Biological Properties and Phenolic Characterization of MetabolAid®: Combination of Plant-Derivate Compound Extracts
Abstract
1. Introduction
2. Materials and Methods
2.1. Extract Preparation
2.2. Phytochemical Characterization
2.3. In Vitro Antioxidant Activity Assays
2.4. UHPLC-ESI-MS/MS Analysis
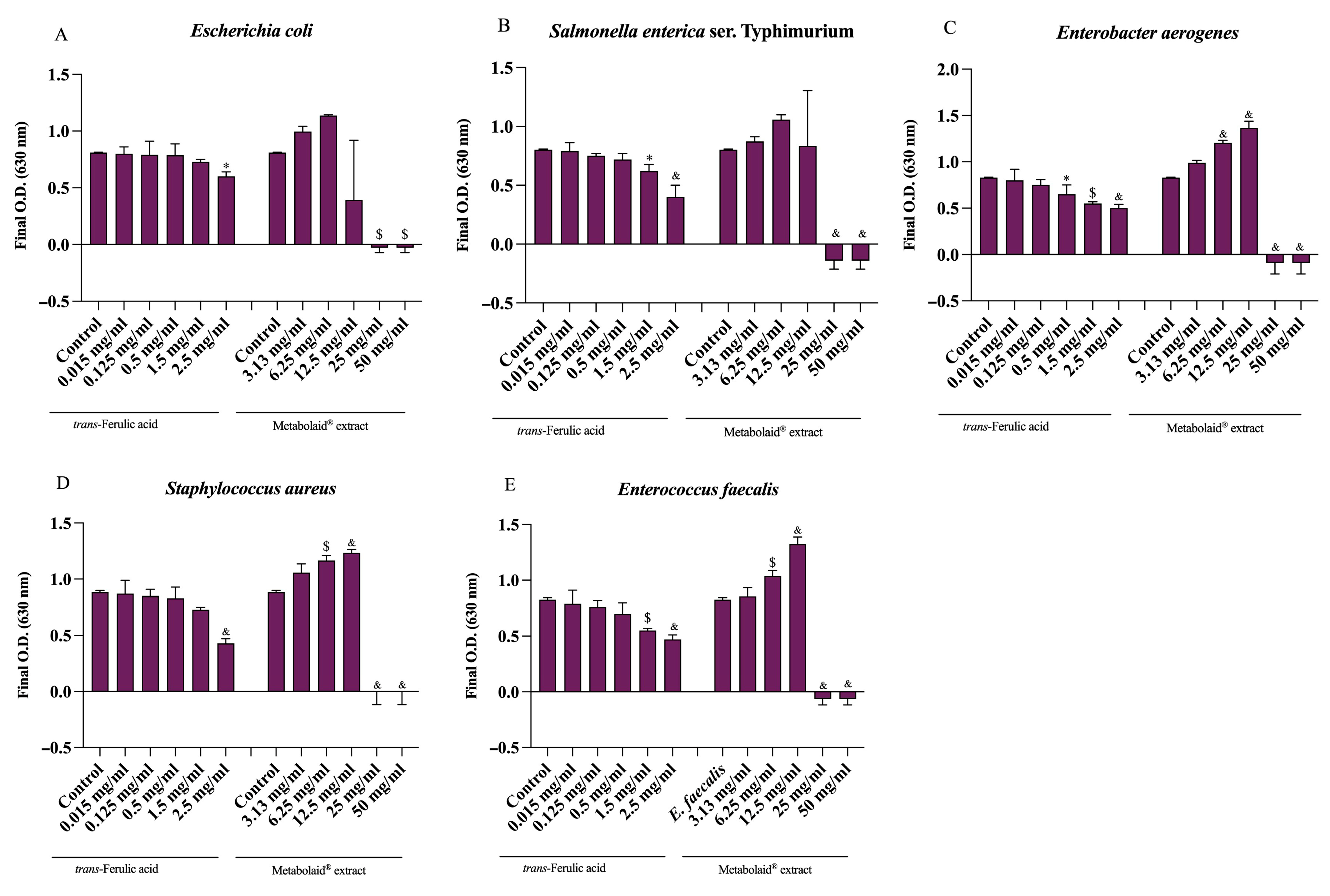
2.5. Antimicrobial Activity
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| GAE | Gallic acid equivalents |
| DM | Dry matter |
| C3GE | Cyanidin-3-glucoside equivalents |
| FRAP | Ferric reducing antioxidant power |
| ORAC | Oxygen radical absorbance capacity |
| MIC | Minimum inhibitory concentration |
| MBC | Minimum bactericidal concentration |
| NF-κB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| PPAR | Peroxisome Proliferator-Activated Receptors |
| AMPK | AMP-activated Protein Kinase |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl assay |
| HCL | Hydrochloric acid |
| APPH | 2,2′-Azobis(2-methylpropionamidine) dihydrochloride |
References
- Olszowy, M. What is responsible for antioxidant properties of polyphenolic compounds from plants? Plant Physiol. Biochem. 2019, 144, 135–143. [Google Scholar] [CrossRef]
- Yahia, Y.; Benabderrahim, M.A.; Tlili, N.; Bagues, M.; Nagaz, K. Bioactive compounds, antioxidant and antimicrobial activities of extracts from different plant parts of two Ziziphus Mill. species. PLoS ONE 2020, 15, e0232599. [Google Scholar] [CrossRef] [PubMed]
- Dikpınar, T.; Süzgeç-Selçuk, S. Antimicrobial activities of medicinal plants containing phenolic compounds. J. Nat. Prod. 2020, 10, 514–534. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Antimicrobial potential of pomegranate peel: A review. Int. J. Food Sci. Technol. 2019, 54, 959–965. [Google Scholar] [CrossRef]
- Lopez-Corona, A.V.; Valencia-Espinosa, I.; González-Sánchez, F.A.; Sánchez-López, A.L.; Garcia-Amezquita, L.E.; Garcia-Varela, R. Antioxidant, Anti-Inflammatory and Cytotoxic Activity of Phenolic Compound Family Extracted from Raspberries (Rubus idaeus): A General Review. Antioxidants 2022, 11, 1192. [Google Scholar] [CrossRef]
- Calderón-Pérez, L.; Llauradó, E.; Companys, J.; Pla-Pagà, L.; Pedret, A.; Rubió, L.; Gosalbes, M.J.; Yuste, S.; Solà, R.; Valls, R.M. Interplay between dietary phenolic compound intake and the human gut microbiome in hypertension: A cross-sectional study. Food Chem. 2021, 344, 128567. [Google Scholar] [CrossRef]
- Perveen, S.; Al-Taweel, A. (Eds.) Pharmacognosy—Medicinal Plants; InthechOpen: London, UK, 2019; pp. 59–72. [Google Scholar]
- World Health Organization (WHO). Traditional medicine: Report of a WHO Expert Committee. WHO Tech. Rep. Ser. 1976, 622, 1–49. Available online: https://apps.who.int/iris/handle/10665/40995 (accessed on 4 June 2025).
- Mickymaray, S. Efficacy and mechanism of traditional medicinal plants and bioactive compounds against clinically important pathogens. Antibiotics 2019, 8, 257. [Google Scholar] [CrossRef]
- Sofowora, A.; Ogunbodede, E.; Onayade, A. The role and place of medicinal plants in the strategies for disease prevention. African Journal of Traditional, Complementary and Alternative Medicines 2013, 10, 210–229. [Google Scholar] [CrossRef]
- Kazemipoor, M.; Hamzah, S.; Hajifaraji, M.; Radzi, C.W.; Cordell, G.A. Slimming and appetite-suppressing effects of caraway aqueous extract as a natural therapy in physically active women. Phytother. Res. 2016, 30, 981–987. [Google Scholar] [CrossRef]
- Duque-Soto, C.; Expósito-Almellón, X.; García, P.; Pando, M.E.; Borrás-Linares, I.; Lozano-Sánchez, J. Extraction, characterization, and bioactivity of phenolic compounds—A case on Hibiscus genera. Foods 2023, 12, 963. [Google Scholar] [CrossRef]
- Ojulari, O.V.; Lee, S.G.; Nam, J.O. Beneficial effects of natural bioactive compounds from Hibiscus sabdariffa L. on obesity. Molecules 2019, 24, 210. [Google Scholar] [CrossRef]
- Takada, K.; Nakano, S.; Nishio, R.; Muku, D.; Mochizuki, S.; Inui, I.; Okita, K.; Koga, A.; Watanabe, K.; Yoshioka, Y.; et al. Medicinal herbs, especially Hibiscus sabdariffa, inhibit oral pathogenic bacteria. J. Oral Biosci. 2024, 66, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, E.M. Antibacterial activity of Hibiscus sabdariffa L. calyces against hospital isolates of multidrug resistant Acinetobacter baumannii. J. Acute Dis. 2016, 5, 512–516. [Google Scholar] [CrossRef]
- Newall, C.A.; Anderson, L.A.; Phillipson, J.D. Herbal Medicines: A Guide for Healthcare Professionals; Pharmaceutical Press: London, UK, 1996; p. 296. [Google Scholar]
- Jamila, F.; Mostafa, E. Ethnobotanical survey of medicinal plants used by people in oriental Morocco to manage various ailments. J. Ethnopharmacol. 2014, 154, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Lenoir, L.; Rossary, A.; Joubert-Zakeyh, J.; Vergnaud-Gauduchon, J.; Farges, M.C.; Fraisse, D.; Texier, O.; Lamaison, J.-L.; Vasson, M.P.; Felgines, C. Lemon verbena infusion consumption attenuates oxidative stress in dextran sulfate sodium-induced colitis in the rat. Dig. Dis. Sci. 2011, 56, 3534–3545. [Google Scholar] [CrossRef]
- Herranz-López, M.; Barrajón-Catalán, E.; Segura-Carretero, A.; Menéndez, J.A.; Joven, J.; Micol, V. Lemon verbena (Lippia citriodora) polyphenols alleviate obesity-related disturbances in hypertrophic adipocytes through AMPK-dependent mechanisms. Phytomedicine 2015, 22, 605–614. [Google Scholar] [CrossRef]
- Alipieva, K.; Korkina, L.; Orhan, I.E.; Georgiev, M. Verbascoside—A review of its occurrence, (bio)synthesis and pharmacological significance. Biotechnol. Adv. 2014, 32, 1065–1076. [Google Scholar] [CrossRef]
- Rossi, R.; Mainardi, E.; Vizzarri, F.; Corino, C. Verbascoside-rich plant extracts in animal nutrition. Antioxidants 2024, 13, 39. [Google Scholar] [CrossRef]
- Carrera-Quintanar, L.; Funes, L.; Vicente-Salar, N.; Blasco-Lafarga, C.; Pons, A.; Micol, V.; Roche, E. Effect of polyphenol supplements on redox status of blood cells: A randomized controlled exercise training trial. Eur. J. Nutr. 2015, 54, 1081–1093. [Google Scholar] [CrossRef]
- Funes, L.; Carrera-Quintanar, L.; Cerdán-Calero, M.; Ferrer, M.D.; Drobnic, F.; Pons, A.; Roche, E.; Micol, V. Effect of lemon verbena supplementation on muscular damage markers, proinflammatory cytokine release and neutrophil oxidative stress in chronic exercise. Eur. J. Appl. Physiol. 2011, 111, 695–705. [Google Scholar] [CrossRef]
- Quirantes-Piné, R.; Herranz-López, M.; Funes, L.; Borrás-Linares, I.; Micol, V.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Phenylpropanoids and their metabolites are the major compounds responsible for blood-cell protection against oxidative stress after administration of Lippia citriodora in rats. Phytomedicine 2013, 20, 1112–1118. [Google Scholar] [CrossRef]
- Funes, L.; Fernández-Arroyo, S.; Laporta, O.; Pons, A.; Roche, E.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Micol, V. Correlation between plasma antioxidant capacity and verbascoside levels in rats after oral administration of lemon verbena extract. Food Chem. 2009, 117, 589–598. [Google Scholar] [CrossRef]
- Kang, J.S.; Lee, W.K.; Lee, C.W.; Yoon, W.K.; Kim, N.; Park, S.K.; Lee, H.S.; Park, H.K.; Han, S.B.; Yun, J.; et al. Improvement of high-fat diet-induced obesity by a mixture of red grape extract, soy isoflavone and L-carnitine: Implications in cardiovascular and non-alcoholic fatty liver diseases. Food Chem. Toxicol. 2011, 49, 2453–2458. [Google Scholar] [CrossRef] [PubMed]
- Walton, R.J.; Whitten, D.L.; Hawrelak, J.A. The efficacy of Hibiscus sabdariffa (rosella) in essential hypertension: A systematic review of clinical trials. Aust. J. Herb. Med. 2016, 28, 48–51. [Google Scholar]
- Eddouks, M.; Maghrani, M.; Lemhadri, A.; Ouahidi, M.-L.; Jouad, H. Ethnopharmacological survey of medicinal plants used for the treatment of diabetes mellitus, hypertension and cardiac diseases in the south-east region of Morocco (Tafilalet). J. Ethnopharmacol. 2002, 82, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Serban, C.; Sahebkar, A.; Ursoniu, S.; Andrica, F.; Banach, M. Effect of sour tea (Hibiscus sabdariffa L.) on arterial hypertension: A systematic review and meta-analysis of randomized controlled trials. J. Hypertens. 2015, 33, 1119–1127. [Google Scholar] [CrossRef]
- Guardiola, S.; Mach, N. Therapeutic potential of Hibiscus sabdariffa: A review of the scientific evidence. Endocrinol. Y Nutr. Órgano De La Soc. Española De Endocrinol. Y Nutr. 2014, 61, 274–295. [Google Scholar] [CrossRef]
- Jiang, F.; Zhang, Y.; Dusting, G.J. NADPH oxidase-mediated redox signaling: Roles in cellular stress response, stress tolerance, and tissue repair. Pharmacol. Rev. 2011, 63, 218–242. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar] [PubMed]
- Boudjou, S.; Oomah, B.D.; Zaidi, F.; Hosseinian, F. Phenolics content and antioxidant and anti-inflammatory activities of legume fractions. Food Chem. 2013, 138, 1543–1550. [Google Scholar] [CrossRef] [PubMed]
- Gabriele, M.; Parri, E.; Felicioli, A.; Sagona, S.; Pozzo, L.; Biondi, C.; Domenici, V.; Pucci, L. The antioxidant capacity of honey varies according to the origin of the flowers used in its production. Food Chem. 2015, 174, 637–643. [Google Scholar]
- Pozzo, L.; Russo, R.; Frassinetti, S.; Vizzarri, F.; Árvay, J.; Vornoli, A.; Casamassima, D.; Palazzo, M.; Croce, C.M.D.; Longo, V. Wild Italian Prunus spinosa L. fruit exerts in vitro antimicrobial activity and protects against in vitro and in vivo oxidative stress. Foods 2020, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Adamez, J.; Fernandez-Leon, M.F.; Velardo-Micharet, B.; Gonzalez-Gomez, D. In vitro assays of the antibacterial and antioxidant activity of aqueous leaf extracts from different Prunus salicina Lindl. Cultivars. Food Chem. Toxicol. 2012, 50, 2481–2486. [Google Scholar] [CrossRef]
- Baliyan, S.; Mukherjee, R.; Priyadarshini, A.; Vibhuti, A.; Gupta, A.; Pandey, R.P.; Chang, C.M. Determination of antioxidants by DPPH radical scavenging activity and quantitative phytochemical analysis of Ficus religiosa. Molecules 2022, 27, 1326. [Google Scholar] [CrossRef]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef]
- Dudonné, S.; Vitrac, X.; Coutière, P.; Woillez, M.; Mérillon, J.M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- Ou, B.; Huang, D.; Hampsch-Woodill, M.; Flanagan, J.A.; Deemer, E.K. Analysis of Antioxidant Activities of Common Vegetables Employing Oxygen Radical Absorbance Capacity (ORAC) and Ferric Reducing Antioxidant Power (FRAP) Assays: A Comparative Study. J. Agric. Food Chem. 2002, 50, 3122–3128. [Google Scholar] [CrossRef]
- Lone, A.S.; Ravindran, K.C.; Jeandet, P. Evaluation of antimicrobial activity and bioactive compound analysis of Verbascum thapsus L.: A folklore medicinal plant. Phytomedicine Plus 2024, 4, 100560. [Google Scholar] [CrossRef]
- Puupponen-Pimiä, R.; Nohynek, L.; Meier, C.; Kähkönen, M.; Heinonen, M.; Hopia, A.; Oksman-Caldentey, K.-M. Antimicrobial properties of phenolic compounds from berries. J. Appl. Microbiol. 2001, 90, 494–507. [Google Scholar] [CrossRef]
| Value | ||
|---|---|---|
| Antioxidant activity | FRAP (mg TE/g) | 0.32 ± 0.06 |
| DPPH (mg TE/g) | 1.32 ± 0.05 | |
| ORAC (mg TE/g) | 474.13 ± 7.59 | |
| Bioactive compounds | Total phenols | 256.10 ± 2.26 |
| Total flavonoids | 48.90 ± 2.95 | |
| Total flavonols | 60.17 ± 7.68 | |
| Anthocyanins | 3.78 ± 0.17 |
| Compound Name | Concentration (µg/100 g) | Concentration (%) |
|---|---|---|
| 3-O-Caffeoylquinic acid (Chlorogenic acid) | 27.63 ± 2.37 | 75.08 |
| Gallic acid | 2.81 ± 0.07 | 7.64 |
| Caffeic acid | 2.81 ± 0.07 | 7.64 |
| p-Coumaric acid | 1.10 ± 0.08 | 2.99 |
| Vanillic acid | 0.89 ± 0.06 | 2.42 |
| trans-Ferulic acid | 0.88 ± 0.06 | 2.39 |
| Rosmarinic acid | 0.68 ± 0.08 | 1.85 |
| 1,3-Dicaffeoylquinic acid (Cynarin) | 0.01 ± 0.00 | 0.03 |
| ∑ Phenolic acids | 36.80 | 100 |
| Quercetin | 6.33 ± 0.05 | 39.74 |
| Quercetin 3-O-glucoside | 0.08 ± 0.03 | 0.50 |
| Quercetin 3-O-rutinoside (Rutin) | 7.15 ± 0.12 | 44.88 |
| Quercetin 3,4-O-diglucoside | 1.09 ± 0.10 | 6.84 |
| Kaempferol 7-O-glucoside | 0.07 ± 0.01 | 0.44 |
| Kaempferol 3-O-glucoside | 0.78 ± 0.08 | 4.90 |
| Kaempferol 3-O-rutinoside | 0.43 ± 0.04 | 2.70 |
| ∑ Flavonols | 15.93 | 100 |
| Cyanidin 3-O-glucoside (Kuromanin) | 7.26 ± 0.30 | 37.75 |
| Cyanidin 3,5-O-diglucoside (Cyanin) | 0.02 ± 0.00 | 0.10 |
| Delphinidin 3,5-O-diglucoside (Delphin) | 11.82 ± 0.09 | 61.47 |
| Malvidin 3-O-glucoside (Oenin) | 0.01 ± 0.01 | 0.05 |
| Petunidin 3-O-glucoside | 0.11 ± 0.03 | 0.57 |
| ∑ Anthocyanins | 19.23 | 100 |
| Apigenin | 0.46 ± 0.04 | 95.83 |
| (+)-Catechin | 0.01 ± 0.01 | 2.08 |
| (−)-Epicatechin | 0.01 ± 0.00 | 2.08 |
| ∑ Flavan-3-ols | 0.48 | 100 |
| Verbascoside | 233.40 ± 20.38 | 98.93 |
| Luteolin | 0.83 ± 0.09 | 0.35 |
| Hydroxytyrosol | 0.56 ± 0.06 | 0.24 |
| Phloretin | 0.46 ± 0.04 | 0.19 |
| Naringenin | 0.30 ± 0.08 | 0.13 |
| Oleuropein | 0.24 ± 0.05 | 0.10 |
| Ligstroside | 0.13 ± 0.02 | 0.5 |
| Phloridzin | 0.02 ± 0.01 | 0.01 |
| Resveratrol | 0.02 ± 0.01 | 0.01 |
| ∑ Others | 235.92 | 100 |
| Bacterial Strains | MIC * (mg/mL) | MBC * (mg/mL) | |
|---|---|---|---|
| Escherichia coli | ATCC 25922 | 12.5 | 25 |
| Salmonella enterica ser. Typhimurium | ATCC 14028 | 25 | 50 |
| Enterobacter aerogenes | ATCC 13048 | 12.5 | 25 |
| Enterococcus faecalis | ATCC 29212 | 12.5 | 25 |
| Staphylococcus aureus | ATCC 25923 | 25 | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vizzarri, F.; Kisova, A.; Spevakova, I.; Raffaelli, A.; Longo, V.; Pozzo, L. Biological Properties and Phenolic Characterization of MetabolAid®: Combination of Plant-Derivate Compound Extracts. Processes 2025, 13, 2405. https://doi.org/10.3390/pr13082405
Vizzarri F, Kisova A, Spevakova I, Raffaelli A, Longo V, Pozzo L. Biological Properties and Phenolic Characterization of MetabolAid®: Combination of Plant-Derivate Compound Extracts. Processes. 2025; 13(8):2405. https://doi.org/10.3390/pr13082405
Chicago/Turabian StyleVizzarri, Francesco, Aneta Kisova, Ivana Spevakova, Andrea Raffaelli, Vincenzo Longo, and Luisa Pozzo. 2025. "Biological Properties and Phenolic Characterization of MetabolAid®: Combination of Plant-Derivate Compound Extracts" Processes 13, no. 8: 2405. https://doi.org/10.3390/pr13082405
APA StyleVizzarri, F., Kisova, A., Spevakova, I., Raffaelli, A., Longo, V., & Pozzo, L. (2025). Biological Properties and Phenolic Characterization of MetabolAid®: Combination of Plant-Derivate Compound Extracts. Processes, 13(8), 2405. https://doi.org/10.3390/pr13082405







