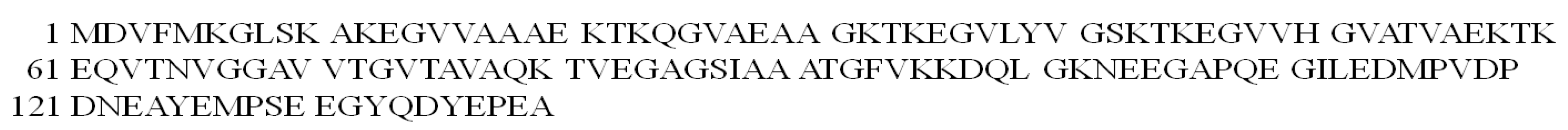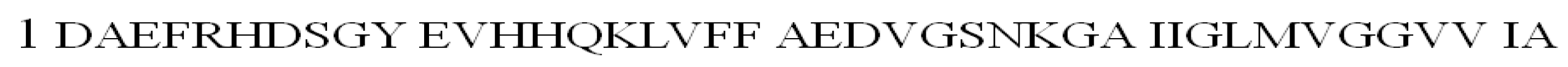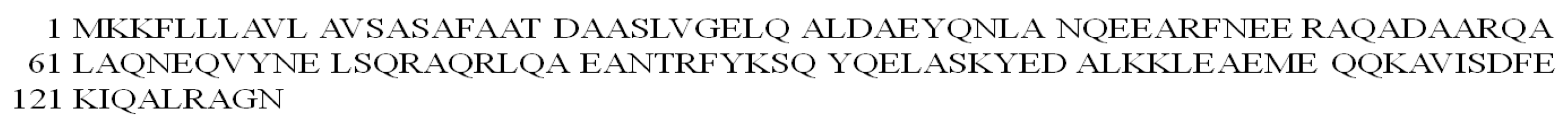Abstract
Copper ions bind to biomolecules (e.g., peptides and proteins) playing an essential role in many biological and physiological pathways in the human body. The resulting complexes may contribute to the initiation of neurodegenerative diseases, cancer, and bacterial and viral diseases, or act as therapeutics. Some compounds can chemically damage biological macromolecules and initiate the development of pathogenic states. Conversely, a number of these compounds may have antibacterial, antiviral, and even anticancer properties. One of the most significant current discussions in Cu biochemistry relates to the mechanisms of the positive and negative actions of Cu ions based on the generation of reactive oxygen species, including radicals that can interact with DNA molecules. This review aims to analyze various peptide–copper complexes and the mechanism of their action.
1. Introduction
Metal ions play a significant role in many physiological processes in the human body. Biologically active metal ions are considered as trace metals; however, their amounts are higher than those suggested in the literature. Copper (Cu), in particular, participates in a wide variety of molecular interactions and also in many catalytic reactions [1]. Disruption of Cu homeostasis is a pathological feature and a potential cause or contributor too many disease states [2]. Then again, copper ions coordinated with the appropriate ligands may show a therapeutic effect [3]. The mechanism of action of copper compounds seems to be still not completely explained [4,5]. However, there is a consensus among researchers that the labile Cu fractions exert their toxicity by generating reactive oxygen species (ROS) via the Haber–Weiss reaction, which is related to the presence of the Cu(II)/Cu(I) redox couple [3,6,7]. Therefore, the Cu(II)/Cu(I) interaction with DNA and oxidative stress induction due to reactive oxygen species (ROS) production are the two most considered modes of copper compounds’ actions [5,8].
ROS are chemical species derived from the partial reduction of O2 that can be either free radicals (superoxide (O2•–), hydroxyl (•OH), and hydroperoxyl (HOO•) radicals) having an unpaired electron, or non-radical species with a high oxidation potential, such as hydrogen peroxide (H2O2) and singlet oxygen (1O2) [9,10,11,12,13,14,15]. Reactive oxygen species (ROS) are involved in many biological and medical processes, ranging from neurodegenerative disorders and cancer to bacterial and viral diseases, and sometimes are of major commercial interest. They are important regulators of and secondary messengers in several cell-signaling pathways, including the reactive oxygen species-mediated death of different cells [16,17,18,19,20,21].
Due to their extremely reactive character, ROS react unselectively with the surrounding molecules [12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34].
Interestingly, reactive oxygen species can have both negative (dark side) and positive (bright side) effects on the human body. On the one hand, they can damage or destroy healthy cells leading to the initiation of many pathogenic processes; on the other hand, they play a crucial role as agents causing pathogen and cancer cell death [35].
Maintaining the balance between the overproduction of ROS and their elimination has a huge impact on the proper functioning of cells and redox processes [36]. However, if the balance is disturbed, e.g., in the case of inflammation, hyperoxia, or when the antioxidant defense fails, the excess of ROS contributes to the initiation of various pathological conditions. The most common of these include neurodegenerative diseases, cancer, and respiratory system diseases [37,38]. Various mechanisms of ROS action, leading to healthy cell destruction have been proposed. ROS can directly interact with lipids, nucleic acids, and proteins and cause their oxidation. During lipid peroxidation, malondialdehyde (MDA) and 4-hydroxynonenal (4-HNE) are formed [39,40]. Additionally, the interaction of MDA with DNA and/or proteins can lead to mutagenesis [37,41]. In turn, the contact of ROS with nucleic acid leads to DNA degradation by single and/or double DNA strand breakage, damage to deoxyribose, and modification of DNA bases. This action results in dysfunctional or maladaptive apoptosis and/or necrosis of healthy cells [37,42,43].
The above mentioned typical biochemical changes in the disease sites were inspiration to exploit unbalanced ROS levels for new drug development [6,7]. In general, low or moderate levels of ROS induce the non-specific damage of proteins, lipids and DNA and destroy bacteria, viruses, cancer, and fungus cells [44]. Sometimes, photosensitizes can be used simultaneously for synergistic therapeutic efficacy, which are one of the representative exogenous ROS sources and have the ability to excite oxygen to its singlet state by using exogenous light energy [45,46]. Other beneficial effects of ROS involve their physiological roles in the functioning of a number of cellular signaling systems [47]. Their production by non-phagocytic NADPH oxidase isoforms plays a key role in the regulation of intracellular signaling cascades in various types of non-phagocytic cells including fibroblasts, endothelial cells, vascular smooth muscle cells, cardiac myocytes, and thyroid tissue [48,49].
This review is related to medicine, where ROS produced by copper complexes can have both deleterious and beneficial effects.
2. Dark Side of Copper–Peptide Complexes
2.1. Neurodegenerative Disorders
2.1.1. Tau (T) Protein
Tau protein is found in the brain and consists of 441 amino acid residues (Figure 1). Among them, there are 12 histidyl residues with high affinity to metal ions. The main function of the T protein is the stabilization of neuronal microtubules, as well as the enhancement of axon transport [50,51]. This shows how important the role that the Tau protein plays is in transmitting messages inside the brain. Research suggests that the T protein may be involved in the development of neurodegenerative disorders, especially Alzheimer’s disease (AD). Various metal ions have been shown to contribute to protein hyperphosphorylation. In the case of the Tau protein, this process results in the formation of neurofibrillary tangles and thus the deposition of the Tau protein in the brain. The concentration of transition metal ions, their oxidation state, and the redox reactions they catalyze have a decisive influence on the degree of T protein aggregation [52,53].

Figure 1.
The amino acid sequence of the Tau protein in the one-letter code.
Moreover, it has been confirmed that oxidative stress is strongly associated with the pathology of the T protein as it can generate reactive oxygen species (ROS) in the mitochondria. Studies have shown that Tau phosphorylation in mice causes mitochondrial dysfunction leading to H2O2 formation, lipid peroxidation, and ultimately loss of neurons [54,55]. The aggregation and misfolding of the T protein cause tauopathies (understood to include all neurodegenerative disorders) [56,57]. The most common tauopathy is Alzheimer’s disease, which mainly affects the elderly.
Therefore, many scientists have studied the effect of metal ion chelation (mainly copper(II) ions) on Tau protein fragments and the impact of the resulting complexes on the initiation of neurodegenerative diseases. The ability of two selected fragments (275VQIINKKLDLSNVQSKCGSKDNIKHVPGGGS305 (L1; Figure S1A) and 306VQIVYKPVDLSKVTSKCGSLGNIHHKPGGGQ336 (L2; Figure S1B)) of the T protein to bind Cu(II) ions was determined. The following were selected as potential donor atoms of both ligands to participate in Cu(II) ion binding: the imidazole nitrogen atom of the histidyl residue, the amide nitrogen atom of the peptide bond, and oxygen-based donor atoms [58]. It was also investigated whether the binding of the Cu(II) ion to the L1 and L2 peptides, being fragments of the Tau protein, stimulates the generation of reactive oxygen species (ROS).
Both peptides, after the coordination of a Cu(II) ion can produce a hydroxyl radical (•OH) and hydrogen peroxide (H2O2). It was also confirmed that during the reaction, Cu(II) ions are reduced to Cu(I) ions and both peptides (L1 and L2) tend to aggregate [58]. Moreover, the L2 ligand is responsible for filament formation after the addition of the metal ion and ascorbic acid (reducing agent) [59]. The obtained results show that metal ion chelation by peptides derived from the Tau protein may influence the initiation of Alzheimer’s disease.
2.1.2. α-Synuclein (αSyn) Protein
The 140 amino acid alpha-synuclein (αSyn) protein is located at the presynaptic terminals in the brain (Figure 2). This protein is responsible for dopamine metabolism, binding to membranes, and the recycling of synaptic vesicles [60,61]. The presence of Lewy bodies (intracellular inclusions) consisting of protein aggregates suggests αSyn neurotoxicity [62]. The formation of prefibrillary oligomers of this protein causes Parkinson’s disease [63].

Figure 2.
The amino acid sequence of the α-synuclein protein in the one-letter code.
Most importantly, the αSyn protein shows a high affinity for Cu(II) ions, binding these metal ions in micromolar concentration solutions [64]. It is well known that the presence of metal ions such as Cu(II) increases the aggregation of αSyn. This mechanism is related to copper redox chemistry and ROS production. Structural protein changes are a common phenomenon seen in the brain of Parkinson’s patients after their death. Therefore, many recent studies have focused on the redox chemistry of Cu(II) complexes with peptides that are fragments of the αSyn protein [65].
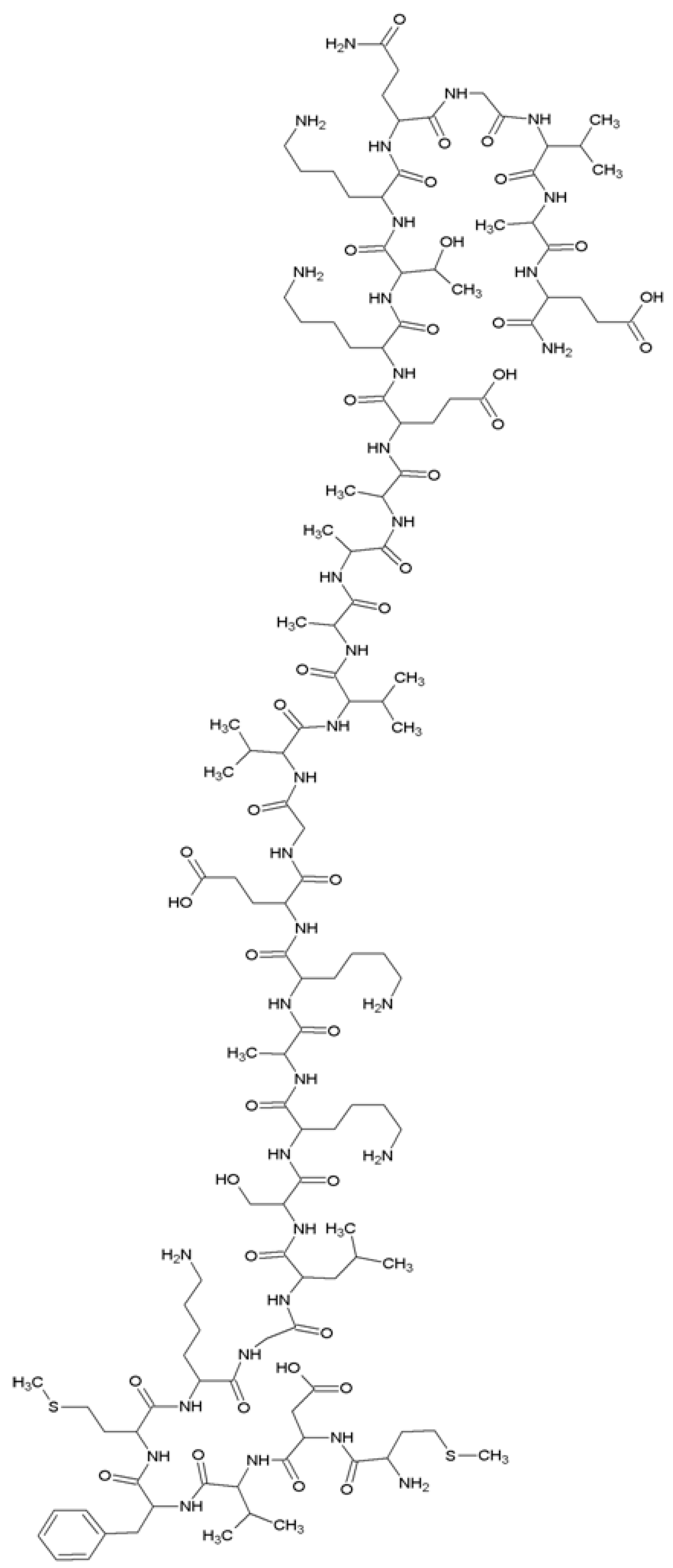
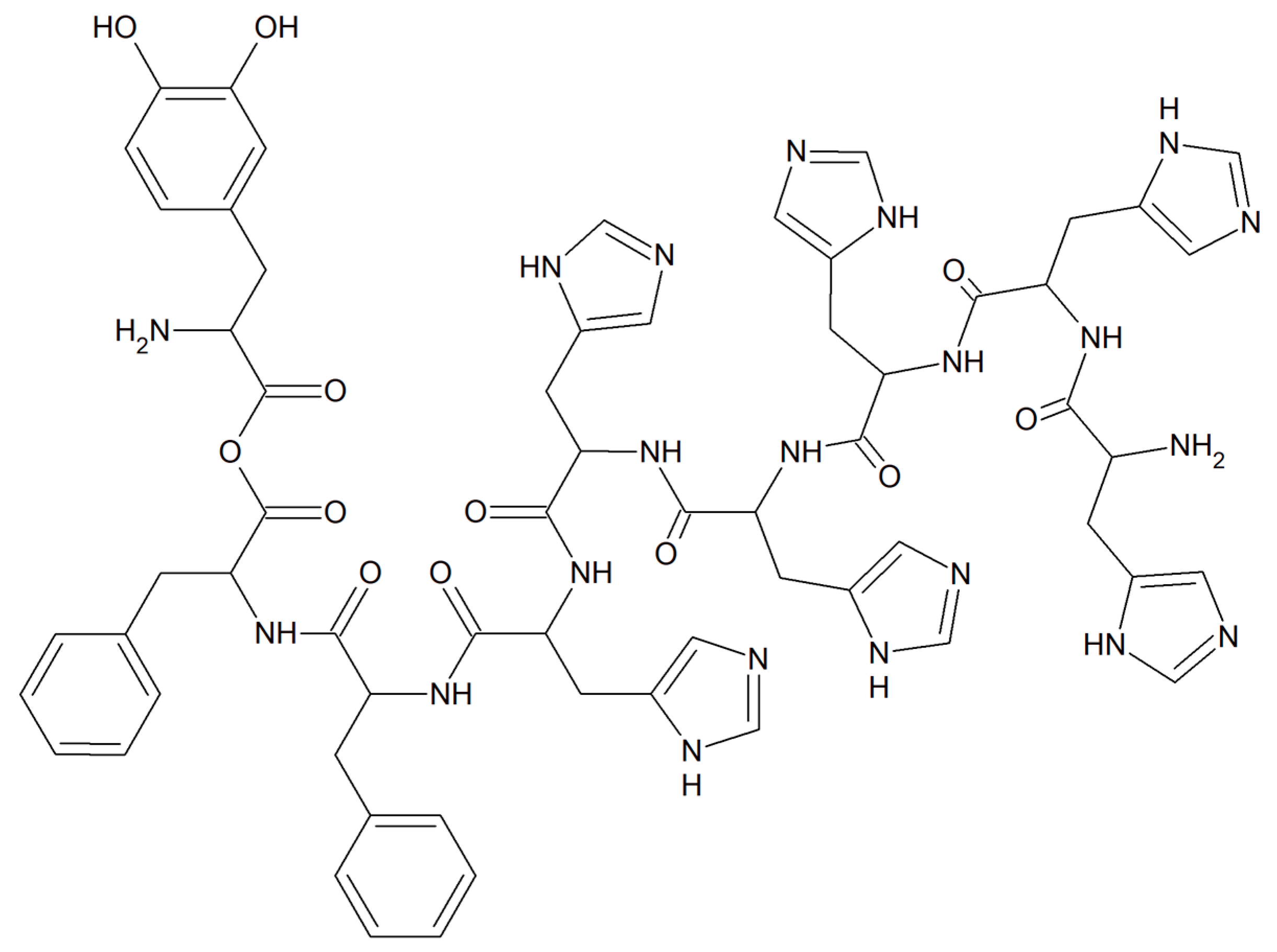
Coordination studies of Cu(II) ions with αSyn protein fragments: MDVFMKGLSKAKEGVVA-NH2 (L3, αSyn 1–17) and MDVFMKGLSKAKEGVVAAAEKTKQGVAE-N7H2 (L4, αSyn 1–28) were carried out (Figure 3).
Figure 3.
The structural formula of the MDVFMKGLSKAKEGVVAAAEKTKQGVAE-NH2 (L4, αSyn 1–28) peptide.
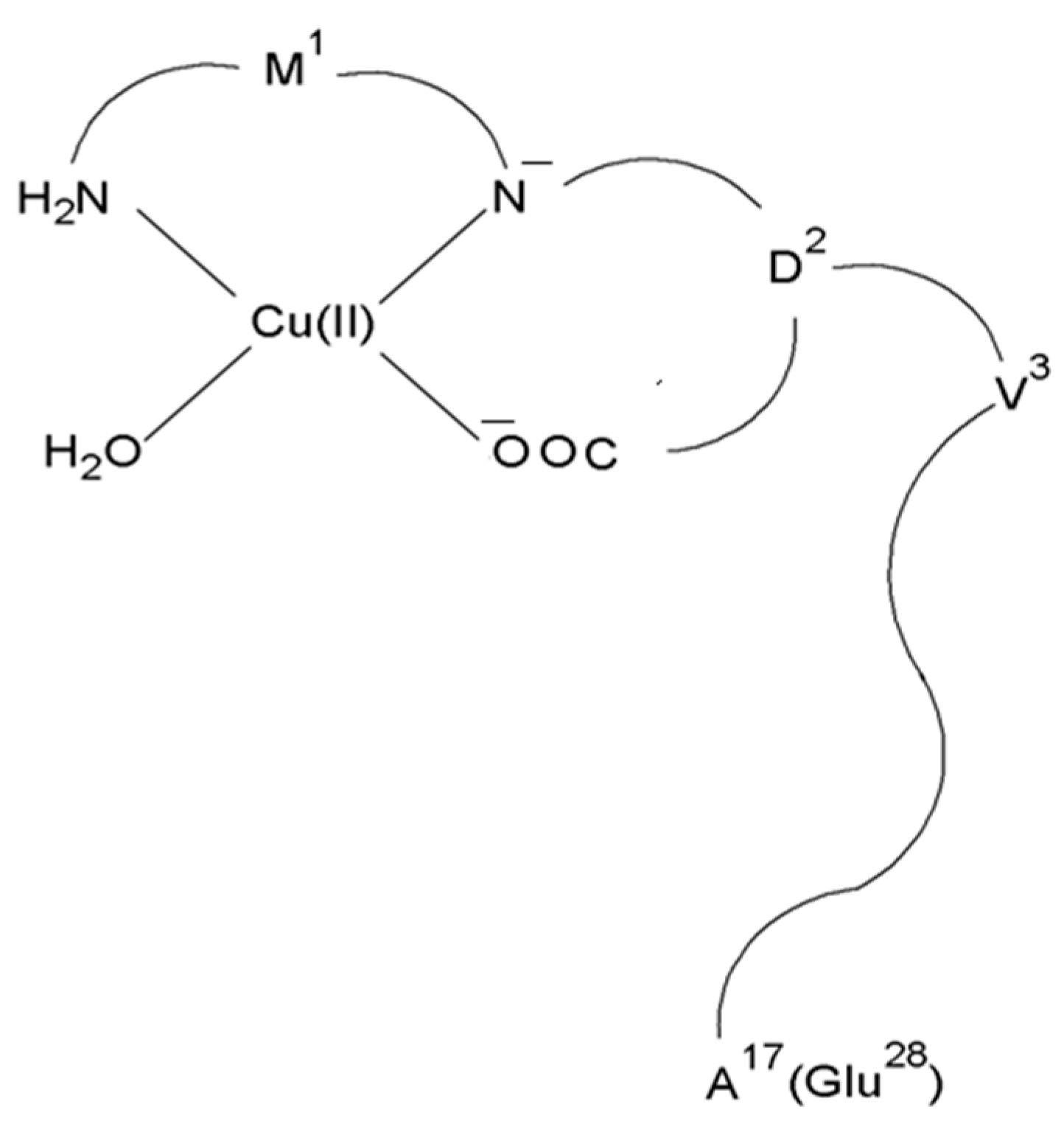
In both cases, the CuH2L complex with the N2O2 (NH2, N−, COO−, H2O) donor set dominates in the solution at pH 7.4 (Figure 4) [66].
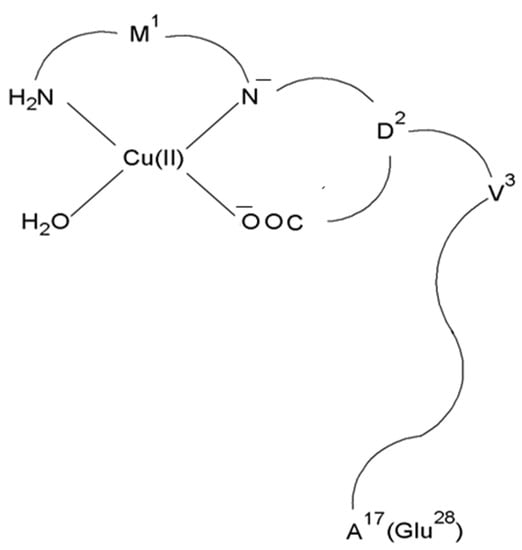
Figure 4.
Schematic representation of the structure of mononuclear CuH2L species of Cu(II)–L3 and Cu(II)–L4 complexes.
To confirm that the L3 and L4 ligands after binding the metal ion are capable of generating ROS (in particular the production of hydroxyl radicals) that immediately oxidize the amino acid residues in the peptide, a metal-ion-catalyzed oxidation (MCO) experiment was performed [67]. Oxidative modifications of peptides are closely related to neurodegenerative disease. Abnormal protein aggregation caused by ROS production contributes to the death of nerve cells. Both methionine residues in the L3 and L4 peptides were oxidized to methionine sulfoxides in the presence of hydrogen peroxide [67]. This step of oxidation was reversible under physiological conditions, but further oxidation (two methionine residues for the L3 ligand and one methionine residue for the L4 peptide) yields the sulfone(s) and its products can then accumulate in the tissues and cause Parkinson’s disease [68].
2.1.3. Presenilin 1 (Prs1) Protein
Presenilin 1 (Prs1) is a protein composed of 467 amino acid residues (Figure 5). This protein is found in the brain in the cell membrane, Golgi apparatus, and endoplasmic reticulum [69]. Mutations in the Prs1 protein have been suggested to cause Familial Alzheimer’s disease (FAD). Mitochondrial dysfunction leading to oxidative stress often occurs in the early stages of Alzheimer’s disease [70,71].

Figure 5.
The amino acid sequence of the Presenilin1 protein in the one-letter code.
The role of the Prs1 protein is to transport Cu(II) ions and maintain its homeostasis [72]. As is well known, the human brain is a source of large amounts of Cu(II) ions. Thus, there is a hypothesis that Cu(II) ions can coordinate to the Prs1 protein and participate in the Fenton reaction causing the formation of hydroxyl radicals [73], which can have implications for the development of Alzheimer’s disease.
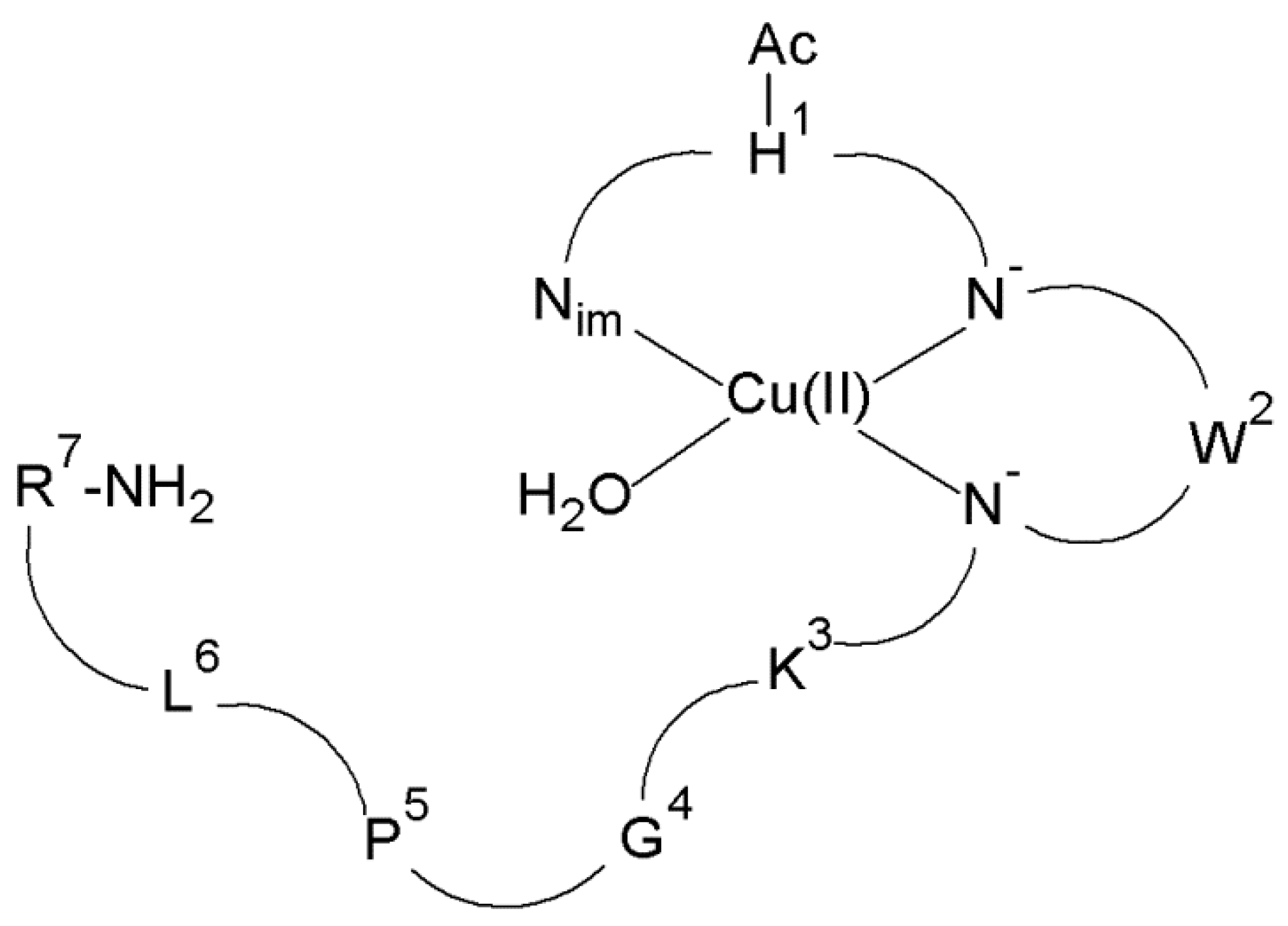
The ability of the Ac-HWKGPLR-NH2 (L5, Prs1 214–220) peptide (Figure 6), being a 214–220 fragment of the Prs1 protein, to produce ROS after binding Cu(II) ions was investigated.
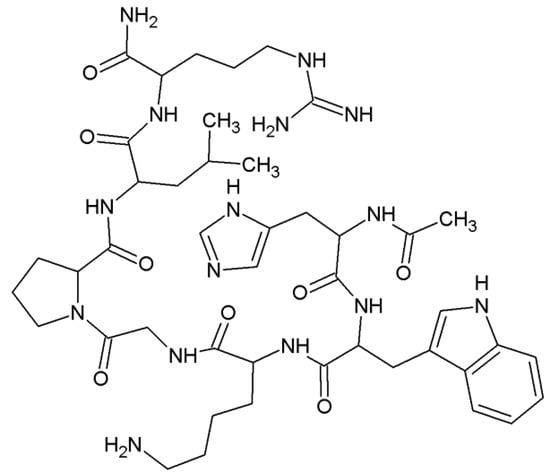
Figure 6.
The structural formula of the Ac-HWKGPLR-NH2 (L5, Prs1 214–220) peptide (Ac—acetyl group).
In the first step, the structure of the complexes present at the intracellular pH of the brain (pH 7.2) was determined. It has been proved that at pH 7.2 there are CuH-1L and CuH-2L species for the Cu(II)–L5 system. In the case of the CuH-1L species, the metal ion is coordinated by three donor atoms (N3 (Nim, 2N−)) (Figure 7), while in the CuH-2L complex, four nitrogen atoms (N4 (Nim, 3N−)) fill the equatorial plane around the Cu(II) ion [73].
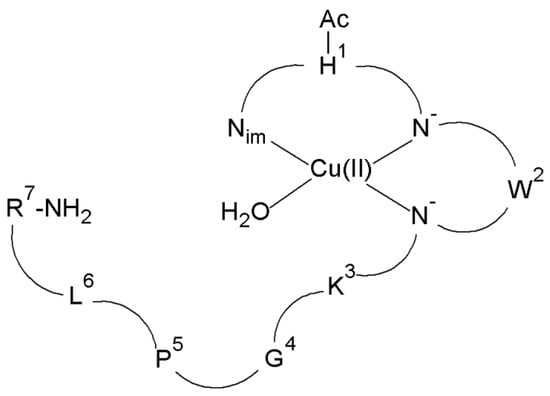
Figure 7.
Schematic representation of the structure of mononuclear CuH-1L species of Cu(II)–L5 complex (Ac—acetyl group).
Interestingly, the Ac-HWKGPLR-NH2 ligand, after binding the metal ion at pH 7.2, produces a hydroxyl radical and singlet oxygen. These two types of ROS are formed during the reaction in the presence of either hydrogen peroxide or ascorbic acid (also present in the brain). Moreover, the Cu(II)–L5 complex participates in the DNA degradation process [73]. The obtained results prove that the binding of the Cu(II) ion by a Prs1 protein fragment activates the generation of ROS and biological macromolecule damage which may be related to the onset of neurodegenerative disease.
2.1.4. β-Amyloid (Aβ) Protein
The β-amyloid peptide is an amyloid precursor protein (APP protein) fragment that consists of 40–43 amino acid residues. Aβ40 is most common in the brain, while Aβ42 is responsible for the formation of senile plaques (a hallmark of AD) in all Alzheimer’s patients (Figure 8) [74,75]. The additional two hydrophobic amino acid residues, isoleucine and alanine, increase fibril formation for Aβ42 and make this peptide more toxic than Aβ40 [75].
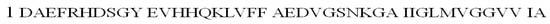
Figure 8.
The amino acid sequence of the β-amyloid 42 (Aβ42) peptide in the one-letter code.
Insoluble amyloid plaques were found in the brain tissues of AD patients. It was confirmed that they consisted mainly of the Aβ protein. β-Amyloid deposition is a key process in nerve death and the triggering of Alzheimer’s disease [76]. Since amyloid plaques contain a high concentration of Cu(II) ions (about a twofold increase in the copper concentration in the cerebrospinal fluid), a mechanism based on the initiation of Alzheimer’s disease after the coordination of Cu(II) ions to Aβ peptide has been postulated [76,77]. It has been suggested that the neurotoxicity of the Cu(II)–Aβ complex in Alzheimer’s disease is due to the production of ROS. The formation of hydrogen peroxide participating in the Fenton reaction is associated with the conversion of Cu(II) ions to Cu(I) ions [77].
Coordination studies of the DAEFRHDSGYEVHHQK (L6, Aβ 1–16) and DAEFRHDSGYEVHHQKLVFFAEDVGSNK (L7, Aβ 1–28) (Figure S2) peptides with Cu(II) ions were conducted. At pH 7.4, the complex with N3O1 (NH2, COO−, 2Nim) donor set is present in the aqueous solution for both tested ligands [76].
In the next step, the ability of Cu(II)–L6 and Cu(II)–L7 complexes to participate in the Fenton reaction was tested. Research has shown that the binding of Cu(II) ions to the peptide and the presence of hydrogen peroxide leads to the formation of free radicals, which by attacking amino acid residues near the bonding center cause their oxidation [74]. Other studies have shown that both Cu(II)–L6 and Cu(II)–L7 complexes can be reduced to Cu(I) complexes. The latter participate in the formation of hydrogen peroxide by catalyzing the reduction of oxygen [78].
2.1.5. Cellular Prion (PrPC) Protein
The unprocessed cellular prion protein (PrPC) in humans comprises 253 amino acid residues (Figure 9) [79]. This protein occurs in many parts of our body, but its highest expression is found in the peripheral and central nervous system [80]. The misfolded PrPC conformer known as PrPSC (SC-scrapie) is responsible for prion diseases known as transmissible spongiform encephalopathies (TSEs). These constitute a family of progressive neurodegenerative disorders in both humans and animals [79]. The pathogenicity of PrPSC is related to its aggregation [81].

Figure 9.
The amino acid sequence of the cellular prion protein (PrPC) in the one-letter code.
Investigations have indicated that metal ions such as copper(II) can not only affect the proper functioning, but also the conformational changes of the prion protein [82]. Therefore, Cu(II) complexes with prion protein fragments have witnessed growing academic interest.
The structure of Cu(II) complexes with Ac-HGGG-NH2 (L8, PrPC 61–64) and Ac-PHGGGWGQ-NH2 (L9, PrPC 60–67; having the –HGGG- sequence) peptides was determined (Figure S3). At physiological pH, both peptides bind Cu(II) ions and form CuH-2L species. In these complexes, three nitrogen atoms (Nim, 2N−) complete the equatorial plane around the metal ion [83].
Moreover, a metal-catalyzed oxidation (MCO) experiment established that reactive oxygen species generated at the metal center oxidize the histidine and tryptophan residues in the L9 peptide [84].
2.2. Cancer
2.2.1. Major Outer Membrane (FomA) Protein
The major outer membrane (FomA) protein of the Fusobacterium nucleatum (Fn) bacterium consists of 370 amino acid residues (Figure 10) [85]. The FomA protein plays an important role in biofilm formation and mediates the co-aggregation of Fn with Gram-negative bacteria, especially Porphyromonasgingivalis, and is directly involved in binding to Streptococussanguis on the tooth surface [86,87].

Figure 10.
The amino acid sequence of the major outer membrane (FomA) protein in the one-letter code.
When the gums are injured, the Fn migrates from the oral cavity to the colon. This bacterium is strongly associated with colorectal cancer [88]. Evidence suggests that the bacterial FomA protein binds Cu(II) ions (the concentration of which increases significantly during inflammation [89]) and in the presence of hydrogen peroxide (present in intestinal areas) is involved in the initiation of colorectal cancer [90].
The structure of the formed Cu(II) complexes with various protein fragments (e.g., Ac-KGHGNGEEGTPTVHNE-NH2 (L10, FomA 203–218) and cyclo(KGHGNGEEGTPTVHNE) (cycloL10, FomA 203–218)) was determined. For both studied ligands, the CuHL and CuH−1L complexes are formed at the pH value of the intestinal environment. For the CuHL species, the following N2 (2Nim) donor set occurs in aqueous solution. The equatorial plane around the Cu(II) ion for the CuH−1L species is built of N4 (2Nim, 2N−) donor set [91].
The L10 and cycloL10 ligands after chelation of Cu(II) ions can produce •OH and singlet oxygen (1O2), which damage DNA. Both stimulate extra- and intracellular ROS formation. The ROS identified inside the mouse colon carcinoma cell (CT26) was the hydroxyl radical [92]. This radical can induce lipid peroxidation (increasing the concentration of malondialdehyde (MDA)) inside the cell. The obtained results therefore suggested that the effect of the binding of the metal ion to the peptide ligand may have negative effects in the form of ROS formation, which may lead to the initiation of the carcinogenesis process at a later stage.
2.2.2. Adhesion (FadA) Protein
The FadA protein is one of the adhesion proteins of the above-mentioned Fusobacterium nucleatum bacterium responsible for interaction with human cells. It comes in two forms: (i) 129 amino acid non-secreted pre-FadA (Figure 11) and (ii) 111 amino acid secreted mature FadA (mFadA) [93,94].
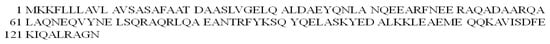
Figure 11.
The amino acid sequence of the pre-FadA protein in the one-letter code.
Recent studies have proven that the FadA protein interacts with E-cadherins and activates β-catenins, triggering the proliferation of cancer cells [95].
There is evidence that ATDAAS-NH2 (L11, FadA 19-24) (Figure S4A) and MKKFL-NH2 (L12, FadA 1–5) (Figure S4B), being fragments of the FadA protein, can produce ROS after chelation of the Cu(II) ion [96].
At the physiological pH of the colon (pH 6.8), the Cu(II) ion is bound by N3O1 (COO−, NH2, 2N−) donor set in CuH-1L species for the ATDAAS-NH2 ligand. In the case of the MKKFL-NH2 peptide, the CuL complex with the N3 (NH2, 2N−) donor set is present in the solution [96]. Both complexes generate singlet oxygen, hydroxyl radical and superoxide anion radical in the system and thus possibly have the potential to induce the process of colorectal carcinogenesis. Interestingly, the Cu(II)–MKKFL-NH2 complex is more effective in facilitating DNA degradation than the Cu(II)–ATDAAS-NH2 compound [96].
2.3. Respiratory System Diseases
Spike (S) Protein
The spike protein, which consists of 1273 amino acid residues, is present on the protein-lipid envelope of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2 virus) (Figure 12) [97]. The S protein interacts with the angiotensin-converting enzyme 2 (ACE2) present on the host cell surface and allows the virus to enter the cell [98]. There has been the suggestion that the pathology of the coronavirus disease 2019 (COVID-19) caused by SARS-CoV-2 is related to the S protein [99].

Figure 12.
The amino acid sequence of the spike (S) protein in the one-letter code.
Of particular concern is that metal ions that bind to viral proteins play a key role in the pathogenesis of the virus [100]. In particular, trace metal ions such as copper influence the course of a viral infection and its consequences. The spike protein of SARS-CoV-2 triggers inflammation [101] and during inflammation, the concentration of Cu(II) ions rises significantly. The possibility of metal ions binding to the spike protein and resulting in Cu(II) complexes might be involved in cell damage [102].
It is well established that the lungs are strongly affected by SARS-CoV-2 [103]. K18-hACE2 transgenic mice injected with the S1 subunit of the spike protein showed histological evidence of lung injury [104]. A high concentration of Cu(II) is observed in lungs when they are infected with a pathogen [102,105]. Studies in mice have shown that these easily accessible Cu(II) ions can bind to the spike protein and initiate ROS formation in lungs. This leads to oxidative stress, cell death (through apoptosis), and subsequent inflammatory reactions. The latter causes pulmonary lesions [102].
An increased ROS level is the major cause of viral replication and the pathological condition. The ROS level was significantly elevated in the cells treated with the spike protein of SARS-CoV-2 and hence ROS are speculated to play a large role in SARS-CoV-2 infection. They are responsible for the aggravation of the disease and its progression. This induces apoptosis of lung cells leading to acute respiratory distress syndrome (ARDS), and even death in COVID-19 patients [106]. The increased ROS level is also a characteristic of other respiratory diseases such as, e.g., acute pneumonia [107]. Research also suggests that oxidative stress is very common in patients infected with RNA viruses [108]. Moreover, in many cases, such as, e.g., in hepatitis C (HCV virus), proteins are strongly associated with extended ROS production. In hepatitis C patients, an abnormally high level of copper was also observed [108,109]. Therefore, it is likely that the spike protein fragments after metal ion chelation may be involved in the Fenton reaction and the induction of oxidative stress. In turn, the oxidative stress can lead to lung damage in COVID-19.
3. Bright Side of Copper–Peptide Complexes
3.1. Cancer Treatment
3.1.1. Copper Complexes with Amino Acids and Peptide
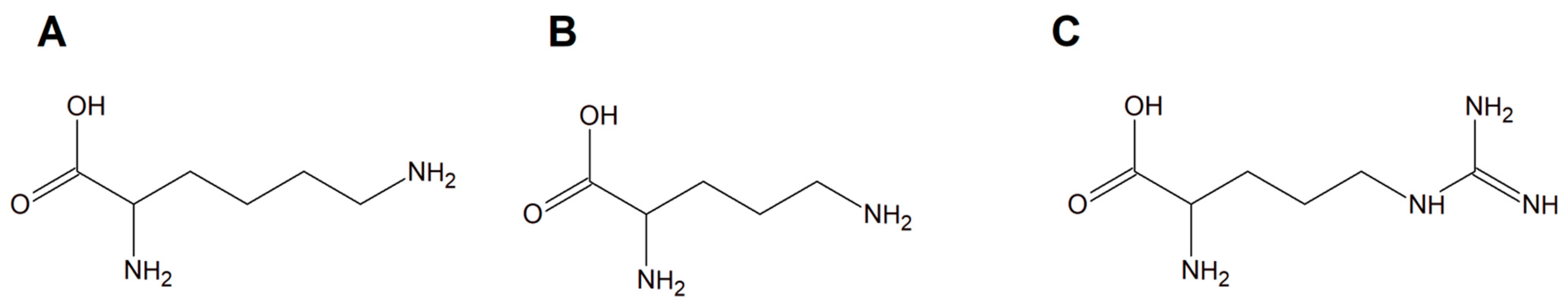
Copper(II) complexes with amino acids (AC) are a group of chemicals extensively studied with respect to various cancer cells. The literature is dominated by many examples of Cu(II) heteroleptic inorganic compounds especially with lysine, arginine (Figure 13A,C), and phenanthroline derivatives. Good examples are those with lysine and ornithine (Figure 13B); phenanthroline and bipyridine as the N,N′ coordinating bases give the classical complexes [Cu(Lys)(phen)(H2O)]2+ and [Cu(Orn)(bipy)(H2O)]2+ having potential anticancer properties.
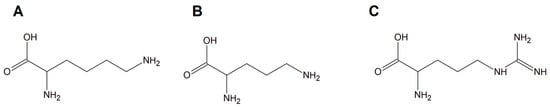
Figure 13.
Schematic structures of amino acids (A) lysine, (B) ornithine, and (C) arginine.
It was proven that some of these inorganic compounds are able to interact with deoxyribonucleic acid molecules by releasing their ligands, leading to Cu(II)/Cu(I) redox processes that initiate the formation of reactive oxygen species (ROS) in vivo, especially hydroxyl radicals [110,111]. This phenomenon suggested that these compounds may act as prodrugs having significant potential towards various cancer cell types [111]. Among the copper(II) complexes, a group with L−arginine (L−Arg; [Cu(L−Arg)2](NO3)2 and [Cu(L−Arg)(B)Cl]Cl⋅2.5H2O complexes (B = heterocyclic base)) was a remarkable idea for potential anticancer drugs [112,113]. Moreover, these compounds exhibited interesting antibacterial and antifungal activity. Importantly, they were found to act as strong minor groove binder agents causing plasmid DNA damage via a ROS-dependent action mode [113,114,115,116,117].
Interestingly, studies on the biological activity of different L−arginine copper(II) complexes with addition of phenanthroline molecules into the coordination sphere i.e., ([Cu(L-Arg)2(μ-4,4′-bpy)]Cl2⋅3H2O}(4,4′−bpy = 4,4′−bipyridine), [CuCl(L-Arg)(phen)]Cl·2H2O (phen = 1,10−phenanthroline) and [Cu(L-Arg)2(H2O)]C2O4·6H2O (C2O42--oxalate counter ion) have shown that these complexes were inactive towards cancer cells but exhibited antimicrobial activity by increasing oxidative stress [112,118,119]. Different groups of L−arginine copper(II) complexes ([Cu(L−Arg)2(NCS)](NCS)·H2O and [Cu(L−Arg)(NCS)2]) were intensively examined due to their therapeutic potential and they exhibited strong anticancer properties towards the A549 cell line (human lung epithelial carcinoma). The authors proved that [Cu(L−Arg)2(NCS)]+ is the only species in aqua solution (pH around 7.0) of the investigated inorganic compound [Cu(L−Arg)2(NCS)](NCS)·H2O. Importantly, it was proven that these species most probably are minor groove-binding agents. Additionally, they are able, in the presence of H2O2, to facilitate reactive oxygen species (ROS) generation and consequently damage of plasmid DNA. ROS are well recognized as mediators of DNA damage. They can cause double strand breaks (DSBs) of DNA through direct high-energy damage to the sugar backbone of DNA, and also through free radicals generated in cells—mostly ●OH [120]. Chemotherapeutics increase the ROS levels, which contributes to their genotoxicity [116,117]. ROS have also been reported to directly induce other forms of deoxyribonucleic acid damage through oxidation of nucleoside bases (e.g., 8-oxo guanine can be formed) [121]. Most probably these processes are the main ones involved in the anticancer mode of action of these copper complexes [122,123,124].
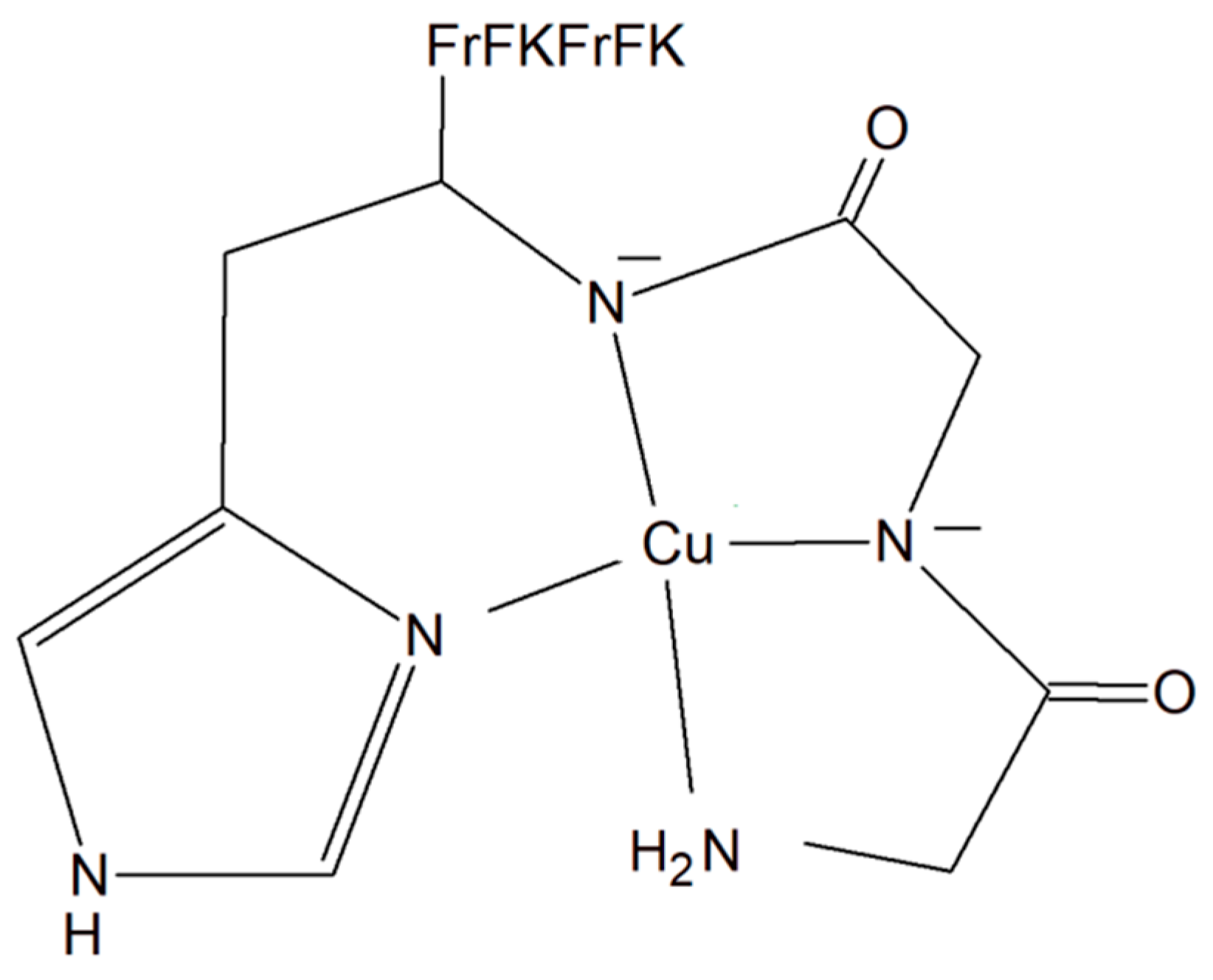
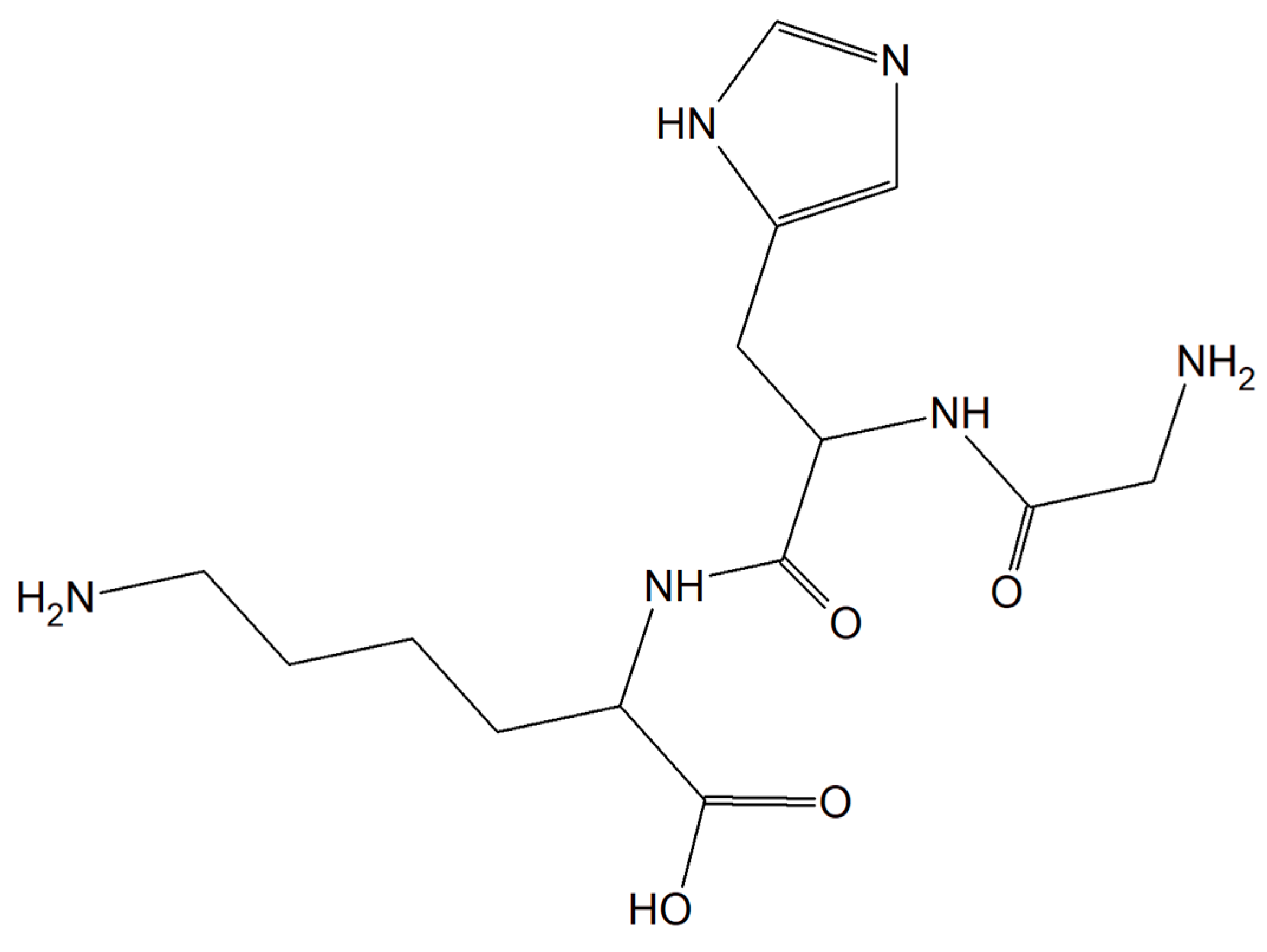
A copper–peptide complex consisting of a domain GGH (Gly-Gly-His) able to efficiently bind copper ions and the peptide (MPP) FrFKFrFK-CONH2 (Phe-r-Phe-Lys-Phe-r-Phe-Lys-CONH2, where r = D-arginine) was designed. It turned out to be well known for its mitochondria-penetrating properties (Figure 14). This inorganic compound is highly active towards various cancer cell lines, especially HeLa cells. It is worth noting that these cells are able to facilitate the selective intracellular uptake of Cu(II) ions. When the Cu–peptide compound reaches the mitochondria, the Fenton reaction is induced. As a result of this reaction, the reactive ●OH radical is produced that can induce cancer cell apoptosis [125].
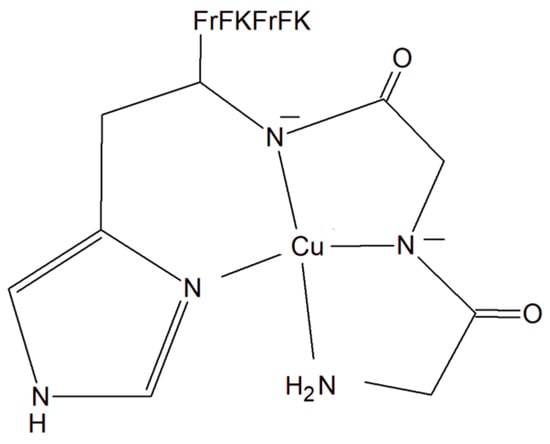
Figure 14.
Copper–peptide complex consisting of GGH (Gly-Gly-His) and mitochondria-penetrating peptide (MPP) FrFKFrFK-CONH2 (Phe-r-Phe-Lys-Phe-r-Phe-Lys-CONH2, where r = D-arginine).
In modern medicine, especially in the field of novel broad-spectrum anticancer drugs, host defense peptides (HDPs) are attracting great attention. Metallating HDPs with Cu2+ is an effective, smart and novel synthetic strategy to increase the cytotoxicity of copper complexes against cancerous cells. It was found that peptidic (piscidins 1 (P1) and 3 (P3)) Cu(II) complexes not only physically but also chemically, with the help of reactive oxygen species, damage lipid membranes [126,127,128,129,130,131]. Interestingly, P1 seems to be a much more potent antimicrobial agent [127,130,132]. Additionally, this fragment is also active towards viruses such as HIV-160, coronaviruses [133], and pseudorabies [134]. However, some cytotoxic properties of both peptide fragments P1 and P3 against several cancerous cell lines were reported by Lin et al. (2012) [134]. The authors found that P1 can induce apoptosis in HT1080 cells supported by radical generation [126].
3.1.2. Copper Complexes with Peptide and Diimines
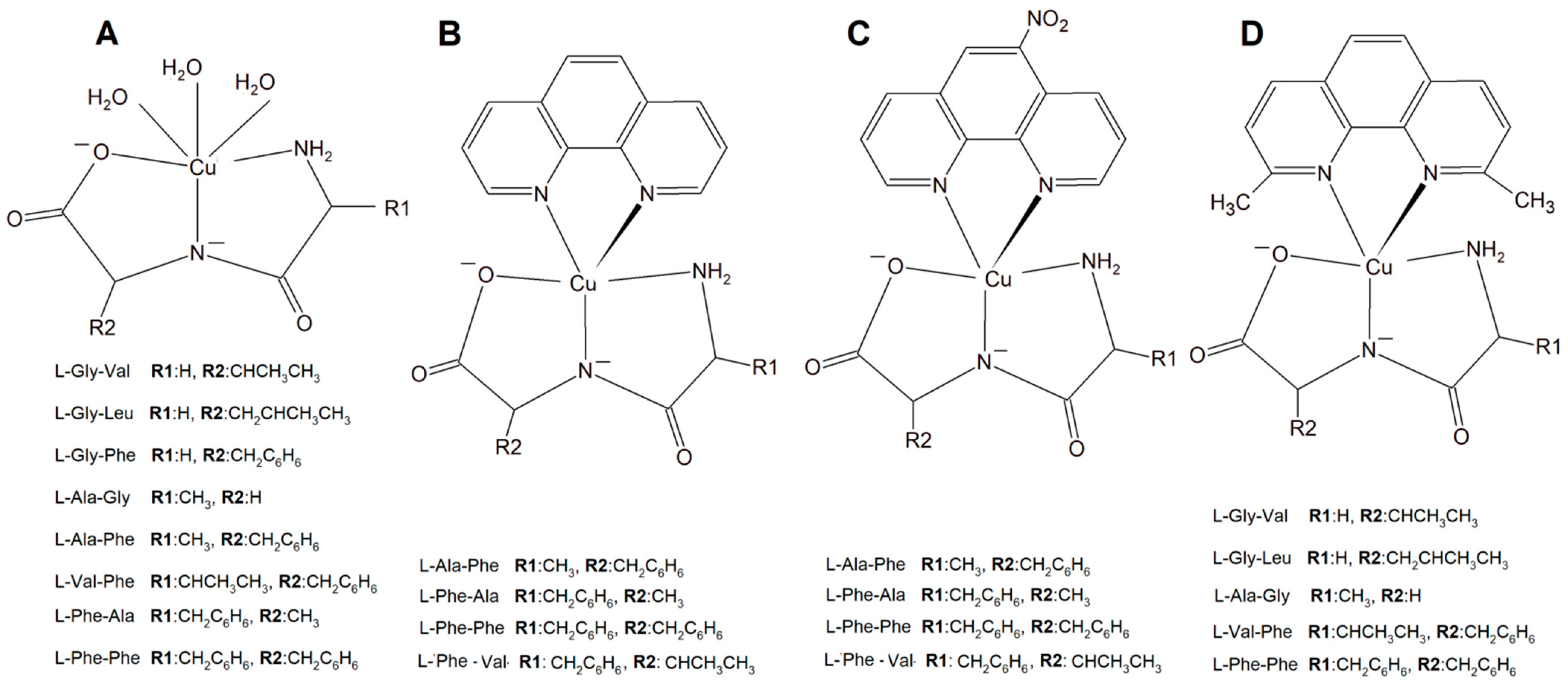
A new large group of Cu complexes with anticancer properties have been reported as a series of Cu(L-dipeptide)]·nH2O (L-dipeptide: L-Gly-Val, L-Gly-Leu, L-Gly-Phe, L-Ala-Gly, L-Ala-Phe, L-Val-Phe, L-Phe-Ala, L-Phe-Phe) [135,136,137], Cu(II)–L-dipeptide–phen (phen = 1,10-phenanthroline, L-dipeptide: Ala-Phe, Phe-Ala, Phe-Val and Phe-Phe [138,139,140,141], and Cu(II)–L-dipeptide–5-NO2-phen (where 5-NO2-phen = 5-NO2-1,10-phenanthroline; L-dipeptide: where L-dipeptide: Ala-Phe, Phe-Ala, Phe-Val and Phe-Phe) (Figure 15) [142]. Heteroleptic inorganic compounds Cu(II)–L-dipeptide–phen are much more active than Cu–phen or Cu–L-dipeptide complexes separately [135,136,137,138,139,140,141,142,143,144,145,146,147,148]. Their mode of cytotoxic action most probably includes DNA binding via phenanthroline ligand (intercalation processes) and also oxidative damage of this acid. The authors obtained similar results while investigating Cu(II)–L-dipeptide–5-NO2-phen.
Figure 15.
Copper(II) complexes (A) Cu–L-dipeptide, (B) Cu(II)–L-dipeptide–phen (phen = 1,10-phenanthroline), (C) Cu(II)–L-dipeptide–5-NO2-phen (5-NO2-phen = 5-NO2-1,10-phenanthroline), and (D) Cu(II)–L-dipeptide–dmp (dmp = 2,9-dimethyl-1,10-phenanthroline).
Another significant group of copper complexes [Cu(L-dipeptide)(dmp)]·nH2O bearing the dipeptide and neocuproine (dmp) ligand ([Cu(Gly-Val)(dmp)]·3H2O (Figure 15), [Cu(Gly-Leu)(dmp)]·H2O, [Cu(Ala-Gly)(dmp)]·4H2O, [Cu(Val-Phe)dmp)]·4.5H2O, and [Cu(Phe-Phe)(dmp)]·3H2O) were studied using various cancer lines [8]. It was proven that they possess better anticancer activity than those with phenanthroline ligands (Figure 15).
The synthesized inorganic compounds exhibited a high cytotoxic effect especially towards a few cancer cell lines: MDA-MB-231, MCF-7 (human metastatic breast adenocarcinomas, the first triple negative), MCF-10A (human normal breast cells), A549 (human lung epithelial carcinoma), and MRC-5 (human lung epithelial cells). Mechanistic studies revealed that the mode of action is mainly connected with DNA partial intercalation causing double strain damage, but also with an increase in the intracellular oxidative stress level [8,149].
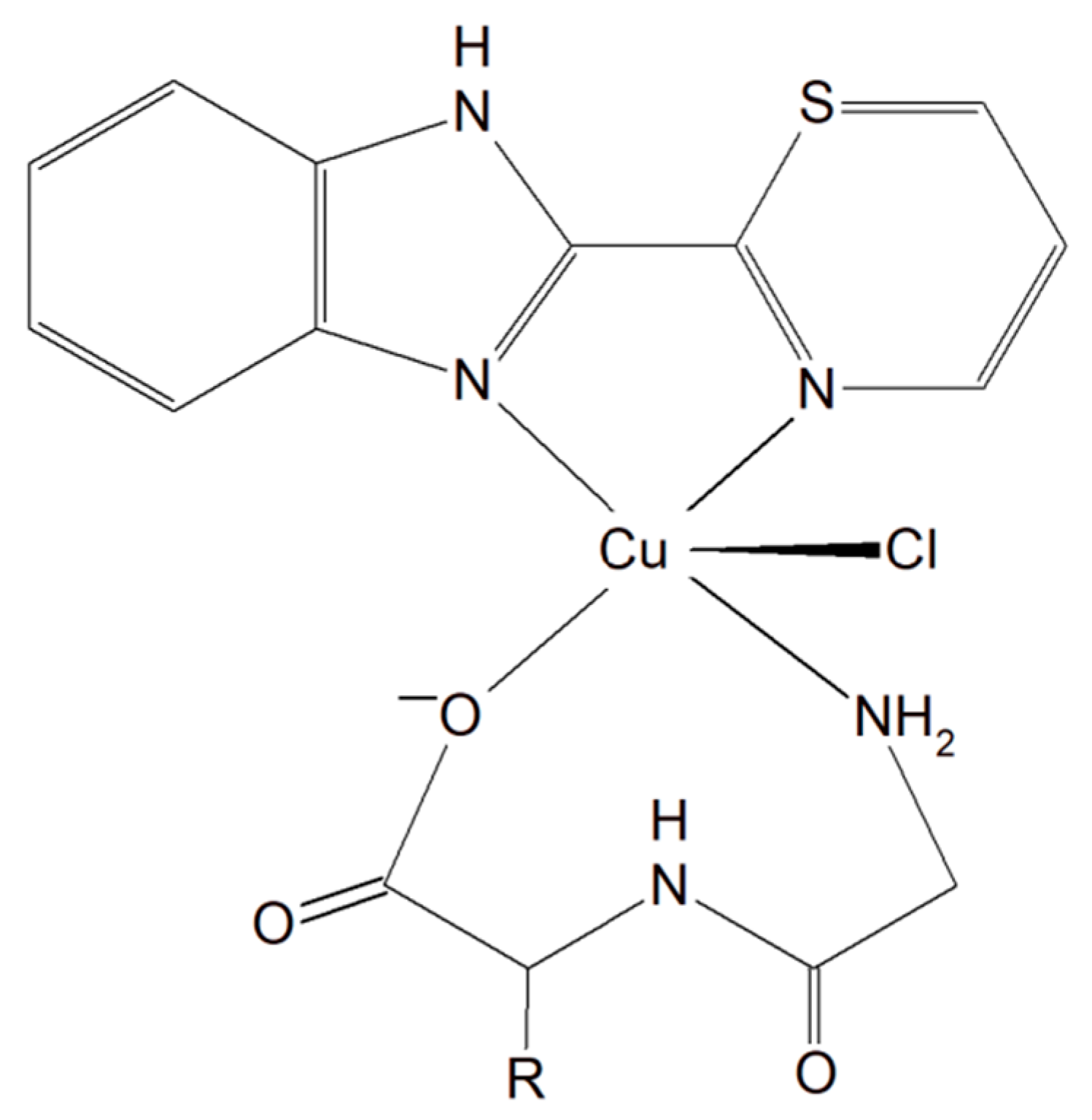
3.1.3. Copper Complexes with Peptide and Imidazole
A group of Cu(II)–dipeptide complexes of 2-(4′-thiazolyl)benzimidazole, [Cu(Gly-Gly)(TBZ)(Cl)]·4H2O and [Cu(Gly-L-Leu)(TBZ)(Cl)]·H2O (Gly-Gly = glycyl-glycine, Gly-L-Leu = glycyl-L-leucine, and TBZ = 2-(4′-thiazolyl)benzimidazole) were synthesized and fully characterized (Figure 16). It was proved that all compounds partially intercalate to calf thymus DNA causing degradation. Interestingly, these complexes in the presence of ascorbic acid (AA), induced ●OH production. The presence of reactive oxygen species can cause peroxidation of lipid and cellular DNA leading to cancer cell death. Moreover, the activity of the complexes described above was tested in vitro towards a few human carcinoma cell lines (HeLa, A549 and HepG2). The analysis of the results showed that the complexes exhibited a significant cytotoxic effect (IC50 from 33.17 to 100 μM) toward HeLa cancer cells. These phenomena undoubtedly indicate that these inorganic compounds have the potential to be effective metallo–peptide anticancer agents [150].
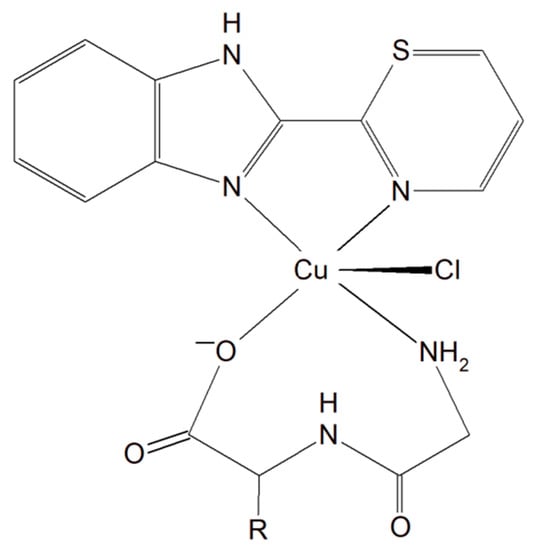
Figure 16.
Cu(II)–dipeptide complexes of 2-(4′-thiazolyl)benzimidazole.
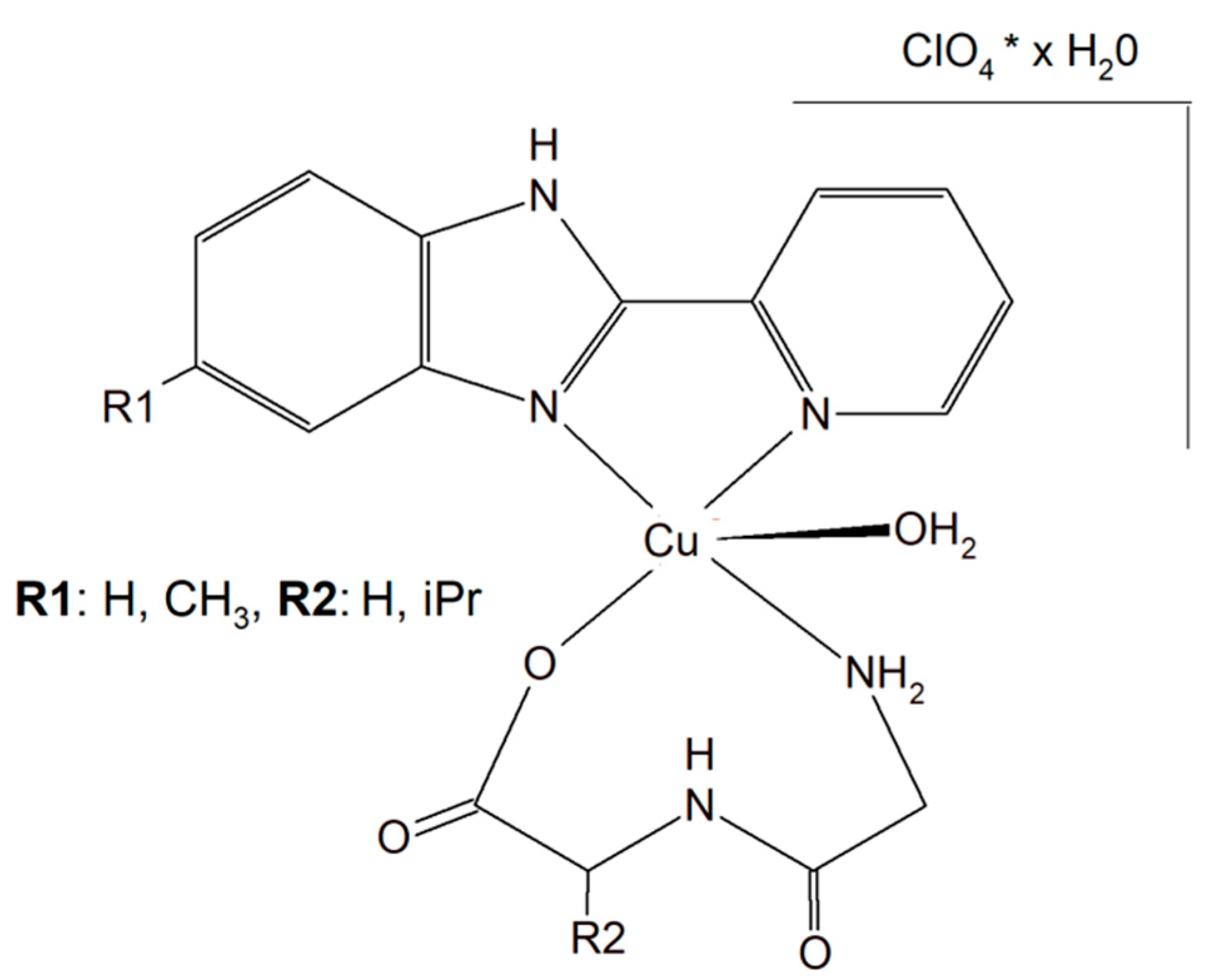
Two different mononuclear peptide–copper(II) complexes with imidazole derivatives, [Cu(Gly-L-val)(HPB)(H2O)]·ClO4·1.5H2O (Figure 17) and [Cu(Gly-L-val)(PBT)(H2O)]·ClO4 (Gly-L-val = glycyl-L-valine, HPB = 2-(2′-pyridyl)benzimidazole, PBT = 2-(2′-pyridyl)benzothiazole), were described. It was found that this type of complex, with imidazole derivatives, can bind to calf thymus DNA through hydrophobic interactions. Importantly, the inorganic compounds displayed, in the presence of ascorbic acid, induced oxidative cleavage of plasmid DNA. This process resulted in reactive oxygen species, especially ●OH production. The cytotoxic study of the Cu(II) complexes against A549, HeLa, and PC-3 tumor cell lines and NIH3T3 (non-tumor cell line) revealed that [Cu(Gly-L-Val)(HPB)(H2O)]·ClO4·1.5H2O exhibited better cytotoxicity towards A549 and PC-3 than [Cu(Gly-L-val)(PBT)(H2O)]·ClO4 and the widely used drug cisplatin [151].
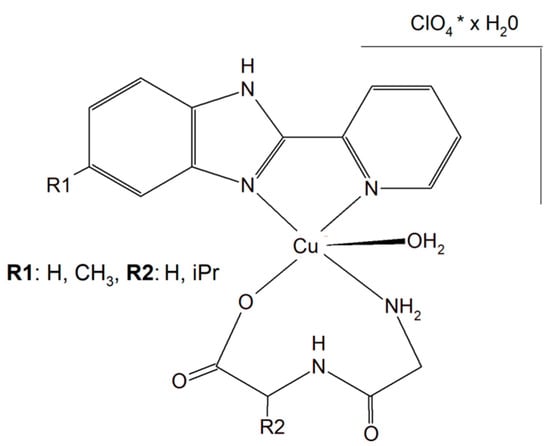
Figure 17.
Mononuclear peptide–copper(II) complexes with imidazole derivatives, [Cu(Gly-L-val)(HPB)(H2O)]·ClO4·1.5H2O and Cu(II) complex of 5-methyl-2-(2′-pyridyl)benzimidazole (HPBM; [Cu(Gly-gly)(HPBM)(H2O)]ClO4·0.5H2O).
The search for better and more selective anticancer drugs led to the synthesis of the Cu(II) complex of 5-methyl-2-(2′-pyridyl)benzimidazole (HPBM; [Cu(Gly-gly)(HPBM)(H2O)]ClO4·0.5H2O (Figure 17) and [Cu(Gly-L-leu)(HPBM)(H2O)]ClO4, where Gly-Gly = Glycyl-glycine, Gly-L-leu = Glycyl-L-leucine). All the complexes turned out to be highly active towards a few types of cancer cells (A549, HeLa, and PC-3). It has been discovered that the possible action mode is related to intracellular reactive oxygen species (ROS) generation with damage of mitochondria and DNA. The results clearly prove that the complexes could induce HeLa cell apoptosis via a ROS-mediated mitochondrial pathway [152].
3.1.4. Copper Complexes with Phosphines
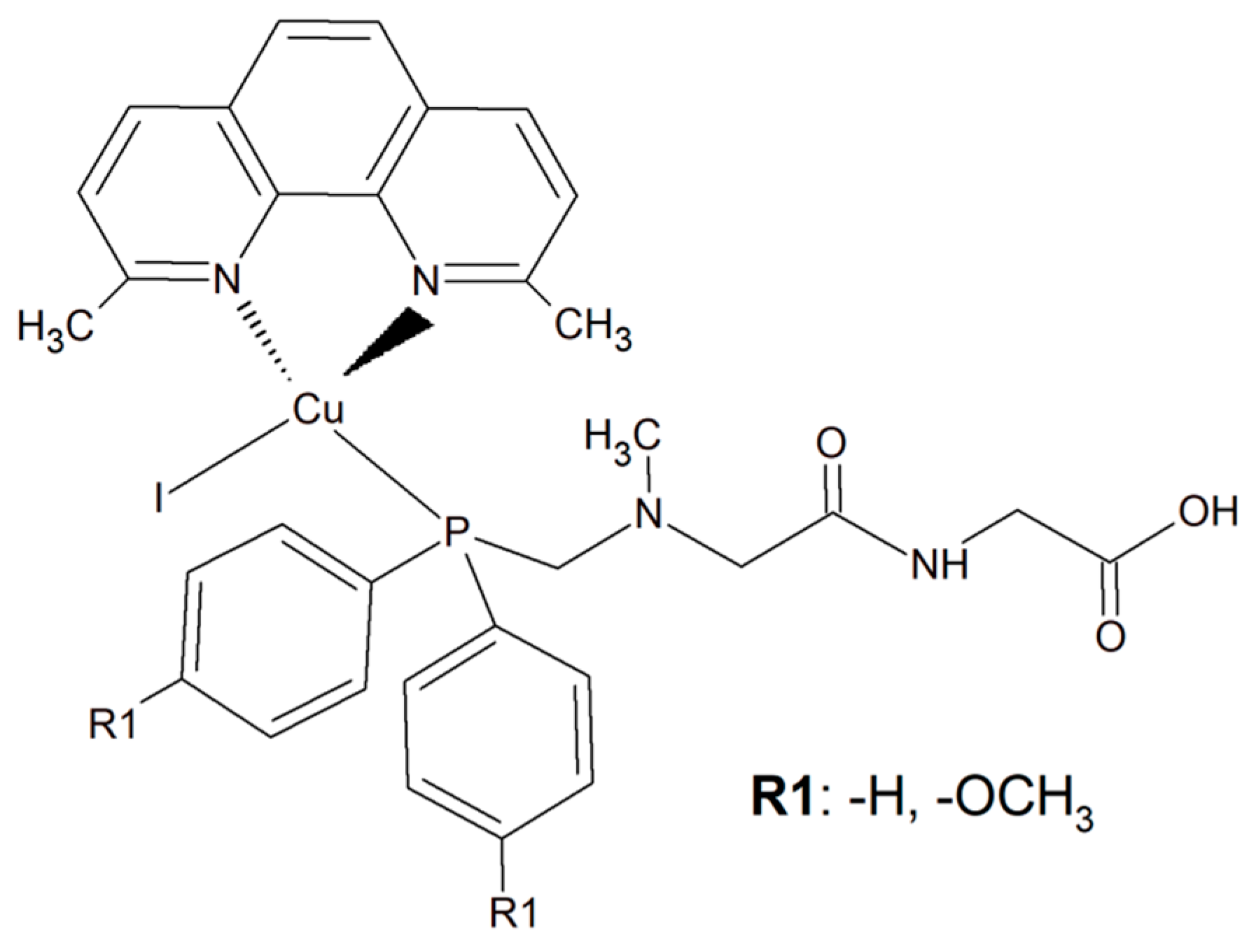
To improve the selectivity of copper(I) complexes, a simple dipeptide motif (sarcosyl-glycine SarGly) was attached to the inorganic compound ([CuI(dmp)(P(Ph)2CH2-SarGly-OH)], where dmp stands for 2,9-dimethyl-1,10-phenanthroline) (Figure 18). The cytotoxic effect of the compounds and cisplatin was tested towards cancer cell lines: mouse colon carcinoma (CT26; 1IC50 = 3.12 ± 0.1 µM), human lung adenocarcinoma (A549; IC50 = 2.01 ± 0.2 µM), and human breast adenocarcinoma (MCF7; IC50 = 0.98 ± 0.2 µM) as well as against a primary line of human pulmonary fibroblasts (MRC-5; IC50 = 78.56 ± 1.1 µM).
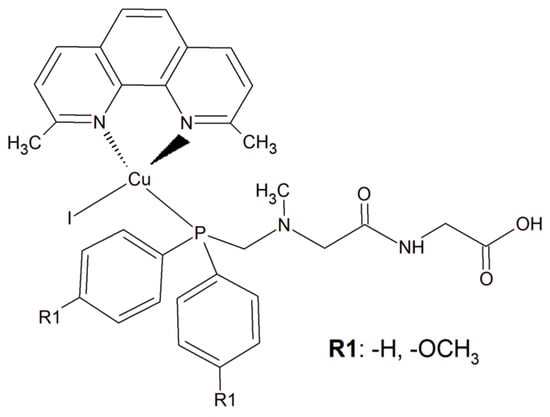
Figure 18.
The inorganic compounds [CuI(dmp)(P(Ph)2CH2-Sar-Gly-OH)] and [Cu(I)(dmp)(P(p-OCH3-Ph)2CH2SarGly)].
Attachment of the peptide motif SarGly to the cytotoxic Cu(I) complex via phosphine motif significantly enhanced the selectivity of this inorganic compound toward the cancerous cells. Insight into the mode of action of this Cu(I) complex showed that it led to apoptotic cell MCF7 death. What is more, a decrease in mitochondrial membrane potential and increase in caspase-9 and -3 activities were observed. Importantly, the investigated compound was able to generate a high level of ROS. Most probably these radicals were the reason for oxidative damage of the sugar–phosphate backbone of DNA [153].
An analogous copper(I) complex [Cu(I)(dmp)(P(p-OCH3-Ph)2CH2SarGly)] was synthesized but with methoxy groups introduced on the phosphine phenyl rings (Figure 18). The cytotoxicity of this inorganic compound was checked in vitro towards colon, lung, breast, pancreatic, and prostate tumor cell lines, as well as towards non-tumor cell lines: lung, kidney, and keratinocyte. The Cu(I) complex turned out to be significantly more effective than cisplatin towards all tested cancerous cell lines. The addition of the methoxy group onto the phenyl rings of the phosphine ligand caused increased cytotoxic activity resulting in damage to breast, pancreatic and prostate tumor cell lines in vitro. Importantly, after the connection of the peptide motif to the metal ion via the phosphine ligand, a significant increase in the selectivity towards cancer cells was observed. Additionally, the described metal complexes were found to be redox active and reactive oxygen species generation was detected [154].
3.2. Neurodegenerative Diseases
Despite the rapid growth of scientific publications describing neurodegenerative disorders, especially Alzheimer’s disease (AD), the exact etiology of this of type issue is still not well understood as we described above in the previous section (Section 2.1). Moreover, there is still no good therapeutic opportunity accessible for this disorder [155]. While there is no treatment, there are five FDA-approved medicines to manage the symptoms of AD. However, they can only prevent the disease from getting much worse with time [156]. It has been proven that in vitro, Cu2+ removal from amyloids prevents its accumulation leading to the inhibition of hydroxyl radical (●OH) production. For the reasons mentioned above, it is suggested that a potential therapy for AD is metal chelation therapy [155,156].
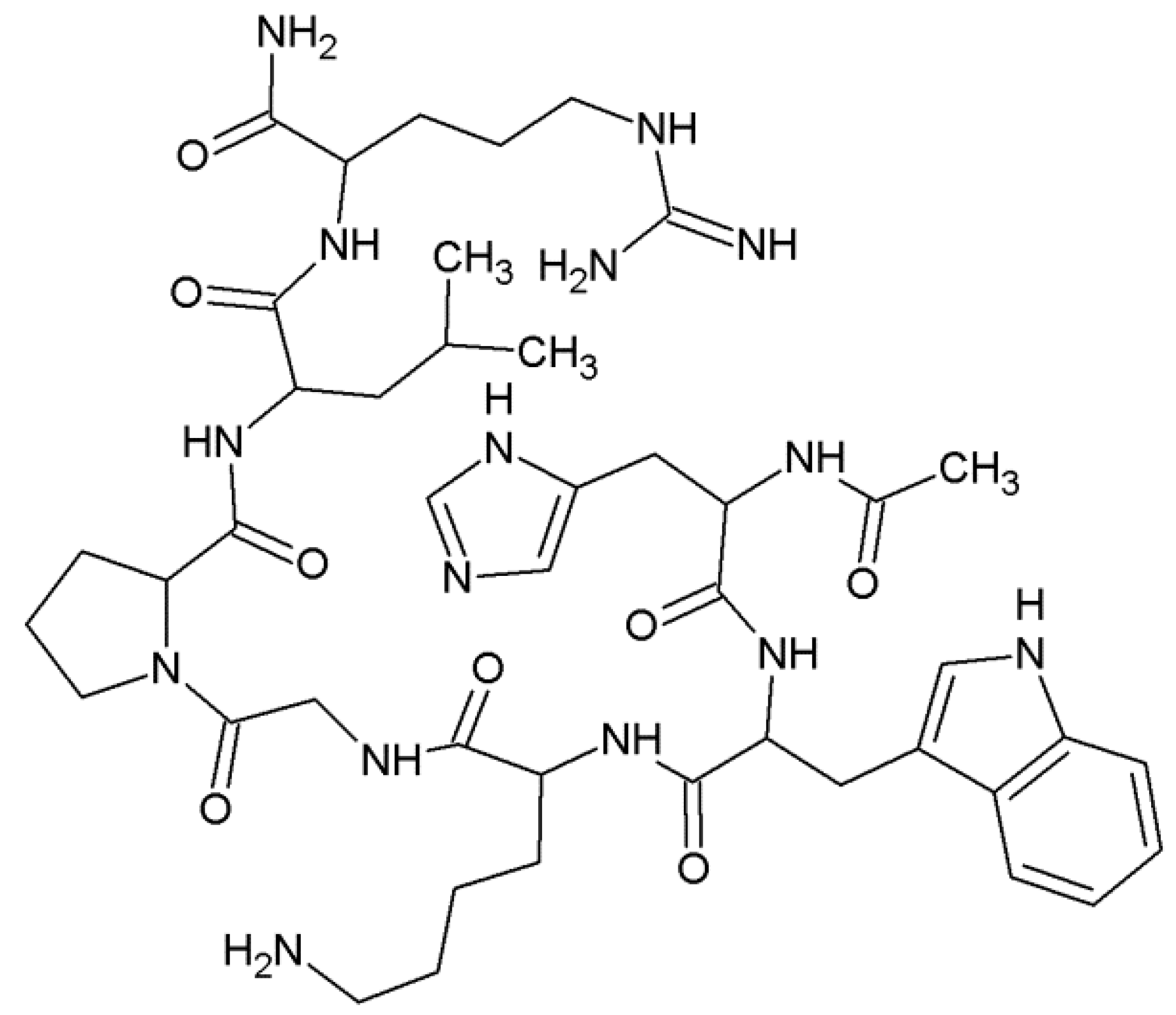
Substances characterized by antioxidant and anti-inflammatory activity and compounds capable of restoring copper balance can prevent age-associated cognitive decline and eliminate, delay, and even cure many common neurodegenerative conditions. The human tripeptide GHK (glycyl-L-histidyl-L-lysine) (Figure 19) was discovered in 1973 as one of the human blood proteins. This peptidic motif is able to form copper inorganic compounds (GHK-Cu). Importantly, the tripeptide GHK is characterized by plenty of different properties such as regenerative and protective actions including antioxidant, anti-inflammatory, and wound healing properties. Current research showed that GHK tripeptide is a very promising agent for the treatment of age-associated neurodegeneration and cognitive decline [157].
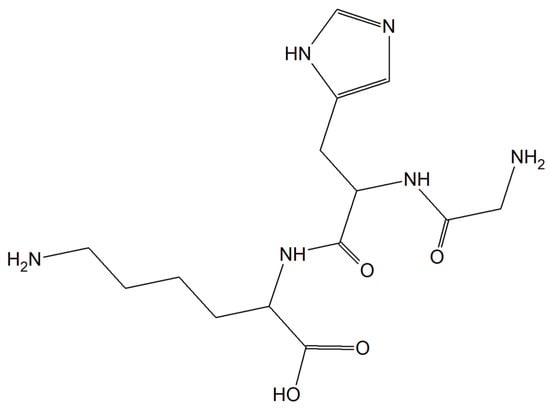
Figure 19.
The human tripeptide GHK (glycyl-L-histidyl-L-lysine).
3.3. Antiviral Properties
Viral infections occur all over the world and have a significant impact on people’s health, finances and quality of life. Currently, short antiviral peptides are a perfect example of a therapeutic agent that binds various metal ions such as Cu [158]. A great example of such a peptide is 3,4-dihydroxyphenylalanine (DOPA) with a peptide motif with adhesive properties (a diphenylalanine motif induces peptide self-assembly; sequence DOPA-(Phe)2-(His)6 is responsible for metal binding, Figure 20) [159].
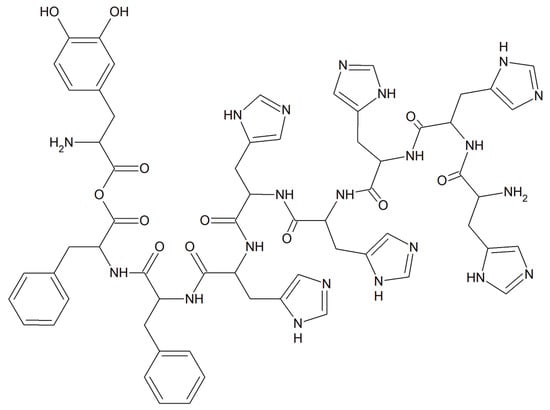
Figure 20.
The metal-binding hexahistidine sequence DOPA-(Phe)2-(His)6.
The authors proved that this compound is able to release monovalent copper ions that interact with hydrogen peroxide leading to ROS generation, giving the compound significant antiviral properties. Additionally, the relief of Cu(I) and H2O2 from the old PCN-coated surface, as well as the probability of renewing these surfaces, enhances the potential of this coating [158].
It is worth emphasizing that copper ions alone exhibited antiviral properties. Interesting research was performed by a few independent scientific groups [160,161,162] showing that copper ions can inactivate viruses (e.g., herpes simplex virus (HSV), feline calicivirus (FCV), bronchitis virus, poliovirus, and human immunodeficiency virus type 1 (HIV-1)) since copper ions can disrupt the activity of certain proteins and are responsible for hydroxyl radical production [162]. However, when the concentration of copper ions was too high, the resulting toxicity was fatal because copper chloride can inactivate specific proteins and produce a large number of reactive oxygen species in cells inducing apoptosis [162].
4. Conclusions
In this short review, we demonstrated the negative and positive consequences of ROS produced by copper(II)–peptide complexes on the human body. It has been documented that ROS can damage healthy cells leading to the initiation of many pathogenic states, e.g., neurodegenerative disorders, cancer, and respiratory system diseases. However, Cu(II)–peptide complexes are well known as agents causing pathogen and cancer cell death through ROS-dependent pathways.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/separations9030073/s1, Figure S1 The structural formulas of the (A)275VQIINKKLDLSNVQSKCGSKDNIKHVPGGGS305 (L1, Tau 275-305) and (B) 306VQIVYKPVDLSKVTSKCGSLGNIHHKPGGGQ336 (L2, 306-336) peptides, Figure S2: The structural formulas of the DAEFRHDSGYEVHHQKLVFFAEDVGSNK (L7, Aβ 1-28) peptide, Figure S3: The structural formulas of the (A) Ac-HGGG-NH2 (L8, PrPC 61-64) and (B)Ac-PHGGGWGQNH2 (L9, PrPC 60-67) peptides (Ac- acetyl group), Figure S4: The structural formulas of the(A) ATDAAS-NH2 (L10, FadA 19-24) and (B) MKKFL-NH2 (L11, FadA 1-5) peptides.
Author Contributions
U.K.K. and M.K.L.: conceptualization, literature review, writing—original draft preparation, writing—review and editing, visualization, and validation; A.B. and M.W.: writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Polish National Science Centre (grant number 2016/23/D/ST5/00269 and 2021/05/X/ST4/00311). We also extend a word of thanks to Rimon Mikhail for language editing on our manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| Ac | acetyl group |
| Dmp | neocuproine; 2,9-dimethyl-1,10-phenatroline |
| SarGly | sarcosyl-glycine |
| DSBs | double strand breaks |
| DNA | deoxyribonucleic acid |
| Phen | 1,10-phenanthroline |
| ROS | reactive oxygen species |
| Prs1 | Presenilin 1 |
| αSyn | alpha-synuclein |
| MDA | malondialdehyde |
| 4-HNE | 4-hydroxynonenal |
| AD | Alzheimer’s disease |
| MCO | metal-ion-catalyzed oxidation |
| FAD | Familial Alzheimer’s disease |
| APP protein | amyloid precursor protein |
| Fn | Fusobacterium nucleatum |
| AC | amino acids |
| DSBs | double strand breaks |
| GGH | Gly-Gly-His |
| MPP | FrFKFrFK-CONH2 (Phe-r-Phe-Lys-Phe-r-Phe-Lys-CONH2, where r = D-arginine) |
| HDPs | host defense peptides |
| MDA-MB-231 | human metastatic breast adenocarcinoma |
| MCF-7 | human metastatic breast adenocarcinoma |
| MCF-10A | human normal breast cells |
| A549 | human lung epithelial carcinoma |
| MRC-5 | human lung epithelial cells |
| AA | ascorbic acid |
| TBZ | 2-(4′-thiazolyl)benzimidazole) |
| DOPA | 3,4-dihydroxyphenylalanine |
| HSV | herpes simplex virus |
| FCV | feline calicivirus |
References
- Harris, E.D. Cellular copper transport and metabolism. Annu. Rev. Nutr. 2000, 20, 291–310. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Guo, Z.J. Copper in medicine: Homeostasis, chelation therapy and antitumor drug design. Curr. Med. Chem. 2006, 13, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Duncan, C.; White, A.R. Copper complexes as therapeutic agents. Metallomics 2012, 4, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Santini, C.; Pellei, M.; Gandin, V.; Porchia, M.; Tisato, F.; Marzano, C. Advances in copper complexes as anticancer agents. Chem. Rev. 2014, 114, 815–862. [Google Scholar] [CrossRef] [PubMed]
- Serment-Guerrero, J.; Bravo-Gomez, M.E.; Lara-Rivera, E.; Ruiz-Azuara, L. Genotoxic assessment of the copper chelated compounds Casiopeinas: Clues about their mechanisms of action. J. Inorg. Biochem. 2017, 166, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T. Radical medicine: Treating ageing to cure disease. Nat. Rev. Mol. Cell Biol. 2005, 6, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; He, Z. ROS-responsive drug delivery systems for biomedical. Asian J. Pharm. Sci. 2018, 13, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, N.D.; Diana Viña, D.; Leite, C.M.; Mendes; Batista, A.A.; Ellena, J.; Costa-Filho, A.J.; Facchin, G. Synthesis and structural characterization of a series of ternary copper(II)-L-dipeptide-neocuproine complexes. Study of their cytotoxicity against cancer cells including MDA-MB-231, triple negative breast cancer cells. J. Inorg. Biochem. 2020, 203, 110930–110941. [Google Scholar] [CrossRef] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharm. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [Green Version]
- Karogodina, T.Y.; Sergeeva, S.V.; Stass, D.V. Stability and reactivity of free radicals: A physicochemical perspective with biological implications. Hemoglobin 2011, 35, 262–275. [Google Scholar] [CrossRef]
- Lushchak, V.I. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem. Biol. Interact. 2014, 224, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Studer, A.; Curran, D.P. Catalysis of radical reactions: A radical chemistry perspective. Angew. Chem. Int. Ed. 2016, 55, 58–102. [Google Scholar] [CrossRef] [PubMed]
- Hayyan, M.; Hashim, M.A.; AlNashef, I.M. Superoxide ion: Generation and chemical implications. Chem. Rev. 2016, 116, 3029–3085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clausen, M.H.; Sassetti, E.; Clausen, M.H.; Laraia, L. Small-Molecule Inhibitors of Reactive Oxygen Species Production. J. Med. Chem. 2021, 64, 5252–5275. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine; Oxford University Press: Oxford, UK, 2015; Volume 5, p. 145. [Google Scholar]
- Thannickal, V.J.; Fanburg, B.L. Reactive oxygen species in cell signaling. Am. J. Physiol. Cell. Mol. Physiol. 2000, 279, 1005–1028. [Google Scholar] [CrossRef] [Green Version]
- Curtin, J.F.; Donovan, M.; Cotter, T.G. Regulation and measurement of oxidative stress in apoptosis. J. Immunol. Methods 2002, 265, 49–72. [Google Scholar] [CrossRef] [Green Version]
- Edge, R. Radiolytic and photolytic production of free radicals and reactive oxygen species: Interactions with antioxidants and biomolecules. In Applied Photochemistry; Evans, R.C., Douglas, P., Burrows, H.D., Eds.; Springer Science: Dordrecht, The Netherlands, 2013; pp. 305–330. [Google Scholar]
- Swartz, H.M.; Mason, R.P.; Hogg, N.; Kalyanaraman, B.; Sarna, T.; Plonka, P.M.; Zareb, M.; Gutierrez, P.L.; Berliner, L.J. Biomedical EPR, Part A: Free Radicals, Metals, Medicine, and Physiology; Springer: Boston, MA, USA, 2005; Volume 23, pp. 25–74. [Google Scholar] [CrossRef]
- Halliwell, B.; Adhikary, A.; Dingfelder, M.; Dizdaroglu, M. Hydroxyl radical is a significant player in oxidative DNA damage in vivo. Chem. Soc. Rev. 2021, 50, 8355–8360. [Google Scholar] [CrossRef]
- Edge, R.; Truscott, T.G. The Reactive Oxygen Species Singlet Oxygen, Hydroxyadicals, and the Superoxide Radical Anion—Examples of Their Roles in Biology and Medicine. Oxygen 2021, 1, 9. [Google Scholar] [CrossRef]
- Ohtsuki, A.; Lei, L.; Tanishima, M.; Goto, A.; Kaji, H. Photocontrolled organocatalyzed living radical polymerization feasible over a wide range of wavelengths. J. Am. Chem. Soc. 2015, 137, 5610–5617. [Google Scholar] [CrossRef]
- Shanmugam, S.; Xu, J.; Boyer, C. Exploiting metalloporphyrins for selective living radical polymerization tunable over visible wavelengths. J. Am. Chem. Soc. 2015, 137, 9174–9185. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.Q.; Gao, F.; Zhang, X.Z. Initiator-loaded gold nanocages as a light-induced free-radical generator for cancer therapy. Angew. Chem. Int. Ed. 2017, 56, 9029–9033. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, H.; Kleiman, M.; Esser-Kahn, A.P. Mechanically controlled radical polymerization initiated by ultrasound. Nat. Chem. 2017, 9, 135. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, K.; Bu, W.; Ni, D.; Liu, Y.; Feng, J.; Shi, J. Marriage of scintillator and semiconductor for synchronous radiotherapy and deep photodynamic therapy with diminished oxygen dependence. Angew. Chem. Int. Ed. 2015, 54, 1770–1774. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Wang, N.; Dong, X.; Wang, C.; Du, Z.; Mei, L.; Yong, Y.; Huang, C.; Li, Y.; Gu, Z.; et al. Graphdiyne nanoparticles with high free radical scavenging activity for radiation protection. ACS Appl. Mater. Interfaces 2019, 11, 2579–2590. [Google Scholar] [CrossRef] [PubMed]
- Buck, A.T.; Paletta, J.T.; Khindurangala, S.A.; Beck, C.L.; Winter, A.H. Anoncovalently reversible paramagnetic switch in water. J. Am. Chem. Soc. 2013, 135, 10594–10597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, B.; Li, W.-Y.; Chang, Y.; Yuan, B.; Wu, Y.; Zhang, M.-T.; Xu, J.-F.; Li, J.; Zhang, X. A supramolecular radical dimer: High-efficiency NIR-II photothermal conversion and therapy. Angew. Chem. 2019, 131, 15672–15677. [Google Scholar] [CrossRef]
- Magennis, E.P.; Fernandez-Trillo, F.; Sui, C.; Spain, S.P.; Bradshaw, D.J.; Churchley, D.; Mantovani1, G.; Winzer, K.; Alexander, C. Bacteria-instructed synthesis of polymers for self-selective microbial binding and labelling. Nat. Mater. 2014, 13, 748–755. [Google Scholar] [CrossRef] [Green Version]
- Niu, J.; Lunn, D.J.; Pusuluri, A.; Yoo, J.I.; O′Malley, M.A.; Mitragotri, S.; Soh, H.T.; Hawker, C.J. Engineering live cell surfaces with functional polymers via cytocompatible controlled radical polymerization. Nat. Chem. 2017, 9, 537. [Google Scholar] [CrossRef]
- Geng, J.; Li, W.; Zhang, Y.; Thottappillil, N.; Clavadetscher, J.; Lilienkampf, A.; Bradley, M. Radical polymerization inside living cells. Nat. Chem. 2019, 11, 578–586. [Google Scholar] [CrossRef] [Green Version]
- Whittaker, J.W. Free radical catalysis by galactose oxidase. Chem. Rev. 2003, 103, 2347–2364. [Google Scholar] [CrossRef]
- Orlando, J.J.; Tyndall, G.S.; Wallington, T.J. The atmospheric chemistry of alkoxy radicals. Chem. Rev. 2003, 103, 4657–4690. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Harmful and Beneficial Role of ROS. Oxid Med. Cell Longev. 2016, 2016, 7909186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef] [PubMed]
- Auten, R.L.; Davis, J.M. Oxygen toxicity and reactive oxygen species: The devil is in the details. Pediatr Res. 2009, 66, 121–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C.C.; Kandaswamy, E.; Zhou, T.; Zuo, L. Role of ROS and Nutritional Antioxidants in Human Diseases. Front. Physiol. 2018, 9, 477. [Google Scholar] [CrossRef] [Green Version]
- Barrera, G.; Pizzimenti, S.; Daga, M.; Dianzani, C.; Arcaro, A.; Cetrangolo, G.P.; Giordano, G.; Cucci, M.A.; Graf, M.; Gentile, F. Lipid Peroxidation-Derived Aldehydes, 4-Hydroxynonenal and Malondialdehyde in Aging-Related Disorders. Antioxidants 2018, 7, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Saieva, C.; Peluso, M.; Palli, D.; Cellai, F.; Ceroti, M.; Selvi, V.; Bendinelli, B.; Assedi, M.; Munnia, A.; Masala, G. Dietary and lifestyle determinants of malondialdehyde DNA adducts in a representative sample of the Florence City population. Mutagenesis 2016, 31, 475–480. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; TsouhFokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Hawkins, C.L.; Davies, M.J. Detection, identification, and quantification of oxidative protein modifications. J. Biol. Chem. 2019, 294, 19683–19708. [Google Scholar] [CrossRef] [Green Version]
- Schieber, M.; Chande, N.S. Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 19, 453–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sai, D.L.; Lee, J.; Nguyen, D.L.; Kim, Y.-P. Tailoring photosensitive ROS for advanced photodynamic therapy. Exp. Mol. Med. 2021, 52, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Escudero, A. Photodynamic therapy: Photosensitizers and nanostructures. Mater. Chem. Front. 2021, 5, 3788–3812. [Google Scholar] [CrossRef]
- Milkovic, L. Overview of ROS as Cell Function Regulators and Their Implications in Therapy Concepts. Cells 2019, 8, 793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forte, M. Targeting Nitric Oxide with Natural Derived Compounds as a Therapeutic Strategy in Vascular Diseases. Oxid. Med. Cell Longev. 2016, 1155, 7364138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lourenço, F.C. Nitric Oxide Pathways in Neurovascular Coupling Under Normal and Stress Conditions in the Brain: Strategies to Rescue Aberrant Coupling and Improve Cerebral Blood Flow. Front. Physiol. 2021, 12, 729201. [Google Scholar] [CrossRef] [PubMed]
- Lukács, M.; Szunyog, G.; Grenács, Á.; Lihi, N.; Kállay, C.; Di Natale, G.; Campagna, T.; Lanza, V.; Tabbi, G.; Pappalardo, G.; et al. Copper(II) Coordination Abilities of the Tau Protein’s N-Terminus Peptide Fragments: A Combined Potentiometric, Spectroscopic and Mass Spectrometric Study. Chempluschem 2019, 84, 1697–1708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muralidar, S.; Ambi, S.V.; Sekaran, S.; Thirumalai, D.; Palaniappan, B. Role of tau protein in Alzheimer’s disease: The prime pathological player. Int. J. Biol. Macromol. 2020, 163, 1599–1617. [Google Scholar] [CrossRef]
- Leal, S.S.; Botelho, H.M.; Gomes, C.M. Metal ions as modulators of protein conformation and misfolding in neurodegeneration. Coord. Chem. Rev. 2012, 256, 2253–2270. [Google Scholar] [CrossRef]
- Sun, X.-Y.; Wei, Y.-P.; Xiong, Y.; Wang, X.-C.; Xie, A.-J.; Wang, X.-L.; Yang, Y.; Wang, Q.; Lu, Y.-M.; Liu, R.; et al. Synaptic released zinc promotes tau hyperphosphorylation by inhibition of protein phosphatase 2A (PP2A). J. Biol. Chem. 2012, 287, 11174–11182. [Google Scholar] [CrossRef] [Green Version]
- Petersen, J.D.; Kaech, S.; Banker, G. Selective microtubule-based transport of dendritic membrane proteins arises in concert with axon specification. J. Neurosci. 2014, 34, 4135–4147. [Google Scholar] [CrossRef] [PubMed]
- Kandimalla, R.; Manczak, M.; Yin, X.; Wang, R.; Reddy, P.H. Hippocampal phosphorylated tau induced cognitive decline, dendritic spine loss and mitochondrial abnormalities in a mouse model of Alzheimer’s disease. Hum. Mol. Genet. 2018, 27, 30–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dash, S. Tau pathology in Alzheimer’s disease and associated hypotheses. Life Res. 2019, 17, 115–124. [Google Scholar] [CrossRef]
- Wang, Y.; Mandelkow, E. Tau in physiology and pathology. Nat. Rev. Neurosci. 2016, 17, 5–21. [Google Scholar] [CrossRef]
- Ahmadi, S.; Zhu, S.; Sharma, R.; Wu, B.; Soong, R.; Dutta Majumdar, R.; Wilson, D.J.; Simpson, A.J.; Kraatz, H.B. Aggregation of Microtubule Binding Repeats of Tau Protein is Promoted by Cu2+. ACS Omega 2019, 4, 5356–5366. [Google Scholar] [CrossRef] [Green Version]
- Golec, C.; Mortensen, S.; Anwar, S.; Martic-Milne, S. Dual roles of tau R peptides on Cu(II)/(I)-mediated reactive oxygen species formation. J. Biol. Inorg. Chem. 2021, 26, 919–931. [Google Scholar] [CrossRef] [PubMed]
- Burré, J.; Sharma, M.; Tsetsenis, T.; Buchman, V.; Etherton, M.R.; Südhof, T.C. Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science 2010, 329, 1663–1667. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.; Uéda, K.; Chan, P. Alpha-synuclein and dopamine metabolism. Mol. Neurobiol. 2005, 31, 243–254. [Google Scholar] [CrossRef]
- Spillantini, M.G.; Schmidt, M.L.; Lee, V.M.; Trojanowski, J.Q.; Jakes, R.; Goedert, M. α-Synuclein in Lewy bodies. Nature 1997, 388, 839–840. [Google Scholar] [CrossRef]
- Lee, H.-J.; Bae, E.-J.; Lee, S.-J. Extracellular α-synuclein—A novel and crucial factor in Lewy body diseases. Nat. Rev. Neurol. 2014, 10, 92–98. [Google Scholar] [CrossRef]
- Binolfi, A.; Quintanar, L.; Bertoncini, C.W.; Griesinger, C.; Fernández, C.O. Bioinorganic chemistry of copper coordination to alpha-synuclein: Relevance to Parkinson’s disease. Coord. Chem. Rev. 2012, 256, 2188–2201. [Google Scholar] [CrossRef] [Green Version]
- Dell’Acqua, S.; Pirota, V.; Anzani, C.; Rocco, M.M.; Nicolis, S.; Valensin, D.; Monzani, E.; Casella, L. Reactivity of copper-α-synuclein peptide complexes relevant to Parkinson’s disease. Metallomics 2015, 7, 1091–1102. [Google Scholar] [CrossRef] [PubMed]
- Kowalik-Jankowska, T.; Rajewska, A.; Wiśniewska, K.; Grzonka, Z.; Jezierska, J. Coordination abilities of N-terminal fragments of alpha-synuclein towards copper(II) ions: A combined potentiometric and spectroscopic study. J. Inorg. Biochem. 2005, 99, 2282–2291. [Google Scholar] [CrossRef] [PubMed]
- Kowalik-Jankowska, T.; Rajewska, A.; Jankowska, E.; Grzonka, Z. Products of Cu(II)-catalyzed oxidation of alpha-synuclein fragments containing M1-D2 and H50 residues in the presence of hydrogen peroxide. Dalton Trans. 2008, 6, 832–838. [Google Scholar] [CrossRef]
- Kowalik-Jankowska, T.; Rajewska, A.; Jankowska, E.; Wiśniewska, K.; Grzonka, Z. Products of Cu(II)-catalyzed oxidation of the N-terminal fragments of α-synuclein in the presence of hydrogen peroxide. J. Inorg. Biochem. 2006, 100, 1623–1631. [Google Scholar] [CrossRef]
- Somavarapu, A.K.; Kepp, K.P. Loss of stability and hydrophobicity of presenilin 1 mutations causing Alzheimer’s disease. J. Neurochem. 2016, 137, 101–111. [Google Scholar] [CrossRef] [Green Version]
- Müller, M.; Cheung, K.H.; Foskett, J.K. Enhanced ROS generation mediated by Alzheimer’s disease presenilin regulation of InsP3R Ca2+ signaling. Antioxid. Redox. Signal. 2011, 14, 1225–1235. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Park, H.; Maharana, C.; Gwon, A.R.; Park, J.; Baek, S.H.; Bae, H.G.; Cho, Y.; Kim, H.K.; Sul, J.H.; et al. Alzheimer’s disease-causing presenilin-1 mutations have deleterious effects on mitochondrial function. Theranostics 2021, 11, 8855–8873. [Google Scholar] [CrossRef]
- Montes, S.; Rivera-Mancia, S.; Diaz-Ruiz, A.; Tristan-Lopez, L.; Rios, C. Copper and Copper Proteins in Parkinson’s Disease. Oxid. Med. Cell. Longev. 2014, 2014, 147251. [Google Scholar] [CrossRef] [Green Version]
- Lesiów, M.K.; Krupa, K. The impact of the histidyl residue position on the formation and stability of Cu(II) complexes and their ability of ROS generation. New J. Chem. 2021, 45, 8543–8556. [Google Scholar] [CrossRef]
- Kowalik-Jankowska, T.; Ruta, M.; Wiśniewska, K.; Łankiewicz, L.; Dyba, M. Products of Cu(II)-catalyzed oxidation in the presence of hydrogen peroxide of the 1–10, 1–16 fragments of human and mouse β-amyloid peptide. J. Inorg. Biochem. 2004, 98, 940–950. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Swomley, A.M.; Sultana, R. Amyloid β-peptide (1-42)-induced oxidative stress in Alzheimer disease: Importance in disease pathogenesis and progression. Antiox. Redox Signal. 2013, 19, 823–835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowalik-Jankowska, T.; Ruta, M.; Wiśniewska, K.; Łankiewicz, L. Coordination abilities of the 1-16 and 1-28 fragments of β-amyloid peptide towards copper(II) ions: A combined potentiometric and spectroscopic study. J. Inorg. Biochem. 2003, 95, 270–282. [Google Scholar] [CrossRef]
- WilochM, Z.; Wawrzyniak, U.E.; Ufnalska, I.; Bonna, A.; Bal, W.; Drew, S.C.; Wróblewski, W. Tuning the Redox Properties of Copper(II) Complexes with Amyloid-β Peptides. J. Electrochem. Soc. 2016, 163, 196–199. [Google Scholar] [CrossRef]
- Jiang, D.; Men, L.; Wang, J.; Zhang, Y.; Chickenyen, S.; Wang, Y.; Zhou, F. Redox reactions of copper complexes formed with different beta-amyloid peptides and their neuropathalogical relevance. Biochemistry 2007, 46, 9270–9282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acevedo-Morantes, C.Y.; Wille, H. The structure of human prions: From biology to structural models-considerations and pitfalls. Viruses 2014, 6, 3875–3892. [Google Scholar] [CrossRef] [PubMed]
- Wulf, M.A.; Senatore, A.; Aguzzi, A. The biological function of the cellular prion protein: An update. BMC Biol. 2017, 15, 34. [Google Scholar] [CrossRef] [Green Version]
- Linden, R. The Biological Function of the Prion Protein: A Cell Surface Scaffold of Signaling Modules. Front. Mol. Neurosci. 2017, 10, 77. [Google Scholar] [CrossRef] [Green Version]
- Zidar, J.; Pirc, E.T.; Hodoscek, M.; Bukovec, P. Copper(II) ion binding to cellular prion protein. J. Chem. Inf. Model 2008, 48, 283–287. [Google Scholar] [CrossRef]
- Bonomo, R.P.; Cucinotta, V.; Giuffrida, A.; Impellizzeri, G.; Magri, A.; Pappalardo, G.; Rizzarelli, E.; Santoro, A.M.; Tabbi, G.; Vagliasindi, L.I. A re-investigation of copper coordination in the octa-repeats region of the prion protein. Dalton Trans. 2005, 1, 150–158. [Google Scholar] [CrossRef]
- Srikanth, R.; Wilson, J.; Burns, C.S.; Vachet, R.W. Identification of the copper(II) coordinating residues in the prion protein by metal-catalyzed oxidation mass spectrometry: Evidence for multiple isomers at low copper(II) loadings. Biochemistry 2008, 47, 9258–9268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pocanschi, C.L.; Apell, H.-J.; Puntervoll, P.; Høgh, B.; Jensen, H.B.; Welte, W.; Kleinschmidt, J.-H. The major outer membrane protein of Fusobacterium nucleatum (FomA) folds and inserts into lipid bilayers via parallel folding pathways. J. Mol. Biol. 2006, 20, 548–561. [Google Scholar] [CrossRef] [Green Version]
- Nobbs, A.H.; Jenkinson, H.F.; Jakubovics, N.S. Stick to your gums: Mechanisms of oral microbial adherence. J. Dent. Res. 2011, 90, 1271–1278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.F.; Shi, W.; Zhu, W.; Smith, J.W.; Hsieh, S.L.; Gallo, R.L.; Huang, C.M. Vaccination targeting surface FomA of Fusobacterium nucleatum against bacterial co-aggregation: Implication for treatment of periodontal infection and halitosis. Vaccine 2010, 28, 3496–3505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Cai, S.; Ma, Y. Association between Fusobacterium nucleatum and colorectal cancer: Progress and future directions. J. Cancer 2018, 9, 1652–1659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hordyjewska, A.; Popiołek, Ł.; Kocot, J. The many “faces” of copper in medicine and treatment. Biometals 2014, 27, 611–621. [Google Scholar] [CrossRef] [Green Version]
- Lesiów, M.K.; Pietrzyk, P.; Kyzioł, A.; Komarnicka, U.K. Cu(II) complexes with fomA protein fragments of Fusobacterium nucleatum increase oxidative stress and malondialdehyde level. Chem. Res. Toxicol. 2019, 32, 2227–2237. [Google Scholar] [CrossRef]
- Lesiów, M.K.; Pietrzyk, P.; Bieńko, A.; Kowalik-Jankowska, T. Stability of Cu(II) complexes with FomA protein fragments containing two His residues in the peptide chain. Metallomics 2019, 11, 1518–1531. [Google Scholar] [CrossRef]
- Lesiów, M.K.; Komarnicka, U.K.; Kyzioł, A.; Bieńko, A.; Pietrzyk, P. ROS-mediated lipid peroxidation as a result of Cu(II) interaction with FomA protein fragments of F. nucleatum: Relevance to colorectal carcinogenesis. Metallomics 2019, 11, 2066–2077. [Google Scholar] [CrossRef]
- Témoin, S.; Wu, K.L.; Wu, V.; Shoham, M.; Han, Y.W. Signal peptide of FadAadhesin from Fusobacterium nucleatum plays a novel structural role by modulating the filament’s length and width. FEBS Lett. 2012, 586, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Guo, P.; Tian, Z.; Kong, X.; Yang, L.; Shan, X.; Dong, B.; Ding, X.; Jing, X.; Jiang, C.; Jiang, N.; et al. FadA promotes DNA damage and progression of Fusobacterium nucleatum-induced colorectal cancer through up-regulation of chk2. J. Exp. Clin. Cancer Res. 2020, 39, 202. [Google Scholar] [CrossRef] [PubMed]
- Gholizadeh, P.; Eslami, H.; Kafil, H.S. Carcinogenesis mechanisms of Fusobacterium nucleatum. Biomed. Pharmacother. 2017, 89, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Krupa, K.; Lesiów, M.; Stokowa-Sołtys, K.; Starosta, R.; Ptaszyńska, N.; Łęgowska, A.; Rolka, K.; Wernecki, M.; Cal, M.; Jeżowska-Bojczuk, M. Copper(II) complexes with Fusobacterium nucleatum adhesin FadA: Coordination pattern, physicochemical properties and reactivity. J. Inorg. Biochem. 2018, 189, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Haque, S.M.; Ashwaq, O.; Sarief, A.; Azad John Mohamed, A.K. A comprehensive review about SARS-CoV-2. Future Virol. 2020, 15, 625–648. [Google Scholar] [CrossRef] [PubMed]
- Ou, X.; Liu, Y.; Lei, X.; Li, P.; Mi, D.; Ren, L.; Guo, L.; Guo, R.; Chen, T.; Hu, J.; et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020, 11, 1620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, Y.J. The viral protein fragment theory of COVID-19 pathogenesis. Med. Hypotheses 2020, 144, 110267. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, U.C.; Shrivastava, R. Interaction of viral proteins with metal ions: Role in maintaining the structure and functions of viruses. FEMS Microbiol. Immunol. 2005, 43, 105–114. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Li, J.; Wang, P.H.; Yang, N.; Huang, J.; Ou, J.; Xu, T.; Zhao, X.; Liu, T.; Huang, X.; et al. SARS-CoV-2 spike promotes inflammation and apoptosis through autophagy by ROS-suppressed PI3K/AKT/mTOR signaling. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166260. [Google Scholar] [CrossRef]
- Raha, S.; Mallick, R.; Basak, S.; Duttaroy, A.K. Is copper beneficial for COVID-19 patients? Med. Hypotheses 2020, 142, 109814. [Google Scholar] [CrossRef]
- Leng, L.; Cao, R.; Ma, J.; Mou, D.; Zhu, Y.; Li, W.; Lv, L.; Gao, D.; Zhang, S.; Gong, F.; et al. Pathological features of COVID-19-associated lung injury: A preliminary proteomics report based on clinical samples, Signal Transduct. Target Ther. 2020, 5, 240. [Google Scholar] [CrossRef]
- Colunga Biancatelli, R.M.L.; Solopov, P.A.; Sharlow, E.R.; Lazo, J.S.; Marik, P.E.; Catravas, J.D. The SARS-CoV-2 spike protein subunit S1 induces COVID-19-like acute lung injury in Κ18-hACE2 transgenic mice and barrier dysfunction in human endothelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2021, 321, L477–L484. [Google Scholar] [CrossRef] [PubMed]
- Besold, A.N.; Culbertson, E.M.; Culotta, V.C. The Yin and Yang of copper during infection. J. Biol. Inorg. Chem. 2016, 21, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Barhoumi, T.; Alghanem, B.; Shaibah, H.; Mansour, F.A.; Alamri, H.S.; Akiel, M.A.; Alroqi, F.; Boudjelal, M. SARS-CoV-2 Coronavirus Spike Protein-Induced Apoptosis, Inflammatory, and Oxidative Stress Responses in THP-1-Like-Macrophages: Potential Role of Angiotensin-Converting Enzyme Inhibitor (Perindopril). Front Immunol. 2021, 12, 728896. [Google Scholar] [CrossRef] [PubMed]
- Miripour, Z.S.; Sarrami-Forooshani, R.; Sanati, H.; Makarem, J.; Taheri, M.S.; Shojaeian, F.; Eskafi, A.H.; Abbasvandi, F.; Namdar, N.; Ghafari, H.; et al. Real-time diagnosis of reactive oxygen species (ROS) in fresh sputum by electrochemical tracing; correlation between COVID-19 and viral-induced ROS in lung/respiratory epithelium during this pandemic. Biosens. Bioelectron. 2020, 165, 112435. [Google Scholar] [CrossRef] [PubMed]
- Reshi, M.L.; Su, Y.C.; Hong, J.R. RNA Viruses: ROS-Mediated Cell Death. Int. J. Cell Biol. 2014, 2014, 467452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Razzaq, Z.; Malik, A. Viral load is associated with abnormal serum levels of micronutrients and glutathione and glutathione-dependent enzymes in genotype 3 HCV patients. BBA Clin. 2014, 2, 72–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, M.; Rathje, O.; Levina, A.; Lay, P.A. High cytotoxicity of vanadium(IV) complexes with 1,10-phenanthroline and related ligands is due to decomposition in cell culture medium. Biol. Inorg. Chem. 2017, 22, 663–672. [Google Scholar] [CrossRef]
- Martínez-Valencia, B.; Corona-Motolinia, N.D.; Sánchez-Laraa, E.; Norieg, L.; Sánchez-Gaytán, B.L.; Castro, M.E.; Meléndez-Bustamante, F.; González-Vergara, E. Cyclo-tetravanadate bridged copper complexes as potential double bullet pro-metallodrugs for cancer treatment. J. Inorg. Biochem. 2020, 208, 111081–111092. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowska, A.; Szuster-Ciesielska, A.; Sztandera, M.; Bregier-Jarzebowska, R.; Jarzab, A.; Rojek, T.; Komarnicka, U.K.; Bojarska-Junak, T.; Jezierska, J. L-argininatocopper(II) complexes in solution exert significant selective anticancer and antimicrobial activities. Appl. Organomet. Chem. 2020, 34, 5698–5706. [Google Scholar] [CrossRef]
- Wojciechowska, A.; Gągor, A.; Zierkiewicz, W.; Jarząb, A.; Dylonga, A.; Duczmala, M. Metal–organic framework in an l-arginine copper(ii) ion polymer: Structure, properties, theoretical studies and microbiological activity. RSC Adv. 2015, 5, 36295–36307. [Google Scholar] [CrossRef]
- Badetti, E.; Calgaro, L.; Falchi, L.; Bonetto, A.; Bettiol, C.; Leonetti, B.; Ambrosi, E.; Zendri, E.; Marcomini, P. Interaction between Copper Oxide Nanoparticles and Amino Acids: Influence on the Antibacterial Activity. Nanomaterials 2019, 9, 792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Hecke, K.; Nam, P.C.; Nguyen, M.T.; Van Meervelt, L. Netropsin interactions in the minor groove of d(GGCCAATTGG) studied by a combination of resolution enhancement and ab initio calculations. FEBS J. 2005, 272, 3531–3539. [Google Scholar] [CrossRef] [PubMed]
- Mashima, T.; Nishikawa, F.; Kamatari, Y.O.; Fujiwara, H.; Saimura, M.; Nagata, T.; Kodaki, T.; Nishikawa, S.; Kuwata, K.; Katahira, M. Anti-prion activity of an RNA aptamer and its structural basis. Nucleic Acids Res. 2013, 41, 1355–1364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macmaster, R.; Sedelnikova, S.; Baker, P.J.; Bolt, E.L.; Lloyd, R.G.; Rafferty, J.B. RusA Holliday junction resolvase: DNA complex structure—Insights into selectivity and specificity. Nucleic Acids Res. 2006, 34, 5577–5582. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.R.; Le, X.Y.; Feng, X.L. Syntheses, characterizations and SOD-like activities of ternary copper(II) complexes with 1,10-phenanthroline and L-α-amino acids. J. Coord. Chem. 2008, 61, 847–854. [Google Scholar] [CrossRef]
- Harada, K.; Franke, A.D. Identification of two novel arginine binding DNAs. EMBO 1995, 14, 5798–5809. [Google Scholar] [CrossRef]
- Conklin, K.A. Chemotherapy-associated oxidative stress: Impact on chemotherapeutic effectiveness. Integr. Cancer Ther. 2004, 3, 294–300. [Google Scholar] [CrossRef]
- Srinivasa, S.U.; Bryce, W.Q.T.; Vellayappan, B.V.; Jeyasekharan, A.D. ROS and the DNA damage response in cancer. Redox Biol. 2019, 25, 101084. [Google Scholar] [CrossRef]
- Wojciechowska, A.; Rojek, T.; Gorzsas, A.; Malik-Gajewska, M.; Duczmal, M. Isothiocyanate controlled architecture, spectroscopic, and magnetic behavior of copper(II) l–arginine complexes. J. Coord. Chem. 2019, 72, 1358–1362. [Google Scholar] [CrossRef]
- Hosseininezhad, S.M.; Hosseinali, R. An analysis of the reactions of L-arginine with Cu(II), Co(II), Fe(III), Zn(II), and Cr(III). Adv. Environ. Biol. 2014, 7, 315. [Google Scholar]
- Wojciechowska, A.; Bregier, R.; Komarnicka, U.K.; Kozieł, S.; Szuster, A.; Sztandera, M.; Jarząb, A.; Staszak, Z.; Witkowska, D.; Bojarska, A.; et al. Isothiocyanate l−argininato copper(II) complexes—Solution structure, DNA interaction, anticancer and antimicrobial activity. Chem. Biol. Interact. 2021, 348, 109636–109641. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hao, S.; Han, A.; Yang, Y.; Fang, G.; Wang, S. Intracellular Fenton reaction based on mitochondria-targeted copper(II)–peptide complex for induced apoptosis. J. Mater. Chem. 2019, 7, 4008–4016. [Google Scholar] [CrossRef]
- Chekmenev, E.Y.; Vollmar, B.S.; Forseth, K.T.; Manion, M.C.; Jones, S.M.; Wagner, T.J.; Endicott, R.M.; Kyriss, B.P.; Homem, L.M.; Pate, M.; et al. Investigating molecular recognition and biological function at interfaces using piscidins, antimicrobial peptides from fish. Biochim. Biophys. Acta 2006, 1758, 1359–1372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chekmenev, E.Y.; Jones, S.M.; Nikolayeva, Y.M.; Vollmar, B.S.; Wagner, T.J.; Gor’kov, P.L.; Brey, W.W.; Manion, M.N.; Cotton, D.M. High-field NMR studies of molecular recognition and structure-function relationships in antimicrobial piscidins at the water-lipid bilayer interface. J. Am. Chem. Soc. 2006, 128, 5308–5309. [Google Scholar] [CrossRef] [PubMed]
- Comert, F.; Heinrich, F.; Chowdhury, A.; Schoeneck, M.; Darling, D.; Anderson, K.W.; Daben, M.; Angeles-Boza, A.M.; Silin, V.; Cotton, M.L.; et al. Copper-binding anticancer peptides from the piscidin family: An expanded mechanism that encompasses physical and chemical bilayer disruption. Sci. Rep. 2021, 11, 12620–12631. [Google Scholar] [CrossRef] [PubMed]
- Mihailescu, M.; Sorci, M.; Seckute, J.; Silin, V.I.; Hammer, J.; Perrin, P.S., Jr.; Hernandez, J.I.; Smajic, N.; Shrestha, A.; Bogardus, K.A.; et al. Structure and function in antimicrobial piscidins: Histidine position, directionality of membrane insertion, and pH-dependent permeabilization. J. Am. Chem. Soc. 2019, 141, 9837–9853. [Google Scholar] [CrossRef] [PubMed]
- Comert, F.; Greenwood, A.; Maramba, J.; Acevedo, R.; Lucas, L.; Kulasinghe, T.; Cairns, L.S.; Wen, Y.; Fu, R.; Hammer, J.; et al. The host-defense peptide piscidin P1 reorganizes lipid domains in membranes and decreases activation energiesin mechanosensitive ion channels. J. Biol. Chem. 2019, 294, 18557–18570. [Google Scholar] [CrossRef] [PubMed]
- Libardo, M.D.J.; Bahar, A.A.; Ma, B.; Fu, R.; McCormick, L.E.; Zhao, J.; McCallum, S.A.; Nussinov, R.; Ren, D.; Angeles-Boza, A.M.; et al. Nuclease activity gives an edge to host-defense peptide piscidin 3 over piscidin 1, rendering it more effective against persisters and biofilms. FEBS J. 2017, 284, 3662–3683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, Z.; Liu, Q.; Zhu, Q.; Yang, B.; Khaliq, H.; Sun, A.; Qi, Y.; Moku, G.K.; Su, Y.; Wang, J.; et al. Comparative pharmacokinetics and preliminary pharmacodynamics evaluation of piscidin 1 against PRV and PEDVin rats. Front Chem. 2018, 6, 244. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.; Guo, N.; Chen, S.; Wang, Y.; Liu, B.; He, O. Antiviral activity of piscidin 1 against pseudorabies virus both in vitro and in vivo. Virol. J. 2019, 16, 95. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.-J.; Huang, T.-C.; Muthusamy, S.; Lee, J.-F.; Duann, Y.-F.; Lin, C.-H. Piscidin-1, an antimicrobial peptide from fish (Hybrid Striped Bass Moronesaxatilis x M. chrysops), induces apoptotic and necrotic activity in HT1080 cells. Zool. Sci. 2012, 29, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Facchin, G.; Torre, M.H.; Kremer, E.; Piro, O.E.; Castellano, E.E.; Baran, E.J. Synthesis and characterization of three new Cu(II)-dipeptide complexes. J. Inorg. Biochem. 2002, 89, 174–180. [Google Scholar] [CrossRef]
- Facchin, G.; Torre, M.E. Cu (II) complexation with His–Gly and His–Ala. X-ray structure of [Cu (his–gly)2(H2O)2]·6H2O. Inorg. Chim. Acta 2003, 355, 408–413. [Google Scholar] [CrossRef]
- Sanchiz, J.; Kremer, C. Magnetic properties of copper (II) complexes containing peptides. Crystal structure of [Cu (phe-leu)]. J. Mol. Struct. 2006, 797, 179–183. [Google Scholar] [CrossRef]
- Facchin, G.; Kremer, E. Interaction of Cu-dipeptide complexes with calf thymus DNA and antiproliferative activity of [Cu (ala-phe)] in osteosarcoma-derived cells. Polyhedron 2009, 28, 2329–2334. [Google Scholar] [CrossRef]
- Iglesias, S.; Alvarez, N.; Ribeiro, R.R.; Barroso, R.P.; Costa-Filho, A.J.; Kramer, M.G.; Facchin, G. Synthesis, structural characterization and cytotoxic activity of ternary copper (II)–dipeptide–phenanthroline complexes. A step towards the development of new copper compounds for the treatment of cancer. J. Inorg. Biochem. 2014, 139, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.; Sinn, E.; Martin, R.B. Crystal structure of a mixed-ligand complex of copper (II), 1, 10-phenanthroline, and glycylglycinedianion: Glycylglycinato (1, 10-phenanthroline) copper (II) trihydrate. Inorg. Chem. 1976, 15, 807–811. [Google Scholar] [CrossRef]
- Bhirud, R.G.; Srivastava, T.S. Synthesis, characterization and superoxide dismutase activity of some ternary copper (II) dipeptide-2,2′-bipyridine, 1,10-phenanthroline and 2,9-dimethyl-1,10-phenanthroline complexes. Inorg. Chim. Acta 1991, 179, 125–131. [Google Scholar] [CrossRef]
- Alvarez, S.N.; Kramer, M.; Torre, M.H.; Kremer, E.; Ellena, J.; Filho, A.; Facchin, G. Structural characterization and cytotoxic activity of heteroleptic copper (II) complexes with L-dipeptides and 5-NO2-phenanthroline. Crystal structure of [Cu(Phe-Ala)(5-NO2-Phen)]·4H2O. Struct. Chem. Crystallogr. Commun. 2015, 1, 1–7. [Google Scholar]
- Vieira, E.D.; Casado, N.M. Weak exchange interaction supported by a biologically relevant long chemical bridge in a Cu-peptide model compound. Inorg. Chem. 2006, 45, 2942–2947. [Google Scholar] [CrossRef]
- Ng, C.; Kong, S.M.; Tiong, Y.L.; Maah, M.J.; Sukram, N.; Ahmad, M.; Khoo, B.A. Selective anticancer copper(II)-mixed ligand complexes: Targeting of ROS and proteasomes. Metallomics 2014, 6, 892–906. [Google Scholar] [CrossRef] [PubMed]
- Facchin, G.; Kremer, E. Structural characterization of a series of new Cu-dipeptide complexes in solid state and in solution. Polyhedron 2006, 25, 2597–2604. [Google Scholar] [CrossRef]
- Facchin, G.; Veiga, N.; Kramer, M.G.; Batista, A.A.; Várnagy, K.; Farkas, E.; Moreno, V.; Torre, M.H. Experimental and theoretical studies of copper complexes with isomeric dipeptides as novel candidates against breast cancer. J. Inorg. Biochem. 2016, 162, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, N.; Noble, C.; Torre, M.H.; Kremer, E.; Costa-Filhod, A.J.; Mendesd, L.F.; Kramere, M.G.; Facchina, G. Synthesis, structural characterization and cytotoxic activity against tumor cells of heteroleptic copper (I) complexes with aromatic diimines and phosphines. Inorg. Chim. Acta 2017, 466, 559–564. [Google Scholar] [CrossRef]
- Sugimori, T.; Shibakawa, K.; Sugimori, T.; Shibakawa, K.; Masuda, H.; Odani, A.; Yamauchi, O. Ternary metal (II) complexes with tyrosine-containing dipeptides. Structures of copper (II) and palladium (II) complexes involving L-tyrosylglycine and stabilization of copper (II) complexes due to intramolecular aromatic ring stacking. Inorg. Chem. 1993, 32, 4951–4959. [Google Scholar] [CrossRef]
- Gaála, A.; Mihucz, V.G.; Bősze, S.; Szabó, I.; Baranyi, M.; Horváth, P.; Streli, C.; Szoboszla, N. Comparative in vitro investigation of anticancer copper chelating agents. Microchem. J. 2018, 136, 227–235. [Google Scholar] [CrossRef]
- Xia-Bing, F.; Zhang, J.-J.; Liu, D.-D.; Gan, Q.; Gao, H.-W.; Mao, Z.-W.; Le, X.-Y. Cu(II)–dipeptide complexes of 2-(4′-thiazolyl)benzimidazole: Synthesis, DNA oxidative damage, antioxidant and in vitro antitumor activity. J. Inorg. Biochem. 2015, 143, 77–87. [Google Scholar]
- Gana, Q.; Zhang, C.-L.; Wang, B.-F.; Xiong, Y.-H.; Fu, Y.-L.; Maoac, Z.-W.; Le, X.-Y. Two new mixed copper(II)–dipeptide complexes of N,N-donor heterocycle ligands: Studies on their non-covalent DNA binding, chemical nuclease, antioxidant and anticancer activities. RSC Adv. 2016, 6, 35952–35965. [Google Scholar] [CrossRef]
- Qi, Y.Y.; Gan, Q.; Liu, Y.X.; Xiong, Y.H.; Mao, Z.W.; Le, X.Y. Two new Cu(II) dipeptide complexes based on 5-methyl-2-(2′-pyridyl)benzimidazole as potential antimicrobial and anticancer drugs: Special exploration of their possible anticancer mechanism. Eur. J. Med. Chem. 2018, 154, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Komarnicka, U.K.; Kozieł, S.; Starosta, R.; Kyzioł, A. Selective Cu(I) complex with phosphine-peptide (SarGly) conjugate contra breast cancer: Synthesis, spectroscopic characterization and insight into cytotoxic action. J. Inorg. Biochem. 2018, 186, 162–175. [Google Scholar] [CrossRef]
- Komarnicka, U.K.; Kozieł, S.; Zabierowski, P.; Kruszyński, R.; Lesiówa, M.K.; Tisato, F.; Porchia, M.; Kyzioł, A. Copper(I) complexes with phosphines P(p-OCH3-Ph)2CH2OH and P(p-OCH3-Ph)2CH2SarGly. Synthesis, multimodal DNA interactions, and prooxidative and in vitro antiproliferative activity. J. Inorg. Biochem. 2020, 203, 110926. [Google Scholar] [CrossRef] [PubMed]
- Hegde, M.L.; Bharathi, P.; Suram, A.; Venugopal, C.; Jagannathan, R.; Poddar, P.; Srinivas, P.; Sambamurti, K.; Rao, K.J.; Scancar, J.; et al. Challenges Associated with Metal Chelation Therapy in Alzheimer’s Disease. J. Alzheimers Dis. 2009, 17, 457–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szeto, J.Y.Y.; Lewis, S.J.G. Current Treatment Options for Alzheimer’s Disease and Parkinson’s Disease Dementia. Curr. Neuropharmacol. 2016, 14, 326–338. [Google Scholar] [CrossRef] [PubMed]
- Pickart, L.; Michelle, J.; Soltero, V.; Margolina, A. The Human Tripeptide GHK-Cu in Prevention of Oxidative Stress and Degenerative Conditions of Aging:Implications for Cognitive Health. Oxidative Med. Cell. Longev. 2012, 5, 8. [Google Scholar]
- Boas, D. A Novel Copper-Binding Peptide That Self-Assembles Into a Transparent Antibacterial and Antiviral Coating Front. Bioeng. Biotechnol. 2021, 20, 11–19. [Google Scholar]
- Andersen, A.; Chen, Y.; Birkedal, H. Bioinspired Metal–Polyphenol Materials: Self-Healing and Beyond. Biomimetics 2019, 4, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sagripanti, J.L.; Routson, L.B.; Bonifacino, A.C.; Lytle, C.D. Mechanism of coppermediatedinactivation of herpes simplex virus. Antimicrob. Agents Chemother. 1997, 41, 812–817. [Google Scholar] [CrossRef] [Green Version]
- Betanzos-Cabrera, G.; Rez, F.J.R.; Oz, J.L.M.; Barrn, B.L.; Maldonado, R. Inactivation ofHSV-2 by ascorbate-Cu (II) and its protecting evaluation in CF-1 miceagainst encephalitis. J. Virol. Methods 2004, 120, 8. [Google Scholar] [CrossRef]
- Guo, W.J.; Ye, S.S.; Cao, N.; Huang, J.; Gao, J.; Chen, Q.Y. ROS-mediatedautophagy was involved in cancer cell death induced by novel copper(II)complex. Exp. Toxicol. Pathol. 2010, 62, 577–582. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).