Determination of Phthalate Esters and Bisphenol A in Pear by Packed-Fiber Solid-Phase Extraction Coupled with Gas Chromatography–Mass Spectrometry
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sample Collection and Preparation
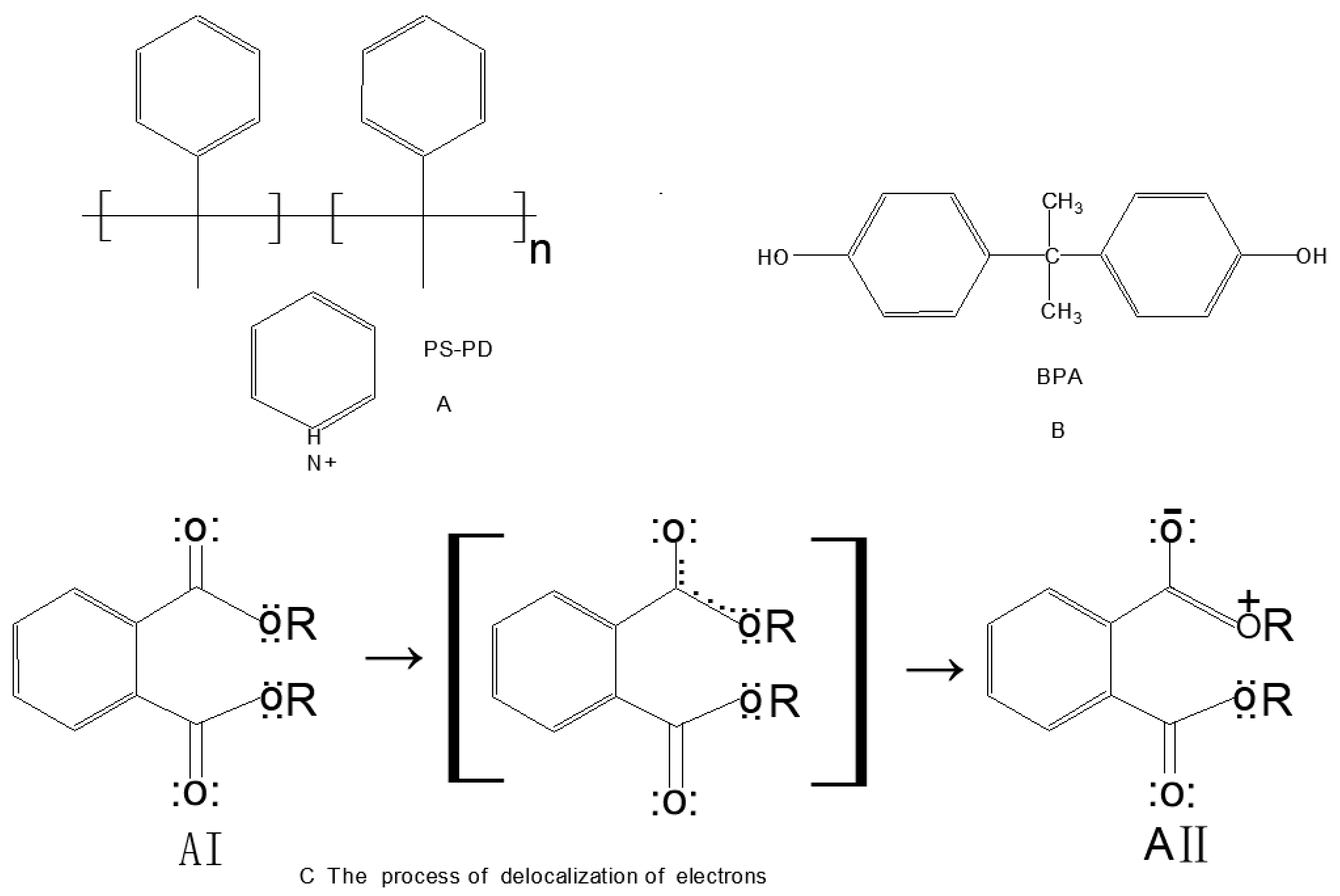
2.3. Preparation of the PS-PD Nanofibers
2.4. PFSPE Procedure
2.5. Chromatographic Analysis
2.6. Quality Assurance and Quality Control (QA/QC)
2.7. Method Validation
3. Results
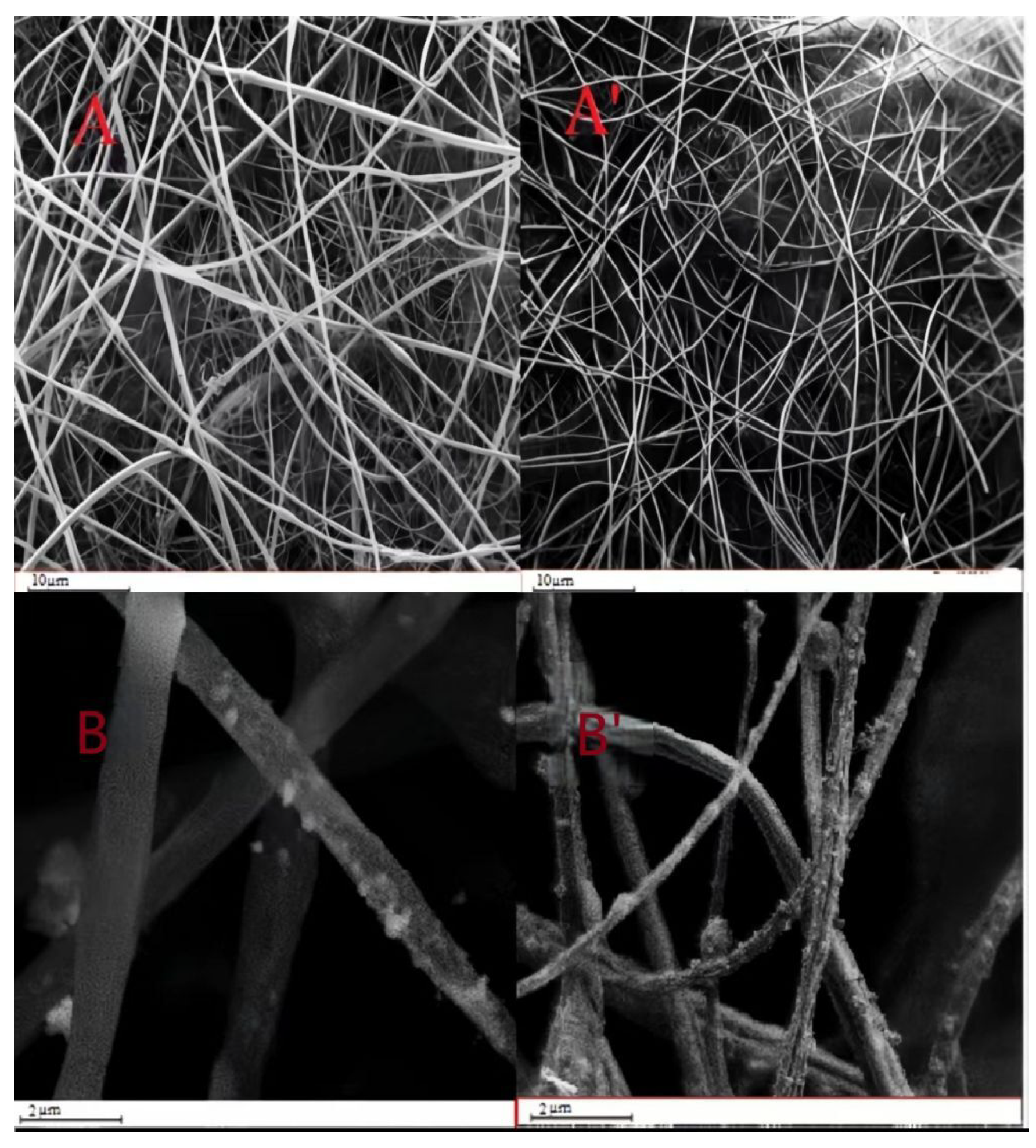
3.1. Characterization of Nanofibers
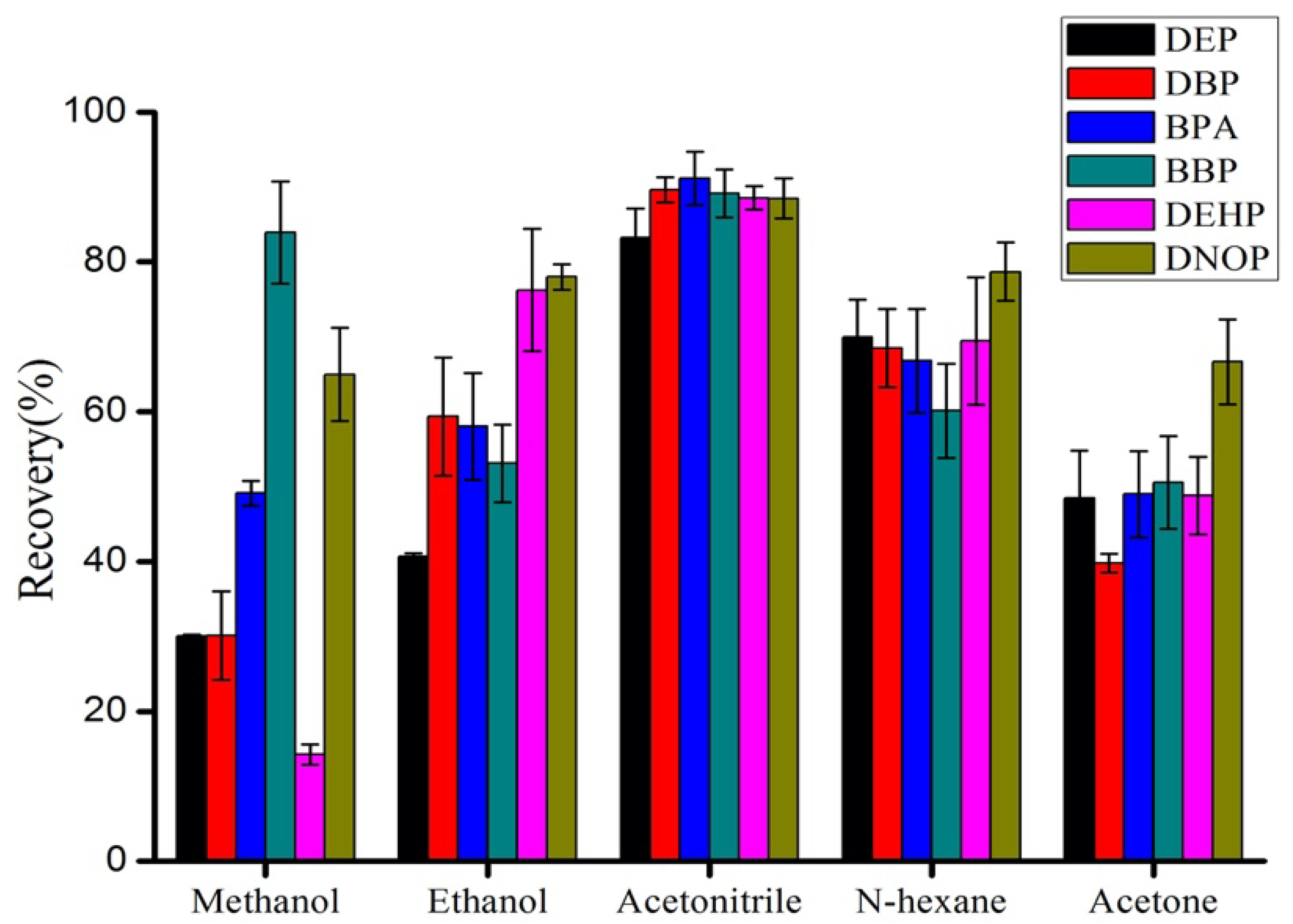
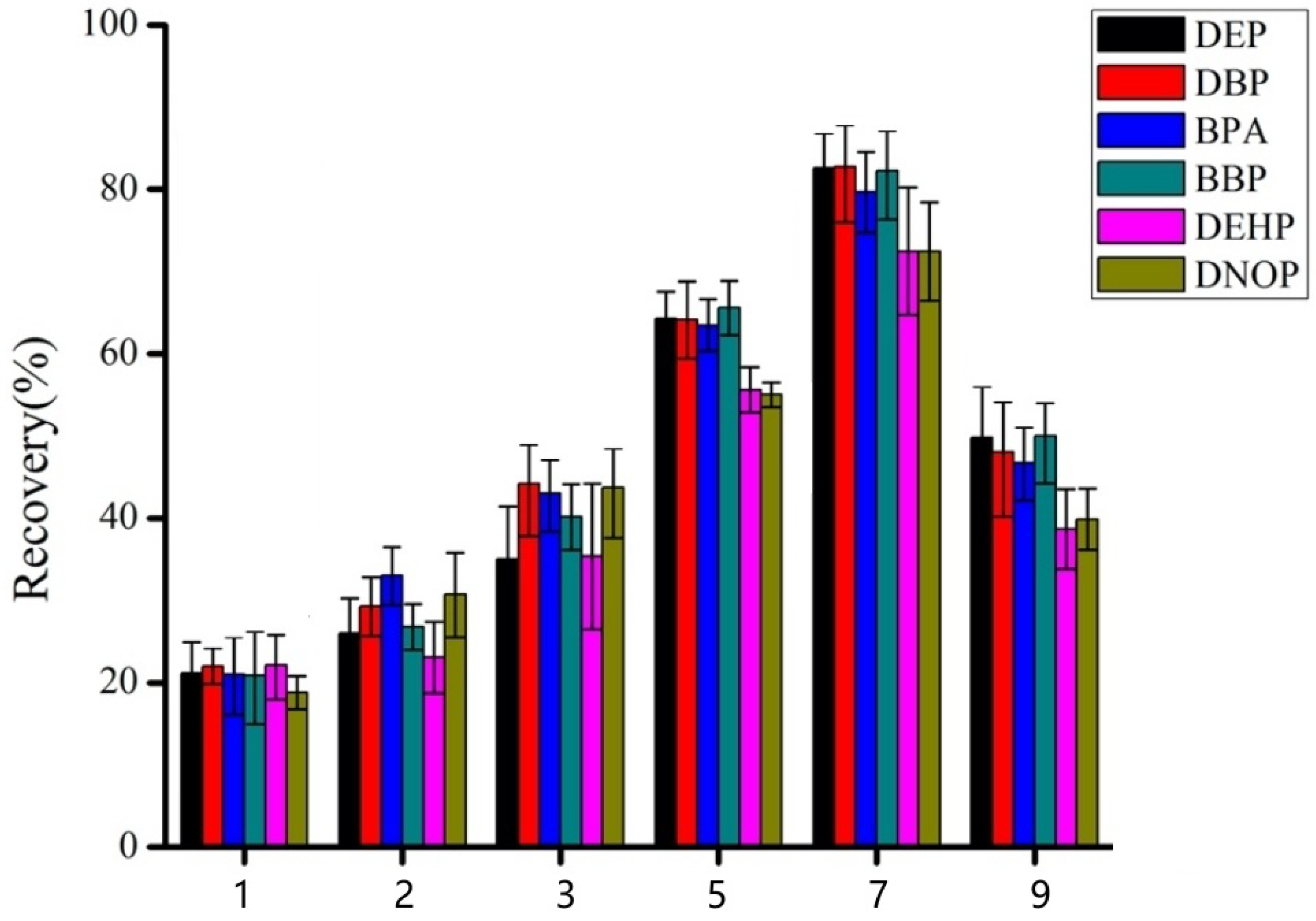
3.2. Optimization of Elution Solvent and PH
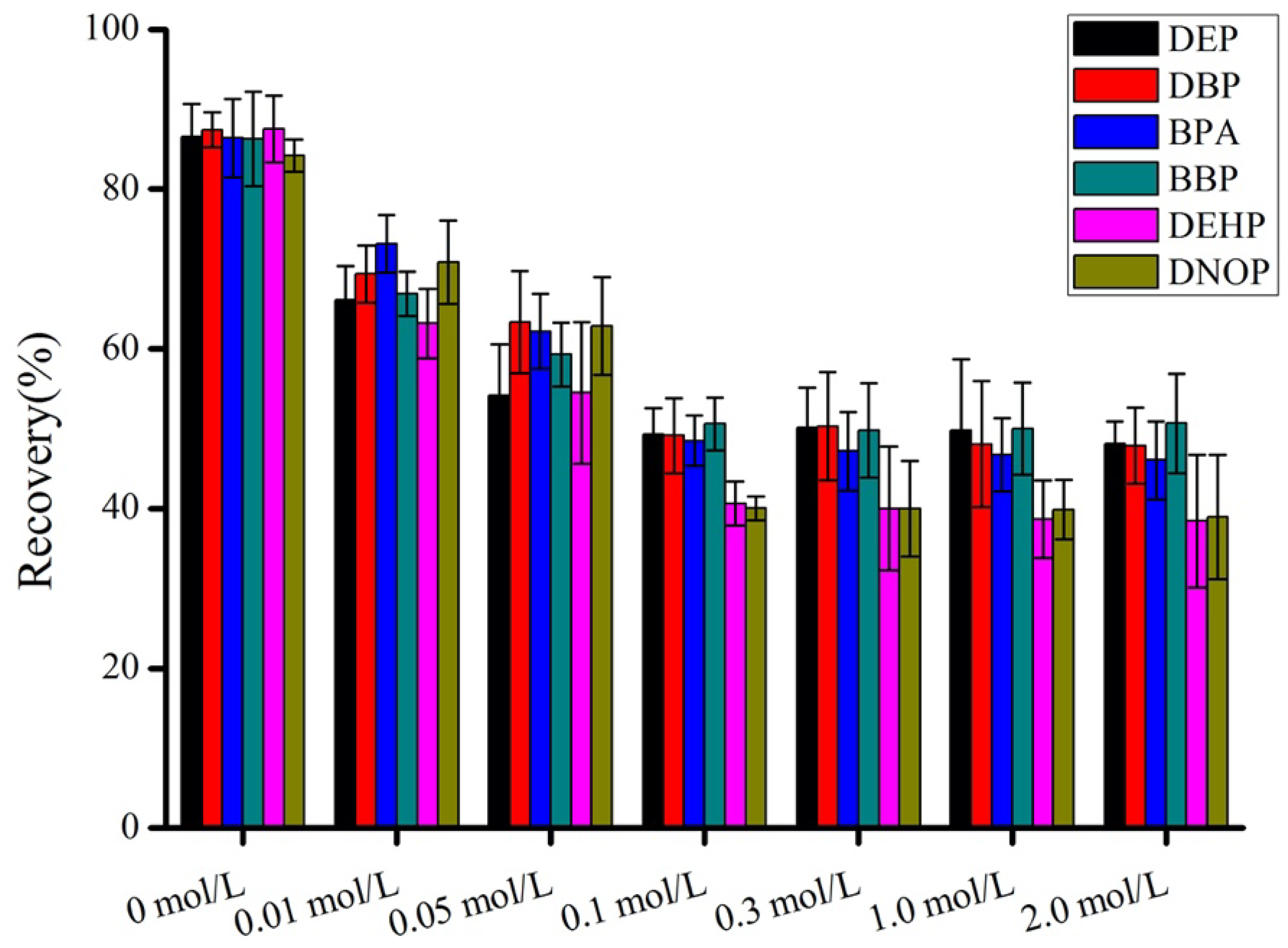
3.3. Optimization of Salt Concentration
3.4. Method Validation
3.4.1. Calibration Curves
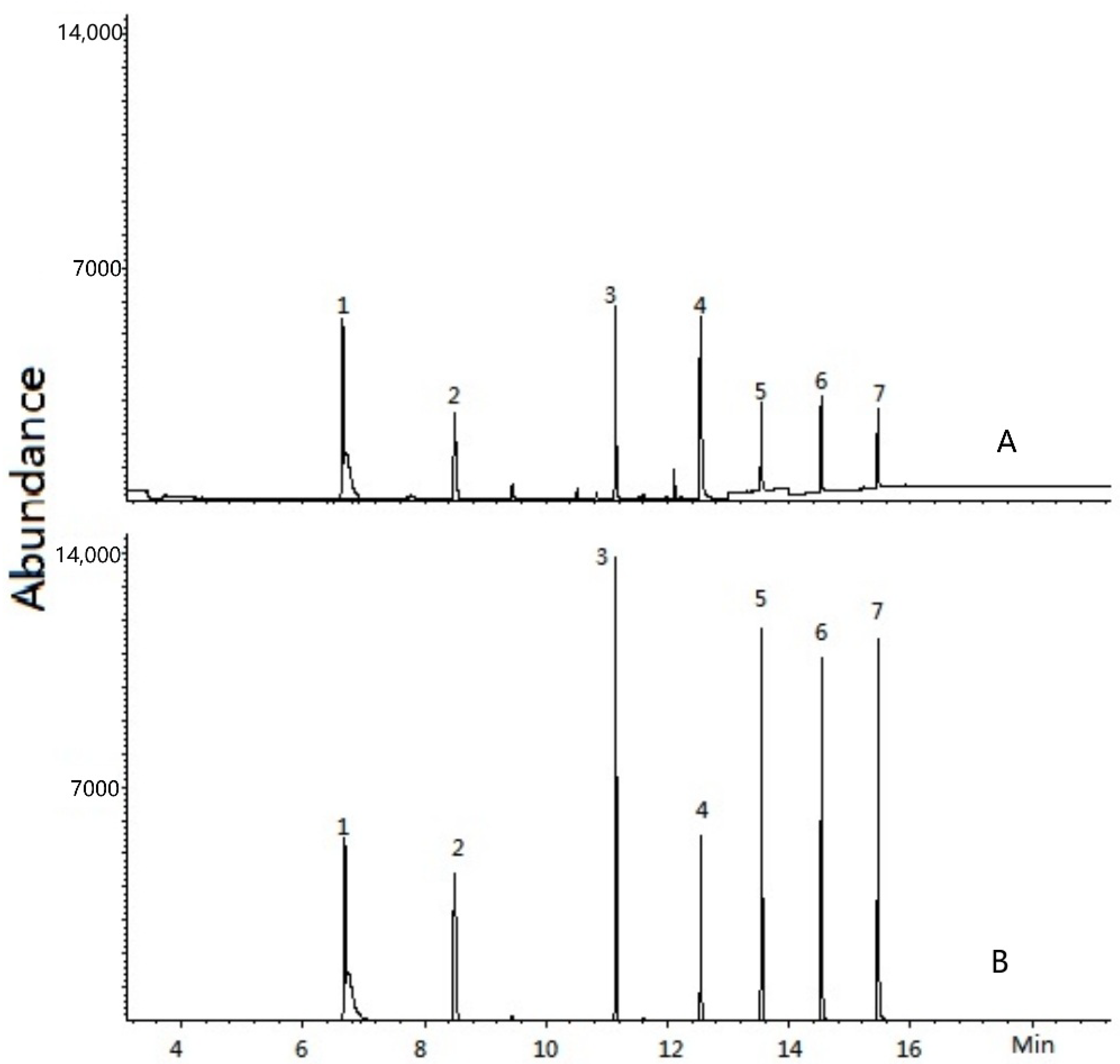
3.4.2. Selectivity and Reproducibility
3.4.3. Precision
3.4.4. Limits of Detection and Quantification
3.5. Matrix Effect
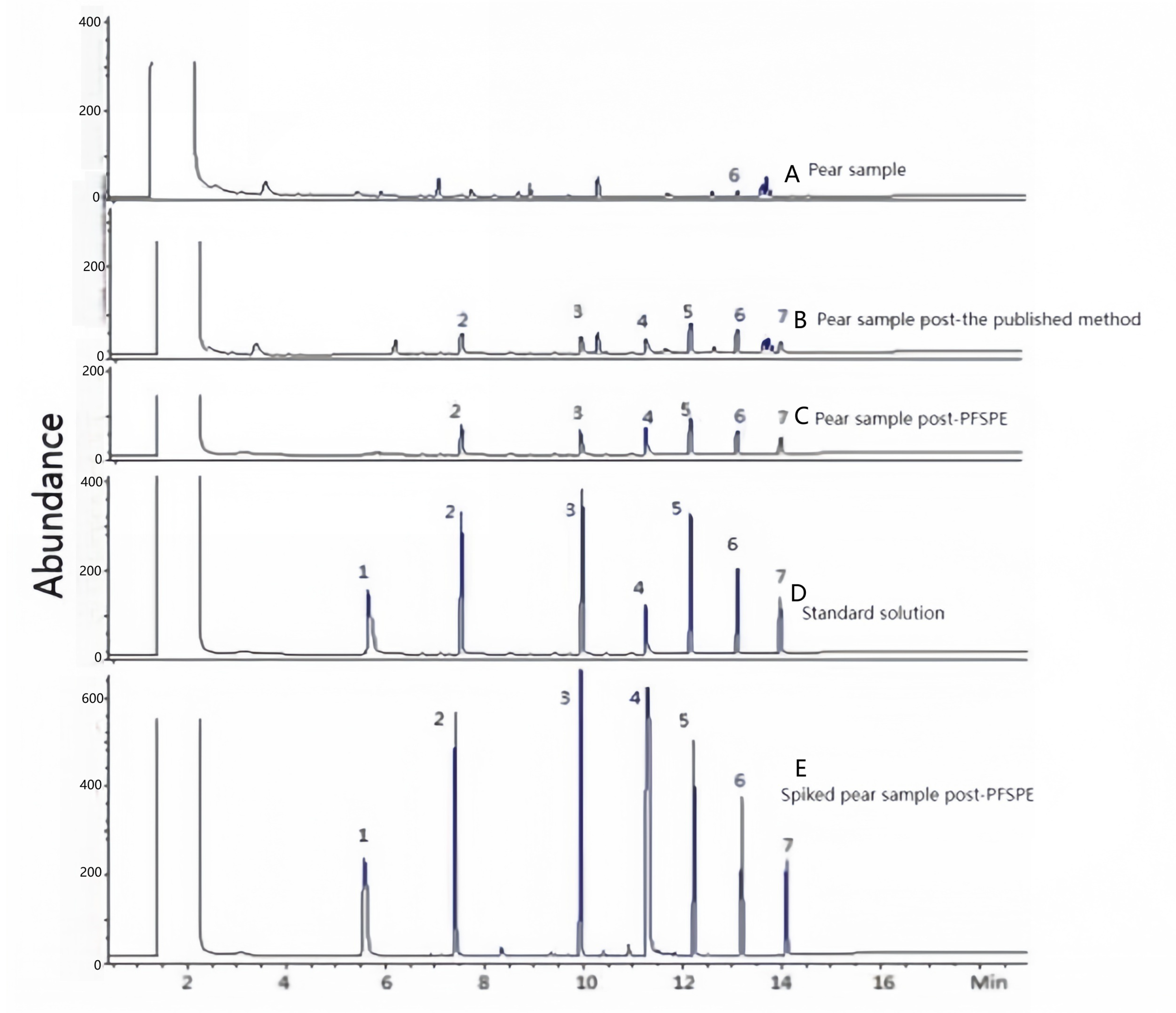
3.6. Application of the Developed Procedure to Real Samples
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gu, Y.Y.; Yu, X.J.; Peng, J.F.; Chen, S.B.; Zhong, Y.Y.; Yin, D.Q.; Hu, X.L. Simultaneous solid-phase extraction coupled with liquid chromatography-tandem mass spectrometry and gas chromatography-tandem mass spectrometry for the highly sensitive determination of 15 endocrine-disrupting chemicals in seafood. J. Chromatogr. B 2014, 965, 164–172. [Google Scholar] [CrossRef]
- Wang, L.X.; Gong, M.Y.; Xu, Y. Phthalates in pear collected from various indoor environments in Beijing, China and resulting non-dietary human exposure. Build. Environ. 2017, 124, 315–322. [Google Scholar] [CrossRef]
- Huang, W.; Li, N.; Xu, R.; Li, T.; Li, C. Determination of nine pyrethroid pesticide residues in tea by gas chromatography-tandem mass spectrometry combined with accelerated solvent extraction and solid-phase extraction. Chin. J. Chromatogr. 2018, 36, 1303–1310. [Google Scholar] [CrossRef]
- Chakraborty, P.; Bharat, G.K.; Gaonkar, O.; Mukhopadhyay, M.; Chandra, S.; Steindal, E.H.; Nizzetto, L. Endocrine-disrupting chemicals used as common plastic additives: Levels, profiles, and human dietary exposure from the Indian food basket. Sci. Total. Environ. 2022, 810, 152200. [Google Scholar] [CrossRef]
- Wu, J.; Lai, Y.; Zhu, H.; Yang, X.; Ye, X.; Zhang, A.; Sun, J. Phthalate esters and their metabolites in paired soil-crop systems from farmland in major provinces of eastern China: Pollution characteristics and implications for human exposure. Sci. Total Environ. 2023, 882, 163645. [Google Scholar] [CrossRef] [PubMed]
- Otero, P.; Saha, S.K.; Moane, S.; Barron, J.; Clancy, G.; Murray, P. Improved method for rapid detection of phthalates in bottled water by gas chromatography-mass spectrometry. J. Chromatogr. B 2015, 997, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Al-Saleh, I.; Elkhatib, R.; Al-Rajoudi, T.; Al-Qudaihi, G. Assessing the concentration of phthalate esters (PAEs) and bisphenol A (BPA) and the genotoxic potential of treated wastewater (final effluent) in Saudi Arabia. Sci. Total Environ. 2017, 578, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Kargarghomsheh, P.; Naghashan, M.; Farhadiyan, S.; Arabameri, M.; Tooryan, F.; Shariatifar, N. Determination of phthalic acid esters (PAEs) along with probabilistic health risk assessment in fruit juice samples in Tehran, Iran. Environ. Sci. Pollut. Res. 2023, 30, 44833–44844. [Google Scholar] [CrossRef]
- Yin, S.; Yang, Y.; Yang, D.; Li, Y.; Jiang, Y.; Wu, L.; Sun, C. Determination of 11 phthalate esters in beverages by magnetic solid-phase extraction combined with high-performance liquid chromatography. J. AOAC Int. 2019, 102, 1624–1631. [Google Scholar] [CrossRef]
- Rastkari, N.; Jeddi, M.Z.; Yunesian, M.; Ahmadkhaniha, R. Effect of sunlight exposure on phthalate migration from plastic containers to packaged juices. J. Environ. Health Sci. Eng. 2018, 16, 27–33. [Google Scholar] [CrossRef]
- Zhang, T.; Ma, B.; Wang, L. Phthalic acid esters in grains, vegetables, and fruits: Concentration, distribution, composition, bioaccessibility, and dietary exposure. Environ. Sci. Pollut. Res. 2023, 30, 2787–2799. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, M.; Yang, A.; Wang, Y.; Hou, S.; Zheng, N.; Dong, D. Health risks of population exposure to phthalic acid esters through the use of plastic containers for takeaway food in China. Sci. Total Environ. 2021, 785, 147347. [Google Scholar] [CrossRef]
- Xu, Q.; Yin, X.; Wu, S.; Wang, M.; Wen, Z.; Gu, Z. Determination of phthalate esters in water samples using Nylon6 nanofibers mat-based solid-phase extraction coupled to liquid chromatography. Microchim. Acta 2010, 168, 267–275. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Ma, Z.R.; Cai, Y.Y.; Li, H.R.; Ying, G.G. Agricultural plastic pollution in China: Generation of plastic debris and emission of phthalic acid esters from agricultural films. Environ. Sci. Technol. 2021, 55, 12459–12470. [Google Scholar] [CrossRef]
- Zhao, R.S.; Chu, L.L.; Wang, Y.; Song, Y.; Liu, P.; Li, C.; Huang, J.J.; Kang, X.J. Application of packed-fiber solid-phase extraction coupled with GC-MS for the determination of short-chain fatty acids in children’s urine. Clin. Chim. Acta 2017, 468, 120–125. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Kang, X.J.; Fang, F. Solid-phase extraction with electrospun nanofibers for determination of retinol and α-tocopherol in plasma. Microchim. Acta 2010, 168, 59–64. [Google Scholar] [CrossRef]
- Tang, Z.G.; Chu, L.L.; Wang, Y.; Song, Y.; Liu, P.; Fan, J.H.; Huang, J.J.; Liu, X.W.; Wei, L.L.; Li, C.; et al. Packed-nanofiber solid-phase extraction coupled with gas chromatography-mass spectrometry for the determination of phthalate esters in urine from children. J. Chromatogr. B 2017, 1061–1062, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.G.; Han, Q.; Xie, L.; Chu, L.L.; Wang, Y.; Sun, Y.; Kang, X.J. Simultaneous determination of five phthalate esters and bisphenol A in milk by packed-nanofiber solid-phase extraction coupled with gas chromatography and mass spectrometry. J. Sep. Sci. 2019, 42, 851–861. [Google Scholar] [CrossRef]
- Liu, D.; Min, S.H.; Song, X. The application of directly suspended droplet microextraction for the evaluation of phthalic acid esters in cow’s milk by gas chromatography-mass spectrometry. J. Chromatogr. A 2016, 1443, 66–74. [Google Scholar] [CrossRef]
- Mousa, A.; Basheer, C.; Al-Arfaj, A.R. Application of electro-enhanced solid-phase microextraction for determination of phthalate esters and bisphenol A in blood and seawater samples. Talanta 2013, 115, 308–313. [Google Scholar] [CrossRef]
- Sajid, M.; Basheer, C.; Alsharaa, A.; Narasimhan, K.; Buhmeida, A.; Qahtani, M.A.; Al-Ahwal, M.S. Development of natural sorbent-based micro-solid-phase extraction for determination of phthalate esters in milk samples. Anal. Chim. Acta 2016, 924, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Mehraie, A.; Shariatifar, N.; Arabameri, M.; Moazzen, M.; Mortazavian, A.M.; Sheikh, F.; Sohrabvandi, S. Determination of phthalic acid esters (PAEs) in bottled water distributed in Tehran: A health risk assessment study. Int. J. Environ. Anal. Chem. 2024, 104, 2417–2431. [Google Scholar] [CrossRef]
- Zhuo, L.Y.; Yin, Y.C.; Fu, W.S.; Qiu, B.; Lin, Z.Y.; Yang, Y.Q.; Zheng, L.M.; Li, J.R.; Chen, G.N. Determination of paralytic shellfish poisoning toxins by HILIC-MS/MS coupled with dispersive solid-phase extraction. Food Chem. 2013, 137, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Almeida, S.; Raposo, A.; Almeida-González, M.; Carrascosa, C. Bisphenol A: Food exposure and impact on human health. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1503–1517. [Google Scholar] [CrossRef]
- GB 9685-2016; Use of Additives in Materials and Products Contacting Food. National Health Commission: Beijing, China, 2016.
- Ji, Y.; Wang, F.; Zhang, L.; Shan, C.; Bai, Z.; Sun, Z.; Liu, L.; Shen, B. A comprehensive assessment of human exposure to phthalates from environmental media and food in Tianjin, China. J. Hazard. Mater. 2014, 279, 133–140. [Google Scholar] [CrossRef]


| Quantification Ion (m/z) | Confirmation Ion (m/z) | Retention Time (min) | |
|---|---|---|---|
| DEP | 149 | 177 | 8.681 |
| DBP | 149 | 223 | 11.34 |
| BPA | 213 | 228 | 12.748 |
| BBP | 149 | 206, 91 | 13.746 |
| DEHP | 149 | 167 | 14.761 |
| DNOP | 149 | 279 | 15.707 |
| Analytes | Linear Range (µg/L) | Recovery (±RSD %) n = 5 | LOD (µg/L) | LOQ (µg/L) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Intraday | Interday | ||||||||
| Spiked Concentration (µg/L) | |||||||||
| 0.1 | 1 | 10 | 0.1 | 1 | 10 | ||||
| DEP | 0.01–10 | 98.3 (3.4) | 98.7 (3.1) | 103.0 (6.9) | 98.6 (4.3) | 108.5 (5.2) | 104.8 (2.5) | 0.03 | 0.13 |
| DBP | 0.01–10 | 101.4 (4.7) | 103.2 (6.7) | 98.3 (9.3) | 109.9 (9.9) | 104.3 (2.8) | 102.3 (6.6) | 0.06 | 0.17 |
| BPA | 0.01–10 | 108.0 (4.9) | 107.0 (2.9) | 100.8 (5.6) | 104.0 (10.9) | 109.0 (1.3) | 100.5 (0.6) | 0.07 | 0.28 |
| BBP | 0.01–10 | 87.0 (5.9) | 88.8 (8.9) | 87.6 (6.3) | 90.5 (10.6) | 87.8 (9.6) | 91.7 (10.9) | 0.1 | 0.34 |
| DEHP | 0.01–10 | 91.8 (6.9) | 92.4 (8.3) | 96.7 (7.7) | 94.3 (8.7) | 91.4 (11.9) | 96.3 (2.8) | 0.01 | 0.034 |
| DNOP | 0.01–10 | 92.9 (7.1) | 91.0 (5.4) | 97.0 (6.1) | 99.2 (4.6) | 98.1 (7.6) | 93.3 (8.9) | 0.06 | 0.19 |
| Analytes | Matrix | Slope | Slope Matrix/Solvent | ME (%) |
|---|---|---|---|---|
| DEP | solution (ACN) of pear | 5.55 | ||
| Water | 5.18 | 1.071 | 107.1 | |
| DBP | solution (ACN) of pear | 7.53 | ||
| Water | 7.33 | 1.027 | 102.7 | |
| BPA | solution (ACN) of pear | 4.81 | ||
| Water | 4.69 | 1.025 | 102.5 | |
| BBP | solution (ACN) of pear | 7.84 | ||
| Water | 7.38 | 1.062 | 106.2 | |
| DEHP | solution (ACN) of pear | 8.37 | ||
| Water | 8.98 | 0.932 | 93.2 | |
| DNOP | solution (ACN) of pear | 5.27 | ||
| Water | 5.20 | 1.013 | 101.3 |
| DEP | DBP | BPA | BBP | DEHP | DNOP | ||
|---|---|---|---|---|---|---|---|
| Pear in supermarket (n = 10) | Median | 0.061 | 0.055 | 0.044 | 0.079 | 0.049 | 0.047 |
| Min | 0.042 | 0.047 | <LOD | <LOD | 0.053 | <LOD | |
| Max | 0.073 | 0.069 | 0.052 | 0.081 | 0.067 | 0.066 | |
| Pear in native place (n = 10) | Median | 0.058 | 0.052 | 0.035 | 0.063 | 0.047 | 0.039 |
| Min | <LOD | <LOD | <LOD | <LOD | 0.023 | <LOD | |
| Max | 0.066 | 0.062 | 0.059 | 0.076 | 0.0054 | 0.049 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.; Wang, L.; Ji, M.; Tang, Z.; Liu, J.; Lan, H.; Zhang, X. Determination of Phthalate Esters and Bisphenol A in Pear by Packed-Fiber Solid-Phase Extraction Coupled with Gas Chromatography–Mass Spectrometry. Separations 2025, 12, 304. https://doi.org/10.3390/separations12110304
Xu W, Wang L, Ji M, Tang Z, Liu J, Lan H, Zhang X. Determination of Phthalate Esters and Bisphenol A in Pear by Packed-Fiber Solid-Phase Extraction Coupled with Gas Chromatography–Mass Spectrometry. Separations. 2025; 12(11):304. https://doi.org/10.3390/separations12110304
Chicago/Turabian StyleXu, Wenhui, Lu Wang, Mengjia Ji, Zigang Tang, Jing Liu, Hangzhen Lan, and Xingtao Zhang. 2025. "Determination of Phthalate Esters and Bisphenol A in Pear by Packed-Fiber Solid-Phase Extraction Coupled with Gas Chromatography–Mass Spectrometry" Separations 12, no. 11: 304. https://doi.org/10.3390/separations12110304
APA StyleXu, W., Wang, L., Ji, M., Tang, Z., Liu, J., Lan, H., & Zhang, X. (2025). Determination of Phthalate Esters and Bisphenol A in Pear by Packed-Fiber Solid-Phase Extraction Coupled with Gas Chromatography–Mass Spectrometry. Separations, 12(11), 304. https://doi.org/10.3390/separations12110304






