, D.; Scheiermann, C.; Chow, A.; Kunisaki, Y.; Bruns, I.; Barrick, C.; Tessarollo, L.; Frenette, P.S. Chemotherapy-induced bone marrow nerve injury impairs hematopoietic regeneration. Nat. Med. 2013, 19, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhen, M.; Zhou, C.; Deng, R.; Yu, T.; Wu, Y.; Shu, C.; Wang, C.; Bai, C. Gadofullerene nanoparticles reverse dysfunctions of pancreas and improve hepatic insulin resistance for type 2 diabetes mellitus treatment. ACS Nano 2019, 13, 8597–8608. [Google Scholar] [CrossRef]
- Jia, W.; Zhen, M.; Li, L.; Zhou, C.; Sun, Z.; Liu, S.; Zhao, Z.; Li, J.; Wang, C.; Bai, C. Gadofullerene nanoparticles for robust treatment of aplastic anemia induced by chemotherapy drugs. Theranostics 2020, 10, 6886–6897. [Google Scholar] [CrossRef]
- Sun, B.; Li, M.; Luo, H.; Shi, Z.; Gu, Z. Electrochemical properties of metallofullerenes and their anions. Electrochim. Acta 2002, 47, 3545–3549. [Google Scholar] [CrossRef]
- Sun, B.; Luo, H.; Shi, Z.; Gu, Z. Electrochemical properties of Gd@C82 and its anions. Electrochem. Commun. 2002, 4, 47–49. [Google Scholar] [CrossRef]
- Takano, Y.; Ishitsuka, M.O.; Tsuchiya, T.; Akasaka, T.; Kato, T.; Nagase, S. Retro-reaction of singly bonded La@C82 derivatives. Chem. Commun. 2010, 46, 8035–8036. [Google Scholar] [CrossRef]
- Zhao, Y.-l.; Zhou, Q.; Lian, Y.-f.; Yu, H.-t. Molecular structures of Pr@C72 and Pr@C72(C6H3Cl2): A combined experimental–theoretical investigation. RSC Adv. 2015, 5, 97568–97578. [Google Scholar] [CrossRef]
- Kareev, I.E.; Bubnov, V.P.; Fedutin, D.N.; Yagubskii, E.B.; Lebedkin, S.F.; Laukhina, E.E.; Kuvychko, I.V.; Strauss, S.H.; Boltalina, O.B. Trifluoromethylation of Endohedral Metallofullerenes M@C82(M = Y, Ce): Synthesis, Isolation and Structure. In Hydrogen Materials Science and Chemistry of Carbon Nanomaterials; Springer: Dordrecht, The Netherlands, 2007; pp. 235–242. [Google Scholar]
- Han, X.; Xin, J.; Yao, Y.; Liang, Z.; Qiu, Y.; Chen, M.; Yang, S. Capturing the Long-Sought Dy@C2v(5)-C80 via Benzyl Radical Stabilization. Nanomaterials 2022, 12, 3291. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Liu, Z.; Zhang, L.; Guo, X.; Cui, R.; Dong, J.; Chang, F.; Jiang, S.; Gao, S.; Sun, B. Paramagnetic properties adjustment for Gd@C2v(9)-C82 by regioselective multi-amination. Carbon 2020, 158, 320–326. [Google Scholar] [CrossRef]
- Shu, C.; Slebodnick, C.; Xu, L.; Champion, H.; Fuhrer, T.; Cai, T.; Reid, J.E.; Fu, W.; Harich, K.; Dorn, H.C.; et al. Highly Regioselective Derivatization of Trimetallic Nitride Templated Endohedral Metallofullerenes via a Facile Photochemical Reaction. J. Am. Chem. Soc. 2008, 130, 17755–17760. [Google Scholar] [CrossRef] [PubMed]
- Li, F.-F.; Pinzón, J.R.; Mercado, B.Q.; Olmstead, M.M.; Balch, A.L.; Echegoyen, L. [2 + 2] Cycloaddition Reaction to Sc3N@Ih-C80. The Formation of Very Stable [5,6]- and [6,6]-Adducts. J. Am. Chem. Soc. 2011, 133, 1563–1571. [Google Scholar] [CrossRef]
- Cai, T.; Slebodnick, C.; Xu, L.; Harich, K.; Glass, T.E.; Chancellor, C.; Fettinger, J.C.; Olmstead, M.M.; Balch, A.L.; Gibson, H.W.; et al. A Pirouette on a Metallofullerene Sphere: Interconversion of Isomers of N-tritylpyrrolidino Ih Sc3N@C80. J. Am. Chem. Soc. 2006, 128, 6486–6492. [Google Scholar] [CrossRef]
- Chen, M.; Guan, R.; Li, B.; Yang, L.; Niu, C.; Jin, P.; Wang, G.-W.; Yang, S. Anomalous Cis-Conformation Regioselectivity of Heterocycle-Fused Sc3N@D3h-C78 Derivatives. Angew. Chem. Int. Ed. 2021, 60, 7880–7886. [Google Scholar] [CrossRef]
- Hu, Y.; Yao, Y.-R.; Liu, X.; Yu, A.; Xie, X.; Abella, L.; Rodríguez-Fortea, A.; Poblet, J.M.; Akasaka, T.; Peng, P.; et al. Unexpected formation of 1,2- and 1,4-bismethoxyl Sc3N@Ih-C80 derivatives via regioselective anion addition: An unambiguous structural identification and mechanism study. Chem. Sci. 2021, 12, 8123–8130. [Google Scholar] [CrossRef]
- Xu, Y.-Y.; Tian, H.-R.; Li, S.-H.; Chen, Z.-C.; Yao, Y.-R.; Wang, S.-S.; Zhang, X.; Zhu, Z.-Z.; Deng, S.-L.; Zhang, Q.; et al. Flexible decapyrrylcorannulene hosts. Nat. Commun. 2019, 10, 485. [Google Scholar] [CrossRef]
- Chen, M.; Zhao, Y.; Jin, F.; Li, M.; Guan, R.; Xin, J.; Yao, Y.-R.; Zhao, X.; Wang, G.-W.; Zhang, Q.; et al. Decisive role of non-rare earth metals in high-regioselectivity addition of μ3-carbido clusterfullerene. Inorg. Chem. Front. 2022, 9, 5688–5696. [Google Scholar] [CrossRef]
- Jin, F.; Xin, J.; Guan, R.; Xie, X.-M.; Chen, M.; Zhang, Q.; Popov, A.A.; Xie, S.-Y.; Yang, S. Stabilizing a three-center single-electron metal–metal bond in a fullerene cage. Chem. Sci. 2021, 12, 6890–6895. [Google Scholar] [CrossRef]
- Zhang, Y.; Guan, R.; Chen, M.; Shen, Y.; Pan, Q.; Lian, Y.; Yang, S. Favorite Orientation of the Carbon Cage and a Unique Two-Dimensional-Layered Packing Model in the Cocrystals of Nd@C82(I,II) Isomers with Decapyrrylcorannulene. Inorg. Chem. 2021, 60, 1462–1471. [Google Scholar] [CrossRef]
- Yang, S.; Zhou, X.; Hu, Y.; Abella, L.; Yao, Y.-R.; Peng, P.; Zhang, Q.; Rodríguez-Fortea, A.; Poblet, J.M.; Li, F.-F. Effects of Solvents on Reaction Products: Synthesis of Endohedral Metallofullerene Oxazoline and Epoxide. J. Org. Chem. 2023, 88, 4234–4243. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Solé-Daura, A.; Yao, Y.-R.; Liu, X.; Liu, S.; Yu, A.; Peng, P.; Poblet, J.M.; Rodríguez-Fortea, A.; Echegoyen, L.; et al. Chemical Reactions of Cationic Metallofullerenes: An Alternative Route for Exohedral Functionalization. Chem. Eur. J. 2020, 26, 1748–1753. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Petersson, G.A.; Al-Laham, M.A. A complete basis set model chemistry. II. Open-shell systems and the total energies of the first-row atoms. J. Chem. Phys. 1991, 94, 6081–6090. [Google Scholar] [CrossRef]
- Petersson, G.A.; Bennett, A.; Tensfeldt, T.G.; Al-Laham, M.A.; Shirley, W.A.; Mantzaris, J. A complete basis set model chemistry. I. The total energies of closed-shell atoms and hydrides of the first-row elements. J. Chem. Phys. 1988, 89, 2193–2218. [Google Scholar] [CrossRef]
- Cao, X.; Dolg, M. Segmented contraction scheme for small-core lanthanide pseudopotential basis sets. J. Mol. Struct. THEOCHEM 2002, 581, 139–147. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A. Gaussian 09, Revision A.01; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Legault, C.Y. CYLview, 1.0b; Université de Sherbrooke: Sherbrooke, QC, Canada, 2009. [Google Scholar]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).