Abstract
Spent automotive catalysts (SAC) not only contain significant amounts of platinum group metals (PGMs) but also hazardous heavy metals, rendering them a solid waste. A harmless technology for the efficient recovery of PGMs through copper smelting has been proposed. By investigating the effects of the CaO/SiO2 mass ratio and Al2O3 content on the properties of the slag, the composition of the slag was adjusted. The influence of copper dosage, Na2B4O7 dosage, smelting temperature, and smelting time on the recovery efficiency of PGMs was also discussed. The determined composition of the target slag was 36.44 wt% CaO, 45.56 wt% SiO2, 12.00 wt% Al2O3, and 6.00 wt% MgO. The optimal processing conditions included 12 wt% Cu, 4 wt% Na2B4O7, smelting temperature 1450 °C, and smelting time 90 min. Ultimately, the recovery efficiency of PGMs reached 99.5%. Compared to traditional plasma furnace smelting methods, PGMs were efficiently recovered at a lower melting temperature. A pilot-scale experiment with a mass of 30 kg also achieved a recovery rate of over 99% for PGMs. TCLP results indicate that the heavy metals were immobilized within the glass slag.
1. Introduction
Platinum group metals (PGMs) are widely utilized in automotive and industrial catalysts due to their excellent physical and chemical properties [1]. In 2023, global demand for PGMs in automotive catalysts was substantial, with 95.3 t Pt, 256.7 t Pd, and 29.5 t Rh consumed. These quantities represent a significant share of the total demand for Pt, Pd, and Rh across all applications, accounting for 41%, 84%, and 90%, respectively [2]. The global supply of PGMs is characterized by small, concentrated reserves, predominantly in South Africa and Russia. Consequently, recovering PGMs from secondary sources has become the main strategy [3]. Spent automotive catalysts (SAC) have increasingly become a major secondary source of PGMs. Compared to primary ores, the concentration of these PGMs is significantly higher in SAC [4]. Therefore, the recycling and utilization of SAC are critical for the sustainable development of PGMs.
The recovery methods for PGMs primarily include pyrometallurgy, hydrometallurgy, and bioleaching [5]. Currently, bioleaching has not yet achieved large-scale application. The core of hydrometallurgy lies in the dissolution of metal elements or compounds, allowing PGMs or carriers to enter the solution in ionic form, thereby facilitating separation. The basic process involves treating SAC powder with acids, bases, or other solutions to selectively leach PGMs or carriers, thereby enriching PGMs [6,7]. However, hydrometallurgy has several drawbacks, including long reaction times and suboptimal recovery rates [4]. Furthermore, the difficulty in treating the resulting hazardous waste residues and waste liquids is also an overlooked issue.
Pyrometallurgical enrichment is a technique that uses high-temperature melting of mechanically pre-treated SAC combined with a capturing agent to enrich PGMs. The pyrometallurgical enrichment process has several significant advantages: lower raw material grade requirements; a simple operational flow; high processing efficiency; and low production costs, resulting in high PGMs recovery rates and making it the mainstream process in industrial applications [8,9]. Iron is a green, economical, and readily available metal that exhibits a strong affinity for PGMs [10]. The method of iron capture is advantageous due to its low pollutant generation and high recovery rate [11]. However, the typical temperature for iron capture ranges from 1600~2000 °C, which leads to the formation of Fe-Si alloy [12]. The presence of Fe-Si alloy presents significant challenges for the subsequent separation and purification of PGMs [1]. Although technologies for low-temperature iron capture of PGMs have been developed to avoid the formation of Fe-Si alloys [13,14], it remains uncertain whether these technologies can be applied at a large scale. Copper capture is a mature technology that is widely used in industrial production. Its capturing mechanism involves the formation of droplets of molten copper, which can alloy with PGMs particles. Moreover, the gravity of alloy particles is greater than that of furnace slag, enabling effective separation, with heavier Pt exhibiting higher recovery rates than Pd and Rh [15]. Compared to iron capture, copper capture offers the following advantages: a lower melting point for copper, leading to reduced smelting temperatures, and a Cu-PGMs alloy that is more amenable to subsequent electrolytic refining for further PGM enrichment. In recent years, numerous copper-containing materials have been developed as capture agents for PGMs enrichment [16,17]. However, these studies have predominantly focused on the types of capturing agents, with insufficient attention given to achieving low-temperature copper capture through slag material ratios and process conditions. Furthermore, SACs typically contain substantial amounts of coke, vanadium, nickel, and other heavy metals, which can lead to surface and water pollution, adversely affecting the survival of flora and fauna and posing severe threats to the ecological environment. Consequently, SACs are categorized as hazardous solid waste [2]. Vitrification technology represents a promising strategy for the sustainable treatment of SAC. Its fundamental principle involves melting at high temperatures while adjusting the slag composition, ultimately aiming to immobilize harmful heavy metals in the glassy slag by-product [18]. Although vitrification technology has been widely applied, research combining low-temperature copper capture with it remains limited.
This study proposes a process that combines low-temperature copper capture with vitrification technology to assess the potential environmental risks of glass slag while accounting for PGMs recovery rates. By investigating the effects of the CaO/SiO2 mass ratio and Al2O3 content on the melting point and viscosity of the slag, the composition of the slag was optimized to achieve efficient capture of PGMs under low-temperature conditions. Additionally, the study examines whether the slag is amorphous. The influence of Cu dosage, Na2B4O7 dosage, smelting temperature, and smelting time on the recovery rates of PGMs was also discussed. Finally, pilot-scale experiments were conducted under optimized conditions to confirm the feasibility of the process and evaluate the environmental harmlessness of the slag.
2. Experimental Process
2.1. Thermodynamic Analysis
The following lists some reactions that may be involved in the reduction in PGMs during the copper smelting and collection process, taking Pd as an example:
The thermodynamic data for the above reaction were calculated using Factsage 7.0. The relationship between Gibbs free energy (ΔG) and temperature is shown in Figure 1. As shown in Figure 1a, Pd undergoes an oxidation reaction with oxygen at low temperatures (600~800 °C) to form the corresponding oxide, PdO. This phenomenon occurs because PGMs’ oxides are thermodynamically more stable at lower temperatures. The oxidation reaction provides intermediate products (oxides) for subsequent reduction and alloying processes, facilitating the reduction in PGMs to their metallic state by reducing agents. A melting temperature above 800 °C indicates that the formation of PGMs’ oxides during the smelting process is inevitable. Even at a relatively low temperature, PdO undergoes a reduction reaction by C and Cu, and the reduction ΔG by C is lower than that by Cu at the same temperature. Therefore, PdO is first reduced by C and then by Cu. This indicates that during the copper smelting and collection process, PdO is particularly prone to reduction to the elemental form. PdS may undergo oxidation reactions with O2 or reduction reactions with C. However, the results show that the ΔG of the reduction reaction is significantly lower than that of the oxidation reaction, indicating that PdS is preferentially reduced by C. For Reaction (6), when the temperature exceeds 1500 °C, ΔG < 0. This indicates that a reduction reaction of SiO2 occurs when the melting temperature surpasses 1500 °C, resulting in the formation of silicon (Si) impurities. To prevent the reduction of SiO2, the smelting temperature should be controlled not to exceed 1500 °C. Reactions (7)~(12) indicate that Pd is more easily reduced as the temperature rises. Therefore, the smelting temperature is set between 1350 °C and 1500 °C to ensure the reduction in PGMs while simultaneously preventing the formation of Si.
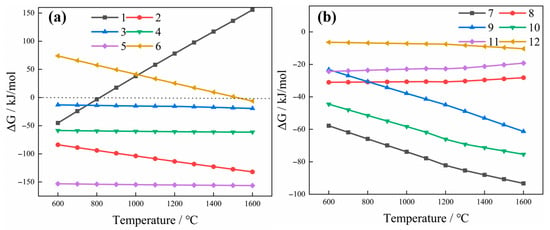
Figure 1.
Standard Gibbs free energy of possible reactions during smelting (a) Reactions (1)~(6); (b) Reactions (7)~(12).
2.2. Slag Design
The SAC used in this experiment was provided by a precious metals recycling company in China. Figure 2 shows the XRD (D8 ADVANCE, BRUKER AXS GMBH, 8°/min) pattern of SAC. As can be seen, the main component of SAC is cordierite (PDF#13-0294). To increase the recovery rate of PGMs, it is necessary to reduce the content of PGMs in the slag phase as much as possible and improve slag–metal separation situation, both of which are related to the slag viscosity. At the same time, the smelting temperature should be reduced as much as possible to achieve low-temperature copper capture. This not only reduces energy consumption but also extends the service life of the equipment.
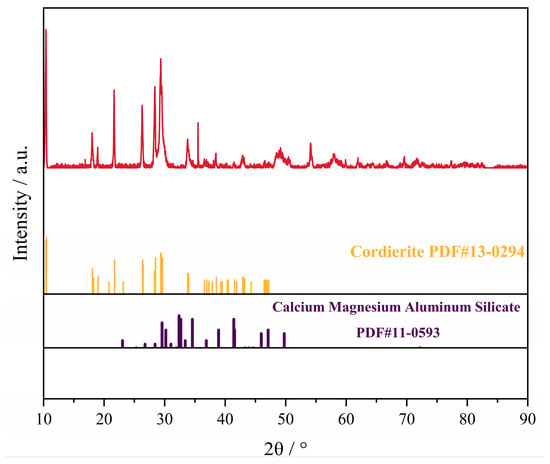
Figure 2.
XRD pattern of SAC.
As shown in Table 1, the main components of SAC are Al2O3 and SiO2, and both exceed 30%. In addition, the SAC contains a small amount of MgO. During the production process, it is essential to adhere to principles of low energy consumption and cost-effectiveness while minimizing the use of expensive, environmentally harmful fluxing agents. Given the significant impact of CaO and SiO2 on the regulation of slag properties, CaO-SiO2-Al2O3-MgO was selected as the basis for the design of the slag system. The pseudo-ternary phase diagram of CaO-SiO2-Al2O3-MgO is illustrated in Figure 3. Based on a predetermined maximum smelting temperature of 1500 °C, different slag compositions with varying CaO/SiO2 mass ratios and Al2O3 contents were selected from the phase diagram, ensuring that the liquidus temperature of the slag does not exceed 1500 °C. The pre-established slag compositions are presented in Table 2. From Figure 3, it can be observed that the liquidus temperatures of the designed slags do not exceed 1500 °C. The study primarily investigates the effects of the CaO/SiO2 mass ratio and Al2O3 content on the properties of the slag. When the CaO/SiO2 mass ratio is too low, it can lead to excessively high slag viscosity, while a ratio that is too high may result in the formation of non-amorphous glassy slag. When the Al2O3 content exceeds 15 wt%, it significantly affects the thermal stability of the slag, causing considerable fluctuations in the thermal viscosity of the molten slag with changes in basicity, which in turn affects the stability of the production process. In this study, such fluctuations during production can affect the recovery rate of PGMs. Given this, and considering the substantial variability in Al2O3 content within the cordierite carrier, the study aims to design the slag such that the Al2O3 content does not exceed 15 wt% to enhance the reference value of the research findings. Analytical reagents were mixed uniformly according to the designed compositions, and 200 g of slag was prepared using a medium-frequency induction furnace. The viscosity of the slag was measured using the RTW10 high-temperature melt property measurement instrument from Northeastern University, employing the rotating cylinder method. Prior to testing, the viscosity constants were calibrated using castor oil of known viscosity. The breaking temperature (TBK) can be determined from the intersection of the temperature–viscosity curve and the tangent line at a 45° angle.

Table 1.
Composition table of SAC.
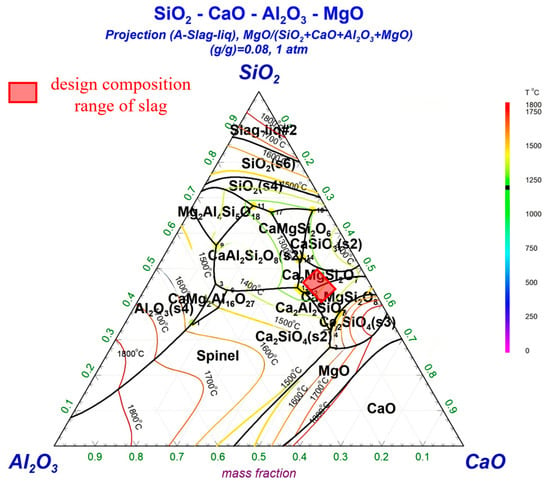
Figure 3.
CaO-Al2O3-SiO2-MgO pseudo-ternary phase diagram.

Table 2.
Selection of experimental slag system.
2.3. Recycling Process
All smelting experiments were conducted in an intermediate frequency induction furnace. The fluxing agents (SiO2, CaO, MgO, and Na2B4O7), collector agents (Cu), and a suitable amount of reducing agent (carbon powder) were mixed with 100 g of SAC. The homogeneous mixture was placed in a graphite crucible (Φ75 mm * Φ60 mm * 80 mm) and positioned within the heating furnace. After preheating the equipment for 10 min, the current was adjusted to raise the temperature to a specified level and maintain it for a designated duration. Since PGMs do not oxidize at high temperatures, the entire experiment was conducted in air. During the collection process, PGM particles entered the copper phase, forming an alloy. After a certain period, the molten sample was rapidly poured into a preheated mold. Due to the significant density difference, the slag and metallic phase can be easily separated. Subsequently, the presence of precious metals in the slag was confirmed using ICP-OES. The process parameters involved in the capture experiment are shown in Table 3. The calculation of the recovery rate of PGMs was performed using the following equations:
where mg is the mass of slag (g); CPGMs is the content of PGMs in slag (g/t); mr is the mass of SAC (g); and is the PGMs content in SAC (g/t).

Table 3.
Process parameters of the capture experiment.
2.4. TCLP Test
To assess the fixation of heavy metals in glass slag, a toxicity characteristic leaching procedure (TCLP) test was conducted [19]. The TCLP leaching procedure utilizes “METHOD 1311”. The analytical precision of this method is reported to be 17~118%RSD. In the experiment, the leachate was prepared by diluting 5.7 mL of acetic acid in 500 mL of distilled water. Subsequently, 64.3 mL (1 mol/L) of sodium hydroxide was added, diluted with distilled water to a final volume of 1 L, and pH was adjusted to 4.93 ± 0.05. Place 100 g of crushed samples with particle sizes less than 10 mm in the leaching bottle and add the leaching solution to maintain a solid–liquid ratio of 20 mL/g. After sealing the leaching bottle, it should be placed in an environment of 25 °C and left to stand for 24 h. The obtained leachate was filtered through a filter membrane smaller than 0.45 µm, and then the concentration of heavy metals (Zn, Cr, As, Pb, Se, Cu, Ag, Cd, Ba, and Hg) in the leachate was determined by inductively coupled plasma optical emission spectrometry (ICP-OES, Optima 8300DV detection limit 10~6 mg/L). The leachate concentrations obtained will be compared with the maximum contaminant levels for toxic characteristics as outlined in the United States Environmental Protection Agency (EPA) regulations (40 CFR Part 261.24) to determine whether the glass slag poses an environmental hazard.
3. Results and Discussion
3.1. Slag Composition Selection
3.1.1. The Influence of the Mass Ratio of CaO/SiO2 on the Physical Properties of Slag
Figure 4a–c shows the viscosity–temperature curves with different mass ratios of CaO/SiO2 under the condition of a fixed Al2O3 content. The figure shows that, as temperature rises, slag viscosity gradually decreases. This is because when the temperature rises, excessive heat energy will also damage the original slag structure, reducing the viscosity of the slag [20].
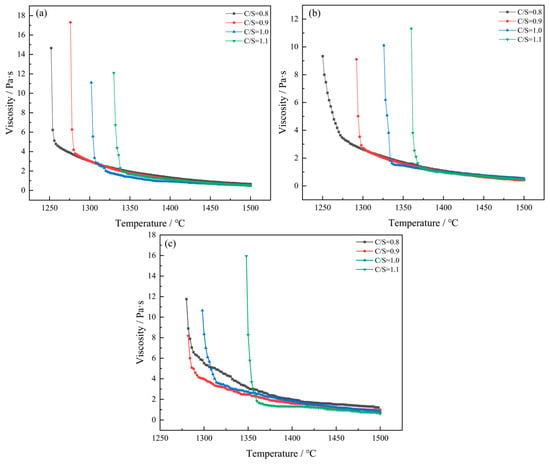
Figure 4.
Viscosity–temperature curves of different CaO/SiO2 mass ratios under the condition of fixed Al2O3 content: (a) 10 wt% Al2O3; (b) 12 wt% Al2O3; (c) 15 wt% Al2O3.
When the mass ratio of CaO/SiO2 increased from 0.8 to 1.1, the viscosity of the slag decreased. For instance, at an Al2O3 content of 15 wt% and a temperature of 1500 °C, as the CaO/SiO2 mass ratio increases from 0.8 to 1.1, the viscosity of the slag decreases from 0.9767 Pa·s to 0.6099 Pa·s. The reason is that the silicate slag is a silicate network mainly composed of [SiO4]4− tetrahedral structures, and each [SiO4]4− tetrahedral structure is connected by bridged oxygen (O0). With the increase in CaO/SiO2, the free oxygen ions (O2−) in the slag increase along with the increase in CaO content. The free oxygen ions (O2−) will react with bridged oxygen (O0), breaking the Si-O bond in the silicate structure and forming non-bridged oxygen, causing the complex silicate structure to depolymerize and eventually depolymerize into simple small units. As the complex silicate structure depolymerizes, the viscosity of the slag will decrease [21].
Figure 5 shows the curve of TBK as a function of the CaO/SiO2 mass ratio for different Al2O3 contents. At different Al2O3 contents, TBK increases with increasing CaO/SiO2 mass ratio. The maximum increase occurs at 12 wt% Al2O3, where TBk rises from 1264 °C to 1370 °C. This is due to the high melting point of CaO and the formation of substances such as Ca2SiO4 (melting point 2130 °C). When the mass ratio of CaO/SiO2 increases from 0.8 to 1.1, the total amount of high-melting-point phases in the slag increases, and the crystallization ability of the slag at high temperatures also increases. Therefore, the TBK increases accordingly.
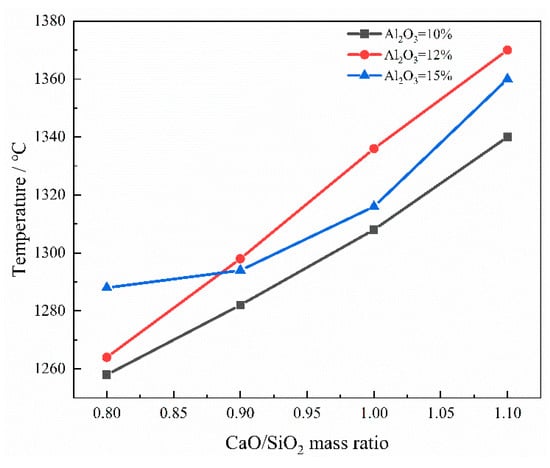
Figure 5.
The curve of TBK varying with CaO/SiO2 mass ratios under different Al2O3 contents.
3.1.2. The Influence of Al2O3 Content on the Physical Properties of Slag
Figure 6a–d shows the viscosity–temperature curves with different Al2O3 contents under the condition of a fixed mass ratio of CaO/SiO2. When the content of Al2O3 increases from 10 wt% to 15 wt%, the viscosity of the slag shows a trend of first decreasing and then increasing and reaches the minimum value when Al2O3 = 12%. For instance, at a CaO/SiO2 mass ratio of 0.9 and a temperature of 1500 °C, the corresponding viscosities for Al2O3 contents of 10 wt%, 12 wt%, and 15 wt% are 0.5393 Pa·s, 0.4198 Pa·s, and 0.939 Pa·s, respectively. Al2O3 is an amphoteric oxide, and the value of XAl2O3/XMxO in the slag is less than 1 (XAl2O3 is the molar fraction of Al2O3, and XMxO is the total molar fraction of the basic oxide). Therefore, Al2O3 in the slag can be regarded as an acidic oxide. Therefore, Al2O3 should exist in two forms: Al3+ and [AlO5]4− [22]. With the continuous increase of Al2O3 content, part of the [SiO4]4− regular tetrahedral structure in the slag is replaced by the [AlO5]4− tetrahedral structure. Due to the weaker bond energy of Al-O compared to the Si-O bond, the stress affecting the free flow of the slag is reduced, making the slag flow more easily and thus reducing its viscosity. As the content of Al2O3 continues to increase, the more complex [AlO5]4− tetrahedral structure will lead to an increase in the degree of polymerization of the viscous units in the slag, making the slag structure more complex, resulting in a deterioration of the slag’s fluidity and subsequently an increase in viscosity. It can also be seen that when Al2O3 = 15%, the viscosity of the slag changes dramatically. This indicates that when the Al2O3 content exceeds 15 wt%, it will have a significant impact on the thermal stability of the slag composition. It is consistent with the previous inference.
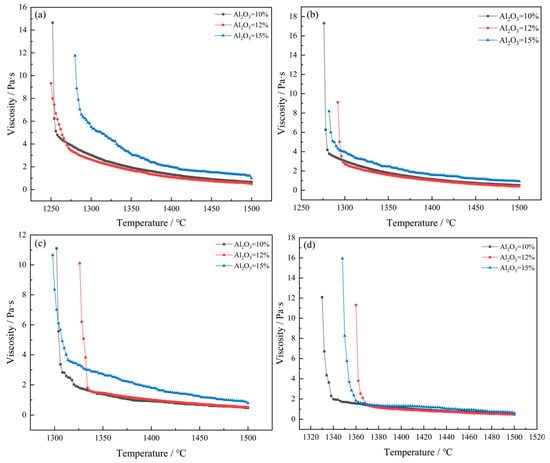
Figure 6.
Viscosity–temperature curves of different contents of Al2O3 under the condition of fixed CaO/SiO2 mass ratios: (a) CaO/SiO2 = 0.8; (b) CaO/SiO2 = 0.9; (c) CaO/SiO2 = 1.0; (d) CaO/SiO2 = 1.1.
The influence of different mass ratios of CaO/SiO2 and Al2O3 content on TBK is shown in Figure 7. When the content of Al2O3 increases from 10 wt% to 12 wt%, the TBK value also increases accordingly. When CaO/SiO2 = 0.9, Al2O3 = 12 wt%, the value of TBk increases from 1282 °C to 1298 °C. This is because, as the content of Al2O3 increases, the formation conditions of high-melting-point phases are improved, such as MgO·Al2O3 (melting point 2135 °C) and CaO·Al2O3 (melting point 1600 °C). Therefore, the content of phases with strong crystallization ability and high melting points in the slag increases, while more heterogeneous phases form. Therefore, when the content of Al2O3 increases, the TBK value of the slag also increases accordingly [23]. When the CaO/SiO2 mass ratio is 0.9~1.1 and Al2O3 content increases to 15 wt%, the value of TBK decreases. When CaO/SiO2 = 0.9, Al2O3 = 12 wt%, the value of TBk decreases from 1298 °C to 1294 °C. It is because Al2O3 reacts with SiO2 to form 3Al2O3·2SiO2 (melting point 1550 °C), and this new compound usually has a relatively low melting point. In addition, the addition of Al2O3 can disrupt the original crystal structure of the slag, making its lattice unstable. This structural disturbance will reduce the TBK value of the slag.
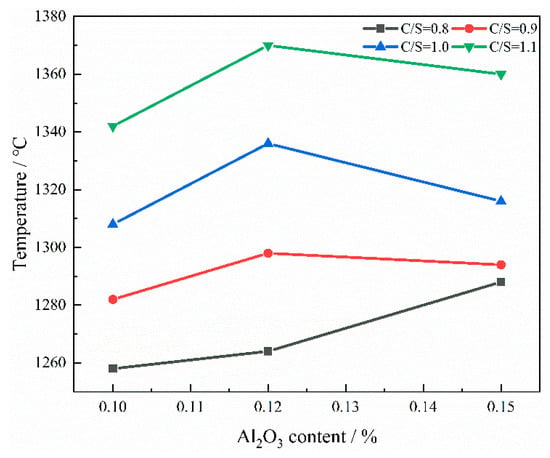
Figure 7.
The curve of TBK varying with Al2O3 contents under different CaO/SiO2 mass ratios.
Considering the physical properties of the slag and the amount of slag produced comprehensively, 36.44 wt% CaO-45.56 wt% SiO2-12.00 wt% Al2O3-6.00 wt% MgO was selected as the target slag type for the capture experiment. An XRD test was conducted on the target slag system, and the result is shown in Figure 8a. No obvious characteristic peaks were observed in the spectrum, indicating that the slag is amorphous glass slag. Figure 8b describes the microscopic morphology of the slag. It can be found that no grains are found on the surface of the slag, which also indicates that the slag is amorphous.
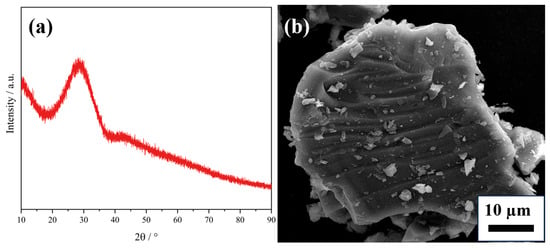
Figure 8.
Characterization of the target slag system: (a) XRD pattern; (b) SEM image.
3.2. Selection of Process Conditions
3.2.1. The Effect of Copper Dosage
As can be seen from Figure 9, when the dosage of the collector Cu is 8%, Cu cannot fully contact the PGMs, thus resulting in a very small recovery rate of Pt, Pd, and Rh, not exceeding 85%. When the amount of metal to be captured is 10 to 12 wt%, the recovery rate significantly increases compared to 8 wt%. This indicates that increasing the amount of the collector can effectively enhance the contact and collection probability of copper with PGMs, thereby significantly improving the recovery efficiency of PGMs. When the captured metal amount reached 12 wt%, the recovery rates for Pt, Pd, and Rh were 96.3%, 96.12%, and 95.52%, respectively. When the Cu content was 14 wt%, the recovery rate did not change much. This phenomenon might be due to excessive Cu use, which led to collector aggregation and reduced the effective contact surface area. From the SEM image and EDS spectrum of the slag at a Cu dosage of 14 wt% presented in Figure 10, it can be observed that, in contrast to the uniform distribution of Al, Ca, and Si within the slag matrix, Cu exhibits a certain degree of aggregation. Moreover, the increased Cu dosage did not correspondingly increase the number of PGMs that could be collected. Additionally, considering the relatively high price of Cu, excessive use of Cu not only increases the production costs. Moreover, it increased the difficulty of separation in the subsequent smelting process. Eventually, it was determined that the amount of copper collector added to the SAC was 12 wt%.
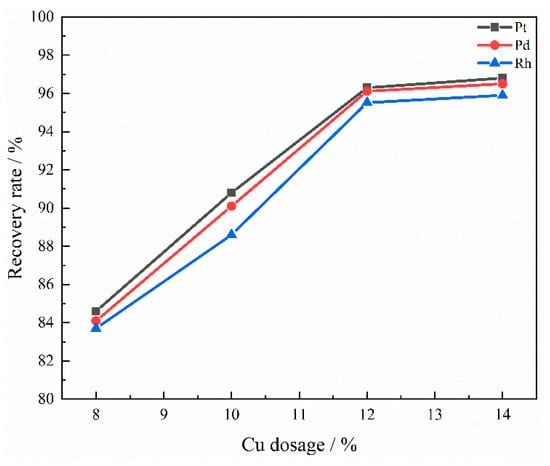
Figure 9.
Influence of Cu dosage on recovery rate.
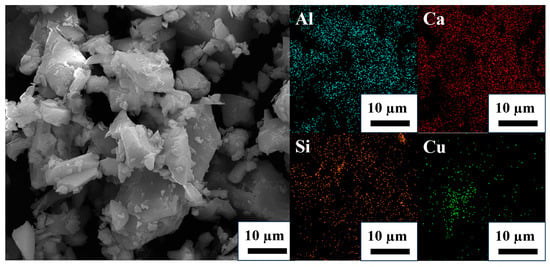
Figure 10.
The SEM image and EDS spectrum of the slag at a Cu dosage of 14 wt%.
3.2.2. The Effect of Na2B4O7 Dosage
Figure 11 illustrates the effect of Na2B4O7 on the recovery rate of PGMs. It can be seen that with the addition of Na2B4O7, the recovery rate of PGMs gradually increases. When the addition amount of Na2B4O7 reached 4 wt%, the recovery rates of Pt, Pd, and Rh reached 99.84%, 99.72%, and 99.51%, respectively. PGMs are recovered by forming alloys with Cu and settling to the bottom of the slag under gravity. The viscosity of the slag is crucial for alloy settling to the bottom. A lower slag viscosity facilitates the easier descent of the alloy [9]. Na2B4O7, as an important flux, can significantly reduce the viscosity of the slag.
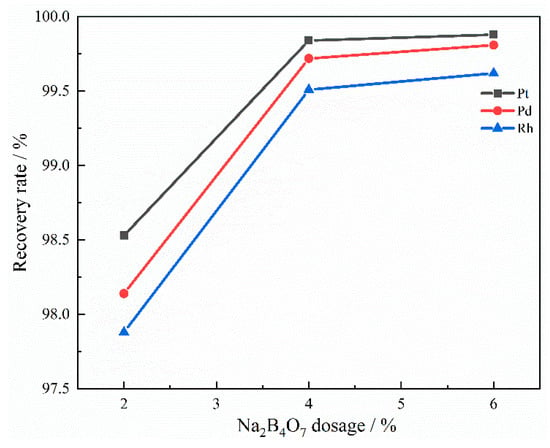
Figure 11.
Influence of Na2B4O7 dosage on recovery rate.
The viscosity of the captured slag was tested, and the results are shown in Figure 12. With the addition of Na2B4O7, the viscosity of the slag decreases. At 1450 °C, the addition of Na2B4O7 resulted in a decrease in the slag viscosity from 1.0111 Pa·s to 0.52 Pa·s. This is because it decomposes into Na2O and B2O3 when heated. The increase in Na2O content will lead to depolymerization of the slag network, thereby reducing its structural complexity. Therefore, the fluidity of the slag is improved and its viscosity is reduced [24]. The addition of B2O3 will produce a [BO3]3− regular trihedron. This leads to simplification of the slag structure and a reduction in slag viscosity [25]. After comprehensive consideration, the additional amount of Na2B4O7 was determined to be 4 wt%.
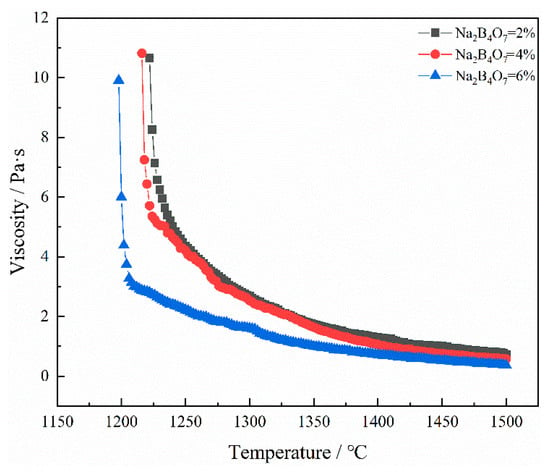
Figure 12.
Viscosity–temperature curve of slag without Na2B4O7 content.
3.2.3. The Effect of Smelting Temperature
It can be seen from Figure 13 that as the smelting temperature rises, the recovery rate of PGMs significantly increases and then tends to stabilize. The recovery rate of PGMs increased significantly within the range of 1350~1450 °C, while the increase was very small above 1450 °C. This is because an increase in temperature will lead to a decrease in the viscosity of the slag. When the smelting temperature is relatively low, PGMs have difficulty flowing in the melt and are hard to recover. However, as the temperature rises, the difficulty of PGM particles settling in the melt decreases. When the temperature reaches 1450 °C, almost all of the PGMs enter the Cu metal phase. Raising the temperature not only has a limited effect on improving the recovery rate of PGMs, but also results in a significant heat loss. Therefore, the optimal smelting temperature is 1450 °C.
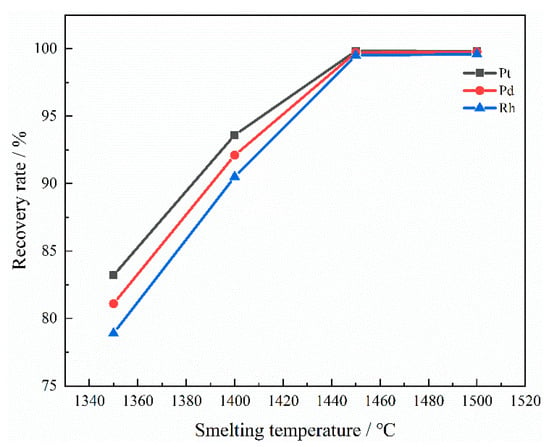
Figure 13.
Influence of smelting temperature on recovery rate.
3.2.4. The Effect of Smelting Time
As shown in Figure 14, when the smelting time is 30 to 60 min, the recovery rate increases significantly. When the smelting time was 90 min, the recovery rate reached its maximum, indicating that the optimal reaction conditions were achieved. However, as smelting time continued to increase, the recovery rate of PGMs decreased slightly. This is attributed to the fact that the recycling process is carried out in an air environment. The Cu-PGMs alloy is prone to oxidation and mixing into the slag in an air environment, resulting in a slight decrease in the recovery rate of PGMs. Moreover, an excessively long smelting time will increase the solubility of PGMs in the slag.
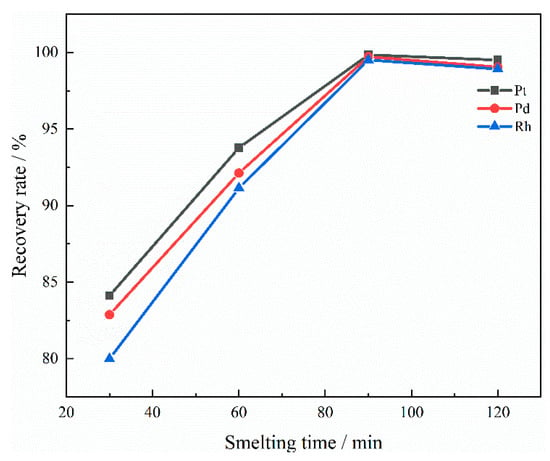
Figure 14.
Influence of smelting time on recovery rate.
Based on the above discussion, the optimized PGMs enrichment process parameters should be Cu 12 wt%; Na2B4O7 4 wt%; smelting temperature 1450 °C; and smelting time 90 min.
3.3. Verification Experiments
To avoid the randomness of the above experimental results, three verification experiments were conducted. The composition of the slag targeted was 36.44 wt% CaO, 45.56 wt% SiO2, 12.00 wt% Al2O3, and 6.00 wt% MgO. The process conditions were set at 12 wt% Cu, 4 wt% Na2B4O7, smelting temperature 1450 °C, and smelting time 90 min. The experimental results are illustrated in Figure 15. The recovery rates for PGMs in the three experiments were 99.62%, 99.71%, and 99.53%, respectively. The recovery rates for Pt, Pd, and Rh were as follows: Pt 99.84 ± 0.22%; Pd 99.72 ± 0.19%; and Rh 99.51±0.08%. These findings indicate that the obtained process parameters exhibit reproducibility.
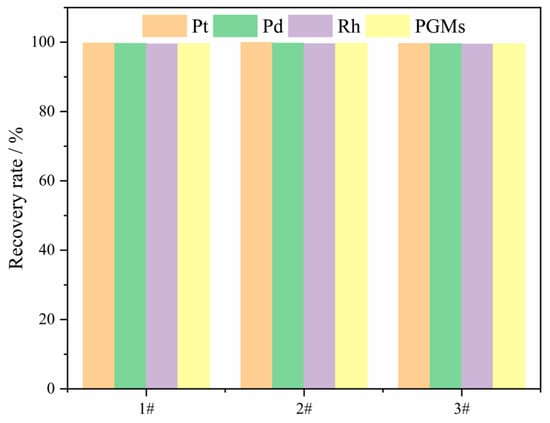
Figure 15.
The recovery rates of PGMs in the verification experiments.
3.4. Pilot-Scale Experiment
To verify the feasibility of optimizing slag type and process conditions, three sets of pilot tests were conducted using the previously optimized slag composition and process parameters. The chemical composition of the SAC used in the pilot-scale experiments is presented in Table 1. The concentrations of Pt, Pd, and Rh are 310, 2140, and 320 g/t, respectively. A total of 30 kg of SAC was added for each experiment. After mixing the SAC with fluxing agents, collecting agent, and reducing agent, the mixture was charged into an electric arc furnace, with the experimental temperature maintained at approximately 1450 °C. After allowing all materials to melt for about 90 min, the slag and metal were discharged from the furnace. The experimental procedure is illustrated in Figure 16. The recovery rates of PGMs are presented in Table 4. The recovery rates of all three groups of experiments exceeded 99%, indicating that this technology has industrial application potential.

Figure 16.
Copper collection experiments on a scale of 30 kg (a) Feeding process; (b) Smelting process; (c) Slag discharge process.

Table 4.
The recovery rate of PGMs in the pilot-scale experiments (%).
XRD tests were conducted on the three groups of slags and alloys obtained, and the results are shown in Figure 17. As can be seen from Figure 17a, all three slag groups are expected to be amorphous glass slag. As can be seen from Figure 17b, the main crystal phase in the alloy is Cu (PDF#04-0836), and no other impurities have been found. The SEM results in Figure 18 indicate that PGMs have been successfully enriched in the Cu matrix.
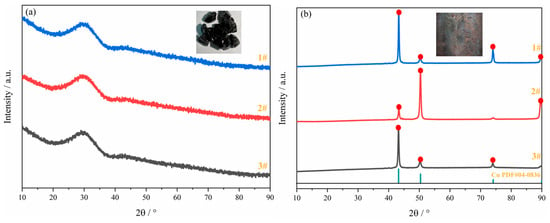
Figure 17.
XRD patterns of the products: (a) glass slag; (b) Cu-PGMs alloy.
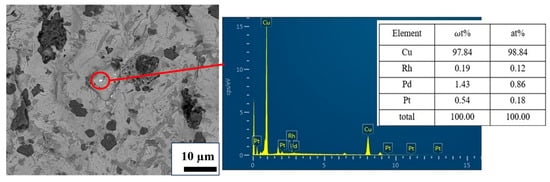
Figure 18.
SEM and EDS results of copper alloys.
The TCLP experiments were conducted on three groups of slags to systematically study the leaching characteristics of heavy metals in glass slag, and the experimental results were compared with the leaching limit values. To ensure the accuracy of the tests, each batch of slag was subjected to three repetitions of the testing process. Table 5 shows the TCLP test results of glass slag. Data show that the leaching content of heavy metals is below the limit value for all samples. Therefore, the obtained glass slag can be regarded as environmentally harmless slag. The fixation mechanism of heavy metals by glass slag includes: being physically encapsulated within the glass phase; replacing cation coordination positions in the crystalline phase and entering lattice positions, forming a solid-solution; and generating new stable crystal phases with the original substances in the original melt [25].

Table 5.
TCLP test results and leaching standards (mg/L).
In addition, the harmless glass slag can be utilized for high-value applications, such as the production of foamed glass ceramics [26] and slag wool fibers [27]. These methods of production not only address the issue of land resource wastage caused by the placement of glass slag but also generate considerable profit, thereby achieving the efficient utilization of low-value resources.
4. Conclusions
This study aims to efficiently recover the PGMs from SAC while simultaneously mitigating the environmental pollution risks associated with SAC. An efficient copper smelting method for enriching PGMs under low-temperature conditions through vitrification technology was proposed, and the principle of slag optimization was discussed. The research found that as the mass ratio of CaO/SiO2 increased, the viscosity of the slag decreased and the TBK increased. With increasing Al2O3 content, the viscosity of the slag first decreases and then increases. TBK first increases and then decreases. Adding Na2B4O7 can reduce the viscosity of the slag and promote the transfer of PGMs in the melt to the alloy phase. Increasing the Cu dosage, raising the smelting temperature, and prolonging the smelting time can all improve the recovery rate of PGMs. The determined target slag type and smelting conditions are: target slag type 36.44 wt% CaO-45.56 wt% SiO2-12.00 wt% Al2O3-6.00 wt% MgO; Cu 12 wt%; Na2B4O7 4 wt%; smelting temperature 1450 °C; and smelting time 90 min. The recovery rate of the optimized PGMs exceeded 99.5%. The recovery efficiency of the pilot-scale experiment exceeded 99%. The final products are impurity-free Cu-PGMs alloy and glass slag, which are less harmful to the environment.
Compared to traditional methods of PGMs recovery via smelting in plasma furnaces, which require melting temperatures of 1600~2000 °C, this study uses an electric arc furnace that can efficiently recover PGMs at a temperature of 1450 °C. This approach significantly reduces energy consumption. Furthermore, future research could consider strategies aimed at lowering production costs. For instance, using alternative types of waste, such as cyanide tailings or nickel-containing slag, as substitutes for flux agents or collectors in the recovery of PGMs. Additionally, employing glass slag as a raw material for high-value utilization could contribute to the development of a circular economy and increase revenue.
Author Contributions
Conceptualization, S.A.; Methodology, G.T.; Software, S.S. and C.S.; Formal analysis, Y.Y. and K.Y.; Investigation, G.T., S.S., F.X., R.S. and C.S.; Data curation, S.A., Y.Y., F.X. and R.S.; Funding acquisition, K.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Key Research and Development Program “Research and Industrial Demonstration of Technology for Metal Recovery and Carrier Reuse of Waste Environmental Protection Catalysts” (2019YFC1907500).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author(s).
Conflicts of Interest
Authors Chengfu Sui and Kuopei Yu were employed by the company Qingdao Qingli Environmental Protectionquipment. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Liu, M.; Zhao, Y.; Li, A.; Chen, C.; Tian, M.; Tian, B.; Xue, T.; Qi, T.; Zhang, H. In Situ Mechanochemical Treatment of the Fe-Si-PGMs Alloy for Enhanced Platinum Group Metal Recovery: Overcoming Thermodynamic and Kinetic Barriers. ACS Sustain. Chem. Eng. 2024, 12, 4506–4516. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, S.; Liu, B.; Zheng, H.; Chang, C.-C.; Ekberg, C. Recovery of precious metals from electronic waste and spent catalysts: A review. Resour. Conserv. Recycl. 2019, 141, 284–298. [Google Scholar] [CrossRef]
- Group, Focus On Catalysts. Platinum group metals market size worth $58 bn in 2032. Focus Catal. 2024, 2024, 1. [Google Scholar] [CrossRef]
- Kukurugya, F.; Wouters, W.; Spooren, J. Microwave-assisted chloride leaching for efficient recovery of platinum group metals from spent automotive catalysts: An approach for chemical reagent reduction. Clean. Eng. Technol. 2025, 24, 100868. [Google Scholar] [CrossRef]
- Karim, S.; Saw, H.M.; Ting, Y.-P. of green chemistry metrics for sustainable recycling of platinum group metals from spent automotive catalysts via bioleaching. Green Chem. 2024, 26, 4112–4126. [Google Scholar] [CrossRef]
- Grilli, M.L.; Slobozeanu, A.E.; Larosa, C.; Paneva, D.; Yakoumis, I.; Cherkezova-Zheleva, Z. Platinum Group Metals: Green Recovery from Spent Auto-Catalysts and Reuse in New Catalysts—A Review. Crystals 2023, 13, 550. [Google Scholar] [CrossRef]
- Paiva, A.P.; Piedras, F.V.; Rodrigues, P.G.; Nogueira, C.A. Hydrometallurgical recovery of platinum-group metals from spent auto-catalysts—Focus on leaching and solvent extraction. Sep. Purif. Technol. 2022, 286, 120474. [Google Scholar] [CrossRef]
- Li, J.; Song, W.; Lyu, J.; Liu, M.; Chen, P.; Liu, Y.; Lyu, X.; Yang, Z. Novel approach for synergistic capturing of platinum group metals from spent automotive catalysts with Pb-Bi alloy. Process. Saf. Environ. Prot. 2025, 197, 106923. [Google Scholar] [CrossRef]
- Shubo, A.; Sun, S.; Tu, G.; Liu, R.; Xiao, F.; Shi, R.; Sui, C.; Yu, K. Iron capture mechanism for harmless recovering platinum group metals from spent automobile catalyst. Environ. Technol. 2025, 46, 1666–1678. [Google Scholar] [CrossRef]
- Morcali, M.H. A new approach to recover platinum-group metals from spent catalytic converters via iron matte. Resour. Conserv. Recycl. 2020, 159, 104891. [Google Scholar] [CrossRef]
- Zheng, H.; Ding, Y.; Wen, Q.; Zhao, S.; He, X.; Zhang, S.; Dong, C. Slag design and iron capture mechanism for recovering low-grade Pt, Pd, and Rh from leaching residue of spent auto-exhaust catalysts. Sci. Total. Environ. 2022, 802, 149830. [Google Scholar] [CrossRef]
- Liu, C.; Sun, S.; Tu, G.; Xiao, F. A novel method for extraction of platinum from spent automotive catalyst: Utilization of spent fluid catalytic cracking catalyst as flux. Environ. Technol. 2021, 44, 139–149. [Google Scholar] [CrossRef]
- He, X.-F.; Yin, X.-P.; Ding, Y.-J.; Shi, Z.-S.; Zhao, B.-H.; Zheng, H.-D.; Jian, J.-X.; Zhang, S.-G.; Chang, C.-C. Slag design and optimization for iron capturing platinum group metals from alumina-based spent catalysts. Rare Met. 2023, 42, 2093–2103. [Google Scholar] [CrossRef]
- Ding, Y.; Zheng, H.; Zhang, S.; Liu, B.; Wu, B.; Jian, Z. Highly efficient recovery of platinum, palladium, and rhodium from spent automotive catalysts via iron melting collection. Resour. Conserv. Recycl. 2020, 155, 104644. [Google Scholar] [CrossRef]
- Xia, J.; Ghahreman, A. Platinum group metals recycling from spent automotive catalysts:metallurgical extraction and recovery technologies. Sep. Purif. Technol. 2023, 311, 123357. [Google Scholar] [CrossRef]
- Zhang, L.; Song, Q.; Liu, Y.; Xu, Z. An integrated capture of copper scrap and electrodeposition process to enrich and prepare pure palladium for recycling of spent catalyst from automobile. Waste Manag. 2020, 108, 172–182. [Google Scholar] [CrossRef]
- Xv, B.; Li, Z.; Zha, G.; Liu, D.; Yang, B.; Jiang, W. Recovery of platinum group metals from spent automotive catalysts: Review of conventional techniques and vacuum metallurgy. Resour. Conserv. Recycl. 2025, 215, 108103. [Google Scholar] [CrossRef]
- Yong, Y.; Hua, W.; Jianhang, H. Co-treatment of electroplating sludge, copper slag, and spent cathode carbon for recovering and solidifying heavy metals. J. Hazard. Mater. 2021, 417, 126020. [Google Scholar] [CrossRef]
- Liu, C.; Sun, S.; Tu, G.; Xiao, F. Co-treatment of spent automotive catalyst and cyanide tailing via vitrification and smelting-collection process for platinum group metals recovery. J. Environ. Chem. Eng. 2021, 9, 105823. [Google Scholar] [CrossRef]
- Hou, Y.; Zhang, S.; Dang, J.; Guo, J.; Zhou, H.; Lü, X. Viscosity and structure relationship with equimolar substitution of CaO with MgO in the CaO-MgO-Al2O3-SiO2 slag melts. Int. J. Miner. Met. Mater. 2024, 32, 70–79. [Google Scholar] [CrossRef]
- Feng, C.; Chu, M.; Tang, J.; Tang, Y.; Liu, Z. Effect of CaO/SiO2 and Al2O3 on Viscous Behaviors of the Titanium earing Blast Furnace Slag. Steel Res. Int. 2016, 87, 1274–1283. [Google Scholar] [CrossRef]
- Sun, C.Y.; Liu, X.H.; Li, J.; Yin, X.T.; Song, S.; Wang, Q. Influence of Al2O3 and MgO on the viscosity and stability of CaO-MgO-SiO2-Al2O3 slags with CaO/SiO2 = 1.0. Isij Int. 2017, 57, 978–982. [Google Scholar] [CrossRef]
- Jiang, C.; Zhang, H.; Xiong, Z.; Chen, S.; Li, K.; Zhang, J.; Liang, W.; Sun, M.; Wang, Z.; Wang, L. Molecular dynamics investigations on the effect of Na2O on the structure and properties of blast furnace slag under different basicity conditions. Mol. Liq. 2019, 299, 112195. [Google Scholar] [CrossRef]
- Rusen, A.; Geveci, A.; Topkaya, Y.A.; Derin, B. Effects of some additives on copper losses to matte smelting slag. JOM 2016, 68, 2323–2331. [Google Scholar] [CrossRef]
- Li, Q.; Liu, B.; Liu, J.; Zhang, Q.; An, C.; Sun, Z.; Pulatov, B.; Wei, S.; Fan, J. Review of glass-ceramics: Solidification of toxic elements and durability. Waste Manag. 2025, 200, 114764. [Google Scholar] [CrossRef] [PubMed]
- Shubo, A.; Sun, S.; Tu, G.; Yan, Y.; Xiao, F.; Liu, R.; Sui, C.; Yu, K. Crystallization behavior of smelting slag for recovery of platinum group metals from spent automotive catalysts and preparation of foamed glass-ceramics. Ceram. Int. 2025, 51, 5930–5939. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, Z.; Zhang, G.; Sun, J.; Zhang, B.; Zhang, J.; Ma, P.; Yan, J. Valorization of municipal solid waste incineration fly ash for mineral wool fiber production: Effects on vitrification process and melt viscosity. J. Environ. Chem. Eng. 2025, 13, 116882. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).