Abstract
Hydroxyurea (HU), a cornerstone treatment for myeloproliferative disorders, is associated with a wide range of cutaneous side effects, from xerosis and hyperpigmentation to more severe conditions like dermatomyositis-like eruptions (DM-LE) and nonmelanoma skin cancers (NMSC), particularly squamous cell carcinoma (SCC). In this review, we present a unique case of HU-induced DM-LE with histological evidence of keratinocyte dysplasia and p53 overexpression, followed by a systematic analysis of similar cases. Our findings reveal that the clinical presentation of DM-LE, while typically considered benign, shares clinical and histological features with hydroxyurea-associated squamous dysplasia (HUSD), a precancerous condition that may progress to SCC in chronically exposed patients. Key insights include the characteristic histopathological findings of DM-LE, the role of chronic HU therapy and UV-induced damage in promoting p53 overexpression, and the overlap between DM-LE and HUSD. Regular dermatologic monitoring, patient education on photoprotection, and the careful assessment of skin lesions in long-term HU users are essential for the early detection and prevention of malignancies. This review underscores the importance of distinguishing between DM-LE, HUSD, and SCC to optimize management and minimize risks associated with HU therapy.
1. Introduction
Hydroxyurea (HU) is a widely used antimetabolite drug for treating myeloproliferative disorders. While it is generally well tolerated, a significant proportion of patients (11–36%) experience cutaneous side effects. These include facial erythema, hyperpigmentation, xerosis, alopecia, skin atrophy, melanonychia, and lower limb ulcers. Less common but clinically significant manifestations, such as dermatomyositis-like eruptions (DM-LE) and nonmelanoma skin cancer (NMSC), have also been reported [1].
A unique presentation associated with chronic HU therapy involves photodistributed erythematous patches and xerosis, resembling photodermatitis or DM-LE. Histologically, these conditions are characterized by nuclear atypia and p53 expression, suggesting a potential premalignant state. This has led to the introduction of the term “hydroxyurea-associated squamous dysplasia” (HUSD) to describe this precancerous condition [2,3,4].
In this paper, we present a patient under long-term therapy with HU who developed a DM-LE with histological features of dermatomyositis and keratinocyte dysplasia, characterized by p53 overexpression. Additionally, we conduct a systematic review of similar cases to further elucidate the clinical and histological characteristics of DM-LE, HUSD, and HU-associated NMSC. This review highlights their potential interrelationships and underscores the importance of vigilant, long-term monitoring for patients undergoing HU therapy.
2. Case Report
A 70-year-old woman with a history of polycythemia vera, treated with hydroxyurea since 2011, presented with a 6-month history of erythematous rash on the face and violaceous papules on the dorsa of her hands. Her medical history also included Raynaud’s phenomenon since 2020, multinodular goiter, and atrial fibrillation. On examination, red-violaceous papules were observed overlying the interphalangeal and metacarpophalangeal joints of the hands (Figure 1b), while the malar region of the face displayed erythema and the eyebrow region exhibited diffuse actinic keratosis (Figure 1a). The dermoscopic evaluation of the nailfolds revealed dilated capillaries and architectural disarray (Figure 1c).
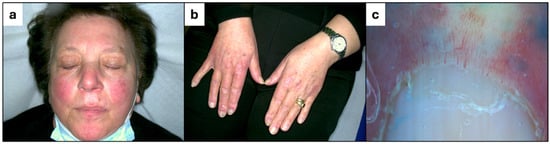
Figure 1.
(a) Erythema of the face. (b) Gottron-like papules on the interphalangeal and metacarpophalangeal joints of the hands. (c) Dilated capillaries and architectural disarray of the nailfolds.
Laboratory exams showed positive antinuclear antibodies (ANAs) at a titer of 1:640 with a homogeneous pattern and elevated erythrocyte sedimentation rate (ESR) at 44 mm/h. A biopsy of the affected skin of the hand was performed and the histologic examination revealed the presence of orthokeratotic hyperkeratosis with slight epidermal atrophy, superficial angioplasia, and a mild perivascular lymphocytic infiltrate (Figure 2a), along with occasional mucin deposits, as detected by Alcian-PAS staining (Figure 2b). At higher magnification keratinocyte atypia, dyskeratosis and nuclear irregularities were also seen (Figure 2c). Immunohistochemical staining showed p53 positivity in the basal and lower epidermal layers (Figure 2d) and p16 positivity in keratinocytes of the lower two-thirds of the epidermis (Figure 2e). Based on the patient’s history of long-term hydroxyurea therapy, the characteristic dermoscopic findings of the nailfolds and histological confirmation, a diagnosis of DM-LE/HSUD induced by hydroxyurea was made. Hydroxyurea was therefore discontinued and at the 4-month follow-up the patient showed a complete remission of the cutaneous findings on the hands and face.
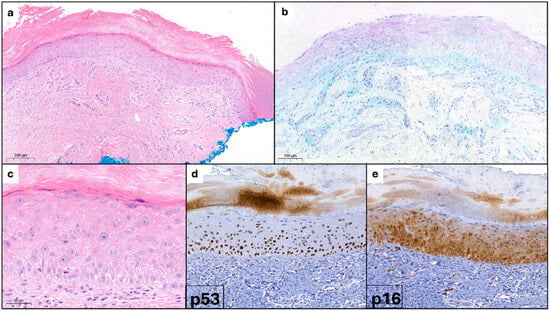
Figure 2.
(a) Orthokeratotic hyperkeratosis with mild epidermal atrophy; angioplasia and vascular ectasia with mild lymphocytic infiltrate. (b) Mucin with Alcian-PAS staining. (c) Keratinocyte atypia, dyskeratosis, and nuclear irregularities. (d) p53 positivity. (e) p16 positivity.
3. Materials and Methods
The review was conducted in compliance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (Figure 3) [5]. We conducted a search across electronic databases, including MEDLINE (PubMed), Scopus, and Web of Science, using keywords such as “hydroxyurea-induced dermatitis”, “hydroxyurea-induced dysplasia”, “hydroxyurea-induced dermatomyositis”, and “hydroxyurea-induced squamous cell carcinoma”. We included studies published from the inception of these databases through July 2024. Abstracts were independently reviewed by two dermatologists according to predefined inclusion and exclusion criteria.
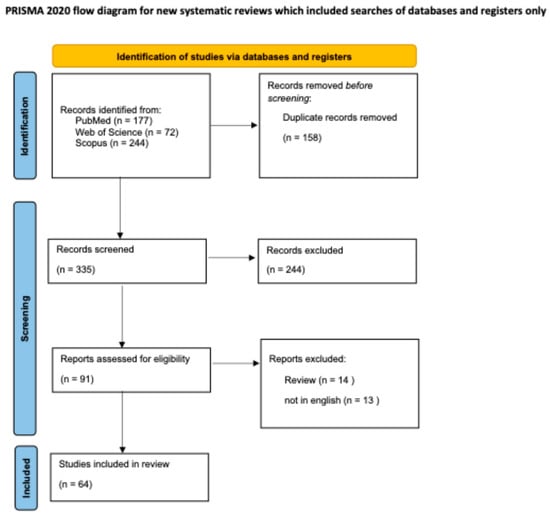
Figure 3.
PRISMA guidelines flow-chart followed to perform this review [5].
Inclusion criteria were as follows:
- Original articles, case series, and case reports on hydroxyurea-induced dermatitis, hydroxyurea-induced dermatomyositis, hydroxyurea-induced dysplasia, and hydroxyurea-induced squamous cell carcinoma.
Exclusion criteria were as follows:
- Review articles;
- Language other than English.
This systematic review was registered in PROSPERO (International Prospective Register of Systematic Reviews) Registration Number: CRD420250653625
The following information was extracted after screening: first author, year of publication, patients included, sex, age, pathology, HU exposure (years), cutaneous manifestations, histopathologic description, evolution after the discontinuation of therapy, and the presence of p53 overexpression.
4. Results
Then, 335 articles were screened after removing duplicates. Based on title and abstract screening, 245 studies were excluded. Therefore, 90 full texts were assessed for eligibility. From these 90 articles, dermatologists independently reviewed abstracts based on set inclusion and exclusion criteria review articles, non-English articles, were excluded, resulting in a total of 63 articles included in our review.
DM-LE
Of the 65 cases analyzed with DM-LE, 54% were female (35 patients) and 46% were male (30 patients). The mean age at diagnosis was 61.4 years (SD: ±13.61). The mean time to onset from the initiation of hydroxyurea therapy was 5.04 years (SD: ±3.23). All patients presented with lesions on their hands (100%), while the face was affected in 25% of cases (16 patients).
Histopathology revealed characteristic findings in most cases, including interface dermatitis, vacuolar alteration of basal keratinocytes, dyskeratotic keratinocytes, melanin incontinence, and vascular ectasia. Unlike classic dermatomyositis, mucin deposition was observed in only 7 cases (10.78%). Additionally, 6 patients exhibited altered expression of p53. After therapy discontinuation, among the 65 patients analyzed, 34 (52.3%) showed improvement and 8 (12.3%) died. Overall, 1 patient (1.5%) maintained a stable condition (STA), outcomes were unknown (U) in 15 cases (23.1%), and 7 (10.8%) participants continued therapy without discontinuation.
HU-Associated Squamous cell carcinoma
Among the 45 cases analyzed with HU-induced squamous cell carcinoma, 40% were female (18 patients) and 60% were male (27 patients). The mean age at diagnosis was 66.07 years (SD: ±10.10). The mean time to onset following the initiation of hydroxyurea therapy was 7.68 years (SD: ±4.15).
The lesions were predominantly located in photodistributed areas, including the face and/or scalp in 47% of cases (21 patients). The involvement of the hands was less frequent, occurring in 7 patients (6.43%). Then, 2 patients exhibited altered expression of p53.
Among the cases analyzed, clinical improvement was observed in 9 patients (20%), while no improvement was reported in 2 patients (4.4%). Unfortunately, 9 patients (20%) died following disease progression or complications.
The results are summarized in Table 1.

Table 1.
DMLE- and HU-induced SCC.
Table 2 and Table 3 summarize the clinical and histopathological features of the hydroxyurea-induced DM-LE (Table 2) and HUSD- and HU-associated NMSC (Table 3).

Table 2.
Cases reported of hydroxyurea-associated dermatomyositis-like eruption.

Table 3.
Cases reported of hydroxyurea-associated squamous dysplasia and hydroxyurea-associated nonmelanoma skin cancer.
5. Discussion
Hydroxyurea therapy, a cornerstone in the management of myeloproliferative disorders, is associated with a spectrum of cutaneous side effects, including dermatomyositis-like eruption, hydroxyurea-associated squamous dysplasia, and nonmelanoma skin cancer, particularly squamous cell carcinoma. Our systematic review highlights the clinical features, histopathology, and outcomes of these HU-induced skin manifestations, shedding light on their potential interrelationships and clinical implications.
DM-LE commonly occurs in patients undergoing long-term HU therapy, typically appearing 25 to 121 months after initiation. Our analysis of 64 cases demonstrated that 100% of patients presented with lesions on the hands, and 25% had facial involvement. The condition clinically resembles dermatomyositis but notably lacks the associated myopathy or malignancy. Key features include xerosis, violaceous papules over the interphalangeal and metacarpophalangeal joints (Gottron’s papules-like), and, rarely, “heliotrope-like” violaceous erythema around the eyes.
Histologically, DM-LE exhibits interface dermatitis, vacuolar alteration of basal keratinocytes, dyskeratotic keratinocytes, melanin incontinence, and vascular ectasia. Mucin deposition, a hallmark of true dermatomyositis, was observed in only 10.78% of cases (7 patients). This distinction is critical, as histology alone cannot differentiate DM-LE from true dermatomyositis. Accurate diagnosis relies heavily on clinical correlation and patient history, particularly in the setting of chronic HU exposure.
Following the discontinuation of HU therapy, DM-LE generally resolves within 11 days to 19 months. Our review found that 52.3% of patients showed improvement, while others exhibited varying outcomes, including persistent lesions, stable conditions, or unknown progression. Notably, skin atrophy may persist even after resolution, highlighting the need for long-term dermatologic monitoring. While DM-LE is considered benign and typically does not necessitate HU discontinuation, its clinical resemblance to dermatomyositis raises the risk of misdiagnosis and unwarranted immunosuppression.
Chronic HU therapy is linked to more aggressive cutaneous conditions, particularly HUSD and SCC, which tend to occur in sun-exposed areas. SCC typically develops 3 to 14 years after HU initiation and is more common in individuals with Fitzpatrick skin types I and II. In our analysis of 45 SCC cases, lesions predominantly involved the face and/or scalp (47%), with hand involvement occurring in a smaller proportion of patients (6.43%).
Recent studies have introduced the term hydroxyurea-associated squamous dysplasia to describe a premalignant condition characterized by photodistributed patches of erythema and xerosis, clinically mimicking photodermatitis or DM-LE. Histologically, HUSD is marked by nuclear atypia and abnormal p53 expression, suggesting early cellular damage that can precede SCC. Interestingly, abnormal p53 expression, not typically tested, was also observed in 6 cases of DM-LE in our review, raising questions about the potential for malignant transformation.
The mechanisms underlying DM-LE-, HUSD-, and HU-induced SCC reflect the complex interplay between HU and UV radiation. Hydroxyurea induces oxidative stress and impairs DNA repair mechanisms, making keratinocytes more susceptible to UV-induced damage. This process promotes the emergence of p53-mutated clones, which can progress to dysplasia and carcinoma over time. The presence of p53 expression in DM-LE highlights a potential overlap with HUSD, suggesting that both conditions may represent stages of a chronic phototoxic process driven by long-term HU therapy and UV exposure.
Given these findings, the historical view of DM-LE as a purely benign condition warrants reevaluation. While DM-LE rarely progresses to malignancy, emerging evidence suggests that its chronicity and the presence of p53 alterations may indicate a premalignant state, particularly in patients with prolonged HU exposure. The clinical and histological overlap between DM-LE and HUSD underscores the importance of careful monitoring, rigorous photoprotection, and the consideration of HU discontinuation in high-risk patients.
6. Conclusions
Our findings emphasize the need for heightened vigilance in patients undergoing long-term HU therapy. Although DM-LE is typically benign and resolves after therapy discontinuation, its resemblance to true dermatomyositis and association with p53 abnormalities necessitate caution. Moreover, the potential for progression to SCC, particularly in sun-exposed areas, highlights the importance of regular dermatologic evaluations, patient education on photoprotection, and early intervention for suspicious lesions.
However, a limitation of our study is that p53 and p16 expression were not tested in the majority of included cases. Addressing this gap in future studies is essential to comprehensively assess these markers, better understand the potential premalignant nature of DM-LE, and refine clinical management strategies to mitigate the long-term cutaneous risks associated with HU therapy.
Author Contributions
Conceptualization, G.D.M. and G.D.B.; methodology, S.G.; software, E.C.; validation, S.G. and F.R.; formal analysis, G.D.M.; investigation, G.D.B.; resources, S.G. and N.R.; data curation, E.C.; writing—original draft preparation, G.D.M.; writing—review and editing, G.D.B.; visualization, G.D.B.; supervision, F.R.; project administration, F.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Janerowicz, D.; Czarnecka-Operacz, M.; Stawny, M.; Silny, W. Dermatomyositis-like eruption induced by hydroxyurea: A case report. Acta Dermatovenerol. Alp. Pannonica Adriat. 2009, 18, 131–134. [Google Scholar] [PubMed]
- Kalajian, A.H.; Cely, S.J.; Malone, J.C.; Burruss, J.B.; Callen, J.P. Hydroxyurea-associated dermatomyositis-like eruption demonstrating abnormal epidermal p53 expression: A potential premalignant manifestation of chronic hydroxyurea and UV radiation exposure. Arch. Dermatol. 2010, 146, 305–310. [Google Scholar]
- De Unamuno, J.; Vilata Corell, J.; Ballester Sanchez, R.; Alegre Miquel, V. Hydroxyurea-induced dermatomyositis-like eruption with abnormal expression of p53. J. Am. Acad. Dermatol. 2013, 68, 676–683. [Google Scholar]
- Sanchez-Palacios, C.; Guitart, J. Hydroxyurea-associated squamous dysplasia. J. Am. Acad. Dermatol. 2004, 51, 293–300. [Google Scholar] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. 2021, 18, e1003583. [Google Scholar] [CrossRef]
- Burns, D.A.; Sarkany, I.; Gaylarde, P. Effects of hydroxyurea therapy on normal skin: A case report. Clin. Exp. Dermatol. 1980, 5, 447–449. [Google Scholar]
- Sigal, M.; Crickx, B.; Blanchet, P.; Perron, J.; Simony, J.; Belaïch, S. Lésions cutanées induites par l’utilisation au long cours de l’hydroxyurée. Ann. Dermatol. Venereol. 1984, 111, 895–900. [Google Scholar] [PubMed]
- Richard, M.; Truchetet, F.; Friedel, J.; Leclech, C.; Heid, E. Skin lesions simulating chronic dermatomyositis during long-term hydroxyurea therapy. J. Am. Acad. Dermatol. 1989, 21, 797–799. [Google Scholar] [CrossRef]
- Chantereau, M.; Lorcerie, B.; Escallier, F. L'hydroxyuree responsable de lesions cutanees de type dormatomyosite. Rev Med Inl 1990, 11, 4196. [Google Scholar]
- Thomas, L.; Ferrier, F.; Moulin, C. Dermatomyositis-like eruption complicating hydroxyurea therapy of chronic myelogenous leukemia. Eur. J. Dermatol. 1992, 2, 492–495. [Google Scholar]
- Senet, P.; Aractingi, S.; Porneuf, M.; Perrin, P.; Duterque, M. Hydroxyurea-induced dermatomyositis-like eruption. Br. J. Dermatol. 1995, 133, 455–459. [Google Scholar] [PubMed]
- Bahadoran, P.; Castanet, J.; Lacour, J.P.; Perrin, C.; Del Giudice, P.; Mannocci, N.; Fuzibet, J.G.; Ortonne, J.P. Pseudo-Dermatomyositis Induced by Long-Term Hydroxyurea Therapy: Report of Two Cases. Br. J. Dermatol. 1996, 134, 1161–1162. [Google Scholar] [PubMed]
- Daoud, M.S.; Gibson, L.E.; Pittelkow, M.R. Hydroxyurea Dermopathy: A Unique Lichenoid Eruption Complicating Long-Term Therapy with Hydroxyurea. J. Am. Acad. Dermatol. 1997, 36, 178–182. [Google Scholar] [PubMed]
- Suehiro, M.; Kishimoto, S.; Wakabayashi, T.; Ikeuchi, A.; Miyake, H.; Takenaka, H.; Okano, A.; Hirai, H.; Shimazaki, C.; Yasuno, H. Hydroxyurea dermopathy with a dermatomyositis-like eruption and a large leg ulcer. Br. J. Dermatol. 1998, 139, 748–749. [Google Scholar]
- Kennedy, B.J. Hydroxyurea-associated leg ulceration. Ann. Intern. Med. 1998, 129, 252. [Google Scholar]
- Varma, S.; Lanigan, V. Dermatomyositis-like eruption and leg ulceration caused by hydroxyurea in a patient with psoriasis. Clin. Exp. Dermatol. 1999, 24, 164–166. [Google Scholar]
- Vélez, A. Hydroxyurea-Induced Leg Ulcers: Is Macroerythrocytosis a Pathogenic Factor? J. Eur. Acad. Dermatol. Venereol. 1999, 12, 243–244. [Google Scholar]
- Vassallo, C.; Passamonti, F.; Merante, S.; Ardigò, M.; Nolli, G.; Mangiacavalli, S.; Borroni, G. Muco-cutaneous changes during long-term therapy with hydroxyurea in chronic myeloid leukaemia. Clin. Exp. Dermatol. 2001, 26, 141–148. [Google Scholar]
- Ruiz-Genao, D.P.; Sanz-Sánchez, T.; Bartolomé-González, B.; Fernández-Herrera, J.; García-Díez, A. Dermatomyositis-like reaction induced by chemotherapeutical agents. Int. J. Dermatol. 2002, 41, 885–887. [Google Scholar]
- Oskay, T.; Kutluay, L.; Ozyilkan, O. Dermatomyositis-like eruption after long-term hydroxyurea therapy for polycythemia vera. Eur. J. Dermatol. 2002, 12, 586–588. [Google Scholar]
- Dacey, M.J.; Callen, J.P. Hydroxyurea-induced dermatomyositis-like eruption. J. Am. Acad. Dermatol. 2003, 48, 439–441. [Google Scholar] [CrossRef] [PubMed]
- Rocamora, V.; Puig, L.; Alomar, A. Dermatomyositis-like eruption following hydroxyurea therapy. J. Eur. Acad. Dermatol. Venereol. 2000, 14, 227–228. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Saito, M.; Amagai, M.; Ikeda, Y. Gottron-like papules induced by hydroxyurea. Clin. Exp. Dermatol. 2005, 30, 191–192. [Google Scholar] [CrossRef]
- Zaccaria, E.; Cozzani, E.; Parodi, A. Secondary cutaneous effects of hydroxyurea: Possible pathogenetic mechanisms. J. Dermatolog. Treat. 2006, 17, 176–178. [Google Scholar] [CrossRef]
- Elliott, R.; Davies, M.; Harmse, D. Dermatomyositis-like eruption with long-term hydroxyurea. J. Dermatolog. Treat. 2006, 17, 59–60. [Google Scholar] [CrossRef]
- Slobodin, G.; Lurie, M.; Munichor, M.; Kovalev, J.; Rosner, I. Gottron’s papules-like eruption developing under hydroxyurea therapy. Rheumatol. Int. 2006, 26, 768–770. [Google Scholar] [CrossRef]
- Haniffa, M.A.; Speight, E.L. Painful leg ulcers and a rash in a patient with polycythaemia rubra vera: Diagnosis—Hydroxyurea-induced leg ulceration and dermatomyositis-like skin changes. Clin. Exp. Dermatol. 2006, 31, 733–734. [Google Scholar] [CrossRef]
- Martorell-Calatayud, A.; Requena, C.; Nagore-Enguídanos, E.; Guillén-Barona, C. Úlceras dolorosas múltiples en la pierna resistentes al tratamiento asociadas a lesiones dermatomiositis-like en las articulaciones interfalángicas de las manos: Hidroxiurea como agente causal. Actas Dermosifiliogr. 2009, 100, 804–807. [Google Scholar] [CrossRef] [PubMed]
- Cook-Norris, R.H.; Mansfield, A.S.; Michaels, J.D.; Davis, M.D.P. Hydroxycarbamide-induced dermopathy. Am. J. Hematol. 2010, 85, 75–76. [Google Scholar] [CrossRef]
- Agrawal, P.; Mahajan, S.; Khopkar, U.; Kharkar, V. Gottron - like papules induced by hydroxyurea. Indian J Dermatol Venereol Leprol 2012, 78, 775. [Google Scholar] [CrossRef]
- Nofal, A.; Salah El-Din, E. Hydroxyurea-induced dermatomyositis: True amyopathic dermatomyositis or dermatomyositis-like eruption? Int. J. Dermatol. 2012, 51, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Zappala, T.M.; Rodins, K.; Muir, J. Hydroxyurea-induced dermatomyositis-like eruption. Australas. J. Dermatol. 2012, 53, e58–e60. [Google Scholar]
- De Unamuno-Bustos, B.; Ballester-Sánchez, R.; Sabater Marco, V.; Vilata-Corell, J.J. Dermatomyositis-like eruption associated with hydroxyurea therapy: A premalignant condition? Actas Dermosifiliogr. 2014, 105, 876–878. [Google Scholar]
- Ito, E.; Muro, Y.; Shibata, A.; Sugiura, K.; Akiyama, M. Hydroxyurea-induced amyopathic dermatomyositis presenting with heliotrope erythema. Dermatol. Online J. 2014, 20, 8. [Google Scholar]
- Koch, L.; Lichem, R.; Cerroni, L.; Aberer, W.; Massone, C. Dermatitis, nonmelanoma skin cancer and leg ulcers. Clin. Exp. Dermatol. 2016, 41, 943–944. [Google Scholar]
- Moreno-Artero, E.; Paricio, J.J.; Antoñanzas, J.; España, A. Dermatomyositis-like eruption in a woman treated with hydroxyurea. Actas Dermosifiliogr. 2019, 110, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Calleja Algarra, A.; Miguel, R.A.; Tous Romero, F.; Maroñas Jiménez, L. Mucocutaneous lesions and nail pigmentation in a patient with essential thrombocytosis. Aust. Fam. Physician. 2017, 46, 222–224. [Google Scholar]
- Marie, I.; Joly, P.; Levesque, H.; Heron, F.; Courville, P.; Cailleux, N.; Courtois, H. Pseudo-dermatomyositis as a complication of hydroxyurea therapy. Clin. Exp. Rheumatol. 2000, 18, 536–537. [Google Scholar]
- Platto, J.; Alexander, C.E.; Kurtzman, D.J.B. A violaceous, photodistributed cutaneous eruption and leg ulcer in a woman with essential thrombocytosis. JAMA Dermatol. 2018, 154, 95–96. [Google Scholar]
- Veraitch, O.; Curto-Garcia, N.; Harrison, C.; Stefanato, C.M.; McGibbon, D. Hydroxyurea-induced dermatomyositis koebnerizing at the site of previous shingles. Clin. Exp. Dermatol. 2019, 44, 546–548. [Google Scholar]
- Pruessmann, W.; Kirfel, J.; Sailer, V.W.; Rose, C. Dermatomyositis-like skin eruptions under hydroxyurea therapy conceal TP53-mutated atypical keratinocytes: A histopathologic and molecular pathologic case series. J. Cutan. Pathol. 2024, 51, 852–859. [Google Scholar] [PubMed]
- Ahdoot, R.; Rew, J.; Reynolds, K.A.; Lowe, L.; Mervak, J.E. Violaceous scaly plaques of the dorsal hands. JAAD Case Rep. 2024, 49, 85–87. [Google Scholar] [PubMed]
- Disdier, P.; Harle, J.R.; Grob, J.J. More rapid development of multiple squamous-cell carcinomas during chronic granulocytic leukemia. Dermatologica 1991, 183, 47–48. [Google Scholar] [PubMed]
- Papi, M.; Didona, B.; Def’ita, O.; Abruzzese, E.; Stasi, R.; Papa, G.; Cavalieri, R. Multiple skin tumors on light-exposed areas during long-term treatment with hydroxyurea. J. Am. Acad. Dermatol. 1993, 28, 485–486. [Google Scholar]
- Angeli-Besson, C.; Koeppel, M.C.; Jacquet, P.; Andrac, L.; Sayag, J. Multiple squamous-cell carcinomas of the scalp and chronic myeloid leukemia. Dermatology 1995, 191, 321–322. [Google Scholar]
- Grange, F.; Couilliet, D.; Audhuy, B.; Krzisch, S.; Schlecht, P.; Guillaume, J.C. Kératoses multiples induites par l’hydroxyurée. Ann. Dermatol. Venereol. 1995, 122, 16–18. [Google Scholar]
- Callot-Mellot, C.; Bodemer, C.; Chosidow, O.; Frances, C.; Azgui, Z.; Varet, B.; de Prost, Y. Cutaneous carcinoma during long-term hydroxyurea therapy: A report of 5 cases. Arch. Dermatol. 1996, 132, 1395–1397. [Google Scholar]
- De Simone, C.; Guerriero, C.; Guidi, B.; Rotoli, M.; Venier, A.; Tartaglione, R. Multiple squamous cell carcinomas of the skin during long-term treatment with hydroxyurea. Eur. J. Dermatol. 1998, 8, 114–115. [Google Scholar]
- Best, P.J.; Petitt, R.M. Multiple skin cancers associated with hydroxyurea therapy. Mayo Clin. Proc. 1998, 73, 961–963. [Google Scholar]
- Young, H.S.; Khan, A.S.; Kendra, J.R.; Coulson, I.H. The cutaneous side effects of hydroxyurea. Clin. Lab. Haematol. 2000, 22, 229–232. [Google Scholar]
- Salmon-Ehr, V.; Leborgne, G.; Vilque, J.P.; Potron, G.; Bernard, P. Secondary cutaneous effects of hydroxyurea: Prospective study of 26 patients from a dermatologic consultation. Rev. Med. Interne 2000, 21, 30–34. [Google Scholar] [CrossRef]
- Aste, N.; Fumo, G.; Biggio, P. Multiple squamous epitheliomas during long-term treatment with hydroxyurea. J. Eur. Acad. Dermatol. Venereol. 2001, 15, 89–90. [Google Scholar] [CrossRef]
- Estève, E.; Georgescu, V.; Heitzmann, P.; Martin, L. Carcinomes cutanés et buccaux multiples après traitement par l’hydroxyurée. Ann. Dermatol. Venereol. 2001, 128, 919–921. [Google Scholar]
- Pamuk, G.E.; Turgut, B.; Vural, O.; Demir, M.; Tek, M.; Altaner, S. Metastatic squamous cell carcinoma of the skin in chronic myeloid leukaemia: Complication of hydroxyurea therapy. Clin. Lab. Haematol. 2003, 25, 329–331. [Google Scholar] [CrossRef]
- De Benedittis, M.; Petruzzi, M.; Giardina, C.; Lo Muzio, L.; Favia, G.; Serpico, R. Oral squamous cell carcinoma during long-term treatment with hydroxyurea. Clin. Exp. Dermatol. 2004, 29, 605–607. [Google Scholar] [CrossRef] [PubMed]
- Schleußinger, T.M.; Dyall-Smith, D.; Field, L.M. Hydroxyurea-associated squamous dysplasia in a monozygotic twin. J. Am. Acad. Dermatol. 2011, 65, 679–680. [Google Scholar] [CrossRef] [PubMed]
- Stone, T.; Berger, A.; Blumberg, S.; O’Neill, D.; Ross, F.; McMeeking, A.; Chen, W.; Pastar, I. A multidisciplinary team approach to hydroxyurea-associated chronic wound with squamous cell carcinoma. Int. Wound J. 2012, 9, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Radić, J.; Batinac, T.; Hadžisejdić, I.; Načinović-Duletić, A.; Valković, T.; Jonjić, N. Concurrent basal cell and squamous cell carcinomas associated with hydroxyurea therapy. Acta Dermatovenerol. Croat. 2011, 19, 183–186. [Google Scholar]
- Neill, B.; Ryser, T.; Neill, J.; Aires, D.; Rajpara, A. A patient case highlighting the myriad of cutaneous adverse effects of prolonged use of hydroxyurea. Dermatol. Online J. 2017, 23, 15. [Google Scholar] [CrossRef]
- Antar, A.; Ishak, R.S.; Otrock, Z.K.; El-Majzoub, N.; Ghosn, S.; Mahfouz, R.; Taher, A.T. Successful treatment of hydroxyurea-associated chronic leg ulcers associated with squamous cell carcinoma. Hematol. Oncol. Stem Cell Ther. 2014, 7, 166–169. [Google Scholar] [CrossRef]
- Uday Kumar, P.; Anshul, S.; Anusha Reddy, S.; Vidyasgar, M.S.; Prahlad, H.Y. Oral cavity squamous cell carcinoma in a case of ichthyosis follicularis, alopecia, and photophobia syndrome with chronic myeloid leukemia on long-term hydroxyurea: A rare presentation. Asian J. Pharm. Clin. Res. 2017, 10, 1–3. [Google Scholar]
- Cantisani, C.; Kiss, N.; Naqeshbandi, A.F.; Tosti, G.; Tofani, S.; Cartoni, C.; Carmosino, I.; Cantoresi, F. Nonmelanoma skin cancer associated with Hydroxyurea treatment: Overview of the literature and our own experience. Dermatol. Ther. 2019, 32, e13043. [Google Scholar] [PubMed]
- Xu, Y.; Liu, J. Hydroxyurea-induced cutaneous squamous cell carcinoma: A case report. World J. Clin. Cases 2019, 7, 4091–4097. [Google Scholar] [PubMed]
- Brown, H.; Lamrock, E. Hydroxyurea-induced squamous cell carcinoma. Australas. J. Dermatol. 2021, 62, 83–84. [Google Scholar]
- Kerdoud, O.; Aloua, R.; Kaouani, A.; Belem, O.; Slimani, F. Squamous cell carcinoma during long-term hydroxyurea treatment: A case report. Int. J. Surg. Case Rep. 2021, 85, 106–160. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the European Society of Dermatopathology. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).