Abstract
This study aims to explore the feasibility of fine-needle aspiration biopsy (FNAB) under dual modal photoacoustic tomography(PAT)/ultrasound (US) imaging. A total of 25 patients who have thyroid nodules with thyroid imaging reporting and data system (TIRADS) 3 and 4 (malignant risk <85%) were recruited. The specimens obtained from the PAT/US-guided FNAB were collected for cytology analysis. Cytological diagnoses for the 25 patients were classified in perspective of the Bethesda system for reporting thyroid cytopathology diagnostic category (DC) I: 4%(1/25); DC II: 12% (3/25); DC III: 20% (5/25); DC IV: 8% (2/25); DC V: 32% (8/25); and DC VI: 24% (6/25). The DC I nodule exhibited inadequate cytology and had structural characteristic of predominant calcifications in PAT/US mapping. The DC V-VI nodules showed lower photoacoustic (PA) signals compared to the DC I-IV nodules. Regions with a high PA signal demonstrated a significant number of erythrocytes in FNAB cytology. Moreover, nodules with microcalcifications did not show a significant difference compared to their surroundings in the PA signal, while nodules with macrocalcifications gave higher PA signals compared to their surroundings. The conclusions are as follows: combining US with PAT can evaluate the structure and function of thyroid nodules in vivo. This study demonstrates that dual modal PAT/US imaging has the potential to be an effective clinical tool to guide FNAB of thyroid nodules.
1. Introduction
Thyroid nodules are common varieties of benign and malignant lesions inside the thyroid and are radiologically different from the surrounding thyroid parenchyma. The overall prevalence of thyroid nodules was 24.83% (95% CI 21.44–28.55), regardless of the diagnostic techniques used [1]. Ultrasonography (US) reveals thyroid nodules in approximately 50–70% of the population [2,3]. Despite high incidence rates, survival rates of papillary thyroid carcinoma (PTC) continue to be as high as 97% [4]. It generally shows indolent behavior with declined mortality. However, a meta-analysis showed that 68.8% of all thyroid nodules undergoing surgical excision were benign [5,6]. The overdiagnosis and overtreatment of the thyroid nodules has caused increasing public health concerns.
Fine-needle aspiration biopsy (FNAB) of the thyroid is a minimally invasive method and is the gold standard in the diagnosis of thyroid nodules [7]. Tissues obtained by FNAB for cytological assessment and classification of malignancy risk, are commonly analyzed using the Bethesda system for reporting thyroid cytopathology (TBSRTC) [8]. The TBSRTC consists of six diagnostic categories, each associated with a specific risk of malignancy and corresponding clinical management recommendation. Inadequate samples are classified as diagnostic category (DC) I, reported as ‘‘nondiagnostic’’ (ND) or ‘‘unsatisfactory’’ (UNS). A repeated FNAB is recommended for DC I nodules, but some nodules remain persistently ND/UNS and excision is considered in these cases.
Several factors may contribute to an inadequate specimen, including the guidance method, nodule’s characteristics, cytological techniques and skill of the operators [9,10,11,12,13]. Previous studies have emphasized the primary diagnostic challenge related to FNAB is the inadequate extraction of specimens during the procedure [14]. As reported, efforts should be spent to minimize the prevalence of insufficient FNAB to the lowest possible extent, ideally falling within the acceptable threshold of 10% [15].
To improve the diagnostic efficacy of FNAB, it is crucial to increase specimen adequacy. Historically, the FNAB has been performed under palpation guidance. The practice of performing FNAB under US guidance has become more common in recent years [16]. The use of US-guided FNAB allows the visualization of the needle in real time, which has largely reduced the rate of inadequate sampling. Consequently, US guidance is recommended as the method with the highest sensitivity and specificity for the preoperative estimation of discriminating benign thyroid nodules from malignant cytologically. Nevertheless, nondiagnostic FNAB results are up to 15–33.6% in all cases, even under US guidance [17,18]. The poor needle contrast with surrounding tissues and artifacts (e.g., reverberation from metal or acoustic clutter) have motivated researchers to investigate guidance under a new imaging method or multi-modality imaging based on US imaging.
PAT is a novel hybrid imaging modality which can provide both important structural and functional information. Over the past decade, several studies confirmed that PAT imaging can identify tumor boundaries, as well as differentiate benign from malignant tumor by detecting the distribution of endogenous chromophores including oxygenated hemoglobin(HbO2), deoxyhemoglobin (HbR), water, collagen and lipids [19]. Moreover, PAT has shown viability for guiding multiple surgeries and procedures both in animals [20] and humans [21]. In recent years, there has been a growing accessibility to the implementation of photoacoustic-guided surgery [22]. Kim C et al., has successfully developed a pioneering handheld photoacoustic probe, marking the first instance of its application. This achievement is attributed to the incorporation of optical fiber bundles, which facilitated the delivery of pulsed laser light for the purpose of PAT image-guided needle biopsy. Notably, this innovative technique allows for the precise insertion of a needle into rat axillary lymph nodes containing accumulated indocyanine green (ICG) [23]. The primary work for PAT-guidance in humans encompasses areas related to the brain [24], spine [25,26], lungs [27], liver [28,29,30], prostate [31,32], breast [33,34], cardiovascular system [35,36], kidneys [37], pancreas [38] and uterus [21].
Recently, PAT has made significant progress in both implementation and technology development, aiming to provide an important clue in the diagnosis of thyroid diseases with reliable anatomical and vascular information. Several studies for thyroid diseases have been conducted using various PAT systems and achieved high-fidelity performance in the human thyroid in vivo [39,40]. The results demonstrated that PAT made it possible to detect the thyroid’s outline, as well as identify vascular features. To further analyze the tissue oxygenation contents in thyroid imaging, multispectral PAT was used. All the functional parameter analyses of thyroid showed encouraging results with statistically differentiable distributions [39,40,41].
More importantly, PAT has the advantage of having no binding barriers which allows it to be integrated with other imaging modalities, like US or NIR fluorescence imaging [39,42,43]. To capture and visualize anatomical structures as well as functional imaging in real time, several PAT/US dual-model imaging systems have already been developed and applied in clinical applications [40]. In an initial clinical study, PAT combined with US provided accurate vascular information in the diagnosis of thyroid diseases [44]. More recently, several groups have successfully visualized PAT/US images of thyroid nodules in real time. Using PAT/US, viable tissue of thyroid nodules can be accurately detected and located, all of which have shown excellent capabilities in the diagnosis of thyroid diseases.
Thus, in this study we aim to test the feasibility of PAT/US guidance for FNAB of thyroid nodules in humans. To the best of our knowledge, it is the first of its kind.
2. Materials and Methods
2.1. Subjects
In this pilot clinical study, a total of 25 patients diagnosed with TIRADS 3 and 4 thyroid nodules were consecutively recruited from both outpatient and inpatient departments at the Fifth People’s Hospital of Chengdu (FPHC) between 1 July 2022 and 31 July 2023 (Table 1). All thyroid nodules underwent a FNABs guided by PAT/US imaging. The examination procedures were approved by the institutional ethics committee of the FPHC. All the patients signed the informed consent form prior to the examinations.

Table 1.
The patients’ characteristic of thyroid nodules.
Body mass index (BMI) of all our patients was calculated by the following formula:
BMI = body weight (kg)/body height2 (m2)
2.2. Experimental System
An imaging system devised by our group was used to perform PAT imaging [45], which enables real-time visualization of thyroid nodules. The schematic diagram is shown in Figure 1. In this system, optical parametric oscillators pumped with Nd: YAG provide the laser illumination with a wavelength range of 680–950 nm and frequency 20 Hz (Surelite, Continuum, Santa Clara, CA, USA). Optical fibers were used to transmit light to the imaging object. The PA signals were received via a 128-element concave transducer array with a 5 MHz central frequency and transferred to 64 data acquisition channels (12-bit sampling progress, sampling frequency 50 MHz). This concave-shaped array of transducers was arranged in a half arc (180° spanning range) with a radius of 50 mm. The demonstrated time resolution was 100 ms by using a multiplexer without taking 10 times average. The spatial resolution was ~150 μm. Image depth of view was demonstrated to be at least 25 mm depending on transducer configuration and concave array. To avoid physiological thyroid movements (e.g., respiratory movements) and improve the signal-to-noise ratio, the signal was averaged ten times (as verified in previous studies) [46]. All gray-scale and color Doppler flow imaging (CDFI) examinations were performed by using 8–15-MHz linear probe (S60; Sonoscape Medical System, Shenzhen, China).
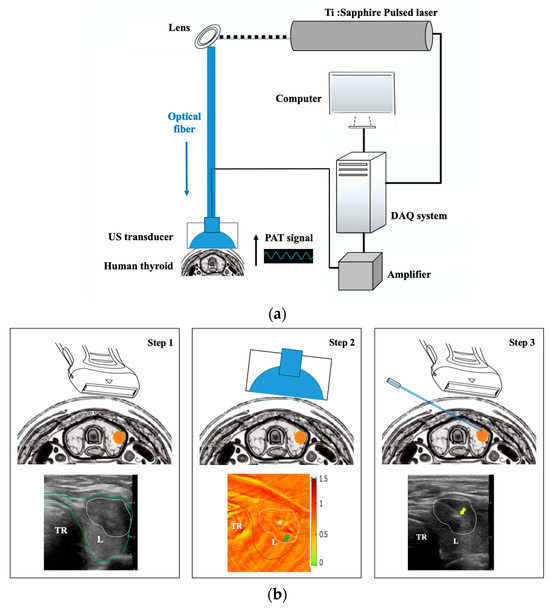
Figure 1.
The PAT/US system for human thyroid imaging. (a) The schematic of the hardware components employed, including the pulsed laser, optical fiber, computer, DAQ system, lens, amplifier, and US transducer. (b) The procedure of PAT/US FNAB for human thyroid in vivo. Step 1: US-guided imaging for nodule localization; step 2: PAT-guided imaging(PA signals for SO2 distribution) for selecting puncture point(white asterisk); step 3: real-time US-guided FNAB for thyroid nodule. PAT: photoacoustic tomography; US: ultrasound; DAQ: data acquisition; L: left thyroid lobe; R: right thyroid lobe; TR: trachea. White asterisk represents viable tissues (moderate-intensity PA signals); green arrow indicates small blood vessels in the thyroid nodule and yellow arrow indicates the needle tip.
2.3. PAT/US guided FNAB
The procedure of PAT/US FNAB for human thyroid in vivo were conducted by following three steps (Figure 1b).
Step 1: Thyroid nodule localization by US.
The thyroid nodules were imaged at axial locations. Nodule location (right lobe, left lobe, or isthmus), depth (vertical distance from skin to the nearest point on the surface of nodule), size (largest diameter), internal component (solid, predominantly solid, predominantly cystic, or pure cystic), echogenicity(hypoechoic, isoechoic, hyperechoic, or anechoic appearance), calcification, and vascularity were assessed by US. Nodule vascularity was assessed by CDFI and classified based on Adler semi-quantitative standard [47].
Step 2: Selecting a puncture point by PAT.
PAT was performed before FNAB. An imaging plane localization was marked on the body’s surface at the location where cross-sectional imaging was acquired by US (Figure 1b). To reduce the disturbance of breathing and movement, all patients were told to keep motionless and breathe calmly. For each US position, a matching set of PAT images was performed using our PAT system, as described above [48]. PAT images were acquired at three wavelengths: 760 nm, 840 nm and 910 nm. In addition, a maximum amplitude projection image was obtained by a multispectral reconstruction algorithm [49], which was used to analyze the oxyhemoglobin saturation(SO2), HbO2 and HbR contents for the region of interest. The SO2 distribution of the thyroid nodule and surrounding parenchyma were revealed with multispectral PAT mapping. Moreover, PA signals for SO2 were categorized according to their intensity, referred to in the scale bar (Figure 2). These were low-intensity (0–0.5), moderate-intensity (0.5–1.0) and high-intensity (1.0–1.5). Moderate-intensity PA signals were selected as the puncture point.
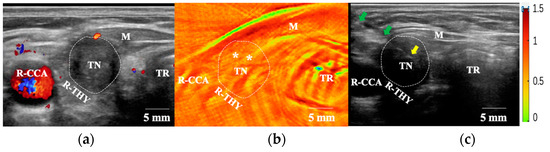
Figure 2.
FNAB procedures under PAT/US-guidance. (a) US(in CDFI mode) shows a blood vessel around the TN. (b) The moderate-intensity PA signals (*) for SO2 inside the TN were selected as puncture points. (c) Needle tip was positioned in the region of TN under US guidance according to the PAT results. FNAB: fine-needle aspiration biopsy; US: ultrasound; PAT: photoacoustic tomography; M: muscle; R-THY: right thyroid lobe; R-CCA: right common carotid artery; TN: thyroid nodular; TR: trachea. White asterisks represent viable tissues (moderate-intensity PA signals); green arrows indicate the needle and yellow arrow indicates the needle tip.
Step 3: The FNAB process by real-time US guidance.
The FNABs were performed by two sonographers with more than 5 years’ experience in thyroid US examination. A 22-gauge 8 cm long needle (CCZD Type, Leapmed Healthcare Corporation) was employed for FNAB [50,51]. A sterile transducer cover was used to avoid the risk of infection. Based on prior research, all FNAB procedures were conducted without local anesthesia [52,53]. The nodule for biopsy was localized in a transverse plane. The needle was punctured obliquely along a path parallel to allow continuous visualization of the tip and shaft during the procedure of the FNAB. According to both the structural and functional parameters of the thyroid nodules, solid components (US-mode) with moderate-intensity signals (PAT-mode) were confirmed in the puncture area, which effectively avoided the feeding vessels, both around and inside the thyroid nodules (Figure 2). Based on the quantity of the specimens, 2–5 passes were performed until a proper amount of material filled the needle hub [54]. All thyroid nodules included in our study were biopsied using the same size needle and smeared with the same method. Additionally, any complication that occurred during or after FNAB procedures were also recorded.
2.4. Cytologic Analysis
Following biopsy, each sample was mounted onto a glass slide immediately after biopsy and fixed in 95% ethanol for pathological analysis. All cases were reviewed by two cytopathologists with a minimum of five years’ experience in pathology. The pathological results are based on the Bethesda reporting system for TBSRTC [55]. Inadequate cytology was reported as DC I, which defined as specimens showing less than 6 groups of well-visualized follicular cells that consisted of less than 10 cells per group, while adequate cytology was reported as DC II to VI. Patients with DC V and DC VI in this study underwent thyroidectomy or hemithyroidectomy surgery. The BRAF V600E gene tests were performed according to the patients’ willingness. In total, twenty-five thyroid nodule samples were analyzed (Table 1).
2.5. Statistical Analysis
Continuous variables are presented as means with standard deviations (mean ± SD). Data variability and group comparisons were represented by using boxplots drawn in R. p < 0.05 was considered statistically significant (ns p > 0.05; * p < 0.05; ** p < 0.01).
3. Results and Discussion
In this pilot clinical study, for the first time to our knowledge, we report the thyroid FNAB using a dual modal PAT/US guidance, which investigate the feasibility of this system for humans in vivo.
A curved array, as opposed to linear array was used in acquiring PA signals. In general, PA waves propagate in all directions, which ideally require detection on a spherical surface that completely encloses the volume illuminated. In this regard, the geometry of the detection array greatly affects the quality of the PAT image. By using curved arrays, it is possible to collect a more complete projection data set, which can be used to represent the light absorption distribution more accurately.
The FNAB procedures were without local anesthesia in this study. In our experience, US-FNABs have been performed without local anesthesia for several years and this does not cause significant pain when compared to procedures with local anesthesia. Furthermore, performing US-FNABs without local anesthesia shortens the procedure duration and can prevent the formation of micro-air-bubbles caused by lidocaine injections that may interfere with the guidance.
In our study, PAT/US dual-modality imaging was performed in 25 cases. There were 5 male and 20 female participants in total, the median age was 46.8 ± 12.4 years (age range, 24–73 year). Twenty-five patients (BMI range, 19.4–27.1 kg/m2; mean size ± SD, 22.2 ± 2.0) underwent PAT/US FNAB for 25 nodules (size range, 4.3–27.8 mm; mean size ± SD, 7.9 ± 5.0 mm; depth range, 5.8–18.2 mm, mean size ± SD, 9.7 ± 2.9 mm). In the literature, female sex, older age, and higher BMI are associated with an increased prevalence of thyroid nodules [56,57]. These results were consistent with our experiment. Female patients aged >40 accounted for the majority of patients with thyroid nodules and an increase in BMI at any age was associated with malignant risk of thyroid nodules in cytology (Figure 3a). The two sonographers graded all PAT/US images observed as having good image quality, independent of the BMI level, in all of 25 cases. In addition, there is no statistically significant difference in nodule depth between DC V-VI and DC I-IV(Figure 3b). This may indicate that nodule depth had no correlation with the malignant risk of thyroid nodules. A quantitative SO2 value (average ROI) of thyroid nodules and their surrounding parenchyma has also been calculated (SO2 values range of thyroid nodules, 0.72–1.44; mean values ± SD, 1.04 ± 0.23; SO2 value range of surrounding thyroid parenchyma, 0.75–1.39; mean values ± SD, 1.07 ± 0.19). There was no significant differences between DC V-VI and DC I-IV in the SO2 values of surrounding thyroid parenchyma (p < 0.05). Since the quantitative SO2 value of the surrounding thyroid parenchyma does not vary among different DC groups with normal thyroid hormone levels, it suggests that the PAT intensity of surrounding thyroid parenchyma can be used as reference values for future studies as well as for clinical routines using this imaging system.
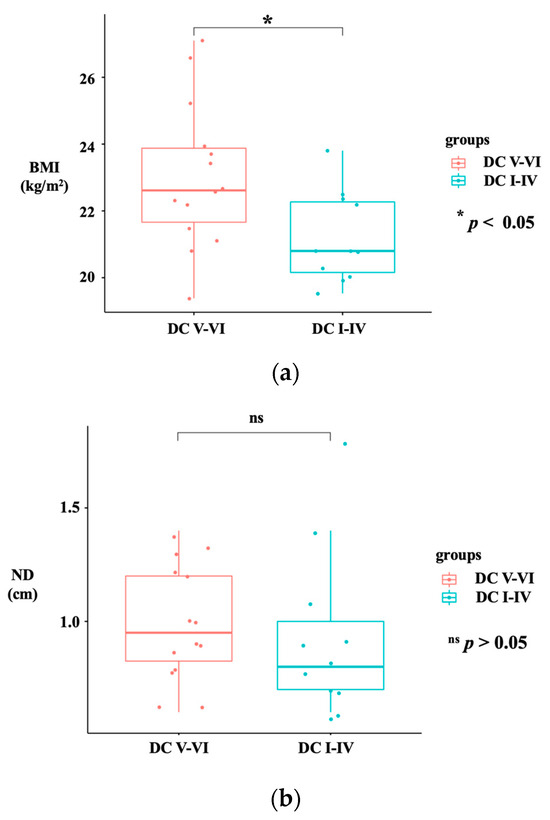
Figure 3.
Boxplot graph showing statistical difference between DC V-VI and DC I-IV in BMI (a) and ND (b). The red boxplot represents the DC V-VI group, and the blue boxplot represents the DC I-IV group. BMI: body mass index; DC: diagnostic category; ND: nodular depth. (ns p > 0.05; * p < 0.05).
Eighteen nodules were found in the right lobe and seven nodules were found in the left lobe. The TIRADS category for 25 nodules were as follows: 2 cases were TIRADS 3 (Malignant risk, ≤5%); 6 cases were TIRADS 4a (Malignant risk, 5~10%); 13 cases were TIRADS 4b (Malignant risk, 10~50%); and 4 cases were TIRADS 4c (Malignant risk, 50~85%). One patient had a prior history of Hashimoto thyroiditis (Case No.24 in Table 1). For 25 nodules, 1 “DC I”, 3 “DC II”, 5 “DC III”, 2 “DC IV”, 8 “DC V” and 6 “DC VI” were identified by cytology based on TBSRTC, accounting for 4%, 12%, 20%, 8%, 32%, and 24% of all nodules, respectively (Table 1). The cytology results showed that 12 out of the 17 nodules with TIRADS ≥ 4b and 2 out of 8 nodules with TIRADS ≤ 4a were DC V (suspicious malignancy) or DC VI (malignancy) in cytology by PAT/US-guided FNAB. Later, patients with DC V and DC VI underwent thyroidectomy or hemithyroidectomy surgery revealed PTC in pathology. As a result, PAT/US-guided FNAB may have the feasibility for differentiating malignant from benign nodules.
To enhance the sample adequacy, areas having moderate-intensity PA signals were selected as FNAB sites. The sample inadequacy of thyroid FNAB is associated with specific nodule characteristics, including hypervascularity, necrosis, sclerosis, calcification and fibrosis [58]. It was found that a high sample adequacy was achieved when both hypervascular and hypovascular site aspirates were evaluated together [59]. However, FNAB in hypervascular site from nodules may result in cytology of DC I in the literature [60,61]. Consistent with our findings, regions with a high-intensity PA signal demonstrated a significant quantity of erythrocytes in FNAB cytology. Non-viable tissues, such as old fibrosis, infarction, and hemorrhage generally show low-intensity PA signals. Therefore, a region with moderate-intensity PA signals was chosen to be the target area of FNAB.
Figure 4 shows results for case No.1 in Table 1, which is a DC III nodule in the right thyroid lobe with the largest diameter of 7.1 mm. The nodular depth (the gel surface to the nearest point on the surface of nodule) is 10.2 mm. Nodular CDFI showed Adler grade 0 blood signals inside the thyroid nodule, while CDFI showed Adler grade I blood signals around the thyroid nodule (Figure 4a). PAT imaging displayed abundant PA signals inside the nodule and its surrounding thyroid parenchyma (Figure 4b), indicating a more vasculature distribution in the thyroid. Annular calcification around this nodule was presented as a hyperechoic feature in the US and high-intensity signal in PAT. According to the trial protocol, we have selected the moderate-intensity PA signals area inside the nodular as the puncture point. Pathological results showed adequate FNAB cytology of the No.1 nodule rendering a conclusive diagnosis possible.

Figure 4.
FNAB procedures under PAT/US-guidance for a calcified nodule. (a) US (in CDFI mode) imaging showed a nodule in the right thyroid lobe represented as an annular calcification with posterior shadow; (b) PAT imaging showed puncture points (asterisks) inside the thyroid nodular, showing moderate-intensity PA signals for SO2; and (c) FNAB under US-guidance according to the PAT imaging in cross-sections of the right thyroid lobe. US: ultrasound; CDFI: colored Doppler flow imaging; PAT: photoacoustic tomography, R-THY: right thyroid lobe; Tr: trachea, M: muscle. R-CCA: right common carotid artery; TN: thyroid nodule. White asterisks represent viable tissues (moderate-intensity PA signals); green arrows indicate the needle and yellow arrow indicates the needle tip.
PAT is an effective imaging modality for observing microvascular perfusion as well as detecting viable tissues [39,42]. A grayscale US and even US with a CDFI mode cannot differentiate between viable and non-viable tissues, especially in predominantly cystic and purely cystic nodules [41]. More vascular signals inside the nodules and surrounding thyroid parenchyma were seen in PAT imaging than in US(CDFI mode) imaging. It was demonstrated PAT/US can image viable tissues by visualizing small vessels and vessels with slow blood flow speed, as well as providing functional imaging besides speed. Alternatively, the DC V~VI nodules in this study showed lower intensity PA signals compared to DC I~IV. DC V, and DC VI represents suspicions for malignancy and malignant sample in cytology, respectively. It was found that vascularity was more frequently seen in benign nodules, since fibrosis within thyroid carcinoma cause the low vascular density [62]. Moreover, a previous study revealed a lower SO2 level in the PTC than in the benign nodule by PAT/US dual-model imaging [41], indicating that this mixed method has a large potential for localizing viable tissues in thyroid tumors. In this regard, it has potential to provide those specific nodule characteristics to improve the sample inadequacy of FNABs.
Nodules with calcified morphology generally resulted in inadequate cytology due to their lack of cellularity [63]. It was reported that 19.8–32.1% of thyroid nodules presented with some type of calcification [64,65]. The prevalence of calcification in thyroid nodules is estimated to be around 40% in malignant and 20% in benign nodules [66]. In US imaging, microcalcifications appear as hyperechoic spots that are ≤2 mm in diameter (named as stromal calcification, psammoma bodies or bone formation), while macrocalcifications were >2 mm with posterior acoustic shadow [67]. Nodules with microcalcifications can be easily identified by US imaging, and easily confirmed the appropriate puncture site for FNAB. However, due to the posterior acoustic shadow of macrocalcifications, it is difficult to identify viable tissues from those dark shadows. In a chicken breast phantom, calcification particles measuring 0.5 mm can be visualized with PAT at a depth of 30 mm [68]. Within this depth range, all 25 nodules were successfully visualized in PAT imaging, including 7 calcified nodules. Only one of whom showed inadequate cytology, classified as DC I (case No. 22). In this regard, the inadequate rate in our work has achieved the acceptable threshold of 10% in the literature [15].
Among seven calcified nodules, three nodules exhibited microcalcifications (punctate hyperechogenic foci) in the US. In the PAT imaging, microcalcification areas showed similar PA signals compared with surrounding nodular tissues. Since PAT is more sensitive to changes of tissue absorption rather than scattering changes, microcalcifications may be difficult to detect in thyroid nodules by PAT alone [69]. Four nodules exhibited macrocalcifications by US. In thyroid nodules, it is generally accepted that microcalcifications represent psammoma bodies and are therefore reliable indicators of malignancy, while central macrocalcifications are usually predictive of benign in pathology. However, there are some different classifications for the types of macrocalcifications associated with malignancy, such as coarse macrocalcifications and interrupted eggshell macrocalcifications [63]. Coarse macrocalcifications are referred to as dystrophic calcifications, which can be found in malignant conditions of the thyroid, as well as benign diseases (e.g., Graves’ disease or nodular goiter) [63]. The tumor infiltration through the broken calcification rim may be presented as the focal interruption of an eggshell calcification. Since those macrocalcifications are associated with malignancy, they lead to FNABs. Case No.22 in our study was classified as TIRADS 4b with the largest diameter of 7.4 mm before FNAB. It was presented as a completely coarse macrocalcification, which presented as a hyperechoic nodule with a wide shadow in US and high-intensity signals in PAT imaging. Thus, it was difficult to detect viable tissues by both US and PAT imaging, resulting in an inadequate cytology(DC I). An eggshell calcification was found in case No.1, presenting as a hypoechoic nodule with a hyperechoic boundary in US imaging and moderate-intensity signal with high-intensity boundary in PAT imaging (Figure 4). The cytology after FNAB revealed it was a DC III thyroid nodule. Therefore, the challenge to FNAB in calcified nodules is to choose an appropriate puncture position, allowing more sufficient samples for cytology. US-guided FNAB makes it difficult to detect viable tissues in macrocalcified nodules, due to the hyperechoic characteristics and posterior wide dark shadow. However, in our investigation, PAT in 760 nm and 840 nm imaging was capable of depicting viable tissues in macrocalcified nodules with both anatomic and functional information.
The distribution of blood and perfusion in PTC are associated with tumor size [70,71]. Smaller nodules measuring less than 10 mm might exhibit poor blood flow signals, whereas larger nodules exceeding 20 mm could demonstrate the contrary pattern. A nodular with the largest diameter of 6.4 mm (case No. 25 in Table 1) is shown in Figure 5. The nodular depth (the gel surface to the nearest point on the surface of nodule) is 12.4 mm. This nodule had an unclear boundary and hypoechoic US feature. Nodular CDFI identified no blood signal (Adler grade 0) inside the thyroid nodule (Figure 5a), while PAT imaging displayed more PA signals inside the nodule and its surrounding thyroid parenchyma (Figure 5b), indicating a higher vasculature distribution in the thyroid nodule. The FNAB result showed adequate cytology (DC V) in Bethesda system, adding confidence on the feasibility of this dual-modality method for FNAB of nodule with small lesions. Finally, the patient underwent a right thyroid lobectomy. The immunohistochemistry result confirmed PTC with BRAF V600E gene mutation, which is a risk factor of lymph node and distant aggressiveness. Consequently, this PAT/US dual-model evaluation of the thyroid nodule before FNAB would assist sonographers to choose puncture paths, depth and site, especially for the smaller size nodules with poor CDFI signals. In addition, PAT imaging is potentially providing information for screening early PTC with a high risk of aggressiveness.
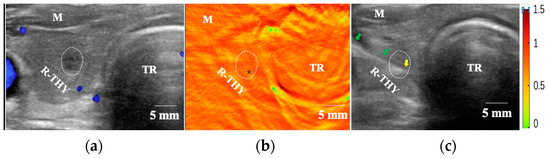
Figure 5.
FNAB procedures under PAT/US-guidance. (a) US(in CDFI mode) imaging showed a nodule in the right thyroid lobe without CDFI signals inside, (b) area with moderate-intensity PA signals (black asterisk) inside the thyroid nodular was selected as the puncture point and (c) FNAB under US-guidance according to the PAT imaging in cross-sections of the right thyroid lobe. Green arrows indicate the needle, and yellow arrow indicates the needle tip in right thyroid lobe. US: Ultrasound; CDFI: colored Doppler flow imaging; PAT: photoacoustic tomography, R-THY: right thyroid lobe; Tr: Trachea, M: muscle.
Given the continuously ultrasonic reflected signals in thyroid nodules shown by PAT, the PAT/US dual modality provides precise guidance for targeted area puncturing. PAT/US guided FNAB may eliminate the effect of both calcified and cystic components to sample adequacy, by increasing the adequate cytology rate of those thyroid nodules. In addition, PAT/US can provide dual-model imaging of the thyroid’s structural and functional information (e.g., HbO2, HbR and SO2 contents) without injection of exogenous contrast agents during FNAB process. Moreover, it takes the advantages of a brief examination duration (~5 min) and radiation-free, which has great potential for clinical application.
Our study had some limitations. First of all, quantitative parameters (e.g., HbO2, HbR and SO2 contents) within the thyroid nodules could not be displayed in real time. Second, the sample size of this study was relatively small; therefore, statistical analysis of thyroid nodules with different components was not performed. Third, PAT and US imaging were performed separately, which might cause inconsistency in the acquisition sections between the two imaging modalities. Forth, the different PA imaging systems may also exhibit different signal strengths for the same samples, meaning there was lack of a standard that can be transferred across different systems. Subsequent developments are needed to further update the PAT/US dual-model system in several regards, as follows: (1) PAT/US-guidance in real-time; (2) multispectral parameters processed; (3) high temporal resolution; (4) three-dimensional fast imaging for vessel information of thyroid tumor; (5) further studies being conducted to explore a more comprehensive quantitative analysis of thyroid nodules, such as PA radio( the ratio of nodule to the normal thyroid tissue), thereby enhancing the value of our studies; and (6) further research on the feasibility of PAT/US guided FNAB will be conducted in a larger sample of thyroid nodules.
Taken together, dual modal PAT/US imaging shows great promise as a real-time diagnosis and biopsy guidance tool for visualization of thyroid nodules and has the potential for FNAB guidance of high-risk inadequate thyroid nodules.
Author Contributions
Conceptualization, Y.W. (Yanting Wen) and H.J.; methodology, D.W.; software, D.W.; formal analysis, Y.X.; investigation, Y.W. (Yun Wu); resources, Y.Y.; writing—original draft preparation, Y.W. (Yanting Wen) and S.J.; writing—review and editing, X.L.; funding acquisition, J.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the program of Chengdu Fifth people’s hospital Fund, grant number KYJJ 2021-29, the Xinglin Scholars research program, grant number 20210067, the Chengdu Medical Research Project, grant number 2022055, the Doctoral Innovative Talents Program of Chongqing University of Posts and Telecommunications, grant number BYJS202117, Chongqing Education Commission, Youth Fund, grant number KJQN202000607 and Chongqing post-doctoral research project (special funding project), grant number 2021XM3040.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Fifth people’s hospital of Chengdu (protocol code AF/72/2019-01.1 and 6 April 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
Data is unavailable due to ethical restrictions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mu, C.; Ming, X.; Tian, Y.; Liu, Y.; Yao, M.; Ni, Y.; Liu, Y.; Li, Z. Mapping global epidemiology of thyroid nodules among general population: A systematic review and meta-analysis. Front. Oncol. 2022, 12, 1029926. [Google Scholar] [CrossRef] [PubMed]
- Yassa, L.; Cibas, E.S.; Benson, C.B.; Frates, M.C.; Doubilet, P.D.; Gawande, A.A.; Moore, F.D.M., Jr.; Kim, B.W.; Nosé, V.; Marqusee, E.; et al. Long-term assessment of a multidisciplinary approach to thyroid nodule diagnostic evaluation. Cancer 2007, 111, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Cronan, J.J. Thyroid nodules: Is it time to turn off the US machines? Radiology 2008, 247, 602–604. [Google Scholar] [CrossRef] [PubMed]
- Uppal, N.; Collins, R.; James, B. Thyroid nodules: Global, economic, and personal burdens. Front. Endocrinol. 2023, 14, 1113977. [Google Scholar] [CrossRef]
- Bongiovanni, M.; Spitale, A.; Faquin, W.C.; Mazzucchelli, L.; Baloch, Z.W. The Bethesda system for reporting thyroid cytopathology: A meta-analysis. Acta. Cytol. 2012, 56, 333–339. [Google Scholar] [CrossRef] [PubMed]
- NICE. Thyroid Cancer: Assessment and Management; National Institute for Health and Care Excellence (NICE): London, UK, 2022. [Google Scholar]
- Larsen, L.V.; Egset, A.V.; Holm, C.; Larsen, S.R.; Nielsen, S.H.; Bach, J.; Helweg-Larsen, J.P.; Wanscher, J.H.; Godballe, C. Thyroid fine-needle aspiration and The Bethesda Classification System. Dan. Med. J. 2018, 65, A5456. [Google Scholar]
- Cibas, E.S. Bethesda System for Reporting Thyroid Cytopathology. Thyroid 2009, 19, 1159–1165. [Google Scholar] [CrossRef]
- Sidiropoulos, N.; Dumont, L.J.; Golding, A.C.; Quinlisk, F.L.; Gonzalez, J.L.; Padmanabha, V. Quality improvement by standardization of procurement and processing of thyroid fine-needle aspirates in the absence of on-site cytological evaluation. Thyroid 2009, 19, 1049–1052. [Google Scholar] [CrossRef]
- Fu, Y.; Sun, Y.; Pei, Q.; Han, X.; Qin, W.; Mei, F.; Tan, S.; Cui, L. Factors Influencing the Sample Adequacy of Ultrasound-Guided Fine-Needle Aspiration from Solid Thyroid Nodules for Liquid-Based Cytology: A Demographic, Sonographic, and Technical Perspective. Medicina 2022, 58, 1639. [Google Scholar] [CrossRef]
- Choi, S.H.; Han, K.H.; Yoon, J.H.; Moon, H.J.; Son, E.J.; Youk, J.H.; Kim, E.K.; Kwak, J.Y. Factors affecting inadequate sampling of ultrasound-guided fine-needle aspiration biopsy of thyroid nodules. Clin. Endocrinol. 2011, 74, 776–782. [Google Scholar] [CrossRef]
- Ullah, M.A.; Iqbal, J.; Ahmed, M.S.; Darira, J.; Lutfi, I.; Hamid, K.; Ali, M. Factors Responsible for Non-Diagnostic Cytology on Ultrasound-Guided Fine-Needle Aspiration of Thyroid Nodules. Cureus 2021, 13, e14955. [Google Scholar]
- Li, Y.; Yu, J.; Du, P.; Xie, Y.; Das, S.K.; Li, B.; Zhang, C. High-Score US-Suspicious Subcentimeter Thyroid Nodules: What Factors Affect Adequate Sampling of US-Guided Fine-Needle Aspiration Biopsy? Int. J. Endocrinol. 2020, 2020, 8464623. [Google Scholar] [CrossRef] [PubMed]
- Baskin, H.J. Ultrasound-guided fine-needle aspiration biopsy of thyroid nodules and multinodular goiters. Endocr. Pract. 2004, 10, 242–245. [Google Scholar] [CrossRef]
- Zhu, Y.; Song, Y.; Xu, G. The Bethesda System for Reporting Thyroid Cytopathology (TBSRTC): A report of 2, 781 cases in a Chinese population. Chin. J. Cancer. Res. 2020, 32, 140–148. [Google Scholar] [CrossRef]
- National Guideline Centre (UK). Ultrasound Guidance for Fine Needle Aspiration: Thyroid Disease: Assessment and Management; National Institute for Health and Care Excellence (NICE): London, UK, 2019. [Google Scholar]
- Pitman, M.B.; Abele, J.; Ali, S.Z.; Duick, D.; Elsheikh, T.M.; Jeffrey, R.B.; Powers, C.N.; Randolph, G.; Renshaw, A.; Scoutt, L. Techniques for thyroid FNA: A synopsis of the National Cancer Institute Thyroid Fine-Needle Aspiration State of the Science Conference. Diagn. Cytopathol. 2008, 36, 407–424. [Google Scholar] [CrossRef] [PubMed]
- Degirmenci, B.; Haktanir, A.; Albayrak, R.; Sahin, D.A.; Sahin, O.; Yucel, A.; Caliskan, G. Sonographically guided fine-needle biopsy of thyroid nodules: The effects of nodule characteristics, sampling technique, and needle size on the adequacy of cytological material. Clin. Radiol. 2007, 62, 798–803. [Google Scholar] [CrossRef]
- Banaka, I.; Kaltsas, G.; Antoniou, S.; Kanakis, G.; Zilos, A.; Baltas, C.S.; Thomas, D. Prognostic value of vascularity index for the diagnosis of autoimmune thyroid disease. J. Belg. Soc. Radiol. 2011, 94, 185–190. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yan, Y.; John, S.; Shaik, T.; Patel, B.; Lam, M.T.; Kabbani, L.; Mehrmohammadi, M. Photoacoustic-guided endovenous laser ablation: Characterization and in vivo canine study. Photoacoustics 2021, 24, 100298. [Google Scholar] [CrossRef]
- Wiacek, A.; Wang, K.C.; Wu, H.; Bell, M.A.L. Photoacoustic-Guided Laparoscopic and Open Hysterectomy Procedures Demonstrated with Human Cadavers. IEEE. Trans. Med. Imaging 2021, 40, 3279–3292. [Google Scholar] [CrossRef]
- Bell, M.A.L. Photoacoustic imaging for surgical guidance: Principles, applications, and outlook. J. Appl. Phys. 2020, 128, 060904. [Google Scholar] [CrossRef]
- Kim, C.; Erpelding, T.N.; Maslov, K.; Jankovic, L.; Akers, W.J.; Song, L.; Achilefu, S.; Margenthaler, J.A.; Pashley, M.D.; Wang, L.V. Handheld array-based photoacoustic probe for guiding needle biopsy of sentinel lymph nodes. J. Biomed. Opt. 2010, 15, 046010. [Google Scholar] [CrossRef]
- Jia, X.; Fan, K.; Zhang, R.; Zhang, D.; Zhang, J.; Gao, Y.; Zhang, T.; Li, W.; Li, J.; Yan, X.; et al. Precise visual distinction of brain glioma from normal tissues via targeted photoacoustic and fluorescence navigation. Nanomedicine 2020, 27, 102204. [Google Scholar] [CrossRef]
- Shubert, J.; Bell, M.A.L. A novel drill design for photoacoustic guided surgeries. In Photons Plus Ultrasound: Imaging and Sensing 2018; SPIE: Bellingham, WA, USA, 2018. [Google Scholar] [CrossRef]
- Shubert, J.; Bell, M.A.L. Photoacoustic imaging of a human vertebra: Implications for guiding spinal fusion surgeries. Phys. Med. Biol. 2018, 63, 144001. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Fujino, K.; Motooka, Y.; Gregor, A.; Bernards, N.; Ujiie, H.; Kinoshita, T.; Chung, K.Y.; Han, S.H.; Yasufuku, K. Photoacoustic imaging to localize indeterminate pulmonary nodules: A preclinical study. PLoS ONE 2020, 15, e0231488. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Huang, S.; Wu, Z.; Zheng, J.; Chen, X.; Nie, L. Label-Free Visualization of Early Cancer Hepatic Micrometastasis and Intraoperative Image-Guided Surgery by Photoacoustic Imaging. J. Nucl. Med. 2020, 61, 1079–1085. [Google Scholar] [CrossRef]
- Kempski, K.M.; Wiacek, A.; Palmer, J.; Graham, M.; González, E.; Goodson, B.; Allman, D.; Hou, H.; Beck, S.; He, J.; et al. In vivo demonstration of photoacoustic-guided liver surgery. In Photons Plus Ultrasound: Imaging and Sensing; International Society for Optics and Photonics: Bellingham, WA, USA, 2019; Volume 10878, p. 108782T. [Google Scholar]
- Miyata, A.; Ishizawa, T.; Kamiya, M.; Shimizu, A.; Kaneko, J.; Shimizu, A.; Kaneko, J.; Ijichi, H.; Shibahara, J.; Fukayama, M.; et al. Photoacoustic Tomography of Human Hepatic Malignancies Using Intraoperative Indocyanine Green Fluorescence Imaging. PLoS ONE 2014, 9, e112667. [Google Scholar] [CrossRef]
- Bungart, B.L.; Lan, L.; Wang, P.; Li, R.; Koch, M.O.; Cheng, L.; Masterson, T.A.; Dundar, M.; Cheng, J.-X. Photoacoustic tomography of intact human prostates and vascular texture analysis identify prostate cancer biopsy targets. Photoacoustics 2018, 11, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Moradi, H.; Tang, S.; Salcudean, S.E. Toward intra-operative prostate photoacoustic imaging: Configuration evaluation and implementation using the da Vinci research kit. IEEE Trans. Med. Imaging 2019, 38, 57–68. [Google Scholar] [CrossRef]
- Xi, L.; Zhou, G.; Gao, N.; Yang, L.; Gonzalo, D.A.; Hughes, S.J.; Jiang, H. Photoacoustic and fluorescence image-guided surgery using a multifunctional targeted nanoprobe. Ann. Surg. Oncol. 2014, 21, 1602–1609. [Google Scholar] [CrossRef]
- Xi, L.; Grobmyer, S.R.; Wu, L.; Chen, R.; Zhou, G.; Gutwein, L.G.; Sun, J.; Liao, W.; Zhou, Q.; Xie, H.; et al. Evaluation of breast tumor margins in vivo with intraoperative photoacoustic imaging. Opt. Express 2012, 20, 8726–8731. [Google Scholar] [CrossRef]
- Iskander-Rizk, S.; van der Steen, A.F.W.; van Soes, G. Photoacoustic imaging for guidance of interventions in cardiovascular medicine. Phys. Med. Biol. 2019, 64, 16TR01. [Google Scholar] [CrossRef] [PubMed]
- Graham, M.; Assis, F.; Allman, D.; Wiacek, A.; Gonzalez, E.; Gubbi, M.; Dong, J.; Hou, H.; Beck, S.; Chrispin, J.; et al. In vivo demonstration of photoacoustic image guidance and robotic visual servoing for cardiac catheter-based interventions. IEEE Trans. Med. Imaging 2020, 39, 1015–1029. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lan, B.; Sankin, G.; Zhou, Y.; Liu, W.; Xia, J.; Wang, D.; Trahey, G.; Zhong, P.; Yao, J.; et al. Simultaneous photoacoustic imaging and cavitation mapping in shockwave lithotripsy. IEEE Trans. Med. Imaging 2020, 39, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Kempski, K.M.; Wiacek, A.; Graham, M.; González, E.; Goodson, B.; Allman, D.; Palmer, J.; Hou, H.; Beck, S.; He, J.; et al. In vivo photoacoustic imaging of major blood vessels in the pancreas and liver during surgery. J. Biomed. Opt. 2019, 24, 1. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhao, L.; He, X.; Su, N.; Zhao, C.Y.; Tang, H.; Hong, T.; Li, W.; Yang, F.; Lin, L.; et al. Photoacoustic/ultrasound dual imaging of human thyroid cancers: An initial clinical study. Biomed. Opt. Express 2017, 8, 3449–3457. [Google Scholar] [CrossRef]
- Dima, A.; Ntziachristos, V. In-vivo handheld optoacoustic tomography of the human thyroid. Photoacoustics 2016, 4, 65–69. [Google Scholar] [CrossRef]
- Kim, J.; Park, B.; Ha, J.; Steinberg, I.; Hooper, S.M.; Jeong, C.; Park, E.Y.; Choi, W.; Liang, T.; Bae, J.S.; et al. Multiparametric photoacoustic analysis of human thyroid cancers in vivo. Cancer. Res. 2021, 81, 4849–4860. [Google Scholar] [CrossRef]
- Roll, W.; Markwardt, N.A.; Masthoff, M.; Helfen, A.; Claussen, J.; Eisenblätter, M.; Hasenbach, A.; Hermann, S.; Karlas, A.; Wildgruber, M.; et al. Multispectral Optoacoustic Tomography of Benign and Malignant Thyroid Disorders: A Pilot Study. J. Nucl. Med. 2019, 60, 1461–1466. [Google Scholar] [CrossRef]
- Kim, J.; Park, E.; Park, B.; Choi, W.; Lee, K.J.; Kim, C. Towards clinical photoacoustic and ultrasound imaging: Probe improvement and real-time graphical user interface. Exp. Biol. Med. 2020, 245, 321–329. [Google Scholar] [CrossRef]
- Dogra, V.S.; Chinni, B.K.; Valluru, K.S.; Moalem, J.; Giampoli, E.J.; Evans, K.; Rao, N.A. Preliminary Results of Ex Vivo Multispectral Photoacoustic Imaging in the Management of Thyroid Cancer. Am. J. Roentgenol. 2014, 202, W552–W558. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, X.; Rong, J.; Jiang, H. Photoacoustic Molecular Imaging Using Combined Acupuncture and Gold Nanorods as a Composite Contrast Agent. J. Innov. Opt. Health. Sci. 2019, 12, 1941004. [Google Scholar] [CrossRef]
- Wang, L.V. Photoacoustic Imaging and Spectroscopy; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Adler, D.D.; Carson, P.L.; Rubin, J.M.; Quinn-Reidl, D. Doppler ultrasound color flow imaging in the study of breast cancer; preliminary findings. Ultrasound Med. Biol. 1990, 16, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Guo, X.; Cui, R.; Wu, M.; Jiang, H. In Vivo hemodynamic Visualization of Berberine-Induced Effect on the Cerebral Cortex of a Mouse by Photoacoustic Tomography. Appl. Opt. 2019, 58, 1–8. [Google Scholar] [CrossRef]
- Jiang, H. Photoacoustic Tomography. Med. Imaging IEEE. Trans. 2015, 34, 2645. [Google Scholar]
- Kim, D.W. How to do it: Ultrasound-guided fine-needle aspiration of thyroid nodules that commonly result in inappropriate cytology. Clin. Imaging 2013, 37, 1–7. [Google Scholar] [CrossRef]
- Kandil, E.; Khalek, M.A.; Alabbas, H.; Moroz, K.; Islam, T.; Friedlander, P.; Jaffe, B.M. Comparison of ultrasound-guided biopsy technique for thyroid nodules with respect to adequacy of cytological material. ORL J. Otorhinolaryngol. Relat. Spec. 2011, 73, 177–181. [Google Scholar] [CrossRef]
- Kim, D.E.; Choo, H.J.; Park, J.S.; Lee, E.J.; Kim, S.H.; Jung, S.J.; Ryu, J.H. Ultrasonography-GuidedFine-Needle Aspiration Cytology for Thyroid Nodules: An Emphasis on One-Sampling and Biopsy Techniques. Diagn. Cytopathol. 2012, 40, E48–E54. [Google Scholar] [CrossRef]
- Todsen, T.; Bennedbæk, F.N.; Kiss, K.; Hegedus, L. Ultrasound guided fine-needle aspiration biopsy of thyroid nodules. Head Neck 2021, 43, 1009–1013. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, D.W.; Jung, S.J.; Baek, H.J. Factors that Influence Sample Adequacy in Liquid-Based Cytology after Ultrasonography-Guided Fine-Needle Aspiration of Thyroid Nodules: A Single-Center Study. Acta Cytol. 2018, 62, 253–258. [Google Scholar] [CrossRef]
- Ali, S.Z.; Cibas, E.S. The Bethesda System for Reporting Thyroid Cytopathology: Definitions, Criteria and Explanatory Notes, 2nd ed.; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Kwong, N.; Medici, M.; Angell, T.E.; Liu, X.; Marqusee, E.; Cibas, E.S.; Krane, J.F.; Barletta, J.A.; Kim, M.I.; Larsen, P.R.; et al. The influence of patient age on thyroid nodule formation, multinodularity, and thyroid cancer risk. J. Clin. Endocrinol. Metab. 2005, 100, 4434–4440. [Google Scholar] [CrossRef]
- Guth, S.; Theune, U.; Aberle, J.; Galach, A.; Bamberger, C.M. Very high prevalence of thyroid nodules detected by high frequency (13 MHz) ultrasound examination. Eur. J. Clin. Investig. 2009, 39, 699–706. [Google Scholar] [CrossRef]
- Kini, S.R. Thyroid Cytopathology: An Atlas and Text, 1st ed.; Lippincott Williams & Wilkins, a Wolters Kluwer Business: Philadelphia, PA, USA, 2008; pp. 17–26. [Google Scholar]
- Ozcan, U.A.; Atahan, S. Ultrasound-guided fine needle aspiration (USFNA) of thyroid nodules; does aspiration site matter? Iran. J. Radiol. 2015, 12, e8307. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Mao, M.; Zhan, W.; Zhou, J.; Zhou, W.; Yao, J.; Hu, Y.; Wang, Y.; Ye, T. Size and ultrasound features affecting results of ultrasound-guided fine-needle aspiration of thyroid nodules. J. Ultrasound. Med. 2018, 37, 1367–1377. [Google Scholar] [CrossRef]
- Moon, W.J.; Baek, J.H.; Choi, J.W.; Kim, Y.J.; Ha, E.J.; Lim, H.K.; Song, D.E.; Lee, J.H.; Shonget, Y.K. The value of gross visual assessment of specimen adequacy for liquid-based cytology during ultrasound guided, fine-needle aspiration of thyroid nodules. Endocr. Pract. 2015, 21, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.J.; Kwak, J.Y.; Kim, M.J.; Son, E.J.; Kim, E.-K. Can Vascularity at Power Doppler US Help Predict Thyroid Malignancy? Radiology 2010, 255, 260–269. [Google Scholar] [CrossRef]
- Ferreira, L.B.; Gimba, E.; Vinagre, J.; Sobrinho-Simões, M.; Soares, P. Molecular Aspects of Thyroid Calcification. Int. J. Mol. Sci. 2020, 21, 7718. [Google Scholar] [CrossRef]
- Lu, Z.; Mu, Y.; Zhu, H.; Luo, Y.; Kong, Q.; Dou, J.; Lu, J. Clinical Value of Using Ultrasound to Assess Calcification Patterns in Thyroid Nodules. World. J. Surg. 2010, 35, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhu, X.; Zou, X.; Yao, J.; Liang, J.; Huang, H.; Li, L.; Lin, L. Retrospective Analysis of Thyroid Nodules by Clinical and Pathological Characteristics, and Ultrasonographically Detected Calcification Correlated to Thyroid Carcinoma in South China. Eur. Surg. Res. 2009, 42, 137–142. [Google Scholar] [CrossRef]
- Kim, D.; Choi, Y.S.; Kwon, H.J.; Lee, J.S.; Heo, J.J.; Han, Y.J.; Park, Y.H.; Kim, J.H. Relationship between patterns of calcification in thyroid nodules and histopathologic findings. Endocr. J. 2012, 60, 155–160. [Google Scholar] [CrossRef]
- Yin, L.; Zhang, W.; Bai, W.; He, W. Relationship Between Morphologic Characteristics of Ultrasonic Calcification in Thyroid Nodules and Thyroid Carcinoma. Ultrasound. Med. Biol. 2020, 46, 20–25. [Google Scholar] [CrossRef]
- Hsiao, T.C.; Cheng, Y.Y.; Tein, W.T.; Luo, S.B.; Chiou, D.Y.; Chung, R.J.; Li, M.L. Deep-penetration photoacoustic array imaging of calcifications. J. Biomed. Opt. 2013, 18, 066002. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kang, J.; Chung, W.Y.; Kang, S.W.; Kwon, H.J.; Yoo, J.; Kim, E.K.; Chang, J.H.; Song, T.K.; Lee, S.; Kwak, J.Y. Ex vivo estimation of photoacoustic imaging for detecting thyroid microcalcifications. PLoS ONE 2014, 9, e113358. [Google Scholar] [CrossRef] [PubMed]
- Bartolotta, T.V.; Midiri, M.; Galia, M.; Runza, G.; Attard, M.; Savoia, G.; Lagalla, R.; Cardinale, A.E. Qualitative and quantitative evaluation of solitary thyroid nodules with contrast-enhanced ultrasound: Initial results. Eur. Radiol. 2006, 16, 2234–2241. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Jiang, J.; Du, X. Value of Contrast-enhanced Ultrasound in Diagnosis of Thyroid Papillary Carcinoma. Chin. J. Ultrasound Med. 2011, 27, 595–597. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).