Abstract
Product safety is important for medicines. For drugs on the market, it must be demonstrated that the levels of toxic contaminants are below the permitted limits. These impurities are used as reagents or are generated during synthesis. N-bromosuccinimide is used as a brominating agent in the synthesis of some active pharmaceutical ingredients. The determination of N-bromosuccinimide is difficult due to its high reactivity. In this work, a high-performance ion chromatographic method was developed for the determination of N-bromosuccinimide. The ion chromatographic measurement can be performed in two ways, one involves the assay of the resulting bromide ion and the other is via the assay of the 3-carbamoyl propanoic acid ion produced from the succinimide. Both acid ions were analyzed on an anion exchange column by gradient elution with potassium hydroxide eluent and detection was performed by a suppressed conductivity detector. During the method development, the results showed that the measurement of bromide ion was more selective than the measurement of 3-carbamoyl propanoic acid ion. Two different types of active pharmaceutical ingredients (API), i.e., prasugrel and favipiravir, were chosen to test the developed method and sample preparation. For both APIs, sample preparation was performed in a vial and consists of liquid–liquid extraction with an alkaline reagent. Finally, the anion exchange ion chromatography method was validated at the limit value level, and harmonized with the guidelines. For prasugrel, the quantification limits and the accuracy at the limit level are 7.2 ppm and 96.4%, while for favipiravir these are 7.5 ppm and 114.7%, respectively.
1. Introduction
N-bromosuccinimide (NBS) is a widely used bromination agent in the organic synthesis, in free-radical substitutions and electrophilic additions of unsaturated systems [1]. Ziegler and his colleagues were the first to use NBS (Wohl–Ziegler bromination) for allylic bromination reaction [2].
NBS is stable, recoverable, and reactive in inert solvents during allylic bromination. It has a low molecular weight and causes minimal side reactions [3]. Because of these beneficial properties, NBS is commonly used for oxidation, dehydrogenation, and ring-opening reactions in the synthesis of different pharmaceutical products.
Previous investigations have shown that NBS is a potentially genotoxic impurity. N-bromosuccinimide can lose easily bromide as a bromination reagent. Bromide can accumulate in the body (especially in the thyroid gland, liver, and kidneys) and it displaces iodine. Bromide can be potentially hazardous to thyroid hormones. The reduction of iodine levels in organs might also cause several types of cancer, such as breast, thyroid gland, ovary, or prostate [4].
During the marketing authorization process, pharmaceutical manufacturers must demonstrate that their products are safe. Proof of safety is that the product does not contain toxic or mutagenic compounds or, if it does, it is below the acceptable intake levels. These critical parameters must be determined and, therefore, appropriate analytical methods must be developed to analyze these impurities. Mutagenic impurities should be monitored in accordance with the ICH M7(R1) guideline. The maximum concentration of NBS in any active pharmaceutical ingredient (API) depends on the maximum daily dose of the API and the duration of the treatment. Taking into account this guideline, the maximum daily intake of NBS is 1.5 μg/day for lifetime exposure [5]. The determined API is the prasugrel. This can be used in combination with acetylsalicylic acid (ASA) to treat atherothrombosis. Atherothrombosis can cause acute coronary syndromes (ACS) and cardiovascular death. Prasugrel API prevents patients from developing heart attacks and strokes [6]. In the synthesis of prasugrel, NBS is used in the second step for bromination (Figure 1).
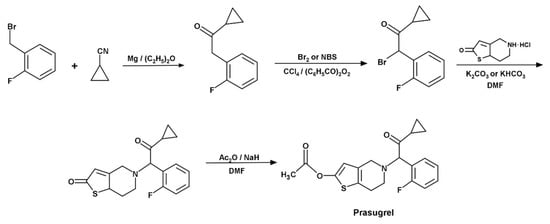
Figure 1.
One of the synthesis routes of prasugrel [7].
In the case of prasugrel API, the maximum daily intake of prasugrel is 60 mg [8]; thus, the maximum allowable limit of NBS in prasugrel API is 25 ppm.
Kaleemullah et al. developed an analytical method for the analysis of NBS by reverse phase HPLC (RP-HPLC) [9]. However, the LQ concentration set by the authorities is lower than the concentration achieved by this method. In addition, the use of this analytical method is limited to sartan-type active substances. For substances with low solubility in a methanol–water solvent, the method cannot be applied directly. The properties of NBS show that it has good water solubility and is suitable for hydrolysis so certain reactions can produce ion bromides that can be measured by ion chromatography selectively and sensitively. The aim of this work was to develop a sample preparation and ion chromatographic method for the determination of NBS in different active pharmaceutical ingredients.
2. Materials and Instrumentation
Dionex ICS 5000 HPIC system with EGC (KOH Eluent Generator Cartridge, Thermo Scientific, Waltham, MA, USA) and a suppressed conductivity detector was used for the ion chromatography measurement (Thermo Scientific, Waltham, MA, USA). The anion exchange column was a Dionex IonPac AS23 (2 mm × 250 mm) with a guard column AG23 (2 mm × 50 mm) and Dionex IonPac AS11HC (2 mm × 250 mm) with a guard column AG11HC (2 mm × 50 mm) (Thermo Scientific, Waltham, MA, USA). The chromatograms were processed using Chromeleon 7. Dionex Version 7.1.3.2425 (Thermo Scientific, Waltham, MA, USA).
Mettler Toledo analytical and precision balances were used for weighing (Mettler Toledo, Greifensee, Switzerland). Eppendorf automatic pipettes were used for liquid handling (Eppendorf SE, Hamburg, Germany).
The sodium hydroxide (Fisher Scientific, Loughborough, UK), sodium thiosulfate (Sigma-Aldrich, Darmstadt, Germany), chloroform (Lach:ner, Neratovice, Czech Republic) and ethyl-acetate (Fisher Scientific, Loughborough, UK) used were of analytical grade. N-bromosuccinimide was also an analytical grade (Sigma-Aldrich, Darmstadt, Germany). Prasugrel API and favipiravir API originated from Egis Pharmaceuticals PLC. Purified water was prepared freshly using an ELGA Purelab system (ELGA, Lane End, UK).
3. Materials and Methods
3.1. Sample Preparation
3.1.1. Hydrolysis of N-Bromosuccinimide
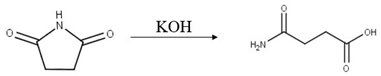
The hydrolysis of NBS in the presence of water leads to the formation of hypobromous acid [10] that can be reduced to a stable bromide by sodium thiosulfate. Accordingly, NBS can be analyzed indirectly by determining the bromide anion formed during the hydrolysis of NBS. The reaction steps used to release the bromide ion are shown in Equations (1) and (2). The hydrolysis is performed by an alkaline solution (solvent solution) containing 60 mg sodium hydroxide and 39.5 mg sodium thiosulfate in 100 mL water. In this solution, sodium hydroxide provides the alkaline hydrolysis and sodium thiosulfate is the reducing agent. The reaction is carried out at room temperature, and after a few minutes the amount of bromide ion does not change even if more reagent is added. The determined active pharmaceutical ingredients are not soluble in sufficient amounts in the anion chromatographic eluent, but they are soluble in organic solvents, so liquid–liquid extraction (LLE) is ideal for the extraction of bromide ions. The matrix elimination and the hydrolysis of the NBS can be done in one step and takes only five minutes.
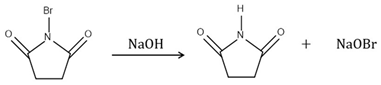

Another way to determine the NBS is to measure the 3-carbamoyl propanoic acid ion, which is converted from the succinimide formed. In this case, only the first reaction step is used from Equation (1). The alkali causes the ring to open up and the 3-carbamoyl propanoic acid is formed and it is ionized under these circumstances (Equation (3)). [11] This formation was proved by LC-MS. Under mild conditions (300 μg/mL succinimide in 1.5 mM NaOH), 3-carbamoyl propanoic is released. This may be a viable route if the sample does not contain interfering impurities near the 3-carbamoyl propanoic acid ion. In the case of prasugrel, the determination of the bromide ion is a better way because the inorganic ion can be detected sensitively and resolved suitably from interfering ions.

3.1.2. Solutions
The following mixtures are required for the measurements.
- Blank solution: contained 5 mL solvent solution (60 mg of sodium hydroxide and 39.5 mg of sodium thiosulfate in 100 mL water) and 5 mL chloroform.
- Limit solution: 5 mL of the NBS solution (2.5 μg/mL NBS in chloroform) and 5 mL solvent solution.
- Limit of quantification: 5 mL of the NBS LQ solution (0.75 μg/mL NBS in chloroform) and 5 mL solvent solution.
- Limit of detection: 5 mL of the NBS LD solution (0.225 μg/mL NBS in chloroform) and 5 mL solvent solution.
- Sample solution: 500 mg of prasugrel is dissolved in 5 mL chloroform and 5 mL solvent solution.
- Spiked sample solution: 500 mg of prasugrel is dissolved in 5 mL NBS solution (2.5 μg/mL NBS in chloroform) and 5 mL solvent solution.
Six LLE shake times (1, 5, 10, 15, 20, 25 min) were tested. The results showed that the shake time had no effect on the extraction efficiency. Accordingly, all the solutions were shaken well for 1 min. The supernatant solutions were transferred into a centrifuge tube and centrifuged for 3 min. After the LLE, the upper aqueous phase contains the bromide ion, which can be directly injected into the HPIC system. A large part of the prasugrel is transferred to the organic phase, so it will not interfere with the analysis and will not precipitate in the chromatographic system.
3.2. Ion Chromatographic Method
The ion chromatographic method was a gradient measurement with a long isocratic section to separate the bromide ion and the other ionic compounds of the solvent solution. The current of the suppressor was modified with the eluent concentration. For the Dionex IonPac AS23 column, a slightly higher flow rate (0.30 mL/min), than recommended was used. The prepared sample was stable at room temperature so it was not necessary to ’thermostate’ the autosampler. The column temperature was 30 °C.
Table 1 shows the parameters of the N-bromosuccinimide measurement in prasugrel API.

Table 1.
HPIC method conditions for the analysis of bromide in prasugrel.
3.3. Method Validation
The validation of the anion exchange method was performed according to the ICH Q2(R1) guideline for limit test [12]. The following measurements were carried out during the limit test validation:
- Selectivity–specificity.The selectivity–specificity of the method was checked by injecting a blank solution and a spiked sample solution. No interference was detected at the retention time of bromide and the other components. The method is considered then as being selective for the determination of bromide ions in prasugrel.
- Limit of quantification (LQ)–limit of detection (LD).The limit of detection (LD)–limit of quantification (LQ) is specified as the minimum concentration, at which the signal of the investigated component is at least three times (LD) and ten times (LQ) greater than the noise level. The LQ level value has to be less than the disregard limit generally applied in pharmacopoeia methods (25 ppm).
- Linearity.The measurements were performed at five concentration levels between LQ (7.2 ppm) and the 120 % (30 ppm) of the nominal concentration.
- System precision.Injections (n = 6) of the limit solution were carried out to determine the statistical error parameters (standard deviation (SD), relative standard deviation (RSD), and confidence interval) of the applied method.
- Accuracy (Recovery).In the study of accuracy, the sample is spiked with NBS at the limit level (25 ppm). The concentrations are determined, and the recoveries of the spiked quantities are calculated in each case.
- Stability.The stability is determined by analyzing the prepared solutions over a period of 24 h in closed plastic vials in the sampler holder.
4. Results
4.1. Validation Results
NBS was determined from prasugrel through the analysis of bromide anion by an anion-exchange chromatography method using suppressed conductivity detection. Typical chromatograms of the anion analyses can be seen in Figure 2. It can be seen that the method is specific to the bromide anion, it can be separated well from the other anions present in the sample solution. It can be concluded that there is no significant matrix effect that would affect the retention of bromide. The shapes of the bromide peaks are symmetric, and the baselines around them are stable and linear.
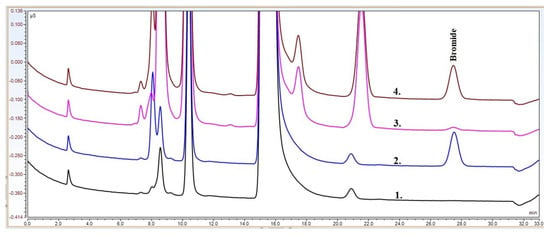
Figure 2.
Typical chromatograms of the anion separation of the LLE extract of prasugrel API. (1) blank, (2) reference, (3) sample, and (4) spiked sample.
The validation results are presented in Table 2. The practical limit of quantitation is 7.2 ppm, which corresponds to 26 in the signal-to-noise ratio. Note that there are rather strict criteria; however, the robustness of the method is improved. The calibration curve was linear in the range of the experiment (7.2–30 ppm). Figure 3 shows the calibration curve and the calibration equation. The relative deviations of retention times are less than 1% at the limit level and LQ level. The precisions of peak areas are better than 3%. The recovery of the NBS was 96.4%, which is well within the limit values. Table 2 shows that the results are within the limits of the method validation criteria for each parameter, confirming the usability of the developed method in the analysis of NBS in prasugrel API.

Table 2.
Validation results summary.
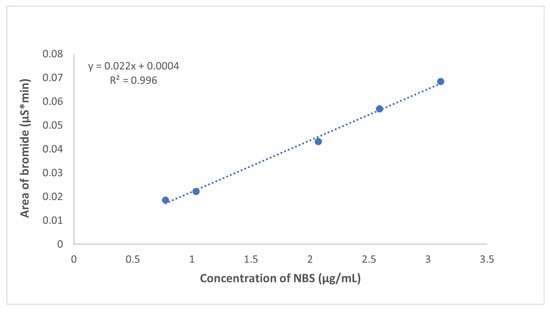
Figure 3.
Calibration curve of determination of NBS.
4.2. Analysis of Favipiravir
The developed sample preparation method was tested with another API, favipiravir, where NBS was also used in the synthesis. This API is an antiviral agent, which inhibits the growth of viruses inside cells. This product has been used successfully in some cases in the clinical treatment of COVID-19. Several papers have been published on the determination of favipiravir API and its impurities by HPLC [13], but NBS is not one of them, despite its use in the second step of a synthetic pathway (Route 3) according to Qi Guo et al. [14].
In the case of favipiravir, the maximum concentration level of the NBS is 37.5 ppm because the dose of treatment varies over time, with the dose changing from 3200 mg on the first day to 1200 mg on the second day [15] and the maximum daily intake of NBS is 120 μg/day.
The preparation of the sample solutions has changed compared to the prasugrel solutions. The chloroform is not usable for this API, so ethyl acetate was used as the solvent.
A separate ion chromatography method was developed for the determination of the bromide favipiravir API, as favipiravir contained an unknown interfering ion immediately before the bromide ion, so a different column (AS11HC) was chosen to separate these ions. The developed anion-exchange chromatography method gave a good separation between the bromide ion and the unknown ion (Figure 4), so the bromide ion can be sensitively measured from the drug favipiravir API, too.
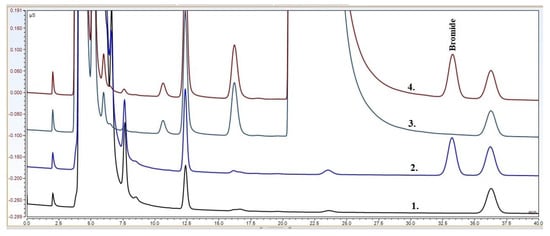
Figure 4.
Typical chromatograms of anion separation of LLE extract of favipiravir API. (1) blank, (2) reference, (3) sample, (4) spiked sample.
In the case of the favipiravir API, the developed method has not been validated at the limit level, but the specificity, the LQ value (7.5 ppm), and the accuracy at the limit level (114.7%) were checked.
5. Conclusions
As the results of this work verify, NBS can be determined through the ion chromatographic analysis of bromide ions formed in the hydrolysis of NBS. In this work, this approach was used for the determination of the NBS content of two different types of APIs–prasugrel and favipiravir. For the prasugrel API, a limit level validation was conducted successfully, all of the parameters tested met the requirements of the pharmaceutical authorities. Accordingly, the developed and validated method can be used for the determination of NBS in prasugrel. The results confirm that the sample preparation and ion chromatography method is selective, specific, stable, precise, and accurate for the analysis of the limit level NBS contamination in the prasugrel active pharmaceutical ingredient. The developed sample preparation method was also applied for the determination of the NBS content of the favipiravir API. The results show that the alkaline reagent works in both cases, despite the fact that the sample preparations of the two different APIs are slightly different. Accordingly, the reaction can be applied to the sample preparation of other pharmaceuticals. The benefit of this sample preparation procedure is that the matrix elimination and the reaction are one step; it takes only a few minutes.
As an alternative to the developed method, NBS can also be determined through the IC analysis of 3-carbamoyl propanoate produced by the opening of the succinimide ring.
Author Contributions
Conceptualization, R.K. and B.P.; methodology, B.P.; software, K.H.; validation, B.P. and I.K.; writing—original draft preparation, B.P. and R.K.; writing—review and editing, K.H.; visualization, B.P and K.H.; supervision, R.K. and K.H.; project administration, K.H.; funding acquisition, K.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been implemented by the TKP2020-NKA-10 project with the support provided by the Ministry of Culture and Innovation of Hungary from the National Research, Development, and Innovation Fund, financed under the 2020 Thematic Excellence Program funding scheme. Financial support from the Hungarian National Research, Development, and Innovation Fund (NKFIH FK128350) is also greatly acknowledged. B.P. acknowledges the support from the Cooperative Doctoral Program (KDP-11-3/PALY-2021) granted by the Ministry of Innovation and Technology from the source of the National Research, Development, and Innovation Fund.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Sample Availability
Samples of the compounds are not available from the authors.
Abbreviations
The following abbreviations are used in this manuscript:
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of Open Access Journals |
| API | active pharmaceutical ingredient |
| CD | conductivity detector |
| EMA | European Medicine Agency |
| EGC | eluent generator cartridge |
| HPIC | high-performance ion chromatography |
| HPLC | high-performance liquid chromatography |
| IC | ion chromatography |
| ICH | International Council for Harmonization |
| LD | limit of detection |
| LQ | limit of quantification |
| LLE | liquid–liquid extraction |
| QbD | quality by design |
| RP-HPLC | reversed-phase high-performance liquid chromatography |
| UV | ultra-violet |
References
- Isac-García, J.; Dobado, J.A.; Calvo-Flores, F.G.; Martínez-García, H. Chapter 11—Microscale Experiments. In Experimental Organic Chemistry; Isac-García, J., Dobado, J.A., Calvo-Flores, F.G., Martínez-García, H., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 371–408. [Google Scholar] [CrossRef]
- Filler, R. Oxidations and dehydrogenations with n-bromosuccinimide and related n-haloimides. Chem. Rev. 1963, 63, 21–43. [Google Scholar] [CrossRef]
- Wang, Z. Comprehensive Organic Name Reactions and Reagents, 3 Volume Set; Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 3067–3072. [Google Scholar]
- du Toit, J.; Casey, N. Iodine as an alleviator of bromine toxicity in thyroid, liver and kidney of broiler chickens. Livest. Sci. 2012, 144, 269–274. [Google Scholar] [CrossRef][Green Version]
- Guideline, I. Assessment and Control of DNA Reactive (Mutagenic) Impurities in Pharmaceuticals to Limit Potential Carcinogenic Risk M7; International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH): Geneva, Switzerland, 2017. [Google Scholar]
- Huber, K.; Yasothan, U.; Hamad, B.; Kirkpatrick, P. Prasugrel. Nat. Rev. Drug Discov. 2009, 8, 449–450. [Google Scholar] [CrossRef] [PubMed]
- Al Omari, M.M.; Qinna, N.A.; Rashid, I.S.; Al-Sou’od, K.A.; Badwan, A.A. Chapter Four—Prasugrel Hydrochloride. In Profiles of Drug Substances, Excipients and Related Methodology; Academic Press: Cambridge, MA, USA, 2015; Volume 40, pp. 195–320. [Google Scholar] [CrossRef]
- Bonhomme, F.; Fontana, P.; Reny, J.L. How to manage prasugrel and ticagrelor in daily practice. Eur. J. Intern. Med. 2014, 25, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Kaleemullah, T.; Ahmed, M.; Sharma, H. Validation of RP-HPLC method for the quantification of N-Bromosuccinimide in Angiotensin II receptor antagonists in Pharmaceuticals. Der Pharma Chem. 2011, 3, 372–380. [Google Scholar]
- Prashanth, K.N.; Basavaiah, K. Sensitive and selective methods for the determination of rizatriptan benzoate in pharmaceuticals using N-bromosuccinimide and two dyes. J. Saudi Chem. Soc. 2015, 19, 233–242. [Google Scholar] [CrossRef]
- Sungur, F.A. Modelling the hydrolysis of succinimide: Formation of aspartate and reversible isomerization of aspartic acid via succinimide. Org. Biomol. Chem. 2003, 1, 2290–2297. [Google Scholar] [CrossRef]
- Guideline, I. ICH Guidance for Industry Q2(R1), Validation of Analytical Procedures: Text and Methodology; International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH): Geneva, Switzerland, 2006. [Google Scholar]
- Hailat, M.; Al-Ani, I.; Zakareia, Z.; Al-Shdefat, R.; Al-Meanazel, O.; Anwer, M.K.; Hamad, M.; Abu Rayyan, W.; Awad, R.; Abu Dayyih, W. Development and Validation of HPLC-DAD Method for the Determination of Favipiravir and Studying the Impact of Vitamin C on the Pharmacokinetics of COVID-19 Antiviral Drug Favipiravir. Separations 2022, 9, 303. [Google Scholar] [CrossRef]
- Guo, Q.; Xu, M.; Guo, S.; Zhu, F.; Xie, Y.; Shen, J. The complete synthesis of favipiravir from 2-aminopyrazine. Chem. Pap. 2019, 73, 1043–1051. [Google Scholar] [CrossRef]
- Manabe, T.; Kambayashi, D.; Akatsu, H.; Kudo, K. Favipiravir for the treatment of patients with COVID-19: A systematic review and meta-analysis. BMC Infect. Dis. 2021, 21, 1–13. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).