Abstract
In this study, two γ-carbon-substituted dialkylphosphinic acids—symmetrical di-(3,5,5-trimethylhexyl)phosphinic acid (P355) and unsymmetrical (2-ethylhexyl)(3,5,5-trimethylhexyl)phosphinic acid (P227-355)—were synthesized via a precise free radical addition method. Their chemical structures were fully characterized using ESI-HRMS, 1H NMR, 31P NMR, and FT-IR. Their middle REE extraction/separation performance, anti-emulsification behavior, and underlying mechanisms were investigated. Key results showed that P355 had better Dy saturation capacity (357.51 mg/L) and good selectivity for middle REEs (their average value of βN + 1/N = 3.18), while P227-355 showed higher back-extraction efficiency (≈90% Dy stripping at ≥0.02 mol/L H2SO4). Methyl n-pentyl ketone (MNPK) eliminated emulsification and boosted saturation capacity (324.18 mg/L Sm and 357.51 mg/L Dy for P355). Mechanistically, the extraction followed cation exchange (Sm3+ + 2(HL)2 ↔ Sm·L3·(HL) + 3H+); MNPK formed hydrogen-bonded associates (HL·MNPK) with free extractants, slightly reducing the effective concentration of (HL)2 but not altering the core cation exchange mechanism.
1. Introduction
Rare earth elements (REEs), comprising 17 elements (lanthanides, Sc, and Y), are indispensable strategic resources for advancing high-tech industries and green energy technologies. Their unique magnetic, optical, and electronic properties enable critical applications in permanent magnets [1], luminescent materials [2], cathode materials, and superconducting devices—making them pivotal to global energy transition and technological innovation [3,4]. Despite their significance, REEs naturally occur as symbiotic mixtures in ores (e.g., ion-adsorbed deposits, bastnasite, monazite), and their separation remains a formidable challenge due to their nearly identical ionic radii, similar chemical reactivity, and overlapping coordination behaviors [4,5].
Solvent extraction has emerged as the dominant industrial technique for REE separation, owing to its high throughput, scalability, and controllable selectivity [6,7]. Traditional organophosphorus extractants—such as di-(2-ethylhexyl) phosphoric acid (P204, D2EHPA), 2-ethylhexyl phosphonic acid mono-2-ethylhexyl ester (EHEHPA/P507/PC88A), and bis(2,4,4-trimethylpentyl) phosphinic acid (Cyanex 272)—have been widely employed for decades. However, these extractants suffer from inherent limitations: D2EHPA exhibits low selectivity for adjacent REEs, necessitating hundreds of extraction stages; P507 requires high concentrations of sulfuric acid (5~6 mol/L) for stripping heavy REEs, and fails to achieve complete stripping of them; and Cyanex 272 is prone to emulsification and third-phase formation at high REE loading, hindering long-term industrial operation [8,9,10].
To address these drawbacks, extensive research has been devoted to developing advanced extractants with enhanced selectivity for adjacent REEs, reduced acid consumption during stripping, and improved environmental compatibility [7]. Representative examples include calix [4] arene-based macrocyclic extractants [11], crown esters [12], α-aminophosphonates/phosphinates/phosphine oxides [13], functional ionic liquids (ILs) [14,15], and synergistic extraction systems (e.g., IL-molecular extractant composites or extractant mixtures like Cyanex 572-TBP) [16,17,18,19]. Despite their promising performance in lab-scale studies, these advanced extractants still face non-negligible limitations that impede industrial translation: calix [4] arene derivatives require multi-step, complex synthesis routes, which increases production costs and scalability challenges; crown ethers suffer from high commercial prices, making them economically unfeasible for large-volume REE processing; functional ILs exhibit pH-dependent chemical stability, narrowing their applicable operation window; and the mechanism of synergistic interactions between extractants (e.g., ionic liquids and molecular extractants) is not fully elucidated, hindering the rational design of high-performance synergistic systems.
In addition, organophosphorus acids and organophosphorus acid-functionalized compounds are the most extensively studied class of ligands for REE extraction, such as propylphosphonic acid butyl ester (-(CH2)3POOH(OC4H9)) [11]/p-phosphonic acid ethyl ester (-POOH(OC2H5)) [20] modified calix [4] arenes, organophosphorus acid-amine mixed extractant systems [16], [N2222][EHEHP] [17] and [A336][P507] [21,22] bifunctional ILs [17], Cyanex 572 [23], P227 [8], and unsymmetrical dialkylphosphinic acids [10,24,25]. Beyond solvent extraction, they are also widely applied and studied in alternative REE separation technologies, including solid–liquid extraction [26,27,28] and liquid membrane extraction [29,30,31], further highlighting their versatility in REE hydrometallurgy.
To address the inherent drawbacks of traditional organophosphorus acid extractants (P204, P507, and Cyanex 272), the development of dialkylphosphinic acids with tailored molecular structures has emerged as a highly promising research direction. Dialkylphosphinic acids, which feature two alkyl chains directly bonded to the central P atom (see Figure 1), have widespread application in the separation of key metal systems, such as REEs [32], Co/Ni [33], Zr/Hf [34]. They have weaker acidity compared to their analogous phosphoric acids (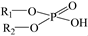 , e.g., P204) and phosphonic acids (
, e.g., P204) and phosphonic acids (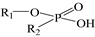 , e.g., P507). This is a key property that increases selectivity and reduces the acid concentration required for stripping REEs.
, e.g., P507). This is a key property that increases selectivity and reduces the acid concentration required for stripping REEs.
 , e.g., P204) and phosphonic acids (
, e.g., P204) and phosphonic acids (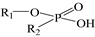 , e.g., P507). This is a key property that increases selectivity and reduces the acid concentration required for stripping REEs.
, e.g., P507). This is a key property that increases selectivity and reduces the acid concentration required for stripping REEs.A pivotal breakthrough in this field was the establishment of a reliable synthetic route for asymmetric dialkylphosphinic acids, in which the two alkyl groups exhibit distinct structures (see Figure 1) [10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35]. This advancement enabled the synthesis of a serious of novel asymmetric dialkylphosphinic acid derivatives, such as INET-3 [9], USTB-1 [24], and USTB-2 [25]. This enables the detailed study of structure-activity relationship of dialkylphosphinic acids in REE extraction—including how variations in alkyl chain length, side chain length and position, and symmetry of R1/R2 affect extraction selectivity, loading capacity, stripping acidity, and anti-emulsification [10,36,37,38]—laying the foundation for the rational design of high-performance dialkylphosphinic acid extractants.
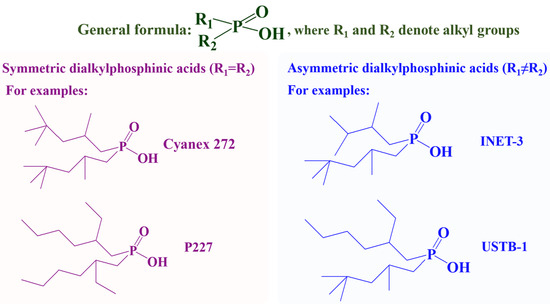
Figure 1.
Structure comparison of symmetric and asymmetric dialkylphosphinic acids.
Building on the aforementioned advances in dialkylphosphinic acid development—particularly the focus on unsymmetrical structures to tailor extraction performance—this study targets the separation of middle REEs (Sm, Eu, Gd, Tb, Dy), a subgroup yet challenging to isolate due to their extremely close physicochemical properties. Two kinds of dialkylphosphinic acids were designed—di-(3,5,5-trimethylhexyl)phosphinic acid (P355) (a symmetrical derivative) and (2-ethylhexyl)(3,5,5-trimethylhexyl)phosphinic acid (P227-355) (an unsymmetrical analog)—which was guided by two key considerations: (1) branched (3,5,5-trimethylhexyl) chain, with its increased carbon chain length relative to Cyanex 272’s branched groups, further boosts lipophilicity of the extractant, avoiding the emulsification issues; and 2-ethylhexyl chain improves selectivity and phase separation; (2) the contrast between symmetrical (P355) and unsymmetrical (P227-355) alkyl chain configurations was intended to elucidate how structural symmetry modulates coordination affinity toward middle REEs, which is rarely systematically explored in existing dialkylphosphinic acid studies.
The synthetic routes for P355 and P227-355 are illustrated in Figure 2, with their chemical structures fully characterized using ESI-HRMS, 1H NMR, 31P NMR, and FT-IR. Batch extraction experiments were conducted to investigate the effects of equilibrium pH, extractant concentration, and type and dosage of organic modifiers on middle REEs extraction and separation behaviors. Additionally, their saturation capacity and stripping acidity were determined. The underlying extraction mechanism was elucidated through slope analysis.
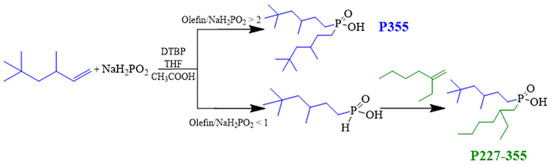
Figure 2.
Synthetic strategy of P355 (MW = 318.47) and P227-355 (MW = 304.45).
2. Materials and Methods
2.1. Materials and Reagents
Sodium hypophosphite (NaH2PO2, A.R.) was bought from Guangdong Wengjiang Chemical Reagent Co., Ltd. (Shaoguan, China). Tetrahydrofuran (THF, ≥99.5%), ethyl ether (≥99.5%), concentrated hydrochloric acid (HCl, G.R.), concentrated sulfuric acid (H2SO4, ≥96.0%), and sodium hydroxide (NaOH, ≥96.0%) were supplied by Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Di-tert-butyl peroxide (DTBP, 97%) was obtained from MACKLIN Biochemical Co., Ltd. Shanghai, China). Glacial acetic acid (CH3COOH, ≥99.5%) was produced by Xilong Scientific Co., Ltd. (Shantou, China). Olefins 3,5,5-trimethyl-1-hexene (>98.0%) and 2-ethyl-1-hexene (>95.0%) were purchased from TCI (Shanghai) Development Co., Ltd. (Shanghai, China). For solvent extraction experiments: diluent kerosene (B.R.), organic modifiers [isooctanol (≥99.0%) and methyl n-pentyl ketone (MNPK, 98%)], and rare earth chloride salts [SmCl3·6H2O (99.99%), EuCl3·6H2O (99.99%), DyCl3·6H2O (≥99.99%)] were provided by Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). Additional rare earth chloride salts, GdCl3·6H2O (99.99%) and TbCl3·6H2O (99.99%), were bought from Shanghai Haohong Biomedical Technology Co., Ltd. (Shanghai, China). Xylene (99%) was supplied by J&K Scientific Limited. (Beijing, China). All chemicals and reagents used in this study were applied without further purification.
2.2. Instrumentation
Synthesis of P355 and P227-355 was conducted using a laboratory-scale home-made shaft furnace integrated with an MS magnetic stirrer system. Solvent removal was facilitated by a R-1001-V rotary evaporator system with DL-400 recirculation cooling and SHB-III vacuum pumping components (Zhengzhou Greatwall Scientific Industrial and Trade Co., Ltd., Zhengzhou, China). Structural characterization was performed through multiple analytical techniques: nuclear magnetic resonance spectroscopy (400 MHz, Bruker, Berlin, Germany), Fourier-transform infrared spectroscopy (Nicolet iS20, Thermo Scientific, Waltham, MA, USA), and high-resolution mass spectrometry (ESI-Q-TOF MS/MS system, Bruker, Berlin, Germany).
Phase-mixing and phase-separation operations were systematically conducted using specialized apparatus: (1) Liquid–liquid contact enhancement was achieved with a KB-5010 Test Tube Shaker (Kylin-Bell, Haimen, China), and (2) Phase disengagement accelerated through a TD4C bench-top Centrifuge (Guanghe, Changzhou, China). Solution acidity monitoring employed a (Mettler Toledo, Zurich, Switzerland) with LE438 triode sensor, while rare earth quantification was performed through inductively coupled plasma optical emission spectrometry (ICP-OES, iCAP 7400 series, Thermo Fisher Scientific, Waltham, MA, USA).
2.3. Synthesis and Purification of P355 and P227-355
2.3.1. P355
The target compound P355 was prepared via free radical reaction method [38] in a PTFE-lined reaction vessel (50 mL) containing 7.30 g 3,5,5-trimethyl-1-hexene, 2.04 g NaH2PO2, 0.20 g DTBP initiator, 2.04 g CH3COOH, and 2 mL THF. Reaction proceeded under controlled conditions (135 °C, 45 h) with periodic DTBP replenishment (0.10 g at 15 h intervals). Post-synthetic workup involved: (1) Solvent and unreacted olefin removal via rotary evaporation; (2) Liquid–liquid extraction with anhydrous diethyl ether; (3) Sequential purification washes (H2O → 4% NaOH → 10% H2SO4 → saturated NaCl); (4) Dehydration with anhydrous MgSO4. Final cobalt salt precipitation yielded 5.67 g crystalline P355 (78.35% yield).
2.3.2. P227-355
The target compound P227-355 was synthesized via a Two-Step Protocol [10,35]: Synthesis of mono-3,5,5-trimethylhexylphosphinic acid, and Synthesis of P227-355.
Step 1: Synthesis of mono-3,5,5-trimethylhexylphosphinic acid
A reaction mixture containing 7.30 g 3,5,5-trimethyl-1-hexene, 7.63 g NaH2PO2, 0.40 g DTBP initiator, 7.63 g CH3COOH, and 15 mL THF was sealed in a 50 mL PTFE-lined reaction vessel and reacted at 120 °C for 8 h. After cooling, THF and unreacted olefin were removed via rotary evaporation. The residue was dissolved in anhydrous diethyl ether, and the product was successively extracted into an aqueous phase using 4% NaOH, acidified with 10% H2SO4, and re-extracted with fresh diethyl ether. The organic phase was washed with saturated NaCl, dehydrated with anhydrous MgSO4, filtered, and concentrated to afford mono-3,5,5-trimethylhexylphosphinic acid (9.12 g, 83.73% yield).
Step 2: Synthesis of P227-355
The mono-3,5,5-trimethylhexylphosphinic acid (8.65 g) was combined with 2-ethyl-1-hexene (7.97 g), DTBP (0.45 g initially, with periodic supplementation of 0.20 g every 8 h), CH3COOH (3.51 g), and THF (10 mL) in a PTFE-lined reaction vessel. The mixture was reacted at 135 °C for 24 h. Post-treatment and purification procedures, analogous to P355, yielded 5.55 g pure P227-355 (40.18% yield).
2.4. Liquid–Liquid Extraction Procedure
Equal volumes of organic phase and aqueous feed solution (phase ratio A/O = 1) were loaded into a 20 mL centrifuge tube, which was subsequently fixed on a Test Tube Shaker and shaken at 240 rpm for 20 min. The extraction process exhibited rapid kinetics, with shaking for merely 3 min sufficient to achieve nearly complete extraction equilibrium. To ensure the attainment of full extraction equilibrium, a shaking duration of 20 min was ultimately adopted in subsequent experiments. Following phase separation via centrifugation (3000 rpm, 5 min), the raffinate was collected for pH measurement and rare earth element (REE) quantification analysis.
The REE concentration in the organic phase after extraction (Co), extraction efficiency (E, %), distribution ratio (D), and separation factor (β) were calculated using Equations (1)–(4), respectively:
where Ca1 and Ca2 denote the concentrations of REEs in the aqueous feed solution (initial aqueous phase before extraction) and raffinate (residual aqueous phase after extraction equilibrium), respectively, mg/L; A/O represents the volumetric ratio of the aqueous phase to the organic phase during the extraction process.
2.5. Saturation Extraction Experiments
The saturation extraction experiments for determining the maximum REE loading capacity of P355 and P227-355 were conducted using separation funnels with manual shaking. Specially, a fixed volume of the organic phase (containing 10 mmol/L P355 or P227-355 dissolved in kerosene, with/without modifiers) was repeatedly contacted with equal volumes of fresh aqueous REE feed solutions (~70 mg/L Sm3+/Dy3+ in HCl media with pH value of 4.00) at an A/O of 1:1. After each extraction cycle, the mixture was allowed to stand for 5 min, and key phenomena—including phase clarity, and the presence of emulsification or third-phase formation—were recorded in detail. If emulsification occurred or phase separation was incomplete, the mixture was transferred to a 50 mL centrifuge tube and centrifuged at a predefined speed to facilitate complete phase separation before proceeding to the next extraction cycle. The total concentration of REEs accumulated in the organic phase (CoT, mg/L) after each cycle—an indicator of approaching saturation—was calculated using Equation (5).
where Ca2n represents the concentration of REE in the aqueous raffinate obtained after the nth extraction stage, mg/L; n denotes the number of extraction stages.
2.6. Back-Extraction
The back-extraction (stripping) experiments were conducted in two sequential stages: REE preloading into the organic phase and subsequent REE stripping into the aqueous phase. To obtain REE preloading organic phase, equal volumes of the organic phase (10 mmol/L P355 or P227-355 in kerosene) and aqueous feed solution (20 mg/L Dy3+ in HCl media) were manually mixed in a separation funnel. After complete phase separation, the Dy3+-loaded organic phase was retained for stripping, while the aqueous raffinate was collected to determine residual Dy concentration. In the stripping step, equal volumes of the Dy3+-loaded organic phase and aqueous stripping solution (0.01~0.1 mol/L H2SO4) were added to a centrifugal tube and thoroughly mixed using the Test Tube Shaker. After phase separation, the aqueous stripping solution was collected to measure Dy concentration. The Dy stripping percentage (S, %) was calculated according to Equation (6).
where Cas denotes the Dy concentration in the stripping solution after back-extraction, mg/L; Cos represents the Dy concentration in the Dy3+-loaded organic phase before back-extraction, mg/L.
3. Results and Discussion
3.1. Characterization
3.1.1. P355
The chemical structures of P355 and P227-355 were comprehensively characterized using ESI-HRMS, 1H NMR, 31P NMR, and FT-IR. The results are presented in Figure 3 and Figure 4, respectively.
Di-(3,5,5-trimethylhexyl)phosphinic acid (P355): ESI-HRMS, m/z: 319.28 (M + H)+, 637.55 (2M + H)+, 955.82 (3M + H)+, 1274.09 (4M + H)+, 1593.37 (5M + H)+, 1949.59 (6M + K)+. 31P NMR (162 MHz, CDCl3-d1), δ: 60.45 ppm. 1H NMR (400 MHz, CDCl3-d1), δ/ppm: 0.87 (s, 18H, 6-CH3), 0.92 (d, 6H, 2-CH3), 1.04 (dd, J1 = 14.0 Hz, J2 = 6.1 Hz, 2H), 1.20 (dd, J1 = 14.0 Hz, J2 = 3.0 Hz, 2H), 1.37~1.58 (m, 6H), 1.57~1.72 (m, 4H), 11.10 (s, 1H, -OH). FT-IR/cm−1: 2866.88~2951.06 (C-H stretching vibrations), 2255.96 (O-H stretching caused by dimer formation), 1677.65 (O-H bending vibration), 1466.16 (C-H bending vibration), 1363.73 (C-H rocking vibration), 1156.24 (P=O stretching vibration), 964.35 (P-O(H) stretching vibration), 812.12 (P-C stretching vibration).
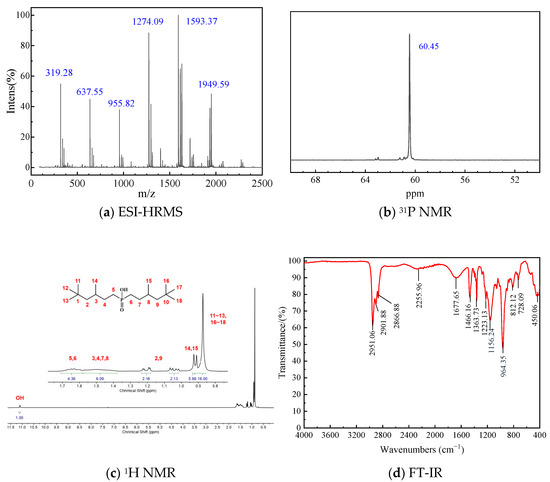
Figure 3.
Characterization results of P355.
3.1.2. P227-355
(2-Ethylhexyl)(3,5,5-trimethylhexyl)phosphinic acid (P227-355): ESI-HRMS, m/z: 305.26 (M + H)+, 609.52 (2M + H)+, 913.77 (3M + H)+, 1218.03 (4M + H)+, 1522.28 (5M + H)+, 1865.48 (6M + K)+. 31P NMR (162 MHz), δ: 60.55 ppm. 1H NMR (400 MHz, CDCl3-d1), δ/ppm: 0.87 (s, 9H, 3-CH3), 0.83~0.90 (m, 6H), 0.92 (d, J = 6.3 Hz, 3H), 1.04 (dd, J1 = 14.0, J2 = 6.1 Hz, 1H), 1.33~1.17 (m, 5H), 1.35~1.54 (m, 6H), 1.55~1.69 (m, 5H), 1.78 (m, 1H), 11.54 (s, 1H, -OH). FT-IR/cm−1: 2867.38~2954.38 (C-H stretching vibrations), 2175.79 (O-H stretching caused by dimer formation), 1676.63 (O-H bending vibration), 1463.03 (C-H bending vibration), 1363.96 (C-H rocking vibration), 1156.97 (P=O stretching vibration), 961.12 (P-O(H) stretching vibration), 819.63 (P-C stretching vibration).
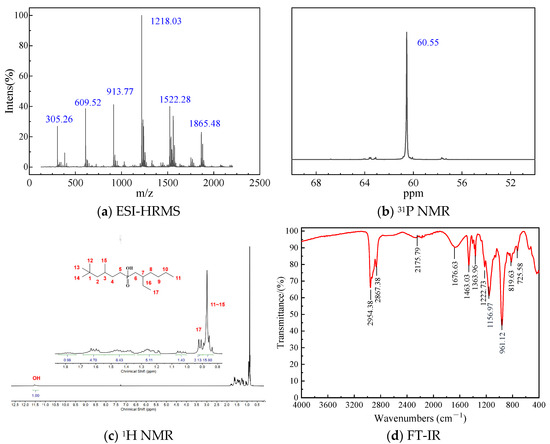
Figure 4.
Characterization results of P227-355.
Collectively, these characterization results confirm the successful synthesis of P355 and P227-355 with the target structures.
3.2. Equilibrium pH
The equilibrium pH (pHequilibrium) of the aqueous raffinate significantly influences REE extraction, as organophosphorus acids typically extract most metal ions (such as Co, Ni, rare earth, Al, Fe) through a cation exchange mechanism. To clarify this effect, we first investigated extraction behaviors of the middle REEs (Sm, Eu, Gd, Tb and Dy) using P355 and P227-355 diluted in kerosene under varying pHequilibrium conditions. In these experiments, both extractants were used at a concentration of 10 mmol/L and diluted in kerosene without any modifiers. To obtain a range of equilibrium pH values, the aqueous feed solutions—comprising a mixture of Sm, Eu, Gd, Tb, and Dy (each with an initial concentration of ~0.1 mmol/L)—were prepared by diluting aliquots of the same stock solution with different ultrapure water of pre-adjusted acidity (from 1.4 to 3) using dilute HCl. The results are presented in Figure 5 and Table 1.

Table 1.
Separation performance of Sm~Dy by P355 and P227-355 at different equilibrium pH values.
As shown in Figure 5a, the extraction efficiency of P355 for middle REEs follows the order: Dy > Tb > Gd > Eu > Sm. At identical experimental conditions, the extraction percentage increases with increasing atomic number of middle REEs—an observation attributed to the Lanthanide Contraction effect. This effect reduces the ionic radius of middle REEs as atomic number rises, enhancing their charge density and thus strengthening their coordination affinity with the -POOH groups of P355, which promotes extraction.
The separation performance of P355 for adjacent middle REEs was evaluated using two key metrics: ΔpH0.5 and separation factor (β). Here, ΔpH0.5 refers to the equilibrium pH at which the extraction percentage of a given REE reaches 50%, while ΔpH0.5 denotes the difference in pH0.5 between two REEs (a larger ΔpH0.5 indicates better separability). For P355, the ΔpH0.5 values of adjacent middle REE pairs follow the sequence: ΔpH0.5Tb−Gd (0.23) > ΔpH0.5Dy−Tb (0.21) > ΔpH0.5Eu−Sm (0.09) > ΔpH0.5Gd−Eu (0.05), as presented in Table 1. This indicates that the Tb-Gd pair is the most easily separated, followed by Dy-Tb, while Gd-Eu exhibits the poorest separability.
Notably, this trend is consistent with the order of separation factors for adjacent middle REE pairs (βTb/Gd > βDy/Tb > βEu/Sm > βGd/Eu) (see Table 1). The consistency between ΔpH0.5 and β further confirms the reliability of P355’s separation performance for middle REEs.
Figure 5.
Extraction behaviors of Sm~Dy by P355 and P227-355 at different equilibrium pH values.
The extraction behaviors and separation performance of P227-355 toward middle REEs Sm~Dy are analogous to those of P355, as illustrated in Figure 5b and Table 1. A key distinction, however, lies in their equilibrium pH at 50% extraction (pH0.5): for all target REEs, the pH0.5 values of P227-355 are consistently higher than those of P355. Specially: pH0.5(P227-355)Sm (2.46) > pH0.5(P355)Sm (2.39), pH0.5(P227-355)Eu (2.37) > pH0.5(P355)Eu (2.30), pH0.5(P227-355)Gd (2.34) > pH0.5(P355)Gd (2.25), pH0.5(P227-355)Tb (2.12) > pH0.5(P355)Tb (2.02), pH0.5(P227-355)Dy (1.94) > pH0.5(P355)Dy (1.81). In the context of organophosphinic acid extraction (governed by the cation exchange mechanism −POOH ⇌ −POO− + H+), a higher pH0.5 indicates that more alkaline conditions are required to promote ligand deprotonation (−POOH → −POO−) and subsequent middle REE3+ coordination. This directly confirms that P227-355 exhibits weaker extraction capacity for Sm3+–Dy3+ compared to P355.
The reduced extraction ability of P227-355 can be attributed to its structural difference from P355: P227-355 features an unsymmetrical alkyl chain configuration (one 2-ethylhexyl group + one 3,5,5-trimethylhexyl group), whereas P355 is symmetric (two 3,5,5-trimethylhexyl groups). Replacing one 3,5,5-trimethylhexyl group with a 2-ethylhexyl group—i.e., substituting the γ-carbon branched structure with a β-carbon branched 2-ethylhexyl group—strengthens the electron-donating effect of the alkyl chains. This increases the electron density of the central P atom in the −POOH moiety, suppressing the dissociation of the acidic proton (H+) and lowering the availability of −POO− ligands for middle REE3+ binding. Collectively, these results demonstrate that γ-carbon branched alkyl chains (e.g., 3,5,5-trimethylhexyl) exert a positive effect on middle REE extraction by organophosphinic acids, while replacing them with β-carbon branched alkyl chains (e.g., 2-ethylhexyl) impairs extraction performance.
For P227-355, the ΔpH0.5 values of adjacent middle REE pairs follow the same sequence to that of P355, namely: ΔpH0.5Tb−Gd (0.22) > ΔpH0.5Dy−Tb (0.18) > ΔpH0.5Eu−Sm (0.09) > ΔpH0.5Gd−Eu (0.03). This trend is also consistent with the order of separation factors for adjacent middle REE pairs (βTb/Gd > βDy/Tb > βEu/Sm > βGd/Eu), as presented in Table 1.
3.3. Extractant Concentration
To investigate the effect of extractant concentration on the extraction of middle REEs, batch extraction experiments were conducted with P355 and P227-355 diluted in kerosene at varying concentrations (5~25 mmol/L). The aqueous feed solution was a mixed middle REE system, with each REE concentration maintaining at ~0.1 mmol/L and pH of 2.0. The results are presented in Figure 6 and Table 2.

Table 2.
Separation performance of Sm~Dy by P355 and P227-355 at different extractant concentrations.
As the concentration of P355 and P227-355 increases, the extraction percentage (E, %) of all REEs rises consistently. At the same extractant concentration, it is maintained as EDy > ETb > EGd > EEu > ESm for both extractants—consistent with the lanthanide effect. Notably, the increments in EDy and ETb (heavier middle REEs) are more pronounced than those in EGd, EEu and ESm (lighter middle REEs) across the tested concentration range (5~25 mmol/L). This trend arises because heavier middle REEs have higher ligand affinity, making their extraction more responsive to increased extractant availability. Additionally, at the same concentration, the E% values of P355 for each middle REE are consistently higher than those of P227-355, further confirming that P355 has stronger middle REE extraction capacity than P227-355.
To ensure reliable separation factor (β) calculation (avoiding deviations from excessively high/low E%), we analyzed the adjacent middle REE separation factors (βN + 1/N) at extractant concentrations where all REE E% values fell within 5%~95%. For both P355 and P227-355, the β order follows βTb/Gd > βDy/Tb > βEu/Sm > βGd/Eu, with βTb/Gd being the highest and βGd/Eu the lowest. This result is consistent with the separation performance trends observed in Table 1 (pH-dependent experiments), verifying the reproducibility of the two extractants’ selectivity for middle REE pairs.
P355 exhibits better separation performance for middle REEs, as its βN + 1/N values are almost all higher than the corresponding ones of P227-355. At an extractant concentration of 20 mmol/L, the average value of adjacent middle REEs separation factors at is 3.01 for P355 and 2.98 for P227-355 (Table 2).
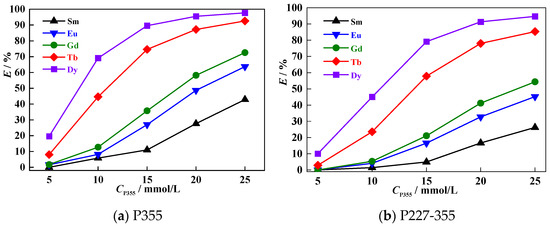
Figure 6.
Extraction behaviors of Sm~Dy at different extractant concentrations.
3.4. Saturation Capacity
The saturation capacity of an extractant is a key metric that reflects its metal-loading efficiency, and it serves as a crucial parameter for the design of industrial REE extraction processes. To determine the saturation capacities of P355 and P227-355, two representative middle REEs—Sm3+ (the lightest REE in the target system) and Dy3+ (the heaviest one)—were selected as model ions. Saturation extraction experiments were then conducted following a cyclic contact protocol, with the detailed experimental procedure provided in Section 2.5 (“Saturation extraction experiments”). The dynamic phenomena observed during each extraction cycle for P355 and P227-355 are presented in Figure 7 and Figure 8, respectively. Meanwhile, the stage-wise accumulated concentrations of REEs in the organic phase are illustrated in Figure 9.
For Sm extraction with P355: Slight emulsification emerged at the organic-aqueous interface during the 2nd extraction stage, accompanied by the generation and aggregation of small bubbles. The 3rd stage extraction was initiated only after these bubbles disappeared completely via prolonged standing; at this stage, more pronounced bubble formation was observed (Figure 7a), and the accumulated Sm concentration in the organic phase reached 191.21 mg/L—corresponding to a P355/Sm molar ratio of 7.86. Although the 4th stage extraction was attempted, severe emulsification prevented effective phase separation, making it impossible to collect the aqueous raffinate for Sm concentration determination.
For Sm extraction with P227-355: Slight emulsification first appeared at the 3rd extraction stage (Figure 8a), and it intensified significantly by the 4th stage. At the point of severe emulsification (4th stage), the accumulated Sm concentration in the organic phase was 183.24 mg/L—lower than that achieved with P355 under comparable conditions (Figure 9).
For Dy extraction: Emulsification was observed at different stages for the two extractants—occurring at the 5th extraction stage when using P355, and at the 4th stage when using P227-355. At the respective emulsification onset stages, the accumulated Dy concentrations in the organic phase were 357.51 mg/L (for P355) and 261.67 mg/L (for P227-355), respectively (Figure 9). Corresponding to these metal-loading levels, the molar ratios of extractant to Dy were calculated as 4.55 (P355/Dy) and 6.21 (P227-355/Dy).
These results confirm that P355 exhibits a higher saturation capacity for both Sm and Dy compared to P227-355. Additionally, as the number of extraction stages increases, the molar ratio of extractant to REE gradually decreases—even falling below 6 in later stages. This trend implies a transformation in the structure of the REE-extraction complexes, as the changing ligand-to-metal ratio reflects a shift from the initial coordination mode to a more compact complex configuration when the organic phase approaches saturation [37].
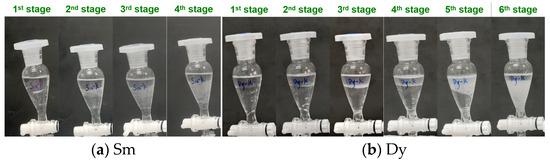
Figure 7.
Extraction phenomena of Sm and Dy by P355 at different stages.
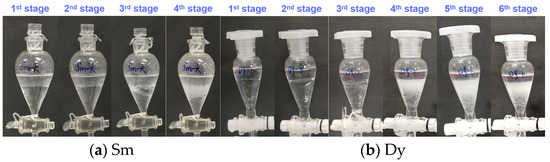
Figure 8.
Extraction phenomena of Sm and Dy by P227-355 at different stages.
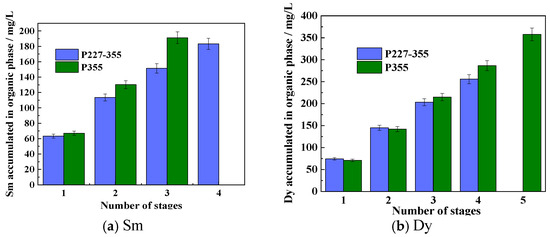
Figure 9.
Accumulated concentrations of REEs in the organic phase across extraction stages.
3.5. Modifier Addition
The formation of emulsions in solvent extraction is a complex phenomenon influenced by multiple factors: It is closely related to the molecular structure of the extractant [37], and the composition and intrinsic properties of both the organic and aqueous phases must also be considered [39]. Once emulsions form, they trigger a series of adverse effects (e.g., hindering phase separation, reducing extraction efficiency, and complicating raffinate analysis).
To mitigate emulsification, we investigated the effect of adding three modifiers—isooctanol, xylene, and MNPK—on the extraction phenomena and efficiency of Sm and Dy. Experiments were conducted following the cyclic contact protocol described in Section 2.5. When using isooctanol and xylene as modifiers, the corresponding extraction efficiencies of Sm and Dy are shown in Figure 10 and Figure 11, where the abbreviations denote: K = kerosene (blank, no modifier), I = isooctanol and X = xylene. The extraction phenomena observed in the presence of different modifiers are presented in Supplementary Figures S1–S4, Figure 12 and Figure 13. When using MNPK as a modifier, the extraction efficiencies are shown in Figure 14 (M = MNPK).
3.5.1. Isooctanol
When 10 vol% isooctanol was added as a modifier (volume ratio of kerosene to isooctanol, Vk:VI = 9:1), no emulsification was observed for either Sm or Dy extraction by P355 or P227-355—even after 10 extraction stages—with only slight turbidity in the organic phase (Supplementary Figures S1a,c and S2a,c). However, compared with the kerosene-only system (no modifier), the stage-wise accumulated concentrations of Sm and Dy in the organic phase decreased significantly for both extractants (Figure 10). For instance, after the 3rd extraction stage: The accumulated Sm concentration in P355’s organic phase dropped sharply from 191.21 mg/L (kerosene-only) to 55.66 mg/L (10 vol% isooctanol); For P227-355, a corresponding decrease was observed, with its accumulated Sm concentration in the organic phase falling from 151.37 mg/L (kerosene-only) to 55.01 mg/L (10 vol% isooctanol). Further increasing the isooctanol dosage to 20 vol% led to a more pronounced reduction in the accumulated Sm and Dy concentrations in the organic phase (relative to both 0 vol% and 10 vol% isooctanol groups) (Figure 10), though emulsification remained absent (Supplementary Figures S1b,d and S2b,d).
The decline in Sm and Dy extraction efficiency after isooctanol addition is attributed to the formation of hydrogen bonds between the hydroxyl groups (-OH) of isooctanol and the phosphinic acid groups (−POOH) of P355/P227-355. These hydrogen bonds compete with the coordination interaction between −POOH and REE3+, weakening the binding affinity of the extractants for Sm and Dy. Thus, isooctanol is not a suitable modifier for REE extraction using P355 and P227-355.
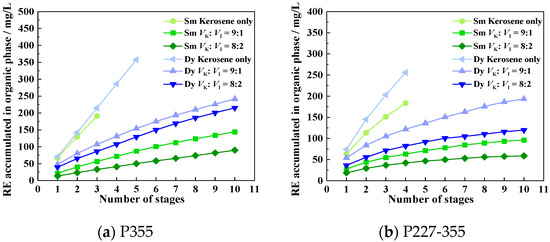
Figure 10.
Accumulated concentrations of REEs in the organic phase across extraction stages with isooctanol as modifier.
3.5.2. Xylene
When xylene was used as a modifier, the emulsification phenomenon during Sm and Dy extraction by P355 and P227-355 was slightly alleviated (Supplementary Figures S3 and S4). Regarding extraction efficiency: the addition of xylene exerted a weak negative effect on ESm, whereas it had negligible impact on EDy (Figure 11).
The slight emulsification alleviation and differential impacts on ESm and EDy with xylene as modifier stem from its physicochemical properties and the lanthanide contraction effect. Xylene is an aromatic hydrocarbon with moderate lipophilicity and low polarity. It might mitigate emulsification by two means: It reduces the viscosity of the kerosene-based organic phase, weakening the aggregation of extractants’ polar −POOH groups; It also moderately adjusts organic-aqueous interfacial tension, destabilizing small emulsified droplets. However, its low polarity limits strong interference with pre-formed emulsions, leading only to slight improvement. Heavier middle REE Dy3+ (smaller ionic radium) forms stable extraction complexes with P355/P227-355. Xylene’s weak solvating effect barely disrupts this strong coordination, leaving EDy unchanged. In contrast, lighter middle REE Sm3+ (larger radium) forms less stable extraction complexes, which is more influenced by xylene’s solvating effect, leading to a slight decrease in ESm.
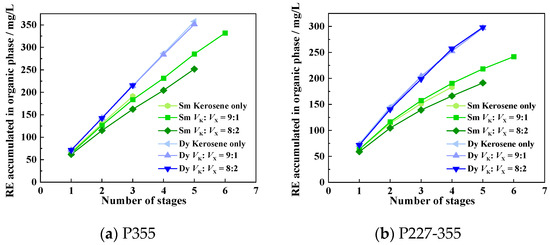
Figure 11.
Accumulated concentrations of REEs in the organic phase across extraction stages with xylene as modifier.
3.5.3. MNPK
MNPK emerges as an effective modifier for preventing emulsification during the extraction of Sm and Dy by both P355 and P227-355, while exerting only a minimal negative impact on their extraction efficiencies. For Sm extraction: When 10 vol% MNPK was added (VK:VM = 9:1), emulsification was largely eliminated in the P355 system (Figure 12a), and only slight emulsification occurred at the 9th extraction stage in the P227-355 system (Figure 12c). Increasing the MNPK dosage to 20 vol% resulted in clear aqueous raffinates across all extraction stages for P355 (Figure 12b), with complete elimination of emulsification in the P227-355 system (Figure 12d). For Dy extraction: the anti-emulsification effect was similarly pronounced with MNPK addition, as evidenced by significant improvements in phase separation behavior (Figure 13).
MNPK contains both lipophilic segments (pentyl and methyl) and a moderated polar group (carbonyl). The lipophilic parts enhance compatibility with the kerosene-based organic phase, reducing phase separation resistance. Its polar group preferentially adsorbs at the organic-aqueous interface, disrupting the stable emulsifying film formed by aggregated −POOH groups of P355/P227-355. This breaks emulsified droplets, with higher MNPK dosage (20 vol%) strengthening this effect to eliminate emulsification.
Unlike isooctanol (forming strong H-bonds with −POOH), MNPK’s weak interaction with extractants only slightly competes with the coordination between −POOH and Sm3+/Dy3+. It also does not drastically alter the organic phase’s solvating ability, so the stability of REE-extractant complexes remains largely unchanged, leading to only a little negative effect on extraction efficiency (Figure 14). Notably, with the addition of 20 vol% MNPK, the saturation capacity of Sm increased to 324.18 mg/L for P355 and 229.12 mg/L for P227-355; concurrently, that of Dy rose to 357.51 mg/L for P355 and 320.80 mg/L for P227-355.
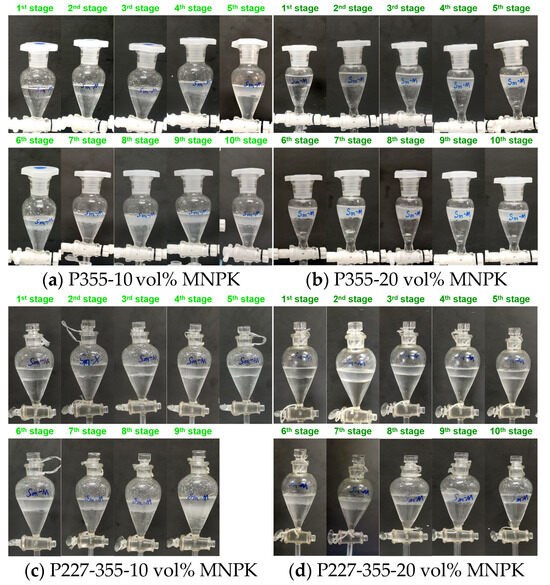
Figure 12.
Phenomena of Sm extraction after adding MNPK by (a,b) P355 and (c,d) P227-355.
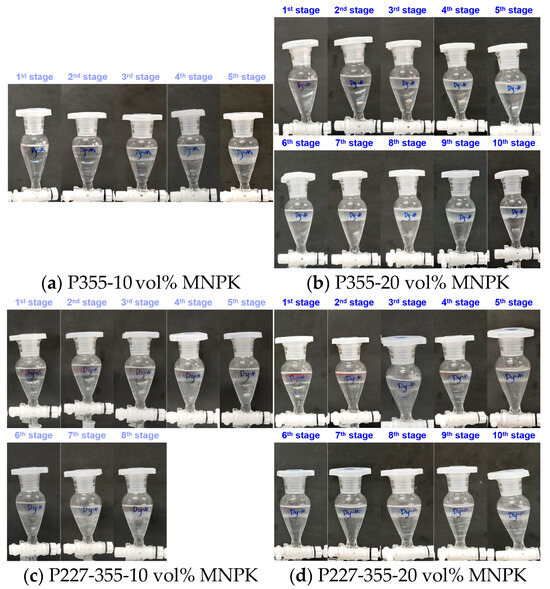
Figure 13.
Phenomena of Dy extraction after adding MNPK by (a,b) P355 and (c,d) P227-355.
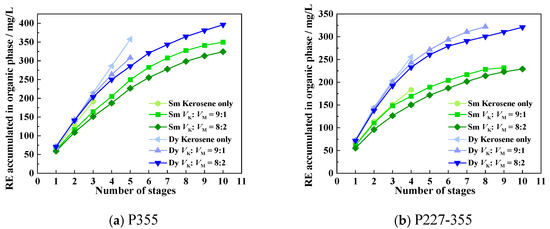
Figure 14.
Accumulated concentrations of REEs in the organic phase across extraction stages with MNPK as modifier.
3.6. Stripping Acidity
Among the investigated middle REEs, Dy3+ exhibits the smallest ionic radius (0.908 Å [40]). According to the hard and soft acids and bases (HSAB) theory, smaller REE ions form stronger coordination complexes with phosphinic acid extractants (P355 and P227-355) due to enhanced charge density. Consequently, Dy3+ requires the harshest stripping conditions, making it a representative model for evaluating the stripping performance of the two extractants. The results are presented in Figure 15.
The stripping percentage of Dy loaded in the P227-355 organic phase (SDy-P227-355) was consistently higher than that in the corresponding P355 organic phase (SDy-P355) under the same conditions. This observation indicates that the Dy-P355 complex requires a higher acidity to achieve effective stripping compared to the Dy-P227-355 complex. The SDy-P227-355 reached a stable plateau at approximately 90% when the H2SO4 concentration was ≥0.02 mol/L; In contrast, SDy-P355 did not stabilize until the H2SO4 concentration reached 0.06 mol/L, with the plateau value remaining at around 80%.
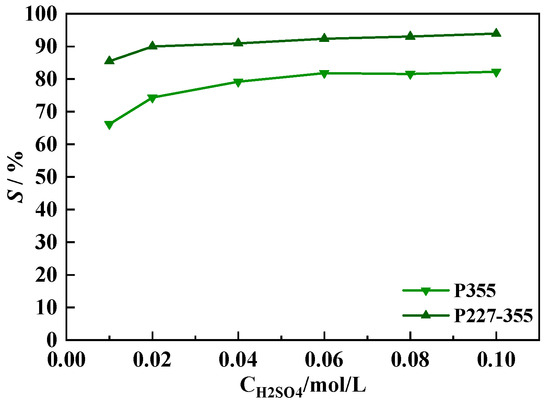
Figure 15.
Stripping behavior of Dy using aqueous H2SO4 solutions of different concentrations.
3.7. Extraction Mechanism
The addition of the ketonic compound MNPK not only effectively eliminated the third-phase formation but also enhanced the saturation capacity of organic phases containing P355 and P227-355. Thus, investigating the extraction mechanism for P355 and P227-355 in the presence of MNPK is imperative. Trivalent REE ions exhibit striking similarities in their physicochemical properties, particularly in aqueous solutions where each ion is surrounded by a coordinated water film. This structural and chemical resemblance results in highly analogous extraction mechanisms across different trivalent REEs. Therefore, Sm was selected as a representative to investigate the extraction behavior of this group. Slope analysis was conducted to clarify the charge balance and ligand coordination ratio in the Sm extraction process, with the aqueous phase maintaining ~70 mg/L Sm and the organic phase containing 10 mmol/L extractant (phase ratio 1:1). The results are presented in Figure 16.
In the absence of MNPK, the fitted slopes of Log D versus equilibrium pH are 2.92 for P355 and 2.86 for P227-355, both close to the theoretical value of 3. This indicates that each Sm3+ ion coordinates with extractants to release 3 protons, confirming cation exchange as the dominant mechanism. Meanwhile, the slopes of Log D versus Log[(HL)2] approach 2 for both extractants. Given that P355 and P227-355 form dimers (HL)2 in kerosene, this result demonstrates that one Sm3+ ion interacts with 2 dimeric extractant species to form a stable complex. The extraction equation can be expressed as:
Sm3+ + 2(HL)2 ↔ Sm·L3·(HL) + 3H+
Upon adding MNPK, the slopes of Log D versus equilibrium pH decrease slightly to 2.61 (P355) and 2.50 (P227-355), while the slopes of Log D versus Log[(HL)2] decline marginally to 2.04 (P355) and 2.01 (P227-355). All slopes remain near the theoretical values of 3 and 2, respectively, proving that MNPK does not alter the core cation exchange mechanism or charge balance. Instead, MNPK form hydrogen-bonded associates (HL·MNPK) with free extractants (HL), reducing the effective concentration of (HL)2 and causing minor slope variations.
Organophosphorus acids feature a -POOH group and two alkyl chains. It has been demonstrated that the two oxygen atoms of the -POOH group are the active sites that coordinate with metal ions to form extraction complexes [41,42]. The structure of the extracted Sm3+ complex can be deduced, as illustrated in Figure 17.
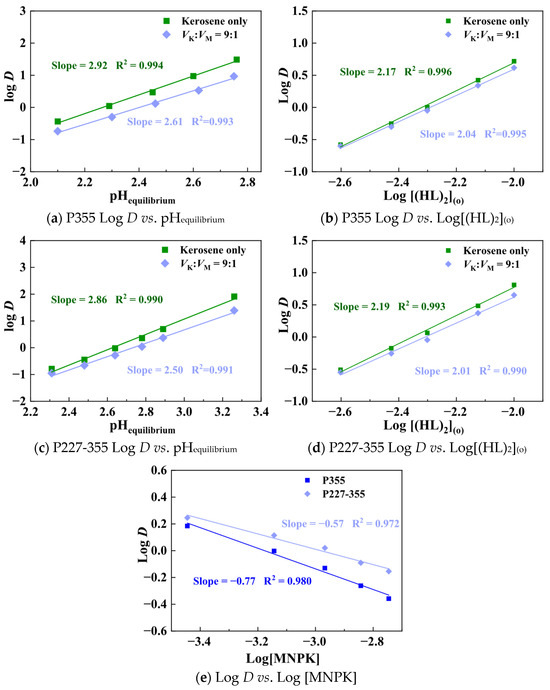
Figure 16.
Slope analysis results of Sm extraction by P335 and P227-355 in the presence of MNPK as a modifier.
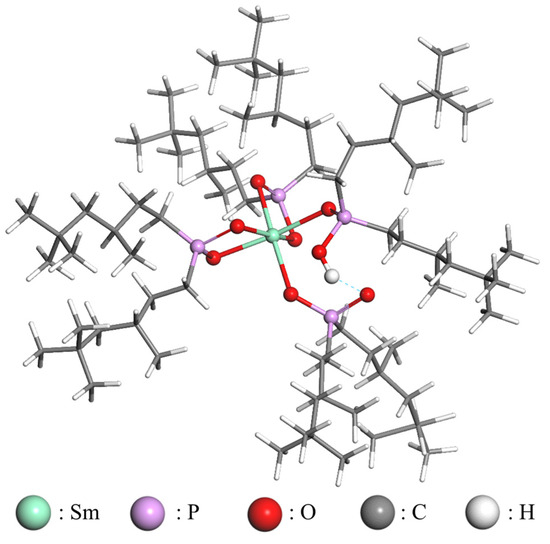
Figure 17.
Structure of the extracted Sm3+ complex.
4. Conclusions
This study systematically investigates the synthesis, middle REE extraction/separation performance, and anti-emulsification mechanism of two novel γ-carbon-substituted dialkylphosphinic acids (P355 and P227-355), yielding the following key conclusions:
(1) The molecular structure of dialkylphosphinic acids directly governs their extraction performance for middle REE. P355 (symmetrical γ-carbon branches) exhibits stronger REE extraction capacity, while P227-355 (unsymmetrical β/γ-carbon branches) offers more favorable stripping behavior, confirming the tunability of extraction performance via structural symmetry and branch position.
(2) Both P355 and P227-355 outperform conventional industrial extractants in middle REE separation selectivity. P355 achieves an average separation factor (β) of 3.01 for adjacent MREE pairs (at 20 mmol/L). P227-355’s average β of 2.98 also exceeds most commercial analogs, highlighting the potential of γ-carbon-substituted dialkylphosphinic acids as high-performance MREE extractants.
(3) Methyl n-pentyl ketone (MNPK) emerges as an optimal modifier for middle REE extraction. Unlike isooctanol (which reduces extraction efficiency via strong H-bonding with −POOH) or xylene (which only slightly alleviates emulsification), 20 vol% MNPK completely eliminates emulsification by disrupting the interfacial film formed by aggregated −POOH groups. More importantly, MNPK promotes the formation of extraction complexes, increasing Sm and Dy saturation capacities to 324.18 mg/L and 357.51 mg/L for P355, respectively—further enhancing the industrial applicability of the extractants.
(4) The middle REE extraction mechanism is clarified as a cation exchange process. Slope analysis confirms that 1 middle REE3+ reacts with 2 extractant dimers (HL)2 to release 3 protons in the absence of MNPK. With addition of MNPK, it does not alter this cation exchange mechanism but forms hydrogen-bonded associates with free HL, moderately adjusting extractant availability without compromising coordination stability.
Overall, this work demonstrates that γ-carbon substitution and structural symmetry modulation are effective strategies for designing high-performance MREE extractants. The developed P355/P227-355-MNPK system balances extraction selectivity, saturation capacity, anti-emulsification, and back-extraction efficiency, providing a solution for efficient middle REE separation and laying the foundation for rational extractant design in sustainable rare earth hydrometallurgy.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/separations12110303/s1, Figure S1: Extraction phenomena of Sm in the presence of isooctanol as modifier: For (a,b) P355 and (c,d) P227-355; Figure S2: Extraction phenomena of Dy in the presence of isooctanol as modifier: For (a,b) P355 and (c,d) P227-355; Figure S3: Extraction phenomena of Sm in the presence of xylene as modifier: For (a,b) P355 and (c,d) P227-355; Figure S4, Extraction phenomena of Dy in the presence of xylene as modifier: For (a,b) P355 and (c,d) P227-355.
Author Contributions
Methodology, R.S., N.S. and J.W.; software, N.S.; validation, Y.X.; formal analysis, R.S. and F.L.; investigation, R.S. and F.L.; resources, Y.L. and J.W.; data curation, Y.X., Y.L. and J.W.; writing—original draft preparation, R.S. and F.L.; writing—review and editing, Y.L. and J.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Guangxi Major Science and Technology Project, grant number Guike AA24206022; and the National Natural Science Foundation of China, grant number 22578027.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Coey, J.M.D. Perspective and Prospects for Rare Earth Permanent Magnets. Engineering 2020, 6, 119–131. [Google Scholar] [CrossRef]
- Gupta, I.; Singh, S.; Bhagwan, S.; Singh, D. Rare Earth (RE) Doped Phosphors and Their Emerging Applications: A Review. Ceram. Int. 2021, 47, 19282–19303. [Google Scholar] [CrossRef]
- Xue, H.; Lv, G.; Wang, L.; Zhang, T.-a. Review of Rare Earth Extraction and Product Preparation Technologies and New Thinking for Clean Utilization. Miner. Eng. 2024, 215, 108796. [Google Scholar] [CrossRef]
- Merroune, A.; Brahim, J.; Berrada, M.; Essakhraoui, M.; Achiou, B.; Mazouz, H.; Beniazza, R. A Comprehensive Review on Solvent Extraction Technologies of Rare Earth Elements from Different Acidic Media: Current Challenges and Future Perspectives. J. Ind. Eng. Chem. 2024, 139, 1–17. [Google Scholar] [CrossRef]
- Huang, X.; Long, Z.; Wang, L.; Feng, Z. Technology Development for Rare Earth Cleaner Hydrometallurgy in China. Rare Met. 2015, 34, 215–222. [Google Scholar] [CrossRef]
- Traore, M.; Gong, A.; Wang, Y.; Qiu, L.; Bai, Y.; Zhao, W.; Liu, Y.; Chen, Y.; Liu, Y.; Wu, H.; et al. Research Progress of Rare Earth Separation Methods and Technologies. J. Rare Earths 2023, 41, 182–189. [Google Scholar] [CrossRef]
- Liu, T.; Chen, J. Extraction and Separation of Heavy Rare Earth Elements: A Review. Sep. Purif. Technol. 2021, 276, 119263. [Google Scholar] [CrossRef]
- Chen, L.; Li, H.; Chen, J.; Li, D.; Liu, T. Separation of Heavy Rare Earths by Di-(2-ethylhexyl) Phosphinic Acid: From Fundamentals to Cascade Extraction Simulation. Miner. Eng. 2020, 149, 106232. [Google Scholar] [CrossRef]
- Wang, J.; Xie, M.; Wang, H.; Xu, S. Solvent Extraction and Separation of Heavy Rare Earths from Chloride Media Using Nonsymmetric (2,3-dimethylbutyl)(2,4,4′-trimethylpentyl)phosphinic Acid. Hydrometallurgy 2017, 167, 39–47. [Google Scholar] [CrossRef]
- Wang, J.; Chen, G.; Xu, S.; Li, L. Synthesis of Novel Nonsymmetric Dialkylphosphinic Acid Extractants and Studies on Their Extraction-Separation Performance for Heavy Rare Earths. Hydrometallurgy 2015, 154, 129–136. [Google Scholar] [CrossRef]
- Ohto, K.; Matsufuji, T.; Yoneyama, T.; Tanaka, M.; Kawakita, H.; Oshima, T. Preorganized, Cone-Conformational Calix [4]arene Possessing Four Propylenephosphonic Acids with High Extraction Ability and Separation Efficiency for Trivalent Rare Earth Elements. J. Incl. Phenom. Macrocycl. Chem. 2011, 71, 489–497. [Google Scholar] [CrossRef]
- Khoshoei, A.; Mokhtarifar, M.; Fazeli, S.; Jean-Fulcrand, A.; Boffito, D.C. Crown Ethers in the Group and Individual Separation of Rare-Earth Elements Through Solvent Extraction: Interactions, Thermodynamics, and Applications. Ind. Eng. Chem. Res. 2025, 64, 12347–12367. [Google Scholar] [CrossRef]
- Kukkonen, E.; Virtanen, E.; Moilanen, J. α-Aminophosphonates, -Phosphinates, and -Phosphine Oxides as Extraction and Precipitation Agents for Rare Earth Metals, Thorium, and Uranium: A Review. Molecules 2022, 27, 3465. [Google Scholar] [CrossRef]
- Okamura, H.; Hirayama, N. Recent Progress in Ionic Liquid Extraction for the Separation of Rare Earth Elements. Anal. Sci. 2021, 37, 119–130. [Google Scholar] [CrossRef]
- Wang, K.; Adidharma, H.; Radosz, M.; Wan, P.; Xu, X.; Russell, C.K.; Tian, H.; Fan, M.; Yu, J. Recovery of Rare Earth Elements with Ionic Liquids. Green Chem. 2017, 19, 4469–4493. [Google Scholar] [CrossRef]
- Abreu, R.D.; Morais, C.A. Study on Separation of Heavy Rare Earth Elements by Solvent Extraction with Organophosphorus Acids and Amine Reagents. Miner. Eng. 2014, 61, 82–87. [Google Scholar] [CrossRef]
- Sun, X.; Waters, K.E. Synergistic Effect between Bifunctional Ionic Liquids and a Molecular Extractant for Lanthanide Separation. ACS Sustain. Chem. Eng. 2014, 2, 2758–2764. [Google Scholar] [CrossRef]
- Hidayah, N.N.; Abidin, S.Z. Extraction of Light, Medium and Heavy Rare-Earth Elements Using Synergist Extractants Developed from Ionic Liquid and Conventional Extractants. Comptes Rendus Chim. 2019, 22, 728–744. [Google Scholar] [CrossRef]
- Dashti, S.; Sadri, F.; Shakibania, S.; Rashchi, F.; Ghahreman, A. Separation and Solvent Extraction of Rare Earth Elements (Pr, Nd, Sm, Eu, Tb, and Er) Using TBP and Cyanex 572 from a Chloride Medium. Miner. Eng. 2021, 161, 106694. [Google Scholar] [CrossRef]
- Lu, Y.; Liao, W. Extraction and Separation of Trivalent Rare Earth Metal ions from Nitrate Medium by p-Phosphonic Acid Calix [4]arene. Hydrometallurgy 2016, 165, 300–305. [Google Scholar] [CrossRef]
- Shen, L.; Chen, J.; Chen, L.; Liu, C.; Zhang, D.; Zhang, Y.; Su, W.; Deng, Y. Extraction of Mid-Heavy Rare Earth Metal Ions from Sulphuric Acid Media by Ionic Liquid A336 P507. Hydrometallurgy 2016, 161, 152–159. [Google Scholar] [CrossRef]
- Quinn, J.E.; Soldenhoff, K.H.; Stevens, G.W. Solvent Extraction of Rare Earth Elements Using a Bifunctional Ionic Liquid. Part 2: Separation of Rare Earth Elements. Hydrometallurgy 2017, 169, 621–628. [Google Scholar] [CrossRef]
- Wang, Y.; Li, F.; Zhao, Z.; Dong, Y.; Sun, X. The Novel Extraction Process Based on CYANEX® 572 for Separating Heavy Rare Earths from Ion-Adsorbed Deposit. Sep. Purif. Technol. 2015, 151, 303–308. [Google Scholar] [CrossRef]
- Wang, J.; Xie, M.; Liu, X.; Wang, H. Extractant (2-ethylhexyl)(2,4,4′-trimethylpentyl)phosphinic Acid (USTB-1): Synthesis and Its Extraction and Separation Behaviors for Rare Earths from Chloride Media. Sep. Purif. Technol. 2018, 194, 188–196. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Fu, J.; Xie, M.; Huang, G.; Wang, H. Novel Extractant (2,4-dimethylheptyl)(2,4,4′-trimethylpentyl)phosphinic Acid (USTB-2) for Rare Earths Extraction and Separation from Chloride Media. Sep. Purif. Technol. 2019, 209, 789–799. [Google Scholar] [CrossRef]
- Sharifian, S.; Wang, N. Resin-Based Approaches for Selective Extraction and Purification of Rare Earth Elements: A Comprehensive Review. J. Environ. Chem. Eng. 2024, 12, 112402. [Google Scholar] [CrossRef]
- Yang, B.; Wu, S.; Liu, X.; Yan, Z.; Liu, Y.; Li, Q.; Yu, F.; Wang, J. Solid-Phase Extraction and Separation of Heavy Rare Earths from Chloride Media Using P227-Impregnated Resins. Rare Met. 2021, 40, 2633–2644. [Google Scholar] [CrossRef]
- Inan, S.; Tel, H.; Sert, S.; Çetinkaya, B.; Sengül, S.; Özkan, B.; Altas, Y. Extraction and Separation Studies of Rare Earth Elements Using Cyanex 272 Impregnated Amberlite XAD-7 Resin. Hydrometallurgy 2018, 181, 156–163. [Google Scholar] [CrossRef]
- Kunthakudee, N.; Sunsandee, N.; Pancharoen, U.; Ramakul, P. Separation of Yttrium from Rare Earth Using Hollow Fiber-Supported Liquid Membrane: Factorial Design Analysis. Desalination Water Treat. 2016, 57, 3985–3994. [Google Scholar] [CrossRef]
- Liu, J.; Huang, K.; Wu, X.; Liu, W.; Song, W.; Liu, H. Extraction of Rare Earths Using Bubbling Organic Liquid Membrane with Un-Saponified P507. Hydrometallurgy 2018, 175, 340–347. [Google Scholar] [CrossRef]
- Li, L.; Davis, K.; King, A.; Dal-Cin, M.; Nicalek, A.; Yu, B. Efficient Separation of Nd(III) and La(III) via Supported Liquid Membrane Using EHEHPA (P507) as a Carrier. J. Sustain. Metall. 2022, 8, 1215–1224. [Google Scholar] [CrossRef]
- Sun, P.P.; Kim, D.H.; Cho, S.Y. Separation of Neodymium and Dysprosium from Nitrate Solutions by Solvent Extraction with Cyanex272. Miner. Eng. 2018, 118, 9–15. [Google Scholar] [CrossRef]
- Koladkar, D.V.; Dhadke, P.M. Cobalt-nickel Separation: The Extraction of Cobalt(II) and Nickel(II) with Bis(2-ethylhexyl)phosphinic Acid (PIA-8) in Toluene. Solvent Extr. Ion Exch. 2001, 19, 1059–1071. [Google Scholar] [CrossRef]
- Wang, J.; Liu, H.; Zhao, H.R.; Wang, H.J. Selective Extraction of Hf over Zr by a Novel Extractant (n-Octyl)(2,4,4′-trimethylpentyl)phosphinic Acid (INET-1) from Sulfuric Acid Media. J. Radioanal. Nucl. Chem. 2023, 332, 2473–2485. [Google Scholar] [CrossRef]
- Wang, J.; Xie, M.; Liu, X.; Xu, S. Synthesis of High Purity Nonsymmetric Dialkylphosphinic Acid Extractants. J. Vis. Exp. 2017, 128, e56156. [Google Scholar] [CrossRef]
- Wang, J.; Xu, W.; Cui, J.; Xu, G.; Chen, Y.; Wang, P.; Chang, Z. The Role of β, γ, δ-Substituent of Dialkylphosphinic Acids in HREE Extraction and Separation Behaviors. J. Rare Earths 2023, 41, 1789–1795. [Google Scholar] [CrossRef]
- Wang, J.; Xu, G.; Xu, W.; Cui, J.; Chen, Y.; Chang, Z. Loading Capacity and Emulsification Phenomena of HREE Extraction by Dialkylphosphinic Acids with Different β,γ,δ-Substituents. J. Rare Earths 2024, 42, 191–199. [Google Scholar] [CrossRef]
- Wang, J.; Xu, S.; Li, L.; Li, J. Synthesis of Organic Phosphinic Acids and Studies on the Relationship Between Their Structure and Extraction-Separation Performance of Heavy Rare Earths from HNO3 Solutions. Hydrometallurgy 2013, 137, 108–114. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Y.; Wang, Z.; Jia, M.; Xia, W.; Wu, G.; Guo, W.; Chi, R.; Huang, K. Review on Micro-Mechanism of Forming Emulsification During Rare Earth Extraction by Acidic Extractants. J. Rare Earths 2025, 43, 9–20. [Google Scholar] [CrossRef]
- Xu, G.X.; Yuan, C.Y. Solvent Extraction of Rare Earths; Science Press: Beijing, China, 2010. [Google Scholar]
- Das, S.; Behera, S.S.; Murmu, B.M.; Mohapatra, R.K.; Mandal, D.; Samantray, R.; Parhi, P.K.; Senanayake, G. Extraction of Scandium(III) from Acidic Solutions Using Organo-Phosphoric Acid Reagents: A Comparative Study. Sep. Purif. Technol. 2018, 202, 248–258. [Google Scholar] [CrossRef]
- Zhang, F.Y.; Dai, J.J.; Wang, A.M.; Wu, W.Y. Investigation of the Synergistic Extraction Behavior Between Cerium (III) and Two Acidic Organophosphorus Extractants Using FT-IR, NMR and Mass Spectrometry. Inorg. Chim. Acta 2017, 466, 333–342. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).