Abstract
High energy demand has led to excessive fuel consumption and high-concentration CO2 production. CO2 release causes serious environmental problems such as the rise in the Earth’s temperature, leading to global warming. Thus, chemical industries are under severe pressure to provide a solution to the problems associated with fuel consumption and to reduce CO2 emission at the source. To this effect, herein, four highly porous aromatic Schiff bases derived from melamine were investigated as potential media for CO2 capture. Since these Schiff bases are highly aromatic, porous, and have a high content of heteroatoms (nitrogen and oxygen), they can serve as CO2 storage media. The surface morphology of the Schiff bases was investigated through field emission scanning electron microscopy, and their physical properties were determined by gas adsorption experiments. The Schiff bases had a pore volume of 0.005–0.036 cm3/g, an average pore diameter of 1.69–3.363 nm, and a small Brunauer–Emmett–Teller surface area (5.2–11.6 m2/g). The Schiff bases showed remarkable CO2 uptake (up to 2.33 mmol/g; 10.0 wt%) at 323 K and 40 bars. The Schiff base containing the 4-nitrophenyl substituent was the most efficient medium for CO2 adsorption and, therefore, can be used as a gas sorbent.
1. Introduction
Fuel consumption has been increasing over the years to meet the high demand for energy required for various human activities. Therefore, CO2 concentration has increased drastically to unprecedented levels in the atmosphere [1]. Fossil fuel combustion is the main contributor (60%) to the increased CO2 concentration level in the environment [2]. Chemical, agro, power, and pharmaceutical industries contribute to approximately 70% greenhouse gas emission, which primarily causes climate changes and global warming [3,4]. The rise of sea and ocean levels, increased acidity of water, and drastic global weather changes are the main environmental problems associated with the increased CO2 emission, which will consequently lead to economic collapse [5,6]. Additionally, the CO2 levels in the environment cannot be lowered rapidly due to the large-scale and high consumption of fuels, which are difficult to be reduced. Novel strategies must be developed to not only resolve the environmental problems arising from global warming but also reduce carbon emission at the source. In addition, it is important to devise new technologies and design novel materials that can be used as a media to capture CO2 effectively [7,8,9,10].
Various technologies have been developed to capture and store CO2 that can efficiently reduce its atmospheric level [11,12,13,14,15,16]. Recently, researchers from both academia and industry directed their attention toward the capture and storage of CO2 [17,18,19]. Various chemical absorbents have been used as media for CO2 capture, in which amines (e.g., ethanolamine) are the most common ones [20]. The use of amines involves a simple process; however, it is limited because of high operational cost, energy requirement, and the use of very volatile chemicals [21]. Therefore, other techniques that involve the use of adsorbents were developed. Such materials reportedly exhibit adsorption capacity of >4.4% by weight, long life duration, recyclability, and reusability [22,23,24].
Chemical adsorption of CO2 is a simple as well as cost and energy effective process. Metal-based adsorbents such as metal oxides are known as common capture media for CO2 because of their basic and ionic nature [25]. For example, calcium and magnesium oxides can adsorb CO2 stoichiometrically to produce the corresponding metal carbonate through an exothermic reaction [26]. However, the adsorption capacity of materials varies on the basis of kinetic factors [25]. The adsorption capacity of calcium oxide is limited but sufficiently high to facilitate its use as an effective medium for CO2 capture. Several other materials such as ionic liquids in a solid matrix [27], zeolites [28], silica [29], and those containing activated carbons [30,31,32] have been evaluated as CO2 sorbents. Some of these materials possess unique thermal properties, high chemical stability, high surface area, tunable chemical structures, recyclability, and reusability. However, zeolites are not suitable for CO2 capture from flue gases because of their excellent hydrophilic properties [33]. In addition, materials containing activated carbon exhibit poor selectivity [34].
Activated carbon has been prepared from different materials such as polymers, resins, and biomass and can be used as an efficient adsorbent for CO2 [30]. Various chemical and physical processes have been conducted to activate and modify the surface area and pore volume of such adsorbents to increase their capacity for CO2 capture. The chemical process of activation requires the use of a base, while the physical one requires an appropriate carbonization gas [35,36]. The adsorption capacity of activated carbon depends on the distribution of the chemical activator within the matrix. Polyacrylonitrile in the presence of a base (e.g., potassium hydroxide; KOH) was used as an effective medium to capture CO2 and exhibited good CO2 uptake at 25 °C and under 1 bar [31]. The CO2 uptake was even higher for the resorcinol–formaldehyde resin at the same temperature and pressure in the presence of potassium carbonate as an activator [37].
Metal–organic frameworks (MOFs), synthesized from different molecular building units, have been investigated as adsorbents for CO2 because of their extended surface area [38,39,40]. The interaction between MOFs and CO2 is strong because it occurs through hydrogen bonding and requires a low heat of adsorption, similar to that observed for zeolites [25]. The CO2 storage capacity of MOFs can be enhanced through the addition of polar residues within their surfaces [41]. Porous-organic polymers (POPs) are highly stable chemically and thermally as well as have low density, tunable structure with a desirable surface area, and different functional groups; therefore, they act as good adsorbents for CO2 [33]. The presence of heteroatoms (e.g., nitrogen, oxygen, sulfur, phosphorus) within the skeleton of POPs enhances CO2 capture capacity [33]. The surface polarity of POPs can be increased by the addition of organic moieties containing polar groups or inorganic ions, which facilitates the strong interaction between CO2 and adsorbent materials [33]. Various POPs showed good CO2 capture capacity; however, the use of metals in the synthesis of POPs produces toxic pollutants. More research is still needed to optimize the synthetic procedures for POP production by employing simple and effective processes [42].
Nitrogen-rich heterocycles such as triazines have potential use in supramolecular applications because they interact with many chemicals through interactions, hydrogen bond formation, and chelation [43]. Melamine has a high nitrogen content (66% by weight) and has been used in various applications such as the production of raw materials with high nitrogen content, plastic, medicinal products, metal-free catalysts, and CO2 adsorbents [44,45,46]. Melamine Schiff bases can be easily synthesized through the reaction of melamine and aromatic carbonyl compounds in the presence of a catalyst. Recently, we have synthesized various Schiff bases and investigated their use as additives to stabilize polymeric films against irradiation [47,48,49,50,51,52,53]. Melamine Schiff bases have all the qualities needed for their use as efficient adsorbents for CO2. In this study, we report the use of melamine Schiff bases, which are highly aromatic and porous, as an efficient media for the capture of CO2 at 40 bars and 323 K.
2. Materials and Methods
2.1. Materials
Chemicals, reagents, and solvents were purchased from Merck (Schnelldorf, Germany) and were used as received.
2.2. Physiochemical Measurements
The surface morphology of Schiff bases was observed through field emission scanning electron microscopy (FESEM, TESCAN MIRA3, Kohoutovice, Czech Republic) at an accelerating voltage of 10 kV. The N2 adsorption–desorption isotherms of the Schiff bases were recorded on a Quantchrome chemisorption analyzer (Quantachrome Instruments, Boynton Beach, FL, USA) at 77 k. The Schiff bases 1–4 were degassed in a vacuum oven for a long period (6 h) at a high temperature (100 °C) under a flow of N2 gas (Cascade TEK, Cornelius, OR, USA) to ensure the removal of any residues or small molecules such as water from the pores of materials. The surface area of the Schiff bases was calculated using the Brunauer–Emmett–Teller (BET) equation at a relative pressure (P/P°) of 0.98. The pore size of the Schiff bases was verified by the Barrett–Joyner–Halenda (BJH) method. The CO2 uptake was measured at 40 bars and 323 K using the H-sorb 2600 high-pressure volumetric adsorption analyzer (Gold APP Instruments Corp., Beijing, China), which has two degassing and analyzing ports that can be simultaneously operated. The experiment of CO2 storage was repeated for at least 10 times for pressure optimization. A known quantity of gas was injected into a measurement tube that contained the Schiff base sample until an equilibrium between the adsorbed gas and the Schiff base sample was established. The final equilibrium pressure was recorded automatically using a software program and the adsorbed quantity of gas was calculated from the obtained data.
2.3. Synthesis of Schiff Bases 1–4
Schiff bases 1–4 were synthesized using a reported procedure by the condensation of melamine and 3 molar equivalents of aromatic aldehydes; 4-nitrobenzaldehyde, 2-hydroxybenzaldehyde, 3-hydroxybenzaldehyde, and 4-hydroxybenzaldehyde, in boiling dimethylformamide containing acetic acid as a catalyst under reflux for 6 h [47].
3. Results
3.1. Synthesis of Schiff Bases 1–4
The spectroscopic data from the 1H-NMR and FT-IR spectra, elemental analysis results, and physical properties (e.g., melting points and colors) of the synthesized Schiff bases 1–4 were identical to those of the previously reported bases [47]. Figure 1 represents the chemical structures of the synthesized Schiff bases 1–4. Schiff base 1 contained a nitro group, while Schiff bases 2–4 contained a hydroxyl group with different arrangements (ortho, meta, and para).
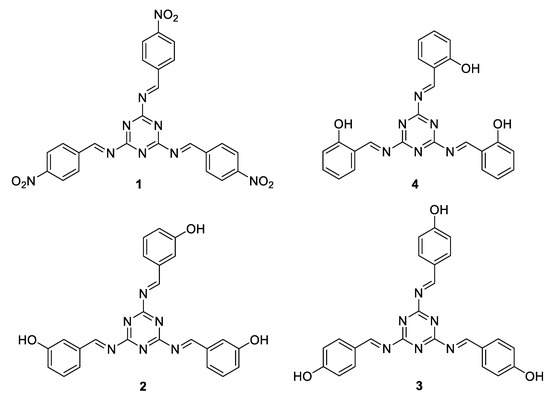
Figure 1.
Schiff bases 1–4 [47].
3.2. FESEM of Schiff Bases 1–4
The morphologies of Schiff bases 1–4 were investigated through FESEM. Figure 2, Figure 3, Figure 4 and Figure 5 show that Schiff bases 1–4 had a relatively uniform and amorphous surface with micro-size particles. The pore dimensions of the Schiff base samples varied and were found to be 20–392 nm. It was clear that the particle size of Schiff base 1 (Figure 2) was smaller than those of Schiff bases 2–4. Schiff bases 2 and 4 (Figure 3, Figure 4 and Figure 5) have the largest pore dimensions. Schiff base 1 had a different morphology compared to the other Schiff bases because it contains a nitro group, which causes more noticeable irregularity in particle size and shape and, thus, a highly porous structure. The presence of the functional group that had a high content of nitrogen (nitro group) could improve not only the porosity but also the surface area and efficiency for CO2 uptake [33].

Figure 2.
Field emission scanning electron microscopy (FESEM) image of Schiff base 1.

Figure 3.
FESEM image of Schiff base 2.
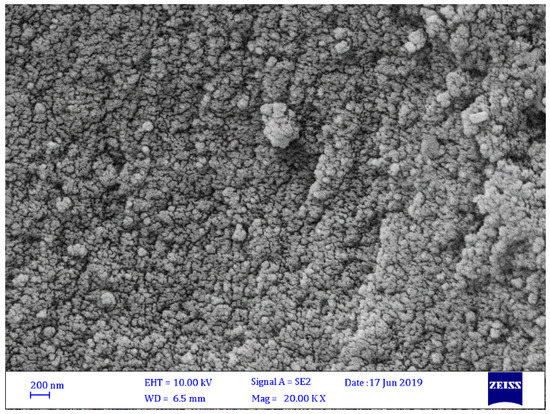
Figure 4.
FESEM image of Schiff base 3.
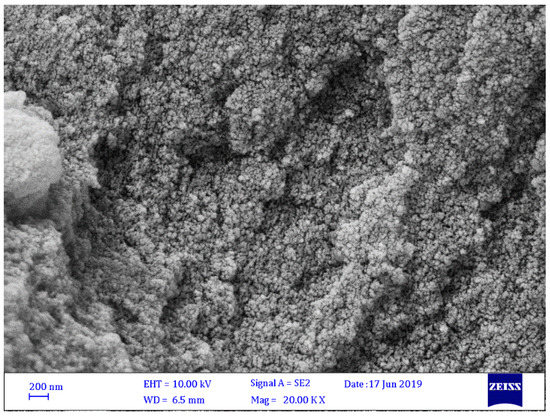
Figure 5.
FESEM image of Schiff base 4.
The pore dimensions of Schiff bases 1–4 were smaller than those reported for some POPs and larger than those for telmisartan tin complexes [54,55,56]. For example, POPs containing polyphosphates derived from 1,4-diaminobenzene showed irregular and porous structures with pore dimensions of 49–981 nm [54]. In addition, polyphosphates derived from benzidine showed porous structures with pore dimensions of 28–806 nm [55]. In contrast, the pore dimensions of telmisartan tin complexes ranged from 20 to 51 nm.
3.3. N2 Adsorption–Desorption of Schiff Bases 1–4
The N2 adsorption–desorption measurements for the Schiff bases 1–4 were conducted at 77 K. The N2 isotherms and pore sizes and volumes of Schiff bases 1–4 are represented in Figure 6, Figure 7, Figure 8 and Figure 9. The shape of the N2 isotherm for 1 was similar to the type IV isotherm. Schiff bases 2–4 showed N2 sorption isotherms that are almost identical to the type III isotherm, in which monolayer formation was not identified.
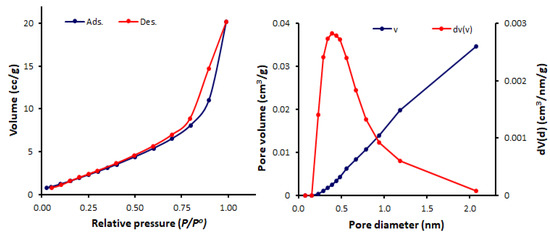
Figure 6.
N2 isotherms and pore size and volume for Schiff base 1.
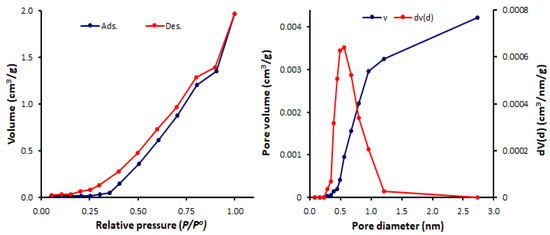
Figure 7.
N2 isotherms and pore size and volume for Schiff base 2.
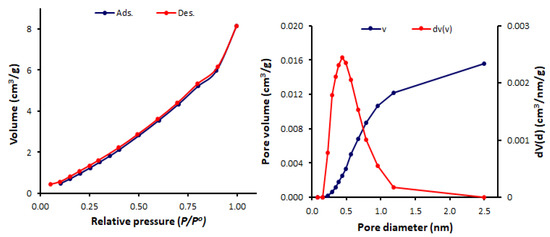
Figure 8.
N2 isotherms and pore size and volume for Schiff base 3.
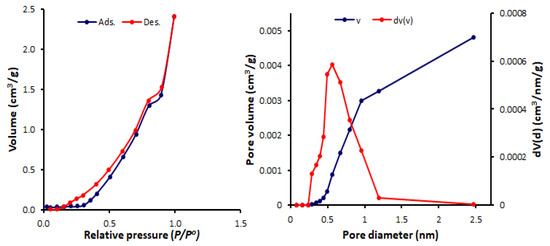
Figure 9.
N2 isotherms and pore size and volume for Schiff base 4.
The BET surface area (SBET), pore volumes, and average pore diameters of Schiff bases 1–4 were calculated (Table 1). Among the synthesized Schiff bases, 1 (containing a nitro group) exhibited the highest surface area (SBET = 11.6 m2/g) and total pore volume (0.036 cm3/gm), but the lowest pore diameter (1.69 nm). Schiff base 1 had a mesoporous structure, while, 2–4 (containing a hydroxy group at ortho-, meta- and para-position of the aryl ring) had microporous structures (pore diameter = 2.44–3.63 nm). Some POPs and tin complexes showed porous structures with similar pore diameters. For example, porous polyphosphates derived from either 1,4-diaminobenzene or benzidine exhibited a pore diameter of 1.96–2.43 nm [54] or 2.43–2.86 nm [55], respectively, compared to that of 2.43 nm for telmisartan tin complexes [56].

Table 1.
Porosity properties of 1–4.
A gravimetric technique was used to detect the gas uptake quantity and, therefore, determine the gas adsorption isotherm [57]. In addition, the gas quantity that has been removed from the gas phase was used to estimate the physisorption isotherms of the gas. The desorption or adsorption branch of the isotherm can be used to calculate the pore size distribution. The CO2 sorption isotherms for Schiff bases 1–4 are shown in Figure 10, Figure 11, Figure 12 and Figure 13 and their CO2 uptake are reported in Table 2.
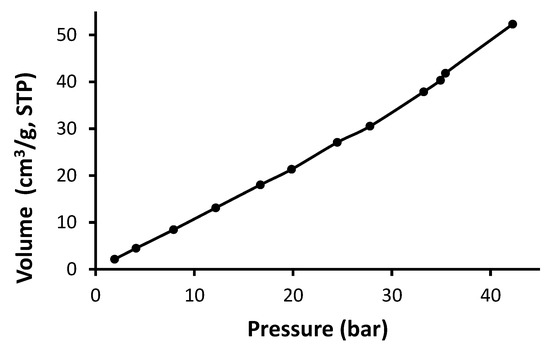
Figure 10.
Adsorption isotherm of CO2 for Schiff base 1.
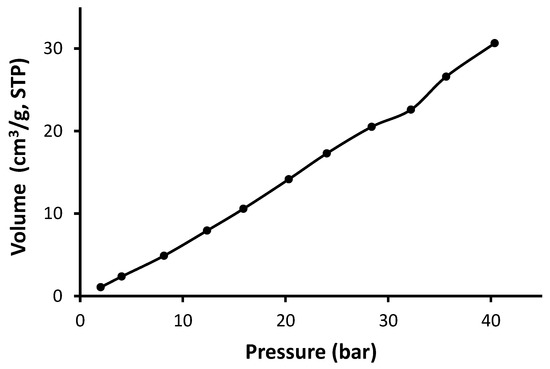
Figure 11.
Adsorption isotherm of CO2 for Schiff base 2.
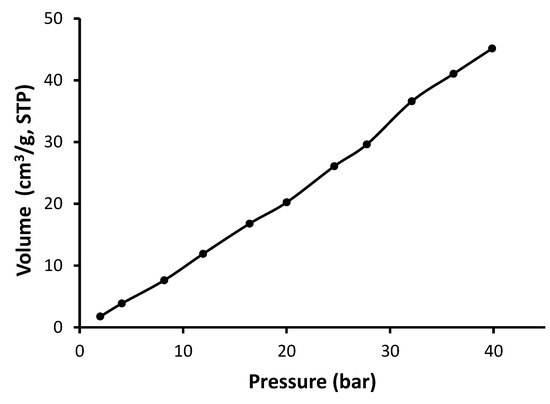
Figure 12.
Adsorption isotherm of CO2 for Schiff base 3.
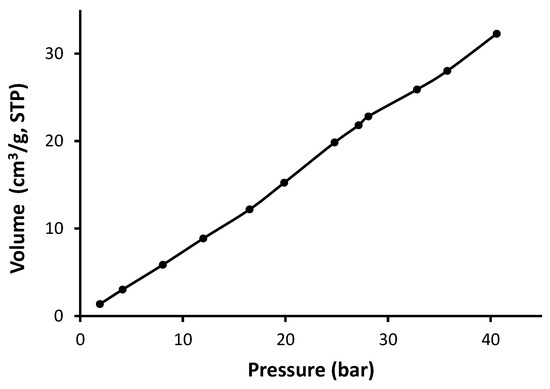
Figure 13.
Adsorption isotherm of CO2 for Schiff base 4.

Table 2.
CO2 adsorption capacity of Schiff bases 1–4 at 323 K and 40 bars.
As seen in Figure 10, Figure 11, Figure 12 and Figure 13, Schiff bases 1–4 do not have an apparent adsorption–desorption hysteresis, which indicates the reversible adsorption of CO2 within the Schiff base pores at the temperature and pressure used (323 K and 40 bars). The CO2 uptake for Schiff bases 1–4 was high (6.1–10.0 wt%), possibly because of the excellent pore diameter and the strong van der Waals interactions and hydrogen bonding between the Schiff bases and CO2. In addition, Schiff bases 1–4 contain strong Lewis base sites that aid the capture of CO2. Indeed, porous materials containing heteroatoms such as oxygen, nitrogen, and phosphorous can selectively capture CO2 over methane and nitrogen gases [54,55,56].
The surface area for the Schiff bases was relatively low (5.2–11.6 m2/g); however, they showed remarkable CO2 uptake (1.36–2.33 mmol/g; 6.1–10.0 wt%). Similar observations have been previously reported at similar temperature and pressure. For example, porous polyphosphates containing benzidine showed low surface area (27.5–30.0 m2/g) and high CO2 uptake (up to 14.0 wt%) [55]. On the other hand, polyphosphates containing 1,4-diaminobenzene exhibited high surface area (82.7–213.5 m2/g), but the CO2 uptake was limited to 0.6 wt% [54]. Telmisartan tin complexes showed surface area of 32.4–130.4 m2/g and up to 7.1 wt% CO2 uptake [56]. Materials with the highest surface area showed the most effective CO2 uptake. Polyacrylonitrile carbon fibers in the presence of a base provided a CO2 uptake of 2.74 mmol/g at room temperature and normal pressure [31]. In contrast, porous nanocarbons with a high surface area (1114 m2/g) in the presence of potassium oxalate and ethylenediamine provided a CO2 uptake as 4.60 mmol/g at a similar temperature and pressure [30]. Porous nanocarbons with a small surface area (439 m2/g) provided a low CO2 uptake (1.94 mmol/g [30]. Ionic liquids in a silica matrix led to materials having a very small surface area (1–9 m2/g) and relatively poor sorption capacity towards CO2 as 0.35 g of CO2 per g of adsorbent [27].
4. Conclusions
Four melamine Schiff bases have been investigated as potential media for CO2 storage at 323 K and 40 bars. These Schiff bases have a relatively low surface area (SBET = 5.2–11.6 m2/g) and varied porous structures, showing pore volumes of 0.004–0.036 cm3/g and diameters of 1.69–2.63 nm. The Schiff bases showed remarkable CO2 uptake (6.1–10.0 wt%), possibly because of their high aromaticity and heteroatom contents. The Schiff base containing a nitro group showed the most effective CO2 uptake (10.0 wt%) owing to the high content of nitrogen (heteroatom) within the porous material. The Schiff bases containing a hydroxy group have a lower surface area and pore volume, but higher pore diameter compared to the one containing a nitro group. Such Schiff base is inexpensive and easily producible in high yield and, therefore, can be used at an industrial scale.
Author Contributions
Conceptualization and experimental design: G.A.E.-H., M.F.A., D.S.A., and E.Y.; Experimental work and data analysis: R.M.O. and E.T.B.A.-T.; writing: G.A.E.-H., D.S.A. and E.Y. All authors discussed the results and have approved the final version of the paper. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are grateful to the Deanship of Scientific Research, King Saud University for funding through Vice Deanship of Scientific Research Chairs.
Acknowledgments
We thank Al-Nahrain and Al-Mansour Universities for the technical support.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Mardani, A.; Streimikiene, D.; Cavallaro, F.; Loganathan, N.; Khoshnoudi, M. Carbon dioxide (CO2) emissions and economic growth: A systematic review of two decades of research from 1995 to 2017. Sci. Total Environ. 2019, 649, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Yaumi, A.L.; Bakar, M.Z.A.; Hameed, B.H. Recent advances in functionalized composite solid materials for carbon dioxide capture. Energy 2017, 124, 461–480. [Google Scholar] [CrossRef]
- Boamah, K.B.; Du, J.; Bediako, I.A.; Boamah, A.J.; Abdul-Rasheed, A.A.; Owusu, S.M. Carbon dioxide emission and economic growth of China—The role of international trade. Environ. Sci. Pollut. Res. 2017, 24, 13049. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Xin, Q.; Ma, Z.; Lan, S. Effects of plant diversity on carbon dioxide emissions and carbon removal in laboratory-scale constructed wetland. Environ. Sci. Pollut. Res. 2019, 26, 5076. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Perez, E.S.; Murdock, C.R.; Didas, S.A.; Jones, C.W. Direct capture of CO2 from ambient air. Chem. Rev. 2016, 116, 11840–11876. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change. Climate Change. Climate Change 2007: Synthesis Report. In Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Pachauri, R.K., Reisinger, A., Eds.; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2008; p. 104. [Google Scholar]
- Okesola, A.A.; Oyedeji, A.A.; Abdulhamid, A.F.; Olowo, J.; Ayodele, B.E.; Alabi, T.W. Direct air capture: A review of carbon dioxide capture from the air. Mater. Sci. Eng. 2018, 413, 12077. [Google Scholar] [CrossRef]
- Shukla, S.K.; Khokarale, S.G.; Bui, T.Q.; Mikkola, J.-P.T. Ionic liquids: Potential materials for carbon dioxide capture and utilization. Front. Mater. 2019, 6, 42. [Google Scholar] [CrossRef]
- Mukherjee, A.; Okolie, J.A.; Abdelrasoul, A.; Niu, C.; Dalai, A.K. Review of post-combustion carbon dioxide capture technologies using activated carbon. J. Environ. Sci. 2019, 83, 46–63. [Google Scholar] [CrossRef]
- Goh, K.; Karahan, H.E.; Yang, E.; Bae, T.-H. Graphene-based membranes for CO2/CH4 separation: Key challenges and perspectives. Appl. Sci. 2019, 9, 2784. [Google Scholar] [CrossRef]
- Variny, M.; Jediná, D.; Kizek, J.; Illés, P.; Lukáč, L.; Janošovský, J.; Lesný, M. An investigation of the techno-economic and environmental aspects of process heat source change in a refinery. Processes 2019, 7, 776. [Google Scholar] [CrossRef]
- Shukrullah, S.; Naz, M.Y.; Mohamed, N.M.; Ibrahim, K.I.; Abd El-Salam, N.M.; Ghaffar, A. CVD synthesis, functionalization and CO2 adsorption attributes of multiwalled carbon nanotubes. Processes 2019, 7, 634. [Google Scholar] [CrossRef]
- Osman, A.; Eltayeb, M.; Rajab, F. Utility paths combination in HEN for energy saving and CO2 emission reduction. Processes 2019, 7, 425. [Google Scholar] [CrossRef]
- Kelektsoglou, K. Carbon capture and storage: A review of mineral storage of CO2 in Greece. Sustainability 2018, 10, 4400. [Google Scholar] [CrossRef]
- Aminua, M.D.; Nabavia, S.A.; Rochelleb, C.A.; Manovica, V. A review of developments in carbon dioxide storage. Appl. Energy 2017, 208, 1389–1419. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef]
- Thomas, D.M.; Mechery, J.; Paulose, S.V. Carbon dioxide capture strategies from flue gas using microalgae: A review. Environ. Sci. Pollut. Res. 2016, 23, 16926. [Google Scholar] [CrossRef]
- Sabouni, R.; Kazemian, H.; Rohani, S. Carbon dioxide capturing technologies: A review focusing on metal organic framework materials (MOFs). Environ. Sci. Pollut. Res. 2014, 21, 5427. [Google Scholar] [CrossRef]
- Samanta, A.; Zhao, A.; Shimizu, G.K.H.; Sarkar, P.; Gupta, R. Post-combustion CO2 capture using solid sorbents: A review. Ind. Eng. Chem. Res. 2012, 51, 1438–1463. [Google Scholar] [CrossRef]
- Luis, P. Use of monoethanolamine (MEA) for CO2 capture in a global scenario: Consequences and alternatives. Desalination 2016, 380, 93–99. [Google Scholar] [CrossRef]
- Figueroa, J.D.; Fout, T.; Plasynski, S.; McIlvried, H.; Srivastava, R.D. Advances in CO2 capture technology—The U.S. department of energy’s carbon sequestration program: A review. Int. J. Greenh. Gas Control 2008, 2, 9–20. [Google Scholar] [CrossRef]
- Asadi-Sangachini, Z.; Galangash, M.M.; Younesi, H.; Nowrouzi, M. The feasibility of cost-effective manufacturing activated carbon derived from walnut shells for large-scale CO2 capture. Environ. Sci. Pollut. Res. 2019, 26, 26542–26552. [Google Scholar] [CrossRef] [PubMed]
- Gibson, J.A.A.; Mangano, E.; Shiko, E.; Greenaway, A.G.; Gromov, A.V.; Lozinska, M.M.; Friedrich, D.; Campbell, E.E.B.; Wright, P.A.; Brandani, S. Adsorption materials and processes for carbon capture from gas-fired power plants: AMPgas. Ind. Eng. Chem. Res. 2016, 551, 33840–33851. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Park, S.-J. A review on solid adsorbents for carbon dioxide capture. J. Ind. Eng. Chem. 2015, 23, 1–11. [Google Scholar] [CrossRef]
- Choi, S.; Drese, J.H.; Jones, C.W. Adsorbent materials for carbon dioxide capture from large anthropogenic point sources. ChemSusChem 2009, 2, 796–854. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Chae, H.J.; Lee, S.J.; Choi, B.Y.; Yi, C.K.; Lee, J.B.; Ryu, C.K.; Kim, J.C. Development of regenerable MgO-based sorbent promoted with K2CO3 for CO2 capture at low temperatures. Environ. Sci. Technol. 2008, 42, 2736–2741. [Google Scholar] [CrossRef]
- Aquino, A.S.; Vieira, M.O.; Ferreira, A.S.D.; Cabrita, E.J.; Einloft, S.; de Souza, M.O. Hybrid ionic liquid–silica xerogels applied in CO2 capture. Appl. Sci. 2019, 9, 2614. [Google Scholar] [CrossRef]
- Hauchhum, L.; Mahanta, P. Carbon dioxide adsorption on zeolites and activated carbon by pressure swing adsorption in a fixed bed. Int. J. Energy Environ. Eng. 2014, 5, 349–356. [Google Scholar] [CrossRef]
- Lu, C.; Bai, H.; Su, F.; Chen, W.; Hwang, J.F.; Lee, H.-H. Adsorption of carbon dioxide from gas streams via mesoporous spherical-silica particles. J. Air Waste Manag. Assoc. 2010, 60, 489–496. [Google Scholar] [CrossRef]
- Staciwa, P.; Narkiewicz, U.; Moszyński, D.; Wróbel, R.J.; Cormia, R.D. Carbon spheres as CO2 sorbents. Appl. Sci. 2019, 9, 3349. [Google Scholar] [CrossRef]
- Chiang, Y.-C.; Yeh, C.Y.; Weng, C.H. Carbon dioxide adsorption on porous and functionalized activated carbon fibers. Appl. Sci. 2019, 9, 1977. [Google Scholar] [CrossRef]
- Al-Ghurabi, E.H.; Ajbar, A.; Asif, M. Enhancement of CO2 removal efficacy of fluidized bed using particle mixing. Appl. Sci. 2018, 8, 1467. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, M.; Yuan, D. Carbon dioxide capture in amorphous porous organic polymers. J. Mater. Chem. A 2017, 5, 1334–1347. [Google Scholar] [CrossRef]
- Wang, R.; Lang, J.; Yan, X. Effect of surface area and heteroatom of porous carbon materials on electrochemical capacitance in aqueous and organic electrolytes. Sci. China Chem. 2014, 57, 1570–1578. [Google Scholar] [CrossRef]
- Wickramaratne, N.P.; Jaroniec, M. Activated carbon spheres for CO2 adsorption. ACS Appl. Mater. Interfaces 2013, 5, 1849–1855. [Google Scholar] [CrossRef] [PubMed]
- Pari, G.; Darmawan, S.; Prihandoko, B. Porous Carbon spheres from hydrothermal carbonization and KOH activation on cassava and tapioca flour raw material. Procedia Environ. Sci. 2014, 20, 342–351. [Google Scholar] [CrossRef]
- Choma, J.; Kloske, M.; Dziura, A.; Stachurska, K.; Jaroniec, M. Preparation and studies of adsorption properties of microporous carbon spheres. Eng. Prot. Environ. 2016, 19, 169–182. [Google Scholar] [CrossRef]
- Dawson, R.; Cooper, A.I.; Adams, D.J. Nanoporous organic polymer networks. Prog. Polym. Sci. 2012, 37, 530–563. [Google Scholar] [CrossRef]
- Férey, G. Hybrid porous solids: Past, present, future. Chem. Soc. Rev. 2008, 37, 191–214. [Google Scholar] [CrossRef]
- Millward, A.R.; Yaghi, O.M. Metal-organic frameworks with exceptionally high capacity for storage of carbon dioxide at room temperature. J. Am. Chem. Soc. 2005, 127, 17998–17999. [Google Scholar] [CrossRef]
- Lu, W.; Yuan, D.; Sculley, J.; Zhao, D.; Krishna, R.; Zhou, H.-C. Sulfonate-grafted porous polymer networks for preferential CO2 adsorption at low pressure. J. Am. Chem. Soc. 2011, 133, 18126–18129. [Google Scholar] [CrossRef]
- Ahmed, D.S.; El-Hiti, G.A.; Yousif, E.; Ali, A.A.; Hameed, A.S. Design and synthesis of porous polymeric materials and their applications in gas capture and storage: A review. J. Polym. Res. 2018, 25, 75. [Google Scholar] [CrossRef]
- Mooibroek, T.J.; Gamez, P. The s-triazine ring, a remarkable unit to generate supramolecular interactions. Inorg. Chim. Acta 2007, 360, 381–404. [Google Scholar] [CrossRef]
- Jurgens, B.; Irran, E.; Senker, J.; Kroll, P.; Muller, H.; Schnick, W. Melem (2,5,8-triamino-tri-s-triazine), an important intermediate during condensation of melamine rings to graphitic carbon nitride: Synthesis, structure determination by X-ray powder diffractometry, solid-state NMR, and theoretical studies. J. Am. Chem. Soc. 2003, 125, 10288–10300. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Pevida, C.; Drage, T.C.; Snape, C.E. Silica-templated melamine–formaldehyde resin derived adsorbents for CO2 capture. Carbon 2008, 46, 1464–1474. [Google Scholar] [CrossRef]
- El-Hiti, G.A.; Alotaibi, M.H.; Ahmed, A.A.; Hamad, B.A.; Ahmed, D.S.; Ahmed, A.; Hashim, H.; Yousif, E. The morphology and performance of poly(vinyl chloride) containing melamine Schiff bases against ultraviolet light. Molecules 2019, 24, 803. [Google Scholar] [CrossRef]
- Yousif, E.; Ahmed, D.S.; El-Hiti, G.A.; Alotaibi, M.H.; Hashim, H.; Hameed, A.S.; Ahmed, A. Fabrication of novel ball-like polystyrene films containing Schiff bases microspheres as photostabilizers. Polymers 2018, 10, 1185. [Google Scholar] [CrossRef]
- Hashim, H.; El-Hiti, G.A.; Alotaibi, M.H.; Ahmed, D.S.; Yousif, E. Fabrication of ordered honeycomb porous poly(vinyl chloride) thin film doped with a Schiff base and nickel(II) chloride. Heliyon 2018, 4, e00743. [Google Scholar] [CrossRef]
- Shaalan, N.; Laftah, N.; El-Hiti, G.A.; Alotaibi, M.H.; Muslih, R.; Ahmed, D.S.; Yousif, E. Poly(vinyl chloride) photostabilization in the presence of Schiff bases containing a thiadiazole moiety. Molecules 2018, 23, 913. [Google Scholar] [CrossRef]
- Ahmed, D.S.; El-Hiti, G.A.; Hameed, A.S.; Yousif, E.; Ahmed, A. New tetra-Schiff bases as efficient photostabilizers for poly(vinyl chloride). Molecules 2017, 22, 1506. [Google Scholar] [CrossRef]
- Ali, G.Q.; El-Hiti, G.A.; Tomi, I.H.R.; Haddad, R.; Al-Qaisi, A.J.; Yousif, E. Photostability and performance of polystyrene films containing 1,2,4-triazole-3-thiol ring system Schiff bases. Molecules 2016, 21, 1699. [Google Scholar] [CrossRef] [PubMed]
- Yousif, E.; El-Hiti, G.A.; Hussain, Z.; Altaie, A. Viscoelastic, spectroscopic and microscopic study of the photo irradiation effect on the stability of PVC in the presence of sulfamethoxazole Schiff’s bases. Polymers 2015, 7, 2190–2204. [Google Scholar] [CrossRef]
- Satar, H.A.; Ahmed, A.A.; Yousif, E.; Ahmed, D.S.; Alotibi, M.F.; El-Hiti, G.A. Synthesis of novel heteroatom-doped porous-organic polymers as environmentally efficient media for carbon dioxide storage. Appl. Sci. 2019, 9, 4314. [Google Scholar] [CrossRef]
- Ahmed, D.S.; El-Hiti, G.A.; Yousif, E.; Hameed, A.S.; Abdalla, M. New eco-friendly phosphorus organic polymers as gas storage media. Polymers 2017, 9, 336. [Google Scholar] [CrossRef] [PubMed]
- Hadi, A.G.; Jawad, K.; Yousif, E.; El-Hiti, G.A.; Alotaibi, M.H.; Ahmed, D.S. Synthesis of telmisartan organotin(IV) complexes and their use as carbon dioxide capture media. Molecules 2019, 24, 1631. [Google Scholar] [CrossRef] [PubMed]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).