Study of Efficiency of Capacity Gradient Ion-Exchange Stationary Phases
Abstract
1. Introduction
2. Methods
2.1. Calculation of Elution Profiles
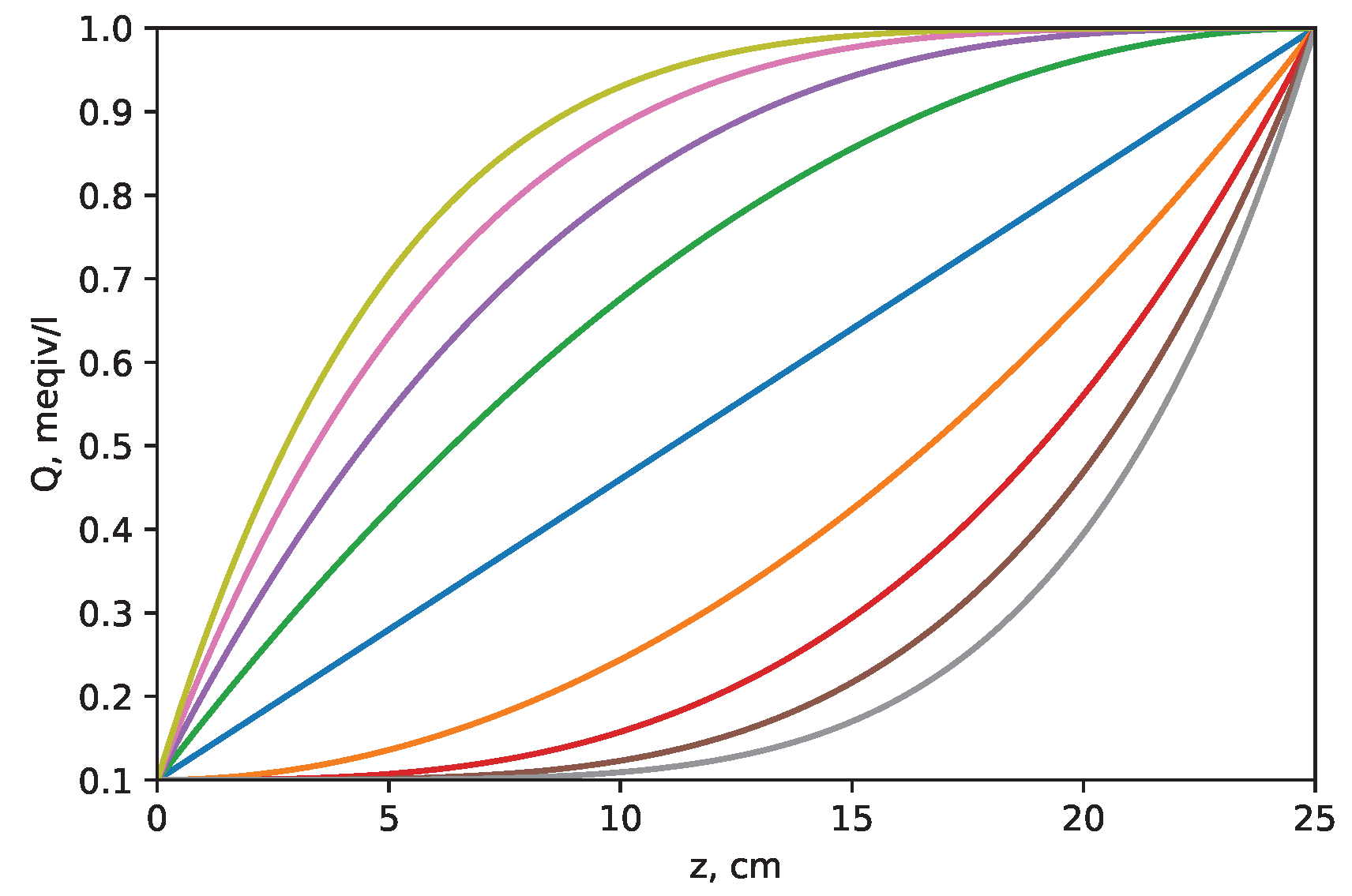
2.2. Ion-Exchange Capacity Gradients
- 1.
- Linear gradient
- 2.
- Convex gradient
- 3.
- Concave gradientwhere and are capacity values at the beginning and end of the column, respectively, and i is a positive integer (i goes 2 to 5 in this work). The profiles of the different capacity gradients are illustrated in Figure 1:
2.3. Determination of Equivalent Ion-Exchange Capacity
2.4. Parameters of Calculations
3. Results
3.1. Resolution Analysis of Non-Uniform Columns
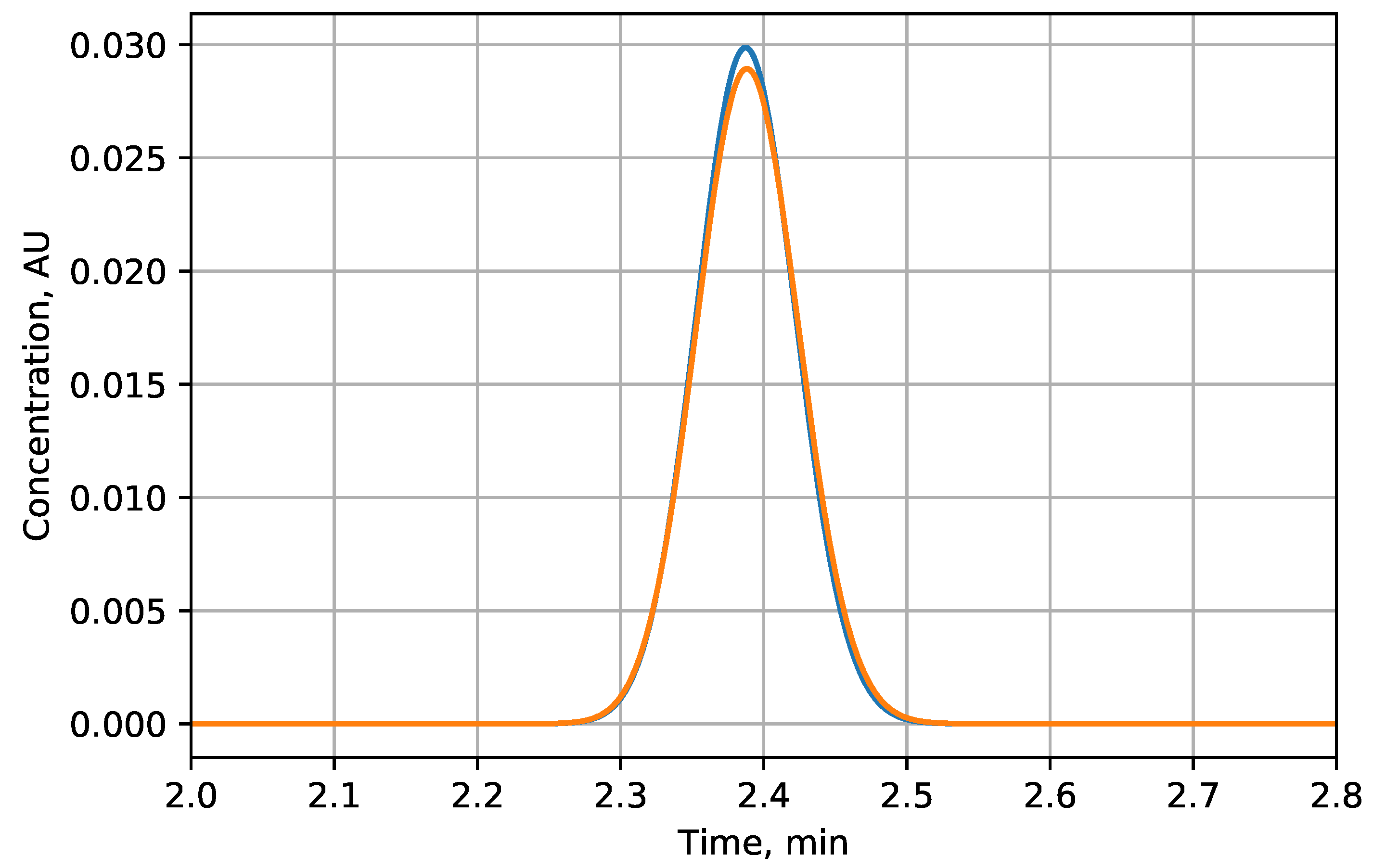
3.1.1. Separation of Ions with the Same Charges
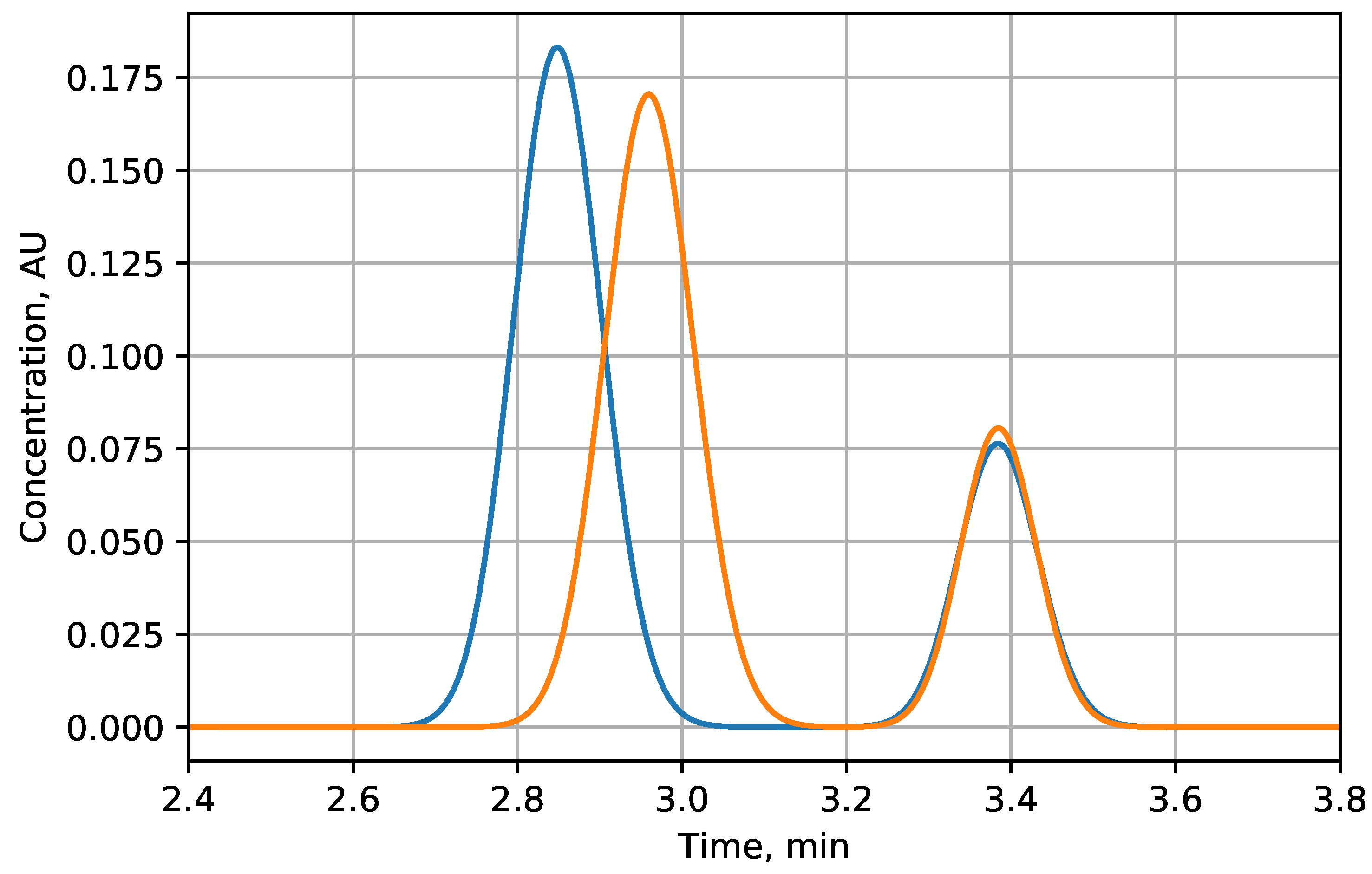
3.1.2. Separation of Ions of Different Charges
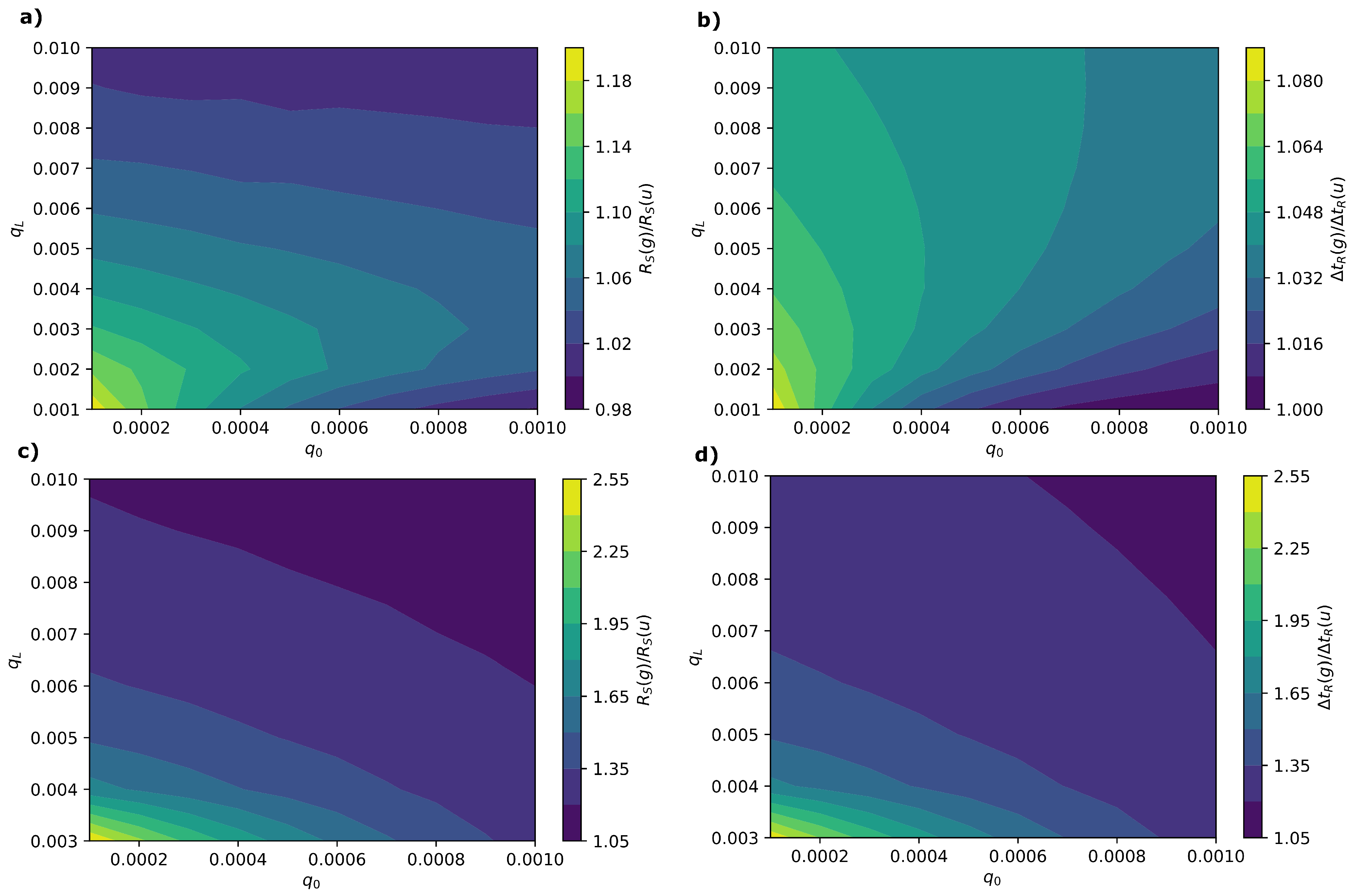
3.1.3. Effect of Capacity Gradient Profile on Resolution
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Glajch, J.; Gluckman, J.; Charikofsky, J.; Minor, J.; Kirkland, J. Simultaneous selectivity optimization of mobile and stationary phases in reversed-phased liquid chromatography for isocratic separations of phenylthiohydantoin amino acid derivatives. J. Chromatogr. A 1985, 318, 23–39. [Google Scholar] [CrossRef]
- Lukulay, P.H.; McGuffin, V.L. Solvent modulation in liquid chromatography: Extension to serially coupled columns. J. Chromatogr. A 1995, 691, 171–185. [Google Scholar] [CrossRef]
- Blasius, E.; Adrian, W.; Janzen, K.P.; Klautke, G. Darstellung und eigenschaften von austauschern auf basis von kronenverbindungen. J. Chromatogr. A 1974, 96, 89–97. [Google Scholar] [CrossRef]
- Takayanagi, T.; Ikeda, I.; Motomizu, S. Analysis of complex formation between crown ethers and potassium ion by determining retention factors in reversed-phase high-performance liquid chromatography. J. Chromatogr. A 2001, 932, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Okada, T. Nonaqueous anion-exchange chromatography II. Changing anion-exchange selectivity by resin surface complex formation of crown ethers. J. Chromatogr. A 1997, 758, 29–35. [Google Scholar] [CrossRef]
- Weiss, J. Handbook of Ion Chromatography, 3rd ed.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2004. [Google Scholar]
- Lamb, J.D.; Smith, R.G. Application of macrocyclic ligands to ion chromatography. J. Chromatogr. A 1991, 546, 73–88. [Google Scholar] [CrossRef]
- Lamb, J.D.; Smith, R.G.; Jagodzinski, J. Anion chromatography with a crown ether-based stationary phase and an organic modifier in the eluent. J. Chromatogr. A 1993, 640, 33–40. [Google Scholar] [CrossRef]
- Blasius, E.; Janzen, K.P. Ion Chromatography and Catalysis of Organic Reactions Using Polymers with Cyclic Polyethers as Anchor Groups. Isr. J. Chem. 1985, 26, 25–34. [Google Scholar] [CrossRef]
- Lim, L.W.; Tokunaga, K.; Takeuchi, T. Development of Chemically Bonded Crown Ether Stationary Phases in Capillary Ion Chromatography. Chromatography 2014, 35, 95–101. [Google Scholar] [CrossRef][Green Version]
- Lamb, J.D.; Smith, R.G.; Anderson, R.C.; Mortensen, M.K. Anion separations on columns based on transition metal-macrocycle complex exchange sites. J. Chromatogr. A 1994, 671, 55–62. [Google Scholar] [CrossRef]
- Lamb, J.D.; Drake, P.A. Chemically suppressed anion chromatography based on macrocycle—Cation complexation. J. Chromatogr. A 1989, 482, 367–380. [Google Scholar] [CrossRef]
- Woodruff, A.; Pohl, C.A.; Bordunov, A.; Avdalovic, N. Adjustable-capacity anion-exchange separator. J. Chromatogr. A 2002, 956, 35–41. [Google Scholar] [CrossRef]
- Bruzzoniti, M.C.; Carlo, R.M.D.; Horvath, K.; Perrachon, D.; Prelle, A.; Tófalvi, R.; Sarzanini, C.; Hajós, P. High performance ion chromatography of haloacetic acids on macrocyclic cryptand anion exchanger. J. Chromatogr. A 2008, 1187, 188–196. [Google Scholar] [CrossRef]
- Lukács, D.; Horváth, K.; Hajós, P. Development of retention mechanism for the separation of carboxylic acids and inorganic anions in cryptand-based ion chromatography. J. Chromatogr. A 2020, 1621, 461066. [Google Scholar] [CrossRef]
- Chen, S.J.; Shih, J.S. The Application of Cryptand 22 as a Bifunctional Stationary Phase for Ion Chromatography. J. Chin. Chem. Soc. 1991, 38, 211–216. [Google Scholar] [CrossRef]
- Yamamoto, A.; Kodama, S.; Matsunaga, A.; Inoue, Y.; Aoyama, T.; Kumagai, Y. Characteristics of a column suitable for capacity gradient chromatography with a borate eluent. Analyst 2001, 126, 465–468. [Google Scholar] [CrossRef]
- Bo, C.; Wang, X.; Wang, C.; Wei, Y. Preparation of hydrophilic interaction/ion-exchange mixed-mode chromatographic stationary phase with adjustable selectivity by controlling different ratios of the co-monomers. J. Chromatogr. A 2017, 1487, 201–210. [Google Scholar] [CrossRef]
- Pucci, V.; Raggi, M.A.; Svec, F.; Fréchet, J.M. Monolithic columns with a gradient of functionalities prepared via photoinitiated grafting for separations using capillary electrochromatography. J. Sep. Sci. 2004, 27, 779–788. [Google Scholar] [CrossRef]
- Forzano, A.V.; Cain, C.N.; Rutan, S.C.; Collinson, M.M. In situ silanization for continuous stationary phase gradients on particle packed LC columns. Anal. Methods 2019, 11, 3648–3656. [Google Scholar] [CrossRef]
- Cain, C.N.; Forzano, A.V.; Rutan, S.C.; Collinson, M.M. Destructive stationary phase gradients for reversed-phase/hydrophilic interaction liquid chromatography. J. Chromatogr. A 2018, 1570, 82–90. [Google Scholar] [CrossRef]
- Fekete, S.; Lauber, M. Studying the possibilities of dual stationary phase gradients to explore alternative selectivities in liquid chromatography. J. Chromatogr. A 2022, 1681, 463492. [Google Scholar] [CrossRef] [PubMed]
- Codesido, S.; Rudaz, S.; Veuthey, J.L.; Guillarme, D.; Desmet, G.; Fekete, S. Impact of particle size gradients on the apparent efficiency of chromatographic columns. J. Chromatogr. A 2019, 1603, 208–2015. [Google Scholar] [CrossRef] [PubMed]
- Horváth, S.; Gritti, F.; Horváth, K. Theoretical study of the efficiency of liquid chromatography columns with particle size gradient. J. Chromatogr. A 2021, 1651, 462331. [Google Scholar] [CrossRef] [PubMed]
- Pohl, C.A. Preparation of ion exchange columns with longitudinal stationary phase gradients. Heliyon 2021, 7, e06961. [Google Scholar] [CrossRef]
- Horváth, K.; Fairchild, J.N.; Kaczmarski, K.; Guiochon, G. Martin-Synge algorithm for the solution of equilibrium-dispersive model of liquid chromatography. J. Chromatogr. A 2010, 1217, 8127–8135. [Google Scholar] [CrossRef]
- van Deemter, J.; Zuiderweg, F.; Klinkenberg, A. Longitudinal diffusion and resistance to mass transfer as causes of nonideality in chromatography. Chem. Eng. Sci. 1956, 5, 271–289. [Google Scholar] [CrossRef]
- Giddings, J. Dynamics of Chromatography; M. Dekker: New York, NY, USA, 1965. [Google Scholar]
- Haarhoff, P.C.; Van der Linde, H.J. Concentration Dependence of Elution Curves in Non-Ideal Gas Chromatography. Anal. Chem. 1966, 38, 573–582. [Google Scholar] [CrossRef]
- Martin, A.; Synge, R. A new form of chromatogram employing two liquid phases: 1. A theory of chromatography 2. Application to the micro-determination of the higher monoamino-acids in proteins. Biochem. J. 1941, 12, 1358–1368. [Google Scholar] [CrossRef]
- Jandera, P.; Churáček, J. Gradient elution in liquid chromatography: II. Retention characteristics (retention volume, band width, resolution, plate number) in solvent-programmed chromatography—Theoretical considerations. J. Chromatogr. A 1974, 91, 223–235. [Google Scholar] [CrossRef]

| Parameter | Value |
|---|---|
| column length (L) | 25 cm |
| mobile phase velocity (u) | 25 cm/min |
| initial eluent concentration () | 0.001 mol/L |
| rate of change in eluent concentration () | 0.010 mol/L/min |
| Anion | |
|---|---|
| monovalent ion 1 (mono1) | 1.51 |
| monovalent ion 2 (mono2) | 5.04 |
| divalent ion 1 (di1) | 29.05 |
| divalent ion 2 (di2) | 66.21 |
| Scenario | i | , meqiv/L | -u | -g | Improvement, % |
|---|---|---|---|---|---|
| Linear | 1 | 0.420 | 2.12 | 2.63 | 24 |
| Convex | 2 | 0.330 | 1.56 | 2.45 | 57 |
| 3 | 0.282 | 1.22 | 2.33 | 90 | |
| 4 | 0.250 | 0.99 | 2.22 | 125 | |
| 5 | 0.228 | 0.81 | 2.12 | 163 | |
| Concave | 2 | 0.520 | 2.67 | 3.02 | 13 |
| 3 | 0.568 | 2.91 | 3.17 | 9 | |
| 4 | 0.596 | 3.03 | 3.25 | 7 | |
| 5 | 0.614 | 3.12 | 3.30 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horváth, S.; Lukács, D.; Farsang, E.; Horváth, K. Study of Efficiency of Capacity Gradient Ion-Exchange Stationary Phases. Separations 2023, 10, 14. https://doi.org/10.3390/separations10010014
Horváth S, Lukács D, Farsang E, Horváth K. Study of Efficiency of Capacity Gradient Ion-Exchange Stationary Phases. Separations. 2023; 10(1):14. https://doi.org/10.3390/separations10010014
Chicago/Turabian StyleHorváth, Szabolcs, Diána Lukács, Evelin Farsang, and Krisztián Horváth. 2023. "Study of Efficiency of Capacity Gradient Ion-Exchange Stationary Phases" Separations 10, no. 1: 14. https://doi.org/10.3390/separations10010014
APA StyleHorváth, S., Lukács, D., Farsang, E., & Horváth, K. (2023). Study of Efficiency of Capacity Gradient Ion-Exchange Stationary Phases. Separations, 10(1), 14. https://doi.org/10.3390/separations10010014







