Abstract
Ion exchange and cementation experiments were done to separate silver(I) from a raffinate containing silver(I), nickel(II), and zinc(II) and small amounts of copper(II) and tin(II). The raffinate resulted from the recovery of gold(III), tin(II) and copper(II) by solvent extraction from a leaching solution of anode slime. Ion exchange with anionic resins was not effective in separating silver(I) because tin(II) and zinc(II) were selectively adsorbed into the anionic resins. It was possible to separate silver(I) by cementation with copper sheet. Treatment of the cemented silver with nitric acid solution increased the purity of silver(I) in the solution from 50.9% to 99.99%. Adjusting the pH of the AgNO3 solution to higher than 6, followed by adding ascorbic acid as a reducing agent, led to the synthesis of silver particles with micron size.
1. Introduction
In recent years, resources recycling has become an important issue in the chemical and metallurgical industries. In order to recover pure metals or compounds with high purity from secondary resources, the valuable components together with some impurities are dissolved in the adequate leaching medium. Therefore, separation of the valuable component from the impurities is necessary to obtain a pure solution of the target component. There are several separation methods, such as solvent extraction, ion exchange, precipitation and cementation. In particular, solvent extraction and ion exchange are widely employed for the separation of chemically similar ions in the solution. In general, ion exchange is employed when the concentration of the impurities is less than 100 ppm [1]. Ion exchange is very useful for the removal of heavy metals from the solution with low metal concentration before discharge to the environment [2,3]. Cementation makes utilization of the difference in the reduction potential among the ions in the solution and can be represented as Equation (1). Since cementation has some advantages such as easy control, low energy consumption and low cost, it is widely employed for metal separation as well as metal production [4].
where M20 and M2n2+ are the metallic forms and ionic of reductive metals and M10 and M1n1+ are forms of noble metals.
M1n1+ + (n1/n2)M20 = M10 + (n1/n2)M2n2+
In our work on the recovery of noble metals present in the anode slimes from the electro-refining of tin, the components in the anode slimes were dissolved in hydrochloric acid solution in the presence of an oxidizing agent [5]. The leach liquor of the anode slimes contained gold(III), silver(I), copper(II), nickel(II), tin(II) and zinc(II). First, gold(III) was separated by solvent extraction with Cyanex 272 (bis(2,4,4-trimethylpentyl)phosphinic acid, Cytec Industries, Woodland Park, NJ, USA) and then copper(II) was selectively extracted by LIX 63 (5,8-diethyl-7-hydroxydodecan-6-oxime, BASF Corporation, Tucson, AZ, USA) from the gold(III) free raffinate [6,7]. After the separation of gold(III) and copper(II), the raffinate contained silver(I), tin(II), nickel(II) and zinc(II) together with a small amount of copper(II). In order to recover the silver(I) present in the raffinate, ion exchange and cementation experiments were performed in this work. Some works have been reported on the cementation of silver(I) by employing metals such as zinc, copper, magnesium and iron from various solutions [8,9,10,11,12]. Employment of these metals leads to high recovery percentage of silver. However, these metals can be applied to the solution with weak acidity, and silver powders were thus obtained, easily covering the surface of reductive metals, resulting in a decrease in the purity of metals, and excessive consumption of reductive metals owing to the reaction with the acid in the solution.
In this work, synthetic hydrochloric solutions were employed in the experiments. In a strong hydrochloric acid solution, soluble silver(I) exists as AgCln1−n. Therefore, several anion exchange resins were employed for the ion exchange experiments and the ion exchange behavior was investigated by varying the concentration of the resins. In cementation experiments, copper sheet was employed and the cementation behavior was investigated by varying reaction temperature, stirring speed, reaction time and the area of copper sheet. An integrated process was proposed for the recovery of silver(I) from the hydrochloric acid solution by comparing ion exchange and cementation results.
2. Materials and Methods
2.1. Chemical Reagents
A synthetic raffinate was prepared by dissolving a certain amount of AgCl (above 99.5%, Daejung Chemicals and Metals Co., Ltd., Shiheung, Korea), CuCl2∙2H2O (97%, Daejung Chemicals and Metals Co., Ltd., Shiheung, Korea), SnCl2∙H2O (97%, Daejung Chemicals and Metals Co., Ltd., Shiheung, Korea) as well as ZnCl2 (90%, Duksan Pure Chemicals Co., Ltd., Ansan-si, Korea) and NiCl2∙6H2O (YAKURI Pure Chemicals Co., Ltd., Kyoto, Japan, 96%) into 5 M HCl solution. A small amount of H2O2 (30%, DaeJung Chemical and Metals Co., Ltd., Shiheung, Korea) was added in the synthetic solution to suppress the formation of some precipitates. The ion exchange resins used in this work are AG 1-X8 (Bio-Rad, Hercules, CA, USA), AG MP-1M (Bio-Rad), Diaion WA21J (Mitsubishi Chemical Corporation, Tokyo, Japan), Lewatit MP-64 (Lanxess Energizing Chemistry), Purolite A500 (Lenntech, Edmonton, Canada), and TEVA (Eichrom, Chicago, IL, USA). All the resins were directly used without further pretreatment. The physical properties of these resins are listed in Table 1.

Table 1.
The physical properties of the resins employed in this work.
2.2. Experimental Procedure
2.2.1. Ion Exchange
Ion exchange experiments were carried out by using the screw bottle containing 20 mL of synthetic solution containing Ag(I), Cu(II), Ni(II), Sn(II) and Zn(II) with certain amounts of resins in a shaking incubator (HB-201SF, Hanbeak Scientific Co., Ltd., Bucheon-si, Korea). The mixtures were shaken at 200 rpm for 6 h at room temperature. After shaking, the loaded resin was separated from the effluent using a filter paper. The metal concentration in the effluent was measured by inductively coupled plasma optical emission spectroscopy (ICP-OES, Spectro Arcos, Cleve, Germany). The metal concentration loaded on the resin was obtained by mass balance.
2.2.2. Cementation of Ag(I) by Copper Sheet
Cementation experiments were carried out in a 100 mL beaker with magnetic stirrer on the hotplate (Daihan scientific Co., Ltd., Wonju-si, Korea). When the temperature reached the desired value, copper sheet was placed in the beaker lying on the wall with the cover to prevent the evaporation of the solution. The copper sheets were polished by 220 cc abrasive cloth before each experiment. A series of copper sheets with different width were cut by diamond cutter (METSAW-LS (RB 205), R&B Inc., Daejeon, Korea) based on the necessity of experiments. After finishing the cementation experiments, the solution was filtered through filter paper. The metal concentration in the solution was measured by ICP-OES (Inductively coupled plasma optical emission spectroscopy, Spectro Arcos, Cleve, Germany). Ag recovery percentage was calculated using Equation (2). The silver powders thus cemented were washed down with distilled water from the copper sheet. The powders were characterized by scanning electron microscopy (SEM, S-3500N Hitachi, Tokyo, Japan).
where m0 and m1 refer to the mass of silver(I) before and after cementation, respectively.
3. Results and Discussion
3.1. Speciation of the Metals in the Raffinate at 5 M HCl
Table 2 shows the composition of the raffinate after the sequential extraction of gold(III) and copper(II) by Cyanex 272 (bis(2,4,4-trimethylpentyl)phosphinic acid, Cytec Industries, Woodland Park, NJ, USA) and LIX 63 (5,8-diethyl-7-hydroxydodecan-6-oxime, BASF Corporation, Tucson, AZ, USA), respectively. The synthetic solution was prepared on the basis of the composition of the metals shown in Table 2.

Table 2.
The composition of the raffinate after selective extraction of gold(III) and copper(II) by Cyanex 272 and LIX 63 at 5 M HCl.
In ion exchange, the size and ionic charge of the reactants are very important. Therefore, the speciation of the metals present in the synthetic solution was investigated. In general, chloride ion has a strong tendency to form complexes with metal ion, and the degree of the complex formation depends on the concentration of chloride ion and the metal ion. In this work, the concentration of HCl in the synthetic solution was fixed at 5 M. According to the literature, silver(I) exists as AgCl2− and AgCl32− in this solution [13]. When HCl concentration is lower than 4 M, most of copper(II) forms mononuclear complexes with chloride ion such as CuCl3− and CuCl42− [3]. However, some of CuCl42− can be polymerized into Cu2Cl62− when HCl concentration is higher than 5 M [3,14]. It has been reported that nickel(II) does not form anionic complex with chloride ion even in 10 M HCl solution and exists as NiCl+ in 5 M HCl solution [15]. The distribution of tin(II) in HCl solution is more complex than that of the above metals and the mole fraction of tin(II) complex is in the following order SnCl42− > SnCl3− > SnCl2(aq) in the 5 M HCl solution [16]. The predominant complex of zinc(II) in 5 M HCl solution is ZnCl42− [17]. The stability constants for the formation of complexes in the HCl solution are shown in Table 3 [18]. Based on the stability constant of these metals, the complexes of silver(I) and nickel(II) are more thermodynamically stable than those of other metals in the solution. In addition, the complexes with lower charge density has a stronger tendency to react with the functional group in the resin than those with higher charge density [19].

Table 3.
The stability constants for the formation of chloro-complexes in HCl solution.
3.2. Purification of Silver(I) by Ion Exchange
In order to investigate the adsorption behavior of the metal ions in the solution, ion exchange experiments were performed. In these experiments, the concentration of the resins was varied from 0.5 g/L to 8 g/L. Figure 1 shows the adsorption behavior of metals into AG 1-X8 from 5 M HCl solution. Tin(II) and zinc(II) were selectively adsorbed into AG 1-X8, while less than 20% of silver(II) was loaded. When AG 1-X8 concentration was higher than 2 g/L, tin(II) was completely adsorbed into the resin. Zn(II) adsorption percentage increased to 60% as resin concentration increased from 0.5 to 8 g/L. Copper(II) and nickel(II) were negligibly adsorbed into AG 1-X8 in these experimental conditions.
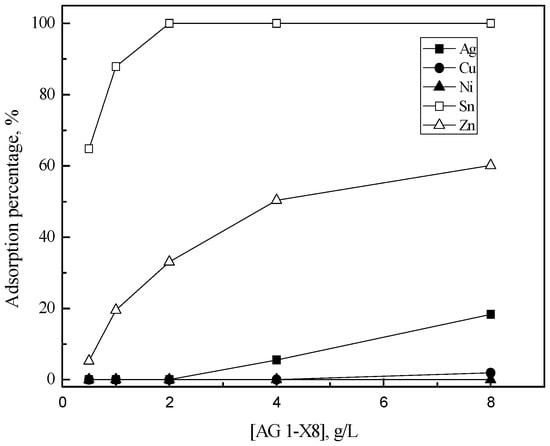
Figure 1.
Effect of AG 1-X8 concentration on the adsorption of metals in 5 M HCl solution. [AG 1-X8] = 0.5–8 (g/L), time = 6 h, shaking speed = 200 rpm.
The chemical structure of AG MP-1M is similar to that of AG 1-X8. This is reflected in Figure 2 which shows the variation of the adsorption percentage of the metals into AG MP-1M. Comparing Figure 1 and Figure 2 indicates that the adsorption behavior of tin(II) and zinc(II) into these two resins was similar, while the adsorption behavior of silver(I) was slightly different. Since AgCl32− is the predominant species of Ag(I) in 5 M HCl solution [21], the adsorption of tin(II), zinc(II) and silver(I) into these two resins might be represented as
where RCl refers to the AG 1-X8 or AG MP-1M and Me refers to tin(II) and zinc(II).
(n − 2)RCl + MeCln2−n = Rn − 2MeCln + (n − 2)Cl−
2RCl + AgCl32− = R2AgCl3 + 2Cl−
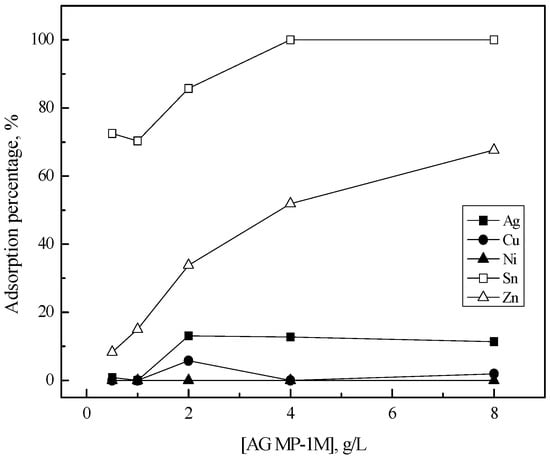
Figure 2.
Effect of AG MP-1M concentration on the adsorption of metals in 5 M HCl solution. [AG MP-1M] = 0.5–8 (g/L), time = 6 h, shaking speed = 200 rpm.
Figure 3 shows the adsorption behavior of the metal ions into Purolite A500. As resin concentration increased from 0.5 to 8 g/L, the adsorption percentage of both tin(II) and zinc(II) increased from 53% to 93% and from 3% to 82%, respectively. However, silver(I), copper(II) and nickel(II) were hardly adsorbed into this resin in these experimental ranges. The adsorption reaction of tin(II) and zinc(II) into Purolite A500 can be represented as
(n − 2) RN(NH3)3Cl + MeCln2−n = [RN(NH3)3]n−2MeCln + (n − 2)Cl−
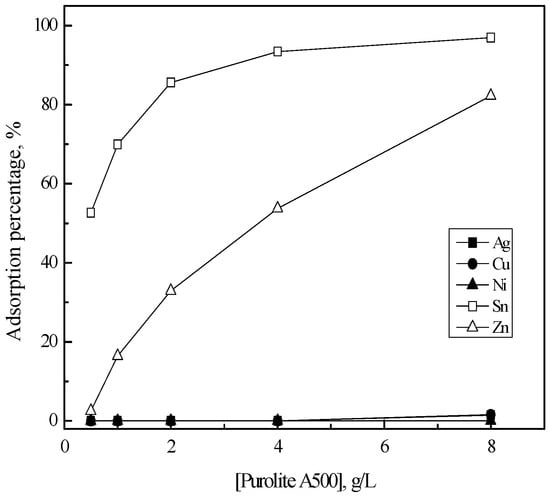
Figure 3.
Effect of Purolite A500 concentration on the adsorption of metals in 5 M HCl solution. [Purolite A500] = 0.5–8 (g/L), time = 6 h, shaking speed = 200 rpm.
Adsorption into Diaion WA21J is shown in Figure 4. As resin concentration increased from 0.5 to 8 g/L, the adsorption percentage of tin(II) and zinc(II) increased almost linearly to 80% and 50%, respectively. However, only 5% of silver(I) was adsorbed into the resin when resin concentration was higher than 2 g/L and most of copper(II) and nickel(II) remained in the solution. Figure 5 shows the adsorption behavior of the metal ions into Lewaitit MP-64. Comparing Figure 4 and Figure 5 indicates that the adsorption behavior of the metal ions into Diaion WA21J and Lewaitit MP-64 was similar to each other. The adsorption percentage of tin(II) and zinc(II) increased from 41% to 92% and from zero to 78% as resin concentration increased from 0.5 to 8 g/L. Less than 5% of silver(I) was adsorbed into Lewaitit MP-64. The adsorption reaction of tin(II) and zinc(II) into Diaion WA21J and Lewaitit MP-64 can be represented as [22].
R4NX + MeClnn−2 = R4NMeCln + X−
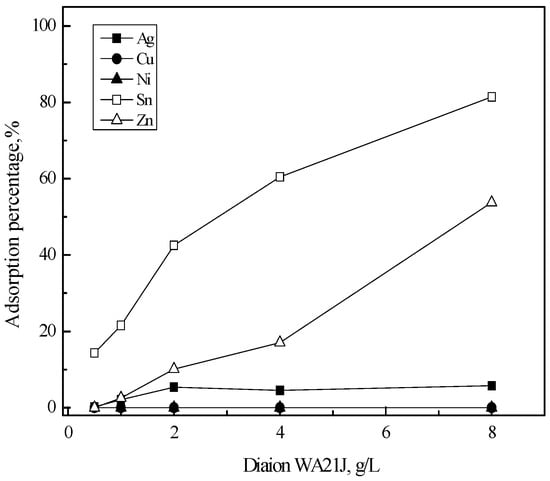
Figure 4.
Effect of Diaion WA21J concentration on the adsorption of metals in 5 M HCl solution. [Diaion WA21J] = 0.5–8 (g/L), time = 6 h, shaking speed = 200 rpm.
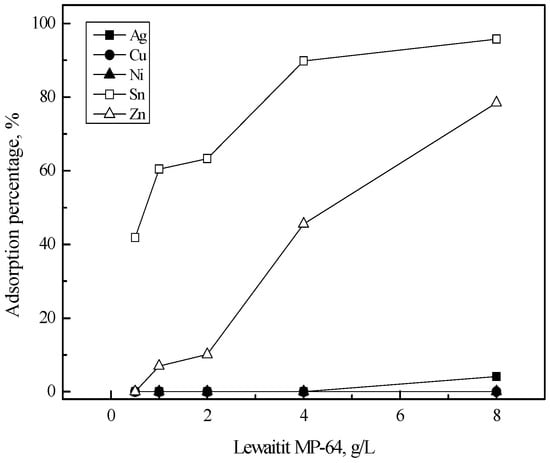
Figure 5.
Effect of Lewaitit MP-64 concentration on the adsorption of metals in 5 M HCl solution. [Lewaitit MP-64] = 0.5–8 (g/L), time = 6 h, shaking speed = 200 rpm.
Figure 6 shows the variation in the adsorption percentage of the metal ions with TEVA resin concentration. Tin(II) was completely adsorbed into TEVA within these experimental ranges. The dependence of zinc(II) adsorption on TEVA concentration can be divided into two regions. When TEVA concentration increased from 0.5 to 2 g/L, the adsorption percentage of zinc(II) increased rapidly and then the increase rate with resin concentration was reduced. Moreover, most of all the Sn(II) could be separated from other metals at 0.5 g/L of TEVA. A small amount of silver(I) was adsorbed when TEVA concentration was 8 g/L, while copper(II) and nickel(II) were not adsorbed at this condition. The adsorption reaction of tin(II) and zinc(II) into TEVA can be represented as [23]
where R3NCH3X refers to TEVA resin and X is Cl− or NO3−, R = C8H17 and C10H21.
R3NCH3X + MeClnn−2 = R3NCH3MeCln + (n − 2) X−
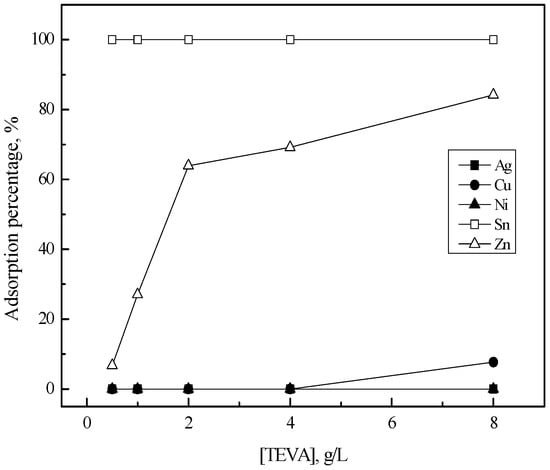
Figure 6.
Effect of TEVA resin concentration on the adsorption of metals in 5 M HCl solution. [TEVA] = 0.5–8 (g/L), time = 6 h, shaking speed = 200 rpm.
From ion exchange experiments, it can be concluded that the anionic resins employed in this work selectively adsorb tin(II) and zinc(II) from the synthetic solution with 5 M HCl. In most of the conditions, a small amount of silver(I) was adsorbed into these resins. Although it may be possible to remove tin(II) and zinc(II) from the solution by ion exchange at high resin concentration, co-adsorption of silver(I) at these conditions makes the separation process complicated. Therefore, it is difficult to separate silver(I) from the solution by ion exchange with the resins employed in this work.
Since there are no nickel(II) anionic complexes in 5 M HCl solution, it is natural that nickel(II) was not at all adsorbed into the resins employed in this work. The selective adsorption of tin(II) and zinc(II) into the resins might be related to the charge density of the complexes of copper(II), silver(I), tin(II), and zinc(II). The charge density of metal chloro-complexes is shown in Table 4. It is indicated that the charge density of metal complexes is in the following order: Ag < Sn < Zn< Cu < Ni. The metal complexes with lower charge density have a stronger tendency to react with the resin than those with higher charge density [19]. Therefore, the adsorption behavior of other metal complexes might follow the above-mentioned order. Namely, tin chloro-complexes could be selectively adsorbed and then the zinc choro-complexes. However, the adsorption percentage of silver(I) was very low in our work and this might be attributed to the characteristics of silver complexes. Silver(I) is classified as soft acid because it has high polarizability and low charge density [24]. Meanwhile, Cu(II) and Zn(II) are considered as borderline acids. Most of tertiary amine is hard base, while chloride ion is borderline base. Table 5 shows the classification of the ions present in the solution according to HSAB (Hard soft acid base). The soft acids are willing to react with soft bases, while hard acids are willing to react with hard bases. Therefore, silver chloro-complexes do not have a strong tendency to react with tertiary amine on the basis of HSAB and thus its adsorption percentage was low in our experiments.

Table 4.
The charge density of metal complexes in this work [25,26].

Table 5.
Classification of ions present in the solution on the basis of hard soft acid base (HSAB) theory.
3.3. Purification of Silver(I) by Cementation with Copper Sheet
According to the ion exchange results in Section 3.2, purification of silver(I) by ion exchange is not easy. The standard reduction potential of the metal ions in the solution is listed in Table 6. Among the metal ions in the synthetic solution, the reduction potential of silver(I) is the highest. Therefore, cementation experiments were done to make utilization of the difference in the standard reduction potential. For this purpose, the copper sheet was employed for the cementation of silver(I) from the synthetic solution with 5 M HCl. The cementation reaction can be described as Equation (8) and parts of copper might react with CuCl42− as represented in Equation (9) [27]. A series of cementation experiments was performed to obtain an optimum condition for silver recovery.
2AgCl2− + Cu(s) = CuCl42− + 2Ag(s)
Cu(s) + CuCl42− = 2CuCl2−

Table 6.
The standard reduction electrode potential of the metal ions present in the solution at 25 °C.
3.3.1. Cementation of Silver(I)
Figure 7 shows the effect of reaction time on the recovery percentage of silver(I) and the amount of copper(II) dissolved during the reaction. The detailed experimental conditions were: 95 °C, stirring speed of 200 rpm and copper sheet with 50 × 12.5 mm2 area. The recovery percentage of silver(I) increased rapidly as reaction time increased to 40 min and then remained constant with the further increase of reaction time to 60 min In cementation experiments, 50 mL of the synthetic solution was employed. In this case, the mass of silver ion in the 50 mL of the solution was about 2 mg. Equation (7) indicates that 0.6 mg of copper is enough to cement 2 mg of silver ion. However, the mass of dissolved copper was around 21 mg, which is much higher than the stoichiometric mass. This might be attributed to the dissolution of oxidized copper in HCl solution [28]. The mass of copper with the volume of 50 × 12.5 × 1 mm3 was around 11.2 g, which was enough for the silver cementation. Although Equation (7) can proceed to completion in terms of thermodynamics, about 88% of silver(I) was cemented at 60 min reaction time. The reason why complete cementation of silver(I) did not occur in these experiments is related to the employment of the copper sheet. In these experiments, the copper sheet with 50 × 12.5 mm2 was employed. When 80% of silver(I) was cemented in these experiments, the mass of cemented silver was 1.6 mg in the case of 50 mL solution. Since the density of silver metal is 10.5 g/cm3, the thickness of silver layer thus cemented on the copper sheet is 2.4 × 10−4 m2. The cemented silver would cover the whole surface of copper sheet employed in the experiments and thus prevent the contact of copper with the silver ions in the solution.
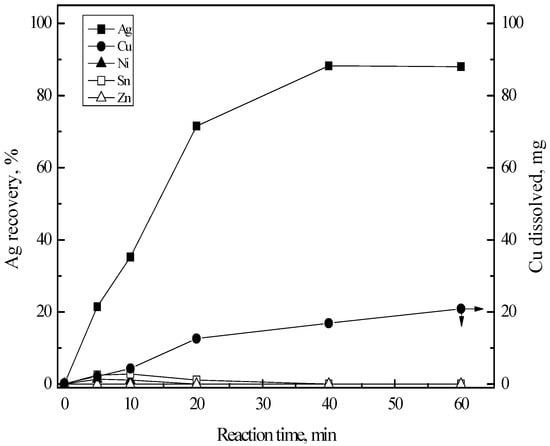
Figure 7.
Effect of reaction time on the Ag recovery by cementation with Cu sheet. Stirring speed = 200 rpm; temperature = 80 °C; time = 0–60 min; copper sheet = 50 × 12.5 mm2.
In cementation, reaction temperature has a significant effect on the cementation kinetics and thus the effect of reaction temperature was investigated. In these experiments, the cementation reactions were done for 60 min at 200 rpm for each reaction temperature. According to Figure 8, the recovery percentage of silver(I) increased linearly with the increase of reaction temperature from 30 to 65 °C and reached 93% at 80 °C and then decreased a little as reaction temperature increased to 95 °C. During the formation of silver film, some base metal ions such as tin(II) and nickel(II) might be incorporated into the rough surface of copper sheet together with cemented silver. Therefore, 80 °C was selected as an optimum temperature.
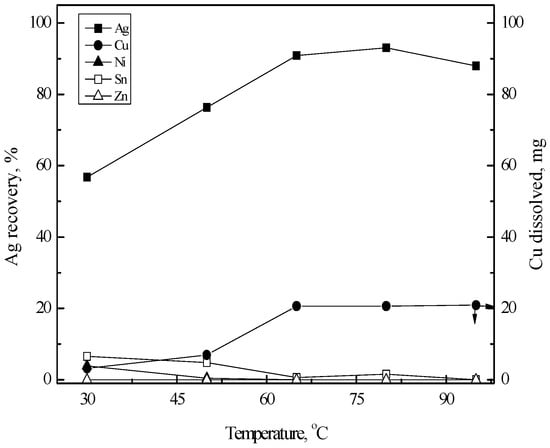
Figure 8.
Effect of temperature on the Ag recovery by cementation with Cu sheet. Stirring speed = 200 rpm; temperature = 30–95 °C; time = 60 min; copper sheet = 50 × 12.5 mm2.
Stirring speed is also one of the important factors in cementation. In order to obtain proper stirring speed, the experiments were carried out by varying stirring speed from zero to 400 rpm. Reaction temperature and time were fixed at 80 °C and 60 min, respectively. Figure 9 shows the effect of stirring speed on the recovery percentage of silver(I). The recovery percentage of silver(I) rapidly increased as the stirring speed increased from zero to 200 rpm. After 200 rpm of stirring speed, the recovery percentage of silver(I) remained constant. These results indicate that the mass transfer step plays a vital role during the cementation of silver(I) with copper sheet, which are in good agreement with the reported results [29].
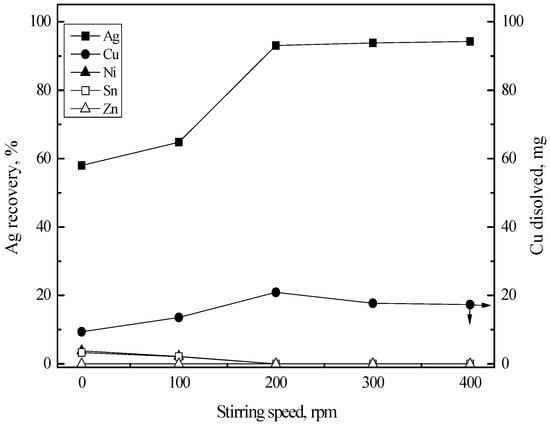
Figure 9.
Effect of stirring speed on the Ag recovery by cementation with Cu sheet. Stirring speed = 0–400 rpm; temperature = 80 °C; time = 60 min; copper sheet = 50 × 12.5 mm2.
The mass of copper sheet might be related to the recovery percentage of silver(I). Therefore, several copper sheets with the same area but different width were employed and the results are shown in Figure 10. As the width of copper sheet increased, the recovery percentage of silver(I) slowly increased. When the width of the copper sheet was 25 mm, around 93% of silver(I) was recovered from the solution.
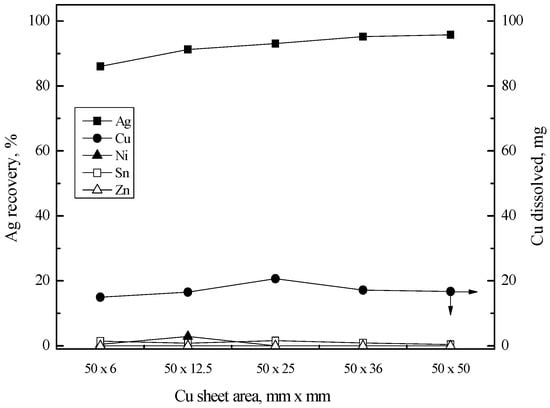
Figure 10.
Effect of Cu sheet area on the Ag recovery by cementation with Cu sheet. Stirring speed = 200 rpm; temperature = 80 °C; time = 60 min; copper sheet = 50 × 6, 50 × 12.5, 50 × 25, 50 × 36; 50 × 50 mm2.
3.3.2. Synthesis of Silver Particles
During the cementation of silver(I) by copper sheet, it is inevitable that trace amount of other metal ions such as copper(II), nickel(II), tin(II) and zinc(II) can be incorporated into the cemented silver. In order to recover the silver metal with high purity, treatment of the cemented silver is necessary. For this purpose, the cemented silver was washed several times by 2% (v/v) hydrochloric acid solution and distilled water. The SEM image of the dried silver powder after washing is shown in Figure 11. The particle size and morphology of the silver metal was not homogeneous. This might be ascribed to the non-smoothness of copper sheet surface, which results in the heterogeneity of the cemented silver [8].

Figure 11.
SEM images of the HCl and water-washed silver particles on the surface of copper sheet. [HCl] = 2% (v/v).
In the filtration and drying, a trace amount of silver powders might form the precipitate of AgCl and be oxidized to Ag2O, which might be incorporated into the cemented silver. Therefore, the cemented silver powders were dissolved into 3 M nitric acid solution to dissolve silver metal and then remove AgCl by filtration. Equation (9) represents the dissolution reaction of Ag2O in HNO3 solution. The purity of silver in the AgNO3 solution thus obtained was 99.99%. Therefore, it can be said that cementation of the synthetic solution with copper sheet followed by HNO3 treatment increased the purity of silver in the solution from 50.9% to 99.99%.
Ag2O + 2HNO3 = 2AgNO3 + H2O
In order to synthesize silver powders with micron size, ascorbic acid was added as a reducing agent to the AgNO3 solution thus obtained and the reduction reaction can be represented as Equation (11) [30,31]. Equation (11) indicates that the reduction of silver ion by ascorbic acid cannot occur in acidic solution. Therefore, solution pH of the AgNO3 solution was adjusted to 6, 8, and 10 by adding ammonia solution. Figure 12 shows the SEM images of the silver powders thus synthesized at three different solution pH values.
C6H8O6 + 2Ag+ = 2Ag0 + C6H8O6 + 2H+
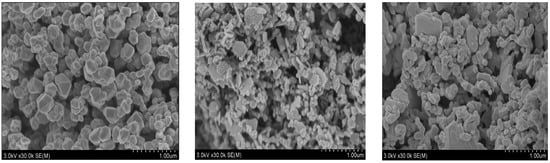
Figure 12.
SEM images of the silver powders reduced by ascorbic acid from the HNO3 solution with several pH (6, 8, 10) at 30 °C.
The shape of the silver particles synthesized at pH 6 consisted of polyhedral crystal blocks and the average diameter of the particles was 2 µm. When solution pH was increased to 8, the shape of the silver particles was changed from polyhedral crystal block into lamellate with the average size lower than 2 µm. As solution pH was further increased to 10, the silver lamellate disappeared and the shape of the particles were changed into cubic and bar with average diameter of 2 µm. Therefore, it can be said that solution pH has a great effect on the shape of the silver particles reduced by ascorbic acid.
3.4. Integrated Proposed Methods
The anode slime resulted from the electro-refining of tin contained gold, silver, copper, nickel, tin and zinc. Leaching of these anode slimes with hydrochloric acid solution in the presence of an oxidizing agent led to the solution containing these metal ions [5]. Solvent extraction with Cyanex 272 followed by LIX 63 separated gold(III) and copper(II) and most of tin(II) [6,7]. From the raffinate after the solvent extraction with the above extractants, silver(I) can be separated by cementation with copper sheet.
Ion exchange with the resins employed in this work could not separate silver(I) from the solution, but only both tin(II) and zinc(II) could be selectively adsorbed into the resins. However, silver(I) cementation with copper sheet was successfully achieved from the solution. Therefore, cementation can be applied to separate silver(I) from the hydrochloric acid solution containing copper(II), nickel(II), tin(II) and zinc(II).
The dissolved Cu(II) during the cementation might be recovered by solvent extraction with LIX 63. In consequence, silver particles can be synthesized by adding ascorbic acid after changing solution pH of the AgNO3 solution.
4. Conclusions
Gold(III), copper(II) and tin(II) can be separated from the hydrochloric acid leaching solution of anode slimes containing nickel(II), silver(I), and zinc(II) by solvent extraction. After the solvent extraction, the raffinate contained nickel(II), silver(I) and zinc(II) together with a small amount of copper(II) and tin(II). Ion exchange and cementation experiments were done in this work to separate silver(I) from this raffinate. In ion exchange with the anionic resins employed in this work, tin(II) and zinc(II) were selectively adsorbed into the resins, while the adsorption percentage of silver(II) was very low. Therefore, it was difficult to separate silver(I) by ion exchange with anionic resins employed in this work. Silver(I) was separated from the solution by cementation with copper sheet. Above 93% of silver was recovered at the optimum condition of 80 °C, 200 rpm and 50 × 25 mm2 copper sheet in 60 min Dissolution of the cemented silver into nitric acid solution indicated that the purity of silver(I) was increased from 50.9% to 99.99% by cementation. Silver powders with micron size were synthesized by reduction of the AgNO3 solution with ascorbic acid at solution pH 6, 8 and 10.
Author Contributions
M.S.L. designed the research and helped to analyze data. W.D.X. performed experiments. S.H.C. discussed the results and made the experimental plan.
Funding
This work was funded by Korea Institute of Energy Technology Evaluation and Planning (KETEP). The grant number is 20165010100810.
Acknowledgments
This work was supported by the Global Excellent Technology Innovation of the Korea Institute of Energy Technology Evaluation and Planning (KETEP), granted financial resource from the Ministry of Trade, Industry & Energy, Republic of Korea (No. 20165010100810).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sulka, G.D.; Jaskuła, M. Effect of sulphuric acid and copper sulphate concentrations on the morphology of silver deposit in the cementation process. Electrochim. Acta 2006, 51, 6111–6119. [Google Scholar] [CrossRef]
- Bodsworth, C. The Extraction and Refining of Metals; CRC Press, Inc.: Boca Raton, FL, USA, 1994. [Google Scholar]
- Kononova, O.; Kuznetsova, M.; Mel’nikov, A.; Karplyakova, N.; Kononov, Y. Sorption recovery of copper (ii) and zinc (ii) from chloride aqueous solutions. J. Serbian Chem. Soc. 2014, 79, 1037–1049. [Google Scholar] [CrossRef]
- Ahmed, E.S.; Fouad, O.A.; El-Midany, A.A.; El-Sabbagh, E.A.; El-Rahman, A.A.; Ibrahim, I.A. Silver nanostructures via cementation on copper: A comparison between experimental data and statistical design model. Surf. Interface Anal. 2010, 42, 730–734. [Google Scholar] [CrossRef]
- Xing, W.; Lee, M. Leaching of gold and silver from anode slime with a mixture of hydrochloric acid and oxidizing agents. Geosyst. Eng. 2017, 20, 216–223. [Google Scholar] [CrossRef]
- Xing, W.; Lee, M.; Senanayake, G. Recovery of metals from chloride leach solutions of anode slime by solvent extraction. Part I: Recovery of gold with cyanex 272. Hydrometallurgy 2018, 180, 58–64. [Google Scholar] [CrossRef]
- Xing, W.D.; Lee, M.S.; Senanayake, G. Recovery of metals from chloride leach solutions of anode slimes by solvent extraction. Part II: Recovery of silver and copper with cyanex 272, lix 63 and alamine 336. Hydrometallurgy 2018, 180, 49–57. [Google Scholar] [CrossRef]
- Kuntyi, O.I.; Zozulya, G.I.; Kurilets, O.G. Silver cementation with magnesium in cyanide solutions. Russ. J. Appl. Chem. 2007, 80, 189–192. [Google Scholar] [CrossRef]
- Timur, S.; Cetinkaya, O.; Erturk, S.; Orhan, G. Investigating silver cementation from nitrate solutions by copper in forced convection systems. Miner. Metall. Process. 2005, 22, 205–210. [Google Scholar]
- Sulka, G.D.; Jaskuła, M. Influence of the sulphuric acid concentration on the kinetics and mechanism of silver ion cementation on copper. Hydrometallurgy 2005, 77, 131–137. [Google Scholar] [CrossRef]
- Grishina, E.P.; Ramenskaya, L.M. Silver cementation on copper in 1-butyl-3-methylimidazolium bromide–silver bromide ionic liquid medium. J. Mol. Liq. 2017, 248, 963–971. [Google Scholar] [CrossRef]
- Lee, H.Y.; Kim, S.G.; Oh, J.K. Cementation behavior of gold and silver onto Zn, Al and Fe powders from acid thiourea solutions. Can. Metall. Q. 2013, 36, 149–155. [Google Scholar]
- Winand, R. Chloride hydrometallurgy. Hydrometallurgy 1991, 27, 285–316. [Google Scholar] [CrossRef]
- Zhao, H.; Chang, J.; Boika, A.; Bard, A.J. Electrochemistry of high concentration copper chloride complexes. Anal. Chem. 2013, 85, 7696–7703. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Nam, S.H. Chemical equilibria of nickel chloride in HCL solution at 25 °C. Bull. Korean Chem. Soc. 2009, 30, 2203–2207. [Google Scholar]
- Lee, M.S.; Ahn, J.G. Separation of Tin(ii) and In(iii) in hydrochloric acid solution by solvent extraction. Korean J. Met. Mater. 2015, 53, 488–494. [Google Scholar]
- Senanayake, G.; Muir, D.M. Competitive solvation and complexation of Cu(i), Cu(ll), Pb(ll), Zn(ll), and Ag(i) in aqueous ethanol, acetonitrile, and dimethylsulfoxide solutions containing chloride ion with applications to hydrometallurgy. Metall. Mater. Trans. B 1990, 21B, 439–448. [Google Scholar] [CrossRef]
- Senanayake, G. Chloride assisted leaching of chalcocite by oxygenated sulphuric acid via Cu(ii)–OH–Cl. Miner. Eng. 2007, 20, 1075–1088. [Google Scholar] [CrossRef]
- Bernardis, F.L.; Grant, R.A.; Sherrington, D.C. A review of methods of separation of the platinum-group metals through their chloro-complexes. React. Funct. Polym. 2005, 65, 205–217. [Google Scholar] [CrossRef]
- Schweitzer, G.K.; Pesterfield, L.L. The Aqueous Chemistry of the Elements; Oxford University Press, Inc.: New York, NY, USA, 2010. [Google Scholar]
- Fritz, J.J. Thermodynamic properties of chloro-complexes of silver chloride in aqueous solution. J. Solut. Chem. 1985, 14, 865–879. [Google Scholar] [CrossRef]
- Alguacil, F.J.; Adeva, P.; Alonso, M. Processing of residual gold(iii) solutions via ion exchange. Gold Bull. 2005, 38, 9–13. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Lee, M.S. Separation of molybdenum (vi) and tungsten (vi) from sulfuric acid solution by ion exchange with teva resin. Sep. Sci. Technol. 2015, 50, 2060–2065. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and soft acids and bases, hsab, part I fundamental principles. J. Chem. Educ. 1968, 45, 581–587. [Google Scholar] [CrossRef]
- Wells, A.F. Structural Inorganic Chemistry, 4th ed.; Oxford University Press: Oxford, UK, 1975; p. 1127. [Google Scholar]
- Canham, G.R.; Overton, T. Descriptive Inorganic Chemistry, 4th ed.; W. H. Freeman and Company: New York, NY, USA, 2006. [Google Scholar]
- Sulka, G.D.; Jaskuła, M. Study of the mechanism of silver ions cementation onto copper from acidic sulphate solutions and the morphology of the silver deposit. Hydrometallurgy 2004, 72, 93–110. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, T.; Fu, G.; He, B.; Huang, Z. Selective recovery of silver from an acidic solution by cementation with copper. J. Cent. South Univ. Technol. 1998, 5, 113–116. [Google Scholar] [CrossRef]
- Puvvada, G.; Tran, T. The cementation of Ag(i) ions from sodium chloride solutions onto a rotating copper disc. Hydrometallurgy 1995, 37, 193–206. [Google Scholar] [CrossRef]
- Malassis, L.; Dreyfus, R.; Murphy, R.J.; Hough, L.A.; Donnio, B.; Murray, C.B. One-step green synthesis of gold and silver nanoparticles with ascorbic acid and their versatile surface post-functionalization. RSC Adv. 2016, 6, 33092–33100. [Google Scholar] [CrossRef]
- Singha, D.; Barman, N.; Sahu, K. A facile synthesis of high optical quality silver nanoparticles by ascorbic acid reduction in reverse micelles at room temperature. J. Coll. Interface Sci. 2014, 413, 37–42. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).