Abstract
Utilizing abundant volcanic rock resources as supplementary cementitious materials is a critical pathway for regional low-carbon construction. However, the high crystallinity of natural volcanic rocks limits their reactivity. This study systematically investigates the regulation mechanisms of Triethanolamine (TEA) and Triisopropanolamine (TIPA) on the hydration kinetics and microstructure of a cement system containing Volcanic Rock Powder (VRP) thermally treated at 700 °C. Dissolution kinetics reveal that both TEA and TIPA inhibit Si release but exhibit distinct structural selectivity in promoting metal ion dissolution: TEA demonstrates superior efficiency in promoting the release of Al and Ca ions due to lower steric hindrance, whereas TIPA exhibits a stronger specific activation capacity for insoluble Fe, which is likely attributed to the electron-donating inductive effect. Macroscopic tests show that TEA at 0.05% dosage significantly improved the 28-day compressive strength by 20.4%, attributed to the synergistic effect of efficient chemical activation and pore structure refinement. In contrast, the stronger surface activity of TIPA introduced substantial detrimental macropores; this deterioration in physical structure severely offset its chemical contributions, leading to slow late-age strength development. The study highlights the critical trade-off between chemical activation and microstructural evolution, confirming that TEA is a more suitable activator than TIPA for the Al/Fe-rich thermally treated VRP.
1. Introduction
In the context of intensifying global climate change and tightening resource and environmental constraints, the construction materials industry is facing significant pressure to undergo a green transition. Portland cement, the most fundamental and widely consumed binder in global infrastructure construction, involves limestone decomposition and fossil fuel combustion during its production. Consequently, it is a primary source of anthropogenic carbon dioxide emissions, accounting for approximately 8% of the global total [1,2,3,4]. To align with the international consensus on carbon emission peaking and carbon neutrality, developing low-carbon, sustainable alternative binders has become a shared pursuit within both academia and industry. The utilization of industrial by-products or natural minerals possessing potential hydraulic or pozzolanic activity as Supplementary Cementitious Materials (SCMs) to partially replace cement clinker is widely recognized as one of the most cost-effective and technically feasible pathways for emission reduction [5,6].
The Xizang region of China, characterized by its unique geographical location and active crustal movement, possesses abundant volcanic rock resources. These resources, predominantly distributed in volcanic clusters such as Makehacuo and Kaerda, are chemically dominated by aluminosilicates, theoretically offering significant potential as alternative cementitious materials [7,8,9,10]. However, the industrial foundation in Xizang region is relatively weak, resulting in a scarcity of high-quality industrial waste residues such as fly ash (FA) and ground granulated blast furnace slag (GGBFS). Transporting these materials over long distances would not only incur high logistics costs but also generate a massive transport-related carbon footprint, which undermines the environmental benefits of using SCMs. Therefore, utilizing local resources to develop and prepare high-performance Volcanic Rock Powder (VRP) is of great strategic significance. This approach addresses the shortage of raw materials for regional engineering construction, reduces project costs, protects the fragile plateau ecosystem, and promotes the low-carbon development of construction materials industry in this area [11].
Despite the superior resource endowment, volcanic rocks in this region (mainly andesite and basalt) face significant technical bottlenecks in practical application. Unlike fly ash glass microspheres formed by rapid quenching, natural volcanic rocks typically undergo a relatively slow cooling process. This results in high crystallinity of internal minerals, where silicon–oxygen and aluminum–oxygen tetrahedra primarily exist in the form of structurally stable crystals such as feldspar and quartz, leading to a relatively low content of reactive vitreous phases [12,13,14]. This high crystallinity results in weak pozzolanic activity of VRP at ambient temperatures. Previous studies indicate that when untreated VRP is directly incorporated as an SCM into cement-based materials, it significantly retards the hydration process, slows early strength development, and increases water demand, thereby severely limiting its application in modern high-volume composite binder systems [15,16].
To overcome this activity bottleneck, effective activation of VRP is imperative. While physical activation (e.g., mechanical grinding) and thermal activation are fundamental methods, they have limitations [16,17,18,19]. Our previous research [20] determined that 700 °C is the optimal thermal activation temperature for VRP. Detailed characterization revealed that at this temperature, the VRP exhibited the highest amorphous phase content and the lowest zeta potential, indicating significant lattice depolymerization. Furthermore, dissolution kinetics showed that the leaching rates of reactive Si and Al peaked at 700 °C, increasing by approximately 70% and 200% respectively compared to the uncalcined sample. This enhanced chemical reactivity was further validated by mechanical testing, where the 700 °C-activated VRP yielded the highest 28-day strength activity index of 78.5%. In contrast, higher temperatures caused the recrystallization of amorphous phases into inert minerals, thereby reducing the potential activity. However, relying solely on physical or thermal activation often involves high energy consumption or hits a ceiling in activity enhancement. Even after treatment at 700 °C, the release rate of active species from VRP may still be insufficient to match the early hydration rate of cement clinker in the early stages. Therefore, introducing chemical activation technology—specifically using small amounts of chemical admixtures to alter the ionic environment of the liquid phase and accelerate the dissolution and depolymerization of SCM particle surfaces—becomes key to further exploiting its reactivity.
Among chemical activators, alkanolamines such as Triethanolamine (TEA) and Triisopropanolamine (TIPA) are notable for their “complexation-promoted dissolution” mechanism. As mature grinding aids and early strength agents, they have accumulated rich application experience in improving the hydration rate and mechanical properties of Portland cement [21,22]. Their hydroxyl (-OH) and amino (-N-) groups form stable water-soluble complexes with metal ions (Al, Fe) on SCM surfaces, increasing the chemical potential gradient and accelerating dissolution [23,24]. However, research on alkanolamines has focused primarily on traditional waste-based SCMs; their effects on thermally activated, high-crystallinity natural VRP remain underexplored. Specifically, for the Fe-rich VRP in Xizang, it is unclear how the steric hindrance and electronic effects arising from the structural differences between TEA and TIPA influence the extraction of Al and Fe. Furthermore, the trade-off between the “positive chemical activation” and the “negative air-entraining effect” typical of these organic surfactants remains to be quantified.
To address these gaps, this study systematically investigates the regulation mechanisms of alkanolamine activators on the thermally activated Xizang VRP-cement composite system. VRP thermally activated at 700 °C for 60 min was selected as the precursor, and the activation efficacy of two typical alkanolamines, TEA and TIPA, was comparatively analyzed at varying dosages. The research focuses on three aspects: (1) Quantifying the release of Si, Al, and Fe in simulated pore solutions to elucidate structure–activity relationships. (2) Evaluating compressive strength development to determine optimal parameters. (3) Utilizing X-ray Diffraction (XRD), Thermogravimetric Analysis (TGA), and Mercury Intrusion Porosimetry (MIP) to analyze phase transformation, reaction degree, and pore structure evolution. This work aims to dissect the competition between chemical activation and physical air-entrainment, providing a theoretical basis for the efficient utilization of volcanic resources in Xizang.
2. Materials and Methods
2.1. Raw Materials
The cement used in this study was P.O 42.5 grade Ordinary Portland Cement (OPC), complying with the Chinese National Standard GB 8076-2008 [25]. It possessed a Brunauer–Emmett–Teller (BET) specific surface area of 1039 m2/kg and a median particle size (d50) of 15.24 µm, as determined by laser particle size analysis.
The VRP originated from the Lhasa region of Xizang, China. The raw ore underwent crushing and mechanical grinding in a planetary ball mill for 15 min (based on the optimized preparation protocol from [20]), followed by thermal treatment in a muffle furnace. The thermal activation protocol involved heating at a rate of 10 °C/min to a target temperature of 700 °C, holding for 60 min, and subsequently cooling to room temperature within the furnace. After thermal activation and grinding, the BET specific surface area of the VRP was determined to be 2651 m2/kg, and the d50 was 25.95 µm. Additionally, the 28-day strength activity index (SAI) of the thermally treated VRP was determined to be 78.5% [20].
The chemical compositions of the cement and VRP, determined by X-ray Fluorescence (XRF), are presented in Table 1.

Table 1.
Chemical composition of cement and VRP.
Analytical-grade Triethanolamine (TEA, ≥99%) and Triisopropanolamine (TIPA, ≥99%), purchased from Macklin Biochemical Co., Ltd. (Shanghai, China), were employed as chemical activators.
2.2. Sample Preparation
A series of mortar and paste mixtures, including a control group and experimental groups with varying activator dosages, were designed (see Table 2). The binder system consisted of 70 wt.% cement and 30 wt.% thermally treated VRP. The water-to-binder ratio (w/b) was fixed at 0.5 for all mixtures. For mortar specimens, the binder-to-sand ratio was set at 1:3 by mass. To prepare the modified specimens, predetermined dosages of TEA or TIPA (0.02%, 0.05%, and 0.08% by mass of the binder, selected based on previous studies [21,22,23,24] and preliminary trials to cover the effective modification range) were pre-dissolved in the mixing water to ensure homogeneous dispersion of the activator within the system.

Table 2.
Mix proportions of mortar and paste mixtures.
The fresh mixtures were cast into 40 mm × 40 mm × 160 mm steel molds and compacted on a vibration table for 60 s to eliminate entrapped air. The mold surfaces were covered with plastic film to prevent moisture evaporation, and the specimens were placed in a standard curing room at 20 ± 1 °C with a relative humidity (RH) greater than 95%. After demolding at 24 h, the specimens were cured in the same environment until the designated testing ages (3, 7, and 28 days).
2.3. Experimental Methods
Dissolution kinetics: To evaluate the ion release behavior of VRP, a 1 mol/L NaOH solution was prepared to serve as a simplified simulated pore solution. Although the authentic liquid phase in cementitious systems is a complex environment with coexisting ions (e.g., K+, Ca2+) and time-varying pH, this simplified alkaline model was adopted to isolate the specific chemical regulation of alkanolamines on VRP dissolution, thereby minimizing the interference of ion precipitation caused by concurrent cement hydration.
For groups containing activators, the corresponding proportion of TEA or TIPA was pre-dissolved in the alkaline solution. 1.00 g of VRP powder was dispersed in 100 mL of the aforementioned solution and placed in a constant-temperature water bath at 40 °C with a magnetic stirring speed of 300 rpm. At reaction intervals of 5, 15, 60, 120, and 300 min, 5 mL of the supernatant was extracted using a syringe and immediately filtered through a 0.22 µm syringe filter to remove solid particles. The filtrate was subsequently acidified and diluted with a 5% (v/v) nitric acid solution to prevent metal ion precipitation. The concentrations of Si, Al, and Ca in the solution were determined using an Inductively Coupled Plasma Optical Emission Spectrometer (ICP-OES, Agilent 5110, Agilent Technologies, Santa Clara, CA, USA), while the Fe concentration was measured using an Inductively Coupled Plasma Mass Spectrometer (ICP-MS, Agilent 7800, Agilent Technologies, CA, USA).
Mechanical strength testing: The compressive strength of the mortar was tested in accordance with the GB/T 17671-2021 standard [26]. At ages of 3, 7, and 28 days, destructive testing was performed using a fully automatic cement pressure testing machine at a loading rate of 2.4 kN/s. Three prismatic specimens were tested for each mix proportion at each age, and the arithmetic mean of six measurements was taken as the final compressive strength result, accurate to 0.1 MPa.
Microstructural characterization: Prior to microscopic analysis, hydration termination was performed on the hardened paste samples. The crushed central portions of the samples were immersed in anhydrous ethanol for 48 h, with the ethanol replaced once during this period, followed by drying to constant weight in a vacuum drying oven at 40 °C. The dried samples were partially ground and passed through a 200-mesh (75 µm) sieve for XRD and TGA testing, while 3–5 mm granules were retained for MIP testing.
X-ray Diffraction (XRD) analysis was conducted using a Bruker D8 Advance (Bruker, Karlsruhe, Germany) equipped with a Cu Kα radiation source, operating at 40 kV and 40 mA. The scanning range (2θ) was 5° to 80°, with a step size of 0.02° and a scanning time of 0.2 s per step.
Thermogravimetric Analysis (TGA) was performed using a Mettler Toledo TGA/DSC 3+ thermal analyzer (Mettler Toledo, Schwerzenbach, Switzerland). Approximately 20 mg of powder sample was placed in an alumina crucible and heated from 30 °C to 800 °C at a rate of 10 °C/min under a high-purity nitrogen flow of 50 mL/min. Based on the TG curve, the decomposition amount of calcium hydroxide (CH) (approximately 400–500 °C) was calculated using the tangent method [24], and the chemically bound water (CW) content was calculated based on the total mass loss, correcting for physical water as proposed in our previous study [20].
Pore structure characteristics were determined using a Micromeritics AutoPore V 9620 mercury porosimeter (Micromeritics Instrument Corp., Norcross, GA, USA). Before testing, block samples were evacuated under low pressure to remove gas from the pores. The test pressure range covered 0.10 psia to 60,000 psia.
3. Results and Discussion
3.1. Dissolution Kinetics and Molecular Mechanism of VRP in Alkaline Environment
The essence of the pozzolanic reaction involves the depolymerization of the aluminosilicate glass network on the surface of active SCMs in an alkaline environment, releasing reactive Si and Al ions, which subsequently react with calcium ions in the liquid phase to form cementitious products. Therefore, the ion dissolution rate is the rate-determining step of the reaction process. In this section, alkaline dissolution tests were conducted to reveal the differential regulation mechanisms of TEA and TIPA on the leaching behavior of Si, Al, Fe, and Ca from thermally treated VRP. The results are presented in Figure 1 and Figure 2.
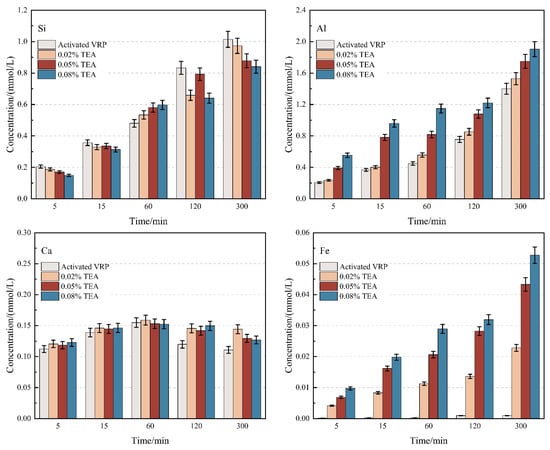
Figure 1.
Effect of TEA on the dissolution behavior of Si, Al, Ca, and Fe from VRP in 1 mol/L NaOH solution.
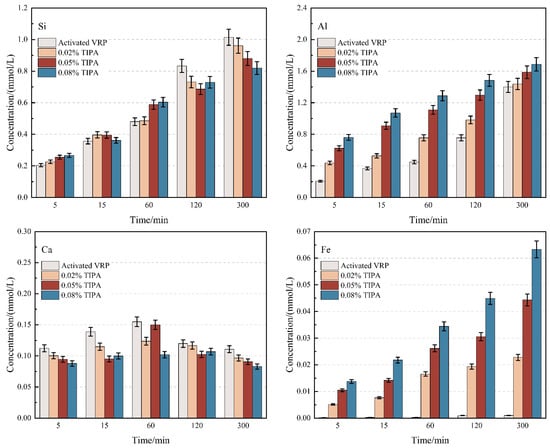
Figure 2.
Effect of TIPA on the dissolution behavior of Si, Al, Ca, and Fe from VRP in 1 mol/L NaOH solution.
Notably, although alkanolamines are generally believed to accelerate mineral dissolution, this study observed a certain inhibitory effect on Si release. At 300 min, the Si concentration in the control group reached 1.01 mmol/L, whereas the addition of 0.08% TEA and TIPA significantly reduced the Si concentration to 0.84 mmol/L and 0.82 mmol/L, respectively. This suppression suggests that alkanolamine molecules may interact physicochemically with the VRP surface. A plausible speculation is that alkanolamine molecules adsorb onto Si-rich sites on the mineral surface, partially hindering the nucleophilic attack of OH− on the Si-O-Si framework. The comparable inhibition levels between the two suggest that this potential surface adsorption mechanism depends primarily on the functional groups rather than the spatial differences in the side chains [21].
In sharp contrast to the inhibition of Si, the dissolution of Al, Ca, and Fe was promoted to varying degrees, demonstrating significant “structural selectivity”. For the main group metals Al and Ca, the promotion efficiency of TEA was markedly superior to that of TIPA. This difference is generally attributed to the steric hindrance effect. Compared to the bulky isopropanol side chains in TIPA, the TEA molecule possesses a more compact structure, which is theoretically more favorable for accessing metal centers and forming stable water-soluble complexes, thus driving the dissolution equilibrium.
However, regarding Fe dissolution, TIPA exhibited a higher activation capacity than TEA. Although iron in the VRP exists primarily as insoluble hematite, the Fe release in the TIPA group remained slightly higher than that in the TEA group. According to coordination chemistry theory, this may be associated with the electron-donating inductive effect of the isopropyl groups in the TIPA molecule [27,28]. This effect likely enhances the electron density of the central nitrogen atom and its affinity for Fe3+, thereby partially offsetting the adverse effects of steric hindrance and maintaining a higher concentration of iron ions in the liquid phase.
To comprehensively evaluate the contribution of different activators to the overall reactivity of VRP, the Leaching Efficiency (LE) index was introduced, defined as the percentage increase in the total amount of eluted elements in the presence of activators relative to the control group. The calculated results are shown in Figure 3. At the same mass dosage, the aggregate LE value of TEA was consistently higher than that of TIPA. For instance, at the high dosage of 0.08%, the LE of TEA reached 28.7%, whereas that of TIPA was only 17.9%. These data strongly demonstrate that for the aluminosilicate-based and Al-rich VRP, TEA exhibits superior overall chemical activation potential due to its more efficient complexation capacity for the dominant aluminum element.
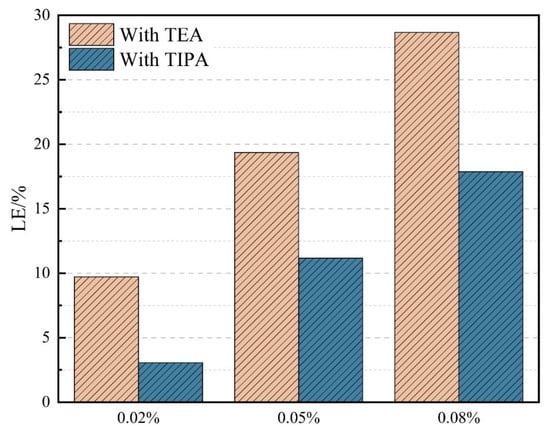
Figure 3.
Effect of TEA and TIPA on the Leaching Efficiency (LE) of VRP.
3.2. Mechanical Property Evolution and Dosage Sensitivity
The evolution of compressive strength following the introduction of activators into the thermally treated VRP–cement composite system provides macroscopic evidence for evaluating the actual efficacy of chemical activation. As illustrated in Figure 4, both TEA and TIPA exhibited significant early-strength enhancement during the early hydration stage (3 days). The addition of 0.05% TEA and TIPA increased the compressive strength by 23.3% and 24.9%, respectively, compared to the control group. This strength improvement at the early stage is not solely dependent on the pozzolanic reaction of VRP but is largely attributed to the catalytic effect of alkanolamines on cement clinker hydration [21,23,24]. They facilitate the rapid dissolution of C3A and C4AF on the clinker surface, accelerating the formation of the AFt skeleton. At this stage, TIPA slightly outperformed TEA in early strength, likely due to its stronger complexation capacity for the iron phase, which more effectively promotes the reaction of the interstitial C4AF phase.
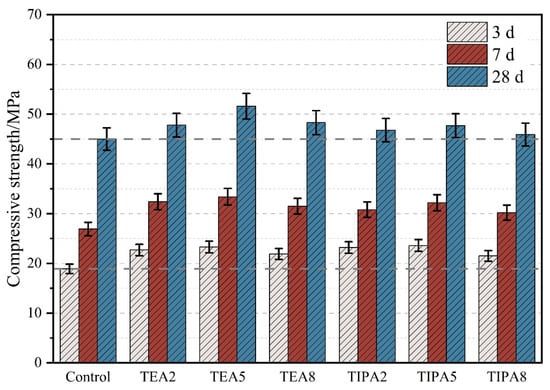
Figure 4.
Effect of TEA and TIPA on the compressive strength of the thermally treated VRP–cement system.
However, as hydration progressed into the later stage (28 days), the performance of the two activators diverged fundamentally. The strength of the TEA group exhibited a parabolic trend with increasing dosage, peaking at 0.05% with a value of 54.2 MPa, representing a significant increase of 20.4% over the control. This indicates that TEA can continuously and effectively stimulate the potential activity of VRP in the later stages, promoting the generation of abundant secondary hydration products that effectively fill the capillary pores of the cement paste, thereby densifying the matrix structure. In contrast, the late-age enhancement of TIPA appeared limited, showing only a 6.0% increase at the same dosage. More notably, TIPA exhibited high “dosage sensitivity”: when the dosage was further increased to 0.08%, the strength not only failed to increase but showed a trend of regression. This substantial disparity in late-age strength implies a fundamental difference in their mechanisms of action: the positive chemical activation effect of TEA predominates in the later stages, whereas TIPA, despite possessing chemical activation capability, is inevitably accompanied by a negative physical effect that intensifies with dosage, severely offsetting the strength gain derived from its chemical activity. This “interplay between positive and negative effects” is the key determinant of the final performance of the composite material.
3.3. Phase Assemblage Transformation and Reaction Degree Quantification
To unravel the chemical nature underlying the differences in strength, qualitative and quantitative analyses of the phase evolution in the hardened pastes were conducted using XRD and TGA techniques. As shown in the XRD patterns in Figure 5, the introduction of TEA and TIPA did not induce the formation of new anomalous hydration products. The primary crystalline phases in the system remained CH, AFt, AFm, and unreacted residual minerals from VRP (such as quartz and albite). However, the relative variations in characteristic peak intensities revealed significant alterations in hydration kinetics.
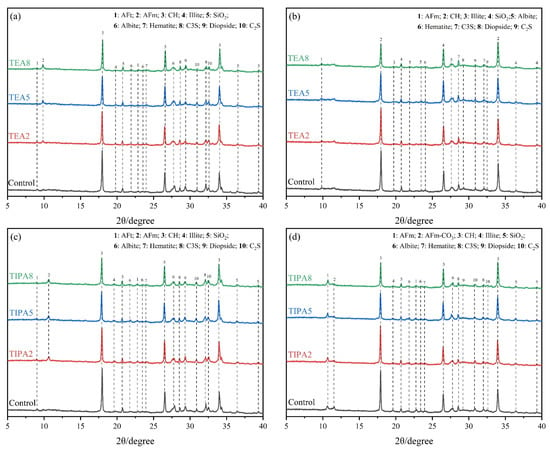
Figure 5.
XRD patterns of the thermally treated VRP–cement system: TEA group at (a) 3 d, (b) 28 d; TIPA group at (c) 3 d, (d) 28 d.
First, regarding aluminate products, compared to the control group, the diffraction peak intensity of AFt in the activator groups slightly decreased at 3 days, while the characteristic peaks of the AFm phase significantly increased. This phenomenon corroborates the results of the dissolution kinetics tests: alkanolamines significantly increased the saturation of Al3+ in the pore solution through complexation. The high concentration of aluminum ions elevated the Al/S ratio of the system, thereby thermodynamically accelerating the transformation of metastable AFt to the more stable AFm.
Second, regarding the CH phase, the intensities of CH characteristic peaks in both TEA and TIPA groups were lower than those of the control group at 28 days, with the TEA group exhibiting the most visually perceptible reduction. Since CH is a byproduct of cement hydration, the reduction in its intensity directly reflects its consumption by the pozzolanic reaction of VRP. Notably, the peak intensities of crystalline phases such as quartz and albite originating from the VRP raw ore remained largely unchanged during the hydration process. This indicates that under the current activation conditions, alkanolamine activators primarily promote the dissolution and reaction of the amorphous aluminosilicate glass in VRP, having a limited destructive effect on the thermodynamically stable crystalline skeleton.
More critical evidence comes from the quantitative analysis of CH consumption at 28 days. To further evaluate the degree of VRP participation in the hydration reaction, thermogravimetric analysis was conducted at 3 and 28 days (original TG/DTG curves are provided in Supplementary Materials), and the content of CH and chemically bound water (CW) within the system was calculated. The results are presented in Figure 6 and Figure 7.
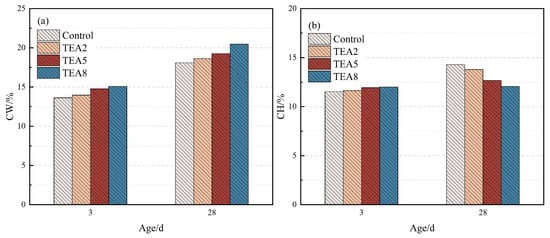
Figure 6.
CW and CH contents of the thermally treated VRP–cement system with TEA: (a) CW, (b) CH.
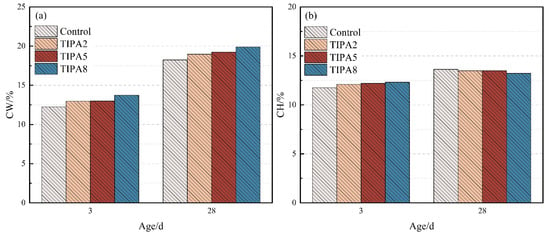
Figure 7.
CW and CH contents of the thermally treated VRP–cement system with TIPA: (a) CW, (b) CH.
CH serves as both a product of cement hydration and a reactant for stimulating the pozzolanic reaction of VRP; thus, a reduction in its content corresponds directly to a deepening of the pozzolanic reaction degree. The TGA data indicate that after incorporating 0.05% TEA, the CH content in the system decreased substantially by 18.0% compared to the control, while the CW, reflecting the total amount of hydration products, increased by 12.5%. The concurrence of substantial CH consumption and CW increase strongly proves that TEA greatly promoted the secondary reaction between the VRP vitreous phase and CH, generating abundant water-rich C-S-H or C-A-S-H gels, which constitutes the chemical basis for the substantial increase in late-age strength.
In contrast, the same dosage of TIPA resulted in only a 6.3% reduction in CH and a 6.2% increase in CW. This result aligns highly with the lower aluminum leaching efficiency of TIPA observed in the aforementioned dissolution kinetics tests, further corroborating that in the complex ionic environment of the cement matrix, the late-age activation capability of TIPA for the aluminosilicate-dominated VRP is indeed weaker than that of TEA due to steric hindrance limitations.
3.4. Microstructural Evolution and Competition Mechanism
The macroscopic mechanical properties of cementitious composites are governed not only by the degree of reaction but more critically by the microstructural pore characteristics [29,30]. MIP results (presented in Figure 8 and Figure 9) revealed substantial differences between TEA and TIPA in modulating the pore structure, providing key microscopic evidence to explain the divergence in their mechanical performance.
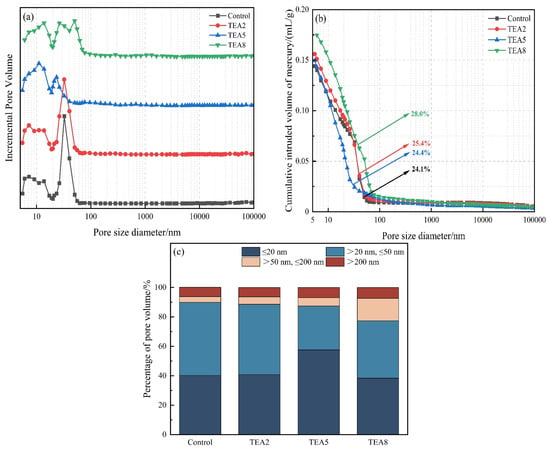
Figure 8.
Pore structure of the thermally treated VRP–cement system with TEA: (a) Incremental pore size distribution; (b) Cumulative pore size distribution; (c) Pore size distribution histogram.
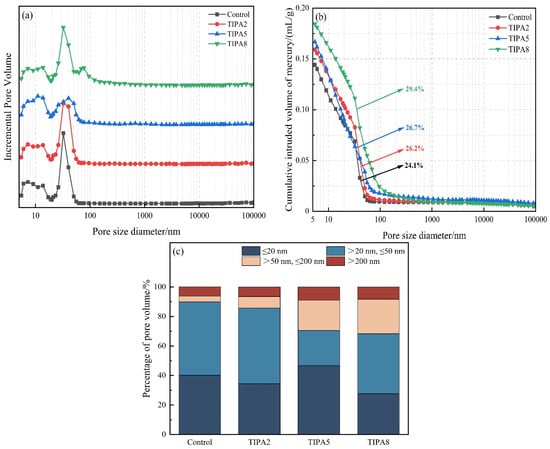
Figure 9.
Pore structure of the thermally treated VRP–cement system with TIPA: (a) Incremental pore size distribution; (b) Cumulative pore size distribution; (c) Pore size distribution histogram.
TEA exhibited a superior pore structure refinement effect at an appropriate dosage (≤0.05%). Its total porosity was comparable to that of the control group, but the pore size distribution curve shifted significantly towards smaller pore diameters, with an increased proportion of gel micropores. This indicates that the pozzolanic reaction products stimulated by TEA effectively filled the originally larger capillary pores, playing a dual role of “grain refinement” and “void filling”.
However, the TIPA group exhibited a distinct trend of pore structure coarsening across all dosages. Particularly at the high dosage of 0.08%, the proportion of detrimental macropores (diameter > 50 nm) increased significantly, and the total porosity surged to 29.4%. To explain this phenomenon, a competitive mechanism model between chemical activation and physical air entrainment is proposed. Alkanolamines serve as organic surfactants with amphiphilic structures; they reduce the surface tension of the mixing water during agitation, inevitably introducing micro-bubbles (air-entraining effect) [31].
TEA possesses a relatively compact molecular structure and higher hydrophilicity, resulting in a comparatively weak air-entraining capacity. Crucially, the potent chemical activation capability of TEA (evidenced by high dissolution efficiency and high CH consumption) generates a substantial quantity of dense gel, which is sufficient to offset and fill the minor defects caused by air bubbles, thereby manifesting macroscopically as an increase in strength.
In contrast, the bulky isopropanol groups in the TIPA molecule impart greater hydrophobicity and surface activity. The significant increase in macropores (>50 nm) observed in MIP results strongly suggests that TIPA induces a more pronounced air-entraining effect compared to TEA. Although TIPA can enhance strength in the early stages through chemical action, at later ages, the limited gel products generated fail to fill the extensive macropore defects likely derived from this intensified physical air-entrainment (a severe negative physical effect). These macropores act as weak points and stress concentration zones within the mechanical structure, dominating the material failure process. This imbalance, where the physical negative effect outweighs the chemical positive effect, constitutes the fundamental reason for the sluggish growth or even regression of late-age strength in the TIPA group.
4. Conclusions
This study systematically investigated the regulation mechanisms of TEA and TIPA on the hydration kinetics, microstructural evolution, and macroscopic mechanical properties of the thermally treated VRP–cement composite binder system. Based on the experimental results and mechanistic analysis, the following main conclusions are drawn.
Both alkanolamines inhibit Si release via surface adsorption but exhibit distinct structural selectivity for metal ions. TEA demonstrates superior efficiency in extracting Al and Ca due to its lower steric hindrance. In contrast, TIPA exhibits a stronger specific activation capacity for insoluble Fe, which is attributed to the electron-donating inductive effect of its isopropyl groups.
TEA at the 0.05% dosage significantly improved the 28-day compressive strength by 20.4%. This enhancement is quantitatively supported by TGA results, which showed an 18.0% reduction in CH content and a 12.5% increase in chemically bound water, confirming that TEA promotes the pozzolanic reaction to form abundant gel products that refine the pore structure.
A critical trade-off was observed between chemical activation and physical air-entrainment. While TIPA is chemically active, its stronger surface activity introduced substantial detrimental macropores (>50 nm), causing the total porosity to surge to 29.4%. This physical deterioration severely offset its chemical contribution to Fe-activation, leading to negligible or negative strength development at later ages.
For the Al-rich and Fe-rich thermally treated VRP, TEA is identified as the superior activator. The selection of alkanolamines must balance the “positive chemical effect” (dissolution) against the “negative physical effect” (air-entraining defects). This study provides a theoretical basis for optimizing chemical admixtures in complex volcanic-based cementitious systems.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pr14010022/s1, Figure S1: TG/DTG curves of the thermally treated VRP–cement system with TEA: (a) 3 d, (b) 28 d. Figure S2: TG/DTG curves of the thermally treated VRP–cement system with TIPA: (a) 3 d, (b) 28 d.
Author Contributions
J.Y.: conceptualization, investigation, methodology, data curation, writing—original draft, writing—review and editing, and supervision. S.W.: supervision, resources, and writing—review and editing. F.M.: formal analysis, data curation, and funding acquisition, and writing—review and editing. Z.S.: investigation, project administration, and data curation. All authors have read and agreed to the published version of the manuscript.
Funding
Supported by the Open Research Fund of Key Laboratory of Water Engineering Materials of Ministry of Water Resources, China Institute of Water Resources and Hydropower Research, Grant No. EMF202610; the National Natural Science Foundation of China, Grant No. 52408284.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author. The data is not publicly available due to continuing research.
Conflicts of Interest
Author Fanyuan Mu was employed by Anhui Conch Group. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Guo, Y.; Luo, L.; Liu, T.; Hao, L.; Li, Y.; Liu, P.; Zhu, T. A review of low-carbon technologies and projects for the global cement industry. J. Environ. Sci. 2024, 136, 682–697. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yi, H.; Tang, X.; Wang, Y.; An, H.; Liu, J. Historical trend and decarbonization pathway of China’s cement industry: A literature review. Sci. Total Environ. 2023, 891, 164580. [Google Scholar] [CrossRef] [PubMed]
- Chaudhury, R.; Sharma, U.; Thapliyal, P.; Singh, L. Low-CO2 emission strategies to achieve net zero target in cement sector. J. Clean. Prod. 2023, 417, 137466. [Google Scholar] [CrossRef]
- Wu, T.; Ng, S.T.; Chen, J. Deciphering the CO2 emissions and emission intensity of cement sector in China through decomposition analysis. J. Clean. Prod. 2022, 352, 131627. [Google Scholar] [CrossRef]
- Amran, M.; Murali, G.; Khalid, N.H.A.; Fediuk, R.; Ozbakkaloglu, T.; Lee, Y.H.; Haruna, S.; Lee, Y.Y. Slag uses in making an ecofriendly and sustainable concrete: A review. Constr. Build. Mater. 2021, 272, 121942. [Google Scholar] [CrossRef]
- Mathapati, M.; Amate, K.; Prasad, C.D.; Jayavardhana, M.; Raju, T.H. A review on fly ash utilization. Mater. Today Proc. 2022, 50, 1535–1540. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, F.; Xu, J.; Zeng, Y.; Wang, B.; Lv, M.; Zhang, L.; Li, M.; Zhang, Z.; Tian, Y. Origin of the volcanic rocks in Dianzhong Formation, central Lhasa Terrane, Tibet: Implication for the genesis of syn-collisional magmatism and Neo-Tethyan slab roll-back. Int. Geol. Rev. 2023, 65, 21–39. [Google Scholar] [CrossRef]
- Lang, X.; Deng, Y.; Wang, X.; Tang, J.; Yin, Q.; Xie, F.; Yang, Z.; Li, Z.; He, Q.; Li, L. Geochronology and geochemistry of volcanic rocks of the Bima Formation, southern Lhasa subterrane, Tibet: Implications for early Neo-Tethyan subduction. Gondwana Res. 2020, 80, 335–349. [Google Scholar] [CrossRef]
- Mao, Z.; Fang, X.; Yang, Y.; Ye, C.; Zhang, T.; Zhang, W.; Zan, J. Geochemical discrimination of the altered volcanic tuff from sediments in the Lunpola Basin, central Tibetan Plateau. Clay Miner. 2020, 55, 303–319. [Google Scholar] [CrossRef]
- Mao, Z.; Fang, X.; Yang, Y.; Ye, C.; Zhang, W.; Zhang, T.; Christidis, G.E. Identification and origin of the Late Oligocene to Miocene pyroclastic rocks in the Lunpola Basin and link with deep geodynamics in the Lhasa terrane, Tibetan Plateau. J. Asian Earth Sci. 2023, 247, 105575. [Google Scholar] [CrossRef]
- Özkan, Ş.; Ceylan, H. The effects on mechanical properties of sustainable use of waste andesite dust as a partial substitution of cement in cementitious composites. J. Build. Eng. 2022, 58, 104959. [Google Scholar] [CrossRef]
- Tran, Q.; Ghosh, P. Influence of pumice on mechanical properties and durability of high performance concrete. Constr. Build. Mater. 2020, 249, 118741. [Google Scholar] [CrossRef]
- Tomoyose, A.; Noguchi, T.; Sodeyama, K.; Higashi, K. Concrete with high-purity volcanic glass fine powder manufactured from pyroclastic deposit. SN Appl. Sci. 2020, 2, 851. [Google Scholar] [CrossRef]
- Zeyad, A.M.; Khan, A.H.; Tayeh, B.A. Durability and strength characteristics of high-strength concrete incorporated with volcanic pumice powder and polypropylene fibers. J. Mater. Res. Technol. 2020, 9, 806–818. [Google Scholar] [CrossRef]
- Khan, K.; Amin, M.N.; Saleem, M.U.; Qureshi, H.J.; Al-Faiad, M.A.; Qadir, M.G. Effect of fineness of basaltic volcanic ash on pozzolanic reactivity, ASR expansion and drying shrinkage of blended cement mortars. Materials 2019, 12, 2603. [Google Scholar] [CrossRef]
- Tang, H.; Xu, L.; Xue, K.; Tian, J.; Yang, J.; Wang, D.; Shu, K.; Zuo, A.; Wu, H. Effect of mechanical activation on hydration properties of natural volcanic rock towards partially replacing cement in composite cementitious materials. J. Build. Eng. 2024, 83, 108427. [Google Scholar] [CrossRef]
- Yang, S.; Wu, Y.; Wang, H.; Yang, G.; Ding, X.; Xia, Z. Activity enhancement study of Xinjiang silica-alumina volcanic rock powder through different activation processes. Appl. Sci. 2024, 14, 7935. [Google Scholar] [CrossRef]
- Lei, S.; Gao, H.; Feng, R.; Dai, H.; Bernardo, E.; Zhang, H.; Cheng, Z.; Zhang, X.; Deng, M.; Li, P. Effect of calcination temperature on the activity of basalt tailings for the lightweight geopolymer from microwave curing. Mater. Chem. Phys. 2024, 328, 129991. [Google Scholar] [CrossRef]
- Baki, V.A.; Ke, X.; Heath, A.; Calabria-Holley, J.; Terzi, C.; Sirin, M. The impact of mechanochemical activation on the physicochemical properties and pozzolanic reactivity of kaolinite, muscovite and montmorillonite. Cem. Concr. Res. 2022, 162, 106962. [Google Scholar] [CrossRef]
- Mu, F.; Zou, S.; Sun, Z.; Yang, J. Evaluation of the pozzolanic reactivity of volcanic rock powder at different calcination temperatures and the performance of sodium silicate-activated volcanic rock powder geopolymer. Constr. Build. Mater. 2025, 475, 141257. [Google Scholar] [CrossRef]
- Jiang, J.; Ye, Z.; Wu, J.; Yang, Q.; Li, Q.; Kong, X. Impact of triethanolamine on the hydration of Portland cement in the presence of high pozzolanic activity supplementary cementitious materials. Cem. Concr. Compos. 2024, 147, 105435. [Google Scholar] [CrossRef]
- Huang, H.; Wang, Q.; Li, X.; Zhou, H.; Yang, C.-h.; Shen, X.-d. Sulfate adjustment for cement with triisopropanolamine: Mechanism of early strength enhancement. Constr. Build. Mater. 2018, 182, 516–522. [Google Scholar] [CrossRef]
- Zhang, T.; Sun, Z.; Yang, H.; Ji, Y.; Yan, Z. Enhancement of triisopropanolamine on the compressive strength development of cement paste incorporated with high content of wasted clay brick powder and its working mechanism. Constr. Build. Mater. 2021, 302, 124052. [Google Scholar] [CrossRef]
- Zhang, T.; Ma, B.; Tan, H.; Liu, X.; Chen, P.; Luo, Z. Effect of TIPA on mechanical properties and hydration properties of cement-lithium slag system. J. Environ. Manag. 2020, 276, 111274. [Google Scholar] [CrossRef]
- GB 8076-2008; Concrete Admixtures. Standards Press of China: Beijing, China, 2008.
- GB/T 17671-2021; Test Method of Cement Mortar Strength (ISO Method). Standards Press of China: Beijing, China, 2021.
- Baral, A.; Pesce, C.; Zhakiyeva, Z.; Snellings, R.; Hanein, T.; Provis, J.L.; Peys, A. Characterisation of iron-rich cementitious materials. Cem. Concr. Res. 2024, 177, 107419. [Google Scholar] [CrossRef]
- Li, J.; Zeng, T.; Chang, J. Microstructure of Fe(OH)3 phase in hydration products of calcium sulfoaluminate cement. Constr. Build. Mater. 2023, 400, 132609. [Google Scholar] [CrossRef]
- Yang, J.; Sun, Z.; De Belie, N.; Snoeck, D. Self-healing ability of cracks in alkali-activated slag systems incorporating superabsorbent polymers. Cem. Concr. Res. 2023, 170, 107183. [Google Scholar] [CrossRef]
- Guo, A.; Mu, F.; Zhang, T.; Wu, J.; Sun, Z.; Yang, J. Monitoring Early-Stage Evolution of Free Water Content in Alkali-Activated Slag Systems by Using 1H Low-Field NMR. Buildings 2024, 14, 3079. [Google Scholar] [CrossRef]
- Li, P.; Li, W.; Sun, Z.; Shen, L.; Sheng, D. Development of sustainable concrete incorporating seawater: A critical review on cement hydration, microstructure and mechanical strength. Cem. Concr. Compos. 2021, 121, 104100. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.