Cross-Linking of Fibrex Gel by Fungal Laccase: Gel Rheological and Structural Characteristics
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Enzymes
2.3. General Experimental Setup
2.4. Sugar Beet Fibre Extraction
2.5. Sugar Beet Fibre Cross-Linking
2.6. Forming Sweetened Fibrex Gels
2.7. Rheology
2.8. Analysing Diferulic Acids in Fibrex Gels
2.8.1. Sample Preparation
2.8.2. High Performance Liquid Chromatography Mass Spectrometry
2.9. Melting Point
2.10. Water-Holding Capacity WHC
2.11. Swelling Ratio
3. Results
3.1. Fibrex Gel Characterization
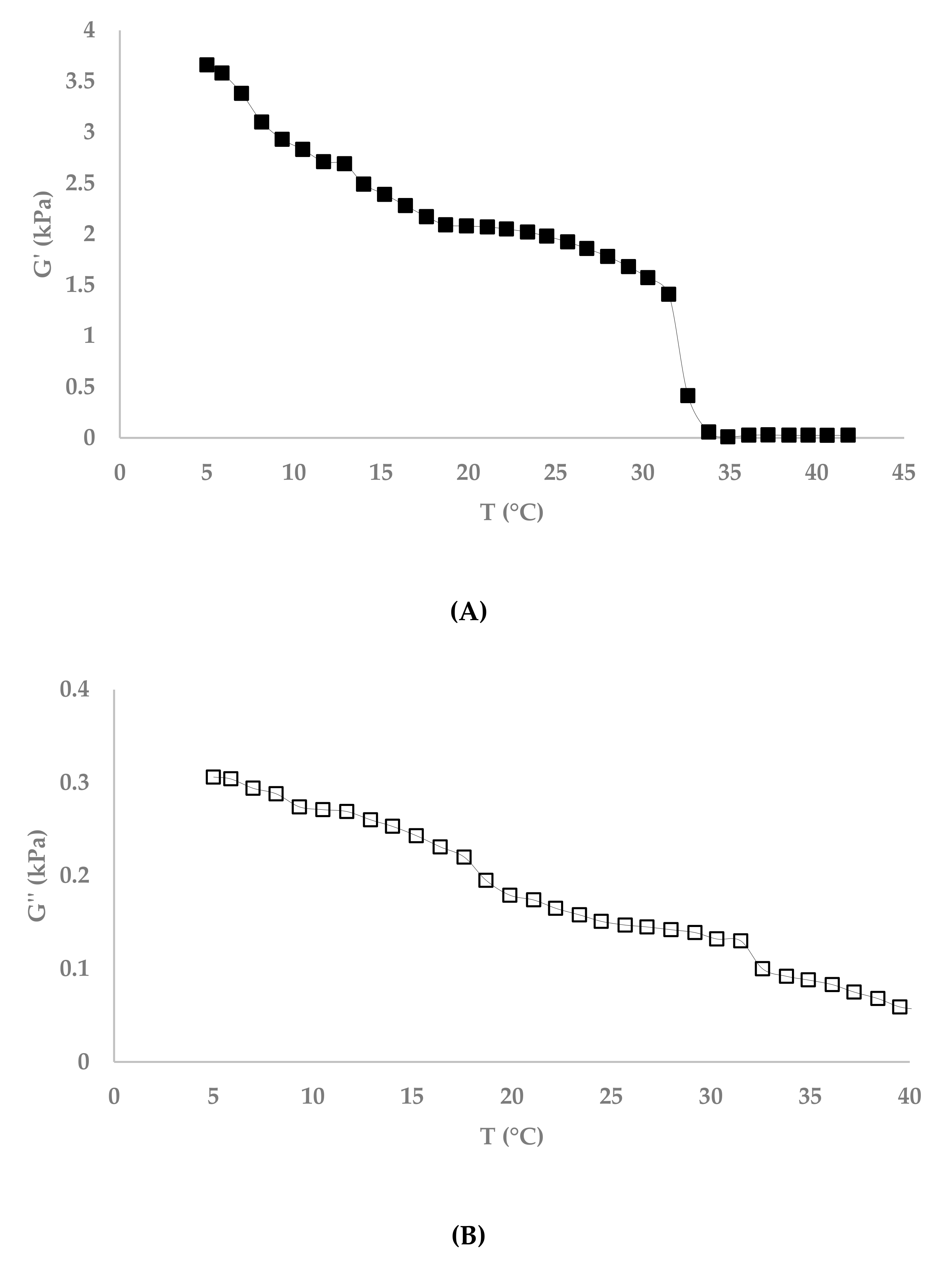
3.1.1. Oscillatory Tests on Fibrex Formed with LccFtr and Standard Gelatin Gels
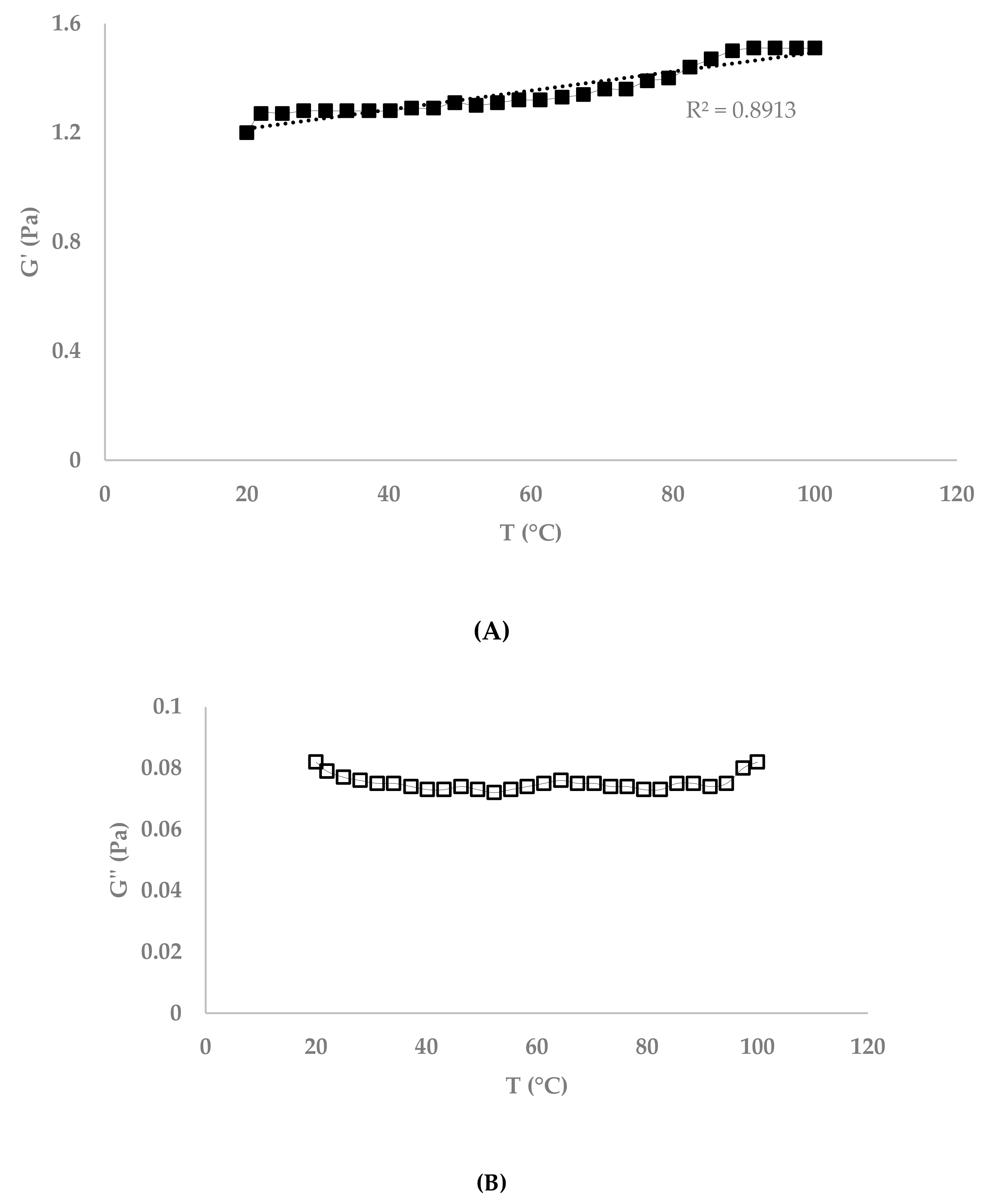
3.1.2. Effect of Citrus Pectin and Vanillin on Fibrex Gels
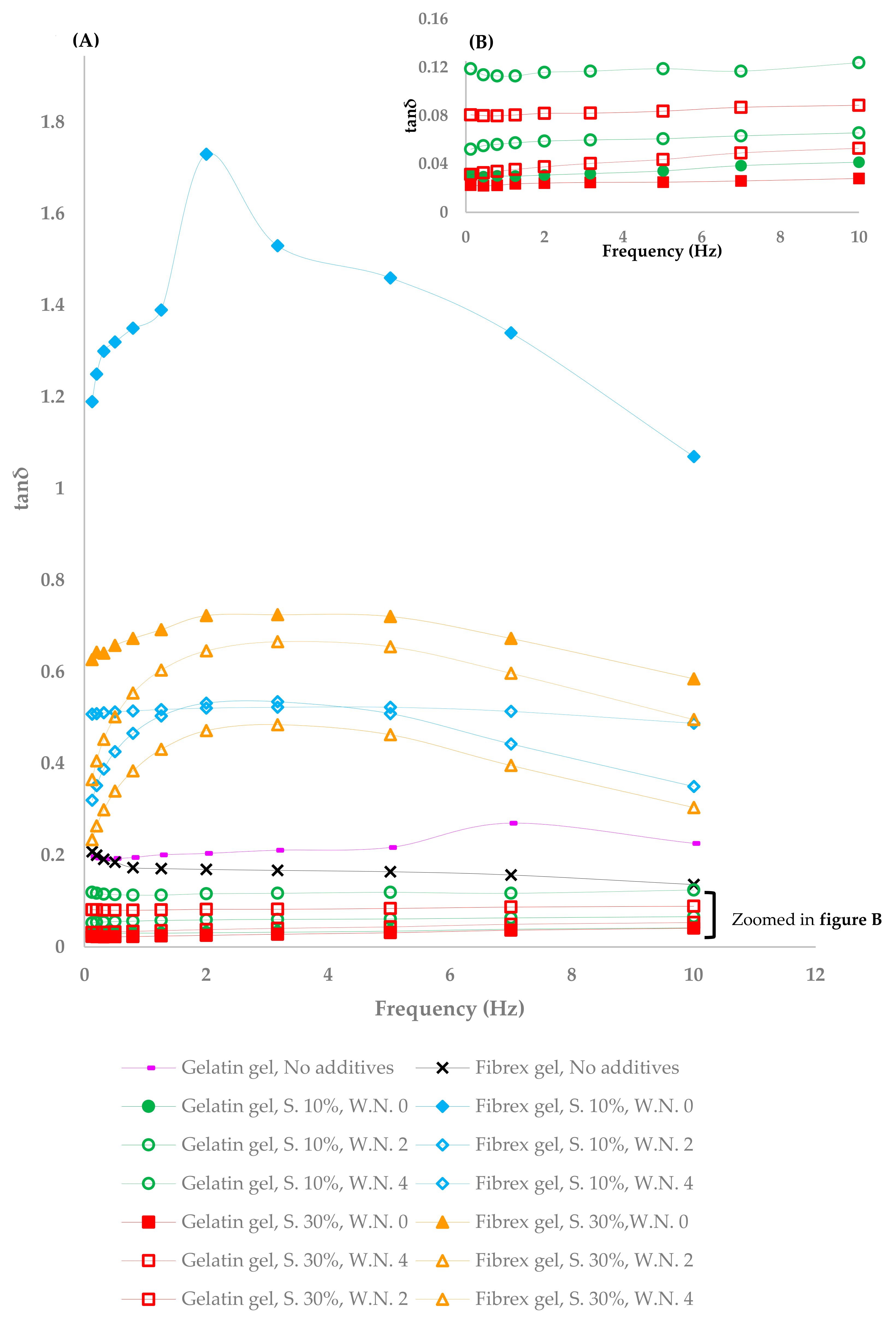
3.2. Viscoelastic Properties of Sweetened Fibrex Gels
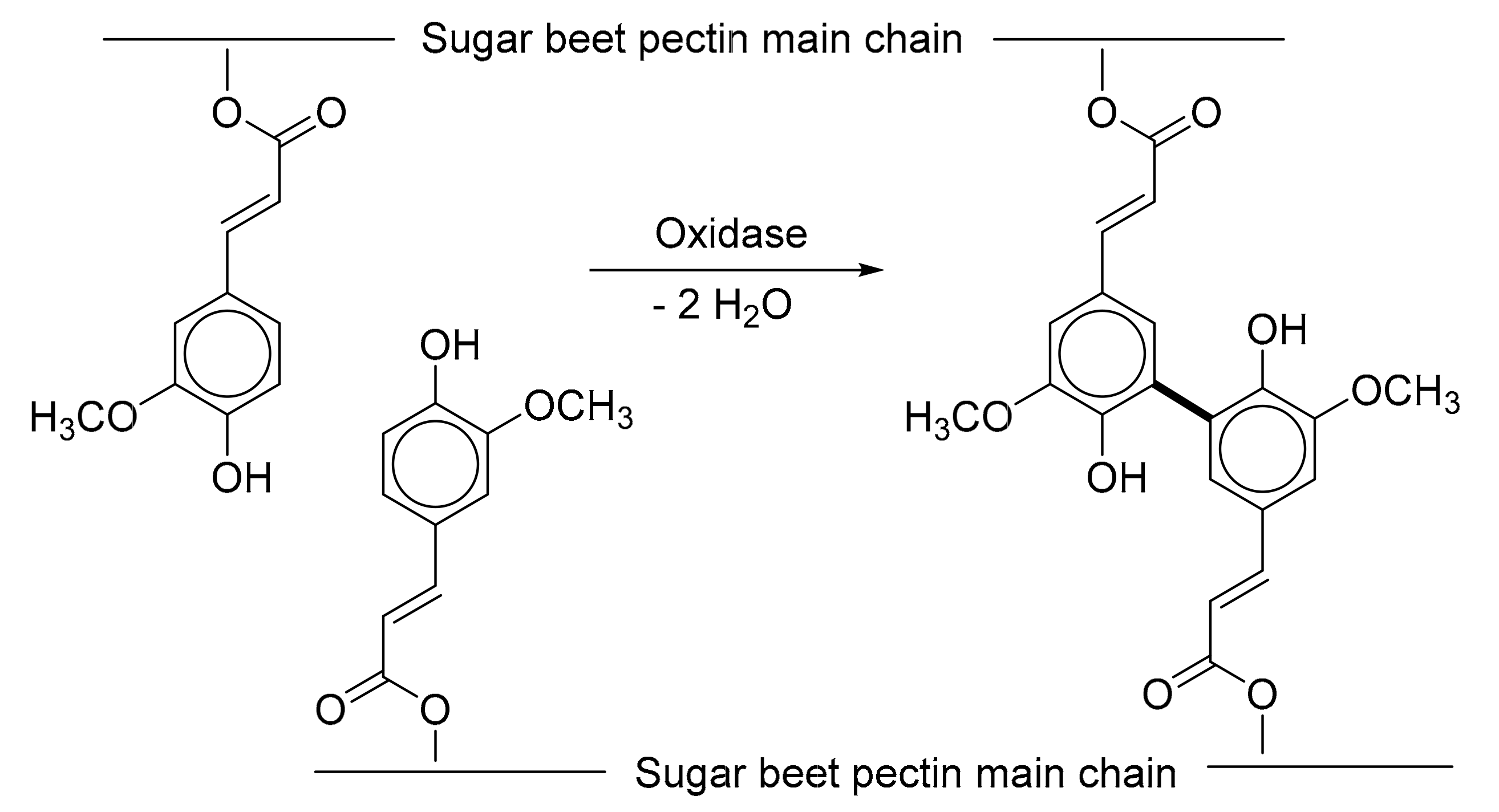
3.3. Identifying Diferulic Acids in Cross-Linked Fibrex Gel
3.4. Melting Point
3.5. Water-Holding Capacity
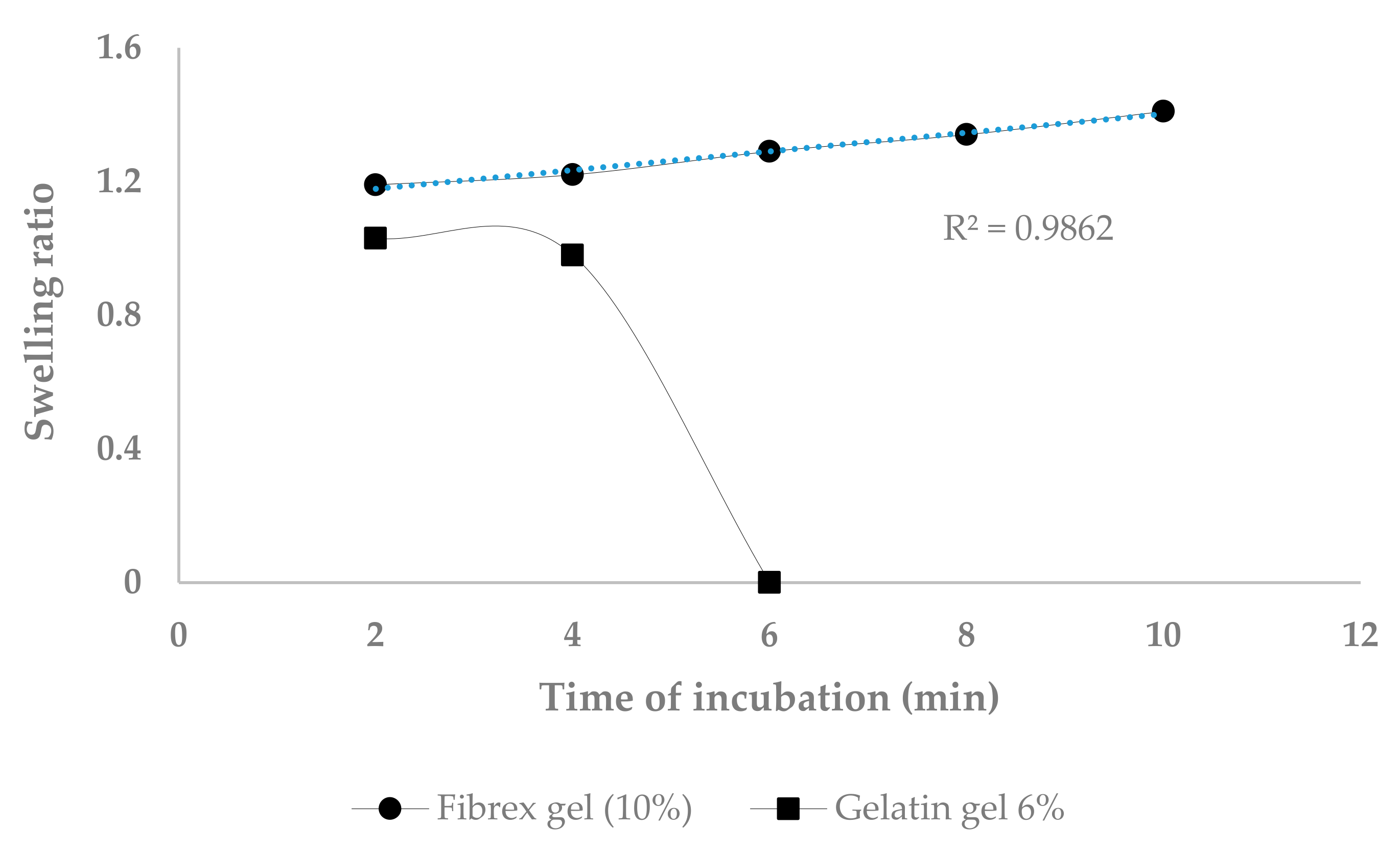
3.6. Swelling Ratio
4. Discussion
4.1. Fibrex and Gelatin Gel Characterization
4.2. Effect of Citrus Pectin and Vanillin on Fibrex Gels
4.3. Viscoelastic Properties of Sweetened Fibrex and Gelatin Gels
4.4. Identifying Diferulic Acids in Cross-Linked Fibrex Gel
4.5. Fibrex and Gelatin Gel Structural Characteristics
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Michel, F.; Thibault, J.; Barryb, J. Preparation and characterisation of dietary fibre from sugar beet pulp. J. Sci. Food Agric. 1987, 42, 77–85. [Google Scholar] [CrossRef]
- Sato, N.; Takano, Y.; Mizuno, M.; Nozaki, K.; Umemura, S.; Matsuzawa, T.; Amano, Y.; Makishima, S. Production of feruloylated arabino-oligosaccharides (FA-AOs) from beet fiber by hydrothermal treatment. J. Supercrital Fluids 2013, 79, 84–91. [Google Scholar] [CrossRef]
- Pacheco, M.; Villamiel, M.; Moreno, R.; Moreno, F. Structural and rheological properties of pectins extracted from industrial sugar beet by-products. Molecules 2019, 24, 392. [Google Scholar] [CrossRef] [PubMed]
- Saulnier, L.; Thibault, J.F. Review ferulic acid and diferulic acid as components of sugar beet pectins and maize bran heteroxylan. J. Sci. Food Agric. 1999, 79, 396–402. [Google Scholar] [CrossRef]
- Holck, J.; Lorentzen, A.; Vigsnaes, L.K.; Licht, T.R.; Mikkelsen, J.D.; Meyer, A.S. Feruloylated and Nonferuloylated Arabino-oligosaccharides from Sugar Beet Pectin Selectively Stimulate the Growth of Bifidobacterium spp. in Human Fecal in Vitro Fermentations. J. Agric. Food Chem. 2011, 59, 6511–6519. [Google Scholar] [CrossRef]
- Liu, L.; Fishman, M.; Kost, J.; Hicks, K. Pectin based systems for colon specific drug delivery via oral route. Biomaterials 2003, 24, 3333–3343. [Google Scholar] [CrossRef]
- Levigne, S.; Ralet, M.C.; Quemener, B.; Thibault, J.F. Isolation of diferulic bridges ester linked to arabinan in sugar beet cell walls. Carbohydr. Res. 2004, 339, 2315–2319. [Google Scholar] [CrossRef]
- Gawkowska, D.; Cybulska, J.; Zdunek, A. Structure-related gelling of pectins and linking with other natural compounds: A review. Polymers 2018, 10, 762. [Google Scholar] [CrossRef]
- Kuuva, T.; Lantto, R.; Reinikainen, T.; Buchert, J.; Autio, K. Rheological properties of laccase-induced sugar beet pectin gels. Food Hydrocoll. 2003, 17, 679–684. [Google Scholar] [CrossRef]
- Baldrian, P. Fungal laccases—occurrence and properties. FEMS Microbiol. Rev. 2006, 30, 215–242. [Google Scholar] [CrossRef]
- Osma, J.F.; Toca-Herrera, J.L.; Rodríguez-Couto, S. Uses of Laccases in the Food Industry. Enzym. Res. 2010, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Soto, F.E.; Serna-Saldivar, S.O.; Welti-Chanes, J. Effect of processing time, temperature and alkali concentration on yield extraction, structure and gelling properties of corn fiber arabinoxylans. Food Hydrocoll. 2016, 60, 21–28. [Google Scholar] [CrossRef]
- Khalighi, S.; Berger, R.G.; Ersoy, F. Cross-linking of wheat bran arabinoxylan by fungal laccases yields firm gels. Processes 2019. under review. [Google Scholar]
- Morrison, N.A.; Clark, R.C.; Chen, Y.L.; Talashek, T.; Sworn, G. Gelatin alternatives for the food industry. In Physical Chemistry and Industrial Application of Gellan Gum; Progress in Colloid and Polymer Science; Nishinari, K., Ed.; Springer: Berlin/Heidelberg, Germany, 1999; Volume 114, ISBN 978-3-540-48349-6. [Google Scholar]
- Struch, M.; Linke, D.; Mokoonlall, A.; Hinrichs, J.; Berger, R.G. Laccase catalyzed crosslinking of skimmed milk yoghurt enhanced by food-grade mediators. Int. Dairy J. 2015, 49, 89–94. [Google Scholar] [CrossRef]
- Kolwek, J.; Behrens, C.; Linke, D.; Krings, U.; Berger, R.G. Cell-free one-pot conversion of (+)-valencene to (+)-nootkatone by a unique dye-decolorizing peroxidase combined with a laccase from Funalia trogii. J. Ind. Microbiol. Biotechnol. 2018, 45, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Guillon, F.; Barry, J.; Thibault, J. Effect of autoclaving sugar-beet fibre on its physico-chemical properties and its in-vitro degradation by human faecal bacteria. J. Sci. Food Agric. 1992, 60, 69–79. [Google Scholar] [CrossRef]
- Yapo, B.M.; Robert, C.; Etienne, I.; Wathelet, B.; Paquot, M. Effect of extraction conditions on the yield, purity and surface properties of sugar beet pulp pectin extracts. Food Chem. 2007, 100, 1356–1364. [Google Scholar] [CrossRef]
- Nieter, A.; Kelle, S.; Linke, D.; Berger, R.G. Feruloyl esterases from Schizophyllum commune to treat food industry side-streams. Bioresour. Technol. 2016, 220, 38–46. [Google Scholar] [CrossRef]
- Barnes, H.A. A Handbook of Elementary Rheology; The University of Wales, Institute of Non-Newtonian Fluid Mechanics, Department of Mathematics: Cardiff, UK, 2000; Chapter 6; ISBN 92-5-101137-0. [Google Scholar]
- Schulz, K.; Nieter, A.; Scheu, A.K.; Copa-Patiño, J.L.; Popper, L.; Berger, R.G. A type D ferulic acid esterase from Streptomyces werraensis affects the volume of wheat dough pastries. Appl. Microbiol. Biotechnol. 2018, 102, 1269–1279. [Google Scholar] [CrossRef]
- Malunga, L.N.; Beta, T. Isolation and identification of feruloylated arabinoxylan mono- and oligosaccharides from undigested and digested maize and wheat. Heliyon 2016, 2, e00106. [Google Scholar] [CrossRef]
- Chen, M.J.; Lin, C.W. Factors Affecting the Water-Holding Capacity of Fibrinogen/Plasma Protein Gels Optimized by Response Surface Methodology. J. Food Sci. 2002, 67, 2579–2582. [Google Scholar] [CrossRef]
- Amal, A.; Hussain, S.; Jalaluddin, M. Preparation of artificial saliva formulation. Int. Conf. ICB Pharma II 2015, A002, 6–12. [Google Scholar]
- Mendez-Encinas, M.A.; Carvajal-Millan, E.; Rascon-Chu, A.; Astiazaran-Garcia, H.F.; Valencia-Rivera, D.E. Ferulated Arabinoxylans and Their Gels: Functional Properties and Potential Application as Antioxidant and Anticancer Agent. Hindawi Oxidative Med. Cell. Longev. 2018, 2018, 2314759. [Google Scholar] [CrossRef] [PubMed]
- Vismeh, R.; Lu, F.; Shishir, P.S.; Chundawat, S.; Humpula, J.F.; Azarpira, A.; Balan, V.; Dale, B.E.; Ralph, J.; Jones, A.D. Profiling of diferulates (plant cell wall cross-linkers) using ultrahigh-performance liquid chromatography mass spectrometry. Analyst 2013, 138, 6683–6692. [Google Scholar] [CrossRef] [PubMed]
- Francis, F.J. Wiley Encyclopaedia of Food Science and Technology, 2nd ed.; Francis, F.J., Ed.; John Wiley & Sons Inc.: London, UK, 1999; Volume 4, ISBN 978-0-471-19285-5. [Google Scholar]
- Zayas, J.F. Water Holding Capacity of Proteins. In Functionality of Proteins in Food; Springer: Berlin/Heidelberg, Germany, 1997; ISBN 978-3-642-63856-5. [Google Scholar]
- Oosterveld, A.; Grabber, J.; Beldman, G.; Ralph, J.; Voragen, A. Formation of ferulic acid dehydrodimers through oxidative cross-linking of sugar beet pectin. Carbohydr. Res. 1997, 300, 179–181. [Google Scholar] [CrossRef]
- Eidam, D.; Kulicke, W.M.; Kuhn, K.; Stute, R. Formation of maize starch gels selectively regulated by the addition of hydrocolloids biosynthesis. Starch Stärke 1995, 47, 378–384. [Google Scholar] [CrossRef]
- Saha, D.; Bhattacharya, S. Hydrocolloids as thickening and gelling agents in food: A critical review. J. Food Sci. Technol. 2010, 47, 587–597. [Google Scholar] [CrossRef]
- Weiss, J.; Mokoonlall, A.; Sykora, L.; Pfannstiel, J.; Stefan, N.; Hinrichs, J. A feasibility study on the application of a laccase-mediator system in stirred yoghurt at the pilot scale. Food Hydrocoll. 2016, 60, 119–127. [Google Scholar]
- Figueroa-Espinoza, M.C.; Rouau, X. Effect of cysteinyl caffeic acid, caffeic acid, and l-dopa on the oxidative cross-linking of feruloylated arabinoxylans by a fungal laccase. J. Agric. Food Chem. 1999, 47, 497–503. [Google Scholar] [CrossRef]
- Yesilada, O.; Birhanli, E.; Ozmem, N.; Ercan, S. Highly stable laccase from repeated-batch culture of Funalia trogii ATCC 200800. Appl. Biochem. Microbiol. 2014, 50, 55–61. [Google Scholar] [CrossRef]
- Tabilo-Munizaga, G.; Saenz-Hernandez, C.; Herrera-Lavados, C. Influence of temperature, calcium and sucrose concentration on viscoelastic properties of Prosopis chilensis seed gum and nopal mucilage dispersions. Int. J. Food Sci. Technol. 2018, 53, 1781–1788. [Google Scholar] [CrossRef]
- Krnic, P.; Truman, P.; Bates, D.; Vilkhu, K. Method for the Production of Jelly Confectionary. U.S. Patent 8895093, 25 November 2014. [Google Scholar]
- Cheng, L.; Yang, H. Effect of salt and sugar addition on the physicochemical properties and nanostructure of fish gelatin. Food Hydrocoll. 2015, 45, 72–82. [Google Scholar]
- Morales-Burgos, A.M.; Carvajal-Millan, E.; Lopez-Franco, Y.; Rascon-Chu, A.; Lizardi-Mendoza, J.; Sotelo-Cruz, N.; Brown-Bojorquez, F.; Burgara-Estrella, A.; Pedroza-Montero, M. Syneresis in gels of highly ferulated arabinoxylans: Characterization of covalent cross-linking, rheology, and microstructure. Polymers 2017, 9, 164. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Rao, M. Rheology and structure development during gelation of low-methoxyl pectin gels: The effect of sucrose. Food Hydrocoll. 2001, 15, 93–100. [Google Scholar] [CrossRef]
- Oakenfull, D.; Scott, A. Stabilization of gelatin gels by sugars and polyols. Food Hydrocoll. 1986, 1, 163–175. [Google Scholar] [CrossRef]
- Burey, P.; Bhandari, R.; Rutgers, R.P.G.; Halley, P.J.; Torley, P.J. Confectionery Gels: A Review on Formulation, Rheological and Structural Aspects. Int. J. Food Prop. 2010, 12, 176–210. [Google Scholar] [CrossRef]
- Kuan, Y.; Mohammadi, A.; Huda, N.; Arif, F.; Karim, A. Effect of sugars on the gelation kinetics and texture of duck feet gelatin. Food Hydrocoll. 2016, 58, 267–275. [Google Scholar] [CrossRef]
- Porayanee, M.; Katemake, P.; Duangmal, K. Effect of gelatin concentrations and glucose syrup to sucrose ratios on textural and optical properties of gelatin gel. Int. J. Food Sci. Technol. 2015, 1, 26–30. [Google Scholar]
- Kamer, D.D.; Palabiyik, I.; Isik, N.O.; Akyuz, F.; Demiric, A.S.; Gumus, T. Effect of confectionery solutes on the rheological properties of fish (Oncorhynchus mykiss) gelatin. LWT Food Sci. Technol. 2019, 101, 499–505. [Google Scholar] [CrossRef]
- Norulfairuz, D.; Zaidel, A.; Chronakis, I.; Meyer, A. Food hydrocolloids enzyme catalysed oxidative gelation of sugar beet pectin: Kinetics and rheology. Food Hydrocoll. 2012, 28, 130–140. [Google Scholar]
- Karim, A.; Bhat, R. Gelatin alternatives for the food industry: Recent developments, challenges and prospects. Trends Food Sci. Technol. 2008, 19, 644–656. [Google Scholar] [CrossRef]
- Singh, J.; Kaur, L.; McCarthy, O.J. Factors influencing the physico-chemical, morphological, thermal and rheological properties of some chemically modified starches for food applications—A review. Food Hydrocoll. 2007, 21, 1–22. [Google Scholar] [CrossRef]
- Carvajal-Millan, E.; Landillon, V.; Morel, M.H.; Rouau, X.; Doublier, J.L.; Micard, V. Arabinoxylan gels: Impact of the feruloylation degree on their structure and properties. Biomacromolecules 2005, 6, 309–317. [Google Scholar] [CrossRef] [PubMed]


| Rheological Parameters | No Additives | Vanillin | Pectin C | |||
|---|---|---|---|---|---|---|
| 2 mM | 4 mM | 1% | 3% | 5% | ||
| G′ | 5.52 | 1.83 | 1.34 | 5.44 | 5.23 | 5.57 |
| G″ | 1.26 | 0.41 | 0.38 | 1.26 | 1.28 | 1.40 |
| tanδ | ≤0.36 | ≤0.76 | ≤0.80 | ≤0.31 | ≤0.29 | ≤0.30 |
| Gels | Additives | Week # | G′ | G″ | tanδ |
|---|---|---|---|---|---|
| Fibrex gel 10% formed with LccFtr | 10% d-sucrose | 0 | 5.48 | 1.34 | ≤1.73 |
| 2 | 5.36 | 1.43 | ≤0.53 | ||
| 4 | 5.75 | 1.50 | ≤0.52 | ||
| 30% d-sucrose | 0 | 5.27 | 1.33 | ≤0.72 | |
| 2 | 5.23 | 1.25 | ≤0.48 | ||
| 4 | 5.86 | 1.49 | ≤0.66 | ||
| Gelatin gel 6% | 10% d-sucrose | 0 | 7.90 | 1.51 | ≤0.19 |
| 2 | 8.22 | 1.40 | ≤0.17 | ||
| 4 | 6.16 | 1.19 | ≤0.20 | ||
| 30% d-sucrose | 0 | 6.86 | 1.44 | ≤0.20 | |
| 2 | 6.85 | 1.36 | ≤0.19 | ||
| 4 | 6.46 | 1.23 | ≤0.19 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalighi, S.; Berger, R.G.; Ersoy, F. Cross-Linking of Fibrex Gel by Fungal Laccase: Gel Rheological and Structural Characteristics. Processes 2020, 8, 16. https://doi.org/10.3390/pr8010016
Khalighi S, Berger RG, Ersoy F. Cross-Linking of Fibrex Gel by Fungal Laccase: Gel Rheological and Structural Characteristics. Processes. 2020; 8(1):16. https://doi.org/10.3390/pr8010016
Chicago/Turabian StyleKhalighi, Sanaz, Ralf G. Berger, and Franziska Ersoy. 2020. "Cross-Linking of Fibrex Gel by Fungal Laccase: Gel Rheological and Structural Characteristics" Processes 8, no. 1: 16. https://doi.org/10.3390/pr8010016
APA StyleKhalighi, S., Berger, R. G., & Ersoy, F. (2020). Cross-Linking of Fibrex Gel by Fungal Laccase: Gel Rheological and Structural Characteristics. Processes, 8(1), 16. https://doi.org/10.3390/pr8010016





